Abstract
Objective
To measure the diagnostic performance of contrast-enhanced mammography (CEM) for the index lesion when it is performed the same day prior to biopsy in patients with suspicious findings at US.
Methods
This IRB-approved retrospective study compared radiologist original reports of the presence or absence of index lesion enhancement on CEM to biopsy results and follow-up. The most suspicious lesion or the larger of equally suspicious lesions recommended for biopsy by US after a diagnostic workup including mammography was considered the index lesion. CEM exams were performed the same day, immediately prior to the scheduled biopsy, as requested by the radiologist recommending the biopsy. Numeric variables were summarized with means and standard deviations, or medians and the minimum and maximum, where appropriate.
Results
Biopsy demonstrated cancer in 64.7% (200/309) of index lesions. Of these, 197/200 demonstrated enhancement for a sensitivity of 98.5% (95% CI: 95.7%–99.7%) (197/200) and the negative predictive value of CEM for non-enhancing index lesions was 95.1% (58/61; 95% CI: 86.1%–98.4%). The three false negative exams were two grade 1 ER+ HER2− invasive ductal cancers that were 6 mm and 7 mm in size, and a 3-mm grade 2 ductal carcinoma in situ in a complex cystic and solid mass. False positive exams made up 20.6% (51/248) of the positive exams.
Conclusion
Diagnostic CEM showed high sensitivity and specificity for cancer in lesions with suspicious US findings. CEM may reduce the need for some biopsies, and negative CEM may support a true negative biopsy result.
Keywords: contrast-enhanced, mammography, ultrasound, biopsy, breast cancer
Key Messages.
Contrast-enhanced mammography (CEM) showed enhancement in 98.5% of suspicious index lesions on US with cancer.
Contrast-enhanced mammography showed no enhancement in 53% of suspicious index lesions with benign biopsy results.
The few false negative CEM exams in our study occurred in lesions that were small and of low grade.
Introduction
Multiple studies report contrast-enhanced mammography (CEM) to be similar in performance to MRI in the detection of breast cancer (1–8), and superior to mammography and US (9–13). However, CEM has some clear limitations compared to MRI. Breast cancer detection by any imaging test requires the cancer to be included in the imaged field of view. Any form of mammography, including CEM, has a limitation for including far posterior lesions, unlike MRI (14–16). Additionally, evaluation of the regional lymph nodes not well evaluated by diagnostic mammography and US may be important for treatment planning for breast cancer, and MRI clearly outperforms CEM in this regard, as noted in a recent review by Lewin et al (17). While these are important relative strengths of MRI over CEM, MRI has a very significant limitation in that it requires much more expensive equipment that is not conveniently located within the typical breast imaging suite. Because of this limitation, and despite the clear superiority of MRI in breast cancer detection over mammography and US alone, the diagnostic use of MRI prior to biopsy documentation of cancer has been controversial, except for very narrow indications (18,19).
Utilizing a contrast-enhanced diagnostic breast imaging exam the same day prior to an imaging-guided needle biopsy of the breast is not a familiar concept to most radiologists because of our inability to use contrast-enhanced breast MRI in this way. According to original Food and Drug Administration approval documents, CEM “can be used as an adjunct following mammography and US to localize a known or suspected lesion” (Contrast Enhanced Spectral Mammography, General Electric, 2011; Contrast Enhanced Digital Mammography, Hologic, 2013) (20,21). As such, CEM can be used for diagnostic purposes immediately prior to biopsy, at a radiologist’s discretion.
Contrast-enhanced mammography has a cost and convenience advantage over MRI in that it uses modified mammography equipment that can perform full field digital mammography and tomosynthesis, as well as various forms of mammography-guided biopsies. If diagnostic CEM is done prior to biopsy of a suspicious lesion or lesions detected by mammography and US in a patient who is subsequently diagnosed with cancer at biopsy, any additional lesions detected whose biopsy assessment is judged to be appropriate for patient management can undergo sampling at the time of the first scheduled biopsy visit. In the patient with cancer, performing biopsies of all appropriate lesions for eventual patient management during the first scheduled biopsy visit should expedite the cancer workup and breast disease team consultation process, compared with performing an extent-of-disease workup starting with breast MRI or CEM after the initial pathology result of cancer becomes available. If the accuracy of diagnostic CEM is sufficient to exclude cancer, this may preclude the need for immediate biopsy or, if the biopsy is performed and benign, support a true negative result.
