Abstract
In this review we summarize multiple aspects of the human immunodeficiency virus (HIV) protease from both structural and functional viewpoints. After an introductory overview, we provide an up-to-date status report on protease inhibitors (PI). This proceeds from a discussion of PI structural design, to how PI are optimally utilized in highly active antiretroviral triple therapy (one PI along with two reverse transcriptase inhibitors), the emergence of PI resistance, and the natural role of secretory leukocyte PI. Then we switch to another focus: the interaction of HIV protease with other genes in acute and persistent infection, which in turn may have an effect on AIDS pathogenesis. We conclude with a discussion on future directions in HIV treatment, involving multiple-target anti-HIV therapy, vaccine development, and novel reactivation-inhibitory reagents.
Proteases (PRs) function critically in a wide variety of cellular and viral processes by exhibiting limited substrate site specificity on their respective precursor proteins. For cellular systems, PRs like chymotrypsin, plasmin, and pepsin are produced after such cleavages, while for viral systems structural proteins as well as enzymes are formed (190, 282).
Further, many cellular PRs are now proving to be essential in abnormal processes related to cancer biology and tumorigenesis, e.g., metastasis and angiogenesis (93). PR inhibitors (PI) which block these processes may eventually prove to be as important as those used in viral diseases such as AIDS. Further, some PRs even help eukaryotic microorganisms such as yeast find mating partners, by secreting a PR that hydrolyzes α factor (14).
In the first part of this review, we focus on retroviral PRs, such as that of human immunodeficiency virus (HIV), and their inhibitors. General examples of viral PR that play a significant role in morphogenesis are hepatitis C virus PR (148), human adenovirus PR (83), and retroviral aspartyl PRs (70, 96, 282). The detailed function of each viral PR is different. For example, although the PR of adenovirus type 2 requires cysteine residues for both activation and catalysis (128), factors required for activation of HIV and other retroviral PRs from their Gag or Gag-Pol precursors are relatively unknown.
Recently, it has been suggested that incorporation and proper folding of the minor (10% of Gag) virion component cyclophilin A is necessary to allow PR dimerization and activation in HIV (341, 342). Alternatively, cyclophilin A may also play a role in viral entry (55). An older model for murine leukemia retroviruses (MLV) suggests that activation of a kinase that phosphorylates Gag or Gag-Pol precursors at specific amino acid residues might be important as well (223). It is also worthwhile to note that since aspartyl PRs function optimally in an acidic (pH 4.5 to 5.0) environment (282), there may be some specific events required for lowering of the pH in retroviral buds.
Based on predicted models from X-ray crystallization and nuclear magnetic resonance spectroscopy data for the capsid (CA) dimer and matrix (MA) trimer proteins, the assembly of Gag protein precursors (Pr55) of HIV has been suggested to simulate a cocked gun in an unfavorable, precursor conformation, awaiting PR activation before providing realignment of “immature” to “mature” Pr55 Gag cleaved proteins in the virus particles (162). This conformational change is consistent with recent models suggesting that the HIV PR also triggers a “myristyl switch” mechanism that alters exposure of the myristyl moiety from its tight (uncleaved Pr55gag) to a loose (cleaved p17 MA) membrane-binding affinity (140, 333). Another aspect of the retroviral morphogenesis process, which is still unknown, involves the role of Pr55gag interactions with the cytoskeleton (224). Recently it was shown that actin molecules can be specifically associated with the nucleocapsid (NC) domain of Pr55gag (308, 366), while other studies have shown that vimentin filaments could be degraded by the HIV PR (149; E. Pichova, personal communication).
In these retroviral assembly models, viral PRs appear to act similarly to cellular aspartyl proenzymes, such as pepsinogen, in that they have to be activated through an autocatalytic mechanism. In cells, pepsin is activated during secretion from gastric cells, while for HIV, PR activation occurs in immature retroviral particles during and/or after budding from the outer plasma membrane. However, both classes of aspartyl PRs differ in a major structural way; HIV and other retroviral PRs are small homodimers of 10 to 14 kDa (282, 293), while pepsin and similar cellular analogs are larger monomers of 25 to 30 kDa that can fold into catalytically active forms (175, 207).
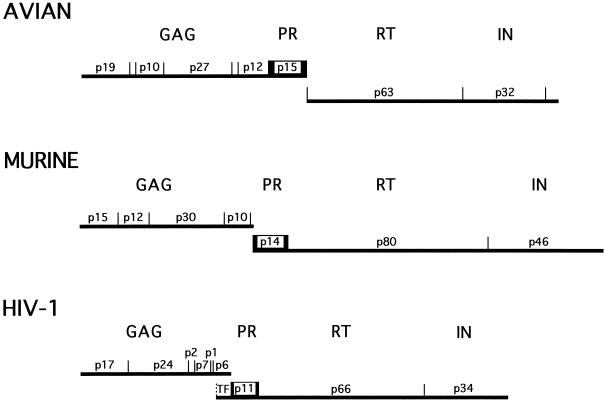
The first retroviral proteolytic enzymes to be studied were those from avian and murine leukosis virus systems. Specifically, von der Helm (55, 358) reported cleavage of avian Pr76gag by a p15 gag-encoded gel filtration fraction prepared by 6 M guanidine–HCl treatment of virions, while Yoshinaka and Luftig (371) reported a similar specific cleavage of the murine precursor protein Pr65gag by a non-gag-encoded gel filtration fraction obtained from Nonidet P-40 detergent-treated virions. Further, with MLV it was observed that cleavage was accompanied by a morphological transition of “immature” to “mature” particles, which correlated with the production of infectious virus (223, 371).
The MLV PR, like the HIV PR, is found only in small quantities in virions because it is derived from a Gag-Pol precursor located at the 5′ terminus of the pol gene (by convention, the PR coding domain of HIV is now named as a separate gene, pro, and thus one refers to the precursor Gag-Pro-Pol) (55). In contrast to MLV and HIV PRs, the avian leukosis virus PR is made in large (≥20-fold) excess, since it is located at the 3′ terminus of the gag gene (Fig. 1). The latter process appears to be unusual, since not only MLV and HIV but also other retroviral PRs, such as feline leukemia virus, feline immunodeficiency virus, and mouse mammary tumor virus, are produced in small quantities. In addition, as noted previously (256), the preference of MLV and HIV PRs for predominant cleavage between Tyr-Pro and Phe-Pro residues on retroviral precursors contrasts with the inefficient hydrolysis of these sites by cellular PRs, including pepsin (141). This viral specificity accounts in part for the success of PIs in treating AIDS patients.
FIG. 1.
Comparison of the gene structure of HIV-1 with those of avian and murine retroviruses. The genes for protease (pro) are boxed.
In this review, we will discuss the role of the retroviral PR in HIV morphogenesis, as well as the potential role of a class of specific PR and accessory gene-mutated particles in enhancing AIDS pathogenesis. As of now, infection of adults who live in most developed countries, including the United States and Europe, occurs predominantly with HIV of the major (M) group, clade B, and leads to AIDS within about 10 to 12 years in the absence of antiretroviral therapy. Specifically, about 20% of such individuals develop AIDS in 5 years, while <2% are long-term (>12 years) nonprogressors (LTNP) (101). For infected individuals, the HIV PR and reverse transcriptase (RT) have been excellent targets for antiviral therapy, since they are both crucial to the formation of infectious, properly assembled particles (69, 70, 256, 331).
It has been well documented over the past several years by several groups that there is a daily HIV-infected versus healthy CD4+ T-cell replacement war occurring in the patient, so that infected, dying cells continually are replaced (54, 142, 294, 295, 363; D. Havlir, S. Eastman, and D. D. Richman, Abstr. 2nd Natl. Conf. Hum. Retroviruses Relat. Infect., abstr. 299, 1995). In addition, as the disease progresses, there is an increase in the number of latently infected cells, producing defective HIV particles (35, 48, 49, 155). This is due in part to the high error rate of reverse transcription (161), leading eventually to the formation of mutations in critical genes. Although definitive proof of the specific role played by the increased number of defective particles (299) in progression to AIDS is lacking, we believe, based on in vitro experiments with a specific class of defective particles, that similar particles, possibly derived during candidiasis infection, may be involved (164–166; P. Hickman, P. L. Fidel, Jr., J. Leigh, R. Mera, and R. B. Luftig, Abstr. 19th ASV Meet., abstr. 145, 2000).
AIDS pathogenesis can grossly be characterized as a progressive loss of immune cells (100, 211). It can be explained in part by direct killing of host cells due to production and secretion in tissue media of HIV gene products, e.g., gp120 and Tat. Also, defective particles may lead to direct T-cell killing, as well as bystander apoptosis (164, 165). AIDS pathogenesis is further complicated by the HIV life cycle, which varies greatly depending on (i) which HIV strains or host cell genotypes are involved; (ii) which coreceptors are used, leading to non-syncytium-inducing (NSI) or syncytium-inducing (SI), as well as slow/low or rapid/high, viruses; (iii) the temporal production of macrophage- or T-tropic viruses; and, finally, (iv) the physiological state of the host cell, e.g., resting versus activated T cells or naive versus memory T cells.
Thus, there are clearly complex combinations of viruses and host cells interacting in HIV-infected patients, which can cause both rapid syncytium formation and/or cell death in CD4+ T cells. This immediately results in short-lived infected cells that are replaced by healthy T cells. Additionally, infection of a small number of quiescent cells may cause establishment of a persistent or latent HIV infection that apparently results in a small percentage of long-lived infected cells.
Further, a major indirect mechanism that accounts for the loss of immune cells is apoptosis, which can be induced by various secreted products or defective particles resulting from HIV infection. Apoptosis can be induced in immune cells of both the CD4+ and CD8+ T-cell lineage from uninfected patients (105, 127, 138, 164, 260) or as an activation-dependent event after mitogenic stimulation (126, 130).
Another level of complexity in understanding AIDS pathogenesis is that clinical staging of the disease appears to be significantly correlated with virus load (39, 245). Although viral replication occurs in HIV carriers throughout all clinical stages (95, 289, 299), as noted above most of the plasma-derived HIV is produced in short-lived infected cells with a half-life of only a few days (142, 294, 295, 363) and only a small portion of cells carrying the HIV genome can establish an inducible latent infection (35, 95, 292). The importance of latent reservoirs for HIV was recognized by studies with potent antiretroviral drugs that block new rounds of infection and produce rapid dramatic drops in plasma viremia (23, 53, 143).
Currently, combinatorial inhibitors with different protein targets are used in highly active antiretroviral therapy (HAART), e.g., two RT nucleoside inhibitors and one PI. The former inhibits HIV replication in newly infected cells but shows no effect on virus production from persistently or latently infected cells, while the latter inhibits the production of infectious HIV particles from both acutely and persistently or latently infected cells. Possibly due to the high mutation rate of HIV RT, mutations can also arise in other genes, such as the PR, that in turn potentially can generate large amounts of “immature”-appearing defective particles. Using an in vitro assay system, we have not found (with four different PI drugs) that PI-produced “immature” particles are any more SI than is the wild-type virus itself (11). We believe that this is a very positive result, in terms of PI therapy. In contrast, a certain class of HIV defective particles called L-2 particles, which have mutations in the PR gene and three additional genes (nef, gp41, and vpr) (10), are highly SI in vitro when exposed to uninfected T-cells (11, 279).
In addition to the accumulation of defective particles, large amounts of free HIV proteins continue to be generated from both acutely and chronically infected cells. The role of these soluble HIV proteins, as well as of mutant and defective particles and immune products stimulated by them (137), needs to be considered as factors in AIDS pathogenesis (155, 222).
Since this is a rapidly expanding field, we have chosen July 2000 as the cutoff date for references cited in this review.
HIV PROTEASE INHIBITORS AND THEIR USE IN THERAPY
The recent development of HAART (G. Moyle, Editorial, Curr. Opin. Infect. Dis. 12:1–4, 1999), also recently called potent antiretroviral therapy (PART) (133), combined with the use of viral load diagnosis led last year for the first time in over a decade to a decrease in the number of AIDS-related deaths in the United States (101). Due to this, certain recently Food and Drug Administration (FDA)-approved drugs developed for treatment of opportunistic infections such as cytomegalovirus retinitis may be less necessary than expected in areas with high AIDS incidence, such as New Orleans (M. Hagensee, personal communication). HAART, as noted above, involves the use of two nucleoside inhibitors and one PI (Table 1), although one recent clinical trial suggests that perhaps a converse treatment, with two PIs plus one RT inhibitor, may be even more effective (101). HAART treatment, however, is not for everyone and can be tolerated or used effectively by only about 50% of AIDS patients. Side effects include a rise in the level of blood sugar, an increased risk of diabetes, and the formation of unusual body fat deposits (218, 236).
TABLE 1.
Potential use of protease inhibitors in HAART
| FDA-approved HIV-1 protease inhibitorsa
|
Recommended companion nucleoside inhibitors for triple therapyb | |
|---|---|---|
| Name (synonym) | Company (code) | |
| Saquinavir (Invirase or Fortovase) | Roche (Ro-31-8959) | AZT + ddI |
| Ritonavir (Norvir) | Abbott (ABT-538) | d4T + ddI |
| Indinavir (Crixivan) | Merck (MK-639,L735) | AZT + ddC |
| Nelfinavir (Viracept) | Agouron (AG-1343) | AZT + 3TC |
Some compounds not yet FDA approved but in the pipeline are DMP-450 (analog of DMP-323 but with good oral bioavailability and a low Ki of 0.3 nM), VX-470 (141W94), and KNI-272 (tight-binding transition state analog containing allophenylnorstatine [12]). Recently, amprenavir (Agenerase) was FDA approved, and the information is described in the text.
This table is from a list that was compiled based on recommendations by several pharmaceutical manufacturers at the 1999 ICAAC Meeting in San Francisco. As noted at the July 2000 AIDS World Congress (D. Moodley, personal communication), nevirapine, a non-nucleoside reverse transcriptase inhibitor (NNRTI), is recommended alone in one dose during labor, one at birth, and one after birth as a low-cost replacement for the more complex AZT treatment. Also, there is currently some controversy among clinicians as to whether efavirenz (Sustiva), another NNRTI, should be used first before PIs, due to its simplicity of dosage (one pill a day because of its long half-life [40 to 55 h]) and its prevention of problems with lipodystrophy, saving the PIs for a time when RT inhibitors fail. Thus, there is some flux about which HIV drugs are optimal, and we have provided only a potential snapshot in this table.
An additional concern about HAART is the eventual selection of nucleoside inhibitor- and PI-resistant HIV strains (17, 19, 28–30, 54, 58, 59, 66, 75, 132, 167; S. Deeks, R. Grant, C. Horton, N. Simmonds, S. Follansbee, and S. Eastman, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother, abstr. I-205, 1997) which may occur in patients that tolerate and most benefit from the treatment (33). Nevertheless, triple therapy has given a large number of AIDS patients a new lease on life (135), and searches are under way for alternative PI and RT inhibitors, as well as vaccines (144), including unexpected sources such as a novel low-molecular-weight (Mr, 447) cytochalasin isolated in fermentations from a bark-inhabiting fungus (84) or oyster protein peptides (205). A plethora of additional, chemically synthesized potentially new PI have also been recently described (93; Program 12th Int. Conf. Antiviral Res., see abstr. A31–A33 and A40–A46, 1999).
Background of Retroviral Proteases
Historically, pepstatin and its dihydroxystere analogs were first identified as inhibitors of avian and murine retroviral aspartyl PRs, about 12 years ago (26, 172, 282, 309). The genome regions and molecular size of PR for avian (p15, avian sarcoma/leukosis virus), murine (p14, MLV), and human (p11, HIV) viruses all differ (Fig. 1).
Specifically, HIV PR is encoded in the pol gene as a homodimer of 99 amino acids, with a molecular mass of about 10 kDa (175, 282). This enzyme is responsible for all of the limited cleavages observed during morphogenesis and permits the separation of Gag, Gag-Pol, and even Nef precursor proteins into their respective, polypeptide components; e.g., Gag is converted to MA, CA, and NC, while Gag-Pol is converted to PR, RT, and integrase (IN). It is even thought that enhanced cleavage of the PR may be provided by NC interactions (326).
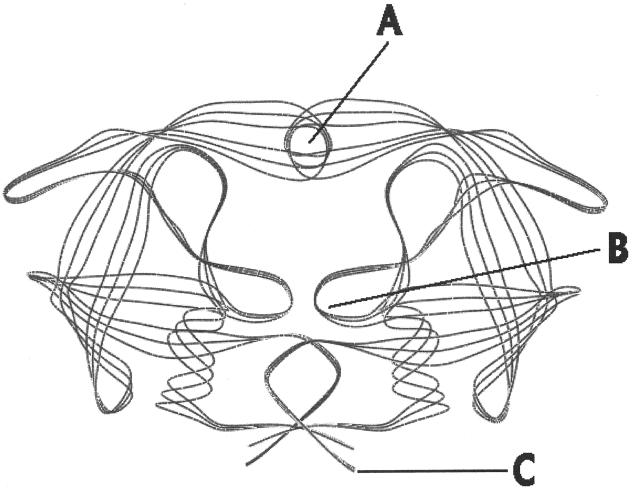
The HIV PR aspartyl “fireman's grip” structure (Fig. 2, area B) results in sensitivity to dihydroxystere analog inhibitors, which is similar to but different from those found among inhibitors of cellular and aspartic PRs. Specifically, these compounds block cleavage of the substrate by binding to several residues at the active site, which includes the highly conserved sequence Leu-(Leu/Val)-Asp-Thr-Asp-Thr-Gly-Ala-Asp-Lys (207, 282, 331). Asp, Thr, and Gly are the most essential amino acids for activity.
FIG. 2.
X-ray crystallographic structure of HIV-1 showing the flap (A), active site (B), and dimer binding (C) regions.
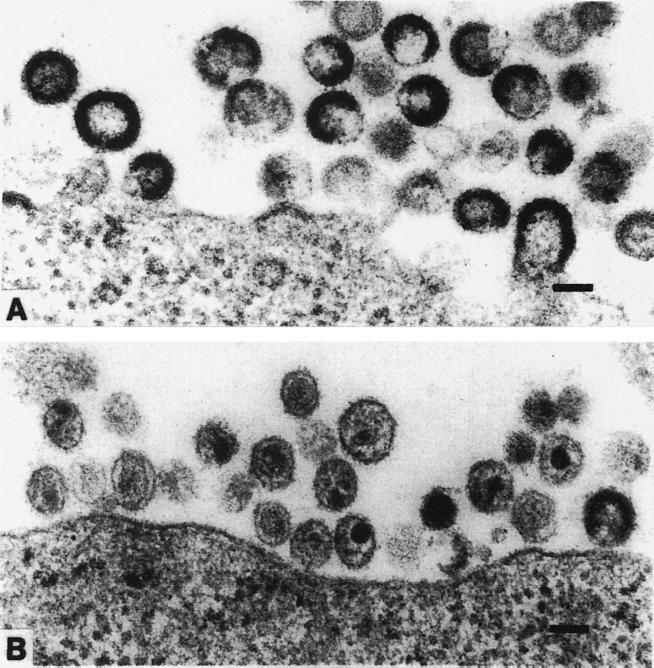
Another way to look at the HIV PR is to consider how it functions in the timing of viral morphogenesis. In all cases, “immature,” uncleaved particles appear (Fig. 3A), and after cleavage by the PR, they appear as “mature” infectious particles (Fig. 3B). In the viral life cycle, the retroviral PR must remain inactive in its precursor form until virus assembly is initiated; otherwise the >1,000 Gag precursors per virion will be abnormally cleaved (199). As noted previously, site-specific mutagenesis has demonstrated that the enzyme is absolutely required for replication of mature, infectious virions; noninfectious particles, containing uncleaved Gag and Gag-Pol polyproteins, are produced if the enzyme is inactivated (191, 219, 231).
FIG. 3.
Electron micrographs of immature (A) and mature (B) HIV-1 particles. Kindly provided by Toshiyuki Goto, Department of Microbiology, Osaka Medical College, Takatsuki, Japan.
Accordingly, an effort was made by many laboratories to use dihdroxystere analogs, such as pepstatin-based compounds, to inhibit avian and murine retroviral assembly as a starting point for the development of HIV-specific PI (256).
Overview of HIV Protease Inhibitors
Currently, although HIV PI appeared among the most effective antiviral agents used to treat HIV infection and AIDS when initially used in vitro and in monotherapy (69, 72), PR-resistant mutants arose and specific PI varied in their effectiveness when used alone or in combination (UCSF Website; Deeks et al., 37th ICAAC). Thus, PI were found to be clinically more efficient when used in combination therapy with certain RT inhibitors (Table 1). PI basically differ from the anti-RT drugs, which inhibit virus production only in acutely infected cells and do not affect virus production in persistently infected cells or activated HIV postintegrated quiescently infected cells.
HIV particles produced in the presence of PI can undergo Gag polyprotein cleavage following removal of the PI, but this process does not appear to occur at a high enough level to restore infectivity in the majority of particles (152).
PI-mediated treatment of HIV infection in SCID mice implanted with human fetal thymus and liver tissue is not completely effective, although treated mice have markedly lower viral loads in the region of the implants and spleens (298). Also, a significant correlation has been found between an increase in viral neutralizing-antibody titer and treatment with PI alone or in combination with zidovudine (AZT) but not with AZT alone (322).
Characteristics of Successful HIV Protease Inhibitors
For HIV PI to be successfully used as drugs in patients, they should be effective in vitro at the nanomolar level, have good oral bioavailability, and exhibit minimal side effects against human cellular aspartyl PR. Several excellent reviews have been written to address this area (70, 368). Genetic and X-ray crystallographic studies (27) have been performed for the HIV PR alone (Fig. 2) or with inhibitors in place, and an extensive database is available (368). This has allowed dissection of the inhibition process into three discrete structural features. (i) The first is modification of amino acid residues at the PR substrate binding pocket (Fig. 2B). This involves replacement of substrate scissile bonds by nonscissile bonds in the inhibitor; use of peptidomimetics has permitted this to be the starting point for design of most first-generation PI. (ii) The second is alteration of the PR flap (325) (Fig. 2A). (iii) The final feature is alteration of the stereochemistry of peptide bond replacements in the active site. Thus far, four major PI have been approved by the FDA and are being used in clinical treatments. Many other PI are in clinical trials or in earlier stages of development. The first four FDA-approved drugs are described in greater detail here.
