Abstract
The pandemic coronavirus disease 2019 (COVID-19) has rapidly spread to all countries worldwide. The emergence of its variants has exacerbated this problem. To date, many variants have been identified across the viral genome; the variants of concern are the focus of attention due to their higher transmissibility and resistance to vaccines, especially the delta variant. The delta variant has become the dominant severe acute respiratory syndrome novel coronavirus (SARS-CoV-2) variant worldwide, causing severe panic as it is highly infectious. A better understanding of these variants may help in the development of possible treatments and save more lives. In this study, we summarize the characteristics of the variants of concern. More importantly, we summarize the results of previous studies on the delta variant. The delta variant has a high transmissibility rate and increases the risk of hospitalization and death. However, it is partially sensitive to vaccines. In addition, nonpharmaceutical interventions are valuable during epidemics. These interventions can be used against the delta variant, but managing this variant should still be taken seriously.
Key words: severe acute respiratory syndrome novel coronavirus, variants of concern, vaccine, delta variant
Introduction
Since the pandemic occurred, severe acute respiratory syndrome novel coronavirus (SARS-CoV-2) has rapidly spread in all countries worldwide, with nearly 200 million confirmed cases and over 4 million deaths reported worldwide.[1] SARS-CoV-2 belongs to the cluster of β-coronaviruses with positive-sense, single-stranded RNA (ssRNA).[2] It is much easier for RNA viruses to mutate due to the intrinsically error-prone nature of RNA polymerase during replication.[3] The gene correction mechanism in coronaviruses such as SARS-CoV-2 increases the replication accuracy and viral transcription; this means that the mutation accumulation rate would be slower than that of other RNA viruses.[4] At present, according to the characteristics of the variants, the US government interagency organizations have divided the variants into three classes: (1) variants of interest (VOI); (2) variants of concern (VOC): B.1.1.7 (alpha variant), B.1.351 (beta variant), B.1.617.2 (delta variant), and P.1 (gamma variant); and (3) variants of high consequence: none of the variants have been classified as variants of high consequence.[5]
Among these variants, delta variant, which was first reported in India, has surpassed the alpha variant and has gradually become the major global pandemic variant since June 2021, with 89% of coronavirus disease 2019 (COVID-19) patients infected with this variant on July 26, 2021.[6,7] Similar to other SARS-CoV-2 variants, the delta variant can attack cells by binding between viral spike proteins and host angiotensin-converting enzyme 2 (ACE2),[8, 9, 10, 11] during which the receptor-binding domain (RBD) on the spike protein plays a fundamental role.[12, 13, 14] RBD is also a dominant immune epitope of the spike protein.[15, 16, 17] The mutations in the spike protein significantly increase the infectivity and transmissibility of the delta variant.[18, 19, 20] Colhoff, staff of the World Health Organization (WHO), stated that the delta variant has now spread to 92 countries worldwide. More than 10,000 new cases per day were confirmed in the UK, 99% of which were caused by the more infectious delta variant; it replaced the alpha variant and became the main British pandemic variant.[21,22] In the USA also, the proportion of COVID-19 cases caused by the delta variant is increasing, which was approximately 31.1% (95% confidence interval [CI], 24.6%–48.3%) on July 20; now, it has exceeded the proportion of COVID-19 cases caused by the alpha variant, with a proportion of 82.2% (95% CI, 78.3%–86.0%).[23] It has become a huge concern in many countries, threatening the control and prevention of the global epidemic, especially in some developing countries with poorly prepared public health systems.[24,25] This study summarizes the characteristics of VOC, especially the results of previous studies on the transmissibility and clinical severity of the delta variant, and the vaccine efficacy. We provide advice on maintaining a positive, but serious attitude in the midst of the COVID-19 pandemic and suggestions on the prevention and control of epidemics.
Reasons of mutation
Mutation is a common phenomenon in the evolution of viruses, and SARS-CoV-2 is no exception. Mutation provides an opportunity for viruses to adapt to a new host and/ or evade the host immune response.[26] Coronavirus has a non-structural protein with exoribonuclease activity, which can perform gene correction.[27,28] It also allows the coronavirus to continuously mutate compared with other RNA viruses,[29] making it easier for a novel coronavirus to invade the host and cause damage.
As the virus spreads, selective pressure drives the retention of certain new mutations.[2] With regard to SARS-CoV-2, the spread of this virus to a huge amount of susceptible population allows virus replication, and thus induces more new mutations. The different strengths of the immune response of patients during host-to-host transmission contribute to selective pressure, which probably promotes the retention of adaptive mutations, facilitating faster spread and longer incubation period and even causing immune evasion.[29] For instance, high population density and inadequate epidemic control in India created opportunities for the emergence of delta variants, which accumulated new mutations such as T19R, T478K, and P681R.[30,31] However, not all mutations have the incredible ability to increase or decrease infectivity. The mutation may be caused by the genetic drift,[32] including the gene hitchhiker or founder effect. A typical example of SARS-CoV-2 mutation is the D614G mutation.[33,34] The D614G mutation may result from replacement of the hitchhiker gene. D614G is accompanied by a mutation in ORF1A/B (P4715L), which may enhance the variant’s adaptability and in turn benefit the advantageous mutation – D614G.[35,36]
With the transmission of the virus among the population, American epidemiologist Fauci stated, “You still have a fixed immunogen and a virus that is changing. Sooner or later, you are going to get a mutant that evades that.”[37] The SARS-CoV-2 variant, like delta variant, may gain a significant adaptation advantage and greatly influence the efficacy of existing therapeutic regimens.[38] To effectively control the variants, more studies on the specific origin and evolution of SARS-CoV-2 variants are warranted in order to develop new methods, such as blocking one step of the evolution of variants.[39]
Global mainstream variant – Delta variant
VOC are more infectious than VOI and can cause severe diseases. In addition, patients infected with VOC have produced significantly less neutralizing antibodies (NAbs) regardless of the status of infection or vaccination, making it difficult for some tests to diagnose the infection and reducing the effectiveness of treatments and vaccines.[5] Herein, we aimed to summarize the characteristics of VOC (Table 1), especially a detailed description of the delta variant in terms of infectivity, severity, and impact on vaccines (Table 2 and 3), which has become a global concern.
