Abstract
The ability to sustain attention is a critical cognitive domain that emerges in infancy and is predictive of a multitude of cognitive processes. Here, we used a heart rate (HR) defined measure of sustained attention to assess corresponding changes in frontal electroencephalography (EEG) power at 3 months of age. Second, we examined how the neural underpinnings of HR-defined sustained attention were associated with sustained attention engagement. Third, we evaluated if neural or behavioral sustained attention measures at 3-months predicted subsequent recognition memory scores at 9 months of age. Seventy-five infants were included at 3 months of age and provided usable attention and EEG data and 25 infants returned to the lab at 9 months and provided usable recognition memory data. The current study focuses on oscillatory power in the theta (4–6 Hz) frequency band during phases of HR-defined sustained attention and inattention phases. Results revealed that theta power was significantly higher during phases of sustained attention. Second, higher theta power during sustained attention was positively associated with proportion of time in sustained attention. Third, longitudinal analyses indicated a significant positive association between theta power during sustained attention on 9-month visual paired comparison scores such that higher theta power predicted higher visual paired comparison scores at 9-months. These results highlight the interrelation of the attention and arousal systems which have longitudinal implications for subsequent recognition memory processes.
Keywords: Infant Attention, EEG, Development, Recognition Memory
1. Introduction
The ability to sustain attention is a critical cognitive domain that emerges in infancy and is predictive of a multitude of cognitive outcomes across child development (Brandes-Aitken et al., 2019; Johansson et al., 2015, Pérez-Edgar et al., 2010). The majority of existing research looking at predictors and longitudinal associations of infant sustained attention have relied on behavioral methodology. However, the use of psychophysiology and neuroimaging may increase our ability to predict individual differences in cognitive outcomes, longitudinally (Farah, 2018). This article has three research questions. First, does a heart-rate (HR) defined measure of sustained attention to correspond to changes in neural oscillations at 3 months of age. Second, how do neural measures of sustained attention correlate with engagement in HR-defined sustained attention. Third, do individual differences in sustained attention and neurophysiology at 3 months predict recognition memory outcomes at 9 months of age.
Attention is a multidimensional cognitive domain, composed of both exogenous (reflexive) and endogenous (non-reflexive) cognitive mechanisms. Sustained attention is a critical manifestation of endogenous attention referring to the ability to focus attention on a given stimulus for a prolonged period in the presence of distractors. Sustained attention shares considerable neurobiological substrates with the general physiological arousal system (Richards, 2011). In general, the autonomic nervous system (ANS) is known to modulate fronto-cortical brain regions that support sustained attention processes (Arnsten, 1998, 2009; Arnsten & Li, 2005). This relation is cortically-mediated via connectivity between the brainstem, thalamus, and cholinergic inputs with the frontal cortex (Reynolds et al., 2013, Reynolds et al., 2013; Sarter et al., 2001). Thus, activation of the ANS triggers downstream effects on an infant’s state of arousal which can lead to optimal or suboptimal ranges for attention and deeper processing to occur.
The interplay of arousal and attentional systems is reflected in distinct and measurable phases of attention (Reynolds & Romano, 2016; Richards, 2011). In particular, a reliable psychophysiological method for measuring sustained attention is characterizing heart-rate decelerations (Richards and Casey, 1991). Richards (1989) found that during periods of heart rate deceleration, 2-month-old infants take twice as long to shift their visual attention away from a central stimulus to a peripheral stimulus, indicating increased focus and decreased distractibility. Integrating observable measures with physiological ones provides a more objective window into periods of active engagement (i.e., versus only measuring looking behavior, which may not reflect engagement) and an added level of specificity about the type of attention that is being indexed. This close relation between heart rate and looking behaviors further emphasizes the physiological underpinnings of sustained attention development. By 3 months of age, associations between HR deceleration and sustained attention become evident, as infants’ anterior attention system and cardiac regulation systems begin to mature and co-modulate one another (Reynolds & Richards, 2008). At 3 months of age, periods of sustained attention are more likely driven by maturing alerting systems (i.e., arousal) than executive systems (i.e., task-driven, top-down, and volitional), because executive skills do not emerge before the end of the first year (Colombo, 2002; Ruff & Rothbart, 2001). Looking at cortical mechanisms in conjunction with ANS mechanisms supporting arousal-attention associations may elucidate information not otherwise evident from measuring behavior alone early in infancy.
Electroencephalography (EEG) is a popular neuroimaging method for characterizing direct neural activity underlying cognitive processes particularly within infancy. EEG measures neural synchronization by indexing oscillatory power in distinct frequency bands that are linked with specific cognitive processes. With regards to sustained attention, theta synchronization (4–7 Hz; Orekhova et al., 1999), alpha desynchronization (6–9 Hz; Ward, 2003) are most commonly examined. Xie and Richards (2017) examined relations between infant sustained attention, indexed through HR deceleration, and EEG oscillations. Using a cross-sectional sample of infants at ages 6, 8, 10 and 12 months, the authors found that 10- and 12-month-old infants’ demonstrated theta synchronization and alpha desynchronization during sustained attention episodes relative to inattention episodes. These findings built off of past work from Richards and Reynolds linking heart-rate defined sustained attention with event related EEG components (Reynolds, Courage, & Richards, 2011; Richards, 2003). This is an important study because it was the first to demonstrate developmental differences in neural oscillatory power associated with heart-rate defined phases of sustained attention. Further research is needed to connect the brain to observable attention behavior.
