ABSTRACT
Detecting latent tuberculosis infection (LTBI) is important, especially in high-risk populations including healthcare workers (HCWs). QuantiFERON-TB Gold Plus (QFT-Plus) is a new version of the interferon-gamma release assays (IGRAs) to replace the QuantiFERON-TB Gold In-tube (QFT-GIT). However, data on the use of QFT-Plus for LTBI detection in high TB-burden countries are limited. This study was conducted in a TB-endemic setting in Thailand. HCWs were enrolled in the study and underwent both tests during the annual health screening. The testing results were compared and the concordance was determined. Of 102 HCWs, 11 (10.78%) were positive according to both tests, and 15 (14.71%) were positive according to QFT-Plus. The overall agreement between assays was 96.08%, with Cohen’s kappa coefficient (k) at 0.82. All four discordant results occurred with QFT-GIT negative and QFT-Plus positive. The comparison between QFT-GIT and QFT-Plus based on each antigen tube (TB1 or TB2) exhibited similar concordance with 99.02% and 95.10% agreement, respectively. The intra-comparison between TB1 and TB2 of QFT-Plus also showed good concordance at 96.08%. Among this group of HCWs, the LTBI prevalence of any positive results in both tests was low. Overall, the study showed good agreement between QFT-Plus and QFT-GIT (k = 0.82) with a minimal difference, suggesting similar assay performance to that mainly carried out in TB-low incidence countries. The results support the use of QFT-Plus for detecting LTBI in a format similar to QFT-GIT.
KEYWORDS: Latent tuberculosis, QFT-Plus, Healthcare workers, TB-endemic, Thailand
INTRODUCTION
Tuberculosis (TB) remains a global health problem, with an estimated 10.6 million new cases and 1.6 million TB-related deaths worldwide in 2021 1 . About a quarter of the global population is estimated to be latently infected with Mycobacterium tuberculosis (MTB) 1,2 , 5 to 15% of whom can progress to develop active TB in their lifetime 3 . According to the World Health Organization (WHO), to achieve the global goal of the End TB Strategy, latent TB infection (LTBI) should be targeted and managed properly, especially in high-risk populations 4 .
There are no gold standard tests for the microbiological diagnosis of LTBI and few immunological tests are available. Currently, the LTBI diagnosis is based on a conventional tuberculin skin test (TST) and interferon-γ (IFN-γ) release assays (IGRAs). The TST is based on the skin’s immune reaction against purified protein derivatives of tuberculin. Its limitations involve cross-reactivity with the BCG vaccine and non-tuberculous mycobacteria (NTM), administrative and interpretative errors, and the requirement for two clinic visits 5 , 6 . IGRAs are blood-based assays that measure the IFN-γ following in vitro stimulation of individual white blood cells with specific MTB antigens. For a decade, two IGRAs have been commercially available, namely T-SPOT.TB and QuantiFERON-TB Gold In-Tube (QFT-GIT) 7 .
The advantages and disadvantages of IGRAs have been reviewed in previous studies 8,9 . By using MTB-derived specific peptides, IGRAs exhibit a more specific immune reaction when compared to TST. However, cross-reactivity is still present with some other mycobacteria, such as Mycobacterium marinum, Mycobacterium kansasii, and Mycobacterium szulgai 7 . Nonetheless, all tests show a reduction in sensitivity for immunocompromised patients and children 10 , and are unable to distinguish between latent and active TB, including recent and old LTBI. Since the risk of developing active TB is high in the first two years following MTB infection 11 , tests for identifying recent LTBI would be advantageous.
QuantiFERON-TB (QFT) is an interferon-gamma release assay (IGRA). In 2015, QFT-Plus was launched to replace the early version, QFT-GIT 7 . According to the manufacturer, the new generation offered higher sensitivity and specificity, including the ability to identify recent LTBI 12 . QFT-Plus has two TB-antigen tubes, TB1 and TB2. Both tubes contain long ESAT-6 and CFP-10 peptides, primarily stimulating CD4+ helper T cells, but the TB7.7 antigen is removed. The extra antigen tube (TB2) contains additional short peptides from ESAT-6 and CFP-10, stimulating both CD4+helper T cells and CD8+ cytotoxic T cells. These peptides have been reported to induce the CD8+ T-cell response associated with recent MTB infection 13 . Therefore, the QFT-Plus test is deemed able to differentiate between recent and past LTBI, and this could support the decision to start preventive treatment, including an improvement in assay sensitivity.
