Abstract
Testosterone has been theorized to direct status-seeking behaviors, including competitive behavior. However, most human studies to date have adopted correlational designs, and findings across studies are inconsistent. This experiment (n = 115) pharmacologically manipulated men’s testosterone levels prior to a mixed-gender math competition and examined basal cortisol (a hormone implicated in stress and social avoidance) and context cues related to an opponent’s perceived status (an opponent’s gender or a win/loss in a prior competition) as factors that may moderate testosterone’s impact on competitive behavior. We test and find support for the hypothesis that testosterone given to low-cortisol men evokes status-seeking behavior, whereas testosterone given to high-cortisol men evokes status-loss avoidance. In the initial rounds of competition, testosterone’s influence on competitive decisions depended on basal cortisol and opponent gender. After providing opponent-specific win-lose feedback, testosterone’s influence on decisions to re-enter competitions depended on basal cortisol and this objective cue to status, not gender. Compared to placebo, men given exogenous testosterone who were low in basal cortisol showed an increased tendency to compete against male and high-status opponents relative to female and low-status opponents (status-seeking). Men given exogenous testosterone who were high in basal cortisol showed the opposite pattern - an increased tendency to compete against female and low-status opponents relative to male and high-status opponents (status-loss avoidance). These results provide support for a context-dependent dual hormone hypothesis: Testosterone flexibly directs men’s competitive behavior contingent on basal cortisol levels and cues that signal an opponent’s status.
Keywords: testosterone administration, cortisol, social status, competition, opponent gender
Introduction
Competitions determine access to valuable resources that are fundamental components of social mobility and societal life: Securing jobs, promotions, and financial compensation are often contingent on an individual’s willingness to enter and ultimately succeed in competitions. Competitive behavior can also be destructive, foster violence and aggression, and lead to toxic social environments (Carré & Olmstead, 2015; Kohn, 1992; Wilson & Daly, 1985). Testosterone is a steroid sex hormone that is theorized to drive status-seeking behavior (Mazur & Booth, 1998), including aggressive, dominant, and competitive behaviors (Archer, 2006). However, most human studies to date have adopted correlational designs, and findings across studies are inconsistent. The primary aim of the present research is to identify dispositional and contextual factors that may account for heterogeneity in testosterone’s association with status-seeking behavior. A secondary aim is to enable causal inference about testosterone’s role in social behavior.
In service of these aims, we pharmacologically manipulated men’s testosterone levels prior to a mixed-gender math competition and examined individual differences in endogenous basal cortisol (a hormone linked to stress and social avoidance) and cues to an opponent’s social status (opponent gender, and a win/loss in a prior competition) as factors that may moderate testosterone’s effect on competitive behavior. We use this design to test a context-dependent dual hormone hypothesis: That testosterone treatment given to men low in basal cortisol will evoke status-seeking motivation, resulting in a preference to compete against high-status relative to low-status opponents; by contrast, testosterone treatment given to men high in basal cortisol is expected to evoke status-loss avoidance motivation, resulting in a preference to compete against low-status relative to high-status opponents.
Testosterone, Cortisol, and Social Behavior
Testosterone is a steroid sex hormone produced and released from Leydig cells in the testes following activation of the hypothalamic-pituitary-gonadal (HPG) axis; in women, testosterone is produced in the ovaries and adrenal cortices. A broad literature has focused on testosterone’s role in directing social behavior, extending particularly from theoretical frameworks focused on status seeking and dominance (Archer, 2006; Mazur & Booth, 1998; Wingfield et al., 1990). Empirical evidence indicates that testosterone is associated with a suite of psychological (e.g., implicit power motives; (Stanton & Schultheiss, 2009), physiological (e.g., reduced cardiovascular indices of fear; (Hermans et al., 2006; Van Honk et al., 2001), and morphological characteristics (e.g., facial cues that signal dominance; (Hodges-Simeon et al., 2016; Swaddle & Reierson, 2002; Welling et al., 2016); (Kordsmeyer et al., 2019) that support the pursuit and maintenance of status and dominance within social hierarchies. Competitive behavior – the act of challenging an opponent over a limited resource (e.g., money) or for the purpose of besting a specific individual (e.g., a rivalry; (Deutsch, 1949; M. Mead, 1937) – is a direct means of seeking status. Specifically, competing against an individual presents an opportunity to boost or affirm the winner’s rank relative to the loser; this rank comparison defines a hierarchy and is an explicit indicator of social status.
Two theoretical frameworks generate predictions for testosterone’s effects on competitive behavior. First, according to the challenge hypothesis, status-relevant conflicts increase testosterone levels in males and these fluctuations in testosterone in turn drive status-seeking behavior, such as competitive or aggressive behaviors (Archer, 2006; Wingfield et al., 1990). Testosterone has been found to rise in anticipation of and during competitions and fluctuate dependent on competitive outcomes (Casto & Edwards, 2016; Cheng et al., 2018; Geniole et al., 2017; Prasad et al., 2021; van der Meij et al., 2011). Testosterone responses to competitions, in turn, have been associated with increases in competitive behavior particularly in males (Carré & McCormick, 2008; Casto et al., 2020; Losecaat Vermeer et al., 2020; Mehta & Josephs, 2006). However, other studies indicate that higher testosterone relates to avoiding competitions in certain situations, presumably to prevent loss of status under conditions of status threat (Mehta et al., 2008; Mehta, Snyder, et al., 2015; Mehta, van Son, et al., 2015) and, in two experiments, testosterone treatment in men did not increase competitive behavior (Nadler et al., 2021). The challenge hypothesis is thus partly supported by human research primarily in correlational studies, but some inconsistencies remain for testosterone’s links to competitive behavior.
The second theoretical framework, the dual-hormone hypothesis (Mehta & Josephs, 2010), provides a possible explanation for these inconsistencies: Testosterone’s link to status-seeking behavior may depend on cortisol, a glucocorticoid hormone produced and released by the hypothalamic-pituitary-adrenal (HPA) axis in response to physical and psychological stress (Dickerson & Kemeny, 2004)1. The dual-hormone hypothesis specifically focuses on basal cortisol, an individual difference factor that is related to exposure or the propensity to respond to recent, chronic, or ongoing stress (McEwen, 2019). According to the dual-hormone hypothesis, testosterone’s influence on status-seeking behavior is posited to be more robust when basal cortisol levels are low; when basal cortisol levels are high, the effect of high testosterone on status-seeking behavior is expected to be inhibited (Knight, Sarkar, et al., 2020; Mehta & Prasad, 2015; Sarkar et al., 2019). Consistent with this hypothesis, higher basal testosterone was positively related to behaviors such as dominant leadership behavior, risk-taking, overbidding in a competitive auction, and higher social status when basal cortisol levels were low but not when basal cortisol levels were high (Edwards & Casto, 2013; Mehta, Welker, et al., 2015; Mehta & Josephs, 2010; Sherman et al., 2016; Van Den Bos et al., 2013)
The dual-hormone hypothesis is informed by the interplay between the psychological motives associated with testosterone and cortisol. High testosterone is theorized to increase the desire for status (Mazur & Booth, 1998). High basal cortisol is associated with stress, anxiety, threat vigilance, and social avoidance, whereas low basal cortisol is linked to decreased stress and social approach (Bertsch et al., 2011; L. L. Brown et al., 1996; Enter et al., 2019; McEwen, 2019; Montoya et al., 2012; Pfattheicher, 2016; Roelofs et al., 2005; Van Honk et al., 1998). Because status-seeking behaviors are approach-oriented in nature, coupling high motivation for social status (high testosterone) and a predisposition toward social approach (low cortisol) may enhance status-seeking behaviors such as dominance and competitive behavior. By contrast, high social avoidance (high cortisol) may inhibit the effect of a high motivation for status (high testosterone) on the expression of status-seeking behaviors.
The dual-hormone hypothesis is also informed by research on the physiological interplay between the HPA and HPG axes (Viau, 2002), which may underlie the psychological mechanisms for status-seeking behavior. For example, cortisol suppresses the activity of the HPG axis, antagonizes the effect of testosterone on target tissues, and downregulates androgen receptors (Burnstein et al., 1995; Chen et al., 1997; Johnson et al., 1992; Mehta & Josephs, 2010; Smith et al., 1985; Tilbrook, 2000; Viau, 2002). The inhibitory effects of cortisol on the HPG axis are particularly evident when cortisol levels are elevated for prolonged periods (i.e., basal cortisol) and are mediated by genomic mechanisms (i.e., hormones bind to and activate receptors, alter genetic transcription and protein synthesis), which occur over relatively long time scales (Tilbrook et al., 2000). Collectively, this evidence suggests that high basal cortisol concentrations may inhibit the effect of testosterone on status-seeking behavior through multiple physiological pathways, and these pathways involve relatively slow genomic mechanisms of action. These physiological pathways may drive an interplay between status and approach-avoidance motivational systems that give rise to status-seeking behaviors
Despite emerging evidence supporting the dual-hormone hypothesis, some studies have revealed different patterns of results. For example, some studies have found higher basal testosterone to be positively related to aggressive behavior, cheating behavior, and psychopathic traits among individuals with high basal cortisol (Denson, Mehta, et al., 2013; Lee et al., 2015; Roy et al., 2019; Welker et al., 2014). These findings were taken as evidence against the standard predictions of the dual-hormone hypothesis because these behaviors were considered status-seeking behaviors. High testosterone coupled with high cortisol levels is also an endocrine pattern associated with socially threatening situations (Knight & Mehta, 2017; Marceau et al., 2015; Scheepers & Knight, 2020; Turan et al., 2015). Overall, basal testosterone’s relationship to social behaviors implicated in the pursuit of status do appear to depend on basal cortisol in several correlational studies, but with some variability in the exact pattern of results (Dekkers et al., 2019).
Context-Dependent Dual Hormone Hypothesis
We introduce a new theoretical framework – the context-dependent dual-hormone hypothesis – that may account for some of the discrepancies in testosterone and cortisol’s links to status-relevant behavior. This framework extends prior theorizing in three related ways. First, and most importantly, the model makes a distinction between two types of status motives – status-seeking and status-loss avoidance. Second, the model makes predictions about dual-hormone profiles that map onto these two motives. Third, according to the model, these two motives should have context-dependent effects on behavior. We introduce the model in Figure 1 and describe it in detail below.
Figure 1.
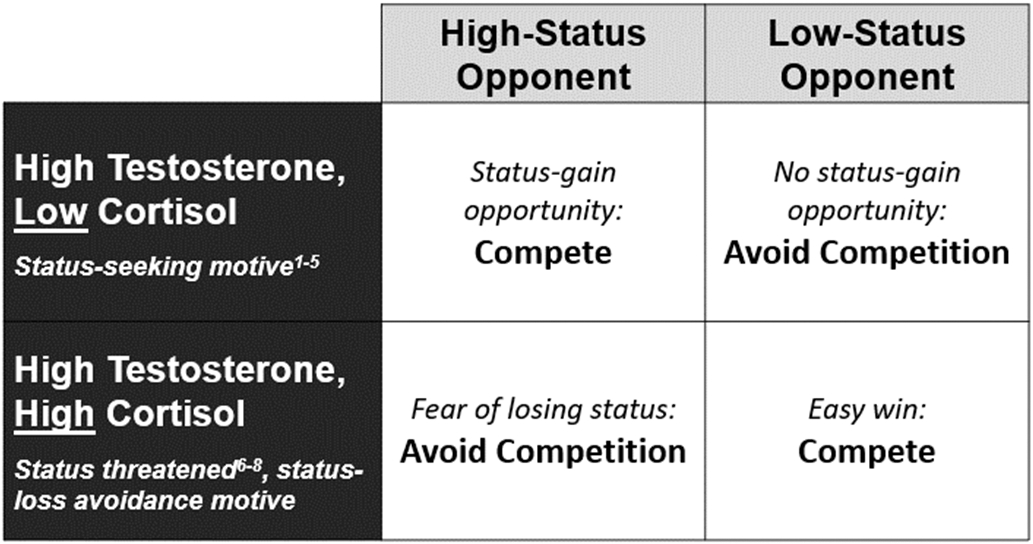
Theoretical model of a context-dependent dual-hormone hypothesis for testosterone’s effects on competitive behavior. References in figure: 1) Mehta & Josephs, 2010; 2) Mehta & Prasad, 2015; 3) Sarkar et al., 2019; 4) Knight et al., 2020; 5) Dekkers et al., 2019; 6) Knight & Mehta, 2017; 7) Marceau et al., 2015; 8) Turan et al., 2015. See Knight et al. (2020) for a broader review.
According to this context-dependent dual-hormone hypothesis, higher testosterone should increase a general motivation for status, which can manifest as status-seeking or status-loss avoidance. Crucially, which type of status motive is dominant within an individual should depend on basal cortisol. Among low-cortisol individuals (low stress and high approach motivation), high testosterone is expected to promote status-seeking. But among high-cortisol individuals (high stress and high avoidance motivation), high testosterone is expected to induce status-loss avoidance. These two status motives should have divergent effects on competitive behavior that depend on an opponent’s perceived status. The status-seeking motive in high testosterone-low cortisol individuals is expected to evoke competitive behavior against opponents perceived to be of high status as a means to earn higher rank in the social hierarchy, but less competitive behavior against low-status opponents because these competitions do not provide a status-gain opportunity. In contrast, the status-loss avoidance motive in high testosterone-high cortisol individuals is expected to evoke a fear of losing competitions, and hence, a preference to avoid competing against high-status opponents and, instead, compete against low-status, less skilled opponents.
These hypothesized interactions between biological and contextual factors extend separate areas of research that have studied hierarchy and competition from different perspectives. Research in behavioral endocrinology has focused primarily on the roles of these hormones in hierarchy-relevant behavior without considering how the social context may alter hormone-behavior associations (Dekkers et al., 2019), whereas research in social psychology and related fields has examined the impact of hierarchy-relevant contextual factors on behavior with little attention to biological moderators (Buser, 2016; Fast & Chen, 2009; N. L. Mead & Maner, 2012). Thus, a gap remains for understanding whether contextual factors such as an opponent’s perceived status alter biological determinants of competitive social behavior as only limited work has examined these factors together in competitive settings.
Consistent with this biology × context interactionist framework, one correlational study found that men with high basal testosterone and low basal cortisol levels who had experienced a competitive defeat tended to compete again against the same opponent – that is, against a higher-status opponent. But this tendency to compete against the same opponent was not seen for high-testosterone, low-cortisol individuals who had experienced a victory (Study 2; (Mehta & Josephs, 2010). In the same study, men with high basal testosterone and high basal cortisol displayed the opposite pattern, a tendency to avoid competitions against high-status but not low-status opponents. Collectively, these results suggest that testosterone’s association with competitive behavior depends on cortisol and on an opponent’s social status: High basal testosterone coupled with low basal cortisol is associated with increased competitive behavior against high-status but not low-status opponents (status-seeking), whereas high basal testosterone coupled with high basal cortisol is associated with avoiding competitions against high-status opponents and increased competitive behavior against low-status opponents (status-loss avoidance).
The Present Research
An important limitation of previous research is its correlational design with regard to the direct and moderated effects of testosterone. Thus, it remains unknown whether testosterone has a direct causal effect on men’s decisions to enter competitions in line with the challenge hypothesis and/or whether testosterone’s causal influence on men’s competitive behavior depends on an opponent’s perceived status and cortisol levels. Understanding testosterone’s causal impact on competitive behavior within the context-dependent dual-hormone hypothesis framework is a crucial step in developing comprehensive theory on the pursuit of social status.
To test hypotheses about the nature of testosterone’s causal role in competitions, the present experiment administered exogenous testosterone or placebo to men prior to a competitive decision-making task. The ideal design for testing the modulatory effects of cortisol would involve simultaneous manipulation of the HPG axis (testosterone) and HPA axis (cortisol). However, such dual-systems pharmacology protocols are not readily available because the validity of a joint manipulation of both testosterone and cortisol levels has not been established. Instead, we adopted a mixed experimental design, where we pharmacologically manipulated testosterone levels and examined the moderating effects of endogenous basal cortisol levels and perceived opponent status. This design represents a novel extension of previous correlational work on hormones and competitive behavior2.
Prior correlational research has also tended to measure competitive behavior with a single decision to compete or not (Carré & McCormick, 2008; Mehta et al., 2008; Mehta & Josephs, 2010), preventing assessment of each participant’s relative propensity to compete against low- and high-status opponents. The present experiment improved on this prior work by using a competition task with multiple decisions to enable within-person comparisons of competitive behavior. Such an approach also increases statistical power compared to paradigms with a single decision.
