Abstract
In the past few decades, obesity in the pediatric population has dramatically increased and is common in many countries. Childhood obesity often causes health problems and increases the risk of cardiometabolic diseases such as type 2 diabetes, nonalcohol fatty liver, and cardiovascular diseases. Obesity in young people has been closely associated with environmental, behavioral, and genetic defects, including the availability of high-energy and sugary food and beverages, sedentary behavior, and hereditary factors. Few drugs are currently available to treat obesity in children and adolescents because it is difficult to demonstrate the safety of these drugs on the growth and development of the youth. Lifestyle modifications, such as diet control and physical exercise, are the primary approaches for preventing and treating childhood obesity. Among them, physical activity is a crucial component. This review summarizes the epidemiology, cardiometabolic risk of obesity, therapeutic strategies, and the benefits of exercise on obesity-related chronic diseases in children and adolescents.
Key words: pediatric obesity, cardiometabolic diseases, sedentary behavior, lifestyle modification, physical exercise
Introduction
Different sources cite different age range numbers for children and adolescents. “Adolescents” are defined as “individuals in the 10–19 years age group” by the World Health Organization (WHO), and the age range of children is up to 18 years. The Food and Drug Administration (FDA) and the US Department of Health and Human Services (HHS) reference approximate age ranges for these life stages are as follows: for “children” “between 2 and 12 years of age” and for “adolescents” “between 12 and 21 years of age.” The American Academy of Pediatrics (AAP) recommends that young people under the age of 21 can seek pediatric care. Pediatrics covers patients through the age of 18 in the UK. Obesity arises from higher energy intake than energy expenditure, leading to excessive energy reservoir and energy imbalance.[1] The occurrence of obesity in children and adolescents is related to various factors, including overnutrition, sedentary behavior, insufficient physical activity, genetic heredity, psychological stress, and the environmental factors, which cause stunted growth, weight gain, early atherosclerosis, and obesity-related cardiometabolic diseases in youth population.[2, 3, 4, 5] Pediatric obesity has become an epidemic globally, and its prevalence is continually increasing. Data from the WHO showed that over 340 million children and adolescents from 5 to 19 years of age globally were overweight or obese in 2016, while an estimated 38.2 million children under 5 years of age were overweight or obese in 2019 worldwide. The global prevalence of obesity among children and adolescents of age 5–19 years was 4% in 1975, whereas it dramatically increased to over 18% in 2016. Obesity mainly results from overnutrition in high-income countries. However, obese youth have also increased dramatically in low- and middle-income countries. Almost half of the obese children under the age of 5 were in Asia in 2019. While in Africa, the number of overweight children under the age of 5 has increased by nearly 24% since 2000. Children in low- or middle-income countries consume high-sugar, high-fat, high-salt, energy-dense foods, which are cheap and have poor nutrient quality, and they are fighting obesity throughout their lives. Unhealthy dietary patterns combined with a sedentary lifestyle leads to significant increases in pediatric obesity, while the undernutrition problem remains unsolved.[6,7] It deserves global attention to develop cost-effective strategies to intervene the epidemic of obesity in children and adolescents. Currently, lifestyle interventions, such as diet control and physical exercise, are the primary approaches to treat pediatric obesity. Among them, physical exercise is considered the critical component in preventing and treating obesity in the young population. Here, in this review, we mainly summarize the epidemiology of obesity, cardiometabolic risks in obese youth, therapeutic strategies, and the benefits of exercise in obesity-related chronic diseases in children and adolescents.
Pediatric obesity and its assessment
With its economic and social development, the modern era has posed a significant challenge to children and adolescents concerning obesity throughout their lives, which poses as a constantly rising health concern that could further augment global public health concerns in the future.[2,6] Obesity is defined by the WHO as “abnormal or excessive fat accumulation that may impair health.” Several classification systems estimate childhood obesity based on the body mass index (BMI).[8] The US Centers for Disease Control and Prevention (CDC) defines the child’s weight status using age- and sex-specific BMI percentiles. The BMI of children between 85th and 94th percentiles on BMI-for-age growth charts is defined as overweight. Pediatric obesity is defined as a BMI equal to or over the 95th percentile. BMI ≥ 120th percentile of the 95th BMI percentile is defined as severe obesity among children and adolescents.[9] Childhood obesity, especially severe obesity, is strongly associated with cardiometabolic diseases, such as type 2 diabetes, non-alcohol fatty liver, and cardiovascular diseases (Figure 1).
Figure 1.
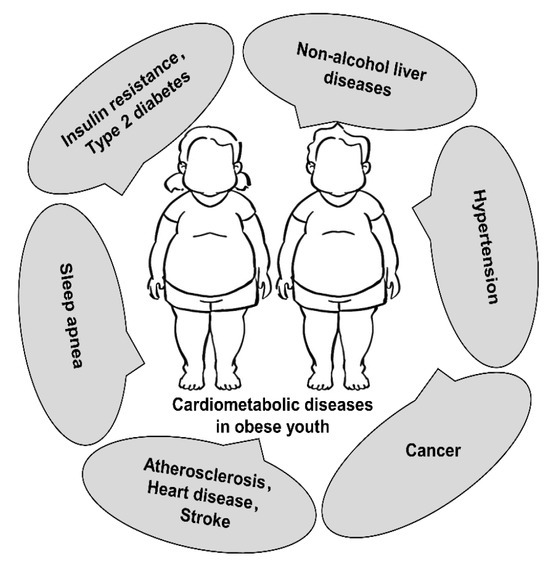
Childhood obesity is strongly associated with multiple cardiometabolic diseases.