Ghaderi et al noted in a recent review that the value of diagnostic CEM compared to mammography and US remains unclear in the evaluation of something as fundamental as a solitary mass and has only been reported in biopsy-proven breast cancer for extent-of-disease workups (22). Image-guided needle biopsies in a breast imaging center are typically performed with US or mammographic (stereotactic or tomosynthesis) guidance, and US guidance is preferable when a suspicious solitary breast mass and other suspicious findings evaluated at diagnostic mammography and US can be reliably seen with US (23–26).
The purpose of this study was to clarify the diagnostic performance of CEM in the case of suspicious solitary masses and other suspicious lesions found at US prior to biopsy proof of cancer. Specifically, it was to determine how accurately the presence or absence of enhancement predicts the index lesion biopsy and follow-up results.
Methods
This retrospective study was approved by our institutional review board and compliant with the Health Insurance Portability and Accountability Act. Informed consent for the biopsy was obtained from all individual participants included in the study.
Contrast-enhanced mammography exams were performed at two university staffed breast centers and interpreted by board-certified radiologists subspecializing in breast imaging between September 2012 and December 2017. Contrast-enhanced mammography exams were identified using clinical report software to search in our picture archiving and communications system for “contrast-enhanced” within the mammography modality filter. Inclusion criteria consisted of patients with CEM exams performed on the same day and prior to a scheduled US-guided biopsy after a diagnostic mammography and US workup. Included patients had Breast Imaging Reporting and Data System (BI-RADS) assessment category 4 or 5 lesions (27). All patients had follow-up of at least 12 months. Included patients required a completed US-guided core-needle biopsy with histopathology result and medical record follow-up, a cyst aspiration through the biopsy cannula with medical record follow-up, or medical record follow-up only if the biopsy was cancelled due to lesion non-visualization at repeat US. Patients were excluded if they had a CEM exam on a day other than that of the scheduled US-guided needle biopsy, or if they underwent stereotactic or other method of biopsy.
The size of the study was determined by the number of patients in our database meeting inclusion criteria at the time this analysis was performed. Contrast-enhanced mammography exams were advised at the discretion of the radiologist who performed the diagnostic workup and recommended the biopsy. Histopathology and follow-up results were not available prior to the radiologist reporting the CEM exam. Follow-up was by review of the electronic medical record, including follow-up breast imaging. The reasons why CEM exams were not performed in patients who would have otherwise met inclusion criteria were not recorded. Reasons may have included the following: lack of patient consent for this extra test, lack of equipment availability or other scheduling issue, lack of peripheral intravenous access, renal insufficiency, marked comorbidity, local recurrence after bilateral mastectomy, and recent PET-CT for stage 4 breast or other malignancy.
Data Collection
Variables were recorded from the electronic medical record and consisted of patient age, whether the workup started as a screening mammogram or not, lesion size, presence or absence of CEM enhancement, histopathologic diagnosis, and follow-up duration. Lesion size was the longest diameter of either the most suspicious or the larger of equally suspicious lesions, termed the index lesion, taken from the US exam report that had been recorded in the medical record at the time of the diagnostic mammography and US workup. The presence or absence of enhancement of the index lesion on high energy subtracted CEM images was taken from the original CEM radiologist report.
Contrast-enhanced mammography studies were considered positive if the index lesion had enhancement reported by the radiologist on the day of the biopsy on the higher energy subtracted images. Likewise, CEM studies were considered negative if there was no reported contrast enhancement of the index lesion on the higher energy subtracted images of the CEM exam. Data on the radiologist’s rationale for recommending CEM exams on individual patients, including risk factors such as breast density and cancer risk assessment including subcategories of BI-RADS 4 and whether a finding was BI-RADS 4 or 5, were not collected. There was no attempt to quantify enhancement in the index lesion or compare its level of enhancement relative to other foci of background enhancement if they were present or to retrospectively assess the presence or absence of index lesion enhancement. The visibility of the index lesion in question on the CEM low energy images, which duplicate a 2D digital mammogram but do not show whether a lesion enhances, was not used for the purposes of this study in deciding whether CEM was positive or negative.
Malignant histopathology included the Nottingham grade and tumor markers including estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2). If there was a discrepancy between core-needle biopsy results and final surgical pathology, the final surgical pathology result was used; the exception to this was for patients undergoing neoadjuvant therapy prior to surgery.
