Abstract
Biofilms are complex communities of microorganisms attached to surfaces or associated with interfaces. Despite the focus of modern microbiology research on pure culture, planktonic (free-swimming) bacteria, it is now widely recognized that most bacteria found in natural, clinical, and industrial settings persist in association with surfaces. Furthermore, these microbial communities are often composed of multiple species that interact with each other and their environment. The determination of biofilm architecture, particularly the spatial arrangement of microcolonies (clusters of cells) relative to one another, has profound implications for the function of these complex communities. Numerous new experimental approaches and methodologies have been developed in order to explore metabolic interactions, phylogenetic groupings, and competition among members of the biofilm. To complement this broad view of biofilm ecology, individual organisms have been studied using molecular genetics in order to identify the genes required for biofilm development and to dissect the regulatory pathways that control the plankton-to-biofilm transition. These molecular genetic studies have led to the emergence of the concept of biofilm formation as a novel system for the study of bacterial development. The recent explosion in the field of biofilm research has led to exciting progress in the development of new technologies for studying these communities, advanced our understanding of the ecological significance of surface-attached bacteria, and provided new insights into the molecular genetic basis of biofilm development.
Our perception of bacteria as unicellular life forms is deeply rooted in the pure-culture paradigm. Since bacteria can, in a strict sense, be diluted to a single cell and studied in liquid culture, this mode of operation has been exploited and used to study many bacterial activities. Although this traditional way of culturing bacteria in liquid medium has been instrumental in the study of microbial pathogenesis and enlightening as to some of the amazing facets of microbial physiology, pure-culture planktonic growth is rarely how bacteria exist in nature. For example, environmental microbiologists have long recognized that complex bacterial communities are responsible for driving the biogeochemical cycling that maintains the biosphere (153). Until recently, the lack of methods for exploring these communities in situ has hampered detailed analyses. Fortunately, recent advances in microscopy and molecular technologies have made it possible to examine such communities in situ in great detail and without the bias of liquid culture. Direct observation of a wide variety of natural habitats has established that the majority of microbes persist attached to surfaces within a structured biofilm ecosystem and not as free-floating organisms (47). Moreover, it is becoming clear that these natural assemblages of bacteria within the biofilm matrix function as a cooperative consortium, in a relatively complex and coordinated manner (22, 47). Hence, although microorganisms can have an independent planktonic existence, an interdependent lifestyle in which they function as an integral part of a population or community is also possible and is, in fact, more typical.
Complex Attached and Aggregated Communities
What constitutes a bacterial community? From an ecological perspective, populations of bacteria arise from individual cells, and metabolically similar populations (e.g., sulfate- and sulfur-reducing bacteria) constitute groupings referred to as guilds. Sets of guilds (e.g., fermentative, sulfate- and sulfur-reducing, and methanogenic bacteria) conducting interdependent physiological processes form microbial communities. In essence, biofilms represent an interdependent community-based existence. Biofilms can be composed of a population that developed from a single species or a community derived from multiple microbial species, and they can form on a vast array of abiotic and biotic surfaces. Microorganisms also form natural assemblages at air-water interfaces and in suspensions, such as anaerobic digestors, in which they preferentially aggregate to form flocs or granules (151, 280). Although the substrata for attachment are difficult to discern in these granules, we view these assemblages as biofilm communities. For the purpose of this review, biofilms are broadly defined as assemblages of microorganisms and their associated extracellular products at an interface and typically attached to an abiotic or biotic surface.
Collective Behavior
Shapiro proposed the view of bacteria as interactive organisms capable of significant collective activity as a general bacterial trait over a decade ago (229–231). Complex differentiation and collective behavior have been demonstrated for a number of different organisms under a variety of different situations (231). Most notable are species of Myxococcus that differentiate when starved to form elaborate fruiting bodies (66), Anabaena during heterocyst development (278), Bacillus subtilis during its metamorphosis into spores (147), Streptomyces coelicolor during its morphological differentiation in response to nutritional conditions (30), and Serratia liquefaciens during the migration of populations by means of swarming motility (66). These examples surely testify to the ability of microorganisms to exploit intercellular interactions and communication to facilitate their adaptation to changing environmental parameters (119). At first glance, however, it is not obvious that the majority of prokaryotes are capable of such coordinated collective behavior unless one considers them in the context of biofilms. Whether single- or multispecies, the development of biofilms requires multicellular behavior. As we will explore below, the development of a biofilm is a complex process that requires collective bacterial behavior. What's more, in contrast to the other examples of development described above, the collective behavior can involve more than one bacterial species. Biofilm formation may require coordination with, interactions of, and communication between multiple bacterial species.
Remarkable discoveries have occurred in biofilm research during this past decade. The application of new microscopic and molecular technologies to biofilm investigations has opened our eyes to this underappreciated area of microbial biology. Using these technologies, researchers have shown that biofilms are not simply organism-containing slime layers on surfaces; instead, biofilms represent biological systems with a high level of organization where bacteria form structured, coordinated, functional communities (184). In a number of recent articles, Caldwell et al. have discussed the complex interactions that form the basis of coexistence in these sessile communities (23, 26). Viewing bacteria from the biofilm community perspective is providing us with novel insights into microbial biology and ecology. Consequently, it seems that the restricted view of bacteria as unicellular life forms is expanding to include their remarkable ability to function as part of a collective (reviewed in references 39, 45 to 49, 184, 192 and 238).
SURFACE-ATTACHED COMMUNITIES IN THE REAL WORLD
The natural habitats of prokaryotes are remarkably diverse (188, 268). Prokaryotes can inhabit any environment that is suitable for higher life forms, as well as a variety of inhospitable settings that the majority of higher life forms would find extremely objectionable (152). Their ability to persist throughout the biosphere is due, in part, to their unequaled metabolic versatility and phenotypic plasticity. One key element of their adaptability is their ability to position themselves in a niche where they can propagate. Numerous positioning mechanisms have been discovered in bacteria. The most common mechanism is flagellar motility and different methods of surface translocation, including twitching, gliding, darting, and sliding (102). However, there are other mechanisms utilized by bacteria to position themselves in response to their environment. Some species are able to affect their position by synthesizing cellulose, thereby forming a fibrous pellicle that places cells near the air-water interface. In addition, cellulose synthesis aids in attachment to surfaces such as plant cells (216). Other bacteria, such as the purple sulfur bacterium Amoebobacter purpureus, modulate their density in order to position themselves. These photosynthetic bacteria position themselves at different levels in the water column in response to light intensity by producing gas vesicles for bouyancy or synthesizing carbohydrates or forming aggregates in order to sink (187). In addition, some species have magnetosomes (intracellular structures consisting of a crystal of a magnetic mineral surrounded by a membrane) that cause the cells to passively align with the Earth's geomagnetic field, thereby restricting lateral excursions (11, 227). One of the most important positioning mechanisms is aggregation or attachment. Aggregation enhances cell-cell interaction as well as the sedimentation rate of cells. Through attachment, the bacteria not only position themselves on a surface; they can form communities and obtain the additional benefit of the phenotypic versatility of their neighbors. Since a surface-attached lifestyle is ubiquitous, it is likely that this type of sessile community-based existence is a critical characteristic for persistence of the bacteria. Organisms can exist in an environment independently, but in many cases they proliferate more effectively by interacting and forming communities (23). Some of the concepts discussed in the following sections are illustrated in Fig. 1.
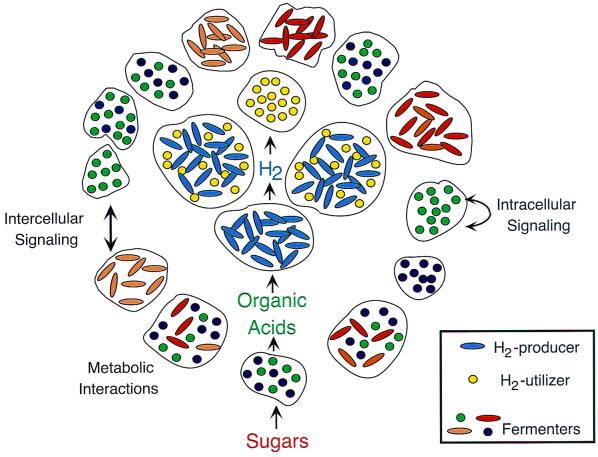
FIG. 1.
Ecology of microbial communities. Top-down view of an idealized surface-attached microbial community, illustrating some of the major concepts pertaining to the ecology of biofilms discussed in the text. The four microcolonies at the center of the figure represent organisms that both generate and consume hydrogen and comprise two organisms that participate in syntrophism (see text). Fermenting organisms produce organic acids used by the hydrogen producers, and these fermenting organisms gain their carbon and energy by utilizing various sugars. In addition to potential metabolic interactions between organisms, signaling molecules may aid in inter- and intraspecies communication. The factors described above (as well as environmental influences) may all contribute to the spatial organization of the biofilm. As shown here, microcolonies in natural communities can comprise either a single or multiple bacterial species. The proximity of different microbes allows the possibility of physical interactions in addition to communication via diffusible factors.
Bacterial communities in nature play a key role in the production and degradation of organic matter, the degradation of many environmental pollutants, and the cycling of nitrogen, sulfur, and many metals. Most of these natural processes require the concerted effort of bacteria with different metabolic capabilities, and it is likely that bacteria residing within biofilm communities carry out many of these complex processes. Studies in bioreactors and enrichment cultures have shown that biofilms are involved in the processing of sewage (see below), in the treatment of groundwater contaminated with petroleum products (155), and in nitrification (58). Biofilms also form in many extreme environments, such as in acid mine drainage (at a pH of 0), where they contribute to the cycling of sulfur (67). Cyanobacterial mat biofilms have been intensively studied in thermal springs (204, 261), and recently, researchers have started to investigate biofilms in the “desert-like” lake ice cover in Antarctica (190). Complex structured communities in these extreme environments have been found to conduct a variety of biological processes, such as photosynthesis, nitrogen fixation, and fermentation.
Another type of biofilm community that is being investigated is the bacterial assemblages associated with suspended particles of organic and inorganic material in the marine environment. Researchers have shown that these macroscopic particles, often referred to as marine snow, are enriched in microbial biomass, nutrients, and trace metals and are involved in biogeochemical transformation of particulate organic carbon in the pelagic environment (28, 189). Although the importance of microbial communities associated with these macroscopic particles has not been thoroughly investigated, methanogenesis (121), nitrogen fixation (191), and sulfide production (228) have been detected in these particles, indicating microbial activity. Moreover, microbial production of methane or sulfide as well as nitrogen fixation only occurs under anoxic conditions; therefore, the data indicate that anaerobic metabolism is being performed in an otherwise oxygenated environment. Also, these aggregates have been examined with oxygen microelectrodes, and steep redox gradients were found in these biofilms, providing additional evidence of anaerobic metabolism (191). In a study by Rath et al., the phylogenetic diversity of the bacterial community associated with marine snow was assessed by amplifying and classifying small-subunit ribosomal DNA (rDNA) fragments from nucleic acids extracted from samples of marine snow collected in the northern Adriatic Sea (208). These experiments showed that bacterial colonization of marine snow can result in diverse and complex assemblages, with specific phyla being associated with the particles. Also, the nature of the associated phylogenetic groups was found to be similar to that of other assemblages found in marine sediments and terrestrial soils.
Biofilm Structure
The application of confocal scanning laser microscopes (CSLM) to biofilm research radically altered our perception of biofilm structure and function (140). Before the use of CSLM, electron microscopy was the method of choice to examine microbial biofilms under high resolution. Unfortunately, sample preparation for electron microscopy results in dehydrated samples. Consequently, this approach provided a deceivingly simplistic view of biofilms, since the biofilm collapsed when water was removed. On the other hand, CSLM, which allows the visualization of fully hydrated samples, has revealed the elaborate three-dimensional structure of biofilms (47, 56, 57). CSLM has been used very effectively to monitor biofilm development in flow cells. Flow cells are small continuous-flow systems with a viewing port that allows direct observation of the biofilm without disrupting the community. These systems are often once-flow, meaning that fresh medium enters the system, passes through the cell, and is collected as waste—the medium is not recycled through the flow cell. A number of descriptions of flow cell and related techniques have been reported (64a).
Interestingly, biofilms formed from single species in vitro and those produced in nature by mixed species consortia exhibit similar overall structural features (47, 52, 264). Most biofilms have been found to exhibit some level of heterogeneity in that patches of cell aggregates, not monolayers, are interspersed throughout an exopolysaccharide matrix that varies in density, creating open areas where water channels are formed. An example of a mature single-species biofilm of Vibrio cholerae is shown in Fig. 2.
FIG. 2.
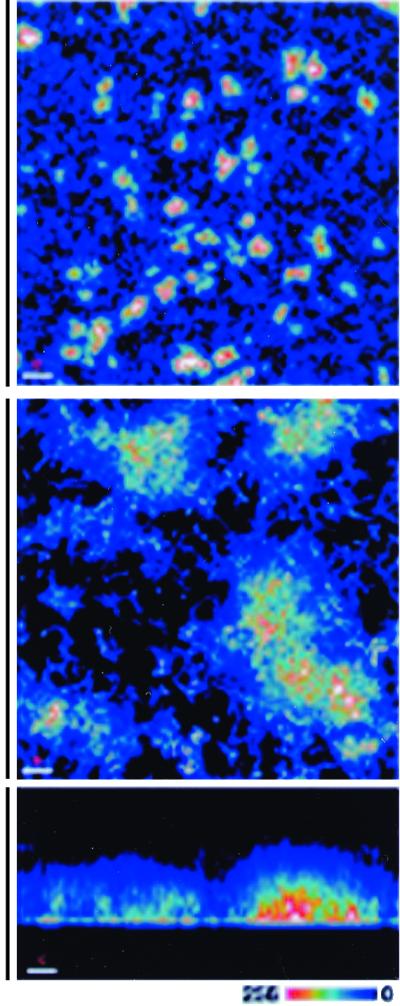
Architecture of a typical biofilm. Three-dimensional reconstruction of V. cholerae biofilms. Bacteria carrying a plasmid constitutively expressing the green fluorescent protein (GFP) were incubated in chambers containing borosilicate glass. At 6 h, the wells were emptied, washed, and examined with a CSLM using 488- and 510-nm excitation and emission wavelengths, respectively. The top and center panels show horizontal (xy or top-down view) projected images at low and high magnification, respectively. Islands of bacterial aggregates are visible on the surface. The bottom panel is a sagittal (xz or side view) view of the same biofilm. The relative intensity of the pseudo-colored images is shown at the lower right corner and correlates with cell density. Bars, 50 μm (top panel) and 10 μm (center and bottom panels). This image was kindly provided by Fitnat Yildiz and Gary Schoolnik. A diagrammatic representation of various biofilms is also shown in Fig. 5. Reproduced with permission from reference 282.
The microcolonies that constitute the biofilm can be composed of single-species populations or multimember communities of bacteria, depending on the environmental parameters under which they are formed. Numerous conditions, such as surface and interface properties, nutrient availability, the composition of the microbial community, and hydrodynamics, can affect biofilm structure (240). For example, under high shear stresses, such as on the surface of teeth during chewing, the biofilm (dental plaque) is typically stratified and compacted (15, 274). Biofilms have also been examined under various hydrodynamic conditions such as laminar and turbulent flows, and it was shown that biofilm structures are altered in response to flow conditions (241). Biofilms grown under laminar flow were found to be patchy and consisted of rough round cell aggregates separated by interstitial voids. Biofilms grown in the turbulent flow cells were also patchy, but elongated “streamers” that oscillated in the bulk fluid were observed. Moreover, by observing biofilm development under continuous flow, this group was able to evaluate the effect of perturbations on established biofilms. They showed that the biofilm was polymorphic and structurally adapted to changes in nutrient availability.
In biofilms formed in upflow anaerobic sludge bed reactors (continuous-flow systems comprising multiple microbial species, where the flow occurs from bottom to top of the vessel), aggregates consisting of complex bacterial communities (referred to as flocs or sludge granules) predominate (151). This is primarily due to the fact that the degradation of organic materials to methane and carbon dioxide is a community-level process that is driven by the close contact of multiple guilds interacting in a food web (159, 224). In addition, through aggregation, the bacteria are advantageously positioned in these reactors. Since there is no surface area for attachment in this type of reactor except for the walls, the formation of granular sludge is a mechanism by which the biofilm communities settle to the bottom of the reactor, which prevents their being washed out of the system. Furthermore, through granular sedimentation, the biomass is more readily exposed to the continuous supply of nutrients being pumped into the bottom of the reactor. Hence, biofilm structure is affected by both the microbial biology and environmental parameters. Structural organization is clearly a hallmark of biofilm communities that differentiates this mode of growth from conventional suspension cultures.
The interstitial voids or channels are also an integral part of the biofilm structure. Using particle-tracking techniques, researchers have been able to demonstrate water flow through these channels (242). Therefore, the channels are, in essence, the lifeline of the system, since they provide a means of circulating nutrients as well as exchanging metabolic products with the bulk fluid layer (45). For instance, in situ measurements of dissolved oxygen using microelectrodes revealed that oxygen is available in the biofilm as far down as the substrata, indicating that the channels are transporting the oxygenated bulk fluid throughout the biofilm to the surface (143). Also, in situ measurements of toluene degradation in a multispecies biofilm indicated that toluene was available to cells deep within the biofilm, indicating transport through channels (168). Presumably the channels are a vital part of the biofilm structure and function, and therefore there are likely to be mechanisms for the formation as well as the maintenance of these structures. This is clearly a key area for future investigations.
Structure and Function Studies
The identification and quantification of members of particular microbial communities, as well as a clear understanding of the functional relationship between members, are required before we can fully appreciate and possibly manage the complex processes that these communities perform. Recent technological advances have aided in attaining this goal. The remarkable breakthrough in rRNA-based phylogenetic analysis (276) has provided a means of developing tools with which to investigate microbial communities. The development of fluorescently labeled rRNA-targeted oligonucleotides, a variety of microsensors, real-time image analysis, and confocal microscopy has provided researchers with noninvasive means to monitor populations in situ (5, 24, 25, 260). In addition, one of the key advances in the study of microbial communities has been the development of various tools for cultivating communities, such as chemostats, continuous-flow slide cultures, microstats, and colonization tracks (22). These techniques have been used to identify and quantify specific populations within a variety of complex microbial mixtures.
As discussed above, the anaerobic degradation of complex organic material to methane and carbon dioxide is a community-level process carried out by multiple microbial populations interacting in a food web (159). This process is one of the most complex interactions between bacterial populations known to exist. Although anaerobic food chains have been studied extensively, our understanding of community-level processes in anaerobic food webs is still limited. Due to the important role of microbes in wastewater treatment, an extensive amount of research and method development has been performed in order to increase our understanding of the processes involved in the degradation of organic materials. Here we will describe some of the research in this area in order to illustrate one of the primary goals of biofilm research, that is, connecting structure and function.
