SUMMARY
S. aureus is a unique microorganism, able to infect every organ system or tissue within the human body. Treatment of acute infection, as well as prevention, are complicated by the ability of S. aureus to exist as a commensal organism while also possessing pathogenic potential. Management of acute infection includes promptly addressing the source of infection and sites of metastatic infection and initiation of effective antibiotics, which should be selected based upon anatomic location of infection, severity of infection, and local antibiotic susceptibility patterns. Additional research is needed to determine the optimal route, length, and agent of antimicrobial therapy for S. aureus infections in children. Topical antimicrobials are frequently employed in healthcare and outpatient settings to eradicate S. aureus and prevent subsequent infection. Given the role of close contacts in S. aureus transmission in hospital and community settings, targeting decolonization to these individuals should also be considered. The development of novel prevention and therapeutic strategies, including lytic agents [112], vaccines [113], probiotics [114], microbiota transplants [115], and phage therapy [116], are exciting avenues for future research.
Keywords: Staphylococcus aureus, MRSA, colonization, decolonization, skin infection, bacteremia, osteomyelitis, pneumonia, meningitis
INTRODUCTION
Staphylococcus aureus is a Gram-positive pathobiont, a common skin commensal with potential to cause a broad spectrum of infections including toxin-mediated disease, skin and soft tissue infections (SSTIs), and invasive, life-threatening infections including bacteremia, endocarditis, pneumonia, and osteomyelitis [1]. S. aureus remains one of the most common pathogens responsible for pediatric healthcare associated infections (HAIs), particularly central line-associated bloodstream infections (CLABSIs) and surgical site infections (SSIs) [2, 3].
S. aureus is continually evolving, developing mechanisms to evade antibiotic treatment. Soon after the introduction of penicillin for widespread use in the 1940s, S. aureus strains resistant to penicillin emerged, causing an epidemic of infections among individuals in healthcare and community settings. To combat these penicillin-resistant S. aureus strains, the semi-synthetic penicillin, methicillin, was introduced. Unfortunately, only several years after the introduction of methicillin, strains resistant to this new “silver bullet” also emerged, and continue to pose challenges for clinicians, particularly as these strains confer resistance not only to methicillin, but to the entire class of β-lactam antibiotics [4]. From the time of their emergence in the 1960s until the 1990s, these methicillin-resistant S. aureus (MRSA) strains predominantly caused nosocomial infections. In the late 1990s, clinically and genetically distinct strains of MRSA emerged, infecting otherwise healthy hosts without the traditional healthcare-associated risk factors for MRSA infections. These strains were coined “Community-Associated (CA)” MRSA; the most frequent circulating strain in the US, determined by pulse-field gel electrophoresis, is the USA300 clone [5, 6]. The emergence of these strains has resulted in an epidemic of skin and soft tissue infections (SSTIs) as well as invasive, necrotizing infections [6, 7].
SSTI is the most frequent entity caused by S. aureus and includes impetigo, folliculitis, cellulitis, and cutaneous abscesses (furuncles and carbuncles). The incidence of SSTIs rose significantly in the early 2000s driven by the emergence of the CA-MRSA USA300 clone. From 1997 to 2005, visits to ambulatory centers (outpatient physician offices and emergency departments) by patients with purulent SSTI rose from 4.6 million to 9.6 million annually [8, 9]. While the incidence of these infections has recently plateaued, and decreased in some populations, the overall burden of these infections remains high (8.4 million ambulatory visits annually) [10, 11]. Recently, there has been an increase in methicillin-susceptible S. aureus (MSSA) strains possessing the USA300 genetic background, though with increased resistance to non-β-lactam antibiotics [12, 13].
S. aureus is one of the most frequent causes of bloodstream infections (BSIs) and musculoskeletal infections in children. The estimated rate of S. aureus bacteremia is 1.5 to 3.5 per 1000 pediatric hospital admissions [14–16]. An investigation of pediatric BSIs using the Premier Healthcare Database revealed that, among children beyond the neonatal period, 25% of BSIs were caused by S. aureus (16% MSSA and 9% MRSA) [17]. The mortality associated with S. aureus bacteremia in children is as high as 6% [18, 19]. Due to great strides in healthcare infection-prevention practices, the incidence of nosocomial MRSA bloodstream infections in the US has decreased. Approximately half of all S. aureus infections in healthcare settings are caused by MSSA, and the incidence of MSSA BSIs has remained steady. In hospitalized patients, the rate of CA-MRSA bacteremia has not changed while CA-MSSA infections have significantly increased [20].
Approximately 30% of healthy people are colonized with S. aureus, and 2–10% with MRSA. Colonization poses risk for subsequent infection in both healthy and hospitalized individuals [21–25]. The anterior nares have traditionally been described as the most common site of S. aureus colonization. In children, however, non-nasal sites (e.g., inguinal folds, axillae, umbilicus, oropharynx) may be more common reservoirs for S. aureus [23, 26]. Thus, among high-risk populations, many hospital infection-prevention programs perform active surveillance (nasal screening) to detect S. aureus colonization among vulnerable populations, to inform targeted decolonization using topical antimicrobials or antiseptics [27]. Importantly, understanding the burden of S. aureus colonization among hospitalized patients promotes antimicrobial stewardship. For example, among adult patients, absence of nasal S. aureus colonization has a high (>96%) negative predictive value for S. aureus infections at other anatomic sites [28].
CLINICAL ENTITIES AND TREATMENT
Antibiotic Overview
Management of S. aureus infections includes prompt initiation of appropriate antibiotics, rapid assessment for the source of infection, and possible sites of metastatic infection, with urgent eradication of infectious source(s) or metastatic foci, when feasible. As geographic variation in antimicrobial susceptibilities exists, empiric therapy should be selected based upon local antibiotic susceptibility patterns. Definitive antibiotic choice and length of therapy depend on the site and severity of infections and the antibiotic susceptibility of the infecting isolate. Intravenous antibiotics provide optimal initial therapy for invasive S. aureus infections, and transition to oral options are appropriate for some infections. Classes of antibiotics utilized for pediatrics include semi-synthetic penicillins (oxacillin, nafcillin), cephalosporins (cefazolin, ceftriaxone, ceftaroline), tetracyclines (doxycycline), oxazolidinone (linezolid), lincosamides (clindamycin), sulfonamides (trimethoprim-sulfamethoxazole [TMP-SMX]), and cyclic lipopeptides (daptomycin) (Table 1). Adjunctive therapy may be indicated for relapsed or prolonged S. aureus infections, although impact on mortality and clinical outcomes remain unclear [29].
Table 1.
Systemic Antibiotics for Staphylococcus aureus Infections and Associated Adverse Effects
| Antibiotic Class | Specific Medications | Activity against MRSA |
Activity against MSSA |
Reported Adverse Effects | Special Considerations |
|---|---|---|---|---|---|
| ß-lactam antibiotics (penicillins, cephalosporins, ß-lactam inhibitors, carbapenems) |
Oxacillin/Nafcillin Cefazolin/Cephalexin Cefepime Ceftriaxone Ceftaroline Amoxicillin-clavulanate Ampicillin-sulbactam Piperacillin-tazobactam Meropenem Ertapenem |
X |
X X X X X X X X X X |
• Bone marrow suppression with prolonged use • Transaminase elevation • Thrombophlebitis • Diarrhea • Clostridioides difficile infection |
• Class of choice for MSSA infections • Inactive against MRSA except ceftaroline |
| Glycopeptide | Vancomycin | X | X | • Tissue penetration variable depending on degree of inflammation • Nephrotoxicity |
• Therapeutic monitoring necessary • Higher MICs (>1) have been associated with treatment failure |
| Lipopeptide | Daptomycin | X | X | • Elevation in creatinine phosphokinase (CPK) | • Inactivated by pulmonary surfactant; should not be used for treatment of pneumonia or left-sided endocarditis • Approved for use in children ≥12 months of age |
| Lincosamides | Clindamycin* | X | X | • Diarrhea • Clostridioides difficile infection |
• Poor taste of oral suspension • Excellent oral bioavailability and tissue/bone penetration, poor CNS penetration • S. aureus may exhibit either constitutive or inducible resistance • Most HA-MRSA isolates resistant to clindamycin |
| Oxazolidinones | Linezolid Tedizolid |
X | X | • Bone marrow suppression (more likely to occur beyond 3 weeks of treatment) • Peripheral and optic neuropathy (more likely to occur beyond 3 weeks of treatment) • Lactic acidosis • Diarrhea, emesis |
• Activity against VISA and VRSA • Excellent oral bioavailability • Use with caution in patients taking serotonergic or adrenergic agents |
| Tetracycline | Doxycycline Minocycline Tigecycline Omadacycline |
X X X X |
X X X X |
• Use caution in children <8 years of age due to tooth enamel discoloration and decreased bone growth; short courses (<3 weeks) are unlikely to result in harm • Photosensitivity |
• Excellent oral bioavailability • Tigecycline has low serum concentration |
| Lipoglycopeptides | Telavancin Dalbavancin Oritavancin |
X X X |
X X X |
Gastrointestinal symptoms (nausea, vomiting) |
• Limited data available in children • Dalbavancin and oritavancin are administered once weekly |
| Quinolones | Delafloxacin Levofloxacin* Moxifloxacin* |
X X X |
X X X |
• Tendinopathies • QT interval prolongation |
• Excellent oral bioavailability |
| Others | Trimethoprim-Sulfamethoxazole (TMP-SMX) Rifampin |
X X |
X X |
• TMP-SMX not recommended in infants younger than 2 months of age due to risk of bilirubin displacement | • Rifampin should not be used as monotherapy due to rapid development of resistance • Rifampin may be combined with another agent for hardware-associated infections |
Notes: Antibiotics included are those available for use in the United States. Choice of antibiotic to treat Staphylococcus aureus infection is dependent upon susceptibility of infecting organism; empiric therapy should be selected based on local antibiotic susceptibility patterns.