Equipment and Technique
All CEM studies and diagnostic mammograms were performed on digital mammography equipment modified to perform CEM (either Senographe ES, General Electric, Milwaukee, WI, or Selenia Dimensions, Hologic, Marlborough, MA). US exams were performed with 12, 17, or 18 MHz transducers (IU 22 or Epiq 7G, Philips, Amsterdam, Netherlands). Women were screened for iodinated contrast contraindications using American College of Radiology guidelines (28). Intravenous iodine was administered as Isovue 300 (Bracco, Milan, Italy) at a dose of 1.5 mL⋅kg−1 with a power injector at 3 mL⋅s−1 through a 20-gauge canula in a peripheral vein, followed by a 10-mL saline flush. Dual energy acquisitions in craniocaudal and mediolateral oblique projections were acquired between two and seven minutes following completion of the contrast injection, and processed CEM images were sent to a dedicated mammography workstation for immediate review by the radiologist, with additional views obtained at the radiologist’s request. US-guided spring-loaded core-needle biopsies were performed using spring-loaded 14-gauge biopsy needles with or without access through a 13-gauge cannula (Care Express Products, Cary, IL, USA).
Statistical Analysis
Numeric variables were summarized with means and standard deviations (SDs), or medians and the minimum and maximum, where appropriate. Categorical data were summarized with frequencies and percentages. The negative predictive value (NPV) was calculated for the overall data. Ninety-five percent confidence intervals (95% CIs) for the NPV were calculated using the Wilson score interval with continuity correction, as the normal approximation method may be inappropriate when the prevalence is either very large or small, as was the case in this study (29).
Results
There were 312 patients in our CEM database meeting our inclusion criteria. Two patients were excluded from our total study group because their lesion was clearly outside the field of view of mammography and CEM standard views and so the ability of the radiologist to know whether the lesion enhanced or not was not possible, and one patient was excluded because of CEM equipment technical failure delaying the acquisition of images well beyond the CEM protocol. The remaining 309 patients made up the study set and had a mean (SD) age of 53.3 (12.1) years.
Study Group Diagnoses
US-guided core-needle biopsy was completed for 95.8% (296/309) of the study population completed, with 67.6% (200/296) of these demonstrating histopathology of cancer. The scheduled US-guided core-needle biopsy was cancelled for 0.6% (2/309) of patients in the study after a non-bloody needle cyst aspiration caused disappearance of the suspicious finding. An additional 3.6% (11/309) of patients had their US-guided core-needle biopsy cancelled after their CEM exam when the initially identified suspicious lesion could not be confirmed at the time of the US biopsy.
Cancer was documented in 64.7% (200/309) of the total study group. Malignant histopathology included invasive ductal carcinoma of no special type in 77.0% (154/200), invasive lobular carcinoma in 14.5% (29/200), 0.5% (1/200) each for mucinous, micropapillary, solid papillary, encapsulated papillary, medullary, tubular, and apocrine breast carcinoma, ductal carcinoma in situ in 3.5% (7/200), and lung cancer metastatic to the breast in 0.5% (1/200).
Benign results were recorded in 35.3% (109/309) of patients. Benign lesion histopathology was fibroadenoma in 24.8% (27/109), fibrocystic change in 11.0% (12/109), papilloma in 8.3% (9/109), stromal fibrosis in 8.3% (9/109), sclerosing adenosis in 8.3% (9/109), pseudo-angiomatous stromal hyperplasia in 5.5% (6/109), and other miscellaneous benign histopathologic diagnoses in 22.0% (24/109) of patients. Benign diagnoses came from non-bloody complicated cyst aspiration resulting in resolution of the finding, confirmed by 12 and 23 months of follow-up in 1.8% (2/109) of patients, respectively. Presumed benign diagnoses were made after the needle biopsy was cancelled in 10.1% (11/109) of patients because of non-visualization by US at the time of biopsy, with a mean medical record follow-up of 29 months (range, 12–45 months) failing to disclose any diagnosis of breast cancer.
Of the study group, 51.5% (159/309) had their workup initiated by screening mammography, and 61.6% (98/159) of screening-initiated patients were diagnosed with cancer in this study. Of the 48.5% (150/309) of patients in the study group with a diagnostic indication because of clinical signs or symptoms of a possible breast cancer who were evaluated by diagnostic mammography and US, 68.0% (102/150) were subsequently proven to have cancer.