Thailand is a high TB-burden country with an estimated 103,000 people developing TB and a TB incidence rate of 143 cases per 100,000 people1. Currently, no national policy is in place to systematically test for LTBI 6 . At present, IGRAs or TST are being used for MTB infection screening in a few groups of high-risk populations, such as TB household contacts, HCWs, or immunocompromised patients receiving immunosuppressants or dialysis. Thailand has published a number of LTBI studies using QFT-GIT, some of which show an agreement between TST and QFT-GIT 14,15 . To obtain a higher agreement between these two tests, the adjustment of the cutoff value for TST has been proposed 6 . Under special considerations, IGRA has been increasingly used. However, the QFT-Plus performance within endemic settings has been documented less. Nonetheless, comparative data on the performance of QFT-GIT and QFT-Plus in LTBI detection are largely based on middle or low TB-incidence settings 7,16 – 18 . Therefore, we aim to compare the two tests in Thailand, where the TB burden is high. The performance of QFT-Plus and the prevalence of LTBI among Thai HCWs were determined in this study.
MATERIALS AND METHODS
Study participants
The HCWs referred for QuantiFERON testing were enrolled. Participants were tested simultaneously with the regular QFT-GIT (Qiagen, Hilden, Germany) and the new QFT-Plus test (Qiagen, Hilden, Germany) for LTBI detection. The testing was performed under the TB screening program in hospitals. HCWs with prior or current TB were excluded. Screening for active TB was routinely performed based on symptoms and a chest radiograph examination. Anonymous data were analyzed with the approval of the Ethical Review Committee of the Ministry of Public Health, Thailand.
Sample size calculation
The sample size was estimated based on an infinite population proportion using the formula n = [(Z21 – (a/2))(p)(1-p)]/d2 where n = sample size; Z21 – (a/2) = Z score (based on the confidence level); p = population proportion and d = margin of error. The proportion of latent tuberculosis infection among healthcare workers from our previous report was around 20% 6 . The confidence level was taken as 95% and the margin of error was 8%. The sample size was then calculated.
Blood collection and QFT-GIT and QFT-Plus procedures
For IFN-γ analysis, whole-blood samples were collected in lithium heparin tubes, with testing conducted according to the manufacturer’s protocol. Afterwards, exact amounts of blood from the heparin tubes were transferred into a set of QFT-GIT and QFT-Plus tubes. Blood samples were incubated for at least 16 to 24 h at 37 °C within 16 h of collection, and then immediately processed or stored at 4 °C, before performing the enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s guidance. The cutoff point of the IFN-γ for positive and negative results was 0.35 IU/ml after correcting for negative control as the background in each test. Due to the QFT-Plus having two antigen tubes, TB-antigen 1 (TB1) and TB-antigen 2 (TB2), the maximum of any two antigen tubes was considered for the testing results. The results of both tests were interpreted and reported as positive, negative, and indeterminate according to the test instructions.
Data and statistical analyses
To assess test concordance, the results of the two tests were compared with a 95% confidence interval (CI). Similar analyses were performed to assess the intra-differences between the TB1 and TB2 results of the QFT-Plus assay. The agreement was analyzed according to the percentage of concordant results and Cohen’s kappa coefficient (k). Kappa values ≥ 0.81 were considered to be very good, 0.61-0.80 good, 0.41-0.60 moderate, 0.21-0.40 fair, and ≤ 0.20 in poor agreement. All statistical analyses were performed using VassarStats: Statistical Computation Freeware (Vassar College, Poughkeepsie, NY, USA).
