Abstract
Background
This study provides a systematic review and meta-analysis of randomized controlled trials, which examined the effect of the selenium supplementation on polycystic ovary syndrome (PCOS).
Methods
Confirmed studies related to selenium supplementation and PCOS were searched from the databases of EMBASE, PubMed and Web of Science. Data were reported as weighted mean difference (WMD) or standard mean difference (SMD) and associated 95% confidence intervals (CIs). Analysis was performed with Stata version 12.0.
Results
A total of 389 cases (selenium group n = 195, control group n = 194) were included in this studies. This meta-analysis showed that selenium supplementation has a positive effect on TAC, and supplementation of selenium does not significantly improve the level of BMI, Weight, LDL, HDL, Triglycerides, Total Testosterone, HOMA-IR, NO, GSH, MDA and FPG.
Conclusion
Although selenium can improve TAC in PCOS patients, it has no significant effect on BMI, Total Testosterone, et al. In terms of the results of this meta-analysis, it is not recommended for patients with PCOS to use selenium as a regular trace element supplement. Based on the improving effect of selenium on TAC, supplementation of selenium may have a positive effect on improving follicle quality for some PCOS patients who have poor follicle quality caused by oxidative stress or who want to undergo IVF.
Keywords: Selenium, Polycystic ovary syndrome, Meta-analysis
Background
POLYCYSTIC OVARY SYNDROME (PCOS) is a common gynecological endocrine disease, which affects 5–20% women of reproductive age worldwide [1–3]. Its harm is not limited to infertility and abnormal menstruation [4–6], but also brings economic burden and long-term health risks to patients [7, 8]. Studies have shown that patients with PCOS often have insulin resistance and abnormal lipid metabolism [9–11]. In addition, patients with PCOS have oxidative stress [12–14]. Excessive oxidative stress and depletion of antioxidants may contribute to ovarian mesenchymal hyperplasia [15]. This affects the quality of oocytes in patients with PCOS, and ultimately leads to undesirable pregnancy [16, 17].
Selenium is an indispensable trace element for the human body and it has antioxidant and anti-inflammatory properties [18, 19]. Selenium operates as an integral part of selenoproteins assisting redox processes as an effective antioxidant [20]. Studies have shown that selenium supplementation has a positive effect on improving reproductive outcomes and inflammation biomarkers [21, 22]. It was proven that supplementing selenium increases the gene expression levels of certain enzymes and may improve lipid metabolism [23].
Many studies have confirmed that selenium supplementation has beneficial effects on patients with PCOS. It is necessary to accurately judge the effect of selenium on PCOS patients. Therefore, all relevant studies were selected and this meta-analysis was done to further confirm the effect of selenium on patients with PCOS.
Methods
Search strategy
A comprehensive literature search was carried out to identify all potentially relevant articles using the PubMed, EMBASE and Web of Science database from their inception to December 2022. All search methods were based on a systematic approach in line with the Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P). And after "snowball search", all relevant literatures were included.
Searches for terms ("Selenium"[Mesh] OR "Selenium"[Text Word] OR “Selenium-80” OR “Selenium 80”) AND ("Polycystic Ovary Syndrome"[Mesh] OR "Polycystic Ovary Syndrome"[Text Word] OR (Ovary Syndrome, Polycystic) OR (Syndrome, Polycystic Ovary) OR “Stein-Leventhal Syndrome” OR “Stein Leventhal Syndrome” OR (Syndrome, Stein-Leventhal) OR “Sclerocystic Ovarian Degeneration” OR (Ovarian Degeneration, Sclerocystic) OR “Sclerocystic Ovary Syndrome” OR “Polycystic Ovarian Syndrome” OR (Ovarian Syndrome, Polycystic) OR “Polycystic Ovary Syndrome 1” OR “Sclerocystic Ovaries” OR (Ovary, Sclerocystic) OR “Sclerocystic Ovary”) were performed. The GRADE approach was followed and uncertainty assessment per-formed for studies included in meta-analysis.
Selection criteria
Two reviewers performed the literature search, evaluated potentially eligible studies for inclusion, and extracted the data independently. When required, disagreements were resolved by consultation with a third reviewer. Authors of the original studies were contacted for additional data, if necessary.
The main selection criteria are as follows:
Randomized controlled clinical trials, case–control studies. Patients took selenium or selenium combined with probiotics in the selenium group. Patients took placebo in the control group.
Study population: patients with PCOS and obesity. PCOS was diagnosed on the basis of the revised Rotterdam 2003 criteria [24]. The presence of 2 out of 3 criteria (oligo or/and anovulation, clinical or/and biochemical signs of hyperandro-genism, and polycystic ovary) was recommended as diagnostic of PCOS.
Aged 20–40 years
Studies that reporting weighted mean difference (WMD) or Standard mean difference (SMD) with corresponding 95% confidence intervals (95% CIs) or providing other ways to calculate or obtain these values.
Data extraction
Two researchers extracted data from the eligible studies independently, and resolved the divergence through discussion. The information collected included author, the year of publication, age of patient, sample size, treatment method, WMD (95% CI) or SMD (95% CI), and controlled variables for matching or used in some multivariable models. The data were entered into the Review Manager software (RevMan 5.3). The quality of selected studies was evaluated by Cochrane score according to the quality standards of the Cochrane scale. The disagreement was resolved through discussion between the two reviewers. If necessary, the disagreement was resolved by consultation with the third reviewer.