Much of the previous work examined associations between endogenous hormones and competitive behavior after an explicit social status manipulation (a previous win/lose experience) in men competing against other men. However, individuals often make decisions to enter competitions lacking explicit information relevant to an opponent’s perceived status. Real-world settings such as academia and other workplaces are also increasingly diverse in terms of gender (Cheryan et al., 2017; Joshi et al., 2015) while notably still lacking equality in terms of power, prestige, and financial compensation (A. J. Brown & Goh, 2016; Gruber et al., 2020; Skitka et al., 2020). Absent explicit evidence of an opponent’s status, gender may be used as a cue to an opponent’s perceived social status based on culturally-determined stereotypes (Datta Gupta et al., 2013; Ellemers, 2018; Fiske et al., 2002; Gneezy et al., 2003; Niederle et al., 2013). Gender stereotypes may be especially relevant in a math-based competition, a domain in which women are stereotyped to perform poorly (Cheryan et al., 2017; Ellemers, 2018; Josephs et al., 2003; Spencer et al., 1999) despite evidence that women can outperform men on math tasks in laboratory settings (Niederle & Vesterlund, 2011). Based on these gender stereotypes, men who are motivated to seek challenging opponents as a means to gain social status (i.e., men with high testosterone and low cortisol) should prefer competing against male opponents relative to female opponents in a math-based competition; conversely, men threatened by the prospect of losing competitions (men with high testosterone and high cortisol) should actively avoid competition against male opponents and pursue competition against female opponents instead.
Stereotypes are particularly likely to guide person perception and behavior in the absence of objective, individuating information based on social experience (Fiske & Neuberg, 1990). This research suggests that a man competing with an unknown woman in a math competition may initially perceive her to be a low-status opponent based on gender stereotypes (Fiske et al., 2002; Spencer et al., 1999). But if additional information based on social experience suggests that she is a high-status competitor (she wins in a prior math competition), this new information may override gender stereotypes when evaluating her social status. Consistent with this general notion, Wozniak, Harbaugh, and Mayr (2014) found that effects of participant’s gender and position in the menstrual cycle in choices to compete in math tasks disappeared once valid performance information was provided. Based on these results, we expected that gender may moderate effects of testosterone and cortisol on competitive behavior only in the absence of objective information of an opponent’s status (win/lose performance feedback). Testing these opponent-status hypotheses in a math-based competition extends prior work on testosterone and cortisol’s interactions with opponent status, which has focused on objective cues to opponent status but has neglected subjective cues to status based on stereotypes.
In sum, the challenge hypothesis predicts that testosterone should cause increased competitive behavior overall (Archer, 2006; Wingfield et al., 1990). However, initial correlational evidence indicates that testosterone and cortisol interact with perceived opponent status to predict status-seeking behavior (Mehta & Josephs, 2010). Hence, exogenous testosterone given to men with low basal cortisol may increase willingness to compete against seemingly high-status opponents, predicated on gender stereotypes or feedback that indicates an opponent’s relative status. Conversely, exogenous testosterone given to men with high basal cortisol may result in avoiding competition against high-status opponents and instead choosing to compete against “easy-to-beat” low-status targets. The present work examined these hypotheses by measuring basal cortisol levels and pharmacologically manipulating testosterone prior to a mixed-gender competition in which men made decisions to enter competitions before and after receiving accurate win/lose feedback.
Methods
Participants
As part of a broader experiment on exogenous testosterone (Knight et al., 2017), men (n = 120) between the ages of 18 and 40 (M = 21.5 years, SD = 3.5 years) were recruited via flyers on and near campus and by contacting email lists (see Figure S1 for full experimental timeline). We maximized the diversity of the sample within the constraints of the local population by recruiting students and community members, on and off campus (final sample, 28% people of color; see Supplemental Materials, Table S1 for diversity evident in socioeconomic indicators). All participants were prescreened for physical and mental health conditions via a telephone interview prior to the laboratory day (see Supplemental Materials for full list). Upon verifying that participants met the requirements to participate, a laboratory session was scheduled, and they were instructed to abstain from eating, drinking, smoking, or brushing their teeth at least two hours prior to the experimental session. The protocol was approved by the University of Oregon’s Institutional Review Board.
Protocol
Participants arrived at the laboratory between 9:00 AM and 11:00 AM. Informed consent was obtained during a 15- to 20-minute resting period to allow participants to acclimate to the laboratory setting, after which participants provided a baseline saliva sample in order to measure pretreatment, basal cortisol values. This approach to basal cortisol measurement is consistent with previous research on the dual-hormone hypothesis, which also measured baseline hormone levels after a similar acclimation period (Mehta & Josephs, 2010). Participants then applied topical testosterone gel or placebo to their shoulders and upper arms under the supervision of an experimenter. Three hours after gel application, participants provided a second saliva sample and then immediately began the competition task. Approximately fifteen minutes after completing the competition task (and immediately after another, unrelated decision-making task), participants provided another saliva sample. Participants received payment at the end of the experimental session (approximately 2 hours after the end of the competition task) for their time in the laboratory. Participants were also paid based on their performance in the competition task and one other decision-making task.
Exogenous Testosterone and Blinding
We chose to manipulate testosterone levels in a placebo-controlled fashion to derive causal inference of testosterone’s role in competitive behavior. This experimental approach extends previous correlational work that measured endogenous hormone levels only (Apicella et al., 2011; Dekkers et al., 2019; Eisenegger et al., 2017; Mehta & Josephs, 2010). Topical testosterone gel (AbbeVie, Inc., Chicago, IL) was portioned into 150-mg doses and placed in blunted-tip syringes with no indication of the contents. The placebo consisted of a gel produced to exactly match the vehicle of the testosterone gel and was placed in syringes in an equivalent volume to the testosterone samples. Half of the participants were told which treatment they were given (single blind), in order to emulate real-world environments in which testosterone is prescribed. The other half of participants were only told they had an equal chance of receiving testosterone or placebo (double blind). This information was conveyed through a letter in a sealed envelope that had been prepared by members of our laboratory who were not involved in data collection. The experimenters never knew which treatment or blinding condition a participant was assigned. The blinding manipulation was included to facilitate measurement and control of potential expectancy effects related to testosterone and social behavior (Eisenegger et al., 2010). All behavioral analyses control for the blinding condition; follow-up analyses reported in the Supplemental Materials explored blinding condition as a moderator to ensure any patterns observed in the main analyses replicated across single- and double-blinded participants.
Pharmacokinetics
In prior testosterone administration research, testosterone levels reached peak levels 3 hours after application of topical gel (Eisenegger et al., 2013) and physiological differences due to testosterone were evident within 3-6 hours after sublingual intake3 (Radke et al., 2015; Tuiten et al., 2000). Thus, the protocol was designed such that the competition task began approximately three hours after gel application (Mean = 2.92, SE = 0.03 hours).
Salivary Hormone Measurement
Participants were instructed to drool approximately 2 mL of saliva into polypropylene tubes, which were immediately frozen in a −20 °C freezer, prior to transportation to a −80°C freezer for longer-term storage. All samples were assayed for testosterone and cortisol in duplicate consistent with standard, published procedures (Schultheiss & Stanton, 2009) using commercial enzyme immunoassay kits (DRG International, New Jersey, USA).
Due to the large dose of exogenous testosterone, 17% of the samples from the testosterone treatment group were above the kit’s maximum testosterone concentration (no samples in the placebo group were above threshold). Prior research has shown that topical testosterone heightens blood-based testosterone concentrations to a high-normal level despite more extreme values evident in saliva (Krebs et al., 2019; Puiu et al., 2019; Schönfelder et al., 2016). Supraphysiological salivary hormone concentrations after topical treatment may result from absorption of the hormone into subcutaneous tissue and transport to the salivary glands via the lymphatic system (Du et al., 2013; Krebs et al., 2019). Due to these concerns with salivary concentrations after testosterone administration, testosterone concentrations in the present experiment are used only to ensure testosterone treatment increased testosterone levels and are not used to predict behavior. Samples with concentrations above the kit’s threshold were replaced with the kit’s maximum (5250 pg/mL) as a conservative approximation of the sample’s testosterone concentration. For cortisol, the average intra-assay coefficient of variation (CV) was 4.68%; the inter-assay CV was 14.8%. For testosterone (ignoring samples above kit threshold), the average intra-assay CV was 6.55% and the inter-assay CV was 16.1%. Testosterone and cortisol concentrations were square-root transformed to correct positively skewed distributions (see Figure S2 in Supplemental Materials for distributions of hormone concentrations).
Basal Cortisol Measurement
Our primary analyses focus on pretreatment basal cortisol in line with previous research on the dual hormone hypothesis, which has focused almost exclusively on basal hormone levels (Dekkers et al., 2019; Mehta & Josephs, 2010). We considered the first saliva sample of the experiment a basal measurement because it occurred after an acclimation period but prior to testosterone or placebo administration, prior to laboratory task instructions, and prior to any laboratory tasks. The second cortisol measurement in the present experiment, collected prior to the competition task, could not be considered a basal measure as it occurred approximately three hours post-administration of testosterone treatment and because testosterone treatment can influence activity across the HPA axis (Rubinow et al., 2005; Viau, 2002). This approach to basal cortisol measurement follows directly from previous research. For example, the previous correlational study upon which we are building also measured basal cortisol with an initial sample that was taken after an acclimation period but before behavioral task instructions (Mehta & Josephs, 2010). Our approach to basal cortisol measurement is consistent with genomic mechanisms of action, whereby pretreatment basal cortisol is expected to moderate the effects of exogenous testosterone treatment on competitive behavior measured several hours later.
Competition Task
The competition task was designed to measure competitive decisions in mixed-gender math competitions before and after receiving win/lose feedback (Figure 2; (Mayr et al., 2012). Performance metrics were also measured in the task to determine whether effects on competitive behavior were or were not explained by performance.
Figure 2.
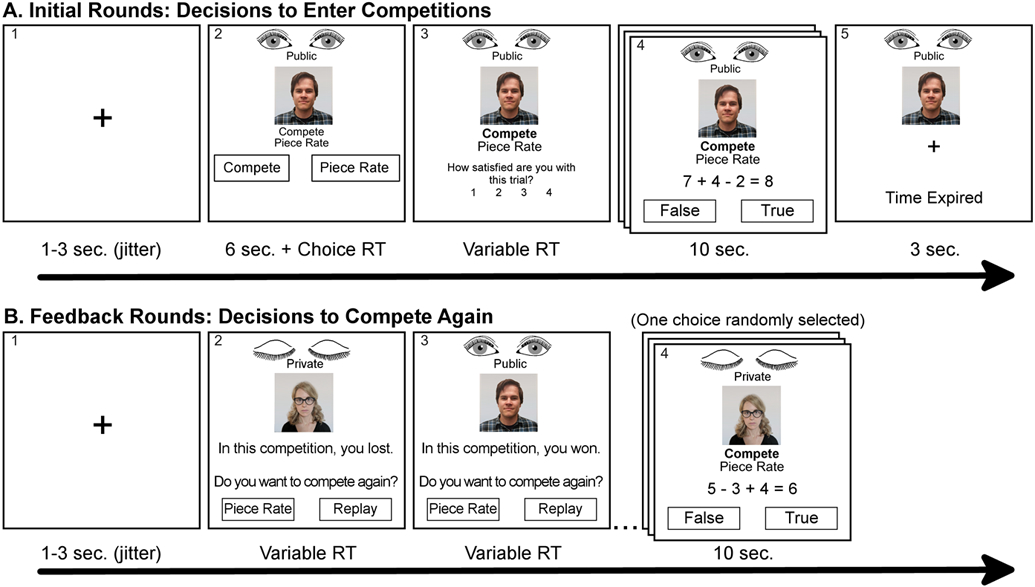
The Competition Task Note. (A) In the early rounds of the competition task, participants made decisions to enter competitions against 16 opponents (n = 8 females) or play for a piece rate instead. Participants also played in 16 mandatory compete and 16 mandatory piece-rate rounds against the same opponents (48 rounds in total). The actual competition consisted of answering as many True/False equations as possible within 10 s. Scores in a given competition trial were compared to the opponent’s actual score on the same set of True/False equations. (B) After completing all 48 rounds, participants were provided feedback from the 16 mandatory compete rounds and asked whether they wanted to compete against that opponent again (“Replay”) or play for a piece rate instead. One of these decisions was randomly selected to be played. Opponent images in this figure were not part of the stimuli but are representative of the types of photos in the opponent pool. The numbers appearing in the upper left corner of each frame are included to be able to describe the task and were not part of the task.
The initial phase consisted of forty-eight rounds of a math task in which participants were given opportunities to compete against other players for points in a winner-take-all payment scheme or to play for a piece rate instead. In each round, subjects were tasked with deciding if simple math equations containing addition and subtraction were true or false. Participants had ten seconds in each round to answer as many equations as possible (panel 4 in Figure 2A and 2B). One point was awarded for every correct answer and one point was deducted for every incorrect answer. Each round presented one of sixteen possible opponents (n = 8 female) who were individuals who had completed the task previously and had their actual scores saved from the same series of equations.
During “compete” rounds, a winner was determined by comparing the number of points earned by the participant to the number of points earned previously by the opponent. For these rounds, each point was worth $4 but only if the participant won the round; if they scored fewer points than their opponents, they earned nothing ($0). In the case of ties, the amount of time required to respond to the round’s equations was used to determine a winner4. During “piece rate” rounds, participants attempted to answer as many math equations as possible, but their score was not compared to that of the opponent. In piece rate rounds, every point the participant earned was worth $2 regardless of how the opponent performed in that round. In rounds in which participants earned negative points (more incorrect than correct responses), participants’ earnings were set to $0.
In one-third of the rounds, participants chose whether to compete against the current opponent or play for a piece rate (i.e., “choice rounds”; panel 2 in Figure 2A). Decisions to play a competition in these choice rounds were coded as competitive behavior. In the remaining rounds, participants were forced to compete or play for a piece rate (“mandatory rounds”). In summary, there were 48 rounds divided into three categories – 16 choice, 16 mandatory compete, and 16 mandatory piece rate – and each opponent appeared once in each category.
After the initial 48 rounds of the task (Figure 2A), participants completed the “Feedback rounds,” in which feedback was provided on whether the participant had won or lost in the sixteen mandatory compete rounds (Figure 2B). Immediately after providing feedback on the outcome of a prior competition, participants were asked whether they preferred to compete against that opponent again in a follow-up round or play a piece rate round instead (panels 2 and 3, Figure 2B). After all of these decisions were made, one of these post-feedback decisions was randomly chosen and participants played a round based on that choice (i.e., as a compete or piece-rate round; panel 4, Figure 2B). Participants’ performance-based payout consisted of winnings based on one randomly chosen choice round, one randomly chosen mandatory competition round, one randomly chosen mandatory piece-rate round, and the randomly chosen post-feedback round.
These instructions were explained to the participant approximately an hour prior to the start of the competition task. The experimenter guided the participant through an interactive demonstration of the task that ended with a practice trial of the math task. Participants also had to successfully complete a short, verbal quiz focused on key features of the task (see Supplemental Materials).
Competition Score, Satisfaction, and Outcome
Prior to the start of each trial’s math task (and after participants made decisions in choice rounds), participants were asked how satisfied they were with the present trial on a scale from 1 (not at all satisfied) to 4 (very satisfied; panel 3, Figure 2A). We also recorded the number of points earned in each trial and the participants’ rate of winning rounds. These additional measures were included to explore the extent to which they did or did not explain hormonal effects on decisions to compete.
Subjective Ratings of the Opponents
To examine whether gender was used as a cue to perceived opponent status in our competition task, participants rated the opponents’ images on a set of nine variables on a Likert scale from 1 (strongly disagree) to 7 (strongly agree). Here we report the extent to which the rater felt the opponent was “good at simple math tasks.” (see Supplemental Materials for analyses of the remaining variables). Participants rated the photos at the end of the laboratory session, several hours after feedback had been provided during the competition task.
Because these ratings are confounded with the feedback provided in the competition task, a small sample (n = 16) of men who did not participate in the competition task also rated each opponent on the “good…at math” item. These follow-up raters consisted of a convenience sample of undergraduate research assistants and graduate students not affiliated with this experiment.
Other Aspects of the Competition Task
The task also contained a social-evaluative manipulation (Figure 2). In half of all trials, participants were instructed that the experimenter could see the participants’ decisions and competitive performances displayed on a screen in another room (Public condition); the public trials contained an outline of open eyes and the word “Public” written at the top of the screen throughout those trials. In the remaining trials, participants’ decisions and performance were not visible to the experimenter (Private condition); the private trials contained an outline of closed eyes and the word “Private” written at the top of the screen. Because few studies have examined a social-evaluative manipulation as a moderator of testosterone’s influence on behavior (Losecaat Vermeer et al., 2020; Wu et al., 2020), on an exploratory basis we tested the hypothesis that social evaluation might enhance testosterone’s effects on behavior by raising the status implications of the competition in trials when there was an audience. Further background and results for this manipulation are discussed in the Supplemental Materials. All behavioral analyses control for the social-evaluative manipulation.
