PURPOSE
Recent studies, including a meta-analysis of 88 trials, have shown higher than expected rates of recurrence and death in hormone receptor–positive breast cancer. These new findings suggest a need to re-evaluate the use of risk-reducing medication to avoid invasive breast cancer and breast cancer death in high-risk women.
METHODS
We adapted an established Cancer Intervention and Surveillance Modeling Network model to evaluate the lifetime benefits and harms of risk-reducing medication in women with a ≥ 3% 5-year risk of developing breast cancer according to the Breast Cancer Surveillance Consortium risk calculator. Model input parameters were derived from meta-analyses, clinical trials, and large observational data. We evaluated the effects of 5 years of risk-reducing medication (tamoxifen/aromatase inhibitors) with annual screening mammography ± magnetic resonance imaging (MRI) compared with no screening, MRI, or risk-reducing medication. The modeled outcomes included invasive breast cancer, breast cancer death, side effects, false positives, and overdiagnosis. We conducted subgroup analyses for individual risk factors such as age, family history, and prior biopsy.
RESULTS
Risk-reducing tamoxifen with annual screening (± MRI) decreased the risk of invasive breast cancer by 40% and breast cancer death by 57%, compared with no tamoxifen or screening. This is equivalent to an absolute reduction of 95 invasive breast cancers, and 42 breast cancer deaths per 1,000 high-risk women. However, these drugs are associated with side effects. For example, tamoxifen could increase the number of endometrial cancers up to 11 per 1,000 high-risk women. Benefits and harms varied by individual characteristics.
CONCLUSION
The addition of risk-reducing medication to screening could further decrease the risk of breast cancer death. Clinical guidelines for high-risk women should consider integrating shared decision making for risk-reducing medication and screening on the basis of individual risk factors.
INTRODUCTION
Estrogen receptor–positive (ER+) breast cancer is generally considered to have a favorable prognosis. However, recent studies have shown that the annual rates of recurrence and breast cancer death could remain up to 3% for almost three decades after an ER+ breast cancer diagnosis.1,2 This new information on the long-term burden of ER+ breast cancer warrants a reconsideration of the lifetime benefits and harms of risk-reducing medication for primary prevention of breast cancer.
CONTEXT
Key Objective
We re-evaluated the use of risk-reducing medication to avoid breast cancer deaths in women with a 3% or greater 5-year risk of developing breast cancer. The key objective of the study was to provide the benefits and harms of risk-reducing medication, screening, and supplemental magnetic resonance imaging to facilitate shared decision making about risk-reducing drugs with high-risk women seen in clinical practice.
Knowledge Generated
The addition of risk-reducing medication to annual mammography screening (± magnetic resonance imaging) could further decrease the risk of breast cancer death in high-risk women. However, these drugs are associated with side effects. The benefits and harms of risk-reducing medication could vary on the basis of individual risk factors such as age, prior biopsy, and family history of breast cancer.
Relevance (K.D. Miller)
-
Chemoprevention to reduce the risk of breast cancer is effective but underused. Many physicians and patients underestimate the benefit and overestimate the side effects. This analysis provides important data to guide discussions on the basis of individual risks.*
*Relevance section written by JCO Senior Deputy Editor Kathy D. Miller, MD.
Several randomized controlled trials have shown that risk-reducing medications such as tamoxifen and aromatase inhibitors (AIs) could decrease the incidence of ER+ breast cancer by 30%-50% in women who are at high risk of developing breast cancer.3-23 However, in clinical practice, the uptake of risk-reducing medication has remained extremely low.3 The reasons for underuse are not fully understood, but some studies have posited that insufficient data on the long-term benefits and harms of risk-reducing drugs, lack of biomarkers to measure response to medication, and fear of rare but serious side effects (eg, endometrial cancer and pulmonary embolism) are deterrents—causing women and their clinicians to conclude that the harms of risk-reducing medications outweigh their potential benefits.24-26 Furthermore, adding to these barriers is the lack of personalized data to help women and their clinicians quantify the net balance of potential benefits and harms of risk-reducing medication in the presence of mammography screening and magnetic resonance imaging (MRI).
We used an established Cancer Intervention and Surveillance Modeling Network (CISNET) model27-30 to re-evaluate the benefits (avoiding invasive breast cancer and breast cancer death) and harms (side effects) of risk-reducing medication, mammography screening, and MRI in high-risk women. A woman's individual risk for breast cancer may depend on her age, family history of breast cancer, genetic predisposition (eg, BRCA1/2), breast density, and prior history of biopsy.31-33 Recognizing that there are specific guidelines for carriers of pathogenic variants in high-risk genes, in this study, we focused on a larger population of women at increased risk because of age, breast density, prior biopsy, and family history. We considered emerging data1,2 on the long-term risk of breast cancer death in ER+ breast cancer to estimate the benefits and harms of risk-reducing medication, screening, and MRI on the basis of individual characteristics of high-risk women. The overarching goal of this study was to provide novel personalized data to facilitate shared decision making about risk-reducing medication with high-risk women seen in clinical practice.
