PURPOSE
Cediranib, a pan-vascular endothelial growth factor receptor inhibitor, suppresses expression of homologous recombination repair (HRR) genes and increases sensitivity to poly-(ADP-ribose) polymerase inhibition in preclinical models. We investigated whether cediranib combined with olaparib improves the clinical outcomes of patients with prostate cancer.
METHODS
Patients with progressive metastatic castration-resistant prostate cancer (mCRPC) were randomly assigned 1:1 to arm A: cediranib 30 mg once daily plus olaparib 200 mg twice daily or arm B: olaparib 300 mg twice daily alone. The primary end point was radiographic progression-free survival (rPFS) in the intention-to-treat patients. The secondary end points were rPFS in patients with HRR-deficient and HRR-proficient mCRPC.
RESULTS
In the intention-to-treat set of 90 patients, median rPFS was 8.5 (95% CI, 5.4 to 12.0) and 4.0 (95% CI, 3.2 to 8.5) months in arms A and B, respectively. Cediranib/olaparib significantly improved rPFS versus olaparib alone (hazard ratio [HR], 0.617; 95% CI, 0.392 to 0.969; P = .0359). Descriptive analyses showed a median rPFS of 10.6 (95% CI, 5.9 to not assessed [NA]) and 3.8 (95% CI, 2.33 to NA) months (HR, 0.64; 95% CI, 0.272 to 1.504) among patients with HRR-deficient mCRPC, and 13.8 (95% CI, 3.3 to NA) and 11.3 (95% CI, 3.8 to NA) months (HR, 0.98; 95% CI, 0.321 to 2.988) among patients with BRCA2-mutated mCRPC in arms A and B, respectively. The incidence of grades 3-4 adverse events was 61% and 18% in arms A and B, respectively.
CONCLUSION
Cediranib combined with olaparib improved rPFS compared with olaparib alone in men with mCRPC. This combination was associated with an increased incidence of grades 3-4 adverse events. BRCA2-mutated subgroups treated with olaparib with or without cediranib were associated with a numerically longer median rPFS.
INTRODUCTION
Poly (ADP-ribose) polymerase (PARP) inhibition has emerged as a standard treatment option for patients with metastatic castration-resistant prostate cancer (mCRPC) with deleterious homologous recombination repair (HRR) gene mutations. A phase III study showed that in patients with mCRPC harboring an HRR gene mutation who progressed after a novel hormonal agent (eg, enzalutamide or abiraterone), olaparib demonstrated a statistically significant improvement in radiographic progression-free survival (rPFS) and overall survival (OS) compared with a physician's choice of treatment with either enzalutamide or abiraterone.1,2 Although variable response rates were reported with different HRR gene mutations, the objective response rate (ORR) to olaparib was highest among patients with BRCA1- or BRCA2-mutated mCRPC. A single-arm phase II study of rucaparib also showed an ORR of 44% in patients with BRCA1- or BRCA2-mutated mCRPC, who had been treated with a novel hormonal agent and a taxane-based chemotherapy.3
CONTEXT
Key Objective
Olaparib, an inhibitor of poly (ADP-ribose) polymerase, demonstrated clinical efficacy in patients with metastatic castration-resistant prostate cancer (mCRPC) with altered DNA repair genes, who represent about 10%-20% of our patients in our clinic. Our randomized study evaluated whether combining an antiangiogenic agent, cediranib, to olaparib can improve the outcomes of patients with mCRPC.
Knowledge Generated
Combination of cediranib and olaparib demonstrated an improved radiographic progression-free survival compared with olaparib alone in patients with mCRPC. However, this combination was associated with an increased rate of significant adverse effects.
Relevance
To our knowledge, this is the first study to demonstrate the potential efficacy of combining an antiangiogenic agent with a poly (ADP-ribose) polymerase inhibitor for patients with mCRPC. Further correlative studies are needed to elucidate the mechanism of synergy between these two agents in prostate cancer and to provide a clearer direction for the clinical development.
Patients with mCRPC with either somatic or germline HRR mutations comprise approximately 10%-20% of the patient population.4,5 To broaden the patient population eligible to receive a PARP inhibitor, strategies that induce an HRR-deficient (HRD) phenotype in tumor cells to enable the synthetic lethality of PARP inhibitors are needed. Preclinical studies in lung, breast, and prostate cancer cell lines including DU145 and PC36–8 demonstrate that a hypoxic tumor microenvironment downregulates the expression of HRR genes, including BRCA1, BRCA2, or RAD51, and thus increases sensitivity to PARP inhibition.7,9,10 Kaplan et al11 reported that cediranib, by inducing tumor hypoxia and by inhibiting platelet-derived growth factor receptor-beta, suppresses the expression of BRCA1, BRCA2, and RAD51 in preclinical models of both BRCA-mutant and wild-type ovarian and breast cancer cell lines. To test the hypothesis that cediranib sensitizes prostate cancers to olaparib independent of deleterious HRR gene mutation status, a randomized phase II trial was designed to compare olaparib alone to olaparib combined with cediranib in patients with mCRPC.
METHODS
Study Design and Oversight
This randomized, open-label, phase II trial was conducted at nine US institutions in the Experimental Therapeutic Clinical Trials Network of the National Cancer Institute (NCI). Oversight of the study was provided by the Clinical Trials Monitoring Service of Theradex twice yearly. Patients were registered and randomly assigned in a 1:1 ratio to arm A: cediranib plus olaparib, or arm B: olaparib alone. Patients continued study treatment until they met any of the following discontinuation criteria: (1) radiographic disease progression as defined by the Prostate Cancer Working Group 3 (PCWG3), (2) grade 3-4 treatment-related events that did not resolve to grade 1 or less within 14 days, according to the NCI's Common Terminology Criteria for Adverse Events version 5.0, or (3) withdrawal of consent. The dosing could be interrupted for up to 14 days for treatment-related toxicities. For patients in the cediranib and olaparib combination arm, the starting doses and dose modification schema were based on the results of a phase I study in patients with ovarian and breast cancer.12 The starting dose of cediranib was 30 mg orally once daily, and dose reductions to 20 mg and 15 mg once daily were allowed. The starting dose of olaparib was 200 mg orally twice daily (tablet formulation) and olaparib dose reductions to 150 mg twice daily and 100 mg twice daily were allowed. For patients on olaparib monotherapy, a starting dose of 300 mg twice daily was chosen because it was considered a maximally effective dose with acceptable tolerability, and it was the dose chosen for other trials including the phase III PROFOUND trial in mCRPC.1 Two dose reductions of olaparib were allowed, to 250 mg twice daily and 200 mg twice daily. Patients in the olaparib monotherapy arm had an option to cross over to receive cediranib/olaparib after radiographic progression.
