Abstract
To probe the diagnostic value of transvaginal color Doppler ultrasonography plus serum β-human chorionic gonadotropin (β-HCG) dynamic monitoring in intrauterine residue after medical abortion.In total, 200 pregnant women undergoing medical abortion in our institution from January 2017 to December 2019 were picked, and assigned to either group A (n = 75, with residue) or group B (n = 125, without residue). We detected serum β-HCG, progesterone (P), follicle stimulating estrogen (FSH) levels and ultrasonic indicators endometrial thickness (ET), peak systolic velocity (PSV), resistance index (RI) values, dissected correlation of indicators using logistic linear regression analysis, and prospected the diagnostic value of relevant indicators in intrauterine residue after medical abortion utilizingreceiver operating characteristic curve.At 7 days after abortion (T3), total vaginal bleeding and visual analogue scalescore in group A were saliently higher in contrast to group B (P < .05). At 72 hours after abortion (T2) and T3, serum β-HCG, P and FSH levels declined strikingly in both groups, but group B held plainly higher decrease rate than group A (P HC.05). At T3, ET and PSV levels in both groups considerably waned, whereas RI levels notedly waxed, and group B owned markedly higher decrease/increase than group A (P wa.05). At T3, serum β-HCG in group A possessed positive association with serum P, FSH, intrauterine ET, PSV levels separately (P HC.05), whereas negative link with RI levels (P , .05). The specificity and sensitivity of β-HCG, P, FSH, β-HCG/ET, β-HCG/PSV and β-HCG/RI in the diagnosis of intrauterine residue after medical abortion were high (P < .05).Serum β-HCG dynamic monitoring plus transvaginal color Doppler ultrasonography is of great value in diagnosing intrauterine residue after medical abortion. Serum β-HCG, P, FSH levels can be combined with the results of intrauterine ET, PSV, RI values, so as to boost the diagnostic accuracy of the intrauterine residue after medical abortion.
Keywords: intrauterine residue, medical abortion, serum β-HCG, transvaginal color Doppler ultrasonography
1. Introduction
Artificial abortion is principally classified into medical abortion as well as surgical abortion. Of those, medical abortion refers to the use of oral mifepristone tablets and misoprostol drugs, aiming at achieving early termination of pregnancy.[1,2] Medical abortion is primarily suitable for pregnant women aged <40 years who have stopped menopause within 7 weeks of intrauterine pregnancy. Compared with surgical termination, medical abortion appears to be associated with a lower risk of inflammatory pelvic disease, possibly due to the avoidance of intrauterine fixation. Also, it is suitable for pregnant women who cannot be operated or have undergone multiple artificial abortions, has the advantages of no need for surgery, less pain, better pregnancy termination and has been broadly applied in the treatment of early termination of pregnancy.[3,4] Nonetheless, after undergoing medical abortion, pregnant women are likely to have intrauterine residue. The failure rate of medical abortion using mifepristone/vaginal prostaglandin analogues is 2% to 6%. Incomplete medical abortion can increase the risk of infection and is also associated with persistent or recurrent discomfort such as bleeding and pain.[5] If they are not removed in time, it may influence menstrual flow and the intrauterine environment, and even induce heavy bleeding, imperiling patient’s life.[6,7] Thus, diagnosing whether there is intrauterine residue after medical abortion early is exceedingly pivotal. Transvaginal color Doppler ultrasonography is currently the cardinal approach for detecting and diagnosing whether there is intrauterine residue after medical abortion, which is leadingly determined by observing whether there are abnormal echoes and lesions in the uterus.[8,9] However, some clinical studies have illuminated that this detection method is greatly impacted by the operator’s subjective judgment, requires higher operator capabilities, and is easy to ignore residue with smaller diameters, which influences the diagnosis.[10] Currently, clinical serum indicators to diagnose whether there is intrauterine residue after medical abortion are lacking. β-human chorionic gonadotropin(β-HCG), whose expression is usually dramatically minified after abortion, is a prevalently utilized clinical indicator for early pregnancy diagnosis.[11] Hereby, this study performed transvaginal color Doppler ultrasonography and serum β-HCG monitoring on 75 pregnant women with intrauterine residue after medical abortion and 125 pregnant women without intrauterine residue in our institution, aiming at delving into the diagnostic value of the combination of the 2.