Data analysis
According to each study, 13 variables were extracted as mean ± standard deviation (SD). In this study, data were analyzed by Stata (version 12.0). P-values were two-sided, and P < 0.05 was considered the limit of statistical significance. In addition, the heterogeneity of these seven studies was assessed. In this meta-analysis, I2 was used to assess the heterogeneity between these included studies, and I2 ≥ 50 was set as significant heterogeneity. The random effects model was used for calculation. The WMD or SMD for continuous variables were used to explain outcomes with the 95% CI. For some data with significant heterogeneity, in-depth research was also conducted on subgroup (or regression) analysis and sensitivity analysis.
Results
Literature search and study characteristics
Figure 1 summarizes the research selection process. A total of 1098 unique references were retrieved through literature search, of which 708 were considered duplicate and irrelevant, following title and abstract screening, 17 were considered duplicate. 13 of these articles were excluded due to inappropriate article type. Of the remaining eight articles, one was excluded because of improper administration of medicine. Finally, in total, seven studies eligible for data extraction were included in the meta-analysis. Table 1 summarizes the characteristics of these studies. After "snowball search", no other literature was included.
Fig. 1.
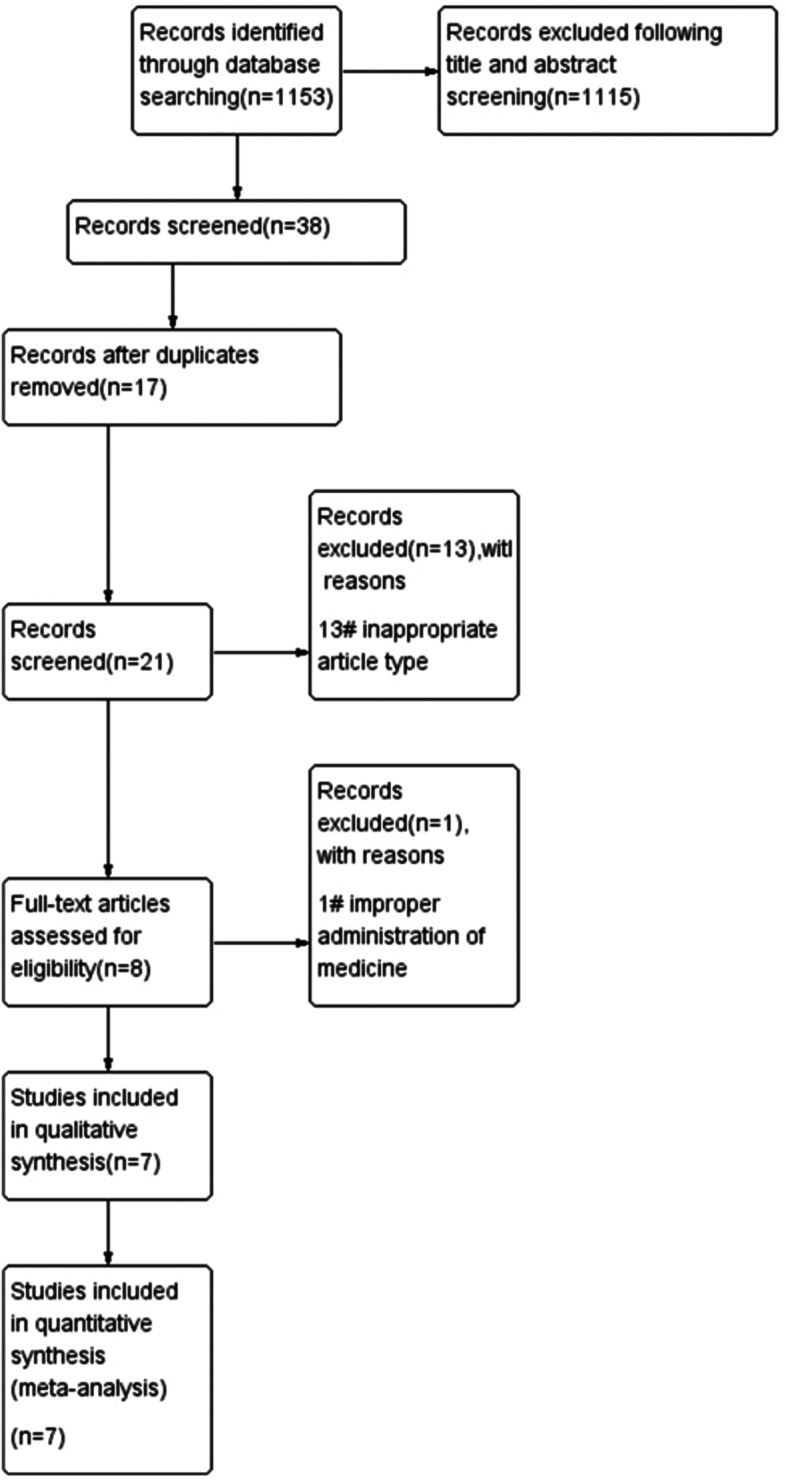
Flow Diagram of Study Selection
Table 1.