Adipose tissue, composition, location, and function
Adipose tissue makes up about 20%–25% of the whole-body weight in healthy subjects, while the ratio is much higher in obese subjects. Adipose tissue is distributed in different places of the whole body, including subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT), and bone marrow adipose tissue. SAT is located beneath the skin and the underlying muscles of the whole body. VAT is present in the internal organs of the abdominal cavity, such as the liver, kidney, intestine, and the peritoneum.[6,10] The adipose tissue is of two types—white adipose tissue and brown adipose tissue. White adipose tissue forms the majority of the adipose tissue found in the human body that stores excessive energy. White adipose tissue comprises white adipocytes, the most abundant adipocytes in the human body, and beige/brite adipocytes. Brown adipose tissue is primarily present in fetal life and newborns and is composed of brown adipocytes. Brown adipose contains more mitochondria than white adipose and can produce heat in cold conditions. Brown adipose tissue is considered a negative indicator of BMI in adults, especially in older people.[11]
Besides adipocytes, adipose tissue contains several other cell types, including fibroblasts, fibroblastic pre-adipocytes, endothelial cells, and immune cells. Now, adipose tissue is recognized as not just an inert tissue for fat storage, but an active organ that regulates various physiological and pathological processes.[12] Adipocytes can produce and release multiple adipokines into circulation, including leptin, adipsin, resistin, adiponectin, and visfatin, which play critical roles in regulating insulin sensitivity, inflammation, immunity, food intake, and vascular sclerosis. Adipocyte enlargement, macrophage infiltration, and increased release of multiple adipokines were observed in the adipose tissue of obese subjects. The two most studied adipokines in children and adults are leptin and adiponectin. Hyperleptinemia and hypoadiponectinemia were more prevalent in obese individuals compared to normal individuals. Circulating leptin levels were inversely correlated with insulin sensitivity, while adiponectin increased insulin sensitivity by stimulating glucose uptake and fatty acid oxidation in the skeletal muscle.[13]
Cardiometabolic risk in pediatric obesity
Genetic defects-related early-onset pediatric obesity may result in complex genetic syndromes, including Prader– Willi syndrome (PWS), Bardet–Biedl syndrome (BBS), and Alstrom syndrome.[14] However, not all childhood obesity will develop cardiometabolic diseases—a lot of children and adolescents with obesity exhibit standard metabolic profiles and are nonsyndromic in early life. One hypothesis to explain this paradox is that fat in the whole body is not the culprit for the adverse metabolic profile.[6,15] Obese adolescents with a high ratio of VAT to SAT were found to have large and small adipocytes coexist, with essential adipogenic genes downregulated and inflammation featured by macrophage infiltration enhanced.[6] Consistently, it has been demonstrated that the risks of increased intra-abdominal fat depots (visceral or central obesity) are far more significant than the risks of subcutaneous accumulation of excess fat (peripheral obesity). Thus, visceral or central obesity, but not peripheral obesity, and excessive fat accumulation in non-adipose tissues such as skeletal muscles and liver are recognized as the determinants of cardiometabolic diseases.[16,17] However, how pediatric obesity impairs health remains to be elucidated.
Studies have shown that the adverse effects of excessive fat accumulation include inflammation, oxidative stress, hypertension, insulin resistance, and endothelial dysfunction (Figure 2), which cause disruption of energy balance and metabolic disturbance, cellular and tissue stress and dysfunction, and musculoskeletal disorders induced by fat mass pressure overload.[18]
Figure 2.
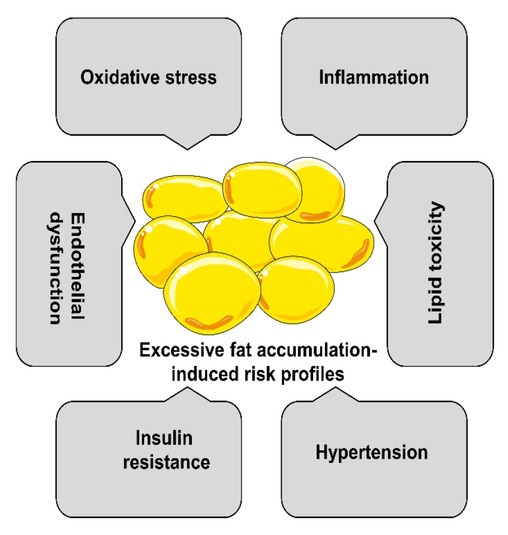
Cardiometabolic risk in obese youth.
In recent years, obesity has been recognized as a systemic and subclinical inflammatory state. Extra nutrients in the body trigger hyperplasia of adipose tissues and hypertrophy of adipocytes. The blood supply cannot match the progressive enlargement of adipose tissue. It causes hypoxia, which is considered an inciting etiology of oxidative damage, macrophage infiltration, overproduction of proinflammatory factors, proliferation of blood vessels and connective tissues, and necrosis of adipose tissue.[19] Immune cells and adipocytes release several cytokines and adipokines in obese subjects. Among them, interleukin (IL)-6, tumor necrosis factor-alpha (TNF-α), IL-1β, C-reactive protein (CRP), leptin, and resistin are recognized as proinflammatory markers. In contrast, adiponectin is recognized as an anti-inflammatory marker. [20]
Inflammation and oxidative stress are strongly related to obesity.[21] The presence of excess adipose tissue, especially white fat, activates monocytes and macrophages and induces the production and release of proinflammatory cytokines. Activated immune cells promote the production of reactive oxygen species (ROS), which, in turn, increase abnormal production of inflammatory factors and adipokines that promote inflammation. Reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), especially NOX4, is the primary enzyme that synthesizes ROS in adipocytes.[22] The antioxidant system is also impaired in obese subjects, which aggravates their susceptibility to oxidative damage. Higher free fatty acid (FFA) levels trigger oxidative damage in obese individuals, which results in mitochondrial oxidation, free radical synthesis, high rate of lipid peroxidation, and impaired glucose metabolism.[23] A source of bioactive adipokines, such as leptin, resistin, and adiponectin, has also been implicated in oxidative stress. Leptin has been found to promote oxidative stress and inflammation, while adiponectin shows high anti-inflammatory function and inhibits ROS release induced by the low-density lipoprotein (LDL).[13]
Historically, childhood hypertension is rare; however, along with the increasing incidence of childhood obesity in the last few decades, the prevalence of primary hypertension in the young population has also correspondingly increased. As a result, the relationship between obesity and hypertension in children and adults has become well established. However, the underlying mechanism of obesity-induced arterial hypertension is still under investigation.[24,25] It is demonstrated that the activation of the sympathetic nervous system, the renin–angiotensin system, and sodium retention contribute to the development of the obesity-associated hypertension.[26,27]
Insulin plays a vital role in reducing blood sugar levels by promoting liver, muscle, and fat cells to utilize circular glucose as fuel and inhibiting glucose production in the liver, intestine, and kidney. Obese youth with altered lipid partitioning and ectopic fat storage show a high prevalence of insulin resistance syndrome by exhibiting altered glucose metabolism and the presence of adverse cardiovascular biomarkers including elevated triglyceride (TG) concentrations, increased oxidized LDL cholesterol small particles, elevated blood pressure, high chronic inflammation, and reduced high-density lipoprotein (HDL) cholesterol and reduced adiponectin levels.[6,28] Insulin resistance occurs due to insulin insensitivity of insulin-responsive tissue, thus leading to a compensatory increase in insulin levels to conquer the high levels of blood glucose. Insulin resistance is considered an important predictive factor of prediabetes and normally exists many years before type 2 diabetes.[29] Obesity is strongly associated with insulin resistance. However, the molecular mechanism of insulin resistance is still not fully understood. Inflammation, mitochondrion dysfunction, oxidative stress, and lipid toxicity might be the major causes of insulin resistance. Low-grade inflammation in obesity impairs the insulin signaling pathway by several mechanisms, including inhibition of insulin receptor and insulin receptor substrate-1 (IRS-1), prevention of peroxisome proliferator-activated receptor γ (PPARγ) activity, increasing plasma FFA through blocking TG synthesis, and stimulation of lipolysis.[30, 31] Mitochondrion dysfunction influences the metabolism and oxidation of glucose and fatty acid, increasing FFA accumulation, disturbing adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK) activity, and decreasing insulin sensitivity.[32] Whether hyperinsulinemia is the cause of insulin resistance or the result of insulin insensitivity is still a strong argument point.[33] Indeed, hyperinsulinemia could induce insulin resistance. Hyperinsulinemia results either from an overproduction of insulin or a reduction of insulin clearance.[34] Increasing numbers of β cells and functions are found in obesity during weight gain, which is related to excessive fatty acids or glucose stimulation. A high insulin level leads to hyperinsulinemia and inhibited IRS-1/2 function due to the negative feedback loop of the insulin signaling pathway and causes insulin resistance. The liver and kidney are responsible for glucose production and insulin clearance, which plays a vital role in maintaining the blood glucose.[35] Insulin degradation relies on the activity of insulin receptors and insulin-degrading enzymes, and the deficiency of these two genes will lead to hyperinsulinemia.[36] Hypoxia induces inflammation and oxidative stress in obesity, promoting insulin resistance through inhibiting insulin signal transduction. Dyslipidemia caused by lipid toxicity is another risk factor for insulin resistance.
