Abstract
Objective:
We hypothesized that, among midlife women with vasomotor and/or genitourinary symptoms of menopause, (1) hormone therapy (HT) compared to complementary and alternative (CAM) will be associated with higher quality of life (QoL), and (2) race/ethnicity would modify associations of HT and CAM with QoL.
Methods:
Cross-sectional and longitudinal analyses of QoL in The Study of Women’s Health Across the Nation participants using HT, CAM or both. Women (n=2514) completed a CAM use questionnaire and QoL assessments at baseline and every 1-2 years from 2002 to 2013. Associations between QoL and treatment, adjusted for covariates, and race/ethnicity-by-treatment interactions, were analyzed using linear and mixed effects regression models.
Results:
During 7.8 (SD 2.9) years follow-up, 732 women (29%) reported HT 2.4 (SD 1.7) years and 798 women (32%) reported CAM use 2.1 (SD 1.4) years. Overall, neither HT nor CAM was associated with QoL. However, the treatment by race/ethnicity interaction was significant for Self-Reported QoL (p=0.034 at baseline, p=0.044 longitudinal). Among White women, Self-Reported QoL was higher in HT only users than in those who used neither (p=0.030, d=0.11, 95%CI [0.01-0.21]). In contrast, Black women using HT only had lower Self-Reported QoL compared to Black women using neither (p=0.027, d=−0.21, 95%CI [−0.40 - −0.02]).
Conclusion:
Comparisons between treatment type within each racial/ethnic group yielded significant differences in Self-Reported QoL. Clinicians should be aware of racial/ethnic differences in treatment preferences when counseling patients on menopausal symptoms treatment options in order to provide optimal care.
Keywords: complementary alternative medicine, integrative medicine, hormone therapy, vasomotor symptoms, vaginal dryness, quality of life
Brief summary of study:
Overall neither CAM nor HT use was associated with QoL measures. However, within racial/ethnic groups significant differences in SRQoL were found between the group using HT only compared with the group using neither HT nor CAM..
Introduction
Menopause is characterized by symptoms that can have a detrimental impact on a woman’s quality of life (QoL)1,2. The duration and severity of these symptoms and treatment preferences vary by individual and along racial/ethnic lines1,3. Whereas scientific evidence suggests that hormone therapy (HT) improves vasomotor symptoms (VMS; hot flashes and night sweats) and genitourinary syndrome of menopause (GSM; vulvovaginal dryness/itching/irritation, dyspareunia, dysuria, urgency and frequent urinary tract infections),4-6 similar benefits for complementary alternative medicine (CAM), also referred to as “Integrative Medicine”, treatment of menopausal symptoms have not been demonstrated7-9. Nevertheless, women seeking to ease the menopause transition spend millions of dollars each year on CAM10. Despite evidence that HT is a safe and effective treatment option, many women are reluctant to use HT, viewing CAM therapies as more congruent with good health11.
A prior Study of Women’s Health Across the Nation (SWAN) analysis found that HT use by women who reported frequent menopausal symptoms was associated with improvement in vitality, as measured by health-related quality of life (HRQoL)12. Women who took HT were more likely to be White, well educated, and of higher socioeconomic status. Similarly, studies analyzing CAM use within the SWAN cohort showed a higher uptake of CAM among women with higher levels of education and higher socioeconomic status13,14. Although studies have evaluated efficacy and safety of CAM use, the association with QoL has not been assessed5,8,12.
We explored the perceived benefit of HT and CAM use on QoL in symptomatic menopausal women participating in SWAN. Specifically, we examined 2 hypotheses. (1) Among women with VMS and/or GSM, higher QoL will be associated with HT when compared to CAM use. (2) Associations of HT and CAM with QoL will vary according to race/ethnicity.
Methods
Study Design and Participants:
SWAN is a multi-site, multi-racial/ethnic study of midlife women’s health during the menopausal transition. Participants were recruited at 7 sites across the United States between 1996 and 1997, and 3302 women, aged 42-52 years old, were enrolled. Each site enrolled a community-based sample of White women and women from a specified non-white racial/ethnic group: Boston, Chicago, Southeast Michigan, and Pittsburgh (Black), Los Angeles (Japanese), Newark, New Jersey (Hispanic), and Oakland, CA area (Chinese). Women who were eligible to participate in SWAN were 42-52 years, premenopausal or early perimenopausal, had an intact uterus and at least 1 ovary, had at least 1 menstrual period in the previous 3 months, and were not pregnant/lactating or using any sex steroid hormones in the 3 months preceding the baseline interview. A comprehensive summary of the SWAN study design has been published15.
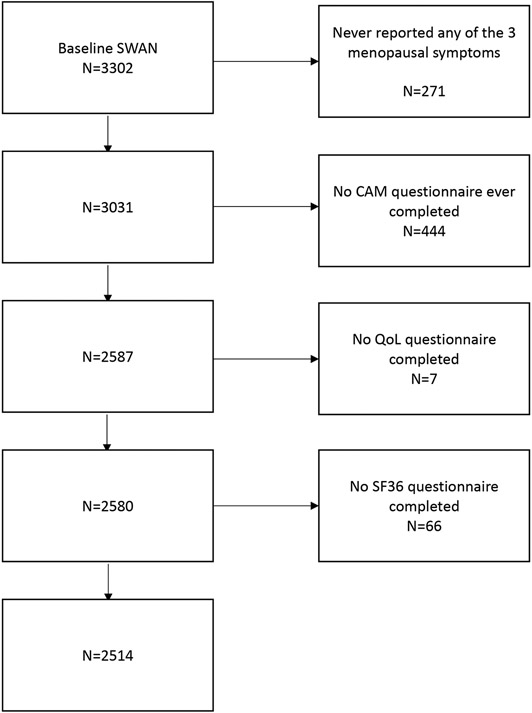
The sample for both the cross-sectional and longitudinal components for this analysis included all women who reported menopausal symptoms of VMS (hot flashes, night sweats) and/or GSM (vaginal dryness), and subsequently completed at least 1 CAM use questionnaire and at least 2 QoL assessments. Although, many organizations have updated the term ‘CAM’ to “Integrative Medicine’, we maintained the term ‘CAM’ for consistency, as SWAN questionnaires and prior studies used the terminology ‘CAM’. Women who never reported menopausal symptoms or did not complete the CAM questionnaire or QoL assessments were excluded. After exclusions, data from 2514 women were included in the analyses (Figure 1).