METHODS
We adapted an extant CISNET breast cancer model (model G-E) for this study.27 The study was approved by the Georgetown University Institutional Review Board and was considered as exempt research on the basis of the use of deidentified data.
Model Overview
The development and validation of model G-E has been described in detail elsewhere.27 In brief, the model simulates life histories for a parallel-universe population that includes breast cancer incidence and survival trends that are specific to ER/human epidermal growth factor receptor 2 (HER2) status, in the absence of risk-reducing medication, screening, MRI, and adjuvant treatment. Life histories are generated for individual women from birth till death or age 100 years to account for her entire potential life history. In this study, a woman's life history also included the trajectory of her individual risk factors such as breast density, family history of breast cancer, and prior history of biopsy conditional on her age, and the joint effects of these risk factors and age on breast cancer incidence. We assumed that 20% of all tumors that would present clinically as ductal carcinoma in situ would never progress to invasive cancer.27 The effects of screening, MRI, risk-reducing medication, and breast cancer treatment could alter a woman's life history and her health outcomes. Screening (± MRI) could reduce breast cancer death through a stage shift and an age shift resulting from early detection. Risk-reducing medication could reduce ER+ breast cancer incidence, which could lead to a reduction in ER+ breast cancer–related death. If a woman was diagnosed with breast cancer, then we applied the effects of hormonal and adjuvant treatment to reduce her risk of breast cancer death. Simulated women could die of breast cancer or other causes.
Model Inputs
The model input parameters and data sources are summarized in Table 1 and described below.
TABLE 1.
Input Parameters Used for Model Development
Breast cancer risk factors and breast cancer incidence.
We used the joint distributions of breast density, family history, and prior biopsy by age in Breast Cancer Surveillance Consortium (BCSC) data34 to simulate the distribution of these risk factors overtime in high-risk women. Breast density was modeled using Breast Imaging Reporting and Data System categories, which included almost entirely fatty, scattered areas of fibroglandular density, heterogeneously dense, or extremely dense.35 Prior history of biopsy included lobular hyperplasia or lobular carcinoma in situ (LCIS), proliferative changes with atypia, proliferative changes without atypia/nonproliferative lesions or none. Family history included one or more first-degree relatives with breast cancer. Breast density, family history, and prior biopsy were first assigned to a woman at age 35 years, and subsequent changes in these risk factors were applied at age 50 years and then at age 65 years. Then, we dynamically updated each simulated woman's risk of breast cancer incidence on the basis of her changing age, density, family history, and prior biopsy by adjusting an age-period-cohort model.36 These adjustments considered breast density, family history, prior biopsy, race/ethnicity, age, and interactions between age and other risk factors (Data Supplement, online only). The joint distribution of ER/HER2 status by age and stage (American Joint Committee on Cancer, version 6) were also obtained from BCSC data.
Screening performance.
We modeled sensitivity and specificity for digital breast tomosynthesis (DBT) by calibrating the performance characteristics of DBT observed in BCSC data.34 The sensitivity and specificity for supplemental screening with MRI + DBT were obtained from a meta-analysis of six screening studies.37 Further details are provided in the Data Supplement.
Effects of risk-reducing medication.
We modeled the effects of a 5-year course of tamoxifen and aromatase inhibitor (AI) on the incidence of breast cancer on the basis of a meta-analysis of trials (Data Supplement).3-23 We assumed that risk-reducing medication did not have an impact on ER-negative (ER−) disease, and the effects of tamoxifen on ER+ disease did not vary by age.3
The side effects attributable to risk-reducing medication were obtained from published data (Data Supplement),3,10,12,38 which included venous thromboembolism; deep vein thrombosis, pulmonary embolism, and superficial phlebitis; coronary heart disease; stroke; and endometrial cancer. We varied the effects of tamoxifen on endometrial cancer risk by age (< 50; ≥ 50 years) according to the age-specific rates reported in trials.10,12,38 Potential death due to these side effects were captured in other-cause mortality. We assumed that the side effects only occurred during the 5-year active treatment period on the basis of published data.8
Breast cancer treatment and other cause mortality.
All women diagnosed with ER+ tumors were assumed to receive 5 years of adjuvant hormonal therapy with a proportion receiving docetaxel and cyclophosphamide.39 We incorporated the late effects of hormonal therapy in ER+ tumors obtained from a meta-analysis of 88 clinical trials.2 Women with ER-tumors received anthracycline-based regimens with a taxane.40 Those with HER2+ tumors received trastuzumab ± pertuzumab in addition to chemotherapy.39 Treatment effectiveness was based on trial data that assumed women received local therapy.40 Age-specific other-cause mortality rates were based on published estimates.41,42 We assumed 100% adherence to isolate the effects of screening and risk-reducing medication.