Susceptibility is variable
Abbreviations: MIC, minimum inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible S. aureus; HA-MRSA, healthcare-associated methicillin-resistant S. aureus; VISA, vancomycin-intermediate S. aureus; VRSA, vancomycin-resistant S. aureus. Adapted from [34, 48].
Bloodstream Infection (BSI)
S. aureus is a common cause of BSI in children, resulting in significant morbidity and mortality (Table 2) [15]. S aureus BSI can be secondary to, or result in, infectious foci at another site, including a central venous catheter (CVC), bone, lung, or skin and soft tissue. However, BSI without an obvious source occurs in 5–30% of cases and is associated with higher mortality [14, 30]. Risk factors for BSI include S. aureus colonization, congenital heart disease, underlying immunosuppression (leukemia/lymphoma, hemoglobinopathy, primary immunodeficiency, solid-organ transplantation), and prematurity [29, 31]. Of note, an increase in rates of S. aureus BSI occurred concurrently with the emergence of USA300 CA-MRSA and subsequent increase in SSTIs [32].
Table 2.
Staphylococcus aureus Infection Entities
| Infection Site | Epidemiology | Clinical Presentation | Duration of Antibiotic Therapy |
|---|---|---|---|
| Bloodstream (Bacteremia) |
S. aureus accounts for 5–30% of BSIs without localizing source and CLABSIs [30, 31, 33]. Increased risk of persistent bacteremia and treatment failure with retained foreign body, >2 infected sites, and endovascular infection [14, 16]. |
Fever Lethargy |
2–6 weeks depending on the source of bacteremia |
| Central Nervous System (Meningitis, Epidural abscess) |
Uncommon (<1% of all pediatric meningitis), <5% of S. aureus invasive infections [65]. Mortality in children is low. Associated with device placement, trauma, prematurity, intraventricular hemorrhage, or CNS malformation. |
Fever Altered mental status Back pain |
Meningitis: 2 weeks Brain, spinal epidural abscess, subdural empyema: 4–6 weeks |
| Lungs (Pneumonia, empyema) | Rare (<1% of all pediatric pneumonias). Bullous lung disease more common among infants. Common cause of necrotizing pneumonia [57]. Increased risk of complications: pleural space involvement and lung abscesses. |
Fever Cough Chest pain |
7–21 days depending on severity of infection and clinical improvement |
| Endovascular (endocarditis, thrombophlebitis, septic embolism) |
Common cause of endocarditis with high mortality and morbidity. Typical signs of endocarditis may not be present in children [42]. |
Fever Chest pain |
4–6 weeks |
| Bone, Muscle, Joint (osteomyelitis, pyomyositis, osteoarthritis) |
Most common cause of musculoskeletal infections in children [45, 47]. | Fever Focal joint or extremity pain Arthralgia |
Septic arthritis: 3–4 weeks Osteomyelitis: 3–6 weeks |
| Skin and Soft Tissue Infection (SSTI) | Represents >60% of S. aureus infections. |
Pain Rash Erythema Swelling Purulence |
7–10 days |
Abbreviations: BSI, bloodstream infection; CLABSI, central line-associated bloodstream infection; CNS, central nervous system; MRSA, methicillin-resistant Staphylococcus aureus.
Prolonged S. aureus bacteremia is associated with an increased risk of complications such as septic emboli, thrombi, and metastatic foci of infection [16]. Each additional day of bacteremia has been associated with a 50% (95% CI: 26–79%) increased odds of bacteremia-related complications [33]. Risk factors for prolonged S. aureus bacteremia include methicillin-resistance, musculoskeletal infection, endovascular infection, and delayed intervention for source control [14, 16]. Children with risk factors for infective endocarditis (e.g., congenital heart disease and fever), clinical features suggestive of infective endocarditis, or persistent bacteremia for greater than 72 hours should be thoroughly assessed for complications using echocardiogram, as well as imaging of other anatomic sites as indicated by swelling, inflammation, or pain [29, 34].
Presence of a CVC may increase the risk of prolonged bacteremia. CVC removal is recommended for patients with S. aureus bacteremia given the ability of S. aureus to adhere to foreign material, making eradication difficult [35]. Efforts have been made to optimize bacteremia prevention strategies among hospitalized patients, including bathing with topical antiseptics to decrease bacterial bioburden on the skin. In a multicenter trial of children in pediatric intensive care units, daily chlorhexidine bathing yielded a 34% reduction in risk of bacteremia compared to daily bathing with soap and water [36].
Vancomycin combined with a beta-lactam is recommended as empiric treatment for S. aureus bacteremia until antimicrobial susceptibility is available [29]. Treatment duration depends on the source of the bacteremia and presence of complications such as endovascular infection or metastatic foci of infection. In pediatrics, 7–14 days of IV antibiotic therapy is generally recommended for uncomplicated bacteremia, though more research is needed to derive the optimal length of IV therapy [29]. Endovascular infection, musculoskeletal infection, critical illness, and initial sub-therapeutic vancomycin levels have been associated with MRSA BSI treatment failure [33, 37].
To assess response to antibiotic therapy, it is essential to document clearance of bacteremia. Thus, it is recommended to obtain daily blood cultures until blood cultures are negative for 2 days [38]. As the yield of positivity is low, additional blood cultures to document sterility following 2 days of negative cultures are not typically indicated unless the patient’s clinical condition deteriorates. Infectious disease consultation for S. aureus BSI has been demonstrated to improve patient management and outcomes, including decreased mortality and recurrence of bacteremia [29, 39].
Endocarditis/Endovascular Infection
S. aureus is the most common cause of infective endocarditis (IE). The overall mortality for S. aureus IE is as high as 66% (Table 2) [40]. In the International Collaboration on Endocarditis Prospective Cohort Study conducted from 2000 to 2003, S. aureus caused 32% of all IE cases, of which 27% were MRSA. MSSA IE cases tended to result in higher incidence of systemic embolization compared to MRSA (26% vs 18%, p=0.06), but MRSA IE was associated with longer duration of bacteremia (43% vs 9%, p<.001) and a trend towards increased mortality [41].
The clinical presentation of IE can be non-specific and indolent with symptoms such as malaise, weight loss, or myalgias. The typical signs of IE, including new-onset heart murmur, congestive heart failure, and embolic phenomenon, are often not present in children [42]. When endocarditis is suspected in a child, it is recommended to obtain three sets of blood cultures, ideally through separate venipunctures, prior to initiation of antibiotics. Unlike adults, transthoracic echocardiography (TTE) can be sufficient in children [43]. However, transesophageal echocardiography (TEE) should be obtained in children who have had prior cardiac surgeries, have congenital anomalies, are at high risk for aortic root abscesses, or in whom TTE does not provide sufficient information [44]. Negative echocardiography does not exclude IE.
Bactericidal, rather than bacteriostatic, antibiotics should be used to treat endovascular infection (endocarditis, septic thrombophlebitis, mycotic aneurysms) for the duration of therapy. S. aureus bacteremia commonly persists after initiation of appropriate therapy, particularly if the source of infection has not been removed (e.g., CVC). For MRSA endocarditis, vancomycin or daptomycin are recommended for a minimum of 6 weeks. For MSSA IE, oxacillin/nafcillin or cefazolin should be used for at least 4–6 weeks and vancomycin can be used as an alternative in those highly allergic to beta-lactam antibiotics. Given the difficulty of eradicating S. aureus from foreign material, if prosthetic material is present, the addition of rifampin for 6 weeks and gentamicin for the initial two weeks of treatment is recommended; patients receiving this regimen should be closely monitored for adverse side effects [44].
Acute Hematogenous Osteomyelitis, Septic Arthritis, Pyomyositis
S. aureus is the most common cause of musculoskeletal infections (Table 2) [45–47]. In children, infections in the bone, joint, and muscle most commonly arise from hematogenous seeding, and more rarely from a contiguous focus of infection, such as a decubitus ulcer, or through direct inoculation from trauma or a surgical procedure. S. aureus accounts for up to 60% of pyomyositis cases in children and commonly affects the pelvic and lower extremity muscles [46]. S. aureus accounts for up to 78% of acute hematogenous osteomyelitis (AHO) in children; 33% of these cases are caused by MRSA [48]. AHO most frequently occurs in the long bones of the lower extremities and the pelvis, followed by the long bones of the upper extremities [49, 50]. Multifocal osteomyelitis has been reported in 5–10% of patients with AHO and up to 35% of patients have contiguous septic arthritis [49, 51]. AHO may be complicated by the formation of an intraosseous or subperiosteal abscess (Figure 1) and has also been associated with thrombotic complications including deep venous thrombosis adjacent to the site of infection and septic pulmonary emboli [52, 53].
Figure 1.

A. MRI of the left thigh and femur with intravenous contrast. Shown is the coronal view demonstrating a T2 hyperintense signal in the left distal femoral metaphysis (arrow) which extends 19 cm proximal to the distal femoral physis consistent with acute MRSA hematogenous osteomyelitis. B. Shown is the sagittal view demonstrating a T2 hyperintense, rim-enhancing subperiosteal abscess measuring 2.8 × 4.5 × 13.0 cm (arrow) and hyperintensity in the vastus intermedius, medialis, lateralis, and semitendinosus muscles, and diffuse subcutaneous edema throughout the left thigh.