Primary Invasive Breast Cancer Characteristics
Of the 192 primary breast malignancies, 25.5% (49/192) were Nottingham grade 1, 48.4% (93/192) were Nottingham grade 2, and 26.0% (50/192) were Nottingham grade 3. Regarding size, 37.5% (72/192) of primary breast malignancies were greater than 20 mm and 62.5% (120/192) were 20 mm or less (Table 1). Tumor markers of the primary invasive breast cancers were as follows: ER+ HER2−, 69.8% (134/192); ER+ HER2+, 14.1% (27/192); ER− HER2+, 4.7% (9/192); ER− HER2−, 11.5% (22/192).
Table 1.
Grade, Size, and Tumor Markers of 192 Primary Invasive Breast Cancers in the Study Group*
| Characteristic | Result, n (%) |
|---|---|
| (N = 192) | |
| Grade | |
| 1 | 49 (25.5%) |
| 2 | 93 (48.4%) |
| 3 | 50 (26.0%) |
| Size | |
| <20 mm | 120 (62.0%) |
| ≥20 mm | 72 (38.0%) |
| Receptor status | |
| ER+ HER2− | 134 (69.8%) |
| ER+ HER2+ | 27 (14.1%) |
| ER− HER2+ | 9 (4.7%) |
| ER− HER2− | 22 (11.5%) |
*There were 200 total breast malignancies in the study group: 192 primary invasive breast cancer, 7 ductal carcinoma in situ cases, and 1 lung cancer metastasis.
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor 2.
CEM Performance
A total of 80.2% (248/309) of index lesions enhanced on CEM and made up the CEM positives, and 19.7% (61/309) that did not enhance comprised the CEM negatives (Table 2). Of the CEM positive exams, 79.4% (197/248) were malignant and true positives, and 20.1% (51/248) were benign and false positives. Of the non-enhancing lesions (CEM negative), 95.1% (58/61) were benign and true negatives, and 4.9% (3/61) were malignant and false negatives. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 98.5% (197/200; 95% CI: 95.7%–99.7%), 53.2% (58/109; 95% CI: 43.4%–62.8%), 79.4% (197/248; 95% CI: 76.0%–82.5%), and 95.1% (58/61; 95% CI: 86.1%–98.4%), respectively. The 3.6% (11/309) of patients with negative CEM exams who had their biopsy cancelled when their previously identified suspicious lesion could not be confirmed on the day of biopsy had no subsequent breast cancer diagnosis over the follow-up period.
Table 2.
Diagnostic Contrast-enhanced Mammography (CEM) Performance
| Parameter | CEM Positive | CEM Negative |
|---|---|---|
| (N = 248) | (N = 61) | |
| Malignant | 197 | 3 |
| Benign | 51 | 58 |
| PPV = 79.4% (95% CI: 76.0%–82.5%) | ||
| NPV = 95.1% (95% CI: 86.1%–98.4%) | ||
| Sensitivity = 98.5% (95% CI: 95.7%–99.7%) | ||
| Specificity = 53.2% (95% CI: 43.4%–62.8%) |
Abbreviations: NPV, negative predictive value; PPV, positive predictive value.
The most common enhancing histologically proven benign lesions resulting in CEM false positive results were fibroadenoma, 74.1% (20/27), fibrocystic changes, 33.3% (4/12), papilloma, 55.6% (5/9), stromal fibrosis, 33.3% (3/9), and sclerosing adenosis, 55.6% (5/9). The false negative lesions were 2 grade 1, ER+, HER2− invasive ductal carcinomas not otherwise specified, which were 6 and 7 mm in longest diameter (Figure 1), and a third, mostly cystic, complex cystic and solid lesion that was atypical ductal hyperplasia at US-guided core-needle biopsy that yielded a 3-mm grade 2 ductal carcinoma in situ at surgical excision. There were no false negative results that were either ER− or HER2 + breast cancer.
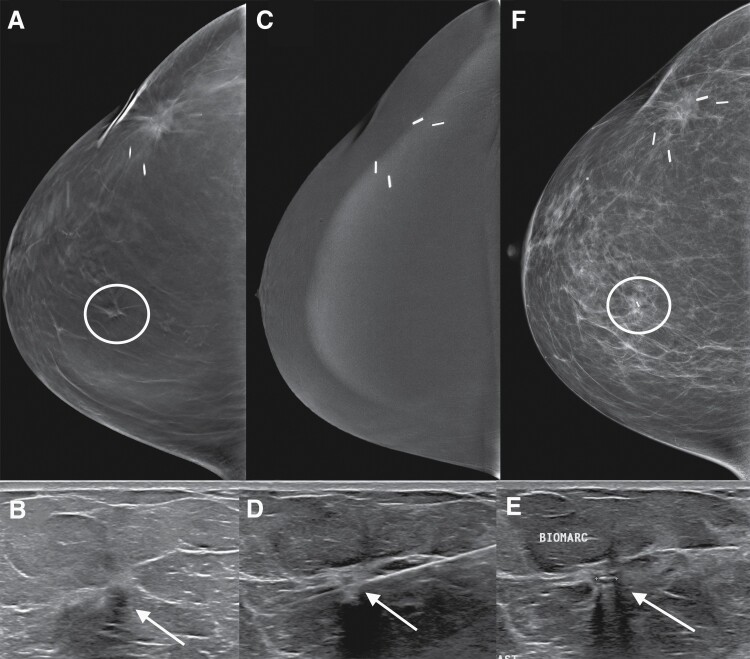
Figure 1.