To access the recent TB infection, the CD8+ T-cell response was analyzed based on the difference between IFN-γ levels in TB1 and TB2 tubes. A difference of 0.6 IU/ml in IFN-γ release by subtracting the TB1 from the TB2 IFN-γ levels was considered to be a true difference and used to determine the CD8+ T-cell response, indicating recent TB infection 19–21 .
RESULTS
Results of QFT-GIT and QFT-Plus
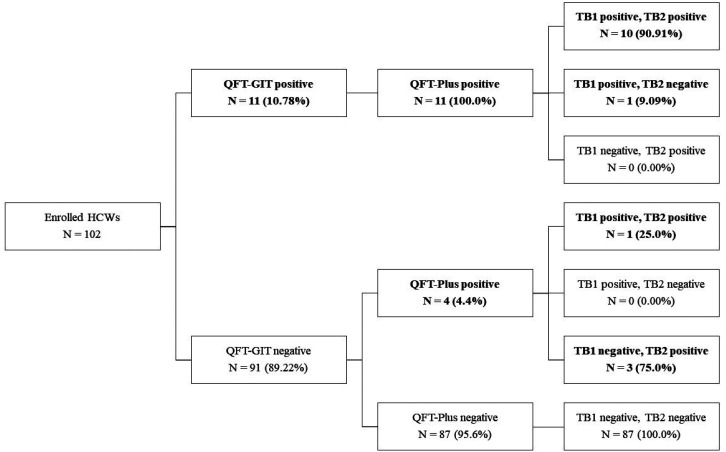
QFT-GIT and QFT-Plus assays were completed on 102 subjects (calculated sample size = 97). No indeterminate results were obtained. The overall LTBI positivity provided by the two tests was 15/102 (14.71%). Of these, 11 (10.78%) and 15 (14.71%) were positive by QFT-GIT and QFT-Plus, respectively (Table 1). The scheme overview of QFT-GIT and QFT-Plus results is shown in Figure 1. Of the 11 positive results provided by both tests, 10 were QFT-Plus positive by both TB1 and TB2, while one was positive by TB1 only. Of the four QFT-GIT negative/QFT-Plus positive results, one was QFT-Plus positive by both TB1 and TB2, while three were QFT-Plus positive by TB2 only (Figure 1). Overall, the disagreement in the four samples (4/102) occurred in the direction of QFT-GIT negative but QFT-Plus positive (Figure 1, Table 1).
Table 1. Qualitative comparison between QFT-GIT and QFT-Plus.
| QFT-Plus | QFT-Plus TB1 | QFT-Plus TB2 | ||||
|---|---|---|---|---|---|---|
| QFT-GIT /result(n) | Positive(15) | Negative(87) | Positive(12) | Negative(90) | Positive(14) | Negative(88) |
| Positive (11) | 11 | 0 | 11 | 0 | 10 | 1 |
| Negative (91) | 4 | 87 | 1 | 90 | 4 | 87 |
| Total (102) | 15 | 87 | 12 | 90 | 14 | 88 |
Figure 1. Schematic overview of QFT-GIT and QFT-Plus results.
Intra-comparison of TB1 and TB2 in the QFT-Plus assay
For the QFT-Plus results, a comparison was also performed between TB1 and TB2. The positive rates of QFT-Plus based on TB1 and TB2 were not equal. Of all 15 QFT-Plus positive results, 12 were TB1 positive and 14 were TB2 positive (Table 1). The concordance of positive results for QFT-Plus in the intra-comparison between TB1 and TB2 was 80.0% (12/15) and 93.33% (14/15), respectively ( Table 1). Therefore, the interpretation outcomes based on the individual TB1 and TB2 were slightly different ( Table 1). Meanwhile, the positive rate of TB2 (14/15) was likely to be higher than those of TB1 (12/15) and TB tubes (11/15).
Agreement analysis of QFT-GIT and QFT-Plus
The assessment of the agreement between the two tests showed that the overall QFT-GIT results were similar to those of QFT-Plus, resulting in an agreement of 96.08% (95% CI; 89.69-98.74) and a Cohen’s kappa coefficient (k) of 0.82 (Table 2). The results for QFT-Plus based on either TB1 or TB2 compared to those of QFT-GIT also showed high agreement at 99.02% (95% CI, 93.88–99.95) and 95.10% (95% CI, 88.39–98.18) with k of 0.95 and 0.77, respectively. The intra-comparison between TB1 and TB2 of QFT-Plus results also showed good concordance at 96.08% (Table 2).
