Abstract
Pseudoangiomatous stromal hyperplasia (PASH) is a benign mesenchymal proliferative lesion of the breast. PASH is postulated to be hormonally induced and predominantly occurs in premenopausal women and postmenopausal women on menopausal hormone therapy. Clinical presentation varies from screen-detected lesions to palpable masses. Imaging findings of PASH are nonspecific. The most common mammographic findings are an oval or round circumscribed non-calcified mass or developing asymmetry. On US, PASH is often seen as an oval hypoechoic mass that may be circumscribed and can have an echogenic rim, or, when manifest as mammographic asymmetry, US may show a corresponding non-mass focal area of echogenic tissue. Limited studies have investigated the MRI appearance, with PASH most often manifesting as non-mass enhancement, or, less often, as an oval or irregular mass with persistent kinetics. Histopathologically, PASH can be mistaken for a fibroadenoma or phyllodes tumor and has features overlapping low-grade angiosarcoma. Assessment of radiologic-pathologic concordance is particularly important as PASH is often an incidental finding, adjacent to the targeted lesion at histopathology. Surgical excision or repeat core-needle biopsy is necessary for discordant suspicious cases. After a benign, concordant diagnosis of PASH, the patient may resume routine screening.
Keywords: radiologic-pathologic correlation, PASH, pseudoangiomatous hyperplasia
Key Messages.
Pseudoangiomatous stromal hyperplasia (PASH) is a benign mesenchymal proliferation of the breast postulated to be hormonally induced. It is typically seen in premenopausal women or postmenopausal women taking hormone supplements.
Imaging findings of PASH are nonspecific. It is most often an incidental finding on screening mammography as an oval or round circumscribed mass or developing asymmetry, a hypoechoic or mixed echogenicity mass on US, and persistent non-mass enhancement on MRI. Pseudoangiomatous stromal hyperplasia can manifest as a palpable mass, including rare cases of rapid growth and bilateral breast involvement.
Pseudoangiomatous stromal hyperplasia is frequently incidental at histopathology and is discordant with suspicious calcifications, architectural distortion, or high suspicion masses.
Introduction
Pseudoangiomatous stromal hyperplasia (PASH) is a benign mesenchymal proliferative lesion of the breast (1). The term “pseudoangiomatous” refers to the slit-like spindle cell–lined spaces that mimic small vessels at histopathology. “Stromal hyperplasia” is recognized when there is expansion of the intralobular stroma, often accompanied by increased cellularity of myofibroblasts, relative to the epithelial component of the breast (2). The clinical presentation of PASH varies from asymptomatic lesions detected on screening to palpable masses (3,4). When symptomatic, it most commonly presents as a palpable unilateral mass (3,4); 33% (8/24) of women in one study had pain or focal tenderness at the site of PASH (1). Additionally, rare cases of rapid growth, synchronous massive breast and axillary tumoral PASH, or bilateral breast enlargement with skin thickening and erythema have been reported (5,6).
The cause of PASH remains unknown, but it is postulated to be hormonally induced (particularly by progesterone) and predominantly occurs in premenopausal women and in postmenopausal women on hormone supplements (7–11). It has also been noted in men with gynecomastia, adolescent and pediatric patients with juvenile hypertrophy, and transgender individuals on hormonal therapy (1,7,9–12). The mean age at presentation ranges from 39 to 51 years across series (range, 9-86) (1,3,7,10–15).
Pseudoangiomatous stromal hyperplasia is commonly seen at histopathology as an incidental finding adjacent to the targeted lesion. In one series of 200 consecutive breast specimens (16), 23% (46/200) contained PASH. In another series of 412 consecutive core-needle biopsies (15), 9% (37/412) included PASH. Pseudoangiomatous stromal hyperplasia alone can form masses and produce mammographic asymmetries and enhancing mass and non-mass lesions on contrast-enhanced imaging. It is therefore particularly important to understand when the histopathologic finding of PASH is an acceptable and concordant explanation for imaging findings and when it is not.
Imaging Findings
It has been reported that in 23% (35/149) of women with breast biopsies yielding PASH as the sole pathologic finding who had had breast imaging within the prior year, no corresponding mammographic or US findings were seen on retrospective review of imaging (9).