Figure 1: Sample selection.
Legend: SWAN (Study of Women’s Health Across the Nation), CAM (Complementary Alternative Medicine), QoL (Quality of Life), SF36 (Short Form–36)
The study was approved by the institutional review boards (IRB) at each SWAN study site and by the lead author’s institution, the University of Chicago. Written informed consent was obtained from all study participants at every study visit.
Procedures and Measures:
Baseline for the cross-sectional analysis was defined as the first visit that the comprehensive CAM questionnaire was completed, which typically occurred at visit 6, between 2002 and 2003. Follow-up assessments for the longitudinal analyses were conducted every 1-2 years from 2003 through 2013.
Symptoms and treatment type, along with medical history, demographics, and QoL were assessed with both self- and interviewer-administered surveys at baseline and at follow-up visits. Menopausal status was defined by bleeding criteria using a nine-level categorical variable: (1) bilateral salpingo-oophorectomy (BSO) not on HT, (2) BSO on HT, (3) post-menopausal not on HT, (4) post-menopausal on HT, (5) late peri-menopausal (had a menstrual cycle > 3 months ago, but < 12 months ago), (6) early peri-menopausal (menstrual period in the previous 3 months with change in regularity of menstrual cycles), (7) pre-menopausal (no change in regularity of menstrual cycles), (8) undetermined due to HT (pre- or perimenopausal at previous visit), (9) undetermined due to hysterectomy (pre- or perimenopausal, without BSO and not on HT)1,15.
Dependent variables were QoL assessments, the Short Form-36 version 1 (SF36) and a self-reported QoL measure. The SF-36 questionnaire was developed and validated for assessing HRQoL in the clinical and research setting16,17. The SF-36 includes 36 questions that are used to determine a mental component score (SF36 MCS) and a physical component score (SF36 PCS). The Self-Reported QoL (SRQoL) was measured with 1 question on a scale of 1-10 with 10 as the highest quality18-20.
Independent variables were CAM use for menopausal symptoms and HT. The CAM questionnaire asked women to “specify CAM modalities they used specifically to treat menopausal symptoms”, listed 21 modalities13, many of which are not traditionally accepted categories of CAM such as diet, exercise, self-help groups, prayer, and multivitamins. For this reason, these CAM modalities were excluded. The 16 CAM modalities included were acupuncture, black cohosh, dehydroepiandrosterone, dong quai, energy healing, flaxseed, ginko biloba, ginseng, glucosamine, Mexican yam/progesterone cream, methyl-sulfonyl-methane, S-adenosyl-L-methionine, soy supplements, St. John’s wort, tai chi, and yoga. Self-reported HT use included any estrogen and progestogen treatment alone or in combination.
Covariates assessed at baseline included race/ethnicity, age, education (high school or less versus more), which were not time-varying, and financial strain (very hard or somewhat hard to pay for basics versus not hard at all) and medical burden, which were time-varying21,22. Medical burden was defined by having 2 or more self-reported medical conditions (diabetes, cardiovascular disease, hypertension, arthritis, hypercholesterolemia, stroke, anemia, migraines, angina, osteoporosis). These specific medical conditions were selected as they may impact HT use4,6,23 and were included in SWAN questionnaires.
Data Analysis:
Continuous variables were summarized as mean (SD), and race/ethnic group comparisons were conducted with analysis of variance (ANOVA). Categorical variables were summarized as N (%) and compared with chi-square tests among race/ethnic groups. Treatment groups were defined as use of both HT and CAM concurrently, HT use only, CAM use only, or none. Baseline associations between treatment groups and QoL were examined with linear models. The base model included race/ethnicity and number of menopausal symptoms as covariates. The fully adjusted model added study site, baseline age, education, financial strain, and medical burden as covariates.
Longitudinal associations were examined with mixed models, with a random intercept to allow each woman her own starting point. The base model included race/ethnicity, number of menopausal symptoms (time-varying covariate), and time since baseline. The fully adjusted model added study site, baseline age, education, financial strain (time-varying), and medical burden (time-varying). To assess whether the effect of treatment varied by race/ethnicity, an interaction term of race/ethnicity and treatment group was included in both cross-sectional and longitudinal models. In case of an overall significant interaction (only true for SRQoL), this was followed by pairwise comparisons within each racial/ethnic group, and results of these comparisons were reported as estimates with 95% confidence intervals as well as treatment group contrasts that were significant at α=0.05.
The New Jersey site, which included the Hispanic cohort, missed visits 7 and 8, and at visit 9 they received an abbreviated CAM use questionnaire that did not include the reasons for CAM use. The results were unchanged when analyses were conducted without the Hispanic women. The results were also unchanged when analyses were conducted without all women from the New Jersey site, and the results of the comparisons among the White women were the same. Therefore, analyses were run using all participants, but Hispanic participants, due to their small numbers, were excluded from the racial/ethnic comparisons.
