Abstract
Kaposi sarcoma is an angioproliferative disease associated with human herpes virus 8 infection. Classic Kaposi sarcoma (CKS) usually develops in older age. Although CKS often does not require systemic therapy, systemic therapy can be administered in progressively symptomatic patients. In this real-life study, we purposed to determine effectiveness and safety of weekly paclitaxel therapy in the first-line treatment of CKS. In this cross-sectional retrospective study, we analyzed the clinical data of 44 patients with CKS who received first-line paclitaxel therapy between January 2000 and December 2020. Paclitaxel was administered by intravenous infusion 80 to 100 mg/weekly. The median age of the patients was 67 years (range, 39–86 years), and majority male (77.2%). All patients had cutaneous involvement in extremities. The median follow-up time from paclitaxel treatment was 39.1 (range, 3.7–173.5) months. The median progression free survival from start of therapy was 35.1 months (range, 2–144 months). Complete response, partial response and stable disease were observed in 7 (15.9%), 28 (63.7%) and 6 (13.6) patients, respectively. Objective control rate was 79.6%, and the median response time after the last dose of paclitaxel was 18.2 months. A total of 4 patients (9.1%) had grade 3 to 4 neutropenia, but it was not complicated by febrile neutropenia. Three patients (6.8%) experienced grade 3 to 4 peripheral neuropathy. No patient had grade 3 to 4 allergic reaction. There was no drug-related death. According to our results, paclitaxel is an effective therapy option with an acceptable safety profile for patients with advanced CKS.
Keywords: angioproliferative disease, chemotherapy, HHV-8 virus, Kaposi sarcoma, paclitaxel
1. Introduction
Kaposi sarcoma (KS) is an angioproliferative disease associated with human herpes virus 8 infection.[1] The disease was named after the Hungarian dermatologist Moritz Kaposi of the University of Vienna, who once described the skin manifestations as “idiopathic multipigmented sarcoma of the skin” in 1872.[2] KS consists of 4 clinical types.[3,4] The first of these is the classical type (classical Kaposi sarcoma), which was first described by Kaposi and usually develops in old age. Others are associated with immunosuppressive conditions and are referred to as iatrogenic, endemic, and AIDS-related type. Classic Kaposi sarcoma (CKS) affects men 3 times more often than women.[5,6] The disease is most commonly diagnosed in individuals from the Mediterranean basin or Eastern European Jewish population.[3–5] CKS usually develops in the 60s and 70s.[5,6] Only < 10% of cases develop in people younger than 50 years.
CKS is a chronic disease with a slow course and is characterized by skin involvement and usually does not cause death.[4,5] Lesions appearing as purplish to reddish-blue well-circumscribed macules, plaques, and nodules usually begin on the distal extremities, particularly the lower extremities and feet.[3,4] Clinically, it can cause lymphedema, edema, pain, ulceration, bleeding, and functional disorders. Rarely, involvement of lymph nodes and visceral organs can be observed in advance cases.[3,4] There is no widely used and internationally accepted staging system for CKS.[7–10] It is generally divided into 4 stages according to a staging system developed by Brambilla et al[10] according to the rate of progression and spread of the disease observed in 300 patients with CKS: stage 1, maculonodular involvement observed in the lower extremity; stage 2, infiltrative stage predominantly associated with plaque and sometimes small nodules on the lower extremities; stage 3, florid stage characterized by ulcerated multiple angiomatous plaques and nodules; and stage 4, the disseminated stage in which multiple angiomatous nodules and plaques extending beyond the lower extremities. Clinically, stages 1 to 2 usually have a slow course, while stages 3 and 4 can have an aggressive course.