Analyses
Endocrine Levels
Group differences in testosterone and cortisol levels were examined at baseline and in terms of overall exposure across the duration of this experiment [area-under-the-curve with respect to ground (AUCG)]. General linear models (GLM) were produced with hormone concentration (baseline or AUCG) regressed on testosterone treatment group to test for group differences. Coefficients representing the difference between the testosterone treatment and placebo groups are reported with 95% confidence intervals (95% CIs), F-tests, and p-values.
Primary Behavioral Analyses
Primary analyses were focused on participants’ decisions to compete or play for a piece rate in the choice rounds of the early phase and in the feedback rounds as a function of the main effects and interactions of testosterone treatment, basal cortisol, and opponent status. In each set of models, the effects of testosterone treatment group and opponent gender in a given trial were analyzed as categorical variables (Testosterone Treatment: testosterone = 1, placebo = 0; Opponent Gender: female opponent = 1, male opponent = 0); basal cortisol was included as a continuous variable.
Binomial logistic multilevel models were constructed in R (3.5.1; R-Core Team, 2018) using the ‘glmer’ function from the lme4 package (Bates et al., 2015). Decisions were binary coded as Compete = 1 and Piece rate = 0. For behavioral analyses, we report odds ratios (ORs) with 95%CIs.
For decisions to enter competitions, our model to test the interaction among testosterone treatment, cortisol, and opponent gender consisted of the following variables across two levels for round i within participant j:
Level 1:
| (1) |
Level 2:
| (2) |
| (3) |
| (4) |
We also examined a model with just the main effects (i.e., removing all interactions of testosterone, cortisol, and gender) as well as a model with just two-way interactions with testosterone treatment (i.e., separate models for testosterone treatment and cortisol, testosterone treatment and gender).
For our primary analyses of decisions to compete again in the feedback rounds, the models contained an additional term representing the prior competition outcome from the mandatory compete round against a given opponent (Won = 1, Lost = 0). Thus, our principal model to test the interaction among testosterone treatment, cortisol, and prior outcome consisted of the following variables across 2 levels:
Level 1:
| (5) |
Level 2:
| (6) |
| (7) |
| (8) |
| (9) |
Similar to the early phase models (Equations 1-4), we examined a model with just the main effects (i.e., testosterone treatment, cortisol, opponent gender, and prior outcome) as well as models with just two-way interactions with testosterone treatment (i.e., testosterone treatment and cortisol, testosterone treatment and prior outcome). We also explored the four-way interaction between testosterone treatment, cortisol, opponent gender, and prior competitive outcome (i.e., this was not considered a primary analysis).
In cases where the initial and feedback phase models could not be satisfactorily fit (i.e., due to a singular fit), the complexity of the model was reduced by sequential removal of the random term for social-evaluative observation, gender, and/or prior outcome and rerun (Nakagawa et al., 2017).
Significant interactions were broken down in two ways. First, a simple slopes approach was used to examine within-person comparisons of high and low status opponents based on opponent gender in the initial phase or prior outcome in the feedback phase among individuals in the testosterone treatment and placebo groups with high (+1 SD) or low (−1SD) cortisol levels (Hughes, 2020; Preacher et al., 2006). Second, we calculated empirical Bayes estimates of the slopes of opponent gender and prior outcome. To estimate these slopes from the initial phase, decisions to compete were regressed on opponent gender with a random slope and intercept for opponent gender per participant, controlling for the social evaluation condition. In the feedback phase, decisions to compete were regressed on prior outcome with random slope and intercept for prior outcome per participant, controlling for opponent gender and social evaluation condition. For each of these sets of slopes, a positive slope indicates a greater propensity to compete against female opponents or prior losers relative to male opponents or prior winners (respectively), and a negative slope value indicates a greater propensity to compete against male opponents or prior winners relative to female opponents or prior losers. In separate models, these slopes were then regressed on the two-way interaction between testosterone treatment condition and basal cortisol.
Follow-up Analyses with Covariates.
We followed up our initial analyses with separate models that controlled for time of day of the laboratory task and time since awakening prior to the first salivary sample. Both of these variables may index diurnal aspects of endocrine functioning. We also included participants’ overall skill in the task – indexed by each participant’s mean points earned on the mandatory piece rate rounds – as a covariate in follow-up analyses.
Secondary Analyses
Behavioral analyses with trait dominance.
Some prior work suggests that trait dominance – defined as the tendency to rely on force, fear, and intimidation to take or defend higher status positions (Cheng et al., 2013) – may accentuate testosterone’s association with status-relevant behavior5. Within this theoretical framework, self-reported trait dominance is considered an explicit component of dominance, whereas testosterone is considered an implicit component of dominance that operates outside conscious awareness (Knight, Sarkar, et al., 2020). Because implicit and explicit forms of a given construct can interactively determine behavior (Slatcher et al., 2011), high levels of testosterone in an individual with high trait dominance may synergistically heighten concern for status and increase status-relevant behaviors.
However, the behavioral effects within the testosterone × trait dominance literature are somewhat nuanced. For example, exogenous testosterone’s effects on competitive motivation were exaggerated among women who were high in trait dominance (Mehta, van Son, et al., 2015) and among men high in trait dominance who were assigned to a low status position (Losecaat Vermeer et al., 2020). However, this latter effect was not observed in a later portion of the same contest and, in another experiment focused on men’s physical persistence in a competition, trait dominance did not moderate the effects of exogenous testosterone (Kutlikova et al., 2021). Among other status-relevant behaviors and contexts, trait dominance enhanced endogenous testosterone’s association with men’s mating behavior (Slatcher et al., 2011) and with men’s aggressive behavior (albeit only after a victory experience; (Carré et al., 2009); trait dominance also enhanced exogenous testosterone’s causal effect on men’s aggressive behavior (Carré et al., 2017). Another experiment found that a personality risk factor that included dominance and other related traits significantly accentuated the effects of exogenous testosterone on men’s aggressive behavior; the moderating effect of trait dominance alone was not significant but was similar in magnitude and direction as the composite personality risk factor (Geniole et al., 2019). In the same sample of men reported here, trait dominance amplified the effects of testosterone on cortisol and negative affect responses to social-evaluative stress (Knight et al., 2017). Other work found that trait dominance did not significantly moderate the association between testosterone and men’s risk-taking behavior, with a directional pattern that was unexpected (Welker et al., 2019).
One previous study also investigated trait dominance in the context of the dual-hormone hypothesis. Trait dominance did not significantly moderate the interactive association of testosterone and cortisol with aggressive behavior in another study, although this report suggests that the high-testosterone, low-cortisol association with aggressive behavior may be more evident in men with higher trait dominance (Pfattheicher, 2017). It therefore remains unknown whether testosterone and cortisol interactions with trait dominance will extend to men’s competitive decisions.
Because of this small but growing literature, we conducted secondary analyses to explore the moderating effect of trait dominance on testosterone and cortisol’s associations with men’s decisions to compete. We indexed trait dominance via the dominance subscale of the Dominance and Prestige scale. The dominance subscale consists of 8 items related to dominance (e.g., “I try to control others rather than permit them to control me.”) on a scale from 1 (not at all) to 7 (very much). Dominance items (Cronbach’s α = .68) were averaged and standardized. To limit the number of tests conducted, we tested testosterone treatment × trait dominance, testosterone treatment × basal cortisol × trait dominance, and testosterone treatment × opponent status cue × trait dominance effects on decisions to enter competitions in each phase of the competition task.
Subjective ratings of the opponents.
We examined perceptions of how “good…at math” each of the opponents were as an implicit index of perceived opponent status in the competition via a multi-level model. Opponent gender was entered as a dummy code in each model (1 = female opponent, 0 = male opponent), with a random intercept and a random slope of opponent gender for each participant and a random intercept for each opponent. We next examined the effect of gender on “good at…math” ratings while controlling for whether the participant won or loss to that opponent in the mandatory compete rounds. In a separate model, we examined the effects of gender on “good at…math” ratings among the follow-up sample. Finally, we examined the similarity of the ratings across the two datasets by pooling data and producing a model that included the effects of opponent gender, source of ratings (participants = −0.5, follow-up raters = 0.5), and the interaction between gender and rating source.
Exploratory Analyses
Dual-hormone effects with other cortisol measures.
Because the dual-hormone hypothesis focuses on basal cortisol, less is known about state cortisol measures as moderators of testosterone’s behavioral effects. The few studies that examined state measures of cortisol within the dual-hormone literature suggest that acute cortisol fluctuations may be a relevant moderator for testosterone’s behavioral effects when an acute stressor is included prior to the measurement of the behavioral outcome measure (Prasad et al., 2017; 2019; Knight et al., 2020). However, the present experiment did not include an acute stressor prior to the competition task. Thus, we did not expect state measures of cortisol to moderate the impact of exogenous testosterone on competitive behavior.
Nevertheless, given that few studies within the dual-hormone literature have examined state cortisol measures, we conducted exploratory analyses with such measures to guide ongoing theory development. Specifically, we explored cortisol fluctuations around the competition task using a difference score from pre- to post-competition cortisol and diurnal cortisol dynamics with AUCG using the variable time durations between samples (Pruessner et al., 2003). AUCG with variable time durations provides a relatively precise index of total cortisol exposure during the period before and concurrent to the competition task that other approaches (e.g., a single measure or averaging across several measures) cannot readily provide (Pruessner et al., 2003). For behavioral analyses, transformed cortisol values were standardized. Readers interested in other cortisol measures not included in this report are invited to explore the open dataset.
Competition score, satisfaction, and outcome.
On an exploratory basis, we investigated whether testosterone treatment, basal cortisol, and opponent gender predicted points earned in each round, self-reported satisfaction, and likelihood of winning competitions during the initial phase of the competition task. These analyses were intended to explore if testosterone, cortisol, and opponent status led to differential performance or satisfaction in this task. These measures are of interest for theory development to determine if testosterone, cortisol, and opponent status predicted differences in competitive behavior with or without subsequent differences in actual performance or satisfaction. Multilevel linear regression models were produced that examined points earned and satisfaction among all choice trials. A binary logistic multilevel model was used to investigate whether testosterone treatment and its interaction with basal cortisol and opponent gender predicted likelihood of winning. A second set of models were analyzed that included the participant’s choice (piece rate = 0; compete = 1) as an additional moderator of testosterone treatment, basal cortisol, and opponent gender in order to explore if participants decisions were associated with score, satisfaction, or likelihood of winning.
False Discovery Rate Correction
Our primary analyses build on previous research and the theoretical framework outlined in Figure 1 to test the context-dependent dual-hormone hypothesis. Specifically, the testosterone × cortisol × prior outcome (i.e., win/lose feedback) interaction has prior support in previous correlational research (Mehta & Josephs, 2010), and we tested this interaction and extended it to opponent gender (i.e., testosterone × cortisol × opponent gender) as a second status cue based on stereotypes in both phases of the competition. As discussed in the introduction, previous research points to additional hypotheses about the influence of testosterone or opponent status cues on competitive behavior. Therefore, we tested eight other effects (representing thirteen results; hence, sixteen results corrected in total) for which support was evident in the prior literature and applied Benjamini-Hochberg (1995) FDR correction. Specifically, primary and secondary analyses also tested the following effects in both stages of the competition (initial and feedback phase, where applicable): the main effects of testosterone (Mehta et al., 2017), opponent gender (Datta Gupta et al., 2013), and prior outcome (Buser, 2016); testosterone × cortisol (Mehta & Josephs, 2010); testosterone × prior outcome (Mehta et al., 2008); testosterone x opponent gender (Josephs et al., 2003); testosterone × trait dominance (Slatcher et al., 2011); and testosterone × trait dominance × prior outcome (Carré et al., 2009; Mehta, van Son, et al., 2015). We report adjusted p-values for these behavioral results and consider q = .05 as a cutoff for FDR corrected statistical significance.
Meta-Analyses
In order to examine the meta-effect evident across this experiment and one prior correlational study (Mehta & Josephs, 2010), we examined a fixed-effects (FE) meta-analysis of the three-way interaction among testosterone, cortisol, and prior competition outcome (win vs. lose) on decisions to compete again. T-test scores from each study’s three-way interaction model were transformed into correlation coefficients, then transformed further to Fisher’s z scores (Dekkers et al., 2019). Degrees of freedom from this experiment’s multilevel models were calculated using the between-within method, which has been shown to maintain appropriate Type I error rates, maintain power, and is robust to small numbers of clusters and variation in cluster size (Li & Redden, 2015). To examine the effects of testosterone, cortisol, and opponent status across both phases of the competition task, a second, random effects (RE) meta-analysis was examined that included both of the primary three-way interactions from the initial and feedback phase and the prior correlational effect. This model included random effects per study in order to account for the non-independence of the two effects from the present experiment.
Justification for Sample Size and Maximizing Power
The sample size for this experiment was determined by power to detect between-group differences in a pharmacological treatment experiment and was as large as possible within the experiment’s budget. The present experiment improves power compared to previous work on testosterone, cortisol, and opponent status (e.g., (Mehta & Josephs, 2010) by 1) doubling the sample size; 2) using a within-subjects comparisons based on multiple trials for each opponent gender and for winning and losing opponents; and 3) administering exogenous testosterone treatment, which was expected to boost effect sizes relative to an observational approach based on endogenous testosterone. Power simulations conducted after data collection was completed indicate that, given our intended sample size (n = 120), a within-subjects approach, and assuming a moderate level of within-subject correlation, this experiment was 80% powered to detect a three-way interaction term equivalent to log(B) = 0.7, OR = 2.0, akin to a moderate effect size (Figure S2; see Supplemental Materials for details of simulation and a comparison of our within-subjects approach to simulations of one-shot study designs). The simulated, 80%-powered effect size was also weaker than an effect size reported in earlier correlational work (Mehta & Josephs, 2010).
Transparent Reporting
This report is part of a larger project examining exogenous testosterone’s effects on social behavior and responses to social stress (e.g., a social stressor occurred after the competition task and participants did not know the nature of the stressor task prior to the competition; (Knight et al., 2017; Knight, McShane, et al., 2020). All data, code, and experimental materials are archived online at the project’s Open Science Framework website (https://osf.io/hvumx/).
Results
Data Attrition and Descriptive Results
Four participants’ data were lost due to equipment or software malfunction during the competition task; one additional participant left the experiment prior to the competition task, leaving a total of n = 115 participants in the analyses. See Table 1 for descriptive statistics of the experiment’s sample.
Table 1:
Descriptive statistics [mean (SD)] of study sample
| Testosterone | Placebo | All | |
|---|---|---|---|
| Sample size | 58 | 57 | 115 |
| Testosterone (pg/mL) | |||
| Baseline | 136.3 (172.3) | 112.5 (188.1) | 124.5 (179.9) |
| Pre-Competition1 | 2768.5 (2130.7) | 108.1 (95.3) | 1449.9 (2014.8) |
| Post-Competition1 | 3229.0 (2167.1) | 244.7 (426.6) | 1750.0 (2164.2) |
| Cortisol (μg/dL) | |||
| Baseline | 0.367 (0.209) | 0.310 (0.181) | 0.339 (0.197) |
| Pre-Competition | 0.213 (0.154) | 0.217 (0.127) | 0.215 (0.141) |
| Post-Competition | 0.154 (0.089) | 0.163 (0.090) | 0.158 (0.090) |
| Decisions to Compete (% of choice trials in Initial Phase) | 67.5 (25.6) | 59.1 (30.1) | 63.3 (28.1) |
| Male Opponents | 60.3 (29.5) | 54.4 (33.9) | 57.4 (31.8) |
| Female Opponents | 74.6 (26.4) | 63.8 (31.7) | 69.2 (29.5) |
| Decisions to Compete Again (% of choices in Feedback Phase) | 48.6 (23.9) | 47.7 (23.5) | 48.2 (23.6) |
| Male Opponents2 | 49.1 (28.1) | 43.9 (27.8) | 46.6 (28.0) |
| Female Opponents2 | 54.1 (28.8) | 57.5 (25.8) | 55.8 (27.3) |
| Prior Winners3 | 47.0 (37.1) | 42.4 (33.6) | 44.7 (35.3) |
| Prior Losers3 | 60.9 (40.8) | 64.1 (38.6) | 62.4 (39.6) |
| Trial Score (all trials in Initial Phase) | 2.59 (0.99) | 2.52 (0.73) | 2.55 (0.87) |
Notes:
A portion of samples in the testosterone treatment group were above the maximum values of the kits used. For the purposes of calculating these means, the missing values were replaced with the kit’s maximum levels.
Regardless of prior win/loss.
Regardless of opponent gender. “Winners” and “losers” in these rows refers to the opponent – e.g., “prior winners” are opponents that won a mandatory compete trial against the participant in the initial phase.