Table 1.
The characteristics of VOC
| VOC | Alpha variant | Beta variant | Gamma variant | Delta variant |
|---|---|---|---|---|
| Alternative name | 20I/501Y.V1 VOC202012/01 B.1.1.7 | 20H/501.V2 B.1.351 | 20C/S:452R CAL.20C P.1 | 20A/S:478K B.1.617.2 |
| First identification | September 2020 | October 2020 | January 2021 | October 2020 |
| Affected nations | UK 182 | South Africa 131 | Japan/Brazil 81 | India 132 |
| Transmissibility | Increase ﹥ 50% | Increase ﹥ 50% | Increase 40%–120% | Increase ﹥ 100% |
| Severity | Increased risk of hospitalization Increased fatality | Increased reinfection rate | Increased mortality Increased reinfection rate | Increased risk of hospitalization and deaths |
| Immunogenicity (neutralization antibody) | Mildly to moderately reduce Significantly reduce with E484K mutation | Significantly reduce | Moderately reduce | Moderately reduce |
| Reference | [3],[5],[70],[71],[72],[73],[74],[75] | [5],[75],[76],[77],[78] | [3],[5],[26],[75] [79],[80] | [5],[18],[22],[49],[63],[75], [81] |
VOC: variants of concern.
Table 2.
The effects of Delta variant on neutralizing antibody
| Vaccine | Serological assay | Samples | Reference objects | Serum specimen | Median age | Country | Result | Reference |
|---|---|---|---|---|---|---|---|---|
| BNT162B2 | Pseudovirus | 20 | Wild type | Two doses | NA | USA | GMT: wild type was 502 B.1.617.2-spike was 355 B.1.617.2-v2-spike was 343 | [22] |
| Live virus | 250 | Wild type | Two doses One dose | 42 | UK | Two doses: NAbTs: 5.8-fold reduced (95% CI 5.0–6.9) One dose: 68% had low NAbTs (IC50* < 40) | [63] | |
| Pseudovirus | 36 | Wild type | Two doses | 48 | Israel | Delta-S1#: NAbTs: 2.6-fold reduced (95% CI, 1.8–3.5) Delta-S2: NAbTs: 2.1-fold reduced (95% CI, 1.7–2.5) | [82] | |
| Pseudovirus | 9 | D614G | Two doses | 31 | USA | NAbTs: 3.6-fold reduced | [83] | |
| ChAdOx1 | Live virus | 106 | BNT162B2 Wild type | Two doses One dose | 34 | UK | Two doses: NAbTs: 2.5-fold reduced (95% CI, 1.4–2.7) relative to BNT162B2 One dose: 85% (95% CI, 68%– 94%) had quantifiable NAbTs; 91% (95% CI, 75%–98%) wild type had quantifiable NAbTs | [63][64] |
| mRNA- 1273 | Pseudovirus | 8 | D614G | Two doses | 41 | USA | NAbTs: fourfold reduced | [83] |
NA: not available; GMT: geometric mean neutralizing titers; NAbT: neutralizing antibody titers. IC50*: 50% inhibitory concentration; #delta sample 1 (S1, hCoV-19/Israel/CVL-12804/2021) and sample 2 (S2, hCoV-19/Israel/CVL-12806/2021).
Table 3.
The efficacy of vaccine
| Vaccine | Samples | Country | Any vaccine | One dose | Two doses | Reference |
|---|---|---|---|---|---|---|
| BNT162B2 | 4,272 | UK | 30.7% (95% CI, 25.2%–35.7%) | 35.6% (95% CI, 22.7%–46.4%) | 88.0% (95% CI, 85.3%–90.1%) | [62] |
| ChAdOx1 | 4,272 | UK | 30.7% (95% CI, 25.2%–35.7%) | 30.0% (95% CI, 24.3%–35.3%) | 67.0% (95% CI, 61.3%–71.8%) | [62] |
| mRNA- 1273 | NA | NA | NA | NA | NA | NA |
NA: not available.
Characteristic mutations in spike protein
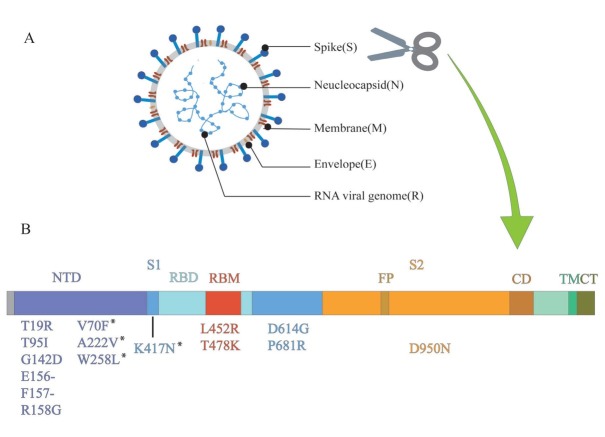
Delta variants have a variety of new mutations, including T19R, T478K, and P681R, which were not found in previous variants (Figure 1). The D614G mutation is shared with other VOC and increases the infection titers.[35] In addition to the D614G mutation, the delta variant has some important mutations such as L452R, T478K, and P681R. Like N501Y, the L452R and T478K mutations are also located in the RBD, suggesting that they may also have an effect on the infectivity of the variant.[40] Deng et al.[26] infected 293T cells, which stably expressed the ACE2 receptor and the TMPRSS2 cofactor of SARS-CoV-2, with a pseudovirus carrying the L452R mutation. In 293T cells, the reproductive number of pseudoviruses carrying the L452R mutation was 5.8–14.7 times higher than that of pseudoviruses carrying the D614G mutation, suggesting a higher infectivity of the L452R mutation. In addition, researchers have employed the TopNetTree model to predict the binding free energy (BFE) changes of RBD and ACE2 induced by mutations. The infectivity of different variants is directly proportional to the BFE between the
Figure 1.