Eligibility
Eligible participants were men age ≥ 18 years with histologically confirmed prostate adenocarcinoma that is progressive, metastatic, and castration-resistant by PCWG3 criteria.13 Participants were required to have received at least one prior therapy for mCRPC, an Eastern Cooperative Oncology Group performance status of ≤ 1 or Karnofsky score ≥ 70%, and adequate organ function including hemoglobin ≥ 10 g/dL without packed red blood cell transfusion for 28 days before enrollment, aspartate transferase and alanine transferase < 3 × upper limit of normal unless liver metastases present, and creatinine clearance > 50 mL/min, calculated using the Cockcroft-Gault formula.14 Prior PARP inhibitor was not allowed. Prior platinum-based chemotherapy was allowed. The study was performed after obtaining approval from the appropriate institutional review board for each participating site and in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. The study was sponsored by the US NCI and registered on ClinicalTrials.gov (identifier: NCT02893917). All patients provided written informed consent before enrollment. The full Protocol (online only) is included in the Data Supplement (online only).
Safety and Toxicity Assessment
Serum chemistry and hematologic assessments were performed every 2 weeks for the first 8 weeks and every 4 weeks thereafter. All patients receiving cediranib were provided with a blood pressure cuff and were required to record their blood pressure twice daily. Patients were instructed to contact the study staff immediately for any blood pressure reading ≥ 140 mm Hg/90 mm Hg.
Biomarker Assessment
All study participants were required to undergo tumor biopsies at baseline and during week 4 of treatment and to provide a whole-blood sample for germline DNA. Extracted nucleic acids were analyzed using the BROCA homologous recombination sequencing assay, a targeted-capture, massively parallel sequencing test developed at the University of Washington.15 Tumors were classified as HRD if a deleterious mutation or biallelic loss was present in any of the following genes: ATM, BARD1, BRCA1, BRCA2, BRIP1, CDK12 (somatic mutations only), NBN, PALB2, RAD51C, and RAD51D. Otherwise, the tumors were classified as HRR-proficient (HRP).
End Point Assessment
The primary end point of the study was the investigator-assessed rPFS. The rPFS was defined as the time interval from random assignment to the first occurrence of any of the following: (1) bone scan progression defined by PCWG3 criteria: (a) the first bone scan with ≥ 2 new lesions compared with baseline observed in < 12 weeks from random assignment and confirmed by a second bone scan taken ≥ 6 weeks later showing ≥ 2 additional new lesions (a total of ≥ 4 new lesions compared with baseline); (b) the first bone scan with ≥ 2 new lesions compared with baseline observed in ≥ 12 weeks from random assignment and verified on the next bone scan ≥ 6 weeks later (a total of ≥ 2 new lesions compared with baseline); (2) soft tissue progression defined by modified RECIST version 1.1 (v1.1)16; or (3) death from any cause. Participants who were alive without disease progression were censored on the date of the last imaging evaluation. Tumor assessments were conducted every 8 weeks by computed tomography or magnetic resonance imaging and technetium-99 whole-body bone scan for the first 24 weeks of the study, then every 12 weeks thereafter.
Secondary end points included rPFS analyses among subgroups by HRR gene mutation status, OS, ORR in patients with measurable disease by RECIST v1.1, prostate-specific antigen (PSA)50 response rate, defined as ≥ 50% PSA decline from baseline confirmed by a second value obtained at least three weeks later, and toxicity as graded by Common Terminology Criteria for Adverse Events version 5.0.
Statistical Analysis
The target accrual was 90 patients for an expected 84 evaluable patients (42 per arm). This provided at least 90% power with a two-sided type I error rate of 5% to detect a hazard ratio (HR) of 0.55, which corresponds to a 4-month improvement in rPFS for the combination arm. An interim futility analysis was performed at 37 progressions/deaths (half of the total required events). The safety analyses included all patients who received at least one dose of the study medication.
The primary end point of rPFS was assessed in the intention-to-treat (ITT) analysis set of all randomly assigned patients between cediranib plus olaparib versus olaparib alone arms. The survival curve was calculated using the Kaplan-Meier method and evaluated using a log-rank test. Statistical significance set at P < .05 was considered statistically significant. HR and its 95% CIs between two groups were estimated using the Cox proportional hazards regression model for rPFS. The study was not powered for secondary end points including OS in the ITT, rPFS in biomarker subgroups, ORR, PSA50 response rate, and safety and toxicity. Descriptive statistics were used to analyze these secondary end points, where P value was not calculated. Descriptive statistics for the patients' baseline clinical characteristics, including the median and range for continuous variables, as well as percentages and frequencies for categorical variables, are presented. Statistical data analysis was performed using R Statistical Software version 4.0.0.17
RESULTS
Patient Characteristics
Overall, 90 patients were enrolled in the study between August 2017 and February 2019: 45 were randomly assigned to cediranib/olaparib and 45 to olaparib (Fig 1). Table 1 presents the baseline patient characteristics. The treatment groups were generally balanced across the baseline characteristics. Ten patients (22%) in each arm had liver metastasis. Twenty (44%) and 12 (26%) received ≥ 2 prior cytotoxic chemotherapies in the cediranib/olaparib and olaparib arms, respectively.
FIG 1.

CONSORT diagram. ITT, intention-to-treat; PD, progression of disease.
TABLE 1.
Demographics and Baseline Patient Characteristics
HRR Gene Status
Of the 90 patients who underwent biopsies, 84 (93%) had tumor samples sufficient for the sequencing of HRR genes using the BROCA homologous recombination assay. Sequencing revealed that 26 (29%) patients had tumors harboring at least one deleterious HRR gene mutation and were categorized as HRD mCRPC: 12 of 45 (26%) and 14 of 45 (31%) patients in the cediranib/olaparib and olaparib monotherapy arms, respectively. As shown in the Data Supplement, the most common HRR gene mutations were in BRCA2 (n = 16; 18%), CDK12 (n = 8; 9%), and ATM (n = 3; 3%). Of the 16 patients with BRCA2 mutations, nine were in the cediranib/olaparib combination and seven in the olaparib monotherapy arms. Four (4%) patients had germline BRCA2 mutations with two patients in each arm. A case of BRCA2 reversion mutation was detected in a 4 week on-treatment biopsy in a patient whose best overall response was a progressive disease. Descriptions of the HRR mutations and treatment responses in evaluable patients are provided in the Data Supplement.
Clinical Outcomes
rPFS in the ITT analysis set.
The final analysis was conducted in October 2020. The data cutoff was October 1, 2020. With a median follow-up of 26.1 months, 79 primary events occurred. Median (95% CI) rPFS was 8.5 (5.4 to 12.0) and 4.0 (3.2 to 8.5) months for the cediranib/olaparib and olaparib arms, respectively (HR, 0.617; 95% CI, 0.392 to 0.969; P = .0359; Fig 2A).