Characterizing the biological basis of sustained attention early in life is critical as infant attention is found to be predictive of a range of cognitive outcomes (Brandes-Aitken et al., 2019, 2020; Johansson et al., 2015). The development of attention is one of the most central and pervasive processes active during infancy (Brandes-Aitken et al., 2019; Diamond, 2009). Attention cascades across development into a multitude of more complex developmental domains including executive functions (Johansson et al., 2015), emotion regulation (Brandes-Aitken et al., 2021; Pérez-Edgar et al., 2010), memory, language, and social communication (Mundy & Jarrold, 2010; Salley et al., 2016). Existing studies looking at the hierarchical nature of sustained attention have studied behavioral measures of observed sustained attention starting around 10 months (Frick et al., 2018; Johansson et al., 2015; Johansson et al., 2016). However, it is possible that the neural architecture supporting emerging sustained attention in the first few months of life infancy may set the foundation for higher-order cognitive abilities longitudinally.
1.1. The current study
In the current study, we investigated patterns of HR-defined sustained attention and neural activity at 3 months of age and longitudinal associations with subsequent recognition memory abilities at 9 months of age. The current study has three research questions:
Do HR-defined measures of sustained attention characterize differentiated neural oscillations at 3-months of age? We hypothesized that neural activity would be higher during phases of HR-defined sustained attention.
Do HR-defined neural oscillations correspond with engagement in HR-defined sustained attention? We hypothesized that neural activity would be associated with greater sustained attention engagement.
Do HR-defined measures of sustained attention and corresponding neural activity at 3-months predict longitudinal recognition memory outcomes at 9-months of age? We hypothesized that greater neural activity during HR-defined sustained attention at 3-months would be associated with higher recognition memory scores at 9-months.
2. Methods
2.1. Participants
The initial sample included 100 infants (63 males; age M = 3.46 months, SD = 0.38) recruited from community events, family services, health care providers, and flyers posted at local businesses around New York City. The final sample only included infants who provided usable EEG data (N = 75; See protocol for attrition related data). Participants were excluded from participating in the present study based on birth before 36 weeks’ gestation, multiple births, or presence of developmental disorders. Families were invited to participate in the study when infants were 3 months of age. See Table 1 for participant demographics on the analytic sample. The present study was conducted according to guidelines laid down in the Declaration of Helsinki, with written informed consent obtained from a parent or guardian for each child before any assessment or data collection. All research procedures were approved by the [MASKED FOR BLINDING] IRB.
Table 1.
Participant demographics.
| Mean (SD) or N (%) | |
|---|---|
| Infant Age at Visit 1 (months) | 3.46 (0.38) |
| Infant Age at Visit 2 (months) | 9.59 (0.51) |
| Gestational age (weeks) | 39.16 (1.24) |
| Income-to-Needs at Visit 1 | 5.60 (5.36) |
| Maternal Education (years) | 15.62 (3.81) |
| Infant sex (male) | 47 (61 %) |
| Ethnicity | |
| Hispanic/Latino | 40 (53 %) |
| Not Hispanic/Latino | 33 (44 %) |
| Unreported | 2 (3 %) |
| Race | |
| Two or More/Other | 29 (39 %) |
| White | 26 (35 %) |
| Black/African-American | 12 (16 %) |
| Asian | 5 (7 %) |
| Unreported | 3 (4 %) |
2.2. Protocol
Infants and their caregivers visited the lab at 3 (Age M = 3.48, SD = 0.39) and 9 months (Age M = 9.48, SD = 0.53). At the 3-month time point infant EEG and ECG was recorded during an attention task and at rest, and responses to socio-demographic questionnaires were collected. At the 9-month time point, a visual recognition memory task was administered, and infant looking data was recorded. Out of the initial sample (N = 100), 7 infants did not provide EEG data due to behavioral issues, 8 EEG files were lost due to data acquisition technical problems, 7 EEG files were unusable due to excessive artifacts, and 3 did not provide sufficient sustained attention EEG data (See Section 2.3.4). Thus, the analytic sample included 75 infants at 3 months. At the 9-month time point, a subset of 25 infants returned to the lab and provided usable recognition memory data. Attrition in the study sample is primarily attributed to the onset of the COVID-19 pandemic. Testing for the current study began in March 2018 and was halted in March 2020. To assess for attrition-related bias, we ran t-tests to assess for differences in covariates between each time point. The 3-month infant sample that returned at 9 months did not significantly differ from the 3-month sample that did not return in terms of income-to-needs, maternal education, or EEG power (t≤1.1, p≥.23).
2.3. Materials and measures
2.3.1. Family and household characteristics
Families were given questionnaires to obtain demographic information including maternal and infant age, race, and ethnicity. Caregivers also reported on their highest level of education attained and annual household income. Family income-to-needs ratio (ITN) is the total household income divided by the federal poverty line for the corresponding number of adults and children in the home and used as the measure of socioeconomic status within the analyses.
