ABSTRACT
In this hybrid review, we have first collected and reviewed available information on the structure and function of the enigmatic cache domains in α2δ proteins. These are organized into two double cache (dCache_1) domains, and they are present in all α2δ proteins. We have also included new data on the key function of these domains with respect to amino acid and gabapentinoid binding to the universal amino acid–binding pocket, which is present in α2δ-1 and α2δ-2. We have now identified the reason why α2δ-3 and α2δ-4 do not bind gabapentinoid drugs or amino acids with bulky side chains. In relation to this, we have determined that the bulky amino acids Tryptophan and Phenylalanine prevent gabapentin from inhibiting cell surface trafficking of α2δ-1. Together, these novel data shed further light on the importance of the cache domains in α2δ proteins.
KEYWORDS: Voltage-gated calcium channel, gabapentinoid, cache domain, amino acid, α2δ protein
Introduction
The identification of a human variant in a Cache domain within α2δ-1 that contributes to a phenotype severely affecting neural development and function [1] has prompted this review of α2δ structure and function, in order to further understand the function of the cache domains in these multi-domain proteins. In addition, we present further experimental data related to the specificity and importance of amino acid and gabapentinoid binding to the amino acid–binding site in the first double cache domain.
Classical role of α2δ in a complex within calcium channels
Voltage-gated calcium channels were first purified and the genes cloned from skeletal muscle in the 1980s [2,3]. The α2δ subunit was identified as one of the subunits, which was associated with the dihydropyridine receptor (α1 subunit) that was identified as a pore-forming subunit of the skeletal muscle calcium channel. Once the α2δ subunits were purified and cloned [4,5], they were also found to associate with N-type and P/Q-type channels, as well as other L-type channels [6,7]. The α2δ subunits are now known to associate with and affect the function of all CaV1 and CaV2 channels [8–11].
α2δ subtypes
The skeletal muscle α2δ protein, termed α2δ-1, is encoded by CACNA2D1, which was the first α2δ gene to be cloned [12,13]. Four α2δ subunit genes were eventually cloned: CACNA2D2, encoding α2δ-2, was identified as a result of finding spontaneous mouse mutations leading to cerebellar ataxia and absence epilepsy [14,15]. CACNA2D3 and CACNA2D4 encoding α2δ-3 [11] and α2δ-4 [16], respectively, were then identified by homology to the α2δ-1 sequence.
α2δ distribution and functions
The skeletal muscle α2δ protein, α2δ-1, was found also to be present extensively in other mainly excitable cell types, including those in the heart, smooth muscle, and brain [17]. In neurons, it is particularly concentrated presynaptically, and it is involved in presynaptic functions including transmitter release, homeostatic plasticity, and synaptic organization [18–21]. In contrast, the tissue distribution of α2δ-2 was found to be mainly in the brain, particularly the cerebellum, but also in other tissues [11,14], and α2δ-3 was expressed widely in the brain, particularly in the caudate-putamen [11]. The selective distribution and importance of α2δ-4 in retinal function was elucidated by virtue of its mutation in hereditary retinal dysfunction [22,23].
Biochemistry and domains within α2δ
All α2δ proteins have similar topology, biochemical properties, and domain architecture (Figure 1). Both α2 and δ are highly N-glycosylated with up to 16 glycosylation sites [13,24,25], in agreement with their extracellular topology. All are proteolytically cleaved into two polypeptides, the larger α2, and the smaller δ [26]. These remain disulfide-bonded together [13]. The C-terminal hydrophobic domain is present in all α2δ pre-protein sequences [26]. Although this hydrophobic domain was originally predicted to be transmembrane [25], it was found to contain key glycosyl-phosphatidylinositol (GPI)-anchor signal motifs for all the α2δ sequences [27], which was confirmed in biochemical, functional, and structural studies [27,28]. Thus, the C-terminal hydrophobic domain and short putative intracellular sequence, translated in the α2δ pre-protein are removed by processing in the endoplasmic reticulum, being replaced by a lipid anchor, and are therefore absent from the mature α2δ protein present in the calcium channel complex [28,29].
Figure 1.
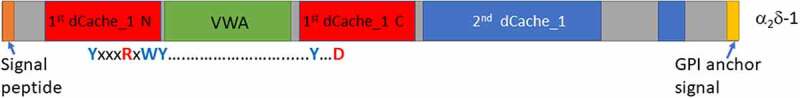
Domain structure of α2δ-1 The amino acid–binding site motif in the first dCache_1 domain is shown beneath the linear domain representation .[35]. The R and D drawn in red in the motif have been mutated in binding studies described here. Modified from Figure 3c in [35].
There is also a von Willebrand factor-A (VWA) domain in α2, [30] which is a well-recognized protein–protein interaction domain, that is also present in many other extracellular proteins, including integrins [31]. The VWA domain in α2δ proteins is required for enhancement of calcium current function [9]. In α2δ-1 and α2δ-2 subunits, the VWA domains have a characteristic completely intact metal ion-dependent adhesion site (MIDAS) motif [30,32]. In other VWA domains, such as those in integrins, this MIDAS motif co-ordinates binding to another protein ligand, which occurs in the presence of a divalent cation, and which results in a conformational change [31]. In α2δ-1 and α2δ-2, disruption of the MIDAS motif prevents the ability of these α2δ subunits to enhance calcium channel currents [9,18]. The main corresponding interaction of the α2δ MIDAS motif with the CaV channels involves an aspartate in extracellular loop I of domain I of the α1 subunit, which coordinates with the α2δ MIDAS motif [28,33]. However, the structure also shows an additional interaction between a loop of the first Cache domain of α2δ-1 with the top of pore loop 5 in domain III, which forms part of the extracellular entrance to the channel pore [28].