Endothelial dysfunction is one of the earliest vascular alterations observed in obesity, which results from inflammation and oxidative stress in adipose tissues.[37] The adverse effects of obesity trigger endothelial cells to undergo transition to a pro-atherosclerotic phenotype and alter endothelial nitric oxide synthase (eNOS) and arginase activities, resulting in lower nitric oxide (NO) bioavailability. Damaged vasculature, in turn, exacerbates ROS and the release of proinflammatory mediators in favor of further development of vascular atherosclerosis.[38]
Pediatric obesity-related type 2 diabetes
Type 2 diabetes is strongly correlated with childhood obesity.[29,39] In parallel to the increasing number of obese youth, the prevalence of prediabetes and type 2 diabetes in children and adolescents has increased significantly in the past few decades.[9] A retrospective cohort study (from 1994 to 2013) of the association of BMI with type 1 and type 2 diabetes events in youth aged less than 15 years was performed. This study found that type 1 diabetes and type 2 diabetes increased in both sexes and ages among children and adolescents in the UK. The incidence of type 2 diabetes in obese individuals (about one-half of total cases) was four times higher than in those with normal BMI. No linear association was found between the incidence of type 1 diabetes and childhood BMI.[40] Another longitudinal study chose 3107 child participants divided into three groups, pre-adolescence (from birth to 8 years old), early adolescence (from 8 to 13 years old), and late adolescence (from 13 to 18 years old), to track the relationship of childhood obesity with type 2 diabetes risks and found that in all growth periods, higher weight and increased speed of weight gain are strongly associated with a higher incidence of type 2 diabetes. Significantly, higher weight and accelerated weight velocity of pre-adolescence jointly correlated with the highest prevalence of type 2 diabetes.[41] In a historic longitudinal retrospective cohort study, 269,913 schoolchildren of 10 years of age with 21,896 established type 2 diabetes in adults and 261,192 schoolchildren of 13 years of age with 21,530 for type 2 diabetes incidence in adulthood were analyzed. It was found that the association of fat mass with type 2 diabetes is much stronger than weight after adjusting for childhood height, suggesting that body fat mass index is a more pronounced predictor for type 2 diabetes than body weight index.[42]
Pediatric obesity-related nonalcoholic fatty liver disease
The incidence of nonalcoholic fatty liver disease (NAFLD) in obese adolescents is about 38%, which is characterized by fat accumulation in the liver.[43] The degree of NAFLD is classified according to the existence of inflammation, fibrosis, and cirrhosis.[44] The prevalence of NAFLD in obese youth increases with BMI. NAFLD increases the risk of cardiometabolic complications, including insulin resistance, type 2 diabetes, and dyslipidemia. However, some studies suggest that childhood obesity-related insulin resistance precedes the development of NAFLD.[45,46] Pediatric obesity-induced fat accumulation in the liver leads to glucose and lipid metabolism imbalance, possibly due to insulin resistance.[45] A total of 503 obese adolescents, including 191 whites, 134 blacks, and 178 Hispanics, were recruited in a prospective cohort study to evaluate the association of clinical and genetic features with pediatric NAFLD. This study found that weight gain and high fasting C-peptide levels were the risk factors for NAFLD in white or Hispanic ethnicity. In contrast, black obese adolescents were relatively resistant to liver steatosis, but were more affected by deleterious glucose metabolism. Moreover, higher insulin levels were found in those developing NAFLD than in those without NAFLD. [46] Another longitudinal cohort study in obese adolescent girls found that a significant determinant of unfavorable alterations in glucose and lipid metabolism is a high ratio of VAT to total SAT and VAT (high VAT/[VAT + SAT]). The underlying mechanism of development of fatty liver and related metabolic disorders may not be due to decreased capacity of TG deposition or adipocyte proliferation. Still, it may result from an increased turnover of mature adipocytes and TGs in both abdominal and gluteal SAT.[47]
Pediatric obesity-related cardiovascular diseases
Cardiovascular diseases are the leading causes of global death. Childhood obesity has become a global epidemic, significantly increasing their risk for cardiovascular disease, a group of heart and blood vessel disorders, including coronary artery diseases, arrhythmias, congenital heart defects, cardiomyopathy, peripheral vascular diseases, heart failure, and stroke.[48] Atherosclerosis is the leading cause of cardiovascular diseases. The development of atherosclerosis has been demonstrated to begin in childhood. Studies have found that childhood obesity is associated with the earlier appearance of atherosclerotic lesions.[1,42] In obese youth, the occurrence of arteriosclerosis is accelerated due to excessive fat accumulation. For most children, the progress of atherosclerosis is relatively slow and asymptomatic, but in some children, atherosclerosis develops rapidly and is caused by the same risk factors as adults.