2.3.2. HR-defined sustained attention measure
This study uses the same stimuli and procedure as Xie et al. (2017) to measure sustained attention. Participants sat on their caregivers’ lap while they were presented with a dynamic Sesame Street video on a large computer monitor. A camera in front of the infant recorded the infants’ faces, while a camera behind the participants recorded the stimulus. The 4-minute video consisted of several characters from Sesame Street, such as ‘Elmo’ and ‘Big Bird,’ that moved from side to side, disappeared, sang, and danced. These videos have been repeatedly demonstrated to elicit periods of sustained attention in young infants (Xie et al., 2017). Visual attention to stimuli was manually coded retroactively with the Net Station 5.1 software. Heart rate data was edited and processed using the software QRSTool to remove artifacts and identify heart beats. The inter-beat intervals (IBIs, or the time in milliseconds between heart beats) were used to evaluate heart rate with longer IBIs indicating slower heart rates, and vice versa. Periods of infant sustained attention were categorized based on infant looking and heart rate deceleration. The criteria for categorizing periods of infant sustained attention required infant visual fixation to the stimulus paired with decreased heart rate (Richards, 2011). Specifically, HR-defined sustained attention phases began when the infant was looking at the screen and the median of five consecutive IBI values was lower than the median of the five IBIs preceding a look onset. HR-defined sustained attention phases ended (and inattention phases began) when the median of five consecutive IBI values were higher than the median of the five IBIs preceding a look onset. All phases of attention occur during looks to the experimental stimuli. To characterize sustained attention engagement, we calculated proportion of time in HR-defined sustained attention phases (seconds in sustained attention/total seconds of visual looking (Tonnsen et al., 2018; Xie & Richards, 2016). We also calculate look-defined sustained attention and inattention phases (based on infant looking or not looking to the computer stimuli) to evaluate value added by incorporating HR deceleration into our characterization of sustained attention.
2.3.3. Recognition memory
At 9 months, infant recognition memory was measured using a visual paired comparison task (Morgan & Hayne, 2006). In this computerized task, infants are seated on the caregiver’s laps in front of a monitor. In this task the computer monitor is a split screen with one stimulus appearing on the left half and one appearing on the right half. First, to capture the infant’s attention, two identical spinning balls appeared on both halves of the screen for 13 s. Next, in the familiarization block, each half of the screen displays an identical blue mailbox shaped face for 10 s. In the first novelty preference block, one side of the screen continued to show a blue mailbox face while the other half was replaced by a circular yellow face for 10 s (See Fig. 1). In the second novelty preference block, the yellow face was replaced by the blue mailbox face, and the other half of the screen presented a squared red face. Research assistants coded this task by reviewing videos frame-by-frame to establish total looking time for each phase. At every 200 ms, the coder determined if the infant was looking to the left half, right half, or neither. From these codes, the ratio of novel looking time relative to total looking time was calculated. Infants with novel looking proportions less than 30 % were flagged as outlier (+/− 3 SD from the mean) and excluded from further analysis (N = 1).
Fig. 1.

VPC Stimuli. Visual Paired Comparison Stimuli. The top panel depicts the familiarization block and the bottom panel depicts the first novelty preference block.
2.3.4. EEG data acquisition and processing
EEG data during the visual attention task and during resting data were acquired while the infants were seated on their caregivers’ laps. The recording room was dimly lit and an experimenter was nearby to soothe the infant with bubbles or a toy if the infant became too fussy. EEG was recorded using a 64-channel HydroCel Geodesic Sensory Net and amplifier (Electrical Geodesic, Inc., Eugene, OR). Electrode impedances were kept below 100 KΩ and the sampling rate was recorded at 1000 Hz.
All EEG files were processed in the Batch EEG Automated Processing Platform (BEAPP) software to ensure standardization in data processing and cleaning across all files (Levin et al. 2018). Continuous resting EEG files were converted from NetStation format to Matlab (2018b) format. Data preprocessing was carried out using the Harvard Automated Processing Pipeline for EEG (HAPPE), an automated preprocessing pipeline designed for infant EEG data (Gabard-Durnam et al. 2018). First, a 1 Hz high-pass and 100 Hz low-pass filter was applied to each EEG dataset. Second, the data, which was originally sampled at 1000 Hz was resampled with interpolation to 250 Hz, following guidelines for further HAPPE processing. The third step involved artifact removal and included CleanLine’s multitaper approach to removing 60 Hz electrical noise, bad channel rejection, and wavelet-enhanced ICA for artifact rejection with automated component rejection through the Multiple Artifact Rejection Algorithm (Winkler et al. 2011) in EEGLAB. A subset of spatially distributed electrodes was selected for analysis with MARA: 2 3 5 6 8 9 10 11 12 13 14 18 20 24 25 28 30 31 34 35 39 40 42 44 48 50 52 57 58 59 60 (NetStation Geodesic 64- Channel Net). Bad channels that were initially rejected were repopulated using spherical interpolation to reduce bias in re-referencing and the signal was mean detrended. Finally, each EEG file was segmented into 1-second windows for power decomposition.
EEG power decomposition was accomplished using Matlab’s fast Fourier transformation using hanning windowing to decompose into power for 1-second segments for each channel. The current study specifically focuses on oscillatory power in the theta (4–6 Hz) and alpha (6–9 Hz) frequency ranges. Segments exceeding 3 SD + /− micro-volts2 from the median were excluded from further analysis (Cuevas et al., 2014). Summed power within each frequency band was averaged across all segments and normalized by a log base 10 transformation. Summed power was then averaged across all channels of interest in the frontal region (electrode #: 2, 3, 5, 6, 9, 10, 11, 12, 13, 14, 57, 59, 60) during the HR-defined phases of sustained attention and inattention phases (See Fig. 2). To ensure there were no differences between brain activity between hemispheres, paired t-test revealed that there were no differences in alpha or theta activity between the right and left hemisphere alpha and theta activity during sustained attention phases and therefore were averaged across hemispheres. Infants with less than 20 s of usable HR-defined sustained attention EEG data were excluded (N = 3). Sustained attention neural activity was calculated by subtracting neural activity during sustained attention phases from neural activity during inattention phases to generate a change score.