FIG 2.
Kaplan-Meier curves in the ITT analysis set and biomarker subgroups: (A) rPFS in the ITT, (B) OS in the ITT, (C) rPFS in the homologous recombination repair-deficient subgroup, and (D) rPFS in the homologous recombination repair-proficient subgroup. (E) An exploratory analysis of rPFS in BRCA2 mutation subgroup. Data cutoff date, October 1, 2020. The number in parentheses refers to the cumulative number censored associated with number at risk. C + O, cediranib plus olaparib; HR, hazard ratio; ITT, intention-to-treat; NA, not assessed; O, olaparib; OS, overall survival; rPFS, radiographic progression free-survival; Total, total analytic unit.
rPFS in HRD, HRP, and BRCA2 mutation subgroups.
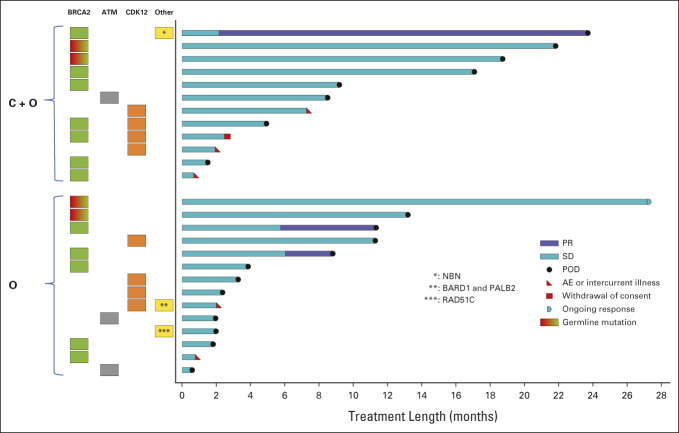
In patients with HRD mCRPC (n = 26), median rPFS was 10.6 (5.9 to not assessed [NA]) and 3.8 (2.33 to NA) months for the cediranib/olaparib and olaparib arms, respectively (Fig 2C). The HR was 0.64 (0.272 to 1.504). The duration is shown in a swimmer plot in Figure 3 for patients with HRD mCRPC. In both arms, the patients with a BRCA2 mutation remained on treatment longer than the others. In patients with HRP mCRPC, median rPFS was 5.5 (3.73 to 11.77) and 4.0 (3.73 to 8.47) months for the cediranib/olaparib and olaparib arms, respectively (Fig 2D). The HR was 0.75 (0.448 to 1.348). An exploratory analysis of rPFS in patients with somatic or germline BRCA2-mutated mCRPC (n = 16) was performed. The median rPFS in BRCA2 mutation subgroup was 13.83 (3.30 to NA) and 11.33 (3.83 to NA) months for the cediranib/olaparib and olaparib arms, respectively (Fig 2E; insufficient samples to estimate the upper bound of 95% CI for median survival). The HR was 0.98 (0.321 to 2.988).
FIG 3.
Swimmer plot of duration of treatment in patients with homologous recombination repair-deficient metastatic castration-resistant prostate cancer subgroup. Each bar represents an individual patient with the length corresponding to length of time on study drug. The panel to the left of the plot shows the homologous recombination deficiency subgroup of each patient and homologous recombination gene mutation type identified in tumor or blood samples. Mutations are somatic in origin unless specified as germline. AE, adverse event; C + O, cediranib plus olaparib; O, olaparib; POD, disease progression; PR, partial response; SD, stable disease.
Overall survival.
At a median follow up of 26.1 months, 42 deaths were reported. Median (95% CI) OS was 11.8 (10.33 to NA) and 17.3 (15.5 to NA) months for the cediranib/olaparib and olaparib arms, respectively (Fig 2B). The HR was 1.30 (0.705 to 2.399). Given 38% (n = 17) patients in the olaparib arm crossed over to the combination arm upon radiographic progression, a sensitivity analysis of the OS was performed by excluding these patients. The result is provided in the Data Supplement.
ORR and duration of response.
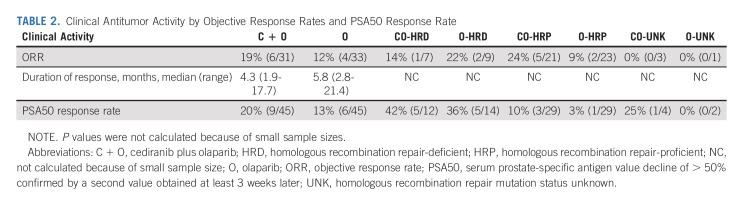
Of the 90 patients randomly assigned, 64 (71%) patients were evaluable for objective response by RECIST v1.1 criteria. The ORRs were 19% and 12% in the cediranib/olaparib and olaparib arms, respectively. The median durations of response were 4.3 months and 5.8 months in the cediranib/olaparib and olaparib arms, respectively. The ORR according to HRR gene mutation status is summarized in Table 2. The change in the tumor burden (waterfall plot) is shown in Figure 4A.
TABLE 2.
Clinical Antitumor Activity by Objective Response Rates and PSA50 Response Rate
FIG 4.
Waterfall plots of (A) best tumor burden changes and (B) best PSA change. (A) Each bar represents an individual patient with the length corresponding to the maximal percentage change from baseline in sum of the target lesions according to RECIST v1.1. (B) Each bar represents an individual with the length corresponding to the best percentage change of PSA from the baseline value. Each bar is color-coded by the HRR gene mutation status. C + O, cediranib plus olaparib; HRP, homologous recombination repair-proficient; HRR, homologous recombination repair; O, olaparib; PSA, prostate-specific antigen.
PSA50 response.
The PSA50 response rates were 20% and 13% in the cediranib/olaparib and olaparib arms, respectively. The PSA50 response rates according to the HRR gene mutation status are summarized in Table 2. The best PSA changes are shown in Figure 4B.
Safety
Of the 90 patients, 89 who received at least one dose of study treatment were included in the safety analysis (Data Supplement). Ninety-four percent (84/89) of patients experienced treatment-related adverse events. The frequency of treatment-related adverse events was similar between the arms: 95% and 93% in the cediranib/olaparib and olaparib monotherapy arms, respectively. Grade 3-4 event rates were higher in cediranib/olaparib arm with 61% compared with 18% in olaparib monotherapy arm. Thirty-seven (84%) and 16 (36%) patients required dose reduction because of an adverse event (AE) in the cediranib/olaparib and olaparib monotherapy arms, respectively. No acute myeloid leukemia or myelodysplastic syndrome was reported in this study. One treatment-related fatal intracranial hemorrhage occurred in a patient who received cediranib/olaparib. This participant had a history of fall before the enrollment, who later died due to a large subdural hematoma on day 5 of the study.