Saquinavir (RO 31-8959, Invirase), developed by Roche, was the first FDA-approved PI. Since the HIV PR readily cleaves highly hydrophobic scissile, e.g., Tyr(Phe)-Pro, amino acid residues in Gag and Gag-Pol precursors, a strategy was developed to create nonscissile analogs of this dipeptide with five additional binding amino acid residues. Saquinavir has a Ki of 0.12 nM at pH 5.5 against the HIV PR, and it was shown by X-ray crystallography that inhibitors bound in an extended conformation, preserving the homodimeric nature of this enzyme. Recently, Fortivase, a new formulation of saquinavir in soft gelatin capsules, has shown enhanced oral bioavailability. This also provides for higher drug exposure and antiviral activity than the hard-tablet form. Both compounds are recommended in HAART with AZT and dideoxyinosine (ddI), and both exhibit excellent evidence of clinical benefit and sustained suppression of the plasma viral load (123).
In addition to saquinavir, three other PI (ritonavir, indinavir, and nelfinavir) have been developed, and more are in the pipeline (70, 123, 259). As mentioned above, all four drugs are currently recommended in HAART for treatment of established HIV infection (Table 1).
In the development of ritonavir, it is of interest that Abbott originally had developed the synthetic compound A-77003, which, based on in vitro studies, appeared to be an excellent PI; this was related to its mirror-image dimeric structural symmetry, ease of crystallization, and high level of antiviral activity (96). However, A-77003 had low oral bioavailability and resistant HIV strains appeared rapidly in tissue culture. A new direction was thus taken when it appeared that symmetric PR inhibitors could bind the HIV PR in an asymmetric fashion (89, 368, 369). This led to a process where shorter, more orally bioavailable, asymmetric analogs of A-77003, such as ritonavir, were constructed. Ritonavir inhibits the cytochrome P-450 system, making its levels in blood very stable and also stabilizing the levels of other PI, such as saquinavir, in blood (178).
Indinavir (Crixivan) was synthesized by the Merck group, using a similar strategy to that taken by Roche involving nonscissile hydroxyethylene bond analogs. This approach was combined with the use of X-ray crystallography and molecular modeling to test leading compounds. Indinavir, with a Ki of 0.52 nM in vitro against the HIV PR, was synthesized as a first-generation PI. Again, as with other PI, there was high specificity for retroviral versus cellular (e.g., renin) enzymes. Indinavir inhibited the replication of HIV-infected cells at 25 nM, was orally bioavailable, caused a major decrease in plasma viral RNA load, and led to an increase in the number of CD4+ cells (91).
Nelfinavir mesylate (Viracept), an FDA-approved PI, was synthesized by the Agouron group using a totally different approach from that taken with other PI. It contains a DIQ (decahydroisoquinoline) group like saquinavir, which permits tight binding to the water molecule near the D-T-G active site. Nelfinavir mesylate has become a major PI in the United States, in part because it was the first one approved for the treatment of pediatric AIDS (70). An oddity, in some ways, is that none of the above HIV PI inhibit the similarly conformed human T-cell leukemia virus type 1 HTLV-1 aspartyl PR; this may be related to differences in substrate subsite (S1 and S1′) recognition (297).
Recently, amprenavir (Agenerase, 141-W94, VX-478) was also approved for treatment of HIV infection in the United States. One potential use for amprenavir is as salvage therapy in patients for whom treatment that includes one (or more) of the above four PI has failed. The presence of the N88S mutation and associated amprenavir hypersensitivity may be useful in predicting an improved clinical response to amprenavir salvage therapy (385). Also, treatment with amprenavir plus AZT, and lamivudine, was shown to reduce the levels of HIV RNA significantly more than did amprenavir monotherapy (261).
Some other new approaches to development of HIV PI involve (i) modeling studies with d-amino acids introduced into the PR P1 and P1′ positions of reduced bond inhibitors (70), (ii) C-2 symmetry-based inhibitors targeted against new variants of A-770003 (96), and (iii) placement of a trisubstituted cyclopropane-derived peptidomimetic into a known HIV PI to stabilize the inhibitor extended structure (96).
Emergence of Resistant Mutants
There is evidence of resistance to PI in some patients under HAART after 1 to 2 years, depending in part on location; e.g., in New Orleans, La., this has taken longer to appear (Hagensee, personal communication), while in Costa Rica, although patients are generally doing well on triple therapy, resistance to some PI appeared relatively quickly, attributable in part to earlier monotherapy or noncompliance (K. Visona, personal communication). Resistance, as with tuberculosis, involves selection in the patient of preexisting or newly formed variants containing amino acid replacements at conserved sites such as near the PR active site, the flap, as well as at other sites which decrease binding of the PI (Fig. 2). There is also a certain level of cross-resistance between different groups of PI (86; Deeks et al., 37th ICAAC). Interestingly, as with the RT, one can find second-site mutants that restore enzyme activity (31, 281).
The emergence of HIV variants with a reduced sensitivity to PI also occurs most rapidly when treatment fails to achieve a sufficiently profound suppression of viral replication (D. J. Kempf, R. Rode, Y. Xu, E. Sun, A. Japour, S. Danner, C. Boucher, J. Leonard, and A. Molla, Abstr. Int. Workshop HIV Drug Resist. Treat. Strategies Eradication, abstr. 62, 1997). The evolution of HIV toward high-level resistance to PI is thus the result of a gradual accumulation of those resistance mutations in the PR (58, 59, 96, 253, 258, 311). Most of the HIV PR mutations associated with decreased sensitivity to PI have been identified, e.g., L10I, K20R, M36I, M46I, F53L, L63P, A71V, V82A, D30N, I84V, I54V, L90M, and G48V (58, 59, 143, 167, 234, 237, 290, 291, 312). These mutations are usually not found in HIV isolates which have not been previously exposed to PI (15, 197, 204, 263, 267, 367).
Recently, Rouzine and Coffin (317) proposed a novel mechanism of evolution for the HIV pro gene. Variation in pro was shown by analyzing a database of 213 sequences restricted to rare variable bases which are highly diverse and differ in location among individuals. The average intrapatient distance per individual variable site is similar for synonymous and nonsynonymous sites, although synonymous sites are twice as abundant. The latter observation excludes selection for diversity as an important, permanently acting factor in the evolution of pro and leaves purifying selection as the only kind of selection. Based on this, a model of evolution was proposed in which there are two possible explanations for the high mutant frequency: (i) the frequency of coinfection in the natural host population may be quite low, and/or (ii) a strong variation of the best-adapted sequence between individuals could be caused by a combination of an immune response present in early infection and coselection.
Studies using in vitro cell cultures in the presence of a PI showed that PI-resistant variants have mutations that are often located close to the enzyme active site (28, 66, 132, 167, 217, 316, 321). In addition, the impairment of HIV replication which results from selection of these resistance mutations can be partially compensated for by secondary mutations that are usually located outside the PR active site (28, 58, 167, 272, 316) or in trans (206). Furthermore, for some HIV variants obtained in culture in the presence of a PI, it has been found that adaptive changes that partly correct resistance-associated loss of HIV infectivity can emerge outside the PR coding sequence, e.g., in PR cleavage sites within the Gag precursor protein, such as p7-p1 and p1–p6 (66, 281; A. Carrillo, H. Sham, D. Norbeck, D. Kempf, W. Kohlbrenner, J. Plattner, J. Leonard, and A. Molla, Abstr. Fourth Conf. Retroviruses Opportunistic Infect., p. 462, 1997). Surprisingly, the p1–p6 mutant sequence found in HIV PI resistance variants can promote ribosomal frameshifting both in vitro and in virus-expressing cells (87).
It should be reiterated that although HAART (or PART) is currently the treatment of choice, PI had been initially used in monotherapy trials, where HIV populations in plasma remained genetically constant prior to drug treatment and viremia decreased 10- to 100-fold and during the 1 to 2 weeks following initiation of therapy. Rapid plasma viremia rebounds frequently occurred, and this was associated with either noncompliance or the rapid emergence of drug-resistant virus (75). Thus, as noted above, the switch was made from monotherapy to triple antiviral therapy. Further, this permitted the use of lower PI levels than with monotherapy (23, 312). Another interesting mechanism to explain the positive effectiveness of PI in HAART is that PI seem to be effective at both early and late stages of infection (241, 266); however, there is still controversy about the role of the PR in early virus replication (168).
Secretory Leukocyte Protease Inhibitor
The virtual absence of oral transmission of HIV (114, 188, 254) and reports of antiviral activity in human saliva (6, 21, 63, 111, 119, 229, 313) led to the identification of secretory leukocyte PI (SLPI) as a potent antiviral factor in saliva (239, 240, 358, 360). SLPI is also found in certain other mucous secretions, e.g., cervical and bronchial secretions (198). SLPI does not appear to act on the virus directly (240); instead, its inhibitory activity is most probably due to interaction with the host cell. SLPI most probably inhibits a step of viral infection that occurs after virus binding but before reverse transcription (240). Thus, it differs from the classic PI involved in blocking viral maturation. SLPI, along with other salivary factors with potential HIV-inhibitory activity, has recently been reviewed (328).
A recent new aspect of the role of the HIV PR in the oral cavity has been reported (P. Hickman, P. Fidel, J. Leigh, R. Mera, and R. B. Luftig, Abstr. 19th ASV Meet., abstr. 145, 2000; R. Luftig, P. Hickman, J. Leigh, R. Mera, P. L. Fidel, Jr., A. Mock, and W. Gallaher, Abstr. 99th. Gen. Meet. Am. Soc. Microbiol. 1999, abstr. T-14, p. 630, 1999). In AIDS-infected individuals with oral pharyngeal candidiasis, a common opportunistic infection, one or more yeast aspartyl proteinases are present in the oral cavity. Most of these are secreted by the yeast Candida albicans (265) and may play an as yet undefined role in the selection of certain classes of HIV PR mutations (Luftig et al., Abstr. 99th Gen. Meet. Am. Soc. Microbiol.). Further, it has been shown that certain PIs can prevent recurrent oral pharyngeal candidiasis (40, 131), and this may indirectly affect the oral flora, including HIV, as well as the course of viral and/or yeast pathogenesis.
ROLE OF HIV-1 PROTEASE AND OTHER GENES IN ACUTE AND PERSISTENT INFECTIONS
Viral Accessory Genes and Their Role in Overall HIV Production
In the previous section, we have presented in detail the role of the HIV PR in virus production. Even partial inhibition of the PR gene can lead to formation of noninfectious particles, which may be relevant to disease. Further, as we show in the next section, there can also be interactions between mutations in the PR and several accessory genes, such as nef and vpr, to create defective particles that may play a significant role in AIDS pathogenesis. Toward this end, we briefly review the role of accessory genes in HIV production.
The HIV genome consists of structural (gag, pol, and env), regulatory (tat and rev), and accessory (vif, vpr, vpu, and nef) genes. Both structural and regulatory genes are essential for replication in vitro, while accessory genes are considered nonessential for replication in tissue culture, because each one can be deleted without destroying virus replication in different cell lines (1, 71, 110, 189, 221, 277, 340, 348).
The assignment of molecular functions to accessory genes was initially based on in vitro experiments with cloned viral mutants. vif and vpu are required for the efficient production of infectious virus particles in certain cell lines (106, 338, 339, 349), while vpr has a slight effect on virus replication in cultured T-cell lines (277). Further, Vpr is located in virions (43, 104) as a serine phosphoprotein in stoichiometric amounts with respect to Gag. It is phosphorylated in several HIV-infected cell lines used by different groups, and the serine phosphorylation at position 79 of Vpr appears important for arresting cells in the G2 phase of the cell cycle (L. Ratner, personal communication; Y. Zhou and L. Ratner, Abstr. 18th Annu. ASV Meet., abstr. 150, 1999). There is still controversy about whether Vif (81) is assembled in particles.
The nef gene, located at the 3′ end of the viral genome (partially overlapping the U3 region of the 3′ long terminal repeat [LTR]), encodes a membrane-associated myristylated protein synthesized from early, multiply spliced mRNA transcripts and displays cytotocix activity (67) or can act as a transcriptional repressor of the HIV-1 LTR (1). The Nef protein also acts as a positive factor serving to augment viral replication, most notably in primary lymphocytes and macrophages (2, 79, 134, 182, 247, 334, 348, 350). Further, Nef can be cleaved in vitro between residues 57 and 58 by the HIV PR, generating two Nef polypeptides (115, 120). The myristylated Nef protein itself is incorporated into HIV virions (34, 286, 364), with 60 to 80% of incorporated Nef cleaved by the HIV PR. However, a recent paper suggests that PR cleavage of the Nef protein in virions may not be necessary for its infectivity (249).
Viral Factors Involved in the HIV Life Cycle
HIV is strongly cytopathic for CD4+ T lymphocytes in a variety of tissue culture systems (209). However, biological properties of HIV, such as its replication rate, cell tropism, and cytopathogenicity, can vary during the course of infection (8, 45, 346, 347). In the early asymptomatic phase, preferentially macrophage-tropic, NSI variants of HIV predominate, whereas later in the course of the infection, more T-cell-tropic variants appear; >50% of AIDS cases are associated with the emergence of SI variants (61, 323, 356, 384).
In vitro systems using CD4+ T cells infected with recombinant HIV variants containing single or multiple accessory gene (vif, vpr, and vpu) mutations have shown a loss of cytopathogenicity leading to viral persistence (184–187, 273). Also, the same nonsense mutations in vpr that can be observed to arise naturally in peripheral blood mononuclear cells (PBMCs) of HIV-seropositive individuals during the early stages of infection increase in number with increasing in vitro serial passage of wild-type virus (268, 274). Further, deletion mutations due to a putative misalignment mechanism have been detected over a region spanning vif and vpr open reading frames after extensive in vitro serial passage of wild-type virus (269). Similarly, a high percentage of extensively deleted defective HIV genomes generated by such a misalignment mechanism have also been identified in PBMCs from HIV-infected individuals (320).
In summary, a wide spectrum of mutations generating multiple defects in accessory genes during HIV replication appear to correlate in vitro and in vivo with viral persistence and loss of cytopathogenicity. This conclusion is consistent with findings from other laboratories that nondefective Vpr plays a role in cell cycle arrest and apoptosis, as well as in nuclear transport of the preintegration complex (139, 226, 314, 359). It should also be noted that a persistent infection with highly passaged (≥50 passages) nef frameshift mutant viruses could not be established, using the cell system described above (117, 274). Thus, the need for wild-type nef, in addition to mutations in other accessory genes, appears to be associated with establishment of persistent HIV infection in vitro.
Host Factors Involved in the HIV Life Cycle
Host factors have been known for some time to alter the rate of HIV progression in individuals, including secretion of cytokines; e.g., proinflammatory cytokines like tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-6 upregulate HIV replication, while transforming growth factor β and IL-10 lead to its downregulation (55, 100). Also, about 3 years ago, an extremely important observation was made that HIV tropism is determined predominantly by second-receptor usage (103, 375). As shown below, this has led to further refinements in antiviral therapy.
The study of coreceptors has been a dynamic area. Many groups have shown that as many as 12 coreceptors, CCR2b, CCR3, CCR5, CCR8, CXCR4, CX3CR1, BONZO/STRL33/Tymstr, BOB/GPR15, GPR1, APJ, HCMV-US28, and BLTR, can function in HIV infection or syncytium formation in tissue culture (3, 4, 47, 52, 73, 76, 85, 88, 92, 99, 103, 112, 138, 147, 215, 220, 284, 300, 319, 330). Recent work has shown that the β chemokine receptor 5 (CCR5) and chemokine receptor 4 (CXCR4) are the two major coreceptors for infection by primary isolates (18, 376, 380). This cytokine family of receptors bind conformationally modified surface glycoproteins (SU) from the HIV M group of subtypes A, B, C, D, and E, as well as the outlier (O) group. Other chemokine receptors play a lesser role in facilitating viral entry into stimulated PBMCs (381). Macrophage-tropic NSI variants use CCR5, T-cell line-tropic SI variants use CXCR4, and primary SI variants can use both receptors (4, 25, 47, 76, 85, 88, 103, 329, 377). Based on the chemokine responsiveness of PBMCs, CXCR4 is thought to be abundantly expressed while CCR5 expression on PBMCs is low (24, 275). This result is consistent with the previously observed broader T-cell host range of SI variants (109).
There is no apparent geographical clustering of CCR5 polymorphism in different ethnic populations, suggesting that CCR5 diversity is not the underlying explanation for differences in the spread of different HIV subtypes. While CXCR4 is also widely expressed on resting T lymphocytes, the expression of CCR5 is low on quiescent T cells and is significantly upregulated only after stimulation (24, 275, 353). Moreover, a largely reciprocal expression of CXCR4 and CCR5 among peripheral blood T cells has been reported (24).
Recently, differential patterns of chemokine receptor cofactor usage have been seen in HIV variants from the lungs and blood (330), as well as inhibition of HIV replication by chemokines (Carrillo et al., Abstr. Fourth Conf. Retroviruses Opportunistic Infect.). CXCR4 is expressed predominantly on an unactivated naive CD26low CD45RA+ CD45RO− T-lymphocyte subset, while CCR5 is found on CD26high CD45RAlow CD45RO+ T lymphocytes thought to represent activated/memory cells (25). Thus, since NSI and SI HIV variants may have different cellular tropisms for activated/memory and resting/naive T cells, respectively, specific coreceptor expression coinciding with the presence of a cellular kinase specific for phosphorylation of certain dideoxynucleotides, may contribute to the virus phenotype-dependent effect of different dideoxynucleotide analogs.
This effect led van't Wout et al. (357) to propose a model whereby AZT is preferentially active in activated CCR5-expressing cells that can be infected by NSI HIV variants while ddI preferentially acts in quiescent CXCR4-expressing cells that support SI HIV infection (74). The combination of AZT and ddI then covers both cell types and is used to inhibit both phenotypic variants. In contrast, PI, e.g., ritonavir, which do not require intracellular phosphorylation, are active in both quiescent and activated T cells (327). Thus, combined use of two nucleoside inhibitors and one PR inhibitor for HAART is also justified in terms of covering HIV infection of multiple host cell phenotypes (Table 1).
Primary SI variants are also capable of using both CCR5 and CXCR4 for entry (329, 377). AZT, which is presumed to preferentially inhibit viral infection of CCR5-expressing cells, partially inhibits the SI virus load as well. Primary SI variants do not seem to escape from ddI and are presumed to inhibit infection preferentially in CXCR4-expressing cells by first replicating in CCR5-expressing cells (357). This is in agreement with the finding that NSI variants always remain present and contribute significantly to the viral load even after the emergence of SI variants (195).
Activation from Latency
After initial infection with HIV in most individuals, an asymptomatic carrier (AC) state is established, where there is productive replication of HIV in most CD4+ T cells (which are then replaced with uninfected CD4+ T cells newly provided from bone marrow) together with a quiescent or latent integrated state of the proviral DNA in a small but increasing number of non-lytically infected cells. This is followed by chronic infection and eventual clinical progression toward AIDS-related complex and then AIDS (238, 303).
The latently integrated state in HIV, although apparently quiescent, can best be described as dynamic, involving interactions between cellular and viral factors, i.e., HIV regulatory and accessory genes on one hand and several internal transcription factors, e.g., NF-κB, and external immune regulatory proteins on the other hand (55, 155). The mechanism of HIV latency has been examined in several cell lines expressing very low levels of viral mRNAs and proteins, and this situation is referred to as postintegration latency (238, 303). Further, different subclones have been established in various laboratories by rescuing and cloning latently or persistently HIV-infected human cell lines such as U937, HL-60, A3.01, Jurkat, and MOLT-4 (37, 51, 107, 116, 118, 296). Under unstimulated conditions, the level of viral transcripts in such subclones is very low (less than 5% of the total cell population) (302). During isolation of HIV from such model cell lines, it was found that virus latency in the human CD4+ T cell line MOLT-4 could be established only by infection with HIV recovered from acutely infected cells but not with virus from persistently infected cells (118).
Activation leading to virus production from postintegration latency in such model cell lines can then be induced by several reagents such as TNF-α, phorbol esters, or other reagents (20, 116). All of these reagents are presumably involved in induction of cellular transcription factors (122, 264). The mechanism of HIV activation from latency has been further characterized and also shown to exhibit a cell cycle dependence for the G2/M phase in phorbol ester-treated cells, while it occurs in a cell cycle-independent manner after TNF-α treatment (351).
The accessory proteins Nef and Vpr appear to play an important role in regulating pathogenesis in vivo (181, 201), while extracellular or soluble Nef (116) and Vpr (209, 210) may function to activate HIV from latency. For soluble Nef this may occur through a signal transduction pathway involving ras, raf, and NF-κB (M. Tobiume and K. Ikuta, unpublished data). The ability of these accessory gene products to activate latent HIV in a manner analogous to that for cellular cytokines could provide a simple and direct means to explain HIV activation.
Further, specific interactions between Nef and the putative Nef binding receptors on T cells (283) and between Vpr and the glucocorticoid receptor type II complex (307) could also be triggers for intracellular signaling pathways that lead to activation of latent HIV proviral DNA, followed by viral antigen expression and particle production.
AIDS PATHOGENESIS
Accessory Gene Products Involved in AIDS Pathogenesis
The nef gene was initially shown to be essential for AIDS pathogenicity of simian immunodeficiency virus (SIV)-infected rhesus macaques (181). Efforts have also been made to characterize genetic features of HIV present in long-term survivors (233). Sequence analyses of viral genomes in long-term survivors revealed defects in both regulatory and accessory genes (68, 156, 183, 246). This was observed initially in an Australian cohort of LTNP infected with HIV through a blood transfusion from a single donor; multiple deletions in nef and the U3 part of LTR were found in all members of this cohort (68, 203). Another study also revealed a high frequency of defective nef alleles in a single LTNP (233). The relative lack of disease in these individuals raised the possibility that one or more specific genetic defects in HIV accounted for the prolonged AC state. This finding appeared similar to those in macaques that remained asymptomatic for a prolonged period after infection with a nef-deleted SIV strain (181).