Fig. 2.
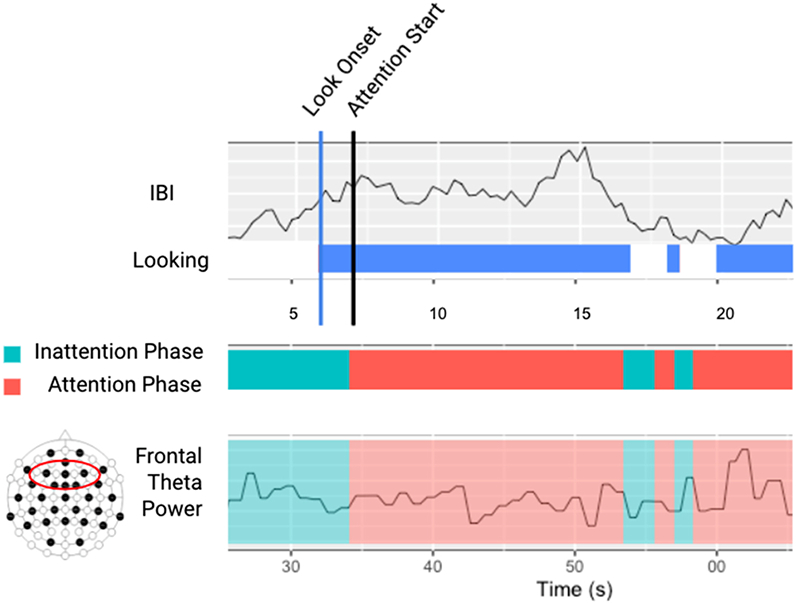
Schematic of the analytic strategy for characterizing phases of inattention and sustained attention.
2.4. Analysis plan
The analytic plan was broken down into four components. First, preliminary analyses included a series of paired t-tests to evaluate significant differences in infant heart rate (HR) and EEG power between HR-defined sustained attention and inattention phases in the hypothesized direction. These differences were compared to sustained attention defined by behavioral looking alone. Next, multiple linear regressions were used to test how sustained attention neural activity corresponded with proportion of time in sustained attention and explore how neural activity predicted subsequent recognition memory outcomes at 9 months. Infant age, gestational age at birth, and ITN were included as covariates in regression models. Gestational age and ITN did not have significant effects on any outcomes and were not included in subsequent models. Full information maximum likelihood (FIML) was used to account for missing data in all analyses, as FIML produces unbiased parameter estimates. All models were run in the R environment.
3. Results
3.1. Preliminary analyses
Table 2 presents descriptive statistics of relevant variables for sustained attention and inattention phases and Table 3 presents correlations between sustained attention neural and behavioral variables with potential covariates of interest.
Table 2.
Descriptives of key attention variables.
| Mean (SD) | Median | Min-Max | |
|---|---|---|---|
| IBI during HR-Defined SA | 440.04 (37.18) | 438.04 | 367.64–600.51 |
| IBI during HR-Defined IA | 419.31 (38.40) | 413.64 | 349.18–624.78 |
| Theta during HR-Defined SA (log10; 4–6 Hz) | −0.46 (0.22) | −0.43 | −0.99–0.03 |
| Theta during HR-Defined IA (log10; 4–6 Hz) | −0.48 (0.22) | −0.47 | −1.05- – 0.01 |
| Alpha during HR-Defined SA (log10; 4–6 Hz) | −0.54 (0.20) | −0.52 | −1.08- – 0.06 |
| Alpha during HR-Defined IA (log10; 6–9 Hz) | −0.54 (0.20) | −0.51 | −1.08 - – 0.09 |
| Proportion of Time in SA | 0.40 (0.20) | 0.19 | 0.11–1.00 |
Note. SA, Sustained attention; IA, Inattention
Table 3.
Correlation table of key attention variables and covariates.
| SA Theta (inattention-attention) | SA Alpha (inattention-attention) | Proportion of time in SA | Age | Gestation | |
|---|---|---|---|---|---|
| Theta (IA-SA) | |||||
| Alpha (IA-SA) | 0.14 | ||||
| Proportion of time in SA | 0.33** | 0.09 | |||
| Age | −0.28* | −0.19 | −0.30 * | ||
| Gestation | 0.02 | 0.09 | 0.05 | −0.19 | |
| Income-to-Needs | 0.00 | 0.15 | 0.06 | −0.22 | −0.01 |
| Computed correlation used pearson-method with pairwise-deletion. | |||||
Note. SA, Sustained attention; IA, Inattention
3.2. Research question 1: Does HR-defined inattention and sustained attention differentiate neural activity at 3-months?
To ensure that our measure of HR-defined sustained measure clearly delineated a HR decrease, we constructed paired t-tests to measure differences in IBI between sustained attention and inattention. Results indicated a significant difference (t(74)= 9.65, p < .001) such that IBI was higher (i.e., lower heart-rate) during sustained attention compared to inattention (Fig. 3).
Fig. 3.

Violin plots of IBI during attention phases. Violin plot representing the means and distribution of infant inter-beat interval (IBI) during HR-defined sustained attention (SA) and HR-defined inattention (inattention) phases.