When visible mammographically, PASH most commonly presents as an oval or round circumscribed non-calcified mass (1,3,4,8–10,13) (Figures 1, 2). Pseudoangiomatous stromal hyperplasia can also present as an asymmetry or developing asymmetry (1,8–10) (Figure 3). In the series of Jones et al (10), after excluding 22% (12) of the 55 cases with no mammographic correlate, a mass was noted in 58% (25/43) of the remaining cases and focal asymmetry in 42% (18/43).
Figure 1.
This 43-year-old woman had left breast cancer six years earlier and developed a palpable mass due to pseudoangiomatous stromal hyperplasia (PASH). A: Craniocaudal (CC) and mediolateral oblique (MLO) right mammograms show a small mass in the outer right breast (white arrows), new in retrospect but not noted and not enhancing on research screening contrast-enhanced mammogram (not shown). The patient had a prior biopsy showing tubular adenoma in the retroareolar right breast (short red arrows). Seven months later (B), the patient noted a palpable lump in the outer right breast (triangle marker), corresponding to a markedly enlarged 3-cm oval mass on CC and MLO mammograms (arrows), with mostly circumscribed margins on CC tomosynthesis (C, arrow). Targeted US (D) shows an oval, slightly hypoechoic mass with focally microlobulated margins (arrow). US-guided core-needle biopsy showed PASH. In view of rapid enlargement, the patient sought excision. E: Histopathology (hematoxylin and eosin, ×10) of the excised mass shows mostly fibrotic stroma and multiple slit-like spaces (arrows) mimicking vessels. F: Staining for CD34 (a vascular progenitor cell marker; ×10) is positive around the slit-like spaces (arrows) and around vessels. Again, findings were consistent with PASH. Pathology images courtesy of Dr Tatiana Villatoro, Department of Pathology, University of Pittsburgh Medical Center.
Figure 2.
This 44-year-old woman was noted to have a right breast mass due to pseudoangiomatous stromal hyperplasia (PASH) on baseline screening mammogram. A: Close-up of craniocaudal (left) and mediolateral oblique (right) tomosynthesis shows an oval circumscribed mass (arrows). B: Transverse (left) and sagittal US (right) demonstrates an oval, mostly hypoechoic mass with an echogenic rim (arrows) and internal hyperechogenicity. C: Histopathology (hematoxylin and eosin, ×2) demonstrates admixed fibrosis and fat (arrows) at the border, with the multiple interfaces producing the echogenic rim and internal hyperechogenicity. Although fibromatosis could be considered due to the slightly irregular border, overall cellularity is low and a fascicular growth pattern is not observed. At higher power, slit-like spaces typical of PASH were evident (not shown). Pathology image courtesy of Dr Beth Clark, Department of Pathology, University of Pittsburgh Medical Center.
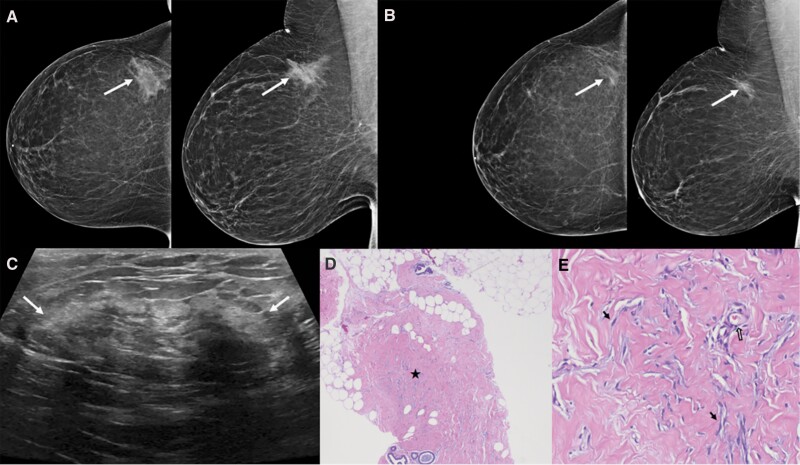
Figure 3.