The importance of cache domains in α2δ proteins
The α2δ proteins were found to contain domains related to those in bacterial chemoreceptors that were termed Cache domains [34], and it was identified structurally that four Cache domains were present in α2δ-1 [28]. In α2δ proteins, as in some prokaryotic proteins, these were found to be organized into double Cache domains (dCache_1), and in bacteria, they are involved in amino acid nutrient binding in chemoreceptors and other signal transduction proteins, leading to intracellular signaling [35,36]. Although these domains are widely found in bacteria and archaea, where they have well-studied roles in nutrient sensing, the only animal proteins in which these dCache domains have been identified are α2δ proteins (Figure 1), and the novel α2δ-like protein Cachd1 [35], which is a transmembrane protein with some α2δ-like properties [33,37,38]
A conserved structural motif including several key residues was found to be essential for amino acid binding in all these dCache_1 domains, including in the first dCache_1 domain in α2δ-1 [35]. This dCache_1 domain is split in α2δ-1, with the VWA domain inserted into it. The presence of the VWA domain also splits the amino acid–binding motif. The motif (using the single letter amino acid code) consists of YxxxRxWY in the first cache domain and Y … D in the second cache domain (Figure 1). The Arg (R) in this motif (in red in Figure 1) was previously identified as being the third Arginine in the triple-Arg sequence that was found to be essential for gabapentin binding and for the function of gabapentinoids in alleviating neuropathic pain [39,40].
Splicing creates variation in cache domains of α2δ proteins
Several different splice variants of the α2δ proteins have been identified [12,41,42]; these have been investigated most extensively in α2δ-1 and involve the cache domains. There are three regions of splicing in α2δ-1, termed A, B, and C; A and C are cassette exons, and B is introduced via an alternative splice acceptor site [43]. A and B are situated in the distal half of the first dCache_1 domain in a loop between β-sheet 6 and α-helix 7, whereas the third splice insertion, region C, is at the start of the second dCache_1 domain (see Figure 2 and Fig. S11, in [35]).
The three splice insertions in α2δ-1 are differentially expressed in different tissues [43,44]. These studies showed region A to be expressed exclusively in skeletal muscle from all the tissues examined. The rat skeletal muscle variant is +A + B ΔC, whereas in the rat brain the main splice variant is ΔA + B + C. A minor splice variant of α2δ-1 lacking region C (ΔA + B ΔC) is differentially up-regulated in rat dorsal root ganglion neurons following neuropathic injury, and it shows lower affinity for gabapentin [43]. The importance of the different splice insertions is unknown; it remains to be determined whether they are important for α2δ-1 structure and interaction with specific calcium channels such as, in the case of region A, the skeletal muscle channel α1S, or for interaction with other potential binding partners of α2δ-1 [45]. In this regard, it is of great interest that exogenous expression in hippocampal neurons of an α2δ-2 splice variant lacking exon 23, which is in an equivalent position to splice site C in α2δ-1 (see alignment in Fig. S11 in [35]), triggers aberrant synapse formation in tissue culture [46].
Importance of α2δ proteins in disease in mouse and other animal models: Relevance to cache domains
Knockout mice have been generated for the different α2δ isoforms. From these studies, it is clear that the observed phenotype of particular α2δ knockout mice depends on the cell types and developmental stages associated with selective expression of the particular isoform, which may then become indispensable. The α2δ-1 knockout mice have a mild phenotype of reduced cardiac function, as α2δ-1 is strongly expressed in ventricular myocytes [47]. They also have a reduced sensation of mechanical pain [48], associated with the finding that α2δ-1 is strongly expressed in sensory neurons and is upregulated following neuropathic injury [49–51]. Furthermore, upregulated α2δ-1 mediates an increase in the trafficking of CaV2.2 particularly in low threshold mechanoreceptors involved in hyperalgesia and allodynia [52]. Related to this, α2δ-1 knockout mice also exhibit delayed development of neuropathic pain-related responses [48]. Furthermore, transgenic mice that constitutively over-express α2δ-1 by random insertion [53] show spontaneous epileptiform behavior observed on EEG [54], and constitutive pain-like behavior [53]. In addition, auto-antibodies recognizing α2δ-1 are present in cases of autoimmune encephalitis [55] and amyotrophic lateral sclerosis with type 2 diabetes [56].
In contrast, α2δ-2 knockout mice [57] have a similar severe phenotype to the spontaneously arising Ducky and entla mutants, including cerebellar ataxia and epilepsy [14,15]. This phenotype relates to the fact that α2δ-2 is very strongly expressed in cerebellar Purkinje cells [14,58]. The phenotype of α2δ-3 knockout mice was more subtle, and included impaired acoustic startle response and hearing disruption [59].
Drosophila melanogaster has two α2δ orthologs, the skeletal muscle ortholog, Ca-Ma2d, and the α2δ-3 ortholog, straightjacket (stg) or dα2δ-3, which is important in neurotransmission [60]. Knockdown of stg gene expression results in impaired heat sensitivity [61]. Furthermore, single nucleotide polymorphisms (SNPs) in the human gene CACNA2D3 have been associated with reduced behavioral noxious thermal sensitivity, likely via a central impairment [61].