It has been discovered that surface-attached biofilms as well as sludge granules readily form in anaerobic reactors (151, 281). Moreover, the development of these biofilm communities results in more efficient processing of contaminants in wastewater. rRNA probes have been used to identify and quantify phylogenetically defined populations of organisms in sludge granules (120, 206, 207, 256, 257). In a recent study by Raskin et al. (207), changes in the composition of two metabolically competitive populations (methanogens and sulfate-reducing bacteria [SRB]) were examined in a biofilm reactor in response to the availability of sulfate. Both of these metabolic types catalyze final stages in the anaerobic mineralization of organic matter, and both depend on other microorganisms (fermentative bacteria) to convert complex organic matter to simpler compounds, such as hydrogen and acetate, which in turn can serve as their substrates. Hence, the methanogens and SRB compete for the same substrates. The generally accepted paradigm of SRB and methanogens in their natural habitats is that of mutual exclusion. Typically, environments that are rich in sulfate select for SRB, and environments that are sulfate depleted select for methanogens. However, it is becoming clear that the interactions of these two groups are more complex than previously envisioned. The coexistence of methanogens and SRB has been observed when sulfate is available (112, 178), and large populations of SRB have been found in sulfate-depleted environments (247).
In order to determine the composition of the SRB and methanogen community under different conditions, a portion of the attached biofilm was removed from the reactor at a specific time point. Experiments were carried out in biofilm reactors, and the populations of bacteria were monitored by quantitation of specific 16S rRNA organisms compared to total 16S rRNA (5). The nucleic acids were extracted from the samples and probed for specific populations (e.g., universal, Archaea, Bacteria, various methanogens, and various SRB). In addition, various metabolic activities, including sulfide and methane production, were assayed. Using this approach, it was found that in the absence of sulfate, certain types of SRB were still present at high levels in the reactors. The authors state that this ability to persist without sulfate may be explained by the ability of certain SRB to function as fermenters or as proton-reducing acetogens, and the potential for SRB to have these metabolic capabilities has been reported previously (91, 245). Upon addition of sulfate to the reactor, the levels of sulfate reduction were found to increase with a concomitant increase in the SRB population. Also, methane production and the methanogenic population decreased immediately following the addition of sulfate. However, the opposite did not occur. When the sulfidogenic reactor was reversed to sulfate-free medium, it took a long time (50 days) for any significant amount of methane to be produced, indicating that under these condition the SRB population can more readily reestablish itself in the environment than the methanogenic bacteria. These experiments illustrate how an rRNA-based approach can be combined with functional assays in order to monitor population dynamics in conjunction with metabolic changes in a biofilm community.
Other researchers have used identification of cells hybridized in situ with fluorescent rRNA-targeted probes to study the diversity and spatial distribution of populations within the community. In a recent study by Amann and colleagues, the microdiversity in a municipal activated-sludge sample was assessed using fluorescent rRNA-targeted probes (4). The primary reason for this study was that high microdiversity (i.e., clusters of closely related yet distinct 16S rRNA sequences with similarities of between 95 and 99%) is commonly reported in complex environmental samples. It has become a concern that this technique may be providing misleading results. Cultivation-independent comparative rRNA analysis relies on PCR amplification of rRNA from nucleic acids extracted from environmental samples (5), and therefore there are several factors at each step of the process that can give rise to artificial sequence diversity, or lack there of, in rRNA gene libraries (5, 196, 203, 210). By using in situ probes and CSLM, these researchers investigated the potential for high microdiversity in a natural microbial community without the selective bias of cultivation, extraction, or amplification. Evidence for high diversity was shown, indicating that high diversity within a relatively narrow phylogenetic group (in this case beta-1 Proteobacteria) is present in this environment.
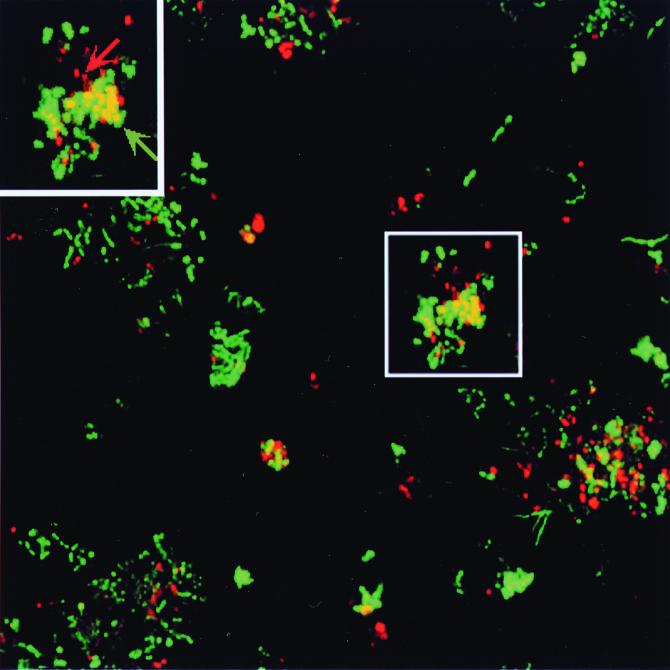
Studies combining fluorescent in situ hybridization (FISH) with microelectrode analysis for determining pH, oxygen, or sulfide profiles have been performed in order to evaluate the distribution of different populations in relationship to chemical profiles (92a, 93, 205, 225, 226). In a study by Harmsen et al. (92a), FISH was used to localize organisms belonging to the bacterial domain (two syntrophic propionate-oxidizing bacteria) and various types of methanogens in sludge granules (Fig. 3). It was shown that the outer layers of the granules were populated with a variety of bacterial colonies most likely involved in hydrolyzing complex organic matter, while the interior of the granule contained methanogenic microcolonies. Moreover, the syntrophic strains, which require low hydrogen partial pressures in order to oxidize propionate, were found to be tightly associated with the methanogens in microcolonies. Consequently, these experiments provided convincing evidence of a layered microbial architecture in sludge granules where the bacteria on the surface of the granule hydrolyze complex organic materials, thereby providing the anaerobic bacteria in the interior of the biofilm with an energy source.
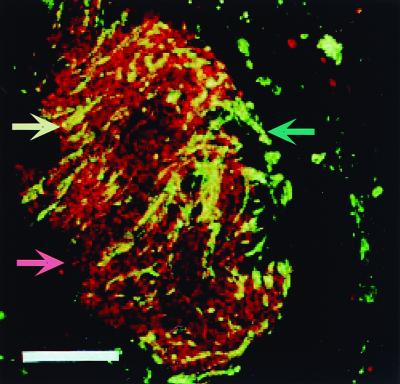
FIG. 3.
Syntrophism in a sludge granule. Photomicrograph of in situ hybridization of a sludge granule obtained from a methanogenic reactor to illustrate biofilm organisms participating in a metabolic interaction. Fluorescein-labeled fluorescent probes were used to identify organisms specific to the order Methanomicrobiales (green and green arrow), and rhodamine-labeled probes were used to localize syntrophic propionate-oxidizing bacteria related to the genus Syntrophus (red and red arrow). The double (red and green) labeling results in yellow fluorescence. The results indicate that the syntrophic microcolonies are intertwined with chains of methanogens (yellow and yellow arrow). The metabolic interactions between these two organisms speed the anaerobic degradation of certain compounds (see text for details). Bar, 20 μm. This figure was modified from Harmsen et al. (92a), with permission to use this image kindly provided by Willem de Vos.
In a recent comprehensive study by Schramn et al., multiple methods were used to investigate the occurrence of anaerobic processes, such as denitrification and sulfate reduction, in well-aerated activated-sludge samples (226). In this set of experiments, microsensors were used to measure oxygen, nitrite, nitrate, and sulfide concentrations, and 15NO3− and 35SO42−were used to measure denitrification (177) and sulfate reduction (76), respectively. In addition, the three-dimensional structure of the flocs was examined with CSLM, and the SRB population was monitored by FISH and by PCR with primers specific for the dissimilatory sulfate reductase gene (258). Also, a newly designed flow system including microelectrodes (199) was used in the experiments. It was discovered that anoxic microniches and denitrification can occur in well-aerated activated sludge, but this potential appeared to be the exception rather than the rule. In four of the six samples examined, no anoxic zones developed during aeration of the granules, indicating that the respiratory capacity of the microbial community is simply not sufficient to create an anoxic environment when they are well aerated. In addition, sulfate reduction was not detected in any of the flocs, but SRB were found to be present, although in very small numbers. These findings are significant because the development of anaerobic niches in aerated sludge granules is detrimental to the degradation of contaminants. Anoxic habitats can support the persistence of SRB, resulting in the production of hydrogen sulfide and subsequent problems in the treatment process.
In addition to the techniques mentioned above, by using hybridization with fluorescent probes or by staining cells with acridine orange (AO), researchers are able to evaluate growth rate by determining cellular ribosome (rRNA) content. The direct correlation between ribosome content and growth rate is based on early observations in microbial physiology (223). Therefore, by using FISH in combination with digital microscopy, researchers have been able to quantify the cellular content of rRNA and thereby estimate the growth rate of cells in a biofilm. Using this technique, it was discovered that cells (the SRB PT2) in a young biofilm (initial colonization in the bioreactor) had a doubling time of 33 h, and cells in long-established (presumably steady-state) biofilms had doubling times of at least 70 h (200). Interestingly, a subset of cells observed in the mature biofilm were significantly more fluorescent (corresponding to a doubling time of 33 h) than any of the other surrounding cells. These data indicate that populations of cells within the biofilm may have different growth rates, which may reflect the heterogeneity of microniches within a biofilm in that some cells may be in a better position to obtain nutrients. One limitation of the rRNA-based quantitation technique is that a standard curve is required in order to quantify ribosome content, and therefore cells must be isolated before their examination. By using AO staining to determine the RNA-DNA ratio, the need for isolation is eliminated. When AO complexes with nucleic acids, it will emit red fluorescence when it is attached to single-stranded templates and green fluorescence if the nucleic acids are double stranded (211). Therefore, AO can be used to differentially stain RNA and DNA in cells. Moreover, the amount of light from AO-stained cells can be quantified by using image analysis software (167). The measurements included determination of cell volume, frequency of dividing cells, and simultaneous quantitative measurement of RNA and DNA by AO staining. Using this combinatorial method, it was shown that Pseudomonas putida cells residing in a biofilm exhibited a constant growth rate that was independent of the dilution rate of the chemostat and, hence, independent of nutrient availability. These data indicate that other factors (e.g., oxygen availability or physical constraints) may be limiting the growth of bacteria in the biofilm.
Researchers have also combined FISH with specific enzyme activity probes (e.g., phosphatase activity) so as to assign functions to certain phylogenetic groups (125). In this study it was discovered that strains that cluster with the cytophaga-flavobacteria group are involved in the release of inorganic phosphate during wastewater treatment. Previously it had been reported that this group of microorganisms was not involved in the removal of phosphate (14), but the combined use of FISH with the phosphatase localization probe method clearly illustrated the colocalization of phosphatase activity and the cytophaga-flavobacteria probe. Moreover, a significant amount (35 to 45%) of the total phosphatase activity was detected associated with the cytophaga and flavobacteria, indicating that this group not only has activity but is responsible for a significant portion of the total phosphatase activity in the sludge. In addition, the authors point out that the synthesis of other precipitating, fluorogenic substrates for various enzymatic activities should be possible, and therefore this approach should prove useful in addressing a variety of biological questions.
Plant-Associated Biofilms
Soils constitute a heterogeneous environment with numerous fluctuating parameters that can affect microbial growth and survival (193). Like many natural environments, soil is nutrient poor (272). Soil organic matter varies in concentration from 0.8 to 2.0%, with the bulk of the carbon in recalcitrant forms, such as humic acids. Therefore, bacteria indigenous to soil must constantly contend with nutrient deprivation (252). The rhizosphere (the root surface and the region immediately surrounding a root, typically ∼2 mm) constitutes an ecological niche in soil where nutrients are more readily available, and certain bacteria have developed mechanisms to take advantage of this niche. Rhizodeposition (the release of organic material from the roots as they grow through the soil) enhances microbial growth and drives the structuring of the microbial communities in the rhizosphere (27). Rhizodeposition consists of a variety of compounds, including (i) exudates, such as amino acids, simple sugars, and organic acids that are passively released from the roots; (ii) actively secreted compounds such as carbohydrates and enzymes; (iii) mucilage (sloughed-off cells and cell lysates); and (iv) gases, such as carbon dioxide and ethylene (267). This deposition accounts for a significant amount of the plant's photosynthate, estimated to be ∼20% of the carbon allocated to the root system. Thus, numerous bacteria are attracted to the rhizosphere and compete in order to colonize this oasis in soil (266). Moreover, the interactions between the plant and the surrounding microorganisms select for the establishment of only certain microbial populations (rhizobacteria). Therefore, structured microbial communities attached to the roots and the surrounding soil particles could be viewed as a biofilm community. This suggests that a highly evolved association may exist between the nutritionally rich photosynthesizing plants and the nutrient-deprived bacteria residing in soil. An example of a biofilm on a plant root is shown in Fig. 4. There are many indications of biofilm communities in the rhizosphere. First of all, it is evident that bacteria attach to roots, and various mechanisms have been described for attachment that involve a variety of cell components, such as outer membrane proteins, wall polysaccharides (capsules), lipopolysaccharide (LPS), and cell surface agglutinin (164). Second, exopolysaccharide (EPS) is produced by bacteria in the rhizosphere (7). This not only provides many advantages to bacterial cells (as described below), it also enhances soil aggregation, which in turn improves water stability, which is critical to the survival of the plant. Hence, there is a strong selective advantage for the production of EPS in the rhizosphere. Third, microcolonies have been observed in the root system (231) along with an increase in the frequency of conjugation between certain bacteria (Pseudomonas species) in the areas immediately adjacent to the roots, indicating cell-cell contact (251).
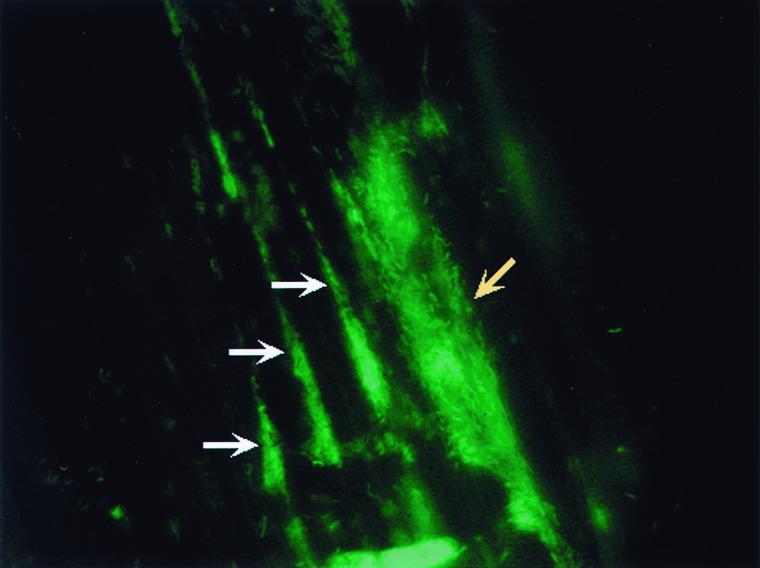
FIG. 4.
Biofilm on a plant root. Biofilm of GFP-labeled Pseudomonas fluorescens WCS365 on the root of a tomato plant. A large microcolony of bacteria is apparent on the root surface and is indicated by the yellow arrow. The white arrows highlight three smaller colonies that have formed at plant root cell boundaries, which may be the site of release of root exudates used by bacteria as nutrient sources. The diffuse appearance of some bacterial cells in the large microcolonies suggests that these bacteria are covered by an EPS. EPS may play a role in formation of these microcolonies (see text), suggesting that these communities have many of the characteristics of typical bacterial biofilms. This image is kindly provided by Guido Bloemberg.
Another part of the plant root system where microbial biofilms are formed is on the surface of symbiotic fungi associated with roots. Arbuscular mycorrhizal fungi form an association with plants in which the fungi colonize the cells of roots obtaining carbon from the plant, and in turn, the fungi develop a network of external hyphae which absorb and transfer phosphates and other minerals from the soil to the root (94). It has been estimated that as much as 80% of the extant species of plants form this type of symbiosis (92), and it follows that bacteria have evolved to take advantage of this common ecological niche. Bacteria described as good root colonizers, i.e., Pseudomonas fluorescens (31), have been shown to form biofilms on mycorrhizal fungi (reference 194 and references therein). Although the significance of microbial attachment to the fungi is not known, it is likely that this is a positioning mechanism that allows the bacteria to more readily obtain nutrients and propagate. Another area of active biofilm research in the plant world is the study of biofilms on leaves of plants (known as the phyllosphere) (169, 170). Early studies have shown that phyllosphere biofilms consist of diverse morphotypes of bacteria embedded in an exopolymer present on a variety of leaf surfaces. However, the nature of the microbial community and the role they play in this unique environment have not yet been determined.
ECOLOGICAL ADVANTAGES: WHY MAKE A BIOFILM?
There has been some speculation about the advantages of forming a biofilm versus living as individual cells. Although it is difficult to test these speculations experimentally, in the section below we offer some reasons why the biofilm strategy has been adopted by so many microbes.
Protection from the Environment
Bacteria experience a certain degree of shelter and homeostasis when residing within a biofilm, and one of the key components of this microniche is the surrounding extrapolymeric substance matrix. This matrix is composed of a mixture of components, such as EPS, protein, nucleic acids, and other substances. The best studied of these components is EPS. Most bacteria are able to produce polysaccharides, either as wall polysaccharides (capsules) or as extracellular excretions into the surrounding environment (EPS). Some bacterial species, such as Klebsiella aerogenes, appear to be limited in the types of polymers that they can synthesize, but members of some genera (e.g., Streptococcus pneumoniae) are able to produce EPS with a wide range of different components (243). Hence, broad generalizations about the function of EPS may be misleading. It is most likely that EPS plays various roles in the structure and function of different biofilm communities. Moreover, it is quite possible that EPS plays a different role in similar microbial communities under different environmental conditions. Here we will describe some of the benefits that are attributed to EPS.
As described above, EPS is clearly an integral part of the structural organization of biofilms. For example, Veiga et al. (254) found that the EPS produced by the two predominant species (Methanobacterium formicium and Methanosarcina mazeii) in granules (multispecies bacterial aggregates) from an anaerobic reactor had the same composition as the extracellular polymers found in the granules. Therefore, based on these experiments as well as earlier reports (280), these authors concluded that the EPS produced by the two methanogens contributed to the polymer matrix of the flocs. In addition, they proposed that the methanogens are producing the EPS, forming an aggregate that then acts as a backbone in which other species can embed. Physiologically, this mode of structuring seems a reasonable strategy, since it would result in the strictly anaerobic methanogens, being situated near the core of the granule where the redox state and oxygen levels would be the lowest. Hence, it appears that EPS plays a critical role in both the formation and the structure of the sludge granules. EPS has also been shown to adsorb dissolved organic compounds, such as diclofop methyl (a herbicide) and other xenobiotics, from the bulk fluid, thereby providing a mechanism by which the community can concentrate essential nutrients and growth components (277 and references therein). However, the mechanisms involved in sorption of molecules as well as the distribution and chemical nature of EPS are largely unknown (12).
