Summary
Regeneration is initiated by wounding, but it is unclear how injury-induced signals precisely convey the identity of the tissues requiring replacement. In the planarian Schmidtea mediterranea, the first event in head regeneration is the asymmetric activation of the Wnt inhibitor notum in longitudinal body-wall muscle cells, preferentially at anterior-facing versus posterior-facing wound sites. However, the mechanism driving this early symmetry breaking event is unknown. We identify a noncanonical Wnt11 and Dishevelled pathway regulating notum polarization, which opposes injury-induced notum-activating Wnt/βcatenin signals and regulates muscle orientation. Using expression analysis and experiments to define a critical time of action, we demonstrate that Wnt11 and Dishevelled signals act prior to injury and in a growth-dependent manner to orient the polarization of notum induced by wounding. In turn, injury-induced notum dictates polarization used in the next round of regeneration. These results identify a self-reinforcing feedback system driving the polarization of blastema outgrowth and indicate that regeneration uses pre-existing tissue information to determine the outcome of wound-induced signals.
Keywords: Wnt, polarity, notum, injury signaling, regeneration, Dishevelled, blastema, planaria
eToc blurb
Planarians regenerate heads or tails at appropriate amputations and control this decision through canonical Wnt signaling. Gittin and Petersen identify a noncanonical Wnt11 and Dishevelled pathway acting before injury to mediate the earliest symmetry-breaking step in regeneration by controlling polarized expression of the Wnt inhibitor notum.
Graphical Abstract
Introduction
Animals capable of whole-body regeneration need mechanisms to specify the identity of tissues formed within blastemas. However, the nature of these signals is not fully understood. The planarian head-versus-tail regeneration decision is a paradigm for studying blastema fate determination. Schmidtea mediterranea regenerates from nearly any surgical injury using neoblast adult pluripotent stem cells, the only planarian adult proliferating cells1–4. In planarians, anterior-facing amputation sites made anywhere along the anteroposterior (AP) axis result in head regeneration, while tail regeneration occurs at any posterior-facing amputation site5. Because these outcomes are direction- and not position-dependent, the orientation of wound sites somehow directs blastema determination.
Head-versus-tail regeneration fates are driven by a canonical Wnt signaling process, because βcatenin-1 RNAi causes ectopic head regeneration at posterior-facing wounds, while pathway overactivation through APC RNAi causes ectopic tail regeneration at anterior-facing wounds6–8. Several planarian Wnts are expressed in nested posterior domains (wntP-2/wnt11–5, wnt11–1, wnt11–2, wnt1, wntP-3)7,9–11 and a βCATENIN−1 protein gradient forms posteriorly 12,13, suggestive of canonical Wnt regulation in head/tail formation. In posterior blastemas, high Wnt signaling forms a signaling center controlled by differentiation of new wnt1+ muscle cells through STRIPAK/mob4, pitx, and islet factors14–16, and posterior Wnt signals restore tail and trunk identity17–19. However, wnt1 is induced early (6–18 hours post-injury) and generically at all injury sites, suggesting head regeneration normally involves early Wnt inhibition at anterior-facing wounds.
The secreted Wnt inhibitor notum is expressed early after injury (6–18 hours) preferentially at anterior-facing and not posterior-facing wounds10. Subsequently during head regeneration, transcription factors foxD, zic-1, prep, and pbx trigger differentiation of notum+ anterior pole muscle cells, forming an organizing center driving head outgrowth and patterning20–24. notum inhibition leads to anterior regeneration phenotypes, including head regeneration failure or ectopic tails10. Injection of notum dsRNA immediately after amputation resulted in regeneration of anterior tails, indicating notum can act after injury10. notum;βcatenin-1(RNAi) animals form ectopic heads, indicating notum acts upstream of βcatenin-1 at an early step to drive head regeneration10. In addition, RNAseq found notum to be the only gene asymmetrically expressed by 6–16 hours, while all injuries generically activate ~120 other genes25. Therefore, notum expression polarity marks the earliest symmetry-breaking event distinguishing anterior-and posterior-facing wounds to drive divergent regeneration behavior.
What is the mechanism driving early notum asymmetry? Injury-induced notum expression and polarization does not depend on stem cells10,20, nor on removal of particular tissues such as the head10. In addition, injury-induced notum is expressed exclusively from pre-existing myoD+ longitudinal muscle cells26, suggesting some latent polarization of muscle or other tissues instructs permissiveness for notum activation. In principle, canonical Wnt signals could be candidates for polarization given that notum is a feedback inhibitor whose expression depends on βcatenin10. However, APC RNAi leads to elevated notum at all injury sites and retention of polarization10. Therefore, although βcatenin signaling controls head-versus-tail blastema identity, it likely does not control notum polarity.
Other pathways involved in head/tail regeneration could potentially contribute to this standing polarity. Hedgehog signaling perturbation affects head-versus-tail regeneration but does so through controlling levels of wnt1 activation and does not regulate notum asymmetry10,27,28. Perturbation of gap junction and calcium signaling impacts head/tail regeneration, but through modulating wnt1 expression and/or notum activation rather than notum polarity29–31. Several additional factors (e.g., foxD, zic-1) control downstream head regeneration but do not influence early notum expression20–22. Activin signaling plays a critical role in regulating notum expression polarity because activin-2 RNAi leads to posterior head regeneration and elevated injury-induced notum expression at posterior-facing injury sites32. However, activin-2 is expressed broadly, so it is unclear whether it instructs polarization32. In addition, the Activin inhibitor follistatin is expressed by any injury to drives mitotic and cell death responses specific to tissue removal and regulates head regeneration by suppressing wnt1 expression independent of notum asymmetry33. Therefore, the nature of tissue polarization leading to injury-induced notum expression asymmetry remains unknown.
Noncanonical βcatenin-independent Wnt signaling pathways mediated by Dishevelled (Dvl) function in numerous developmental contexts, including planar cell polarity34–37. In planarians, AP polarization of ventral ciliated epidermal cells are mediated by Dishevelled38,39, but it is unknown whether such a pathway controls polarity of injury-induced notum expression instructing blastema fate determination. In addition, whether some or all of the planarian Wnts function equally in tissue polarization and/or the activation of notum is not known.
We analyze the roles for Wnt signals in notum expression regulation and find two systems that activate and repress notum at wound sites, leading to polarized injury-induced behavior. A noncanonical pathway involving two Wnt11s (wnt11–1 and wnt11-2) signaling through Dishevelled (Dvl) suppresses notum expression at posterior-facing wounds, while two distinct Wnts (wnt1 and wntP-2) promote notum expression at anterior-facing wounds. Wnt11 and Dvl signals emerge from the posterior and regulate muscle architecture homeostatically. The re-expression of Wnt11s through regeneration occurs after the notum polarity decision, and Wnt11 and Dvl RNAi phenotypes require long-term stem cell dependent turnover prior to injury, together suggesting these signals act prior to injury to establish latent polarity cues which dictate asymmetry of notum expression following injury.