Mutations in Cacna2d4 result in disruption of retinal ribbon synapses in mice, as a result of both rod and cone dysfunction [23].
Effect of human mutations in CACNA2D genes and relevance to cache domains
Neurological disease
Several recent reviews cover the involvement of CaV channels in neurological and psychiatric disorders [62,63] and only a summary of recent studies relating to CACNA2D genes is provided here. In CACNA2D2, rare biallelic loss-of-function variation has been reported in individuals with developmental epileptic encephalopathy, including cerebellar atrophy [64–67]. Rare homozygous truncating mutations of CACNA2D4 have been reported, which result in recessive, slowly progressing cone dystrophy and hereditary night blindness [22].
In CACNA2D1, biallelic loss-of-function mutations have also recently been reported in two patients with developmental epileptic encephalopathy, which is associated with cerebral cortical rather than cerebellar atrophy [1]. These individuals were also reported to be insensitive to pain. In one patient, there was a homozygous frameshift mutation, resulting in a marked reduction in CACNA2D1 mRNA measured in the patient fibroblasts. The other patient was compound heterozygous for a very early frameshift mutation on one allele, and a point mutation (Gly209-Asp) on the other allele. This Gly209 was in a highly conserved residue in the first dCache_1 domain of α2δ-1 [1]. We found that this mutation rendered α2δ-1 nonfunctional, in that the mutant protein did not traffic to the cell surface. Our evidence further suggested that the mutant α2δ-1 was retained in the endoplasmic reticulum, since it was not proteolytically cleaved into α2 and δ, a process that occurs mainly in the Golgi apparatus [1,68].
Genetic variation in CACNA2D1: Implications for cardiac disease in humans
In humans, heterozygous missense variations in CACNA2D1 have previously been associated with cardiac dysfunction, with Brugada [69] and short QT [70] syndromes. However, these dominant associations with cardiac dysfunction have recently been called into question [1].
Mechanism of action of gabapentinoid drugs and basis for their selectivity with respect to α2δ proteins
Gabapentin and pregabalin were first developed in drug discovery programs to identify novel antiepileptic drugs mimicking or promoting the function of the inhibitory neurotransmitter GABA [71]. These drugs were then identified to bind to α2δ-1 rather than their originally intended mechanism of action [72]. Mutational analysis then found the Arg mentioned above to be involved in gabapentinoid binding and function [39,40,73]. More recently, a key aspartate (Asp, D, Figure 1) was also identified as being essential to coordinate amino acid binding in this binding pocket, which is in the dCache_1 domain of α2δ-1 [35].
Regarding the mechanism of action of the gabapentinoids, we identified that gabapentin reduced the trafficking of α2δ-1 and α2δ-2 [74,75] and also disrupted the trafficking of associated calcium channels, and their function [75–78]. Within α2δ-1, both the key Arg241 [75,78] and also Asp491 [35] residues in the dCache_1 amino acid–binding site of α2δ-1 are important for the ability of gabapentin to inhibit α2δ-1 trafficking and function.
Of interest, α2δ-3 (and also α2δ-4) does not contain the triple Arg sequence that was thought to be implicated in gabapentin binding (it is Arg-Asn-Arg in α2δ-3), and neither α2δ-3 nor α2δ-4 binds gabapentin [79]. Furthermore, α2δ-3 is not recycled to the plasma membrane via a Rab11-dependent pathway [80].
Our analysis of the structures modeled by AlphaFold [81] shows that α2δ-3 and α2δ-4 do contain an amino acid–binding site, in an analogous position to that identified in the first dCache domain of α2δ-1 [35] (Figure 2). We conducted molecular docking in AutoDock Vina [82] with AlphaFold models of α2δ-2, α2δ-3 and α2δ-4 proteins using gabapentin, pregabalin, mirogabalin, and amino acids, and found that in case of α2δ-3 and α2δ-4, only small amino acids bind to the pocket, while gabapentinoids and bulky amino acids do not (structural models of α2δ-2, α2δ-3, and α2δ-4 proteins with docked ligands and docking simulation parameters can be found at this link: https://github.com/ToshkaDev/Alpha2Delta-proteins-review). Interestingly, all proteinogenic amino acids and gabapentinoids were bound to α2δ-2, but tryptophan (Trp) was found to bind only in a certain pose and with low affinity, which contrasts with its high affinity binding to α2δ-1. Our structural analysis shows that the first two Arg residues of the above-mentioned triple-Arg motif are directed away from the pocket and in fact do not directly contribute to the formation of the ligand-binding interface (Figure 2a). Only the third Arg in this sequence, which is part of the universal amino acid–binding motif, is directed toward the inside of the pocket and binds ligands (Figure 2b, [35]). Thus, the two first residues of the triple-Arg sequence do not play a role in ligand binding and, therefore, replacement of the second Arg to Asn in this motif observed in α2δ-3 and α2δ-4 is not the reason for their inability to bind gabapentinoids. Our subsequent examination allowed us to identify the “culprit” – Phenylalanine (Phe) at a specific position within the ligand-binding pocket of α2δ-3 and α2δ-4 that creates a steric hindrance interfering with the binding of bulky ligands (Figure 2b). In α2δ-1 and α2δ-2, alanine (Ala217) and threonine (Thr257), respectively, are located at this position (see Figure 2), and they do not impede ligand binding.
Figure 3.