DISCUSSION
This randomized phase II trial demonstrated that olaparib combined with cediranib improved median rPFS by 4.5 months with a 38% reduction in the risk of radiographic progression or death compared with olaparib alone in 90 patients with mCRPC. To our knowledge, this is the first trial of a PARP inhibitor combined with a vascular endothelial growth factor receptor (VEGFR) inhibitor in mCRPC that demonstrated an improvement of rPFS with the combination over a PARP inhibitor alone. However, this was accompanied by increased rates of grades 3 or 4 toxicities.
Our study found that 29% of the patients had HRR gene-mutated mCRPC including 18% BRCA2 mutation. These seemingly high percentages of the HRR gene mutation and BRCA2 mutation may have been enriched by investigators' knowledge of patients' mutation status whenever available by other testing and preferential enrollment to our study. Our report may not reflect the real-world prevalence of HRR gene alteration.
Descriptive rPFS analyses in the prespecified biomarker subgroups indicated no significant difference between the arms among the HRP subgroup. We, however, observed a numerical difference among the HRD subgroup (10.6 v 3.83 months in combination v monotherapy arms, respectively, HR 0.64 [95% CI, 0.272 to 1.504]). To dissect this further, we performed an exploratory analysis of rPFS by BRCA2 mutation. Other trials of a PARP inhibitor in patients with mCRPC with BRCA1 or BRCA2 mutations reported median rPFS ranging from 8.3 to 11.2 months.1,3,18–20 Our study showed a median rPFS of 11.3 months in patients with BRCA2 mutation treated with olaparib monotherapy and 13.8 months for those treated with olaparib with cediranib. Our data are consistent with the observation that a deleterious BRCA2 mutation is the strongest predictor of the benefit of olaparib in mCRPC. Whether a VEGFR inhibitor provides any added benefit for a BRCA2- or other HRR gene-mutated mCRPC remains a question.
Furthermore, this exploratory rPFS analysis by BRCA2 mutation status provides a likely explanation for why the HRD subgroup performed less well than expected with olaparib monotherapy. Of the14 patients with HRD mCRPC treated with olaparib monotherapy, seven (50%) had a BRCA2 mutation and the remaining had CKD12 (n = 4), ATM (n = 2), and RAD51C (n = 1) without a co-occurring BRCA2 mutation. None of the patients with non–BRCA2-mutated HRD mCRPC (n = 10) achieved an objective response in either arm. Although the number was too small to make inferences, the minimal clinical activity in the non-BRCA2 mutation subgroups aligns with other studies.1,19,21
Safety analyses of the cediranib/olaparib combination were consistent with previous reports in other tumors without any unexpected serious AEs.12,22 Compared with olaparib monotherapy, the combination was associated with increased incidence of grade 3 or 4 AEs (61% v 18%), including hypertension (23%), fatigue/asthenia (16%), and diarrhea (9%) as well as an increased incidence of other AEs of all grades including anorexia (48%) and weight loss (25%). Although these AEs were manageable with support care, these AEs were the common reason for dose interruption and explained the higher rates of dose reduction (84%) and discontinuation (25%). The AE profile of olaparib monotherapy was also consistent with other studies on prostate cancer.1,18,19
A key limitation of our study was that it was not powered for subgroup analyses by biomarkers. The small sample sizes in each biomarker subgroup and wide CIs limited our ability to evaluate the observed clinical activity using the biomarkers with statistical confidence. Thus, our biomarker analyses are descriptive, and should only be interpreted as hypothesis-generating.
Another weakness is that we did not have strong preclinical data in prostate cancer models to support the hypothesis. Preclinical data demonstrating the synergy between cediranib and olaparib were primarily from ovarian and breast cancer models. More robust preclinical and correlative studies are needed to strengthen our hypothesis and to provide a clearer direction for the next step in clinical development. To this end, additional correlative studies, including whole exome and transcriptome analyses from the baseline and on-treatment tumor biopsy samples, are planned and will be reported separately.
In conclusion, our study is, to our knowledge, the first randomized study to demonstrate the potential of clinical efficacy of adding a VEGFR inhibitor to a PARP inhibitor in 90 patients with mCRPC. Unfortunately, the combination of cediranib and olaparib is associated with increased toxicity rates. Although they were manageable with supportive care, treatment interruptions were frequent. Descriptive analyses of rPFS in biomarker subgroups indicated that a deleterious BRCA2 mutation is the best predictor of olaparib efficacy. Whether cediranib adds any benefit to olaparib in BRCA2-mutated mCRPC remains unclear.
For future direction of this combination, the following points should be considered. One is that an antiangiogenic agent with easier tolerability would enable better clinical testing. Another is the need for more robust preclinical data in HRD and HRP mCRPC models to show the mechanism of synergy. The other is the identification of biomarkers to select patients who derive benefit from the combination versus PARP inhibitor monotherapy. We speculate that our ongoing whole-exome and transcriptome analyses may help identify a biomarker by examining the genetic landscape beyond HRR genes and evaluating the changes of the gene expression profile with the treatment.