The EPS matrix also has the potential to physically prevent access of certain antimicrobial agents into the biofilm by acting as an ion exchanger, thereby restricting diffusion of compounds from the surrounding milieu into the biofilm (87). This characteristic largely depends on the nature of both the agent and the EPS matrix. The effect appears to be most pronounced with antibiotics that are hydrophilic and positively charged, such as the aminoglycosides (173, 174, 176). EPS has also been reported to sequester metals, cations, and toxins (59, 72). In one study, the copper-binding characteristics of capsular polysaccharide from an unidentified bacterium isolated from metal-laden sediments were examined (165). The authors found that a highly purified EPS preparation was capable of binding copper. Furthermore, in order to evaluate the relevance of the adsorption to the binding potential in situ, these studies were conducted at copper concentrations, pHs, and temperatures that simulated the conditions where the isolate was obtained. Another interesting ecological study on biofilms and metals was reported by Farag et al. (70). In this study, the concentrations of metals (Ar, Cd, Pb, Hg, and Zn) in various food web components (bacterial biofilms, sediments, invertebrates, and fish) of the Coeur d'Alene River Basin in Idaho were examined. The aim of this study was to trace the transfer of metals between the different components, and it was consistently found that biofilms obtained from the rocks along the shore contained the highest concentrations of metals. Moreover, the authors discovered that the accumulation of metals in the invertebrates correlated with the mechanisms used for feeding. Certain invertebrates use a grazing-scraping feeding mechanism, and these organisms, referred to as shredders and scrapers, feed on biofilms (163). Therefore, not only are biofilms able to accumulate metals, they can also be a key link in the transfer of metals through an ecosystem.
EPS has also been reported to provide protection from a variety of environmental stresses, such as UV radiation, pH shifts, osmotic shock, and desiccation (72). One study reported by Elasir and Miller (68) utilized a whole-cell bioluminescent biosensor to investigate the response of Pseudomonas aeruginosa biofilms to UV radiation. It had been shown previously that the recA gene of P. aeruginosa was induced by DNA-damaging agents. A P. aeruginosa strain containing a transcriptional fusion of the P. aeruginosa recA gene to the lux operon from Vibrio fischeri was constructed, creating a bioluminescent biosensor for monitoring the response to DNA damage. When they immobilized the biosensor strain in alginate to mimic a biofilm, they found that the EPS matrix protected the cells from DNA damage, as indicated by the lack of induction of the biosensor. An additional study by Ophir and Gutnick examined the role of EPS in protection from desiccation (182). In these experiments, mucoid strains of Escherichia coli, Acinetobacter calcoaceticus, and Erwinia stewartii were compared to nonmucoid variants in their resistance to desiccation. It was demonstrated that EPS-producing mucoid strains of all three bacteria showed better survival under conditions of dehydration.
Nutrient Availability and Metabolic Cooperativity
The highly permeable water channels interspersed throughout the biofilm in the areas surrounding the microcolonies have been compared to a primitive circulatory system. They provide an effective means of exchanging nutrients and metabolites with the bulk aqueous phase, enhancing nutrient availability as well as removal of potentially toxic metabolites (47). The metabolic characteristics of bacteria within a biofilm community are distinct from those of their planktonic counterparts. The elaborate architecture provides the opportunity for metabolic cooperation, and niches are formed within these spatially well-organized systems. Consequently, the bacteria are exposed to an array of distinct environmental signals within a biofilm. For instance, cells situated near the center of a microcolony are more likely to experience low oxygen tensions.
Although it is not always the case, in multimember films, microcolonies often consist of a mixture of species (105, 151). These multispecies microconsortia can result from an association between metabolically cooperative organisms, and their proximity facilitates interspecies substrate exchange and the removal or distribution of metabolic products. For example, the degradation of complex organic matter into methane and carbon dioxide during anaerobic digestion requires the interaction of at least three guilds. Fermentative bacteria initiate the catabolism, producing acids and alcohols that are then readily utilized as substrates by acetogenic bacteria. Finally, the methanogens obtain energy from converting acetate, carbon dioxide, and hydrogen to methane. Hence, very efficient cooperations and mutual dependence can evolve within a biofilm. In fact, biofilms provide an ideal environment for the establishment of syntrophic relationships. Syntrophism is a special case of symbiosis in which two metabolically distinct types of bacteria depend on each other to utilize certain substrates, typically for energy production. Syntrophic associations have been well studied with regard to methanogenic degradation (224). A classic example of such an interaction was discovered and described by Bryant et al. in 1967 (18). A culture that was thought to consist of a single strain was found to contain two different organisms, strain S and strain M.o.H. In this coculture, the two strains syntrophically interact to convert ethanol to acetate and methane by interspecies hydrogen transfer. The fermenting bacterium is not able to grow on ethanol unless the hydrogen partial pressure is kept sufficiently low (because the fermenting organism carries out a reaction that is endergonic under standard conditions), and the methanogen relies on the fermentative bacteria to provide it with an energy source. Therefore, the first reaction can only occur and provide energy for the methanogen if the hydrogen-scavenging methanogen maintains a low hydrogen partial pressure. Therefore, neither partner can grow on ethanol alone, but together they both efficiently derive energy.
Acquisition of New Genetic Traits
Horizontal gene transfer is important for the evolution and genetic diversity of natural microbial communities. The importance of studying gene transfer in natural environments has recently been emphasized by the emergence of multidrug-resistant bacteria (55), the extensive use of antibiotics to promote growth in domestic animals (275), and the use of genetically engineered microorganisms in industrial processes (114, 220). The prevalence of plasmids in bacteria from diverse habitats is well established, and gene transfer by conjugation is one of the best understood mechanisms for dissemination of genetic information. Since most bacteria in natural settings reside within biofilms, it follows that conjugation is a likely mechanism by which bacteria in biofilms transfer genes within or between populations. Although there may be fewer incidences of mating events within a biofilm, the “fixed” close quarters are likely to favor conjugation, especially within a population. Researchers have investigated the role that conjugation may play in the spread of genetic information in biofilms.
Novel plasmids have been isolated from biofilms in marine environments (50) by the exogenous isolation method (77). In this study, plasmids conferring mercury resistance were isolated from bacteria residing in biofilms by combining nutrient-deprived recipient cells (a strain of Pseudomonas putida) with cells resuspended from biofilm communities and depositing the mixture on filters floating on artificial seawater medium without the addition of nutrients. The starvation conditions were chosen in order to better simulate the more typical environmental parameters found in marine waters, and in fact, it was found that gene transfer occurred on the artificial seawater and not on selective plates. In addition, the isolated plasmids were found to have novel replication and/or incompatibility systems, different from those of commonly used plasmids.
Another recent study examined gene transfer in a microcosm dental plaque. These experiments were performed by creating a Streptococcus biofilm in a constant-depth fermentor (a device which uses physical scraping of the biofilm to keep the community at a constant depth). A Bacillus subtilis strain harboring a conjugative transposon which confers resistance to tetracycline was introduced to the system, and the resistance profile of the biofilm bacteria was assessed (212). It was found that transfer of the conjugative transposon occurred within the biofilm, resulting in a Streptococcus species that now harbored the transposon. This was the first demonstration of gene transfer in an oral microbe growing in a biofilm, and these findings indicate that nonoral bacteria have the potential to transfer genes to oral commensals.
In another study, Lebaron et al. demonstrated that plasmid transfer occurred between E. coli strains in single-species biofilms formed on glass beads within a reactor vessel (141). A goal of this study was to investigate the potential for the dissemination of genetic information after accidental release of genetically engineered microorganisms. Although plasmids used in genetic engineering are usually devoid of transfer functions so as to limit their dispersal, transfer may still occur via mobilization by either trans interactions (mobilization by donation) or formation of a cointegrate with a self-transmissible plasmid that is already present in organisms in the environment (36). In order to examine the potential for this type of gene transfer, a variety of different model microcosms, including biofilms, were examined. In the biofilm experiments, recipient cells were allowed to attach to glass beads in a fixed-bed reactor. These biofilms were then exposed to donor cells harboring all three of the following plasmids: pCE325 (oriT+), pUB2380 (mob+), and R388 (tra+). The numbers of transconjugants containing the different plasmids were determined, and it was shown that all three plasmids carried by planktonic organisms were transferred into the biofilm population. In addition, the analysis of transconjugants carrying R388 showed that mobilization by donation was the likely mechanism used for transfer, since no cointegrates were formed.
Another research group has investigated the transfer of the TOL plasmid, carrying genes for the degradation of toluene and benzyl alcohol, into a biofilm community growing on benzyl alcohol as the sole carbon and energy source (33). In this study, the biofilm community consisted of three different organisms, P. putida, Acinetobacter, and an unidentified isolate. All three isolates are able to mineralize benzyl alcohol, but only P. putida is able to propagate the TOL plasmid. To monitor the occurrence and growth of P. putida transconjugants, a gfp-tagged TOL plasmid was created. In addition, the lacI gene was inserted into the chromosome of the donor strain, also a P. putida strain, resulting in repression of gfp expression from the plasmid in this strain. Consequently, expression of the gfp gene was induced only if the plasmid was transferred to the recipient P. putida strain that does not contain the lacI gene encoding the repressor (zygotic induction of fluorescence). CSLM was used to identify the specific starting strains and transconjugants in the community by using 16S rRNA hybridization probes and expression of green fluorescence. Using these tools, this group was able to monitor conjugation in the biofilm. Data from these experiments indicated that the frequency of horizontal plasmid transfer was low; instead, growth of an occasional recipient bacterium into a microcolony (known as vertical transfer) accounted for the establishment of the plasmid in the biofilm. Another recent study examining the kinetics of gene transfer by conjugation in the mouse intestine showed transfer kinetics similar to that of a biofilm (144).
Virus-mediated transduction is another mode of gene transfer. In the late 1980s, it was discovered that there is a very high abundance of viruses (as high as 108 per ml) in both limnetic (fresh water) and marine systems and that the majority of these viruses are bacteriophages (13, 202). Various procedures have been used to evaluate the impact of viruses on microbial mortality and gene transfer. The data indicate that viral lysis is a major contributor to bacterial mortality (as reviewed in reference 279). As much as 10 to 20% of the bacterial population is lysed daily by phages (244). Hence, phages can have a significant impact on the microbial food web by increasing death rates and/or by decreasing growth rates at all trophic levels (248). For example, an intriguing study recently reported by van Hannen et al. supported the idea that phage can structure or “restructure” microbial communities (253). In these studies, almost complete lysis of a cyanobaterial population was observed in two laboratory-scale enclosures filled with lake water (130 liters each). They concluded that phage-like particles were responsible for the lysis. The group then used denaturing gradient gel electrophoresis of 16S rDNA fragments (171) to qualitatively monitor the prokaryotic as well as the eukaryotic community composition. Using this technique, they observed that soon after lysis of the cyanobacteria occurred, new species of bacteria capable of degrading organic carbon emerged. Hence, phage lysis drove community structure changes.
There is little information with regard to bacteriophage infecting biofilms. A recent article by Doolittle et al. (63) showed data indicating that Escherichia coli biofilms on polyvinyl chloride coupons or disks are susceptible to T4 phage attack. In this study, a modified Robbins device (a continuous-flow system with sampling ports that allow removal of samples) was used to create E. coli biofilms, and then the films were exposed to the phage by pumping a solution of phage through the system. In order to determine the extent of infection and lysis of the biofilms by the bacteriophage, coupons were removed at various time points after exposure, and viable-cell counts as well as plaque assays were performed. An even distribution of infected cells throughout the sampling ports on the modified Robbins device was observed, indicating that diffusion of the phage from the bulk fluid into the biofilm occurred at a relatively constant rate and that the E. coli biofilm, under the conditions used, was susceptible to phage attack. Both the EPS matrix associated with biofilms and the significant physiological changes that occur when cells enter the biofilm mode of growth have the potential to hinder successful infection by phage. These researchers state that it is possible that the conditions used in this set of experiments resulted in the synthesis of significantly less or chemically different EPS, and therefore the phage were better able to penetrate the biofilm. It is evident that additional studies are required before we understand the role of phage in structuring biofilm communities. However, if bacteria growing in biofilms in nature are more resistant to lysis by phage, the selective pressure to form a biofilm may be quite considerable. There is no evidence for transduction in biofilms, although the high concentration of phage in aquatic systems indicates that the potential is certainly there. In addition, it was recently demonstrated that high transduction frequencies can occur in marine environments (32), and therefore the impact of transduction on gene exchange in biofilms may be more significant then we presently envision.
ROLE OF SURFACE-ATTACHED BACTERIA IN DISEASE
Bacterial Biofilm Infections
One of the greatest accomplishments in modern medicine has been our progress against infectious disease. As a result of scientific ingenuity, most modern-day acute infections can be treated effectively with antibiotics. However, there are two important exceptions to this rule. First are bacteria that are innately antibiotic resistant, and the second pertains to bacteria that reside within a biofilm. Biofilm bacteria can be up to 1,000-fold more resistant to antibiotic treatment than the same organism grown planktonically (87), but the mechanisms by which the biofilm-grown bacteria attain this resistance are still a matter of speculation. Mechanisms of resistance that are considered likely include (i) phenotypic changes in bacteria resulting in resistance occuring within the biofilm environment, (ii) inactivation of the antibiotics by extracellular polymers or modifying enzymes, and (iii) nutrient limitation resulting in slowed growth rate (87). Antibiotic resistance is an intensive area of investigation in biofilm research, but a detailed treatment of this field is outside the scope of this review (see references 9, 43, 107, 108, and 113 and references therein).
Clinical biofilm infections are marked by symptoms that typically recur even after repeated treatments with antibiotics. Standard antibiotic therapy is only able to eliminate the planktonic cells, leaving the sessile forms to propagate within the biofilm and to continue to disseminate when the therapy is terminated. Moreover, biofilm infections are rarely resolved by the host's immune system. Biofilm bacteria release antigens and stimulate the production of antibodies, yet bacteria residing in biofilms are resistant to these defense mechanisms (49). In fact, this immune response may even cause damage to the surrounding tissue. Therefore, a better understanding of biofilm formation is required to develop novel strategies for dealing with these infections.
Implant-Based Infections
The role of biofilms in the contamination of medical implants has been well established. Early electron microscopy studies of medical implants revealed signs of bacteria residing in biofilms on these abiotic surfaces (21, 90, 172, 175). Table 1 lists examples of implants prone to contamination and the organisms that can cause such biofilm-based implant infections. It is evident that bacterial biofilms on prosthetic valves are the leading cause of endocarditis in patients who have undergone heart valve replacement. Among patients who develop these infections, the mortality rate is as high as 70% (110). Millions of catheters (e.g., central line, intravenous, and urinary catheters) are inserted into patients every year, and these implants serve as a potential surface for biofilm formation. Biofilm formation can also occur on contact lenses, and these biofilms are thought to contribute to keratitis (69, 88, 162). Overall, it is thought that upwards of 60% of all nosocomial infections are due to biofilms. These biofilm-based infections can increase hospital stays by 2 to 3 days and cost upwards of $1 billion per year in added costs (10).
TABLE 1.
Examples of common implant infections
| Implant | Organism(s) found | Associated disease or consequences | Reference(s) |
|---|---|---|---|
| Prosthetic valve | S. epidermidis, S. sanguis | Prosthetic valve endocarditis | 101, 106, 145 |
| Contact lenses | P. aeruginosa, S. epidermidis | Keratitis | 69 |
| Intravascular catheters | S. epidermidis, S. aureus | Septicemia, endocarditis | 232, 250; reviewed in 122 |
| Total artificial heart | P. aeruginosa, S. epidermidis, S. aureus | Septicemia, device failure | 90, 138 |
| Urinary catheters | E. coli, P. aeruginosa, E. faecalis, Proteus mirabilis | Bacteriuria | 175, 236, 239, 262 |
| Joint replacement | S. epidermidis, S. aureus | Septicemia, device failure | Reviewed in 64 |
| Endotracheal tube | P. aeruginosa, E. coli, S. epidermidis, S. aureus | Pneumonia | 111, 235 |
| Voice prostheses | Streptococci staphylococci | Prosthesis failure | 172 |
Biofilms and Pathogenesis
Although the role of biofilms in implant infections has been demonstrated in numerous systems, the role of biofilms in nonimplant disease is less well established. One example of a disease in which biofilms are thought to play a prominent role is the occurrence of lung infections by P. aeruginosa in patients with cystic fibrosis (CF). Individuals with this inherited genetic disorder are susceptible to chronic P. aeruginosa infections. The basis of this susceptibility is not known, but the direct consequence of the P. aeruginosa infection is a hyperactive inflammatory response in the lung that eventually destroys lung function and leads to the death of the patient (41, 197).
Two lines of evidence are consistent with a role for P. aeruginosa biofilms in the CF lung. Microcolonies of bacteria have been observed in sections of lung from CF patients, and these may represent biofilm-grown cells (89). Second, many isolates of P. aeruginosa from the CF lung are mucoid due to the overexpression of an EPS called alginate. Numerous studies have explored the molecular basis of this mucoid phenotype (reviewed in references 16, 78, 89, and 156). It is widely believed that alginate is the key EPS required for establishing biofilm architecture in P. aeruginosa, rendering this organism recalcitrant to antimicrobial treatment. However, to date, there is no direct evidence establishing a role for alginate in biofilm architecture and resistance to biocides. A recent publication by Stewart and colleagues (40) assayed biocide resistance in an algT mutant, which is defective in the expression of the alginate biosynthesis genes and is unable to produce detectable levels of alginate. On two different surfaces for biofilm development, alginate beads (which yield a relatively thin biofilm) and glass (which yields a thicker biofilm), the algT mutant was not altered in its resistance to hydrogen peroxide and monocholoramine, two oxidative biocides, at the 48-h time point. At 24 h, the algT mutant was more sensitive to hydrogen peroxide treatment than the wild type, but there was no difference between these two strains when treated with monocholoramine. These data call into question the importance of alginate in conferring protection to the biofilm against this class of biocides. Furthermore, algT codes for a sigma factor that may have targets other than the alginate biosynthesis genes, leaving open the possibility that the increased resistance attributed to alginate in these experiments is due to other uncharacterized AlgT-mediated changes in gene expression. It has become increasingly clear that detailed analysis of the role of alginate in biofilm development and resistance to various biocides should be a top priority.
Another example of a likely biofilm-mediated infection is chronic ear infection (otitis media). These infections are often caused by biofilm bacteria (62). In addition, it has been a quandary for some time why patients with chronic otitis media do not appear to have infections when tested by routine culture methods. This is likely due to the fact that biofilm bacteria can be difficult to culture by routine methods (47).
Periodontitis is another example of a biofilm-mediated disease that results in chronic inflammation of the tissue supporting the gums and can eventually lead to tooth loss. The main microbe associated with this disease is Porphyromonas gingivalis (reviewed in reference 139). This bacterium can colonize a number of surfaces in the oral cavity, including various mucosal surfaces and the tooth surface, either directly or via interactions with primary colonizers of the tooth surface such as Streptococcus gordonii and Streptococcus sanguis. The binding of one bacterium to another, or coaggregation, is a well-studied aspect of tooth colonization and will be explored in more detail below. Colonization of surfaces may permit the bacteria to invade mucosal cells, alter calcium flux in epithelial cells, and release toxins. These bacteria are thought to produce proteases and other exoproducts that interfere with cytokine signaling pathways and other host factors used to mount a defense response against the bacterial invader (17, 42, 73, 198). Data to date are consistent with a role for biofilm formation in this disease.