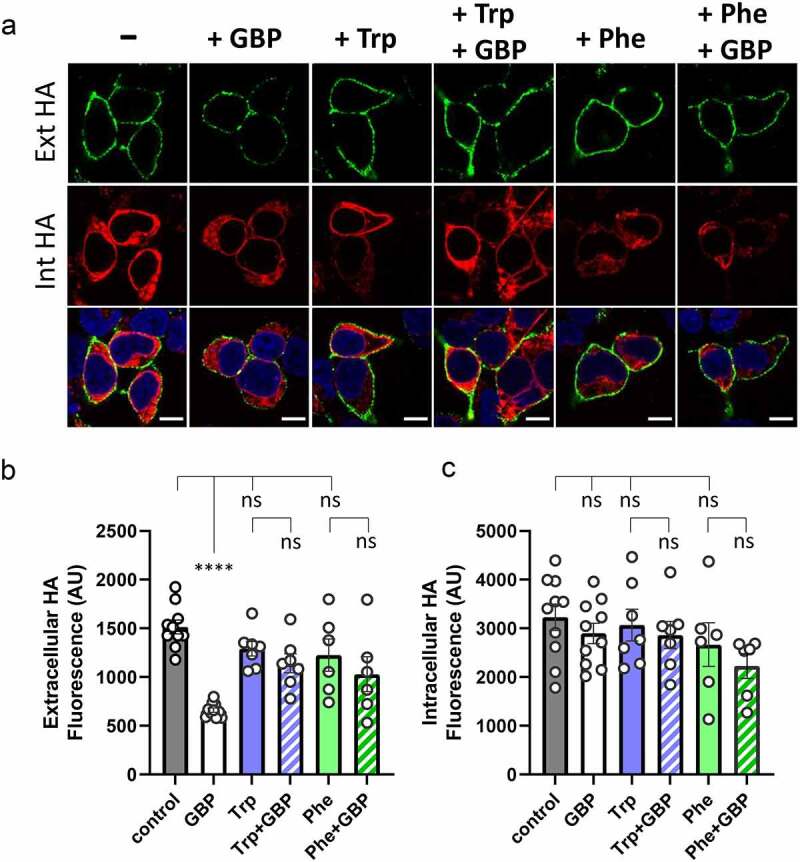
Tryptophan and Phenylalanine prevent the inhibition of cell surface expression of HA-tagged α2δ-1 by gabapentin. Experiments were performed as described previously described [35]. (a) Representative images of tsA-201 cells expressing hemagglutinin (HA)-tagged α2δ-1 subunit in the absence of gabapentin or additional amino acids (control, -) or the presence of 1 mM gabapentin (+ GBP) alone, 1 mM L-Tryptophan (+ Trp) alone, 1 mM L-Tryptophan + 1 mM gabapentin (+ Trp + GBP), 1 mM L-Phenylalanine (+ Phe) alone or 1 mM L-Phenylalanine + 1 mM gabapentin (+ Phe + GBP), incubated in serum-free media for 24 h. Top row (green, Ext HA) shows cell surface α2δ-1-HA staining in the nonpermeabilized condition; middle row (red, Int HA) shows intracellular α2δ-1-HA staining after permeabilization with 0.1% Triton X-100; bottom row shows merged images with the nuclei stained with DAPI (blue). Scale bars: 10 µm. (b) Bar chart (mean ± SEM, with individual data-points each showing the mean of more than 35 cells from 6–10 different transfections in three independent experiments), showing cell surface expression of α2δ-1-HA in the absence (control, gray) or presence of 1 mM GBP (white), 1 mM Trp (blue), 1 mM Trp + 1 mM GBP (blue and white stripes), 1 mM Phe (green), 1 mM Phe + 1 mM GBP (green and white stripes). Statistical significance was determined using one-way ANOVA and Šídák’s multiple comparison post-hoc test; **** P < 0.0001, ns: no statistical significance (P > 0.2). (c) As for (B) but showing intracellular HA staining after permeabilization of the cells. Cell surface expression of α2δ-1-HA is reduced by GBP to 44% of control levels but this reduction is not seen in the presence of additional L-Trp or L-Phe.
Bacterial chemoreceptors bind both agonists and antagonists at this universal amino acid–binding site within the dCache_1 domain [83,84]. For α2δ-1, the amino acid leucine was found previously to bind to the same binding site and compete with gabapentin, although the function of this binding was not known [85]. In our recent study, the binding affinity of various amino acids including Trp and Phe to α2δ-1 was calculated from docking analysis to be higher than that of leucine, and as high as that of the gabapentinoids [35].
We therefore examined here, using techniques already described [35], whether Trp or Phe would either inhibit α2δ-1 trafficking in the same way as gabapentin or, alternatively, act as agonists and enhance its trafficking. We found that although an elevated concentration (1 mM) of either Trp or Phe alone did not affect α2δ-1 cell surface expression in cultured cells, both these amino acids did inhibit the ability of gabapentin in this regard (Figure 3a-b). The cell surface expression of α2δ-1 was reduced by 56% by 1 mM gabapentin, as we have described previously [78,80], whereas this reduction was prevented by the additional presence of 1 mM Trp or Phe (Figure 3a, b). There were no effects of any of the manipulations on intracellular α2δ-1 expression (Figure 3a, c).
Figure 2.