Although CKS often does not require systemic therapy, in advance cases chemoterapy can be administered in progressively symptomatic patients to reduce the number and size of lesions and alleviate disease-related severe symptoms reducing quality of life such as bleeding, pain, lymphedema, and functional disorders.[7,8,11] In advanced cases, pegylated liposomal doxorubicin (Lip-D) and paclitaxel are the most preferred agents as first- and second line therapy, regardless of KS subtype.[7,8,11] Other chemotherapeutic agents for KS treatment include oral etoposide, vinblastine, bleomycin, vinorelbine, and gemcitabine.[7,8] Data on the efficacy of paclitaxel in CKS were obtained from retrospective studies[12–15] and have been inferred from data supporting the use of paclitaxel in AIDS-related KS.[16–18] Previous studies show that paclitaxel is well tolerated and effective in controlling the disease in the elderly population.[12–15]
In the literature review, studies showing effectiveness and safety of weekly paclitaxel therapy as a first-line chemotherapy for CKS were very few and conducted in a very small sample size.[11–14] In this real-life study we purposed to investigate the effectiveness and safety of paclitaxel therapy in the first-line treatment of CKS in a large sample size with 20 years of experience in our tertiary cancer center.
2. Methods
In this cross-sectional retrospective study, we evaluated the clinical data of 44 patients with CKS who received first-line paclitaxel therapy between January 2000 and December 2020 at a single cancer center. The inclusion criteria of the study were as follows: age of > 18 years and histologically confirmed KS. Patients with HIV-positive or immunosuppression (iatrogenic KS) were excluded from this study.
The baseline clinicodemographic and laboratory data such as; age, gender, comorbidities (diabetes mellitus, hypertension, chronic heart failure), eastern cooperative oncology group (ECOG) performance status, stage, tumor location, and response to treatment were recorded from our institutional cancer registry. Tumor-related complications included pain, functional impairment, ulceration, bleeding, and lymphedema. The disease was staged according to the classification proposed by Brambilla et al[10] for CKS.
Paclitaxel was administered by intravenous infusion 80 to 100 mg/weekly and continued up to complete response (CR) was achieved or maximum clinical response. It was recommended that paclitaxel therapy be discontinued, if occur in case of serious side effects or disease progression.
3. Response to treatment
There is no globally accepted response assessment and staging system for CKS, and the only approved system is from the AIDS Clinical Trials Group Oncology Committee.[16,19] While these criteria are certainly utility useful for HIV-related KS, they are not entirely useful for CKS from its different clinical features. Therefore, we preferred the revised World Health Organization criteria.[20] This response assessment system was based on the number of skin lesions usually present when there was an objective reduction in skin lesions and reductions in tumor-related complications. Treatment response assessment included a detailed clinical examination, physical observation, and review of old photographs of the disease. Thus, tumor-related complications were followed in each paclitaxel cycle. Objective treatment response assessment was performed every 3 weeks. A CR was defined as the disappearance of all clinical manifestations. A partial response (PR) was defined as the absence of new lesions and at least 25% regression of previous lesions. A progressive disease (PD) was defined as an increase of ≥ 25% in the size of previously existing lesions or development of new lesions. Any response that did not meet the criteria for CR, PR, or PD was defined as stable disease (SD). The objective response rate (ORR) was defined as CR + PR, whereas the disease control rate (DCR) was defined as CR + PR + SD. The severity of adverse events was graded using the Common Terminology Criteria for Adverse Events (version 5.0) scale.
4. Statistical analysis
Descriptive statistical analyses were performed including all data collected for this study. Statistical Package for the Social Sciences version 25.0 was used for all statistical analyses, and P values of < .05 were used to denote statistical significance. Survival analysis was performed using the Kaplan–Meier method. Overall survival was defined as the time from the start of the first-line treatment until death due to any cause. Progression-free survival was calculated from the beginning of chemotherapy treatment to disease progression or death from any cause.
5. Results
In this retrospective study, we evaluated 44 patients receiving paclitaxel as the first-line therapy for CKS. The average age of the patients was 67 years (range, 39–86 years), with a male preponderance (77.2%). Table 1 presents detailed the demographic and clinical characteristics of the patients. Except for 5 (11.3%) patients with an ECOG-PS of 2, all patients ECOG-PS was 0 or 1. Moreover, 63.6% of patients had 1 or more comorbidities: 19 patients (43.1%) had hypertension, 11 patients (24%) had diabetes mellitus, and 5 patients (11.3%) had cardiac failure. Family history of KS was not identified in any of the patients.
Table 1.