A cohort study in Spain enrolled 212 children and adolescents aged 6–17 years and divided them into three groups: average weight (n = 106), overweight (n = 57), and obesity (n = 9). All these subjects performed echocardiography to assess cardiac morphology and function. Compared to average weight, larger left ventricular mass, greater left ventricular end-diastolic/systolic volumes, and smaller ejection fraction were found in overweight and obesity. Although not clinically pathological, alterations in cardiac structure and function in obese youth are unfavorable incipient alterations and risk factors.[49] Another 30-year longitudinal and cross-sectional study investigated the risk of cardiovascular disease events among adolescents with different BMI values and grouped the study subjects as follows: average weight (n = 247), overweight (n = 54), obesity (n = 131), severe obesity without metabolic and bariatric surgery (n = 302), and severe obesity receiving metabolic and bariatric surgery (n = 215). A higher risk for cardiovascular disease events was found in adolescents with severe obesity, and after metabolic and bariatric surgery, the risk was significantly reduced.[50] A comparison study was performed on 6328 subjects with high adiposity or normal BMI from childhood to adulthood. An increased risk of type 2 diabetes, hypertension, higher levels of LDL cholesterol and TG, reduced levels of HDL cholesterol, and increased intima–media thickness of the carotid artery atherosclerosis were found in obese compared with normal BMI subjects. The risks of these outcomes were reduced when obese youth became nonobese adults, suggesting the importance of weight loss in obese children.[51] Weight loss reduces ectopic lipid deposition in the body, including in the liver and muscle, increases insulin sensitivity, and is expected to reverse the risk factors of obesity-related cardiovascular diseases. Clinical trials of dietary modifications, physical activity, and home-directed therapy have shown that weight loss or maintaining a stable level of weight significantly improves metabolism.[6]
Treating pediatric obesity
Overweight and obesity, as well as their related concomitant diseases, are harmful, but are also preventable. It is highly needed to intervene in the increasing pediatric obesity in time and reduce the global burden of obesity-related chronic diseases.[7] The purpose of the treatment of pediatric obesity is to reduce body fat mass, reduce the development of physical and psychosocial complications, and prevent the development of cardiometabolic diseases.[52] Treatment choice typically depends on the severity of obesity in children and adolescents, their age, family conditions, clinical competency, and health-care system.[53] Treatment of obesity integrates many components, including exercise therapy, diet control therapy, psychological therapy, medication, and surgery. Effective therapies primarily rely on the parents for young children, while for adolescents, a more significant degree of self-management may be required.
Pharmacotherapy is quite limited, and few validated drugs are currently available to treat obese youth in clinical practice due to safety concerns of such drugs in their growth and development.[54] Among the limited medications in clinical settings, liraglutide is one drug approved by both US FDA and European Medicines Agency for treating chronic obesity in adolescents between 12 and 18 years of age. Liraglutide is the agonist of the glucagon-like peptide-1(GLP-1) receptor. It is given by subcutaneous injection, and it induces weight loss by reducing appetite and gastric motility and enhancing satiety through binding its receptor in the hypothalamus.[55] In a randomized, double-blind clinical trial, 251 adolescents aged 12–18 years were recruited to test the effect of liraglutide on obesity.[56] In addition to lifestyle therapy, 125 participants were given 3 mg liraglutide, and the others were given a placebo subcutaneously once daily. The liraglutide group showed significant reduction in BMI and body weight; however, it showed even stronger gastrointestinal adverse effects than the placebo group. Considerable improvements in cardiometabolic risk factors were not observed between the liraglutide and placebo groups. Several other medications have been evaluated or are under evaluation in clinical trials, including orlistat,[57] topiramate,[58] metformin,[59] phentermine,[60] and semaglutide.[61] To evaluate the efficacy and safety of orlistat for the treatment of pediatric obesity, 539 obese adolescents aged 12–16 years were enrolled in a randomized, double-blind study; 357 subjects were given orlistat and 182 subjects were given placebo three times daily for 1 year, combined with diet, exercise, and behavior therapy. At the end of the study, BMI, body fat mass, and waist circumference decreased in the orlistat group without inducing major safety problems. However, mild to moderate gastrointestinal adverse effects occurred.[57] To assess the efficacy and safety of topiramate in BMI reduction, adolescents aged 12–18 years with severe obesity were recruited to a randomized and double-blind clinical trial. Participants were first treated with 4 weeks of meal therapy and then 24 weeks of topiramate or placebo. At the end of this pilot study, BMI was not significantly reduced, but visceral fat and very low-density lipoprotein cholesterol were decreased in the topiramate group.[58] The effect and safety of metformin in weight management in obese youth have been evaluated in many clinical settings. BMI, body weight, waist circumference, and body fat mass were found to be significantly decreased following the administration of metformin.[59,62] Phentermine is widely used to treat adulthood obesity, but little is known about its effect on childhood obesity. A retrospective chart review of obesity in adolescents was conducted to examine the effects of phentermine on weight loss in the young population, combined with standard of care (SOC) life modification therapy. Compared to sole SOC therapy, phentermine combined SOC therapy may promote weight loss in obese adolescents with higher efficiency.[60]
Compared to other types of therapy, metabolic and bariatric surgery is considered the most effective and durable strategy for treating obesity in both adulthood and childhood. Approximately 25%–40% BMI reduction is found in obesity 3–9 years after Roux-en-Y gastric bypass or vertical sleeve gastrectomy surgery. Cardiometabolic health and weight-related quality of life are also improved post-surgery.[63, 64, 65] However, the use of bariatric surgery to treat obese youth is minimal. Only severe forms of obesity combined with cardiometabolic complications are reserved for surgical procedures due to the worrisome long-term safety, invasiveness, and irreversibility of side effects of the surgery on the growth and development of obese youth.
Among different therapies for obesity, healthy dietary patterns and regular physical exercise are the primary strategies to prevent and treat childhood obesity. Helping children develop good habits of physical exercise and eating a healthy diet is very important to prevent them from becoming obese.[5,66,67] The imbalance between energy intake and consumption in children and adolescents leads to excessive energy storage and weight gain. The less time children and adolescents exercise, the more significant is the increase in body fat mass. To maintain a healthy weight in the young population, physical activity is considered a much better strategy than the dietary restriction method.[3] First, children and adolescents are in a period of exuberant physical growth. Limiting energy intake may cause malnutrition and adverse effects on their health. Second, research shows that when reducing the energy intake, the body changes to slow the metabolism and burns less energy, rather than promoting body weight loss. On the opposite, physical exercise enhances the metabolism, induces fat mass loss by increasing fat oxidation, and makes the body resistant to weight gain.[68] Regular physical activity by children and adolescents could help them lose weight, get in good shape, and relieve tension and pressure.[69] Thus, physical exercise is considered the critical component in preventing and treating obesity in the young population.