Population and Subgroups
Risk assessment tools such as the BCSC Breast Cancer Risk Assessment Calculator43 can be used to estimate a woman's 5-year risk of developing breast cancer. In this study, we analyzed the benefits and harms for women when they first would have a ≥ 3% 5-year risk of developing breast cancer on the basis of the BCSC risk calculator. These women are at high risk of developing breast cancer according to current guidelines.44
We also provided results for several subgroups on the basis of the prevalence of individual risk factors in the population (Data Supplement). These subgroups included (1) 35-year-olds with a history of LCIS and family history of breast cancer; (2) 50-year-olds with a history of nonproliferative/proliferative changes without atypia and a family history; and (3) 65-year-olds with a history of nonproliferative/proliferative changes without atypia and no family history of breast cancer.
Strategies
We examined the benefits and harms for 5 strategies, including (1) annual mammography screening (with DBT) alone; (2) 5 years of risk-reducing medication combined with annual screening; (3) annual screening with supplemental MRI; (4) risk-reducing medication combined with annual screening and MRI; and (5) no screening, MRI, or risk-reducing medication. An annual interval was defined as 9-18 months between examinations. We considered tamoxifen for all women in the primary analysis as it is the only risk-reducing medication approved for premenopausal women. We considered the effects of AI in (postmenopausal) women age 50 and 65 years.
Analysis
We simulated 10 million life histories for each strategy described above and summarized the number of invasive (ER+/ER−) breast cancers, breast cancer deaths, side effects of risk-reducing drugs, false positives, and overdiagnosis per 1,000 high-risk women undergoing each strategy. False positives were defined as screens resulting in additional imaging that did not result in a breast cancer diagnosis within 12 months.45,46 We defined overdiagnoses as breast cancers that would not have been clinically detected in the absence of screening because of lack of progressive potential or preceding death from competing causes other than breast cancer.46 Therefore, overdiagnosis included screen-detected nonprogressive ductal carcinoma in situ and some ER+ invasive breast cancers that would not have surfaced to clinically detected tumors before the woman's death from other causes. All outcomes (except for side effects) were summarized from the starting age of a given strategy till death or age 100 years.
The incremental benefits for screening, MRI, or risk-reducing medication strategies (ie, strategies 1-4) were calculated in comparison with the strategy with no risk-reducing medication or screening or MRI (strategy 5). We estimated the absolute and relative (ie, %) reductions in invasive (ER+/ER−) breast cancers and breast cancer deaths. Harms of 5 years of risk-reducing medication were calculated as the difference between the number of adverse events and the background rate in untreated women.3 Harms of screening included the number of false positives and overdiagnoses per 1,000 women screened with DBT ± MRI. The benefits and harms in terms of ER+ tumors were calculated separately.
Sensitivity Analysis
In our primary analysis we assumed that risk-reducing drugs decreased the underlying risk of invasive breast cancer beyond the discontinuation of medication according to follow-up data provided by IBIS-I7-9 and Marsden trials.13,14 However, the effect of risk-reducing medication could potentially decline over time. Therefore, in a sensitivity analysis, we decreased the impact of risk-reducing medication by 10% every 5 years up to 15 years following initiation by applying a step function that diminished the drug effects on the underlying risk of breast cancer. The 10% reduction was selected to capture the variation of drug efficacy seen in trials.47
Exploratory Analysis: 2 Years of Tamoxifen
In practice, women may opt for a shorter duration (eg, 1-2 years) of tamoxifen. However, currently there are no data on the effects of a shorter regimen of risk-reducing drugs. Therefore, we modeled the benefits and harms of 2 years of risk-reducing tamoxifen using data from the Stockholm trial,48 which shows the effects of 2 years of adjuvant tamoxifen on contralateral breast cancer in postmenopausal early-stage breast cancer (Data Supplement).
Validation
Independent validation of results was performed to confirm model accuracy.49 The oncologist coinvestigators (A.W.K. and C.I.) reviewed the face validity of the model structure, inputs, and results. To assess the external validity of the model, we simulated a modern trial (Marsden trial14) and then we compared simulated trial outcomes with the actual trial results.
