Abstract
OBJECTIVES
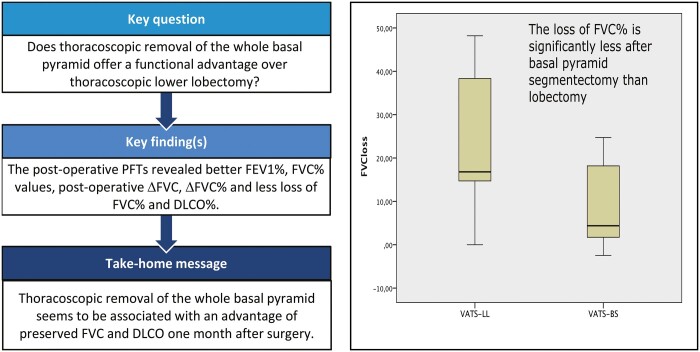
The functional impact of thoracoscopic basal segmentectomy in comparison with lower lobectomy has not been investigated in-depth and the aim of this study was to clarify this topic.
METHODS
We retrospectively analysed a cohort of patients who underwent surgery between 2015 and 2019 for non-small-cell lung cancer, peripherally located lung nodules, far enough from both the apical segment and the lobar hilum to allow an oncologically safe thoracoscopic lower lobectomy or basal segmentectomy. Pulmonary function tests (PFTs) including spirometry and plethysmography were performed 1 month after surgery and forced expiratory volume in 1 s, forced vital capacity (FVC) and diffusing capacity for carbon monoxide (DLCO) were collected; the difference, the loss and the recovery rate of pulmonary function were calculated and compared with the Wilcoxon–Mann–Whitney test.
RESULTS
During the study period, n = 45 and n = 16 patients for video-assisted thoracoscopic surgery (VATS) lower lobectomy and for VATS basal segmentectomy, respectively, completed the study protocol: the 2 groups were homogeneous as to preoperative variables and PFT values. Postoperative outcomes were similar and PFTs revealed significant differences between postoperative forced expiratory volume in 1 s %, FVC%, ΔFVC and ΔFVC%. The loss percentage of FVC%, DLCO% and the recovery rate was better for FVC and DLCO in the VATS basal segmentectomy group.
CONCLUSIONS
Thoracoscopic basal segmentectomy seems to be associated with a more preserved lung function, maintaining more FVC and DLCO levels than lower lobectomy, and could be performed in selected cases ensuring also adequate oncological margins.
Keywords: Thoracoscopic basal segmentectomy, Functional assessment, Spirometry, Pulmonary function tests
Lobectomy and systematic lymph node dissection is the standard treatment for early-stage non-small-cell lung cancer (NSCLC) according to the results of a prospective randomized multi-institutional trial conducted by the Lung Cancer Study Group in 1995 [1] which showed that sublobar resection has a significative increased risk of recurrence compared to lobectomy.
INTRODUCTION
Lobectomy and systematic lymph node dissection is the standard treatment for early-stage non-small-cell lung cancer (NSCLC) according to the results of a prospective randomized multi-institutional trial conducted by the Lung Cancer Study Group in 1995 [1] which showed that sublobar resection has a significative increased risk of recurrence compared to lobectomy. From that moment, pulmonary sublobar resection (both wedge and segmentectomy) had a controversial role in the treatment of early-stage NSCLC and segmentectomy has been applied only in patients not able to tolerate lobectomy. On the other hand, some recent retrospective experiences demonstrated that segmentectomy for small (<2 cm) stage IA NSCLC had an overall survival and disease-free survival comparable to lobectomy [2–5]. Two prospective randomized controlled trials (JCOG0802/WJOG4607L and CALBG/Alliance 140503) were conducted to outline the role of segmentectomy for small and peripheral NSCLC and early postoperative results reported that segmentectomy is not inferior to lobectomy [6, 7]. Recently, long-term results of the JCOG0802/WJOG4607L study have been published showing that segmentectomy was not inferior in terms of oncological outcomes, such as overall survival and disease-free survival [8]. One of the secondary end points of the JCOG0802/WJOG4607L study was the assessment of the lung function after 6 and 12 months, and the authors demonstrated a significative advantage in preserving lung function for segmentectomy, although the difference of forced expiratory volume in 1 s (FEV1) was lesser than expected (−13.1% vs −10.4 at 6 months and −12% vs −8.5% at 1 year). Unfortunately, this study did not directly compare the loss of pulmonary function between lobectomy and the corresponding segmentectomy and the removal of the whole basal pyramid was not included as a procedure in the study, so the functional changes after this procedure are lacking. To our knowledge, the functional impact of basal segmentectomy is not well explored and all the body of literature about the functional changes after segmentectomy or lobectomy mainly consisted of studies on patients treated through thoracotomy. Thus, the functional data of thoracoscopic segmentectomy in comparison with lobectomy are weak [9–11].