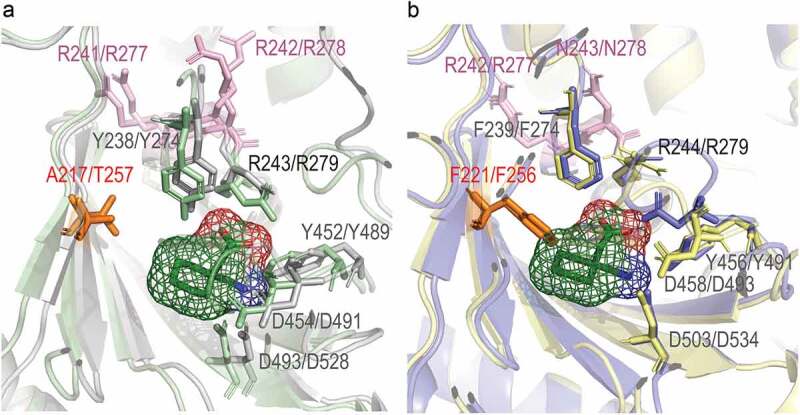
Gabapentinoid and amino acid–binding pockets of α2δ-1 – α2δ-4. Phe221/Phe256 (F221/F256) creates steric hindrance in the ligand-binding pocket of α2δ-3 and α2δ-4 proteins. Superimposed structures of α2δ-1 and α2δ-2 (a) and α2δ-3 and α2δ-4 (b) ligand-binding pockets. α2δ-1 is the rabbit protein cryo-EM structure, α2δ-2 to α2δ-4 are the AlphaFold models. Gabapentin is docked to the binding pocket of α2δ-1. Each of α2δ-2 to α2δ-4 was superimposed on α2δ1; α2δ-3 and α2δ-4 then were extracted and placed on panel B for clarity. A figure with all the proteins simultaneously superimposed on α2δ-1 can be found on GitHub at the following link https://github.com/ToshkaDev/Alpha2Delta-proteins-review. In pink font the first two residues of the RRR (in α2δ-1 and α2δ-2)/RNR (in α2δ-3 and α2δ-4) sequence are shown – as can be seen they are not involved in ligand binding. Other residues, except for the residue corresponding to Asp454 (D454) in α2δ-1, denote the amino acid–binding motif .[35]
These results indicate that, although endogenous amino acids are likely to occupy the universal amino acid–binding site in α2δ-1, we were unable to detect any effect of the binding of high concentrations of Trp or Phe on cell surface expression of α2δ-1, indicating that under the conditions used here they did not act alone as either agonists or antagonists, although they are able to prevent the effect of gabapentin, presumably by occupying the binding site. This may represent one mechanism that contributes to the variable efficacy of gabapentinoid drugs.
Conclusion
Within Metazoa, cache domains are only found in α2δ proteins and in Cachd1. In these proteins, the four cache domains are organized into two double Cache (dCache_1) domains, and contain a universal amino acid–binding pocket, which in α2δ-1 and α2δ-2 also accommodates gabapentinoid drugs. Here we have examined, from a structural point of view, why α2δ-3 and α2δ-4 do not bind gabapentinoids or amino acids with bulky side chains. Furthermore, we have determined that the bulky amino acids Trp and Phe prevent gabapentin from inhibiting cell surface expression of α2δ-1. Altogether, this illustrates the importance of the cache domains in α2δ proteins. It also highlights that novel interactions of these cache domains are likely to be found in the future.
Funding Statement
This work was supported by the National Institutes of Health [R35GM131760] and Wellcome Trust [098360/Z/12/Z].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
Structural data are available at https://github.com/ToshkaDev/Alpha2Delta-proteins-review. Other data will be made available upon reasonable request.
References
- [1].Dahimene S, von Elsner L, Holling T, et al. Biallelic CACNA2D1 loss-of-function variants cause early-onset developmental epileptic encephalopathy. Brain. 2022;awac081. DOI: 10.1093/brain/awac081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tanabe T, Takeshima H, Mikami A, et al. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987;328:313–10. [DOI] [PubMed] [Google Scholar]
- [3].Curtis BM, Catterall WA.. Purification of the calcium antagonist receptor of the voltage-sensitive calcium channel from skeletal muscle transverse tubules. Biochemistry. 1984;23:2113–2118. [DOI] [PubMed] [Google Scholar]
- [4].Jay SD, Sharp AH, Kahl SD, et al. Structural characterization of the dihydropyridine-sensitive calcium channel α2-subunit and the associated F064 peptides. J Biol Chem. 1991;266:3287–3293. [PubMed] [Google Scholar]
- [5].De Jongh KS, Merrick DK, Catterall WA. Subunits of purified calcium channels: a 212-kDa form of α1 and partial amino acid sequence of a phosphorylation site of an independent β subunit. Proc Natl Acad Sci U S A. 1989;86:8585–8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Witcher DR, De Waard M, Sakamoto J, et al. Subunit identification and reconstitution of the N-type Ca2+ channel complex purified from brain. Science. 1993;261:486–489. [DOI] [PubMed] [Google Scholar]
- [7].Liu H, De Waard M, Scott VES, et al. Identification of three subunits of the high affinity w-conotoxin MVIIC-sensitive Ca2+ channel. J Biol Chem. 1996;271:13804–13810. [PubMed] [Google Scholar]
- [8].Qin N, Olcese R, Stefani E, et al. Modulation of human neuronal α1E-type calcium channel by α2δ-subunit. Am J Physiol. 1998;274:C1324–C31. [DOI] [PubMed] [Google Scholar]
- [9].Canti C, Nieto-Rostro M, Foucault I, et al. The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of alpha2delta subunits is key to trafficking voltage-gated Ca2+ channels. Proc Natl Acad Sci USA. 2005;102:11230–11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Canti C, Davies A, Dolphin AC. Calcium channel alpha2delta subunits: structure, function and target site for drugs. Curr Neuropharmacol. 2003;1:209–217. [Google Scholar]
- [11].Klugbauer N, Lacinova L, Marais E, et al. Molecular diversity of the calcium channel α2-δ subunit. J Neurosci. 1999;19:684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ellis SB, Williams ME, Ways NR, et al. Sequence and expression of mRNAs encoding the α1 and α2 subunits of a DHP-sensitive calcium channel. Science. 1988;241:1661–1664. [DOI] [PubMed] [Google Scholar]