The clinical presentation of S. aureus musculoskeletal infection in children includes fever, pain, erythema, warmth, point tenderness, and limited range of motion. For children with lower extremity osteomyelitis, patients frequently present with limp or inability to bear weight [47]. Detailed clinical evaluation should be prioritized in children with osteomyelitis. While procalcitonin, a common inflammatory biomarker, is not recommended, the initial diagnostic investigation should include serum C-reactive protein (CRP). The baseline CRP value can be followed during the clinical course to assess response to therapy [48]. Blood cultures should be obtained prior to antimicrobial therapy. Although the sensitivity of early plain radiographs for suspected AHO infection is low, it is reasonable to start with plain radiography of the affected limbs. However, further imaging such as magnetic resonance imaging (MRI) to confirm the diagnosis is often needed [54]. End of treatment imaging studies should not be routinely obtained in uncomplicated AHO unless there is involvement of physis; end of therapy imaging is recommended for patients with complicated AHO [48].
When feasible, a bone biopsy or aspirate of purulent material obtained via surgical procedure or interventional radiology should be performed to obtain microbiological studies that can guide targeted antimicrobial therapy. While blood cultures may reveal the causative organism in ~50% of patients with S. aureus musculoskeletal infection, aspirate or biopsy increases the yield and may be the only specimen revealing the organism [48, 51, 54]. Surgical debridement and drainage of a joint space or subperiosteal or intraosseous abscess should be performed to prevent long-term morbidity. If patients are clinically stable and an upcoming surgical procedure is planned, waiting to start antibiotics until after the procedure is reasonable as the yield of cultures obtained within 24 to 48 hours of antibiotic initiation is similar to those obtained prior to the onset of antibiotic therapy [48]. However, for rapidly deteriorating patients, prompt administration of antibiotics reduces the risk of mortality.
Empiric antimicrobial therapy should include coverage against S. aureus and CA-MRSA based on patient risk factors and local susceptibility data [48]. Definitive treatment should be based on microbiologic study results. Early transition from IV to oral antibiotics with good bioavailability and bone concentration has been demonstrated to be effective in children with uncomplicated musculoskeletal infections who are clinically stable [45, 48]. However, a paucity of data exist to guide the optimal length of hospitalization and IV antibiotic therapy prior to transitioning to oral antibiotic therapy. In one retrospective study, patients who were discharged home and successfully treated with oral antibiotics received a median of 7 (interquartile-range 5–10) days of IV antibiotic therapy prior to hospital discharge. Ultimately, the optimal duration and route of antibiotic therapy should be individualized based on the patient’s clinical response; some patients with a complicated course, including multifocal or deep-seated infection, prolonged bacteremia, immunocompromised state, and very young age may require a longer course of IV antibiotics [53]. Recently published clinical practice guidelines for pediatric AHO state that three to four weeks of total antibiotic therapy may be adequate for children with uncomplicated AHO who have shown clinical response to initial therapy, while longer courses of antibiotics may be needed with more virulent pathogens, extensive infection, or complicated cases [48]. Three to four weeks of antibiotics are generally recommended for treatment of septic arthritis, although concomitant osteomyelitis is not uncommon and may warrant longer treatment.
Up to 10% of children with S. aureus AHO will experience acute complications (e.g., treatment failure within 6 weeks of antibiotic therapy initiation, prolonged hospitalization) as well as long-term morbidity (e.g., growth arrest of the affected limb or limb length discrepancy, pathologic fractures, avascular necrosis, chronic dislocation, chronic osteomyelitis) [51, 53]. The development of orthopedic complications following AHO has been associated with prolonged fever, delayed source control, need for multiple surgical procedures, bacteremia, and AHO with concomitant septic arthritis (Box) [45, 51, 53]. In two large pediatric retrospective studies, methicillin resistance was not associated with adverse outcomes; however, methicillin resistance was associated with decreased range of motion at end of therapy in one pediatric study compared to individuals with non-S. aureus musculoskeletal infection [45, 51, 53]. The S. aureus strain type may be more important than antibiotic susceptibility (i.e., methicillin resistant vs. susceptible) [51]. A multidisciplinary approach to S. aureus musculoskeletal infections and the development of clinical practice guidelines can decrease time to diagnostic MRI, increase the proportion of patients with tissue culture and identification of the infecting organism, increase prescription of targeted antibiotic therapy, and decrease hospital length of stay and hospital readmission rates [54]. Severity of illness scores have been devised to predict patients at high risk of developing AHO complications, which may inform treatment and follow-up plans [53].
Box.
Factors Associated with Complications in Pediatric Patients with Acute Hematogenous Osteomyelitis
|
Factors Associated with Acute Complications [53] Fever (body temperature >100.4ºF) for more than 48 hours after starting antibiotics Bone abscess Associated suppurative arthritis Disseminated disease Delayed source control (surgical intervention more than 3 days after hospital admission) |
|
Factors Associated with Chronic Complications [51, 53] Prolonged fever (body temperature >100.4ºF) for more than 4 days following hospital admission Disseminated disease Delayed source control (surgical intervention more than 3 days after hospital admission) Requirement for bone debridement C-reactive protein (CRP) ≥100 mg/L 2–4 days after antibiotic initiation S. aureus strain type (agr group III) |
Abbreviations: agr, accessory gene regulator
Pneumonia
S. aureus is an important cause of community-acquired pneumonia (CAP) and can be rapidly progressive, resulting in severe, fulminant respiratory failure (Table 2) [55]. S. aureus pneumonia is associated with increased disease severity and worse outcomes, with increased length of hospital stay, need for intensive care unit (ICU) admission, and use of mechanical ventilation [56, 57]. S. aureus is also an important contributor to hospital-acquired pneumonia (HAP), ventilator-associated pneumonia (VAP), and healthcare-associated pneumonia (HCAP). Risk factors for S. aureus pneumonia in children include underlying cystic fibrosis and mechanical ventilation.
Children typically present with severe pneumonia with or without effusion, necrotizing or cavitary infiltrates, or empyema (Figure 2) [57]. Viral co-detection is common, and children may present with preceding or concurrent upper respiratory tract infection. In particular, necrotizing pneumonia caused by S. aureus strains producing Panton-Valentine leucocidin has been associated with preceding flu-like illness [58]. Influenza-S. aureus co-detection has high morbidity and mortality rates. MRSA co-infection of the lungs during the 2009 influenza A (H1N1) outbreak resulted in an 8-fold increased relative risk of mortality among previously healthy children [59].
Figure 2.
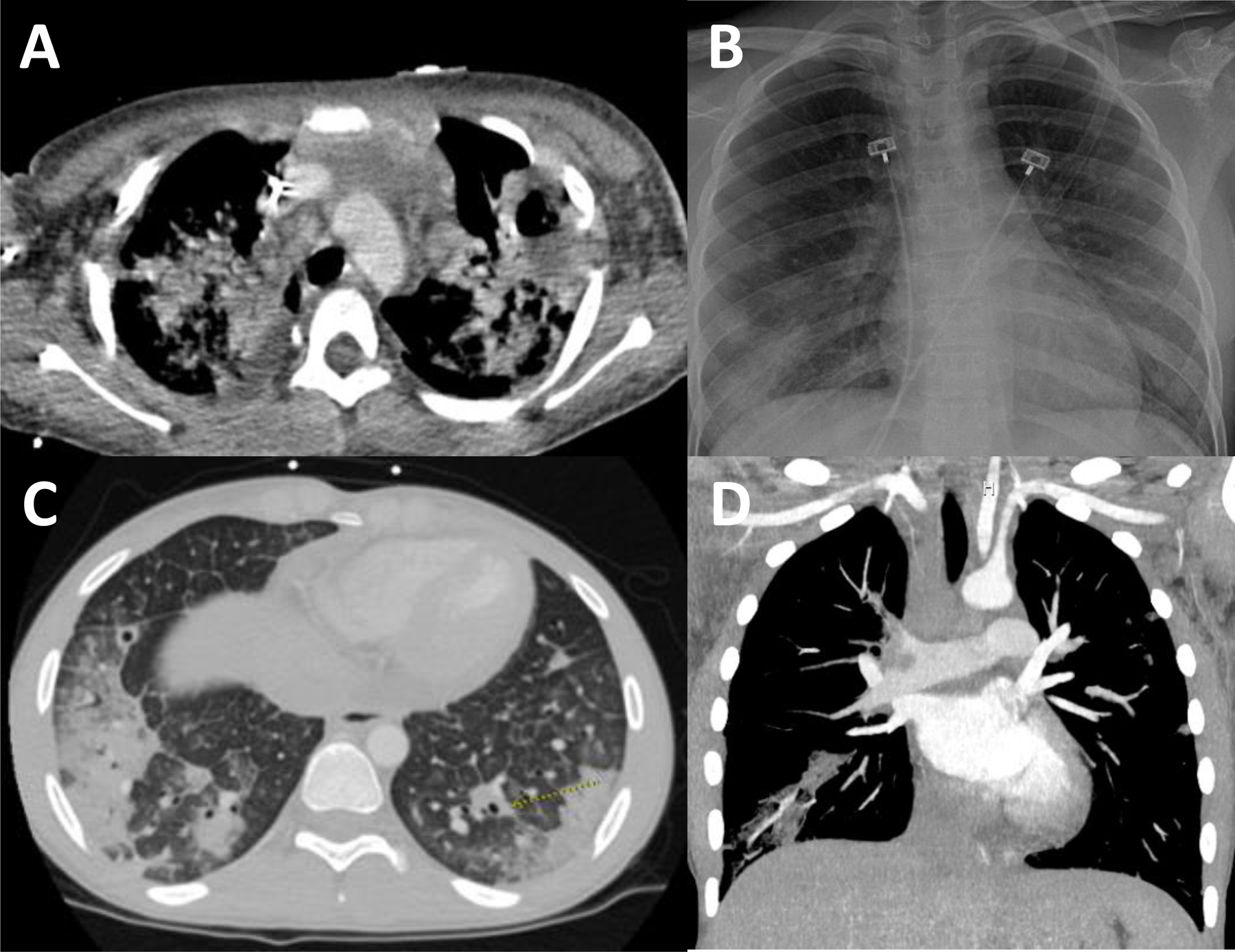
A. Chest CT with contrast. Shown is a patient with extensive MRSA multifocal necrotizing pneumonia throughout both lungs with a lung abscess in the left upper lobe measuring 4.0 × 2.6 × 3.9 cm and additional cavitary lesions in the right middle lobe. There is also moderate global cardiomegaly with a possible vegetation measuring 12 mm along the lateral leaflet of the mitral valve, and moderate pericardial effusion with pericardial enhancement. B. Chest radiograph with scattered bilateral infiltrates from a patient with MSSA bacteremia. C, D. Chest CT angiography with contrast showing bilateral opacities with areas of early cavitation, multiple septic pulmonary emboli including non-occlusive thrombi within the pulmonary arteries.