2. Methods
2.1. Patient clinical data
200 cases of pregnant women receiving medical abortion in our hospital from January 2017 to December 2019 were selected as the research objects. According to whether there was intrauterine residue after abortion diagnosed by sonohysterography, they were allocated to either group A (n = 75, with residue) or group B (n = 125, without residue). Medical Ethics Committee had authorized this research, and all pregnant women had submitted consent form.
2.2. Inclusion and exclusion criteria
2.2.1. Inclusion criteria.
All pregnant women underwent medical abortion in our hospital and the process went smoothly; Undergoing clinical diagnosis, patients in group A had intrauterine residue after medical abortion, while group B had no residue[12]; Patients without history of allergies or other adverse reactions to the instrument and equipment used in this study; Pregnant women aged ≥ 18 years; Pregnant women have good nutritional level and physical health, and have no anemia, malnutrition or other underlying diseases.
2.2.2. Exclusion criteria.
Pregnant women with congenital immune deficiency or severe infectious diseases; Patients with severe heart, liver, or kidney dysfunction; Patients with mental illness or poor mental health; Patients with incomplete clinical data; Patients with poor research cooperation or non-cooperation.
2.3. Research methods
Relevant tests were performed on the 2 groups of patients 1 day before abortion (T1), 72 hours after abortion (T2), and 7 days after abortion (T3).
Serum index detection: at T1, T2, and T3, we collected 6mL of fasting venous blood from 2 groups, and left to settle lasting 30 minutes. After the whole blood had spontaneously coagulated and the serum was precipitated, centrifugation was completed at about 1000g to 2000g lasting 10 minutes at 4°C to obtain supernatant, namely serum. The serum β-HCG, progesterone (P), and follicle stimulating estrogen (FSH) levels were tested using automatic immunoassay analyzer (ORGENTEC, Germany, ORG 300).
Transvaginal color Doppler ultrasonography: during T1 and T3, we measured the endometrial thickness (ET), peak systolic velocity (PSV) and resistance index (RI) adopting GE color Doppler ultrasound instrument (General Electric Company). The probe frequency was 3.0 to 5.0 MHz, and the operation was strictly in compliance with the instrument manual.
2.4. Observation indicators
Vaginal bleeding and abdominal pain after abortion: we observed and recorded days of vaginal bleeding and total vaginal bleeding after abortion, and used visual analogue scale for comparing the abdomen pain degree of both groups at T3. The score was 0 to 10 points, and the higher the score, the more obvious the pain.
Serum index levels: the serum β-HCG, P and FSH levels of both groups at T1, T2 and T3.
Ultrasonic examination: ET, PSV and RI values of both groups at T1 and T3.
We examined the association between serum β-HCG and P, FSH, ET, PSV and RI of pregnant women in group A at T3.
We observed the sensitivity and specificity of β-HCG, P, FSH, ET, PSV, and RI in diagnosing the intrauterine residue after medical abortion.
2.5. Statistical analysis
Exploiting SPSS 25.0 software (IBM SPSS Inc., Chicago), we implemented statistical analysis, in which the measurement data was exhibited as mean ± SD (x ± s). Two independent samples nonparametric test was fulfilled for inter-group analysis. Count data were manifested as rate (%). We employed chi-square test for inter-group comparison, logistic linear regression for dissecting the correlation of relevant indicators, and receiver operating characteristic (ROC) curve for drawing the diagnostic value of relevant indicators in intrauterine residue after medical abortion. *P < .05. We applied GradpadPrism7.0 software (Graphpad Software, Inc., Chicago) for mapping.
3. Results
3.1. General information
In all, 200 pregnant women were embraced in the investigation, and divided into 2 groups according to whether there was intrauterine residue after medical abortion. No statistical significance was unveiled in pregnant women’s age, BMI, gestational age, primipara and other general data (P an.05), but they were comparable. We followed up the pregnant women for survival, and 1 and 3 lost cases were presented in groups A and B, separately. See Table 1, Figure 1.
Table 1.