Demographic and clinical characteristics of the patients.
| Characteristics | N | % | |
|---|---|---|---|
| Age, median | 67 (range;39–86) | ||
| Sex | Female | 10 | 22.8 |
| Male | 34 | 77.2 | |
| ECOG Performance Status | 0–1 | 39 | 88.7 |
| 2 | 5 | 11.3 | |
| Involvement body sites | Lower extremities | 40 | 90.9 |
| Extremities | 44 | 100 | |
| Extremities, trunk | 5 | 2.3 | |
| Extremities, trunk, head | 2 | 6.8 | |
| Stage | Maculonodular | 0 | 0 |
| İnfiltrative | 18 | 40.9 | |
| Florid | 22 | 50 | |
| Disseminated | 4 | 9.1 | |
| Complication of disease | Edema | 23 | 52.2 |
| Pain | 21 | 47.7 | |
| Hemorrhage | 15 | 34 | |
| Ulceration | 12 | 27.2 | |
| Functional impairment | 7 | 15.9 | |
| Subsequent therapy | Second line | 20 | 45.4 |
| Third or higher line | 11 | 25 | |
| Number of cycles, median | 13.9 (range, 3–26) | ||
| Median follow-up time (mo) | 39.1 months (3.7–173.5) | ||
ECOG = eastern cooperative oncology group.
All patients had cutaneous involvement in extremities: 26 (59.1%) patients, only lower extremities; 14 (31.8%) patients, lower and upper extremities; and 4 (9.1%) patients, only upper extremities. Extremity and trunk involvement was noted in 5 patients (11.3%), while extremity and trunk and head involvement was noted in 2 patients (4.5%). Noncutaneous involvement was present in the following patients: 4 patients (9%), lymph node; 2 patients (4.5%), visceral involvement; and 3 patients (6.8%), mucosal involvement.
The cutaneous stage consisted of the following: maculonodular, 0 (0%); infiltrative, 18 (40.9%); florid, 22 (50%); and disseminated, 4 (9.1%). In the pretreatment clinical evaluation of patients, there was edema in 23 (52.2%) patients, pain in 21(47.7%) patients, hemorrhage in 15 (34.1%) patients, ulceration in 12 (27.2) patients, and functional deterioration in 10 (22.7) patients. Twenty patients received second line treatment, and 11 patients received third- or higher line treatment. Oral etoposide, Lip-D, vinorelbine, and capecitabine treatments were used in the second and other lines of therapy. Paclitaxel rechallenge treatment was used in 5 patients.