- [13].De Jongh KS, Warner C, Catterall WA. Subunits of purified calcium channels α2 and δ are encoded by the same gene. J Biol Chem. 1990;265:14738–14741. [PubMed] [Google Scholar]
- [14].Barclay J, Balaguero N, Mione M, et al. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J Neurosci. 2001;21:6095–6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brill J, Klocke R, Paul D, et al. entla, a novel epileptic and ataxic Cacna2d2 mutant of the mouse. J Biol Chem. 2004;279:7322–7330. [DOI] [PubMed] [Google Scholar]
- [16].Qin N, Yagel S, Momplaisir ML, et al. Molecular cloning and characterization of the human voltage-gated calcium channel α2δ-4 subunit. Mol Pharmacol. 2002;62:485–496. [DOI] [PubMed] [Google Scholar]
- [17].Ahlijanian MK, Westenbroek RE, Catterall WA. Subunit structure and localization of dihydropyridine-sensitive calcium channels in mammalian brain, spinal cord, and retina. Neuron. 1990;4:819–832. [DOI] [PubMed] [Google Scholar]
- [18].Hoppa MB, Lana B, Margas W, et al. alpha2delta expression sets presynaptic calcium channel abundance and release probability. Nature. 2012;486:122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pilch KS, Ramgoolam KH, Dolphin AC. Involvement of Ca V 2.2 channels and α 2 δ-1 in homeostatic synaptic plasticity in cultured hippocampal neurons. J Physiol. 2022;600(24):5333–5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bikbaev A, Ciuraszkiewicz-Wojciech A, Heck J, et al. Auxiliary alpha2delta1 and alpha2delta3 subunits of calcium channels drive excitatory and inhibitory neuronal network development. J Neurosci. 2020;40:4824–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schӧpf CL, Ablinger C, Geisler SM, et al. Presynaptic alpha2delta subunits are key organizers of glutamatergic synapses. Proc Natl Acad Sci U S A. 2021;2021:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wycisk KA, Zeitz C, Feil S, et al. Mutation in the auxiliary calcium-channel subunit CACNA2D4 causes autosomal recessive cone dystrophy. Am J Hum Genet. 2006;79:973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wycisk KA, Budde B, Feil S, et al. Structural and functional abnormalities of retinal ribbon synapses due to Cacna2d4 mutation. Invest Ophthalmol Vis Sci. 2006;47:3523–3530. [DOI] [PubMed] [Google Scholar]
- [24].Kadurin I, Alvarez-Laviada A, Ng SF, et al. Calcium currents are enhanced by alpha2delta-1 lacking its membrane anchor. J Biol Chem. 2012;1287:33554–33566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gurnett CA, De Waard M, Campbell KP. Dual function of the voltage-dependent Ca2+ channel α2δ subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–440. [DOI] [PubMed] [Google Scholar]
- [26].Davies A, Hendrich J, Van Minh AT, et al. Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels. Trends Pharmacol Sci. 2007;28:220–228. [DOI] [PubMed] [Google Scholar]
- [27].Davies A, Kadurin I, Alvarez-Laviada A, et al. The alpha(2)delta subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc Natl Acad Sci U S A. 2010;107:1654–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wu J, Yan Z, Li Z, et al. Structure of the voltage-gated calcium channel Cav1.1at 3.6 A resolution. Nature. 2016;537. 191–196. [DOI] [PubMed] [Google Scholar]
- [29].Gao S, Yao X, Yan N. Structure of human Cav2.2 channel blocked by the painkiller ziconotide. Nature. 2021;596(7870):143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Whittaker CA, Hynes RO. Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol Biol Cell. 2002;13:3369–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yang W, Shimaoka M, Salas A, et al. Intersubunit signal transmission in integrins by a receptor-like interaction with a pull spring. Proc Natl Acad Sci U S A. 2004;101:2906–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dahimene S, Page KM, Kadurin I, et al. The alpha2delta-like protein cachd1 increases n-type calcium currents and cell surface expression and competes with alpha2delta-1. Cell Rep. 2018;25:1610–21 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Anantharaman V, Aravind L. Cache-a signalling domain common to animal Ca channel subunits and a class of prokaryotic chemotaxis receptors. Trends Biochem Sci. 2000;25:535–537. [DOI] [PubMed] [Google Scholar]
- [35].Gumerov VM, Andrianova EP, Matilla MA, et al. Amino acid sensor conserved from bacteria to humans. PNAS. 2022; 19: e2110415119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Upadhyay AA, Fleetwood AD, Adebali O, et al. Cache domains that are homologous to, but different from pas domains comprise the largest superfamily of extracellular sensors in prokaryotes. PLoS Comput Biol. 2016;12:e1004862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cottrell GS, Soubrane CH, Hounshell JA, et al. CACHD1 is an α2δ-like protein that modulates Ca V 3 voltage-gated calcium channel activity. J Neurosci. 2018;38:9186–9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ablinger C, Eibl C, Geisler SM, et al. alpha(2)delta-4 and Cachd1 Proteins Are Regulators of Presynaptic Functions. Int J Mol Sci, 2022. 2022:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Field MJ, Cox PJ, Stott E, et al. Identification of the α2δ-1 subunit of voltage-dependent calcium channels as a novel molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci USA. 2006;103:17537–17542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Brown JP, Gee NS. Cloning and deletion mutagenesis of the α2δ calcium channel subunit from porcine cerebral cortex. J Biol Chem. 1998;273:25458–25465. [DOI] [PubMed] [Google Scholar]