Comparison of the general information of both groups.
| Group | A (n = 75) | B (n = 125) | X2/T | P | |
|---|---|---|---|---|---|
| Age (yr) | 26.42 ± 3.59 | 27.35 ± 3.42 | 0.594 | .527 | |
| BMI (kg/m2) | 22.17 ± 1.24 | 22.42 ± 1.14 | 0.625 | .463 | |
| Gestation wk (wk) | 4.59 ± 1.32 | 4.31 ± 1.27 | 0.641 | .432 | |
| Primipara | Yes | 49 | 81 | 0.539 | .526 |
| No | 26 | 44 | |||
Figure 1.
Research processes.
3.2. Comparison of related clinical symptoms after abortion
At T3, days of vaginal bleeding, total vaginal bleeding and visual analogue scalescore in group A were patently higher than those in group B (P in.05). See Figure 2, Table 2. In contrast to group B, the symptoms of vaginal bleeding and abdominal pain after abortion in group A were more serious.
Figure 2.
Comparison of vaginal bleeding and abdominal pain after medical abortion. (A) Days of vaginal bleeding. (B) Total vaginal bleeding. (C) Visual analogue scale (VAS), **P < .001. VAS = visual analogue scale.
Table 2.
Comparison of vaginal bleeding and abdominal pain after medical abortion ( ± s).
| Group | A (n = 75) | B (n = 125) | T | P |
|---|---|---|---|---|
| Days of vaginal bleeding (d) | 16.95 ± 4.38 | 7.49 ± 2.53 | 6.472 | .001 |
| Total vaginal bleeding volume (mL) | 275.58 ± 24.62 | 95.42 ± 6.18 | 7.384 | .001 |
| Pain score (scores) | 6.48 ± 2.13 | 2.51 ± 0.87 | 6.425 | .001 |
3.3. Comparison of serum β-HCG, P, FSH
During T1, no significant difference was uncloaked in serum β-HCG, P, FSH levels between the 2 groups of pregnant women (P ro.05). At T2 and T3, the serum β-HCG, P, and FSH levels of both groups diminished brilliantly, and group B harbored blatantly higher decrease rate than group A (P bl.05). See Figure 3, Table 3.
Figure 3.
Comparison of serum β-HCG, P and FSH levels. (A) Serum β-human chorionic gonadotropin (β-HCG). (B) Serum progesterone (P), (C) Serum follicle stimulating estrogen (FSH) levels, 1 day before abortion (T1), 72 hours after abortion (T2) and 7 days after abortion (T3), **P < .001. FSH = follicle stimulating estrogen, P = progesterone, β-HCG = β-human chorionic gonadotropin.
Table 3.
Comparison of serum β-HCG, P and FSH levels ( ± s).
| Group | A (n = 75) | B (n = 125) | T | P | |
|---|---|---|---|---|---|
| β-HCG (mIU/mL) | T1 | 2526.13 ± 437.16 | 2524.73 ± 436.29 | 0.574 | .528 |
| T2 | 1947.45 ± 352.29* | 1352.36 ± 261.46* | 6.731 | .001 | |
| T3 | 1372.52 ± 273.49* | 372.69 ± 138.47* | 7.895 | .001 | |
| P (ug/L) | T1 | 45.19 ± 8.37 | 46.52 ± 8.41 | 0.653 | .429 |
| T2 | 38.34 ± 6.53* | 27.61 ± 4.72* | 6.425 | .001 | |
| T3 | 29.15 ± 4.73* | 13.54 ± 2.84* | 7.931 | .001 | |
| FSH (mIU/mL) | T1 | 234.25 ± 42.83 | 236.14 ± 43.52 | 0.642 | .431 |
| T2 | 172.26 ± 23.47* | 98.61 ± 16.95* | 6.539 | .001 | |
| T3 | 138.62 ± 15.58* | 45.32 ± 11.37* | 7.842 | .001 | |
FSH = follicle stimulating estrogen, P = progesterone, β-HCG = β-human chorionic gonadotropin.
* P < .05.
3.4. Comparison of ultrasonic indicators
During T1, no significant difference was unveiled in ET, PSV, and RI levels between both groups (P ur.05). At T3, ET and PSV levels compellingly waned, whereas RI levels tellingly waxed, and group B held higher decrease/increase rate than group A (P . .05). See Table 4, Figure 4.
Table 4.