Results
Dishevelled negatively regulates polarization of injury-induced notum from longitudinal muscle
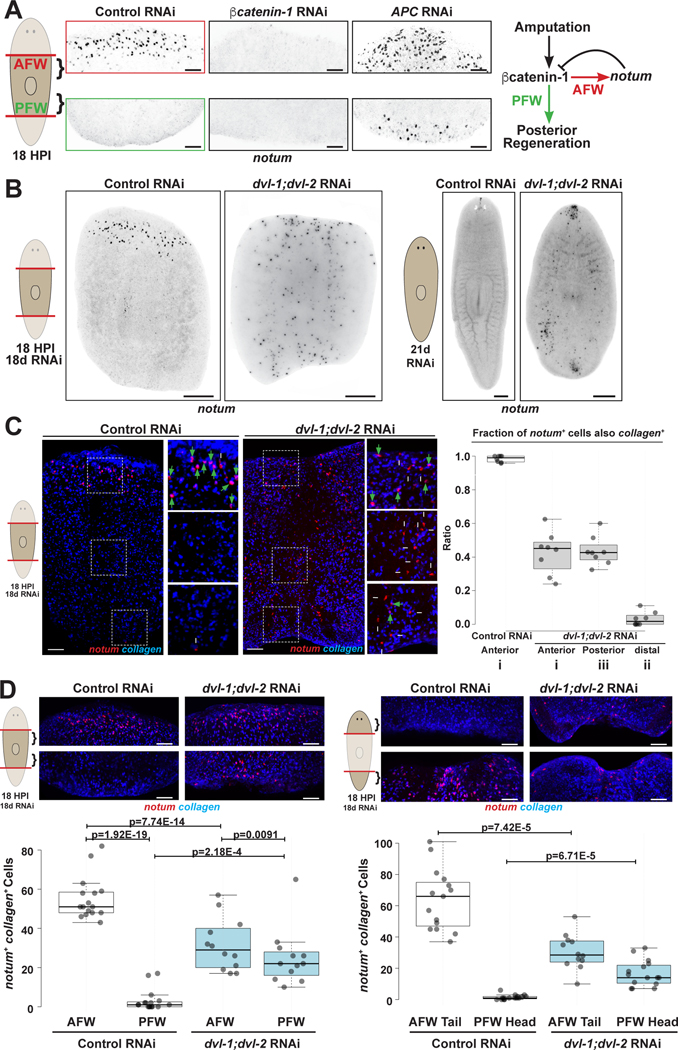
We tested whether Wnt-related pathways might control notum polarity. As observed previously10, βcatenin-1 inhibition prevented notum expression, while APC inhibition elevated notum at both anterior- and posterior-facing wounds while retaining expression asymmetry (Figure 1A). To perturb Wnt-related pathways more broadly, we inhibited planarian Dishevelled homologs dvl-1 and dvl-2, resulting in a distinct phenotype of ectopic notum expression (Figure 1B). Dvls likely act upstream of βcatenin-1 to activate canonical Wnt signaling6,37, indicating Dvl must additionally exert a βcatenin-1-independent role to suppress notum expression. Uninjured dvl-1;dvl-2(RNAi) worms also had ectopic notum+ cells (Figure 1B), so we examined the types of cells expressing notum (Figure 1C). Whereas >95% of notum-expressing cells were collagen+ muscle in controls, 40% of notum+ cells at either wound site in dvl-1;dvl-2(RNAi) trunk fragments were collagen+ (Figure 1C, insets i and iii), indicating populations of muscle and non-muscle among ectopic notum expressing cells. By contrast, in dvl-1;dvl-2(RNAi) animals, notum+ cells away from the wound site (Figure 1C, inset ii), or in the prepharyngeal region of uninjured animals (Figure S1A), were collagen-negative. We tested whether these might be chat+ neurons based on notum’s expression in the brain of normal animals40, but 15% of notum+ cells in dvl-1;dvl-2(RNAi) animals were chat+ (Figure S1B), accounting for only 25% of collagen-negative cells. Together, Dvl inhibition causes expression of notum in chat+ neurons and in unidentified cells, and results in inappropriate notum expression in collagen+ muscle proximal to wounds.
Figure 1. Dishevelled negatively regulates notum expression in muscle near wound sites.
(A) Left, FISH detecting notum expression asymmetry at 18 hours post-injury (HPI) in anterior-facing wounds (AFW) versus posterior-facing wounds (PFW) after indicated treatments. Right, model of head/tail blastema determination. (B) FISH detecting notum after indicated treatments. (C) Left, double-FISH detecting notum+ collagen+ muscle (green arrows) and notum+ collagen− cells (white arrows). 300% zoomed insets depict locations (i) at anterior-facing wounds, (ii) distal from wounds, and (iii) at posterior-facing wounds. Right, quantification of fraction of notum+ cells expressing collagen. (D) Double-FISH detecting notum+ collagen+ cells after indicated treatments. Box plots show median values (middle bars) and first-to-third interquartile ranges (boxes); whiskers indicate 1.5× interquartile ranges, dots show individual datapoints. P-values, two-tailed unpaired t-tests as indicated. Bars, 100 (A,C,D) or 300 microns (B). See also Figure S1. See also Data S1.
We counted notum+collagen+ cells at wounds and found Dvl inhibition elevated notum at posterior-facing wounds and reduced it at anterior-facing wounds, decreasing polarization (Figure 1D). Furthermore, Dvl inhibition similarly reduced notum polarization at amputations from different axis locations (Figure 1D), indicating Dvl broadly regulates notum expression orientation.
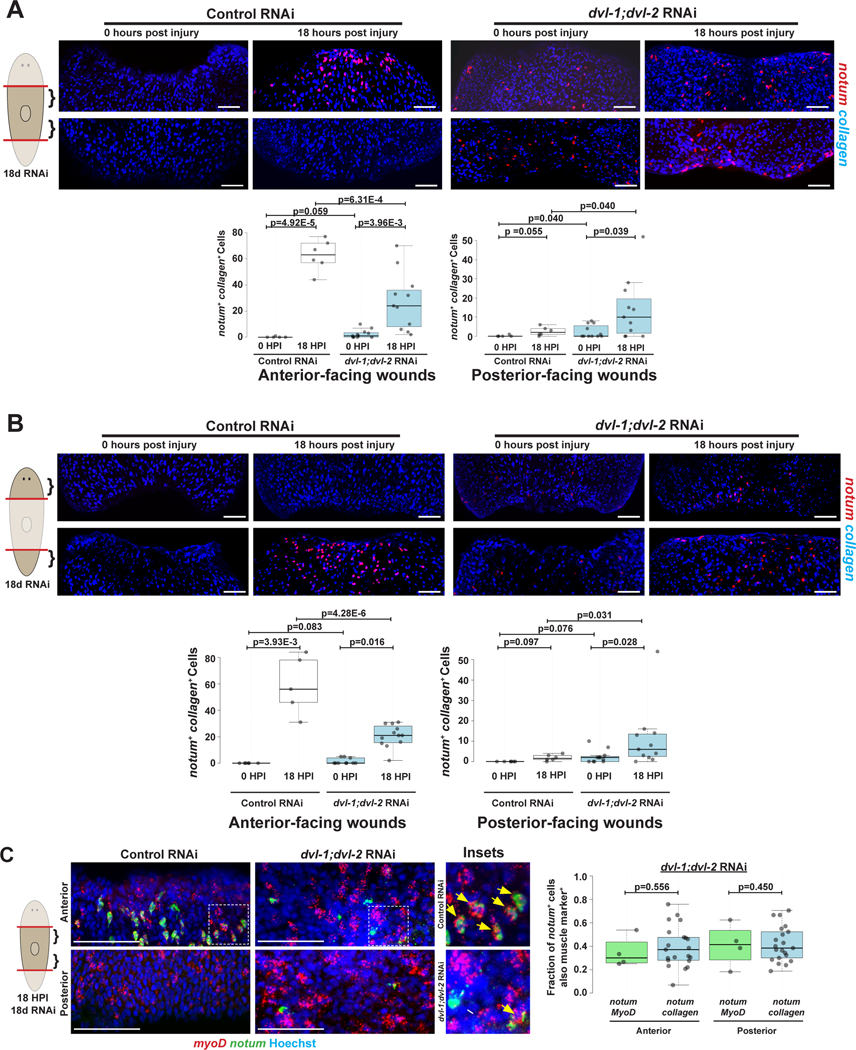
We tested whether notum misexpression occurred in response to injury (Figures 2A–B). dvl-1;dvl-2(RNAi) animals fixed at 18- versus 0-hours had elevated notum+collagen+ cells at both anterior- and posterior-facing wound sites and at different AP axis locations (Figure 2A–B). Therefore, Dvl inhibition affects the polarity of notum expression induced in muscle by injury. By contrast, non-muscle notum-expressing cells (notum+collagen-) were not significantly upregulated in injured dvl-1;dvl-2(RNAi) animals (Figure S2; Trunk AFWs p=0.374, Trunk PFW p=0.934, Tail AFW p=0.204, Trunk PFW p=0.438). Additionally, dvl-1;dvl-2(RNAi) animals expressed notum from myoD+ longitudinal muscle cells at posterior-facing wounds (Figure 2C), with similar fractions of notum+myoD+ cells and notum+collagen+ cells versus total wound-proximal notum+ cells (~40% in each case). Therefore, Dvl polarizes injury-induced notum, in longitudinal muscle, and across the body axis.
Figure 2. Dvl RNAi reduces polarization of injury-induced notum from longitudinal muscle.
(A-B) Double FISH detecting notum+ collagen+ muscle as indicated. (C) Double FISH detecting notum+ myoD+ longitudinal muscle (yellow arrows) versus notum+ myoD− cells (white arrow), with insets (boxes) indicated. Right, fraction of notum+ cells colocalizing myoD compared to colocalization of notum and collagen from Figure 1 data. A similar fraction of notum+ cells co-expressed myoD or collagen (~40%) in dvl-1;dvl-2(RNAi) animals. Box plot shows median values (middle bars) and first to third interquartile ranges (boxes); whiskers indicate 1.5× interquartile ranges, dots show datapoints obtained from separate individuals. P-values, two-tailed unpaired t-tests as indicated. Bars, 100 (A-B) or 50 (C) microns. See also Figure S2. See also Data S1.
