PURPOSE
To investigate efficacy and safety of trastuzumab deruxtecan (T-DXd) in human epidermal growth factor receptor 2 (HER2)–low gastric or gastroesophageal junction (GEJ) adenocarcinoma.
METHODS
Patients with locally advanced or metastatic HER2-low (cohort 1, immunohistochemistry 2+/in situ hybridization–negative; cohort 2, immunohistochemistry 1+) gastric/GEJ adenocarcinoma treated with at least two prior regimens, including fluoropyrimidine and platinum, but anti-HER2 therapy naive, received T-DXd 6.4 mg/kg intravenously once every 3 weeks. The primary end point was confirmed objective response rate by independent central review.
RESULTS
Among 21 patients enrolled in cohort 1 and 24 enrolled in cohort 2, 19 and 21 patients, respectively, had central HER2 confirmation, received T-DXd, and had measurable tumors at baseline. The confirmed objective response rate was 26.3% (95% CI, 9.1 to 51.2) from five partial responses in cohort 1 and 9.5% (95% CI, 1.2 to 30.4) from two partial responses in cohort 2. Thirteen patients (68.4%) in cohort 1 and 12 (60.0%) in cohort 2 experienced reduced tumor size. The median overall survival was 7.8 months (95% CI, 4.7 to nonevaluable) in cohort 1 and 8.5 months (95% CI, 4.3 to 10.9) in cohort 2; the median progression-free survival was 4.4 months (95% CI, 2.7 to 7.1) and 2.8 months (95% CI, 1.5 to 4.3), respectively. The most common grade ≥ 3 treatment-emergent adverse events in cohorts 1 and 2 were anemia (30.0% and 29.2%), decreased neutrophil count (25.0% and 29.2%), and decreased appetite (20.0% and 20.8%). Drug-related interstitial lung disease/pneumonitis occurred in one patient in each cohort (grade 1 or 2). No drug-related deaths occurred.
CONCLUSION
This study provides preliminary evidence that T-DXd has clinical activity in patients with heavily pretreated HER2-low gastric/GEJ adenocarcinoma.
INTRODUCTION
Human epidermal growth factor receptor 2 (HER2) is a transmembrane tyrosine kinase receptor that regulates signaling pathways in cell cycle progression, cell proliferation, differentiation and survival, and angiogenesis.1 Overexpression or amplification of HER2 occurs in approximately one-fifth of gastric/gastroesophageal junction (GEJ) cancers.2 HER2 expression levels are heterogeneous within and between tumors and can change after anti-HER2 treatment.2-4
CONTEXT
Key Objective
To investigate trastuzumab deruxtecan (T-DXd) in patients with gastric or gastroesophageal junction adenocarcinoma who have low expression of human epidermal growth factor receptor 2.
Knowledge Generated
T-DXd demonstrated clinical activity in patients with heavily pretreated human epidermal growth factor receptor 2–low gastric/gastroesophageal junction adenocarcinoma and safety was consistent with the manageable safety profile of T-DXd.
Relevance (E.M. O'Reilly)
-
The results from this phase II study of trastuzumab deruxtecan in HER2-low esophagogastric malignancies provide support for the observation that this antibody-drug conjugate has potential value in a broader patient population of immunohistochemistry 2+/in situ hybridization-cancers; however, further evaluation is needed.*
*Relevance section written by JCO Associate Editor Eileen M. O’Reilly, MD.
In the ToGA study of the anti-HER2 antibody trastuzumab, overall survival (OS) was longer with trastuzumab plus chemotherapy versus chemotherapy in patients with HER2-positive gastric/GEJ cancer.5 Consequently, recommended first-line therapy for HER2-positive (immunohistochemistry [IHC] 3+ or 2+ and in situ hybridization-positive [ISH+]) gastric/GEJ cancer was trastuzumab plus chemotherapy for over a decade. Pembrolizumab was approved in the United States in 2021 on the basis of the KEYNOTE-811 trial, which showed a higher objective response rate (ORR) with pembrolizumab added to trastuzumab plus chemotherapy.6
Although HER2 positivity was primarily defined as IHC 3+ or fluorescence in situ hybridization (FISH)+ in ToGA, a benefit of trastuzumab was observed in patients with IHC 3+/IHC 2+ and FISH+ tumors but not in patients with IHC 0/IHC 1+ and FISH+ tumors.2,5 The proportion of patients with HER2-low gastric cancer defined as IHC 2+/ISH– or IHC 1+ is not well documented but estimated at 5.4% or 18.6%, respectively.2 There is an unmet medical need for treatments of HER2-low gastric/GEJ cancer.
Trastuzumab deruxtecan (T-DXd; DS-8201; [fam]-trastuzumab deruxtecan-nxki in the United States) is an antibody-drug conjugate comprising a humanized anti-HER2 immunoglobulin G1 monoclonal antibody with the same amino acid sequence as trastuzumab, a cleavable tetrapeptide-based linker, and a cytotoxic topoisomerase I inhibitor.7,8 T-DXd improved ORR and OS versus physician's choice irinotecan or paclitaxel in patients with HER2-positive (IHC 3+ or IHC 2+/ISH+) gastric/GEJ adenocarcinoma who had progressed on a trastuzumab-containing regimen in primary analysis of the DESTINY-Gastric01 trial.9 T-DXd is approved in multiple countries for the treatment of patients with locally advanced or metastatic HER2-positive gastric/GEJ adenocarcinoma who have received a trastuzumab-based regimen. T-DXd is approved in the United States, with boxed warnings for interstitial lung disease (ILD) and embryo-fetal toxicity.10
T-DXd has a drug-to-antibody ratio of approximately 8, and the topoisomerase I inhibitor payload is highly membrane permeable, signifying it can penetrate neighboring tumor cells that do not necessarily express HER2 or have low expression (bystander antitumor effect); these properties make T-DXd a potential treatment for patients with HER2-low tumors.3,7,11 T-DXd demonstrated antitumor activity in HER2-low cells and mouse xenografts,7,12 in a phase I trial that included patients with HER2-low breast cancer and one patient with HER2-low gastric cancer,13 and in phase I and III trials in HER2-low breast cancer.14,15 We report the efficacy and safety of T-DXd in exploratory cohorts of patients with HER2-low (IHC 2+/ISH– or IHC 1+) gastric/GEJ adenocarcinoma who had not received anti-HER2 treatment in DESTINY-Gastric01.
METHODS
Study Design and Patients
DESTINY-Gastric01 (ClinicalTrials.gov identifier: NCT03329690) was a multicenter, open-label, three-cohort phase II trial of T-DXd in patients from Japan and South Korea with HER2-expressing advanced gastric/GEJ adenocarcinoma that progressed after at least two previous therapies. The trial design and enrollment criteria are published.9 Eligible patients were age 20 years or older with pathologically documented locally advanced or metastatic gastric/GEJ adenocarcinoma, had measurable disease on the basis of RECIST v1.1, and had Eastern Cooperate Oncology Group performance status (ECOG PS) scores of 0 or 1.
Patients had tumors that progressed during or after treatment with at least two previous therapies including fluoropyrimidine and platinum-containing regimens. Patients were excluded if ILD or pneumonitis was present or suspected or if they had a history of noninfectious ILD/pneumonitis treated with glucocorticoids.