Table 2. Qualitative agreement between QFT-GIT and QFT-Plus.
| Comparison | Agreement (%, 95% CI) | Kappa (95% CI) |
|---|---|---|
| QFT-GIT vs QFT-Plus | 98/102 (96.08, 89.69–98.74) | 0.82 (0.66–0.99) |
| QFT-GIT vs QFT-Plus TB1 | 101/102 (99.02, 93.88–99.95) | 0.95 (0.86–1.00) |
| QFT-GIT vs QFT-Plus TB2 | 97/102 (95.10, 88.39–98.18) | 0.77 (0.58–0.96) |
| QFT-Plus TB1 vs QFT-Plus TB2 | 98/102 (96.08, 89.69–98.74) | 0.82 (0.66–0.99) |
Quantitative detecting results of QFT-GIT and QFT-Plus
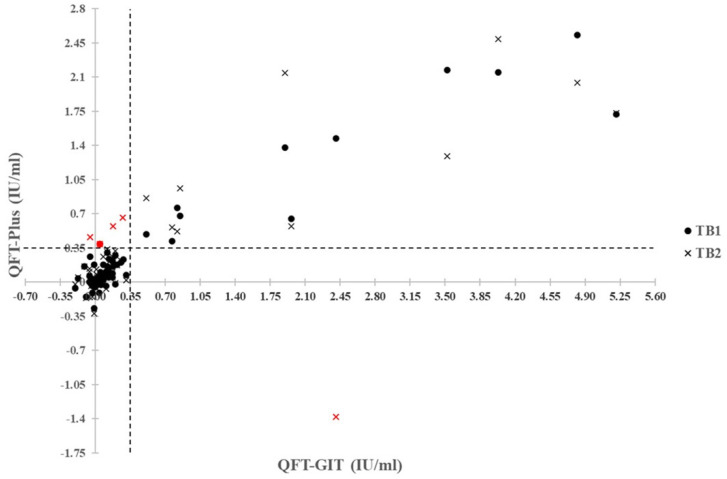
For the quantitative analysis, IFN-γ levels of all subjects were obtained, as displayed in the scatter plot ( Figure 2). The reference lines show the IFN-γ cutoff point for QFT-GIT and QFT-Plus at 0.35 µg/ml. The scatter plot could show the difference in IFN-γ release among both tests.Table 3 displays the IFN-γ levels in all 15 positive HCWs by QFT-GIT or QFT-Plus. The released IFN-γ levels of the discordant results in four HCWs are underlined in Table 3. Based on IFN-γ levels, the difference occurred in the borderline. All discrepant results exhibited TB2 positive for LTBI, and IFN-γ levels in TB2 were all higher than those in the TB tube. One subject exhibited TB1 and TB2 positive (HCW9) and the remainder TB2 positive but TB1 negative (HCW2, 13, 14). The difference in IFN-γ release in TB2 minus TB1 was further determined.
Figure 2. Quantitative results of QFT-GIT and QFT-Plus results in healthcare workers. Scatter plots show quantitative results for QFT-GIT versus QFT-Plus in healthcare workers. Discordances are shown in red (1 for TB1 and TB2, and 4 for TB2 only). The dashed reference lines at 0.35 IU/ml are the assay cutoffs.