Joseph W. Kim
Stock and Other Ownership Interests: Abiomed (I), Celgene (I), Johnson & Johnson (I)
Consulting or Advisory Role: Voluntis, Sanofi, EMD Serono, Clovis Oncology
Research Funding: Immune Design (Inst), Hummingbird Bioscience (Inst), Exelixis (Inst), ADC Therapeutics (Inst), Regeneron (Inst), Hoffmann‐La Roche, Ltd (Inst)
Rana R. McKay
Consulting or Advisory Role: Janssen, Novartis, Tempus, Exelixis, Pfizer, Bristol Myers Squibb, Astellas Medivation, Dendreon, Bayer, Sanofi, Merck, Vividion Therapeutics, Calithera Biosciences, AstraZeneca, Myovant Sciences, Caris Life Sciences, Sorrento Therapeutics, AVEO, Seattle Genetics
Research Funding: Pfizer (Inst), Bayer (Inst), Tempus (Inst)
Mary-Ellen Taplin
Honoraria: Janssen-Ortho, Clovis Oncology, UpToDate, Research to Practice, Pfizer, AstraZeneca, Roivant, AbbVie, Arcus Biosciences, Constellation Pharmaceuticals, Epizyme, Targeted Oncology, Arvinas, Blue Earth Diagnostics, Hengrui Therapeutics
Consulting or Advisory Role: Janssen-Ortho, Bayer, Best Doctors Inc, UpToDate, Clovis Oncology, Research to Practice, Myovant Sciences, Pfizer, AstraZeneca, Arcus Ventures
Research Funding: Janssen-Ortho (Inst)
Travel, Accommodations, Expenses: APCCC meeting
Nancy B. Davis
Consulting or Advisory Role: Janssen Biotech
Research Funding: AstraZeneca (Inst), Roche (Inst), Pfizer (Inst), Merck (Inst), Incyte (Inst), Mirati Therapeutics (Inst), Seattle Genetics/Astellas (Inst), Calithera Biosciences (Inst), Immunomedics (Inst), Bristol Myers Squibb (Inst), Exelixis (Inst), Gilead Sciences (Inst)
Paul Monk
Honoraria: Sanofi/Aventis
Consulting or Advisory Role: Dendreon, Sanofi, Myovant Sciences, Pfizer/NCCN
Speakers' Bureau: Janssen
Leonard J. Appleman
Consulting or Advisory Role: AADi
Research Funding: Pfizer (Inst), Exelixis (Inst), Bristol Myers Squibb (Inst), Astellas Pharma (Inst), Novartis (Inst), Bayer (Inst), Merck (Inst), Genentech/Roche (Inst), AVEO (Inst), Peloton Therapeutics (Inst), Calithera Biosciences (Inst), Seattle Genetics (Inst), Inovio Pharmaceuticals (Inst), Eisai (Inst), Lilly (Inst), Amgen (Inst), Surface Oncology (Inst), BioNTech (Inst), Epizyme (Inst), Janssen Oncology
Other Relationship: Pfizer
Uncompensated Relationships: Exelixis
Primo N. Lara Jr
Consulting or Advisory Role: Janssen, Calithera Biosciences (Inst)
Research Funding: Janssen Biotech (Inst), Taiho Pharmaceutical (Inst)
Ulka N. Vaishampayan
Consulting or Advisory Role: Pfizer, Exelixis, Bayer, Bristol Myers Squibb/Medarex, Merck Serono, Advanced Accelerator Applications, Aveo, Gilead Sciences, Sanofi/Aventis
Speakers' Bureau: Pfizer, Bayer, Exelixis
Research Funding: Bristol Myers Squibb, Merck KGaA
Jingsong Zhang
Honoraria: Sanofi, AstraZeneca/MedImmune, Bayer, Seattle Genetics, Sanofi/Aventis, Pfizer, EMD Serono, Dendreon
Consulting or Advisory Role: AstraZeneca/MedImmune, Bayer, Dendreon, Pfizer, EMD Serono
Speakers' Bureau: Sanofi, AstraZeneca, Seattle Genetics, Dendreon
Asit K. Paul
Consulting or Advisory Role: Tempus, Genzyme, Bayer, Cardinal Health
Travel, Accommodations, Expenses: Cardinal Health
Eliezer M. Van Allen
Stock and Other Ownership Interests: Syapse, Tango Therapeutics, Genome Medical, Microsoft, Ervaxx, Monte Rosa Therapeutics, Manifold Bio, Genomic Life
Consulting or Advisory Role: Syapse, Roche, Third Rock Ventures, Takeda, Novartis, Genome Medical, InVitae, Illumina, Tango Therapeutics, Ervaxx, Janssen, Monte Rosa Therapeutics, Manifold Bio, Genomic Life
Speakers' Bureau: Illumina
Research Funding: Bristol Myers Squibb, Novartis, Sanofi (Inst)
Patents, Royalties, Other Intellectual Property: Patent on discovery of retained intron as source of cancer neoantigens (Inst), Patent on discovery of chromatin regulators as biomarkers of response to cancer immunotherapy (Inst), Patent on clinical interpretation algorithms using cancer molecular data (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Ying Huang
Employment: Novartis Institutes for BioMedical Research
Stock and Other Ownership Interests: Novartis
Travel, Accommodations, Expenses: Novartis Institutes for BioMedical Research
Massimo Loda
Stock and Other Ownership Interests: Methylex
Patents, Royalties, Other Intellectual Property: cdx2 antibodies—royalties
Geoffrey I. Shapiro
Consulting or Advisory Role: G1 Therapeutics, Lilly, Pfizer, Roche, Merck Serono, Sierra Oncology, Cybrexa Therapeutics, Ipsen, Bayer, Fusion Pharmaceuticals, Bicycle Therapeutics, Almac Diagnostics, Astex Pharmaceuticals, Daiichi Sankyo, Angiex, Seattle Genetics, Artios, Boehringer Ingelheim, Concarlo, Atrin Pharmaceuticals, Syros Pharmaceuticals, Zentalis, CytomX Therapeutics, Blueprint Medicines, Kymera, Janssen Oncology, Xinthera, ImmunoMet
Research Funding: Pfizer (Inst), Genentech (Inst), Bayer (Inst), Immune Design (Inst), Vertex (Inst), Millennium (Inst), Puma Biotechnology (Inst), Tensha Therapeutics (Inst), Covidien (Inst), Novartis (Inst), Cellceutix (Inst), Sanofi (Inst), Cyclacel (Inst), Mirati Therapeutics (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Lilly (Inst), Aileron Therapeutics (Inst), PharmaMar (Inst), PTC Therapeutics (Inst), Roche (Inst), CanBas (Inst), Tesaro (Inst), Merck Serono (Inst), Sierra Oncology (Inst), Syros Pharmaceuticals (Inst), Curis (Inst), Merck (Inst), Array BioPharma (Inst), Seattle Genetics (Inst), Clovis Oncology (Inst), Exelixis (Inst), Boehringer Ingelheim (Inst), Esperas Pharma (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), Tango Therapeutics (Inst), Senhwa (Inst), Biosplice (Inst), Cyteir (Inst), Abbvie (Inst)
Patents, Royalties, Other Intellectual Property: Patent #: 9872874 Title: Dosage regimen for sapacitabine and seliciclib Issue Date: January 23, 2018, Provisional Patent #:62/538,319 Title: Compositions and methods for predicting response and resistance to CDK4/6 inhibition Filed: July 28, 2017
Travel, Accommodations, Expenses: Lilly, Pfizer, Bicycle Therapeutics, G1 Therapeutics, Sierra Oncology, Bayer
Peter M. Glazer
Stock and Other Ownership Interests: Cybrexa Therapeutics, Gennao Bio
Consulting or Advisory Role: Cybrexa Therapeutics, pHLIP, Gennao Bo
Research Funding: Gennao Bio