Next, we ran paired t-tests to examine the extent to which theta and alpha power differed from HR-defined inattention to sustained attention. Paired t-tests revealed theta power was significantly higher during HR-defined phases of sustained attention (t(74)= 4.65, p < .001), but there were no differences in alpha power (t(74)= 0.84, p = .40; Fig. 4). Interestingly, when we looked for differences in theta and alpha power during behavioral looking-defined sustained attention and inattention (i.e., looking at the stimuli or not looking at the stimuli), paired t-tests did not show any significant differences in theta (t(74) = 1.46, p = .15) or alpha power (t(74) = −0.70, p = .48). For this reason, all subsequent analyses examine theta activity during HR-defined phases of sustained attention.
Fig. 4.

Topoplots of Theta and alpha power in sustained attention. Topoplots of change in theta and alpha from HR-defined inattention (inattention) to HR-defined sustained attention (SA). Values were derived by subtracted log10 absolute values of HR-defined sustained attention from HR-defined inattention.
3.3. Research question 2: how HR-defined sustained attention neural activity correspond with engagement in HR-defined sustained attention?
Next, we were interested in assessing associations between HR-defined sustained attention theta change score and proportion of time in sustained attention. Regressions revealed a significant positive association between HR-defined sustained attention theta and proportion of time in sustained attention (B = 0.33, p = .003; Fig. 5).
Fig. 5.

Topoplots of theta power for infants with high vs. low attention engagement. Topoplot of change in theta from HR-defined inattention (inattention) to HR-defined sustained attention (SA) for infants with high proportion of time in sustained attention compared to infants with low proportion of time in sustained attention. High and low average proportion of time in sustained attention were designated using a median split.
3.4. Research question 3: Does 3-month neural activity during hr-defined sustained attention predict 9-month recognition memory outcomes?
Finally, the longitudinal effect of 3-month HR-defined sustained attention variables on 9-month recognition memory was assessed. We regressed visual paired comparison (VPC) scores at 9 months on infant age on sustained attention theta change score, proportion of time spent in sustained attention, and infant age at 9-months. Results indicated a significant positive association between sustained attention theta change score at 3 months and 9-month VPC scores (B = 0.48, p = .02) such that sustained attention theta power at 3 months predicted higher VPC scores at 9 months (See Fig. 6). Proportion of time spent in sustained attention or age at 9-months was not significantly associated with VPC scores at 9 months.
Fig. 6.

Scatterplot of 3-month HR-defined sustained attention theta power change score on 9-month visual paired comparison (VPC) scores.
4. Discussion
The present study characterized the neural correlates of a HR-defined sustained attention measure at 3 months of age and investigated longitudinal relations with recognition memory abilities at 9 months of age in a sociodemographically diverse sample. A primary goal of this study was understanding whether a HR-defined measure of sustained attention could differentiate neural activity as early as 3-months of age. Secondarily, we aimed to explore neural activity correlated with sustained attention engagement (proportion of time in sustained attention). Our final goal was to examine how 3 month neural and behavioral HR-defined sustained attention measures predict 9-month recognition memory. To the best of our knowledge, this is the first study to investigate individual differences in HR-defined sustained attention at 3-months of age and explore longitudinal associations with 9-month cognitive outcomes.
We were first interested in examining the extent to which the HR-defined sustained attention measure categorized infant heart rate and neural oscillations into inattention and sustained attention phases. Results indicated that HR-defined sustained attention significantly differentiated heart-rate between inattention phases and sustained attention phases. The decrease in heart rate which co-occurs with sustained attention indexes the onset and presence of psychophysiological arousal to promote processing of stimulus information (Richards & Casey, 1991). Indeed, prior research has suggested that measures of the parasympathetic nervous system can improve our ability to sensitively measure sustained attention at an early age (Conte & Richards, 2021; Berg & Richards, 1997; Reynolds et al., 2013, Reynolds et al., 2013; Richards, 1995, Richards & Casey, 1992; Richards & Hunter, 1998). Our neural findings support this idea, given that only HR-defined but not looking-defined sustained attention differentiated EEG power. These findings align with prior research demonstrating that heart rate deceleration is a more sensitive measure of sustained attention over behavioral looking alone (Brez & Colombo, 2012; Tonnsen et al., 2018). This suggests that using physiological indices in tandem with behavioral looking may aid our measurement and understanding of sustained attention in infancy when these processes are still developing and are difficult to measure.
Specifically, we found that HR-defined measures of sustained attention differentiated frontal EEG synchronization in theta but not alpha oscillations. This finding parallels the work of Xie et al. (2018), but extends their results by using a larger, younger, and more diverse sample. While Xie and colleagues, showed HR-defined sustained attention theta synchronization patterns by 10 months, we demonstrated a similar result at 3 months of age. Similarly, our null effect of alpha is consistent with studies noting an earlier emergence of theta synchronization relative to more delayed onset of alpha (Marshall et al., 2002; Xie et al., 2018). While research suggests that endogenous sustained attention becomes well established around the first year of life, our results point to neural indicators of a maturing anterior attention system supporting the subsequent development of attentional control. Task-related theta oscillations are reflective of goal-oriented or active attention (Begus & Bonawitz, 2020; Orekhova et al., 1999). Theta waves are thought to originate from cortical-hippocampal feedback loops, which facilitate information encoding. In relation to our findings, frontal theta activity partitioned out during sustained attention is likely linked to a developing goal-motivated, endogenous sustained attention system (Cohen et al., 2012).