The recent increased interest in biofilms has led to further study into whether if and how biofilms play a role in pathogenesis. The examples mentioned above highlight just a few examples of possible roles for surface-attached communities in pathogenesis. A great deal of additional work is necessary to establish a direct link between functions required for biofilm development and those factors required to cause disease in a human host.
GENETIC DISSECTION OF BIOFILM FORMATION
Although mixed-population biofilms are more prevalent in nature, single-species biofilms are of particular interest due to their clinical importance. Single-species biofilms develop on medical implants as well as dead and living tissue, contributing to a variety of persistent infections (49). The formation of biofilms by single species is a well-regulated developmental process that results in a complex population of cell types. Although many species-specific behaviors exist that reflect the unique requirement of each microorganism, some general concepts hold true in the formation of most bacterial biofilms (reviewed in reference 184). Four organisms, P. aeruginosa, P. fluorescens, E. coli, and V. cholerae, have become prominent model organisms for biofilm research. In order to illustrate the complexity involved in patterns of development even when only a single species is involved, we will compare and contrast several stages in biofilm formation by these gram-negative organisms.
Role of Environmental Signals
Many species have shown distinct developmental steps in biofilm formation, which include (i) initial attachment to a surface, followed by (ii) the formation of microcolonies, and finally (iii) maturation of microcolonies into an EPS-encased mature biofilm. These basic steps leading to the formation of a single-species biofilm are outlined in Fig. 5. The process is believed to begin when bacteria sense certain environmental parameters that trigger the transition from planktonic growth to life on a surface (75, 180, 183, 186, 200, 237, 259). The environmental cues that control this transition vary greatly among organisms. P. aeruginosa will form biofilms under most conditions that allow growth (185), but some strains of E. coli K-12 will not form biofilms in minimal medium unless supplemented with amino acids (201), and E. coli O157:H7 has been reported to make a biofilm only under low-nutrient conditions (60).
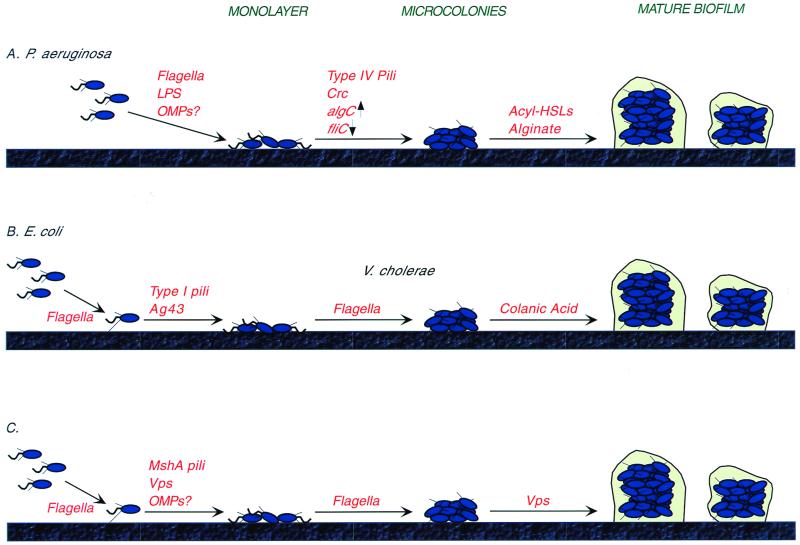
FIG. 5.
Biofilm development in gram-negative organisms. This figure outlines the current models for the early stages in biofilm formation in three of the best-studied model organisms, P. aeruginosa, E. coli, and V. cholerae. (A) In P. aeruginosa, flagella are required to bring the bacterium into proximity with the surface, and LPS mediates early interactions, with an additional possible role for outer membrane proteins (OMPs). Once bacteria are on the surface in a monolayer, type IV pilus-mediated twitching motility is required for the cells to aggregate into microcolonies. The production of pili is regulated at least in part by nutritional signals via Crc. Documented changes in gene expression at this early stage include upregulation of the alginate biosynthesis genes and downregulation of flagellar synthesis. The production of cell-to-cell signaling molecules (acyl-HSLs) is required for formation of the mature biofilm. Alginate may also play a structural role in this process. (B.) In E. coli, flagellum-mediated swimming is required for both approaching and moving across the surface. Organism-surface interactions require type I pili and the outer membrane protein Ag43. Finally, the EPS known as colanic acid is required for development of the normal E. coli biofilm architecture. (C) V. cholerae, like E. coli, utilizes the flagella to approach and spread across the surface. The MshA pili, and possibly one or more unidentified outer membrane proteins, are required for attachment to the surface. This initial surface attachment appears to be stabilized by EPS. Formation of the mature biofilm, with its associated three-dimensional structure, also requires production of EPS. Vps refers to the EPS produced by V. cholerae.
P. fluorescens can also form a biofilm under every condition tested (186). Interestingly, a genetic analysis of biofilm formation by this organism revealed that it utilizes multiple genetic pathways to initiate biofilm development. For example, mutants unable to form a biofilm when grown on glucose were rescued for this defect by growth on citrate, suggesting an alternative citrate-dependent pathway for biofilm formation (186). V. cholerae also appears to utilize different pathways for initial attachment depending on the surface to which the organism attaches. For example, in vivo the Tcp pilus is required for colonization of the intestine (104). However, Tcp appears to play no role in attachment to abiotic surfaces, which is probably one environment exploited by V. cholerae when not in its human host. Here, it is the pilus encoded by the msh locus (having no role in pathogenesis [246]) that is required for attachment to abiotic surfaces. Abiotic surfaces can be further subdivided into nonnutritive (plastic, glass, metal, etc.) and nutritive (e.g., chitin). While mshA is required for colonization of nonnutritive abiotic surfaces, an mshA mutant colonizes cellulose as well as the wild-type strain (263), suggesting the presence of a third set of functions required for the colonization of nutritive surfaces. Other environmental signals that can also influence initial attachment are osmolarity, pH, iron availability, oxygen tension, and temperature (74, 180, 185, 186, 201). Although the details of the environmental signals triggering biofilm development may vary from organism to organism, it is clear that environmental parameters have a profound impact on the transition between planktonic and biofilm growth.
Initiation of Biofilm Formation
The use of well-characterized mutant strains studied with phase contrast microscopy has proven instrumental in determining the mechanisms by which bacteria can initiate biofilm formation. A simple genetic screen has been implemented utilizing plastic 96-well microtiter dishes as a substrate for biofilm development, allowing the large-scale isolation of mutants defective in biofilm formation among a wide variety of organisms (83, 97, 98, 150, 180, 185, 186, 201). These biofilm-defective mutants have been used both to identify functions required for biofilm development and to define the various stages in this process. A number of P. aeruginosa mutants, designated sad for surface attachment defective, have been described (185). One group of strains is defective in flagellum-mediated motility—these strains appeared to be blocked in the initial interactions with a surface. A second class of sad mutants are defective in the biogenesis of type IV pili, which are known to be involved in surface-associated movement referred to as twitching motility. Strains unable to make functional type IV pili attach to the surface and form a monolayer much as the wild type does, yet they are unable to form the microcolonies that are a hallmark of early biofilm development in P. aeruginosa. Therefore, twitching motility is required for the assembly of a monolayer of P. aeruginosa cells into microcolonies (185). Time-lapse movies illustrating twitching motility and microcolony formation can be viewed at http://www.dartmouth.edu/∼gotoole/movies.html. The crc locus, which codes for the catabolite repressor protein, is also involved in biofilm development. The Crc protein was originally identified as being required for the repression of sugar metabolism in the presence of organic acids, the preferred carbon and energy source for Pseudomonas. Recently, Crc was also shown to regulate pilA and pilB, encoding the main structural protein of type IV pili and an accessory factor required for pilus assembly, respectively (183). Although the mechanism by which Crc regulates carbon metabolism and pilus biosynthesis is unknown, these data draw a link between nutrient availability and biofilm formation as well as identify the first component of a signal transduction pathway required for biofilm development in P. aeruginosa. LPS, an important component of the bacterial outer membrane, also plays a role in initial surface attachment. Of the two major species of LPS produced, the loss of the B-band LPS (but not A-band LPS) reduced the cell's ability to interact with hydrophilic surfaces (153). No phase contrast microscopy was performed in this study, so the exact nature of the defect in attachment is not clear. Alterations in LPS have also been shown to alter attachment in the related organism P. fluorescens (273).
E. coli has also been found to require flagella and pili to initiate the early attachment processes (83, 201). Type I pili are absolutely essential for the initial attachment event to proceed but do not appear to play a role in moving the bacteria across the surface. The major phase-variable outer membrane protein of E. coli, known as Ag43, is also required for biofilm formation and may play a direct role in the interaction of the bacterial cell with a surface (51). Furthermore, as in P. aeruginosa, loss of LPS in E. coli results in a decreased ability to attach (82). However, because these LPS mutants are also defective in flagellum-mediated motility and type I pilus production, it is difficult to determine if the loss of LPS has a direct or indirect role in biofilm development. Finally, the proteinaceous cell surface structures known as curli have also been implicated in early attachment events (255).
The biofilm phenotype of E. coli flagellum mutants is distinctly different from that of the P. aeruginosa mutants described above. Attachment is not completely eliminated in E. coli flagellar mutants (although it is severely defective), and the biofilm that forms consists of isolated microcolonies (201). These data were interpreted to mean that once the E. coli cells are in close proximity to the surface, flagellum-mediated motility is required for movement parallel to the surface (in addition to bringing the bacteria into proximity to the surface). Therefore, the roles that flagella play in the formation of biofilms of P. aeruginosa and E. coli are quite different (201). Furthermore, although a number of cell surface structures have been shown to be important in early attachment events, their exact role in biofilm development may differ greatly from organism to organism.
The role of surface structures in V. cholerae appears to be similar to what has been observed for E. coli. Flagella are important for bringing bacteria into close proximity with a surface and for bacterial spread across the surface. The MshA pilus also appears to speed the attachment of bacteria to the surface. The analysis of mature biofilms formed by flagellar and mshA mutants using CSLM revealed that, although they are slightly delayed in biofilm formation, the mature biofilm formed by mutants lacking these surface structures is indistinguishable from that formed by the wild-type strain. Although these three organisms use flagella and pili in the early stages of biofilm development, each organism has adapted the use of these surface structures to its own particular needs.
Maturation of the Biofilm
With time, microcolonies develop into a mature biofilm that is often associated with the production of EPS. Alginate has been implicated as a likely EPS in biofilm development in P. aeruginosa, based in large part on the fact that isolates of this organism from the lung of CF patients are mucoid (e.g., overexpress alginate) (89). Despite the great interest in alginate and its role in P. aeruginosa biofilms, there is no direct evidence that lack of alginate production leads to alterations in the structure of the biofilm. However, we will discuss some of the data suggesting a role for this EPS in biofilm structure.
In P. aeruginosa, the transcription of algC, a key gene involved in the biosynthesis of alginate, is induced soon after the bacteria attach to the surface (53). A recent result reported by Wozniak and colleagues linked the downregulation of flagellum synthesis with the upregulation of alginate synthesis (81). Isolates of P. aeruginosa from the CF lung are typically mucoid and nonmotile. These mucoid strains have a mutation in the mucA gene, which leads to increased levels of the sigma factor ς22, which is encoded by the algT gene. These workers showed that inactivation of the algT gene restored flagellum-mediated motility, which these authors interpreted as meaning that ς22 plays an indirect role (probably via an as yet to be identified intermediary) in regulating flagellum synthesis. Although the exact link between ς22 and the regulation of flagellar synthesis remains to be elucidated, these data point to coordinate regulation of two important players in biofilm development. As cells adjust to an immobile life on a surface, they lose their flagella and increase the production of EPS. It is not clear if there is a causal link between downregulation of flagellar synthesis and upregulation of the genes required for alginate synthesis. In Vibrio parahaemolyticus, it has been shown that interfering with flagellar rotation (i.e., by the cell's being in close proximity to the surface) directly leads to the induction of a signal transduction cascade that upregulates the expression of a second flagellar machinery required for surface swarming (157, 158).
Another important step in biofilm development is the formation of the characteristic biofilm architecture. Although numerous techniques have been utilized to document the biofilm architecture of bacteria, until recently it was not clear if this structural complexity was regulated or the consequence of stochastic processes. The observation that a mutant of P. aeruginosa unable to synthesize the major quorum-sensing molecules acylhomoserine lactones (acyl-HSLs) was radically altered in biofilm architecture clearly demonstrated that these molecules regulate the formation of biofilm structures in this organism. As visualized in a continuous-flow system, the lasI mutant (which is defective in acyl-HSL production) formed a biofilm without the usually well-spaced microcolonies (attaining heights of over 100 μm) and resistance to 0.2% sodium dodecyl sulfate (SDS) treatment typically seen in the wild-type strain (54). Instead, the biofilm formed by the lasI mutant was a homogeneous layer of cells approximately 20 μm thick that had completely lost the ability to resist SDS treatment. An exposure to SDS for as little as 5 min stripped the biofilm from the glass slide on which it formed. The typical biofilm architecture and resistance to SDS could be restored by the addition of exogenous acyl-HSLs (54). These data strongly suggest that cell-cell communication is essential for this bacterium to establish a well-ordered surface community. As discussed above, this structural and spatial organization can have a profound impact on biofilm ecology. A recent report of the genetic analysis of S. gordonii, an oral microbe, suggests that cell-to-cell communication may also be important for biofilm development in this gram-positive organism (see the next section for details). The role of cell-cell signaling molecules in the biofilms of other microbes needs to be explored in more detail.
An essential step in the study of other bacterial models of biofilm development is the demonstration that these organisms form biofilms and the evaluation of the biofilm architecture characteristics of each strain. A recent report by Danese and coworkers showed that the laboratory workhorse strain E. coli makes a biofilm with architecture reminiscent of that produced by pseudomonads (52). Furthermore, a strain defective in colanic acid production, the major EPS synthesized by this organism, does not develop normal biofilm architecture (52). However, initial attachment to the surface is not affected in this mutant, strongly suggesting that colanic acid is not acting as an adhesin during early attachment events. Additional studies are required to determine if the properties often associated with biofilms (such as drug resistance) are observed in these biofilms and are affected by the loss of colanic acid.
As is the case with E. coli, EPS appears to be important for the development of the typical biofilm architecture associated with V. cholerae. Like P. aeruginosa, V. cholerae growing on a surface makes large microcolonies separated by channels essentially devoid of bacteria (264, 282). Two lines of evidence support the notion that EPS is essential for the development of biofilm architecture in V. cholerae. Watnick and Kolter showed that their wild-type strain produces a biofilm whose architecture is very reminiscent of that made by Pseudomonads (264). A mutant unable to make EPS is not competent to produce the typical biofilm architecture. These workers suggest that EPS may stabilize bacterial interactions with the surface and contribute to the formation of the architecture that is a hallmark of a wild-type V. cholerae biofilm (P. I. Watnick and R. Kolter, unpublished data). Yildiz and Schoolnik (282) showed that their wild-type strain, which produces very little EPS, makes a very thin biofilm devoid of observable architecture. However, spontaneous variants of the wild type make an increased amount of EPS (this strain is referred to as a rugose variant), and these EPS-producing strains make a biofilm (166, 282). It is not clear if the rugose variants arise due to phase variation, random mutation, or some as yet undescribed mechanism. However, the observation that the rugose and nonrugose strains can interconvert suggests that regulation via a phase variation mechanism is likely (282). Yildiz and Schoolnik propose that the increased EPS production of the rugose variants may afford these strains a means by which V. cholerae can survive outside of the host (282). This idea is intriguing because it suggests that biofilm formation can play a role in both pathogenesis (see above) and survival of the bacterium outside of its host. Thus, the role of biofilms in pathogenesis and natural environments may be two sides of the same coin. It is possible that biofilms that form in vivo and those that form in environmental settings may require very different genetic pathways.
Molecular Genetics of Oral Biofilms
One of the most-studied biofilm communities is dental plaque. This system is particularly complex because it consists of hundreds of bacterial species, and new species are still being isolated, including known bacterial pathogens not typically associated with the oral cavity (20, 136, 217, 283). Therefore, as opposed to the genetic systems described above, many studies of oral biofilms are concerned with understanding the interactions among the organisms comprising this community. An example of a reconstituted mixed-species oral biofilm is shown in Fig. 6. A number of recent reviews have covered the structure and composition of oral communities (19, 61, 128, 136, 154, 233, 270), and therefore, we will focus our efforts here on summarizing the advances in understanding the molecular mechanisms required for the formation of oral biofilms on teeth.
FIG. 6.
Mixed-species oral biofilm. Confocal image using live-dead stain (Molecular Probes, Inc.) of a mixed-species dental biofilm formed overnight in a flow cell. The inoculum used was saliva, and the chamber was incubated at 37°C with a flow of saliva at 0.2 ml/min. This image is a 0.5-μm slice through the 20-μm biofilm; the slice is between 1.0 and 1.5 μm from the substratum (saliva-coated glass). The green staining indicates live cells, while red bacteria either are dead or have a compromised membrane. The inset in the upper left corner of the figure is a higher magnification of the boxed area in the center of the image. The red arrow points to individual dead or damaged cells, and the green arrow points to a microcolony of live cells. This biofilm comprises a variety of oral microbes that have been reconstituted in an in vitro system. The image is kindly provided by Paul Kolenbrander and Rob Palmer.
Generally speaking, the formation of biofilms on tooth surfaces involves three steps: (i) formation of the conditioning film or acquired pellicle on the tooth enamel, (ii) subsequent cell-to-surface attachment of the primary colonizers, and (iii) cell-to-cell interactions of the mid- and late colonizers with one another as well as primary colonizers. After the tooth surface is thoroughly cleaned, it rapidly becomes coated with a complex mixture of components that include glycoproteins, acidic proline-rich proteins, mucins, bacterial cell debris, exoproducts (such as α-amylase), and sialic acid (65, 84, 86, 221, 222). The acquired pellicle serves as a substrate for the first wave of bacteria, known as the primary colonizers. The primary colonizers include organisms such as S. gordonii, S. sanguis, and Streptococcus parasanguis (179), with this family of organisms making up ∼60 to 80% of the early bacterial population (182). These early events have been reviewed in some detail (95, 115, 116, 118). A recent study by Ganeshkumar and colleagues used polystyrene dishes as a model of an abiotic surface to assess the biofilm-forming ability of the pioneer species S. gordonii and to search for bacterial factors required for these early attachment events (146). Using this polystyrene plate system, they showed that biofilm formation in S. gordonii is influenced by a number of environmental parameters, including osmolarity, carbon source, and pH. A library of 25,000 transposon insertion mutants was screened, and 18 strains defective in biofilm formation were isolated. Among the genes identified in this screen are those that code for functions required for peptidoglycan biosynthesis (PBP 2B, PBP 5, glmM, and bacA), oligopeptide transport (appC), DNA replication-repair (mutT), and competence (comD). Interestingly, comD, which codes for a histidine kinase required for the development of competence in Streptococcus (148), is the receptor for a peptide quorum signal (96, 195). These data suggest that, as is the case for the gram-negative organism P. aeruginosa (54), quorum sensing may also be important for biofilm formation in gram-positive organisms. In addition to the factors described above, pili have been found to bind to proline-rich salivary protein 1 and statherin (6, 142). This result is particularly interesting in light of the finding that many gram-negative organisms also require fimbriae (or pili) for initial interactions with a surface (185, 201, 263). The approach described above and first applied to Staphylococcus aureus and Staphylococcus epidermidis (97–100, 149, 150, 284) represents an excellent way to dissect the molecular genetic basis of the early events in biofilm development in the medically important gram-positive bacteria.