Results
Baseline characteristics are presented in Table 1. Demographic characteristics were similar across all racial/ethnic groups except Hispanic women, who were older, and reported the lowest education attainment and highest financial strain. Hispanic and Black women reported a high prevalence of medical conditions. Overall, 75% of women reported at least 1 menopausal symptom at baseline, with a preponderance of Black and Hispanic women reporting all 3 menopausal symptoms (hot flashes, night sweats, vaginal dryness). The other 25% had reported experiencing at least 1 menopausal symptom before baseline.
Table 1,
Baseline Characteristics, Overall and by Race/Ethnicity
| Total | Black | Chinese | Japanese | Hispanic | White | P-value | ||
|---|---|---|---|---|---|---|---|---|
| N (%) | 2514 (100) | 704 (28.0) | 214 (8.5) | 244 (9.7) | 136 (5.4) | 1216 (48.4) | ||
| Site, N (%) | ||||||||
| Southeast Michigan | 424 (16.4) | 256 (35.5) | 168 (13.5) | |||||
| Boston | 385 (14.9) | 166 (23.0) | 219 (17.6) | |||||
| Chicago | 343 (13.3) | 177 (24.5) | 166 (13.3) | |||||
| Oakland Area | 379 (14.7) | 214 (100) | 165 (13.2) | |||||
| Los Angeles | 440 (17.1) | 246 (100) | 194 (15.6) | |||||
| Newark | 233 (9.0) | 152 (100) | 81 (6.5) | |||||
| Pittsburgh | 376 (14.6) | 123 (17.0) | 253 (20.3) | |||||
| Age, mean (SD) | 53.3 (3.3) | 52.9 (3.1) | 52.9 (2.8) | 53.2 (2.8) | 56.5 (5.0) | 53.2 (3.3) | <0.001 | |
| Education, N (%) | ||||||||
| ≤high school | 548 (21.8) | 185 (26.3) | 61 (28.5) | 39 (16.0) | 89 (65.4) | 174 (14.3) | <0.001 | |
| Some college | 819 (32.6) | 287 (40.8) | 46 (21.5) | 89 (36.5) | 30 (22.1) | 367 (30.2) | ||
| College/grad degree | 1128 (44.9) | 233 (31.7) | 107 (50.0) | 116 (47.5) | 13 (9.6) | 669 (55.0) | ||
| missing | 19 (0.8) | 9 (1.3) | 0 (0.0) | 0 (0.0) | 4 (2.9) | 6 (0.5) | ||
| Financial straina, N (%) | 744 (29.6) | 284 (40.3) | 39 (18.2) | 66 (27.0) | 111 (81.6) | 244 (20.1) | <0.001 | |
| Medical burdenb | 1474 (58.6) | 518 (73.6) | 80 (37.4) | 92 (37.7) | 114 (83.8) | 670 (55.1) | <0.001 | |
| Menopausal status, N (%) | <0.001 | |||||||
| BSOc, no HTd use | 54 (2.1) | 27 (3.8) | 3 (1.4) | 1 (0.4) | 4 (2.9) | 19 (1.6) | ||
| BSO & HT use | 66 (2.6) | 19 (2.7) | 5 (2.3) | 7 (2.9) | 1 (0.7) | 34 (2.8) | ||
| Post menopause, no HT | 926 (36.8) | 277 (39.3) | 81 (37.9) | 84 (34.4) | 90 (66.2) | 394 (32.4) | ||
| Post menopause & HT use | 249 (9.9) | 49 (7.0) | 14 (6.5) | 25 (10.2) | 2 (1.5) | 159 (13.1) | ||
| Late Peri-menopause | 248 (9.9) | 72 (10.2) | 22 (10.3) | 26 (10.7) | 11 (8.1) | 117 (9.6) | ||
| Early Peri-menopause | 653 (26.0) | 187 (26.6) | 62 (29.0) | 75 (30.7) | 20 (14.7) | 309 (25.4) | ||
| Pre-menopause | 57 (2.3) | 18 (2.6) | 6 (2.8) | 5 (2.0) | 0 (0.0) | 28 (2.3) | ||
| Undetermined due to HT | 198 (7.9) | 23 (3.3) | 21 (9.8) | 17 (7.0) | 3 (2.2) | 134 (11.0) | ||
| Undetermined due to Hysterectomy | 63 (2.5) | 32 (4.5) | 0 (0.0) | 4 (1.6) | 5 (3.7) | 22 (1.8) | ||
| Number of Menopausal Symptomsc, N (%) | <0.001 | |||||||
| 0 | 639 (25.4) | 128 (18.2) | 60 (28.0) | 78 (32.0) | 34 (25.0) | 339 (27.9) | ||
| 1 | 775 (30.8) | 181 (25.7) | 84 (39.3) | 85 (34.8) | 48 (35.3) | 377 (3.0) | ||
| 2 | 694 (27.6) | 257 (36.5) | 43 (20.1) | 48 (19.7) | 26 (19.1) | 320 (26.3) | ||
| 3 | 406 (16.1) | 138 (19.6) | 27 (12.6) | 33 (13.5) | 28 (20.6) | 180 (14.8) | ||
| Night sweats, N (%) | <0.001 | |||||||