The objectives of our study were (i) to evaluate the changes in pulmonary function of thoracoscopic lower lobectomies in comparison with thoracoscopic basal segmentectomies after 1 month from surgery and (ii) to compare the postoperative results of the 2 procedures in a well-selected cohort of patients affected by early-stage NSCLC.
MATERIAL AND METHODS
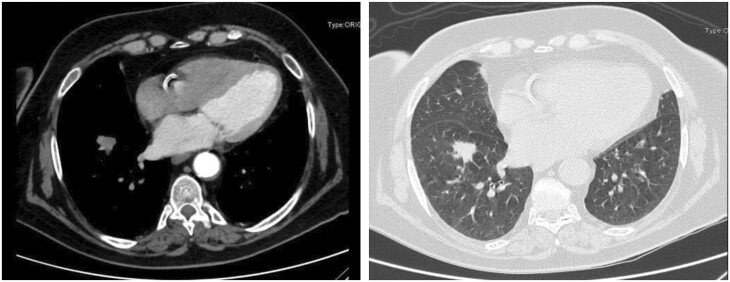
We retrospectively analysed a cohort of patients with suspected or confirmed clinical stage I NSCLC of the basal segments of the lower lobes who underwent surgery at the Careggi University Hospital from 2015 to 2019. The study population consisted of peripherally located lung nodules, with preoperative diagnosis of NSCLC or highly suspicious for that, and located far enough from the superior segment and from the lobar hilum to allow an oncologically safe thoracoscopic basal segmentectomy (VATS-BS) or lower lobectomy (VATS-LL) (Fig. 1). The cohort was homogeneously selected and the choice between these 2 procedures was made by the surgeon preoperatively and in the operating theatre:
Figure 1:
Nodule of 1.6 cm × 2 cm in the basal pyramid of the right lower lobe, far enough from both the apical segment and the lobar hilum ensuring an oncologically safe basal pyramid resection or lower lobectomy.
The preoperative phase included a deep screen of the imaging with three-dimensional computed tomography (CT) scan reconstruction analysing the dimensions of the tumour, the consolidation-to-tumour ratio, the segment/s involved and the evaluation of the distance between the tumour and the superior segment.
The intraoperative phase consisted of a first thoracoscopic exploration (exclusion of pleural implants, visceral pleural involvement, adenopathy/ies, completeness of the fissure); second, the results of the frozen section on the lobar and intersegmental lymph nodes and lastly the adequacy of the parenchymal margins. If the patients did not have pleural implants, visceral pleural involvement, hilar or segmental adenopathies and adequate surgical margins, then he/she underwent to VATS-BS; otherwise, we performed VATS-LL.
Patients affected by NSCLC in locally advanced stage, with lung metastasis from other cancers, benign lesions, patients converted to open procedures or not able to tolerate a lobectomy were excluded from this study.
All patients underwent conventional preoperative examinations, including cardiological assessment and pulmonary function tests (PFTs), contrast enhanced thoracic and abdominal CT scan, brain CT scan and positron emission tomography–CT scan. In case of mediastinal lymph node, CT enlargement or positron emission tomography–CT scan hyperactivity, endobronchial ultrasound transbronchial needle aspiration or mediastinoscopic biopsy was performed before surgery. Clinical and pathological stages were resumed with the American Joint Committee on Cancer 8th Edition TNM Classification [12]. All patients were evaluated by our institutional Multidisciplinary Tumour Board and the individual treatment was decided based on clinical stage, patient performance and the most recent international guidelines [13].
VATS-LL or VATS-BS was performed under general anaesthesia with one-lung ventilation using a standardized Copenhagen three-port approach [14, 15] (utility incision at the fifth intercostal space and 2 ports above the diaphragm for camera and assistant) with individual dissection of the pulmonary artery, bronchus and veins and all the bronchovascular structures were transected with endoscopic staplers or energy devices. In all cases, a systematic lymph node dissection was performed in accordance with the ESTS guidelines [16]. During segmentectomy, segmental lymph node station was dissected and sent for frozen section in every case and if positive, the procedure was converted to lobectomy. Inflation/deflation technique was used to identify the intersegmental plane which was separated with staplers.