Dishevelled RNAi causes ectopic head regeneration, failed outgrowth, and muscle fiber disorganization
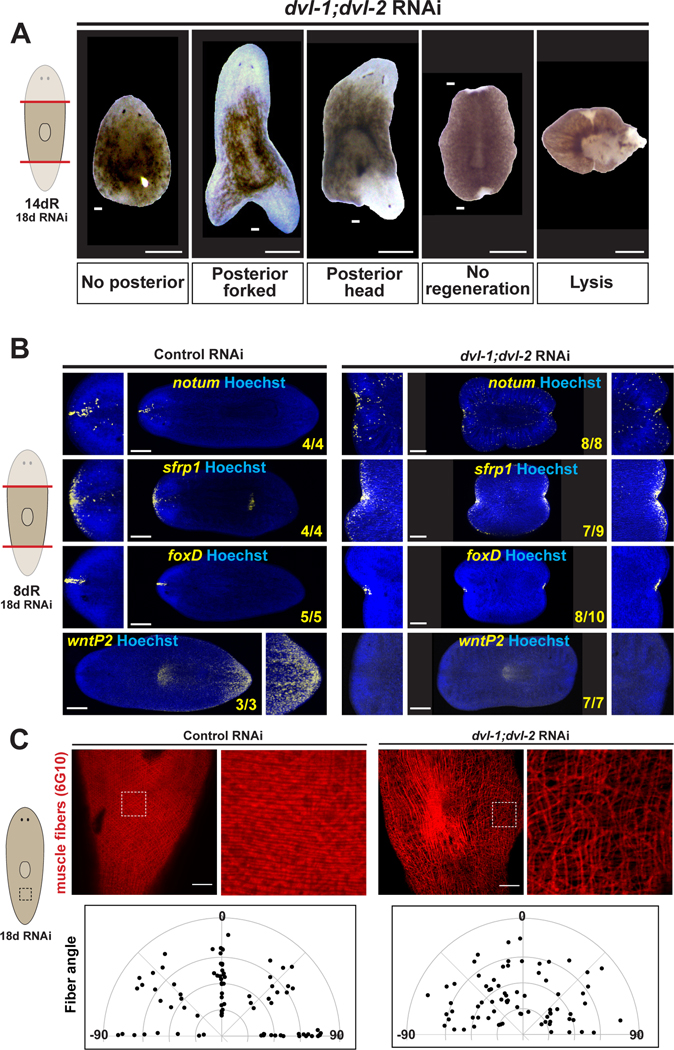
We examined regeneration outcomes under the Dvl RNAi conditions leading to ectopic notum. 21% (12/57) of dvl-1;dvl-2(RNAi) animals regenerated posterior-facing heads, 21% formed posterior forked-tail blastemas (12/57), 12% failed posterior regeneration (7/57), 33% failed all regeneration (19/57), and 11% underwent lysis and death (6/57) (Figure 3A), as observed previously6,38,39. Dishevelled likely functions in multiple pathways, with posterior head formation involving silencing sufficient to alter polarity without compromising regenerative capacity. By 8 days, dvl-1;dvl-2(RNAi) trunk fragments generated two anterior poles expressing notum, sfrp-1, and foxD, even when blastemas were absent, while also not expressing posterior marker wntP-2 (Figure 3B). Therefore, some fragments made incorrect head/tail polarity determinations but subsequently failed to proceed with regeneration39. By contrast, βcatenin-1 RNAi causes posterior head regeneration and not failed regeneration7.
Figure 3. Dishevelled inhibition causes ectopic head regeneration, failed outgrowth, and muscle fiber disorganization.
(A) Regeneration phenotypes from dvl-1;dvl-2 RNAi. (B) FISH detecting anterior (notum, sfrp1, foxD) or posterior (wntP-2) identity, with insets showing 200% zoom of animal anterior/posterior. (C) Immunofluorescence using 6G10 antibody detecting muscle fiber organization, with 400% zoomed insets (boxes). Quantifications of muscle fiber angles from 3–4 20×20-micron fields-of-view per condition. Dots represent individual fiber/AP-axis angles (degrees), jittered over random radial positions to display similar measurements. Control fibers clustered by muscle subtype: longitudinal (0), circular (+/− 90), and diagonal (+/− 45). dvl-1;dvl-2(RNAi) spread angle clusters. Bars, 100 (C) or 300 microns (A-B). See also Figure S3. See also Data S1.
dvl-1 and dvl-2 both had broad expression by FISH (Figure S3A), consistent with prior single-cell RNAseq atlases showing broad expression across celltypes (Figure S3B)41. Dishevelleds were expressed broadly throughout regeneration and in collagen+ muscle (Figure S3C–D). Therefore, Dishevelleds could function in many tissues, including muscle.
Given the spectrum of Dvl RNAi phenotypes including notum mispolarization, we reasoned Dvl inhibition may affect muscle organization. We stained animals with the 6G10 antibody42, labeling circular and diagonal muscle and weakly marking longitudinal muscle. In controls, muscle fibers angles binned according to subtype (longitudinal ~0 degrees, diagonal +/− 45 degrees, circular +/− 90 degrees). By contrast, dvl-1;dvl-2(RNAi) animals had fiber disorganization, with reduced abundance and broad distribution of angles (Figure 3C). Although no markers of muscle fiber polarity are known, these results suggest Dvl inhibition leads to body transformations involving muscle disorganization which could contribute to mispolarized notum.
wnt1 and wntP-2 positively regulate injury-induced notum expression
We hypothesized that subsets of the nine planarian Wnt genes might transduce separate activities of notum suppression or activation and tested this using RNAi (Figures 4–5, S4). wnt1 RNAi reduced but did not eliminate notum expression at anterior-facing wounds and had no effect at posterior-facing wounds (Figure 4A). wnt1 acts with wntP-2, a gene expressed in an animal-wide posterior-to-anterior gradient, to suppress head formation11. Phylogenetic analysis has either placed wntP-2 (also referred to as wnt11-5) as either a member of Wnt119 or Wnt443 subfamilies. 75% of wnt1(RNAi) animals regenerated posterior heads, whereas 100% of wnt1;wntP-2(RNAi) animals regenerated posterior heads (Figure S4A)11, so we tested co-inhibition of these genes on notum expression. wntP-2(RNAi) animals modestly decreased notum expression, and wnt1;wntP-2(RNAi) animals had more severely reduced notum at anterior-facing wounds without affecting posterior-facing wounds (Figure 4A). In addition, wnt1;wntP-2 RNAi similarly reduced injury-induced notum at across AP axial amputation sites, suggesting the function of these genes on notum expression is not regionally specific (Figures 4A–B). The re-establishment of wntP-2 expression during regeneration occurs after injury-induced notum expression9, so wntP-2’s role might either precede injury or involve post-transcriptional activation. wntP-2 expression could be detected far into the anterior (Figure 4C) and is expressed in both circular and longitudinal muscle cells26, while wnt1 is activated near wound sites from 6–18 hours (Figure 4C) and is co-expressed with notum10. These results suggest notum expression is activated by wound-induced wnt1 and homeostatic wntP-2 acting with βcatenin-1 following injury.
Figure 4. wnt1 and wntP-2 positively regulate injury-induced notum expression.
Single-channel images from double-FISH detecting notum and collagen in (A) trunk or (B) head and tail fragments as indicated. Graphs, quantification of notum+ collagen+ cells. Box plots show median values (middle bars) and first-to-third interquartile ranges (boxes); whiskers indicate 1.5× interquartile ranges and dots show datapoints from individual animals. p-values, two-tailed unpaired t-tests between each condition and corresponding control. (C) FISH detecting wnt1 and wntP-2 in uninjured animals or 18-hour trunk fragments (5/5 animals as shown). Bars, 100 (A-B) or 300 microns (C). See also Figure S4. See also Data S1.
Figure 5. wnt11–1 and wnt11–2 negatively regulate notum at posterior-facing wounds.
(A, B, E) Single-channel images from double-FISH detecting notum and collagen after Wnt11 RNAi 18 (A, E) or 30 days (B) before amputation as indicated, and graphs show quantification of notum+ collagen+ cells. (C) Double-FISH detecting notum+myoD+ longitudinal muscle (yellow arrows); quantifications show fractions of notum+ cells as collagen+ or myoD+. (D) FISH detecting wnt11–2/wnt11–1 in uninjured animal posterior (5/5 animals as shown). (E) Left, Wnt11 RNAi elevated notum expression by FISH at posterior-facing wounds in the animal anterior; right, quantification of notum+ collagen+ cells. (F) Left, wnt11–1;wnt11–2(RNAi) animals formed posterior-facing heads (3/17 animals, arrow) or failed posterior regeneration (14/17, arrow) after 30 days of RNAi. Right, FISH detecting anterior marker sfrp-1 marking posterior head of wnt11–1;wnt11–2(RNAi) animal (arrow). Box plots show median values (middle bars) and first-to-third interquartile ranges (boxes); whiskers indicate 1.5× interquartile ranges and dots are datapoints from individual animals. p-values, two-tailed unpaired t-tests between each condition and corresponding controls or indicated by brackets. Bars, 300 (D), 100 (A, B, E) or 50 microns (C). See also Figure S5. See also Data S1.