| None | 1387 (55.3) | 305 (43.4) | 148 (69.5) | 162 (66.4) | 78 (57.4) | 694 (57.3) | ||
| 1-5 days | 733 (29.2) | 263 (37.4) | 49 (23.0) | 57 (23.4) | 35 (25.7) | 329 (27.1) | ||
| 6-8 days | 140 (5.6) | 50 (7.1) | 2 (0.9) | 7 (2.9) | 10 (7.4) | 71 (5.9) | ||
| 9-13 days | 82 (3.3) | 21 (3.0) | 6 (2.8) | 4 (1.6) | 3 (2.2) | 48 (4.0) | ||
| Every day | 166 (6.6) | 64 (9.1) | 8 (3.8) | 14 (5.7) | 10 (7.4) | 70 (5.8) | ||
| Hot flashes, N (%) | <0.001 | |||||||
| None | 1130 (45.1) | 217 (30.9) | 110 (51.9) | 127 (52.0) | 87 (64.0) | 589 (48.6) | ||
| 1-5 days | 771 (30.8) | 261 (37.2) | 68 (32.1) | 67 (27.5) | 22 (16.2) | 353 (29.1) | ||
| 6-8 days | 190 (7.6) | 68 (9.7) | 17 (8.0) | 14 (5.7) | 10 (7.4) | 81 (6.7) | ||
| 9-13 days | 145 (5.8) | 50 (7.1) | 5 (2.4) | 16 (6.6) | 5 (3.7) | 69 (5.7) | ||
| Every day | 271 (10.8) | 106 (15.1) | 12 (5.7) | 20 (8.2) | 12 (8.8) | 121 (10.0) | ||
| Vaginal dryness, N (%) | <0.001 | |||||||
| None | 1619 (64.7) | 476 (67.8) | 125 (59.8) | 163 (66.8) | 59 (43.4) | 796 (65.7) | ||
| 1-5 days | 504 (20.1) | 147 (20.9) | 38 (18.2) | 50 (20.5) | 44 (32.4) | 225 (18.6) | ||
| 6-8 days | 120 (4.8) | 23 (3.3) | 15 (7.2) | 12 (4.9) | 15 (11.0) | 55 (4.5) | ||
| 9-13 days | 72 (2.9) | 19 (2.7) | 6 (2.9) | 3 (1.2) | 7 (5.1) | 37 (3.1) | ||
| Every day | 187 (7.5) | 37 (5.3) | 25 (12.0) | 16 (6.6) | 11 (8.1) | 98 (8.1) | ||
| CAM/HT use, N (%) | <0.001 | |||||||
| Neither | 1628 (64.8) | 501 (71.2) | 142 (66.4) | 149 (61.1) | 125 (91.9) | 711 (58.5) | ||
| CAM only | 361 (14.4) | 108 (15.3) | 32 (15.0) | 45 (18.4) | 5 (3.7) | 171 (14.1) | ||
| HT only | 422 (16.8) | 76 (10.8) | 30 (14.0) | 39 (16.0) | 5 (3.7) | 272 (22.4) | ||
| Both | 103 (4.1) | 19 (2.7) | 10 (4.7) | 11 (4.5) | 1 (0.7) | 62 (5.1) | ||
| Self-reported QoL, mean (SD) | 7.4 (1.7) | 7.3 (1.8) | 7.2 (1.5) | 7.4 (1.7) | 7.5 (2.0) | 7.6 (1.6) | <0.001 | |
| SF36 Physical Component, mean (SD) | 50.2 (9.4) | 47.7 (10.7) | 50.5 (7.7) | 52.2 (6.4) | 49.6 (9.3) | 51.3 (9.1) | <0.001 | |
| SF36 Mental Component, mean (SD) | 50.0 (10.0) | 50.0 (10.2) | 49.6 (10.0) | 51.0 (9.0) | 47.1 (10.5) | 50.2 (9.9) | 0.005 |
BSO: Bilateral Salpingo-oophorectomy; CAM: Complementary Alternative Medicine use for menopausal symptom relief; HT: Hormone Therapy; QoL: Quality of Life: SF36: Short Form-36.
Financial strain: table entry shows number (percent) endorsing ‘very hard’ or ‘somewhat hard’ to pay for basics versus ‘not hard at all.
Medical burden: 2 or more medical conditions. Medical conditions include: diabetes, cardiovascular disease, hypertension, arthritis, hypercholesterolemia, stroke, anemia, migraines, angina, osteoporosis
Number of symptoms reported in the previous 2 weeks
Table 1 shows that of the 2514 women included at baseline, 14% (n=361) of women reported only CAM use, and 17% (n=422) of women reported only HT use. Concurrent HT and CAM use was reported by 4% (n=103) of all women. White women reported the highest use of HT (alone or in combination with CAM) (27%), while Black (13%) and Hispanic (4%) women reported the lowest use.
Overall, soy supplements (n=257, 10.2%) and black cohosh (n=145, 5.8%) were the most commonly reported CAM treatments for menopausal symptoms across all racial/ethnic groups at baseline (Table 2). However, Chinese women reported higher use of Dong quai (n=12, 5.6%) and Tai Chi (n=6, 2.8%), while Japanese women reported the highest use of yoga (n=13, 5.3%) for menopausal symptom management.