The characteristics of studies included in this meta-analysis
| Study identifier | Yr | Sample | Age(yr) | Intervention | Duration of intervention | BMI at study baseline (kg/m2) | Weight at study baseline (kg) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N(s) | N(c) | Age(s) | Age(c) | BMI(s) | BMI(c) | W(s) | W(c) | ||||
| Mehri Jamilian, et al. [25] | 2018 | 30 | 30 | 26.0 ± 5.3 | 25.6 ± 3.8 | intake 8 × 109 CFU/day probiotic plus 200 μg/day selenium supplements or placebo | 12 weeks | 24.6 ± 3.3 | 24.0 ± 3.0 | 63.9 ± 9.3 | 63.4 ± 7.7 |
| M. Razavi, et al. [26] | 2015 | 32 | 32 | 25.1 ± 4.5 | 25.4 ± 4.9 | Selenium group received 200 μg daily selenium tablet as selenium yeast and Placebo group received the placebo | 8 weeks | 24.7 ± 3.5 | 25.3 ± 4.3 | not available | not available |
| Fatemeh Mohammad Hosseinzadeh, et al. [27] | 2016 | 26 | 27 | 29.23 ± 4.9 | 28.90 ± 6.1 | received 200 µg selenium as a selenium-enriched yeast tablet or placebo | 12 weeks | 27.4 ± 4.5 | 28.39 ± 3.7 | 70.2 ± 13.8 | 72.6 ± 13.7 |
| Mehri Jamilian, et al. [28] | 2015 | 35 | 35 | 25.4 ± 5.1 | 25.7 ± 4.8 | receive 200 µg per day selenium supplements or placebo | 8 weeks | 25.0 ± 3.7 | 25.2 ± 4.1 | 66.7 ± 10.0 | 67.1 ± 11.0 |
| Shahrzad Zadeh Modarres, et al. [29] | 2017 | 20 | 20 | 31.1 ± 4.7 | 31.4 ± 3.6 | receive either 200-μg selenium as selenium yeast-free other supplements such as zinc and copper or placebo per day | 8 weeks | 26.5 ± 4.1 | 27.3 ± 2.6 | 69.8 ± 10.7 | 70.7 ± 7.1 |
| Zahra Heidar, et al. [30] | 2019 | 18 | 18 | 32.1 ± 4.7 | 32.6 ± 3.5 | intake either 200 μg/day selenium as selenium yeast (Nature Made, California, USA) or placebo (Barij Essence, Kashan, Iran) | 8 weeks | 27.2 ± 3.1 | 28.6 ± 2.5 | 74.5 ± 8.0 | 76.8 ± 6.5 |
| Batool Hossein Rashidi, et al. [31] | 2019 | 34 | 32 | 29.4 ± 5.3 | 28.6 ± 5.5 | receive either a daily dose of 200 μg selenium as a selenium-enriched yeast tablet or placebo | 12 weeks | 28.3 ± 5.2 | 29.5 ± 5.4 | 71.9 ± 14.3 | 74.6 ± 14.2 |
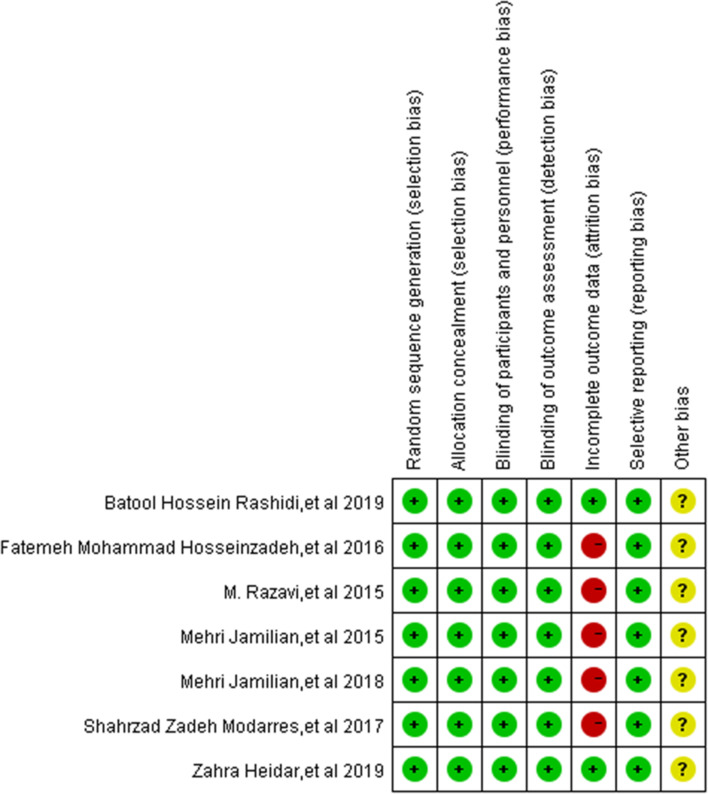
Risk of bias for all studies
For each randomized and prospective non-randomized clinical study which was selected, the risk of bias was assessed according to the criteria described in the Cochrane Reviewers Handbook [32]. Figure 2 shows the summary of the risk of bias for each included study.
Fig. 2.