RESULTS
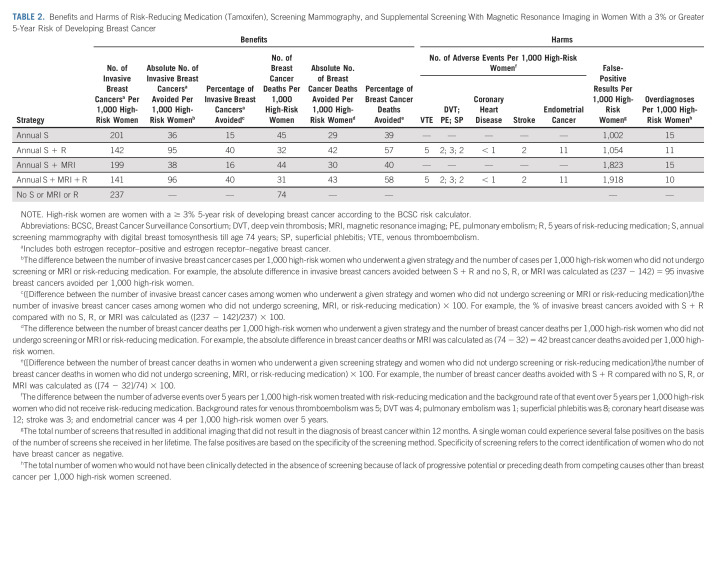
Overall, 5 years of risk-reducing tamoxifen and screening (± MRI) helped avoid 40% of invasive (ER+/ER−) breast cancers, and 57%-58% of breast cancer deaths in high-risk women compared with no screening or risk-reducing tamoxifen (Table 2). This is equivalent to an absolute reduction of 95-96 invasive breast cancer cases, and 42-43 breast cancer deaths per 1,000 high-risk women. In absolute terms, 5 years of risk-reducing tamoxifen alone was attributable to avoiding 58-59 invasive breast cancers and 13 breast cancer deaths per 1,000 women. Tamoxifen primarily reduced ER+ tumors and related deaths (Data Supplement). Over a 5-year period, tamoxifen resulted in 11 endometrial cancers per 1,000 women. The majority (98%) of women in the overall high-risk population were ≥ 50 years (Data Supplement). As a result, the endometrial cancer events in the overall population reflect the risk in older women. The addition of MRI resulted in more false positives compared with screening alone.
TABLE 2.
Benefits and Harms of Risk-Reducing Medication (Tamoxifen), Screening Mammography, and Supplemental Screening With Magnetic Resonance Imaging in Women With a 3% or Greater 5-Year Risk of Developing Breast Cancer
Subgroup Analysis
The benefits and harms varied by age, prior biopsy, and family history (Table 3). More than 95% of 35-year-old high-risk women had LCIS on a prior biopsy (Data Supplement). Tamoxifen with screening (± MRI) could avoid 191-195 invasive breast cancers and 98-100 breast cancer deaths per 1,000 thirty-five-year-old women with a history of LCIS and a family history of breast cancer (Table 3). A reduction in 100-102 invasive breast cancers and 19-20 breast cancer deaths per 1,000 women were attributable to risk-reducing tamoxifen alone. The benefits were primarily seen in ER+ disease (Data Supplement). However, tamoxifen was associated with five venous thromboembolisms and five endometrial cancers per 1,000 women.
TABLE 3.
Benefits and Harms of Risk-Reducing Medication (Tamoxifen), Screening Mammography, and Supplemental Screening With Magnetic Resonance Imaging in 35-, 50-, and 65-Year-Old Women on the Basis of History of Breast Biopsy and Family History of Breast Cancer
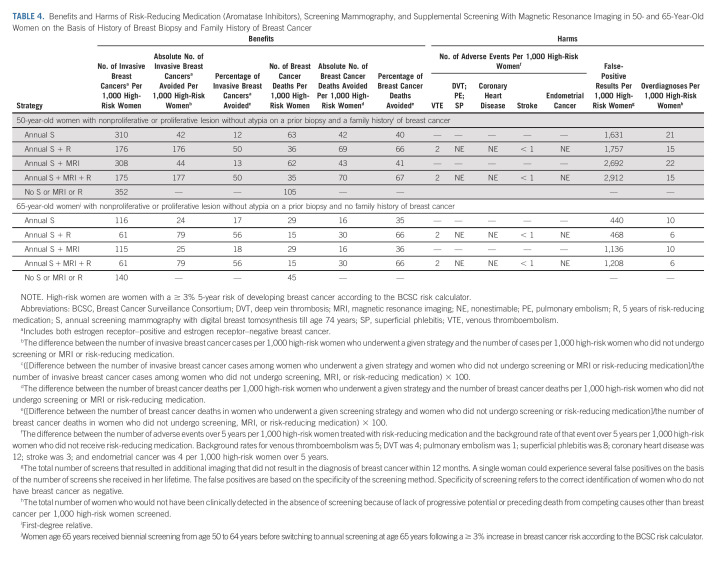
The majority of 50-year-old high-risk women had a history of proliferative changes without atypia (68%; Data Supplement), and a family history (75%; Data Supplement). These women could avoid 126-128 invasive breast cancers and 59-60 breast cancer deaths per 1,000 women with tamoxifen and screening (± MRI) (Table 3). However, their endometrial cancers went up to 11 events per 1,000 women. Most high-risk women ≥ 65 years had a history of proliferative changes without atypia (79%), and no family history (55%; Data Supplement). Tamoxifen and screening could avoid up to 60 invasive breast cancers and 25 breast cancer deaths per 1,000 women. However, tamoxifen also increased the number of thromboembolisms and endometrial cancers (Table 3).