Screening mammogram in this 40-year-old woman showed a developing right breast asymmetry due to pseudoangiomatous stromal hyperplasia (PASH). A: Right breast mammography shows a developing asymmetry in the upper outer right breast (arrows). B: In retrospect, the asymmetry was present but smaller on screening mammogram five years earlier (arrows). C: Longitudinal US shows a corresponding heterogeneous regional area of mixed echogenicity that is not very discrete with peripheral echogenic rim (arrows). US-guided core-needle biopsy showed PASH. D: Histopathology [hematoxylin and eosin (H&E), ×2] demonstrates breast tissue involved by extensive PASH, with ducts separated by collagenization of the stroma (star). E: Histopathology (H&E, ×20) shows small uniform myofibroblasts with flat nuclei (arrows), resembling endothelial cells, surrounding the complex network of slit-like spaces within collagenous stroma. A true capillary lined by endothelial cells is also noted (open arrow).
Mammographic findings of architectural distortion (1,9,13), calcifications (4,9,13), and an irregular mass with spiculated margins (3,4) have been reported on core-needle biopsies yielding PASH. Importantly, however, careful radiologic-pathologic concordance evaluation in several series revealed that those imaging findings were attributable to other concurrent pathologies and that PASH was incidental (4,9).
When discernable on US, PASH is most often seen as a circumscribed hypoechoic oval mass (Figure 1) (1,8–10,12,13). The sonographic appearance ranges from homogeneously hypoechoic, isoechoic, or hyperechoic solid oval (Figure 1), round, or even irregular masses with or without cystic components to also include heterogeneous or echogenic non-mass focal areas that are less discrete (Figure 3), with hypoechoic foci and irregular or poorly defined borders (1,8,10,12). A hyperechoic rim (Figures 2, 3) may be present (8,12) because of admixed nests of fibrosis and fat.
After excluding 14% (8/56) of sonographically occult cases, Jones et al (10) reported a circumscribed oval hypoechoic mass in 77% (37/48) of PASH lesions (rarely containing a cystic component) and, less commonly, that 23% (11/48) manifested as heterogeneous or echogenic areas with hypoechoic central areas (Figure 2). Hargaden et al (9) reported that 85% (44/52) of visible PASH cases presented sonographically as oval masses (Figure 1) and 83% (43/52) had circumscribed margins. In the same series, the echogenicity was usually hypoechoic (56%, 29/52) or isoechoic (38%, 20/52) and, uncommonly, complex cystic and solid (6%, 3/52). Choi et al (12) described 61% (28/46) of PASH cases as oval masses, 41% (19/46) as having circumscribed margins, and 22% (10/46) as having an echogenic rim. Of the 46, 39% (18/46) were complex cystic and solid, 37% (17/46) hypoechoic, 22% (10/46) isoechoic, and 2% (1/46) hyperechoic.
Few studies have investigated the MRI appearance of PASH. In a study with 69 PASH lesions, the majority (59%, 41/69) were seen as non-mass enhancement (14) (Figure 4). Masses (28%, 19/69) and foci (13%, 9/69) were less frequent. Kinetics were most often persistent (65%, 45/69), followed by washout (29%, 20/69) or plateau (6%, 4/69). Multiple slit-like and/or cystic spaces of high intensity on T2-weighted imaging have been reported (17). Both enhancement (18) and lack of enhancement (19) have been reported in a few cases of PASH on contrast-enhanced mammography, though further study is warranted.
Figure 4.
This 43-year-old woman at high risk for breast cancer due to family history had a screening MRI showing non-mass enhancement (NME) due to pseudoangiomatous stromal hyperplasia (PASH). Recent screening mammogram (not shown) was negative. A: Axial post-contrast T1-weighted subtraction image shows clumped NME in a linear distribution in the central left breast (arrow). B: Axial T2-weighted image with fat saturation demonstrates minimal T2 hyperintensity (arrow). C: Delayed post-contrast sagittal fat-suppressed T1-weighted image of the left breast again shows the clumped NME (arrow). D: Axial kinetic overlay shows persistent kinetics (arrow). MRI-guided vacuum-assisted biopsy showed PASH. E: Histopathology (hematoxylin and eosin, ×10) demonstrates complex anastomosing spaces in dense collagenous stroma (star). These spaces are lined by spindle-shaped myofibroblasts (arrows) and are associated with benign breast ductules (open arrowheads).