After surgery, all the patients were referred to respiratory physiotherapy service from postoperative day 1 to the discharge and encouraged to continue the physiotherapy exercises at home. In this cohort, no patients were readmitted, and the postoperative surveillance included 2 outpatient visits and a chest X-ray in the first month.
PFTs were performed according to American Thoracic Society standards [17] and recorded 2–4 weeks before surgery and 4 weeks after surgery including 3 parameters: forced vital capacity (FVC) in litres and percentage of the predicted value, FEV1 in litres and percentage of the predicted value and diffusing capacity for carbon monoxide (DLCO) percentage of the predicted. Every measurement was done with the same spirometer, plethysmograph and for the measurement of the diffusion capacity for carbon monoxide.
Ethical statement
Our institutional review board granted approval and waived the requirement for specific informed consent for this retrospective analysis.
Statistical analysis
Statistical analysis was performed using SPSS 24.0 (IBM SPSS Statistics for Macintosh, Version 24.0, Armonk, NY, USA). Standard descriptive statistics have been used to summarize data, with respect to demographic and oncological characteristics. Continuous variables, expressed as median and interquartile range, were compared with the Wilcoxon–Mann–Whitney test; categorical variables were analysed using the χ2 test or the Fisher exact test as appropriate. A P-value of below 0.05 was considered as statistically significant.
The difference in the pulmonary function value was calculated as follows: FEV1pre − FEV1post.
The loss of pulmonary function value was calculated as follows: (FEV1pre − FEV1post)/FEV1pre × 100.
The recovery rate of pulmonary function was defined as: FEV1post/FEV1pre.
RESULTS
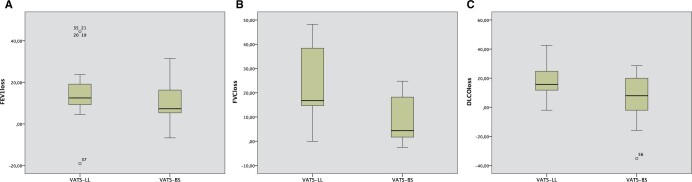
During the study period, n = 45 and n = 16 patients, respectively, for VATS-LL and VATS-BS met the inclusion criteria of this study including the preoperative and postoperative PFTs at our institution (Careggi University Hospital). Demographical, clinical, periprocedural and pathological data are depicted in Table 1. In the VATS-LL group, n = 26 (57.8%) were right lower lobectomies, whereas n = 19 (42.2%) were left lower lobectomies; in the VATS-BS group, we operated on n = 8 patients per side. The 2 groups were homogeneous with respect to age, sex, performance status, chronic obstructive pulmonary disease incidence, smoking history, NSCLC clinical stage, tumour diameter and PFT values. In particular, the inter-group difference between FEV1% values (82.5% vs 96%) was apparently wide, although not significant. The postoperative outcomes reported in Table 2 showed that there are no differences in terms of the hospital length of stay, chest tube duration, complication rate and grade using the Clavien–Dindo classification [18]. Each patient repeated PFTs approximatively after a month (Table 1) revealing a significant difference between FEV1% (72.5% vs 79.5%) and FVC% (86% vs 96.5%) while the difference between the FVC in litres was marginally not significative (2.03 vs 2.61). ΔFVC and the ΔFVC% values (Table 3) were different between the 2 groups and ΔDLCO% had a trend of significance. Moreover, the loss percentage of the pulmonary values was different for FVC% (16.8 vs 4.37) and DLCO% (16.7 vs 7.95). Figure 2 visually reports this difference. The median recovery rate for FVC% and DLCO% was significantly higher in the VATS-BS group, as shown in Table 3. Moreover, the FVC and DLCO recovery rates were significantly better in the VATS-BS group.