Table 3. Individual data for quantitative results of positive QFT-GIT and/or QFT-Plus from healthcare workers.
| Subject Nº | TB, IU/ml (QFT-GIT) | TB1, IU/ml (QFT-Plus) | TB-TB1, IU/ml | TB2, IU/ml (QFT-Plus) | TB2-TB1, IU/ml |
|---|---|---|---|---|---|
| HCW1 | 0.82 | 0.76 | 0.06 | 0.52 | -0.24 |
| HCW2 | 0.18 | 0.16 | 0.02 | 0.57 | 0.41 |
| HCW3 | 0.51 | 0.49 | 0.02 | 0.86 | 0.37 |
| HCW4 | 4.82 | 2.53 | 2.29 | 2.04 | -0.49 |
| HCW5 | 5.21 | 1.72 | 3.49 | 1.73 | 0.01 |
| HCW6 | 4.03 | 2.15 | 1.88 | 2.49 | 0.34 |
| HCW7 | 1.90 | 1.38 | 0.52 | 2.14 | 0.76 |
| HCW8 | 0.85 | 0.68 | 0.17 | 0.96 | 0.28 |
| HCW9 | 0.05 | 0.39 | -0.34 | 0.39 | 0 |
| HCW10 | 2.41 | 1.47 | 0.94 | -1.38 | -2.85 |
| HCW11 | 0.77 | 0.42 | 0.35 | 0.56 | 0.14 |
| HCW12 | 3.52 | 2.17 | 1.35 | 1.29 | -0.88 |
| HCW13 | 0.28 | 0.23 | 0.05 | 0.66 | 0.43 |
| HCW14 | -0.05 | 0.26 | -0.31 | 0.46 | 0.2 |
| HCW15 | 1.96 | 0.65 | 1.31 | 0.57 | -0.08 |
| Average | 1.82 | 1.03 | 0.79 | 0.92 | -0.11 |
The bold digits represent the values used for reporting the testing result; the underlined elements represent the discordant results of QFT-GIT vs QFT-Plus.
The difference in IFN-γ release in TB2 and TB1 tubes (TB2–TB1)
The difference in IFN-γ release in TB2 and TB1 tubes (TB2–TB1) > 0.6 IU/ml was considered a true difference and used to determine recent exposure to TB 7,19 . In this study, only one HCW exhibited a true difference in TB1 positive/TB2 positive results (HCW7). No true difference in the IFN-γ levels between TB1 and TB2 was found among the discrepant results (Table 3).
DISCUSSION
This study showed an overall good agreement between the two tests during the screening process for TB infection of HCWs in Thailand. The concordant results were similar to previous studies conducted in low TB incidence and high-income countries 7,17,18,22 . The findings revealed high agreement between the QFT-Plus and QFT-GIT results at 96.08% (95% CI; 89.69-98.74) and k at 0.82 (95% CI; 0.66-0.99), ranging from 89.9%-96.0% for agreement with the kappa values ranging from 0.80-0.91, as summarized by Shafeque et al. 16 . The data support the potential use of QFT-Plus in the same format as QFT-GIT for LTBI testing in TB-endemic settings. Its utility would depend on other factors such as availability, accessibility, and appropriateness in each location.
QFT-Plus could detect more responders among infected persons and provide greater positivity. It should be noted that all discordant results were QFT-Plus positive but QFT-GIT negative and were observed to be in the borderline range. Another observation was that only one positive subject had a true difference in IFN-γ release, suggesting a recent infection. However, this study could not show an association with recent TB infection, as specific information on TB exposure or risk factors and data on LTBI serial testing were not available. It has recently been reported that QFT-Plus cannot distinguish between recent and past infections, suggesting that it should not be used for contact tuberculosis tracing 23 . This might indicate the need for further evidence. In addition, a lower level of IFN-γ was observed in the TB1 tubes when compared to TB tubes based on the average values (Table 3). Since the TB tube contained an additional antigen, TB7.7, the IFN-γ release in the TB tubes was likely higher than that of the TB1 tubes. This observation was similar to those reported in previous studies 7,24,25 . Furthermore, considering that TB1 exhibits a CD4+ T-cell reaction and TB2 poses both CD4+ and CD8+ T-cell responses, the positive rate of the TB2 tube should be higher than that of the TB1 tube. In this study, a higher IFN-γ release in TB2 tubes compared to TB1 tubes was likely observed, in similarity to previous studies 24,25 .