The addition of MRI increased false positives in all three subgroups. For example, in 65-year-old women, the number of false positives nearly tripled with MRI.
AIs Versus Tamoxifen in 50- and 65-Year-Old Women
AIs resulted in higher benefits and lower harms compared with tamoxifen in 50- and 65-year-old women (Table 4; Data Supplement for ER+). The absolute reduction attributable to AIs alone in 50- and 65-year-olds were 133-134 and 84 invasive breast cancers, and 54-55 and 14 breast cancer deaths per 1,000 women, respectively.
TABLE 4.
Benefits and Harms of Risk-Reducing Medication (Aromatase Inhibitors), Screening Mammography, and Supplemental Screening With Magnetic Resonance Imaging in 50- and 65-Year-Old Women on the Basis of History of Breast Biopsy and Family History of Breast Cancer
Sensitivity Analysis
Screening and the diminishing effects of risk-reducing medication over time resulted in a lower absolute reduction of 92 invasive breast cancers and 41 breast cancer deaths (Data Supplement). In 50- and 65-year-olds, the absolute reduction attributable to AIs alone reduced to 91 and 22 invasive breast cancers, and 18 and five breast cancer deaths per 1,000 women, respectively (Data Supplement).
Exploratory Analysis: 2 Years of Tamoxifen
Annual screening with 2 years of tamoxifen could potentially avoid 61 invasive breast cancer cases and 35 breast cancer deaths per 1,000 women (Data Supplement).
Validation
The model closely replicated the estimates observed in the original Marsden trial14 (Data Supplement).
DISCUSSION
Recent studies with long-term follow-up data have shown higher-than-expected rates of recurrence and death in ER+ breast cancer.1,2 In light of this new understanding of the long-term burden of ER+ disease, it is more important than previously recognized for clinicians to introduce medical risk reduction when counseling women at high risk of developing breast cancer. Our results show that the addition of risk-reducing medication to annual screening could further reduce the risk of breast cancer death in high-risk women. The benefits and harms of risk-reducing medication and screening may vary on the basis of individual risk factors such as age, family history of breast cancer, and prior history of biopsy. For instance, consistent with trial data,10,12,38 our results showed that risk-reducing tamoxifen was associated with an increase in endometrial cancers in older women (≥ 50 years). Therefore, AIs may be more suitable for postmenopausal older women.
Current clinical guidelines for high-risk women provide limited information on the basis of individual risk factors or the benefits of adding risk-reducing medication to screening.44,50 Our results suggest that shared decision making regarding risk-reducing medication and mammography screening should be integrated into clinical guidelines for high-risk women, considering their individual risk factors. In a future study, these model results could be developed into a web-based decision tool to further facilitate shared decision making about risk-reducing drugs and mammography screening in clinical practice.
Several studies have shown that one of the major barriers to the uptake of risk-reducing medication is the concern among women and their clinicians that the risks of therapy will not outweigh its benefits.24-26 Some of these concerns could be addressed by data on the long-term impact of risk-reducing medication on breast cancer death; combined effects of risk-reducing medication, screening, and MRI; and variation of harms and benefits of these strategies on the basis of individual risk factors. At present, to our knowledge, there is no single data source that could provide all the information needed to quantify the long-term benefits and harms of risk-reducing medication with screening (± MRI) on the basis of individual characteristics. To the best of our knowledge, currently there are no such planned or ongoing trials. In such situations, the Institute of Medicine has recommended mathematical modeling as a virtual laboratory to synthesize existing knowledge and extrapolate results from trials to provide novel data that could help inform clinical guidelines and practice.51 This study was conducted using a well-established CISNET mathematical model (model G-E), which has been used to inform breast cancer screening guidelines and practice.28,29,52-55
Our model results should be considered within the context of the limitations of the data sources and the assumptions used for model development. There were limited data to model the direct effects of risk-reducing medication on breast density. Although studies have shown that risk-reducing medication could reduce breast density,56 there are limited data on the long-term impact of tamoxifen/AI on changing breast density.57 A decrease in breast density because of tamoxifen/AI could increase the sensitivity of screening/MRI, which could lead to an early detection of breast cancer and a further reduction in breast cancer death. Therefore, our results could be considered conservative estimates of the impact of risk-reducing drugs on breast cancer death. We also did not have data to model side effects beyond the treatment period or side effects considering medical history. There were limited data on the effects of a shorter duration of risk-reducing tamoxifen/AI in high-risk women. Future studies should explore the dose-response relationship between risk-reducing medication and breast cancer in high-risk women.
Overall, our results show that risk-reducing medication could help avoid breast cancer deaths in high-risk women. To our knowledge, this is the first study to incorporate the current understanding of the long-term trajectory of ER+ breast cancer and emphasize the substantial value of preventing a breast cancer diagnosis with medical risk reduction. These results will enable physicians to counsel patients more effectively about the benefits of risk-reducing medication, and potentially save lives.