Table 1:
Demographical, clinical, periprocedural and pathological data
| Variable | VATS-LL (n = 45) | VATS-BS (n = 16) | P-Value |
|---|---|---|---|
| RLL, n (%) | 26 (57.8) | ||
| LLL, n (%) | 19 (42.2) | ||
| RBS, n (%) | 8 (50) | ||
| LBS, n (%) | 8 (50) | ||
| Sex male, n (%) | 27 (60) | 12 (75) | <0.99 |
| Age, median (IQR) | 68 (11) | 67 (10) | 0.8 |
| ASA score, n (%) | 0.556 | ||
| 1 | 6 (13.3) | 4 (25) | |
| 2 | 23 (51.1) | 7 (43.8) | |
| 3 | 16 (35.6) | 5 (31.3) | |
| PS, n (%) | 0.621 | ||
| 0 | 19 (42.2) | 5 (31.3) | |
| 1 | 19 (42.2) | 7 (43.8) | |
| 2 | 7 (15.6) | 4 (25) | |
| COPD, n (%) | 9 (20) | 4 (25) | 0.675 |
| CCI, median (range) | 1 (0–3) | 2 (0–3) | 0.725 |
| Smoking history, n (%) | 0.44 | ||
| Never smoker | 7 (15.6) | 1 (6.3) | |
| Former smoker | 18 (40) | 9 (56.3) | |
| Actual smoker | 20 (44.4) | 6 (37.5) | |
| NSCLC clinical stage, n (%) | 0.07 | ||
| 1a | 34 (75.5) | 16 (100) | |
| 1b | 11 (24.5) | ||
| Maximun tumour diameter, median (IQR) | 20 (15) | 18 (9) | 0.35 |
| Minimum tumour diameter, median (IQR) | 15 (10) | 13 (5) | 0.48 |
| Preoperative PFTs, n (%) | |||
| FEV1 l | 1.68 (0.73) | 1.825 (0.58) | 0.9 |
| FEV1% | 82.5 (14) | 96 (28.25) | 0.078 |
| FVC l | 2.66 (0.86) | 2.63 (1) | 0.49 |
| FVC% | 99 (28) | 111.5 (18.4) | 0.3 |
| DLCO% | 73.5 (20) | 69.5 (40.75) | 0.3 |
| Postoperative PFTs, n (%) | |||
| FEV1 l | 1.47 (0.37) | 1.51 (0.65) | 0.26 |
| FEV1% | 72.5 (10) | 79.5 (27.5) | 0.016 |
| FVC l | 2.03 (0.84) | 2.61 (1.12) | 0.056 |
| FVC% | 86 (11) | 96.5 (29) | 0.015 |
| DLCO% | 63 (18.5) | 67 (21.5) | 0.22 |
ASA: American Society of Anesthesiologists; CCI: Charlson Comorbidity Index; COPD: chronic obstructive pulmonary disease; DLCO: diffusing capacity for carbon monoxide; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; IQR: interquartile range; LBS: left basal segmentectomy; LLL: left lower lobectomy; NSCLC: non-small-cell lung cancer; PFTs: pulmonary function tests; PS: performance status score; RBS: right basal segmentectomy; RLL: right lower lobectomy; VATS-BS: video-assisted thoracoscopic surgery basal segmentectomy; VATS-LL: video-assisted thoracoscopic surgery lower lobectomy.
Table 2:
Postoperative outcomes, complications and pathology and staging
| Variable | VATS-LL (n = 45) | VATS-BS (n = 16) | P-Value |
|---|---|---|---|
| HS, median (IQR) | 5.5 (5) | 5 (2) | 0.57 |
| Chest tube duration, median (IQR) | 5 (2) | 5 (2) | 0.84 |
| Patients with at least 1 complication, n (%) | 17 (37.8) | 2 (12.5) | 0.11 |
| Complication rate (%) | 40 | 18.75 | 0.12 |
| Clavien–Dindo classification, n (%) | 0.27 | ||
| 2 | 12 (26.7) | 3 (18.75) | |
| 3a | 4 (8.9) | ||
| 3b | 1 (2.2) | ||
| Complications in detail, n (%) | |||
| Atrial fibrillation | 8 (17.7) | 1 (6.3) | |
| Pneumonia | 3 (6.7) | 1 (6.3) | |
| PAL | 4 (8.9) | ||
| Chylothorax | 1 (2.2) | ||
| Bleeding requiring reoperation | 1 (2.2) | ||
| Increased troponin | 1 (2.2) | ||
| Bleeding requiring transfusions | 0 | 1 (6.3) | |
| Pathology, n (%) | 0.75 | ||
| ADC | 29 (65) | 10 (62.5) | |
| SCC | 12 (27) | 4 (25) | |
| NET | 4 (8) | 2 (12.5) | |
| NSCLC pathological stage, n (%) | 0.88 | ||
| 1a | 33 (73.4) | 12 (75) | |
| 1b | 6 (13.4) | 1 (6.25) | |
| 2a | 2 (4.4) | 0 | |
| 2b | 2 (4.4) | 1 (6.25) | |
| 3a | 2 (4.4) | 2 (12.5) | |
ADC: adenocarcinoma; HS: hospital stay; IQR: interquartile range; NET: neuroendocrinal tumour; NSCLC: non-small-cell lung cancer; PAL: prolonged air leak; SCC: squamous cell carcinoma; VATS-BS: video-assisted thoracoscopic surgery basal segmentectomy; VATS-LL: video-assisted thoracoscopic surgery lower lobectomy.