Table 2:
Type of Complementary Alternative Medicine (CAM) used to treat menopausal symptoms overall and by race/ethnicity at baseline: N (%)
| Overall | Black | White | Chinese | Japanese | Hispanic | |
|---|---|---|---|---|---|---|
| N | 2514 | 704 | 1216 | 214 | 244 | 136 |
| Any CAM for menopausal Symptoms | 464 (18.5) | 127 (18.0) | 233 (19.2) | 42 (19.6) | 56 (23.0) | 6 (4.4) |
| Soy supplements | 257 (10.2) | 73 (10.4) | 123 (10.1) | 26 (12.1) | 32 (13.1) | 3 (2.2) |
| Black Cohosh | 145 (5.8) | 54 (7.7) | 77 (6.3) | 3 (1.4) | 10 (4.1) | 1 (0.7) |
| Mexican Yam/Progesterone Cream | 60 (2.4) | 14 (2.0) | 35 (2.9) | 2 (0.9) | 8 (3.3) | 1 (0.7) |
| Flaxseed | 58 (2.3) | 17 (2.4) | 33 (2.7) | 4 (1.9) | 4 (1.6) | 0 (0.0) |
| Yoga | 58 (2.3) | 12 (1.7) | 30 (2.5) | 2 (0.9) | 13 (5.3) | 1 (0.7) |
| Dong Quai | 40 (1.6) | 13 (1.8) | 9 (0.7) | 12 (5.6) | 5 (2.0) | 1 (0.7) |
| Energy Healing | 26 (1.0) | 9 (1.3) | 12 (1.0) | 3 (1.4) | 2 (0.8) | 0 (0.0) |
| Glucosamine | 14 (0.6) | 3 (0.4) | 7 (0.6) | 1 (0.5) | 3 (1.2) | 0 (0.0) |
| Acupuncture | 13 (0.5) | 2 (0.3) | 8 (0.7) | 1 (0.5) | 2 (0.8) | 0 (0.0) |
| Ginseng | 12 (0.5) | 3 (0.4) | 5 (0.4) | 3 (1.4) | 1 (0.4) | 0 (0.0) |
| Tai Chi | 11 (0.4) | 3 (0.4) | 1 (0.1) | 6 (2.8) | 1 (0.4) | 0 (0.0) |
| St. John's Wort | 10 (0.4) | 1 (0.1) | 8 (0.7) | 0 (0.0) | 1 (0.4) | 0 (0.0) |
| Dehydroepiandrosterone | 8 (0.3) | 1 (0.1) | 7 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gingko Biloba | 8 (0.3) | 1 (0.1) | 4 (0.3) | 2 (0.9) | 1 (0.4) | 0 (0.0) |
| S-adenosyl-L-methionine | 4 (0.2) | 1 (0.1) | 3 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Methyl-sulfonyl-methane | 3 (0.1) | 3 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Baseline quality of life:
Neither CAM nor HT, used alone or in combination, was significantly associated with the SF36 PCS or MCS or the SRQoL (p>0.05 for all analyses). White women reported the highest SRQoL, but there was no difference in SRQoL among treatment groups. The interaction of treatment group and race/ethnicity was significant at baseline only for SRQoL(p=0.034). Comparisons within each racial/ethnic group at baseline showed that Chinese women who used HT only had lower SRQoL compared to Chinese women who reported use of neither (p=0.002, d=−1.0, 95%CI [−1.6 - −0.4]). SRQoL did not differ significantly across treatment groups within Black, White, or Japanese women (Table 3). The sample size for the analyses included in Table 3 is 2378 because the Hispanic women were excluded from the comparisons.
TABLE 3:
Relationship of treatment of menopausal symptoms and self-reported quality of life (SRQoL score, possible range 1-10 with higher values = better QoL) within racial/ethnic groups: model-based estimates and significant contrasts with 95% confidence intervals
| Baseline Analyses (N=2378) | |||||
|---|---|---|---|---|---|
| Neither | CAM only | HT only | Both | Contrast, p | |
| Black | 7.5 (7.3 - 7.7) N=501 |
7.6 (7.3 - 8.0) N=108 |
7.2 (6.8 - 7.6) N=76 |
7.1 (6.4 - 7.9) N=19 |
|
| Chinese | 7.3 (7.0 - 7.7) N=142 |
7.0 (6.4 - 7.6) N=32 |
6.3 (5.7 - 7.0) N=30 |
6.7 (5.7 - 7.7) N=10 |
HT only<Neither −1.0 (−1.6 - −0.4), p=0.002 |
| Japanese | 7.3 (6.9 −7.6) N=149 |
7.4 (6.9 - 7.9) N=45 |
7.6 (7.0 - 8.1) N=39 |
7.1 (6.1 - 8.1) N=11 |
|
| White | 7.5 (7.3 - 7.7) N=711 |
7.7 (7.4 - 8.0) N=171 |
7.6 (7.3 - 7.9) N=272 |
7.6 (7.1 - 8.0) N=62 |
|
| Longitudinal Analyses (total number of observations = 13223) | |||||
| Neither | CAM only | HT only | Both | Contrast, p | |
| Black | 7.6 (7.4 - 7.7) n=3100 |
7.6 (7.3 - 7.8) n=419 |
7.4 (7.1 - 7.6) n=242 |
7.2 (6.8 - 7.6) n=43 |
HT only <Neither −0.21 (−0.40 - −0.02), 0.027 |
| Chinese | 7.3 (7.1 - 7.6) n=1026 |
7.3 (7.0 - 7.7) n=99 |
7.1 (6.7 - 7.4) n=111 |
6.9 (6.2 - 7.6) n=12 |
|
| Japanese | 7.4 (7.2 - 7.6) n=1070 |
7.3 (7.1 - 7.6) n=206 |
7.4 (7.1 - 7.7) n=158 |
7.1 (6.6 - 7.7) n=29 |
|
| White | 7.6 (7.4 - 7.7) n=4851 |
7.6 (7.5 - 7.8) n=694 |
7.7 (7.5 - 7.9) n=982 |
7.6 (7.4 - 7.9) n=181 |
HT only >Neither 0.11 (0.01 - 0.21), 0.030 |
CAM: Complementary Alternative Medicine use for menopausal symptom relief; HT: Hormone Therapy.
N of women = n of observations
Analyses adjusted for study site, baseline age, education, financial strain and medical burden.