Patients in the exploratory cohorts had HER2-low (exploratory cohort 1, IHC 2+/ISH–; exploratory cohort 2, IHC 1+) gastric/GEJ adenocarcinoma confirmed by central laboratory testing and had not received anti-HER2 therapy. All patients received T-DXd 6.4 mg/kg by intravenous infusion once every 3 weeks, as determined by previous pharmacokinetic, efficacy, and safety data,16 until occurrence of disease progression, withdrawal of consent, or unacceptable adverse events.
The study was designed by Daiichi Sankyo, which also oversaw the conduct of the study. Independent ethics committees or institutional review boards at each site reviewed and approved the Protocol (online only). The study was conducted in accordance with the principles of the Declaration of Helsinki, the International Conference on Harmonisation guidelines for Good Clinical Practice, other local regulations where applicable, and the study protocol. Written informed consent was provided by all patients before enrollment.
Assessments and End Points
Computed tomography or magnetic resonance imaging scans of the chest, abdomen, and pelvis were performed at screening and every 6 weeks (± 7 days) from the first dose until progressive disease (PD), initiation of new cancer treatment, or study withdrawal. Tumor assessment was performed by independent central review (ICR) and investigator assessment according to RECIST v1.1. Safety was assessed as the incidences of treatment-emergent adverse events (TEAEs) and serious adverse events graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 4.03, adverse events of special interest (ILD/pneumonitis, QT prolongation, left ventricular ejection fraction decrease, infusion-related reaction), and discontinuations due to TEAEs or adverse events of special interests. Potential ILD/pneumonitis was evaluated by an independent adjudication committee.
The primary end point in the exploratory cohorts was confirmed ORR (sum of the proportion of patients with complete response [CR] and partial response [PR] lasting ≥ 4 weeks) by ICR. Secondary end points included OS, progression-free survival (PFS) by ICR, duration of response (DoR) by ICR, disease control rate (DCR; proportion of patients who achieved a best overall response of CR or PR or stable disease [SD]) by ICR, time to treatment failure (TTF) by ICR, ORR by investigator assessment, and safety. Exploratory end points included time to response (TTR) by ICR, best percentage change in the sum of diameters of measurable tumors by ICR, PFS by investigator assessment, and time to deterioration of ECOG PS score of 2 or higher.
Statistical Analysis
A sample size of approximately 20 patients in each exploratory cohort was planned to provide at least 75% power to detect a difference between the expected ORR of 30% and the null hypothesis ORR of 15% with a one-sided alpha of .20. If more than four responders were observed of 20 patients (ORR > 20%), T-DXd would be supported as having promising activity. ORR and DCR were analyzed in the response evaluable set (all patients who received at least one dose of study treatment and had measurable tumors by ICR at baseline), other efficacy analyses were performed in the full analysis set (all patients who received at least one dose of study treatment), and safety analyses were performed in the safety analysis set (all patients who received at least one dose of study treatment according to treatment received). ORRs and two-sided 95% exact binomial CIs were estimated for each cohort. End points were defined as follows: OS, time from registration to date of death (censored at the last known date alive if death not reported before the data cutoff); PFS, time from registration to the earliest date of first radiographic evidence of PD or death (censored at the last imaging assessment if clinical diagnosis of PD not confirmed by central imaging); DoR, time from first objective response (CR or PR) to PD or death measured in responders; TTF, time from registration to radiographic PD, death, or treatment discontinuation; and TTR, time from first dose of study treatment to first documented objective response (CR or PR) measured in responders. OS, PFS, DoR, TTF, and TTR were summarized with median event times and two-sided 95% CIs, using Brookmeyer and Crowley methods. Kaplan-Meier estimates were assessed at fixed time points. Descriptive statistics were used for best percentage change from baseline in the sum of tumor diameters and for safety analyses.
RESULTS
Patients
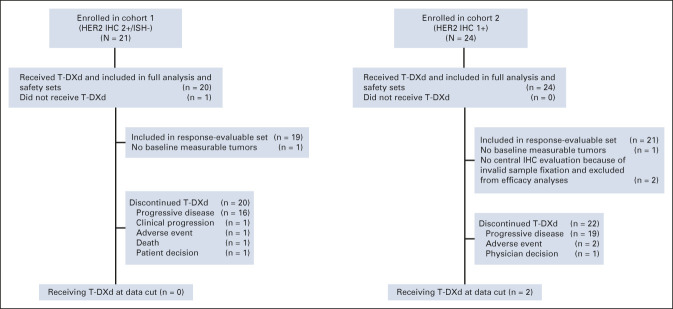
Between February 2018 and February 2019, 21 patients were enrolled in cohort 1 (HER2 IHC 2+/ISH–) and 24 patients were enrolled in cohort 2 (IHC 1+) across 27 sites in Japan and South Korea; 20 patients (95.2%) in cohort 1 and 24 patients (100%) in cohort 2 received T-DXd (Fig 1). At the November 8, 2019, data cutoff, no patients in cohort 1 and two patients in cohort 2 remained on treatment. The most common reason for discontinuation was disease progression (16 of 20 [80%] in cohort 1; 19 of 24 [79.2%] in cohort 2). The median treatment duration was 4.2 (range, 1.3‐10.5) months in cohort 1 and 2.8 (range, 0.7-14.9) months in cohort 2. The full analysis and safety sets included 20 patients in cohort 1 and 24 patients in cohort 2, and the response evaluable set included 19 and 21 patients, respectively (two patients in cohort 2 were excluded because they did not have HER2 status confirmed by central laboratory; one patient in each cohort was excluded because of no baseline measurable lesion by ICR).
FIG 1.
CONSORT diagram. HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; ISH, in situ hybridization; T-DXd, trastuzumab deruxtecan.