Our main sustained attention variables of interest in the current analysis were proportion of time in HR-defined sustained attention (a measure of sustained attention engagement) and sustained attention theta change score (a measure of neural activity during sustained attention). HR-defined sustained attention phases index when deep information processing is occurring during visual attention (Richards & Casey, 1991). Proportion of sustained attention episodes reflects the amount of time an infant spent in a HR-defined sustained attention relative to inattention. The proportion of time in sustained attention could reflect the magnitude of information processing occurring (Courage et al., 2006). On the other hand, neural activity during HR-defined sustained attention episodes tells us more directly about the underlying mechanisms supporting active and goal-oriented information processing.
Several theories have been suggested about the functional role of EEG power during sustained attention. It is hypothesized that investigating EEG power during sustained attention phases may represent neural efficiency underlying the cognitive processes involved with sustaining attention (Kulke et al., 2016; Richards et al. 2010). Theoretical accounts of attention development predict that increased neural activation may reflect more efficient cognitive processing (Richards et al. 2010) while new skills are developing. Specifically, increased neural activity in infancy may reflect increased efficiency to compensate for under-developed myelinated axons (Deoni et al., 2012). In relation to the current analysis, assessing EEG power during sustained attention offers unique insights into the underlying neural process of sustained attention above behavior alone.
To connect the brain back to behavior, we examined how theta during HR-defined sustained attention coupled with proportion of time in HR-defined sustained attention. We found that increased theta power was associated with greater proportion of time in sustained attention. Prior studies have found that HR-defined sustained attention is characterized by prolonged visual engagement and increased theta synchronization (Reynolds & Romano, 2016; Xie et al., 2018), thus it is expected for the proportion of time in sustained attention phases and theta power to be correlated. Collectively, the neural and engagement measures of sustained attention offer unique information about underlying cognitive processes by which infants deploy attention.
To evaluate the predictive ability of sustained attention at 3 months to recognition memory at 9 months, we investigated longitudinal associations between HR-defined sustained attention at 3 months and recognition memory at 9 months. We found that there was a positive association between theta power during HR-defined sustained attention and subsequent recognition memory at 9 months of age, such that higher theta power was associated with increased looking at a novel stimulus during the recognition memory task. Recognition memory performance requires more complex executive attention as infants must preferentially distribute selective attention towards novel stimuli for information processing. As such, these findings indicate that early patterns of theta activity during sustained attention may have predictive value as a biomarker for later higher order recognition memory abilities. Given that theta power has been linked to active learning in infancy, the early organization of task-based theta synchronization may set the foundation for longitudinal information processing phenotypes to emerge (Begus & Bonawitz, 2020; Orekhova et al., 1999).
These findings are broadly aligned with emerging research that have found that early indices of infant brain function measured via EEG predict subsequent cognitive outcomes later in development (Brito et al., 2016; Cainelli et al., 2021; Gou et al., 2011; Tarullo et al., 2017; Wilkinson et al., 2020). Interestingly, proportion of time in sustained attention did not predict subsequent VPC performance, despite prior research documenting that sustained attention in infancy is predictive of subsequent higher order cognition, longitudinally (Colombo & Mitchell, 1990). In addition, much of the prior research connecting behavioral infant attention to later cognition have studied infants at 6 months or older. As such, the current findings point to the utility of an early infant neural biomarker for predicting trajectories of cognitive development beginning in the first few months of life.
4.1. Limitations
While this study expands our knowledge of infant attention in multiple ways, our findings should be interpreted with consideration to its limitations. Although a strength of the current study is the internal validity offered from a lab-based measurement of infant sustained attention, this measure has limited ecological validity. Attention is a difficult construct to measure especially early in infancy, thus a secondary, perhaps more naturalistic, measure of sustained attention would increase our construct validity. In addition, although our sample size at 3 months is larger than many previous studies looking at attention in infancy, our sample size suffered from attrition at the 9-month visit resulting in a smaller subsequent sample size. Replication of this study with a larger longitudinal sample is necessary before more conclusive interpretations can be drawn. Finally, causal inferences of sustained attention to subsequent cognitive outcomes cannot be concluded given the correlational nature of this study. It is possible that unobserved confounding variables, such as infant temperament, could be contributing to the longitudinal association of EEG power during sustained attention at 3 months and outcomes at 9 months (Rothbart et al., 2006). Future research should aim to include a repeated measures component of attention and EEG power across infancy to allow for within-person longitudinal inferences. Intraindividual associations help negate some issues related to bias introduced from confounding between-person variables.
5. Conclusion
In this study we examined the psychophysiological basis and hierarchical nature of sustained attention at 3-months in infancy. We used a measure that couples visual attention with shifts in general psychophysiological arousal to characterize the multidimensionality of sustained attention and measure corresponding change in underlying neural activity. Results demonstrated that early variation in oscillatory power during sustained attention at 3-months predicted subsequent recognition memory at 9-months. Altogether, this study highlights the importance of characterizing the multifaceted nature of attention and underlying neural function at an early age to better understand individual differences in trajectories of cognitive development.
Acknowledgements
This work was funded by National Institute of Health R00HD086255 to Natalie H. Brito.
Footnotes
CrediT authorship contribution statement
Annie Brandes-Aitken: Conceptualization, Methodology, Writing, Software, Formal analysis Maya Metser: Writing, Investigation, Data curation Stephen Braren: Data curation, Investigation, Methodology, Writing- Review & Editing Sarah Vogel: Investigation, Methodology, Writing- Review & Editing Natalie Brito: Conceptualization, Supervision, Project administration, Funding acquisition.