ACKNOWLEDGMENT
The authors acknowledge the data provided by the Breast Cancer Surveillance Consortium, and data analysis conducted by Linn Abraham and Joanna Eavey with guidance from Ellen O'Meara and Charlotte Gard to develop the input parameters on breast cancer risk, screening performance, and stage distributions.
Suzanne O'Neill
Research Funding: Pfizer
Allison W. Kurian
Research Funding: Myriad Genetics (Inst)
Other Relationship: Ambry Genetics, Color Genomics, GeneDx/BioReference, InVitae, Genentech
Claudine Isaacs
Consulting or Advisory Role: Pfizer, Genentech/Roche, Novartis, Puma Biotechnology, Seattle Genetics, Sanofi/Aventis, Eisai, Ion Solutions, bioTheranostics, AstraZeneca/MedImmune, Gilead Sciences
Research Funding: Tesaro (Inst), Merck (Inst), Seattle Genetics (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), AstraZeneca (Inst), Novartis (Inst), Genentech/Roche (Inst), Bristol Myers Squibb/Celgene (Inst)
Patents, Royalties, Other Intellectual Property: McGraw Hill Publishing, UpToDate—Wolters Kluwer—Author of chapters, Elsevier—Editor of Book
Other Relationship: Side-Out Foundation
No other potential conflicts of interest were reported.
DISCLAIMER
The contents and views in this manuscript are those of the authors and should not be construed to represent the views of the National Institutes of Health. Opinions and comments expressed in this article belong to the authors and do not necessarily reflect those of the US Government, Department of Health and Human Services, National Institutes of Health, or the National Institute on Minority Health and Health Disparities.
PRIOR PRESENTATION
Presented in part at the 2022 ASCO annual meeting, Chicago, IL, June 3-7, 2022.
SUPPORT
Supported by the National Cancer Institute of the National Institutes of Health under award number K99CA241397 and R03CA259896 to J.J.; and a pilot award to J.J. supported by the Georgetown University Lombardi Cancer Center support grant (5P30CA051008-28). J.J. was also partly supported by the Division of Intramural Research at the National Institute on Minority Health and Health Disparities of the National Institutes of Health, and the National Institutes of Health Distinguished Scholars Program.
Breast Cancer Surveillance Consortium (http://www.bcsc-research.org) data collection was supported by the National Cancer Institute (P01CA154292, U54CA163303), Patient-Centered Outcomes Research Institute (PCS-1504-30370), and Agency for Health Research and Quality (R01 HS018366-01A1). J.M.'s effort was supported by R35 CA197289, CA152958, and CA199218.
The study funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
A.W.K. and C.I. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Jinani Jayasekera, Kathryn Lowry, Jeanne Mandelblatt, Allison W. Kurian, Claudine Isaacs
Collection and assembly of data: Jinani Jayasekera, Amy Zhao
Data analysis and interpretation: Jinani Jayasekera, Amy Zhao, Clyde Schechter, Jennifer M. Yeh, Karen J. Wernli, Natasha Stout, Jeanne Mandelblatt, Allison W. Kurian, Claudine Isaacs
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Reassessing the Benefits and Harms of Risk-Reducing Medication Considering the Persistent Risk of Breast Cancer Mortality in Estrogen Receptor–Positive Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Suzanne O'Neill
Research Funding: Pfizer
Allison W. Kurian
Research Funding: Myriad Genetics (Inst)
Other Relationship: Ambry Genetics, Color Genomics, GeneDx/BioReference, InVitae, Genentech
Claudine Isaacs
Consulting or Advisory Role: Pfizer, Genentech/Roche, Novartis, Puma Biotechnology, Seattle Genetics, Sanofi/Aventis, Eisai, Ion Solutions, bioTheranostics, AstraZeneca/MedImmune, Gilead Sciences
Research Funding: Tesaro (Inst), Merck (Inst), Seattle Genetics (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), AstraZeneca (Inst), Novartis (Inst), Genentech/Roche (Inst), Bristol Myers Squibb/Celgene (Inst)
Patents, Royalties, Other Intellectual Property: McGraw Hill Publishing, UpToDate—Wolters Kluwer—Author of chapters, Elsevier—Editor of Book
Other Relationship: Side-Out Foundation
No other potential conflicts of interest were reported.