Inhibition of five other Wnt genes had either no effect on notum expression, small effects, or effects specific to axis position (Figures S4B–C). Inhibition of wnt5, wntP-3, and wnt2–1 had no effect on notum at anterior- or posterior-facing wounds. wntP-4 RNAi caused a small (15% fewer) but statistically significant (p=2.86E-3) reduction to notum at trunk fragment anterior-facing wounds and impaired tail regeneration (Figure S4A). Inhibition of wnt11–6 (also termed wntA)40,44,45 did not change notum expression at anterior-facing wounds or at trunk fragment posterior-facing wounds, but elevated notum expression at posterior-facing wounds of head fragments (Figures S4B–C). Therefore, wnt11–6 has a regional-specific suppressive role to limit notum expression, but likely cannot account for notum polarization at all injury sites. wnt11–6/wntA functions in anterior head and eye patterning and not in posterior identity determination, consistent with this factor influencing regional patterning40,44,45.
wnt11–1 and wnt11–2 negatively regulate notum expression at posterior-facing wound sites
RNAi of wnt11–1 and wnt11–2 resulted in ectopic notum expression at posterior-facing wounds and had no effect at anterior-facing wounds (Figure 5A). wnt11–1;wnt11–2 RNAi produced stronger notum overexpression, suggesting these genes act together. WNT11–1 and WNT11–2 are highly similar proteins, consistent with possibly redundant functions9. Treatments with wnt11–1;wnt11–2 dsRNAs for 18 days caused notum overexpression at posterior-facing wounds but relatively few notum+ cells (~15) versus normal anterior-facing wounds (~60 cells) (Figure 5A), suggesting insufficient knockdown timing for a full-strength phenotype. wnt11–1;wnt11–2 RNAi for an extended time (30 days) before injury increased notum+ cells at posterior-facing wounds (~40 cells) and had no effect at anterior-facing wounds (Figure 5B). We conclude that long-term wnt11–1;wnt11–2 RNAi progressively depletes a process suppressing notum induction at posterior-facing wounds.
notum was expressed almost entirely from myoD+ longitudinal muscle cells in wnt11–1;wnt11–2(RNAi) animals, similar in frequency to expression in collagen+ cells (Figure 5C). Therefore, Wnt11s suppress notum expression in longitudinal muscle not in other muscle cell types. We tested whether Wnt11s or Dvls regulate numbers of longitudinal muscle cells to examine whether elevated notum expression could arise indirectly from higher numbers of myoD+ cells. dvl-1;dvl-2(RNAi) animals had reduced myoD+ cells, consistent with lower overall muscle fiber density in these animals (Figure 3C), in spite of increasing notum-expressing cells at posterior-facing wounds (Figure S5A). Furthermore, wnt11–1;wnt11–2(RNAi) animals had normal myoD+ cell numbers, indicating Wnt11 and Dvl signaling does not control notum polarization through regulating muscle density (Figure S5A).
wnt11–1 and wnt11–2 are expressed in graded domains from the posterior prior to injury (Figure 5D)9. Previous studies determined these factors are expressed in body-wall muscle cells26,46, and single-cell RNAseq from a planarian cell atlas41 and double-FISH confirmed they are expressed in collagen+ muscle (Figures S5B–C). wnt11–1 is expressed specifically in nkx1.1+ circular muscle cells and in an nkx1.1-dependent manner, while wnt11–2 expression was depleted only by co-inhibition of myoD and nkx1.126. Despite their prominent posterior expression and non-detection by FISH in the anterior, wnt11–1;wnt11–2 inhibition caused notum expression at posterior-facing wound sites throughout the AP axis, including in head fragments (Figure 5E). Therefore, Wnt11s exert influence across the body and could be polarizing cues for negative regulation of notum after injury.
We examined the regeneration consequences of dual wnt11–1;wnt11–2 inhibition. Administration of wnt11–2 dsRNAs for 18 days prior to amputation led to tail regeneration failure and a posterior bulged morphology reminiscent of dvl-1;dvl-2(RNAi) phenotypes, and wnt11–1 caused tail narrowing, as shown previously17,44. wnt11–1;wnt11–2 RNAi for 18 days caused tail retraction and bulged posterior phenotypes before amputation, followed by failed tail but normal head regeneration (Figure S5D). Animals with failed posterior regeneration did not express anterior markers sfrp-1, foxD, or notum in the posterior and maintained posterior wntP-2 expression (Figure S5E). In these animals, muscle architecture appeared normal in the anterior but was altered in the posterior (Figure S5F); however, musculature was much more disorganized in dvl-1;dvl-2 RNAi (Figure 3C). Given that notum mis-polarization occurs in the anterior of wnt11–1;wnt11–2(RNAi) animals with apparently normal muscle fibers, Wnt11 inhibition can likely affect undetected aspects of muscle polarized responsiveness. By contrast, extended inhibition of wnt11–1;wnt11–2 for 30 days before injury caused regeneration of posterior heads (17%, 3/17 animals) which were sfrp-1+, or posterior bulges and regeneration failure (83%, 14/17 animals) (Figure 5F). Therefore, long-term wnt11–1;wnt11–2 RNAi increases the severity of notum mis-polarization and inverts posterior blastema identity. Because βcatenin-1 inhibition causes head/tail transformations while eliminating notum expression, we suggest wnt11–1 and wnt11–2 likely control blastema and notum polarity through a Dvl-dependent, βcatenin-independent noncanonical Wnt pathway.
Wnt11 and Dishevelled signaling likely acts prior to injury to control injury-induced notum
We reasoned Dvl and Wnt11 factors could either act after injury in signaling induced by wounding, or alternatively might set up axis polarization before injury. dsRNA dosing experiments revealed the notum polarity phenotype increased in severity between 9 and 18 days of dvl-1;dvl-2 RNAi, suggesting progressive emergence of this phenotype (Figure S6A) similar to wnt11–1;wnt11–2 RNAi (Figures 5A–B). These results suggest Dvl and Wnt11 homologs act over a longer term to establish notum polarity, but could instead be explained by slow Dvl and/or Wnt11 protein turnover.
We reasoned that any Wnt11 and Dvl signaling triggered by wounding would likely occur through regulating muscle cells already present at the time of injury rather than produced during the first 6–18 hours after surgery. Indeed, injury-induced notum activation occurs normally in irradiated animals depleted of neoblasts that cannot form new muscle20. Instead, the decision to activate notum takes place in longitudinal muscle already present at the time of injury. We therefore sought to determine whether Dvl and Wnt11 RNAi notum mispolarization phenotypes were similarly independent of tissue production by neoblasts. We first tested the effects of Dishevelled inhibition in animals subjected to sublethal X-rays doses (1350 rads), known to decrease neoblast abundance and temporarily halt production of new muscle cells32. We initiated this irradiation treatment on day two of an 18-day dsRNA dosing period, followed by amputation and fixation 18-hours post-injury (Figure 6A, irradiation “early”). As reported previously20, irradiation caused moderate reduction to notum expression at control anterior-facing wounds without altering posterior-facing wounds, confirming normal injury-induced notum polarization can take place under reduced cell turnover (Figure S6B). This treatment decreased muscle fiber density without apparently altering overall orientations (Figure S6C). This early irradiation treatment completely suppressed the excess notum phenotype at posterior-facing wound sites of dvl-1;dvl-2(RNAi) animals (p=3.53E-11), reducing notum levels to those of matched irradiated control animals (p=0.654) (Figure 6A, irradiated “early”). By contrast, the ectopic notum expression phenotype occurred (p=9.07E-4) in Dvl RNAi animals administered radiation the day prior to injury (Figure 6A, irradiated “late”), leading to similar ectopic notum+ cells as unirradiated Dvl RNAi animals (p=0.988) (Figure 6A). Therefore, the Dvl RNAi phenotype mis-polarizing notum expression involves an irradiation-sensitive step occurring between 1 and 17 days prior to injury. Similarly, the wnt11–1;wnt11–2(RNAi) phenotype was also eliminated by irradiation at the onset of an 18 day dsRNA administration before injury (Figure 6B). The irradiation sensitivity of the notum repolarization effect also occurred in either Dvl or Wnt11 RNAi wound sites in the animal’s anterior, so these effects were not region-specific (Figures S6D–E). Additionally, irradiation also prevented posterior reduction and widening in wnt11–1;wnt11–2 RNAi, and lateral ruffling in dvl-1;dvl-2 RNAi (Figure S6F), indicating these phenotypes correlate with mis-polarized notum.
Figure 6. Wnt11 and Dishevelled function before injury and require neoblasts to polarize notum activation after injury.