False-negative contrast-enhanced mammogram (CEM) in a 59-year-old patient who was treated seven years prior for breast cancer with lumpectomy and radiation therapy. Screening mammography tomosynthesis right craniocaudal view (A) shows a subtle mass that had developed at the 12:00 position (circle) for which additional diagnostic imaging was recommended. Diagnostic US (B) confirmed the presence of an appropriately sized and located solid suspicious mass (arrow). Right craniocaudal view from CEM (C) obtained immediately prior to biopsy did not show abnormal enhancement at the site of the mass. US-guided core-needle biopsy (D) of the mass (arrow) was performed. Post-biopsy US (E) and mammogram (F) demonstrates the biopsy marker within the mass (arrow and circle, respectively), which was a 6-mm grade 1 ER+ HER2− infiltrating ductal cancer.
Discussion
In the diagnostic evaluation and clinical management of women with breast lesions identified at breast imaging, a high NPV (the probability of the lesion being benign) based on the imaging alone is crucial to exclude the necessity of histologic diagnosis. According to the current American College of Radiology BI-RADS lexicon, an NPV of less than 98% based on imaging findings should result in a BI-RADS assessment category of either 4 or 5 with a biopsy recommendation, while findings meeting criteria for 98% or greater chance of being benign should be assigned a BI-RADS assessment category of 3 with a recommendation of short-term follow-up (27). The BI-RADS lexicon also states that certain risk-tolerant patients may elect to undergo short-term follow-up instead of biopsy if the predicted NPV is 90% to 98% (27). Our study of patients with suspicious findings at US and no enhancement on CEM had an NPV of 95.1%, which falls into the BI-RADS category 4a range.
Contrast-enhanced mammography demonstrated no enhancement in 1.5% (3/200) of cancers in our study. The two invasive cancers that were missed were grade 1, less than a centimeter, with favorable tumor markers being ER+ and HER2−. There are two other reports we found that measured CEM performance specifically in those with suspicious US findings. Dromain et al reported six false negative CEM studies of 80 malignancies, with the four invasive cancers being grade 1 or 2 (12). The six false negative exams reported by Dromain et al, together with the 37 benign lesions reported to not enhance, leads to an NPV for their study of 86.0% (12). A study by Yuzkan et al compared CEM with mammography and MRI in patients with suspicious findings at US (8). In their study of 41 cancers and 21 benign lesions, all cancers enhanced at CEM and 7 of 21 benign lesions did not enhance, giving an NPV of 100% (8). If larger studies can confirm a high NPV in lesions with suspicious findings at US, especially if false negatives are small and low-grade malignancies, this may reduce the number of biopsies performed. It is important that the radiologist confirms that a lesion in question is not too posterior to know whether it enhances or not on CEM.
Malignancy rates of cancelled MRI biopsies and stereotactic biopsies have been reported. A review by Niell et al in 2013 showed that the biopsy cancellation rate in MRI-guided biopsies due to lesion non-visualization varied from 8% to 13% over eight studies, with four of the seven studies that reported follow-up data demonstrating no subsequent malignancy in the ipsilateral quadrant. However, the three largest studies showed a 2% to 10% malignancy rate (30). Cancellation of stereotactic biopsies because of target non-visualization has also been reported to be associated with the subsequent detection of breast cancer (31,32). We were unable to find similar studies documenting malignancy rates in cancelled US biopsies due to non-visualization at the time of biopsy. In our study, 3.6% (11/309) of patients had a previously identified suspicious lesion at US that could not be identified at the time of scheduled biopsy. None of these patients developed breast cancer in either breast after their negative CEM study and cancelled biopsy over the follow-up period. If confirmed with further studies, a negative CEM exam prior to a scheduled US biopsy in which there is inability to confirm a previously identified suspicious US lesion on the day of the biopsy will add confidence concerning the absence of malignancy, especially higher-grade malignancy.