Longitudinal quality of life:
Women were followed for an average of 7.8 (SD2.9) years. CAM use was reported by 798 women (32%) for a mean (SD) duration of 2.1 (SD 1.4) years, and HT use was reported by 732 women (29%) for 2.4 (SD 1.7) years. Overall, none of the 3 QoL measures differed by treatment group in unadjusted or adjusted models. However, similar to baseline, the interaction of treatment group and race/ethnicity was significant for SRQoL (p=0.044). Among White women who used HT, SRQoL was higher than in those who reported use of neither HT nor CAM (p=0.030, d=0.11, 95%CI [0.01 - 0.21]). In contrast, Black women who reported use of HT only had lower SRQoL compared to Black women who used neither (p=0.027, d=−0.21, 95%CI [−0.40 - −0.02]).
Discussion
In this analysis of SWAN participants reporting HT and/or CAM use for menopausal symptoms of VMS and/or GSM, White women had higher SRQoL than the other racial/ethnic groups. CAM was used more than HT to treat menopausal symptoms by all racial/ethnic groups except White women, who used HT more than CAM. Despite more frequent use of CAM, it was not associated with significantly higher QoL on any of the 3 measures. However, when investigating these associations within individual race/ethnic groups, there were some differences, particularly for HT. Longitudinally, among White women, SRQoL was higher in the group that used HT than in the group that reported using neither CAM nor HT. The findings were just the opposite for Chinese and Black women. HT use was associated with lower reported SRQoL at baseline in Chinese women and in Black women HT use was associated with lower SRQoL in the longitudinal analysis, both in comparison to no therapy.
Overall use of HT was low throughout the study period. Baseline analysis began at visit 6, which occurred just after the publication of the Women’s Health Initiative findings in 2002, showing increased risks of breast cancer and cardiovascular disease with HT4. These findings may have negatively impacted prescribing patterns and patient acceptance and could explain why overall HT use was low and CAM use was higher in this cohort24-26. Use of HT has been shown to improve bothersome VMS and GSM, and has been aligned with improved QoL4,6,23,27. The 2022 NAMS HT position statement specifically addresses the benefit of HT on menopause-specific quality of life, citing reduction in frequency of symptoms with HT use as the potential reason for improved QoL23.
Black women are more likely to report having more bothersome VMS and have been shown to experience symptoms for a longer duration of time compared to other racial/ethnic groups3,28-30. However, while Black women report more menopausal symptoms, which are more troubling and of longer duration, several studies have shown that use of HT is low, especially compared to White women12,31,32. Discrepancies in clinician prescribing patterns and counseling around HT has been cited as factors, with some studies showing that White women were more than twice as likely to receive a prescription for HT33,34. Many have postulated that the differences in HT prescribing is due to higher associated risks and/or contraindications in Black women34,35, while others cite their decreased acceptance of HT or disparate access to healthcare33,36,37. Given that Black and Hispanic women, reported more medical conditions, it is plausible that lower use of HT in these women in our sample was related to higher associated risks. However, due to their small numbers, Hispanic women were excluded from race/ethnic comparisons.
Higher use of CAM in most racial/ethnic groups within the SWAN cohort is consistent with other studies showing increased popularity of CAM despite the lack of proven efficacy, safety and cost effectiveness11. It is probable that the use of CAM in SWAN is even higher than what was reported. Investigators at the New Jersey site suspected use of herbal remedies was higher than what was captured on the CAM use questionnaire. At visit 13 (2011-2013), the New Jersey cohort, consisting of Hispanic and non-Hispanic white women, completed an additional 48-item survey inquiring about specific herbal remedies, attitudes and beliefs around use, and whether use was disclosed to clinicians38. They found Hispanic women had much higher use of herbal remedies than what was previously reported on the CAM questionnaire and, while both groups were less likely to disclose use of CAM to clinicians, lack of disclosure was much higher among Hispanics. Notably, use of multiple herbal remedies was high even in those taking prescription medications.
In the North American Menopause Society’s (NAMS) 2015 position statement on non-hormonal VMS treatment options, most CAM modalities were not recommended for treatment of VMS as they found benefit was indistinguishable from placebo7. A systematic review evaluating prevalence of CAM use by menopausal women worldwide found that over 50% of women reported CAM use to treat their menopausal symptoms11. Of concern, many did not disclose use of CAM to their clinician and cited the internet as their primary source for knowledge. The widespread use of these unregulated products, especially in certain high risk populations, raises safety concerns regarding drug interactions, toxicity, contamination, and the potential for other life threatening adverse events39.
Strengths of the SWAN database include a large, diverse, well-characterized, cohort of midlife women and a wealth of longitudinal data that allowed an average of 7.8 years of longitudinal follow-up of QoL outcomes related to menopausal symptom treatment. However, there were limitations in our analysis. For one, data were available only for the occurrence of menopausal symptoms, but not for their severity or the degree of burden caused. Moreover, SWAN assessments were conducted only every 1-2 years, which could compromise recall and determination of continuous patterns of use. As previously mentioned, the number of SWAN participants reporting HT use was small, especially within certain racial/ethnic groups. This limited interpretation of data and made it difficult to investigate differences in some groups. Due to small numbers, we only present overall average effects of treatment on SRQoL in each racial/ethnic group, not the change in treatment. A much larger sample in the treatment groups would be necessary to detect effects of change. Lastly, SWAN participants tended to have higher educational attainment and were followed for several years, showing consistency and their dedication to the research process. They may not be representative of the general population.
Acceptance of CAM by menopausal women illustrates a desire for safe alternative treatments to improve overall health and well-being and alleviate menopausal symptoms 10,11. Clinicians must help patients differentiate between healthy lifestyle practices and treatments known to be ineffective and possibly harmful, including education about the benefits and risks of HT and CAM to help them make informed menopause care choices. Our findings also highlight the complex relationship between racial/ethnic differences in treatment preference and QoL. Clinicians may find these data useful when counseling patients on menopausal treatment options.