Comparison of ET, PSV and RI levels of both groups ( ± s).
| Group | A (n = 75) | B (n = 125) | T | P | |
|---|---|---|---|---|---|
| ET (mm) | T1 | 13.48 ± 1.84 | 14.25 ± 1.79 | 0.642 | .435 |
| T3 | 9.53 ± 1.07* | 5.82 ± 0.76* | 6.248 | .001 | |
| PSV (cm/s) | T1 | 21.25 ± 6.84 | 20.93 ± 7.04 | 0.569 | .514 |
| T3 | 15.73 ± 4.52* | 10.58 ± 3.24* | 6.372 | .001 | |
| RI | T1 | 0.42 ± 0.07 | 0.43 ± 0.06 | 0.548 | .523 |
| T3 | 0.52 ± 0.08* | 0.64 ± 0.09* | 6.024 | .001 | |
ET = endometrial thickness, PSV = peak systolic velocity, RI = resistance index.
* P < .05.
Figure 4.
Comparison of serum ET, PSV, and RI levels. (A) Endometrial thickness (ET) during T1, (B) ET during T3, (C) peak systolic velocity (PSV) during T1, (D) PSV during T3, (E) resistance index (RI) during T1, (F) RI during T3, 1 day before abortion (T1), and 7 days after abortion (T3). **P < .001.ET = endometrial thickness, PSV = peak systolic velocity, RI = resistance index.
3.5. Correlation of serum β-HCG with serum P, FSH, intrauterine ET, PSV and RI
During T3, in group A, serum β-HCG owned positive relation with ET (R = 0.573, P .5.05); serum β-HCG possessed positive link with PSV (R = 0.682, P .6.05); serum β-HCG held negative association with RI (r = −0.637, P .6.05); serum β-HCG possessed positive relationship with serum P (R = 0.594, P .5.05); serum β-HCG had positive correlation with serum FSH (R = 0.615, P .6.05). See Figure 5.
Figure 5.
Correlation of serum β-HCG with P, FSH, ET, PSV, and RI levels. (A) Logistic regression analysis evinced that serum β-HCG and ET harbored positive association, R = 0.573, P < .05. (B) Serum β-HCG held positive link with PSV, R = 0.682, P < .05. (C) Serum β-HCG possessed negative relation with RI, r = −0.637, P < .05. (D) Serum β-HCG owned positive relationship with serum P, R = 0.594, P < .05. (E) Serum β-HCG had positive correlation with serum FSH, R = 0.615, P < .05. ET = endometrial thickness, FSH = follicle stimulating estrogen,P = progesterone, PSV = peak systolic velocity, RI = resistance index, β-HCG = β-human chorionic gonadotropin.
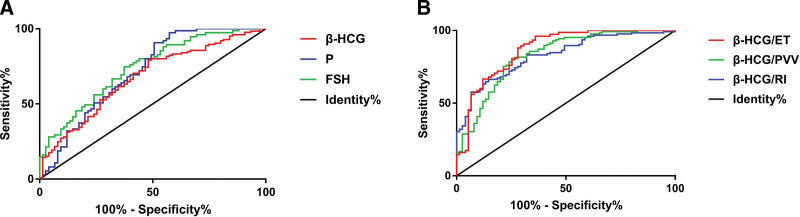
3.6. ROC curve analysis of associated indicators in diagnosing the intrauterine residue after medical abortion
β-HCG, P, FSH, β-HCG/ET, β-HCG/PSV, β-HCG/RI all had high specificity and sensitivity in diagnosing intrauterine residue after medical abortion (P H .05). See Table 5, Figure 6. The specificity and sensitivity of serum β-HCG were 64.26% and 77.59%; serum P were 65.38%, 79.41%; serum FSH were 67.82%, 80.15%; β-HCG/ET were 73.18%, 86.33%; β-HCG/PSV were 71.49%, 81.52%; β-HCG/RI were 69.52%, 80.47%.
Table 5.