Blood cultures should be obtained in children requiring hospitalization for CAP, those with severe or complicated CAP, or those who fail to improve after initial antibiotic therapy. Of the children enrolled in the prospective Etiology of Pneumonia in the Community surveillance study diagnosed with S. aureus CAP, 26% also had bacteremia, 52% had a positive pleural fluid culture, and 39% had positive culture from bronchoalveolar lavage fluid or endotracheal aspirate [60]. Sputum sample or tracheal aspirate for Gram stain and culture should also be obtained in children with severe CAP. Drainage of pleural effusion or empyema, if present, should be considered based on the size of the effusion and the degree of respiratory compromise [61].
Empiric coverage for S. aureus is recommended in cases of severe pneumonia defined as requirement for ICU admission, necrotizing or cavitary infiltrates, or presence of empyema [62]. For initial treatment of MRSA or MSSA pneumonia, vancomycin or clindamycin (based on local susceptibility data) in addition to a beta-lactam is recommended, although linezolid and ceftaroline fosamil are alternative antibiotics with MRSA coverage [61]. Daptomycin should not be administered for pneumonia given inactivation by pulmonary surfactant.
Central Nervous System (CNS) Infection (Meningitis, Epidural Abscess, Ventricular Shunt Infection)
S. aureus CNS infection is uncommon and typically occurs as a complication of neurosurgical procedures, CNS instrumentation (e.g., ventricular shunt), bacteremia, or extension from a parameningeal focus. S. aureus has been implicated in 13–25% of ventricular shunt infections and is the most common cause of spinal epidural abscesses (Table 2) [63, 64]. In a prospective surveillance study of S. aureus at a large children’s hospital, CNS infections accounted for 5% of all invasive S. aureus infections [65]. The majority (70%) of these cases were associated with a CNS device, typically a ventriculoperitoneal shunt, 13% were associated with hematogenous meningitis, and 10% were associated with spinal epidural abscess. MSSA accounted for 67% of infections in the case series. Overall mortality and morbidity in children was low [65].
S. aureus CNS infections typically present with fever, headaches, neck or back pain, nausea, vomiting, and neurologic symptoms. Epidural abscesses classically present with back pain, fever, and neurologic symptoms (Figure 3). CNS shunt infections present with fever, and signs of increased intracranial pressure [66, 67]. Given the severity of S. aureus CNS infections, a high degree of clinical suspicion is needed. Imaging studies should be considered, particularly if a child presents with neurologic signs and symptoms accompanied by S. aureus bacteremia.
Figure 3.

A. MRI brainstem and spine with contrast. Shown is the sagittal view of an extensive MRSA spinal epidural abscess with T2 hyperintense fluid collection and peripheral enhancement extending from the level of C3 to the distal sacrum/coccyx, measuring up to 8 mm in thickness in the mid thoracic spine in maximum diameter (arrow). B. Shown is a T2 hyperintense paraspinal abscess extending from the level of C6-T2 along the left side (arrow).
Management of S. aureus CNS infection typically requires surgical intervention. Due to potential spinal cord compression, emergent surgical intervention is critical in management of spinal epidural abscesses to prevent long-term neurologic sequelae. For treatment of CNS shunt infections, it is essential to remove all components of an infected device, place an external CSF drain, and initiate antibiotic treatment [62]. Antibiotic choice in treatment of CNS infection should take into account cerebrospinal fluid (CSF) penetration. An anti-staphylococcal beta-lactam antibiotic such as nafcillin or oxacillin is recommended for MSSA CNS infections and vancomycin for MRSA [68]. However, vancomycin has relatively poor CSF penetration even in the presence of inflamed meninges, with a median CSF to serum ratio of 3% and high inter-subject pharmacokinetic variability. Linezolid has relatively good CSF penetration [69]. Rifampin has similar penetration for inflamed and non-inflamed meninges and achieves bactericidal concentrations in the CSF. Given poor CSF penetration of most antibiotics, vancomycin with rifampin is recommended by some experts, although randomized clinical trials have not been performed [62]. Ceftaroline for treatment of MRSA CNS infection has been reported in limited case reports [70]. Phase 1 clinical trials for the use of ceftaroline in pediatric CNS infections are in progress (NCT00633126, NCT02600793) [71].
Skin and Soft Tissue Infection (SSTI)
S. aureus is a leading cause of SSTI, manifesting as cellulitis, cutaneous abscesses, folliculitis, and impetigo (Table 2, Figure 4) [72]. SSTIs are a leading cause of pediatric hospitalizations and healthcare utilization, especially given the high likelihood for recurrence, reported to range from 20–70% [15, 26, 73–76]. Risk factors for the development and recurrence of SSTIs include S. aureus colonization, particularly persistent colonization, colonization of multiple anatomic sites, underlying dermatologic conditions (i.e. eczema), personal history of prior SSTI or close contact with someone with SSTIs, participation in contact sports, and certain chronic medical conditions [21, 77, 78].
Figure 4.

Staphylococcus aureus skin and soft tissue infections. Shown is a toddler with a left posterior thigh abscess with central head and surrounding area of cellulitis.
Incision and drainage (I&D) has traditionally been the mainstay for uncomplicated S. aureus SSTI [62, 79]. I&D evacuates infective material which can be sent for microbiologic culture, and provides pain relief and faster wound healing. Recently, several large, multicenter, placebo-controlled, randomized trials have demonstrated that I&D in conjunction with systemic antibiotic therapy results in higher likelihood of clinical cure, regardless of the abscess size. Moreover, administering systemic antibiotics in conjunction with I&D reduces the incidence of recurrent skin infection, likely due to eradication of S. aureus colonization by systemic antibiotics. (Figure 5). [72, 80–83]. Despite robust evidence to support the use of systemic antibiotics in conjunction with I&D, antibiotics are prescribed for 69% of ambulatory and ED SSTI visits and only 60% of surveyed pediatric infectious disease providers would recommend antibiotics for treatment of uncomplicated skin abscesses [10, 84].
Figure 5.

Recommended approach to the management of patients presenting with skin abscesses based on current evidence). Management of the acute infection includes incision and drainage, culture of the purulent material for organism identification and susceptibility testing, and systemic antibiotic therapy. Decolonization should be recommended for patients who experience recurrent skin abscesses or in settings of ongoing transmission (e.g., SSTI in multiple household members) despite optimizing hygiene measures. Specifically, the recommended decolonization regimen is a 5-day protocol consisting of intranasal application of mupirocin (approximately a pea-sized amount to each nostril, applied with a sterile cotton-tipped applicator) twice daily, and daily antimicrobial body washes with either chlorhexidine or dilute bleach water baths. Chlorhexidine should be applied with a clean washcloth to the neck and below (contact with the face and ears should be avoided as ocular and ototoxicity may occur) and should be rinsed off after 1–3 minutes. Dilute bleach baths should consist of ¼ cup of bleach per ¼ filled bathtub for a standard sized bathtub or 1 teaspoon of bleach per gallon of bathwater for a non-standard bathtub; individuals should soak in the dilute bleach water for 15 minutes.
Cultures from abscesses and purulent SSTIs should be obtained to help guide antibiotic therapy. Empiric choice for treatment of S. aureus SSTIs should be based on disease severity and local antibiotic susceptibility patterns, which may change over time [12]. In hospitalized children, β-lactams or vancomycin can be started empirically depending on local antimicrobial susceptibility. Clindamycin, linezolid, daptomycin, tetracyclines, or TMP-SMX are alternative options [62]. There is a paucity of data regarding optimal length of antibiotic therapy for cellulitis and skin abscesses. The optimal duration of treatment should be based on clinical response, and a 7–10 day course is generally thought to be sufficient. A randomized-controlled trial comparing 3 versus 10 days of TMP-SMX after drainage for treatment of MRSA skin abscesses found that 3 days of antibiotics resulted in increased treatment failure and subsequent recurrence within 1 month [85]. A subgroup analysis of a randomized trial demonstrated that that each additional day of antibiotic therapy (up to 10 days of either clindamycin or TMP-SMX) resulted in increased likelihood for cure and decreased incidence of SSTI recurrence 6 weeks following the acute infection [82].