Structure of SARS-CoV-2 and mutations of delta variant. (A) SARS-CoV-2 forms coated spherical particles with diameters of 100–160 nm. It contains a 27–32 kb positive ssRNA genome. Envelope glycoproteins spike protein (S), envelope (E), membrane (M), and nucleocapsid (N) are encoded by 3' terminal genome. (B) Spike protein is critical for the virus to invade the host, which is also a key focus in the research on SARS-CoV-2. S1 and S2 subunits and a transmembrane domain constitute a spike protein. Scissor cuts at the boundary of S1 and S2. RBD containing the core-RBD and RBM, and NTD are on S1, which undertakes the role of binding to the host cell receptor, especially RBD, while S2 consists of FP, CD, and CT, contributing to membrane fusion. Mutations of delta variant are marked in the figure. ssRNA: single-stranded RNA; NTD: amino-terminal domain; RBD: receptor-binding domain; RBM: receptor-binding motif; FP: fusion peptide; CD: connecting domain; TM: transmembrane domain; CT: cytoplasmic tail; SARS-CoV-2: severe acute respiratory syndrome novel coronavirus. *Detected in some sequences of delta variant, but not all.
RBD of the variants and ACE2. Results revealed that T478K mutation had the largest BFE change, which meant that T478K might be vital to the high infectivity of the delta variant.[41] Double mutation, like L452R and T478K, is more likely to be dominant in enhancing the binding affinity of the spike protein to ACE2.[42] A review of SARS-CoV-2 mutation sites, identified by CoV-GLUES, suggested that P681R, like D614G, was also adjacent to the RBD in the S1 subunit and could switch the RBD to an “open” conformation, promoting the binding between RBD and ACE2.[32,40] Cherian et al.[43] analyzed the P681R structure at the furoin cleavage site and revealed its role in enhancing the alkalinity of polybasic stretching. This may contribute to the increased infectivity by increasing the rate of membrane fusion internalization.
The critical mutations of the delta variant mainly occur in the spike protein. Among them, mutations in and adjacent to the RBD, such as L452R, T478K, D614G, and P681R, are due to their ability to invade the host. In addition, the accumulation of these mutations modifies the characteristics of the delta variant, offering a new way to understand its traits.
Transmissibility of the delta variant
Campbell et al.[44] analyzed 1,722,652 sequences of SARS-CoV-2 uploaded to the hCOV-19 database of the Global Initiative of Sharing All Influenza Data. They applied a competitive growth multinomial logistic model to estimate the effective reproduction rate of each variant. Compared with VOI, the effective reproduction rate of the delta variant was 97% (95% CI, 76%–117%). In this study, the delta variant was the VOC with the highest effective reproduction number, implying that this variant has extremely high transmissibility and might also be the variant with the highest transmissibility to date. Allen et al.[45] assessed the probability of familial transmission (≥ 2 cases within 14 days) of the delta variant in a case–control study conducted in the UK. A total of 3,765 clustered families were sequenced to match 7,530 sporadic cases (a single case in each family). After adjusting for age, sex, ethnicity, index of multiple deprivation, and inoculation status of indicative cases, the odds ratio of household transmission of the delta variant compared with that of the alpha variant was 1.64 (95% CI, 1.26–2.13). In a study published in the Chinese Center for Disease Control and Prevention, Zhang et al.[46] reported the clear chain of transmission of the initial 68 infections in Guangdong and found that the mean incubation period was 4.4 days (95% CI, 3.9–5.0), with a mean interval of transmission of 2.9 days (95% CI, 2.4–3.3), which was shorter than the initial mean incubation period of 5.2 days (95% CI, 4.1–7.2) and the mean interval of transmission of 7.5 days (95% CI, 5.3–19) for SARS-CoV-2 virus.[47]
The delta variant is the most prevalent variant worldwide, with remarkably high transmissibility. Regardless of the parameter indicating transmissibility, the delta variant shows incredible superiority compared with the alpha variant, which was once preponderant. To control the damage caused by the delta variant, effective measures to control the transmission are warranted.
Impacts of the delta variant on clinical severity
A highly transmissible variant, such as the delta variant, may rapidly increase the number of confirmed cases within a short period of time as well as the number of hospital admissions. Inadequate medical personnel and facilities may contribute to the increased mortality rates.