- [41].Brust PF, Simerson S, McCue AF, et al. Human neuronal voltage-dependent calcium channels: studies on subunit structure and role in channel assembly. Neuropharmacology. 1993;32:1089–1102. [DOI] [PubMed] [Google Scholar]
- [42].Barclay J, Rees M. Genomic organization of the mouse and human α2δ2 voltage-dependent calcium channel subunit genes. Mammalian Genome. 2000;11:1142–1144. [DOI] [PubMed] [Google Scholar]
- [43].Lana B, Schlick B, Martin S, et al. Differential upregulation in DRG neurons of an alpha2delta-1 splice variant with a lower affinity for gabapentin after peripheral sensory nerve injury. Pain. 2014;155:522–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Angelotti T, Hofmann F. Tissue-specific expression of splice variants of the mouse voltage-gated calcium channel α2/δ subunit. FEBS Lett. 1996;397:331–337. [DOI] [PubMed] [Google Scholar]
- [45].Dolphin AC. Voltage-gated calcium channel alpha 2delta subunits: an assessment of proposed novel roles. F1000Res. 2018;7:1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Geisler S, Schopf CL, Stanika R, et al. Presynaptic alpha2delta-2 calcium channel subunits regulate postsynaptic GABAA receptor abundance and axonal wiring. J Neurosci. 2019;39:2581–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Fuller-Bicer GA, Varadi G, Koch SE, et al. targeted disruption of the voltage-dependent Ca2+ Channel alpha2delta-1 Subunit. Am J Physiol Heart Circ Physiol. 2009;297:H117–H24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Patel R, Bauer CS, Nieto-Rostro M, et al. alpha2delta-1 gene deletion affects somatosensory neuron function and delays mechanical hypersensitivity in response to peripheral nerve damage. J Neurosci. 2013;33:16412–16426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bauer CS, Nieto-Rostro M, Rahman W, et al. The increased trafficking of the calcium channel subunit α2δ-1 to presynaptic terminals in neuropathic pain is inhibited by the α2δ ligand pregabalin. J Neurosci. 2009;29:4076–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Newton RA, Bingham S, Case PC, et al. Dorsal root ganglion neurons show increased expression of the calcium channel alpha2delta-1 subunit following partial sciatic nerve injury. Brain Res Mol Brain Res. 2001;95:1–8. [DOI] [PubMed] [Google Scholar]
- [51].Luo ZD, Calcutt NA, Higuera ES, et al. Injury type-specific calcium channel alpha 2 delta-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J Pharmacol Exp Ther. 2002;303:1199–1205. [DOI] [PubMed] [Google Scholar]
- [52].Nieto-Rostro M, Patel R, Dickenson AH. Nerve injury increases CaV2.2 trafficking in dorsal root ganglion mechanoreceptor central terminals. Pain. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Li CY, Zhang XL, Matthews EA, et al. Calcium channel alpha(2)delta(1) subunit mediates spinal hyperexcitability in pain modulation. Pain. 2006;125:20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Faria LC, Gu F, Parada I, et al. Epileptiform activity and behavioral arrests in mice overexpressing the calcium channel subunit alpha2delta-1. Neurobiol Dis. 2017;102:70–80. [DOI] [PubMed] [Google Scholar]
- [55].Lee ST, Lee BJ, Bae JY, et al. CaV alpha2delta Autoimmune Encephalitis: a Novel Antibody and its Characteristics. Ann Neurol. 2021;89:740–752. [DOI] [PubMed] [Google Scholar]
- [56].Shi Y, Park KS, Kim SH, et al. IgGs from patients with amyotrophic lateral sclerosis and diabetes target CaValpha2delta1 subunits impairing islet cell function and survival. Proc Natl Acad Sci U S A. 2019;116:26816–26822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ivanov SV, Ward JM, Tessarollo L, et al. Cerebellar ataxia, seizures, premature death, and cardiac abnormalities in mice with targeted disruption of the Cacna2d2 gene. Am J Pathol. 2004;165:1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Brodbeck J, Davies A, Courtney J-M, et al. The ducky mutation in Cacna2d2 results in altered Purkinje cell morphology and is associated with the expression of a truncated a2d-2 protein with abnormal function. J Biol Chem. 2002;277:7684–7693. [DOI] [PubMed] [Google Scholar]