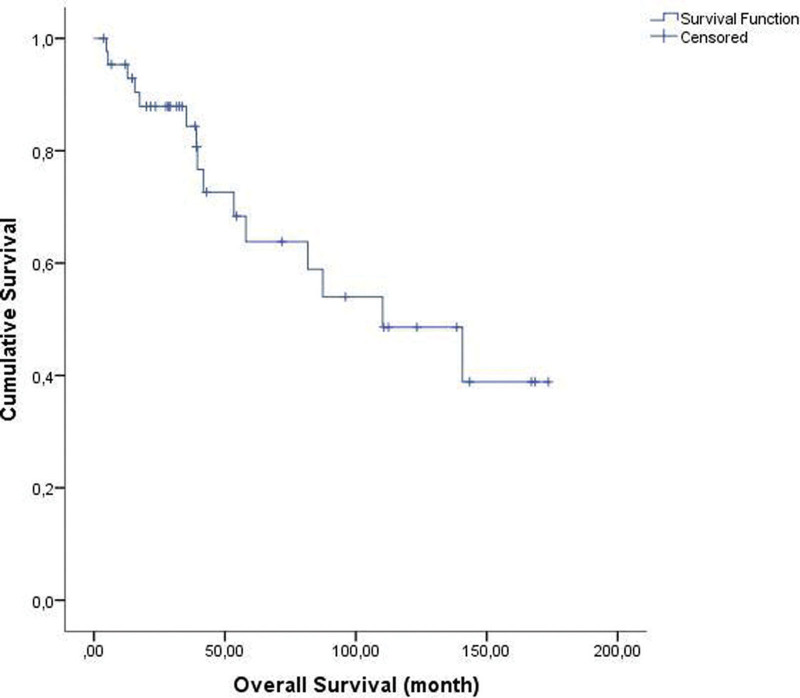
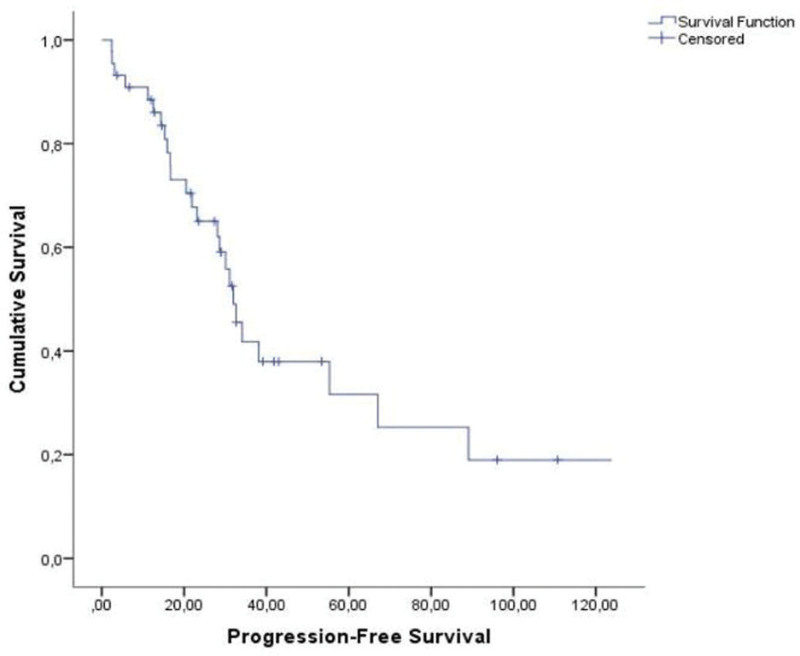
The mean time interval between the diagnosis of KS and the start of paclitaxel therapy was 2.7 years. The median follow-up duration from paclitaxel therapy was 39.1 (range, 3.7–173.5) months. The response to therapy of the 44 evaluable patients is summarized in Table 2. CR and PR were observed in 7 (15.9%) and 28 (63.7%) patients, respectively, giving an OR rate of 79.6%. SD was noted in 6 patients (13.6%), and PD in 3 patients (6.8%). DCR was observed in 93.1%. The median overall survival was 110.2 months (range, 49.9–170.5 months) (Fig. 1). The median progression free survival from the start of therapy was 35.1 months (range, 2–144 months) (Fig. 2). The median duration of response was 18.2 months.
Table 2.
Response to treatment.
| Variables | n/% |
|---|---|
| Best response to therapy (n/%) | |
| Complete response n (%) | 7/15.9 |
| Partial response n (%) | 28/63.7 |
| Stable disease n (%) | 6/15.9 |
| Progressive disease n (%) | 3/6.8 |
| Disease control rate n (%) | 41/93.1 |
| Objective response rate n (%) | 34/79.6 |
| Median overall survival (mo) | 110.2 (49.9–170.5 mo) |
| Median progression-free survival (mo) | 31.9 (27.2–36.6 mo) |
| Median duration of response (mo) | 18.2 |
Figure 1.
Median overall survival was 110.2 ± 30.7 months (range; 49.9–170.5).
Figure 2.
Median progression-free survival was 31.9 ± 2.3 months (range; 27.2–36.6).
Patients generally tolerated the treatment well. Major toxicities are shown in Table 3. Patients received an average of 13.9 cycles (range, 3–26) of paclitaxel treatment. The most common hematological side effects were grade 1 to 2 neutropenia (43.1%) and anemia (31.8%), while the most common non-hematological grade 1 to 2 side effects were fatigue (68.1%), hair loss (54.5%), and peripheral neuropathy (45.4). A total of 4 patients (9.1%) had grade 3 to 4 neutropenia, but it was not complicated by febrile neutropenia. Three patients (6.8%) had grade 3 to 4 peripheral neuropathy. No patient had grade 3 to 4 allergic reaction. There was no drug-related death.
Table 3.