ROC curve analysis of related indicators in diagnosing the intrauterine residues after medical abortion.
| Indicator | AUC | 95% CI | Specificity | Sensitivity | P |
|---|---|---|---|---|---|
| β-HCG | 0.62 | 0.545–0.732 | 64.26% | 77.59% | .01 |
| P | 0.64 | 0.551–0.750 | 65.38% | 79.41% | .01 |
| FSH | 0.66 | 0.559–0.764 | 67.82% | 80.15% | .01 |
| β-HCG/ET | 0.78 | 0.663–0.821 | 73.18% | 86.33% | .01 |
| β-HCG/PSV | 0.72 | 0.641–0.786 | 71.49% | 81.52% | .01 |
| β-HCG/RI | 0.71 | 0.634–0.782 | 69.52% | 80.47% | .01 |
AUC, area under the curve, CI = confidence interval, ET = endometrial thickness, FSH = follicle stimulating estrogen, P = progesterone, PSV = peak systolic velocity, RI = resistance index, ROC = receiver operating characteristic, β-HCG = β-human chorionic gonadotropin.
Figure 6.
ROC curve of β-HCG, P, FSH, β-HCG/ET, β-HCG/PSV, β-HCG/RI in the intrauterine residue after medical abortion. (A) ROC curve of β-HCG, P and FSH in the intrauterine residue after medical abortion. (B) ROC curve of β-HCG/ET, β-HCG/PSV and β-HCG/RI in the intrauterine residue after medical abortion. ET = endometrial thickness, FSH = follicle stimulating estrogen, P = progesterone, PSV = peak systolic velocity, RI = resistance index,ROC = receiver operating characteristic, β-HCG = β-human chorionic gonadotropin.
4. Discussion
In recent years, with the change of people’s concept of fertility, the number of pregnant women who choose abortion clinically has been mounting.[13] Medical abortion, with high safety and relatively stable abortion effect as features, has been extensively employed in the abortion treatment of early pregnancy pregnant women.[14] Nevertheless, medical abortion has certain limitations for some pregnant women, and it is prone to incomplete abortion and intrauterine residue. Intrauterine residue may lead to increased vaginal bleeding, leading to more severe abdominal pain in pregnant women, and may even cause heavy bleeding, requiring timely uterine evacuation.[15,16] The early diagnosis of intrauterine residue after medical abortion has always been a hot topic in clinical research.[17] Currently, the clinical diagnosis prevailingly uses transvaginal color Doppler ultrasonography to detect abnormal echo and whether there are lesions, so as to determine whether there is intrauterine residue of pregnant women after medical abortion.[18]However, the use of ultrasound alone to detect intrauterine residues after medical abortion may lead to a higher rate of missed detection, especially for small residues. In this work, we focused on looking into the ET and hemodynamics of the pregnant women.
Human chorionic gonadotropin (HCG), dominantly including α-HCG and β-HCG, is a glycoprotein secreted by the trophoblast cells of the placenta. It has the functions of protecting the corpus luteum, controlling the activity level of lymphocytes in pregnant women and preventing fetus rejection and other effects.[19–21] HCG level in pregnant women’s serum is dominatingly assessed by detecting their serum β-HCG level. Serum β-HCG level can be observably elevated in the early pregnancy. After 40 days of pregnancy, its level can reach 2000mIU/ml, and then gradually diminish. After miscarriage, serum β-HCG level will rapidly decline.[22,23] Serum P and FSH are the staple estrogen and progesterone indicators in pregnant women. These hormones can modulate genes and metabolism levels through neurotransmitters in the brain, foster the sensitivity and activity of the central nervous system, and ultimately promote smoothness of pregnancy, so the serum P and FSH levels in pregnant women are usually highly expressed.[24–27] According to pertinent investigation, after medical abortion, pregnant woman’ body has undergone major changes physiologically, the central nervous system, gene and metabolic levels have decreased sensibly, and the serum estrogen and progesterone levels have also minified accordingly.[28]Honkanenet al found that the decline in β-HCG after a medically induced abortion is inversely correlated with the time taken to abort, Which means the absolute and the relative values of β-HCG as well as the ET after medical abortion were higher in women who turned out to be late failures than in successfully treated women.[29]Additionally, Cleeve A et alhave corroborated that the decrease range of serum estrogen and progesterone levels of some pregnant women with intrauterine residue after medical abortion is notably lower than that of pregnant women with complete abortion.[30] In this study, group A harbored noticeably lower decrease range of serum β-HCG, P and FSH levels after abortion than group B. This was predominantly due to the presence of residue sending wrong signals to the body, causing the ovaries to still secrete more estrogen and progesterone to maintain the central nervous system function and gene and metabolic levels of pregnant women.