Maturation of the biofilm relies on cell-to-cell interactions called coaggregation. Coaggregation can be defined as “the recognition and adhesion between genetically distinct bacteria” (270) and was first described 30 years ago (85). Kolenbrander and colleagues performed an elegant series of mixing experiments wherein they coincubated pairs of organisms and assayed coaggregation by monitoring the rapid settling of strains out of suspension (126, 130, 131, 134). As is the case for initial adhesion events, studies describing coaggregation in oral bacteria have been reviewed in some detail (135, 136, 270). These interactions do not require viable cells, may be disrupted by the addition of selected soluble sugars, and can occur between organisms of the same or different genera (34, 86, 117, 124, 126, 129, 132–135, 161). The results of the coaggregation assays have allowed the development of a model for bacterial interactions in oral plaque. For example, the pioneer colonizer S. gordonii can coaggregate with the gram-negative bacterium Fusobacterium nucleatum but not with the late colonizer Actinobacillus actinomycetemcomitans (133). Therefore, F. nucleatum, which can aggregate with both species, acts to bridge early and late colonizers. Bacteroides (Porphyromonas) can also serve as a bridging species in oral biofilms (132). A number of lines of evidence suggest that coaggregation occurs as a result of relatively specific interactions. For example, bacteria isolated from certain parts of the oral cavity tend to coaggregate with other bacteria isolated from the same location (e.g., bacteria isolated from the tongue coaggregate best with other tongue-localized bacteria [109]), suggesting a direct spatial organization in the formation of oral biofilms. Finally, coaggregation can be interrupted by the addition of certain sugars such as lactose and galactose, suggesting specific receptor-ligand interactions (29, 137, 265).
The application of molecular and genetic techniques to the study of coaggregation has also been a very fruitful approach. Both spontaneous and transposon-generated mutants defective in coaggregation have been isolated (8, 17, 124, 219, 271). These mutants have helped define functions required for intrageneric and intergeneric coaggregation. For example, S. gordonii mutants that were defective for coaggregating with other streptococci were identified, but these mutants were fully proficient for coaggregating with organisms of other genera (37, 269). These data strongly suggest that oral bacteria have multiple adhesins for interaction with different bacteria. Examples of adhesins isolated include FimA (181), ScaA (8, 130), ScbA (44), PsaA (218), and SsaB (79, 80). Recent studies have shown that many of these adhesins are lipoproteins and, interestingly, are part of ATP-binding cassette (ABC) transporter systems. ScaA is predicted to be a surface-localized lipoprotein that is part of a manganese uptake ABC transporter system required for growth on low Mn concentrations (127). ScbA from Streptococcus crista is also an ABC transporter, and although the substrate transported by this system has not yet been determined, based on its sequence similarity to ScaA (∼93% at the amino acid level), ScbA may also play a role in metal transport (8). The FimA protein also appears to be part of an ABC transporter (71). All of these lipoproteins belong to the LraI (lipoprotein receptor antigen I) family of lipoproteins and have been shown to be involved not only in coaggregation (130), but also in binding components of the salivary pellicle (115). Furthermore, there is evidence that some of these family members are found in the extracellular medium and therefore may be secreted (71, 180). These data strongly suggest that essential cellular functions have been recruited to play a second role in cell-to-cell interactions among the oral bacteria. Recent studies of proteins like SspA and SspB of P. gingivalis have gone a step further and identified a ∼100-amino-acid domain conserved between these proteins that is required for binding to the partner bacterium S. gordonii (17). Another surface protein, encoded by gbpC, was identified in a hunt for mutant strains that no longer aggregated in the presence of dextran (219). GbpC is one example of a class of probable surface proteins required for glucan-dependent binding (219). These adhesins may recognize glucan-like molecules on the surfaces of bacteria with which they coaggregate.
The receptor partners of the adhesins are believed to be cell wall-associated polysaccharides (possibly lipoproteins) which have been identified in a number of organisms, including S. sanguis (29) and Streptococcus oralis (160). These receptors are linear cell wall polysaccharides typically containing characteristic repeating units, including N-acetylglucosamine (1–3, 34, 35, 209; reviewed in reference 135).
Coaggregation has also been shown to require specific amino acid modifications of lipoteichoic acids (LTA). Loss of the gene required for the d-alanylation of the LTA of S. gordonii resulted in a strain that is defective for intrageneric coaggregation, although intergeneric coaggregation appeared to be unaffected (38). It is possible that the effects seen in the strain with modified LTA are indirect effects in presenting receptors or adhesins. For example, LTA can bind calcium (214, 215), and calcium has been shown to be important for coaggregation (134, 161, 213, 214).
Taken together, the studies described above for oral biofilms may provide the ideal system for linking a broad ecological perspective of biofilms with the power of molecular genetics to identify and dissect genetic determinants required for the formation of surface-attached communities.
CONCLUSIONS
Although researchers such as Henrici and Zobell recognized and studied surface-attached bacteria almost 70 years ago (103, 285–287), we are only just beginning to fully realize the significance of biofilm communities. Moreover, viewing bacteria from the perspective of multicellular behavior is, in essence, altering our view of microbiology. It is evident that bacterial cells have the ability to aggregate into particular three-dimensional assemblages, differentiate and hence divide labor within these assemblages, and then disperse as part of their life cycle. Moreover, whether single-species or mixed-species biofilms, intercellular interactions and communication are undoubtedly required for biofilm development and persistence. Dissecting these interactions provides one of the future challenges in biofilm research. Particularly challenging is the attempt to understand the complexity of the interactions within the biofilm community. Communication between species may include extracellular compounds whose sole role is to influence gene expression, metabolic cooperativity and competition (possibly encompassing global changes in gene expression and metabolism), physical contact, and the production of antimicrobial exoproducts. One or all of these interactions may be occurring simultaneously.
One of the keys to studying complex biological systems is to develop accurate and realistic models of natural communities in the laboratory. Scientists have demonstrated remarkable ingenuity in the development of tools for studying biofilm systems, and further development in this area will certainly be required. Progress has already been made in designing an artificial mouth (123, 234) as well as a model to study catheter-induced bladder infections (239). Hence, as is the case with most biological investigations as we enter the 21st century, the future of biofilm research will surely rely upon concerted efforts from scientists in a variety of disciplines. Only with such a collaborative effort will we be able to fully explore these complex systems of the microbial world.
ACKNOWLEDGMENTS
We gratefully acknowledge Guido Bloemberg, Paul Kolenbrander, Rob Palmer, Willem de Vos, and Fitnat Yildiz for providing the images used in this article. We also thank Steve Finkel for a critical review of this manuscript. This work was supported with funds from NIH Training Grant T32 AI07519 (to M.E.D), NSF Career grant MCB-9984521, and the Pew Charitable Trusts (to G.A.O.). G.A.O. is a Pew Scholar in the Biomedical Sciences.
REFERENCES
- 1.Abeygunawardana C, Bush C A, Cisar J O. Complete structure of the cell surface polysaccharide of Streptococcus oralis ATCC 10557: a receptor for lectin-mediated interbacterial adherence. Biochemistry. 1991;30:6528–6540. doi: 10.1021/bi00240a025. [DOI] [PubMed] [Google Scholar]
- 2.Abeygunawardana C, Bush C A, Cisar J O. Complete structure of the cell surface polysaccharide of Streptococcus oralis C104: a 600-MHz NMR study. Biochemistry. 1991;30:8568–8577. doi: 10.1021/bi00099a012. [DOI] [PubMed] [Google Scholar]
- 3.Abeygunawardana C, Bush C A, Cisar J O. Complete structure of the polysaccharide from Streptococcus sanguis J22. Biochemistry. 1990;29:234–248. doi: 10.1021/bi00453a032. [DOI] [PubMed] [Google Scholar]
- 4.Amann R, Snaidr J, Wagner M, Ludwig W, Schleifer K H. In situ visualization of high genetic diversity in a natural microbial community. J Bacteriol. 1996;178:3496–3500. doi: 10.1128/jb.178.12.3496-3500.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amann R I, Ludwig W, Schleifer K-H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amano A, Sharma A, Lee J Y, Sojar H T, Raj P A, Genco R J. Structural domains of Porphyromonas gingivalis recombinant fimbrillin that mediate binding to salivary proline-rich protein and statherin. Infect Immun. 1996;64:1631–1637. doi: 10.1128/iai.64.5.1631-1637.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amellal N, Burtin G, Bartoli F, Heulin T. Colonization of wheat roots by an exopolysaccharide-producing Pantoea agglomerans strain and its effect on rhizosphere soil aggregation. Appl Environ Microbiol. 1998;64:3740–3747. doi: 10.1128/aem.64.10.3740-3747.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson R N, Ganeshkumar N, Kolenbrander P E. Cloning of the Streptococcus gordonii PK488 gene, encoding an adhesin which mediates coaggregation with Actiomyces naselundii PK606. Infect Immun. 1993;61:981–987. doi: 10.1128/iai.61.3.981-987.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anwar H, Starp J L, Costerton W J. Establishment of aging biofilms: possible mechanism of bacterial resistance to antimicrobial therapy. Antimicrob Agents Chemother. 1992;36:1347–1351. doi: 10.1128/aac.36.7.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Archibald L K, Gaynes R P. Hospital acquired infections in the United States: the importance of interhospital comparisons. Nosocomial Infect. 1997;11:245–255. doi: 10.1016/s0891-5520(05)70354-8. [DOI] [PubMed] [Google Scholar]
- 11.Bazylinski D A. Structure and function of the bacterial magnetosome. ASM News. 1995;61:337–343. [Google Scholar]
- 12.Bellin C A, Rao P S C. Impact of bacterial biomass on contaminant sorption and transport in a subsurface soil. Appl Environ Microbiol. 1993;59:1813–1820. doi: 10.1128/aem.59.6.1813-1820.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergh O, Borsheim K Y, Bratbak G, Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989;340:467–468. doi: 10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- 14.Bond P L, Hugenholtz P, Keller J, Blackall L L. Bacterial community structures of phosphate-removing and non-phosphate-removing activated sludges from sequencing batch reactors. Appl Environ Microbiol. 1995;61:1910–1916. doi: 10.1128/aem.61.5.1910-1916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowden G H W, Li Y H. Nutritional influences on biofilm development. Adv Dent Res. 1997;11:81–99. doi: 10.1177/08959374970110012101. [DOI] [PubMed] [Google Scholar]
- 16.Boyd A, Chakrabarty A M. Pseudomonas aeruginosa biofilms: role of the alginate exopolysaccharide. J Ind Microbiol. 1995;15:162–168. doi: 10.1007/BF01569821. [DOI] [PubMed] [Google Scholar]
- 17.Brooks W, Demuth D R, Gil S, Lamont R J. Identification of a Streptococcus gordonii SspB domain that mediates adhesion to Porphyromonas gingivalis. Infect Immun. 1997;65:3753–3758. doi: 10.1128/iai.65.9.3753-3758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bryant M P, Wolin E A, Wolin M J, Wolfe R S. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch Mickrobiol. 1967;59:20–31. doi: 10.1007/BF00406313. [DOI] [PubMed] [Google Scholar]
- 19.Burne R A, Quivey R G, Jr, Marquis R E. Physiologic homeostasis and stress responses in oral biofilms. Methods Enzymol. 1999;310:441–460. doi: 10.1016/s0076-6879(99)10035-1. [DOI] [PubMed] [Google Scholar]
- 20.Bussac G. Helicobacter pylori and the oral environment. Pract Periodontics Aesthet Dent. 1999;11:918. , 920, 922. [PubMed] [Google Scholar]
- 21.Busscher H J, Bruinsma G, Weissenbruch R v, Leunisse C, v. d. Mei H C, Dijk F, Albers F W J. The effect of buttermilk consumption on biofilm formation on silicone rubber voice prostheses in an artificial throat. Eur Arch Otorhinolaryngol. 1998;255:410–413. doi: 10.1007/s004050050088. [DOI] [PubMed] [Google Scholar]
- 22.Caldwell D E. Cultivation and study of biofilm communities. In: Lappin Scott H M, Costerton J W, editors. Microbial biofilms. Cambridge, U.K: University Press; 1995. pp. 64–79. [Google Scholar]
- 23.Caldwell D E, Atuku E, Wilkie D C, Wivcharuk K P, Karthiken S, Korber D R, Schmid D F, Wolfaardt G M. Germ theory vs. community theory in understanding and controlling the proliferation of biofilms. Adv Dent Res. 1997;11:4–13. doi: 10.1177/08959374970110011501. [DOI] [PubMed] [Google Scholar]
- 24.Caldwell D E, Korber D R, Lawrence J R. Confocal laser microscopy and digital image anaylsis in microbial ecology. Adv Microb Ecol. 1992;12:1–67. [Google Scholar]
- 25.Caldwell D E, Korber D R, Lawrence J R. Imaging of bacterial cells by fluorescence exclusion using scanning confocal microscopy. J Microbiol Methods. 1992;15:249–261. [Google Scholar]
- 26.Caldwell D E, Wolfaardt G M, Korber D R, Lawrence J R. Do bacterial communities transcend darwinism? Adv Microb Ecol. 1997;15:105–191. [Google Scholar]
- 27.Campbell R, Greaves M P. Anatomy and community structure of the rhizosphere. In: Lynch J M, editor. The rhizosphere. West Sussex, U.K: John Wiley & Sons; 1990. pp. 11–34. [Google Scholar]
- 28.Caron D A, Davis P G, Madin L P, Sieburth J M. Enrichment of microbial populations in macroaggregates (marine snow) from surface waters of the North Atlantic. J Mar Sci. 1986;44:543–565. [Google Scholar]
- 29.Cassels F J, London J. Isolation of a coaggregation-inhibiting cell wall polysaccharide from Streptococcus sanguis H1. J Bacteriol. 1989;171:4019–4025. doi: 10.1128/jb.171.7.4019-4025.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chater K F. Genetics of differentiation in Streptomyces. Annu Rev Microbiol. 1993;47:685–713. doi: 10.1146/annurev.mi.47.100193.003345. [DOI] [PubMed] [Google Scholar]
- 31.Chin-A-Woeng T F C, de Priester W, van der Bij A J, Lugtenberg B J J. Description of the colonization of a gnotobiotic tomato rhizosphere by Pseudomonas fluorescens biocontrol strain WCS365 using scanning electron microscopy. Mol Plant-Microbe Interact. 1997;10:79–86. [Google Scholar]
- 32.Chiura H X. Generalized gene transfer by virus-like particles from marine bacteria. Aquat Microb Ecol. 1997;13:75–83. [Google Scholar]
- 33.Christensen B B, Sternberg C, Andersen J B, Eberl L, Moller S, Givskov M, Molin S. Establishment of new genetic traits in a microbial biofilm community. Appl Environ Microbiol. 1998;64:2247–2255. doi: 10.1128/aem.64.6.2247-2255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cisar J O, Sandberg A L, Abeygunawardana C, Reddy G P, Bush C A. Lectin recognition of host-like saccharide motifs in streptococcal cell wall polysaccharides. Glycobiology. 1995;5:655–662. doi: 10.1093/glycob/5.7.655. [DOI] [PubMed] [Google Scholar]
- 35.Cisar J O, Sandberg A L, Reddy G P, Abeygunawardana C, Bush C A. Structural and antigenic types of cell wall polysaccharides from viridans group streptococci with receptors for oral actinomyces and streptococcal lectins. Infect Immun. 1997;65:5035–5041. doi: 10.1128/iai.65.12.5035-5041.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark A J, Warren J. Conjugal transmission of plasmids. Annu Rev Genet. 1979;13:99–125. doi: 10.1146/annurev.ge.13.120179.000531. [DOI] [PubMed] [Google Scholar]
- 37.Clemans D L, Kolenbrander P E. Identification of a 100-kilodalton putative coaggregation-mediating adhesin of Streptococcus gordonii DL1 (Challis) Infect Immun. 1995;63:4890–4893. doi: 10.1128/iai.63.12.4890-4893.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clemans D L, Kolenbrander P E, Debabov D V, Zhang Q, Lunsford R D, Sakone H, Whittaker C J, Heaton M P, Neuhaus F C. Insertional inactivation of genes responsible for the d-alanylation of lipoteichoic acid in Streptococcus gordonii DL1 (Challis) affects intrageneric coaggregations. Infect Immun. 1999;67:2464–2474. doi: 10.1128/iai.67.5.2464-2474.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coburn J, Frank D W. Macrophages and epithelial cells respond differently to the Pseudomonas aeruginosa type III secretion system. Infect Immun. 1999;67:3151–3154. doi: 10.1128/iai.67.6.3151-3154.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cochran W L, Suh S J, McFeters G A, Stewart P S. Role of RpoS and AlgT in Pseudomonas aeruginosa biofilm resistance to hydrogen peroxide and monochloramine. J Appl Microbiol. 2000;88:546–553. doi: 10.1046/j.1365-2672.2000.00995.x. [DOI] [PubMed] [Google Scholar]
- 41.Collins F S. Cystic fibrosis: molecular biology and therapeutic implications. Science. 1992;256:774–779. doi: 10.1126/science.1375392. [DOI] [PubMed] [Google Scholar]
- 42.Cook G S, Costerton J W, Lamont R J. Biofilm formation by Porphyromonas gingivalis and Streptococcus gordonii. J Periodont Res. 1998;33:323–327. doi: 10.1111/j.1600-0765.1998.tb02206.x. [DOI] [PubMed] [Google Scholar]
- 43.Coquet L, Junter G-A, Jouenne T. Resistance of artificial biofilms of Pseudomonas aeruginosa to imipenem and tobramycin. J Antimicrob Chemother. 1998;42:755–760. doi: 10.1093/jac/42.6.755. [DOI] [PubMed] [Google Scholar]