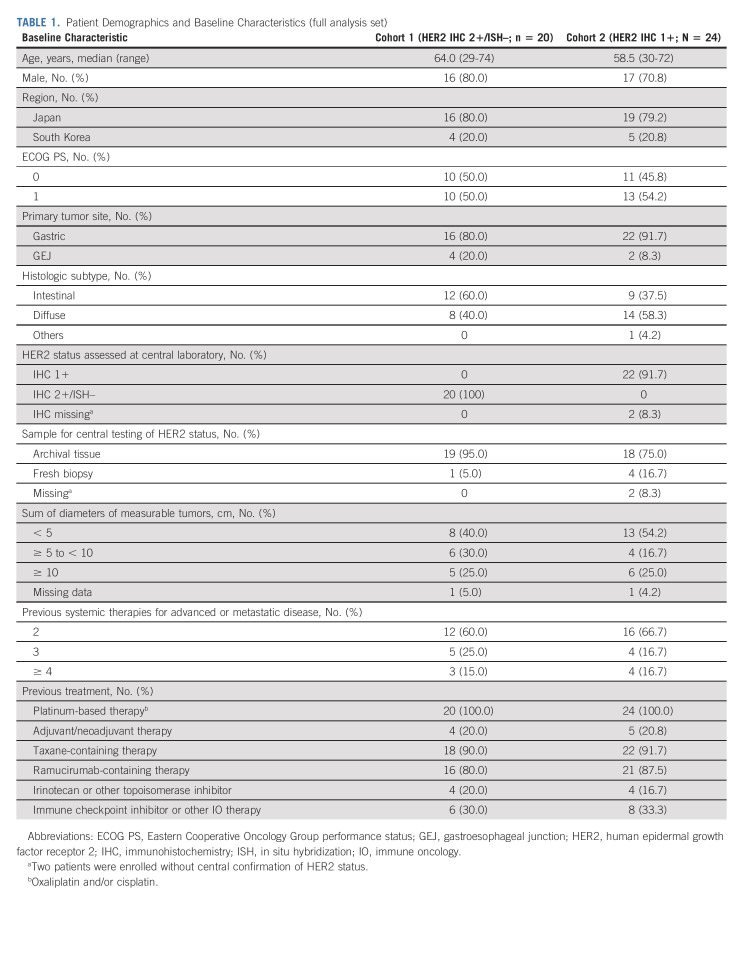
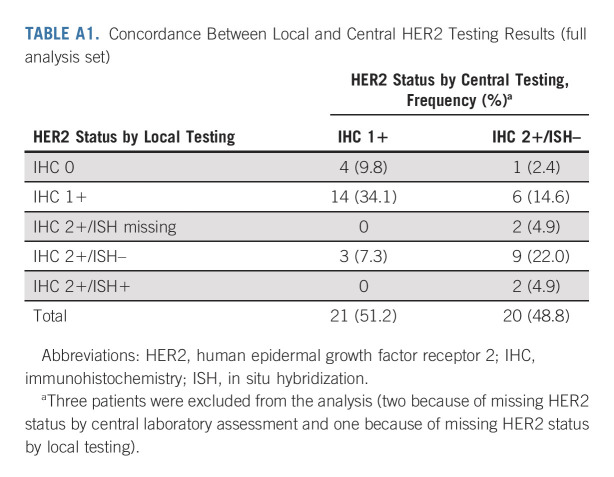
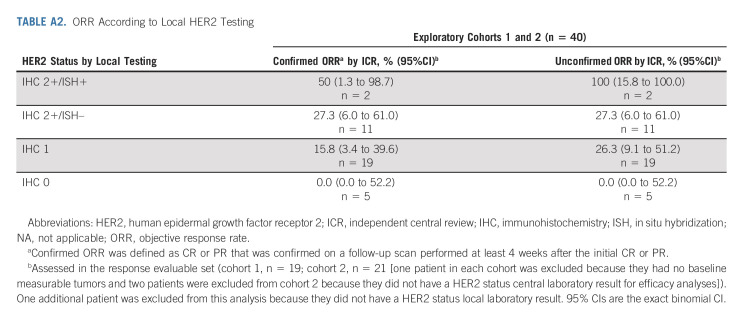
Baseline demographics and disease characteristics (Table 1) were comparable between cohort 1 and cohort 2 and the primary cohort,9 except for a higher proportion of patients with diffuse histological subtype in the exploratory cohorts (cohort 1, 40.0%; cohort 2, 58.3%) than the primary cohort (24.6%). Other differences in patient characteristics and previous treatments were expected given the difference in HER2 status between the cohorts and because the exploratory cohorts had not received trastuzumab. The concordance rate between local and central HER2 testing results was 56.1% (Appendix Table A1, online only); 34.1% for IHC 1+ and 22.0% for IHC 2+/ISH–, whereas the concordance rate of HER2 low gastric/GEJ cancer was 78.0%. ORR according to HER2 status by local testing is in Appendix Table A2 (online only).
TABLE 1.
Patient Demographics and Baseline Characteristics (full analysis set)
Efficacy
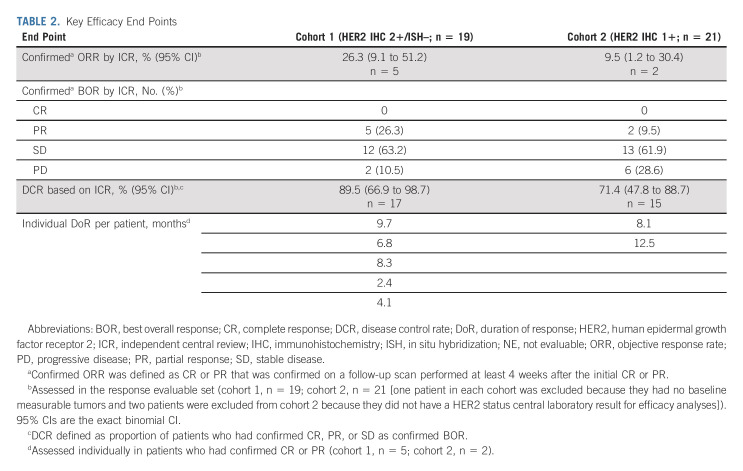
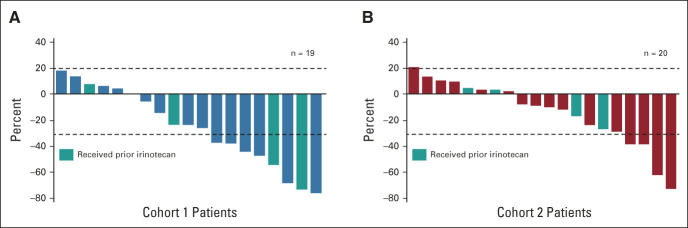
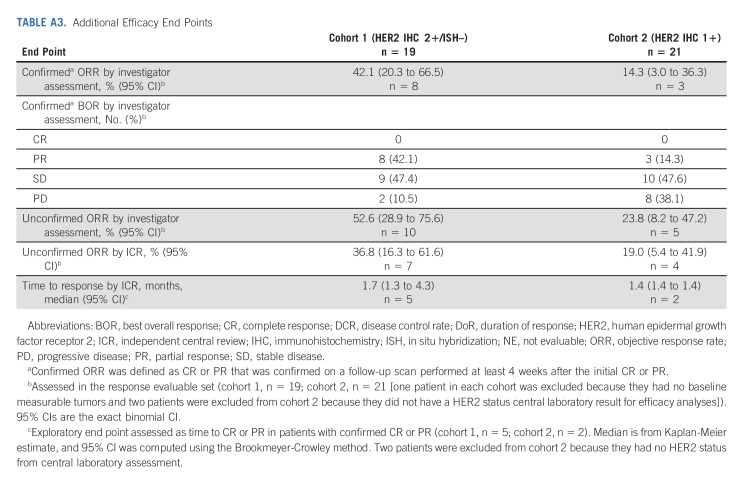
Confirmed ORR by ICR showed antitumor activity in cohort 1 and cohort 2 (Table 2). Confirmed ORR was 26.3% (95% CI, 9.1 to 51.2) from five PRs in cohort 1 and 9.5% (95% CI, 1.2 to 30.4) from two PRs in cohort 2. No patients in either cohort had CR. The unconfirmed ORR was 36.8% (95% CI, 16.3% to 61.6%) from seven PRs in cohort 1 and 19.0% (95% CI, 5.4 to 41.9) from four PRs in cohort 2. Confirmed ORR by investigator assessment was 42.1% in cohort 1 and 14.3% in cohort 2 (Appendix Table A3, online only). Approximately two thirds of patients in cohort 1 (13 of 19 [68.4%]) and cohort 2 (12 of 20 [60%]) had reduction in tumor size assessed by ICR, including three patients in cohort 1 and two patients in cohort 2 who had previously received irinotecan (Figs 2A and 2B).
TABLE 2.
Key Efficacy End Points
FIG 2.