In contrast to the above result, a cohort of LTNP in the United States were infected with HIV in which the nef gene was both genetically and functionally intact (150, 151). Detailed sequence analyses of vif, vpr, and vpu in these viruses also revealed the integrity of these genes (379). In the 5′ LTR sequences, all, except for that in one individual which carried G-to-A hypermutations throughout the entire region, shared nearly identical consensus sequences in the binding sites for NF-κB, Sp1, and the viral trans-activator Tat (378). Env proteins have also been shown to have functional abnormalities in long-term survivors (60). Taken together, these results suggest that it is unlikely that a single common genetic determinant is responsible for the well-being of LTNP.
When paired isolates obtained by coculture with PBMCs from HIV-infected individuals before and after the panning of CD8+ T cells were compared, it was found that a clear distinction at nef occurred between HIV paired isolates from AC but not from LTNP, indicating the importance of wild-type Nef-specific CD8+ T-cell selection pressure for maintaining a stable asymptomatic state (383).
In contrast, the role of vpr in development of AIDS is controversial: on one hand, vpr gene mutations when combined with nef mutations in SIV decreases simian AIDS pathogenesis in monkeys (201); on the other hand, vpr deletion mutants of SIV themselves can still induce simian AIDS in infected monkeys (145). Also, molecular analysis of HIV strains derived from the blood and plasma of an HIV-infected long-term-surviving (>13 years) mother-child pair showed the presence of defects (insertions and deletions) and polymorphisms in vpr (362).
Examination of the interaction between HIV regulatory gene products and the host immune system is fundamental in understanding the pathogenesis of HIV (225) and could reveal possible targets for AIDS treatment. The HIV tat gene is also a potential candidate for this type of strategy. In transgenic mice harboring tat, there is an effect on the immune system, i.e., enhanced TNF production (32). Also, the addition of synthetic Tat peptides, but not those from recombinant Nef and Vif proteins, inhibits proliferative responses of CD4+ tetanus antigen-specific, IL-2-dependent T-cell clones in a dose-dependent manner (46).
CD4 Cell Count and HIV Viral Load in Plasma as Markers of AIDS Progression
CD4 cell counts are widely used to predict disease progression in HIV-infected patients (336) and have been employed as a surrogate marker to provide evidence for the effectiveness of therapeutic agents. In addition, viral load or the HIV RNA level determined by PCR is an excellent predictor of the prognosis for patients infected with HIV (121, 242, 276). By investigation of the relationship between these markers, it was shown that the return of the CD4+ cell count to higher levels was significantly related to both the baseline CD4 count and the decline in HIV RNA PCR-determined viral load (101). However, it has also been shown that sole use of a return to high levels of the CD4 cell count can be misleading in monitoring disease progression, since changes in CD4 count after therapy are determined more by the starting CD4 count than by the change in viral load (243), and large increases can occur with minimal antiviral effect (91).
The emergence of SI variants has been shown by many groups to correlate with both accelerated decline in CD4+ T-cell counts and accelerated disease progression in the natural course of HIV infection (171, 192, 197, 310), as well as during treatment (29, 170, 176, 193, 336). Moreover, individuals harboring only NSI HIV variants were shown to benefit more from treatment with AZT than were individuals also harboring SI variants (193). In fact, a preferential inhibition of NSI variants by AZT was observed (355), while ddI treatment resulted in a loss of SI variants in bulk cocultures (74, 382). Also, it has been shown that neither AZT therapy alone nor AZT in combination with alpha interferon or ddI prevents the acquisition of SI strains (352).
On the other hand, it was also known that approximately half of people infected with clade B HIV who develop AIDS never acquire detectable CXCR4 binding (X4) HIV quasispecies (7, 45, 193, 346). It is enigmatic that most studies of primary CCR5 binding (R5) isolates of HIV have detected little if any pathogenesis attributable to these viral isolates in tissue culture yet many AIDS patients die from infection with exclusively R5 HIV-1 quasispecies. Many R5 isolates of HIV replicate more slowly and to lower titers in stimulated PBMCs than do R5X4 or X4 isolates (61, 354). Nevertheless, some R5 isolates found later in the course of infection, particularly after an AIDS diagnosis has been made, replicate more rapidly and to higher titers in tissue culture than do typical R5 isolates found early in the course of infection (354).
Activation of latently infected or quiescent T cells during HIV infection is becoming another active area of study in AIDS pathogenesis. The levels of CD4+ and/or CD8+ T cells expressing activation markers such as HLA-DR (9, 16, 179, 180, 227), CD25 (146, 227), or CD38 (158, 179, 180, 228, 370) are elevated in HIV infection. However, the mechanism of activation is not yet understood; e.g., it could reflect an antigen-induced T-cell activation in vivo or may be secondary to HIV-induced cytokine production (157, 158).
Treatment with AZT has been shown to reduce activation significantly (17, 194). In addition, treatment with PI, such as ritonavir, reduces the percentage of CD4 and CD8 lymphocytes expressing CD38 (177). Recent cocapping experiments have revealed the gp120-induced association of CD4, the major receptor for HIV, with CD38, suggesting that assembly of abnormal multimolecular complexes could be involved in either gp120-CD4+ T-cell dysfunction or viral entry into cells (33, 82, 102). The association with CD38, but not with chemokine receptors, was observed at increased percentages in both CD45RA+ naive and memory cells (102). Using monoclonal antibody panning and flow cytometry, it was possible to separate PBMCs into CD4+ CD38+ and CD4+ CD38− subsets, which show susceptibility to HIV with different tropism (146a). This may eventually allow one to examine these populations as potential reservoirs of HIV, even in HAART patients. However, there is still little information about whether the differentially activated CD4+ T cells described above differentially express coreceptors which could contribute to varied stages in the HIV life cycle.
Role of Dendritic Cells
It has been known for some time that the acute phase of HIV infection is followed by an extended period in which humoral and cell-mediated immune responses occur, viremia decreases, and the major virus reservoir is represented by germinal centers in lymph nodes and possibly other peripheral lymphoid tissues (94, 287, 288). Thus, monitoring the status of the viral burden and of trapping and replication of viruses in lymph nodes during combination antiretroviral drug treatment may be critical to an assessment of therapy (56). Plasma cells producing antibodies to HIV have been identified in germinal centers of lymph nodes from asymptomatic subjects (344). Therefore, extracellular immunoglobulin may form immune complexes, as shown by the presence of HIV-specific antibodies, HIV particles, and complement components. In addition, receptor-mediated endocytosis of viral particles by follicular dendritic cells (DC), when virus is abundant in the cytoplasm, leading potentially to the formation of a hidden reservoir, has been observed (144). In this location, HIV may escape recognition by cytotoxic T lymphocytes (CTLs). In contrast, virus budding has seldom been seen, indicating that productive infection of HIV in follicular DC would be rare.
DC play a critical role in the activation of primary immune responses since they are specialized to present antigens to naive T cells in vivo (374). Immature DC capture antigen at peripheral sites such as the skin, airways, mucosa, or gut and then migrate via afferent lymphatics to T-cell-enriched regions in secondary lymphoid organs (57). During this homing process, immature DC lose their capacity to process antigen but upregulate costimulatory molecules (CD86, CD80, and ICAM-1) on their surfaces and display a mature phenotype. These mature DC are then able to select antigen-specific T cells from the circulating pool and stimulate them (42, 374). The yield of DC derived from monocyte-depleted PBMC by culture in human IL-4 and human granulocyte-macrophage colony-stimulating factor was lower in HIV-infected than seronegative subjects, and the lower yield of cells correlates with smaller numbers of peripheral blood CD4+ T cells and higher plasma viral load (98).
Since Langerhans cells express CD4, the major cellular receptor for HIV, their presence in the urogenital mucosa gives rise to the possibility that DC are involved in sexual transmission of HIV, as well as SIV, infection (78, 271, 306, 332). In rhesus monkeys, SIV can be efficiently transferred to lymph nodes within 2 days following mucosal inoculation of the virus (161, 335). Evidence that DC could be directly involved in HIV transmission was deduced from the observation that HIV readily associates with DC and can cause DC and T cells to generate syncytia, which represent sites of vigorous viral replication in vitro and in vivo (38, 304, 305). Both macrophages and DC of mucosal epithelia are among the first cells to be associated with virus following sexual contact involving HIV (113, 248, 250, 374). DC are also capable of transmitting HIV to CD4+ cells of the monocytoid lineage (163).
Recently it was observed that PR-defective, gp120-enriched L-2 particles can bind to DC prepared from PBMC of healthy donors, using IL-4 and granulocyte-macrophage colony-stimulating factor and cause them to acquire an effector function, leading to apoptosis of bystander CD4+ and CD8+ T cells (343).
Accumulation of Infected Cells Producing Defective Particles
According to a deterministic model of HIV population genetics, the frequency of any particular mutation in the differentiated viral quasispecies found a few years after initial primary infection is primarily a function of its production rate and the relative replication fitness it confers on the genome (53, 54). According to this model, any single-nucleotide mutation associated with drug resistance may be found on as many as 0.1 to 1% of the viral genomes in the fully differentiated quasispecies which have evolved following several years of infection. This modeling assumes that 108 to 109 cells are infected per day (142, 363). Mutation rates are highly dependent on sequence context (17, 161, 234), with an average rate of 3 × 10−5 bp/replication cycle (234).
Interestingly, a novel proposal has been suggested which takes advantage of this high mutation rate by showing that promutagenic nucleosides, such as 5-hydroxydeoxycytidine (5-OH-dC), lead to additional “lethal” mutations and/or loss of viral replication during passage in human CEM cells (219). The fitness cost associated with some drug resistance-associated mutations in the absence of drug selection has been estimated, from their frequency in untreated patients (204, 267) and competition between variants in vivo (125), to be approximately 1% (54). Using such assumptions, a steady-state total of 108 cells are expected to carry any particular single-site drug resistance-associated mutation (53, 54). Following the application of drug selection, the continued replication of such a large drug-resistant mutant population would be expected to maintain the genetic composition of the quasispecies at other (nonselected) loci. The emergence of PI-resistant viruses in plasma therefore reflects a highly complex process. Several explanations for this phenomenon have been proposed, including variable levels of drug efficacy at different anatomical sites of virus production, as well as the selection and amplification of a few drug-resistant lineages, so that the frequency distribution of nonselected gene variants is altered (75).
Apoptosis of Host Immune Cell Populations
Several reports have focused on the possible function of HIV-related proteins such as soluble gp120 and/or Tat to prime signals for induction of apoptosis in bystander cells (13, 213, 285, 365). Although these reports have revealed the ability of these proteins to induce apoptosis by using the Fas/FasL system in CD4+ T cells (169), they mostly examined the effect on T-cell lines after exposure to large amounts of such recombinant HIV proteins. Thus, soluble gp120 was shown to prime apoptosis only in T-cell lines or activated PBMCs but not in freshly prepared resting PBMCs (108, 164, 235). In addition, HIV Tat protein was shown to induce cell death by apoptosis in a T-cell line and in cultured PBMCs from uninfected donors (213). These results thus suggest that the depletion of immune cells by apoptosis in vivo can occur by both direct and indirect mechanisms (5, 244).
Several features have been ascribed to the process of apoptosis in HIV-infected individuals. First, apoptosis can be induced in patient PBMCs as an activation-dependent event after mitogenic stimulation (126, 130). Studies on the surface expression of activation markers on apoptotic and nonapoptotic cells from patient material have shown that in the chronic phase of HIV infection, 50 to 60% of apoptotic cells exhibited activated phenotypes (HLA-DR+, CD38+, CD45RO+, and Fas+) and have led to the suggestion that the CD45RO+ T-cell subset was more prone to apoptosis in HIV-infected individuals (127). Second, HIV can induce apoptosis in HIV genome-negative, uninfected cells as a bystander effect (105). In fact, apoptosis has been observed not only in CD4+ T cells but also in CD8+ T cells (105, 127, 165, 212, 260). Third, apoptosis in uninfected cells can be mediated by Fas/Fas ligand (FasL) interactions and/or an imbalance of Th1 and Th2 cytokines (50, 173). Finally, mRNA for FasL is up-regulated in PBMCs from HIV-seropositive individuals and can be expressed in CD4+ T cells (252).
HIV-induced apoptosis has also been correlated with fusion activity of the virus (230). Therefore, the propensity of HIV for apoptosis can in part be estimated by measuring its SI activity. As noted above, SI viruses also predominantly appear in patients who progress most rapidly to AIDS (104, 129). In addition, although continuous virus production is observed even at early infection times as well as during the course of disease (142, 289, 294, 363), a large number of HIV particles in the peripheral blood of infected individuals at late stages of disease are noninfectious (299). Therefore, the possible role of SI-type, PR-defective gp120-containing HIV particles in induction of apoptosis of healthy donor PBMCs, through an activation-dependent mechanism, has also been examined (164, 165).
A subclone (named L-2), which produces noninfectious HIV particles (124, 153, 373) and carries a provirus with a 1-base insertion in the pol PR (10), thus is a producer of the doughnut-shaped, RT-negative, and noninfectious HIV particles (similar to those seen in Fig. 3A). These L-2 particles exhibited a higher fusion activity for CD4+ T cells, as shown by their syncytium formation in virus-to-cell fusion, than did the parental wild-type HIV LAI particles (279) and induced apoptosis in PBMC-derived CD4+ and CD8+ T cells in an activation-dependent manner (164). The trigger for apoptosis is related to acquisition of nonspecific killer activity by a subpopulation of CD4+ CD38− T cells after adsorption with L-2 particles (165). In addition, two unrelated Thai primary isolates of HIV (clade E) exhibited a similar killer activity (165). Also, a CD4+ CD38− T-cell subset derived from 16 HIV-1 carriers showed significantly higher effector activities than did a subset from HIV-seronegative healthy donors against healthy-donor-derived PBMCs (166). All of these results suggest to us that HIV and its associated disease AIDS is unique (345) and will be around for quite some time in the human population. The epidemic has tended to increase not only among young adult males but also among adolescents (77) and drug users (41). Hopefully, new antiviral approaches, including vaccine development to diminish its virulence, will be developed along with current HAART and immune replacement therapy, but as we learned with multiple cancer therapy, these vaccine methods (see the following section) may not eliminate the virus, as was effectively done 20 years ago by the smallpox vaccine.
FUTURE DIRECTIONS
Multiple-Target Anti-HIV Therapy
HIV inhibitor treatment eventually leads to the selection of HIV variants with resistant mutations, whether they be to nucleoside inhibitors or PI. One advantage of HAART is that for the ≥50% of AIDS patients who tolerate treatment, there is an improvement in life-style, as well as longevity. Generally, mutant formation has been relatively slow to occur for PR, in contrast to nucleoside inhibitors (55, 155; Havlir et al., Abstr. 2nd Natl. Conf. Hum. Retroviruses Relat. Infect.). Thus, one goal is to utilize yet additional protein enzyme targets in the HIV replication cycle for inhibitor design, such as IN, which is involved in integration of proviral DNA, so that by potent quadruple HIV enzyme inhibitor therapy, mutant selection by resistant inhibitors can be further delayed.
Also, one can target sites other than the active site on the PR for drug development, since in bacterial systems, such as that in tuberculosis, it appears that multiple inhibitors are useful for synergistic effects. In a preliminary study, we showed a number of years ago that pepstatin (binds to active site of HIV PR) and cerulenin (binds to an unknown site(s) (perhaps the cysteine-SH group on HIV), when used at 10-fold-lower doses of inhibitor, exhibited a synergistic effect (R. B. Luftig and M. Bu, unpublished data). This approach, however, could have its drawbacks in that binding of inhibitors, such as cerulenin (154, 173), to sites other than the active site either could lead to higher Kis for active-site inhibition and/or may be toxic to cells, so that nontoxic analogs need to be developed (26). On the other hand, such synergy may be beneficial and may imply possible use of smaller amounts of active-site PI, so that there is a longer period before resistance appears in the AIDS patient.
Another strategy would be to select inhibitory compounds, such as loviride (an RT inhibitor) or quinoxiline (a nonnucleoside RT inhibitor) that can be used at high concentrations (0.1 or 2.5 μg/ml, respectively, in CEM cells) to completely suppress HIV replication, so that development of resistance is greatly minimized (70).
Finally, an idea that was propagated several years ago and is winning renewed interest is to transduce cells with DNA encoding single-chain variable-fragment antibodies (“intrabodies”), thus causing intracellular formation of heavy- and light-chain variable domains, joined through a synthetic linker and directed via protein-trafficking signals to specific cellular sites for HIV proteins, such as gp120, Rev, and PR (315). In the latter case, one can take advantage of the fact that there are different monoclonal antibodies to PR flap epitopes, which undergo dramatic conformational changes on inhibitor binding (65, 251), as well as to NH2-terminal regions leading to monomer formation (J. Sedlacek and M. Fabry, personal communication).
Vaccine Trials
The development of anti-HIV drugs represents a great effort to reduce the viral load and to prevent the onset of AIDS development. However, most HIV-infected individuals will never benefit from such therapeutic agents. The spread of the AIDS epidemic is concentrated in regions of the world where insufficient financial resources are available to allow access to these drugs.
It is believed by most investigators within the AIDS research community that eventually the only acceptable worldwide treatment for the epidemic will be through formulation of an inexpensive, effective vaccine that induces both humoral and cellular immunity (208). In fact, toward this end the National Institutes of Health (NIH) have created a new Vaccine Research Office, headed by Nobel laureate David Baltimore, the codiscoverer of HIV RT. One may also take some solace in the words of the other RT codiscoverer, Howard Temin, that even if a vaccine or treatment for HIV is not discovered soon, HIV, although different, may not be a unique virus (345). Further, a new NIH Vaccine Research Center has been constructed under the Directorship of Gary Nabel, who recently reported on vaccine strategies for HIV and other emerging viruses (G. Nabel, ASV Satellite Symp. Frontiers HIV Res., 2000).
There is evidence that neutralizing antibodies can protect against HIV infection in certain animal models (22, 36). However, it will not be easy to induce antibodies of the right quality in sufficient quantity by vaccination. Also, there is a wealth of data showing a correlation between the appearance of CTL activity and the containment of viral infection (159, 280, 318). In fact, the early containment of HIV replication in infected individuals coincides temporally with the emergence of a virus-specific CTL response (44, 196, 288). In addition, in individuals chronically infected with HIV, a high-frequency CTL response is correlated with the maintenance of low virus load and a stable clinical status (262, 278). Thus, it is believed that both antibodies and CTL responses will have to be induced for an HIV vaccine to be effective.
Historically, most successful vaccines have been made of attenuated or killed virus. However, both of these approaches appear problematic for HIV. Although attenuated viruses are likely to be quite effective (80), there are genuine safety concerns, as emphasized by the report of morbidity in monkeys after vaccination with attenuated (nef-deleted) SIVmac (97).
Recently, as noted above, several HIV vaccines have been made (202, 216, 256, 257), including one from nonenveloped, Gag-containing inactivated HIV particles and shown to give an enhanced benefit in β-chemokine stimulation in HIV-seropositive subjects. It is also hypothesized that the vaccine-increased production of β-chemokines in turn may block the binding of HIV particles to chemokine coreceptors (256, 257).
Other groups are attempting to develop envelope protein vaccines, e.g., gp120 alone or in a poxvirus vaccine vector, or to use other strategies to block the HIV fusion step, e.g., passive immunization with human monoclonal antibodies to gp120 or use of peptides to block gp41 fusion. A particularly novel approach generates “fusion-component” HIV vaccine whole-cell immunogens, which initially capture transient envelope-CD4 coreceptor structures into a complex. Then the complex is used as a formaldehyde-fixed vaccine to elicit antibodies which potentially can neutralize diverse primary isolates (200).
In our view, another possible approach for trials of vaccines against HIV may be the use of PR-defective particles, similar to the multiply mutant L-2 particles described above, for a vaccine immunogen. Subunit envelope vaccines using recombinant forms of the HIV envelope glycoprotein (particularly gp120) are safe but have been disappointing (62, 136). To date, these proteins have not elicited significant neutralizing-antibody responses to representative primary viruses (136), almost certainly because epitope exposure differs between recombinant proteins and the mature oligomer found on the virus (36, 255). Therefore, native forms of the envelope protein in defective particles may play a role as immunogens.
Regulation of Reactivation from Latency
The current guidelines for use of antiretroviral agents in HIV-infected adults and adolescents (101) are very reasonable and suggest that HAART should be introduced in persons acutely infected by primary HIV infection before extensive immune system damage has occurred. It is these patients, who number over 100,000 in the United States, and patients with advanced late-stage disease (CD4+ count < 50 cells/mm) who derive the greatest benefit from HAART even though large increases in CD4+ T-cell counts may not occur. Individuals currently infected with HIV and living in a condition where many proviral DNA copies have already been incorporated into quiescent T cells may present a treatment concern in that such virus can become reactivated and lead to the production of HIV particles, which in turn can enhance AIDS pathogenesis.
To eliminate these possibilities, other approaches must be developed, e.g., using ribozymes or antisense oligonucleotides, which can effectively inhibit reactivation at the DNA level. Thus far, such new approaches have not yet been successful in reaching phase II trials, although there have been positive outcomes in tissue culture, suggesting that these modalities have merit.
Ribozymes are enzymatic RNA molecules that can recognize and cleave other RNA molecules in a specific manner (64). By targeting HIV mRNA, one can block its translation (372). Ribozymes are being considered in both antiviral and anticancer therapy (301, 324). There are three such ribozymes in use, e.g., hammerhead, hairpin, and hepatitis delta ribozymes. Ribozymes are thought to be more advantageous than proteins in gene therapy, since they are less likely to generate an immune response. They are, however, limited in that transcription units on plasmids need to be designed so that larger amounts of the ribozyme are produced intracellularly in order to permit adequate binding and cleavage.