- 44.Correia F F, DiRienzo J M, McKay T L, Rosan B. scbA from Streptococcus crista CC5A: an atypical member of the IraI gene family. Infect Immun. 1996;64:2114–2121. doi: 10.1128/iai.64.6.2114-2121.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costerton J W. Overview of microbial biofilms. J Ind Microbiol. 1995;15:137–140. doi: 10.1007/BF01569816. [DOI] [PubMed] [Google Scholar]
- 46.Costerton J W, Cheng K-J, Geesey G G, Ladd T I, Nickel J G, Dasgupta M, Marrie T J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 47.Costerton J W, Lewandowski Z, Caldwell D E, Korber D R, Lappin-Scott H M. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 48.Costerton J W, Lewandowski Z, De Beer D, Caldwell D, Korber D, James G. Biofilms, the customized microniche. J Bacteriol. 1994;176:2137–2142. doi: 10.1128/jb.176.8.2137-2142.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Costerton J W, Stewart P S, Greenberg E P. Bacterial biofilms: a common cause of persistant infections. Science. 1999;284:318–322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 50.Dahlberg C, Linberg C, Torsvik V L, Hermansson M. Conjugative plasmids isolated from bacteria in marine environments show various degrees of homology to each other and are not closely related to well-characterized plasmids. Appl Envirn Microbiol. 1997;63:4692–4697. doi: 10.1128/aem.63.12.4692-4697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Danese P N, Pratt L A, Dove S, Kolter R. The outer-membrane protein, Ag43, mediates cell-to-cell interactions in E. coli biofilms. Mol Microbiol. 2000;37:424–432. doi: 10.1046/j.1365-2958.2000.02008.x. [DOI] [PubMed] [Google Scholar]
- 52.Danese P N, Pratt L A, Kolter R. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J Bacteriol. 2000;182:3593–3596. doi: 10.1128/jb.182.12.3593-3596.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davies D G, Geesey G G. Regulation of the alginate biosynthesis gene algC in Pseudomonas aeruginosa during biofilm development in continuous culture. Appl Environ Microbiol. 1995;61:860–867. doi: 10.1128/aem.61.3.860-867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davies D G, Parsek M R, Pearson J P, Iglewski B H, Costerton J W, Greenberg E P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 55.Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science. 1994;264:375–382. doi: 10.1126/science.8153624. [DOI] [PubMed] [Google Scholar]
- 56.de Beer D, Stoodley P. Relation between the structure of an aerobic biofilm and mass transport phenomena. Water Sci Technol. 1995;32:11–18. [Google Scholar]
- 57.de Beer D, Stoodley P, Lewandowski Z. Effects of biofilm structures on oxygen distribution and mass transport. Biotechnol Bioeng. 1994;43:1131–1138. doi: 10.1002/bit.260431118. [DOI] [PubMed] [Google Scholar]
- 58.de Boer W, Klein Gunnewick R J A, Veenhuis M, Bock E, Laanbroeck H J. Nitrification at low pH by aggregated chemolithotrophic bacteria. Appl Environ Microbiol. 1991;57:3600–3604. doi: 10.1128/aem.57.12.3600-3604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Decho A W. Microbial exopolymer secretions in ocean environments: their role(s) in food webs and marine processes. Oceanogr Mar Biol Annu Rev. 1990;28:73–153. [Google Scholar]
- 60.Dewanti R, Wong A C L. Influence of culture conditions on biofilm formation by Escherichia coli O157:H7. Int J Food Microbiol. 1995;26:147–164. doi: 10.1016/0168-1605(94)00103-d. [DOI] [PubMed] [Google Scholar]
- 61.Dibdin G, Wimpenny J. Steady-state biofilm: practical and theoretical models. Methods Enzymol. 1999;310:296–322. doi: 10.1016/s0076-6879(99)10025-9. [DOI] [PubMed] [Google Scholar]
- 62.Dingman J R, Rayner M G, Mishra S, Zhang Y, Ehrlich M D, Post J C, Ehrlich G D. Correlation between presence of viable bacteria and presence of endotoxin in middle-ear effusions. J Clin Microbiol. 1998;36:3417–3419. doi: 10.1128/jcm.36.11.3417-3419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doolittle M M, Cooney J J, Caldwell D E. Lytic infection of Escherichia coli biofilms by bacteriophage T4. Can J Microbiol. 1995;41:12–18. doi: 10.1139/m95-002. [DOI] [PubMed] [Google Scholar]
- 64.Dougherty S H. Pathobiology of infection in prosthetic devices. Rev Infect Dis. 1988;10:1102–1117. doi: 10.1093/clinids/10.6.1102. [DOI] [PubMed] [Google Scholar]
- 64a.Doyle R J, editor. Methods in enzymology. New York, N.Y: Academic Press; 1999. [Google Scholar]
- 65.Duan Y, Fisher E, Malamud D, Golub E, Demuth D. Calcium-binding properties of SSP-5, the Streptococcus gordonii M5 receptor for salivary agglutinin. Infect Immun. 1994;62:5220–5226. doi: 10.1128/iai.62.12.5220-5226.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dworkin M. Recent advances in the social and developmental biology of the myxobacteria. Microbiol Rev. 1996;60:70–102. doi: 10.1128/mr.60.1.70-102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Edwards K J, Bond P L, Gihring T M, Banfield J. An archaeal iron-oxidizing extreme acidophile important in acid mine drainage. Science. 2000;287:1796–1799. doi: 10.1126/science.287.5459.1796. [DOI] [PubMed] [Google Scholar]
- 68.Elasir M O, Miller R V. Study of the response of a biofilm bacterial community to UV radiation. Appl Environ Microbiol. 1999;65:2025–2031. doi: 10.1128/aem.65.5.2025-2031.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elder M J, Stapleton F, Evans E, Dart J K G. Biofilm-related infections in ophtalmology. Eye. 1995;9:102–109. doi: 10.1038/eye.1995.16. [DOI] [PubMed] [Google Scholar]
- 70.Farag A M, Woodward D F, Goldstein J N, Brumbaugh W, Meyer J S. Concentrations of metals associated with mining waste in sediments, biofilm, benthic macroinvertebrates, and fish from the Coeur d'Alene River Basin, Idaho. Arch Environ Contam Toxicol. 1998;34:119–127. doi: 10.1007/s002449900295. [DOI] [PubMed] [Google Scholar]
- 71.Fenno J C, Shaikh A, Spatafora G, Fives-Taylor P. The fimA locus of Streptococcus parasanguis encodes an ATP-binding membrane transport system. Mol Microbiol. 1995;15:849–863. doi: 10.1111/j.1365-2958.1995.tb02355.x. [DOI] [PubMed] [Google Scholar]
- 72.Flemming H-C. Biofilms and environmental protection. Water Sci Technol. 1993;27:1–10. [Google Scholar]
- 73.Fletcher J, Nair S, Poole S, Henderson B, Wilson M. Cytokine degradation by biofilms of Porphyromonas gingivalis. Curr Microbiol. 1998;36:216–219. doi: 10.1007/s002849900297. [DOI] [PubMed] [Google Scholar]
- 74.Fletcher M. Bacterial attachment in aquatic environments: a diversity of surfaces and adhesion strategies. In: Fletcher M, editor. Bacterial adhesion: molecular and ecological diversity. New York, N.Y: John Wiley & Sons, Inc.; 1996. pp. 1–24. [Google Scholar]
- 75.Fletcher M, Pringle J H. Influence of substratum hydration and absorbed macromolecules on bacterial attachment to surfaces. Appl Environ Microbiol. 1986;51:1321–1325. doi: 10.1128/aem.51.6.1321-1325.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fossing H, Jørgensen B B. Measurement of bacterial sulfate reduction in sediments: evaluation of a single-step chromium reduction method. Biogeochemistry. 1996;8:205–222. [Google Scholar]
- 77.Fry J C, Day M J. Bacterial genetics in natural environments. 1st ed. London, United Kingdom: Chapman & Hall, Ltd.; 1990. [Google Scholar]
- 78.Gacesa P. Bacterial alginate biosynthesis—recent progress and future prospects. Microbiology. 1998;144:1133–1143. doi: 10.1099/00221287-144-5-1133. [DOI] [PubMed] [Google Scholar]
- 79.Ganeshkumar N, Hannam P M, Kolenrander P E, McBride B C. Nucleotide sequence of a gene coding for a saliva-binding protein (SsaB) from Streptococcus sanguis 12 and possible role of the protein in coaggregation with actinomyces. Infect Immun. 1991;59:1093–1099. doi: 10.1128/iai.59.3.1093-1099.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ganeshkumar N, Song M, McBride B C. Cloning of a Streptococcus sanguis adhesin which mediates binding to saliva-coated hydroxyapatite. Infect Immun. 1988;56:1150–1157. doi: 10.1128/iai.56.5.1150-1157.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garrett E S, Perlegas D, Wozniak D J. Negative control of flagellum synthesis in Pseudomonas aeruginosa is modulated by the alternative sigma factor AlgT (AlgU) J Bacteriol. 1999;181:7401–7404. doi: 10.1128/jb.181.23.7401-7404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Genevaux P, Bauda P, DuBow M S, Oudega B. Identification of Tn10 insertions in the rfaG, rfaP, and galU genes involved in lipopolysaccharide core biosynthesis that affect Escerichia coli adhesion. Arch Microbiol. 1999;172:1–8. doi: 10.1007/s002030050732. [DOI] [PubMed] [Google Scholar]
- 83.Genevaux P, Muller S, Bauda P. A rapid screening procedure to identify mini-Tn10 insertion mutants of Escherichia coli K-12 with altered adhesion properties. FEMS Microbiol Lett. 1996;142:27–30. doi: 10.1111/j.1574-6968.1996.tb08402.x. [DOI] [PubMed] [Google Scholar]
- 84.Gibbons R J, Hay D I, Schlesinger D H. Delineation of a segment of adsorbed salivary acidic proline-rich proteins which promotes adhesion of Streptococcus gordonii to apatitic surfaces. Infect Immun. 1991;59:2948–2954. doi: 10.1128/iai.59.9.2948-2954.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gibbons R J, Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970;15:1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- 86.Gibbons R J, van Houte J. Bacterial adherence and the formation of dental plaques. In: Beachey E H, editor. Bacterial adherence. London, U.K: Chapman and Hall; 1980. pp. 63–104. [Google Scholar]
- 87.Gilbert P, Das J, Foley I. Biofilms susceptibility to antimicrobials. Adv Dent Res. 1997;11:160–167. doi: 10.1177/08959374970110010701. [DOI] [PubMed] [Google Scholar]
- 88.Gorlin A I, Gabriel M M, Wilson L A, Ahearn D G. Effect of adhered bacteria on the binding of Acanthamoeba to hydrogel lenses. Arch Ophthalmol. 1996;114:576–580. doi: 10.1001/archopht.1996.01100130568013. [DOI] [PubMed] [Google Scholar]
- 89.Govan J R W, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gristina A G, Dobbins J J, Giammara B, Lewis J C, DeVreies W C. Biomaterial-centered sepsis and the total artificial heart. JAMA. 1988;259:870–874. [PubMed] [Google Scholar]
- 91.Hansen T A. Carbon metabolism of sulfate-reducing bacteria. In: Odom J M, Singleton J R, editors. The sulfate-reducing bacteria: contemporary perspectives. New York, N.Y: Springer-Verlag; 1993. pp. 21–40. [Google Scholar]
- 92.Harley J L, Smith S E. Mycorrhizal symbiosis. New York, N.Y: Academic Press; 1983. [Google Scholar]
- 92a.Harmsen H J M, Akkermans A D L, Stams A J M, de Vos W M. Population dynamics of propionate-oxidizing bacteria under methanogenic and sulfidogenic conditions in anaerobic granular sludge. Appl Environ Microbiol. 1996;62:2163–2168. doi: 10.1128/aem.62.6.2163-2168.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Harmsen H J, Kengen H M, Akkermans A D, Stams A J, de Vos W M. Detection and localization of syntrophic propionate-oxidizing bacteria in granular sludge by in situ hybridization using 16S rRNA-based oligonucleotide probes. Appl Environ Microbiol. 1996;62:1656–1663. doi: 10.1128/aem.62.5.1656-1663.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harrison M J. The arbuscular mycorrhizal symbiosis: and underground association. Trends Plant Sci. 1997;2:54–60. [Google Scholar]
- 95.Hasty D L, Ofek I, Courtney H S, Doyle R J. Multiple adhesins of streptococci. Infect Immun. 1992;60:2147–2152. doi: 10.1128/iai.60.6.2147-2152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Havarstein L S, Gaustad P, Nes I F, Morrison D A. Identification of the streptococcal competence-pheromone receptor. Mol Microbiol. 1996;21:863–869. doi: 10.1046/j.1365-2958.1996.521416.x. [DOI] [PubMed] [Google Scholar]
- 97.Heilmann C, Gerke C, Perdreau-Remington F, Gotz F. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect Immun. 1996;64:277–282. doi: 10.1128/iai.64.1.277-282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heilmann C, Gotz C. Further characterization of Staphylococcus epidermidis transposon mutants deficient in primary attachment or intercellular adhesion. Zentvalbl Bakteriol. 1998;287:69–83. doi: 10.1016/s0934-8840(98)80149-7. [DOI] [PubMed] [Google Scholar]
- 99.Heilmann C, Hussain M, Peters G, Gotz F. Evidence for autolysin mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol. 1997;24:1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- 100.Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Gotz F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol. 1996;20:1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 101.Heimberger T S, Duma R J. Infections of prosthetic heart valves and cardiac pacemakers. Infect Dis Clin N Am. 1989;3:221–245. [PubMed] [Google Scholar]
- 102.Henrichsen J. Bacterial surface translocation: a survey and a classification. Microbiol Rev. 1972;36:478–503. doi: 10.1128/br.36.4.478-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Henrici A T. Studies of freshwater bacteria. I. A direct microscopic technique. J Bacteriol. 1933;25:277–287. doi: 10.1128/jb.25.3.277-287.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Herrington D A, Hall R H, Losonsky G, Mekalanos J J, Taylor R K, Levine M M. Toxin, toxin-coregulated pilus, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988;168:1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hirsch P. Microcolony formation and consortia. In: Marshall K C, editor. Microbial adhesion and aggregation. Berlin, Germany: Springer; 1984. pp. 373–393. [Google Scholar]
- 106.Horstkotte D, Weist K, Ruden H. Better understanding of the pathogenesis of prosthetic valve endocarditis—recent perspectives for prevention strategies. J Heart Valve Dis. 1998;7:313–315. [PubMed] [Google Scholar]
- 107.Hoyle B, Alcantara J, Costerton J W. Pseudomonas aeruginosa biofilm as a diffusion barrier to piperacillin. Antimicrob Agents Chemother. 1992;36:2054–2056. doi: 10.1128/aac.36.9.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hoyle B D, Costerton W J. Bacterial resistance to antibiotics: the role of biofilms. Progress Drug Res. 1991;37:91–105. doi: 10.1007/978-3-0348-7139-6_2. [DOI] [PubMed] [Google Scholar]
- 109.Hughes C V, Kolenbrander P E, Andersen R N, Moore L V. Coaggregation properties of human oral Veillonella spp.: relationship to colonization site and oral ecology. Appl Environ Microbiol. 1988;54:1957–1963. doi: 10.1128/aem.54.8.1957-1963.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hyde J A J, Darouiche R O, Costerton J W. Strategies for prophylaxis against prosthetic valve endocarditis: a review article. J Heart Valve Dis. 1998;7:316–326. [PubMed] [Google Scholar]
- 111.Inglis T J J, Tit-Meng L, Mah-Lee N, Ee-Koom T, Hui K-P. Structural features of tracheal tube biofilm formed during prolonged mechanical ventilation. Chest. 1995;108:1049–1052. doi: 10.1378/chest.108.4.1049. [DOI] [PubMed] [Google Scholar]
- 112.Isa Z, Grusenmeyer S, Verstraete W. Sulfate reduction relative to methane production in high-rate anaerobic digestion: microbiological aspects. Appl Environ Microbiol. 1986;51:580–587. doi: 10.1128/aem.51.3.580-587.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ishida H, Ishida Y, Kurosaka Y, Otani T, Sato K, Kobayashi H. In vitro and in vivo activities of levofloxacin against biofilm-producing Pseudomonas aeruginosa. Antimicrob Agenets Chemother. 1998;42:1641–1645. doi: 10.1128/aac.42.7.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jansson J K, Prosser J I. Quantification of the presence and activity of specific microorganisms in nature. Mol Biotechnol. 1997;7:103–120. doi: 10.1007/BF02761746. [DOI] [PubMed] [Google Scholar]
- 115.Jenkinson H F. Adherence and accumulation of oral streptococci. Trends Microbiol. 1994;2:209–212. doi: 10.1016/0966-842x(94)90114-k. [DOI] [PubMed] [Google Scholar]
- 116.Jenkinson H F. Cell surface protein receptors in oral streptococci. FEMS Microbiol Lett. 1994;121:133–140. doi: 10.1111/j.1574-6968.1994.tb07089.x. [DOI] [PubMed] [Google Scholar]
- 117.Jenkinson H F. Cell-surface proteins of Streptococcus sanguis associated with cell hydrophobicity and coaggregation properties. J Gen Microbiol. 1986;132:1571–1589. doi: 10.1099/00221287-132-6-1575. [DOI] [PubMed] [Google Scholar]
- 118.Jenkinson H F. Genetic analysis of adherence by oral streptococci. J Ind Microbiol. 1995;15:186–192. doi: 10.1007/BF01569824. [DOI] [PubMed] [Google Scholar]
- 119.Kaiser D, Losick R. How and why bacteria talk to each other. Cell. 1993;73:873–885. doi: 10.1016/0092-8674(93)90268-u. [DOI] [PubMed] [Google Scholar]
- 120.Kämpfer P, Erhart R, Beimfohr C, Böhringer M, Wagner M, Amann R. Characterization of bacterial communities from activated sludge: culture-dependent numerical identification versus in situ identification using group- and genus-specific rRNA-targeted oligonucleotide probes. Microb Ecol. 1996;32:101–121. doi: 10.1007/BF00185883. [DOI] [PubMed] [Google Scholar]
- 121.Karl D M, Tilbrook B D. Production and transport of methane in oceanic particulate organic matter. Nature. 1994;368:732–734. [Google Scholar]
- 122.Khardori N, Yassien M. Biofilms in device-related infections. J Ind Microbiol. 1995;15:141–147. doi: 10.1007/BF01569817. [DOI] [PubMed] [Google Scholar]
- 123.Kinniment S L, Wimpenny J W T, Adams D, Marsh P D. Development of a steady-state oral microbial biofilm community using the constant-depth film fermenter. Microbiology. 1996;142:631–638. doi: 10.1099/13500872-142-3-631. [DOI] [PubMed] [Google Scholar]
- 124.Klier C M, Kolenbrander P E, Roble A G, Marco M L, Cross S, Handley P S. Identification of a 95 kDa putative adhesin from Actinomyces serovar WVA963 strain PK1259 that is distinct from type 2 fimbrial subunits. Microbiology. 1997;143:835–846. doi: 10.1099/00221287-143-3-835. [DOI] [PubMed] [Google Scholar]
- 125.Kloeke F V O, Geesey G G. Localization and identification of populations of phosphatase-active bacterial cells associated with activated sludge flocs. Microb Ecol. 1999;38:201–214. doi: 10.1007/s002489900170. [DOI] [PubMed] [Google Scholar]
- 126.Kolenbrander P E. Intergeneric coaggregation among human oral bacteria and ecology of dental plaque. Annu Rev Microbiol. 1988;42:627–656. doi: 10.1146/annurev.mi.42.100188.003211. [DOI] [PubMed] [Google Scholar]