Table 3:
Comparison of the pulmonary functional changes after video-assisted thoracoscopic surgery lower lobectomy and video-assisted thoracoscopic surgery basal segmentectomy 1 month after surgery
| Variable | VATS-LL (n = 45) | VATS-BS (n = 16) | P-Value |
|---|---|---|---|
| ΔFEV1 l diff, n (%) | 0.21 (0.21) | 0.19 (0.19) | 0.23 |
| ΔFEV1% diff, n (%) | 13 (13.5) | 7.2 (10.5) | 0.47 |
| ΔFVC litres diff, n (%) | 0.41 (0.76) | 0.125 (0.37) | <0.01 |
| ΔFVC% diff, n (%) | 24 (41.75) | 5.5 (16.8) | 0.049 |
| ΔDLCO% diff, n (%) | 14 (10) | 6.5 (21) | 0.055 |
| FEV1loss#, n (%) | 12.5 (9) | 7.3 (11.52) | 0.17 |
| FVCloss#, n (%) | 16.8 (28.13) | 4.37 (16.94) | 0.013 |
| DLCOloss#, n (%) | 16.7 (13.78) | 7.95 (22.89) | <0.01 |
| FEV1recovery rate, n (%) | 0.83 (0.14) | 0.90 (0.11) | 0.22 |
| FVC recovery rate, n (%) | 0.77 (0.34) | 0.95 (0.17) | 0.03 |
| DLCO recovery rate, n (%) | 0.84 (0.14) | 0.92 (0.23) | <0.01 |
DLCO: diffusing capacity for carbon monoxide; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; VATS-BS: video-assisted thoracoscopic surgery basal segmentectomy; VATS-LL: video-assisted thoracoscopic surgery lower lobectomy.
Figure 2:
Scatter box plot representative of the postoperative loss of FEV1% (A), FVC% (B) and DLCO% (C) between VATS-LL and VATS-BS. DLCO: diffusing capacity for carbon monoxide; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; VATS-BS: video-assisted thoracoscopic surgery basal segmentectomy; VATS-LL: video-assisted thoracoscopic surgery lower lobectomy.
DISCUSSION
Over the past 2 decades, thoracic surgeons have increasingly discussed whether a sublobar resection, and more specifically an anatomically segmentectomy, is feasible in early-stage NSCLC in terms of both oncological and functional outcomes. The lung cancer study group (LCSG) study demonstrated poor oncological results for sublobar resection (both segmentectomy and wedge), but, however, sublobar resection had a limited impact on pulmonary function and this advantage was predominant in the first 6 months after surgery [1]. From that moment, sublobar resection was indicated only in high-risk patients for comorbid conditions or limited lung function due to its lower oncological adequacy [10], but in the last years, a renewed interest in segmentectomy for small both solid nodules and for GGOs has been demonstrated [4].
Recently, the randomized controlled trial JCOG0802/WJOG4607L [8] showed that segmentectomy is not inferior to lobectomy in OS and recurrence-free survival (RFS) for small (<2 cm) and peripheral NSCLC, thus laying the foundations for considering segmentectomy as the gold standard for this kind of tumours. The 6-month assessment of the postoperative lung function showed an advantage in preserving the function in the segmentectomy group, even if not significative. However, in this study, the authors excluded patients who underwent basal segmentectomy because in these cases less lung parenchyma was preserved. Furthermore, a recently published meta-analysis [19], including only 14 studies (13 retrospective and 1 prospective observational) showed that thoracoscopic segmentectomy preserves more pulmonary function than lobectomy in all the evaluated parameters (FEV1 l, FEV1%, FVC l, FVC%, FEV1/FVC, DCLO%). In light of this lack of evidence about the functional assessment of the basal pyramid removal, we started the collection of pulmonary function values in patients treated with thoracoscopic basal segmentectomy or lower lobectomy. The primary aim of our study was to investigate if the VATS-BS could have a lesser impact on pulmonary function than VATS-LL and we demonstrated that VATS-BS seems to be associated with a more preserved lung function than VATS-LL as early as a month after surgery.