Reducing HIV infection by ribozyme cleavage of target RNA has been a major goal of many laboratories. One example is the development of a hairpin ribozyme (60 to 70 nucleotides) to cleave HIV RNA in the 5′ region of the LTR sequence and then use it to inhibit HIV replication of primary PBMCs, including T cells, or in progenitor stem cells (372). Recently, cleavage of the MLV RT gene by a double hammerhead ribozyme was reported, where the virion content was decreased by >80% in acutely infected cells (J. Tulpinski and B. K. Pal, Abstr. ASV 19th. Annu. Meet., abstr. P29-4, p. 175, 2000).
Finally, in addition to ribozymes, there is a whole class of HIV genetic antiviral strategies, involving RNA decoy and antisense oligonucleotides (phosphothiorates) among others, which all attempt to eliminate the latent, integrated proviral DNA that cannot be targeted by HAART (90, 214, 270).
One recent drug, called a “molecular tong,” has been designed which inhibits homodimerization of the 2 aspartyl PR monomers (Fig. 2). Another possible antiviral drug, methionine enkephalin, has also been described (N. P. Plotnikoff, Abstr. Am. Soc. Biochem. Mol. Biol. and Am. Soc. Pharmacol. Exp. Ther., abstr. A18, 2000). Other approaches suggest that combining several ribozymes and decoys to target multiple sites on HIV RNA may be most effective for treating infection from multiple M-group HIV clades (A to E) (214).
In conclusion, we believe that with the plethora of new PI, as well as other antiviral drugs including small molecular inhibitors against novel targets and, eventually, the development of one or more effective vaccines, the future looks bright for AIDS patients.
ACKNOWLEDGMENTS
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports and Culture of Japan, a Grant-in-Aid for AIDS Research from the Ministry of Health and Welfare of Japan, a JSPS fellowship, S-K grant NA97FD0062(NOAA) to R.B.L., and LSU Medical Center Institutional Funds.
We thank Ron Swanstrom, Tony Fauci, John Erickson, and Steve Oroszlan for providing reprints and/or preprints, as well as Jeanine Campbell for persistence in typing the manuscript.
REFERENCES
- 1.Ahmad N, Venkatesan S. Nef protein of HIV-1 is a transcriptional repressor of HIV-1 LTR. Science. 1988;241:1481–1485. doi: 10.1126/science.3262235. [DOI] [PubMed] [Google Scholar]
- 2.Aldrovandi G, Gao L, Bristos G, Zack J A. Regions of human immunodeficiency virus type 1 nef required for function in vivo. J Virol. 1998;72:7032–7039. doi: 10.1128/jvi.72.9.7032-7039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alkhatib G, Berger E A, Murphy P M, Pease J E. Determinants of HIV-1 coreceptor function on CC chemokine receptor 3. Importance of both extracellular and transmembrane/cytoplasmic regions. J Biol Chem. 1997;272:20420–20426. doi: 10.1074/jbc.272.33.20420. [DOI] [PubMed] [Google Scholar]
- 4.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR-5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 5.Ameisen J C, Estaquier J, Idziorek T, De Bels F. The relevance of apoptosis to AIDS pathogenesis. Trends Cell Biol. 1995;5:27–32. doi: 10.1016/s0962-8924(00)88933-3. [DOI] [PubMed] [Google Scholar]
- 6.Archibald D W, Cole G A. In vitro inhibition of HIV-1 infectivity by human salivas. AIDS Res Hum Retrovir. 1990;6:1425–1432. doi: 10.1089/aid.1990.6.1425. [DOI] [PubMed] [Google Scholar]
- 7.Åsjö B, Morfeldt-Manson L, Albert J, Biberfeld G, Karlsson A, Lidman K, Fenyö E M. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet. 1986;ii:660–662. [PubMed] [Google Scholar]
- 8.Asjö B, Barin F, Biberfeld G, Bradac J, Buve A, Dielly S. HIV-subtypes: implications for epidemiology, pathogenicity, vaccines and diagnostics. Workshop Report from European Community and Joint United National Program on HIV/AIDS. AIDS. 1997;11:17–36. [PubMed] [Google Scholar]
- 9.Autran B, Giorgi J V. Activated CD8+ cell in HIV-related diseases. In: Janossy G, Autran B, Miedema F, editors. Immunodeficiency in HIV infection and AIDS. S. Basel, Switzerland: Karger; 1992. pp. 171–184. [Google Scholar]
- 10.Bahmani M K, Kameoka M, Nakaya T, Fujinaga K, Zhong Q, Takahashi H, Nakano T, Nakai M, Ueda S, Jones I M, Luftig R B, Ikuta K. Production of doughnut-shaped, protease-defective particles from a human T-cell clone carrying a provirus with specific mutations in the env, pol, vpr and nef genes. AIDS Res Hum Retrovir. 1997;13:523–526. doi: 10.1089/aid.1997.13.523. [DOI] [PubMed] [Google Scholar]
- 11.Bahmani M K, Kameoka M, Goto T, Sano K, Luftig R B, Ikuta K. Fusion of uninfected T-cells occurs with immature HIV-1 protease-mutant, but not morphologically similar protease inhibitor derived particles. Virus Res. 2000;66:131–137. doi: 10.1016/s0168-1702(99)00132-x. [DOI] [PubMed] [Google Scholar]
- 12.Baldwin E T, Bhat T H, Gulnik S, Liu B, Topol I A, Kiso Y, Mimoto T, Mitsuya H, Erickson J W. Structure of HIV-1 protease with KNI-272, a tight-binding transition-state analog containing allophenylnorstatine. Structure. 1995;3:581–590. doi: 10.1016/s0969-2126(01)00192-7. [DOI] [PubMed] [Google Scholar]
- 13.Banda N K, Bernier J, Kurahara D K, Kurrle R, Haigwood N, Sekaly R P, Finkel T H. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J Exp Med. 1992;176:1099–1106. doi: 10.1084/jem.176.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barkai N, Rose M D, Wingreen N S. Protease helps yeast find mating partner. Nature. 1998;396:422–423. doi: 10.1038/24760. [DOI] [PubMed] [Google Scholar]
- 15.Barrie K A, Perez E E, Lamers S L, Farmerie W G, Dunn B M, Sleasman J W, Goodenow M M. Natural variation in HIV-1 protease, Gag p7 and p6, and protease cleavage sites within Gag/Pol polyproteins: amino acid substitutions in the absence of protease inhibitors in mothers and children infected by human immunodeficiency virus type 1. Virology. 1996;219:407–416. doi: 10.1006/viro.1996.0266. [DOI] [PubMed] [Google Scholar]
- 16.Bass H Z, Hardy W D, Mitsuyasu R T, Wang Y X, Cumberland W, Fahey J L. Immune changes in HIV infection: significant correlation and differences in serum markers and lymphoid phenotypic antigens. Clin Immunol Immunopathol. 1992;64:63–70. doi: 10.1016/0090-1229(92)90060-2. [DOI] [PubMed] [Google Scholar]
- 17.Bass H Z, Hardy W D, Mitsuyasu R T, Wang Y-X, Cumberland W, Fahey J L. Eleven lymphoid phenotypic markers in HIV infection: selective changes induced by zidovudine treatment. J Acquir Immune Defic Syndr. 1992;5:890–897. [PubMed] [Google Scholar]
- 18.Bazan H A, Alkhatib G, Broder C C, Berger E A. Patterns of CCR5, CXCR4, and CCR3 usage by envelope glycoproteins from human immunodeficiency virus type 1 primary isolates. J Virol. 1998;72:4485–4491. doi: 10.1128/jvi.72.5.4485-4491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bebenek K, Abbotts J, Wilson S H, Kunkel T A. Error-prone polymerization by HIV-1 reverse transcriptase. Contribution of template-primer misalignment, miscoding, and termination probability to mutational hot spots. J Biol Chem. 1993;268:10324–10334. [PubMed] [Google Scholar]
- 20.Bednarik D P, Folks T M. Mechanisms of HIV-1 latency. AIDS. 1992;6:3–16. doi: 10.1097/00002030-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Bergey E J, Cho M-I, Hammarskjoid M-L, Rekosh D, Levine M J, Blumberg B M, Epstein L G. Aggregation of human immunodeficiency virus type 1 by human salivary secretions. Crit Rev Oral Biol Med. 1993;4:467–474. doi: 10.1177/10454411930040033001. [DOI] [PubMed] [Google Scholar]
- 22.Berman P W, editor. New perspectives on AIDS vaccine development. AIDS Res Hum Retrovir. 1998;14:S185–S333. [Google Scholar]
- 23.Blankson J, Persaud D, Siliciano R F. Latent reservoirs for HIV. Curr Opin Infect Dis. 1999;12:5–12. doi: 10.1097/00001432-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–832. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 25.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blumenstein J J, Luftig R, Bu M, Copeland T D, Michejda C J, Oroszlan S. Non-peptide inhibitors of HIV-1 proteinases: synthetic analogs of cerulenin. Cold Spring Harbor Banbury Conference on Viral Proteinases as Targets for Chemotherapy. Curr Commun Mol Biol. 1989;1989:211–214. [Google Scholar]
- 27.Blundell T L, Cooper J, Foundling S I, Jones D M, Trash B A, Szelke M. On the rational design of renin inhibitors: X-ray studies of aspartic proteinases complexed with transition-state analogues. Biochemistry. 1982;26:5585–5590. doi: 10.1021/bi00392a001. [DOI] [PubMed] [Google Scholar]
- 28.Borman A, Paulous S, Clavel F. Resistance of HIV-1 to protease inhibitors: selection of resistance mutations in the presence and in the absence of the drug. J Gen Virol. 1996;77:419–426. doi: 10.1099/0022-1317-77-3-419. [DOI] [PubMed] [Google Scholar]
- 29.Boucher C A B, Lange J M A, Miedema F, Weverling G J, Koot M, Mulder J W, Goudsmit J, Kellam P, Larder B A, Tersmette M. HIV-1 biological phenotype and development of zidovudine resistance in relation to disease progression in asymptomatic individuals during treatment. AIDS. 1992;6:1259–1264. doi: 10.1097/00002030-199211000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Bouras A, Boggetto N, Benatalah Z, DeRosny E, Sicsic S, Reboud-Ravaux M. Design synthesis is an evaluation of conformationally constrained tongs, new inhibitors of HIV-1 protease determination. J Med Chem. 1999;42:957–962. doi: 10.1021/jm9803976. [DOI] [PubMed] [Google Scholar]
- 31.Boyer P L, Gao H O, Hughes S H. A mutation at position 190 of human immunodeficiency virus type 1 reverse transcriptase interacts with mutations at position 74 and 75 via the template primer. Antimicrob Agents Chemother. 1998;42:447–452. doi: 10.1128/aac.42.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brady H J M, Abraham D J, Pennington D J, Miles C G, Jenkins S, Dzierzak E A. Altered cytokine expression in T lymphocytes from human immunodeficiency virus Tat transgenic mice. J Virol. 1995;69:7622–7629. doi: 10.1128/jvi.69.12.7622-7629.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bragardo M, Buonfiglio D, Feito M J, Bonissoni S, Redoglia V, Rajo J M, Ballester S, Portoles P, Garbarino G, Malavasi F, Dianzani U. Modulation of lymphocyte interaction with endothelium and homing by HIV-1 gp120. J Immunol. 1997;159:1619–1627. [PubMed] [Google Scholar]
- 34.Bukovsky A A, Dorfman T, Weimann A, Göttlinger H G. Nef association with human immunodeficiency virus type 1 virions and cleavage by the viral protease. J Virol. 1997;71:1013–1018. doi: 10.1128/jvi.71.2.1013-1018.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bukrinsky M I, Stanwick T L, Dempsey M P, Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991;254:423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burton D R. A vaccine for HIV type 1: the antibody perspective. Proc Natl Acad Sci USA. 1997;94:10018–10023. doi: 10.1073/pnas.94.19.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butera S T, Perez V L, Wu B-Y, Nabel G J, Folks T M. Oscillation of the human immunodeficiency virus surface receptor is regulated by the state of viral activation in a CD4+ cell model of chronic infection. J Virol. 1991;65:4645–4653. doi: 10.1128/jvi.65.9.4645-4653.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cameron P U, Freudenthal P S, Barker J M, Gezelter S, Inaba K, Steiman R M. Dendritic cells of in vitro human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 39.Cao Y, Ho D D, Todd J, Kokka R, Urdea M, Lifson J D, Piatak M, Jr, Chen S, Hahn B H, Saag M S, Shaw G M. Clinical evaluation of branched DNA signal amplification for quantifying HIV type 1 in human plasma. AIDS Res Hum Retrovir. 1995;11:353–361. doi: 10.1089/aid.1995.11.353. [DOI] [PubMed] [Google Scholar]
- 40.Cauda R, Tacconelli E, Tubarello M, Morace G, DeBernardis F, Torosantucci A, Cassone A. Role of protease inhibitors in preventing recurrent oral candidosis in patients with HIV infection: a prospective case-control study. J Acquir Immune Defic Syndr. 1999;21:20–25. doi: 10.1097/00126334-199905010-00003. [DOI] [PubMed] [Google Scholar]
- 41.Celentano D D, Jittiwutikorn J, Hodge M J, Beyrer C, Nelson K E. Epidemiology of HIV-1 infection in opiate users in northern Thailand. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:73–78. doi: 10.1097/00042560-199801010-00011. [DOI] [PubMed] [Google Scholar]
- 42.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 43.Checroune F, Yao X J, Göttlinger H G, Bergeron D, Cohen E A. Incorporation of Vpr into human immunodeficiency virus type 1: role of conserved regions within the p6 domain of Pr55gag. J Acquir Immune Defic Syndr. 1995;10:1–7. [PubMed] [Google Scholar]
- 44.Chen Z W, Kou Z C, Lekutis C, Shen L, Zhou D, Halloran M, Li J, Sodroski J, Lee-Parritz D, Letvin N L. T cell receptor V beta repertoire in an acute infection of rhesus monkeys with simian immunodeficiency viruses and a chimeric simian-human immunodeficiency virus. J Exp Med. 1995;182:21–31. doi: 10.1084/jem.182.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng-Mayer C, Seto D, Tateno M, Levy J A. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988;240:80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- 46.Chirmule N, Than S, Khan S A, Pahwa S. Human immunodeficiency virus Tat induces functional unresponsiveness in T cells. J Virol. 1995;69:492–498. doi: 10.1128/jvi.69.1.492-498.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 48.Chun T-W, Finzi D, Margolick J, Chadwich K, Schwartz D, Siliciano R F. Fate of HIV-1 infected cells in vivo: rates of transition to stable latency. Nat Med. 1995;1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 49.Chun T W, Carruth L, Finzi D, Shen X, DiGiuseppe J A, Taylor H. Quantitation of latent tissue reservoirs and total body load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 50.Clerici M, Sarin A, Coffman R L, Wynn T A, Blatt S P, Hendrix C W, Wolf S F, Shearer G M, Henkart P A. Type 1/type 2 cytokine modulation of T-cell programmed cell death as a model for human immunodeficiency virus pathogenesis. Proc Natl Acad Sci USA. 1994;91:11811–11815. doi: 10.1073/pnas.91.25.11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clouse K A, Powell D, Washington I, Poli G, Strebel K, Farrar W, Barstad B, Kovacs J, Fauci A S, Folks T M. Monokine regulation of HIV-1 expression in a chronically infected human T-cell clone. J Immunol. 1989;142:431–438. [PubMed] [Google Scholar]
- 52.Cocchi F, DeVico A L, Garzino-Demo A, Cara A, Gallo R C, Lusso P. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 53.Coffin J M. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 54.Coffin J M. Population dynamics of HIV drug resistance. In: Richman D D, editor. Antiviral drug resistance. New York, N.Y: John Wiley & Sons, Inc.; 1996. pp. 279–303. [Google Scholar]
- 55.Coffin J M, Hughes S H, Varmus H E. Retroviruses. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1998. [PubMed] [Google Scholar]
- 56.Cohen O J, Kinter A, Fauci A S. Host factors in the pathogenesis of HIV disease. Immunol Rev. 1997;159:31–48. doi: 10.1111/j.1600-065x.1997.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 57.Cohen O J, Pantaleo G, Lam G R, Fauci A S. Studies on lymphoid tissue from HIV-infected individuals: implications for the design of therapeutic strategies. Springer Semin Immunopathol. 1997;18:305–320. doi: 10.1007/BF00813500. [DOI] [PubMed] [Google Scholar]
- 58.Condra J H, Holder D J, Schleif W A, Blahy O M, Danovich R M, Gabryelski L J, Graham D J, Laird D, Quintero J C, Rhodes A, Robbins H L, Roth E, Shivaprakash M, Yang T, Chodakewitz J A, Deutsch P J, Leavitt R Y, Massari F E, Mellors J W, Squires K E, Steigbigel R T, Teppler H, Emini E A. Genetic correlates of in vivo viral resistance to Indinavir, a human immunodeficiency virus type 1 protease inhibitor. J Virol. 1996;70:8270–8276. doi: 10.1128/jvi.70.12.8270-8276.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Condra J H, Schleif W A, Blahy O M, Gabryelski L J, Graham D J, Quintero J C, Rhodes A, Hobbins H L, Roth E, Shivaprakash M, Titus D L, Yang T, Teppler H, Squires K E, Deutsch P J, Emini E A. In vivo emergence of HIV-1 variants resistant to multiple protease inhibitors. Nature. 1995;374:569–571. doi: 10.1038/374569a0. [DOI] [PubMed] [Google Scholar]
- 60.Connor R, Sheridan K E, Lai C, Zhang L, Ho D D. Characterization of the functional properties of env genes from long-term survivors of human immunodeficiency virus type 1 infection. J Virol. 1996;70:5306–5311. doi: 10.1128/jvi.70.8.5306-5311.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Connor R I, Mohri H, Cao Y, Ho D D. Increased viral burden and cytopathicity correlate temporally with CD4+ T-lymphocyte decline and clinical progression in human immunodeficiency virus type 1-infected individuals. J Virol. 1993;67:1772–1777. doi: 10.1128/jvi.67.4.1772-1777.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Connor R I, Korber B T, Graham B S, Hahn B H, Ho D D, Walker B D, Neumann A U, Vermund S H, Mestecky J, Jackson S, Fenamore E, Cao Y, Gao F, Kalams S, Kunstman K J, McDonald D, McWilliams N, Trkola A, Moore J P, Wolinsky S M. Immunological and virological analyses of persons infected by human immunodeficiency virus type 1 while participating in trials of recombinant gp120 subunit vaccines. J Virol. 1998;72:1552–1576. doi: 10.1128/jvi.72.2.1552-1576.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coppenhaver D H, Sriyuktasuth-Woo P, Baron S, Barr C E, Qureshi M N. Correlation of nonspecific antiviral activity with the ability to isolate infectious HIV-1 from saliva. N Engl J Med. 1994;330:1314–1315. doi: 10.1056/NEJM199405053301815. [DOI] [PubMed] [Google Scholar]
- 64.Couture L A, Stinchcomb D T. Antigene therapy: the use of ribozymes to inhibit gene function. Trends Genet. 1996;12:510–515. doi: 10.1016/s0168-9525(97)81398-4. [DOI] [PubMed] [Google Scholar]
- 65.Croix D A, Yeh H Y, Sedlacek J, Luftig R B, Gottlieb P D. A dominant epitope of HIV-1 protease recognized by hamster monoclonal antibodies. J Acquir Immune Defic Syndr. 1993;6:558–566. [PubMed] [Google Scholar]
- 66.Croteau G, Doyon L, Thibeault D, McKercher G, Pilote L, Lamarre D. Impaired fitness of human immunodeficiency virus type 1 variants with high-level resistance to protease inhibitors. J Virol. 1997;71:1089–1096. doi: 10.1128/jvi.71.2.1089-1096.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Curtain C C, Lowe M G, Arunagiri C K, Mobley D W, Macreadie I G, Azad A A. Cytotoxic activity of the amino terminal region of HIV-1 Nef protein. AIDS Res Hum Retrovir. 1997;13:1213–1220. doi: 10.1089/aid.1997.13.1213. [DOI] [PubMed] [Google Scholar]
- 68.Deacon N J, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker D J, McPhee D A, Greenway A L, Ellett A, Chatfield C, Lawson V A, Dwyer S, Dowton D, Mills J. Genomic structure of an attenuated quasispecies of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 69.Debouck C. The HIV-1 protease as a therapeutic target for AIDS. AIDS Res Hum Retrovir. 1992;8:153–164. doi: 10.1089/aid.1992.8.153. [DOI] [PubMed] [Google Scholar]
- 70.DeClerq E. New perspectives for the treatment of HIV infections. Collect Czech Chem Commun. 1998;63:449–479. [Google Scholar]
- 71.Dedera D, Hu W, Heyden N Y, Ratner L. Viral protein R of human immunodeficiency virus types 1 and 2 is dispensable for replication and cytopathogenicity in lymphoid cells. J Virol. 1989;63:3205–3208. doi: 10.1128/jvi.63.7.3205-3208.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deeks S D, Smith M, Holodny M, Kahn J O. HIV-1 protease inhibitors: a review for clinicians. JAMA. 1997;277:145–153. [PubMed] [Google Scholar]
- 73.Delezay O, Koch N, Yahi N, Hammamache D, Tourres C, Tamalet C. Co-expression of CXCR4/fusin and galactosylceramide in the human intestinal epithial cell line HT-29. AIDS. 1997;11:1311–1318. doi: 10.1097/00002030-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 74.Delforge M-L, Liesnard C, Debaisieux L, Tchetcheroff M, Farber C-M, Van Vooren J-P. In vivo inhibition of syncytium-inducing variants of HIV in patients treated with didanosine. AIDS. 1995;9:89–101. [PubMed] [Google Scholar]