- [59].Pirone A, Kurt S, Zuccotti A, et al. alpha2delta3 is essential for normal structure and function of auditory nerve synapses and is a novel candidate for auditory processing disorders. J Neurosci. 2014;34:434–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Dickman DK, Kurshan PT, Schwarz TL. Mutations in a Drosophila alpha2delta voltage-gated calcium channel subunit reveal a crucial synaptic function. J Neurosci. 2008;28:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Neely GG, Hess A, Costigan M, et al. A genome-wide Drosophila screen for heat nociception identifies alpha2delta3 as an evolutionarily conserved pain gene. Cell. 2010;143:628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ablinger C, Geisler SM, Stanika RI, et al. Neuronal alpha2delta proteins and brain disorders. Pflugers Arch. 2020;472:845–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Andrade A, Brennecke A, Mallat S, et al. Genetic associations between voltage-gated calcium channels and psychiatric disorders. Int J Mol Sci. 2019:20. doi: 10.3390/ijms21010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Pippucci T, Parmeggiani A, Palombo F, et al. A novel null homozygous mutation confirmsCACNA2D2 as a gene mutated in epileptic encephalopathy. PLoS ONE. 2013;8:e82154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Edvardson S, Oz S, Abulhijaa FA, et al. Early infantile epileptic encephalopathy associated with a high voltage gated calcium channelopathy. J Med Genet. 2013;50:118–123. [DOI] [PubMed] [Google Scholar]
- [66].Butler KM, Holt PJ, Milla SS, et al. Cerebellar Atrophy resulting from compound heterozygous CACNA2D2 Variants. Case Rep Genet. 2018;2018:6308283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Punetha J, Karaca E, Gezdirici A, et al. Biallelic CACNA2D2 variants in epileptic encephalopathy and cerebellar atrophy. Ann Clin Transl Neurol. 2019;6:1395–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kadurin I, Rothwell SW, Lana B, et al. LRP1 influences trafficking of N-type calcium channels via interaction with the auxiliary alpha2delta-1 subunit. Sci Rep. 2017;7:43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Burashnikov E, Pfeiffer R, Barajas-Martinez H, et al. Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm. 2010;7:1872–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Templin C, Ghadri JR, Rougier JS, et al. Identification of a novel loss-of-function calcium channel gene mutation in short QT syndrome (SQTS6). Eur Heart J. 2011;32:1077–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Crawford P, Ghadiali E, Lane R, et al. Gabapentin as an antiepileptic drug in man. J Neurol Neurosurg Psychiatry. 1987;50(6):682–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gee NS, Brown JP, Dissanayake VUK, et al. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the α2δ subunit of a calcium channel. J Biol Chem. 1996;271:5768–5776. [DOI] [PubMed] [Google Scholar]
- [73].Lotarski SM, Donevan S, El Kattan A, et al. Anxiolytic-like activity of pregabalin in the Vogel conflict test in alpha2delta-1 (R217A) and alpha2delta-2 (R279A) mouse mutants. J Pharmacol Exp Ther. 2011;338:615–621. [DOI] [PubMed] [Google Scholar]
- [74].Tran-Van-Minh A, Dolphin AC. The alpha2delta ligand gabapentin inhibits the Rab11-dependent recycling of the calcium channel subunit alpha2delta-2. J Neurosci. 2010;30:12856–12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Hendrich J, Tran-Van-Minh A, Heblich F, et al. Pharmacological disruption of calcium channel trafficking by the α2δ ligand gabapentin. Proc Natl Acad Sci USA. 2008;105:3628–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Hendrich J, Bauer CS, Dolphin AC. Chronic pregabalin inhibits synaptic transmission between rat dorsal root ganglion and dorsal horn neurons in culture. Channels (Austin). 2012;6:124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Heblich F, Tran-Van-Minh A, Hendrich J, et al. Time course and specificity of the pharmacological disruption of the trafficking of voltage-gated calcium channels by gabapentin. Channels. 2008;2:4–9. [DOI] [PubMed] [Google Scholar]
- [78].Cassidy JS, Ferron L, Kadurin I, et al. Functional exofacially tagged N-type calcium channels elucidate the interaction with auxiliary alpha2delta-1 subunits. Proc Natl Acad Sci U S A. 2014;111:8979–8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Marais E, Klugbauer N, Hofmann F. Calcium channel alpha(2)delta subunits - Structure and gabapentin binding. Mol Pharmacol. 2001;59:1243–1248. [DOI] [PubMed] [Google Scholar]
- [80].Meyer JO, Dolphin AC. Rab11-dependent recycling of calcium channels is mediated by auxiliary subunit alpha2delta-1 but not alpha2delta-3. Sci Rep. 2021;11:10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Jumper J, Evans R, Pritzel A, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Martin-Mora D, Ortega A, Perez-Maldonado FJ, et al. The activity of the C4-dicarboxylic acid chemoreceptor of Pseudomonas aeruginosa is controlled by chemoattractants and antagonists. Sci Rep. 2018;8:2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Johnson KS, Elgamoudi BA, Jen FE-C; Johnson KS, Elgamoudi BA, Jen FE, Day CJ, Sweeney EG, Pryce ML, et al . The dCache chemoreceptor TlpA of Helicobacter pylori binds multiple attractant and antagonistic ligands via distinct sites. mBio. 2021;12:e0181921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Brown JP, Dissanayake VU, Briggs AR, et al. Isolation of the [3H]gabapentin-binding protein/alpha 2 delta Ca2+ channel subunit from porcine brain: development of a radioligand binding assay for alpha 2 delta subunits using [3H]leucine. Anal Biochem. 1998;255:236–243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Structural data are available at https://github.com/ToshkaDev/Alpha2Delta-proteins-review. Other data will be made available upon reasonable request.