Patents, Royalties, Other Intellectual Property: Patents related to gene editing and gene therapy via peptide nucleic acids. Patents related to oncometabolites and DNA repair for cancer therapy. Patents related to antibody-mediated cancer therapy
Patricia M. LoRusso
Stock and Other Ownership Interests: BAKX Therapeutics
Honoraria: Five Prime Therapeutics
Consulting or Advisory Role: Genentech, CytomX Therapeutics, Roche/Genentech, Halozyme, Five Prime Therapeutics, Agenus, Agios, Cybrexa Therapeutics, Sotio, AbbVie, Genmab, Takeda, TYME, IQvia, Trial to Reduce IDDM in the Genetically at Risk (TRIGR), Pfizer, ImmunoMet, Black Diamond Therapeutics, GlaxoSmithKline, QED Therapeutics, AstraZeneca, EMD Serono, Shattuck Labs, Astellas Pharma, Salarius Pharmaceuticals, Silverback Therapeutics, Macrogenics, Kyowa Kirin International, Kineta, Zentalis, Molecular Templates, Molecular Templates, ABL Bio, SK Life Sciences, ST Cube, Bayer, I-Mab, Seattle Genetics, ImCheck Therapeutics, Relay Therapeutics, Stemline Therapeutics, Mekanistic Therapeutics, Compass Therapeutics, BAKX Therapeutics, Scenic Biotech, Qualigen Therapeutics, Roivant, NeuroTrials
Research Funding: Genentech (Inst)
Travel, Accommodations, Expenses: Genentech
Yu Shyr
Consulting or Advisory Role: Janssen Research & Development, Novartis, Roche/Genentech, Pfizer, GlaxoSmithKline, AstraZeneca
Patents, Royalties, Other Intellectual Property: Royalty for TNBCType for Insight Genetics. TNBCType is a web-based subtyping tool TNBCtype for candidate TNBC samples using our gene expression meta data and classification method
Elizabeth M. Swisher
Leadership: IDEAYA Biosciences
Daniel P. Petrylak
Stock and Other Ownership Interests: Bellicum Pharmaceuticals, TYME
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Roche, Astellas Pharma, AstraZeneca, Lilly, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Clovis Oncology, Incyte, Janssen, Pharmacyclics, Seattle Genetics, Urogen Pharma, Advanced Accelerator Applications, Ipsen, Bicycle Therapeutics, Mirati Therapeutics, Monopteros Therapeutics, Regeneron, Gilead Sciences
Research Funding: Progenics (Inst), Sanofi (Inst), Endocyte (Inst), Genentech (Inst), Merck (Inst), Astellas Medivation (Inst), Novartis (Inst), AstraZeneca (Inst), Bayer (Inst), Lilly (Inst), Innocrin Pharma (Inst), MedImmune (Inst), Pfizer (Inst), Roche (Inst), Seattle Genetics (Inst), Clovis Oncology (Inst), Bristol Myers Squibb (Inst), Advanced Accelerator Applications (Inst), Agensys (Inst), BioXCel therapeutics (Inst), Eisai (Inst), Mirati Therapeutics (Inst), Replimune (Inst), Medivation (Inst), Gilead Sciences (Inst)
Expert Testimony: Celgene, Sanofi
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2020 ASCO Genitourinary Cancer Symposium, San Francisco, CA, February 13-15, 2020; and the 2021 ASCO Genitourinary Cancer Symposium, virtual, February 11-13, 2021.
SUPPORT
Supported by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI) and supported by UM1 CA186689 and UM1 CA186709. The study drugs and automated blood pressure cuff were provided by the CTEP of the NCI via the Cooperative Research & Development Agreements (CRADA) with AstraZeneca. Tissue processing and nucleic acid extractions were funded by a Biomarker Supplement to UM1 CA186709 (M.L. and G.I.S., Project Leaders), NCI P50CA211024, DoD PC160357, DoD PC180582, and the Prostate Cancer Foundation (M.L.). The biomarker work by EMS was funded by a contract with the CTEP. J.W.K. and P.M.L. were supported by UM1 CA186689. R.R.M. was supported by PCFYI2015. M.-E.T., E.M.V.A., M.L. and G.I.S. were supported by UM1 CA186709. P.M.G. was supported by R35CA197574. The protocol was developed at the AACR/ASCO Methods in Clinical Cancer Research Workshop in Vail, CO in 2015.
CLINICAL TRIAL INFORMATION
NCT02893917 (NCI/CTEP/ETCTN 9984)
DATA SHARING STATEMENT
Deidentified data from this article will be available for 1 year from the time of publication for data sharing proposal by submitting request to the corresponding author, who will review the proposal with the sponsor, the NCI.
AUTHOR CONTRIBUTIONS
Conception and design: Joseph W. Kim, Rana R. McKay, Mary-Ellen Taplin, Geoffrey I. Shapiro, Peter M. Glazer, Patricia M. LoRusso, S. Percy Ivy, Daniel P. Petrylak
Financial support: Primo N. Lara Jr, Geoffrey I. Shapiro, Patricia M. LoRusso
Administrative support: Joseph W. Kim, Primo N. Lara Jr, Patricia M. LoRusso, S. Percy Ivy
Provision of study materials or patients: Joseph W. Kim, Rana R. McKay, Mary-Ellen Taplin, Nancy B. Davis, Paul Monk, Leonard J. Appleman, Primo N. Lara Jr, Ulka N. Vaishampayan, Glenn Bubley, Massimo Loda, Geoffrey I. Shapiro, S. Percy Ivy, Elizabeth M. Swisher, Daniel P. Petrylak
Collection and assembly of data: Joseph W. Kim, Rana R. McKay, Marc R. Radke, Mary‐Ellen Taplin, Paul Monk, Leonard J. Appleman, Primo N. Lara Jr, Ulka N. Vaishampayan, Jingsong Zhang, Asit K. Paul, Glenn Bubley, Eliezer M. Van Allen, Serhan Unlu, Ying Huang, Massimo Loda, Elizabeth M. Swisher
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Trial of Olaparib With or Without Cediranib for Metastatic Castration-Resistant Prostate Cancer: Results From National Cancer Institute 9984
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Joseph W. Kim
Stock and Other Ownership Interests: Abiomed (I), Celgene (I), Johnson & Johnson (I)
Consulting or Advisory Role: Voluntis, Sanofi, EMD Serono, Clovis Oncology
Research Funding: Immune Design (Inst), Hummingbird Bioscience (Inst), Exelixis (Inst), ADC Therapeutics (Inst), Regeneron (Inst), Hoffmann‐La Roche, Ltd (Inst)
Rana R. McKay
Consulting or Advisory Role: Janssen, Novartis, Tempus, Exelixis, Pfizer, Bristol Myers Squibb, Astellas Medivation, Dendreon, Bayer, Sanofi, Merck, Vividion Therapeutics, Calithera Biosciences, AstraZeneca, Myovant Sciences, Caris Life Sciences, Sorrento Therapeutics, AVEO, Seattle Genetics
Research Funding: Pfizer (Inst), Bayer (Inst), Tempus (Inst)
Mary-Ellen Taplin
Honoraria: Janssen-Ortho, Clovis Oncology, UpToDate, Research to Practice, Pfizer, AstraZeneca, Roivant, AbbVie, Arcus Biosciences, Constellation Pharmaceuticals, Epizyme, Targeted Oncology, Arvinas, Blue Earth Diagnostics, Hengrui Therapeutics