Studies reporting the nuclear medicine imaging appearance of PASH are scarce. If visualized, PASH lesions have been observed as focal areas of abnormal 99mTc-sestamibi uptake on molecular breast imaging and increased activity on 18F-fluorodeoxyglucose PET (18F-FDG PET) (10).
Pathologic Findings
Pseudoangiomatous stromal hyperplasia is a benign mammary stromal proliferation of myofibroblasts with variable expression of myoid and fibroblastic features. When evident on gross examination, PASH is a circumscribed tumor with a smooth surface, typically lacking hemorrhage or necrosis (20).
Microscopically, PASH appears as an expansion of dense collagenous stroma by complex anastomosing slit-like channels lined by slender bland spindle cells, mimicking vasoformative proliferation. Although epithelial proliferation or atypia is not part of PASH, nonspecific proliferative epithelial changes are commonly seen (21).
Pseudoangiomatous stromal hyperplasia can also be found microscopically admixed with high-risk or malignant lesions (11,15). Often, PASH is an inconspicuous change seen incidentally in the background breast stroma. It can involve the stroma of fibroadenomas or phyllodes tumors and, rarely, when manifesting as a mass, be confused with these entities because of sampling heterogeneity in the latter entities on core-needle biopsy (12). Fibroadenomas and phyllodes tumors have biphasic proliferation of both stromal and glandular elements, whereas PASH is purely a stromal proliferation. In PASH, nests of stroma can be admixed with the surrounding fat (Figure 2), whereas fibroadenomas have a distinct interface with surrounding tissue. Benign phyllodes tumors have a characteristic leaf-like stromal architecture covered by epithelial lining that is not seen in PASH.
The spindle cells in PASH are positive for vimentin, CD34 (a vascular progenitor marker, not specific for PASH), BCL2, and desmin. Pseudoangiomatous stromal hyperplasia most commonly shows a lobulocentric growth pattern; however, a fascicular growth pattern with associated hypercellularity may be observed, and this can mimic fibromatosis or low-grade angiosarcoma (2). Pseudoangiomatous stromal hyperplasia and many other stromal proliferations retain CD34 staining, but this is lost in fibromatosis; beta catenin is lost in PASH and retained in fibromatosis (22). The endothelial markers CD31 and factor VIII are lost in PASH and retained in low-grade angiosarcoma (1). Pseudoangiomatous stromal hyperplasia is also negative for cytokeratins, nuclear stain beta catenin, and smooth muscle actin, although these are not specific for PASH (23). The stromal cells in PASH are usually positive for progesterone receptor and, to a lesser degree, estrogen receptor (24).
Management and Discussion
The correct diagnosis of PASH has important treatment implications for patients. Because PASH is often admixed with other entities, including malignancies (11), careful correlation of clinical, imaging, and histopathologic findings is especially critical in establishing concordance when the only finding reported at histopathology is PASH.
While PASH is a benign lesion without malignant potential (9), it cannot be reliably recognized in the absence of biopsy (4,13). Following a percutaneous core-needle biopsy, a result of PASH is concordant with a circumscribed mass or developing asymmetry, and the patient may resume routine screening and clinical follow-up (9,25). However, a core-needle biopsy result of PASH is discordant with high suspicion imaging findings such as architectural distortion, an irregular spiculated mass, or fine linear or branching calcifications. In these situations, repeat biopsy or surgical excision is warranted (7,9). Surgical excision may also be desirable in patients with large or symptomatic lesions (4,26). In one review (7), recurrence of PASH after surgical excision was reported in 9% to 21% of patients, though most series have not observed any recurrences (4,8,9).
In conclusion, PASH is a benign lesion with nonspecific imaging findings. Careful evaluation of imaging features with pathologic correlation is recommended to ensure optimal diagnosis.