Endocrine Levels
Testosterone
Baseline differences in testosterone were not robust between treatment groups (B = 0.230, 95%CI [−0.048, 0.508], F(1,113) = 2.68, p = .104). Analysis of testosterone AUCG revealed substantial differences between treatment groups, indicating that the pharmacological manipulation increased testosterone levels (B = 5639.7, [4735.9, 6543.5], F(1,111) = 3.50, p <.001; see Knight et al., 2017 for group differences based on full time series of testosterone data).
Cortisol
Robust differences between treatment groups were not evident in baseline cortisol (B = 0.051, [−0.010, 0.111], F(1,112) = 2.76, p = .099) or overall cortisol exposure as indexed by AUCG (B = 5.26, [−4.70, 15.21], F(1,111) = 0.725, p = .298).
Initial Decisions to Enter Competitions
For our primary behavioral analyses, we examined decisions to enter competitions against a mixed-gender pool of opponents in the initial phase of the competition. All analytical models6 were satisfactorily fit with random terms for both within-participant variables in the initial phase (i.e., gender and social-evaluative observation).
Main Effect of Testosterone Treatment7
According to the challenge hypothesis, testosterone should directly increase decisions to compete. Controlling for all other study variables, exogenous testosterone did not substantially affect decisions to enter competitions compared to placebo, though the effect was in the direction of testosterone causing increased competitive behavior (OR = 1.67, [0.86, 3.27], p = .132, pFDR = 0.303; Table S2; Figure 3, upper panel).
Figure 3.
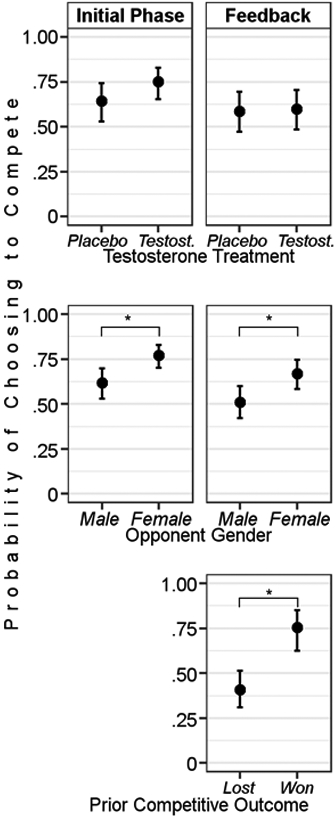
Estimated marginal means of main effects of key study variables on probability of choosing to compete. Charts in left column are from decisions to enter competitions in the Initial Phase; charts in right column are from Feedback Phase decisions to re-enter competitions. *indicates 95%CI of difference does not contain zero.
Main Effect of Opponent Gender (Perceived Opponent Status Cue)
In the same model, we tested whether opponent gender predicted decisions to compete. We found that participants were over twice as likely to compete against female opponents compared to male opponents (OR = 2.09, [1.56, 2.79], p < .001, pFDR < .001; Table S2; Figure 3, middle panel), consistent with prior research (Datta Gupta et al., 2013). This result supports the hypothesis that opponent gender was used as a cue to perceived opponent status that influenced decisions to compete in this task.
Testosterone × Opponent Gender
In a separate model, we examined the extent to which testosterone treatment interacted with the gender of an opponent to cause competitive behavior. Testosterone treatment and opponent gender did not robustly interact to predict competitive behavior (OR = 1.40, [0.81, 2.42], p = .227, pFDR = .404; Table S2).
Dual-Hormone Hypothesis
Next, we tested the original, concise variant of the dual-hormone hypothesis that does not take opponent status cues into consideration. According to this hypothesis, testosterone treatment should increase competitive behavior, but only among men with low basal cortisol. The model’s estimate of a two-way interaction between testosterone treatment and basal cortisol did not support this hypothesis (OR = 1.13, [0.54, 2.34], p = .753, pFDR = .926; Table S2).
Context-Dependent Dual-Hormone Hypothesis
We next assessed our context-dependent dual-hormone hypothesis, which predicts that the effect of testosterone treatment on competitive decision should be moderated by basal cortisol and cues to an opponent’s perceived status (i.e., opponent gender in the initial phase of our task). In support of this hypothesis, the testosterone treatment × basal cortisol × opponent gender interaction was found to predict decisions to enter competitions (OR = 2.54, [1.47, 4.37], p<.001, pFDR = .003; Table S2; Figure 4).
Figure 4.
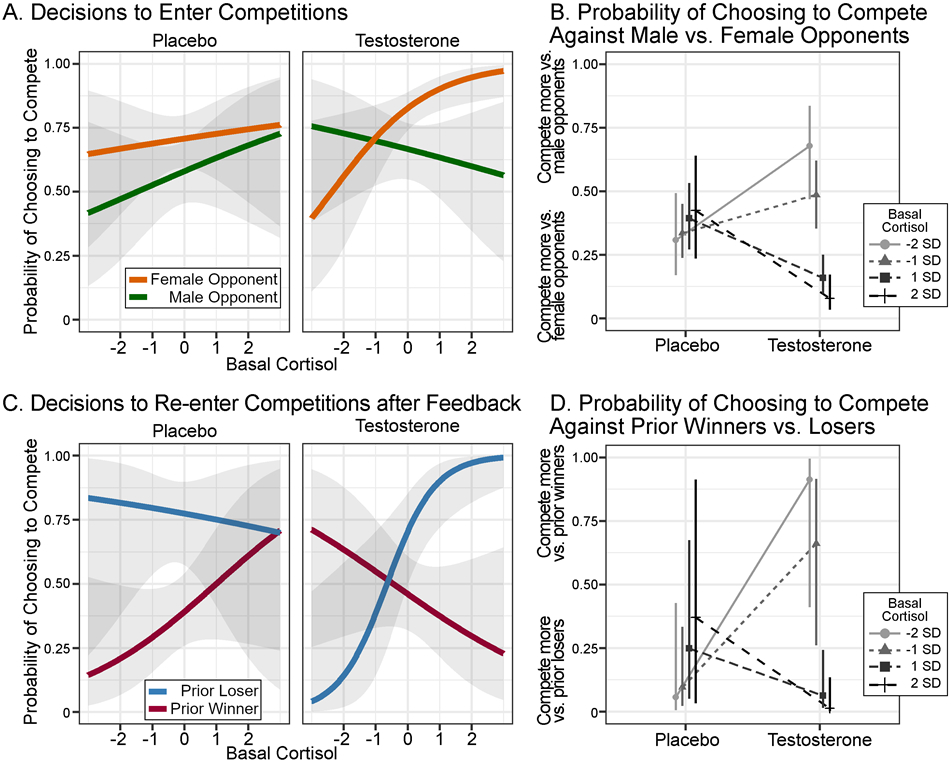
Estimated Marginal Probability of Choosing to Compete as a Function of Testosterone Treatment, Basal Cortisol, and Social Contextual Factors Note. All values on every panel are derived from the main analytical models (e.g., estimated marginal means or simple slopes). Error bands represent 95% CI of model estimates. Panels A and C represent the full range of basal cortisol values, whereas the simple slope analyses in text and Panels B and D present results from ±1 and 2 SD. For illustrative purposes and to better match the prior literature, the probabilities calculated from simple slope logits extracted from the three-way interaction were inverted, that is, “1 − p(Compete),” in Panels B and D. (A) Probability of choosing to enter competitions from the initial phase, conditional on the gender of an opponent. (B) Probability of competing against female opponents versus male opponents for testosterone treatment and placebo groups at several basal cortisol levels. Positive values on this chart indicate that the probability to compete against male opponents is higher than competing against female opponents. (C) Probability of choosing to re-enter competitions after feedback, conditional on prior competitive outcomes. (D) Probability of competing after winning versus after losing for testosterone treatment and placebo groups at several basal cortisol levels. Positive values on this chart indicate that the probability of choosing to compete against a prior winner is higher than competing against a prior loser.
To break down this three-way interaction, we examined the simple slopes associating opponent gender with likelihood to compete at high (+1SD) and low cortisol levels (−1SD) for each treatment group (Preacher et al., 2006). In the placebo group, men competed more against female opponents (perceived low status) than against male opponents (perceived high status), but this effect was roughly equivalent at high (OR = 1.54, [0.882, 2.70]) and low basal cortisol levels (OR = 1.99, [1.23, 3.22]; Figure 4B). In the testosterone group, men who had high basal cortisol levels were substantially more likely to compete against female opponents compared to male opponents (OR = 5.30, [3.01, 9.34]), whereas in men with low basal cortisol levels, the pattern of competing more against female than male opponents was inhibited, due primarily to an increased preference for competing against male opponents (OR = 1.06, [0.611, 1.84]; Figure 4B).
As another strategy to break down this three-way interaction, we examined slopes linking the probability of competing against female and male opponents. A positive slope indicates a greater propensity to compete against female relative to male opponents, and a negative slope value indicates a greater propensity to compete against male relative to female opponents. We regressed opponent gender slope scores on testosterone treatment condition, basal cortisol, and their interaction. The testosterone treatment × basal cortisol interaction was significant (B = 0.184, t(111) = 2.96, p = 0.004). We conducted simple slopes analyses by examining the effect of testosterone treatment (compared to placebo treatment) on the opponent gender slope one standard deviation above and below the basal cortisol mean. Similar to the pattern depicted in Figure 4 (Panel B), these analyses revealed that the effect of testosterone versus placebo on the opponent gender slope went in the opposite directions for those high and low in basal cortisol: There was a positive effect of testosterone compared to placebo on the opponent gender slope for high-cortisol individuals (B = 0.24, [0.07, 0.42]) and a directionally negative effect of testosterone compared to placebo on the opponent gender slope for low-cortisol individuals (B = −0.13, [−0.29, 0.04]). We interpret the somewhat stronger effect of testosterone versus placebo on the opponent gender slope in high-cortisol relative to low-cortisol individuals as noise that is unlikely to be theoretically meaningful (for additional discussion regarding this interpretation, see Supplementary Material)8.
Although overall men are more likely to compete against female opponents compared to male opponents (main effect of opponent gender), these results show that men who were administered testosterone and who had high basal cortisol displayed an increased tendency to compete against women relative to men (i.e., prefer competing against perceived low-status opponents over perceived high-status opponents based on stereotypes), while men given testosterone with low basal cortisol showed the opposite pattern. We also note that the critical three-way interaction between testosterone, basal cortisol, and perceived opponent status replicated across the two blinding conditions, indicating the robustness of this complex pattern of results (see full results in the Supplemental Material).
Other Covariates
In follow-up analyses, variables related to diurnal aspects of endocrine functioning (time of day and time since awakening) and underlying participant skill level (mean points earned in the mandatory piece rate trials) did not substantially alter the results (Table S3).
Decisions to Compete After Win-Loss Feedback
We next examined decisions to re-enter competitions against a mixed-gender pool of opponents in the Feedback rounds, in which participants made decisions after being provided explicit win-loss results from prior rounds in the competition task. The added complexity of these models required removal of the random terms for social-evaluative observation and opponent gender to achieve satisfactory fit. We note that none of the primary behavioral effects or interpretations were meaningfully altered between the more complex and simpler models (Table S11).
Main Effect of Testosterone Treatment
Testosterone did not robustly predict increased decisions to compete after receiving feedback (OR = 1.04, [0.54, 2.02], p = .906, pFDR = .952; Table S4; Figure 3, upper panel). This result does not provide support for the challenge hypothesis.
Main Effects of Opponent Gender and Win-Lose Feedback (Opponent Status Cues)
Participants were still more likely to compete against female compared to male opponents after receiving feedback (OR = 1.90, [1.45, 2.49], p < .001, pFDR < .001; Table S4, Figure 3, middle panel). Participants were also more likely to compete against an opponent that they had previously beaten (controlling for opponent gender) compared to opponents that had previously beaten the participant (OR = 4.48, [1.91, 10.52], p = .001, pFDR = .003; Table S4; Figure 3, lower panel). This result indicates that win-lose feedback was also a cue to opponent status that predicted decisions to compete again.
Testosterone × Opponent Status Cues
Neither of the testosterone treatment interactions with opponent status cues were significantly related to decisions to compete again (testosterone treatment × opponent gender: OR = 0.68, [0.40, 1.16], p = 0.156, pFDR = 0.311; testosterone treatment × prior outcome: OR = 0.56, [0.10, 3.00], p = 0.498, pFDR = 0.725; Table S4).
Dual-Hormone Hypothesis
As in the initial phase, strong support was not evident for a concise dual-hormone hypothesis that does not account for opponent status in the feedback rounds. The effect of testosterone treatment on competitive behavior was not robustly moderated by basal cortisol (OR = 0.93, [0.46, 1.88], p = .840, pFDR = .952; Table S4).
Context-Dependent Dual-Hormone Hypothesis
We next tested the context-dependent dual-hormone hypothesis in the feedback rounds. Building on previous correlational work (Mehta & Josephs, 2010, Study 2), we expected that testosterone, cortisol, and win-lose feedback (a cue to opponent status) would interact to predict decisions to re-enter competitions. A robust testosterone treatment × basal cortisol × prior outcome (win/lose) interaction was found to predict decisions to compete again (OR = 9.55, [1.75, 52.20], p = .009, pFDR = .030; Figure 4; Table S4).
To break down this three-way interaction, we examined the simple slopes comparing likelihood of competing after wins and losses for each treatment group at high (+1SD) and low cortisol (−1SD). In the placebo group, men competed more against losers (objectively lower-status opponents) compared to winners (objectively higher-status opponents) regardless of whether the men had high (OR = 3.02, [0.48, 18.81]) or low cortisol values (OR = 9.49, [2.00, 44.92]). In the testosterone group, men who had high basal cortisol levels were substantially more likely to compete again against losers (lower-status opponents) compared to winners (higher-status opponents; OR = 14.90, [3.13, 70.85]), whereas men with low basal cortisol were less likely to compete against losers compared to winners (OR = 0.51, [0.093, 2.85]). This pattern of results – that is, testosterone and cortisol linked with competitive behavior conditional on an opponent’s status – is consistent with previous correlational research on decisions to compete against the same opponent (Study 2,(Mehta & Josephs, 2010)9.
As with the initial phase, we used another strategy to break down this three-way interaction by extracting slopes linking probability of competing against low- and high-status opponents. A positive slope value indicates a greater propensity to compete against low-status (prior losers) relative to high-status opponents (prior winners), and a negative value indicates a greater propensity to compete against high-status relative to low-status opponents. We regressed testosterone treatment condition, basal cortisol, and their interaction on opponent status slope scores, which revealed a significant treatment × basal cortisol interaction (B = 1.41, t(109) = 2.45, p = .016). We conducted simple slopes analyses by examining the effect of testosterone treatment (compared to placebo treatment) on opponent status slope scores one standard deviation above and below the basal cortisol mean. Similar to the pattern shown in Figure 4D, these analyses revealed that testosterone’s effect on the opponent status slope went in opposite directions for those low and high in basal cortisol: There was a negative effect of testosterone versus placebo on the opponent status slope for low-cortisol individuals (B = −1.84, [−3.41, −0.28]) and a directionally positive effect of testosterone versus placebo on the opponent status slope for high-cortisol individuals (B = 0.97, [−0.64, 2.58]). We interpret the somewhat stronger effect of testosterone on the opponent status slope in low-cortisol relative to high-cortisol individuals as noise that is unlikely to be theoretically meaningful (for additional discussion regarding this interpretation, see Supplemental Material)10.
Overall, these analyses reveal that testosterone treatment coupled with low basal cortisol levels was associated with a relative preference to compete more against men and compete more against higher status opponents (prior winners). Testosterone treatment coupled with high basal cortisol levels was associated with a relative preference to compete more against women and compete more against lower status opponents (prior losers). We note, again, that this pattern replicated across both blinding conditions (see Supplemental Material).
Notably, the testosterone treatment × basal cortisol × opponent gender interaction was not robustly linked to decisions to compete again in the feedback rounds, controlling for competition outcome (OR = 0.93, [0.52, 1.65], p = .796, pFDR = .910; Table S4). The exploratory four-way interaction among testosterone treatment, cortisol, opponent gender, and outcome was not robust (OR = 1.02, [0.28, 3.72], p = .976; Table S4). Thus, after receiving explicit opponent status information via relative performance feedback (win/loss feedback), men given testosterone who had high basal cortisol were more likely to compete against lower status opponents (prior losers), rather than compete discriminately against female opponents. Men given testosterone who had low basal cortisol showed the opposite pattern. An objective indicator of relative opponent status – that is, prior victory or defeat – seems to override gender as a status cue.
Other Covariates
Controlling for time of day, time since awakening, and participant skill level did not substantially alter the three-way interaction between testosterone treatment, cortisol, and prior competitive outcome on decisions to re-enter competitions after feedback (Table S5).
Secondary Analyses
Trait dominance
We did not find strong evidence that trait dominance moderated the effect of testosterone, either alone or in interaction with basal cortisol or opponent status cues, on men’s decisions to compete (Table S9). See Supplemental Materials for additional discussion on this topic.