PREVENTION OF STAPHYLOCOCCUS AUREUS INFECTIONS
Transmission
To devise effective prevention strategies, it is essential to understand the dynamics of S. aureus acquisition and transmission. S. aureus transmission can occur through close personal contact. Households serve as important reservoirs for S. aureus transmission and indeed, household contacts of individuals with S. aureus SSTI have a higher prevalence of colonization and SSTI than the general population [78, 86–89]. In addition to close personal contact, S. aureus transmission among household members also occurs through the sharing of personal hygiene items, towels, and beds [88].
In healthcare and community settings, environmental surfaces and fomites serve as important reservoirs for S. aureus acquisition and transmission [90, 91]. In a comprehensive investigation of S. aureus household transmission dynamics among households affected by S. aureus infections, environmental surfaces, particularly those commonly shared between household members (e.g., hand towels, television remote control, video game controller), as well as bed linens, were frequently contaminated with S. aureus, and specifically, with a strain molecularly concordant with the S. aureus strain that caused the index patient’s infection. Importantly, these surfaces were persistently contaminated over time, perpetuating the cycle of transmission and reacquisition among household members, thus posing risk for recurrent infection [78, 87–89]. Thus, integrating environmental hygiene measures may be an important component of S. aureus eradication and infection prevention; this strategy is currently under study (NCT02572791).
The role of companion animals in S. aureus transmission dynamics and recurrent SSTIs in owners and veterinary personnel is an important consideration. In the aforementioned household investigation, while S. aureus colonization of pet dogs and cats was detected (24% of dogs and 13% of cats), and human to pet transmission was observed, these companion animals were rarely a primary source of a transmitted S. aureus strain to their owners [88, 89]. Thus, while pet dogs and cats may contribute to overall household transmission dynamics, they likely do not represent natural hosts for S. aureus colonization, and their carriage often spontaneously resolves [92, 93]. Strategies to reduce transmission between companion animals and their household members include hand washing before and after pet contact, isolating the pet temporarily from a patient undergoing treatment for an active infection, and disinfecting the pet’s crate or kennel and washing the pet’s bedding [92, 94].
Decolonization
S. aureus colonization, and particularly colonization at multiple anatomic sites, is a known risk factor for S. aureus infection, though infection typically does not occur without disruption of the skin through inflammatory lesions (e.g., eczema), trauma, or microabrasions [21, 75, 95]. Prevention measures for outpatients will be discussed here (Figure 5), and specific risk factors and infection prevention strategies for neonates and immunocompromised hosts are described below. The primary component of S. aureus infection prevention is optimization of hygiene measures, including keeping open wounds covered, frequent hand hygiene, regular bathing, using a barrier between bare skin and shared surfaces (e.g., athletic or gym equipment), and avoiding sharing personal hygiene items (e.g., razors, deodorant, cosmetics) and towels. As bed linens serve as a reservoir of S. aureus, they should be laundered weekly. Additionally, patients can be encouraged to use lotions in pump or pour bottles to avoid contamination, liquid rather than bar soap, avoiding bath or shower loofas, laundering towels and washcloths after each use, and keeping fingernails trimmed short and clean [62, 73, 75]. Optimizing the treatment of underlying skin disorders (e.g., eczema) is also essential.
Up to 70% of patients with SSTI will experience a recurrence, predominantly with the same molecular strain of S. aureus as the primary infection and the strain with which the patient is colonized [73–76, 96]. Thus, to prevent recurrent infections, decolonization strategies should be considered for individuals who have optimized hygiene strategies and experience a recurrent SSTI, or in settings in which there is ongoing transmission between household members or other close contacts (e.g., infection occurring in multiple household members or athletic team members) [62]. These strategies include the application of topical antibiotics or antiseptics to eradicate or decrease the burden of S. aureus carriage. Multiple agents and regimens have been investigated for S. aureus eradication and have been recently reviewed [26]. A combined decolonization approach of intranasal antibiotic application (e.g., mupirocin) and bathing with a topical antiseptic or biocide (e.g., chlorhexidine gluconate and dilute bleach water [sodium hypochlorite]) has been most effective for preventing SSTI [73–75, 97].
Mupirocin is a topical antimicrobial agent effective against Gram-positive microorganisms. Mupirocin is frequently used in hospital settings to prevent nosocomial infections including SSIs and CLABSIs [98, 99]. Chlorhexidine gluconate is a broad-spectrum biocide that is frequently used in healthcare settings for topical disinfection prior to invasive procedures and for decolonization among select populations to prevent nosocomial infections [100]. Chlorhexidine comes in several formulations, including a topical liquid, oral rinse, and impregnated cloths. Sodium hypochlorite, or bleach, has broad-spectrum antimicrobial activities. Dermatologists have traditionally recommended bathing in dilute bleach water for patients with eczema, given its effectiveness to improve skin flares, likely due to decreasing the burden of S. aureus on the skin [101].
As S. aureus household transmission is an important contributor to persistent colonization or reacquisition and development of recurrent SSTI, several trials have been conducted to evaluate the effectiveness of decolonizing household contacts in addition to the index patient with SSTI. A randomized clinical trial enrolling 183 healthy children with S. aureus SSTI and colonization and their household contacts compared a household decolonization approach (i.e., decolonization regimen performed by all household members) vs. decolonization of the index patient alone [73]. The incidence of cumulative SSTI 12 months following the intervention was significantly lower among index patients, as well as household contacts, assigned to the household decolonization arm. A decolonization regimen performed by all household members can be burdensome and costly, especially for large families, and may have the untoward effects of driving antimicrobial resistance and disrupting the commensal skin microbiota. As individuals experiencing SSTI are at increased risk for developing recurrent SSTI, another approach to addressing household transmission and infection prevention is targeting decolonization to household members with a history of SSTI. In a pragmatic, non-inferiority trial enrolling 102 healthy children with CA-MRSA skin abscesses and their household contacts, a household decolonization approach, performed by all household members, was compared to a personalized decolonization approach performed by the index patient and household contacts who had experienced an SSTI in the prior year [102]. The incidence of cumulative SSTI 3 months following the decolonization intervention (the primary study outcome) demonstrated non-inferiority of the personalized approach to the household approach; these findings persisted over the 12-month longitudinal study period. Adherence to the regimen was higher among participants randomized to the personalized decolonization approach. Given these findings, when prescribing a decolonization regimen for a patient with recurrent SSTI, these measures should also be recommended for household contacts experiencing SSTI in the prior year (Figure 5). Importantly, in both trials, eradication of S. aureus carriage and prevention of SSTI waned over time, likely due to ongoing S. aureus exposure and reacquisition. Thus, a discreet, 5-day decolonization regimen may be inadequate for long-term infection prevention. For patients experiencing ongoing recurrent SSTIs despite optimizing hygiene measures and performing a discreet decolonization regimen, periodic decolonization, performed for 3 months, may provide more sustained protection (NCT02572791). Periodic decolonization incudes intranasal application of mupirocin twice daily for 5 consecutive days each month and performing antimicrobial body washes twice weekly (Figure 5).
Neonates and Patients in the Neonatal Intensive Care Unit (NICU)
S. aureus colonization and infections are uniquely problematic in neonatal intensive care units (NICUs) due to prolonged hospitalizations of premature infants with friable skin or underlying medical conditions. These infants frequently require invasive procedures and placement of indwelling devices and have immature immune systems. These factors potentially increase the risk of S. aureus exposure and acquisition. Neonatal S. aureus infections are frequently preceded by nasal colonization. In recent years, MSSA infection incidence among these vulnerable infants has outpaced the incidence of MRSA infections. Importantly, the rates of morbidity and mortality are similar between infants with MSSA and MRSA infections [64, 98, 103].
Among neonates, active S. aureus surveillance and contact isolation are associated with decreased colonization and infection [104]. In addition to protective isolation to prevent S. aureus transmission in the NICU, the risk for invasive infection is further reduced through eradicating or decreasing the burden of S. aureus skin colonization. The most studied agents for neonatal topical decolonization are mupirocin and chlorhexidine gluconate. Given the recent trends in increasing MSSA infections among neonates, one center implemented an active surveillance, isolation, and decolonization policy targeting MSSA in addition to MRSA. Review of over 2700 children before and after policy implementation demonstrated a 73% decrease in MSSA infections immediately after the successful rollout of the intervention, with a sustained 21% quarterly decrease in cultures detecting MSSA [98]. Similar findings have been replicated across multiple studies, further supporting the utility of surveillance and decolonization for S. aureus prevention in neonatal units [105]. Among these patients, recolonization frequently occurs – up to 39% in one study of decolonized infants – with the same colonizing bacterial strain identified prior to decolonization [106]. Additive strategies may be necessary for NICUs tackling ongoing S. aureus outbreaks or in highly endemic settings. These strategies may include antimicrobial stewardship strategies as well as addressing the potential for exposure to caregiver colonization.