REFERENCES
- 1.Pedersen RN, Esen BÖ, Mellemkjær L, et al. : The incidence of breast cancer recurrence 10-32 years after primary diagnosis. J Natl Cancer Inst 114:391-399, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan H, Gray R, Braybrooke J, et al. : 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 377:1836-1846, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson HD, Fu R, Zakher B, et al. : Medication use for the risk reduction of primary breast cancer in women: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 322:868-886, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Vogel VG, Costantino JP, Wickerham DL, et al. : Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295:2727-2741, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Land SR, Wickerham DL, Costantino JP, et al. : Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295:2742-2751, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Vogel VG, Costantino JP, Wickerham DL, et al. : Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 trial: Preventing breast cancer. Cancer Prev Res (Phila) 3:696-706, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuzick J, Forbes J, Edwards R, et al. : First results from the International Breast Cancer Intervention Study (IBIS-I): A randomised prevention trial. Lancet 360:817-824, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Cuzick J, Sestak I, Cawthorn S, et al. : Tamoxifen for prevention of breast cancer: Extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol 16:67-75, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuzick J, Forbes JF, Sestak I, et al. : Long-term results of tamoxifen prophylaxis for breast cancer—96-Month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst 99:272-282, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Fisher B, Costantino JP, Wickerham DL, et al. : Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 90:1371-1388, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Veronesi U, Maisonneuve P, Rotmensz N, et al. : Tamoxifen for the prevention of breast cancer: Late results of the Italian randomized tamoxifen prevention trial among women with hysterectomy. J Natl Cancer Inst 99:727-737, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Fisher B, Costantino JP, Wickerham DL, et al. : Tamoxifen for the prevention of breast cancer: Current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 97:1652-1662, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Powles T, Eeles R, Ashley S, et al. : Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet 352:98-101, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Powles TJ, Ashley S, Tidy A, et al. : Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst 99:283-290, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Veronesi U, Maisonneuve P, Costa A, et al. : Prevention of breast cancer with tamoxifen: Preliminary findings from the Italian randomised trial among hysterectomised women: Italian Tamoxifen Prevention Study. Lancet 352:93-97, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Veronesi U, Maisonneuve P, Sacchini V, et al. : Tamoxifen for breast cancer among hysterectomised women. Lancet 359:1122-1124, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Veronesi U, Maisonneuve P, Rotmensz N, et al. : Italian randomized trial among women with hysterectomy: Tamoxifen and hormone-dependent breast cancer in high-risk women. J Natl Cancer Inst 95:160-165, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Decensi A, Maisonneuve P, Rotmensz N, et al. : Effect of tamoxifen on venous thromboembolic events in a breast cancer prevention trial. Circulation 111:650-656, 2005 [DOI] [PubMed] [Google Scholar]
- 19.DeCensi A, Bonanni B, Maisonneuve P, et al. : A phase-III prevention trial of low-dose tamoxifen in postmenopausal hormone replacement therapy users: The HOT study. Ann Oncol 24:2753-2760, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Richardson H, Johnston D, Pater J, et al. : The National Cancer Institute of Canada Clinical Trials Group MAP.3 trial: An international breast cancer prevention trial. Curr Oncol 14:89-96, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spagnolo F, Sestak I, Howell A, et al. : Anastrozole-induced carpal tunnel syndrome: Results from the International Breast Cancer Intervention Study II prevention trial. J Clin Oncol 34:139-143, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Maunsell E, Goss PE, Chlebowski RT, et al. : Quality of life in MAP.3 (Mammary Prevention 3): A randomized, placebo-controlled trial evaluating exemestane for prevention of breast cancer. J Clin Oncol 32:1427-1436, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goss PE, Ingle JN, Alés-Martínez JE, et al. : Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 364:2381-2391, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Smith SG, Sestak I, Forster A, et al. : Factors affecting uptake and adherence to breast cancer chemoprevention: A systematic review and meta-analysis. Ann Oncol 27:575-590, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hum S, Wu M, Pruthi S, et al. : Physician and patient barriers to breast cancer preventive therapy. Curr Breast Cancer Rep 8:158-164, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noonan S, Pasa A, Fontana V, et al. : A survey among breast cancer specialists on the low uptake of therapeutic prevention with tamoxifen or raloxifene. Cancer Prev Res (Phila) 11:38-43, 2018 [DOI] [PubMed] [Google Scholar]