- 127.Kolenbrander P E, Andersen R N, Baker R A, Jenkinson H F. The adhesion-associated sca operon in Streptococcus gordonii encodes an inducible high-affinity ABC transporter for Mn2+ uptake. J Bacteriol. 1998;180:290–295. doi: 10.1128/jb.180.2.290-295.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kolenbrander P E, Andersen R N, Kazmerzak K, Wu R, Palmer R J., Jr Spatial organization of oral bacteria in biofilms. Methods Enzymol. 1999;310:322–332. doi: 10.1016/s0076-6879(99)10026-0. [DOI] [PubMed] [Google Scholar]
- 129.Kolenbrander P E, Anderson R A. Inhibition of coaggregation between Fusobacterium nucleatum and Porphyromonas (Bacteroides) gingivalis by lactose and related sugars. Infect Immun. 1989;57:3204–3209. doi: 10.1128/iai.57.10.3204-3209.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kolenbrander P E, Anderson R N. Characterization of Streptococcus gordonii (S. sanguis) PK488 adhesion-mediated coaggregation with Actinomyces naeslundii PK606. Infect Immun. 1990;58:3064–3072. doi: 10.1128/iai.58.9.3064-3072.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kolenbrander P E, Anderson R N. Multigenic aggregations among oral bacteria: a network of independent cell-to-cell interactions. J Bacteriol. 1986;168:851–859. doi: 10.1128/jb.168.2.851-859.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kolenbrander P E, Anderson R N, Holdemna L V. Coaggregation of oral Bacteroides species with other bacteria: central role in coaggregation bridges and competitions. Infect Immun. 1985;48:741–746. doi: 10.1128/iai.48.3.741-746.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kolenbrander P E, Anderson R N, Moore L V. Coaggregation of Fusobacterium nucleatum, Selenomonas flueggei, Selenomonas inflix, Selenomonas noxia, and Selenomonas sputigena with strains from 11 genera of oral bacteria. Infect Immun. 1989;57:3194–3203. doi: 10.1128/iai.57.10.3194-3203.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kolenbrander P E, Anderson R N, Moore L V H. Intragenic coaggregation among strains of the human oral bacteria: potential role in primary colonization of tooth surface. Appl Environ Microbiol. 1990;56:3890–3894. doi: 10.1128/aem.56.12.3890-3894.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kolenbrander P E, Ganeshkumar N, Cassels F J, Hughes C V. Coaggregation: specific adherence among human oral plaque bacteria. FASEB J. 1993;7:406–413. doi: 10.1096/fasebj.7.5.8462782. [DOI] [PubMed] [Google Scholar]
- 136.Kolenbrander P E, London J. Adhere today, here tomorrow: oral bacterial adherence. J Bacteriol. 1993;175:3247–3252. doi: 10.1128/jb.175.11.3247-3252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kolenbrander P E, London J. Ecological significance of coaggregation among oral bacteria. Adv Microb Ecol. 1992;12:183–217. [Google Scholar]
- 138.Kunin C M, Dobbins J J, Melo J C, Levinson M M, Love K, Joyce L D, DeVries W. Infectious complications in four long-term recipients of the Jarvik-7 artificial heart. JAMA. 1988;259:860–864. [PubMed] [Google Scholar]
- 139.Lamont R J, Jenkinson H F. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244–1263. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lawrence J R, Korber D R, Hoyle B D, Costerton J W, Caldwell D E. Optical sectioning of microbial biofilms. J Bacteriol. 1991;173:6558–6567. doi: 10.1128/jb.173.20.6558-6567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lebaron P, Bauda P, Lett M C, Duval-Iflah Y, Simonet P, Jacq E, Frank N, Roux B, Baleux B, Faurie G, Hubert J C, Normand P, Prieur D, Schmitt S, Block J C. Recombinant plasmid mobilization between E. coli strains in seven sterile microcosms. Can J Microbiol. 1997;43:534–540. doi: 10.1139/m97-076. [DOI] [PubMed] [Google Scholar]
- 142.Lee J Y, Sojar H T, Bedi G S, Genco R J. Synthetic peptides analogous to the fimbrillin sequence inhibit adherence of Porphyromonas gingivalis. Infect Immun. 1992;60:1662–1670. doi: 10.1128/iai.60.4.1662-1670.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lewansowski Z, Altobelli S A, Fukushima E. NMR and microelectrode studies of hydrodynamics and kinetics in biofilms. Biotechnol Prog. 1993;9:40–45. [Google Scholar]
- 144.Licht T R, Christensen B B, Krogfelt K A, Molin S. Plasmid transfer in the animal intestine and the other dynamic populations: the role of community structure and environment. Microbiology. 1999;145:2615–2622. doi: 10.1099/00221287-145-9-2615. [DOI] [PubMed] [Google Scholar]
- 145.Littler W A. Antimicrobial therapy for bacterial endocarditis on native valves. J Infect. 1998;36:137–139. doi: 10.1016/s0163-4453(98)80001-7. [DOI] [PubMed] [Google Scholar]
- 146.Loo C Y, Corliss D A, Ganeshkumar N. Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes. J Bacteriol. 2000;182:1374–1382. doi: 10.1128/jb.182.5.1374-1382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Losick R, Youngman P, Piggot P J. Genetics of endospore formation in Bacillus subtilis. Annu Rev Genet. 1986;20:625–669. doi: 10.1146/annurev.ge.20.120186.003205. [DOI] [PubMed] [Google Scholar]
- 148.Lunsford R D, London J. Natural genetic transformation in Streptococcus gordonii: comX imparts spontaneous competence on strain Wicky. J Bacteriol. 1996;178:5831–5835. doi: 10.1128/jb.178.19.5831-5835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. The intracellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol. 1996;178:175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mack D, Nedelmann M, Krokotsch A, Schwarzkopf A, Heesemann J, Laufs R. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intracellular adhesin. Infect Immun. 1994;62:3244–3253. doi: 10.1128/iai.62.8.3244-3253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.MacLeod F A, Guiot S R, Costerton J W. Layered structure of bacterial aggregates produced in an upflow anaerobic sludge bed and filter reactor. Appl Environ Microbiol. 1990;56:1598–1607. doi: 10.1128/aem.56.6.1598-1607.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Madigan M T, Martinko J M, Parker J. Brock biology of microorganisms. 8th ed. Upper Saddle River, N.J: Prentice Hall, Inc.; 1997. Microbial ecology; pp. 532–605. [Google Scholar]
- 153.Makin S A, Beveridge T J. The influence of A-band and B-band lipopolysaccharide on the surface characteristics and adhesion of Pseudomonas aeruginosa to surfaces. Microbiology. 1996;142:299–307. doi: 10.1099/13500872-142-2-299. [DOI] [PubMed] [Google Scholar]
- 154.Marsh P D. Microbiologic aspects of dental plaque and dental caries. Dent Clin N Am. 1999;43:599–614. , v–vi. [PubMed] [Google Scholar]
- 155.Massol-Deya A A, Whallon J, Hickey R F, Tiedje J M. Channel structure in aerobic biofilms of fixed film reactors treating contaminated groundwater. Appl Environ Microbiol. 1995;61:769–777. doi: 10.1128/aem.61.2.769-777.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.May T B, Shinabarger D, Maharaj R, Kato J, Chu L, de Vault J D, Roychoudhury S, Zielinski N A, Berry A, Rothmel R K, Misra T K, Chakrabarty A M. Alginate synthesis by Pseudomonas aeruginosa: a key pathogenic factor in chronic pulmonary infections of cystic fibrosis patients. Clin Microbiol Rev. 1991;4:191–206. doi: 10.1128/cmr.4.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.McCarter L, Hilmen M, Silverman M. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell. 1988;54:345–351. doi: 10.1016/0092-8674(88)90197-3. [DOI] [PubMed] [Google Scholar]
- 158.McCarter L, Silverman M. Surface-induced swarmer cell differentiation of Vibrio parahaemolyticus. Mol Microbiol. 1990;4:1057–1062. doi: 10.1111/j.1365-2958.1990.tb00678.x. [DOI] [PubMed] [Google Scholar]
- 159.McInerney M J. Transient and persistant associations among prokaryotes. In: Poindexter J S, Leadbetter E R, editors. Bacteria in nature. Vol. 2. New York, N.Y: Plenum Publishing Corp.; 1986. pp. 293–338. [Google Scholar]
- 160.McIntire F C, Crosby L K, Vatter A E, Cisar J O, McNeil M R, Bush C A, Tjoa S S, Fennessey P V. A polysaccharide from Streptococcus sanguis 34 that inhibits coaggregation of S. sanguis 34 with Actinomyces viscosus T14V. J Bacteriol. 1988;170:2229–2235. doi: 10.1128/jb.170.5.2229-2235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McIntire F C, Vatter A E, Baros J, Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978;21:978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.McLaughlin-Borlace L, Stapleton F, Matheson M, Dart J K G. Bacterial biofilm on contact lenses and lens storgae cases in wearers with microbial keratitis. J Appl Microbiol. 1998;84:827–838. doi: 10.1046/j.1365-2672.1998.00418.x. [DOI] [PubMed] [Google Scholar]
- 163.Merritt R W, Cummins K W. An introduction to the aquatic insects of North America. Dubuque, Iowa: Kendall/Hunt Publishing Co.; 1978. p. 441. [Google Scholar]
- 164.Michiels K W, Croes C L, Vanderleyden J. Two different modes of attachment of Azospirillum brasilense Sp7 to wheat roots. J Gen Microbiol. 1991;137:2241–2246. [Google Scholar]
- 165.Mittleman M W, Geesey G G. Copper-binding characteristics of exopolymers from a fresh water-sediment bacterium. Appl Environ Microbiol. 1985;49:846–851. doi: 10.1128/aem.49.4.846-851.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mizunoe Y, Wai S N, Takade A, Yoshida S I. Isolation and characterization of rugose form of Vibrio cholerae O139 strain MO10. Infect Immun. 1999;67:958–963. doi: 10.1128/iai.67.2.958-963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Møller S, Kristensen C S, Poulsen L K, Carstensen J M, Molin S. Bacterial growth on surfaces: automated image analysis for quantification of growth rate-related parameters. Appl Environ Microbiol. 1995;61:741–748. doi: 10.1128/aem.61.2.741-748.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Møller S, Pederson A R, Poulsen L K, Arvin E, Molin S. Activity and three-dimensional distribution of toluene-degrading Pseudomonas putida in a multispecies biofilm assessed by quantitative in situ hybridization and scanning confocal laser microscopy. Appl Environ Microbiol. 1996;62:4632–4640. doi: 10.1128/aem.62.12.4632-4640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Morris C E, Monier J-M, Jacques M-A. Methods for observing microbial biofilms directly on leaf surfaces and recovering them for isolation of culturable microorganisms. Appl Environ Microbiol. 1997;63:1570–1576. doi: 10.1128/aem.63.4.1570-1576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Morris C E, Monier J-M, Jacques M-A. A technique to quantify the population size and composition of the biofilm component in the communities of bacteria in the phyllosphere. Appl Environ Microbiol. 1998;64:4789–4795. doi: 10.1128/aem.64.12.4789-4795.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Muyzer G. DGGE/TGGE, a method for identifying genes from natural ecosystems. Curr Opin Microbiol. 1999;2:317–322. doi: 10.1016/S1369-5274(99)80055-1. [DOI] [PubMed] [Google Scholar]
- 172.Neu T R, Verkerke G J, Herrmann I F, Schutte H K, Van der Mei H C, Busscher H J. Microflora on explanted silicone rubber voice protheses: taxonomy, hydrophobicity and electrophoretic mobility. J Appl Bacteriol. 1994;76:521–528. doi: 10.1111/j.1365-2672.1994.tb01111.x. [DOI] [PubMed] [Google Scholar]
- 173.Nichols W W, Dorrington S M, Slack M P E, Walmsley H L. Inhibition of tobramycin diffusion by binding to alginate. Antimicrob Agents Chemother. 1988;32:518–523. doi: 10.1128/aac.32.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nichols W W, Evans M J, Slack M P E, Walmsley H L. The penetration of antibiotics into aggregates of mucoid and non-mucoid Pseudomonas aeruginosa. J Gen Microbiol. 1989;135:1291–1303. doi: 10.1099/00221287-135-5-1291. [DOI] [PubMed] [Google Scholar]
- 175.Nickel J C, Downey J A, Costerton J W. Ultrastructural study of microbiologic colonization of urinary catheters. Urology. 1989;34:284–291. doi: 10.1016/0090-4295(89)90327-0. [DOI] [PubMed] [Google Scholar]
- 176.Nickel J C, Ruseska I, Wright J B, Costerton J W. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary tract catheter. Antimicrob Agents Chemother. 1985;27:619–624. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nielsen L P. Denitrification in sediment determined from nitrogen isotope pairing. FEMS Microbiol Ecol. 1992;86:357–362. [Google Scholar]
- 178.Nielsen P H. Biofilm dynamics and kinetics during high-rate sulfate reduction under anaerobic conditions. Appl Environ Microbiol. 1987;53:27–32. doi: 10.1128/aem.53.1.27-32.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nyvad B, Kiian M. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand J Dent Res. 1987;95:369–380. doi: 10.1111/j.1600-0722.1987.tb01627.x. [DOI] [PubMed] [Google Scholar]
- 180.Nyvad B, Kilian M. Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res. 1990;24:267–272. doi: 10.1159/000261281. [DOI] [PubMed] [Google Scholar]
- 181.Oligino L, Fives-Taylor P. Overexpression and purification of a fimbria-associated adhesin of Streptococcus parasanguis. Infect Immun. 1993;61:1016–1022. doi: 10.1128/iai.61.3.1016-1022.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ophir T, Gutnick D L. A role for exopolysaccharide in the protection of microorganisms from desiccation. Appl Environ Microbiol. 1994;60:740–745. doi: 10.1128/aem.60.2.740-745.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.O'Toole G A, Gibbs K A, Hager P W, Phibbs P V, Jr, Kolter R. The global carbon metabolism regulator Crc is a component of a signal transduction pathway required for biofilm development by Pseudomonas aeruginosa. J Bacteriol. 2000;182:425–431. doi: 10.1128/jb.182.2.425-431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.O'Toole G A, Kaplan H, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 185.O'Toole G A, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 186.O'Toole G A, Kolter R. The initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Mol Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 187.Overmann J, Pfennig N. Buoyancy regulation and aggregate formation in Amoebobacter purpureus from Mahoney lake. FEMS Microbiol Ecol. 1992;101:67–79. [Google Scholar]
- 188.Pace N R. A molecular view of microbial diversity and the biosphere. Science. 1997;276:734–740. doi: 10.1126/science.276.5313.734. [DOI] [PubMed] [Google Scholar]
- 189.Paerl H W, Pinckney J L. A minireview of microbial consortia: their roles in aquatic production and biogeochemical cycling. Microb Ecol. 1996;31:225–247. doi: 10.1007/BF00171569. [DOI] [PubMed] [Google Scholar]
- 190.Paerl H W, Priscu J C. Microbial phototrophic, heterotrophic, and diazotrophic activities associated with aggregates in the permanent ice cover of Lake Bonney, Antarctica. Microb Ecol. 1998;36:221–230. doi: 10.1007/s002489900109. [DOI] [PubMed] [Google Scholar]
- 191.Paerl H W, Prufert L E. Oxygen-poor microzones as potential sites of microbial N2 fixation in nitrogen-depleted aerobic marine waters. Appl Environ Microbiol. 1987;53:1078–1087. doi: 10.1128/aem.53.5.1078-1087.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Palmer Jr R J, White D C. Developmental biology of biofilms: implications for treatment and control. Trends Microbiol. 1997;5:435–440. doi: 10.1016/S0966-842X(97)01142-6. [DOI] [PubMed] [Google Scholar]
- 193.Paul E A, Clark F E. Soil microbiology and biochemistry. San Diego, Calif: Academic Press; 1989. [Google Scholar]
- 194.Perotto S, Bonfante P. Bacterial associations with mycorrhizal fungi: close and distant friends in the rhizosphere. Trends Microbiol. 1997;5:496–501. doi: 10.1016/S0966-842X(97)01154-2. [DOI] [PubMed] [Google Scholar]
- 195.Pestova E V, Havarstein L S, Morrison D A. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol Microbiol. 1996;21:853–862. doi: 10.1046/j.1365-2958.1996.501417.x. [DOI] [PubMed] [Google Scholar]
- 196.Picard C, Ponsonnet C, Paget E, Nesme X, Simonet P. Detection and enumeration of bacteria in soil by direct DNA extraction and polymerase chain reaction. Appl Environ Microbiol. 1992;58:2717–2722. doi: 10.1128/aem.58.9.2717-2722.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pier G. Pseudomonas aeruginosa: a key problem in cystic fibrosis. ASM News. 1998;64:339–347. [Google Scholar]
- 198.Pike R N, Potempa J, MaGraw W, Coetzer T H T, Travis J. Characterization of the binding activities of proteinase-adhesin complexes from Porphyromonas gingivalis. J Bacteriol. 1996;178:2876–2882. doi: 10.1128/jb.178.10.2876-2882.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ploug H, Jörgensen B B. A net-jet flow system for mass transfer and microsensor studies of sinking aggregates. Mar Ecol Prog Ser. 1999;176:279–290. [Google Scholar]
- 200.Poulsen L K, Ballard G, Stahl D A. Use of rRNA fluorescence in situ hybridization for measuring the activity of single cells in young and established biofilms. Appl Environ Microbiol. 1993;59:1354–1360. doi: 10.1128/aem.59.5.1354-1360.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pratt L A, Kolter R. Genetic analysis of Escherichia coli biofilm formation: defining the roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–294. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 202.Proctor L M, Fuhrman J A. Viral mortality of marine bacteria and cyanobacteria. Nature. 1990;343:60–62. [Google Scholar]
- 203.Rainey F A, Ward N, Sly L I, Stackebrandt E. Dependence of the taxon composition of clone libraries for PCR amplified, naturally occurring 16S rDNA on the primer pair and the cloning system used. Experientia. 1994;50:796–797. [Google Scholar]
- 204.Ramsing N B, Ferris M J, Ward D M. Highly ordered vertical structure of Synechococcus populations within the 1-millimeter-thick photic zone of a hot spring cyanobacterial mat. Appl Environ Microbiol. 2000;66:1038–1049. doi: 10.1128/aem.66.3.1038-1049.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ramsing N B, Kuhl M, Jorgensen B B. Distribution of sulfate-reducing bacteria, O2, and H2S in photosynthetic biofilms determined by oligonucleotide probes and microelectrodes. Appl Environ Microbiol. 1993;59:3840–3849. doi: 10.1128/aem.59.11.3840-3849.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Raskin L, Poulsen L K, Noguera D R, Rittmann B E, Stahl D A. Quantification of methanogenic groups in anaerobic biological reactors by oligonucleotide probe hybridization. Appl Environ Microbiol. 1994;60:1241–1248. doi: 10.1128/aem.60.4.1241-1248.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Raskin L, Rirrman B E, Stahl D A. Competition and coexistence of sulfate-reducing and methanogenic populations in anaerobic biofilms. Appl Environ Microbiol. 1996;62:3847–3857. doi: 10.1128/aem.62.10.3847-3857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rath J, Wu K Y, Herndl G J, DeLong E F. High phylogenetic diversity in a marine-snow-associated bacterial assemblage. Aquat Microb Ecol. 1998;14:261–269. [Google Scholar]