Subjective Ratings of Opponents
In line with prior work on gender stereotypes (Spencer et al., 1999), female opponents were rated lower than male opponents on “good at…math” by the participants who completed the competition task (B = −0.41, [−0.68, −0.15], p = .021). This effect of opponent gender was robust to controlling for previously winning or losing to a given opponent (B = −0.36, [−0.68, −0.05], p = .039). In the follow-up sample of men who did not participate in the competition task, an effect of gender on “good at…math” was again observed (B = −0.625, [−1.18, −0.07], p = .038). Supplementary analyses with other subjective ratings (e.g. intelligent, dominant) reveal that these opponent gender effects were specific to math-ability ratings (Table S10). These results are suggestive that female opponents were perceived as lower-status opponents compared to male opponents in the math-based competition.
Exploratory Analyses
Dual Hormone Effects with Other Cortisol Measures
We repeated analyses on decisions to enter and re-enter competitions using cortisol change from before to after the competition task and cortisol AUCG, a measure of diurnal cortisol exposure across the experimental period that encompasses the basal measure taken prior to testosterone treatment (or placebo) and the two measures taken immediately before and after the competition task. Cortisol change scores from before to after the competition task did not robustly moderate the interaction between testosterone treatment and opponent status cues on competitive behavior (Tables S6 and S7). These exploratory analyses did reveal an unexpected, weak two-way interaction between testosterone treatment and cortisol change scores in both phases of the competition task. However, the effect was even weaker and the estimates more variable in models that did not include the higher-level interactions with opponent status cues (see Supplementary Materials). Cortisol AUCG showed similar though weaker moderation effects compared to our primary analyses that focused on basal cortisol. The weaker effects in these analyses compared to primary basal cortisol analyses are consistent with previous work on the dual-hormone hypothesis, which suggests that basal cortisol is a key moderator of testosterone’s behavioral effects in the absence of acute stress, compared to state measures of cortisol (Knight, Sarkar, et al., 2020).
Competition Score, Satisfaction, and Outcome
We next explored if the three-way interaction between testosterone treatment, basal cortisol, and opponent gender was associated with points scored, likelihood of winning, or trial satisfaction. These analyses did not reveal robust effects of the three-way interaction on any of these measures in the choice trials (Table S8). These results indicate that although testosterone treatment interacted with basal cortisol and opponent gender to predict decisions to compete, this three-way interaction did not predict performance outcomes or trial-by-trial satisfaction.
Meta-analyses
We examined the meta-effect of the interaction of testosterone, cortisol, and prior competition outcome across this experiment and the previously published correlational study (Mehta & Josephs, 2010). In the fixed-effects model, a significant three-way interaction meta-effect was evident (Fisher’s Z = 0.272, [0.119, 0.425], Z = 3.48, p < 0.001; Figure 5A). We also examined a random-effects model with both of the three-way interactions from the initial and feedback rounds included (i.e., testosterone treatment × basal cortisol × opponent gender and testosterone treatment × basal cortisol × prior outcome, respectively). This model also provided support for a three-way interaction among testosterone, cortisol, and opponent status (Fisher’s Z = 0.293, [0.175, 0.411], Z = 4.86, p <.0001; Figure 5B).
Figure 5.
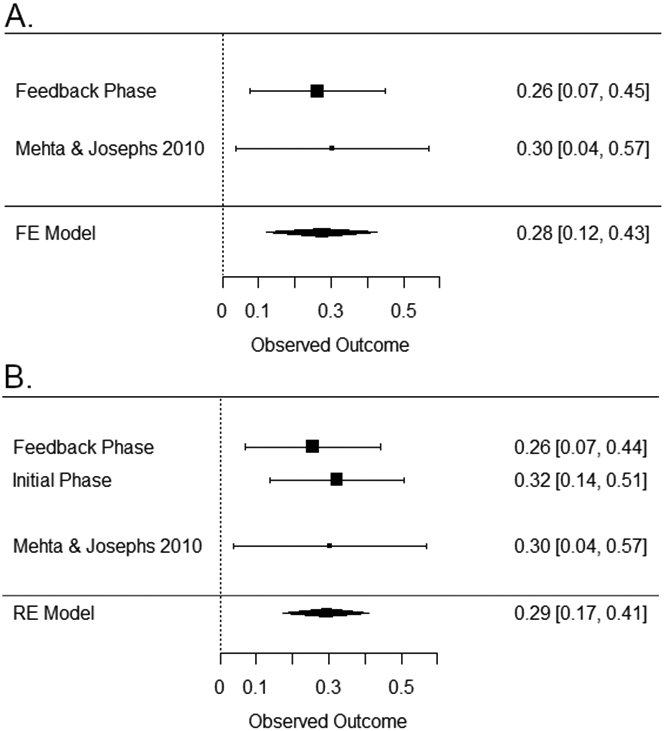
Meta-analyses of testosterone × cortisol × opponent status in two studies. These figures depict Fisher’s z values. A) Fixed effects (FE) model with prior outcome as indicator of opponent status in the present experiment. B) Random effects (RE) model that includes both prior outcome and opponent gender as indices of perceived opponent status.
Discussion
This experiment examined the causal effects of testosterone on men’s competitive behavior within a context-dependent dual-hormone framework by measuring basal cortisol levels and pharmacologically manipulating testosterone prior to a math-based competition against male and female opponents. Prior research inconsistently linked testosterone and testosterone-cortisol interactions to competitive behavior (Dekkers et al., 2019; Nadler et al., 2021). Our results indicate that the context-dependence of testosterone-cortisol interactions is critical in explaining these inconsistencies.
Specifically, the causal effects of testosterone on competitive behavior depended on basal cortisol and opponent gender in the competition’s initial rounds. After win-lose feedback was provided, testosterone’s influence on decisions to re-enter competitions depended on basal cortisol and this objective opponent status cue, not gender. Compared to men given placebo, exogenous testosterone given to men low in basal cortisol promoted status-seeking behavior – competing more against male and prior-winning opponents compared to female and prior-losing opponents. Exogenous testosterone given to men high in basal cortisol induced status-loss avoidance behavior – competing more against female and prior-losing opponents compared to male and prior-winning opponents. In the present experiment, testosterone treatment’s direct effect on competitive behavior was not robust; a robust effect of testosterone was evident only when examined within a context-dependent dual-hormone framework.
Our results extend existing theoretical frameworks (Archer, 2006; Wingfield et al., 1990) and correlational research ((Henry et al., 2017; Mehta & Josephs, 2010) by providing experimental evidence that testosterone’s influence on men’s competitive behavior depends on basal cortisol levels and cues to an opponent’s perceived status11. The present experiment used an innovative design that included pharmacological hormone administration, within-subject comparisons of competitive decisions toward high- and low-status opponents, multiple cues of an opponent’s status, and a larger sample size compared to prior work. Further, supplemental (meta-)analyses (i) confirmed an internal replication of the context-dependent dual-hormone interaction across two opponent status cues and two blinding conditions, and (ii) provided robust evidence for this interaction across the present experiment and previous work.
Opponent gender was likely used as a status cue because the competition task was math-based and contained no explicit status indicators in the initial rounds. This interpretation is supported by subjective ratings and stereotypes of women as less skilled in math (Cheryan et al., 2017; Ellemers, 2018; Spencer et al., 1999). Moreover, objective opponent status information eliminated the testosterone, cortisol, opponent gender interaction, consistent with evidence that stereotypes influence person perception and behavior in the absence of objective, individuating information about a target (Fiske & Neuberg, 1990). The importance of gender as a status cue likely varies across competitions; other domain-specific cues may outweigh gender in certain contexts, such as cues to intelligence in a trivia contest (Talamas et al., 2016).
Possible Mechanisms
Threat and reward may explain testosterone and cortisol’s associations with status-seeking or status-loss-avoidance. Elevated testosterone and cortisol levels have been linked to psychological and neural markers of social threat (Denson, Ronay, et al., 2013; Enter et al., 2019; Goetz et al., 2014; Knight & Mehta, 2017; Mehta & Beer, 2010; Van Honk et al., 1998). High-testosterone, high-cortisol men may have been threatened by high-status opponents, resulting in avoiding status loss by eschewing high-status opponents and pursuing competition against lower-status opponents. Further, some previous research has linked high testosterone and low cortisol to psychological and neural markers of reward (Duell et al., 2021; Hermans et al., 2010; Montoya et al., 2014; Op De Macks et al., 2011; Welker et al., 2015). A high-testosterone, low-cortisol individual facing a high-status opponent may have experienced reward anticipation due to the potential for gaining status, rather than a threat response.
These reward and threat mechanisms may be further informed by the biopsychosocial model of challenge and threat (Tomaka et al., 1993). Challenge and threat states – the perceived availability or lack of resources (respectively) to deal with the demands of a situation – are associated with approach and avoidant behavior, respectively (Blascovich, 2008; Mendes et al., 2001). We speculate that high testosterone and low cortisol levels may activate a challenge state, prompting individuals to approach competitions against high-status opponents relative to low-status opponents. High testosterone and high cortisol levels may instead activate a threat state, prompting individuals to avoid high-status opponents and approach competitions against lower-status opponents. Future research can test these hypotheses by examining psychological and cardiovascular indices of challenge and threat states (Blascovich et al., 2003; Scheepers & Knight, 2020; Tomaka et al., 1993), in conjunction with dual hormone profiles.
An additional mechanism related to reward and threat may involve risk taking (Knutson et al., 2008). Two correlational studies found that testosterone levels were positively related to risk taking among men with low basal cortisol, but were unrelated or negatively related to risk taking among men with high basal cortisol (Mehta, Welker, et al., 2015; Ronay et al., 2018). Competing against a high-status opponent may be considered risky because the chances of losing are greater relative to competing against a low-status opponent.
These proximate mechanisms may inform a broader evolutionary framework that explains the impact of stress on the hormonal reproductive axis. When stress is low, it may be evolutionarily adaptive for elevated testosterone to promote status-seeking behavior focused on challenging high-status individuals. But when stress is high, status-seeking may be inhibited because such behaviors are metabolically costly and potentially dangerous (Buchanan et al., 2003; Haselton & Buss, 2000; Maner et al., 2012), such that testosterone may instead promote status-loss avoidance behavior.
Theoretical Implications
The Social Neuroendocrinology of Status
Several studies have provided support for the dual-hormone hypothesis on attainment of social status. Specifically, higher basal testosterone is related to higher status among individuals with lower levels of basal cortisol, whereas higher basal testosterone is either unrelated or negatively related to status among individuals with higher levels of basal cortisol (Casto et al., 2019; Dekkers et al., 2019; Edwards & Casto, 2013; Ponzi et al., 2016; Sherman et al., 2016; cf. Mazur et al., 2015). This conditional theorizing aligns with nonhuman primate work, in which a search for “the” gonadal profile of status was argued to be unproductive given the strong influences of individual differences and the social context on social status outcomes (Sapolsky, 1991). The present results suggest a behavioral mechanism that may explain the testosterone-cortisol interaction as a predictor of social status. High testosterone-low cortisol individuals may attain higher status because competing against high-status opponents provides these individuals with increased opportunities to rise in the social hierarchy. Conversely, high testosterone-high cortisol individuals may fail to attain high status because they avoid competitions against high-status competitors.
Social Psychology of Hierarchies and Competition
Prior social psychological research has focused primarily on how hierarchies influence psychology and behavior, including competitive and aggressive behavior, but has largely ignored biological factors (Buser, 2016; Fast & Chen, 2009; Hays & Bendersky, 2015; Keltner et al., 2003; N. L. Mead & Maner, 2012; van Kleef & Cheng, 2020). The present experiment points to the value of studying hormones in behavioral studies of hierarchy and competition.
Specifically, the present results suggest that social status does not indiscriminately increase or decrease competitive behavior; rather, strategic preferences to compete depend on interactions among testosterone and cortisol. Basal testosterone and cortisol levels can be considered biological individual differences (Liening et al., 2010; Mehta et al., 2008; Sellers et al., 2007) that weakly correlate with self-report measures (Grebe et al., 2019; Sundin et al., 2021) and operate largely outside of conscious awareness (Akinola et al., 2016; Josephs et al., 2006; Schultheiss et al., 2005; Terburg et al., 2012). Hence, hormones may be critical to advance theories of hierarchy and competition given the unique role of hormones in influencing behavior beyond standard self-report measures.
Mixed-gender Hierarchies
This experiment fills an important empirical gap by studying men engaging in a math-based competition against male and female opponents. The present findings may have implications for real world hierarchies and the representation and advancement of women within them. Women are underrepresented in many hierarchies, including in science, technology, engineering, and math (STEM) fields, and are less likely to advance to high-status positions, in part due to hyper-competitive and other caustic behaviors directed toward them (Berdahl, 2007; Cheryan et al., 2009, 2017; Dasgupta & Stout, 2014; Flory et al., 2015; Glick & Fiske, 2001; Gruber et al., 2020; London et al., 2012; Vandello et al., 2008; Welde & Laursen, 2011). Our results imply that high-testosterone, high-cortisol men may engage in maladaptive competitive behaviors (e.g., aggression, derogation, harassment) directed towards women and low-status individuals and deferential behaviors towards men and high-status individuals. This line of research at the intersection of biological and social factors that guide status-relevant behavior may help improve gender representation and working conditions in mixed-gender environments.
Limitations and Future Directions
We studied male participants due to restrictions on the use of exogenous testosterone in the experiment’s location. Future work should test the generalizability of the present results to female participants (see Henry et al., 2017 for related work in females).
We measured basal cortisol levels because the dual-hormone hypothesis specifically focuses on basal cortisol as a dispositional measure (Dekkers et al., 2019). Future work may consider several options to improve measurement of trait-like basal cortisol, including collecting multiple samples prior to testosterone administration, examining diurnal endocrine rhythms over multiple days, or assaying hormones from hair (Grotzinger et al., 2018; Ronay et al., 2018).
The present experiment manipulated testosterone levels because of the foundational theory on testosterone and competitive behavior (Archer, 2006; Wingfield et al., 1990). An important next step is to conduct dual-systems pharmacology experiments in which the HPG and HPA axes are manipulated simultaneously. Such dual-systems protocols are not readily available and therefore will require rigorous pharmacokinetics testing before theory-driven psychological studies are conducted and interpreted12. A complementary approach is to manipulate testosterone levels indirectly via contextual manipulations rather than pharmacology (Kordsmeyer & Penke, 2019; Roney et al., 2007). Pharmacological and contextual approaches have strengths and limitations; we encourage adoption of both approaches or hybrid designs to build cumulative knowledge about testosterone-cortisol interactions on social behavior.
Cortisol levels can also be contextually manipulated via laboratory stressors (Dickerson & Kemeny, 2004),and cortisol reactions in the context of acute stress have been shown to moderate testosterone’s association with status-relevant behavior (Nitschke & Bartz, 2020; Prasad et al., 2017, 2019)13. Despite the focus of prior dual-hormone studies and the present experiment on basal cortisol and its slower, genomic mechanisms (Dekkers et al., 2019; Tilbrook, 2000), acute cortisol reactions to stress may impact testosterone’s association with behavior via faster, nongenomic mechanisms (Makara & Haller, 2001; Moore & Evans, 1999). Some evidence even suggests opposing effects of basal and state cortisol on behavior (Montoya et al., 2012) . Although ‘trait versus state’ (i.e., basal versus acute cortisol) hormone distinctions are foundational to endocrine research, the psychosocial implications of these distinctions need further examination.
We chose to examine testosterone and cortisol because they have been widely studied across species in the context of hierarchy and competition. Yet, the HPG and HPA axes are complex and highly integrated (Rubinow et al., 2005; Viau, 2002). Thus, it will be important for future work to study other factors within these hormone axes, such as hypothalamic and pituitary hormones that regulate the release of cortisol and testosterone, other gonadal hormones like estradiol (Blake et al., 2017; Stanton & Schultheiss, 2007; Tackett et al., 2015), and androgen and glucocorticoid receptors. The HPA and HPG axes are also linked with the autonomic nervous system. For example, the sympathetic nervous system physiologically mediates coactivation of cortisol and testosterone responses in the context of threat by lowering HPG axis sensitivity to glucocorticoids (Chichinadze & Chichinadze, 2008; Wingfield & Sapolsky, 2003). Thus, we recommend that future studies build on this prior work by examining potential integration with the autonomic nervous system.
Conclusion
According to prevailing theories, increased levels of testosterone should directly increase competitive behavior (Archer, 2006; Wingfield et al., 1990). The results of our experiment indicate that a more nuanced perspective on the role of this hormone in regulating competitive behavior is needed. Using a mixed-gender math-based competition, we found that testosterone’s influence on men’s competitive decisions depends on basal cortisol and cues to an opponent’s perceived status. Testosterone treatment in low-cortisol men evoked status-seeking behavior: An increased preference to compete against male opponents and previous winners (cues to high perceived status) compared to female opponents and previous losers (cues to low perceived status). Testosterone treatment in high-cortisol men evoked status-loss avoidance behavior: An increased preference to compete against female opponents and previous losers compared to male opponents and previous winners. Key next steps include identifying mechanisms and testing for similar interactions between biology and social context in real-world, mixed-gender hierarchies.