- 75.Delwart E L, Pan H, Neumann A, Markowitz M. Rapid, transient changes at the env locus of plasma human immunodeficiency virus type 1 populations during the emergence of protease inhibitor resistance. J Virol. 1998;72:2416–2421. doi: 10.1128/jvi.72.3.2416-2421.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 77.Denning P H, Jones J L, Ward J W. Recent trends in the HIV epidemic in adolescent and young adult gay and bisexual men. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:374–379. doi: 10.1097/00042560-199712150-00011. [DOI] [PubMed] [Google Scholar]
- 78.De Panfilis G, Manara G C, Ferrari C, Torresani C. Simultaneous colloidal gold immunoelectronmicroscopy labeling of CD1a, HLA-DR, and CD4 surface antigens of human epidermal Langerhans cells. J Investig Dermatol. 1998;91:547–552. doi: 10.1111/1523-1747.ep12476912. [DOI] [PubMed] [Google Scholar]
- 79.De Ronde A, Klaver B, Keulen W, Smit L, Goudsmit J. Natural HIV-1 Nef accelerates virus replication in primary human lymphocytes. Virology. 1992;188:391–395. doi: 10.1016/0042-6822(92)90772-h. [DOI] [PubMed] [Google Scholar]
- 80.Desrosiers R C. Non-human primate models for AIDS vaccines. AIDS. 1995;9(Suppl. A):S137–S141. [PubMed] [Google Scholar]
- 81.Dettenhofer M, Yu X F. Highly purified human immunodeficiency virus reveals a virtual absence of Vif in virions. J Virol. 1999;73:1460–1467. doi: 10.1128/jvi.73.2.1460-1467.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dianzani U, Bragardo M, Buonfilio D, Redoglia V, Funaro A, Portoles P, Rojo J, Malavasi F, Pileri A. Modulation of CD4 lateral interaction with lymphocyte surface molecules induced by HIV-1 gp120. Eur J Immunol. 1995;25:1306–1310. doi: 10.1002/eji.1830250526. [DOI] [PubMed] [Google Scholar]
- 83.Ding J, McGrath W J, Sweet R M, Mangel W F. Crystal structure of the human adenovirus proteinase with its 11 amino acid cofactor. EMBO J. 1996;15:1778–1783. [PMC free article] [PubMed] [Google Scholar]
- 84.Dombrowski A, Bills G F, Sabnis G, Koupal L R, Meyer R, Ondeyka J G, Gicobbe R A, Monaghan R L, Lingham R B. Inhibition of HIV protease by a novel cytochalasin. J Antibiot. 1992;45:671–678. doi: 10.7164/antibiotics.45.671. [DOI] [PubMed] [Google Scholar]
- 85.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3 and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 86.Doyon L, Poulin F, Pilote L, Clouette C, Thibeault D, Croteau G, Lamarre D. Second locus involved in human immunodeficiency virus type 1 resistance to protease inhibitors. J Virol. 1996;70:3763–3769. doi: 10.1128/jvi.70.6.3763-3769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Doyon L, Payant C, Brakier-Gingras L, Lamarre D. Novel Gag-Pol frameshift site in human immunodeficiency virus type 1 variants resistant to protease inhibitors. J Virol. 1998;72:6146–6150. doi: 10.1128/jvi.72.7.6146-6150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 89.Dreyer G B, Boehm J C, Chenera P, DesJarlais R L, Hassell A M. A symmetric inhibitor binds HIV-1 protease asymmetrically. Biochemistry. 1993;32:937–947. doi: 10.1021/bi00054a027. [DOI] [PubMed] [Google Scholar]
- 90.Dropulic B, Jeang K T. Gene therapy for HIV infection: genetic antiviral strategies and targets for intervention. Hum Gene Ther. 1994;5:927–939. doi: 10.1089/hum.1994.5.8-927. [DOI] [PubMed] [Google Scholar]
- 91.Drusano G L, Stein D S. Mathematical modeling of the interrelationship of CD4 lymphocyte count and viral load changes induced by the protease inhibitor Indinavir. Antimicrob Agents Chemother. 1998;42:358–361. doi: 10.1128/aac.42.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Edinger A L, Hoffman T L, Sharron M, Lee B, Yi Y, Choe W, Kolson D L, Mitrovic B, Zhou Y, Faulds D, Collman R G, Hesselgesser J, Horuk R, Doms R W. An orphan seven-transmembrane domain receptor expressed widely in the brain functions as a coreceptor for human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol. 1998;72:7934–7940. doi: 10.1128/jvi.72.10.7934-7940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Edwards D R, Murphy G. Proteases—invasion and more. Nature. 1998;394:527–528. doi: 10.1038/28961. [DOI] [PubMed] [Google Scholar]
- 94.Embretson J, Zupancic M, Ribas J L, Burke A, Racz P, Tenner-Racz K, Haase A T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 95.Embretson J, Zupancic M, Beneke J, Till M, Wolinsky S, Ribas J L, Burke A, Haase A T. Analysis of human immunodeficiency virus-infected tissues by amplification of in situ hybridization reveals latent and permissive infections at single-cell resolution. Proc Natl Acad Sci USA. 1993;90:357–361. doi: 10.1073/pnas.90.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Erickson J W. The not-so-great escape. Nat Struct Biol. 1995;2:523–529. doi: 10.1038/nsb0795-523. [DOI] [PubMed] [Google Scholar]
- 97.Ezzell C. Tha monkey's got AIDS: what now for live AIDS vaccine? J NIH Res. 1997;9:21–22. [Google Scholar]
- 98.Fan Z, Huang X-L, Zheng L, Wilson C, Borowski L, Liebmann J, Gupta P, Margolick J, Rinaldo C. Cultured blood dendritic cells retain HIV-1 antigen-presenting capacity for memory CTL during progressive HIV-1 infection. J Immunol. 1997;159:4973–4982. [PubMed] [Google Scholar]
- 99.Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fauci A S. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science. 1993;262:1011–1018. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- 101.Feinberg M B, Carpenter C, Fauci A S, Stanley L K, Cohen O, Bartlett J G, Kaplan J E, Abrutyn E. Report of the NIH panel to define principles of therapy of HIV infection and guidelines for the use of anti-retroviral agents in HIV-infected adults and adolescents. Ann Intern Med. 1998;128:1057–1100. doi: 10.7326/0003-4819-128-12_part_2-199806151-00002. [DOI] [PubMed] [Google Scholar]
- 102.Feito M J, Bragardo M, Buonfiglio D, Bonissoni S, Bottarel F, Malavasi F, Dianzani U. gp120's derived from four syncytium-inducing HIV-1 strains induce different patterns of CD4 association with lymphocyte surface molecules. Int Immunol. 1997;9:1141–1147. doi: 10.1093/intimm/9.8.1141. [DOI] [PubMed] [Google Scholar]
- 103.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 104.Fenyö E M, Månson L M, Chiodi F, Lind B, von Gegerfelt A, Albert J, Olausson E, Åsjö B. Distinct replicative and cytopathic characteristics of human immunodeficiency virus isolates. J Virol. 1988;62:4414–4419. doi: 10.1128/jvi.62.11.4414-4419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Finkel T H, Tudor-Williams G, Banda N K, Cotton M F, Curiel T, Monks C, Baba T W, Ruprecht R M, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 106.Fisher A G, Ensoli B, Ivanoff L, Chamberlain M, Petteway S, Ratner L, Gallo R C, Wong-Staal F. The sor gene of HIV-1 is required for efficient virus transmission in vitro. Science. 1987;237:888–893. doi: 10.1126/science.3497453. [DOI] [PubMed] [Google Scholar]
- 107.Folks T, Justement J, Kinter A, Dinarello C A, Fauci A S. Cytokine-induced expression of HIV-1 in a chronically infected promonocytic cell line. Science. 1987;238:800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- 108.Foster S, Beverley P, Aspinall R. gp120-induced programmed cell death in recently activated T cells without subsequent ligation of the T cell receptor. Eur J Immunol. 1995;25:1778–1782. doi: 10.1002/eji.1830250644. [DOI] [PubMed] [Google Scholar]
- 109.Fouchier R A M, Meyaard L, Brouwer M, Hovenkamp E, Schuitemaker H. Broader tropism and higher cytopathogenicity for CD4+ T cells of a syncytium-inducing compared to a non-syncytium-inducing HIV-1 isolate as a mechanism for accelerated CD4+ T cell decline in vivo. Virology. 1996;219:87–95. doi: 10.1006/viro.1996.0225. [DOI] [PubMed] [Google Scholar]
- 110.Fouchier R A, Simon J H, Jaffe A B, Malim M H. Human immunodeficiency virus type 1 Vif does not influence expression or virion incorporation of gag-, pol-, and env-encoded proteins. J Virol. 1996;70:8263–8269. doi: 10.1128/jvi.70.12.8263-8269.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fox P C, Wolff A, Yeh C-K, Atkinson J C, Baum B J. Saliva inhibits HIV-1 infectivity. J Am Dent Assoc. 1988;116:635. doi: 10.14219/jada.archive.1988.0002. [DOI] [PubMed] [Google Scholar]
- 112.Frade J M R, Llorente M, Mellado M, Alcami J, Gutierrez-Ramos J C, Zaballos A, Real G, Martinez-A C. The amino-terminal domain of the CCR2 chemokine receptor acts as coreceptor for HIV-1 infection. J Clin Investig. 1997;100:497–502. doi: 10.1172/JCI119558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Frankel S S, Wenig B M, Burke A P, Mannan P, Thompson L D R, Abbondanzo S L, Nelson A M, Pope M, Steinman R M. Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid. Science. 1996;272:115–117. doi: 10.1126/science.272.5258.115. [DOI] [PubMed] [Google Scholar]
- 114.Freidland G H, Salzman B R, Rogers M F, Kahl P A, Lesser M L, Meyers M M, Klein R S. Lack of household transmission of HTLV-III infection. N Engl J Med. 1986;314:344. doi: 10.1056/NEJM198602063140604. [DOI] [PubMed] [Google Scholar]
- 115.Freund J, Kellner R, Konvalinka J, Wolber V, Kräusslich H-G, Kalbitzer H R. A possible regulation of Nef activity of HIV-1 by the viral protease. Eur J Biochem. 1994;223:589–593. doi: 10.1111/j.1432-1033.1994.tb19029.x. [DOI] [PubMed] [Google Scholar]
- 116.Fujinaga K, Zhong Q, Nakaya T, Kameoka M, Meguro T, Yamada K, Ikuta K. Extracellular Nef protein regulates productive HIV-1 infection from latency. J Immunol. 1995;155:5289–5298. [PubMed] [Google Scholar]
- 117.Fujinaga K, Nakamura Y, Zhong Q, Nakaya T, Ikuta K. Growth dominance of a revertant virus generated during in vitro serial passage of a nef frameshift mutant of HIV-1. Biochem Biophys Res Commun. 1996;229:96–101. doi: 10.1006/bbrc.1996.1763. [DOI] [PubMed] [Google Scholar]
- 118.Fujinaga K, Nakaya T, Ikuta K. Generation of endogenous tumour necrosis factor-α in MOLT-4 cells during the acute replication phase of human immunodeficiency virus type 1 determined the subsequent latent infection. J Gen Virol. 1998;79:221–229. doi: 10.1099/0022-1317-79-2-221. [DOI] [PubMed] [Google Scholar]
- 119.Fultz P N. Components of saliva inactivate human immunodeficiency virus. Lancet. 1986;ii:1215. doi: 10.1016/s0140-6736(86)92218-x. [DOI] [PubMed] [Google Scholar]
- 120.Gaedigk-Nitschko K, Schon A, Wachinger G, Erfle V, Kohleisen B. Cleavage of recombinant and cell derived HIV-1 Nef protein by HIV-1 protease. FEBS Lett. 1995;357:275–278. doi: 10.1016/0014-5793(94)01370-g. [DOI] [PubMed] [Google Scholar]
- 121.Galetto-Lacour A, Yerly S, Perneger T V, Baumberger C, Hirschel B, Perrin L the Swiss HIV Cohort Study Group. Prognosis value of viremia in patients with long-standing human immunodeficiency virus infection. J Infect Dis. 1996;173:1388–1393. doi: 10.1093/infdis/173.6.1388. [DOI] [PubMed] [Google Scholar]
- 122.Gaynor R. Cellular transcription factors involved in the regulation of HIV-1 gene expression. AIDS. 1992;6:347–363. doi: 10.1097/00002030-199204000-00001. [DOI] [PubMed] [Google Scholar]
- 123.Gazzard B G, Moyle G J, Weber J, Johnson M, Bingham J S, Bettle R, Churchill D, Fisher M, Griffin G, Jeffries D, Gormer K E, Lee C, Pozniak A, Smith J R, Tudor-Williams G, Williams I. British HIV Association guidelines for antiretroviral treatment of HIV seropositive individuals. Lancet. 1997;349:1086–1091. [Google Scholar]
- 124.Goto T, Nakai M, Ikuta K. The life-cycle of human immunodeficiency virus type 1. Micron. 1998;29:123–138. doi: 10.1016/s0968-4328(98)00002-x. [DOI] [PubMed] [Google Scholar]
- 125.Goudsmit J, De Rondo A, Ho D D, Perelson A S. Human immunodeficiency virus fitness in vivo: calculations based on a single zidovudine resistance mutation at codon 215 of reverse transcriptase. J Virol. 1996;70:5662–5664. doi: 10.1128/jvi.70.8.5662-5664.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gougeon M-L, Garcia S, Heeney J, Tschopp R, Lecoeur H, Guetard D, Rame V, Dauguet C, Montagnier L. Programmed cell death in AIDS-related HIV and SIV infections. AIDS Res Hum Retrovir. 1993;9:553–563. doi: 10.1089/aid.1993.9.553. [DOI] [PubMed] [Google Scholar]
- 127.Gougeon M-L, Lecoeur H, Dulioust A, Enouf M-G, Crouvoiser M, Goujard C, Debord T, Montagnier L. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996;156:3509–3520. [PubMed] [Google Scholar]
- 128.Grierson A W, Nicholson R, Talbot P, Webster A, Vemp G. The protease of adenovirus serotype 2 requires cysteine residues for both activation and catalysis. J Gen Virol. 1994;75:2761–2764. doi: 10.1099/0022-1317-75-10-2761. [DOI] [PubMed] [Google Scholar]
- 129.Groenink M, Fouchier R A M, de Goede R E Y, de Wolf F, Gruters R A, Cuypers H T M, Huisman H G, Tersmette M. Phenotypic heterogeneity in a panel of infectious molecular human immunodeficiency virus type 1 clones derived from a single individual. J Virol. 1991;65:1968–1975. doi: 10.1128/jvi.65.4.1968-1975.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Groux H, Torpier G, Monte D, Mouton Y, Capron A, Ameisen J C. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992;175:331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gruber A, Speth C, Lukasser-Vogl E, Zangerle R, Borg-von Zepelin M, Dierich M P, Würzner R. Human immunodeficiency virus type 1 protease inhibitor attenuates Candida albicans virulence properties in vitro. Immunopharmacology. 1999;41:227–234. doi: 10.1016/s0162-3109(99)00035-1. [DOI] [PubMed] [Google Scholar]
- 132.Gulnik S V, Suvorov L I, Liu B, Yu B, Anderson B, Mitsuya H, Erickson J W. Kinetic characterization and cross-resistance patterns of HIV-1 protease mutants selected under drug pressure. Biochemistry. 1995;34:9282–9287. doi: 10.1021/bi00029a002. [DOI] [PubMed] [Google Scholar]
- 133.Günthard H F, Wong J K, Ignacio C C, Guatelli J C, Riggs N L, Havlir D V, Richman D D. Human immunodeficiency virus replication and genotypic resistance in blood and lymph nodes after a year of potent antiretroviral therapy. J Virol. 1998;72:2422–2428. doi: 10.1128/jvi.72.3.2422-2428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hammes S R, Dixon E P, Malim M H, Cullen B R, Greene W C. Nef protein of human immunodeficiency virus type 1: evidence against its role as a transcriptional inhibitor. Proc Natl Acad Sci USA. 1989;86:9549–9553. doi: 10.1073/pnas.86.23.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Havlir D V, Marschner I C, Hirsch M S, Collier A C, Tebas P, Bassett R L. Maintenance antiretroviral therapies in HIV infected subjects with undetectable plasma HIV RNA after triple-drug therapy. N Engl J Med. 1998;339:1261–1268. doi: 10.1056/NEJM199810293391801. [DOI] [PubMed] [Google Scholar]
- 136.Haynes B F. HIV vaccines: where we are and where we are going. Lancet. 1996;348:933–937. doi: 10.1016/S0140-6736(96)09339-7. [DOI] [PubMed] [Google Scholar]
- 137.Haynes B F, Pantaleo G, Fauci A S. Toward an understanding of the correlates of protective immunity to HIV infection. Science. 1996;271:324–328. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 138.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 139.Heinzinger N K, Bukrinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M-A, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hermida-Matsumoto L, Resh M D. Human immunodeficiency virus type 1 protease triggers a myristoyl switch that modulates membrane binding of Pr55gag and p17MA. J Virol. 1999;73:1902–1908. doi: 10.1128/jvi.73.3.1902-1908.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hill R L. Hydrolysis of proteins. Adv Protein Chem. 1965;20:37–107. doi: 10.1016/s0065-3233(08)60388-5. [DOI] [PubMed] [Google Scholar]
- 142.Ho D, Toyoshima T, Mo H, Kempf D, Norbeck D, Chen C, Wideburg N, Burt S, Erickson J, Singh M. Characterization of human immunodeficiency virus type 1 variants with increased resistance to a C2-symmetric protease inhibitor. J Virol. 1994;68:2016–2020. doi: 10.1128/jvi.68.3.2016-2020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 144.Ho D D. Toward HIV eradication or remission: the task ahead. Science. 1998;280:1866–1868. doi: 10.1126/science.280.5371.1866. [DOI] [PubMed] [Google Scholar]
- 145.Hoch J, Lang S M, Weeger M, Stahl-Hennig C, Coulibaly C, Dittmer U, Hunsmann G, Fuchs D, Müller J, Sopper S, Fleckenstein B, Überla K T. vpr deletion mutant of simian immunodeficiency virus induces AIDS in rhesus monkeys. J Virol. 1995;69:4807–4813. doi: 10.1128/jvi.69.8.4807-4813.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hofmann B, Nishanian P, Fahey J L. Serum increases and lymphoid cell surface losses of IL-2R (CD25) in HIV infection: distinctive parameters of HIV-induced change. Clin Immunol Immunopathol. 1991;61:212–224. doi: 10.1016/s0090-1229(05)80025-x. [DOI] [PubMed] [Google Scholar]
- 146a.Horikoshi, H., F. Sasao, M. Kinomoto, T. Mukai, R. B. Luftig, and K. Ikuta. Differential susceptibility of resting CD4+ T lymphocytes to a T-tropic and a macrophage (M)-tropic human immunodeficiency virus type 1 is associated with their surface expression of CD38 molecules. Virus Res., in press. [DOI] [PubMed]
- 147.Horuk R, Hesselgesser J, Zhou Y, Faulds D, Halks-Miller M, Harvey S, Taub D, Samson M, Parmentier M, Rucker J, Doranz B J, Doms R W. The CC chemokine I-309 inhibits CCR8-dependent infection by diverse HIV-1 strains. J Biol Chem. 1998;273:386–391. doi: 10.1074/jbc.273.1.386. [DOI] [PubMed] [Google Scholar]
- 148.Houghton M. Hepatitis C viruses. In: Fields B N, Knipe D M, Howey P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1035–1058. [Google Scholar]
- 149.Höver B, Sherman R L, Traub P. Degradation of cytoskeletal proteins by the HIV-1 protease. Cell Biol Int Rep. 1992;16:603–612. doi: 10.1016/s0309-1651(06)80002-0. [DOI] [PubMed] [Google Scholar]
- 150.Huang Y, Zhang L, Ho D D. Characterization of nef sequences in long-term survivors of human immunodeficiency virus type 1 infection. J Virol. 1995;69:93–100. doi: 10.1128/jvi.69.1.93-100.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huang Y, Zhang L, Ho D D. Functional analysis of nef alleles derived from long-term survivors. J Virol. 1995;69:8142–8146. doi: 10.1128/jvi.69.12.8142-8146.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Humphrey R W, Öhagen A, Davis D A, Fukazawa T, Hayashi H, Hoglung S, Mitsuya H, Yarchoan R. Removal of human immunodeficiency virus type 1 (HIV-1) protease inhibitors from preparations of immature HIV-1 virions does not result in an increase in infectivity or the appearance of mature morphology. Antimicrob Agents Chemother. 1997;41:1017–1023. doi: 10.1128/aac.41.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ikuta K, Morita C, Nakai M, Yamamoto N, Kato S. Defective human immunodeficiency virus (HIV) particles produced by cloned cells of HTLV-1-carrying MT-4 cells persistently infected with HIV. Jpn J Cancer Res. 1988;79:418–423. doi: 10.1111/j.1349-7006.1988.tb01607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ikuta K, Luftig R B. Inhibition of cleavage of Moloney murine leukemia virus gag and env coded precursor polypeptides by cerulenin. Virology. 1986;154:195–206. doi: 10.1016/0042-6822(86)90441-1. [DOI] [PubMed] [Google Scholar]
- 155.lkuta K, Kameoka M, Luftig R B. AIDS pathogenesis: the role of accessory gene mutations, leading to formation of long-term persistently infected cells and/or apoptosis-inducing HIV-1 particles. Virus Res. 1997;52:145–156. doi: 10.1016/s0168-1702(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 156.Iversen A K N, Shpaer E G, Rodrigo A G, Hirsch M S, Walker B D, Sheppard H W, Merigan R C, Mullins J I. Persistence of attenuated rev genes in a human immunodeficiency virus type 1-infected asymptomatic individuals. J Virol. 1995;69:5743–5753. doi: 10.1128/jvi.69.9.5743-5753.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Janossy G, Borthwick N, Lomnitzer R. Proliferative defects of CD4 and CD8 lymphocytes in HIV-1 infection. In: Janossy G, Autran B, Miedema F, editors. Immunodeficiency in HIV infection and AIDS. S. Basel, Switzerland: Karger; 1992. pp. 96–114. [Google Scholar]