Consulting or Advisory Role: Janssen-Ortho, Bayer, Best Doctors Inc, UpToDate, Clovis Oncology, Research to Practice, Myovant Sciences, Pfizer, AstraZeneca, Arcus Ventures
Research Funding: Janssen-Ortho (Inst)
Travel, Accommodations, Expenses: APCCC meeting
Nancy B. Davis
Consulting or Advisory Role: Janssen Biotech
Research Funding: AstraZeneca (Inst), Roche (Inst), Pfizer (Inst), Merck (Inst), Incyte (Inst), Mirati Therapeutics (Inst), Seattle Genetics/Astellas (Inst), Calithera Biosciences (Inst), Immunomedics (Inst), Bristol Myers Squibb (Inst), Exelixis (Inst), Gilead Sciences (Inst)
Paul Monk
Honoraria: Sanofi/Aventis
Consulting or Advisory Role: Dendreon, Sanofi, Myovant Sciences, Pfizer/NCCN
Speakers' Bureau: Janssen
Leonard J. Appleman
Consulting or Advisory Role: AADi
Research Funding: Pfizer (Inst), Exelixis (Inst), Bristol Myers Squibb (Inst), Astellas Pharma (Inst), Novartis (Inst), Bayer (Inst), Merck (Inst), Genentech/Roche (Inst), AVEO (Inst), Peloton Therapeutics (Inst), Calithera Biosciences (Inst), Seattle Genetics (Inst), Inovio Pharmaceuticals (Inst), Eisai (Inst), Lilly (Inst), Amgen (Inst), Surface Oncology (Inst), BioNTech (Inst), Epizyme (Inst), Janssen Oncology
Other Relationship: Pfizer
Uncompensated Relationships: Exelixis
Primo N. Lara Jr
Consulting or Advisory Role: Janssen, Calithera Biosciences (Inst)
Research Funding: Janssen Biotech (Inst), Taiho Pharmaceutical (Inst)
Ulka N. Vaishampayan
Consulting or Advisory Role: Pfizer, Exelixis, Bayer, Bristol Myers Squibb/Medarex, Merck Serono, Advanced Accelerator Applications, Aveo, Gilead Sciences, Sanofi/Aventis
Speakers' Bureau: Pfizer, Bayer, Exelixis
Research Funding: Bristol Myers Squibb, Merck KGaA
Jingsong Zhang
Honoraria: Sanofi, AstraZeneca/MedImmune, Bayer, Seattle Genetics, Sanofi/Aventis, Pfizer, EMD Serono, Dendreon
Consulting or Advisory Role: AstraZeneca/MedImmune, Bayer, Dendreon, Pfizer, EMD Serono
Speakers' Bureau: Sanofi, AstraZeneca, Seattle Genetics, Dendreon
Asit K. Paul
Consulting or Advisory Role: Tempus, Genzyme, Bayer, Cardinal Health
Travel, Accommodations, Expenses: Cardinal Health
Eliezer M. Van Allen
Stock and Other Ownership Interests: Syapse, Tango Therapeutics, Genome Medical, Microsoft, Ervaxx, Monte Rosa Therapeutics, Manifold Bio, Genomic Life
Consulting or Advisory Role: Syapse, Roche, Third Rock Ventures, Takeda, Novartis, Genome Medical, InVitae, Illumina, Tango Therapeutics, Ervaxx, Janssen, Monte Rosa Therapeutics, Manifold Bio, Genomic Life
Speakers' Bureau: Illumina
Research Funding: Bristol Myers Squibb, Novartis, Sanofi (Inst)
Patents, Royalties, Other Intellectual Property: Patent on discovery of retained intron as source of cancer neoantigens (Inst), Patent on discovery of chromatin regulators as biomarkers of response to cancer immunotherapy (Inst), Patent on clinical interpretation algorithms using cancer molecular data (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Ying Huang
Employment: Novartis Institutes for BioMedical Research
Stock and Other Ownership Interests: Novartis
Travel, Accommodations, Expenses: Novartis Institutes for BioMedical Research
Massimo Loda
Stock and Other Ownership Interests: Methylex
Patents, Royalties, Other Intellectual Property: cdx2 antibodies—royalties
Geoffrey I. Shapiro
Consulting or Advisory Role: G1 Therapeutics, Lilly, Pfizer, Roche, Merck Serono, Sierra Oncology, Cybrexa Therapeutics, Ipsen, Bayer, Fusion Pharmaceuticals, Bicycle Therapeutics, Almac Diagnostics, Astex Pharmaceuticals, Daiichi Sankyo, Angiex, Seattle Genetics, Artios, Boehringer Ingelheim, Concarlo, Atrin Pharmaceuticals, Syros Pharmaceuticals, Zentalis, CytomX Therapeutics, Blueprint Medicines, Kymera, Janssen Oncology, Xinthera, ImmunoMet
Research Funding: Pfizer (Inst), Genentech (Inst), Bayer (Inst), Immune Design (Inst), Vertex (Inst), Millennium (Inst), Puma Biotechnology (Inst), Tensha Therapeutics (Inst), Covidien (Inst), Novartis (Inst), Cellceutix (Inst), Sanofi (Inst), Cyclacel (Inst), Mirati Therapeutics (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Lilly (Inst), Aileron Therapeutics (Inst), PharmaMar (Inst), PTC Therapeutics (Inst), Roche (Inst), CanBas (Inst), Tesaro (Inst), Merck Serono (Inst), Sierra Oncology (Inst), Syros Pharmaceuticals (Inst), Curis (Inst), Merck (Inst), Array BioPharma (Inst), Seattle Genetics (Inst), Clovis Oncology (Inst), Exelixis (Inst), Boehringer Ingelheim (Inst), Esperas Pharma (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), Tango Therapeutics (Inst), Senhwa (Inst), Biosplice (Inst), Cyteir (Inst), Abbvie (Inst)
Patents, Royalties, Other Intellectual Property: Patent #: 9872874 Title: Dosage regimen for sapacitabine and seliciclib Issue Date: January 23, 2018, Provisional Patent #:62/538,319 Title: Compositions and methods for predicting response and resistance to CDK4/6 inhibition Filed: July 28, 2017
Travel, Accommodations, Expenses: Lilly, Pfizer, Bicycle Therapeutics, G1 Therapeutics, Sierra Oncology, Bayer
Peter M. Glazer
Stock and Other Ownership Interests: Cybrexa Therapeutics, Gennao Bio
Consulting or Advisory Role: Cybrexa Therapeutics, pHLIP, Gennao Bo
Research Funding: Gennao Bio
Patents, Royalties, Other Intellectual Property: Patents related to gene editing and gene therapy via peptide nucleic acids. Patents related to oncometabolites and DNA repair for cancer therapy. Patents related to antibody-mediated cancer therapy
Patricia M. LoRusso
Stock and Other Ownership Interests: BAKX Therapeutics
Honoraria: Five Prime Therapeutics
Consulting or Advisory Role: Genentech, CytomX Therapeutics, Roche/Genentech, Halozyme, Five Prime Therapeutics, Agenus, Agios, Cybrexa Therapeutics, Sotio, AbbVie, Genmab, Takeda, TYME, IQvia, Trial to Reduce IDDM in the Genetically at Risk (TRIGR), Pfizer, ImmunoMet, Black Diamond Therapeutics, GlaxoSmithKline, QED Therapeutics, AstraZeneca, EMD Serono, Shattuck Labs, Astellas Pharma, Salarius Pharmaceuticals, Silverback Therapeutics, Macrogenics, Kyowa Kirin International, Kineta, Zentalis, Molecular Templates, Molecular Templates, ABL Bio, SK Life Sciences, ST Cube, Bayer, I-Mab, Seattle Genetics, ImCheck Therapeutics, Relay Therapeutics, Stemline Therapeutics, Mekanistic Therapeutics, Compass Therapeutics, BAKX Therapeutics, Scenic Biotech, Qualigen Therapeutics, Roivant, NeuroTrials