It has been claimed that QFT-Plus exhibited greater sensitivity in the detection of MTB infection than QFT-GIT 12 . Due to no reference standard being available for LTBI diagnosis, the sensitivity for detecting active TB has been used as a surrogate reference for evaluating diagnostic performance 5 . Previous studies have reported QFT-Plus sensitivity of 82.5–91% and specificity of 84–98% for detecting active tuberculosis 20,24–26 , while the sensitivity of QFT-GIT for active TB diagnosis in Thailand has been reported at 84.2% 27 . Horne et al. 28 documented that the sensitivities of the two tests for detecting active TB were not significantly different. Since patients with active tuberculosis were not included in this study, the sensitivity of QFT-Plus could not be determined.
The prevalence of LTBI in the study group of HCWs was low (14.71%). This positive IGRA prevalence was lower than that reported previously in other endemic countries such as Vietnam (47%) 29 , India (40%) 30 , Brazil (27%) 31 , and even Thailand (18.8–20%) 6,32 . The low positive rate of LTBI in the group under this study is probably a consequence of effective TB prevention and control practices or the previous annual check-up program resulting in the exclusion of persons with long-term LTBI. Overall, screening for LTBI in HCWs seems to be useful. According to the 2022 WHO LTBI guidelines, TST and IGRAs, including QFT-Plus, are valid for the detection of TB infection or LTBI 33 . In each setting, the use of QFT-Plus should be considered based on cost and benefit to ensure that it would be able to fulfill the clinical requirements and suit local conditions.
This study has some limitations, including the enrollment of a small number of subjects. In addition, most of the HCWs were nurses and nurse assistants and most probably had previous TB contact both inside and outside the hospital. Therefore, the results might not be applicable to other groups of HCWs or settings with differences in HCWs’ characteristics. Another limitation is that TST has not been performed in the comparison. Despite the limitations, the results could demonstrate a high agreement between the two tests. This study is among a few others carried out in TB-high-burden countries. The results support the use of QFT-Plus in a similar way to QFT-GIT. The risk of TB infection should be assessed individually and then people at high-risk should be prioritized for IGRA or QFT testing. This would provide insight into the effectiveness of TB infection prevention and control measures in hospitals.
CONCLUSIONS
The agreement between QFT-Plus and QFT-GIT was good (k = 0.82), suggesting that the use of QFT-Plus might be similar to QFT-GIT and those in TB-low prevalence settings. Overall, the use of QFT-Plus in high TB-burden countries might be appropriate for LTBI detection, with special consideration for its use in each location.
ACKNOWLEDGMENTS
The authors thank all subjects for their willingness to participate in this study. The work was supported by hospitals and teams including clinical nurses and medical technicians. The support from the National Institute of Health, Thailand, was acknowledged.
Footnotes
FUNDING
No funding to declare.
REFERENCES
- 1.World Health Organization . Global tuberculosis report 2022. Geneva: WHO; 2022. [[[cited 2022 Dec 7]]. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 . [Google Scholar]
- 2.Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modeling. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002152. e1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trauer JM, Moyo N, Tay EL, Dale K, Ragonnet R, McBryde ES, et al. Risk of active tuberculosis in the five years following infection….15% Chest. 2016;149:516–525. doi: 10.1016/j.chest.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . Guidelines on the management of latent tuberculosis infection. Geneva: WHO; 2015. [[[cited 2022 Dec 7]]. Available from: https://www.who.int/publications/i/item/9789241548908 . [PubMed] [Google Scholar]