Considering the abovementioned factors, whether the delta variant can cause serious clinical consequences remains a concern. Sheikh et al.[48] evaluated the risk of hospitalization associated with the delta variant. In their study, inpatients with COVID-19 were defined as those who tested positive for SARS-CoV-2 within 14 days of admission. Patients with hospital-acquired COVID-19 were excluded from the study. In the EAVE II platform, a Scotland-wide COVID-19 surveillance platform, they found that delta variant-related cases were associated with an increased risk of COVID-19 hospitalization, compared with the alpha variant-related cases (hazard ratio, 1.85; 95% CI, 1.39–2.47), as reported in a cohort analysis after adjustment of cofounders. A study from the UK showed that the number of COVID-19 cases in the UK increased, as was the number of hospital admissions. On June 9, more than 1,000 people were admitted to the hospital daily due to COVID-19. With regard to the previous outbreak, the number had dropped to a few hundreds by mid-May. Figures from Public Health England showed that 90% of cases in the UK were caused by this variant, with more than 42,000 cases reported, suggesting that this variant might be associated with a higher risk of hospitalization.[9] Ong et al.[49] conducted a retrospective cohort study comparing the outcomes of patients infected with the delta variant in early 2021 with those of patients infected with the wild-type virus in Singapore. From January 1, 2021 to May 22, 2021, data of 838 cases of VOC infection were collected. After adjusting for age and sex, the delta variant-related infections were associated with higher oxygen consumption, intensive care unit (ICU) admission, or death (adjusted odds ratio [aOR]: 4.90; 95% CI, 1.43–30.78). For the 157 patients with VOC admitted to their center, the aOR of pneumonia related to the delta variant was 1.88 (95% CI, 0.95–3.76). In addition, the delta variant was incredibly associated with a longer estimated median duration (18 vs. 13 days) after adjusting for age, sex, comorbidities, and vaccination. This variant seems to have the potential to increase the severity of disease. Another retrospective cohort study in Ontario constructed a mixed-effects logistic regression model using ICU admission and death as outcome variables.[50] After adjustment, in contrast to non-VOC strains, the risk rates of poor clinical outcome due to infection with the delta variant were 120% (95% CI, 93%–153%) for hospitalization, 287% (95% CI, 198%–399%) for ICU admission, and 137% (95% CI, 50%–230%) for deaths. The trial lacked data on vaccination status at the individual level, and the confounding factors were difficult to exclude due to the ongoing vaccination programs. In addition, some Indian doctors have suggested that the delta variant may cause gangrene, hearing loss, loss of appetite, and other atypical symptoms in infected patients.[51]
Overall, delta variants with high transmissibility augment the risks of hospitalization and death. However, a comprehensive evaluation tool for assessing disease severity is warranted; moreover, close attention should be paid to the different symptoms of patients infected with the delta variant and those infected with the wild-type variant, especially individuals with more severe symptoms.
Efficacy of vaccines on the delta variant
Given that the delta variant is more transmissible than the alpha variant, is the vaccine still protective? To date, many vaccines have been introduced, such as BNT162B2[52,53] and Chadox1,[54,55] and many biotechnology companies are manufacturing these vaccines.[56, 57, 58, 59, 60]
A prospective cohort study was conducted in Scotland to investigate the effects of vaccines before the emergence of the delta variant. It is estimated the hospitalization rate of patients infected with SARS-CoV-2 after the first dose of vaccine by fitting a time-dependent Cox model and a Poisson regression model with adverse propensity weights. Among 1,331,993 people with a mean age of 65 years (standard deviation: 16.2) who received the vaccine (from December 8, 2020 to February 22, 2021), the first dose of BNT162B2 reduced the hospitalization rates by 91% (95% CI, 85%–94%) on days 28–34 after vaccination, while Chadox1 reduced the risk by 88% (95% CI, 75%–94%); these results suggest that vaccination can greatly reduce the risk of hospitalization.[61] After the global spread of the delta strain, Bernal et al.[62] explored the efficacy of two vaccines, BNT162B2 and Chadox1, on symptomatic delta variant-infected patients in a test-negative, case–control design. On the contrary, regardless of the type of vaccine, the efficacy of one vaccine dose was 30.7% (95% CI, 25.2%–35.7%) in delta variant-infected patients, which was considerably lower than that in alpha variant-infected patients (48.7%; 95% CI, 45.5%–51.7%). By contrast, the efficacy of two doses of BNT162B2 against the delta variant was 88.0% (95% CI, 85.3%–90.1%), while the efficacy of two doses of Chadox1 against the delta variant was 67.0% (95% CI, 61.3%–71.8%).
The neutralization effect of the vaccines on the delta variant was also explored. Liu et al.[22] assessed antibody neutralization by introducing different mutations in spike protein genes into the wild-type SARS-CoV-2 variant. Although the neutralization effect of BNT162B2 on B. 1.617.1 was weak, it had an effective neutralization effect on other B.1.617 lineage variants, including the delta variant. Wall et al.[63] found that for the delta variant, two doses of BNT162B2 induced a 5.8-fold reduction in serum antibody neutralization titer (95% CI, 5.0–6.9) in a high-throughput SARS-CoV-2 virus assay, which was significantly higher than that of the alpha variant (2.6-fold; 95% CI, 2.2–3.1). Additionally, increase in age was markedly correlated with a decrease in the production of NAbs in all variants. In addition, a high-throughput live-virus SARS-CoV-2 neutralization assay showed that the delta variant reduced the expression of Chadox1-induced NAbs, in contrast to BNT162B2. Data from 106 participants after they received one dose (median time: after the first dose of 41 days) or two doses of Chadox1 (median time: after the second dose, 31 days) were included, and the median interval between doses was 63 days. Two doses of Chadox1 vaccine generated a 2.5-fold (1.4–2.7) reduction in NAb activity against the delta variant, compared with two doses of the BNT162b2 vaccine. Two doses of the BNT162B2 vaccine seemed to be more effective against the delta variant.[64]
Although the BNT162B2 and Chadox1 vaccines are less effective against the delta variant than against the wild-type SARS-CoV-2 variant, the vaccines are still protective, based on the findings of NAb titer experiments and large clinical trials. Despite the high transmissibility and high risk of hospitalization and death caused by infection with the delta variant, the BNT162B2 and Chadox1 vaccines remain effective and protective.
Reacting optimistically, but cautiously
As mentioned above, despite the fact that the delta variant is highly transmissible and increases the risk of hospitalization, the vaccines remain effective and protective. Vaccination popularization must be accelerated to achieve herd immunity. In addition, a brief report by Krammer et al.[65] mentioned that in patients who had been immunized against SARS-CoV-2, especially those who were infected with SARS-CoV-2, the immune response to a single dose of vaccine exceeded that of uninfected individuals who received two doses of vaccine; the frequency of systemic side effects was higher than that in uninfected individuals. This means that people who were infected in the past probably only need a single dose of vaccine, which, given the limited supply of vaccine, could increase the number of people who can be vaccinated and amplify coverage. This is good news and raises realistic hope for countries with vaccination plans to encourage universal vaccination. We should not be overly alarmed and excessively anxious about the emergence of the delta variant and should be more optimistic.