Yang W et al[18] have authenticated that the ET of pregnancy women preponderatingly relies on progesterone, the higher the expression of progesterone, the greater ET, and the 2 are positively correlated, which is consistent with our findings. The increase in estrogen and progesterone level of pregnant women will advance the growth and development of the corpus luteum, meanwhile, the intrauterine neovascularization will gradually increase, the perfusion capacity of the corpus luteum will intensify, the blood flow resistance will diminish subsequently and the PSV will elevate accordingly.[31,32] Here, we found that intrauterine residue group owned prominently higher estrogen and progesterone levels, eminently higher ET and PSV levels and lower RI levels than residue-free group. Moreover, serum β-HCG possessed positive relation with ET and PSV, whereas negative relationship with RI.Rùrbye et al have found the prognostic value of β-HCG combined with ultrasound as a predictor of late failure at a 2-week follow-up of medical abortion.[5] This further supports the conclusions of this study.
Also, this study dug into the diagnostic value of serum β-HCG monitoring plus transvaginal color Doppler ultrasonography in the intrauterine residue after medical abortion through ROC curve analysis. As a result, serum β-HCG, P, and FSH levels all harbored high specificity and sensitivity in diagnosing intrauterine residue after medical abortion, especially when serum β-HCG levels were combined with intrauterine ET, PSV or RI values, its sensitivity and specificity could be further improved. Thus, serum β-HCG level and other indicators combined with intrauterine ET, PSV and RI detection, plus the examination results of abnormal echoes and lesions in the vagina after abortion can be used for the clinical diagnosis of intrauterine residue after medical abortion, so as to improve the accuracy and timeliness of the diagnosis and facilitate the development of follow-up treatment.
To sum up, serum β-HCG dynamic monitoring plus transvaginal color Doppler ultrasonography harbors a high application value in diagnosing intrauterine residue after medical abortion. Clinically, serum β-HCG, P and FSH levels can be combined with intrauterine ET, PSV and RI results to improve the diagnostic accuracy of intrauterine residue after medical abortion. There are still some deficiencies in this research. For instance, there are certain differences in the size of intrauterine residue after medical abortion, and the impact of different lesion sizes on pregnant women may be varying. However, this study did not classify them. Hence, the lesions can be classified according to their diameters in the future study in order to offer a more reliable diagnosis basis for the clinic.
Author contributions
Conceptualization: Yanbo Liu.
Data curation: Yanbo Liu, Wen Lv.
Formal analysis: Yanbo Liu, Wen Lv.
Investigation: Yanbo Liu, Wen Lv.
Methodology: Yanbo Liu, Wen Lv.
Project administration: Yanbo Liu, Wen Lv.
Resources: Yanbo Liu, Wen Lv.
Software: Yanbo Liu, Wen Lv.
Supervision: Yanbo Liu, Wen Lv.
Validation: Yanbo Liu, Wen Lv.
Visualization: Yanbo Liu, Wen Lv.
Writing – original draft: Yanbo Liu, Wen Lv.
Writing – review & editing: Yanbo Liu, Wen Lv.
Abbreviations:
- ET
- endometrial thickness
- FSH
- follicle stimulating estrogen
- P
- progesterone
- PSV
- peak systolic velocity
- RI
- resistance index
- ROC
- receiver operating characteristic
- β-HCG
- β-human chorionic gonadotropin
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This work was supported by the Zhejiang Medical and Health Science and Technology Program Project (NO.2020367726).
Written informed consent was obtained from all participants and the present study was approved by Litongde Hospital of Zhejiang Province (Hangzhou, China).
The authors have no conflicts of interest to disclose.
How to cite this article: Liu Y, Lv W. The diagnostic value of transvaginal color Doppler ultrasonography plus serum β-HCG dynamic monitoring in intrauterine residue after medical abortion. Medicine 2023;102:5(e31217).