As parents may serve as reservoirs for S. aureus transmission to their infants, particularly in NICUs where skin-to-skin contact is highly encouraged, targeting decolonization directly to parents and/or caregivers may be an effective strategy to prevent neonatal infections. TREAT PARENTS was a double-blind randomized clinical trial assessing the effectiveness of treating S. aureus-colonized parents with a 5-day regimen of intranasal mupirocin and topical chlorhexidine, compared to placebo, to reduce S. aureus transmission from parents to their hospitalized infants. Over the 90-day longitudinal study period, 40% of enrolled infants acquired S. aureus colonization; 57% of infants with S. aureus acquisition became colonized with a S. aureus strain concordant with their colonized parents’ strains. Importantly, treating parents with topical decolonization significantly reduced the risk of concordant S. aureus strain acquisition in their infants [107]. Further confirmation of these findings could support widespread utility in the NICU.
Immunocompromised Hosts
Children with immunocompromising conditions are at increased risk for S. aureus infection. Recurrent skin infections are often the first sign of an underlying primary immunodeficiency such as chronic granulomatous disease, leukocyte adhesion defect, or hyperimmunoglobulin E syndrome [108]. Children with malignancy and those who have undergone solid organ or hematopoietic stem cell transplant (HSCT) are at increased risk for S. aureus infection and complications due to comorbid conditions, presence of central venous catheters, immunosuppressive medications, and prolonged hospitalizations [108–110]. Despite high-risk for complicated infections, mortality directly attributable to S. aureus bacteremia is low.
General infection prevention principles such as hand hygiene, use of personal protective equipment, and appropriate care of CVCs are essential to prevent S. aureus infections in immunocompromised children. In contrast to the reduction of S. aureus infections in critically-ill patients through decolonization, chlorhexidine bathing and nasal mupirocin were not effective in preventing S. aureus infections in children with cancer or undergoing HSCT [111]. Further research is needed in this area given the high morbidity from S. aureus in immunocompromised individuals.
Key Points:
S. aureus is a common and challenging cause of infection in children, ranging in severity from asymptomatic colonization, to skin and soft tissue infection, to bacteremia, osteomyelitis, necrotizing pneumonia, and endocarditis.
S. aureus is the leading cause of skin and soft tissue infections, for which systemic antibiotics should be administered in addition to drainage to optimize cure and prevent recurrence.
Management of S. aureus infection entails prompt assessment of the source of infection and initiation of appropriate antibiotics based on local antibiotic susceptibility patterns.
S. aureus skin colonization is common, and transmission can occur through close personal contact with colonized contacts/household members, environmental surfaces, and pets.
In outpatient settings, preventive strategies include optimization of hygiene measures and decolonization regimens performed by all household members with a history of SSTI in the prior year.
In hospitals, preventive strategies, including active surveillance and decolonization of both MRSA- and MSSA-colonized critically-ill infants with expected prolonged hospitalizations, can reduce morbidity and mortality.
Synopsis:
S. aureus is a common skin commensal with potential to cause severe infections resulting in significant morbidity and mortality. Up to 30% of individuals are colonized with S. aureus, though infection typically does not occur without skin barrier disruption. Infection management includes promptly addressing the source of infection, including sites of metastatic infection, and initiation of effective antibiotics, which should be selected based upon local antibiotic susceptibility patterns. Given that S. aureus colonization is a risk factor for infection, preventive strategies are aimed at optimizing hygiene measures and decolonization regimens for outpatients and critically-ill children with prolonged hospitalizations.
CLINICAL CARE POINTS.
Optimal management of S. aureus invasive infection includes addressing the source of infection, including drainage of purulent collections and removal of infected CVCs or ventricular shunts.
Antibiotic selection should be based upon local susceptibility patterns as well as consideration of antibiotic penetration into the infected space (e.g., CNS infection).
Optimal route and duration of antibiotics should take into consideration severity of infection, complications, and clinical improvement.
Prolonged S. aureus bacteremia is associated with increased risk of complications such as septic emboli, thrombi, and metastatic foci of infection. Daily blood cultures should be obtained until demonstration of two days of negative blood cultures to document clearance of bacteremia.
A multidisciplinary approach to S. aureus musculoskeletal infections and implementation of institutional clinical practice guidelines results in more efficient and effective care, leading to decreased hospital length of stay and readmission.
Systemic antibiotics in addition to I&D should be administered to optimize cure and to prevent recurrence of S. aureus SSTIs.
For children with recurrent S. aureus SSTIs, prevention should be aimed at improving hygiene measures and implementing decolonization strategies in all household members with a history of SSTI in the prior year. For those with continued recurrence, periodic decolonization for 3 months is recommended.
Disclosure Statement:
This work was supported in part by a grant from the National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (OT2-HD107559) and the Agency for Healthcare Research and Quality (AHRQ, R01-HS024269). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or AHRQ. ICK reports consulting fees from IPEC Experts, LLC. CMK reports clinical trials research funding from Pfizer and Merck. SAF reports clinical trials research funding from Merck.
Contributor Information
Ibukunoluwa C. Kalu, Department of Pediatrics, Division of Pediatric Infectious Diseases, Medical Director, Pediatric Infection Prevention, Duke University Medical Center, Durham, North Carolina, USA.
Carol M. Kao, Department of Pediatrics, Division of Pediatric Infectious Diseases, Washington University School of Medicine, St. Louis, Missouri, USA.
Stephanie A. Fritz, Department of Pediatrics, Division of Pediatric Infectious Diseases, Washington University School of Medicine, St. Louis, Missouri, USA.
REFERENCES
- 1.Lowy FD, Staphylococcus aureus infections. N Engl J Med, 1998. 339(8): p. 520–32. [DOI] [PubMed] [Google Scholar]
- 2.Weiner-Lastinger LM, et al. , Antimicrobial-resistant pathogens associated with pediatric healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect Control Hosp Epidemiol, 2020. 41(1): p. 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akinboyo IC, et al. , Burden of healthcare-associated infections among hospitalized children within community hospitals participating in an infection control network. Infect Control Hosp Epidemiol, 2021: p. 1–3. [DOI] [PubMed]
- 4.Chambers HF and Deleo FR, Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol, 2009. 7(9): p. 629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herold BC, et al. , Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. Jama, 1998. 279(8): p. 593–8. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan SL, et al. , Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis, 2005. 40(12): p. 1785–91. [DOI] [PubMed] [Google Scholar]
- 7.Naimi TS, et al. , Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA, 2003. 290(22): p. 2976–84. [DOI] [PubMed] [Google Scholar]
- 8.Edelsberg J, et al. , Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis, 2009. 15(9): p. 1516–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hersh AL, et al. , National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med, 2008. 168(14): p. 1585–91. [DOI] [PubMed] [Google Scholar]
- 10.Fritz SA, Shapiro DJ, and Hersh AL, National Trends in Incidence of Purulent Skin and Soft Tissue Infections in Patients Presenting to Ambulatory and Emergency Department Settings, 2000–2015. Clin Infect Dis, 2020. 70(12): p. 2715–2718. [DOI] [PubMed] [Google Scholar]
- 11.Miller LG, et al. , Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005–2010. BMC Infect Dis, 2015. 15: p. 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutter DE, et al. , Changing Susceptibility of Staphylococcus aureus in a US Pediatric Population. Pediatrics, 2016. 137(4). [DOI] [PubMed] [Google Scholar]
- 13.Orscheln RC, et al. , Contribution of genetically restricted, methicillin-susceptible strains to the ongoing epidemic of community-acquired Staphylococcus aureus infections. Clin Infect Dis, 2009. 49(4): p. 536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardenas-Comfort C, et al. , Follow-up Blood Cultures in Children With Staphylococcus aureus Bacteremia. Pediatrics, 2020. 146(6). [DOI] [PubMed] [Google Scholar]
- 15.Gerber JS, et al. , Trends in the incidence of methicillin-resistant Staphylococcus aureus infection in children’s hospitals in the United States. Clin Infect Dis, 2009. 49(1): p. 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamdy RF, et al. , Risk Factors for Complications in Children with Staphylococcus aureus Bacteremia. J Pediatr, 2019. 208: p. 214–220 e2. [DOI] [PubMed] [Google Scholar]
- 17.Spaulding AB, et al. , Epidemiology of Bloodstream Infections in Hospitalized Children in the United States, 2009–2016. Clin Infect Dis, 2019. 69(6): p. 995–1002. [DOI] [PubMed] [Google Scholar]
- 18.Iwamoto M, et al. , Trends in invasive methicillin-resistant Staphylococcus aureus infections. Pediatrics, 2013. 132(4): p. e817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMullan BJ, et al. , Epidemiology and mortality of Staphylococcus aureus bacteremia in Australian and New Zealand children. JAMA Pediatr, 2016. 170(10): p. 979–986. [DOI] [PubMed] [Google Scholar]