- 158.Janossy G, Jiang J D, Roboz J P. Activated CD45RO, CD8+ T lymphocytes in HIV infection. In: Janossy G, Autran B, Miedema F, editors. Immunodeficiency in HIV infection and AIDS. S. Basel, Switzerland: Karger; 1992. pp. 195–210. [Google Scholar]
- 159.Jassoy C, Walker B D. HIV-1-specific cytotoxic T lymphocytes and the control of HIV-1 replication. Springer Semin Immunopathol. 1997;18:341–354. doi: 10.1007/BF00813502. [DOI] [PubMed] [Google Scholar]
- 160.Ji J, Loeb L A. Fidelity of HIV-1 reverse transcriptase copying a hypervariable region of the HIV- 1 env gene. Virology. 1994;199:323–330. doi: 10.1006/viro.1994.1130. [DOI] [PubMed] [Google Scholar]
- 161.Joag S V, Adany I, Li Z, Foresman L, Pinson D M, Wang C, Stephens E B, Raghavan R, Narayan O. Animal model of mucosally transmitted human immunodeficiency virus type 1 disease: intravaginal and oral deposition of simian/human immunodeficiency virus in macaques results in systemic infection, elimination of CD4+ T cells, and AIDS. J Virol. 1997;71:4016–4023. doi: 10.1128/jvi.71.5.4016-4023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jones I, Stuart D. Journey to the core of HIV. Nat Struct Biol. 1996;3:818–820. doi: 10.1038/nsb1096-818. [DOI] [PubMed] [Google Scholar]
- 163.Kacani L, Frank I, Spruth M, Schwendinger M G, Mullauer B, Sprinzl G M, Steindl F, Dierich M P. Dendritic cells transmit human immunodeficiency virus type 1 to monocytes and monocyte-derived macrophages. J Virol. 1998;72:6671–6677. doi: 10.1128/jvi.72.8.6671-6677.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kameoka M, Kimura T, Zheng Y-H, Suzuki S, Fujinaga K, Luftig R B, Ikuta K. Protease-defective, gp120-containing human immunodeficiency virus type 1 particles induce apoptosis more efficiently than does wild-type virus or recombinant gp120 protein in healthy donor-derived peripheral blood T cells. J Clin Microbiol. 1997;35:41–47. doi: 10.1128/jcm.35.1.41-47.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kameoka M, Suzuki S, Kimura T, Fujinaga K, Auwanit W, Luftig R B, Ikuta K. Exposure of resting peripheral blood T-cells to HIV-1 particles generates CD25+ killer cells in a small subset, leading to induction of apoptosis in bystander cells. Int Immunol. 1997;9:1453–1462. doi: 10.1093/intimm/9.10.1453. [DOI] [PubMed] [Google Scholar]
- 166.Kameoka M, Auwanit W, Suzuki S, Horikoshi H, Khlai-Khlam N, Meguro T, Yamada K, Tanaka Y, Yoshihara K, Luftig R B, Ikuta K. A specific T-cell subset with CD4+/CD38− markers derived from HIV-1 carriers induces apoptosis in healthy donor-derived T-lymphocytes. Virus Res. 1998;56:115–122. doi: 10.1016/s0168-1702(98)00052-5. [DOI] [PubMed] [Google Scholar]
- 167.Kaplan A, Michael S, Wehbie E, Knigge M, Paul D, Everitt L, Kempf D, Norbeck D, Erickson J, Swanstrom R. Selection of multiple human immunodeficiency virus type 1 variants that encode viral proteases with decreased sensitivity to an inhibitor of the viral protease. Proc Natl Acad Sci USA. 1994;91:5597–5601. doi: 10.1073/pnas.91.12.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kaplan A H, Krogstad P, Kempf D J, Norbeck D W, Swanstrom R. Human immunodeficiency virus type 1 virions composed of unprocessed Gag and Gag-Pol precursors are capable of efficiently reverse transcribing viral genomic RNA. Antimicrob Agents Chemother. 1994;38:2929–2933. doi: 10.1128/aac.38.12.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kaplan D, Sieg S. Role of the Fas/FasL ligand apoptotic pathway in human immunodeficiency virus type 1 disease. J Virol. 1998;72:6279–6282. doi: 10.1128/jvi.72.8.6279-6282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Karlsson A, Parsmyr K, Aperia K, Sandstrom E, Fenyö E M, Albert J. MT-2 cell tropism of human immunodeficiency virus type 1 isolates as a marker for response to treatment and development of drug resistance. J Infect Dis. 1994;170:1367–1375. doi: 10.1093/infdis/170.6.1367. [DOI] [PubMed] [Google Scholar]
- 171.Karlsson A, Parsmyr K, Sandstrom E, Fenyö E M, Albert J. MT-2 cell tropism as prognostic marker for disease progression in human immunodeficiency virus type 1 infection. J Clin Microbiol. 1994;32:364–370. doi: 10.1128/jcm.32.2.364-370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Katoh I, Yasunaga T, Ikawa Y, Yoshinaka Y. Inhibition of retroviral protease activity by an aspartyl proteinase inhibitor. Nature. 1987;329:654–657. doi: 10.1038/329654a0. [DOI] [PubMed] [Google Scholar]
- 173.Katoh Y, Yoshinaka Y, Luftig R B. The effect of cerulinen on Moloney murine leukemia virus morphogenesis. Virus Res. 1986;5:265–276. doi: 10.1016/0168-1702(86)90023-7. [DOI] [PubMed] [Google Scholar]
- 174.Katsikis P D, Wunderlich E S, Smith C A, Herzenberg L, Herzenberg L A. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J Exp Med. 1995;181:2029–2036. doi: 10.1084/jem.181.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Katz R A, Skalka A M. The retroviral enzymes. Annu Rev Biochem. 1994;63:133–173. doi: 10.1146/annurev.bi.63.070194.001025. [DOI] [PubMed] [Google Scholar]
- 176.Katzenstein D A, Hammer S M, Hughers M D, Gundacker H, Jackson J B, Fiscus S, Rasheed S, Elbeik T, Reichman R, Japour A J, Merigan T C, Hirsch M S AIDS Clinical Trials Group 175 Virology Study Team. The relation of virologic and immunologic markers to clinical outcomes after nucleoside therapy in HIV-infected adults with 200 to 500 CD4 cells per cubic millimeter. N Engl J Med. 1996;335:1091–1098. doi: 10.1056/NEJM199610103351502. [DOI] [PubMed] [Google Scholar]
- 177.Kelleher A D, Carr A, Zaunders J, Cooper D A. Alterations in the immune response of human immunodeficiency virus (HIV)-infected subjects treated with an HIV-specific protease inhibitor, ritonavir. J Infect Dis. 1996;173:321–329. doi: 10.1093/infdis/173.2.321. [DOI] [PubMed] [Google Scholar]
- 178.Kempf D J, Marsh K C, Kumar G, Rodrigues A D, Denissen J F, McDonald E, Kukulka M J, Hsu A, Granneman G R, Baroldi P A, Sun E, Pizzuti D, Plattner J J, Norbeck D W, Leonard J M. Pharmacokinetic enhancement of inhibitors of the human immunodeficiency virus protease by coadministration with ritonavir. Antimicrob Agents Chemother. 1997;41:654–660. doi: 10.1128/aac.41.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kestens L, Vanham G, Gigase P, Young G, Hannet I, Vanlangendonck F, Hulstaert F, Back B A. Expression of activation antigens, HLA-DR and CD38, on CD8 lymphocytes during HIV-1 infection. AIDS. 1992;6:793–797. doi: 10.1097/00002030-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 180.Kestens L, Vanham G, Vereecken C, Vandenbruaene M, Vercauteren G, Colebunders R L, Gigase P L. Selective increase of activation antigens HLA-DR and CD38 on CD4+CD45RO+ T lymphocytes during HIV-1 infection. Clin Exp Immunol. 1994;95:436–441. doi: 10.1111/j.1365-2249.1994.tb07015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kestler H W, Ringler D J, Mori K, Panicali D, Sehgal P K, Daniel M D, Desrosiers R D. Importance of the nef gene for maintenance of high virus loads and/or development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 182.Kim S Y, Ikeuchi K, Byrn R, Groopman J, Baltimore D. Lack of a negative influence of viral growth by the nef gene of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:9544–9548. doi: 10.1073/pnas.86.23.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kirchhoff F, Greenough T C, Brettler D B, Sullivan J L, Desrosiers R C. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 184.Kishi M, Nishino Y, Sumiya M, Ohki K, Kimura T, Goto T, Nakai M, Kakinuma M, Ikuta K. Cells surviving infection by human immunodeficiency virus type 1 (HIV-1): vif or vpu mutants produce non-infectious or markedly less cytopathic viruses. J Gen Virol. 1992;73:77–87. doi: 10.1099/0022-1317-73-1-77. [DOI] [PubMed] [Google Scholar]
- 185.Kishi M, Nishino Y, Ohki K, Kimura T, Ikuta K. Persistently human immunodeficiency virus type 1-infected T cell clone expressing only doubly spliced mRNA exhibits reduced cell surface CD4 expression. Jpn J Cancer Res. 1993;84:153–162. doi: 10.1111/j.1349-7006.1993.tb02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kishi M, Tokunaga K, Zheng Y-H, Bahmani M K, Kakinuma M, Nonoyama M, Lai P K, Ikuta K. Superinfection of a defective human immunodeficiency virus type-1 provirus-carrying T cell clone with vif or vpu mutants gives cytopathic virus particles by homologous recombination. AIDS Res Hum Retrovir. 1995;11:45–53. doi: 10.1089/aid.1995.11.45. [DOI] [PubMed] [Google Scholar]
- 187.Kishi M, Zheng Y-H, Bahmani M K, Tokunaga K, Takahashi H, Kakinuma M, Lai P K, Nonoyama M, Luftig R B, Ikuta K. Naturally occurring accessory gene mutations lead to persistent human immunodeficiency virus type 1 infection of CD4-positive T cells. J Virol. 1995;69:7507–7518. doi: 10.1128/jvi.69.12.7507-7518.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Klein R S, Phelan J A, Freeman P H, Schable M S, Friedland G H, Trieger N, Steigbigel N H. Low occupational risk of human immunodeficiency virus infection among dental professionals. N Engl J Med. 1988;318:86. doi: 10.1056/NEJM198801143180205. [DOI] [PubMed] [Google Scholar]
- 189.Klimkait T, Strebel K, Hoggan M D, Martin M A, Orenstein J M. The human immunodeficiency virus type 1-specific protein Vpu is required for efficient virus maturation and release. J Virol. 1990;64:621–629. doi: 10.1128/jvi.64.2.621-629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Koch G, Richter D, editors. Biosynthesis, modification and processing of cellular and viral proteins. New York, N.Y: Academic Press, Inc.; 1980. [Google Scholar]
- 191.Kohl N E, Emini E A, Schlief W A, Davis L J, Heimback J C, Dixon R A, Scolnick E M, Sigal I S. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci USA. 1988;85:4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Koot M, Vos A H V, Keet R P M, De Goede R E Y, Dercksen W, Terpstra F G, Coutinho R A, Miedema F, Tersmette M. HIV-1 biological phenotype in long term infected individuals, evaluated with an MT-2 cocultivation assay. AIDS. 1992;6:49–54. doi: 10.1097/00002030-199201000-00006. [DOI] [PubMed] [Google Scholar]
- 193.Koot M, Keet I P M, Vos A H V, De Goede R E Y, Roos M T L, Coutinho R A, Miedema F, Schellekens P T A, Tersmette M. Prognostic value of human immunodeficiency virus type 1 biological phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med. 1993;118:681–688. doi: 10.7326/0003-4819-118-9-199305010-00004. [DOI] [PubMed] [Google Scholar]
- 194.Koot M, Schellekens P T A, Mulder J W, Lange J M A, Roos M T L, Coutinho R A, Tersmette M, Miedema F. Viral phenotype and T-cell reactivity in human immunodeficiency virus type 1-infected asymptomatic men treated with zidovudine. J Infect Dis. 1993;168:733–736. doi: 10.1093/infdis/168.3.733. [DOI] [PubMed] [Google Scholar]
- 195.Koot M, van 'tWout A B, Kootstra N A, De Goede R E Y, Tersmette M, Schuitemaker H. Relation between changes in cellular load, evolution of viral phenotype, and the clonal composition of virus populations in the course of human immunodeficiency virus type 1 infection. J Infect Dis. 1996;173:349–354. doi: 10.1093/infdis/173.2.349. [DOI] [PubMed] [Google Scholar]
- 196.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kozal M, Shah N, Shen N, Yang R, Fucini R, Merigan T, Richman D, Morris D, Hubbel E, Chee M, Gingeras T. Extensive polymorphisms observed in HIV-1 clade B protease genes using high-density oligonucleotide arrays. Nat Med. 1996;2:753–759. doi: 10.1038/nm0796-753. [DOI] [PubMed] [Google Scholar]
- 198.Kramps J A, Franken C, Dijkman J G. ELISA for quantitative measurement of low-molecular-weight bronchial protease inhibitor in human sputum. Am Rev Respir Dis. 1984;129:959–962. doi: 10.1164/arrd.1984.129.6.959. [DOI] [PubMed] [Google Scholar]
- 199.Kräusslich H G, Wimmer E. Viral proteinases. Annu Rev Biochem. 1988;57:701–754. doi: 10.1146/annurev.bi.57.070188.003413. [DOI] [PubMed] [Google Scholar]
- 200.La Casse R A, Follis K E, Trahey M, Scarborough J D, Littman D R, Nunberg J H. Fusion-competent vaccines: broad neutralization of primary isolates of HIV. Science. 1999;283:357–362. doi: 10.1126/science.283.5400.357. [DOI] [PubMed] [Google Scholar]
- 201.Lang S M, Weeger M, Stahl-Hennig C, Coulibaly C, Hunsmann G, Muller J, Muller-Hermelink H, Fuchs D, Wachter H, Daniel M M, Desrosiers R C, Fleckenstein B. Importance of vpr for infection of rhesus monkeys with simian immunodeficiency virus. J Virol. 1993;67:902–912. doi: 10.1128/jvi.67.2.902-912.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lanza P, Moss R B, Giermakowska W, Hancock R B, Richieri S P, Theofa G, Jensen F C, Salk P L, Carlo D J. Whole-killed gp120-depleted HIV-1 antigen in a murine model for prophylactic vaccination. Vaccine. 1998;16:727–731. doi: 10.1016/s0264-410x(97)00256-9. [DOI] [PubMed] [Google Scholar]
- 203.Learmont J, Tindall B, Evans L, Cunningham A, Cunningham P, Wells J, Penny R, Kaldor J, Cooper D A. Long-term symptomless HIV-1 infection in recipients of blood products from a single donor. Lancet. 1992;340:863–867. doi: 10.1016/0140-6736(92)93281-q. [DOI] [PubMed] [Google Scholar]
- 204.Lech W, Wang G, Yang Y, Chee Y, Dorman K, McCrae D, Lazzeroni L, Erickson J, Sinsheimer J, Kaplan A. In vivo sequence diversity of the protease of human immunodeficiency virus type 1: presence of protease inhibitor-resistant variants in untreated subjects. J Virol. 1996;70:2038–2043. doi: 10.1128/jvi.70.3.2038-2043.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lee T G, Maruyama S. Isolation of HIV-1 protease inhibiting peptides from thermolysin hydrolysate of oyster proteins. Biochem Biophys Res Commun. 1998;253:604–608. doi: 10.1006/bbrc.1998.9824. [DOI] [PubMed] [Google Scholar]
- 206.Le Grice S F J, Mills J, Mous J. Active site mutagenesis of the AIDS virus protease and its alleviation by trans complementation. EMBO J. 1988;7:2547–2553. doi: 10.1002/j.1460-2075.1988.tb03103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Leis J, Weber I, Wlodawer A, Skalka A M. Structure-function analysis of the Rous sarcoma virus-specific proteinase. ASM News. 1990;56:77–81. [Google Scholar]
- 208.Letvin N L. Progress in the development of an HIV-1 vaccine. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 209.Levy D N, Refaeli Y, MacGregor R R, Weiner D B. Serum Vpr regulates productive infection and latency of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1994;91:10873–10877. doi: 10.1073/pnas.91.23.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Levy D N, Refaeli Y, Weiner D B. Extracellular Vpr protein increases cellular permissiveness to human immunodeficiency virus replication and reactivates virus from latency. J Virol. 1995;69:1243–1252. doi: 10.1128/jvi.69.2.1243-1252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Levy J A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lewis D E, D, Tang S N, Adu-Oppong A, Schober W, Rodgers J R. Allergy and apoptosis in CD8+ T cells from HIV-infected persons. J Immunol. 1994;153:412–420. [PubMed] [Google Scholar]
- 213.Li C J, Friedman D J, Wang C, Metelev V, Pardee A B. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science. 1995;268:429–431. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 214.Li X, Gervaix A, Kang D, Law P, Spector S A, Hoe A D, Wong-Staal F. Gene therapy targeting cord blood-derived CD34+ cells for HIV-exposed infants: preclinical studies. Gene Ther. 1998;5:233–239. doi: 10.1038/sj.gt.3300582. [DOI] [PubMed] [Google Scholar]
- 215.Liao F, Alkhatib G, Peden K W, Sharma G, Berger E A, Farber J M. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Limsuwan A, Churdboonchart V, Moss R B, Sirawaraporn W, Satthent R, Smutharaks B, Glidden D, Trauger R, Theofan G, Carlo D. Safety and immungenicity of Remune™ in HIV-infected Thai subjects. Vaccine. 1997;16:142–149. doi: 10.1016/s0264-410x(97)88327-2. [DOI] [PubMed] [Google Scholar]
- 217.Lin Y, Lin X, Hong L, Foundling S, Heinrickson R L, Thaisrivongs S, Leelamanit W, Raterman D, Shah M, Dunn B M, Tang J. Effect of point mutations on the kinetics and the inhibition of human immunodeficiency virus type 1 protease: relationship to drug resistance. Biochemistry. 1995;34:1143–1152. doi: 10.1021/bi00004a007. [DOI] [PubMed] [Google Scholar]
- 218.Lipsky J J. Abnormal fat accumulation in patients with HIV-1 infection. Lancet. 1998;351:847–848. doi: 10.1016/S0140-6736(05)70281-6. [DOI] [PubMed] [Google Scholar]
- 219.Loeb D D, Swanstrom R, Everitt L, Manchester M, Stamper S E, Hutchison C A. Complete mutagenesis of the HIV-1 protease. Nature. 1989;340:397–400. doi: 10.1038/340397a0. [DOI] [PubMed] [Google Scholar]
- 220.Loetscher M, Amara A, Oberlin E, Brass N, Legler D, Loetscher P, D'Apuzzo M, Meese E, Rousset D, Virelizier J L, Baggiolini M, Arenzana-Seisdedos F, Moser B. TYMSTR, a putative chemokine receptor selectively expressed in activated T cells, exhibits HIV-1 coreceptor function. Curr Biol. 1997;7:652–660. doi: 10.1016/s0960-9822(06)00292-2. [DOI] [PubMed] [Google Scholar]
- 221.Luciw P A, Cheng-Mayer C, Levy J A. Mutational analysis of the human immunodeficiency virus: the orf-B region down-regulates virus replication. Proc Natl Acad Sci USA. 1987;84:1434–1438. doi: 10.1073/pnas.84.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Luftig R B, Ikuta K. Are defective, HIV protease-deficient particles the real culprit in AIDS? ASM News. 1994;60:417–419. [Google Scholar]
- 223.Luftig R B, Ikuta K, Bu M, Calkins P. Terminal stages of retroviral morphogenesis. In: Pearl L H, editor. Retroviral proteases: control of maturation and morphogenesis. London, United Kingdom: Macmillan; 1990. pp. 141–148. [Google Scholar]
- 224.Luftig R B, Lupo L. Viral interactions with the host-cell cytoskeleton: the role of retroviral proteases. Trends Microbiol. 1994;2:178–182. doi: 10.1016/0966-842x(94)90669-6. [DOI] [PubMed] [Google Scholar]
- 225.Luftig R B, Pieniazek D, Pieniazek N J. Update in viral pathogenesis. ASM News. 1990;56:366–368. [Google Scholar]
- 226.Mahalingam S, Collman R G, Patel M, Monken C E, Srinivasan A. Functional analysis of HIV-1 Vpr: identification of determinants essential for subcellular localization. Virology. 1995;212:331–339. doi: 10.1006/viro.1995.1490. [DOI] [PubMed] [Google Scholar]
- 227.Mahalingam M, Peakman M, Davies E T, Pozniak A, McManus T J, Vergani D. T cell activation and disease severity in HIV infection. Clin Exp Immunol. 1993;93:337–343. doi: 10.1111/j.1365-2249.1993.tb08182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mahalingam M, Pozniak A, McManus T J, Vergani D, Peakman M. Cell cycling in HIV infection: analysis of in vivo activated lymphocytes. Clin Exp Immunol. 1995;102:481–486. doi: 10.1111/j.1365-2249.1995.tb03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Malamud D, Davis C, Berthold P, Roth E, Friedman H. Human submandibular saliva aggregates HIV. AIDS Res Hum Retrovir. 1993;9:633–637. doi: 10.1089/aid.1993.9.633. [DOI] [PubMed] [Google Scholar]
- 230.Maldarelli F, Sato H, Berthold E, Orenstein J, Martin M A. Rapid induction of apoptosis by cell-to-cell transmission of human immunodeficiency virus type 1. J Virol. 1995;69:6457–6465. doi: 10.1128/jvi.69.10.6457-6465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Manchester M, Everitt L, Loeb D D, Hutchison C A, Swanstrom R. Identification of temperature-sensitive mutants of human immunodeficiency virus type 1 protease through saturation mutagenesis. Amino acid requirements for temperature sensitivity. J Biol Chem. 1994;269:7689–7695. [PubMed] [Google Scholar]