Major toxicities of therapy.
| Grade 1–2 (n/%) | Grade 3–4 (n/%) | |
|---|---|---|
| Anemia | 14 (31.8) | 1 (4.4) |
| Neutropenia | 19 (43.1) | 4 (9.1) |
| Febrile neutropenia | - | - |
| Thrombocytopenia | 4 (9.1) | - |
| Fatigue | 30 (68.1) | 4 (9.1) |
| Alopecia | 24 (54.5) | - |
| Constipation | 17 (38.6) | - |
| Vomiting/nausea | 12 (27.2) | - |
| Diarrhea | 4 (9.1) | - |
| Peripheral neuropathy | 20 (45.4) | 3 (6.8) |
6. Discussion
In this real-world study, we investigated the efficacy and safety of weekly paclitaxel of first-line treatment of CKS. According to our results, the ORR and DCR were 79.6% and 93.1%, respectively. The median duration of response was 18.2 months.
CKS usually occurs in older age and has a slow course. Although its incidence varies geographically, it is increased in the Mediterranean population.[5,8] Systemic treatment is often not required in the treatment of this chronic neoplasm, which rarely causes mortality.[7,8] Although there is no clear accepted approach on when to start systemic therapy, the general approach is during symptomatic visceral or lymph node involvement, development of diffuse symptomatic lesions on multiple body parts (not suitable for local treatments modality), or diffuse nodular disease of a large part of an extremity or widespread involvement[7,8,11]
The main objectives in the treatment of CKS is not only to prolong survival and control of the disease but also to increase the quality of life of patients by improving complications, such as tumor-related edema-pain-ulceration-bleeding, functional deterioration, and lymphedema[7,8,11]
Paclitaxel is an antimicrotubule agent inhibiting the anti-apoptotic effect of bcl-2, a well-known proto-oncogene that is highly expressed in KS.[21] Data on the use of paclitaxel in CKS were obtained from several retrospective studies in CKS treatment and experience in the treatment of HIV-associated KS.[12–18]
In the study of Brambilla et al[13] in 2008 with 17 patients, weekly 100-mg paclitaxel treatment was applied in the first series treatment of CKS, and the ORR was 14/17 (82.3%). The mean time to recurrence after treatment was 4.5 months.
In the study of Denis et al[12] conducted with 10 patients in 2016, 80 to 100 mg weekly paclitaxel treatment was administered, and PR was obtained in 9 patients and stable response in 1 patient. During the follow-up period, 5 of 10 patients had progression, and the median time of progression was 14 months.
In a recently published retrospective study with a large sample size conducted by Tourlaki et al,[14] 58 patients were examined, and 11 of these patients received the first series of 100 mg/week paclitaxel and 47 of them received a second series of paclitaxel. In the whole population, an average of 13.5 infusions was administered, and the ORR was found to be 94.6%. CR was obtained in 7 of 11 patients who received the first series of paclitaxel, and PR was obtained in 4 (ORR, 100%).
In our study, the ORR was 79.6%, similar to those in the literature[12–15] In our study, the median follow-up period from the beginning of paclitaxel treatment was 39.1 months, and the patients received an average of 13.9 cycles. The median duration of response was 18.2 months. These results were similar to those of previous retrospective studies.[12–15]
There is no consensus on which agent should be selected first in the first-line systemic treatment of CKS. The relatively low incidence of the disease, the fact that it usually occurs in old age, and the abundance of comorbidities limit prospective studies. There are differences in the choice of treatment from center to center, and besides institutional experience, experience in the treatment of HIV-related KS is used.[7,8,10] Accordingly, the more accepted first-line treatment worldwide is Lip-D.[7,8,10] However, unlike HIV-related KS, CKS is seen in the elderly population and patients with several comorbidities with low cardiac reserve, and this is an important limiting factor for cardiac safety in the long-term use of Lip-D.[7,8,13,14]
In our study, twenty patients (45.4%) received second line treatment, and 11 patients (25%) received third- or higher line treatment. Paclitaxel rechallenge treatment was used in 5 patients in second-line setting. Oral etoposide, Lip-D, vinorelbine, and capecitabine treatments were used in the second and other lines of therapy. There is no standard approach in the selection of treatment in patients who have progressed after first-line Paclitaxel therapy. In addition to the patient performance status and comorbidities, institutional experience may be decision in the treatment choose.