Conflict of interest
The authors report no conflicts of interest.
Data availability
Data will be made available on request.
References
- Arnsten AF (1998). Catecholamine modulation of prefrontal cortical cognitive function. Trends in Cognitive Sciences, 2(11), 436–447. [DOI] [PubMed] [Google Scholar]
- Arnsten AF (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature reviews neuroscience, 10(6), 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, & Li B-M (2005). Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biological Psychiatry, 57(11), 1377–1384. [DOI] [PubMed] [Google Scholar]
- Begus K, & Bonawitz E (2020). The rhythm of learning: theta oscillations as an index of active learning in infancy. Developmental Cognitive Neuroscience, 45, Article 100810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg WK, & Richards JE (1997). Attention across time in infant development. Attention and Orienting: Sensory and Motivational Processes, 347–368. [Google Scholar]
- Brandes-Aitken A, Braren S, Swingler M, Voegtline K, & Blair C (2019). Sustained attention in infancy: A foundation for the development of multiple aspects of self-regulation for children in poverty. Journal of Experimental Child Psychology, 184, 192–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes-Aitken A, Braren S, Gandhi J, Perry RE, Rowe-Harriott S, & Blair C (2020). Joint attention partially mediates the longitudinal relation between attuned caregiving and executive functions for low-income children. Developmental Psychology. 10.1037/dev0001089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes-Aitken A, Braren S, Vogel SC, Perry RE, Brito NH, & Blair C (2021). Within-person changes in basal cortisol and caregiving modulate executive attention across infancy. Development and Psychopathology, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brez CC, & Colombo J (2012). Your eyes say “no,” but your heart says “yes”: Behavioral and psychophysiological indices in infant quantitative processing. Infancy: The Official Journal of the International Society on Infant Studies, 17(4), 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito NH, Fifer WP, Myers MM, Elliott AJ, & Noble KG (2016). Associations among family socioeconomic status, EEG power at birth, and cognitive skills during infancy. Developmental Cognitive Neuroscience, 19, 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cainelli E, Vedovelli L, Wigley ILCM, Bisiacchi PS, & Suppiej A (2021). Neonatal spectral EEG is prognostic of cognitive abilities at school age in premature infants without overt brain damage. European Journal of Pediatrics, 180(3), 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Bour L, Mantione M, Figee M, Vink M, Tijssen MAJ, van Rootselaar A-F, van den Munckhof P, Schuurman PR, & Denys D (2012). Top-down-directed synchrony from medial frontal cortex to nucleus accumbens during reward anticipation. Human Brain Mapping, 33(1), 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo J (2002). Infant attention grows up: The emergence of a developmental cognitive neuroscience perspective early theory. Current Directions in Psychological Science, 11(6), 196–200. [Google Scholar]
- Conte S, & Richards J (2021). Attention in early development. Oxford Research Encyclopedia of Psychology. 10.1093/acrefore/9780190236557.013.52 [DOI] [Google Scholar]
- Courage ML, Reynolds GD, & Richards JE (2006). Infants’ attention to patterned stimuli: developmental change from 3 to 12 months of age. Child Development, 77(3), 680–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, Cannon EN, Yoo K, & Fox NA (2014). The infant EEG mu rhythm: methodological considerations and best practices. Developmental Review, 34(1), 26–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SCL, Dean DC 3rd, O’Muircheartaigh J, Dirks H, & Jerskey BA (2012). Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. NeuroImage, 63(3), 1038–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A (2009). The interplay of biology and the environment broadly defined. Developmental Psychology, 45(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ (2018). Socioeconomic status and the brain: Prospects for neuroscience-informed policy. Nature Reviews Neuroscience, 1. [DOI] [PubMed] [Google Scholar]
- Frick MA, Forslund T, Fransson M, Johansson M, Bohlin G, & Brocki KC (2018). The role of sustained attention, maternal sensitivity, and infant temperament in the development of early self-regulation. British Journal of Psychology, 109(2), 277–298. [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Mendez Leal AS, Wilkinson CL, & Levin AR (2018). The harvard automated processing pipeline for electroencephalography (HAPPE): Standardized processing software for developmental and high-artifact data. Frontiers in Neuroscience, 12, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou Z, Choudhury N, & Benasich AA (2011). Resting frontal gamma power at 16, 24 and 36 months predicts individual differences in language and cognition at 4 and 5 years. Behavioural Brain Research, 220(2), 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M, Marciszko C, Brocki K, & Bohlin G (2016). Individual differences in early executive functions: A longitudinal study from 12 to 36 months. Infant and Child Development, 25(6), 533–549. [Google Scholar]
- Johansson M, Marciszko C, Gredebäck G, Nyström P, & Bohlin G (2015). Sustained attention in infancy as a longitudinal predictor of self-regulatory functions. Infant Behavior & Development, 41, 1–11. [DOI] [PubMed] [Google Scholar]
- Kulke LV, Atkinson J, & Braddick O (2016). Neural differences between covert and overt attention studied using EEG with simultaneous remote eye tracking. Frontiers in Human Neuroscience, 10, 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin AR, Méndez Leal AS, Gabard-Durnam LJ, & O’Leary HM (2018). BEAPP: The batch electroencephalography automated processing platform. Frontiers in Neuroscience, 12, 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, & Fox NA (2002). Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 113(8), 1199–1208. [DOI] [PubMed] [Google Scholar]