Best percentage change from baseline in tumor size by ICR in (A) cohort 1 and (B) cohort 2 (full analysis set). Dashed lines at 20% indicate progressive disease; dashed lines at –30% indicate PR. Includes patients in both cohorts who had both baseline and postbaseline target lesion assessments by ICR. One patient in each cohort was excluded because there was no baseline measurable disease by ICR. Three additional patients in the IHC 1+ cohort were excluded because there was no postbaseline assessment (n = 1) or a missing HER2 status by central laboratory (n = 2). Among the four patients in cohort 1 who received prior irinotecan, none responded to prior irinotecan (three SD and one unknown), and two had confirmed response to T-DXd (confirmed ORR = 50%). Among the four patients in cohort 2 who received prior irinotecan, one responded to prior irinotecan (PR), three did not respond to prior irinotecan (one SD, one progressive disease, and one unknown), and none had confirmed response to T-DXd (confirmed ORR = 0%). ICR, independent central review; IHC, immunohistochemistry; ISH, in situ hybridization; ORR, objective response rate; PR, partial response; SD, stable disease; T-DXd, trastuzumab deruxtecan.
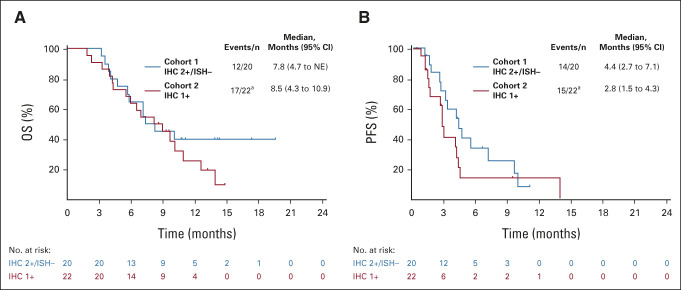
The DCR was 89.5% in cohort 1 and 71.4% in cohort 2. The median (95% CI) DoR in patients with confirmed ORR was 7.6 (4.1 to nonevaluable) months in cohort 1 and 12.5 (nonevaluable to nonevaluable) months in cohort 2 on the basis of Kaplan-Meier estimates. The median OS was 7.8 (95% CI, 4.7 to nonevaluable) months in cohort 1 and 8.5 (95% CI, 4.3 to 10.9) months in cohort 2 (Fig 3A). The median PFS by ICR was 4.4 (95% CI, 2.7 to 7.1) months in cohort 1 and 2.8 (95% CI, 1.5 to 4.3) months in cohort 2 (Fig 3B). TTF, change in tumor size over time, and time to deterioration of ECOG PS ≥ 2 (Appendix Figures A1-A3, online only) were numerically better in cohort 1 than in cohort 2; however, statistical comparison between cohorts was not conducted.
FIG 3.
(A) OS and (B) PFS on the basis of ICR (full analysis set). Vertical lines show censored data: (A) eight patients (40%) in cohort 1 and five patients (23%) in cohort 2 had their data censored; (B) six patients (30%) in cohort 1 and seven patients (32%) in cohort 2 had their data censored. aTwo patients were excluded from the analysis because of a missing HER2 status by central laboratory assessment. HER2, human epidermal growth factor receptor 2; ICR, independent central review; IHC, immunohistochemistry; ISH, in situ hybridization; NE, not evaluable; OS, overall survival; PFS, progression-free survival.
Safety
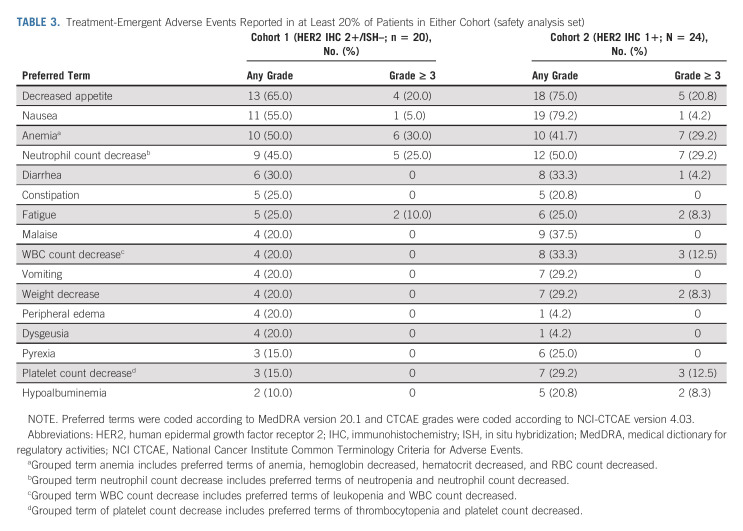
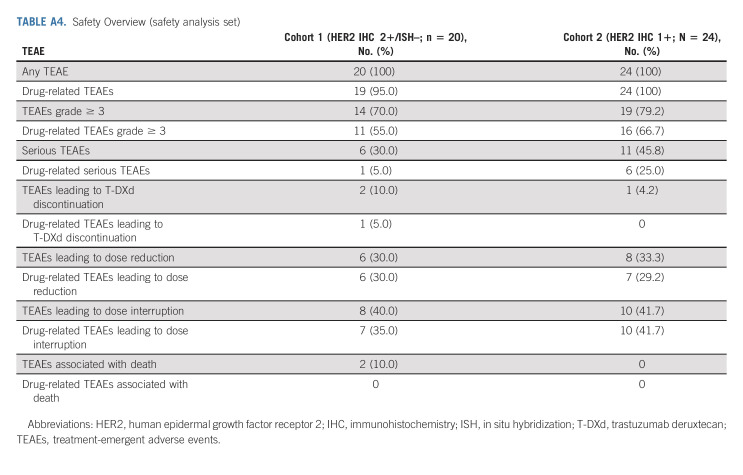
All patients in the safety analysis set experienced at least one TEAE (Appendix Table A4, online only); 70.0% in cohort 1 and 79.2% in cohort 2 had TEAEs of grade 3 or higher. The most common TEAEs (reported in ≥ 20% of patients in either cohort) by Medical Dictionary for Regulatory Activities preferred term in cohorts 1 and 2 were gastrointestinal (decreased appetite [65.0% and 75.0%], nausea [55.0% and 79.2%], diarrhea [30.0% and 33.0%], constipation [25.0% and 20.8%], vomiting [20.0% and 29.2%]), or hematologic disorders (anemia [50.0% and 41.7%], neutrophil count decrease [45.0% and 50.0%], WBC count decreased [20.0% and 33.3%], platelet count decrease [15.0% and 29.2%]; Table 3). The most common grade ≥ 3 TEAEs in cohorts 1 and 2 were anemia (30.0% and 29.2%), neutrophil count decrease (25.0% and 29.2%), and decreased appetite (20.0% and 20.8%). TEAEs associated with study drug discontinuation, dose reduction, or dose interruption were reported in two (10.0%), six (30.0%), and eight (40.0%) patients, respectively, in cohort 1 and in one (4.2%), eight (33.3%), and 10 (41.7%) patients, respectively, in cohort 2 (Appendix Table A4). One patient in cohort 1 had a drug-related TEAE (preferred term ILD) that led to drug discontinuation. There were no drug-related TEAEs associated with death. One patient in each cohort had drug-related ILD/pneumonitis determined by the independent adjudication committee; a grade 1 event in cohort 1 occurred after 248 days, and a grade 2 event in cohort 2 occurred after 171 days. No patients in either cohort had a TEAE of ejection fraction decrease and decrease in left ventricular ejection fraction ≥ 20%. One patient in cohort 2 had a grade 1 TEAE of QT prolongation. There were no infusion-related reactions in either cohort.