In our study, paclitaxel treatment was well tolerated. The most common side effects of grades 3 to 4 were neutropenia, fatigue, and peripheral neuropathy. There was no febrile neutropenia, grade 3 to 4 allergic reaction, or drug-related death, these results were consistent with those of previous retrospective studies.[12–15]
This study has several limitations. this study was retrospective and conducted in a heterogenous patient group unlike randomized trials with strict inclusion criteria. But these findings may provide a realistic picture of what is observed in daily clinical practice.
In conclusion, based on the study findings, we recommend paclitaxel as an effective therapy option with an acceptable safety profile for patients with advanced CKS.
Acknowledgments
We would like to acknowledge MD, Simay OKAY from Istanbul University, Faculty of Medicine for their profreading provided for this manuscript.
Author contributions
Conceptualization: Nail Paksoy, Ni̇jat Khanmammadov, İzzet Doğan, Ferhat Ferhatoğlu, Sule Karaman.
Data curation: Nail Paksoy, Ni̇jat Khanmammadov, İzzet Doğan, Ferhat Ferhatoğlu, Melin Aydan Ahmed.
Formal analysis: Nail Paksoy, Sule Karaman, Adnan Aydiner.
Funding acquisition: Nail Paksoy.
Investigation: Nail Paksoy, İzzet Doğan, Ferhat Ferhatoğlu, Melin Aydan Ahmed, Adnan Aydiner.
Methodology: Nail Paksoy, Ni̇jat Khanmammadov, İzzet Doğan, Ferhat Ferhatoğlu, Sule Karaman.
Project administration: Nail Paksoy, Adnan Aydiner.
Resources: Nail Paksoy, Melin Aydan Ahmed.
Software: Nail Paksoy, Ferhat Ferhatoğlu.
Supervision: Nail Paksoy, Sule Karaman, Adnan Aydiner.
Validation: Nail Paksoy, Ni̇jat Khanmammadov, İzzet Doğan, Ferhat Ferhatoğlu, Melin Aydan Ahmed, Sule Karaman, Adnan Aydiner.
Visualization: Nail Paksoy, Sule Karaman, Adnan Aydiner.
Writing – original draft: Nail Paksoy, Adnan Aydiner.
Writing – review & editing: Nail Paksoy, Sule Karaman, Adnan Aydiner.
Abbreviations:
- CKS
- classic Kaposi sarcoma
- CR
- complete response
- DCR
- disease control rate
- ECOG
- eastern cooperative oncology group
- KS
- Kaposi sarcoma
- Lip-D
- pegylated liposomal doxorubicin
- ORR
- objective control rate
- PD
- progressive disease
- PR
- partial response
- SD
- stable disease
Retrospective analyses of clinical data were approved by the Academic Committee of Istanbul University (file no: 2021/2175). The committee had agreed to the retrospective analysis of routinely collected clinical data without the prior informed consent of patients.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Paksoy N, Khanmammadov N, Doğan <, Ferhatoğlu F, Ahmed MA, Karaman S, Aydiner A. Weekly paclitaxel treatment in the first-line therapy of classic Kaposi sarcoma: A real-life study. Medicine 2023;102:5(e32866).
Contributor Information
Nijat Khanmammadov, Email: nicatxanmemmedli@gmail.com.
Ferhat Ferhatoğlu, Email: drferhatoglu@gmail.com.
Melin Aydan Ahmed, Email: drmelinahmed@yahoo.com.
Sule Karaman, Email: karamansule@yahoo.com.
Adnan Aydiner, Email: aydineradnan@gmail.com.