Contributor Information
Megan E Speer, The University of Texas MD Anderson Cancer Center, Department of Breast Imaging, Houston, TX, USA.
Esther C Yoon, The University of Texas MD Anderson Cancer Center, Department of Pathology, Houston, TX, USA.
Wendie A Berg, Magee-Womens Hospital of University of Pittsburgh Medical Center, Department of Radiology, Pittsburgh, PA, USA; University of Pittsburgh School of Medicine, Department of Radiology, Pittsburgh, PA, USA.
Lauren Q Chang Sen, The University of Texas MD Anderson Cancer Center, Department of Breast Imaging, Houston, TX, USA.
Funding
This work was supported in part by the National Institutes of Health/ National Cancer Institute under award number P30CA016672, which supports the MD Anderson Cancer Center Clinical Trials Office.
Conflict of Interest Statement
W.A.B. discloses a departmental research grant from Koios Medical, Inc., for which she is the principal investigator, and she is voluntary Chief Scientific Advisor to DenseBreast-info.org. W.A.B. is an Associate Editor of the Journal of Breast Imaging and was blinded to the review process. M.E.S., E.C.Y., and L.Q.C.S. do not have any conflicts of interest.
References
- 1. Bowman E, Oprea G, Okoli J, et al. Pseudoangiomatous stromal hyperplasia (PASH) of the breast: a series of 24 patients. Breast J 2012;18(3):242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Virk RK, Khan A.. Pseudoangiomatous stromal hyperplasia: an overview. Arch Pathol Lab Med 2010;134(7):1070–1074. [DOI] [PubMed] [Google Scholar]
- 3. Cohen MA, Morris EA, Rosen PP, Dershaw DD, Liberman L, Abramson AF.. Pseudoangiomatous stromal hyperplasia: mammographic, sonographic, and clinical patterns. Radiology 1996;198(1):117–120. [DOI] [PubMed] [Google Scholar]
- 4. Kurt E, Turanli S, Markoc F, Berberoglu U.. How to manage pseudoangiomatous stromal hyperplasia: our clinical experience. Turk J Med Sci 2017;47(5):1410–1415. [DOI] [PubMed] [Google Scholar]
- 5. Shimpi TR, Baksa Reynolds V, Shikhare S, Srinivasan S, Clarke MJ, Peh WCG.. Synchronous large tumoral pseudoangiomatous stromal hyperplasia (PASH) in the breast and axilla with subsequent carcinoma in the contralateral breast: routine and strain imaging with histopathological correlation. BJR Case Rep 2015;1(3):20150017. doi: 10.1259/bjrcr.20150017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoo K, Woo OH, Yong HS, et al. Fast-growing pseudoangiomatous stromal hyperplasia of the breast: report of a case. Surg Today 2007;37(11):967–970. [DOI] [PubMed] [Google Scholar]
- 7. Gresik CM, Godellas C, Aranha GV, Rajan P, Shoup M.. Pseudoangiomatous stromal hyperplasia of the breast: a contemporary approach to its clinical and radiologic features and ideal management. Surgery 2010;148(4):752–758. [DOI] [PubMed] [Google Scholar]
- 8. Mercado CL, Naidrich SA, Hamele-Bena D, Fineberg SA, Buchbinder SS.. Pseudoangiomatous stromal hyperplasia of the breast: sonographic features with histopathologic correlation. Breast J 2004;10(5):427–432. [DOI] [PubMed] [Google Scholar]
- 9. Hargaden GC, Yeh ED, Georgian-Smith D, et al. Analysis of the mammographic and sonographic features of pseudoangiomatous stromal hyperplasia. AJR Am J Roentgenol 2008;191(2):359–363. [DOI] [PubMed] [Google Scholar]
- 10. Jones KN, Glazebrook KN, Reynolds C.. Pseudoangiomatous stromal hyperplasia: imaging findings with pathologic and clinical correlation. AJR Am J Roentgenol 2010;195(4):1036–1042. [DOI] [PubMed] [Google Scholar]