This benefit was particularly evident for FVC and DLCO values and minorly for FEV1, reflecting the different functional pattern of PFT parameters. We observed a change of −4.37% vs −16.8% (P = 0.01) and −7.95% vs 16.7% (P < 0.01) for FVC% and DCLO%, respectively. These changes could be associated with the less portion of lung parenchyma removed during VATS-BS and with a significant functional role of the lower lobes’ apical segment. As supposed by other authors, the FVC change could be determined by the amount of lung tissue resected, whereas the DLCO% change could be associated with the capillary surface available for gas diffusion and then correlated with the amount of parenchyma spared [20, 21]. Conversely, since FEV1 is considered an indicator of airway resistance and ventilation mechanics, it could be less affected by the adoption of the minimally invasive thoracoscopic technique.
Other authors showed that segmental resection offers a better preservation of pulmonary function than lobectomy, with a lesser reduction in FEV1, FVC and DLCO [9] or only in FEV1 and FVC [2, 11]. However, these studies have been published before the introduction of minimally invasive technique and are therefore affected by the strong influence of the thoracotomy on the respiratory mechanics.
In 2015, Kim et al. [22] compared functional outcomes of VATS lobectomy and VATS sublobar resections and demonstrated a greater postoperative pulmonary function in patients who underwent VATS sublobar resections. Unfortunately, this study do not distinguish between segmentectomy and wedge resections.
We chose to perform PFTs 1 month after surgery to exclude every possible bias of reduced compliance at the forced manoeuvres such as pain or other postoperative complications. Furthermore, the data obtained from these PFTs could be very useful to understand the true reduced values after lower lobectomy and basal segmentectomy. Recently, some different ways to investigate the pulmonary function after surgery have been explored [23, 24], but we need to consider that these measurements are completely undirect and originate from models and algorithms that could under- or over-estimate the postoperative lung function.
In our study, we excluded patients with impaired lung function as such a comparison would be entirely biased, despite even if some papers have demonstrated the safety, in terms of mortality and complications, of VATS lobectomy also in patients with impaired lung function [25, 26]. However, these studies did not evaluate the changes in pulmonary function over time nor the probable functional impact on the quality of life. Furthermore, considering that the incidence of metachronous and contralateral lung cancer [27, 28] is higher in previously treated patients, the first parenchymal-sparing surgery could be considered an advantage because the preserved lung function could allow future treatments in a safer manner both by radiotherapy and surgery.
Limitations
Our study has several limitations. First, this is a single institution retrospective analysis on a small cohort of consecutive patients. The dimension of the cohort is directly dependent on the location of the tumour that should be far enough from both the apical segment and the lobar hilum to ensure an oncologically safe resection. Moreover, the execution of PFTs after 1 month from surgery is not the standard of care of our postoperative follow-up and the medical costs of this protocol and the pandemic breakout of COVID-19 had a strong impact on the study population. Oncological and long-term results of this population are not included into the scope of the study and therefore not reported, even if the overall survival and disease-free survival were similar between the 2 groups [29]. The absence of completely objective criteria for selecting the preferred procedure is another limit that could affect the reproducibility of this study or performing a randomized controlled trial on this topic.
The single measurement could be interpreted as another limit, but the progressive recovery of the lung function in the first 3 or 12 months from surgery is well-demonstrated [30] and, therefore, our single value, 1 month after surgery, could be interpreted as a value close to the real pulmonary function after surgery and predictive for complications or mortality.
CONCLUSION
In conclusion, thoracoscopic basal segmentectomy appears to be associated with a more preserved lung function, mostly with a less impact on FVC and DLCO in comparison with lower lobectomy, and could be performed in selected cases since that it is a less technically demanding procedure than individual segmentectomy of the lower lobe and the oncological margins could be wider.
Conflict of interest: none declared.
Glossary
ABBREVIATIONS
- CT
Computed tomography
- DLCO
Diffusing capacity for carbon monoxide
- FEV1
Forced expiratory volume in 1 s
- FVC
Forced vital capacity
- NSCLC
Non-small-cell lung cancer
- PFTs
Pulmonary function tests
- VATS-BS
Video-assisted thoracoscopic surgery basal segmentectomy
- VATS-LL
Video-assisted thoracoscopic surgery lower lobectomy
Contributor Information
Stefano Bongiolatti, Thoracic Surgery Unit, Careggi University Hospital, Florence, Italy.