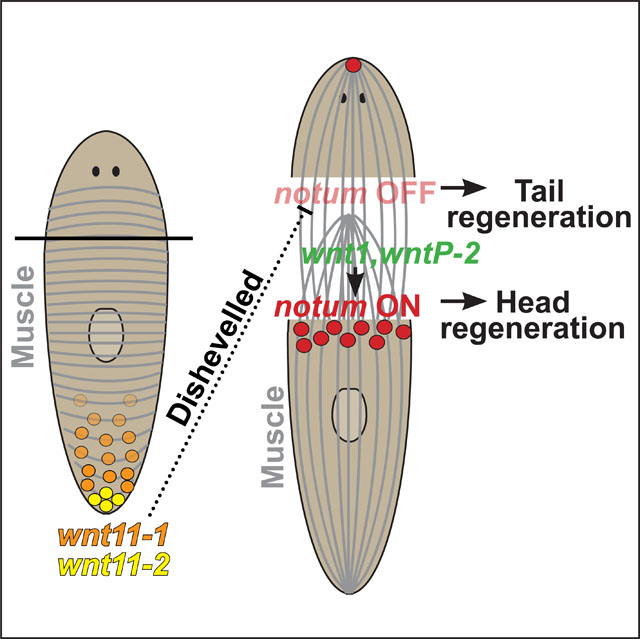
(A) Examining effects of timing sublethal irradiation on expression of notum after control or dvl-1;dvl-2(RNAi). Animals treated with 1350 Rads sublethal X-ray radiation at either the beginning (“early”) or the end (“late”) of 18 days of six dsRNA feedings (f), before amputation (cut), and fixation (fix) 18-hours post-injury. Double-FISH images and quantifications detecting notum+ collagen+ cells at posterior-facing wounds of trunk fragments. (B) “Early” irradiation dose treatment as in (A) testing irradiation sensitivity of wnt11–1;wnt11–2 RNAi phenotypes on notum expression. (A-B) Box plots show median values (middle bars) and first-to-third interquartile ranges (boxes); whiskers indicate 1.5× interquartile ranges and dots show datapoints from individual animals. p-values, two-tailed unpaired t-tests between conditions indicated by brackets. (C) Time-course FISH detecting first appearance (arrows) of new expression of each factor in regeneration, indicating wnt11–1 and wnt11–2 re-expression occurs after notum activation. Panels represent 5/5 animal fragments tested. Scale bars, 100 microns (A-C). (D) Model depicting the roles of Wnt genes in activating and polarizing notum in longitudinal muscle cells early in regeneration. Standing gradients of wnt11–1, wnt11–2, and wntP-2 are present prior to injury. Signals activating expression of notum include the wnt1 gene induced by injury as well as wntP-2, expressed in a body-wide gradient. wnt11–1, wnt11–2, and Dishevelled homologs repress expression of notum at posterior-facing wound sites. Based on timing of expression in regeneration, irradiation sensitivity of their RNAi phenotypes, and exerting influence across the body, we suggest Wnt11 and Dvl control notum polarity prior to injury through initiating or propagating an anterior tissue polarization. wnt11–1 is expressed from circular muscle and injury-induced notum is expressed from longitudinal muscle26. By 18h, polarized notum expression suppresses wnt1 activity at anterior-facing wounds and drives anterior regeneration, while low notum at posterior-facing wounds permits higher wnt1 activity and posterior regeneration. By 72 hours, tail blastemas re-express Wnt11 while head blastemas do not. Therefore, notum and Wnt11 mutually antagonize in order to sustain polarization across successive rounds of regeneration. See also Figure S6. See also Data S1.
One possible interpretation is that Dvl and Wnt11 RNAi causes newly differentiating muscle to form in a mispolarized manner, leading to subsequent mispolarization of notum. Alternatively, irradiation could prevent the Dvl or Wnt11 RNAi phenotype progression in some other way. Control animals show irradiation does not intrinsically block all injury-induced notum in muscle present at the time of wounding (Figure 6B). In addition, irradiation did not affect Dishevelled or wnt11–1/wnt11–2 mRNA expression, suggesting these factors are already expressed in muscle cells at the time of wounding (Figure S6G). We cannot rule out the possibility that depletion of Dvl and Wnt11 proteins depends on neoblasts. However, RNAi experiments in sublethally irradiated planarians successfully uncovered genes controlling self-renewal/differentiation of remaining neoblasts47–49, indicating RNAi can reduce gene function in irradiated planarians, but Dvl and/or Wnt11 proteins may be exceptions. However, this interpretation also implies Dvl and Wnt11 protein cohorts engaged in polarizing notum activation would likely have been produced prior to injury. Together, these experiments argue against, but do not fully rule out, a possible model in which injury elicits Wnt11 and/or Dvl signals that directly control notum polarization. Instead, we suggest Wnt11 expression from the posterior indirectly establishes axis polarization prior to injury, resulting in asymmetric expression of notum after injury.
We further examined the Wnt11 and notum order of action through analysis of their expression dynamics after injury. In animals amputated pre-pharyngeally, asymmetric notum expression activated by 5-hours and peaked at 18-hours, while wnt11–1 and wnt11–2 expression near the wound-site was absent during these early times and only emerged later by 72-hours (Figure 6C). These experiments, together with timed irradiation sensitivity, argue that Wnt11s signal via a noncanonical Wnt pathway from the posterior prior to injury to control symmetry breaking through notum expression after injury.
Discussion
Our analysis indicates Wnts have distinct roles in notum activation (Figure 6D). Injury-induced wnt1 and an animal-wide gradient of wntP-2 provide activating cues for notum after injury dependent on βcatenin-1 (Figure 6D). wnt11–1/wnt11–2 likely signal through Dvl-dependent, βcatenin-independent processes prior to injury to suppress notum at posterior-facing wounds (Figure 6D). Wnt11 and Dvl could polarize longitudinal muscle cells, or alternatively other tissue such as epidermis, known to be polarized by Dishevelled39. However, given that Wnt11s are expressed from muscle and influence notum polarization in muscle, this communication likely takes place across muscle.
Our study advances on prior work implicating planarian Dishevelled in epidermal polarization38,39 by showing specific function with non-canonical Wnt11 factors in suppressing injury-induced notum to determine head-versus-tail blastema identity. The decrease in notum expression at anterior-facing wounds in Dvl RNAi likely occurs through its role in canonical Wnt signaling to activate notum, or alternatively Dvl RNAi randomizes notum expression with respect to direction. By contrast, wnt11–1/wnt11–2 suppress notum specifically at posterior-facing wounds while wnt1/wntP-2 activate expression at anterior-facing wounds. wnt1 could provide a generic injury cue attempting to activate notum, but pre-existing information from wnt11–1/wnt11–2 and wntP-2 enable longitudinal muscles to distinguish orientation of wound sites. Furthermore, the irradiation sensitivity of Dvl and Wnt11 RNAi phenotypes suggests tissue turnover reinforces polarization, possibly by enabling appropriate polarization of newly born longitudinal muscle cells. Pre-existing signals have been implicated in the placement of newly born notum+foxD+ anterior pole cells in head regeneration, regulated by BMP and Wnt5 signals which are present prior to injury, but it is unclear whether this regulation is through the new or pre-existing component of these signals39. Our data defining a critical time for Dvl and Wnt11 activity on notum expression asymmetry, based on the timing of irradiation sensitivity, as well as timeseries expression data of Wnt11 versus notum in regeneration, demonstrates that signals important for instructing regeneration can act prior to injury.
Our results implicate Wnt11s as polarizing determinants used for regeneration. Vertebrate Wnt11s can act through canonical50 or noncanonical51–53 signals in development via Dvl signaling and are required for axis polarization and formation. Additionally, the neural tube expresses Wnt11 critical for aligning myofiber lateral outgrowth54, indicating Wnt11s may have ancient roles in muscle polarization. AP axis regeneration in the acoel Hofstenia miamia also uses separate injury-induced and constitutive Wnt signals from muscle, so this strategy could be ancient in bilaterian whole-body regeneration55–58.
Our results point to the importance of orthogonal muscle in wound polarization. wnt11–1 is expressed primarily from circular muscle cells26 and influences notum in longitudinal muscles, whereas wnt11–2 expression requires both circular and longitudinal muscle26. Additionally, depletion of circular muscle through nkx1.1 RNAi elevated notum expression at posterior-facing wound sites26. nkx1.1 RNAi also depletes expression of activin-2, which regulates notum polarization26. These results suggest circular muscle can control polarized responses of longitudinal muscle.