- 20.Kourtis AP, et al. , Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections - United States. MMWR Morb Mortal Wkly Rep, 2019. 68(9): p. 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fritz SA, et al. , Skin infection in children colonized with community-associated methicillin-resistant Staphylococcus aureus. J Infect, 2009. 59(6): p. 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis MW, et al. , Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis, 2004. 39(7): p. 971–9. [DOI] [PubMed] [Google Scholar]
- 23.Kluytmans J, van Belkum A, and Verbrugh H, Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev, 1997. 10(3): p. 505–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Creech CB 2nd, et al. , Increasing rates of nasal carriage of methicillin-resistant Staphylococcus aureus in healthy children. Pediatr Infect Dis J, 2005. 24(7): p. 617–21. [DOI] [PubMed] [Google Scholar]
- 25.Fritz SA, et al. , Prevalence of and risk factors for community-acquired methicillin-resistant and methicillin-sensitive Staphylococcus aureus colonization in children seen in a practice-based research network. Pediatrics, 2008. 121(6): p. 1090–8. [DOI] [PubMed] [Google Scholar]
- 26.McNeil JC and Fritz SA, Prevention Strategies for Recurrent Community-Associated Staphylococcus aureus Skin and Soft Tissue Infections. Curr Infect Dis Rep, 2019. 21(4): p. 12. [DOI] [PubMed] [Google Scholar]
- 27.Akinboyo IC, et al. , SHEA neonatal intensive care unit (NICU) white paper series: Practical approaches to Staphylococcus aureus disease prevention. Infect Control Hosp Epidemiol, 2020. 41(11): p. 1251–1257. [DOI] [PubMed] [Google Scholar]
- 28.Mergenhagen KA, et al. , Determining the Utility of Methicillin-Resistant Staphylococcus aureus Nares Screening in Antimicrobial Stewardship. Clin Infect Dis, 2020. 71(5): p. 1142–1148. [DOI] [PubMed] [Google Scholar]
- 29.McMullan BJ, et al. , Clinical Management of Staphylococcus aureus Bacteremia in Neonates, Children, and Adolescents. Pediatrics, 2020. 146(3). [DOI] [PubMed] [Google Scholar]
- 30.Ligon J, et al. , Staphylococcus aureus bacteremia without a localizing source in pediatric patients. Pediatr Infect Dis J, 2014. 33(5): p. e132–4. [DOI] [PubMed] [Google Scholar]
- 31.McNeil JC, et al. , Staphylococcus aureus Infections in Children With Congenital Heart Disease. J Pediatric Infect Dis Soc, 2013. 2(4): p. 337–44. [DOI] [PubMed] [Google Scholar]
- 32.Tattevin P, et al. , Concurrent epidemics of skin and soft tissue infection and bloodstream infection due to community-associated methicillin-resistant Staphylococcus aureus. Clin Infect Dis, 2012. 55(6): p. 781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamdy RF, et al. , Epidemiology of Methicillin-Resistant Staphylococcus aureus Bacteremia in Children. Pediatrics, 2017. 139(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu C, et al. , Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis, 2011. 52(3): p. e18–55. [DOI] [PubMed] [Google Scholar]
- 35.Mermel LA, et al. , Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis, 2009. 49(1): p. 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milstone AM, et al. , Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet, 2013. 381(9872): p. 1099–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsu AJ, et al. , Association Between Vancomycin Trough Concentrations and Duration of Methicillin-Resistant Staphylococcus aureus Bacteremia in Children. J Pediatric Infect Dis Soc, 2018. 7(4): p. 338–341. [DOI] [PubMed] [Google Scholar]
- 38.Ten Oever J, et al. , Development of quality indicators for the management of Staphylococcus aureus bacteraemia. J Antimicrob Chemother, 2019. 74(11): p. 3344–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duguid RC, et al. , Impact of Infectious Diseases Consultation on Management and Outcome of Staphylococcus aureus Bacteremia in Children. J Pediatric Infect Dis Soc, 2021. 10(5): p. 569–575. [DOI] [PubMed] [Google Scholar]
- 40.Tong SY, et al. , Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev, 2015. 28(3): p. 603–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fowler VG Jr., et al. , Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA, 2005. 293(24): p. 3012–21. [DOI] [PubMed] [Google Scholar]
- 42.Le Moing V, et al. , Staphylococcus aureus Bloodstream Infection and Endocarditis--A Prospective Cohort Study. PLoS One, 2015. 10(5): p. e0127385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Humpl T, McCrindle BW, and Smallhorn JF, The relative roles of transthoracic compared with transesophageal echocardiography in children with suspected infective endocarditis. J Am Coll Cardiol, 2003. 41(11): p. 2068–71. [DOI] [PubMed] [Google Scholar]
- 44.Baltimore RS, et al. , Infective Endocarditis in Childhood: 2015 Update: A Scientific Statement From the American Heart Association. Circulation, 2015. 132(15): p. 1487–515. [DOI] [PubMed] [Google Scholar]
- 45.Yi J, et al. , Clinical Epidemiology and Outcomes of Pediatric Musculoskeletal Infections. J Pediatr, 2021. 234: p. 236–244 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vij N, et al. , Primary Bacterial Pyomyositis in Children: A Systematic Review. J Pediatr Orthop, 2021. [DOI] [PubMed]
- 47.McNeil JC, Acute Hematogenous Osteomyelitis in Children: Clinical Presentation and Management. Infect Drug Resist, 2020. 13: p. 4459–4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woods CR, et al. , Clinical Practice Guideline by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America: 2021 Guideline on Diagnosis and Management of Acute Hematogenous Osteomyelitis in Pediatrics. J Pediatric Infect Dis Soc, 2021. [DOI] [PubMed]
- 49.McNeil JC, et al. , Role of Operative or Interventional Radiology-Guided Cultures for Osteomyelitis. Pediatrics, 2016. 137(5). [DOI] [PubMed] [Google Scholar]
- 50.Keren R, et al. , Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr, 2015. 169(2): p. 120–8. [DOI] [PubMed] [Google Scholar]
- 51.McNeil JC, et al. , Clinical and Microbiologic Variables Predictive of Orthopedic Complications Following Staphylococcus aureus Acute Hematogenous Osteoarticular Infections in Children. Clin Infect Dis, 2019. 69(11): p. 1955–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonzalez BE, et al. , Venous thrombosis associated with staphylococcal osteomyelitis in children. Pediatrics, 2006. 117(5): p. 1673–9. [DOI] [PubMed] [Google Scholar]
- 53.Alhinai Z, et al. , Prediction of Adverse Outcomes in Pediatric Acute Hematogenous Osteomyelitis. Clin Infect Dis, 2020. 71(9): p. e454–e464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Copley LA, et al. , The impact of evidence-based clinical practice guidelines applied by a multidisciplinary team for the care of children with osteomyelitis. J Bone Joint Surg Am, 2013. 95(8): p. 686–93. [DOI] [PubMed] [Google Scholar]
- 55.Self WH, et al. , Staphylococcus aureus Community-acquired Pneumonia: Prevalence, Clinical Characteristics, and Outcomes. Clin Infect Dis, 2016. 63(3): p. 300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frush JM, et al. , Prevalence of Staphylococcus aureus and Use of Antistaphylococcal Therapy in Children Hospitalized with Pneumonia. J Hosp Med, 2018. 13(12): p. 848–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carrillo-Marquez MA, et al. , Staphylococcus aureus pneumonia in children in the era of community-acquired methicillin-resistance at Texas Children’s Hospital. Pediatr Infect Dis J, 2011. 30(7): p. 545–50. [DOI] [PubMed] [Google Scholar]
- 58.Gillet Y, et al. , Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet, 2002. 359(9308): p. 753–9. [DOI] [PubMed] [Google Scholar]
- 59.Randolph AG, et al. , Critically ill children during the 2009–2010 influenza pandemic in the United States. Pediatrics, 2011. 128(6): p. e1450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jain S, et al. , Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med, 2015. 372(9): p. 835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bradley JS, et al. , The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis, 2011. 53(7): p. e25–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu C, et al. , Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis, 2011. 52(3): p. 285–92. [DOI] [PubMed] [Google Scholar]
- 63.Huang PY, et al. , Spinal epidural abscess in adults caused by Staphylococcus aureus: clinical characteristics and prognostic factors. Clin Neurol Neurosurg, 2012. 114(6): p. 572–6. [DOI] [PubMed] [Google Scholar]
- 64.Shane AL, et al. , Methicillin-resistant and susceptible Staphylococcus aureus bacteremia and meningitis in preterm infants. Pediatrics, 2012. 129(4): p. e914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vallejo JG, et al. , Staphylococcus aureus Central Nervous System Infections in Children. Pediatr Infect Dis J, 2017. 36(10): p. 947–951. [DOI] [PubMed] [Google Scholar]
- 66.Davis DP, et al. , The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med, 2004. 26(3): p. 285–91. [DOI] [PubMed] [Google Scholar]
- 67.Schoenbaum SC, Gardner P, and Shillito J, Infections of cerebrospinal fluid shunts: epidemiology, clinical manifestations, and therapy. J Infect Dis, 1975. 131(5): p. 543–52. [DOI] [PubMed] [Google Scholar]
- 68.Tunkel AR, et al. , 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin Infect Dis, 2017. 64(6): p. e34–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blassmann U, et al. , CSF penetration of vancomycin in critical care patients with proven or suspected ventriculitis: a prospective observational study. J Antimicrob Chemother, 2019. 74(4): p. 991–996. [DOI] [PubMed] [Google Scholar]
- 70.Balouch MA, Bajwa RJ, and Hassoun A, Successful use of ceftaroline for the treatment of MRSA meningitis secondary to an infectious complication of lumbar spine surgery. J Antimicrob Chemother, 2015. 70(2): p. 624–5. [DOI] [PubMed] [Google Scholar]
- 71.Corey A and So TY, Current Clinical Trials on the Use of Ceftaroline in the Pediatric Population. Clin Drug Investig, 2017. 37(7): p. 625–634. [DOI] [PubMed] [Google Scholar]