Summary of risk for each included study + ,low risk of bias;?,unknown risk of bias;-,high risk of bias
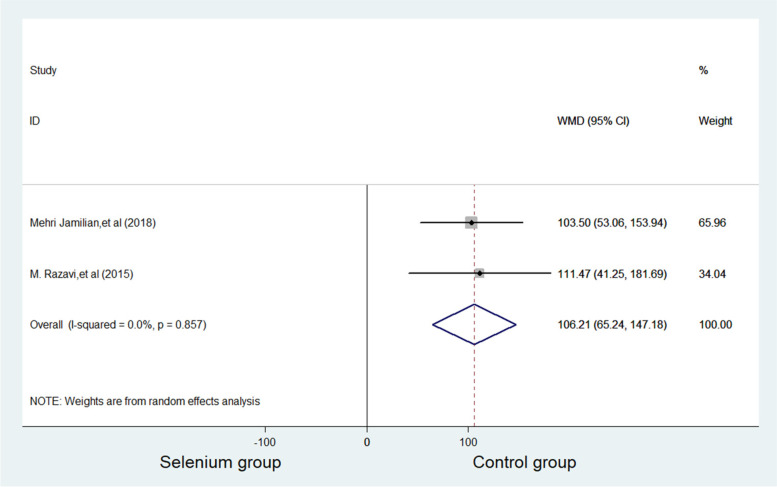
Effects on TAC
TAC was reported in two studies with 124 subjects, with 62 patients in the selenium group and 62 patients in control group. Compared with control group, the TAC level of selenium group increased significantly (WMD = 106.213 mmol/L, 95%CI 65.24 to 147.18, p ≤ 0.001). No significance was considered for heterogeneity. (p = 0.857, I2 = 0.0%) (Fig. 3).
Fig. 3.
Forest plots of selenium supplementation on TAC in patients with PCOS
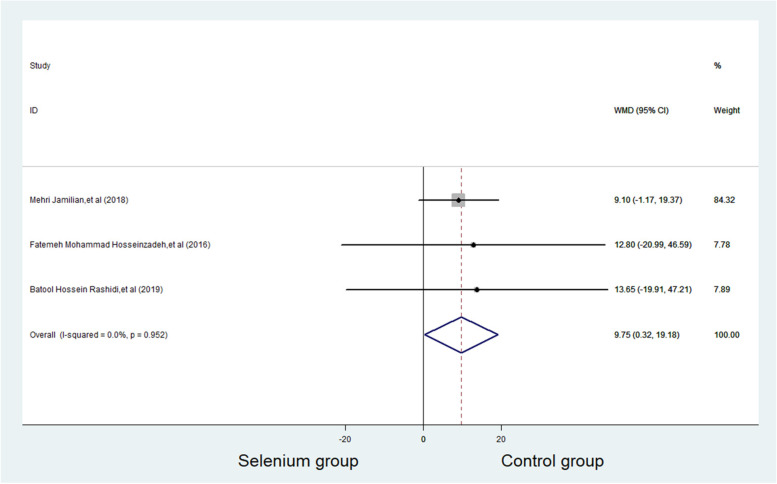
Effects on SHBG
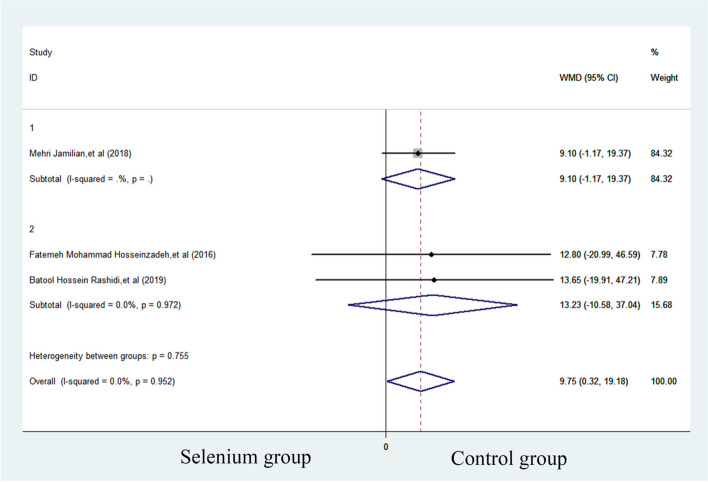
SHBG was reported in three studies with 179 subjects, with 90 patients in selenium group and 89 patients in control group. Compared with control group, the SHBG level of selenium group increased significantly (WMD = 9.747 nmol/L, 95%CI 0.32 to 19.18, p = 0.043). Heterogeneity was considered non-significant. (p = 0.952, I2 = 0.0%) (Fig. 4). In the subgroup analysis, no significant effects were observed in the subgroup of trials using selenium combined with probiotics supplements (WMD = 9.100, 95%CI -1.168 to 19.368 to 0.71) and those administrated with selenium as a single supplement (WMD = 13.228, 95% CI -10.583 to 37.039) (Fig. 5).
Fig. 4.
Forest plots of selenium supplementation on SHBG in patients with PCOS
Fig. 5.
Subgroup analysis of selenium supplementation on SHBG in patients with PCOS
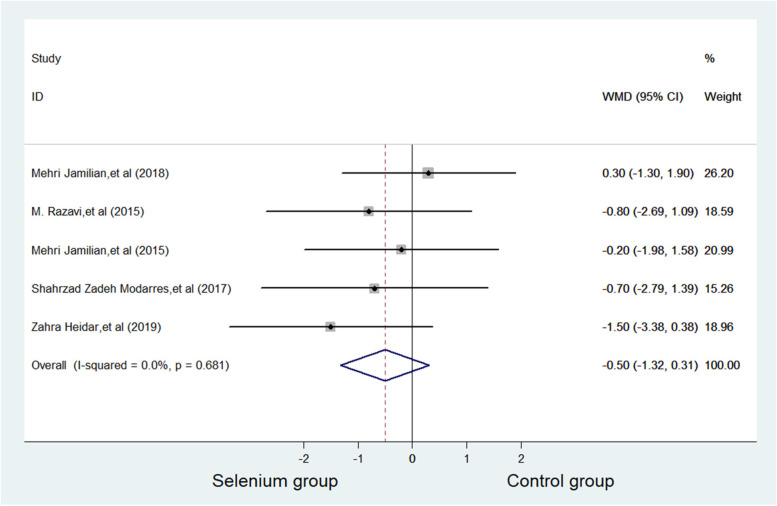
Effects on BMI
BMI was reported in five studies with 270 subjects, with 135 patients in selenium group and 135 patients in control group. The current meta-analysis showed that no difference in BMI was witnessed between selenium group and control group (WMD = -0.503 kg/m2, 95% CI -1.32 to 0.31, p = 0.227). No significance was considered in heterogeneity (p = 0.681, I2 = 0.0%) (Fig. 6).