Polarity conveyance could occur by several possible mechanisms. Polarity could propagate cell-to-cell from Wnt11 sources via alternate signals, similar to planar cell polarity. Alternatively, an axis-wide Wnt11 protein gradient could orient polarization of distant receiving longitudinal muscle. Given that low amounts of wnt11–1 expression have been observed into the anterior13, longitudinal muscle could perhaps polarize from direct contact with Wnt11-expressing cells. Finally, longitudinal muscle might lack polarization and instead is instructed by polarized tissue such as epidermis38,39. Higher-resolution analyses of muscle cells and identification of additional polarity regulators could resolve these mechanisms.
Our results suggest a self-assembly mechanism in which regeneration relies upon and also reinforces tissue polarity. At posterior-facing wounds, homeostatic Wnt11 inhibits notum activation, leading to tail regeneration and consequently re-expression of Wnt11 to instruct the next round of regeneration. At anterior-facing wounds, notum expression is permitted, leading to head regeneration and lack of new Wnt11 activation. The mutual inhibition between notum and Wnt11, bridging information before and after injury, would sustain the fidelity of regeneration outcomes across potentially many rounds of asexual reproduction.
Regeneration involves inputs from injuries, including gene expression states induced by wounding10,11,25,59,60. Our results additionally indicate a critical role for signals operating prior to injury that instruct wound-induced outputs. Adult pattern formation is essential for regeneration to restore the appropriate missing parts following tissue removal. Our results suggest regenerative ability may arise from the coupling of constitutive patterning information with Injury-induced signals.
STAR Methods
RESOURCE AVAILABAILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Christian Petersen (christian-p-petersen@northwestern.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
Data used to generate the figures are presented in Data S1.
Data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Asexual Schmidtea mediteraanea animals (CIW4 strain) were kept in 1x Montjuic salts61 (1.6 mmol/l NaCl, 1.0 mmol/l CaCl2, 1.0 mmol/l MgSO4, 0.1 mmol/l MgCl2, 0.1 mmol/l KCl and 1.2 mmol/l NaHCO3 prepared in Milli-Q water) at 18–20°C. Animals were fed bi-weekly in calf liver (Western Veal Calf Liver Non-Formula Feed Product 2180, Golden Gate Meat Co, San Francisco CA) pureed in a Juiceman JM400 Juiceman Jr. 2-Speed Electric Juicer and stored in aliquots at −80. Animals were starved at least 7 days before the start of experiments.
METHOD DETAILS
Cloning, riboprobes and dsRNA
Genes were cloned from planarian cDNA generated by reverse transcription of bulk RNA preparations as previously described10, and PCR fragments were cloned into pGEM-T-easy. Primers are listed in Table S1. Constructs to inhibit wnt11–1, wnt11–2, wntP-2, wnt1, βcatenin-1, APC, dvl-1, and dvl-2 were previously described6,7,10,11. Riboprobes were generated by in vitro antisense transcription using digoxigenin- or fluorescein-labeled nucleotides and isolated by ethanol precipitation. dsRNA was generated by in vitro transcription to produce sense and antisense transcripts which were ethanol precipitated and then annealed at 72C.
RNAi
RNAi was performed by feeding worms a mixture of dsRNA (16% v/v), red food dye (4% v/v) and homogenized liver paste (80% v/v) and cultured in gentamycin (100 uM) in 1xMontjuic salts. Unless otherwise noted, animals were starved at least 7 days before RNAi feeding, then fed every third day for six feedings spanning 18 days or as indicated fed every third day spanning 30 days. A 3-day waiting period followed the end of dsRNA feeding prior to either fixation or amputations. dsRNA coding for C. elegans unc-22 was used as a negative control, as this sequence is absent in the S. mediterranea genome.
Fluorescence in situ hybridization
Animals were killed in 5% NAC in PBS, fixed in 4% formaldehyde (w/v) and bleached in 6% H2O2 in methanol. Animals were transitioned into riboprobe solution and hybridization was conducted overnight at 56°C. Secondary labeling was conducted using anti-digoxigenin-POD (at 1:1000) or anti-fluorescein-POD (at 1:2000) antibodies in MABT containing 10% horse serum and 10% (v/v) Western Blocking Reagent (Roche). Signal was developed by tyramide amplification with 1:1000 rhodamine-tyramide (DIG probes) or 1:2000 fluorescein-tyramide (FL probes). For double FISH secondary antibody binding and tyramide amplification steps were performed sequentially, separated by enzyme quenching in either 4% formaldehyde (w/v) or 100 mM sodium azide for 45 minutes. Nuclear counterstaining was performed using Hoechst 33342 at 1:1000 in PBSTx.
Immunostaining
Animals were killed in 0.75M HCl, fixed in Carnoy’s solution (60% v/v ethanol, 30% v/v chloroform, 10% v/v glacial acetic acid) and bleached in 6% H2O2 (v/v) in methanol. Animals were blocked for 6 hours in PBST (1x PBS, .3% Triton X-100) plus 10% horse serum (v/v) followed by overnight incubation with primary antibody mouse monoclonal 6G10 at 1:1000 in blocking solution, washout, and overnight incubation with secondary antibody rabbit anti-mouse HRP at 1:1000 in blocking solution. Signal was developed by tyramide amplification with 1:1000 rhodamine-tyramide. Nuclear counterstaining was performed using Hoechst 33342 at 1:1000 in PBSTx.
Image acquisition and cell counting
Live images were obtained on a Leica M210F dissecting microscope equipped with a Leica DFC295 camera. Adjustments to brightness and contrast made using Fiji/ImageJ. FISH images obtained on a Leica DM5500B compound microscope with Optigrid structured illumination or a Leica TCS SPE confocal microscope. Images are maximum projections of a z-stack adjusted for brightness and contrast using Fiji/ImageJ. For quantification of notum+ cells, the ~200-micron region near wound sites were imaged at 10x on a Leica TCS SPE confocal microscope with one-micron Z-stacks. Animals were imaged both dorsally and ventrally, then notum+ cells were manually counted, annotated, and scored in ImageJ for colocalization with either collagen, chat, or myoD as indicated, and cell counts from each side were summed for a total count of cells per wound site. For muscle fiber angle analysis, angles of ~20 fibers with respect to the AP axis were measured from ~2-micron regions of fibers in 20×20-micron fields-of-view taken from 3–4 animals per condition.
Irradiation assay
Animals were irradiated using a Radsource RS-2000 X-ray irradiator to deliver 1350 rads to worms maintained in 1x Montjuic salts at 20°C.
QUANTIFICATION AND STATISTICAL ANALYSIS
Sample sizes (n) are indicated and defined in each legend and/or in Data S1 as either number of animals, cells or muscle fibers as indicated. Box plots show median values (middle bars) and first-to-third interquartile ranges (boxes); whiskers indicate 1.5× interquartile ranges, and dots are individual datapoints obtained by either individual animals, cell, or muscle fibers as indicated in each legend. Data used to generate figures are presented in Data S1, including sample sizes. p-values were computed by two-tailed unpaired t-tests either comparing treatments to the corresponding control or between conditions indicated by brackets in each figure. Numeric p-values are displayed in each figure, and p<0.05 was considered a significant difference. See also Data S1.
Supplementary Material
Data S1. Data used for plotting figures of the study. Related to STAR methods and Figures 1, 2, 3, 4, 5, 6, S1, S2, S3, S4, S5, S6
A) Data plotted in Figure 1C, B) Data plotted in Figure 1D left graph, C) Data plotted in Figure 1D right graph, D) Data plotted in Figure S1A, E) Data plotted in Figure S1A, F) Data plotted in Figure 2A left graph, G) Data plotted in Figure 2A right graph, H) Data plotted in Figure 2B left graph, I) Data plotted in Figure 2B right graph, J) Data plotted in Figure 2C, K) Data plotted in Figure 2A top left graph, L) Data plotted in Figure 2A top right graph, M) Data plotted in Figure 2A bottom left graph, N) Data plotted in Figure 2A bottom right graph, O) Data plotted in Figure 4A left graph, P) Data plotted in Figure 4A right graph, Q) Data plotted in Figure 4B left graph, R) Data plotted in Figure 4B right graph, S) Data plotted in Figure S4A top left graph, T) Data plotted in Figure S4A top right graph, U) Data plotted in Figure S4A bottom graph, V) Data plotted in Figure S4B left graph, W) Data plotted in Figure S4B right graph, X) Data plotted in Figure S4C left graph, Y) Data plotted in Figure S4C right graph, Z) Data plotted in Figure 5A left graph, AA) Data plotted in Figure 5A right graph, AB) Data plotted in Figure 5B left graph, AC) Data plotted in Figure 5B right graph, AD) Data plotted in Figure 5C, AE) Data plotted in Figure 5E, AF) Data plotted in Figure S5A, AG) Data plotted in Figure S5D first graph from left to right, AH) Data plotted in Figure S5D second graph from left to right, AI) Data plotted in Figure S5D third graph from left to right, AJ) Data plotted in Figure S5D fourth graph from left to right, AK) Data plotted in Figure S5D rightmost graph, AL) Data plotted in Figure 6A, AM) Data plotted in Figure 6B Left graph, AN) Data plotted in Figure 6B Right graph, AO) Data plotted in Figure S6A first graph from left to right, AP) Data plotted in Figure S6A second graph from left to right, AQ) Data plotted in Figure S6A third graph from left to right, AR) Data plotted in Figure S6A rightmost graph, AS) Data plotted in Figure S6B, AT) Data plotted in Figure S6D, AU) Data plotted in Figure S6E, AV) Data plotted in Figure S6F left graph, AW) Data plotted in Figure S6F Right graph
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-digoxigenin-POD, Fab fragments | 11 207 733 910 | RRID: AB_514500 |
| anti-fluorescein-POD, Fab fragments | 11 426 346 910 | RRID: AB_840257 |
| Bacterial and virus strains | ||
| Biological samples | ||
| Chemicals, peptides, and recombinant proteins | ||
| Critical commercial assays | ||
| Deposited data | ||
| Experimental models: Cell lines | ||
| Experimental models: Organisms/strains | ||
| Schmidtea mediterranea, clonal strain CIW4, asexual | Laboratory of Christian Petersen | |
| Oligonucleotides | ||
| Listed in Table S1 | This study | NA |
| Recombinant DNA | ||
| Software and algorithms | ||
| Image J (FIJI) | https://fiji.sc | |
| Metamorph | Molecular Devices | https://www.moleculardevices.com/products/cellular-imaging-systems/acquisition-and-analysis-software/metamorph-microscopy#gref |
| Leica LAS | Leica | https://www.leica-microsystems.com/products/microscope-software/p/leica-las-x-ls/ |
| R Studio | R Studio | https://rstudio.com/ |
| Other | ||
Highlights.