Research Funding: Genentech (Inst)
Travel, Accommodations, Expenses: Genentech
Yu Shyr
Consulting or Advisory Role: Janssen Research & Development, Novartis, Roche/Genentech, Pfizer, GlaxoSmithKline, AstraZeneca
Patents, Royalties, Other Intellectual Property: Royalty for TNBCType for Insight Genetics. TNBCType is a web-based subtyping tool TNBCtype for candidate TNBC samples using our gene expression meta data and classification method
Elizabeth M. Swisher
Leadership: IDEAYA Biosciences
Daniel P. Petrylak
Stock and Other Ownership Interests: Bellicum Pharmaceuticals, TYME
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Roche, Astellas Pharma, AstraZeneca, Lilly, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Clovis Oncology, Incyte, Janssen, Pharmacyclics, Seattle Genetics, Urogen Pharma, Advanced Accelerator Applications, Ipsen, Bicycle Therapeutics, Mirati Therapeutics, Monopteros Therapeutics, Regeneron, Gilead Sciences
Research Funding: Progenics (Inst), Sanofi (Inst), Endocyte (Inst), Genentech (Inst), Merck (Inst), Astellas Medivation (Inst), Novartis (Inst), AstraZeneca (Inst), Bayer (Inst), Lilly (Inst), Innocrin Pharma (Inst), MedImmune (Inst), Pfizer (Inst), Roche (Inst), Seattle Genetics (Inst), Clovis Oncology (Inst), Bristol Myers Squibb (Inst), Advanced Accelerator Applications (Inst), Agensys (Inst), BioXCel therapeutics (Inst), Eisai (Inst), Mirati Therapeutics (Inst), Replimune (Inst), Medivation (Inst), Gilead Sciences (Inst)
Expert Testimony: Celgene, Sanofi
No other potential conflicts of interest were reported.
REFERENCES
- 1.de Bono J, Mateo J, Fizazi K, et al. : Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 382:2091-2102, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Hussain M, Mateo J, Fizazi K, et al. : Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med 383:2345-2357, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Abida W, Patnaik A, Campbell D, et al. : Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol 38:3763-3772, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pritchard CC, Mateo J, Walsh MF, et al. : Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med 375:443-453, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson D, Van Allen EM, Wu YM, et al. : Integrative clinical genomics of advanced prostate cancer. Cell 161:1215-1228, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bindra RS, Gibson SL, Meng A, et al. : Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res 65:11597-11604, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Chan N, Pires IM, Bencokova Z, et al. : Contextual synthetic lethality of cancer cell kill based on the tumor microenvironment. Cancer Res 70:8045-8054, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bindra RS, Schaffer PJ, Meng A, et al. : Down-regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol Cell Biol 24:8504-8518, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hegan DC, Lu Y, Stachelek GC, et al. : Inhibition of poly(ADP-ribose) polymerase down-regulates BRCA1 and RAD51 in a pathway mediated by E2F4 and p130. Proc Natl Acad Sci USA 107:2201-2206, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumareswaran R, Chaudary N, Jaluba K, et al. : Cyclic hypoxia does not alter RAD51 expression or PARP inhibitor cell kill in tumor cells. Radiother Oncol 116:388-391, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Kaplan AR, Gueble SE, Liu Y, et al. : Cediranib suppresses homology-directed DNA repair through down-regulation of BRCA1/2 and RAD51. Sci Transl Med 11:eaav4508, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu JF, Tolaney SM, Birrer M, et al. : A phase 1 trial of the poly(ADP-ribose) polymerase inhibitor olaparib (AZD2281) in combination with the anti-angiogenic cediranib (AZD2171) in recurrent epithelial ovarian or triple-negative breast cancer. Eur J Cancer 49:2972-2978, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scher HI, Morris MJ, Stadler WM, et al. : Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol 34:1402-1418, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MD+ CALC: Creatinine Clearance (Cockcroft-Gaut Equation). https://www.mdcalc.com/creatinine-clearance-cockcroft-gault-equation#evidence
- 15.Walsh T, Lee MK, Casadei S, et al. : Detection of inherited mutations for breast and ovarian cancer using genomic capture and massively parallel sequencing. Proc Natl Acad Sci USA 107:12629-12633, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 17.The R Project for Statistical Computing. www.r-project.org
- 18.Mateo J, Carreira S, Sandhu S, et al. : DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med 373:1697-1708, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mateo J, Porta N, Bianchini D, et al. : Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol 21:162-174, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Bono JS, Mehra N, Scagliotti GV, et al. : Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): An open-label, phase 2 trial. Lancet Oncol 22:1250-1264, 2021 [DOI] [PubMed] [Google Scholar]
- 21.Abida W, Campbell D, Patnaik A, et al. : Non-BRCA DNA damage repair gene alterations and response to the PARP inhibitor rucaparib in metastatic castration-resistant prostate cancer: Analysis from the phase II TRITON2 study. Clin Cancer Res 26:2487-2496, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JW, Cardin DB, Vaishampayan UN, et al. : Clinical activity and safety of cediranib and olaparib combination in patients with metastatic pancreatic ductal adenocarcinoma without BRCA mutation. Oncologist 26:e1104-e1109, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data from this article will be available for 1 year from the time of publication for data sharing proposal by submitting request to the corresponding author, who will review the proposal with the sponsor, the NCI.