Alberto Salvicchi, Thoracic Surgery Unit, Careggi University Hospital, Florence, Italy.
Giovanni Mugnaini, Thoracic Surgery Unit, Careggi University Hospital, Florence, Italy.
Eduart Vokrri, Thoracic Surgery Unit, Careggi University Hospital, Florence, Italy.
Domenico Viggiano, Thoracic Surgery Unit, Careggi University Hospital, Florence, Italy.
Alessandro Gonfiotti, Thoracic Surgery Unit, Careggi University Hospital, Florence, Italy; Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy.
Federico Lavorini, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy.
Luca Voltolini, Thoracic Surgery Unit, Careggi University Hospital, Florence, Italy; Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy.
Presented at the 33rd EACTS (European Association for Cardio-Thoracic Surgery) Annual Meeting, Lisbon, Portugal, 3–5 October 2019.
DATA AVAILABILITY
The data underlying this article will be shared on reasonable request to the corresponding authors.
Author contributions
Stefano Bongiolatti: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Validation; Visualization; Writing—original draft; Writing—review & editing. Alberto Salvicchi: Data curation; Investigation. Giovanni Mugnaini: Data curation; Investigation. Eduart Vokrri: Data curation. Domenico Viggiano: Validation. Alessandro Gonfiotti: Resources; Validation; Visualization. Federico Lavorini: Data curation; Resources; Supervision. Luca Voltolini: Methodology; Supervision; Validation; Writing—review & editing.
Reviewer information
Interdisciplinary CardioVascular and Thoracic Surgery thanks Hitoshi Igai and the other anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1. Ginsberg RJ, Rubinstein LV.. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615–23; discussion 622–3. [DOI] [PubMed] [Google Scholar]
- 2. Okada M, Koike T, Higashiyama M, Yamato Y, Kodama K, Tsubota N.. Radical sublobar resection for small-sized non-small cell lung cancer: a multicentre study. J Thorac Cardiovasc Surg 2006;132:769–75. [DOI] [PubMed] [Google Scholar]
- 3. Martin-Ucar AE, Nakas A, Pilling JE, West KJ, Waller DA.. A case-matched study of anatomical segmentectomy versus lobectomy for stage I lung cancer in high-risk patients. Eur J Cardiothorac Surg 2005;27:675–9. [DOI] [PubMed] [Google Scholar]
- 4. Hwang Y, Kang CH, Kim HS, Jeon JH, Park IK, Kim YT.. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy on the patients with non-small cell lung cancer: a propensity score matching study. Eur J Cardiothorac Surg 2015;48:273–8. [DOI] [PubMed] [Google Scholar]
- 5. Winckelmans T, Decaluwé H, De Leyn P, Van Raemdonck D.. Segmentectomy or lobectomy for early-stage non-small-cell lung cancer: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2020;57:1051–60. [DOI] [PubMed] [Google Scholar]
- 6. Suzuki K, Saji H, Aokage K, Watanabe S-I, Okada M, Mizusawa J, Japan Clinical Oncology Group et al Comparison of pulmonary segmentectomy and lobectomy: safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895–907. [DOI] [PubMed] [Google Scholar]
- 7. Altorki NK, Wang X, Wigle D, Gu L, Darling G, Ashrafi AS et al Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med 2018;6:915–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K et al; West Japan Oncology Group and Japan Clinical Oncology Group. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607–17. [DOI] [PubMed] [Google Scholar]
- 9. Keenan RJ, Landreneau RJ, Maley RH Jr, Singh D, Macherey R, Bartley S et al Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228–33; discussion 228–33. [DOI] [PubMed] [Google Scholar]
- 10. Takizawa T, Haga M, Yagi N, Terashima M, Uehara H, Yokoyama A et al Pulmonary function after segmentectomy for small peripheral carcinoma of the lung. J Thorac Cardiovasc Surg 1999;118:536–41. [DOI] [PubMed] [Google Scholar]
- 11. Saito H, Nakagawa T, Ito M, Imai K, Ono T, Minamiya Y.. Pulmonary function after lobectomy versus segmentectomy in patients with stage I non-small cell lung cancer. World J Surg 2014;38:2025–31. [DOI] [PubMed] [Google Scholar]
- 12. Chansky K, Detterbeck FC, Nicholson AG, Rusch VW, Vallières E, Groome P et al; IASLC Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions. The IASLC Lung Cancer Staging Project: external validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2017;12:1109–21. [DOI] [PubMed] [Google Scholar]
- 13. National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 1.2023). http://www.nccn.org/.