Beneficial effects of physical activity on pediatric obesity-related cardiometabolic disease
Numerous studies have evaluated the protective effects of physical exercise in obese youth (Figure 3). Physical exercise has multiple beneficial effects on health promotion and treatment of diseases. Regular exercise could improve energy metabolism, strengthen the function of various organs, including the heart, blood vessels, lung, brain, bone, and muscle, improve cardiovascular disease risk profile, reduce cardiovascular events, and decrease hospitalization of patients with obesity-related diseases.[70, 71, 72] There are two significant types of exercise, aerobic exercise and resistance exercise. Aerobic exercise induces multiple beneficial effects, including improvement in maximal oxygen consumption (VO2max) and cardiorespiratory fitness without significantly affecting the muscle strength.[3] In contrast, resistance exercise induces neuromuscular adaptation and results in muscle strength improvement, but has little effect on cardiorespiratory fitness. Both aerobic exercise and resistance exercise could improve blood pressure and reduce fasting glucose levels, LDL levels, and TGs in obese youth. Most studies on physical activity in children and adolescents with obesity have focused on aerobic exercises, such as running and cycling, primarily to increase energy expenditure. However, studies found that a combination of aerobic exercise and resistance exercise in treating obesity improved both cardiorespiratory fitness and life quality in patients with obesity-related cardiometabolic diseases.[71]
Figure 3.
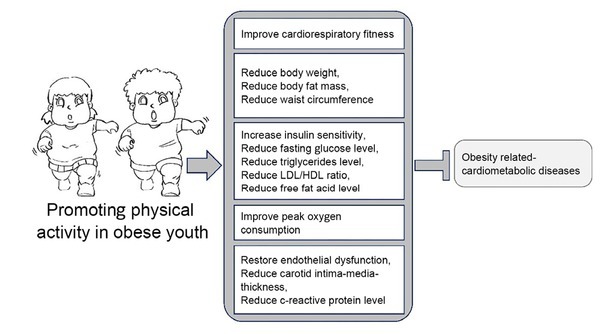
The protective effects of physical activity against childhood cardiometabolic diseases caused by pediatric obesity. HDL: high-density lipoprotein; LDL: low-density lipoprotein.
Numerous clinical studies examined the effect of exercise therapy alone or combined lifestyle intervention therapies, including excise therapy, nutrition limitation, and surgical procedures. A total of 109 children with a mean age of 11 years were enrolled in a clinical study to assess 5 weeks of exercise training. They were divided into four groups: moderate-intensity continuous training (MICT) group, high-intensity interval training (HIIT) group, combined MICT and HIT group, and no training group. Compared to the no training group, the MICT, HIT, and combined MICT and HIT groups exhibited better cardiometabolic health, including improved resting heart rate, fasting glucose, peak oxygen consumption, and C-reactive protein. Compared to the HIT or MICT groups, the combined MITC and HIT group showed reduced waist circumference and waist-to-hip ratio.[73] One hundred adolescents aged 11–17 years with overweight or obesity were recruited to assess the protective effects of physical education in reducing liver fat mass and improving cardiometabolic profiles in school settings. They were assigned to different forms and duration of physical education to elucidate the links between physical education and the hepatic and adipose metabolism.[74] Obese subjects (n = 67) aged 12–16 were randomly assigned to 6 months of exercise training or non-exercise control group to assess the effects of exercise on endothelial integrity and cardiovascular risk. Compared to the control group, exercise training was found to restore endothelial function, reduce carotid intima–media thickness and early atherosclerotic lesions, and improve cardiovascular profiles, which included causing a significant reduction in blood pressure, insulin resistance, TG level, LDL to HDL ratio, C-reactive protein, and body weight.[75] Children aged 7–16 years with obesity were enrolled in a clinical study to elucidate whether 12 weeks of HIIT exhibited higher efficiency than MICT or nutrition restriction in improving cardiorespiratory fitness and mitigating cardiometabolic risk. This study found that 12 weeks of HIIT exerted beneficial effects on cardiorespiratory fitness, but had no impact on abdominal and total body fat mass and blood biomarkers.[76] However, high-intensity intermittent exercise has been shown in other studies to have protective effects on a variety of risk factors associated with obesity-related cardiometabolic diseases.[77] To assess the impact of 1-year physical exercise combined lifestyle program on the anthropometric and metabolic profile and glycemic pattern in pediatric obesity, 142 overweight or obese subjects aged 7–18 years were recruited and 115 participants completed this clinical study. This study showed that BMI, waist-to-height ratio, waist circumference, and body fat mass were significantly reduced. Metabolic risk markers, including mean glucose levels, HbA1c, and FFA levels, improved considerably.[78]
Conclusion
Pediatric obesity is strongly associated with different types of chronic diseases that threaten the health and life quality of obese youth. There are several strategies to prevent and treat obesity in the young, such as dietary intervention, drugs, and surgery. Currently, treating pediatric obesity is neither cost-effective in clinical practice nor has shown very encouraging results. Therefore, novel practical strategies for preventing and treating obesity in children and adolescents are highly required.
Evidence from multiple clinical trials supports that exercise training could reduce the risk factors and exert therapeutic effects nearly without side effects in preventing and treating various cardiometabolic diseases. Exercise training has direct and indirect protective impacts on multiple organs relying on cell adaptation and interorgan interactions mediated by nearby and remote signal transduction.
Young people and adults generally lack exercise in today’s life; some people cannot even exercise. Although physical inactivity has been considered the fourth highest risk factor for global morbidity and mortality, sedentary behavior has no such claim. However, nowadays, sedentary behavior with low energy expenditure, such as reading, playing computer games, watching videos, and conducting a longitudinal study, occupies a large portion of the daily life of the young population and contributes to their poor health condition. Besides, the lifestyle behaviors of young population and adults have been changed a lot by the global pandemic of coronavirus disease 2019 (COVID-19). To contain and mitigate the spread of COVID-19, children and adolescents were deprived of school activities and confined at home, with online classes for many hours without sufficient physical activities, which has adverse effects on pediatric health.[79] A longitudinal study was conducted in Italy to evaluate the effects of the COVID-19 pandemic on the lifestyle behaviors of children and adolescents during a non-school lockdown period. Findings from this study showed that sleep time of these subjects significantly increased, time spent in sports activities significantly decreased, and high-energy food intake, such as potato chips and sugary drinks, increased significantly, indicating that the COVID-19 pandemic lockdown may have a lasting impact on adiposity level among children and adolescents.[80] A systematic review and meta-analysis was conducted to investigate the effects of COVID-19 pandemic lockdown on body weight in children and adolescents. This meat-analysis showed that the body weight and BMI of the pediatric population increased significantly during school closures due to the COVID-19 pandemic.[81] Currently, not only obese youth, but also children and adolescents with normal BMI are encouraged to spend more time performing physical activity. Childhood is a critical period in contributing to one’s lifelong health conditions and a crucial stage wherein one could be trained to develop good physical exercise habits and prevent obesity and cardiometabolic diseases.
Moreover, as children and adolescents spend much time at school, schools should assume a leadership role in promoting positive, healthy behaviors in the youth and ensuring adequate physical activity each day. Furthermore, it is essential to elucidate the mechanism and critical sensors that mediate the beneficial effects of exercise on cardiometabolic diseases in obese children and adolescents. The critical exercise sensors could be used as novel drug targets and to develop novel therapeutic strategies for preventing and treating obesity-related chronic diseases.