Notably, the delta variant can be transmitted through the respiratory tract, hands, contaminants, and aerosols.[1] Therefore, wearing of face masks, maintaining social distance, and avoiding high-traffic areas are essential NPIs. More attention should be paid to the functions of NPIs. Currently, there are no effective drugs for the treatment of delta variants. A previous WHO trial pointed out that currently used antiviral drugs, such as remdesivir, hydroxychloroquine, lopinavir, and interferon regimens, had little effect on COVID-19–hospitalized patients, with no significant improvement in the overall mortality or length of hospitalization.[66] At present, vaccination and NPIs are the two powerful tools. Moreover, vaccination alone cannot sufficiently control the epidemic. Moore et al.[67] developed a mathematical model to study the vaccination status and NPIs according to age and region of the UK, which was consistent with a series of epidemiological data from the UK. In terms of vaccination, relaxation of prevention and control measures would still trigger a peak in the number of COVID-19 cases. Even if the protection rate of vaccination was assumed as 85%, the complete relaxation of prevention and control would likely increase the mortality rate. The model incorporated a planned two-dose vaccination schedule (12 weeks apart, with protection beginning 14 days after vaccination). For some countries that were considered as the center of the pandemic outbreak, it is of referential significance to implement strict NPIs.
Furthermore, with the transmission among large numbers of people, new mutations and variants might better adapt to the host. Although current vaccines are effective, a small percentage of fully vaccinated people can still be infected when exposed to the virus. These cases are referred to as vaccine breakthroughs.[68] Delta variant has been proved to be capable of causing vaccine breakthrough and dominates vaccine breakthrough infections with higher respiratory viral loads in India.[69] While facing an unknown future, all these demonstrate that NPIs are now essential in controlling the epidemic. However, precautionary measures should still be implemented during this epidemic, prevention and control policies should not be easily loosened, and vaccination should be continued.
It is not a war in one country or region. Several national organizations are implementing measures to win the invincible war, such as the Centers for Disease Control and Prevention in the USA (CDC), Public Health England (PHE), and the Chinese CDC. More countries are invited to join them, not only in aggregating regional confirmed cases but also in understanding the different traits of the variants and keeping track of the latest developments in the mutation of the variants, so that more reasonable and valid public health decisions and vaccine recommendations can be determined and applied globally. Furthermore, a brighter future is expected with the arrival of more effective drugs.
Conclusion
The delta variant is the most prevalent SARS-CoV-2 variant worldwide. This variant has high transmissibility and increases the risk of hospitalization and death, probably causing more severe diseases, which needs to be confirmed by conducting additional studies. The delta variant partially reduces the effectiveness of vaccines, but it does not mean that these vaccines are invalid. Vaccines and NPIs have the potential to control the pandemic, which greatly relieves our concerns. The future of the delta variant is unknown; we should stay optimistic, but remain cautious.
Funding Statement
This study was supported by the Specialized Department Foundation of the Minhang District (No.2020 MWTZB02) and Shanghai Municipal Science and Technology Commission (No. 20ZR1443700).
Footnotes
Conflict of Interest
None declared.
References
- 1.WHO Coronavirus (COVID-19) Situation Dashboard. https://covid19.who.int/ https://covid19.who.int/ World Health Organization. Available at. Accessed on August 2, 2021.
- 2.Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–92. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janik E, Niemcewicz M, Podogrocki M, Majsterek I, Bijak M. The Emerging Concern and Interest SARS-CoV-2 Variants. Pathogens. 2021;10:633. doi: 10.3390/pathogens10060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morens DM, Fauci AS. Emerging Pandemic Diseases: How We Got to COVID-19. Cell. 2020;183:837. doi: 10.1016/j.cell.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SARS-CoV-2 variant classifications and definitions. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html Centers for Disease Control and Prevention. Available at. Accessed on August 2, 2021.
- 6.VariantGraphs (Enabled by data from GISAID) https://covid19dashboard.regeneron.com/?tab=Variant_Graphs.Accessed. https://covid19dashboard.regeneron.com/?tab=Variant_Graphs.Accessed Regeneron. Available at. on August 2, 2021.
- 7.European Centre for Disease Prevention and Control. Threat Assessment Brief: Emergence of SARS-CoV-2 B.1.617 variants in India and situation in the EU/EEA - 11 May 2021: ECDC. https://www.ecdc.europa.eu/sites/default/files/documents/Emergence-of-SARS-CoV-2-B.1.617-variants-in-India-and-situation-in-the-EUEEA_0.pdf. https://www.ecdc.europa.eu/sites/default/files/documents/Emergence-of-SARS-CoV-2-B.1.617-variants-in-India-and-situation-in-the-EUEEA_0.pdf Available at. Accessed on May 11, 2021.