TABLE 3.
Treatment-Emergent Adverse Events Reported in at Least 20% of Patients in Either Cohort (safety analysis set)
DISCUSSION
In this study of T-DXd in patients with locally advanced or metastatic HER2-low gastric/GEJ adenocarcinoma after progression on at least two previous fluoropyrimidine and platinum-containing regimens, the confirmed ORR was 26.3% in cohort 1 (HER2 IHC 2+/ISH–) and 9.5% in cohort 2 (HER2 IHC 1+). The primary HER2-positive cohort (HER2 IHC 3+ or IHC 2+/ISH+) had confirmed ORR of 43% with T-DXd and 12% with chemotherapy.9 Although ORR was higher among HER2-positive patients treated with T-DXd, these results suggest T-DXd is effective in patients with low-level HER2 expression (IHC 2+/ISH–) and may identify a new therapeutic target. HER2-low patients demonstrated a DCR of 89.5% in cohort 1 and 71.4% in cohort 2 and had durable responses lasting a median of 7.6 and 12.5 months, respectively. In the primary cohort, the DCR was 86% and the median DoR was 11.3 months with T-DXd, versus 62% and 3.9 months, respectively, with chemotherapy9; however, the small size of the exploratory cohorts and differences in patient populations (primary cohort had prior trastuzumab) limit comparison. Although the sample size is too small to draw conclusions for time-to-event end points, the median OS of 7.8 months in cohort 1 and 8.5 months in cohort 2 was shorter than 12.5 months with T-DXd in the primary cohort, as was median PFS at 4.4 months for cohort 1, 2.8 months for cohort 2, and 5.6 months for the primary cohort.9 Safety results in the exploratory HER2-low cohorts were consistent with those of the primary cohort, and, overall, T-DXd demonstrated a manageable safety profile. Consistent with previous T-DXd studies, the most common drug-related TEAEs were gastrointestinal and hematologic disorders. Two cases of independently adjudicated ILD/pneumonitis were reported, one in each exploratory cohort; both were actively monitored and managed with dose modification or discontinuation, corticosteroid treatment, and supportive care per protocol. In the primary cohort of 125 patients, 12 had ILD (nine grade 1 or 2; three grade 3 or 4), with a median time to onset of 84.5 days.9 ILD/pneumonitis is an important risk that requires careful monitoring and management in patients treated with T-DXd.
There are no treatments specifically for HER2-low gastric cancer, and effective therapies are needed. HER2 expression levels are heterogeneous in gastric/GEJ cancer and change over time2; HER2 positivity is often lost or diminished after trastuzumab and continued trastuzumab therapy is not effective.17 Other treatments for third-line or subsequent therapy for gastric/GEJ cancer are trifluridine and tipiracil.18-20 However, the ORR is 4% for trifluridine/tipiracil in this setting.21 The ORR with third-line or later T-DXd therapy observed in patients with low-level HER2 expression in our study was 26.3% and 9.5% in cohorts 1 and 2, respectively, suggesting T-DXd affects growth of tumors with low HER2 expression. However, cohort 2 (IHC 1+) did not meet prespecified ORR criteria and requires further investigation considering the lower ORR in approved standard of care.21 Activity in HER2-low tumors might be attributed to the high membrane permeability of T-DXd, which enables it to permeate neighboring cells that do not express HER2 or express it at low levels. The bystander antitumor effect, high drug-antibody ratio, and clinical activity in HER2-low tumors provide evidence for T-DXd as a potential treatment option. T-DXd demonstrated significantly longer progression-free and OS versus chemotherapy in HER2-low breast cancer in a phase III trial.15
This study supports preclinical studies and early-phase clinical investigation that indicated an antitumor effect of T-DXd in HER2-low tumors, including gastric/GEJ cancer.7,13 Other published studies on potential treatments for HER2-low gastric/GEJ cancer are mainly in the preclinical setting. ARX788, another anti-HER2 antibody-drug conjugate, demonstrated superior activity to trastuzumab in cancer cells with HER2-low expression and in patient-derived xenograft models including HER2-low gastric cancer.22 Trastuzumab duocarmazine demonstrated preliminary antitumor activity in HER2-low breast cancer and evidence of tumor size reduction in a patient with HER2-low gastric cancer.23 RC48 (disitamab vedotin) showed clinical response and survival benefit in a phase II trial in gastric/GEJ cancer including HER2 IHC 2+ (regardless of ISH status) or IHC 3+ patients24 and is approved for third-line treatment of HER2-positive gastric cancer in China.
Limitations include the small patient numbers and lack of comparator in the exploratory cohorts. Patients had low disease burden (47.7% had baseline sum of measurable tumors < 5 cm), which could limit response assessment. Patients were from Japan and South Korea, and the results should be confirmed in other populations with heavily pretreated gastric/GEJ cancer. Promising antitumor activity of second-line T-DXd was reported in HER2-positive patients with gastric/GEJ cancer from the United States and Europe.25 Another limitation is discrepancy between archived and fresh tissue results for determination of HER2 status, which could result from a lack of clear definitions in HER2 IHC testing regarding the influence of changes in HER2 expression over time, tumor heterogeneity, and discrepancy between laboratories or readers.14 There were inconsistencies between central and local testing; central testing, used to determine HER2 status for enrollment, categorized all patients in cohort 1 as IHC 2+/ISH– and 22 patients in cohort 2 as IHC 1+, whereas there was considerable discrepancy in local IHC/ISH test results, with a concordance rate of 56.1% between local and central scoring; however, the concordance rate of HER2 low gastric/GEJ cancer was high (78.0%).
This study provides preliminary evidence that T-DXd has clinical activity in patients with heavily pretreated HER2-low (IHC 2+/ISH–) gastric/GEJ adenocarcinoma. Additional randomized controlled trials in larger cohorts are required to determine the efficacy and safety of T-DXd in HER2-low gastric/GEJ adenocarcinoma.
ACKNOWLEDGMENT
We thank the patients who participated in this study, as well as their families and caregivers. We also thank the staff and investigators at all the study sites. Under the guidance of the authors, assistance in medical writing and editorial support was provided by Sara Duggan, PhD, and Alya Raphael, PhD, of ApotheCom, and was funded by Daiichi Sankyo Co, Ltd.
APPENDIX
FIG A1.
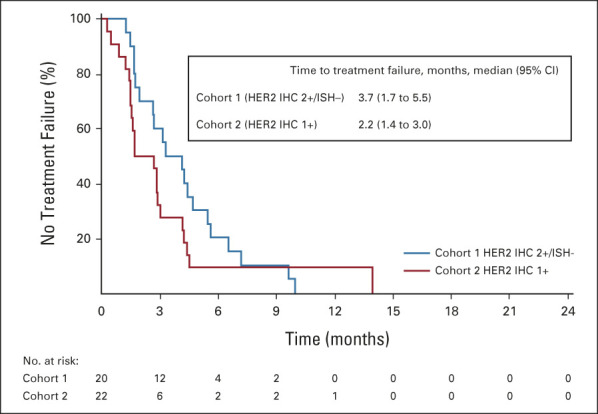
Time to treatment failure on the basis of independent central review (full analysis set). Two patients were excluded from cohort 2 because they had no HER2 status result in central laboratory assessment. HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; ISH, in situ hybridization.
FIG A2.