- 232.Mansky L M, Temin H M. Lower in vivo mutation rate of human immunodeficiency virus type 1 than that predicted from the fidelity of purified reverse transcriptase. J Virol. 1995;69:5087–5094. doi: 10.1128/jvi.69.8.5087-5094.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Mariana R, Kirchhoff F, Greenough T C, Sullivan J L, Desrosiers R C, Skowronski J. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J Virol. 1996;70:7752–7764. doi: 10.1128/jvi.70.11.7752-7764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Markowitz M, Mo H, Kempf D, Norbeck D, Bhat T, Erickson J, Ho D. Selection and analysis of human immunodeficiency virus type 1 variants with increased resistance to ABT-538, a novel protease inhibitor. J Virol. 1995;69:701–706. doi: 10.1128/jvi.69.2.701-706.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Martin S J, Matear P M, Vyakarnam A. HIV-1 infection of human CD4+ T cells in vitro. Differential induction of apoptosis in these cells. J Immunol. 1994;152:330–342. [PubMed] [Google Scholar]
- 236.Martinez E, Gatell J. Metabolic abnormalities and use of HIV-1 protease inhibitors. Lancet. 1998;352:821–822. doi: 10.1016/S0140-6736(05)60719-2. [DOI] [PubMed] [Google Scholar]
- 237.Maschera B, Furfine E, Blair E D. Analysis of resistance to human immunodeficiency virus type 1 protease inhibitors by using matched bacterial expression and proviral infection vectors. J Virol. 1995;69:5431–5436. doi: 10.1128/jvi.69.9.5431-5436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.McCune J M. Viral latency in HIV disease. Cell. 1995;82:183–188. doi: 10.1016/0092-8674(95)90305-4. [DOI] [PubMed] [Google Scholar]
- 239.McNeely T B, Dealy M, Dripps D J, Orenstein J M, Elsienberg S P, Wahl S M. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Investig. 1995;96:456–464. doi: 10.1172/JCI118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.McNeely T B, Shugars D C, Rosendahl M, Tucker C, Eisenberg S P, Wahl S M. Inhibition of human immunodeficiency virus type 1 infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood. 1997;90:1141–1149. [PubMed] [Google Scholar]
- 241.Medina D J, Tung P P, Nelson C J, Sathya B, Casareole D, Strair R K. Characterization and use of a recombinant retroviral system for the analysis of drug resistant HIV. J Virol Methods. 1998;71:169–176. doi: 10.1016/s0166-0934(97)00212-7. [DOI] [PubMed] [Google Scholar]
- 242.Mellors J W, Rinaldo C R, Gupta P, White R M, Todd J A, Kingsley L A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasm. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 243.Mellors J W, Munoz A, Giorgia J V. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 244.Meyaard L, Otto S A, Jonker R R, Mijnster M J, Keet R P M, Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 245.Michael N L, Mo T, Merzouki A, O'Shaughnessy M, Oster C, Burke D S, Redfield R R, Birx D L, Cassol S A. Human immunodeficiency virus type 1 cellular RNA load and splicing patterns predict disease progression in a longitudinally studied cohort. J Virol. 1995;69:1868–1877. doi: 10.1128/jvi.69.3.1868-1877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Michael N L, Change G, d'Arcy L A, Ehrenberg P K, Mariani R, Busch M P, Birx D L, Schwartz D H. Defective accessory genes in a human immunodeficiency virus type 1-infected long-term survivor lacking recoverable virus. J Virol. 1995;69:4228–4236. doi: 10.1128/jvi.69.7.4228-4236.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Miller C J, Vogel P, Alexander N J, Dandekar S, Hendrickx A G, Marx P A. Pathology and localization of simian immunodeficiency virus in the reproductive tract of chronically infected male rhesus macaques. Lab Investig. 1994;70:255–262. [PubMed] [Google Scholar]
- 248.Miller C J, Vogel P, Alexander N J, Sutjipto S, Hendrickx A G, Marx P A. Localization of SIV in the genital tract of chronically infected female rhesus macaques. Am J Pathol. 1992;141:655–660. [PMC free article] [PubMed] [Google Scholar]
- 249.Miller M D, Warmerdam M T, Ferrell S S, Benitez R, Greene W C. Intravirion generation of the C-terminal core domain of HIV-1 Nef by the HIV-1 protease is insufficient to enhance viral infectivity. Virology. 1997;234:215–225. doi: 10.1006/viro.1997.8641. [DOI] [PubMed] [Google Scholar]
- 250.Miller M D, Warmerdam M T, Gaston I, Greene W C, Feinberg M B. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Miller M J, Schreider B K, Sathyanarayana B K. Structure of a complex of synthetic HIV-1 protease with a substrate-based inhibitor at 2.3 Å resolution. Science. 1989;245:1149–1152. doi: 10.1126/science.2686029. [DOI] [PubMed] [Google Scholar]
- 252.Mitra D, Steiner M, Lynch D H, Staiano-Coico L, Laurence J. HIV-1 upregulates Fas ligand expression in CD4+ T cells in vitro and in vivo: association with Fas-mediated apoptosis and modulation by aurintricarboxylic acid. Immunology. 1996;87:581–585. doi: 10.1046/j.1365-2567.1996.510589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Molla A, Kempf D, Korneyeva M, Gao Q, Shipper P, Mo H, Markowitz M, Vasavanonda S, Chernyavskyi T, Niu P, Lyons N, Hsu A, Granneman G, Ho D, Boucher C, Leonard J, Norbeck D. Ordered accumulation of mutations in HIV protease confers resistance to ritonavir. Nat Med. 1996;2:760–766. doi: 10.1038/nm0796-760. [DOI] [PubMed] [Google Scholar]
- 254.Moore B E, Flaitz C M, Coppenhaver D H, Nichols C M, Kalmaz G D, Bessman J D, Cloyd M W, Lynch D P, Prabhakar B S, Baron S. HIV recovery from saliva before and after dental treatment: inhibitors may have critical role in viral inactivation. J Am Dent Assoc. 1993;124:67–74. doi: 10.14219/jada.archive.1993.0197. [DOI] [PubMed] [Google Scholar]
- 255.Moore J P, Ho D D. HIV-1 neutralization: the consequences of viral adaptation to growth on transformed T cells. AIDS. 1995;9(Suppl. A):S117–S136. [PubMed] [Google Scholar]
- 256.Moss R B, Wallace M R, Lanza P, Giermakowska W K, Jensen F C, Theofan G, Chamberlin C, Richieri S P, Carlo D J. In vitro p24 antigen-stimulated lymphocyte proliferation and β-chemokine production in human immunodeficiency virus type 1 (HIV-1)-seropositive subjects after immunization with an inactivated gp 120-depleted HIV-1 immunogen (Remune) Clin Diagn Lab Immunol. 1998;5:308–312. doi: 10.1128/cdli.5.3.308-312.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Moss R B, Li L, Giermakowska W K, Lanza P, Turner J L, Wallace M R, Jensen F C, Richieri S P, Daigle A E, Theofan G, Carlo D J. Tumor necrosis factor α and HIV-specific functional immune responses after immunization with gp120-depleted, inactivated HIV-1 in incomplete Freund's adjuvant (Remune) in HIV-1 seropositive subjects. J Hum Virol. 1998;1:77–81. [PubMed] [Google Scholar]
- 258.Moutouh L, Corbell J, Richman D D. Recombination leads to the rapid emergence of HIV-1 dually resistant mutants under selective drug pressure. Proc Natl Acad Sci USA. 1996;93:6106–6111. doi: 10.1073/pnas.93.12.6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Moyle G. HIV infection and AIDS. Curr Opin Infect Dis. 1999;12:1–27. doi: 10.1097/00001432-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 260.Muro-Cacho C A, Pantaleo G, Fauci A S. Analysis of apoptosis in lymph nodes of HIV-infected persons. Intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J Immunol. 1995;154:5555–5566. [PubMed] [Google Scholar]
- 261.Murphy R L, Gulick R M, DeGruttola V, D'Aquila R T, Eron J J, Sommadossi J P, Currier J S, Smeaton L, Frank I, Caliendo A M, Gerber J G, Tung R, Kuritzkes D R. Treatment with amprenavir alone or amprenavir with zidovudine and lamivudine in adults with human immunodeficiency virus infection. AIDS Clinical Trials Group 347 Study Team. J Infect Dis. 1999;179:808–816. doi: 10.1086/314668. [DOI] [PubMed] [Google Scholar]
- 262.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath M J. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1267–1274. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 263.Myers G, Wain-Hobson S, Henderson L, Korber B, Jeang K, Pavlakis G. Human retroviruses and AIDS. Sequence bank. Los Alamos, N.M: Los Alamos National Laboratory; 1995. [Google Scholar]
- 264.Nabel G J. The role of cellular transcription factors in the regulation of human immunodeficiency virus gene expression. In: Cullen B R, editor. Human retroviruses. Oxford, United Kingdom: IRL Press; 1993. pp. 49–73. [Google Scholar]
- 265.Naglik J R, Newport G, White T C, Fernandez-Naglik L L, Greenspan J S, Greenspan D, Sweet S P, Challaconbe S J, Agabian N. In vivo analysis of secreted aspartyl proteinase expression in human oral candidiasis. Infect Immun. 1999;67:2482–2490. doi: 10.1128/iai.67.5.2482-2490.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Nagy K, Young M, Baboonian C, Merson J, Whittle P, Oroszlan S. Antiviral effect of human immunodeficiency virus protease inhibitors in a single cycle of infection: evidence for a role of protease in the early phase. J Virol. 1994;68:757–765. doi: 10.1128/jvi.68.2.757-765.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Najera I, Holguin A, Quinones-Mateu M E, Munoz-Fernandez M A, Najera R, Lopez-Galindez C, Domingo E. pol gene quasispecies of human immunodeficiency virus: mutations associated with drug resistance in virus from patients undergoing no drug therapy. J Virol. 1995;69:23–31. doi: 10.1128/jvi.69.1.23-31.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Nakaya T, Fujinaga K, Kishi M, Oka S, Kurata T, Jones I M, Ikuta K. Nonsense mutations in the vpr gene of HIV-1 during in vitro virus passage and in HIV-1 carrier-derived peripheral blood mononuclear cells. FEBS Lett. 1994;354:17–22. doi: 10.1016/0014-5793(94)01074-9. [DOI] [PubMed] [Google Scholar]
- 269.Nakaya T, Fujinaga K, Doi H, Suzuki S, Takahashi H, Nishino Y, Kishi M, Azuma I, Luftig R B, Ikuta K. Serial passage of human immunodeficiency virus type 1 generates misalignment deletions in non-essential accessory genes. Virus Res. 1996;46:139–147. doi: 10.1016/s0168-1702(96)01396-2. [DOI] [PubMed] [Google Scholar]
- 270.Nakaya T, Iwai S, Fujinaga K, Sato Y, Otsuka E, Ikuta K. Decoy approach using RNA-DNA chimera oligonucleotides to inhibit the regulatory function of human immunodeficiency virus type 1 Rev protein. Antimicrob Agents Chemother. 1997;41:319–325. doi: 10.1128/aac.41.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Niedecken H, Lutz G, Bauer R, Kreysel H W. Langerhans cell as primary target and vehicle for transmission of HIV. Lancet. 1987;ii:519–520. doi: 10.1016/s0140-6736(87)91843-5. [DOI] [PubMed] [Google Scholar]
- 272.Nijhuis M, Schuurman R, Boucher C A B. Homologous recombination for rapid phenotyping of HIV. Curr Opin Infect Dis. 1997;10:475–479. [Google Scholar]
- 273.Nishino Y, Kishi M, Sumiya M, Ogawa K, Adachi A, Maotani-Imai K, Kato S, Hirai K, Ikuta K. Human immunodeficiency virus type 1 vif, vpr, and vpu mutants can produce persistently infected cells. Arch Virol. 1991;120:181–192. doi: 10.1007/BF01310474. [DOI] [PubMed] [Google Scholar]
- 274.Nishino Y, Nakaya T, Fujinaga K, Kishi M, Azuma I, Ikuta K. Persistent infection of MT-4 cells by human immunodeficiency virus type 1 becomes increasingly likely with in vitro serial passage of wild-type but not nef mutant virus. J Gen Virol. 1994;75:2241–2251. doi: 10.1099/0022-1317-75-9-2241. [DOI] [PubMed] [Google Scholar]
- 275.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J-L, Arenzana-Seisdedos F, Schwartz O, Heard J-M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 276.O'Brien W A, Hartigan P M, Martin D, Esenhardt J, Hill A, Benoit S, Rubin M, Simberkoff M S, Hamilton J D the Veterans Affairs Cooperative Study Group on AIDS. Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts and the risk of progression to AIDS. N Engl J Med. 1996;334:426–431. doi: 10.1056/NEJM199602153340703. [DOI] [PubMed] [Google Scholar]
- 277.Ogawa K, Shibata R, Kiyomasu T, Higuchi I, Kishida Y, Ishimoto A, Adachi A. Mutational analysis of the human immunodeficiency virus vpr open reading frame. J Virol. 1989;63:4110–4114. doi: 10.1128/jvi.63.9.4110-4114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 279.Ohki K, Kishi M, Nishino Y, Sumiya M, Kimura T, Goto T, Nakai M, Ikuta K. Noninfectious doughnut-shaped human immunodeficiency virus type 1 can induce syncytia mediated by fusion of the particles with CD4-positive cells. J Acquir Immune Defic Syndr. 1991;4:1233–1240. [PubMed] [Google Scholar]
- 280.Oldstone M B. HIV versus cytotoxic T lymphocytes—the war being lost. N Engl J Med. 1997;337:1306–1308. doi: 10.1056/NEJM199710303371811. [DOI] [PubMed] [Google Scholar]
- 281.Olivares I, Sánchez-Merino V, Martinez M A, Domingo E, López-Galindez C, Menéndez-Arias L. Second-site reversion of a human immunodeficiency virus type 1 reverse transcriptase mutant that restores enzyme function and replication capacity. J Virol. 1999;73:6293–6298. doi: 10.1128/jvi.73.8.6293-6298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Oroszlan S, Luftig R B. Retroviral proteinases. In: Swanstrom R, Vogt P K, editors. Retroviruses: strategies of replication. New York, N.Y: Springer-Verlag; 1990. pp. 153–186. [Google Scholar]
- 283.Otake K, Fujii Y, Nakaya T, Nishino Y, Zhong Q, Fujinaga K, Kameoka M, Ohki K, Ikuta K. The carboxyl-terminal region of Nef protein is a cell surface domain that can interact with CD4+ T cells. J Immunol. 1994;153:5826–5837. [PubMed] [Google Scholar]
- 284.Owman C, Garzino-Demo A, Cocchi F, Popovic M, Sabirsh A, Gallo R C. The leukotriene B4 receptor functions as a novel type of coreceptor mediating entry of primary HIV-1 isolates into CD4-positive cells. Proc Natl Acad Sci USA. 1998;95:9530–9534. doi: 10.1073/pnas.95.16.9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Oyaizu N, McCloskey T W, Coronesi M, Chirmule N, Pahwa S. Accelerated apoptosis in peripheral blood mononuclear cells (PBMCs) from human immunodeficiency virus type-1 infected patients and in CD4 cross-linked PBMCs from normal individuals. Blood. 1993;82:3075–3080. [PubMed] [Google Scholar]
- 286.Pandori M W, Fitch N J S, Craig H M, Richman D D, Spina C A, Guatelli J C. Producer-cell modification of human immunodeficiency virus type 1: Nef is a virion protein. J Virol. 1996;70:4283–4290. doi: 10.1128/jvi.70.7.4283-4290.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Pantaleo G, Fauci A S. New concepts in the immunopathogenesis of HIV infection. Annu Rev Immunol. 1995;13:487–512. doi: 10.1146/annurev.iy.13.040195.002415. [DOI] [PubMed] [Google Scholar]
- 288.Pantaleo G, Demarest J F, Soudeyns H, Graziosi C, Denis F, Adelsberger J W, Borrow P, Saag M S, Shaw G M, Sekaly R P, Fauci A. Major expansion of CD8+ T cells with a predominant V beta usage during the primary immune response to HIV. Nature. 1994;370:463–467. doi: 10.1038/370463a0. [DOI] [PubMed] [Google Scholar]
- 289.Pantaleo G, Graziosi C, Demarest J F, Butini L, Montroni M, Fox C H, Orenstein J M, Kotler D P, Fauci A S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 290.Partaledis J, Yamaguchi K, Tisdale M, Blair E, Falcione C, Maschera B, Myers R, Pazhanisamy S, Futer O, Cullinan A, Stuver C, Byrn R, Livingston D. In vitro selection and characterization of human immunodeficiency virus type 1 (HIV-1) isolates with reduced sensitivity to hydroxyethylaminosulfonamide inhibitors of HIV-1 aspartyl protease. J Virol. 1995;69:5228–5235. doi: 10.1128/jvi.69.9.5228-5235.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Patick A, Rose R, Greytok J, Bechtold C, Hermsmeier M, Chen P, Barrish J, Zahler R, Colonno R, Lin P. Characterization of a human immunodeficiency virus type 1 variant with reduced sensitivity to an aminodiol protease inhibitor. J Virol. 1995;69:2148–2152. doi: 10.1128/jvi.69.4.2148-2152.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Patterson B K, Till M, Otto P, Goolsby C, Furtado M R, McBride L J, Wolinsky S M. Detection of HIV-1 DNA and messenger RNA in individual cells by PCR-driven in situ hybridization and flow cytometry. Science. 1993;260:976–979. doi: 10.1126/science.8493534. [DOI] [PubMed] [Google Scholar]
- 293.Pearl L H, Taylor W R. A structural model for the retroviral proteases. Nature. 1987;329:351–354. doi: 10.1038/329351a0. [DOI] [PubMed] [Google Scholar]
- 294.Perelson A S, Neumann A U, Markowitz M, Leonard J M, Ho D D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 295.Perelson A S, Essunger P, Cao Y, Vasenen M, Hurley A, Saksela K, Markowitz M, Ho D D. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 296.Perez V L, Rowe T, Justement J S, Butera S T, June C H, Folks T. An HIV infected T-cell clone defective in IL-2 production and Ca++ mobilization following CD3 stimulation. J Immunol. 1991;147:3145–3148. [PubMed] [Google Scholar]
- 297.Pettit S C, Sanchez R, Smith T, Wehbie R, Derse D, Swanstrom R. HIV type 1 protease inhibitors fail to inhibit HTLV-I Gag processing in infected cells. AIDS Res Hum Retrovir. 1998;14:1007–1014. doi: 10.1089/aid.1998.14.1007. [DOI] [PubMed] [Google Scholar]
- 298.Pettoello-Mantovani M, Kollmann T R, Raker C, Kim A, Yurasov S, Tudor R, Wiltshire H, Goldstein H. Saquinavir-mediated inhibition of human immunodeficiency virus (HIV) infection in SCID mice implanted with human fetal thymus and liver tissue: an in vivo model for evaluating the effect of drug therapy on HIV infection in lymphoid tissues. Antimicrob Agents Chemother. 1997;41:1880–1887. doi: 10.1128/aac.41.9.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Piatak M, Jr, Saag M S, Yang L C, Clark S J, Kappes J C, Luk K C, Hahn B H, Shaw G M, Lifson J D. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993;259:1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- 300.Pleskoff O, Treboute C, Brelot A, Heveker N, Seman M, Alizon M. Identification of a chemokine receptor encoded by human cytomegalovirus as a cofactor for HIV-1 entry. Science. 1997;276:1874–1878. doi: 10.1126/science.276.5320.1874. [DOI] [PubMed] [Google Scholar]
- 301.Poeschla E, Wong-Staal F. Antiviral and anticancer ribozymes Curr. Opin Oncol. 1994;6:601–606. doi: 10.1097/00001622-199411000-00012. [DOI] [PubMed] [Google Scholar]
- 302.Pomerantz R J, Trono D, Feinberg M B, Baltimore D. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: a molecular model for latency. Cell. 1990;61:1271–1276. doi: 10.1016/0092-8674(90)90691-7. [DOI] [PubMed] [Google Scholar]
- 303.Pomerantz R J, Bagasra O, Baltimore D. Cellular latency of human immunodeficiency virus type 1. Curr Opin Immunol. 1992;4:475–480. doi: 10.1016/s0952-7915(06)80042-7. [DOI] [PubMed] [Google Scholar]
- 304.Pope M, Betjes G H, Romani N, Hirmand H, Cameron P U, Hoffman L, Gezelter S, Schuler G, Steinman R M. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection of HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 305.Pope M, Gezelter S, Gallo N, Hoffman L, Steinman R M. Low levels of HIV-1 infection in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ T cells. J Exp Med. 1995;182:2045–2056. doi: 10.1084/jem.182.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Pope M, Elmore D, Ho D, Marx P. Dendritic cell-T cell mixtures, isolated from the skin and mucosa of macaques, support the replication of SIV. AIDS Res Hum Retrovir. 1997;13:819–827. doi: 10.1089/aid.1997.13.819. [DOI] [PubMed] [Google Scholar]
- 307.Refaeli Y, Levy D N, Weiner D B. The glucocorticoid receptor type II complex is a target of the HIV-1 vpr gene product. Proc Natl Acad Sci USA. 1995;92:3621–3625. doi: 10.1073/pnas.92.8.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Rey O, Canon J, Krogstad P. HIV-1 Gag protein associates with F-actin present in microfilaments. Virology. 1996;220:530–534. doi: 10.1006/viro.1996.0343. [DOI] [PubMed] [Google Scholar]
- 309.Richards A D, Roberts R, Dunn B M, Graves M C, Kay J. Selective blocking of HIV-1 proteinase activity by characteristic inhibitors of aspartic proteinases. FEBS Lett. 1989;247:113–117. doi: 10.1016/0014-5793(89)81251-7. [DOI] [PubMed] [Google Scholar]
- 310.Richman D D, Bozzette S A. The impact of the syncytium-inducing phenotype of human immunodeficiency virus on disease progression. J Infect Dis. 1994;169:968–974. doi: 10.1093/infdis/169.5.968. [DOI] [PubMed] [Google Scholar]