- 8.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3. doi: 10.1038/s41586-020-2012-7. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D, Lee JY, Yang JS, Kim JW, Kim VN, Chang H. The Architecture of SARS-CoV-2 Transcriptome. Cell. 2020;181:914–21. doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell. 2020;181:894–904. doi: 10.1016/j.cell.2020.03.045. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–20. doi: 10.1038/s41586-020-2180-5. et al. [DOI] [PubMed] [Google Scholar]
- 12.Hasan A, Paray BA, Hussain A, Qadir FA, Attar F, Aziz FM. A review on the cleavage priming of the spike protein on coronavirus by angiotensin-converting enzyme-2 and furin. J Biomol Struct Dyn. 2021;39:3025–33. doi: 10.1080/07391102.2020.1754293. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnes CO, West AP Jr, Huey-Tubman KE, Hoffmann MAG, Sharaf NG, Hoffman PR. Structures of Human Antibodies Bound to SARS-CoV-2 Spike Reveal Common Epitopes and Recurrent Features of Antibodies. Cell. 2020;182:828–42. doi: 10.1016/j.cell.2020.06.025. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–80. doi: 10.1016/j.cell.2020.02.052. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djomkam ALZ, Olwal CO, Sala TB, Paemka L. Commentary: SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Front Oncol. 2020;10:1448. doi: 10.3389/fonc.2020.01448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baum A, Ajithdoss D, Copin R, Zhou A, Lanza K, Negron N. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science. 2020;370:1110–5. doi: 10.1126/science.abe2402. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brouwer PJM, Caniels TG, van der Straten K, Snitselaar JL, Aldon Y, Bangaru S. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020;369:643–50. doi: 10.1126/science.abc5902. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahase E. Delta variant: What is happening with transmission, hospital admissions, and restrictions? BMJ. 2021;373:n1513. doi: 10.1136/bmj.n1513. [DOI] [PubMed] [Google Scholar]
- 19.Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–4. doi: 10.1038/s41586-020-2179-y. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scudellari M. How the coronavirus infects cells - and why Delta is so dangerous. Nature. 2021;595:640–4. doi: 10.1038/d41586-021-02039-y. [DOI] [PubMed] [Google Scholar]
- 21.SARS-CoV-2 variants of concern and variants under investigation in England, Technical briefing 13. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/990339/Variants_of_Concern_VOC_Technical_Briefing_13_England.pdf. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/990339/Variants_of_Concern_VOC_Technical_Briefing_13_England.pdf Public Health England. Available at. Accessed on May 27, 2021.
- 22.Liu J, Liu Y, Xia H, Zou J, Weaver SC, Swanson KA. BNT162b2-elicited neutralization of B. 1.617 and other SARS-CoV-2 variants. Nature. 2021;596:273–5. doi: 10.1038/s41586-021-03693-y. et al. [DOI] [PubMed] [Google Scholar]
- 23.Variant Proportions. https://covid.cdc.gov/covid-data-tracker/#variant-proportions. https://covid.cdc.gov/covid-data-tracker/#variant-proportions Centers for Disease Control and Prevention. Available at. Accessed on August 2, 2021.
- 24.Bhatia N. Delta variant spreads in Iran and North Africa. 2021. https://www.meed.com/delta-variant-spreads-in-iran-and-north-africa. https://www.meed.com/delta-variant-spreads-in-iran-and-north-africa Available at. Accessed on August 2, 2021.
- 25.Salem M. Delta variant surges in Middle East and North Africa as region braces for ‘catastrophic consequences. 2021. https://abc17news.com/news/national-world/cnn-europe-mideast-africa/2021/07/15/delta-variant-surges-in-middle-east-and-north-africa-as-region-braces-for-catastrophic-consequences/ https://abc17news.com/news/national-world/cnn-europe-mideast-africa/2021/07/15/delta-variant-surges-in-middle-east-and-north-africa-as-region-braces-for-catastrophic-consequences/ Available at. Accessed on July 15, 2021.
- 26.Deng X, Garcia-Knight MA, Khalid MM, Servellita V, Wang C, Morris MK. Transmission, infectivity, and neutralization of a spike L452R SARS-CoV-2 variant. Cell. 2021;184:3426–37. doi: 10.1016/j.cell.2021.04.025. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith EC, Blanc H, Surdel MC, Vignuzzi M, Denison MR. Coronaviruses lacking exoribonuclease activity are susceptible to lethal mutagenesis: evidence for proofreading and potential therapeutics. PLoS Pathog. 2013;9:e1003565. doi: 10.1371/journal.ppat.1003565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sevajol M, Subissi L, Decroly E, Canard B, Imbert I. Insights into RNA synthesis, capping, and proofreading mechanisms of SARS-coronavirus. Virus Res. 2014;194:90–9. doi: 10.1016/j.virusres.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plante JA, Mitchell BM, Plante KS, Debbink K, Weaver SC, Menachery VD. The variant gambit: COVID-19’s next move. Cell Host Microbe. 2021;29:508–15. doi: 10.1016/j.chom.2021.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saito A, Irie T, Suzuki R, Maemura T, Nasser H, Uriu K. Enhanced fusogenicity and pathogenicity of SARS-CoV-2 Delta P681R mutation. Nature. 2022;602:300–6. doi: 10.1038/s41586-021-04266-9. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aleem A, Akbar Samad AB, Slenker AK. Emerging Variants of SARS-CoV-2 And Novel Therapeutics Against Coronavirus (COVID-19) Treasure Island: StatPearls Publishing; 2022. [Google Scholar]
- 32.Plante JA, Liu Y, Liu J, Xia H, Johnson BA, Lokugamage KG. Spike mutation D614G alters SARS-CoV-2 fitness. Nature. 2021;592:116–21. doi: 10.1038/s41586-020-2895-3. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou B, Thao TTN, Hoffmann D, Taddeo A, Ebert N, Labroussaa F. SARS-CoV-2 spike D614G variant confers enhanced replication and transmissibility. 2020. https://www.biorxiv.org/content/10.1101/2020.10.27.357558v1. https://www.biorxiv.org/content/10.1101/2020.10.27.357558v1 et al. Available at. Accessed on August 2, 2021.