Supplementary Material
Acknowledgements
This experiment was supported by NSF grant #1063561 (Mayr and Harbaugh) and by NSF grant #1451848 (Mehta). Dr. Knight received support from NIH grant T32 AG049676 (via Pennsylvania State University) during production of the preprint of this report. The authors wish to thank Cassandra Brandes, Dr. Stephanie Kramer, Bethany Lassetter, Dr. Jason Isbell, and Jeffrey D. Whitaker for their help with data collection. We thank Dr. Craig Davidson for his advice throughout the study preparation and execution.
Footnotes
Our primary analyses focused on basal cortisol as a dispositional factor that was expected to modulate testosterone’s effects on competitive behavior. Another theoretical framework, which has received less attention in previous work than the challenge hypothesis or dual-hormone hypothesis, suggests that testosterone’s associations with status-relevant behaviors may be stronger among individuals high in self-reported trait dominance. We examined this possibility in secondary analyses. See the Methods section for further background.
In line with previous theory and research on the dual-hormone hypothesis, our primary analyses examined basal cortisol as a dispositional factor that was expected to moderate exogenous testosterone’s behavioral effects. Indeed, the physiological evidence guiding the dual-hormone hypothesis suggests that chronically elevated cortisol (basal cortisol) robustly affects HPG axis functioning, rather than acute changes in cortisol (Tilbrook, 2000). In line with this physiological evidence, previous research on the dual-hormone hypothesis has primarily examined basal hormone concentrations as predictors of status-seeking behaviors (Dekkers et al., 2019; Knight, Sarkar, et al., 2020). Finally, the previous correlational study that tested the context-dependent dual-hormone hypothesis found that basal cortisol moderated basal testosterone’s association with competitive behavior, as opposed to state measures of cortisol taken during the study (Mehta & Josephs, 2010). Acute cortisol changes seem to be a more critical modulator of testosterone-behavior associations after an acute stressor (Prasad et al., 2017, 2019), but the present experiment did not include an acute stressor prior to the competition task. Overall, prior research indicates that basal cortisol is expected to moderate exogenous testosterone’s impact on competitive behavior in the present experiment, rather than state measures of cortisol. Nevertheless, because little is known about state cortisol within the dual-hormone hypothesis literature, in the supplemental material we report exploratory analyses with state measures of cortisol and have made the raw data open for further exploration.
This delay of several hours between testosterone treatment and behavioral testing is consistent with relatively slow, genomic mechanisms for hormonal effects on behavior. Later research published after these data were collected suggests that topical testosterone treatment increases testosterone and produces measurable physiological differences within an hour post-gel administration (Bird et al., 2016; Carré et al., 2017). Hormonal influences on behavior over this shorter time period may be occurring through more rapid, non-genomic mechanisms (Makara & Haller, 2001; Moore & Evans, 1999).
For example, assume the participant and opponent each scored 5 points, but the participant earned those points in 8.9 seconds compared to the opponent, who required 9.2 seconds. In this case, the participant (8.9 < 9.2) won that round.
Trait dominance × testosterone interactions have been studied using different definitions of dominance – a possible “jingle fallacy” (Block, 1995) – and therefore different scales to measure trait dominance. In the supplementary material, we discuss how these issues may be contributing to heterogeneous results across studies.
See supplement for model fit statistics for all models.
See supplement for reporting of main effects of basal cortisol and experimental blinding manipulation, each of which was not a primary focus of this experiment and each of which demonstrated non-significant associations with decisions to compete in the initial and feedback round of the competition task.
We also conducted simple slopes analyses by examining the association of basal cortisol with the opponent gender slope in the testosterone treatment and placebo conditions. These simple slopes analyses indicated that basal cortisol was unrelated to this opponent gender slope in the placebo group (B = −0.02, [−0.11, 0.07]), whereas basal cortisol was positively related to the opponent gender slope in the testosterone group (B = 0.16, [0.08, 0.25]). This pattern is consistent with the simple slopes reported in the main text. Collectively, these results are consistent with the context-dependent dual-hormone hypothesis: Basal cortisol moderates the causal effect of testosterone on decisions to enter competitions against female relative to male opponents.
This previous study experimentally manipulated opponent status as a between-subjects factor, and therefore, the three-way interaction between testosterone, cortisol, and opponent status was interpreted by examining hormone-behavior patterns separately in each opponent status condition. Nevertheless, to facilitate comparison with the present experiment, we went back to this previous study and examined the directional patterns for competitive behavior towards high- and low-status opponents as a function of testosterone and cortisol levels. The directional patterns were consistent with the present experiment’s findings. For example, high-testosterone low-cortisol individuals showed an increased propensity to compete against high-status opponents relative to low-status opponents, whereas high-testosterone high-cortisol individuals were more likely to avoid competitions against high-status relative to low-status opponents. Therefore, we conclude that the interaction between testosterone, cortisol, and opponent status was consistent across the two studies, and both studies are consistent with the context-dependent dual hormone hypothesis.
We also conducted simple slopes analyses by examining the association of basal cortisol with opponent-status slope scores (i.e., the difference in competing against high- vs. low-status opponents) in the testosterone treatment and placebo conditions. Simple slopes analyses indicated that basal cortisol was not strongly related to the opponent status slope in the placebo group (B = −0.40, [−1.22, 0.42]), whereas basal cortisol was positively related to the opponent status slope in the testosterone group (B = 1.00, [0.23, 1.78]). This pattern is consistent with the simple slopes reported in the main text. Collectively, these results are consistent with the context-dependent dual-hormone hypothesis: Basal cortisol moderates the causal effect of testosterone on decisions to enter competitions against low-status relative to high-status opponents.
We did not find evidence in support of trait dominance accentuating the effects of testosterone on competitive behavior. In the supplemental materials, we discuss several possibilities that might explain this lack of support and future directions to continue to investigate the interaction between testosterone and trait dominance.
It is not clear that manipulating both hormones simultaneously is feasible. Simultaneous manipulation could result in strong, negative feedback that reduces associations between biology and behavior or otherwise produces unexpected results (Rubinow et al., 2005). This experimental work also would need to aim to manipulate longer-term, basal HPA axis functioning to accommodate the focus on basal cortisol in the dual-hormone hypothesis.
Although we did not find evidence that cortisol levels rose during the competition task in the present experiment, future work may consider other competitive tasks to reduce the possibility of math eliciting a cortisol response.
References
- Akinola M, Page-Gould E, Mehta PH, & Lu JG (2016). Collective hormonal profiles predict group performance. Proceedings of the National Academy of Sciences, 113(35), 9774–9779. 10.1073/pnas.1603443113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer J (2006). Testosterone and human aggression: An evaluation of the challenge hypothesis. Neuroscience & Biobehavioral Reviews, 30(3), 319–345. 10.1016/j.neubiorev.2004.12.007 [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker BM, & Walker SC (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Berdahl JL (2007). Harassment Based on Sex: Protecting Social Status in the Context of Gender Hierarchy. Academy of Management Review, 32(2), 641–658. 10.5465/amr.2007.24351879 [DOI] [Google Scholar]
- Bertsch K, Böhnke R, Kruk MR, Richter S, & Naumann E (2011). Exogenous cortisol facilitates responses to social threat under high provocation. Hormones and Behavior, 59(4), 428–434. 10.1016/J.YHBEH.2010.12.010 [DOI] [PubMed] [Google Scholar]
- Bird BM, Welling LLM, Ortiz TL, Moreau BJP, Hansen S, Emond M, Goldfarb B, Bonin PL, & Carré JM (2016). Effects of exogenous testosterone and mating context on men’s preferences for female facial femininity. Hormones and Behavior. 10.1016/j.yhbeh.2016.08.003 [DOI] [PubMed] [Google Scholar]
- Blake KR, Bastian B, O’Dean SM, & Denson TF (2017). High estradiol and low progesterone are associated with high assertiveness in women. Psychoneuroendocrinology. 10.1016/j.psyneuen.2016.10.008 [DOI] [PubMed] [Google Scholar]
- Blascovich J (2008). Challenge and Threat. In Elliot AJ (Ed.), Handbook of Approach and Avoidance Motivation (pp. 431–445). Taylor & Francis. 10.4324/9780203888148.ch25 [DOI] [Google Scholar]
- Blascovich J, Mendes WB, Tomaka J, Salomon K, & Seery M (2003). The Robust Nature of the Biopsychosocial Model Challenge and Threat: A Reply to Wright and Kirby. Personality and Social Psychology Review, 7(3), 234–243. 10.1207/S15327957PSPR0703_03 [DOI] [PubMed] [Google Scholar]
- Block J (1995). A contrarian view of the five-factor approach to personality description. Psychological Bulletin, 117(2), 187–215. 10.1037/0033-2909.117.2.187 [DOI] [PubMed] [Google Scholar]
- Brown AJ, & Goh JX (2016). Some Evidence for a Gender Gap in Personality and Social Psychology. Social Psychological and Personality Science, 7(5), 437–443. 10.1177/1948550616644297 [DOI] [Google Scholar]
- Brown LL, Tomarken AJ, Orth DN, Loosen PT, Kalin NH, Davidson RJ, Whitney M, Fraser M, Morgan S, Nicholson WE, Hawkins B, Farley G, & Turner J (1996). Individual differences in repressive-defensiveness predict basal salivary cortisol levels. Journal of Personality and Social Psychology, 70(2), 362–371. [DOI] [PubMed] [Google Scholar]
- Buchanan KL, Evans MR, & Goldsmith AR (2003). Testosterone, dominance signalling and immunosuppression in the house sparrow, Passer domesticus. Behavioral Ecology and Sociobiology, 55(1), 50–59. 10.1007/s00265-003-0682-4 [DOI] [Google Scholar]
- Burnstein KL, Maiorino CA, Dai JL, & Cameron DJ (1995). Androgen and glucocorticoid regulation of androgen receptor cDNA expression. Molecular and Cellular Endocrinology, 115(2), 177–186. 10.1016/0303-7207(95)03688-1 [DOI] [PubMed] [Google Scholar]
- Buser T (2016). The Impact of Losing in a Competition on the Willingness to Seek Further Challenges. Management Science, 62(12), 3439–3449. 10.1287/mnsc.2015.2321 [DOI] [Google Scholar]
- Carré JM, Geniole SN, Ortiz TL, Bird BM, Videto A, & Bonin PL (2017). Exogenous testosterone rapidly increases aggressive behavior in dominant and impulsive men. Biological Psychiatry. 10.1016/j.biopsych.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Carré JM, & McCormick CM (2008). Aggressive behavior and change in salivary testosterone concentrations predict willingness to engage in a competitive task. Hormones and Behavior, 54(3), 403–409. 10.1016/j.yhbeh.2008.04.008 [DOI] [PubMed] [Google Scholar]
- Carré JM, & Olmstead NA (2015). Social neuroendocrinology of human aggression: Examining the role of competition-induced testosterone dynamics. In Neuroscience. 10.1016/j.neuroscience.2014.11.029 [DOI] [PubMed] [Google Scholar]
- Carré JM, Putnam SK, & McCormick CM (2009). Testosterone responses to competition predict future aggressive behaviour at a cost to reward in men. Psychoneuroendocrinology, 34(4), 561–570. 10.1016/j.psyneuen.2008.10.018 [DOI] [PubMed] [Google Scholar]
- Casto KV, Edwards DA, Akinola M, Davis C, & Mehta PH (2020). Testosterone reactivity to competition and competitive endurance in men and women. Hormones and Behavior, 123, 104665. 10.1016/J.YHBEH.2019.104665 [DOI] [PubMed] [Google Scholar]
- Casto KV, & Edwards DA (2016). Testosterone, cortisol, and human competition. In Hormones and Behavior. 10.1016/j.yhbeh.2016.04.004 [DOI] [PubMed] [Google Scholar]
- Casto KV, Hamilton DK, & Edwards DA (2019). Testosterone and Cortisol Interact to Predict Within-Team Social Status Hierarchy among Olympic-Level Women Athletes. Adaptive Human Behavior and Physiology. 10.1007/s40750-019-00115-2 [DOI] [Google Scholar]
- Chen S, Wang J, Yu G, Liu W, & Pearce D (1997). Androgen and Glucocorticoid Receptor Heterodimer Formation. Journal of Biological Chemistry, 272(22), 14087–14092. 10.1074/jbc.272.22.14087 [DOI] [PubMed] [Google Scholar]
- Cheng JT, Kornienko O, & Granger DA (2018). Prestige in a large-scale social group predicts longitudinal changes in testosterone. Journal of Personality and Social Psychology. 10.1037/pspi0000126 [DOI] [PubMed] [Google Scholar]
- Cheng JT, Tracy JL, Foulsham T, Kingstone A, & Henrich J (2013). Two ways to the top: Evidence that dominance and prestige are distinct yet viable avenues to social rank and influence. Journal of Personality and Social Psychology. 10.1037/a0030398 [DOI] [PubMed] [Google Scholar]
- Cheryan S, Plaut VC, Davies PG, & Steele CM (2009). Ambient belonging: How stereotypical cues impact gender participation in computer science. Journal of Personality and Social Psychology, 97(6), 1045–1060. 10.1037/a0016239 [DOI] [PubMed] [Google Scholar]
- Cheryan S, Ziegler SA, Montoya AK, & Jiang L (2017). Why are some STEM fields more gender balanced than others? Psychological Bulletin. 10.1037/bul0000052 [DOI] [PubMed] [Google Scholar]
- Chichinadze K, & Chichinadze N (2008). Stress-induced increase of testosterone: Contributions of social status and sympathetic reactivity. Physiology and Behavior. 10.1016/j.physbeh.2008.03.020 [DOI] [PubMed] [Google Scholar]
- Dasgupta N, & Stout JG (2014). Girls and Women in Science, Technology, Engineering, and Mathematics. Policy Insights from the Behavioral and Brain Sciences. 10.1177/2372732214549471 [DOI] [Google Scholar]
- Datta Gupta N, Poulsen A, & Villeval MC (2013). Gender matching and competitiveness: Experimental evidence. Economic Inquiry. 10.1111/j.1465-7295.2011.00378.x [DOI] [Google Scholar]
- Dekkers TJ, van Rentergem JAA, Meijer B, Popma A, Wagemaker E, & Huizenga HM (2019). A meta-analytical evaluation of the dual-hormone hypothesis: Does cortisol moderate the relationship between testosterone and status, dominance, risk taking, aggression, and psychopathy? In Neuroscience and Biobehavioral Reviews. 10.1016/j.neubiorev.2018.12.004 [DOI] [PubMed] [Google Scholar]
- Denson TF, Mehta PH, & Ho Tan D (2013). Endogenous testosterone and cortisol jointly influence reactive aggression in women. Psychoneuroendocrinology. 10.1016/j.psyneuen.2012.07.003 [DOI] [PubMed] [Google Scholar]
- Denson TF, Ronay R, von Hippel W, & Schira MM (2013). Endogenous testosterone and cortisol modulate neural responses during induced anger control. Social Neuroscience. 10.1080/17470919.2012.655425 [DOI] [PubMed] [Google Scholar]
- Deutsch M (1949). A Theory of Co-operation and Competition. Human Relations. 10.1177/001872674900200204 [DOI] [Google Scholar]