- 11. Drinka EK, Bargaje A, Ersahin CH, et al. Pseudoangiomatous stromal hyperplasia (PASH) of the breast: a clinicopathological study of 79 cases. Int J Surg Pathol 2012;20(1):54–58. [DOI] [PubMed] [Google Scholar]
- 12. Choi YJ, Ko EY, Kook S.. Diagnosis of pseudoangiomatous stromal hyperplasia of the breast: ultrasonography findings and different biopsy methods. Yonsei Med J 2008;49(5):757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Celliers L, Wong DD, Bourke A.. Pseudoangiomatous stromal hyperplasia: a study of the mammographic and sonographic features. Clin Radiol 2010;65(2):145–149. [DOI] [PubMed] [Google Scholar]
- 14. Nia ES, Adrada BE, Whitman GJ, et al. MRI features of pseudoangiomatous stromal hyperplasia with histopathological correlation. Breast J 2021;27(3):242–247. [DOI] [PubMed] [Google Scholar]
- 15. Kelten Talu C, Boyaci C, Leblebici C, Hacihasanoglu E, Bozkurt ER.. Pseudoangiomatous stromal hyperplasia in core needle biopsies of breast specimens. Int J Surg Pathol 2017;25(1):26–30. [DOI] [PubMed] [Google Scholar]
- 16. Ibrahim RE, Sciotto CG, Weidner N.. Pseudoangiomatous hyperplasia of mammary stroma. Some observations regarding its clinicopathologic spectrum. Cancer 1989;63(6):1154–1160. [DOI] [PubMed] [Google Scholar]
- 17. Solomou E, Kraniotis P, Patriarcheas G.. A case of a giant pseudoangiomatous stromal hyperplasia of the breast: magnetic resonance imaging findings. Rare Tumors 2012;4(2):e23. doi: 10.4081/rt.2012.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patel BK, Naylor ME, Kosiorek HE, et al. Clinical utility of contrast-enhanced spectral mammography as an adjunct for tomosynthesis-detected architectural distortion. Clin Imaging 2017;46:44–52. [DOI] [PubMed] [Google Scholar]
- 19. Zuley ML, Bandos AI, Abrams GS, et al. Contrast enhanced digital mammography (CEDM) helps to safely reduce benign breast biopsies for low to moderately suspicious soft tissue lesions. Acad Radiol 2020;27(7):969–976. [DOI] [PubMed] [Google Scholar]
- 20. Castro CY, Whitman GJ, Sahin AA.. Pseudoangiomatous stromal hyperplasia of the breast. Am J Clin Oncol 2002;25(2):213–216. [DOI] [PubMed] [Google Scholar]
- 21. Magro G. Differential diagnosis of benign spindle cell lesions. Surg Pathol Clin 2018;11(1):91–121. [DOI] [PubMed] [Google Scholar]
- 22. Kuba MG, Lester SC, Giess CS, Bertagnolli MM, Wieczorek TJ, Brock JE.. Fibromatosis of the breast: diagnostic accuracy of core needle biopsy. Am J Clin Pathol 2017;148(3):243–250. [DOI] [PubMed] [Google Scholar]
- 23. Magro G, Spadola S, Motta F, et al. STAT6 expression in spindle cell lesions of the breast: an immunohistochemical study of 48 cases. Pathol Res Pract 2018;214(10):1544–1549. [DOI] [PubMed] [Google Scholar]
- 24. Powell CM, Cranor ML, Rosen PP.. Pseudoangiomatous stromal hyperplasia (PASH). A mammary stromal tumor with myofibroblastic differentiation. Am J Surg Pathol 1995;19(3):270–277. [DOI] [PubMed] [Google Scholar]
- 25. Monticciolo DL, Hajdik RL, Hicks MG, et al. Six-month short-interval imaging follow-up for benign concordant core needle biopsy of the breast: outcomes in 1444 cases with long-term follow-up. AJR Am J Roentgenol 2016;207(4):912–917. [DOI] [PubMed] [Google Scholar]
- 26. Yoon KH, Koo B, Lee KB, et al. Optimal treatment of pseudoangiomatous stromal hyperplasia of the breast. Asian J Surg 2020;43(7):735–741. [DOI] [PubMed] [Google Scholar]