- 34.Hou YJ, Chiba S, Halfmann P, Ehre C, Kuroda M, Dinnon KH 3rd. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science. 2020;370:1464–8. doi: 10.1126/science.abe8499. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812–27. doi: 10.1016/j.cell.2020.06.043. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weissman D, Alameh MG, de Silva T, Collini P, Hornsby H, Brown R. D614G Spike Mutation Increases SARS CoV-2 Susceptibility to Neutralization. Cell Host Microbe. 2021;29:23–31. doi: 10.1016/j.chom.2020.11.012. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin R. COVID-19 Vaccines vs Variants—Determining How Much Immunity Is Enough. JAMA. 2021;325:1241–3. doi: 10.1001/jama.2021.3370. [DOI] [PubMed] [Google Scholar]
- 38.Mlcochova P, Kemp SA, Dhar MS, Papa G, Meng B, Ferreira IATM. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599:114–9. doi: 10.1038/s41586-021-03944-y. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fareh M, Zhao W, Hu W, Casan JML, Kumar A, Symons J. Reprogrammed CRISPR-Cas13b suppresses SARS-CoV-2 replication and circumvents its mutational escape through mismatch tolerance. Nat Commun. 2021;12:4270. doi: 10.1038/s41467-021-24577-9. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409–24. doi: 10.1038/s41579-021-00573-0. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang R, Chen J, Gao K, Wei GW. Vaccine-escape and fast-growing mutations in the United Kingdom, the United States, Singapore, Spain, India, and other COVID-19-devastated countries. Genomics. 2021;113:2158–70. doi: 10.1016/j.ygeno.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan MI, Baig MH, Mondal T, Alorabi M, Sharma T, Dong JJ. Impact of the Double Mutants on Spike Protein of SARS-CoV-2 B.1.617 Lineage on the Human ACE2 Receptor Binding: A Structural Insight. Viruses. 2021;13:2295. doi: 10.3390/v13112295. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cherian S, Potdar V, Jadhav S, Yadav P, Gupta N, Das M. Convergent evolution of SARS-CoV-2 spike mutations, L452R, E484Q and P681R, in the second wave of COVID- 19 in Maharashtra, India. Microorganisms. 2021;9:1542. doi: 10.3390/microorganisms9071542. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell F, Archer B, Laurenson-Schafer H, Jinnai Y, Konings F, Batra N. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Euro Surveill. 2021;26:2100509. doi: 10.2807/1560-7917.ES.2021.26.24.2100509. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen H, Vusirikala A, Flannagan J, Twohig KA, Zaidi A, Chudasama D. Increased household transmission of COVID-19 cases associated with SARS-CoV-2 Variant of Concern B.1.617.2: a national case-control study. Lancet Reg Health Eur. 2022;12:100252. doi: 10.1016/j.lanepe.2021.100252. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang M, Xiao J, Deng A, Zhang Y, Zhuang Y, Hu T. Transmission Dynamics of an Outbreak of the COVID-19 Delta Variant B.1.617.2 – Guangdong Province, China, May – June 2021. China CDC Weekly. 2021;3:584–6. doi: 10.46234/ccdcw2021.148. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199–207. doi: 10.1056/NEJMoa2001316. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheikh A, McMenamin J, Taylor B, Robertson C. Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ong SWX, Chiew CJ, Ang LW, Mak TM, Lin C, Toh MPHS. Clinical and virological features of SARS-CoV-2 variants of concern: a retrospective cohort study comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta) Clin Infect Dis. 2021. p. ciab721. et al. [DOI] [PMC free article] [PubMed]
- 50.Fisman DN, Tuite AR. Progressive Increase in Virulence of Novel SARS-CoV-2 Variants in Ontario, Canada. 2021. https://www.medrxiv.org/content/10.1101/2021.07.05.21260050v2. https://www.medrxiv.org/content/10.1101/2021.07.05.21260050v2 Available at. Accessed on August 2, 2021. [DOI] [PMC free article] [PubMed]
- 51.Shrivastava B, Sanjai PR, Kay C. Gangrene, Hearing Loss Show Delta Variant May Be More Severe. 2021. https://www.bloomberg.com/news/articles/2021-06-07/gangrene-hearing-loss-point-to-delta-variant-being-more-severe. https://www.bloomberg.com/news/articles/2021-06-07/gangrene-hearing-loss-point-to-delta-variant-being-more-severe Available at. Accessed on June 8, 2021.
- 52.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–15. doi: 10.1056/NEJMoa2034577. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2021;590:E26. doi: 10.1038/s41586-020-03098-3. et al. [DOI] [PubMed] [Google Scholar]
- 54.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.AZD1222 Vaccine Met Primary Efficacy Endpoint in Preventing COVID-19. 2020. https://www.astrazeneca.com/media-centre/pressreleases/2020/azd1222hlr.html. https://www.astrazeneca.com/media-centre/pressreleases/2020/azd1222hlr.html AstraZeneca. Available at. 2020. Accessed on November 23, 2020.
- 56.Corbett KS, Flynn B, Foulds KE, Francica JR, Boyoglu-Barnum S, Werner AP. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N Engl J Med. 2020;383:1544–55. doi: 10.1056/NEJMoa2024671. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Novavax COVID-19 vaccine demonstrates 89.3% efficacy in UK phase 3 trial. 2021. https://ir.novavax.com/newsreleases/news-release-details/novavax-covid-19-vaccinedemonstrates-893-efficacy-uk-phase-3. https://ir.novavax.com/newsreleases/news-release-details/novavax-covid-19-vaccinedemonstrates-893-efficacy-uk-phase-3 Novavax. Available at. Accessed on January 28, 2021.
- 58.Johnson & Johnson announces single-shot Janssen COVID- 19 vaccine candidate met primary endpoints in interim analysis of its phase 3 ENSEMBLE trial. 2021. https://www.janssen.com/johnson-johnson-announces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints. https://www.janssen.com/johnson-johnson-announces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints Janssen. Available at. Accessed on January 29, 2021.