References
- [1].Solheim IH, Kahabuka C, Pembe A, et al. Beyond the law: misoprostol and medical abortion in dares salaam, Tanzania. Soc Ence Med 2020;245:112676. [DOI] [PubMed] [Google Scholar]
- [2].Reeves MF, Monmaney JA, Creinin MD. Predictors of uterine evacuation following early medical abortion with mifepristone and misoprostol. Contraception.2016;93:119–25. [DOI] [PubMed] [Google Scholar]
- [3].Finch RE, Mcgeechan K, Johnstone A, et al. Impact of self-administration of misoprostol for early medical abortion: a prospective observational cohort study. BMJ Sex Rep 2019;43:200278. [DOI] [PubMed] [Google Scholar]
- [4].Xie K, Zhang W, Fang W, et al. The analgesic efficacy of oxycodone hydrochloride versus fentanyl during outpatient artificial abortion operation. Medicine (Baltimore).2017;96:e7376e7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rùrbye C, Nùrgaard M, Nilas L. Prediction of late failure after medical abortion from serial β-HCG measurements and ultrasonography. Human Reproduction, 2004;19:85–9. [DOI] [PubMed] [Google Scholar]
- [6].Patil E, Edelman A. Medical abortion: use of mifepristone and misoprostol in first and second trimesters of pregnancy. CurrObstet Gyn.2015;4:69–78. [Google Scholar]
- [7].Gatter M, Cleland K, Nucatola DL. Efficacy and safety of medical abortion using mifepristone and buccal misoprostol through 63 days. Contraception.2015;91:269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Grossman D, White K, Harris L, et al. Continuing pregnancy after mifepristone and “reversal” of first-trimester medical abortion: a systematic review. Contraception.2015;73:206–11. [DOI] [PubMed] [Google Scholar]
- [9].Oppegaard KS, Qvigstad E, Fiala C, et al. Clinical follow-up compared with self-assessment of outcome after medical abortion: a multicentre, non-inferiority, randomised, controlled trial. Lancet.2015;385:698–704. [DOI] [PubMed] [Google Scholar]
- [10].Iyengar K, Klingberg-Allvin M, Iyengar SD, et al. Home use of misoprostol for early medical abortion in a low resource setting: secondary analysis of a randomized controlled trial. Acta Obstet Gyn Scan.2016;73:15. [DOI] [PubMed] [Google Scholar]
- [11].Raymond EG, Weaver MA, Louie KS, et al. Effects of depot medroxyprogesterone acetate injection timing on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol.2016;128:739–45. [DOI] [PubMed] [Google Scholar]
- [12].Ruixia D, Yulan G, Obstetrics DO. Transvaginal ultrasound with color doppler imaging in the examination of intrauterine residue and hemodynamics of residual tissue blood flow after drug abortion. Medi Reca.2017;52:24. [Google Scholar]
- [13].Conkling K, Karki C, Tuladhar H, et al. A prospective open-label study of home use of mifepristone for medical abortion in Nepal. Int J Gyn Obs.2015;128:220–3. [DOI] [PubMed] [Google Scholar]
- [14].Sonalkar S, Mcclusky J, Hou MY, et al. Administration of depot medroxyprogesterone acetate on the day of mifepristone for medical abortion: a pilot study. Contraception.2015;91:174–7. [DOI] [PubMed] [Google Scholar]
- [15].Platais I, Tsereteli T, Grebennikova G, et al. Prospective study of home use of mifepristone and misoprostol for medical abortion up to 10weeks of pregnancy in Kazakhstan. Int J Gynecol Obstet.2016;73:268–71. [DOI] [PubMed] [Google Scholar]
- [16].Tsereteli T, Chong E, Louie K, et al. Acceptability and feasibility of 400μg buccal misoprostol after 200mg mifepristone for early medical abortion in Georgia. Eur J ContracepRepr.2016;51:1–5. [DOI] [PubMed] [Google Scholar]
- [17].Ruixia D, Yulan G, Obstetrics DO. Transvaginal ultrasound with color doppler imaging in the examination of intrauterine residue and hemodynamics of residual tissue blood flow after drug abortion. Medi Reca.2017;52:31. [Google Scholar]
- [18].Besbaci M, Abdelli A, Minviel JJ, et al. Association of pregnancy per artificial insemination with gonadotropin-releasing hormone and human chorionic gonadotropin administered during the luteal phase after artificial insemination in dairy cows: a meta-analysis. JourdairyEnce.2020;103:2006–18. [DOI] [PubMed] [Google Scholar]