- 72.Daum RS, et al. , A Placebo-Controlled Trial of Antibiotics for Smaller Skin Abscesses. N Engl J Med, 2017. 376(26): p. 2545–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fritz SA, et al. , Household versus individual approaches to eradication of community-associated Staphylococcus aureus in children: a randomized trial. Clin Infect Dis, 2012. 54(6): p. 743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fritz SA, et al. , Effectiveness of measures to eradicate Staphylococcus aureus carriage in patients with community-associated skin and soft-tissue infections: a randomized trial. Infect Control Hosp Epidemiol, 2011. 32(9): p. 872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaplan SL, et al. , Randomized trial of “bleach baths” plus routine hygienic measures vs. routine hygienic measures alone for prevention of recurrent infections. Clin Infect Dis, 2014. 58(5): p. 679–82. [DOI] [PubMed] [Google Scholar]
- 76.Bocchini CE, et al. , Recurrent community-associated Staphylococcus aureus infections in children presenting to Texas Children’s Hospital in Houston, Texas. Pediatr Infect Dis J, 2013. 32(11): p. 1189–93. [DOI] [PubMed] [Google Scholar]
- 77.Creech CB, Al-Zubeidi DN, and Fritz SA, Prevention of Recurrent Staphylococcal Skin Infections. Infect Dis Clin North Am, 2015. 29(3): p. 429–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hogan PG, et al. , Environmental Methicillin-resistant Staphylococcus aureus Contamination, Persistent Colonization, and Subsequent Skin and Soft Tissue Infection. JAMA Pediatr, 2020. [DOI] [PMC free article] [PubMed]
- 79.Singer AJ and Talan DA, Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med, 2014. 370(11): p. 1039–47. [DOI] [PubMed] [Google Scholar]
- 80.Talan DA, et al. , Subgroup Analysis of Antibiotic Treatment for Skin Abscesses. Ann Emerg Med, 2018. 71(1): p. 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Talan DA, et al. , Trimethoprim-Sulfamethoxazole versus Placebo for Uncomplicated Skin Abscess. N Engl J Med, 2016. 374(9): p. 823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lake JG, Miller LG, and Fritz SA, Antibiotic Duration, but Not Abscess Size, Impacts Clinical Cure of Limited Skin and Soft Tissue Infection After Incision and Drainage. Clin Infect Dis, 2020. 71(3): p. 661–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hogan PG, et al. , Impact of systemic antibiotics on Staphylococcus aureus colonization and recurrent skin infection. Clin Infect Dis, 2017. [DOI] [PMC free article] [PubMed]
- 84.Parrish KL, et al. , Skin and Soft Tissue Infection Treatment and Prevention Practices by Pediatric Infectious Diseases Providers. J Pediatric Infect Dis Soc, 2020. 9(6): p. 760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holmes L, et al. , Trimethoprim-Sulfamethoxazole Therapy Reduces Failure and Recurrence in Methicillin-Resistant Staphylococcus aureus Skin Abscesses after Surgical Drainage. J Pediatr, 2016. 169: p. 128–34 e1. [DOI] [PubMed] [Google Scholar]
- 86.Fritz SA, et al. , Staphylococcus aureus colonization in children with community-associated Staphylococcus aureus skin infections and their household contacts. Arch Pediatr Adolesc Med, 2012. 166(6): p. 551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mork RL, et al. , Comprehensive modeling reveals proximity, seasonality, and hygiene practices as key determinants of MRSA colonization in exposed households. Pediatr Res, 2018. 84(5): p. 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mork RL, et al. , Longitudinal, strain-specific Staphylococcus aureus introduction and transmission events in households of children with community-associated meticillin-resistant S aureus skin and soft tissue infection: a prospective cohort study. Lancet Infect Dis, 2020. 20: p. 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hogan PG, et al. , Interplay of personal, pet, and environmental colonization in households affected by community-associated methicillin-resistant Staphylococcus aureus. J Infect, 2019. 78(3): p. 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Knox J, et al. , Environmental contamination as a risk factor for intra-household Staphylococcus aureus transmission. PLoS ONE, 2012. 7(11): p. e49900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Price JR, et al. , Transmission of Staphylococcus aureus between health-care workers, the environment, and patients in an intensive care unit: a longitudinal cohort study based on whole-genome sequencing. Lancet Infect Dis, 2017. 17(2): p. 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Davis MF, et al. , Household transmission of meticillin-resistant Staphylococcus aureus and other staphylococci. Lancet Infect Dis, 2012. 12(9): p. 703–16. [DOI] [PubMed] [Google Scholar]
- 93.Davis MF, et al. , Genome sequencing reveals strain dynamics of methicillin-resistant Staphylococcus aureus in the same household in the context of clinical disease in a person and a dog. Vet Microbiol, 2015. 180(3–4): p. 304–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morris DO, et al. , Recommendations for approaches to meticillin-resistant staphylococcal infections of small animals: diagnosis, therapeutic considerations and preventative measures.: Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet Dermatol, 2017. 28(3): p. 304–e69. [DOI] [PubMed] [Google Scholar]
- 95.Wertheim HF, et al. , Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet, 2004. 364(9435): p. 703–5. [DOI] [PubMed] [Google Scholar]
- 96.Al-Zubeidi D, et al. , Molecular epidemiology of recurrent cutaneous methicillin-resistant Staphylococcus aureus infections in children. Journal of the Pediatric Infectious Diseases Society, 2014. 3(3): p. 261–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ellis MW, et al. , Hygiene strategies to prevent methicillin-resistant Staphylococcus aureus skin and soft tissue infections: a cluster-randomized controlled trial among high-risk military trainees. Clin Infect Dis, 2014. 58(11): p. 1540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Popoola VO, et al. , Active Surveillance Cultures and Decolonization to Reduce Staphylococcus aureus Infections in the Neonatal Intensive Care Unit. Infect Control Hosp Epidemiol, 2016. 37(4): p. 381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kohler P, et al. , Effect of perioperative mupirocin and antiseptic body wash on infection rate and causative pathogens in patients undergoing cardiac surgery. Am J Infect Control, 2015. 43(7): p. e33–8. [DOI] [PubMed] [Google Scholar]
- 100.Huang SS, et al. , Targeted versus universal decolonization to prevent ICU infection. N Engl J Med, 2013. 368(24): p. 2255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang JT, et al. , Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics, 2009. 123(5): p. e808–14. [DOI] [PubMed] [Google Scholar]
- 102.Hogan PG, et al. , HOME2: Household vs. Personalized Decolonization in Households of Children with Methicillin-Resistant Staphylococcus aureus Skin and Soft Tissue Infection - A Randomized Clinical Trial. Clin Infect Dis, 2020. [DOI] [PMC free article] [PubMed]
- 103.Schuetz CR, et al. , Factors associated with progression to infection in methicillin-resistant Staphylococcus aureus-colonized, critically ill neonates. J Perinatol, 2021. [DOI] [PMC free article] [PubMed]
- 104.Pierce R, et al. , Meticillin-resistant Staphylococcus aureus (MRSA) acquisition risk in an endemic neonatal intensive care unit with an active surveillance culture and decolonization programme. Journal of Hospital Infection, 2017. 95(1): p. 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wisgrill L, et al. , Active Surveillance Cultures and Targeted Decolonization Are Associated with Reduced Methicillin-Susceptible Staphylococcus aureus Infections in VLBW Infants. Neonatology, 2017. 112(3): p. 267–273. [DOI] [PubMed] [Google Scholar]
- 106.Akinboyo IC, et al. , Epidemiology and risk factors for recurrent Staphylococcus aureus colonization following active surveillance and decolonization in the NICU. Infect Control Hosp Epidemiol, 2018. 39(11): p. 1334–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Milstone AM, et al. , Effect of Treating Parents Colonized With Staphylococcus aureus on Transmission to Neonates in the Intensive Care Unit A Randomized Clinical Trial. Jama-Journal of the American Medical Association, 2020. 323(4): p. 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McNeil JC, Staphylococcus aureus - antimicrobial resistance and the immunocompromised child. Infect Drug Resist, 2014. 7: p. 117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McNeil JC, et al. , Staphylococcus aureus infections in pediatric oncology patients: high rates of antimicrobial resistance, antiseptic tolerance and complications. Pediatr Infect Dis J, 2013. 32(2): p. 124–8. [DOI] [PubMed] [Google Scholar]
- 110.McNeil JC, et al. , Staphylococcus aureus infections among children receiving a solid organ transplant: clinical features, epidemiology, and antimicrobial susceptibility. Transpl Infect Dis, 2015. 17(1): p. 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zerr DM, et al. , Chlorhexidine gluconate bathing in children with cancer or those undergoing hematopoietic stem cell transplantation: A double-blinded randomized controlled trial from the Children’s Oncology Group. Cancer, 2020. 127(1): p. 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fowler VG Jr., et al. , Exebacase for patients with Staphylococcus aureus bloodstream infection and endocarditis. J Clin Invest, 2020. 130(7): p. 3750–3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Clegg J, et al. , Staphylococcus aureus Vaccine Research and Development: The Past, Present and Future, Including Novel Therapeutic Strategies. Front Immunol, 2021. 12: p. 705360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gluck U and Gebbers JO, Ingested probiotics reduce nasal colonization with pathogenic bacteria (Staphylococcus aureus, Streptococcus pneumoniae, and beta-hemolytic streptococci). Am J Clin Nutr, 2003. 77(2): p. 517–20. [DOI] [PubMed] [Google Scholar]
- 115.Nakatsuji T, et al. , Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med, 2021. 27(4): p. 700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Petrovic Fabijan A, et al. , Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat Microbiol, 2020. 5(3): p. 465–472. [DOI] [PubMed] [Google Scholar]