Conclusion
Consistent with other reported studies, we found racial/ethnic differences in use of HT and found overall higher use of CAM modalities to treat menopausal symptoms. Overall neither CAM nor HT use was associated with QoL measures; however, within each racial/ethnic group, there were differences in SRQoL in relation to the specific menopausal treatment used. Whereas in White women use of HT seems to be positively related to SRQoL, Black and Hispanic women reported more frequent menopausal symptoms, but also had the lowest use of HT. Understanding racial/ethnic differences in menopause treatment preferences and how it impacts overall well-being is integral to optimal care of midlife women.
Acknowledgments:
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495 and U19AG063720). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor – Carrie Karvonen-Gutierrez, PI 2021 – present, Siobán Harlow, PI 2011 – 2021, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA – Sherri-Ann Burnett-Bowie, PI 2020 – Present; Joel Finkelstein, PI 1999 – 2020; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Imke Janssen, PI 2020 – Present; Howard Kravitz, PI 2009 – 2020; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Elaine Waetjen and Monique Hedderson, PIs 2020 – Present; Ellen Gold, PI 1994 - 2020; University of California, Los Angeles – Arun Karlamangla, PI 2020 – Present; Gail Greendale, PI 1994 - 2020; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Rebecca Thurston, PI 2020 – Present; Karen Matthews, PI 1994 - 2020.
NIH Program Office: National Institute on Aging, Bethesda, MD – Rosaly Correa-de-Araujo 2020 – present Chhanda Dutta 2016-present; Winifred Rossi 2012–2016; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair, Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
We also thank Kristen Wroblewski MS, Gail Isenberg and Ernst Lengyel M.D. for critical review of the manuscript.
Financial Support:
Faculty Diversity Career Advancement Grant awarded to Dr. Monica Christmas, and internal funds from the Obstetrics & Gynecology Department University of Chicago.
Footnotes
Conflict of Interests:
Dr. Janssen, Dr. Kravitz and Dr. Upchurch each reports no relevant financial disclosures. Dr. Christmas reports Board of Trustees for the North American Menopause Society (NAMS), NAMS Scientific Program Committee, NAMS Editorial Board, Steering Committee for Core OutcoMes in MenopAuse (COMMA), Speaker Alliance Chicago and FDA Bone, Reproductive and Urologic Division (BRUDAC) Committee. Dr. Joffe receives grant support from the National Institutes of Health, Merck and Pfizer. She serves/has served as a consultant to Bayer, Jazz and Eisai. Her spouse is an employee at Arsenal Biosciences and has equity in Merck. Dr. Santoro reports Advisory Board Member for Astellas, Que Oncology, amazon (Ember), Consultant for Ansh Labs, NAMS Program Committee, Past President Society for Reproductive Investigation, and Nominating Committee Endocrine Society.
Presentations at Meetings:
Oral Presenter at 16 World Congress on Menopause. Race/Ethnic Differences in Treatment of Menopausal Symptoms and Perceived Quality of Life in the Study of Women’s Health Across the Nation (SWAN), June 6, 2018, Vancouver Canada.
Abstract Poster North American Menopause Society (NAMS) Annual Meeting. Race/Ethnic Variation in Treatment of Menopausal Symptoms: A Longitudinal Analysis of the Initiation, Duration, and Quality of Life in the Study of Women’s Health Across the Nation (SWAN), October 4, 2018, San Diego, CA.
References
- 1.El Khoudary SR et al. The menopause transition and women's health at midlife: a progress report from the Study of Women's Health Across the Nation (SWAN). Menopause 26, 1213–1227, doi: 10.1097/GME.0000000000001424 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell CM & Waetjen LE Genitourinary Changes with Aging. Obstetrics and gynecology clinics of North America 45, 737–750, doi: 10.1016/j.ogc.2018.07.010 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Avis NE et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA internal medicine 175, 531–539, doi: 10.1001/jamainternmed.2014.8063 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lobo RA Hormone-replacement therapy: current thinking. Nat Rev Endocrinol 13, 220–231, doi: 10.1038/nrendo.2016.164 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Kaunitz AM & Manson JE Management of Menopausal Symptoms. Obstet Gynecol 126, 859–876, doi: 10.1097/AOG.0000000000001058 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The, N. H. T. P. S. A. P. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 24, 728–753, doi: 10.1097/GME.0000000000000921 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Carpenter J et al. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause 22, 1155–1172; quiz 1173-1154, doi: 10.1097/GME.0000000000000546 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Moore TR, Franks RB & Fox C Review of Efficacy of Complementary and Alternative Medicine Treatments for Menopausal Symptoms. J Midwifery Womens Health 62, 286–297, doi: 10.1111/jmwh.12628 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Nedrow A et al. Complementary and alternative therapies for the management of menopause-related symptoms: a systematic evidence review. Arch Intern Med 166, 1453–1465, doi: 10.1001/archinte.166.14.1453 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Ventola CL Current Issues Regarding Complementary and Alternative Medicine (CAM) in the United States: Part 1: The Widespread Use of CAM and the Need for Better-Informed Health Care Professionals to Provide Patient Counseling. P T 35, 461–468 (2010). [PMC free article] [PubMed] [Google Scholar]
- 11.Posadzki P et al. Prevalence of complementary and alternative medicine (CAM) use by menopausal women: a systematic review of surveys. Maturitas 75, 34–43, doi: 10.1016/j.maturitas.2013.02.005 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Hess R et al. The impact of hormone therapy on health-related quality of life: longitudinal results from the Study of Women's Health Across the Nation. Menopause 15, 422–428, doi: 10.1097/gme.0b013e31814faf2b (2008). [DOI] [PubMed] [Google Scholar]