Fig. 6.
Forest plots of selenium supplementation on BMI in patients with PCOS
Other outcomes
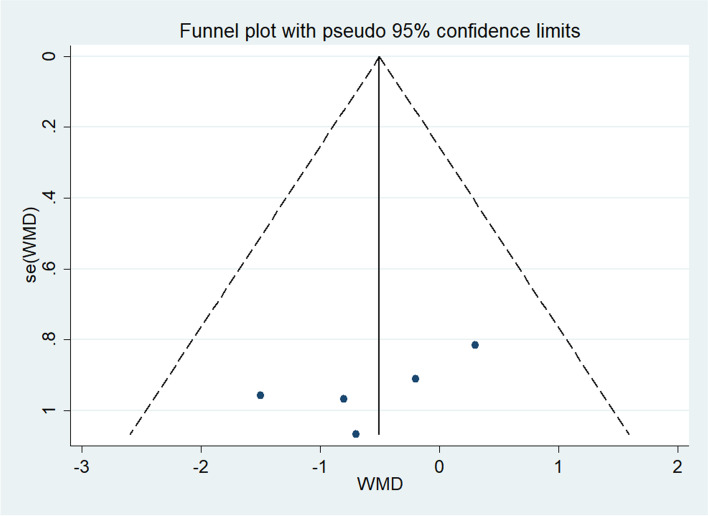
Table 2 shows a summary of the all the results of the meta-analysis, which includes the following: all outcomes: Total Testosterone, SHBG, HOMA-IR, BMI, Weight, NO, TAC, GSH, MDA, LDL, HDL, Triglycerides and FPG. Table 2 shows the results of publication bias. Figure 7 shows Begg's funnel plots estimating publication bias (Table 3).
Table 2.
Summary of meta-analysis outcomes
| Outcome | No. of studies | No. of participants | Type of meta-analysis | Effect estimate (95% CI) | P value | I2 (%) | Egger's test (P value) |
|---|---|---|---|---|---|---|---|
| Total Testosterone | 3 | 179 | WMD (fixed) | -0.14,0.11 | 0.767 | 31.8 | 0.991 |
| SHBG | 3 | 179 | WMD (fixed) | 0.32,19.18 | 0.043 | 0.0 | 0.075 |
| HOMA-IR | 3 | 189 | WMD (random) | -1.12,0.37 | 0.326 | 53.3 | 0.687 |
| BMI | 5 | 270 | WMD (fixed) | -1.32,0.31 | 0.227 | 0.0 | 0.182 |
| Weight | 4 | 206 | WMD (fixed) | -3.25,1.54 | 0.485 | 0.0 | 0.588 |
| NO | 2 | 124 | WMD (random) | -8.78,5.31 | 0.630 | 69.9 | \ |
| TAC | 2 | 124 | WMD (fixed) | 65.24,147.18 | ≤ 0.001 | 0.0 | \ |
| GSH | 2 | 124 | WMD (random) | -39.30,91.68 | 0.433 | 64.1 | \ |
| MDA | 2 | 124 | WMD (random) | -1.16,0.43 | 0.373 | 54.3 | \ |
| LDL | 2 | 136 | SMD (fixed) | -0.49,0.18 | 0.359 | 0.0 | \ |
| HDL | 2 | 136 | SMD (fixed) | -0.48,0.19 | 0.405 | 0.0 | \ |
| Triglycerides | 2 | 136 | SMD (random) | -0.84,0.62 | 0.769 | 78.6 | \ |
| FPG | 2 | 123 | SMD (random) | -1.38,0.90 | 0.678 | 89.7 | \ |
Values are mean ± SD; (s): selenium group (c): control group;
Fig. 7.
Funnel plots of publication bias. a Publication bias on TAC. b Publication bias of SHBG. c Publication bias of BMI
Table 3.
GRADE approach
| Study identifier | Type of Study | Factor of downgrade | Factor of escalation | Level of evidence | ||||
|---|---|---|---|---|---|---|---|---|
| Risk of bias | inconsistency | indirectness | imprecision | Publication bias | ||||
| Mehri Jamilian, et al. [25] | RCT | 0 | -1 | 0 | 0 | 0 | None | Medium |
| M. Razavi, et al. [26] | RCT | -1 | 0 | 0 | -1 | 0 | None | Low |
| Fatemeh Mohammad Hosseinzadeh, et al. [27] | RCT | -1 | 0 | 0 | 0 | 0 | None | Medium |
| Mehri Jamilian, et al. [28] | RCT | -1 | 0 | 0 | 0 | 0 | None | Medium |
| Shahrzad Zadeh Modarres, et al. [29] | RCT | -1 | 0 | 0 | 0 | 0 | None | Medium |
| Zahra Heidar, et al. [30] | RCT | -1 | 0 | 0 | -1 | 0 | None | Low |
| Batool Hossein Rashidi, et al. [31] | RCT | 0 | 0 | 0 | -1 | 0 | None | Medium |
0 Values are mean “not lower the level”, -1 Values are mean “lower one level”
Discussion
Selenium has a significant effect of increasing TAC in patients with PCOS. The results of the study were not heterogeneous, which affirmed the antioxidant effect of selenium. There was oxidative stress in patients with polycystic ovary [9–11]. Fenkci et al. studied the TAC levels of PCOS patients and compared them with a control group matched with age, body mass index, and smoking status. They proved that TAC levels in PCOS patients were significantly reduced [33]. Oxidative stress is a state characterized by an imbalance between pro-oxidant molecules including reactive oxygen and nitrogen species, and antioxidant defenses [34]. The increased level of reactive oxygen species (ROS) deteriorates oocyte quality by inducing apoptosis [35–39]. In light of the important role that oxidative stress plays in the aetiology of oocyte function [40], it is possible that the antioxidant effects of selenium have a therapeutic role in the context of both in vitro fertilization outcomes and controlling the impact of PCSO on fertility.