- 209.Reddy G P, Abeygunawardana C, Bush C A, Cisar J O. The cell wall polysaccharide of Streptococcus gordonii 38: structure and immunochemical comparison with the receptor polysaccharides of Streptococcus oralis 34 and Streptococcus mitis J22. Glycobiology. 1994;4:183–192. doi: 10.1093/glycob/4.2.183. [DOI] [PubMed] [Google Scholar]
- 210.Reysenbach A, Giver L J, Wickham G S, Pace N R. Differential amplification of rRNA genes by polymerase chain reaction. Appl Environ Microbiol. 1992;58:3417–3418. doi: 10.1128/aem.58.10.3417-3418.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rigler R J. Microfluorometric characterization of intracellular nucleic acids and nucleoproteins by acridine orange. Acta Physiol Scand. 1966;67:1–122. [PubMed] [Google Scholar]
- 212.Roberts A P, Pratten J, Wilson M, Mullany P. Transfer of a conjugative transposon, Tn5397 in a model oral biofilm. FEMS Microbiol Lett. 1999;177:63–66. doi: 10.1111/j.1574-6968.1999.tb13714.x. [DOI] [PubMed] [Google Scholar]
- 213.Rose R K. The role of calcium in oral streptococcal aggregation and the implications for biofilm formation and retention. Biochim Biophys Acta. 2000;1475:76–82. doi: 10.1016/s0304-4165(00)00048-9. [DOI] [PubMed] [Google Scholar]
- 214.Rose R K, Hogg S D. Competitive binding of calcium and magnesium to streptococcal lipoteichoic acid. Biochim Biophys Acta. 1995;1245:94–98. doi: 10.1016/0304-4165(95)00073-k. [DOI] [PubMed] [Google Scholar]
- 215.Rose R K, Hogg S D, Shellis R P. A quantitative study of calcium binding by isolated streptococcal cell walls and lipoteichoic acid: comparison with whole cells. J Dent Res. 1994;73:1742–1747. doi: 10.1177/00220345940730111001. [DOI] [PubMed] [Google Scholar]
- 216.Ross P, Mayer R, Benziman M. Cellulose biosynthesis and function in bacteria. Microbiol Rev. 1991;55:35–58. doi: 10.1128/mr.55.1.35-58.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Russell S L, Boylan R J, Kaslick R S, Scannapieco F A, Katz R V. Respiratory pathogen colonization of the dental plaque of institutionalized elders. Spec Care Dentist. 1999;19:128–134. doi: 10.1111/j.1754-4505.1999.tb01413.x. [DOI] [PubMed] [Google Scholar]
- 218.Sampson J S, O'Connor S P, Stinson A R, Tarpe J A, Russel H. Cloning and nucleotide sequence analysis of psaA, the Streptococus pneumoniae gene encoding a 37-kilodalton protein homologous to previously reported Streptococcus sp. adhesins. Infect Immun. 1994;62:319–324. doi: 10.1128/iai.62.1.319-324.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sato Y, Yamamoto Y, Kizaki H. Cloning and sequence analysis of the gbpC gene encoding a novel glucan-binding protein of Streptococcus mutans. Infect Immun. 1997;65:668–675. doi: 10.1128/iai.65.2.668-675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sayler G S, Ripp S. Field applications of genetically engineered microorganisms for bioremediation processes. Curr Opin Biotechnol. 2000;11:286–289. doi: 10.1016/s0958-1669(00)00097-5. [DOI] [PubMed] [Google Scholar]
- 221.Scannapieco F A, Bergey E J, Reddy M S, Levine M J. Characterization of salivary α-amylase binding to Streptococcus sanguis. Infect Immun. 1989;57:2853–2863. doi: 10.1128/iai.57.9.2853-2863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Scannapieco F A, Torres G I, Levine M J. Salivary amylase promotes adhesion of oral Streptococci to hydroxyapatite. J Dent Res. 1995;74:1360–1366. doi: 10.1177/00220345950740070701. [DOI] [PubMed] [Google Scholar]
- 223.Schaechter M, Maaøe O, Kjeldgaard N O. Dependency on medium and temperature of cell size and chemical composition during balanced growth of Salmonella typhimurium. J Gen Microbiol. 1958;19:592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- 224.Schink B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev. 1997;61:262–280. doi: 10.1128/mmbr.61.2.262-280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Schramm A, De Beer D, Wagner M, Amann R. Identification and activities in situ of Nitrosospira and Nitrospira spp. as dominant populations in a nitrifying fluidized-bed reactor. Appl Environ Microbiol. 1998;64:3480–3485. doi: 10.1128/aem.64.9.3480-3485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Schramm A, Santegoeds C M, Nielsen H K, Ploug H, Wagner M, Pribyl M, Wanner J, Amann R, de Beer D. On the occurrence of anoxic microniches, denitrification, and sulfate reduction in aerated activated sludge. Appl Environ Microbiol. 1999;65:4189–4196. doi: 10.1128/aem.65.9.4189-4196.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Schuler D, Frankel R B. Bacterial magnetosomes: microbiology, biomineralization and biotechnological applications. Appl Microbiol Biotechnol. 1999;52:464–473. doi: 10.1007/s002530051547. [DOI] [PubMed] [Google Scholar]
- 228.Shanks A L, Reeder M L. Reducing microzones and sulfide production in marine snow. Mar Ecol Prog Ser. 1993;96:43–47. [Google Scholar]
- 229.Shapiro J A. Bacteria as multicellular organisms. Sci Am. 1988;256:82–89. [Google Scholar]
- 230.Shapiro J A. Thinking about bacterial populations as multicellular organisms. Annu Rev Microbiol. 1998;52:81–104. doi: 10.1146/annurev.micro.52.1.81. [DOI] [PubMed] [Google Scholar]
- 231.Shapiro J A, Dworkin M. Bacteria as multicellular organsims. New York, N.Y: Oxford University Press, Inc.; 1997. [Google Scholar]
- 232.Sheridan R L, Weber J M, Peterson H F, Tompkins R G. Central venous catheter sepsis with weekly catheter change in paediatric burn patients: an analysis of 221 catheters. Burns. 1995;21:127–129. doi: 10.1016/0305-4179(95)92137-2. [DOI] [PubMed] [Google Scholar]
- 233.Singleton S, Treloar R, Warren P, Watson G K, Hodgson R, Allison C. Methods for microscopic characterization of oral biofilms: analysis of colonzation, microstructure, and molecular transposrt phenomena. Adv Dent Res. 1997;11:133–149. doi: 10.1177/08959374970110010401. [DOI] [PubMed] [Google Scholar]
- 234.Sissons C H. Artificial dental plaque biofilm model systems. Adv Dent Res. 1997;11:110–126. doi: 10.1177/08959374970110010201. [DOI] [PubMed] [Google Scholar]
- 235.Sottile F D, Marrie T J, Prough D S, Hobgood C D, Gower D J, Webb L X, Costerton J W, Gristina A G. Nosocomial pulmonary infection: possible etiologic significance of bacterial adhesion to endotracheal tubes. Crit Care Med. 1986;14:265–270. [PubMed] [Google Scholar]
- 236.Stamm W. Catheter-associated urinary tract infections: epidemiology, pathogenesis, prevention. Am J Med. 1991;91(3B):65S–71S. doi: 10.1016/0002-9343(91)90345-x. [DOI] [PubMed] [Google Scholar]
- 237.Stanley P M. Factors affecting the irreversible attachment of Pseudomonas aeruginosa to stainless steel. Can J Microbiol. 1983;29:1493–1499. doi: 10.1139/m83-230. [DOI] [PubMed] [Google Scholar]
- 238.Stickler D. Biofilms. Curr Opin Microbiol. 1999;2:270–275. doi: 10.1016/S1369-5274(99)80047-2. [DOI] [PubMed] [Google Scholar]
- 239.Stickler D J, King J B, Winters C, Morris S L. Blockage of urethral catheters by bacterial biofilms. J Infect. 1993;27:133–135. doi: 10.1016/0163-4453(93)94620-q. [DOI] [PubMed] [Google Scholar]
- 240.Stoodley P, Boyle J D, Dodds I, Lappin-Scott H M. Consensus model of biofilm structure. In: Wimpenny J W T, Handley P S, Gilbert P, Lappin-Scott H M, Jones M, editors. Biofilms: community interactions and controls. Cardiff, U.K: BioLine; 1997. pp. 1–9. [Google Scholar]
- 241.Stoodley P, Boyle J D, Dodds I, Lappin-Scott H M. Influence of hydrodynamics and nutrients on biofilm structure. J Appl Microbiol Symp Ser. 1998;85:19S–28S. doi: 10.1111/j.1365-2672.1998.tb05279.x. [DOI] [PubMed] [Google Scholar]
- 242.Stoodley P, DeBeer D, Lewandowski Z. Liquid flow in biofilm systems. Appl Environ Microbiol. 1994;60:2711–2716. doi: 10.1128/aem.60.8.2711-2716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sutherland J W. Biosynthesis and composition of gram-negative bacterial extracellular and wall polysaccharides. Annu Rev Microbiol. 1985;39:243–270. doi: 10.1146/annurev.mi.39.100185.001331. [DOI] [PubMed] [Google Scholar]
- 244.Suttle C A. The significance of viruses to mortality in aquatic microbial communities. Microb Ecol. 1994;28:237–243. doi: 10.1007/BF00166813. [DOI] [PubMed] [Google Scholar]
- 245.Tasaki M, Kamagato Y, Nakamura K, Okamura K, Minami K. Acetogenesis from pyruvate and differences in pyruvate metabolism among three sulfate-reducing bacteria in the absence of sulfate. FEMS Microbiol Lett. 1993;106:259–264. [Google Scholar]
- 246.Thelin K H, Taylor R K. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect Immun. 1996;64:2853–2856. doi: 10.1128/iai.64.7.2853-2856.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Thiele J H, Chartrain M, Zeikus J G. Control of interspecies electron flow during anaerobic digestion: role of floc formation in syntrophic methanogenesis. Appl Environ Microbiol. 1988;54:10–19. doi: 10.1128/aem.54.1.10-19.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Thingstad T F, Heldal M, Bratbak G, Dundas I. Are viruses important partners in pelagic food webs? Trends Ecol Evol. 1993;8:209–213. doi: 10.1016/0169-5347(93)90101-T. [DOI] [PubMed] [Google Scholar]
- 249.Reference deleted.
- 250.Toltzis P. Current issues in central venous catheter infection. Annu Rev Med. 1990;41:169–176. doi: 10.1146/annurev.me.41.020190.001125. [DOI] [PubMed] [Google Scholar]
- 251.van Elsas J D, Trevor J T, Starodub M E. Bacterial conjugation between pseudomonads in the rhizosphere of wheat. FEMS Microbiol Lett. 1988;53:299–306. [Google Scholar]
- 252.van Elsas J D, van Overbeek L S. Bacterial responses to soil stimuli. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. pp. 55–79. [Google Scholar]
- 253.van Hannen E J, Zwart G, van Agterveld M P, Gons H J, Ebert J, Laanbroek H J. Changes in bacterial and eukaryotic community structure after mass lysis of filamentous cyanobacteria associated with viruses. Appl Environ Microbiol. 1999;65:795–801. doi: 10.1128/aem.65.2.795-801.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Veiga M C, Jain M K, Wu W-M, Hollingsworth R I, Zeikus J G. Composition and role of extracellular polymers in methanogenic granules. Appl Environ Microbiol. 1997;63:403–407. doi: 10.1128/aem.63.2.403-407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Vidal O, Longin R, Prigent-Combaret C, Dorel C, Hooreman M, Lejune P. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: involvement of a new ompR allele that increases curli expression. J Bacteriol. 1998;180:2442–2449. doi: 10.1128/jb.180.9.2442-2449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wagner M, Amann R, Lemmer H, Schleifer K H. Probing activated sludge with oligonucleotides specific for proteobacteria: inadequacy of culture-dependent methods for describing microbial community structure. Appl Environ Microbiol. 1993;59:1520–1525. doi: 10.1128/aem.59.5.1520-1525.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wagner M, Erhart R, Manz W, Amann R, Lemmer H, Wedi D, Schleifer K-H. Development of an rRNA-targeted oligonucleotide probe specific for the genus Acinetobacter and its application for in situ monitoring in activated sludge. Appl Environ Microbiol. 1994;60:792–800. doi: 10.1128/aem.60.3.792-800.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wagner M, Roger A M, Flax J L, Brusseau G A, Stahl D A. Phylogeny of dissimilatory sulfite reductases supports an early origin of sulfate respiration. J Bacteriol. 1998;180:2975–2982. doi: 10.1128/jb.180.11.2975-2982.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wang J, Lory S, Ramphal R, Jin S. Isolation and characterization of Pseudomonas aeruginosa genes inducible by respiratory mucus derived from cystic fibrosis patients. Mol Microbiol. 1996;22:1005–1012. doi: 10.1046/j.1365-2958.1996.01533.x. [DOI] [PubMed] [Google Scholar]
- 260.Ward D M, Bateson M M, Weller R, Ruff-Roberts A L. Ribosomal RNA analysis of microorganisms as they occur in nature. Adv Microb Ecol. 1992;12:219–286. [Google Scholar]
- 261.Ward D M, Ferris M J, Nold S C, Bateson M M. A natural view of microbial biodiversity within hot spring cyanobacterial mat communities. Microbiol Mol Biol Rev. 1998;62:1353–1370. doi: 10.1128/mmbr.62.4.1353-1370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Warren J M. Catheter-associated urinary tract infections. Infect Dis Clin N Am. 1997;11:609–622. doi: 10.1016/s0891-5520(05)70376-7. [DOI] [PubMed] [Google Scholar]
- 263.Watnick P I, Fullner K J, Kolter R. A role for the mannose-sensitive hemagglutinin in biofilm formation by Vibrio cholerae El Tor. J Bacteriol. 1999;181:3606–3609. doi: 10.1128/jb.181.11.3606-3609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Watnick P I, Kolter R. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol Microbiol. 1999;34:586–595. doi: 10.1046/j.1365-2958.1999.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Weiss E I, Eli I, Shenitzki B, Smorodinsky N. Identification of the rhamnose-sensitive adhesin of Capnocytophaga ochracea ATCC 33596. Arch Oral Biol. 1990;35(Suppl.):127S–130S. doi: 10.1016/0003-9969(90)90142-w. [DOI] [PubMed] [Google Scholar]
- 266.Weller D M, Thomashow L S. Current challenges in introducing beneficial microorganisms into the rhizosphere. In: O'Gara F, Dowling D N, Boesten B, editors. Molecular ecology of rhizosphere microorganisms. New York, N.Y: VCH; 1994. pp. 1–18. [Google Scholar]
- 267.Whipps J M. Carbon economy. In: Lynch J M, editor. The rhizosphere. West Sussex, U.K: John Wiley & Sons; 1990. pp. 59–97. [Google Scholar]
- 268.Whitman W B, Coleman D C, Wiebe W J. Prokaryotes: the unseen majority. Proc Natl Acad Sci USA. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Whittaker C J, Clemans D L, Kolenbrander P E. Insertional inactivation of an intrageneric coaggregation-relevant adhesin locus from Streptococcus gordonii DL1 (Challis) Infect Immun. 1996;64:4137–4142. doi: 10.1128/iai.64.10.4137-4142.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Whittaker C J, Klier C M, Kolenbrander P E. Mechanisms of adhesion by oral bacteria. Annu Rev Microbiol. 1996;50:513–552. doi: 10.1146/annurev.micro.50.1.513. [DOI] [PubMed] [Google Scholar]
- 271.Whittaker C J, Kolenbrander P E. Identification and further characterization of a locus coding for a hypothetical 33.6-kDa protein involved in intrageneric coaggregation of oral streptococci. Adv Exp Med Biol. 1997;418:695–698. doi: 10.1007/978-1-4899-1825-3_162. [DOI] [PubMed] [Google Scholar]
- 272.Williams S T. Oligotrophy in soil: fact or fiction. In: Fletcher M, Floodgate G D, editors. Bacteria in their natural environment. Orlando, Fla: Academic Press Inc.; 1985. pp. 81–110. [Google Scholar]
- 273.Williams V, Fletcher M. Pseudomonas fluorescens adhesion and transport through porous media are affected by lipopolysaccharide composition. Appl Environ Microbiol. 1996;62:100–104. doi: 10.1128/aem.62.1.100-104.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wimpenny J W T, Colasanti R. A unifying hypothesis for the structure of microbial biofilms based on cellular automaton models. FEMS Microbiol Ecol. 1997;22:1–16. [Google Scholar]
- 275.Witte W. Medical consequences of antibiotic use in agriculture. Science. 1998;279:996–997. doi: 10.1126/science.279.5353.996. [DOI] [PubMed] [Google Scholar]
- 276.Woese C R. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wolfaardt G M, Lawrence J R, Robarts R D, Caldwell D E. In situ characterization of biofilm exopolymers involved in the accumulation of chlorinated organics. Microb Ecol. 1998;35:213–223. doi: 10.1007/s002489900077. [DOI] [PubMed] [Google Scholar]
- 278.Wolk C P. Heterocyst formation. Annu Rev Genet. 1996;30:59–78. doi: 10.1146/annurev.genet.30.1.59. [DOI] [PubMed] [Google Scholar]
- 279.Wommack K E, Colwell R R. Virioplankton: viruses in aquatic ecosystems. Microbiol Mol Biol Rev. 2000;64:69–114. doi: 10.1128/mmbr.64.1.69-114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wu W-M, Jain M K, Zeikus J G. Formation of fatty acid-degrading, anaerobic granules by defined species. Appl Environ Microbiol. 1996;62:2037–2044. doi: 10.1128/aem.62.6.2037-2044.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Wyndham R C, Kennedy K J. Microbial consortia in industrial wastewater treatment. In: Lappin-Scott H M, Costerton J W, editors. Microbial biofilms. Cambridge, U.K: University Press; 1995. pp. 183–195. [Google Scholar]
- 282.Yildiz F H, Schoolnik G K. Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc Natl Acad Sci USA. 1999;96:4028–4033. doi: 10.1073/pnas.96.7.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zhu H, Willcox M D, Knox K W. A new species of oral Streptococcus isolated from Sprague-Dawley rats, Streptococcus orisratti sp. nov. Int J Syst Evol Microbiol. 2000;50:55–61. doi: 10.1099/00207713-50-1-55. [DOI] [PubMed] [Google Scholar]
- 284.Ziebuhr W, Hielmann C, Gotz F, Meyer P, Wilms K, Straube E, Hacker J. Detection of the intracellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermidis blood culture strains and mucosal isolates. Infect Immun. 1997;65:890–896. doi: 10.1128/iai.65.3.890-896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Zobell C E. The effects of solid surfaces upon bacterial activity. J Bacteriol. 1943;46:39–56. doi: 10.1128/jb.46.1.39-56.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zobell C E. The influence of solid surfaces upon the physiological activities of bacteria in sea water. J Bacteriol. 1937;33:86. [Google Scholar]
- 287.Zobell C E, Allen E C. The significance of marine bacteria in the fouling of submerged surfaces. J Bacteriol. 1935;29:239–251. doi: 10.1128/jb.29.3.239-251.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]