Injury triggers AP-polarized notum expression in muscle, driving head regeneration
notum expression depends on beta-catenin, injury-induced wnt1 and wntP-2
notum polarization depends on Dvl and two Wnt11s expressed from posterior muscle
Wnt11s likely act prior to injury to influence notum polarity after injury
Acknowledgements
We thank members of the Petersen lab for critical comments. The authors acknowledge funding from National Institutes of Health, USA (NIGMS R01GM129339 and R01GM130835) (C.P.P.) and Northwestern’s NIH Cellular and Molecular Basis of Disease Training Program 2T32GM008061 (D.G.).
Footnotes
Declaration of Interests
The authors declare no competing interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Newmark PA, and Sanchez Alvarado A.(2000). Bromodeoxyuridine specifically labels the regenerative stem cells of planarians. Dev Biol 220, 142–153. [DOI] [PubMed] [Google Scholar]
- 2.Reddien PW., Oviedo NJ., Jennings JR., Jenkin JC., and Sanchez Alvarado A. (2005). SMEDWI-2 is a PIWI-like protein that regulates planarian stem cells. Science 310, 1327–1330. [DOI] [PubMed] [Google Scholar]
- 3.Elliott SA, and Sanchez Alvarado A.(2013). The history and enduring contributions of planarians to the study of animal regeneration. Wiley Interdiscip Rev Dev Biol 2, 301–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddien PW (2018). The Cellular and Molecular Basis for Planarian Regeneration. Cell 175, 327–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddien PW, and Sanchez Alvarado A.(2004). Fundamentals of planarian regeneration. Annu Rev Cell Dev Biol 20, 725–757. [DOI] [PubMed] [Google Scholar]
- 6.Gurley KA, Rink JC, and Sanchez Alvarado A.(2008). Beta-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science 319, 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen CP, and Reddien PW (2008). Smed-betacatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science 319, 327–330. [DOI] [PubMed] [Google Scholar]
- 8.Iglesias M, Gomez-Skarmeta JL, Salo E, and Adell T.(2008). Silencing of Smed-betacatenin1 generates radial-like hypercephalized planarians. Development 135, 1215–1221. [DOI] [PubMed] [Google Scholar]
- 9.Gurley KA, Elliott SA, Simakov O, Schmidt HA, Holstein TW, and Sanchez Alvarado A.(2010). Expression of secreted Wnt pathway components reveals unexpected complexity of the planarian amputation response. Developmental biology 347, 24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen CP, and Reddien PW (2011). Polarized notum activation at wounds inhibits Wnt function to promote planarian head regeneration. Science 332, 852–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen CP, and Reddien PW (2009). A wound-induced Wnt expression program controls planarian regeneration polarity. Proc Natl Acad Sci U S A 106, 17061–17066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sureda-Gomez M, Martin-Duran JM, and Adell T.(2016). Localization of planarian beta-CATENIN-1 reveals multiple roles during anterior-posterior regeneration and organogenesis. Development 143, 4149–4160. [DOI] [PubMed] [Google Scholar]
- 13.Stuckemann T, Cleland JP, Werner S, Thi-Kim Vu H, Bayersdorf R, Liu SY, Friedrich B, Julicher F, and Rink JC (2017). Antagonistic Self-Organizing Patterning Systems Control Maintenance and Regeneration of the Anteroposterior Axis in Planarians. Developmental cell 40, 248–263 e244. [DOI] [PubMed] [Google Scholar]
- 14.Schad EG, and Petersen CP (2020). STRIPAK Limits Stem Cell Differentiation of a WNT Signaling Center to Control Planarian Axis Scaling. Current biology : CB 30, 254–263 e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marz M, Seebeck F, and Bartscherer K.(2013). A Pitx transcription factor controls the establishment and maintenance of the serotonergic lineage in planarians. Development 140, 4499–4509. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi T., Motoishi M., Yazawa S., Itomi K., Tanegashima C., Nishimura O., Agata K., and Tarui H. (2011). A LIM-homeobox gene is required for differentiation of Wnt-expressing cells at the posterior end of the planarian body. Development 138, 3679–3688. [DOI] [PubMed] [Google Scholar]
- 17.Sureda-Gomez M, Pascual-Carreras E, and Adell T.(2015). Posterior Wnts Have Distinct Roles in Specification and Patterning of the Planarian Posterior Region. International journal of molecular sciences 16, 26543–26554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scimone ML, Cote LE, Rogers T, and Reddien PW (2016). Two FGFRL-Wnt circuits organize the planarian anteroposterior axis. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lander R, and Petersen CP (2016). Wnt, Ptk7, and FGFRL expression gradients control trunk positional identity in planarian regeneration. eLife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasquez-Doorman C, and Petersen CP (2014). zic-1 Expression in Planarian neoblasts after injury controls anterior pole regeneration. PLoS Genet 10, e1004452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogg MC, Owlarn S, Perez Rico YA, Xie J, Suzuki Y, Gentile L, Wu W, and Bartscherer K.(2014). Stem cell-dependent formation of a functional anterior regeneration pole in planarians requires Zic and Forkhead transcription factors. Dev Biol 390, 136–148. [DOI] [PubMed] [Google Scholar]
- 22.Scimone ML, Lapan SW, and Reddien PW (2014). A forkhead transcription factor is wound-induced at the planarian midline and required for anterior pole regeneration. PLoS Genet 10, e1003999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blassberg RA, Felix DA, Tejada-Romero B, and Aboobaker AA (2013). PBX/extradenticle is required to re-establish axial structures and polarity during planarian regeneration. Development 140, 730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen CC, Wang IE, and Reddien PW (2013). pbx is required for pole and eye regeneration in planarians. Development 140, 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wurtzel O, Cote LE, Poirier A, Satija R, Regev A, and Reddien PW (2015). A Generic and Cell-Type-Specific Wound Response Precedes Regeneration in Planarians. Dev Cell 35, 632–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scimone ML, Cote LE, and Reddien PW (2017). Orthogonal muscle fibres have different instructive roles in planarian regeneration. Nature 551, 623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rink JC, Gurley KA, Elliott SA, and Sanchez Alvarado A.(2009). Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science 326, 1406–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yazawa S, Umesono Y, Hayashi T, Tarui H, and Agata K.(2009). Planarian Hedgehog/Patched establishes anterior-posterior polarity by regulating Wnt signaling. Proceedings of the National Academy of Sciences of the United States of America 106, 22329–22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang D, Chan JD, Nogi T, and Marchant JS (2011). Opposing roles of voltage-gated Ca2+ channels in neuronal control of regenerative patterning. J Neurosci 31, 15983–15995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oviedo NJ, Morokuma J, Walentek P, Kema IP, Gu MB, Ahn JM, Hwang JS, Gojobori T, and Levin M.(2010). Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev Biol 339, 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durant F, Bischof J, Fields C, Morokuma J, LaPalme J, Hoi A, and Levin M.(2019). The Role of Early Bioelectric Signals in the Regeneration of Planarian Anterior/Posterior Polarity. Biophysical journal 116, 948–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cloutier JK, McMann CL, Oderberg IM, and Reddien PW (2021). activin-2 is required for regeneration of polarity on the planarian anterior-posterior axis. PLoS Genet 17, e1009466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tewari AG., Stern SR., Oderberg IM., and Reddien PW. (2018). Cellular and Molecular Responses Unique to Major Injury Are Dispensable for Planarian Regeneration. Cell Rep 25, 2577–2590 e2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma M, Castro-Piedras I, Simmons GE Jr., and Pruitt K.(2018). Dishevelled: A masterful conductor of complex Wnt signals. Cell Signal 47, 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devenport D.(2014). The cell biology of planar cell polarity. J Cell Biol 207, 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aw WY, and Devenport D.(2017). Planar cell polarity: global inputs establishing cellular asymmetry. Curr Opin Cell Biol 44, 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallingford JB, and Habas R.(2005). The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development 132, 4421–4436. [DOI] [PubMed] [Google Scholar]