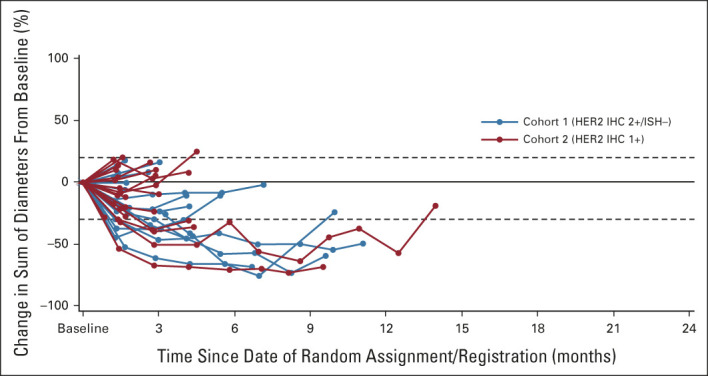
Percentage of change in the sum of diameters for each patient. Baseline was defined as the last measurement before the first dose of study drug. Two patients were excluded from cohort 2 because they had no HER2 status result in central laboratory assessment. Dashed lines at 20% indicate progressive disease; dashed lines at –30% indicate partial response. HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; ISH, in situ hybridization.
FIG A3.
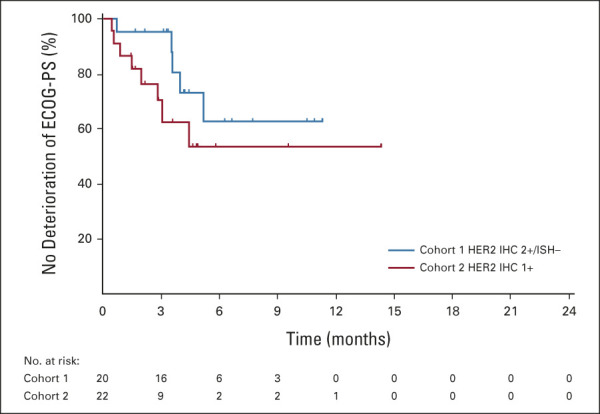
Time to deterioration of ECOG PS ≥ 2 (full analysis set). Vertical lines represent censored data. ECOG PS, Eastern Cooperative Oncology Group performance status; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; ISH, in situ hybridization.
TABLE A1.
Concordance Between Local and Central HER2 Testing Results (full analysis set)
TABLE A2.
ORR According to Local HER2 Testing
TABLE A3.
Additional Efficacy End Points
TABLE A4.
Safety Overview (safety analysis set)
Kensei Yamaguchi
Consulting or Advisory Role: Bristol Myers Squibb Japan, Daiichi Sankyo
Speakers' Bureau: Chugai Pharma, Bristol Myers Squibb Japan, Takeda, Taiho Pharmaceutical, Lilly, Ono Pharmaceutical, Daiichi Sankyo, Merck
Research Funding: Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Daiichi Sankyo (Inst), Lilly (Inst), Gilead Sciences (Inst), Yakult Honsha (Inst), Chugai Pharma (Inst), Boehringer Ingelheim (Inst), Eisai (Inst), MSD Oncology (Inst), Sanofi (Inst), Bristol Myers Squibb (Inst)
Yung-Jue Bang
Consulting or Advisory Role: Hanmi, Astellas Pharma, Samyang, Alexo Therapeutics, Daewoong Pharmaceutical, Amgen
Satoru Iwasa
Honoraria: Taiho Pharmaceutical, Lilly Japan, Chugai Pharma, Ono Pharmaceutical, Daiichi Sankyo, Bristol Myers Squibb Japan
Research Funding: Daiichi Sankyo (Inst), Bristol Myers Squibb (Inst), Eisai (Inst), Merck Serono (Inst), Bayer (Inst), Ono Pharmaceutical (Inst), Astellas Pharma (Inst), Pfizer (Inst), Seattle Genetics (Inst), Zymeworks (Inst), Taiho Pharmaceutical (Inst), AstraZeneca (Inst)
Naotoshi Sugimoto
Research Funding: MSD (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Lilly Japan (Inst), Daiichi Sankyo (Inst), Dainippon Sumitomo Pharma (Inst), Chugai Pharma (Inst), BeiGene (Inst), Solasia Pharma (Inst), Astellas Pharma (Inst), Eisai (Inst)
Min-Hee Ryu
Honoraria: DAEHWA Pharmaceutical, Bristol Myers Squibb, Lilly, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, Daiichi Sankyo, AstraZeneca
Consulting or Advisory Role: DAEHWA Pharmaceutical, Bristol Myers Squibb, Lilly, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, Daiichi Sankyo, AstraZeneca
Daisuke Sakai
Speakers' Bureau: Chugai Pharma, Daiichi Sankyo/UCB Japan
Research Funding: Chugai Pharma (Inst), Yakult Honsha (Inst), Ono Pharmaceutical (Inst), Daiichi Sankyo, Lilly Japan, Astellas Pharma (Inst), Incyte (Inst), Taiho Pharmaceutical (Inst), Eisai (Inst)
Hyun Cheol Chung
Employment: MD Biolab
Consulting or Advisory Role: Taiho Pharmaceutical, Celltrion, MSD, Lilly, Quintiles, Bristol Myers Squibb, Merck Serono, Gloria Biosciences, Amgen, Zymeworks, Beigene
Speakers' Bureau: Merck Serono, Lilly, Foundation medicine
Research Funding: Lilly (Inst), GlaxoSmithKline (Inst), MSD (Inst), Merck Serono (Inst), Bristol Myers Squibb (Inst), Taiho Pharmaceutical (Inst), Amgen (Inst), Beigene (Inst), Incyte (Inst), Zymeworks (Inst)
Hisato Kawakami
Honoraria: Chugai/Roche, Taiho Pharmaceutical, Ono Pharmaceutical, Bristol Myers Squibb Japan, Yakult Pharmaceutical, Takeda, MSD K.K, Merck Serono, Lilly Japan, Daiichi Sankyo, Bayer
Consulting or Advisory Role: Taiho Pharmaceutical, Bristol Myers Squibb Japan, Ono Pharmaceutical, Lilly Japan, Daiichi Sankyo Co. Ltd,
Research Funding: Chugai Pharma (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Kobayashi Pharmaceutical Co, Ltd (Inst)
Jeeyun Lee
Consulting or Advisory Role: Oncologie, Mirati Therapeutics
Research Funding: AstraZeneca, Merck Sharp & Dohme
Keun-Wook Lee
Honoraria: Ono Pharmaceutical, Boryung, JW Pharmaceutical
Consulting or Advisory Role: Bayer, Daiichi Sankyo, Bristol Myers Squibb, MSD, Vifor Pharma, Sanofi/Aventis, Metafines, Ono Pharmaceutical
Research Funding: Macrogenics (Inst), MSD (Inst), Ono Pharmaceutical (Inst), GC Pharma (Inst), AstraZeneca/MedImmune (Inst), Five Prime Therapeutics (Inst), LSK BioPharma (Inst), Merck KGaA (Inst), Pharmacyclics (Inst), Pfizer (Inst), ALX Oncology (Inst), Zymeworks (Inst), BeiGene (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), ABL Bio (Inst), Y-BIOLOGICS (Inst), Oncologie (Inst), Seattle Genetics (Inst), Bolt Biotherapeutics (Inst), Trishula Therapeutics (Inst), InventisBio (Inst), Leap Therapeutics (Inst), Astellas Pharma (Inst), MedPacto (Inst), Ildong Pharmaceutical (Inst), Roche (Inst), Amgen (Inst), Genome & Company (Inst), Arcus Biosciences (Inst)
Kaku Saito
Employment: Daiichi Sankyo, Inc
Yoshinori Kawaguchi
Employment: Daiichi Sankyo, Inc
Stock and Other Ownership Interests: Daiichi Sankyo, Inc
Takahiro Kamio
Employment: Daiichi Sankyo/AstraZeneca
Akihito Kojima
Employment: Daiichi Sankyo Co, Ltd
Stock and Other Ownership Interests: Daiichi Sankyo Co, Ltd
Masahiro Sugihara
Employment: Daiichi-Sankyo Co, Ltd
Kohei Shitara
Honoraria: Bristol Myers Squibb, Takeda, Janssen
Consulting or Advisory Role: Lilly, Bristol Myers Squibb, Takeda, Pfizer, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, AbbVie, GlaxoSmithKline, Daiichi Sankyo, Boehringer Ingelheim, Amgen, Astellas Pharma, Guardant Health Japan
Research Funding: MSD (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), Ono Pharmaceutical (Inst), Astellas Pharma (Inst), Eisai (Inst), Amgen (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2020 European Society for Medical Oncology (ESMO) congress, virtual, September 19-21, 2020.