Funding Statement
This work was supported by the National Key R&D Program of China (No. 2020YFA0803800 to Li J), National Natural Science Foundation of China (No. 82020108002 and 81911540486 to Xiao J, No. 82170390 to Gao J), the grant from Science and Technology Commission of Shanghai Municipality (No. 20DZ2255400 and 21XD1421300 to Xiao J, No. 21ZR1422700 to Gao J), and the “Dawn” Program of Shanghai Education Commission (No. 19SG34 to Xiao J).
Footnotes
Authors’ Contribution
Xiao J had the idea for the article. Gao J and Li J performed the literature search and analysis. Gao J, Lu Y, Gokulnath P, Vulugundam G, and Li G drafted and critically revised the paper.
Conflict of Interest
The authors have no conflicts of interest to disclose.
Reference
- 1.Bleich SN, Ku R, Wang YC. Relative contribution of energy intake and energy expenditure to childhood obesity: a review of the literature and directions for future research. Int J Obes (Lond) 2011;35:1–15. doi: 10.1038/ijo.2010.252. [DOI] [PubMed] [Google Scholar]
- 2.Chung ST, Onuzuruike AU, Magge SN. Cardiometabolic risk in obese children. Ann N Y Acad Sci. 2018;1411:166–83. doi: 10.1111/nyas.13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watts K, Jones TW, Davis EA, Green D. Exercise training in obese children and adolescents: current concepts. Sports Med. 2005;35:375–92. doi: 10.2165/00007256-200535050-00002. [DOI] [PubMed] [Google Scholar]
- 4.Barnett TA, Kelly AS, Young DR, Perry CK, Pratt CA, Edwards NM. Sedentary Behaviors in Today’s Youth: Approaches to the Prevention and Management of Childhood Obesity: A Scientific Statement From the American Heart Association. Circulation. 2018;138:e142–59. doi: 10.1161/CIR.0000000000000591. et al. [DOI] [PubMed] [Google Scholar]
- 5.Parker K, Timperio A, Salmon J, Villanueva K, Brown H, Esteban-Cornejo I. Activity-related typologies and longitudinal change in physical activity and sedentary time in children and adolescents: The UP&DOWN Study. J Sport Health Sci. 2021;10:447–53. doi: 10.1016/j.jshs.2020.02.004. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caprio S, Santoro N, Weiss R. Childhood obesity and the associated rise in cardiometabolic complications. Nat Metab. 2020;2:223–32. doi: 10.1038/s42255-020-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jebeile H, Kelly AS, O’Malley G, Baur LA. Obesity in children and adolescents: epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. 2022;10:351–65. doi: 10.1016/S2213-8587(22)00047-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren J, Wu NN, Wang S, Sowers JR, Zhang Y. Obesity cardiomyopathy: evidence, mechanisms, and therapeutic implications. Physiol Rev. 2021;101:1745–807. doi: 10.1152/physrev.00030.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendor CD, Bardugo A, Pinhas-Hamiel O, Afek A, Twig G. Cardiovascular morbidity, diabetes and cancer risk among children and adolescents with severe obesity. Cardiovasc Diabetol. 2020;19:79. doi: 10.1186/s12933-020-01052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–35. doi: 10.1053/meta.2001.21693. et al. [DOI] [PubMed] [Google Scholar]
- 11.Rosell M, Kaforou M, Frontini A, Okolo A, Chan YW, Nikolopoulou E. Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. Am J Physiol Endocrinol Metab. 2014;306:E945–64. doi: 10.1152/ajpendo.00473.2013. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pyrzak B, Ruminska M, Popko K, Demkow U. Adiponectin as a biomarker of the metabolic syndrome in children and adolescents. Eur J Med Res. 2010;15:147–51. doi: 10.1186/2047-783X-15-S2-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stern JH, Rutkowski JM, Scherer PE. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016;23:770–84. doi: 10.1016/j.cmet.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstone AP, Beales PL. Genetic obesity syndromes. Front Horm Res. 2008;36:37–60. doi: 10.1159/000115336. [DOI] [PubMed] [Google Scholar]
- 15.Cristi-Montero C, Courel-Ibanez J, Ortega FB, Castro-Pinero J, Santaliestra-Pasias A, Polito A. Mediation role of cardiorespiratory fitness on the association between fatness and cardiometabolic risk in European adolescents: The HELENA study. J Sport Health Sci. 2021;10:360–7. doi: 10.1016/j.jshs.2019.08.003. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taksali SE, Caprio S, Dziura J, Dufour S, Cali AM, Goodman TR. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes. 2008;57:367–71. doi: 10.2337/db07-0932. et al. [DOI] [PubMed] [Google Scholar]
- 17.Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. N Engl J Med. 2015;373:1307–17. doi: 10.1056/NEJMoa1502821. [DOI] [PubMed] [Google Scholar]
- 18.Levy E, Saenger AK, Steffes MW, Delvin E. Pediatric Obesity and Cardiometabolic Disorders: Risk Factors and Biomarkers. EJIFCC. 2017;28:6–24. [PMC free article] [PubMed] [Google Scholar]
- 19.Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13:851–63. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marseglia L, Manti S, D’Angelo G, Nicotera A, Parisi E, Di Rosa G. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci. 2014;16:378–400. doi: 10.3390/ijms16010378. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Codoner-Franch P, Valls-Belles V, Arilla-Codoner A, Alonso-Iglesias E. Oxidant mechanisms in childhood obesity: the link between inflammation and oxidative stress. Transl Res. 2011;158:369–84. doi: 10.1016/j.trsl.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 23.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61. doi: 10.1172/JCI21625. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang SZ, Lu W, Zong XF, Ruan HY, Liu Y. Obesity and hypertension. Exp Ther Med. 2016;12:2395–9. doi: 10.3892/etm.2016.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wuhl E. Hypertension in childhood obesity. Acta Paediatr. 2019;108:37–43. doi: 10.1111/apa.14551. [DOI] [PubMed] [Google Scholar]
- 26.Obesity-related hypertension. Ochsner J. 2009;9:133–6. Re RN. [PMC free article] [PubMed] [Google Scholar]