- 13.Gold EB et al. Cross-sectional analysis of specific complementary and alternative medicine (CAM) use by racial/ethnic group and menopausal status: the Study of Women's Health Across the Nation (SWAN). Menopause 14, 612–623, doi: 10.1097/gme.0b013e31802d975f (2007). [DOI] [PubMed] [Google Scholar]
- 14.Bair YA et al. Use of complementary and alternative medicine during the menopause transition: longitudinal results from the Study of Women's Health Across the Nation. Menopause 15, 32–43, doi: 10.1097/gme.0b013e31813429d6 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Sowers M et al. in Menopause: Biology and Pathobiology (eds Lobo RA, Kelsey J, & Marcus R) 175–188 (Academic Press, 2000). [Google Scholar]
- 16.McHorney CA, Ware JE Jr. & Raczek AE The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 31, 247–263, doi: 10.1097/00005650-199303000-00006 (1993). [DOI] [PubMed] [Google Scholar]
- 17.Mishra GD, Hockey R & Dobson AJ A comparison of SF-36 summary measures of physical and mental health for women across the life course. Qual Life Res 23, 1515–1521, doi: 10.1007/s11136-013-0586-3 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Cantril H The pattern of human concerns. (Rutgers University Press, 1965). [Google Scholar]
- 19.Bankowski BJ et al. The association between menopausal symptoms and quality of life in midlife women. Fertil Steril 86, 1006–1008, doi: 10.1016/j.fertnstert.2006.03.031 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Bowling A Measuring Health: a review of quality of life measurement scales. Third edition. (Open University Press, 2004). [Google Scholar]
- 21.Green R & Santoro N Menopausal symptoms and ethnicity: the Study of Women's Health Across the Nation. Womens Health (Lond) 5, 127–133, doi: 10.2217/17455057.5.2.127 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall MH et al. Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. Sleep 32, 73–82 (2009). [PMC free article] [PubMed] [Google Scholar]
- 23.The 2022 hormone therapy position statement of The North American Menopause Society. Menopause 29, 767–794, doi: 10.1097/GME.0000000000002028 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Ettinger B et al. Evolution of postmenopausal hormone therapy between 2002 and 2009. Menopause 25, 1306–1312, doi: 10.1097/GME.0000000000001233 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Barbaglia G et al. Trends in hormone therapy use before and after publication of the Women's Health Initiative trial: 10 years of follow-up. Menopause 16, 1061–1064, doi: 10.1097/gme.0b013e3181a02b44 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Crawford SL et al. Menopausal hormone therapy trends before versus after 2002: impact of the Women's Health Initiative Study Results. Menopause 26, 588–597, doi: 10.1097/GME.0000000000001282 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Utian WH & Woods NF Impact of hormone therapy on quality of life after menopause. Menopause 20, 1098–1105, doi: 10.1097/GME.0b013e318298debe (2013). [DOI] [PubMed] [Google Scholar]
- 28.Thurston RC et al. Beyond frequency: who is most bothered by vasomotor symptoms? Menopause 15, 841–847, doi: 10.1097/gme.0b013e318168f09b (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freeman EW, Sammel MD, Lin H, Liu Z & Gracia CR Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol 117, 1095–1104, doi: 10.1097/AOG.0b013e318214f0de (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richard-Davis G & Wellons M Racial and ethnic differences in the physiology and clinical symptoms of menopause. Seminars in reproductive medicine 31, 380–386, doi: 10.1055/s-0033-1348897 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Solomon DH et al. Medication Use by Race and Ethnicity in Women Transitioning Through the Menopause: A Study of Women's Health Across the Nation Drug Epidemiology Study. J Womens Health (Larchmt) 25, 599–605, doi: 10.1089/jwh.2015.5338 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thurston RC & Joffe H Vasomotor symptoms and menopause: findings from the Study of Women's Health across the Nation. Obstetrics and gynecology clinics of North America 38, 489–501, doi: 10.1016/j.ogc.2011.05.006 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weng HH et al. Racial differences in physician recommendation of hormone replacement therapy. Prev Med 33, 668–673, doi: 10.1006/pmed.2001.0943 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Marsh JV, Brett KM & Miller LC Racial differences in hormone replacement therapy prescriptions. Obstet Gynecol 93, 999–1003, doi: 10.1016/s0029-7844(98)00540-7 (1999). [DOI] [PubMed] [Google Scholar]
- 35.Friedman-Koss D, Crespo CJ, Bellantoni MF & Andersen RE The relationship of race/ethnicity and social class to hormone replacement therapy: results from the Third National Health and Nutrition Examination Survey 1988-1994. Menopause 9, 264–272, doi: 10.1097/00042192-200207000-00007 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Shelton AJ, Lees E & Groff JY Perceptions of hormone replacement therapy among African American women. J Health Care Poor Underserved 13, 347–359, doi: 10.1353/hpu.2010.0678 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Rice VM Strategies and issues for managing menopause-related symptoms in diverse populations: ethnic and racial diversity. Am J Med 118 Suppl 12B, 142–147, doi: 10.1016/j.amjmed.2005.09.048 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Green RR, Santoro N, Allshouse AA, Neal-Perry G & Derby C Prevalence of Complementary and Alternative Medicine and Herbal Remedy Use in Hispanic and Non-Hispanic White Women: Results from the Study of Women's Health Across the Nation. J Altern Complement Med 23, 805–811, doi: 10.1089/acm.2017.0080 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ventola CL Current Issues Regarding Complementary and Alternative Medicine (CAM) in the United States: Part 2: Regulatory and Safety Concerns and Proposed Governmental Policy Changes with Respect to Dietary Supplements. P T 35, 514–522 (2010). [PMC free article] [PubMed] [Google Scholar]