SUPPORT
Supported by Daiichi Sankyo Co, Ltd and AstraZeneca.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Anonymized individual participant data and applicable supporting clinical trial documents may be available upon request at the Vivli website (https://vivli.org). In cases where clinical trial data and supporting documents are provided pursuant to our company policies and procedures, Daiichi Sankyo, Inc, will continue to protect the privacy of our clinical trial participants. Details on data sharing criteria and the procedure for requesting access can be found at Vivli's Daiichi Sankyo web page (https://vivli.org/ourmember/daiichi-sankyo).
AUTHOR CONTRIBUTIONS
Conception and design: Kensei Yamaguchi, Yung-Jue Bang, Hiroshi Yabusaki, Kaku Saito, Yoshinori Kawaguchi, Akihito Kojima, Masahiro Sugihara, Kohei Shitara
Administrative support: Hiroshi Yabusaki, Tatsu Shimoyama, Yoshinori Kawaguchi
Provision of study materials or patients: Yung-Jue Bang, Satoru Iwasa, Min-Hee Ryu, Daisuke Sakai, Hyun Cheol Chung, Hiroshi Yabusaki, Tatsu Shimoyama, Yoshinori Kawaguchi, Kohei Shitara
Collection and assembly of data: Kensei Yamaguchi, Satoru Iwasa, Naotoshi Sugimoto, Min-Hee Ryu, Daisuke Sakai, Hyun Cheol Chung, Hiroshi Yabusaki, Jeeyun Lee, Tatsu Shimoyama, Keun-Wook Lee, Kaku Saito, Yoshinori Kawaguchi, Akihito Kojima, Kohei Shitara
Data analysis and interpretation: Kensei Yamaguchi, Yung-Jue Bang, Min-Hee Ryu, Hyun Cheol Chung, Hisato Kawakami, Hiroshi Yabusaki, Kaku Saito, Yoshinori Kawaguchi, Takahiro Kamio, Akihito Kojima, Masahiro Sugihara, Kohei Shitara
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Trastuzumab Deruxtecan in Anti–Human Epidermal Growth Factor Receptor 2 Treatment–Naive Patients With Human Epidermal Growth Factor Receptor 2–Low Gastric or Gastroesophageal Junction Adenocarcinoma: Exploratory Cohort Results in a Phase II Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kensei Yamaguchi
Consulting or Advisory Role: Bristol Myers Squibb Japan, Daiichi Sankyo
Speakers' Bureau: Chugai Pharma, Bristol Myers Squibb Japan, Takeda, Taiho Pharmaceutical, Lilly, Ono Pharmaceutical, Daiichi Sankyo, Merck
Research Funding: Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Daiichi Sankyo (Inst), Lilly (Inst), Gilead Sciences (Inst), Yakult Honsha (Inst), Chugai Pharma (Inst), Boehringer Ingelheim (Inst), Eisai (Inst), MSD Oncology (Inst), Sanofi (Inst), Bristol Myers Squibb (Inst)
Yung-Jue Bang
Consulting or Advisory Role: Hanmi, Astellas Pharma, Samyang, Alexo Therapeutics, Daewoong Pharmaceutical, Amgen
Satoru Iwasa
Honoraria: Taiho Pharmaceutical, Lilly Japan, Chugai Pharma, Ono Pharmaceutical, Daiichi Sankyo, Bristol Myers Squibb Japan
Research Funding: Daiichi Sankyo (Inst), Bristol Myers Squibb (Inst), Eisai (Inst), Merck Serono (Inst), Bayer (Inst), Ono Pharmaceutical (Inst), Astellas Pharma (Inst), Pfizer (Inst), Seattle Genetics (Inst), Zymeworks (Inst), Taiho Pharmaceutical (Inst), AstraZeneca (Inst)
Naotoshi Sugimoto
Research Funding: MSD (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Lilly Japan (Inst), Daiichi Sankyo (Inst), Dainippon Sumitomo Pharma (Inst), Chugai Pharma (Inst), BeiGene (Inst), Solasia Pharma (Inst), Astellas Pharma (Inst), Eisai (Inst)
Min-Hee Ryu
Honoraria: DAEHWA Pharmaceutical, Bristol Myers Squibb, Lilly, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, Daiichi Sankyo, AstraZeneca
Consulting or Advisory Role: DAEHWA Pharmaceutical, Bristol Myers Squibb, Lilly, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, Daiichi Sankyo, AstraZeneca
Daisuke Sakai
Speakers' Bureau: Chugai Pharma, Daiichi Sankyo/UCB Japan
Research Funding: Chugai Pharma (Inst), Yakult Honsha (Inst), Ono Pharmaceutical (Inst), Daiichi Sankyo, Lilly Japan, Astellas Pharma (Inst), Incyte (Inst), Taiho Pharmaceutical (Inst), Eisai (Inst)
Hyun Cheol Chung
Employment: MD Biolab
Consulting or Advisory Role: Taiho Pharmaceutical, Celltrion, MSD, Lilly, Quintiles, Bristol Myers Squibb, Merck Serono, Gloria Biosciences, Amgen, Zymeworks, Beigene
Speakers' Bureau: Merck Serono, Lilly, Foundation medicine
Research Funding: Lilly (Inst), GlaxoSmithKline (Inst), MSD (Inst), Merck Serono (Inst), Bristol Myers Squibb (Inst), Taiho Pharmaceutical (Inst), Amgen (Inst), Beigene (Inst), Incyte (Inst), Zymeworks (Inst)
Hisato Kawakami
Honoraria: Chugai/Roche, Taiho Pharmaceutical, Ono Pharmaceutical, Bristol Myers Squibb Japan, Yakult Pharmaceutical, Takeda, MSD K.K, Merck Serono, Lilly Japan, Daiichi Sankyo, Bayer
Consulting or Advisory Role: Taiho Pharmaceutical, Bristol Myers Squibb Japan, Ono Pharmaceutical, Lilly Japan, Daiichi Sankyo Co. Ltd,
Research Funding: Chugai Pharma (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Kobayashi Pharmaceutical Co, Ltd (Inst)
Jeeyun Lee
Consulting or Advisory Role: Oncologie, Mirati Therapeutics
Research Funding: AstraZeneca, Merck Sharp & Dohme
Keun-Wook Lee
Honoraria: Ono Pharmaceutical, Boryung, JW Pharmaceutical
Consulting or Advisory Role: Bayer, Daiichi Sankyo, Bristol Myers Squibb, MSD, Vifor Pharma, Sanofi/Aventis, Metafines, Ono Pharmaceutical
Research Funding: Macrogenics (Inst), MSD (Inst), Ono Pharmaceutical (Inst), GC Pharma (Inst), AstraZeneca/MedImmune (Inst), Five Prime Therapeutics (Inst), LSK BioPharma (Inst), Merck KGaA (Inst), Pharmacyclics (Inst), Pfizer (Inst), ALX Oncology (Inst), Zymeworks (Inst), BeiGene (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), ABL Bio (Inst), Y-BIOLOGICS (Inst), Oncologie (Inst), Seattle Genetics (Inst), Bolt Biotherapeutics (Inst), Trishula Therapeutics (Inst), InventisBio (Inst), Leap Therapeutics (Inst), Astellas Pharma (Inst), MedPacto (Inst), Ildong Pharmaceutical (Inst), Roche (Inst), Amgen (Inst), Genome & Company (Inst), Arcus Biosciences (Inst)
Kaku Saito
Employment: Daiichi Sankyo, Inc
Yoshinori Kawaguchi
Employment: Daiichi Sankyo, Inc
Stock and Other Ownership Interests: Daiichi Sankyo, Inc
Takahiro Kamio
Employment: Daiichi Sankyo/AstraZeneca
Akihito Kojima
Employment: Daiichi Sankyo Co, Ltd
Stock and Other Ownership Interests: Daiichi Sankyo Co, Ltd
Masahiro Sugihara
Employment: Daiichi-Sankyo Co, Ltd
Kohei Shitara
Honoraria: Bristol Myers Squibb, Takeda, Janssen