- 5.Goletti D, Sanduzzi A, Delogu G. Performance of tuberculin and interferon-gamma release assays: an update on the accuracy, cutoff stratification and new potential immune-based approaches. J Rheumatol Suppl. 2014;91:24–31. doi: 10.3899/jrheum.140099. [DOI] [PubMed] [Google Scholar]
- 6.Khawcharoenporn T, Apisarnthanarak A, Sangkitporn S, Rudeeaneksin J, Srisungngam S, Bunchoo S, et al. Tuberculin skin test and QuantiFERON® -TB Gold In-Tube for diagnosing latent tuberculosis infection among Thai healthcare workers. Jpn J Infect Dis. 2016;69:224–230. doi: 10.7883/yoken.JJID.2015.181. [DOI] [PubMed] [Google Scholar]
- 7.Pieterman ED, Liqui Lung FG, Verbon A, Bax HI, Ang CW, Berkhout J, et al. A multicentre verification study of the QuantiFERON-TB Gold Plus assay. Tuberculosis (Edinb) 2018;108:136–142. doi: 10.1016/j.tube.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014;27:3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banaei N, Gaur RL, Pai M. Interferon gamma release assays for latent tuberculosis: what are the sources of variability? J Clin Microbiol. 2016;54:845–850. doi: 10.1128/JCM.02803-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rangaka MX, Wilkinson KA, Glynn JR, Ling D, Menzies D, Mwansa-Kambafwile J, et al. Predictive value of interferon- gamma release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:45–55. doi: 10.1016/S1473-3099(11)70210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borgdorff MW, Sebek M, Geskus RB, Kremer K, Kalisvaart N, van Soolingen D. The incubation period distribution of tuberculosis estimated with a molecular epidemiological approach. Int J Epidemiol. 2011;40:964–970. doi: 10.1093/ije/dyr058. [DOI] [PubMed] [Google Scholar]
- 12.QuantiFERON-TB Gold Plus (QFT-Plus) ELISA [package insert] Hilden: Qiagen; 2015. [Google Scholar]
- 13.Nikolova M, Markova R, Drenska R, Muhtarova M, Todorova Y, Dimitrov V. Antigen-specific CD4- and CD8-positive signatures in different phases of Mycobacterium tuberculosis infection. Diagn Microbiol Infect Dis. 2013;75:277–281. doi: 10.1016/j.diagmicrobio.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Tieu HV, Suntarattiwong P, Puthanakit T, Chotpitayasunondh T, Chokephaibulkit K, Sirivichayakul S, et al. Comparing interferon-gamma release assays to tuberculin skin test in Thai children with tuberculosis exposure. PLoS One. 2014;9:e105003. doi: 10.1371/journal.pone.0105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ar-karachaiphong K, Chaiear N, Reechaipichitkul W, Faksri K, Lerdruampattana S. Agreement of tuberculin skin test and QuantiFERON® -TB Gold-In-Tube for screening Mycobacterium tuberculosis infection in healthcare workers in a university hospital. J Med Assoc Thai. 2019;102(Suppl 1):S13–S19. [Google Scholar]
- 16.Shafeque A, Bigio J, Hogan CA, Pai M, Banaei N. Fourth- generation QuantiFERON-TB Gold Plus: what is the evidence? J Clin Microbiol. 2020;58:e01950-19. doi: 10.1128/JCM.01950-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallegos Morales EN, Knierer J, Schablon A, Nienhaus A, Kersten JF. Prevalence of latent tuberculosis infection among foreign students in Lubeck, Germany tested with QuantiFERON-TB In-Tube and QuantiFERON-TB Gold Plus. J Occup Med Toxicol. 2017;12:12–12. doi: 10.1186/s12995-017-0159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Maertelaere E, Vandendriessche S, Verhasselt B, Coorevits L, André E, Padalko E, et al. Evaluation of QuantiFERON-TB Gold Plus on Liaison XL in a low-incidence setting. J Clin Microbiol. 2020;58:e00159-20. doi: 10.1128/JCM.00159-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petruccioli E, Chiacchio T, Pepponi I, Vanini V, Urso R, Cuzzi G, et al. First characterization of the CD4 and CD8 T-cell responses to QuantiFERON-TB Plus. J Infect. 2016;73:588–597. doi: 10.1016/j.jinf.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Petruccioli E, Vanini V, Chiacchio T, Cuzzi G, Cirillo DM, Palmieri F, et al. Analytical evaluation of QuantiFERON-Plus and QuantiFERON-Gold In-tube assays in subjects with and without tuberculosis. Tuberculosis. 2017;106:38–43. doi: 10.1016/j.tube.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Barcellini L, Borroni E, Brown J, Brunetti E, Codecasa L, Cugnata F, et al. First independent evaluation of QuantiFERON-TB Plus performance in contact screening. Eur Respir J. 2016;47:1587–1590. doi: 10.1183/13993003.02033-2015. [DOI] [PubMed] [Google Scholar]