- Morgan K, & Hayne H (2006). The effect of encoding time on retention by infants and young children. Infant Behavior and Development, 29(4), 599–602. [DOI] [PubMed] [Google Scholar]
- Mundy P, & Jarrold W (2010). Infant joint attention, neural networks and social cognition. Neural Networks: The Official Journal of the International Neural Network Society, 23(8–9), 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, & Posikera IN (1999). theta synchronization during sustained anticipatory attention in infants over the second half of the first year of life. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 32(2), 151–172. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, McDermott JNM, Korelitz K, Degnan KA, Curby TW, Pine DS, & Fox NA (2010). Patterns of sustained attention in infancy shape the developmental trajectory of social behavior from toddlerhood through adolescence. Developmental Psychology, 46(6), 1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, & Richards JE (2008). Infant heart rate: A developmental psychophysiological perspective. In Schmidt LA (Ed.), Developmental psychophysiology: Theory, systems, and methods (pp. 173–212). Cambridge University Press; (xxii, pp). [Google Scholar]
- Reynolds GD, & Romano AC (2016). The development of attention systems and working memory in infancy. Frontiers in Systems Neuroscience, 10, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Courage ML, & Richards JE , (2013). The Development of Attention. In Oxford Handbooks; Online. 10.1093/oxfordhb/9780195376746.013.0063. [DOI] [Google Scholar]
- Reynolds GD, Zhang D, & Guy MW (2013). Infant attention to dynamic audiovisual stimuli: Look duration from 3 to 9 months of age. Infancy: The Official Journal of the International Society on Infant Studies, 18(4), 554–577. [Google Scholar]
- Richards JE (1989). Sustained visual attention in 8-week-old infants. Infant Behavior & Development, 12(4), 425–436. [Google Scholar]
- Richards JE (1995). Infant Cognitive Psychophysiology. In Advances in clinical child psychology (pp. 77–107). Boston, MA.Chicago: Springer. [Google Scholar]
- Richards JE (2003). Attention affects the recognition of briefly presented visual stimuli in infants: An ERP study. Developmental Science, 6(3), 312–328.Chicago. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE, & Casey BJ (1991). Heart rate variability during attention phases in young infants. Psychophysiology, 28(1), 43–53. [DOI] [PubMed] [Google Scholar]
- Richards JE, & Hunter SK (1998). Attention and eye movement in young infants: Neural control and development. In Cognitive neuroscience of attention (pp. 141–172). Psychology Press. [Google Scholar]
- 2010 version 9.4.0 (R2018b). (2010). Natick, Massachusetts: The MathWorks Inc. [Google Scholar]
- Richards JE, Reynolds GD, & Courage ML (2010). The neural bases of infant attention. Current Directions in Psychological Science, 19(1), 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE (2011). Infant attention, arousal, and the brain. In Infant perception and cognition (pp. 27–50). Oxford University Press, New York, NY, USA. 10.1093/acprof:oso/9780195366709.003.0002. [DOI] [Google Scholar]
- Richards JE, & Casey BJ (1992). Development of sustained visual attention in the human infant. Attention and information processing in infants and adults: Perspectives from human and animal research, 30–60.Chicago. [Google Scholar]
- Ruff HA, & Rothbart MK (2001). Increasing independence in the control of attention. Attention in Early Development: Themes and Variations, 2001, 1–34. [Google Scholar]
- Salley B, Sheinkopf SJ, Neal-Beevers AR, Tenenbaum EJ, Miller-Loncar CL, Tronick E, Lagasse LL, Shankaran S, Bada H, Bauer C, Whitaker T, Hammond J, & Lester BM (2016). Infants’ early visual attention and social engagement as developmental precursors to joint attention. Developmental Psychology, 52(11), 1721–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Givens B, & Bruno JP (2001). The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Research Reviews, 35(2), 146–160. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Obradović J, Keehn B, Rasheed MA, Siyal S, Nelson CA, & Yousafzai AK (2017). Gamma power in rural Pakistani children: Links to executive function and verbal ability. Developmental Cognitive Neuroscience, 26, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnsen BL, Richards JE, & Roberts JE (2018). Heart rate-defined sustained attention in infants at risk for autism. Journal of Neurodevelopmental Disorders, 10 (1), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward LM (2003). Synchronous neural oscillations and cognitive processes. Trends in Cognitive Sciences, 7(12), 553–559. [DOI] [PubMed] [Google Scholar]
- Wilkinson CL, Gabard-Durnam LJ, Kapur K, Tager-Flusberg H, Levin AR, & Nelson CA (2020). Use of longitudinal EEG measures in estimating language development in infants with and without familial risk for autism spectrum disorder. Neurobiology of Language, 1(1), 33–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler I, Haufe S, & Tangermann M (2011). Automatic classification of artifactual ICA-components for artifact removal in EEG signals. Behavioral and Brain Functions, 7, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, & Richards JE (2016). Effects of interstimulus intervals on behavioral, heart rate, and event-related potential indices of infant engagement and sustained attention. Psychophysiology, 53(8), 1128–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Mallin BM, & Richards JE (2017). Development of infant sustained attention and its relation to EEG oscillations: An EEG and cortical source analysis study. Developmental Science, October, 2016, Article e12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Mallin BM, & Richards JE (2018). Development of infant sustained attention and its relation to EEG oscillations: an EEG and cortical source analysis study. Developmental science, 21(3), Article e12562.Chicago. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