Consulting or Advisory Role: Lilly, Bristol Myers Squibb, Takeda, Pfizer, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, AbbVie, GlaxoSmithKline, Daiichi Sankyo, Boehringer Ingelheim, Amgen, Astellas Pharma, Guardant Health Japan
Research Funding: MSD (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), Ono Pharmaceutical (Inst), Astellas Pharma (Inst), Eisai (Inst), Amgen (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Iqbal N, Iqbal N: Human epidermal growth factor receptor 2 (HER2) in cancers: Overexpression and therapeutic implications. Mol Biol Int 2014:852748, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Bang YJ, Feng-Yi F, et al. : HER2 screening data from ToGA: Targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 18:476-484, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki M, Iwasa S, Boku N: Trastuzumab deruxtecan for the treatment of HER2-positive advanced gastric cancer: A clinical perspective. Gastric Cancer 24:567-576, 2021 [DOI] [PubMed] [Google Scholar]
- 4.Pietrantonio F, Caporale M, Morano F, et al. : HER2 loss in HER2-positive gastric or gastroesophageal cancer after trastuzumab therapy: Implication for further clinical research. Int J Cancer 139:2859-2864, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Bang YJ, Van Cutsem E, Feyereislova A, et al. : Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 376:687-697, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Janjigian YY, Kawazoe A, Yanez PE, et al. : Pembrolizumab plus trastuzumab and chemotherapy for HER2+ metastatic gastric or gastroesophageal junction (G/GEJ) cancer: Initial findings of the global phase 3 KEYNOTE-811 study. J Clin Oncol 39, 2021. (suppl 15; 4013) [Google Scholar]
- 7.Ogitani Y, Aida T, Hagihara K, et al. : DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res 22:5097-5108, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Nakada T, Sugihara K, Jikoh T, et al. : The latest research and development into the antibody-drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem Pharm Bull (Tokyo) 67:173-185, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Shitara K, Bang YJ, Iwasa S, et al. : Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med 382:2419-2430, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Enhertu (fam-trastuzumab deruxtecan-nxki) for injection, for intravenous use. Prescribing information. Basking Ridge, NJ, Daiichi Sankyo, 2022 [Google Scholar]
- 11.Ogitani Y, Hagihara K, Oitate M, et al. : Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci 107:1039-1046, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takegawa N, Tsurutani J, Kawakami H, et al. : [fam-] trastuzumab deruxtecan, antitumor activity is dependent on HER2 expression level rather than on HER2 amplification. Int J Cancer 145:3414-3424, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Doi T, Shitara K, Naito Y, et al. : Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody–drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: A phase 1 dose-escalation study. Lancet Oncol 18:1512-1522, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Modi S, Park H, Murthy RK, et al. : Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: Results from a phase Ib study. J Clin Oncol 38:1887-1896, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modi S, Jacot W, Yamashita T, et al. : Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 387:9-20, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shitara K, Iwata H, Takahashi S, et al. : Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: A dose-expansion, phase 1 study. Lancet Oncol 20:827-836, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Makiyama A, Sukawa Y, Kashiwada T, et al. : Randomized, phase II study of trastuzumab beyond progression in patients with HER2-positive advanced gastric or gastroesophageal junction cancer: WJOG7112G (T-ACT Study). J Clin Oncol 38:1919-1927, 2020 [DOI] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology: Gastric Cancer, Version 4.2021. Plymouth Meeting, PA, National Comprehensive Cancer Network, 2021 [Google Scholar]
- 19.National Comprehensive Cancer Network : Esophageal and Esophagogstric Junctions Cancers. version 4.2021. Plymouth Meeting, PA, National Comprehensive Cancer Network, 2021 [Google Scholar]
- 20.Japanese Gastric Cancer Association : Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 17:1-19, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shitara K, Doi T, Dvorkin M, et al. : Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 19:1437-1448, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Skidmore L, Sakamuri S, Knudsen NA, et al. : ARX788, a site-specific anti-HER2 antibody-drug conjugate, demonstrates potent and selective activity in HER2-low and T-DM1-resistant breast and gastric cancers. Mol Cancer Ther 19:1833-1843, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Banerji U, van Herpen CML, Saura C, et al. : Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol 20:1124-1135, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Peng Z, Liu T, Wei J, et al. : A phase II study of efficacy and safety of RC48-ADC in patients with locally advanced or metastatic HER2-overexpressing gastric or gastroesophageal junction cancers. J Clin Oncol 38, 2020. (suppl 15; abstr 4560) [Google Scholar]
- 25.Van Cutsem E, Di Bartolomeo M, Smyth E, et al. : Primary analysis of a phase II single-arm trial of trastuzumab deruxtecan (T-DXd) in western patients (Pts) with HER2-positive (HER2+) unresectable or metastatic gastric or gastroesophageal junction (GEJ) cancer who progressed on or after a trastuzumab-containing regimen. Ann Oncol 32, 2021. (suppl 5; abstr LBA55) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized individual participant data and applicable supporting clinical trial documents may be available upon request at the Vivli website (https://vivli.org). In cases where clinical trial data and supporting documents are provided pursuant to our company policies and procedures, Daiichi Sankyo, Inc, will continue to protect the privacy of our clinical trial participants. Details on data sharing criteria and the procedure for requesting access can be found at Vivli's Daiichi Sankyo web page (https://vivli.org/ourmember/daiichi-sankyo).