- 38.Vu HT, Mansour S, Kucken M, Blasse C, Basquin C, Azimzadeh J, Myers EW, Brusch L, and Rink JC (2019). Dynamic Polarization of the Multiciliated Planarian Epidermis between Body Plan Landmarks. Developmental cell 51, 526–542 e526. [DOI] [PubMed] [Google Scholar]
- 39.Almuedo-Castillo M, Salo E, and Adell T.(2011). Dishevelled is essential for neural connectivity and planar cell polarity in planarians. Proceedings of the National Academy of Sciences of the United States of America 108, 2813–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hill EM, and Petersen CP (2015). Wnt/Notum spatial feedback inhibition controls neoblast differentiation to regulate reversible growth of the planarian brain. Development 142, 4217–4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fincher CT, Wurtzel O, de Hoog T, Kravarik KM, and Reddien PW (2018). Cell type transcriptome atlas for the planarian Schmidtea mediterranea. Science 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross KG, Omuro KC, Taylor MR, Munday RK, Hubert A, King RS, and Zayas RM (2015). Novel monoclonal antibodies to study tissue regeneration in planarians. BMC developmental biology 15, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riddiford N, and Olson PD (2011). Wnt gene loss in flatworms. Development genes and evolution 221, 187–197. [DOI] [PubMed] [Google Scholar]
- 44.Adell T, Salò E, Boutros M, and Bartscherer K.(2009). Smed-Evi/Wntless is required for β-catenin-dependent and-independent processes during planarian regeneration. Development 136, 905–910. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi C, Saito Y, Ogawa K, and Agata K.(2007). Wnt signaling is required for antero-posterior patterning of the planarian brain. Developmental biology 306, 714–724. [DOI] [PubMed] [Google Scholar]
- 46.Witchley JN, Mayer M, Wagner DE, Owen JH, and Reddien PW (2013). Muscle cells provide instructions for planarian regeneration. Cell Rep 4, 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagner DE, Ho JJ, and Reddien PW (2012). Genetic regulators of a pluripotent adult stem cell system in planarians identified by RNAi and clonal analysis. Cell Stem Cell 10, 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan A, Ma S, Pearson BJ, and Chan D.(2021). Collagen IV differentially regulates planarian stem cell potency and lineage progression. Proceedings of the National Academy of Sciences of the United States of America 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lei K, Thi-Kim Vu H, Mohan RD, McKinney SA, Seidel CW, Alexander R, Gotting K, Workman JL, and Sanchez Alvarado A.(2016). Egf Signaling Directs Neoblast Repopulation by Regulating Asymmetric Cell Division in Planarians. Dev Cell 38, 413–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, and Heasman J.(2005). Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell 120, 857–871. [DOI] [PubMed] [Google Scholar]
- 51.Heisenberg CP., Tada M., Rauch GJ., Saude L., Concha ML., Geisler R., Stemple DL., Smith JC., and Wilson SW. (2000). Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405, 76–81. [DOI] [PubMed] [Google Scholar]
- 52.Matsui T, Raya A, Kawakami Y, Callol-Massot C, Capdevila J, Rodriguez-Esteban C, and Izpisua Belmonte JC (2005). Noncanonical Wnt signaling regulates midline convergence of organ primordia during zebrafish development. Genes Dev 19, 164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Witzel S, Zimyanin V, Carreira-Barbosa F, Tada M, and Heisenberg CP (2006). Wnt11 controls cell contact persistence by local accumulation of Frizzled 7 at the plasma membrane. J Cell Biol 175, 791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gros J, Serralbo O, and Marcelle C.(2009). WNT11 acts as a directional cue to organize the elongation of early muscle fibres. Nature 457, 589–593. [DOI] [PubMed] [Google Scholar]
- 55.Srivastava M, Mazza-Curll KL, van Wolfswinkel JC, and Reddien PW (2014). Whole-body acoel regeneration is controlled by Wnt and Bmp-Admp signaling. Curr Biol 24, 1107–1113. [DOI] [PubMed] [Google Scholar]
- 56.Tewari AG, Owen JH, Petersen CP, Wagner DE, and Reddien PW (2019). A small set of conserved genes, including sp5 and Hox, are activated by Wnt signaling in the posterior of planarians and acoels. PLoS Genet 15, e1008401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramirez AN, Loubet-Senear K, and Srivastava M.(2020). A Regulatory Program for Initiation of Wnt Signaling during Posterior Regeneration. Cell Rep 32, 108098. [DOI] [PubMed] [Google Scholar]
- 58.Raz AA, Srivastava M, Salvamoser R, and Reddien PW (2017). Acoel regeneration mechanisms indicate an ancient role for muscle in regenerative patterning. Nat Commun 8, 1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wenemoser D, Lapan SW, Wilkinson AW, Bell GW, and Reddien PW (2012). A molecular wound response program associated with regeneration initiation in planarians. Genes Dev 26, 988–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benham-Pyle BW, Brewster CE, Kent AM, Mann FG Jr., Chen S, Scott AR, Box AC, and Sanchez Alvarado A.(2021). Identification of rare, transient post-mitotic cell states that are induced by injury and required for whole-body regeneration in Schmidtea mediterranea. Nature cell biology 23, 939–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cebria F., and Newmark PA. (2005). Planarian homologs of netrin and netrin receptor are required for proper regeneration of the central nervous system and the maintenance of nervous system architecture. Development 132, 3691–3703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Data used for plotting figures of the study. Related to STAR methods and Figures 1, 2, 3, 4, 5, 6, S1, S2, S3, S4, S5, S6
A) Data plotted in Figure 1C, B) Data plotted in Figure 1D left graph, C) Data plotted in Figure 1D right graph, D) Data plotted in Figure S1A, E) Data plotted in Figure S1A, F) Data plotted in Figure 2A left graph, G) Data plotted in Figure 2A right graph, H) Data plotted in Figure 2B left graph, I) Data plotted in Figure 2B right graph, J) Data plotted in Figure 2C, K) Data plotted in Figure 2A top left graph, L) Data plotted in Figure 2A top right graph, M) Data plotted in Figure 2A bottom left graph, N) Data plotted in Figure 2A bottom right graph, O) Data plotted in Figure 4A left graph, P) Data plotted in Figure 4A right graph, Q) Data plotted in Figure 4B left graph, R) Data plotted in Figure 4B right graph, S) Data plotted in Figure S4A top left graph, T) Data plotted in Figure S4A top right graph, U) Data plotted in Figure S4A bottom graph, V) Data plotted in Figure S4B left graph, W) Data plotted in Figure S4B right graph, X) Data plotted in Figure S4C left graph, Y) Data plotted in Figure S4C right graph, Z) Data plotted in Figure 5A left graph, AA) Data plotted in Figure 5A right graph, AB) Data plotted in Figure 5B left graph, AC) Data plotted in Figure 5B right graph, AD) Data plotted in Figure 5C, AE) Data plotted in Figure 5E, AF) Data plotted in Figure S5A, AG) Data plotted in Figure S5D first graph from left to right, AH) Data plotted in Figure S5D second graph from left to right, AI) Data plotted in Figure S5D third graph from left to right, AJ) Data plotted in Figure S5D fourth graph from left to right, AK) Data plotted in Figure S5D rightmost graph, AL) Data plotted in Figure 6A, AM) Data plotted in Figure 6B Left graph, AN) Data plotted in Figure 6B Right graph, AO) Data plotted in Figure S6A first graph from left to right, AP) Data plotted in Figure S6A second graph from left to right, AQ) Data plotted in Figure S6A third graph from left to right, AR) Data plotted in Figure S6A rightmost graph, AS) Data plotted in Figure S6B, AT) Data plotted in Figure S6D, AU) Data plotted in Figure S6E, AV) Data plotted in Figure S6F left graph, AW) Data plotted in Figure S6F Right graph
Data Availability Statement
Data used to generate the figures are presented in Data S1.
Data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.