SHBG can be improved with selenium, but this conclusion contradicts many studies. According to the subgroup analysis, it is clear that the overall results of the combined studies are meaningful, but two independent subgroup studies have shown that selenium supplementation alone has no effect on SHBG, and the combination of selenium and probiotics has no effect on SHBG. The reason may be that dividing the subgroups will reduce the sample size in each group. In addition, the 95% CI shows that selenium and probiotics (-1.17, 19.37) may have a more positive effect on SHBG than selenium alone (-10.58, 37.04). And it has been proven that probiotics have a significant improvement effect on SHBG [25, 41, 42]. Therefore, it is inferred that this positive result should be caused by the addition of probiotics in subgroup 1. However, it is still not clear whether the combination of probiotics and selenium has a better effect than probiotics alone.
Some limitations should be considered when the results of this meta-analysis are examined. Significant heterogeneity was founded in eligible studies on HOMA-IR, NO, GSH, MDA, Triglycerides and FPG, which has a negative impact on the meaningful results of the current meta-analysis. And because these indicators involve a small number of studies, it is impossible to accurately determine the heterogeneity source. And, the protocol of this study has not been pre-registered in PROSPERO. In addition, publication bias may slightly bias our conclusions due to the insufficient number of included studies.
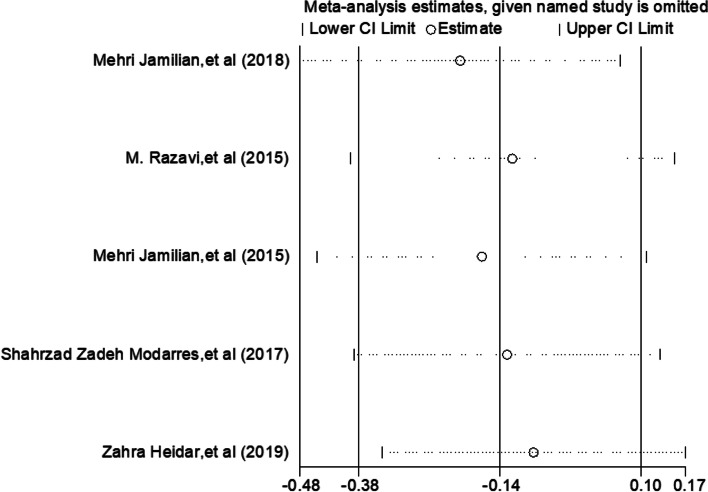
Despite these limitations, all existing studies on the treatment of PCOS with selenium are included, and the conclusions obtained are more valuable than a single study. And sensitivity analysis shows that the studies Mehri Jamilian, et al. [25–31] have significant sensitivity (Fig. 8) However, it is necessary to accurately determine the therapeutic effect of selenium on PCOS patients for the treatment of polycystic ovary. Therefore, more randomized controlled clinical trials of selenium in the treatment of PCOS are suggested to prove the effects of selenium on all aspects of PCOS patients.
Fig. 8.
Sensitivity analysis
Conclusion
Regardless of its positive effect on TAC, this meta-analysis shows that supplementation of selenium does not significantly improve the level of BMI, Weight, LDL, HDL, Triglycerides, Total Testosterone, HOMA-IR, NO, GSH, MDA and FPG. Therefore, in terms of the results of this meta-analysis, it is not recommended for patients with PCOS to use selenium as a regular trace element supplement. As for some PCOS patients who have poor follicle quality caused by oxidative stress or who want to undergo IVF, supplementation of selenium may have a positive effect on improving follicle quality.
Acknowledgements
None.
Abbreviations
- CI
Confidence interval
- PRISMA
Preferred Reporting Items Systematic Reviews and Meta-Analyses
- WMD
Weighted mean difference
- SMD
Standard mean difference
- RCT
Randomized controlled trial
- PubMed
National Library of Medicine
- EMBASE
Excerpta Medica Database
- PCOS
Polycystic ovary syndrome
- LDL
Low-density lipoprotein
- SHBG
Sex hormone binding globulin
- BMI
Body mass index
- HDL
High-density lipoprotein
- HOMA-IR
Homeostasis model of assessment-insulin resistance
- FPG
Fasting plasma glucose
- NO
Nitric oxide
- MDA
Malondialdehyde
- TAC
Total antioxidant capacity
- GSH
Total glutathione
Authors’ contributions
ZH L,LF D and XH S conceived and designed the study; ZH L, JD Z and Y W searched the related articles; ZH L,XH S,Y W and LJ L analyzed the data; ZH L,XH S, LF D and LJ L wrote the manuscript. TT L and JX L supervised the whole process. The author(s) read and approved the final manuscript.