Infiltrating lobular carcinoma was present in 14.5% (29/200) of malignancies in our study, and none were missed by CEM. These were all initially detected by a diagnostic workup of mammography and with suspicious findings at US. Infiltrating lobular carcinoma (ILC) was present in 14.5% (29/200) of malignancies in our study, and none were missed by CEM. These were all initially detected by a diagnostic workup of mammography and with suspicious findings at US. In a study comparing mammography and CEM, Luczynska et al reported all nine of the 114 malignant lesions that were ILC in their study of 152 patients with 173 lesions were detected by CEM (13). Amato et al reported on 31 patients with ILC who were evaluated for preoperative staging with CEM and found no false negatives (33). These are relatively small numbers of patients, and it remains to be seen if CEM would be accurate when used to identify ILC not visible on US.
A cancer rate of 64.7% (200/309) was present in this study. There is no established benchmark for the PPV of diagnostic CEM for the index lesion when CEM is performed prior to US. Our study did not include patients who underwent US-guided biopsy but had not undergone diagnostic mammography, usually those aged 30 years or less with lesions that would most commonly be subcategorized as BI-RADS 4a. The results of this study were not an analysis of CEM performance in these younger patients. Since 20 of the 27 index lesions that were fibroadenomas enhanced in our study, including this group would likely increase the false positive rate of CEM and lower the cancer rate compared to the rates in our study.
The use of diagnostic CEM prior to biopsy opens future possibilities for research. Correlation of CEM performance based on estimate stratification, such as BI-RADS 4 subcategories, should be considered. The performance of diagnostic CEM prior to biopsy could be correlated with breast density, considering the well-established effect of breast density on cancer detection sensitivity. A trial comparing the detection of additional sites of ipsilateral and contralateral cancer detected by CEM performed prior to biopsy that would lead to treatment failure if not found, to consensus guidelines for breast MRI indications once breast cancer has been detected, may also be useful considering the effect of the lower cost of CEM compared to MRI on any cost-benefit analysis. A time study and patient satisfaction survey that compares diagnostic CEM performed prior to biopsy and MRI performed after cancer detection to evaluate extent of disease would likely be revealing.
Our study has several limitations. The study was retrospective, utilizing the breast radiologist faculty reports from a single academic institution. The retrospective design may have led to selection bias, including which patients with suspicious US findings were recommended to have CEM or not. The study was retrospective, utilizing the breast radiologist faculty reports from a single academic institution. It is an analysis of a specific group of patients who were scheduled for US-guided biopsies after a diagnostic mammography workup and did not include analysis of diagnostic CEM performance prior to stereotactic or other forms of biopsy. Prospective trials would be appropriate before changing specific management recommendations of whether to biopsy a lesion or not based on the use of diagnostic CEM prior to biopsy.
Our study was not a screening study, so the results would not apply to lesion detection at CEM used for screening purposes. In our study, the radiologist interpreted and reported the CEM with full knowledge of the presence, location, and recent mammographic and US appearance of the index lesion, unlike a screening study. There was no attempt to quantify enhancement in the index lesion or compare index lesion level of enhancement relative to other foci of background enhancement, if they were present, which would be necessary for differentiating which areas of enhancement require further evaluation in a CEM screening exam.
This was not a study of CEM performance for any lesion other than the defined index lesion, such as any additional ipsilateral and contralateral findings detected on mammography, US, or CEM. This also was not a study in which stereotactic biopsy was recommended for the index lesion. This study did not analyze information from low energy images, which have been shown to perform like 2D digital mammography (34–36). Low energy CEM images better depict suspicious calcifications than subtracted high energy images that show enhancement (17), and if calcifications are suspicious, the absence of enhancement on CEM should not discourage biopsy according to a recent review (37).
Conclusion
Based on the presence or absence of enhancement in a suspicious index lesion at US, CEM excluded the presence of cancer in 95.1% of index lesions in those without enhancement. Our study also showed that CEM demonstrated enhancement for all cancers 1 cm or larger, grade 2 or 3 invasive cancers, or cancers that were ER− or HER2+. Over half of the lesions with benign results in our study group had no enhancement on CEM. The use of CEM may minimize the use of invasive procedures and may aid in biopsy concordance should additional studies confirm our results.
Contributor Information
Tim Emory, University of Minnesota, Department of Radiology, Minneapolis, MN, USA.