- 311.Ridky T W, Kikonyogo A, Leis J, Gulnik S, Copeland T, Erickson J, Wlodawer A, Kurinov J, Harrison R W, Weber I T. Drug-resistant HIV-1 proteases identify enzyme residues important for substrate selection and catalytic rate. Biochemistry. 1998;37:13835–13845. doi: 10.1021/bi980612k. [DOI] [PubMed] [Google Scholar]
- 312.Roberts N A. Drug-resistance patterns of saquinavir and other HIV protease inhibitors. AIDS. 1995;9:S27–S32. [PubMed] [Google Scholar]
- 313.Robinovitch M R, Iversen J M, Resnick L. Anti-infectivity activity of human salivary secretions toward human immunodeficiency virus. Crit Rev Oral Biol Med. 1993;4:455–459. doi: 10.1177/10454411930040032801. [DOI] [PubMed] [Google Scholar]
- 314.Rogel M E, Wu L I, Emerman M. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J Virol. 1995;69:882–888. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Rondon I J, Marasco W A. Intracellular antibodies for gene therapy of infectious disease. Annu Rev Microbiol. 1997;51:257–283. doi: 10.1146/annurev.micro.51.1.257. [DOI] [PubMed] [Google Scholar]
- 316.Rose R, Gong Y, Greytok J, Bechtold C, Terry B, Robinson B, Alam M, Colonno R, Lin P. Human immunodeficiency virus type 1 viral background plays a major role in development of resistance to protease inhibitors. Proc Natl Acad Sci USA. 1996;93:1648–1653. doi: 10.1073/pnas.93.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Rouzine I M, Coffin J M. Search for the mechanism of genetic variation in the pro gene of human immunodeficiency virus. J Virol. 1999;73:8167–8178. doi: 10.1128/jvi.73.10.8167-8178.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Rowland-Jones S, Tan R, McMichael A. Role of cellular immunity in protection against HIV infection. Adv Immunol. 1997;65:277–346. [PubMed] [Google Scholar]
- 319.Rucker J, Edinger A L, Sharron M, Samson M, Lee B, Berson J F, Yi Y, Margulies B, Collman R G, Doranz B J, Parmentier M, Doms R W. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Sanchez G, Xu X, Chermann J-C, Hirsch I. Accumulation of defective viral genomes in peripheral blood mononuclear cells of human immunodeficiency virus type 1-infected individuals. J Virol. 1997;71:2233–2240. doi: 10.1128/jvi.71.3.2233-2240.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Sardana V V, Schlaback A J, Graham P, Bush B L, Condra J H, Culberson J C, Gotlib L, Graham D J, Kohl N E, LeFemina R L, Schneider C L, Wolanski B S, Wolanski J A, Wolfgang J A, Emini E A. Human immunodeficiency virus type 1 protease inhibitors: evaluation of resistance engendered by amino-acid substitutions in the enzyme substrate binding site. Biochemistry. 1994;33:2004–2010. doi: 10.1021/bi00174a005. [DOI] [PubMed] [Google Scholar]
- 322.Sarmati L, Nicastri E, El-Sawaf G, Ventura L, Salanitro A, Ercoli L, Vella S, Andreoni M. Increase in neutralizing antibody titer against sequential autologous HIV-1 isolates after 16 weeks saquinavir (Invirase) treatment. J Med Virol. 1997;53:313–318. [PubMed] [Google Scholar]
- 323.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, De Goede R E Y, Van Steenwijk R P, Lange J M A, Eeftink Schattenkerk J K M, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus populations. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Sczakiel G, Nedbal W. The potential of ribozymes as antiviral agents. Trends Microbiol. 1995;3:213–217. doi: 10.1016/s0966-842x(00)88927-1. [DOI] [PubMed] [Google Scholar]
- 325.Shao W, Everitt L, Manchester M, Loeb D D, Hutchison III C A, Swanstrom R. Sequence requirements of the HIV-1 protease flap region determined by saturation mutagenesis and kinetic analysis of flap mutants. Proc Natl Acad Sci USA. 1997;94:2243–2248. doi: 10.1073/pnas.94.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sheng N, Pettit S C, Tritch R J, Ozturk D H, Rayner M M, Swanstrom R, Erickson-Vittanen S. Determinants of the human immunodeficiency virus type 1 p15NC-RNA interaction that affect enhanced cleavage by protease. J Virol. 1997;71:5723–5732. doi: 10.1128/jvi.71.8.5723-5732.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Shirasaka T, Chokekijchai S, Yamada A, Gosselin G, Imbach J-L, Mitsuya H. Comparative analysis of anti-human immunodeficiency virus type 1 activation of dideoxynucleoside analogs in resting and activated peripheral blood mononuclear cells. Antimicrob Agents Chemother. 1995;39:2555–2559. doi: 10.1128/aac.39.11.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Shugars D C, Wahl S M. The role of the oral environment in HIV-1 transmission J. Am Dent Assoc. 1998;129:851–858. doi: 10.14219/jada.archive.1998.0349. [DOI] [PubMed] [Google Scholar]
- 329.Simmons G, Wilkinson D, Reeves J D, Dittmar M T, Beddows S, Weber J, Carnegie G, Desselberger U, Gray P W, Weiss R A, Clapham P R. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either LESTR or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Singh A, Besson G, Mobasher A, Collman R O. Patterns of chemokine receptor fusion cofactor utilization by human immunodeficiency virus type 1 variants from the lungs and blood. J Virol. 1999;73:6680–6690. doi: 10.1128/jvi.73.8.6680-6690.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Skalka A M. Retroviral proteases: first glimpses at the anatomy of a processing machine. Cell. 1989;56:911–913. doi: 10.1016/0092-8674(89)90621-1. [DOI] [PubMed] [Google Scholar]
- 332.Soto-Ramirez L E, Renjifo B, McLane M F, Marlink R, O'Hara C, Sutthent R, Wasi C, Vithayasai P, Vithayasai V, Apichartpiyakul C, Auewarakul P, Cruz V P, Chui D-S, Osathanondh R, Mayer K, Lee T-H, Essex M. HIV-1 Langerhans' cell tropism associated with heterosexual transmission of HIV. Science. 1996;271:1291–1293. doi: 10.1126/science.271.5253.1291. [DOI] [PubMed] [Google Scholar]
- 333.Spearman P, Horton R, Ratner L, Kuli-Zade I. Membrane binding of human immunodeficiency virus type 1 matrix protein in vivo supports a conformational myristyl switch mechanism. J Virol. 1997;71:6582–6592. doi: 10.1128/jvi.71.9.6582-6592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Spina C A, Kwoh T J, Chowers M Y, Guatelli J C, Richman D D. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Spira A I, Marx P A, Patterson B K, Mahoney J, Koup R A, Wolinsky S M, Ho D D. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.St. Clair M H, Hartigan P M, Andrews J C, Vavro C L, Simberkoff M S, Hamilton J D Veterans Administration Cooperative Study Group. Zidovudine resistance, syncytium-inducing phenotype, and HIV disease in a case-control study. J Acquir Immune Defic Syndr. 1993;6:891–897. [PubMed] [Google Scholar]
- 337.Stein D S, Korvick J A, Vermund S H. CD4 lymphocyte cell enumeration for prediction of clinical course of HIV disease: a review. J Infect Dis. 1992;165:352–363. doi: 10.1093/infdis/165.2.352. [DOI] [PubMed] [Google Scholar]
- 338.Strebel K, Daugherty D, Cohen D, Folks T, Martin M A. The HIV “A” (sor) gene product is essential for virus infectivity. Nature. 1987;38:728–730. doi: 10.1038/328728a0. [DOI] [PubMed] [Google Scholar]
- 339.Strebel K, Klimkait T, Martin M A. A novel gene of HIV-1, vpu, and its 16-kilodalton product. Science. 1988;241:1221–1223. doi: 10.1126/science.3261888. [DOI] [PubMed] [Google Scholar]
- 340.Strebel K, Klimkait T, Maidarelli F, Martin M A. Molecular and biochemical analyses of human immunodeficiency virus type 1 vpu protein. J Virol. 1989;63:3784–3791. doi: 10.1128/jvi.63.9.3784-3791.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Streblow D N, Kitabwalla M, Malkowsky M, Pauza C D. Cyclophilin A modulates processing of HIV type I p55 Gag: mechanism for antiviral effects of cyclosporin A. Virology. 1998;245:197–202. doi: 10.1006/viro.1998.9155. [DOI] [PubMed] [Google Scholar]
- 342.Streblow D N, Kitabwalla M, Pauza C D. Gag protein from human immunodeficiency virus type 1 assembles in the absence of cyclophilin A. Virology. 1998;252:228–234. doi: 10.1006/viro.1998.9468. [DOI] [PubMed] [Google Scholar]
- 343.Suzuki S, Tobiume M, Kameoka M, Sato K, Takahashi T A, Mukai T, Ikuta K. Exposure of normal monocyte-derived dendritic cells to human immunodeficiency virus type-1 particles leads to the induction of apoptosis in co-cultured CD4+ as well as CD8+ T cells. Microbiol Immunol. 2000;44:111–121. doi: 10.1111/j.1348-0421.2000.tb01254.x. [DOI] [PubMed] [Google Scholar]
- 344.Tacchetti C, Favre A, Moresco L, Meszaros P, Luzzi P, Truini M, Rizzo F, Grossi C E, Ciccone E. HIV is trapped and masked in the cytoplasm of lymph node follicular dendritic cells. Am J Pathol. 1997;150:533–542. [PMC free article] [PubMed] [Google Scholar]
- 345.Temin H A. Is HIV unique or merely different? J Acquir Immun Defic Syndr. 1989;2:1–9. [PubMed] [Google Scholar]
- 346.Tersmette M, De Goede R E Y, Al B J M, Winkel I N, Gruters R A, Cuypers H T M, Huisman H G, Miedema F. Differential syncytium-inducing capacity of human immunodeficiency virus isolates: frequent detection of syncytium-inducing isolates in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J Virol. 1988;62:2026–2032. doi: 10.1128/jvi.62.6.2026-2032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Tersmette M, Gruters R A, De Wolf F, De Goede R E Y, Lange J M A, Schellekens P T A, Goudsmit J, Huisman J G, Miedema F. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J Virol. 1989;63:2118–2125. doi: 10.1128/jvi.63.5.2118-2125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Terwilliger E F. The accessory gene functions of the primate immunity viruses. In: Koff W C, Wong-Staal F, Kennedy R C, editors. AIDS research reviews. Vol. 2. New York, N.Y: Marcel Dekker, Inc.; 1992. pp. 3–27. [Google Scholar]
- 349.Terwilliger E F, Cohen E A, Lu Y, Sodrosky J G, Haseltine W A. Functional role of human immunodeficiency virus type 1 vpu. Proc Natl Acad Sci USA. 1989;86:5163–5167. doi: 10.1073/pnas.86.13.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Terwilliger E F, Langhoff E, Gabuzda D, Zazopoulos E, Haseltine W A. Allelic variation in the effects of the nef gene on replication of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1991;88:10971–10975. doi: 10.1073/pnas.88.23.10971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Tobiume M, Fujinaga K, Kameoka M, Kimura T, Nakaya T, Yamada T, Ikuta K. Dependence on host cell cycle for activation of human immunodeficiency virus type 1 gene expression from latency. J Gen Virol. 1998;79:1363–1371. doi: 10.1099/0022-1317-79-6-1363. [DOI] [PubMed] [Google Scholar]
- 352.Torres Y, Leal M, Rey C, Medrano F J, Sanchez-Quijiano A, Lissen E. Acquisition of syncytium-inducing HIV-1 strains during therapy with zidovudine alone or combined with alpha interferon or didanosine. Eur J Clin Microbiol Dis. 1996;15:324–327. doi: 10.1007/BF01695665. [DOI] [PubMed] [Google Scholar]
- 353.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 354.Van't Wout A B, Blaak H, Ran L J, Brouwer M, Kuiken C, Schuitemaker H. Evolution of syncytium-inducing and non-syncytium-inducing biological virus clones in relation to replication kinetics during the course of human immunodeficiency virus type 1 infection. J Virol. 1998;72:5099–5107. doi: 10.1128/jvi.72.6.5099-5107.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Van't Wout A B, De Jong M D, Kootstra N A, Veenstra J, Lange J M A, Boucher C A B, Schuitemaker H. Changes in cellular virus load and zidovudine resistance of syncytium-inducing and non-syncytium-inducing human immunodeficiency virus populations under zidovudine pressure: a clonal analysis. J Infect Dis. 1996;174:845–849. doi: 10.1093/infdis/174.4.845. [DOI] [PubMed] [Google Scholar]
- 356.Van't Wout A B, Kootstra N A, Mulder-Kampinga G A, Albrecht van Lent N, Scherpbier H J, Veenstra J, Boer K, Coutinho R A, Miedema F, Schuitemaker H. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parental and vertical transmission. J Clin Investig. 1994;94:2060–2067. doi: 10.1172/JCI117560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Van't Wout A B, Ran L J, De Jong M D, Bakker M, Van Leeuwen R, Notemans D W, Loeliger A E, De Wolf F, Danner S A, Reiss P, Boucher C A B, Lange J M A. Selective inhibition of syncytium-inducing and nonsyncytium-inducing HIV-1 variants in individuals receiving didanosine or zidovudine, respectively. J Clin Investig. 1997;100:2325–2332. doi: 10.1172/JCI119771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.von der Helm K. Cleavage of Rous sarcoma viral polypeptide precursor into internal structural proteins in vitro involves viral protein p15. Proc Natl Acad Sci USA. 1977;74:911–915. doi: 10.1073/pnas.74.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.von Schwedler U, Kornbluth R S, Trono D. The nuclear localization signal of the matrix protein of human immunodeficiency virus type 1 allows the establishment of infection in macrophages and quiescent T lymphocytes. Proc Natl Acad Sci USA. 1994;91:6992–6996. doi: 10.1073/pnas.91.15.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Wahl S M, McNeely T B, Janoff E N, Shugars D, Worley P, Tucker C, Orenstein J M. Secretory leukocyte protease inhibitor (SLPI) in mucosal fluids inhibits HIV-1. Oral Dis. 1997;3(Suppl.):S64–S69. doi: 10.1111/j.1601-0825.1997.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 361.Wahl S M, Worley P, Jin W W, McNeely T B, Eisenberg S, Orenstein J M, Janoff E. Anatomic dissociation between HIV-1 and its endogenous inhibitor in mucosal tissue. Am J Pathol. 1997;150:1275–1284. [PMC free article] [PubMed] [Google Scholar]
- 362.Wang B, Ge Y C, Palasanthiran P, Xiang S-H, Ziegler J, Dwyer D E, Randle C, Dowton D, Cunningham A, Saksena N K. Gene defects clustered at the C-terminus of the vpr gene of HIV-1 in long-term nonprogressing mother and child pairs: in vivo evolution of vpr quasispecies in blood and plasma. Virology. 1996;223:224–232. doi: 10.1006/viro.1996.0471. [DOI] [PubMed] [Google Scholar]
- 363.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Novak M A, Hahn B H, Saag M S, Shaw G M. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–126. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 364.Welker R, Kottler H, Kalbitzer H R, Kräusslich H-G. Human immunodeficiency virus type 1 Nef protein is incorporated into virus particles and specifically cleaved by the viral proteinase. Virology. 1996;219:228–236. doi: 10.1006/viro.1996.0240. [DOI] [PubMed] [Google Scholar]
- 365.Westendrop M O, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin K-M, Krammer P H. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 366.Wilk T, Gowen B, Fuller S D. Actin associates with the nucleocapsid domain of the human immunodeficiency virus Gag polyprotein. J Virol. 1999;73:1931–1946. doi: 10.1128/jvi.73.3.1931-1940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Winslow D L, Stack S, King P, Scarnati H, Bincsik A, Otto M J. Limited sequence diversity in the HIV type 1 protease gene from clinical isolates and in vivo susceptibility to HIV protease inhibitors. AIDS Res Hum Retrovir. 1995;11:107–113. doi: 10.1089/aid.1995.11.107. [DOI] [PubMed] [Google Scholar]
- 368.Wlodawer A, Vondrasek J. Inhibitors of HIV-1 protease: a major success of structure-assisted drug design. Annu Rev Biophys Biomol Struct. 1998;27:249–284. doi: 10.1146/annurev.biophys.27.1.249. [DOI] [PubMed] [Google Scholar]
- 369.Wlodawer A, Erickson J W. Structure-based inhibitors of HIV-1 protease. Annu Rev Biochem. 1993;62:543–585. doi: 10.1146/annurev.bi.62.070193.002551. [DOI] [PubMed] [Google Scholar]
- 370.Yagi M J, Chu F-N, Jiang J D, Wallace J, Mason P, Liu Y, Carafa J, Bekesi J G. Increase in soluble CD8 antigen in plasma, and CD8+ and CD8+CD38+ cells in human immunodeficiency virus type-1 infection. Clin Immunol Immunopathol. 1992;63:126–134. doi: 10.1016/0090-1229(92)90004-8. [DOI] [PubMed] [Google Scholar]
- 371.Yoshinaka Y, Luftig R B. Murine leukemia virus morphogenesis: cleavage of p70 in vitro can be accompanied by a shift from a concentrically coiled internal strand (“immature”) to a collapsed (“mature”) form of the virus core. Proc Natl Acad Sci USA. 1977;74:3446–3450. doi: 10.1073/pnas.74.8.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Yu M, Leavitt M C, Maruyama M, Yamada O, Young D, Ho A D, Wong-Staal F. Intracellular immunization of human fetal cord blood stem/progenitor cells with a ribozyme against HIV-1. Proc Natl Acad Sci USA. 1995;92:699–703. doi: 10.1073/pnas.92.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Yunoki M, Maotani-Imai K, Kusuda H, Motoyama M, Miyake S, Imai H, Shin Y S, Kato S, Sano K, Morita C, Nakai M, Hirai K, Ikuta K. Production of infectious particles from defective human immunodeficiency virus type 1 (HIV-1)-producing cell clones by superinfection with infectious HIV-1. Arch Virol. 1991;116:143–158. doi: 10.1007/BF01319238. [DOI] [PubMed] [Google Scholar]
- 374.Zambruno G, Giannetti A, Bertazzoni U, Girolomoni G. Langerhans cells and HIV infection. Immunol Today. 1995;16:520–524. doi: 10.1016/0167-5699(95)80044-1. [DOI] [PubMed] [Google Scholar]
- 375.Zhang L, Carruthers C D, He T, Huang Y, Cao Y, Wang G, Hahn B, Ho D D. HIV type 1 subtypes, coreceptor usage, and CCR5 polymorphism. AIDS Res Hum Retrovir. 1997;13:1357–1366. doi: 10.1089/aid.1997.13.1357. [DOI] [PubMed] [Google Scholar]
- 376.Zhang L, He T, Huang Y, Chen Z, Guo Y, Wu S, Kunstman K J, Brown R C, Phair J P, Neumann A U, Ho D D, Wolinsky S M. Chemokine coreceptor usage by diverse primary isolates of human immunodeficiency virus type 1. J Virol. 1998;72:9307–9312. doi: 10.1128/jvi.72.11.9307-9312.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Zhang L, Huang Y, He T, Cao Y, Ho D D. HIV-1 subtype and second-receptor use. Nature. 1996;383:768. doi: 10.1038/383768a0. [DOI] [PubMed] [Google Scholar]
- 378.Zhang L, Huang Y, Yuan H, Chen B K, Ip J, Ho D D. Genetic and phenotypic characterization of long terminal repeat sequences from long-term survivors of human immunodeficiency virus type 1 infection. J Virol. 1997;71:5608–5613. doi: 10.1128/jvi.71.7.5608-5613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Zhang L, Huang Y, Yuan H, Tuttleton S, Ho D D. Characterization of vif, vpr and vpu sequences from long-term survivors of human immunodeficiency virus type 1 infection. Virology. 1997;228:340–349. doi: 10.1006/viro.1996.8378. [DOI] [PubMed] [Google Scholar]
- 380.Zhang Y J, Dragic T, Cao Y, Kostrikis L, Kwon D S, Littman D R, Kewal-Ramani V N, Moore J P. Use of coreceptors other than CCR5 by non-syncytium-inducing adult and pediatric isolates of human immunodeficiency virus type 1 is rare in vitro. J Virol. 1998;72:9337–9344. doi: 10.1128/jvi.72.11.9337-9344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Zhang Y-M, Imamichi H, Imamichi T, Lane H C, Falloon J, Vasudevachari M B, Salzman N P. Drug resistance during indinavir therapy is caused by mutations in the protease gene and in its Gag substrate cleavage sites. J Virol. 1997;71:6662–6670. doi: 10.1128/jvi.71.9.6662-6670.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Zheng N N, McQueen P W, Hurren L, Evans L A, Law M G, Forde S, Barker S, Cooper D A. Changes in biologic phenotype of human immunodeficiency virus during treatment of patients with didanosine. J Infect Dis. 1996;173:1092–1096. doi: 10.1093/infdis/173.5.1092. [DOI] [PubMed] [Google Scholar]
- 383.Zhong Q, Nakaya T, Tateno Y, Fujinaga K, Kameoka M, Tateno M, Ikuta K. A clearer distinction between HIV-1 paired isolates from peripheral blood mononuclear cells of asymptomatic carriers with and without CD8+ T-cells at nef rather than env V3 loci. Vaccine. 1997;15:497–510. doi: 10.1016/s0264-410x(97)00223-5. [DOI] [PubMed] [Google Scholar]
- 384.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 in patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 385.Ziermann R, Limoli K, Das K, Arnold E, Petropoulos C J, Parkin N T. A mutation in human immunodeficiency virus type 1 protease, N88S, that causes in vitro hypersensitivity to amprenavir. J Virol. 2000;74:4414–4419. doi: 10.1128/jvi.74.9.4414-4419.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]