- 27.Brady TM. Obesity-Related Hypertension in Children. Front Pediatr. 2017;5:197. doi: 10.3389/fped.2017.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viner RM, Segal TY, Lichtarowicz-Krynska E, Hindmarsh P. Prevalence of the insulin resistance syndrome in obesity. Arch Dis Child. 2005;90:10–4. doi: 10.1136/adc.2003.036467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 30.Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A. Insulin/ IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest. 2001;107:181–9. doi: 10.1172/JCI10934. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzawa M, Takada I, Yanagisawa J, Ohtake F, Ogawa S, Yamauchi T. Cytokines suppress adipogenesis and PPAR-gamma function through the TAK1/TAB1/NIK cascade. Nat Cell Biol. 2003;5:224–30. doi: 10.1038/ncb942. et al. [DOI] [PubMed] [Google Scholar]
- 32.Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009;9:407–16. doi: 10.1016/j.cmet.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Jeanrenaud B. Hyperinsulinemia in obesity syndromes: its metabolic consequences and possible etiology. Metabolism. 1978;27:1881–92. doi: 10.1016/s0026-0495(78)80006-7. [DOI] [PubMed] [Google Scholar]
- 34.Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. 2008;31:S262–8. doi: 10.2337/dc08-s264. [DOI] [PubMed] [Google Scholar]
- 35.Kim MK, Reaven GM, Chen YD, Kim E, Kim SH. Hyperinsulinemia in individuals with obesity: Role of insulin clearance. Obesity (Silver Spring) 2015;23:2430–4. doi: 10.1002/oby.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meistas MT, Margolis S, Kowarski AA. Hyperinsulinemia of obesity is due to decreased clearance of insulin. Am J Physiol. 1983;245:E155–9. doi: 10.1152/ajpendo.1983.245.2.E155. [DOI] [PubMed] [Google Scholar]
- 37.Freemark M. Endothelial dysfunction and cardiovascular disease in childhood obesity. J Pediatr (Rio J) 2019;95:503–5. doi: 10.1016/j.jped.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Meyers MR, Gokce N. Endothelial dysfunction in obesity: etiological role in atherosclerosis. Curr Opin Endocrinol Diabetes Obes. 2007;14:365–9. doi: 10.1097/MED.0b013e3282be90a8. [DOI] [PubMed] [Google Scholar]
- 39.Twig G, Zucker I, Afek A, Cukierman-Yaffe T, Bendor CD, Derazne E. Adolescent Obesity and Early-Onset Type 2 Diabetes. Diabetes Care. 2020;43:1487–95. doi: 10.2337/dc19-1988. et al. [DOI] [PubMed] [Google Scholar]
- 40.Abbasi A, Juszczyk D, van Jaarsveld CHM, Gulliford MC. Body Mass Index and Incident Type 1 and Type 2 Diabetes in Children and Young Adults: A Retrospective Cohort Study. J Endocr Soc. 2017;1:524–37. doi: 10.1210/js.2017-00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olaiya MT, Knowler WC, Sinha M, Kobes S, Nelson RG, Baier LJ. Weight tracking in childhood and adolescence and type 2 diabetes risk. Diabetologia. 2020;63:1753–63. doi: 10.1007/s00125-020-05165-w. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hudda MT, Aarestrup J, Owen CG, Cook DG, Sorensen TIA, Rudnicka AR. Association of Childhood Fat Mass and Weight With Adult-Onset Type 2 Diabetes in Denmark. JAMA Netw Open. 2021;4:e218524. doi: 10.1001/jamanetworkopen.2021.8524. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–93. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 44.Shaunak M, Byrne CD, Davis N, Afolabi P, Faust SN, Davies JH. Nonalcoholic fatty liver disease and childhood obesity. Arch Dis Child. 2021;106:3–8. doi: 10.1136/archdischild-2019-318063. [DOI] [PubMed] [Google Scholar]
- 45.D’Adamo E, Cali AM, Weiss R, Santoro N, Pierpont B, Northrup V. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care. 2010;33:1817–22. doi: 10.2337/dc10-0284. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trico D, Caprio S, Rosaria Umano G, Pierpont B, Nouws J, Galderisi A. Metabolic Features of Nonalcoholic Fatty Liver (NAFL) in Obese Adolescents: Findings From a Multiethnic Cohort. Hepatology. 2018;68:1376–90. doi: 10.1002/hep.30035. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nouws J, Fitch M, Mata M, Santoro N, Galuppo B, Kursawe R. Altered In Vivo Lipid Fluxes and Cell Dynamics in Subcutaneous Adipose Tissues Are Associated With the Unfavorable Pattern of Fat Distribution in Obese Adolescent Girls. Diabetes. 2019;68:1168–77. doi: 10.2337/db18-1162. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bridger T. Childhood obesity and cardiovascular disease. Paediatr Child Health. 2009;14:177–82. doi: 10.1093/pch/14.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cabeza JF, Aristizabal-Duque CH, Sanchez IMB, Ortiz MR, Almodovar AR, Ortega MD. Relationship between overweight and obesity and cardiac dimensions and function in a paediatric population. Eur J Pediatr. 2022;181:1943–9. doi: 10.1007/s00431-022-04384-0. et al. [DOI] [PubMed] [Google Scholar]
- 50.Ryder JR, Xu P, Inge TH, Xie C, Jenkins TM, Hur C. Thirty-Year Risk of Cardiovascular Disease Events in Adolescents with Severe Obesity. Obesity (Silver Spring) 2020;28:616–23. doi: 10.1002/oby.22725. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–85. doi: 10.1056/NEJMoa1010112. et al. [DOI] [PubMed] [Google Scholar]
- 52.Gabrys L, Baumert J, Heidemann C, Busch M, Finger JD. Sports activity patterns and cardio-metabolic health over time among adults in Germany: Results of a nationwide 12-year follow-up study. J Sport Health Sci. 2021;10:439–46. doi: 10.1016/j.jshs.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baur LA, Hazelton B, Shrewsbury VA. Assessment and management of obesity in childhood and adolescence. Nat Rev Gastroenterol Hepatol. 2011;8:635–45. doi: 10.1038/nrgastro.2011.165. [DOI] [PubMed] [Google Scholar]
- 54.Matson KL, Fallon RM. Treatment of obesity in children and adolescents. J Pediatr Pharmacol Ther. 2012;17:45–57. doi: 10.5863/1551-6776-17.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond) 2014;38:784–93. doi: 10.1038/ijo.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelly AS, Auerbach P, Barrientos-Perez M, Gies I, Hale PM, Marcus C. A Randomized, Controlled Trial of Liraglutide for Adolescents with Obesity. N Engl J Med. 2020;382:2117–28. doi: 10.1056/NEJMoa1916038. et al. [DOI] [PubMed] [Google Scholar]
- 57.Chanoine JP, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293:2873–83. doi: 10.1001/jama.293.23.2873. [DOI] [PubMed] [Google Scholar]
- 58.Fox CK, Kaizer AM, Rudser KD, Nathan BM, Gross AC, Sunni M. Meal replacements followed by topiramate for the treatment of adolescent severe obesity: A pilot randomized controlled trial. Obesity (Silver Spring) 2016;24:2553–61. doi: 10.1002/oby.21633. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sadeghi A, Mousavi SM, Mokhtari T, Parohan M, Milajerdi A. Metformin Therapy Reduces Obesity Indices in Children and Adolescents: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Child Obes. 2020;16:174–91. doi: 10.1089/chi.2019.0040. [DOI] [PubMed] [Google Scholar]