Funding
None.
Availability of data and materials
All the data in this paper support the results of this study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Junde Zhao and Lingfen Dong contributed to the work equally and should be regarded as co-first authors.
Jinxing Liu and Tingting Liu contributed to the work equally and should be regarded as co-corresponding authors.
Contributor Information
Junde Zhao, Email: zhaojunde2020@163.com.
Tingting Liu, Email: 2879408812@qq.com.
Jinxing Liu, Email: Ljx276@sina.com.
References
- 1.Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057. [DOI] [PubMed]
- 2.Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–284. doi: 10.1038/nrendo.2018.24. [DOI] [PubMed] [Google Scholar]
- 3.Polak K, Czyzyk A, Simoncini T, Meczekalski B. New markers of insulin resistance in polycystic ovary syndrome. J Endocrinol Invest. 2017;40(1):1–8. doi: 10.1007/s40618-016-0523-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fauser BC. Reproductive endocrinology: revisiting ovulation induction in PCOS. Nat Rev Endocrinol. 2014;10(12):704–705. doi: 10.1038/nrendo.2014.156. [DOI] [PubMed] [Google Scholar]
- 5.Balen AH, Morley LC, Misso M, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. 2016;22(6):687–708. doi: 10.1093/humupd/dmw025. [DOI] [PubMed] [Google Scholar]
- 6.Skiba MA, Islam RM, Bell RJ, Davis SR. Understanding variation in prevalence estimates of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2018;24(6):694–709. doi: 10.1093/humupd/dmy022. [DOI] [PubMed] [Google Scholar]
- 7.Alhilali MJ, Parham A, Attaranzadeh A, Amirian M, Azizzadeh M. Polycystic ovary syndrome develops the complications of assisted reproductive technologies. Arch Razi Inst. 2022;77(4):1467–1472. doi: 10.22092/ARI.2022.358889.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brutocao C, Zaiem F, Alsawas M, Morrow AS, Murad MH, Javed A. Psychiatric disorders in women with polycystic ovary syndrome: a systematic review and meta-analysis. Endocrine. 2018;62(2):318–325. doi: 10.1007/s12020-018-1692-3. [DOI] [PubMed] [Google Scholar]
- 9.Luque-Ramírez M, Nattero-Chávez L, Ortiz Flores AE, Escobar-Morreale HF. Combined oral contraceptives and/or antiandrogens versus insulin sensitizers for polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2018;24(2):225–241. doi: 10.1093/humupd/dmx039. [DOI] [PubMed] [Google Scholar]
- 10.Pan JX, Tan YJ, Wang FF, et al. Aberrant expression and DNA methylation of lipid metabolism genes in PCOS: a new insight into its pathogenesis. Clin Epigenetics. 2018;10:6. doi: 10.1186/s13148-018-0442-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szczuko M, Hawryłkowicz V, Kikut J, Drozd A. The implications of vitamin content in the plasma in reference to the parameters of carbohydrate metabolism and hormone and lipid profiles in PCOS. J Steroid Biochem Mol Biol. 2020;198:105570. doi: 10.1016/j.jsbmb.2019.105570. [DOI] [PubMed] [Google Scholar]
- 12.Murri M, Luque-Ramírez M, Insenser M, Ojeda-Ojeda M, Escobar-Morreale HF. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Hum Reprod Update. 2013;19(3):268–288. doi: 10.1093/humupd/dms059. [DOI] [PubMed] [Google Scholar]
- 13.Barrea L, Marzullo P, Muscogiuri G, et al. Source and amount of carbohydrate in the diet and inflammation in women with polycystic ovary syndrome. Nutr Res Rev. 2018;31(2):291–301. doi: 10.1017/S0954422418000136. [DOI] [PubMed] [Google Scholar]
- 14.Lu J, Wang Z, Cao J, Chen Y, Dong Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2018;16(1):80. doi: 10.1186/s12958-018-0391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duleba AJ, Foyouzi N, Karaca M, Pehlivan T, Kwintkiewicz J, Behrman HR. Proliferation of ovarian theca-interstitial cells is modulated by antioxidants and oxidative stress. Hum Reprod. 2004;19(7):1519–1524. doi: 10.1093/humrep/deh299. [DOI] [PubMed] [Google Scholar]
- 16.Ascari IJ, Alves NG, Jasmin J, et al. Addition of insulin-like growth factor I to the maturation medium of bovine oocytes subjected to heat shock: effects on the production of reactive oxygen species, mitochondrial activity and oocyte competence. Domest Anim Endocrinol. 2017;60:50–60. doi: 10.1016/j.domaniend.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Youssef MA, Abdelmoty HI, Elashmwi HA, et al. Oral antioxidants supplementation for women with unexplained infertility undergoing ICSI/IVF: randomized controlled trial. Hum Fertil (Camb) 2015;18(1):38–42. doi: 10.3109/14647273.2014.927595. [DOI] [PubMed] [Google Scholar]
- 18.Wrobel JK, Power R, Toborek M. Biological activity of selenium: Revisited. IUBMB Life. 2016;68(2):97–105. doi: 10.1002/iub.1466. [DOI] [PubMed] [Google Scholar]
- 19.Wang N, Tan HY, Li S, Xu Y, Guo W, Feng Y. Supplementation of micronutrient selenium in metabolic diseases: its role as an antioxidant. Oxid Med Cell Longev. 2017;2017:7478523. doi: 10.1155/2017/7478523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kipp AP. Selenium and selenoproteins in redox signaling. Free Radical Biol Med. 2017;108:S1–S3. doi: 10.1016/j.freeradbiomed.2017.04.355. [DOI] [Google Scholar]