- 59.Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID- 19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:670. doi: 10.1016/S0140-6736(21)00234-8. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu FC, Guan XH, Li YH, Huang JY, Jiang T, Hou LH. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID- 19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–88. doi: 10.1016/S0140-6736(20)31605-6. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID- 19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397:1646–57. doi: 10.1016/S0140-6736(21)00677-2. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S. Effectiveness of Covid- 19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021;385:585–94. doi: 10.1056/NEJMoa2108891. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wall EC, Wu M, Harvey R, Kelly G, Warchal S, Sawyer C. Neutralising antibody activity against SARS-CoV-2 VOCs B. 1.617.2 and B. 1.351 by BNT162b2 vaccination. Lancet. 2021;397:2331–3. doi: 10.1016/S0140-6736(21)01290-3. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wall EC, Wu M, Harvey R, Kelly G, Warchal S, Sawyer C. AZD1222-induced neutralising antibody activity against SARS-CoV-2 Delta VOC. Lancet. 2021;398:207–9. doi: 10.1016/S0140-6736(21)01462-8. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krammer F, Srivastava K. Robust spike antibody responses and increased reactogenicity in seropositive individuals after a single dose of SARS-CoV-2 mRNA vaccine. 2021. https://www.medrxiv.org/content/10.1101/2021.01.29.21250653v1. https://www.medrxiv.org/content/10.1101/2021.01.29.21250653v1 the Paris team, Simon V. Available at. Accessed on August 2, 2021.
- 66.Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. WHO Solidarity Trial Consortium. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moore S, Hill EM, Tildesley MJ, Dyson L, Keeling MJ. Vaccination and non-pharmaceutical interventions for COVID-19: a mathematical modelling study. Lancet Infect Dis. 2021;21:793–802. doi: 10.1016/S1473-3099(21)00143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Breakthrough cases. https://www.cdc.gov/vaccines/covid-19/health-departments/breakthrough-cases.html. https://www.cdc.gov/vaccines/covid-19/health-departments/breakthrough-cases.html Centers for Disease Control and Prevention. Available at. Accessed on August 2, 2021.
- 69.Mlcochova P, Kemp S, Dhar MS, Papa G, Agrawal A. SARS-CoV-2 B.1.617.2 Delta variant emergence and vaccine breakthrough. 2021. https://www.researchsquare.com/article/rs-637724/v1.Accessed. https://www.researchsquare.com/article/rs-637724/v1.Accessed Available at. on August 2, 2021.
- 70.Davies NG, Abbott S, Barnard RC, Jarvis CI, Kucharski AJ, Munday JD. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372:eabg3055. doi: 10.1126/science.abg3055. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horby P, Huntley C, Davies N, Edmunds J, Ferguson N, Medley G. NERVTAG note on B.1.1.7 severity. New & Emerging Threats Advisory Group. 2021. https://www.gov.uk/government/publications/nervtag-paper-on-covid-19-variant-of-concern-b117. https://www.gov.uk/government/publications/nervtag-paper-on-covid-19-variant-of-concern-b117 et al. Available at. Accessed on January 22, 2021.
- 72.Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021;372:n579. doi: 10.1136/bmj.n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davies NG, Jarvis CI, CMMID COVID-19 Working Group, Edmunds WJ, Jewell NP, Diaz-Ordaz K. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021;593:270–4. doi: 10.1038/s41586-021-03426-1. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shen X, Tang H, McDanal C, Wagh K, Fischer W, Theiler J. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe. 2021;29:529–39. doi: 10.1016/j.chom.2021.03.002. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Global Variant Report Map. https://covid.cdc.gov/covid-data-tracker/#global-variant-report-map. https://covid.cdc.gov/covid-data-tracker/#global-variant-report-map Centers for Disease Control and Prevention. Available at. Accessed on August 2, 2021.
- 76.Pearson CAB, Russell TW, Davies NG, Kucharski AJ, CMMID COVID-19 working group, Edmunds WJ. Estimates of severity and transmissibility of novel South Africa SARS-CoV-2 variant. 2021. https://cmmid.github.io/topics/covid19/reports/sa-novel-variant/2021_01_11_Transmissibility_and_severity_of_501Y_V2_in_SA.pdf. https://cmmid.github.io/topics/covid19/reports/sa-novel-variant/2021_01_11_Transmissibility_and_severity_of_501Y_V2_in_SA.pdf et al. Available at. Accessed on January 15, 2021.
- 77.Gómez CE, Perdiguero B, Esteban M. Emerging SARS-CoV-2 Variants and Impact in Global Vaccination Programs against SARS-CoV-2/ COVID-19. Vaccines (Basel) 2021;9:243. doi: 10.3390/vaccines9030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang GL, Wang ZY, Duan LJ, Meng QC, Jiang MD, Cao J. Susceptibility of Circulating SARS-CoV-2 Variants to Neutralization. N Engl J Med. 2021;384:2354–6. doi: 10.1056/NEJMc2103022. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garcia-Beltran WF, Lam EC, St Denis K, Nitido AD, Garcia ZH, Hauser BM. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184:2523. doi: 10.1016/j.cell.2021.04.006. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Faria NR, Mellan TA, Whittaker C, Claro IM, Candido DDS, Mishra S. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science. 2021;372:815–21. doi: 10.1126/science.abh2644. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Torjesen I. Covid-19: Delta variant is now UK’s most dominant strain and spreading through schools. BMJ. 2021;373:n1445. doi: 10.1136/bmj.n1445. [DOI] [PubMed] [Google Scholar]
- 82.Lustig Y, Zuckerman N, Nemet I, Atari N, Kliker L, Regev-Yochay G. Neutralising capacity against Delta (B.1.617.2) and other variants of concern following Comirnaty (BNT162b2, BioNTech/Pfizer) vaccination in health care workers, Israel. Euro Surveill. 2021;26:2100557. doi: 10.2807/1560-7917.ES.2021.26.26.2100557. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tada T, Zhou H, Samanovic MI, Dcosta BM, Cornelius A, Mulligan MJ. Comparison of Neutralizing Antibody Titers Elicited by mRNA and Adenoviral Vector Vaccine against SARS-CoV-2 Variants. 2021. https://www.biorxiv.org/content/10.1101/2021.07.19.452771v3. https://www.biorxiv.org/content/10.1101/2021.07.19.452771v3 et al. Available at. Accessed on August 2, 2021.