Noelle Hoven, University of Minnesota, Department of Radiology, Minneapolis, MN, USA.
Michael Nelson, University of Minnesota, Department of Radiology, Minneapolis, MN, USA.
An L Church, University of Minnesota, Department of Radiology, Minneapolis, MN, USA.
Nathan Rubin, University of Minnesota, Department of Radiology, Minneapolis, MN, USA.
Jessica Kuehn-Hajder, University of Minnesota, Department of Radiology, Minneapolis, MN, USA.
Funding
NIH P30 CA77598 and UL1-TR002494 for one author (N.R.).
Conflict of Interest Statement
None declared.
References
- 1. Jochelson M, Dershaw D, Sung J, et al. Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 2013;266(3):743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fallenberg E, Dromain C, Diekmann F, et al. Contrast-enhanced spectral mammography versus MRI: initial results in the detection of breast cancer and assessment of tumour size. Eur Radiol 2014;24(1):256–264. [DOI] [PubMed] [Google Scholar]
- 3. Chou C, Lewin J, Chiang C, et al. Clinical evaluation of contrast-enhanced digital mammography and contrast enhanced tomosynthesis—comparison to contrast-enhanced breast MRI. Eur J Radiol 2015;84(12):2501–2508. [DOI] [PubMed] [Google Scholar]
- 4. Luczyńska E, Heinze-Paluchowska S, Hendrick E, et al. Comparison between breast MRI and contrast-enhanced spectral mammography. Med Sci Monit 2015;21(May 12):1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li L, Roth R, Germaine P, et al. Contrast-enhanced spectral mammography (CESM) versus breast magnetic resonance imaging (MRI): a retrospective comparison in 66 breast lesions. Diagn Interv Imaging 2017;98(2):113–123. [DOI] [PubMed] [Google Scholar]
- 6. Fallenberg E, Schmitzberger F, Amer H, et al. Contrast-enhanced spectral mammography vs. mammography and MRI—clinical performance in a multi-reader evaluation. Eur Radiol 2017;27(7):2752–2764. [DOI] [PubMed] [Google Scholar]
- 7. Xing D, Lv Y, Sun B, et al. Diagnostic value of contrast-enhanced spectral mammography in comparison to magnetic resonance imaging in breast lesions. J Comput Assist Tomogr 2019;43(2):245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yüzkan S, Cengiz D, Hekimsoy I.. Diagnostic performance of contrast-enhanced mammography: comparison with MRI and mammography. J Breast Imaging 2021;3(4):448–454. [DOI] [PubMed] [Google Scholar]
- 9. Luczyńska E, Heinze S, Adamczyk A, Rys J, Mitus JW, Hendrick E.. Comparison of the mammography, contrast-enhanced spectral mammography and ultrasonography in a group of 116 patients. Anticancer Res 2016;36(8):4359–4366. [PubMed] [Google Scholar]
- 10. Travieso-Aja M, Maldonado-Saluzzi D, Naranjo-Santana P, et al. Diagnostic performance of contrast-enhanced dual-energy spectral mammography (CESM): a retrospective study involving 644 breast lesions. Radiol Med 2019;124(10):1006–1017. [DOI] [PubMed] [Google Scholar]
- 11. Lalji U, Houben I, Prevos R, et al. Contrast-enhanced spectral mammography in recalls from the Dutch breast cancer screening program: validation of results in a large multireader, multicase study. Eur Radiol 2016;26(12):4371–4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dromain C, Thibault F, Muller S, et al. Dual-energy contrast-enhanced digital mammography: initial clinical results. Eur Radiol 2011;21(3):565–574. [DOI] [PubMed] [Google Scholar]
- 13. Luczyńska E, Heinze-Paluchowska S, Dyczek S, Blecharz P, Rys J, Reinfuss M.. Contrast-enhanced spectral mammography: comparison with conventional mammography and histopathology in 152 women. Korean J Radiol 2014;15(6):689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tardivel AM, Balleyguier C, Dunant A, et al. Added value of contrast-enhanced spectral mammography in postscreening assessment. Breast J 2016;22(5):520–528. [DOI] [PubMed] [Google Scholar]
- 15. Tennant S, James J, Cornford E, et al. Contrast-enhanced spectral mammography improves diagnostic accuracy in the symptomatic setting. Clin Radiol 2016;71(11):1148–1155. [DOI] [PubMed] [Google Scholar]
- 16. Bassett L, Pagani J, Gold R.. Pitfalls in mammography: demonstrating deep lesions. Radiology 1980;136(3):641–645. [DOI] [PubMed] [Google Scholar]
- 17. Lewin J, Patel B, Tanna A.. Contrast-enhanced mammography: a scientific review. J Breast Imaging 2020;2(1):7–15. [DOI] [PubMed] [Google Scholar]
- 18. Kuhl C. Current status of breast MR imaging. Part 2. Clinical applications. Radiology 2007;244(3):672–691. [DOI] [PubMed] [Google Scholar]
- 19. DeMartini W, Lehman C.. A review of current evidence-based clinical applications for breast magnetic resonance imaging. Top Magn Reson Imaging 2008;19(3):143–150. [DOI] [PubMed] [Google Scholar]
- 20. GE Healthcare 510(k) Premarket Notification Submission. Available at: https://www.accessdata.fda.gov/cdrh_docs/pdf10/K103485.pdf. Accessed September 26, 2022.