- 27.Schechter CB, Near AM, Jayasekera J, et al. : Structure, function, and applications of the georgetown-einstein (GE) breast cancer simulation model. Med Decis Making 38:66s-77s, 2018. (1_Suppl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandelblatt JS, Stout NK, Schechter CB, et al. : Collaborative modeling of the benefits and harms associated with different U.S. breast cancer screening strategies. Ann Intern Med 164:215-225, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandelblatt JS, Cronin KA, Bailey S, et al. : Effects of mammography screening under different screening schedules: Model estimates of potential benefits and harms. Ann Intern Med 151:738-747, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jayasekera J, Li Y, Schechter CB, et al. : Simulation modeling of cancer clinical trials: Application to omitting radiotherapy in low-risk breast cancer. J Natl Cancer Inst 110:1360-1369, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tyrer J, Duffy SW, Cuzick J: A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 23:1111-1130, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Colditz GA, Rosner BA, Chen WY, et al. : Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst 96:218-228, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Hedenfalk I, Duggan D, Chen Y, et al. : Gene-expression profiles in hereditary breast cancer. N Engl J Med 344:539-548, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Ballard-Barbash R, Taplin SH, Yankaskas BC, et al. : Breast Cancer Surveillance Consortium: A national mammography screening and outcomes database. AJR Am J Roentgenol 169:1001-1008, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Sickles EA, D’Orsi CJ, Bassett LW, et al. : ACR BI-RADS® mammography, in ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA, American College of Radiology, 2013 [Google Scholar]
- 36.Gangnon RE, Sprague BL, Stout NK, et al. : The contribution of mammography screening to breast cancer incidence trends in the United States: An updated age-period-cohort model. Cancer Epidemiol Biomarkers Prev 24:905-912, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phi XA, Houssami N, Obdeijn IM, et al. : Magnetic resonance imaging improves breast screening sensitivity in BRCA mutation carriers age >/= 50 years: Evidence from an individual patient data meta-analysis. J Clin Oncol 33:349-356, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Iqbal J, Ginsburg OM, Wijeratne TD, et al. : Endometrial cancer and venous thromboembolism in women under age 50 who take tamoxifen for prevention of breast cancer: A systematic review. Cancer Treat Rev 38:318-328, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Caswell-Jin JL, Plevritis SK, Tian L, et al. : Change in survival in metastatic breast cancer with treatment advances: Meta-analysis and systematic review. JNCI Cancer Spectrum 2:pky062, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Early Breast Cancer Trialists' Collaborative Group, Peto R Davies C et al. : Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379:432-444, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trentham-Dietz A, Chapman CH, Bird J, et al. : Recent changes in the patterns of breast cancer as a proportion of all deaths according to race and ethnicity. Epidemiology 32:904-913, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gangnon RE, Stout NK, Alagoz O, et al. : Contribution of breast cancer to overall mortality for US women. Med Decis Making 38:24s-31s, 2018. (1_Suppl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breast Cancer Surveillance Consortium (BCSC) : Breast Cancer Surveillance Consortium risk calculator. BCSC Website, 2015. https://tools.bcsc-scc.org/bc5yearrisk/calculator.htm [Google Scholar]
- 44.Owens DK, Davidson KW, Krist AH, et al. : Medication use to reduce risk of breast cancer: US Preventive Services Task Force recommendation statement. JAMA 322:857-867, 2019 [DOI] [PubMed] [Google Scholar]
- 45.Mandelblatt JS, Near AM, Miglioretti DL, et al. : Common model inputs used in CISNET collaborative breast cancer modeling. Med Decis Making 38:9s-23s, 2018. (1_Suppl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chapman CH, Schechter CB, Cadham CJ, et al. : Identifying equitable screening mammography strategies for black women in the United States using simulation modeling. Ann Intern Med 174:1637-1646, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson HD, Fu R, Cantor A, et al. : Effectiveness of breast cancer screening: Systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann Intern Med 164:244-255, 2016 [DOI] [PubMed] [Google Scholar]
- 48.Rutqvist LE, Johansson H: Long-term follow-up of the randomized Stockholm trial on adjuvant tamoxifen among postmenopausal patients with early stage breast cancer. Acta Oncol 46:133-145, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Eddy DM, Hollingworth W, Caro JJ, et al. : Model transparency and validation: A report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making 32:733-743, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Visvanathan K, Hurley P, Bantug E, et al. : Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 31:2942-2962, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Institute of Medicine : The national academies collection: Reports funded by National Institutes of Health, in Integrating Large-Scale Genomic Information Into Clinical Practice: Workshop Summary. Washington, DC, National Academies Press (US), National Academy of Sciences, 2012 [PubMed] [Google Scholar]
- 52.Jayasekera J, Sparano JA, O'Neill S, et al. : Development and validation of a simulation model–based clinical decision tool: Identifying patients where 21-gene recurrence score testing may change decisions. J Clin Oncol 39:2893-2902, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burnside ES, Lee SJ, Bennette C, et al. : Using collaborative simulation modeling to develop a web-based tool to support policy-level decision making about breast cancer screening initiation age. MDM Policy Pract 2: 10.1177/2381468317717982, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ozanne EM, Schneider KH, Soeteman D, et al. : onlineDeCISion.org: A web-based decision aid for DCIS treatment. Breast Cancer Res Treat 154:181-190, 2015 [DOI] [PubMed] [Google Scholar]
- 55.Soeteman DI, Stout NK, Ozanne EM, et al. : Modeling the effectiveness of initial management strategies for ductal carcinoma in situ. J Natl Cancer Inst 105:774-781, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cuzick J, Warwick J, Pinney E, et al. : Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: A nested case-control study. J Natl Cancer Inst 103:744-752, 2011 [DOI] [PubMed] [Google Scholar]
- 57.Brentnall AR, Warren R, Harkness EF, et al. : Mammographic density change in a cohort of premenopausal women receiving tamoxifen for breast cancer prevention over 5 years. Breast Cancer Res 22:101, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]