- 60.Ryder JR, Kaizer A, Rudser KD, Gross A, Kelly AS, Fox CK. Effect of phentermine on weight reduction in a pediatric weight management clinic. Int J Obes (Lond) 2017;41:90–3. doi: 10.1038/ijo.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clement K, van den Akker E, Argente J, Bahm A, Chung WK, Connors H. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020;8:960–70. doi: 10.1016/S2213-8587(20)30364-8. et al. [DOI] [PubMed] [Google Scholar]
- 62.Park MH, Kinra S, Ward KJ, White B, Viner RM. Metformin for obesity in children and adolescents: a systematic review. Diabetes Care. 2009;32:1743–5. doi: 10.2337/dc09-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. N Engl J Med. 2016;374:113–23. doi: 10.1056/NEJMoa1506699. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olbers T, Beamish AJ, Gronowitz E, Flodmark CE, Dahlgren J, Bruze G. Laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity (AMOS): a prospective, 5-year, Swedish nationwide study. Lancet Diabetes Endocrinol. 2017;5:174–83. doi: 10.1016/S2213-8587(16)30424-7. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elhag W, El Ansari W. Durability of Cardiometabolic Outcomes Among Adolescents After Sleeve Gastrectomy: First Study with 9-Year Follow-up. Obes Surg. 2021;31:2869–77. doi: 10.1007/s11695-021-05364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pate RR, Davis MG, Robinson TN, Stone EJ, McKenzie TL, Young JC. Promoting physical activity in children and youth: a leadership role for schools: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism (Physical Activity Committee) in collaboration with the Councils on Cardiovascular Disease in the Young and Cardiovascular Nursing. Circulation. 2006;114:1214–24. doi: 10.1161/CIRCULATIONAHA.106.177052. et al. [DOI] [PubMed] [Google Scholar]
- 67.Xin F, Zhu Z, Chen S, Chen H, Hu X, Ma X. Prevalence and correlates of meeting the muscle-strengthening exercise recommendations among Chinese children and adolescents: Results from 2019 Physical Activity and Fitness in China-The Youth Study. J Sport Health Sci. 2021;11:358–66. doi: 10.1016/j.jshs.2021.09.010. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Melanson EL, MacLean PS, Hill JO. Exercise improves fat metabolism in muscle but does not increase 24-h fat oxidation. Exerc Sport Sci Rev. 2009;37:93–101. doi: 10.1097/JES.0b013e31819c2f0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen ST, Liu Y, Tremblay MS, Hong JT, Tang Y, Cao ZB. Meeting 24-h movement guidelines: Prevalence, correlates, and the relationships with overweight and obesity among Chinese children and adolescents. J Sport Health Sci. 2021;10:349–59. doi: 10.1016/j.jshs.2020.07.002. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vina J, Sanchis-Gomar F, Martinez-Bello V, Gomez-Cabrera MC. Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol. 2012;167:1–12. doi: 10.1111/j.1476-5381.2012.01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salamt N, Muhajir M, Aminuddin A, Ugusman A. The effects of exercise on vascular markers and C-reactive protein among obese children and adolescents: An evidence-based review. Bosn J Basic Med Sci. 2020;20:149–56. doi: 10.17305/bjbms.2019.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bei Y, Wang L, Ding R, Che L, Fan Z, Gao W. Animal exercise studies in cardiovascular research: Current knowledge and optimal design-A position paper of the Committee on Cardiac Rehabilitation, Chinese Medical Doctors’ Association. J Sport Health Sci. 2021;10:660–74. doi: 10.1016/j.jshs.2021.08.002. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Biljon A, McKune AJ, DuBose KD, Kolanisi U, Semple SJ. Do Short-Term Exercise Interventions Improve Cardiometabolic Risk Factors in Children? J Pediatr. 2018;203:325–9. doi: 10.1016/j.jpeds.2018.07.067. [DOI] [PubMed] [Google Scholar]
- 74.Gonzalez-Ruiz K, Correa-Bautista JE, Izquierdo M, Garcia-Hermoso A, Dominguez-Sanchez MA, Bustos-Cruz RH. Effects of an exercise program on hepatic metabolism, hepatic fat, and cardiovascular health in overweight/obese adolescents from Bogota, Colombia (the HEPAFIT study): study protocol for a randomized controlled trial. Trials. 2018;19:330. doi: 10.1186/s13063-018-2721-5. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meyer AA, Kundt G, Lenschow U, Schuff-Werner P, Kienast W. Improvement of early vascular changes and cardiovascular risk factors in obese children after a six-month exercise program. J Am Coll Cardiol. 2006;48:1865–70. doi: 10.1016/j.jacc.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 76.Dias KA, Ingul CB, Tjonna AE, Keating SE, Gomersall SR, Follestad T. Effect of High-Intensity Interval Training on Fitness, Fat Mass and Cardiometabolic Biomarkers in Children with Obesity: A Randomised Controlled Trial. Sports Med. 2018;48:733–46. doi: 10.1007/s40279-017-0777-0. et al. [DOI] [PubMed] [Google Scholar]
- 77.Cooper SB, Dring KJ, Nevill ME. High-Intensity Intermittent Exercise: Effect on Young People’s Cardiometabolic Health and Cognition. Curr Sports Med Rep. 2016;15:245–51. doi: 10.1249/JSR.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 78.Bluher S, Petroff D, Wagner A, Warich K, Gausche R, Klemm T. The one year exercise and lifestyle intervention program KLAKS: Effects on anthropometric parameters, cardiometabolic risk factors and glycemic control in childhood obesity. Metabolism. 2014;63:422–30. doi: 10.1016/j.metabol.2013.11.016. et al. [DOI] [PubMed] [Google Scholar]
- 79.Nicodemo M, Spreghini MR, Manco M, Wietrzykowska Sforza R, Morino G. Childhood Obesity and COVID-19 Lockdown: Remarks on Eating Habits of Patients Enrolled in a Food-Education Program. Nutrients. 2021;13:383. doi: 10.3390/nu13020383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pietrobelli A, Pecoraro L, Ferruzzi A, Heo M, Faith M, Zoller T. Effects of COVID-19 Lockdown on Lifestyle Behaviors in Children with Obesity Living in Verona, Italy: A Longitudinal Study. Obesity (Silver Spring) 2020;28:1382–5. doi: 10.1002/oby.22861. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang TH, Chen YC, Chen WY, Chen CY, Hsu WY, Chou Y. Weight Gain Associated with COVID-19 Lockdown in Children and Adolescents: A Systematic Review and Meta-Analysis. Nutrients. 2021;13:3668. doi: 10.3390/nu13103668. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]