- 22.Oh CE, Ortiz-Brizuela E, Bastos ML, Menzies D. Comparing the diagnostic performance of QuantiFERON-TB Gold Plus to other tests of latent tuberculosis infection: a systemic review and meta-analysis. Clin Infect Dis. 2021;73:e1116–e1125. doi: 10.1093/cid/ciaa1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pérez-Recio S, Pallarès N, Grijota-Camino MD, Sánchez- Montalvá A, Campos-Barcia L, Gutiérrez S, et al. Identification of recent tuberculosis exposure using QuantiFERON-TB Gold Plus, a multicenter study. Microbiol Spectr. 2021;9:e0097221. doi: 10.1128/Spectrum.00972-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann H, Avsar K, Göres R, Mavi SC, Hofmann-Thiel S. Equal sensitivity of the new generation QuantiFERON-TB Gold Plus in direct comparison with the previous version QuantiFERON- TB Gold IT. Clin Microbiol Infect. 2016;22:701–703. doi: 10.1016/j.cmi.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Yi L, Sasaki Y, Nagai H, Ishikawa S, Takamori M, Sakashita K, et al. Evaluation of QuantiFERON-TB Gold Plus for detection of Mycobacterium tuberculosis infection in Japan. Sci Rep. 2016;6:30617–30617. doi: 10.1038/srep30617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JK, Lee HW, Heo EY, Yim JJ, Kim DK. Comparison of QuantiFERON-TB Gold Plus and QuantiFERON-TB Gold In-Tube tests for patients with active and latent tuberculosis: a prospective cohort study. J Infect Chemother. 2021;27:1694–1699. doi: 10.1016/j.jiac.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Phetsuksiri B, Srisungngam S, Rudeeaneksin J, Boonchu S, Klayut W, Norrarat R, et al. QuantiFERON-TB Gold In-Tube test in active tuberculosis patients and healthy adults. Rev Inst Med Trop Sao Paulo. 2018;60:e56. doi: 10.1590/S1678-9946201860056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horne DJ, Jones BE, Kamada A, Fukushima K, Winthrop KL, Siegel SA, et al. Multicenter study of QuantiFERON-TB Gold Plus in patients with active tuberculosis. Int J Tuberc Lung Dis. 2018;22:617–621. doi: 10.5588/ijtld.17.0721. [DOI] [PubMed] [Google Scholar]
- 29.Lien LT, Hang NT, Kobayashi N, Yanai H, Toyota E, Sakurada S, et al. Prevalence and risk factors for tuberculosis infection among hospitals workers in Hanoi. Viet Nam. PLos One. 2009;4:e6798. doi: 10.1371/journal.pone.0006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pai M, Gokhale K, Joshi R, Dogra S, Kalantri S, Mendiratta DK, et al. Mycobacterium tuberculosis infection in health care workers in rural India: comparison of a whole-blood interferon gamma assay with tuberculin testing. JAMA. 2005;293:2746–2755. doi: 10.1001/jama.293.22.2746. [DOI] [PubMed] [Google Scholar]
- 31.Prado TN, Riley LW, Sanchez M, Fregona G, Nóbrega RL, Possuelo LG, et al. Prevalence and risk factors for latent tuberculosis infection among primary health care workers in Brazil. Cad Saude Publica. 2017;33:e00154916. doi: 10.1590/0102-311X00154916. [DOI] [PubMed] [Google Scholar]
- 32.Nonghanphithak D, Reechaipichitkul W, Chaiyasung T, Faksri K. Risk factors for latent tuberculosis infection among health-care workers in northeastern Thailand. Southeast Asian J Trop Med Public Health. 2016;47:1198–1208. [PubMed] [Google Scholar]
- 33.World Health Organization . Use of alternative interferon-gamma release assays for the diagnosis of TB infection. Geneva: WHO; 2022. [[[cited 2022 Dec 7]]. Available from: https://www.who.int/publications/i/item/9789240042346 . [Google Scholar]