- 14. Hansen HJ, Petersen RH, Christensen M.. Video-assisted thoracoscopic surgery (VATS) lobectomy using a standardized anterior approach. Surg Endosc 2011;25:1263–9. [DOI] [PubMed] [Google Scholar]
- 15. Gonfiotti A, Bongiolatti S, Borgianni S, Borrelli R, Jaus MO, Politi L et al Development of a video-assisted thoracoscopic lobectomy program in a single institution: results before and after completion of the learning curve. J Cardiothorac Surg 2016;11:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Leyn P, Dooms C, Kuzdzal J, Lardinois D, Passlick B, Rami-Porta R et al Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787–98. [DOI] [PubMed] [Google Scholar]
- 17. Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 1995;152:1107–36. [DOI] [PubMed] [Google Scholar]
- 18. Dindo D, Demartines N, Clavien PA.. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu Y, Qin Y, Ma D, Liu H.. The impact of segmentectomy versus lobectomy on pulmonary function in patients with non-small-cell lung cancer: a meta-analysis. J Cardiothorac Surg 2022;17:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harada H, Okada M, Sakamoto T, Matsuoka H, Tsubota N.. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005;80:2041–5. [DOI] [PubMed] [Google Scholar]
- 21. Gu Z, Wang H, Mao T, Ji C, Xiang Y, Zhu Y et al Pulmonary function changes after different extent of pulmonary resection under video-assisted thoracic surgery. J Thorac Dis 2018;10:2331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim SJ, Lee YJ, Park JS, Cho YJ, Cho S, Yoon HI et al Changes in pulmonary function in lung cancer patients after video-assisted thoracic surgery. Ann Thorac Surg 2015;99:210–7. [DOI] [PubMed] [Google Scholar]
- 23. Tane S, Nishio W, Nishioka Y, Tanaka H, Ogawa H, Kitamura Y et al Evaluation of the residual lung function after thoracoscopic segmentectomy compared with lobectomy. Ann Thorac Surg 2019;108:1543–50. [DOI] [PubMed] [Google Scholar]
- 24. Bae SY, Lee H, Na KJ, Na B, Park S, Park IK et al Computed tomography volumetric analysis for predicting postoperative lung function for segmentectomy. Interact CardioVasc Thorac Surg 2022;35:ivac195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bongiolatti S, Gonfiotti A, Vokrri E, Borgianni S, Crisci R, Curcio C, Italian VATS Group et al Thoracoscopic lobectomy for non-small-cell lung cancer in patients with impaired pulmonary function: analysis from a national database. Interact CardioVasc Thorac Surg 2020;30:803–11. [DOI] [PubMed] [Google Scholar]
- 26. Ceppa DP, Kosinski AS, Berry MF, Tong BC, Harpole DH, Mitchell JD et al Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bongiolatti S, Corzani R, Borgianni S, Meniconi F, Cipollini F, Gonfiotti A et al Long-term results after surgical treatment of the dominant lung adenocarcinoma associated with ground-glass opacities. J Thorac Dis 2018;10:4838–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Voltolini L, Rapicetta C, Luzzi L, Ghiribelli C, Paladini P, Granato F et al Surgical treatment of synchronous multiple lung cancer located in a different lobe or lung: high survival in node-negative subgroup. Eur J Cardiothorac Surg 2010. ;37:1198–204. [DOI] [PubMed] [Google Scholar]
- 29. Bongiolatti S, Salvicchi A, Indino R, Vokrri E, Gonfiotti A, Borgianni S et al Post-operative and early oncological results of simple and complex full thoracoscopic segmentectomies for non-small-cell lung cancer. Asian Cardiovasc Thorac Ann 2022. doi: 10.1177/02184923221138502. [DOI] [PubMed] [Google Scholar]
- 30. Salati M, Brunelli A, Xiumè F, Monteverde M, Sabbatini A, Tiberi M et al Video-assisted thoracic surgery lobectomy does not offer any functional recovery advantage in comparison to the open approach 3 months after the operation: a case matched analysis. Eur J Cardiothorac Surg 2017;51:1177–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding authors.