- Dickerson SS, & Kemeny ME (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. In Psychological Bulletin. 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- Du JY, Sanchez P, Kim L, Azen CG, Zava DT, & Stanczyk FZ (2013). Percutaneous progesterone delivery via cream or gel application in postmenopausal women. Menopause, 20(11), 1169–1175. 10.1097/GME.0b013e31828d39a2 [DOI] [PubMed] [Google Scholar]
- Duell N, van Hoorn J, McCormick EM, Prinstein MJ, & Telzer EH (2021). Hormonal and neural correlates of prosocial conformity in adolescents. Developmental Cognitive Neuroscience, 48, 100936. 10.1016/j.dcn.2021.100936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DA, & Casto KV (2013). Women’s intercollegiate athletic competition: Cortisol, testosterone, and the dual-hormone hypothesis as it relates to status among teammates. Hormones and Behavior. 10.1016/j.yhbeh.2013.03.003 [DOI] [PubMed] [Google Scholar]
- Eisenegger C, Naef M, Snozzi R, Heinrichs M, & Fehr E (2010). Prejudice and truth about the effect of testosterone on human bargaining behaviour. Nature, 463(7279), 356–359. 10.1038/nature08711 [DOI] [PubMed] [Google Scholar]
- Eisenegger C, von Eckardstein A, Fehr E, & von Eckardstein S (2013). Pharmacokinetics of testosterone and estradiol gel preparations in healthy young men. Psychoneuroendocrinology, 38(2), 171–178. 10.1016/j.psyneuen.2012.05.018 [DOI] [PubMed] [Google Scholar]
- Ellemers N (2018). Gender Stereotypes. Annual Review of Psychology, 69(1), 275–298. 10.1146/annurev-psych-122216-011719 [DOI] [PubMed] [Google Scholar]
- Enter D, Hutschemaekers MHM, & Roelofs K (2019). Neuroendocrinological aspects of social anxiety and aggression-related disorders. In Schultheiss OC & Mehta PH (Eds.), Routledge International Handbook of Social Neuroendocrinology (pp. 635–655). Routledge. 10.4324/9781315200439-35 [DOI] [Google Scholar]
- Fast NJ, & Chen S (2009). When the Boss Feels Inadequate. Psychological Science, 20(11), 1406–1413. 10.1111/j.1467-9280.2009.02452.x [DOI] [PubMed] [Google Scholar]
- Fiske ST, Cuddy AJC, Glick P, & Xu J (2002). A model of (often mixed) stereotype content: Competence and warmth respectively follow from perceived status and competition. Journal of Personality and Social Psychology, 82(6), 878–902. 10.1037/0022-3514.82.6.878 [DOI] [PubMed] [Google Scholar]
- Fiske ST, & Neuberg SL (1990). A Continuum of Impression Formation, from Category-Based to Individuating Processes: Influences of Information and Motivation on Attention and Interpretation. Advances in Experimental Social Psychology. 10.1016/S0065-2601(08)60317-2 [DOI] [Google Scholar]
- Flory JA, Leibbrandt A, & List JA (2015). Do Competitive Workplaces Deter Female Workers? A Large-Scale Natural Field Experiment on Job Entry Decisions. The Review of Economic Studies. 10.1093/restud/rdu030 [DOI] [Google Scholar]
- Geniole SN, Bird BM, Ruddick EL, & Carré JM (2017). Effects of competition outcome on testosterone concentrations in humans: An updated meta-analysis. Hormones and Behavior, 92, 37–50. 10.1016/j.yhbeh.2016.10.002 [DOI] [PubMed] [Google Scholar]
- Geniole SN, Procyshyn TL, Marley N, Ortiz TL, Bird BM, Marcellus AL, Welker KM, Bonin PL, Goldfarb B, Watson NV, & Carré JM (2019). Using a Psychopharmacogenetic Approach To Identify the Pathways Through Which—And the People for Whom—Testosterone Promotes Aggression. Psychological Science, 30(4), 481–494. 10.1177/0956797619826970 [DOI] [PubMed] [Google Scholar]
- Glick P, & Fiske ST (2001). An ambivalent alliance: Hostile and benevolent sexism as complementary justifications for gender inequality. American Psychologist, 56(2), 109–118. 10.1037/0003-066X.56.2.109 [DOI] [PubMed] [Google Scholar]
- Gneezy U, Niederle M, & Rustichini A (2003). Performance in Competitive Environments: Gender Differences. The Quarterly Journal of Economics, 118(3), 1049–1074. 10.1162/00335530360698496 [DOI] [Google Scholar]
- Goetz SMM, Tang L, Thomason ME, Diamond MP, Hariri AR, & Carré JM (2014). Testosterone rapidly increases neural reactivity to threat in healthy men: A novel two-step pharmacological challenge paradigm. Biological Psychiatry. 10.1016/j.biopsych.2014.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebe NM, Del Giudice M, Emery Thompson M, Nickels N, Ponzi D, Zilioli S, Maestripieri D, & Gangestad SW (2019). Testosterone, cortisol, and status-striving personality features: A review and empirical evaluation of the Dual Hormone hypothesis. Hormones and Behavior, 109, 25–37. 10.1016/j.yhbeh.2019.01.006 [DOI] [PubMed] [Google Scholar]
- Grotzinger AD, Mann FD, Patterson MW, Tackett JL, Tucker-Drob EM, & Harden KP (2018). Hair and Salivary Testosterone, Hair Cortisol, and Externalizing Behaviors in Adolescents. Psychological Science, 29(5), 688–699. 10.1177/0956797617742981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Mendle J, Lindquist KA, Schmader T, Clark LA, Bliss-Moreau E, Akinola M, Atlas L, Barch DM, Barrett LF, Borelli JL, Brannon TN, Bunge SA, Campos B, Cantlon J, Carter R, Carter-Sowell AR, Chen S, Craske MG, … Williams LA (2020). The Future of Women in Psychological Science. Perspectives on Psychological Science, 174569162095278. 10.1177/1745691620952789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselton MG, & Buss DM (2000). Error management theory: A new perspective on biases in cross-sex mind reading. Journal of Personality and Social Psychology, 78(1), 81–91. 10.1037/0022-3514.78.1.81 [DOI] [PubMed] [Google Scholar]
- Hays NA, & Bendersky C (2015). Not all inequality is created equal: Effects of status versus power hierarchies on competition for upward mobility. Journal of Personality and Social Psychology, 108(6), 867–882. 10.1037/pspi0000017 [DOI] [PubMed] [Google Scholar]
- Henry A, Sattizahn JR, Norman GJ, Beilock SL, & Maestripieri D (2017). Performance during competition and competition outcome in relation to testosterone and cortisol among women. Hormones and Behavior. 10.1016/j.yhbeh.2017.03.010 [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Bos PA, Ossewaarde L, Ramsey NF, Fernández G, & van Honk J (2010). Effects of exogenous testosterone on the ventral striatal BOLD response during reward anticipation in healthy women. NeuroImage, 52(1), 277–283. 10.1016/j.neuroimage.2010.04.019 [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Putman P, Baas JM, Koppeschaar HP, & van Honk J (2006). A Single Administration of Testosterone Reduces Fear-Potentiated Startle in Humans. Biological Psychiatry. 10.1016/j.biopsych.2005.11.015 [DOI] [PubMed] [Google Scholar]
- Hodges-Simeon CR, Hanson Sobraske KN, Samore T, Gurven M, & Gaulin SJC (2016). Facial width-to-height ratio (fWHR) is not associated with adolescent testosterone levels. PLoS ONE. 10.1371/journal.pone.0153083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J (2020). reghelper: Helper Functions for Regression Analysis (1.0.0). [Google Scholar]
- Johnson EO, Kamilaris TC, Chrousos GP, & Gold PW (1992). Mechanisms of stress: A dynamic overview of hormonal and behavioral homeostasis. Neuroscience & Biobehavioral Reviews, 16(2), 115–130. 10.1016/S0149-7634(05)80175-7 [DOI] [PubMed] [Google Scholar]
- Josephs RA, Newman ML, Brown RP, & Beer JM (2003). Status, testosterone, and human intellectual performance: Stereotype Threat as Status Concern. Psychological Science. 10.1111/1467-9280.t01-1-01435 [DOI] [PubMed] [Google Scholar]
- Josephs RA, Sellers JG, Newman ML, & Mehta PH (2006). The mismatch effect: When testosterone and status are at odds. Journal of Personality and Social Psychology, 90(6), 999–1013. 10.1037/0022-3514.90.6.999 [DOI] [PubMed] [Google Scholar]
- Joshi A, Neely B, Emrich C, Griffiths D, & George G (2015). Gender research in AMJ: An overview of five decades of empirical research and calls to action. In Academy of Management Journal. 10.5465/amj.2015.4011 [DOI] [Google Scholar]
- Keltner D, Gruenfeld DH, & Anderson C (2003). Power, approach, and inhibition. Psychological Review, 110(2), 265–284. 10.1037/0033-295X.110.2.265 [DOI] [PubMed] [Google Scholar]
- Knight EL, Christian CB, Morales PJ, Harbaugh WT, Mayr U, & Mehta PH (2017). Exogenous testosterone enhances cortisol and affective responses to social-evaluative stress in dominant men. Psychoneuroendocrinology. 10.1016/j.psyneuen.2017.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight EL, McShane BB, Kutlikova HH, Morales PJ, Christian CB, Harbaugh WT, Mayr U, Ortiz TL, Gilbert K, Ma-Kellams C, Riečanský I, Watson NV, Eisenegger C, Lamm C, Mehta PH, & Carré JM (2020). Weak and Variable Effects of Exogenous Testosterone on Cognitive Reflection Test Performance in Three Experiments: Commentary on Nave, Nadler, Zava, and Camerer (2017). Psychological Science. 10.1177/0956797619885607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight EL, & Mehta PH (2017). Hierarchy stability moderates the effect of status on stress and performance in humans. Proceedings of the National Academy of Sciences of the United States of America. 10.1073/pnas.1609811114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight EL, Sarkar A, Prasad S, & Mehta PH (2020). Beyond the challenge hypothesis: The emergence of the dual-hormone hypothesis and recommendations for future research. In Hormones and Behavior. 10.1016/j.yhbeh.2019.104657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Wimmer GE, Kuhnen CM, & Winkielman P (2008). Nucleus accumbens activation mediates the influence of reward cues on financial risk taking. NeuroReport. 10.1097/WNR.0b013e3282f85c01 [DOI] [PubMed] [Google Scholar]
- Kohn A (1992). No contest: The case against competition. Houghton Mifflin. [Google Scholar]
- Kordsmeyer TL, Freund D, Pita SR, Jünger J, & Penke L (2019). Further Evidence that Facial Width-to-Height Ratio and Global Facial Masculinity Are Not Positively Associated with Testosterone Levels. Adaptive Human Behavior and Physiology. 10.1007/s40750-018-0105-4 [DOI] [Google Scholar]
- Kordsmeyer TL, & Penke L (2019). Effects of male testosterone and its interaction with cortisol on self- and observer-rated personality states in a competitive mating context. Journal of Research in Personality, 78, 76–92. 10.1016/j.jrp.2018.11.001 [DOI] [Google Scholar]
- Krebs A, Clement H-W, Zimmerer J, Schulz E, Doerfer J, Wurm M, Brichta C, van der Werf-Grohmann N, & Schwab K (2019). Transfer of Topical Testosterone to Subcutaneous Microdialysate, Blood and Saliva in Healthy Young Men. Experimental and Clinical Endocrinology & Diabetes, 127(05), 289–294. 10.1055/a-0650-4115 [DOI] [PubMed] [Google Scholar]
- Kutlikova HH, Geniole SN, Eisenegger C, Lamm C, Jocham G, & Studer B (2021). Not giving up: Testosterone promotes persistence against a stronger opponent. Psychoneuroendocrinology, 128, 105214. 10.1016/j.psyneuen.2021.105214 [DOI] [PubMed] [Google Scholar]
- Lee JJ, Gino F, Jin ES, Rice LK, & Josephs RA (2015). Hormones and ethics: Understanding the biological basis of unethical conduct. Journal of Experimental Psychology: General, 144(5), 891–897. 10.1037/xge0000099 [DOI] [PubMed] [Google Scholar]
- Li P, & Redden DT (2015). Comparing denominator degrees of freedom approximations for the generalized linear mixed model in analyzing binary outcome in small sample cluster-randomized trials. BMC Medical Research Methodology, 15(1), 38. 10.1186/s12874-015-0026-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liening SH, Stanton SJ, Saini EK, & Schultheiss OC (2010). Salivary testosterone, cortisol, and progesterone: Two-week stability, interhormone correlations, and effects of time of day, menstrual cycle, and oral contraceptive use on steroid hormone levels. Physiology & Behavior, 99(1), 8–16. 10.1016/j.physbeh.2009.10.001 [DOI] [PubMed] [Google Scholar]
- London B, Downey G, Romero-Canyas R, Rattan A, & Tyson D (2012). Gender-based rejection sensitivity and academic self-silencing in women. Journal of Personality and Social Psychology, 102(5), 961–979. 10.1037/a0026615 [DOI] [PubMed] [Google Scholar]
- Losecaat Vermeer AB, Krol I, Gausterer C, Wagner B, Eisenegger C, & Lamm C (2020). Exogenous testosterone increases status-seeking motivation in men with unstable low social status. Psychoneuroendocrinology, 113, 104552. 10.1016/j.psyneuen.2019.104552 [DOI] [PubMed] [Google Scholar]
- Makara GB, & Haller J (2001). Non-genomic effects of glucocorticoids in the neural system. Progress in Neurobiology, 65(4), 367–390. 10.1016/S0301-0082(01)00012-0 [DOI] [PubMed] [Google Scholar]
- Maner JK, Gailliot MT, Menzel AJ, & Kunstman JW (2012). Dispositional Anxiety Blocks the Psychological Effects of Power. Personality and Social Psychology Bulletin, 38(11), 1383–1395. 10.1177/0146167212453341 [DOI] [PubMed] [Google Scholar]
- Marceau K, Ruttle PL, Shirtcliff EA, Hastings PD, Klimes-Dougan B, & Zahn-Waxler C (2015). Within-person coupling of changes in cortisol, testosterone, and DHEA across the day in adolescents. Developmental Psychobiology, 57(6), 654–669. 10.1002/dev.21173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr U, Wozniak D, Davidson C, Kuhns D, & Harbaugh WT (2012). Competitiveness across the life span: The feisty fifties. Psychology and Aging. 10.1037/a0025655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur A, & Booth A (1998). Testosterone and dominance in men. In Behavioral and Brain Sciences. 10.1017/S0140525X98001228 [DOI] [PubMed] [Google Scholar]
- Mazur A, Welker KM, & Peng B (2015). Does the biosocial model explain the emergence of status differences in conversations among unacquainted men? PLoS ONE. 10.1371/journal.pone.0142941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2019). What is the confusion with cortisol? Chronic Stress, 3, 1–3. 10.1177/2470547019833647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead M (1937). Co-operation and competition in primitive societies. McGraw-Hall. [Google Scholar]
- Mead NL, & Maner JK (2012). On keeping your enemies close: Powerful leaders seek proximity to ingroup power threats. Journal of Personality and Social Psychology, 102(3), 576–591. 10.1037/a0025755 [DOI] [PubMed] [Google Scholar]
- Mehta PH, & Beer J (2010). Neural Mechanisms of the Testosterone–Aggression Relation: The Role of Orbitofrontal Cortex. Journal of Cognitive Neuroscience, 22(10), 2357–2368. 10.1162/jocn.2009.21389 [DOI] [PubMed] [Google Scholar]
- Mehta PH, Jones AC, & Josephs RA (2008). The social endocrinology of dominance: Basal testosterone predicts cortisol changes and behavior following victory and defeat. Journal of Personality and Social Psychology, 94(6), 1078–1093. 10.1037/0022-3514.94.6.1078 [DOI] [PubMed] [Google Scholar]
- Mehta PH, & Josephs RA (2006). Testosterone change after losing predicts the decision to compete again. Hormones and Behavior, 50(5), 684–692. 10.1016/j.yhbeh.2006.07.001 [DOI] [PubMed] [Google Scholar]
- Mehta PH, & Josephs RA (2010). Testosterone and cortisol jointly regulate dominance: Evidence for a dual-hormone hypothesis. Hormones and Behavior. 10.1016/j.yhbeh.2010.08.020 [DOI] [PubMed] [Google Scholar]
- Mehta PH, Lawless DesJardins NM, van Vugt M, & Josephs RA (2017). Hormonal underpinnings of status conflict: Testosterone and cortisol are related to decisions and satisfaction in the hawk-dove game. Hormones and Behavior, 92, 141–154. 10.1016/j.yhbeh.2017.03.009 [DOI] [PubMed] [Google Scholar]
- Mehta PH, & Prasad S (2015). The dual-hormone hypothesis: A brief review and future research agenda. In Current Opinion in Behavioral Sciences. 10.1016/j.cobeha.2015.04.008 [DOI] [Google Scholar]
- Mehta PH, Snyder NA, Knight EL, & Lassetter B (2015). Close Versus Decisive Victory Moderates the Effect of Testosterone Change on Competitive Decisions and Task Enjoyment. Adaptive Human Behavior and Physiology. 10.1007/s40750-014-0014-0 [DOI] [Google Scholar]
- Mehta PH, van Son V, Welker KM, Prasad S, Sanfey AG, Smidts A, & Roelofs K (2015). Exogenous testosterone in women enhances and inhibits competitive decision-making depending on victory-defeat experience and trait dominance. Psychoneuroendocrinology. 10.1016/j.psyneuen.2015.07.004 [DOI] [PubMed] [Google Scholar]