- [19].Yang W, Zhang T, Li Z, et al. Combined analysis of endometrial thickness and pattern in predicting clinical outcomes of frozen embryo transfer cycles with morphological good-quality blastocyst: a retrospective cohort study. Medicine (Baltimore).2018;97:e9577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hafezi M, Madani T, Arabipoor A, et al. The effect of intrauterine human chorionic gonadotropin flushing on live birth rate after vitrified-warmed embryo transfer in programmed cycles: a randomized clinical trial. Arch Gynecol Obstet.2018;53:36. [DOI] [PubMed] [Google Scholar]
- [21].Sauss K, Ehrentraut S, Zenclussen AC, et al. The pregnancy hormone human chorionic gonadotropin differentially regulates plasmacytoid and myeloid blood dendritic cell subsets. Am J Reprod Immunol.2018;73:e12837. [DOI] [PubMed] [Google Scholar]
- [22].Komischke K, Searle K, Enders G. Maternal serum alpha-fetoprotein and human chorionic gonadotropin in pregnant women with acute parvovirus B19 infection with and without fetal complications. Prenatal Diag.2015;17:1039–46. [PubMed] [Google Scholar]
- [23].Wirleitner B, Schuff M, Vanderzwalmen P, et al. Intrauterine administration of human chorionic gonadotropin does not improve pregnancy and life birth rates independently of blastocyst quality: a randomised prospective study. Reprod Biol Endocrin.2015;73:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shimada M, Nishibori M, Yamashita Y. Effects of adding luteinizing hormone to a medium containing follicle stimulating hormone on progesterone-induced differentiation of cumulus cells during meiotic resumption of porcine oocytes. ANIM J.2015;75:515–23. [Google Scholar]
- [25].Dan LI, Juan LI, Mingming Y, et al. Effect of wenjing decoction on serum follicle stimulating hormone, luteinizing hormone,estradiol,progesterone and testosterone of patients with menopathy of excess-cold syndrome. J Clin Med Pra.2017;73:15. [Google Scholar]
- [26].Hwajeong L, Joung CH, Moon YK, et al. Efficacy of luteal estrogen administration and an early follicular Gonadotropin-releasing hormone antagonist priming protocol in poor responders undergoing in vitro fertilization. Obs Gyn.2018;61:102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Takaya Y, Matsubayashi H, Kitaya K, et al. Minimum values for midluteal plasma progesterone and estradiol concentrations in patients who achieved pregnancy with timed intercourse or intrauterine insemination without a human menopausal gonadotropin. BMC Res Note.2018;11:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yah D, Ojo NA, Mshelia GD. Effects of dexamethasone on progesterone and estrogen profiles and uterine progesterone receptor localization during pregnancy in Sahel goat in Semi-Arid region. J Anim Sci Tech.2017;57:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cleeve A, Byamugisha J, Gemzell-Danielsson K, et al. Women’s acceptability of misoprostol treatment for incomplete abortion by midwives and physicians - Secondary outcome analysis from a randomized controlled equivalence trial at district level in Uganda. PLoS One.2016;11:e0149172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Honkanen H, Ranta S, Ylikorkala O, et al. The kinetics of serum hCG and progesterone in response to oral and vaginal administration of misoprostol during medical termination of early pregnancy. Hum Reprod.2002;17:2315–9. [DOI] [PubMed] [Google Scholar]
- [31].Klingberg-Allvin M, Cleeve A, Atuhairwe S, et al. Comparison of treatment of incomplete abortion with misoprostol by physicians and midwives at district level in Uganda: a randomised controlled equivalence trial. Lancet.2018;385:2392–8. [DOI] [PubMed] [Google Scholar]
- [32].Gaur M, Purohit G. Follicular dynamics and color Doppler vascularity evaluations of follicles and corpus luteum in relation to plasma progesterone during the estrous cycle of Surti buffaloes. ReprodDomest Anim.2019;73:14. [DOI] [PubMed] [Google Scholar]
- [32].Abdelnaby EA, Amal M, Abo E. Effect of the side of ovulation on the uterine morphometry, blood flow, progesterone, estradiol, and nitric oxide during spontaneous and induced estrus in lactating dairy cows. ReprodDomest Anim.2020;62:14. [DOI] [PubMed] [Google Scholar]