- 21. Contrast Enhanced Digital Mammography 510(k) Premarket Notification. Available at: https://www.accessdata.fda.gov/cdrh_docs/pdf12/K123873.pdf. Accessed September 26, 2022.
- 22. Ghaderi K, Phillips J, Perry H, et al. Contrast-enhanced mammography: current applications and future directions. Radiographics 2019;39(7):1907–1920. [DOI] [PubMed] [Google Scholar]
- 23. Soo M, Baker J, Rosen E.. Sonographic detection and sonographically guided biopsy of breast microcalcifications. AJR Am J Roentgenol 2003;180(4):941–948. [DOI] [PubMed] [Google Scholar]
- 24. O’Flynn E, Wilson A, Mitchell M.. Image-guided breast biopsy: state-of-the-art. Clin Radiol 2010;65(4):259–270. [DOI] [PubMed] [Google Scholar]
- 25. Helbich T, Matzek W, Fuchsjager M.. Stereotactic and ultrasound-guided breast biopsy. Eur Radiol 2004;14(3):383–393. [DOI] [PubMed] [Google Scholar]
- 26. Liberman L, Feng T, Dershaw D, et al. US-guided core breast biopsy: use and cost-effectiveness. Radiology 1998;208(3):717–723. [DOI] [PubMed] [Google Scholar]
- 27. Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS® Mammography. In: ACR BI-RADS®Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 28. American College of Radiology. ACR Manual on Contrast Media. Available at: https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed September 26, 2022.
- 29. Newcombe R. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998;17(8):857–872. [DOI] [PubMed] [Google Scholar]
- 30. Niell B, Lee J, Johansen C, et al. Patient outcomes in canceled MRI-guided breast biopsies. AJR Am J Roentgenol 2014;202(1):223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brennan S, D’Alessio D, Liberman L, et al. Cancelled stereotactic biopsy of calcifications not seen using the stereotactic technique: do we still need to biopsy? Eur Radiol 2014;24(4):907–912. [DOI] [PubMed] [Google Scholar]
- 32. Philpotts L, Lee C, Horvath L, et al. Canceled stereotactic core-needle biopsy of the breast: analysis of 89 cases. Radiology 1997;205(2):423–428. [DOI] [PubMed] [Google Scholar]
- 33. Amato F, Bicchierai G, Cirone D, et al. Preoperative loco-regional staging of invasive lobular carcinoma with contrast-enhanced digital mammography (CEDM). Radiol Med 2019;124(12):1229–1237. [DOI] [PubMed] [Google Scholar]
- 34. Francescone M, Jochelson M, Dershaw D, et al. Low energy mammogram obtained in contrast-enhanced digital mammography (CEDM) is comparable to routine full-field digital mammography (FFDM). Eur J Radiol 2014;83(8):1350–1355. [DOI] [PubMed] [Google Scholar]
- 35. Lalji U, Jeukens C, Houben I, et al. Evaluation of low-energy contrast-enhanced spectral mammography images by comparing them to full-field digital mammography using EUREF image quality criteria. Eur Radiol 2015;25(10):2813–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Konstantopoulos C, Mehta T, Brook A, et al. Cancer conspicuity on low-energy images of contrast-enhanced mammography compared with 2D mammography. J Breast Imaging 2022;4(1):31–38. [DOI] [PubMed] [Google Scholar]
- 37. Phillips JFZ, Slanetz P.. Pearls and pitfalls of contrast-enhanced mammography. J Breast Imaging 2019;1(1):64–72. [DOI] [PubMed] [Google Scholar]