- Mehta PH, Welker KM, Zilioli S, & Carré JM (2015). Testosterone and cortisol jointly modulate risk-taking. Psychoneuroendocrinology. 10.1016/j.psyneuen.2015.02.023 [DOI] [PubMed] [Google Scholar]
- Mendes WB, Blascovich J, Major B, & Seery M (2001). Challenge and threat responses during downward and upward social comparisons. European Journal of Social Psychology. 10.1002/ejsp.80 [DOI] [Google Scholar]
- Montoya ER, Bos PA, Terburg D, Rosenberger LA, & van Honk J (2014). Cortisol administration induces global down-regulation of the brain’s reward circuitry. Psychoneuroendocrinology. 10.1016/j.psyneuen.2014.04.022 [DOI] [PubMed] [Google Scholar]
- Montoya ER, Terburg D, Bos PA, & van Honk J (2012). Testosterone, cortisol, and serotonin as key regulators of social aggression: A review and theoretical perspective. Motivation and Emotion, 36(1), 65–73. 10.1007/s11031-011-9264-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore FL, & Evans SJ (1999). Steroid hormones use non-genomic mechanisms to control brain functions and behaviors: A review of evidence. Brain, Behavior and Evolution, 54(1), 41–50. 10.1159/000006610 [DOI] [PubMed] [Google Scholar]
- Nadler A, Wibral M, Dohmen T, Falk A, Previtero A, Weber B, Camerer C, Dreber A, & Nave G (2021). Does testosterone increase willingness to compete, confidence, and risk-taking in men? Evidence from two randomized placebo-controlled experiments. PsyArXiv. 10.31234/osf.io/62af7 [DOI] [Google Scholar]
- Nakagawa S, Johnson PCD, & Schielzeth H (2017). The coefficient of determination R 2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. Journal of The Royal Society Interface, 14(134), 20170213. 10.1098/rsif.2017.0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederle M, Segal C, & Vesterlund L (2013). How costly is diversity? Affirmative action in light of gender differences in competitiveness. Management Science. 10.1287/mnsc.1120.1602 [DOI] [Google Scholar]
- Niederle M, & Vesterlund L (2011). Gender and competition. In Annual Review of Economics. 10.1146/annurev-economics-111809-125122 [DOI] [Google Scholar]
- Nitschke JP, & Bartz JA (2020). Lower digit ratio and higher endogenous testosterone are associated with lower empathic accuracy. Hormones and Behavior, 119, 104648. 10.1016/j.yhbeh.2019.104648 [DOI] [PubMed] [Google Scholar]
- Op De Macks ZA, Moor BG, Overgaauw S, Gürolu B, Dahl RE, & Crone EA (2011). Testosterone levels correspond with increased ventral striatum activation in response to monetary rewards in adolescents. Developmental Cognitive Neuroscience. 10.1016/j.dcn.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfattheicher S (2016). Testosterone, cortisol and the Dark Triad: Narcissism (but not Machiavellianism or psychopathy) is positively related to basal testosterone and cortisol. Personality and Individual Differences, 97, 115–119. 10.1016/J.PAID.2016.03.015 [DOI] [Google Scholar]
- Pfattheicher S (2017). Illuminating the dual-hormone hypothesis: About chronic dominance and the interaction of cortisol and testosterone. Aggressive Behavior. 10.1002/ab.21665 [DOI] [PubMed] [Google Scholar]
- Ponzi D, Zilioli S, Mehta PH, Maslov A, & Watson NV (2016). Social network centrality and hormones: The interaction of testosterone and cortisol. Psychoneuroendocrinology. 10.1016/j.psyneuen.2016.02.014 [DOI] [PubMed] [Google Scholar]
- Prasad S, Knight EL, & Mehta PH (2019). Basal testosterone’s relationship with dictator game decision-making depends on cortisol reactivity to acute stress: A dual-hormone perspective on dominant behavior during resource allocation. Psychoneuroendocrinology. 10.1016/j.psyneuen.2018.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Knight EL, Sarkar A, Welker KM, Lassetter B, & Mehta PH (2021). Testosterone fluctuations in response to a democratic election predict partisan attitudes toward the elected leader. Psychoneuroendocrinology, 133. 10.1016/J.PSYNEUEN.2021.105396 [DOI] [PubMed] [Google Scholar]
- Prasad S, Narayanan J, Lim VKG, Koh GCH, Koh DSQ, & Mehta PH (2017). Preliminary evidence that acute stress moderates basal testosterone’s association with retaliatory behavior. Hormones and Behavior. 10.1016/j.yhbeh.2016.10.020 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, & Bauer DJ (2006). Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 10.3102/10769986031004437 [DOI] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, & Hellhammer DH (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28(7), 916–931. 10.1016/S0306-4530(02)00108-7 [DOI] [PubMed] [Google Scholar]
- Puiu AA, Radke S, Votinov M, Habel U, Herpertz-Dahlmann B, Turetsky B, & Konrad K (2019). Serum Testosterone and Cortisol Concentrations After Single-Dose Administration of 100-Mg Transdermal Testosterone in Healthy Men. Frontiers in Pharmacology, 10(1397). 10.3389/fphar.2019.01397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke S, Volman I, Mehta P, Van Son V, Enter D, Sanfey A, Toni I, De Bruijn ERA, & Roelofs K (2015). Testosterone biases the amygdala toward social threat approach. Science Advances. 10.1126/sciadv.1400074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs K, Elzinga BM, & Rotteveel M (2005). The effects of stress-induced cortisol responses on approach–avoidance behavior. Psychoneuroendocrinology, 30(7), 665–677. 10.1016/J.PSYNEUEN.2005.02.008 [DOI] [PubMed] [Google Scholar]
- Ronay R, van der Meij L, Oostrom JK, & Pollet TV (2018). No Evidence for a Relationship Between Hair Testosterone Concentrations and 2D:4D Ratio or Risk Taking. Frontiers in Behavioral Neuroscience, 12. 10.3389/fnbeh.2018.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roney JR, Lukaszewski AW, & Simmons ZL (2007). Rapid endocrine responses of young men to social interactions with young women. Hormones and Behavior, 52(3), 326–333. 10.1016/j.yhbeh.2007.05.008 [DOI] [PubMed] [Google Scholar]
- Roy ARK, Cook T, Carré JM, & Welker KM (2019). Dual-hormone regulation of psychopathy: Evidence from mass spectrometry. Psychoneuroendocrinology. 10.1016/j.psyneuen.2018.09.006 [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Roca CA, Schmidt PJ, Danaceau MA, Putnam K, Cizza G, Chrousos G, & Nieman L (2005). Testosterone Suppression of CRH-Stimulated Cortisol in Men. Neuropsychopharmacology, 30(10), 1906–1912. 10.1038/sj.npp.1300742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM (1991). Testicular function, social rank and personality among wild baboons. In Psychoneuroendocrinology. 10.1016/0306-4530(91)90015-L [DOI] [PubMed] [Google Scholar]
- Sarkar A, Mehta PH, & Josephs RA (2019). The dual-hormone approach to dominance and status-seeking. In Schultheiss OC & Mehta PH (Eds.), Routledge International Handbook of Social Neuroendocrinology (1st ed., pp. 113–132). Routledge. [Google Scholar]
- Scheepers D, & Knight EL (2020). Neuroendocrine and cardiovascular responses to shifting status. In Current Opinion in Psychology. 10.1016/j.copsyc.2019.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönfelder M, Hofmann H, Schulz T, Engl T, Kemper D, Mayr B, Rautenberg C, Oberhoffer R, & Thieme D (2016). Potential detection of low-dose transdermal testosterone administration in blood, urine, and saliva. Drug Testing and Analysis, 8(11-12), 1186–1196. 10.1002/dta.2110 [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, & Stanton SJ (2009). Assessment of salivary hormones. In Harmon-Jones E & Beer J (Eds.), Methods in Social Neuroscience (pp. 17–44). The Guilford Press. [Google Scholar]
- Schultheiss OC, Wirth MM, Torges CM, Pang JS, Villacorta MA, & Welsh KM (2005). Effects of Implicit Power Motivation on Men’s and Women’s Implicit Learning and Testosterone Changes After Social Victory or Defeat. Journal of Personality and Social Psychology, 88(1), 174–188. 10.1037/0022-3514.88.1.174 [DOI] [PubMed] [Google Scholar]
- Sellers JG, Mehl MR, & Josephs RA (2007). Hormones and personality: Testosterone as a marker of individual differences. Journal of Research in Personality, 41(1), 126–138. 10.1016/j.jrp.2006.02.004 [DOI] [Google Scholar]
- Sherman GD, Lerner JS, Josephs RA, Renshon J, & Gross JJ (2016). The interaction of testosterone and cortisol is associated with attained status in male executives. Journal of Personality and Social Psychology. 10.1037/pspp0000063 [DOI] [PubMed] [Google Scholar]
- Skitka LJ, Melton ZJ, Mueller AB, & Wei KY (2020). The Gender Gap: Who Is (and Is Not) Included on Graduate-Level Syllabi in Social/Personality Psychology. Personality and Social Psychology Bulletin, 014616722094732. 10.1177/0146167220947326 [DOI] [PubMed] [Google Scholar]
- Slatcher RB, Mehta PH, & Josephs RA (2011). Testosterone and self-reported dominance interact to influence human mating behavior. Social Psychological and Personality Science. 10.1177/1948550611400099 [DOI] [Google Scholar]
- Smith RG, Syms AJ, Nag A, Lerner S, & Norris JS (1985). Mechanism of the glucocorticoid regulation of growth of the androgen-sensitive prostate-derived R3327H-G8-A1 tumor cell line. Journal of Biological Chemistry, 260(23), 12454–12463. 10.1016/S0021-9258(17)38894-4 [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Steele CM, & Quinn DM (1999). Stereotype Threat and Women’s Math Performance. Journal of Experimental Social Psychology. 10.1006/jesp.1998.1373 [DOI] [Google Scholar]
- Stanton SJ, & Schultheiss OC (2007). Basal and dynamic relationships between implicit power motivation and estradiol in women. Hormones and Behavior. 10.1016/j.yhbeh.2007.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton SJ, & Schultheiss OC (2009). The hormonal correlates of implicit power motivation. In Journal of Research in Personality. 10.1016/j.jrp.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin ZW, Chopik WJ, Welker KM, Ascigil E, Brandes CM, Chin K, Ketay S, Knight EL, Kordsmeyer TL, McLarney-Vesotski AR, Prasad S, Reese ZA, Roy ARK, Sim L, Stern J, Carré JM, Edelstein RS, Mehta PH, Penke L, … Tackett JL (2021). Estimating the Associations between Big Five Personality Traits, Testosterone, and Cortisol. Adaptive Human Behavior and Physiology. 10.1007/s40750-020-00159-9 [DOI] [Google Scholar]
- Swaddle JP, & Reierson GW (2002). Testosterone increases perceived dominance but not attractiveness in human males. Proceedings of the Royal Society B: Biological Sciences. 10.1098/rspb.2002.2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackett JL, Reardon KW, Herzhoff K, Page-Gould E, Harden KP, & Josephs RA (2015). Estradiol and cortisol interactions in youth externalizing psychopathology. Psychoneuroendocrinology. 10.1016/j.psyneuen.2015.02.014 [DOI] [PubMed] [Google Scholar]
- Talamas SN, Mavor KI, Sundelin T, Axelsson J, & Perrett DI (2016). Eyelid-Openness and mouth curvature influence perceived intelligence beyond attractiveness. Journal of Experimental Psychology: General. 10.1037/xge0000152 [DOI] [PubMed] [Google Scholar]
- Terburg D, Aarts H, & van Honk J (2012). Testosterone affects gaze aversion from angry faces outside of conscious awareness. Psychological Science, 23(5), 459–463. 10.1177/0956797611433336 [DOI] [PubMed] [Google Scholar]
- Tilbrook A (2000). Effects of stress on reproduction in non-rodent mammals: The role of glucocorticoids and sex differences. Reviews of Reproduction, 5(2), 105–113. 10.1530/ror.0.0050105 [DOI] [PubMed] [Google Scholar]
- Tomaka J, Blascovich J, Kelsey RM, & Leitten CL (1993). Subjective, physiological, and behavioral effects of threat and challenge appraisal. Journal of Personality and Social Psychology, 65(2), 248–260. 10.1037/0022-3514.65.2.248 [DOI] [Google Scholar]
- Tuiten A, Van Honk J, Koppeschaar H, Bernaards C, Thijssen J, & Verbaten R (2000). Time course of effects of testosterone administration on sexual arousal in women. Archives of General Psychiatry. 10.1001/archpsyc.57.2.149 [DOI] [PubMed] [Google Scholar]
- Turan B, Tackett JL, Lechtreck MT, & Browning WR (2015). Coordination of the cortisol and testosterone responses: A dual axis approach to understanding the response to social status threats. Psychoneuroendocrinology. 10.1016/j.psyneuen.2015.07.166 [DOI] [PubMed] [Google Scholar]
- Van Den Bos W, Golka PJM, Effelsberg D, & McClure SM (2013). Pyrrhic victories: The need for social status drives costly competitive behavior. Frontiers in Neuroscience. 10.3389/fnins.2013.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meij L, Almela M, Buunk AP, Fawcett TW, & Salvador A (2011). Men with elevated testosterone levels show more affiliative behaviours during interactions with women. Proceedings of the Royal Society B: Biological Sciences. 10.1098/rspb.2011.0764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Honk J, Tuiten A, Hermans E, Putman P, Koppeschaar H, Thijssen J, Verbaten R, & Van Doornen L (2001). A single administration of testosterone induces cardiac accelerative responses to angry faces in healthy young women. Behavioral Neuroscience. 10.1037/0735-7044.115.1.238 [DOI] [PubMed] [Google Scholar]
- Van Honk J, Tuiten A, Van Den Hout M, Koppeschaar H, Thijssen J, De Haan E, & Verbaten R (1998). Baseline salivary cortisol levels and preconscious selective attention for threat: A pilot study. Psychoneuroendocrinology, 23(7), 741–747. 10.1016/S0306-4530(98)00047-X [DOI] [PubMed] [Google Scholar]
- van Kleef GA, & Cheng JT (2020). Power, status, and hierarchy: Current trends and future challenges. Current Opinion in Psychology, 33, iv–xiii. 10.1016/j.copsyc.2020.03.011 [DOI] [PubMed] [Google Scholar]
- Vandello JA, Bosson JK, Cohen D, Burnaford RM, & Weaver JR (2008). Precarious manhood. Journal of Personality and Social Psychology, 95(6), 1325–1339. 10.1037/a0012453 [DOI] [PubMed] [Google Scholar]
- Viau V (2002). Functional Cross-Talk Between the Hypothalamic-Pituitary-Gonadal and -Adrenal Axes. Journal of Neuroendocrinology, 14(6), 506–513. 10.1046/j.1365-2826.2002.00798.x [DOI] [PubMed] [Google Scholar]
- De Welde K, & Laursen S (2011). The glass obstacle course: Informal and formal barriers for women Ph. D. students in STEM fields. International Journal of Gender, Science and Technology. [Google Scholar]
- Welker KM, Gruber J, & Mehta PH (2015). A positive affective neuroendocrinology approach to reward and behavioral dysregulation. In Frontiers in Psychiatry. 10.3389/fpsyt.2015.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker KM, Lozoya E, Campbell JA, Neumann CS, & Carré JM (2014). Testosterone, cortisol, and psychopathic traits in men and women. Physiology and Behavior. 10.1016/j.physbeh.2014.02.057 [DOI] [PubMed] [Google Scholar]
- Welker KM, Roy ARK, Geniole S, Kitayama S, & Carré JM (2019). Taking risks for personal gain: An investigation of self-construal and testosterone responses to competition. Social Neuroscience, 14(1), 99–113. 10.1080/17470919.2017.1407822 [DOI] [PubMed] [Google Scholar]
- Welling LLM, Moreau BJP, Bird BM, Hansen S, & Carré JM (2016). Exogenous testosterone increases men’s perceptions of their own physical dominance. Psychoneuroendocrinology. 10.1016/j.psyneuen.2015.11.016 [DOI] [PubMed] [Google Scholar]
- Wilson M, & Daly M (1985). Competitiveness, risk taking, and violence: The young male syndrome. Ethology and Sociobiology. 10.1016/0162-3095(85)90041-X [DOI] [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, & Ball GF (1990). The "challenge hypothesis’: Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. American Naturalist. 10.1086/285134 [DOI] [Google Scholar]
- Wingfield JC, & Sapolsky RM (2003). Reproduction and resistance to stress: When and how. In Journal of Neuroendocrinology. 10.1046/j.1365-2826.2003.01033.x [DOI] [PubMed] [Google Scholar]
- Wozniak D, Harbaugh WT, & Mayr U (2014). The menstrual cycle and performance feedback alter gender differences in competitive choices. Journal of Labor Economics. 10.1086/673324 [DOI] [Google Scholar]
- Wu Y, Zhang Y, Ou J, Hu Y, & Zilioli S (2020). Exogenous testosterone increases the audience effect in healthy males: Evidence for the social status hypothesis. Proceedings of the Royal Society B, 287(1931). 10.1098/RSPB.2020.0976 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