References
- [1].Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-sssociated Kaposi sarcoma. Science. 1994;266:1865–9. [DOI] [PubMed] [Google Scholar]
- [2].Kaposi M. Idiopathisches multiples pigmentsarkom der haut. Archiv für Dermatologie und Syphilis. 1872;4:265–73. [Google Scholar]
- [3].Friedman-Birnbaum R, Bergman R, Bitterman-Deutsch O, et al. Classic and iatrogenic Kaposi sarcoma. Histopathological patterns as related to clinical course. Am J Dermatopathol. 1993;15:523–7. [PubMed] [Google Scholar]
- [4].Schwartz RA. Kaposi sarcoma: an update. J Surg Oncol. 2004;87:146–51. [DOI] [PubMed] [Google Scholar]
- [5].Iscovich J, Boffetta P, Franceschi S, et al. Classic Kaposi sarcoma: epidemiology and risk factors. Cancer. 2000;88:500–17. [PubMed] [Google Scholar]
- [6].Goedert JJ, Vitale F, Lauria C, et al. Risk factors for classical Kaposi sarcoma. J Natl Cancer Inst. 2002;94:1712–8. [DOI] [PubMed] [Google Scholar]
- [7].Régnier-Rosencher E, Guillot B, Dupin N. Treatments for classic Kaposi sarcoma: a systematic review of the literature. J Am Acad Dermatol. 2013;68:313–31. [DOI] [PubMed] [Google Scholar]
- [8].Lebbe C, Garbe C, Stratigos AJ, et al. Diagnosis and treatment of Kaposi sarcoma: European consensus-based interdisciplinary guideline (EDF/EADO/EORTC). Eur J Cancer. 2019;114:117–27. [DOI] [PubMed] [Google Scholar]
- [9].Krigel RL, Laubenstein LJ, Muggia FM. Kaposi sarcoma: A new staging Classification1, 2. Cancer Treatment Rep. 1983;67:531–4. [PubMed] [Google Scholar]
- [10].Brambilla L, Boneschi V, Taglioni M, et al. Staging of classic Kaposi sarcoma: a useful tool for therapeutic choices. Eur J Dermatol. 2003;13:83–6. [PubMed] [Google Scholar]
- [11].Valantin MA, Royston L, Hentzien M, et al. Therapeutic perspectives in the systemic treatment of Kaposi sarcoma. Cancers. 2022;14:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Denis D, Régnier-Rosencher E, Kramkimel N, et al. First-line treatment with paclitaxel for non-HIV-related Kaposi sarcoma: experience in 10 cases. Br J Dermatol. 2016;174:905–8. [DOI] [PubMed] [Google Scholar]
- [13].Brambilla L, Romanelli A, Bellinvia M, et al. Weekly paclitaxel for advanced aggressive classic Kaposi sarcoma: experience in 17 cases. Br J Dermatol. 2008;158:1339–44. [DOI] [PubMed] [Google Scholar]
- [14].Tourlaki A, Germiniasi F, Rossi LC, et al. Paclitaxel as first- or second-line treatment for HIV-negative Kaposi sarcoma: a retrospective study of 58 patients. J Dermatol Treatment. 2020;31:183–5. [DOI] [PubMed] [Google Scholar]
- [15].Fardet L, Stoebner PE, Bachelez H, et al. Treatment with taxanes of refractory or life-threatening Kaposi sarcoma not associated with human immunodeficiency virus infection. Cancer. 2006;106:1785–9. [DOI] [PubMed] [Google Scholar]
- [16].Cianfrocca M, Lee S, Von Roenn J, et al. Randomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcoma: evidence of symptom palliation from chemotherapy. Cancer. 2010;116:3969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Welles L, Saville MW, Lietzau J, et al. Phase II trial with dose titration of paclitaxel for the therapy of human immunodeficiency virus-associated Kaposi sarcoma. J Clin Oncol. 1998;16:1112–21. [DOI] [PubMed] [Google Scholar]
- [18].Tulpule A, Groopman J, Saville MW, et al. Multicenter trial of low-dose paclitaxel in patients with advanced AIDS-related Kaposi sarcoma. Cancer. 2002;95:147–54. [DOI] [PubMed] [Google Scholar]
- [19].Krown SE, Metroka C, Wernz JC. Kaposi sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group oncology committee. J Clin Oncol. 1989;7:1201–7. [DOI] [PubMed] [Google Scholar]
- [20].Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors (RECIST guidelines). J Natl Cancer Inst. 2000;92:205–16. [DOI] [PubMed] [Google Scholar]
- [21].Sgadari C, Toschi E, Palladino C, et al. Mechanism of paclitaxel activity in Kaposi sarcoma. J Immunol. 2000;165:509–17. [DOI] [PubMed] [Google Scholar]