- 21.Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2012;16(7):705–743. doi: 10.1089/ars.2011.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duntas LH. Selenium and inflammation: underlying anti-inflammatory mechanisms. Horm Metab Res. 2009;41(6):443–447. doi: 10.1055/s-0029-1220724. [DOI] [PubMed] [Google Scholar]
- 23.Steinbrenner H. Interference of selenium and selenoproteins with the insulin-regulated carbohydrate and lipid metabolism. Free Radic Biol Med. 2013;65:1538–1547. doi: 10.1016/j.freeradbiomed.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 24.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 25.Jamilian M, Mansury S, Bahmani F, Heidar Z, Amirani E, Asemi Z. The effects of probiotic and selenium co-supplementation on parameters of mental health, hormonal profiles, and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. J Ovarian Res. 2018;11(1):80. doi: 10.1186/s13048-018-0457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Razavi M, Jamilian M, Kashan ZF, et al. Selenium Supplementation and the effects on reproductive outcomes, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome. Horm Metab Res. 2016;48(3):185–190. doi: 10.1055/s-0035-1559604. [DOI] [PubMed] [Google Scholar]
- 27.Mohammad Hosseinzadeh F, Hosseinzadeh-Attar MJ, Yekaninejad MS, Rashidi B. Effects of selenium supplementation on glucose homeostasis and free androgen index in women with polycystic ovary syndrome: a randomized, double blinded, placebo controlled clinical trial. J Trace Elem Med Biol. 2016;34:56–61. doi: 10.1016/j.jtemb.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Jamilian M, Razavi M, Fakhrie Kashan Z, Ghandi Y, Bagherian T, Asemi Z. Metabolic response to selenium supplementation in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol (Oxf) 2015;82(6):885–891. doi: 10.1111/cen.12699. [DOI] [PubMed] [Google Scholar]
- 29.Zadeh Modarres S, Heidar Z, Foroozanfard F, Rahmati Z, Aghadavod E, Asemi Z. The effects of selenium supplementation on gene expression related to insulin and lipid in infertile polycystic ovary syndrome women candidate for in vitro fertilization: a randomized, double-blind placebo-controlled trial. Biol Trace Elem Res. 2018;183(2):218–225. doi: 10.1007/s12011-017-1148-2. [DOI] [PubMed] [Google Scholar]
- 30.Heidar Z, Hamzepour N, Zadeh Modarres S, et al. The effects of selenium supplementation on clinical symptoms and gene expression related to inflammation and vascular endothelial growth factor in infertile women candidate for in vitro fertilization. Biol Trace Elem Res. 2020;193(2):319–325. doi: 10.1007/s12011-019-01715-5. [DOI] [PubMed] [Google Scholar]
- 31.Rashidi BH, Mohammad Hosseinzadeh F, Alipoor E, Asghari S, Yekaninejad MS, Hosseinzadeh-Attar MJ. Effects of selenium supplementation on asymmetric dimethylarginine and cardiometabolic risk factors in patients with polycystic ovary syndrome. Biol Trace Elem Res. 2020;196(2):430–437. doi: 10.1007/s12011-019-01954-6. [DOI] [PubMed] [Google Scholar]
- 32.Higgins J, Green SR. Cochrane Handbook for Systematic Review of InterventionsVersion 5.1.0. 2011.
- 33.Fenkci V, Fenkci S, Yilmazer M, Serteser M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil Steril. 2003;80(1):123–127. doi: 10.1016/S0015-0282(03)00571-5. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaube SK, Prasad PV, Thakur SC, Shrivastav TG. Hydrogen peroxide modulates meiotic cell cycle and induces morphological features characteristic of apoptosis in rat oocytes cultured in vitro. Apoptosis. 2005;10(4):863–874. doi: 10.1007/s10495-005-0367-8. [DOI] [PubMed] [Google Scholar]
- 37.Tamura H, Takasaki A, Miwa I, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44(3):280–287. doi: 10.1111/j.1600-079X.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 38.Revelli A, DellePiane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009;7:40. doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Combelles CM, Gupta S, Agarwal A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod Biomed Online. 2009;18(6):864–880. doi: 10.1016/S1472-6483(10)60038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aitken RJ. Impact of oxidative stress on male and female germ cells: implications for fertility. Reproduction. 2020;159(4):R189–R201. doi: 10.1530/REP-19-0452. [DOI] [PubMed] [Google Scholar]
- 41.Heshmati J, Farsi F, Yosaee S, et al. The effects of probiotics or synbiotics supplementation in women with polycystic ovarian syndrome: a systematic review and meta-analysis of randomized clinical trials. Probiotics Antimicrob Proteins. 2019;11(4):1236–1247. doi: 10.1007/s12602-018-9493-9. [DOI] [PubMed] [Google Scholar]
- 42.Shamasbi SG, Ghanbari-Homayi S, Mirghafourvand M. The effect of probiotics, prebiotics, and synbiotics on hormonal and inflammatory indices in women with polycystic ovary syndrome: a systematic review and meta-analysis. Eur J Nutr. 2020;59(2):433–450. doi: 10.1007/s00394-019-02033-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data in this paper support the results of this study.