Abstract
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrotic lung disease with poor prognosis and cough is the one of most common and major symptoms in IPF. The aim of this study was to evaluate the clinical efficacy of a Mixture of Ivy Leaf Extract and Coptidis rhizome (Synatura®) in patients with IPF. This was a prospective, open-label, single-center, and single-arm study in Korea from October 2019 to September 2020. IPF patients with chronic bronchitis were enrolled. Between baseline and eight weeks after use of Synatura®, clinical measures regarding cough and health-related quality of life, and the systemic inflammatory markers was prospectively collected. Thirty patients were enrolled. Median age was 73 years and 86.7% were men. The median gender-age-pulmonary function stage of IPF was 3. Baseline total score of Leicester cough questionnaire (LCQ) and St. George respiratory questionnaire (SGRQ) were 104.5 and 30.59 respectively. After eight weeks, there was no significant improvement in LCQ (16.8 [15.6–19.1] vs 17.5 [15.2–18.9], P = .772) and SGRQ (30.6 [19.4–37.8] vs 29.9 [19.6–41.8], P = .194) scores. Also, there was no significant difference of systemic inflammatory markers. In analysis of minimal clinically important differences (MCID), one third (33.3%) patients fulfilled the criteria of MCID (1.3) in LCQ scores and median differences was 14 (range: 10–18). In terms of SGRQ, 6 patients (20%) reached MCID (4.0) without significant predictive factors. In our study, use of Synatura® during 8 weeks improved cough-specific life quality in one third patients with IPF. Large-scale, randomized, double-blind, and placebo-controlled clinical trials are needed.
Keywords: cough, idiopathic pulmonary fibrosis, minimal clinically important difference, quality of life
1. Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrotic interstitial lung disease with a poor prognosis and high mortality rate.[1] IPF causes high burden of symptoms, and cough is the most frequent and debilitating symptom, affecting up to 80% of patients with IPF.[2] The presence of cough reduces quality of life and is associated with disease progression regardless of disease severity.[3,4] The 2 anti-fibrotic drugs have been approved by the food and drug administration and recommended in the American thoracic society, European respiratory society, Japanese respiratory society, and Latin American thoracic association international IPF therapy guideline. These drugs are widely used to slow disease progression and increase survival of patients with IPF.[5] Previous major trials demonstrating the effect of anti-fibrotic drugs to contribute to food and drug administration approval have not reported data on the impact of treatment on cough.[6,7] Although some studies show efficacy of the anti-fibrotic drugs to reduce cough, evidence is limited, and the antitussive effect of anti-fibrotic agents is unknown.[8,9] Cough associated with IPF remains an unresolved problem, and the evidence supporting management of chronic cough in IPF is limited.
A mixture of ivy leaf extract and coptidis rhizome (Synatura®) was developed by a South Korean pharmaceuticals company (Ahn-Gook Pharmaceuticals Co., Ltd.) and formulated with a dried 30% ethanolic extract of Heddera helix L. (ivy, Lamiaceae) leaves and a dried water-saturated butanolic extract of the Coptis chinensis French (Ranunculaceae) rhizome (3:1). This drug was approved by the Korean Ministry of Food and Drug Safety in 2011 for patients with chronic bronchitis and improved quality of life and reduced cough in patients with chronic lung disease and chronic bronchitis.[10,11] Patients with IPF usually describe a persistent dry cough. In addition, smoking history is a potential risk factor of IPF, and anyone with a smoking history has a 60% higher risk of developing IPF.[1,12] This study was conducted because we hypothesized that this study drug shown to be effective in patients with chronic bronchitis or history of smoking would have an antitussive effect in IPF patients with chronic bronchitis.
2. Materials and methods
2.1. Study design
This study was an 8-week, prospective, single-center, single-arm, and open label study in Korea from October 2019 to September 2020. The primary outcome of this study was clinical improvement of cough, assessed by the Leicester cough questionnaire (LCQ), and health-related quality of life, and assessed by the St. George respiratory questionnaire (SGRQ) attributed to the study drug. The secondary outcome was the change of systemic inflammation as indexed by levels of interleukin-6, C-reactive protein, and white blood cells. Adverse events, safety, and tolerability were also assessed.
This study was approved by the institutional review board of Haeundae-Paik Hospital (approval number: 2019-06-008-013), and written informed consent was obtained from all patients before enrollment.
2.2. Study subjects and interventions
IPF was diagnosed according to the 2018 American thoracic society/European respiratory society/Japanese respiratory society/Latin American thoracic association statement at multidisciplinary discussion including pulmonologist, rheumatologist, radiologist, and pathologist.[12] Chronic bronchitis was defined as cough and sputum for more than 3 months in 2 consecutive years.[13]
All eligible patients received a mixture of ivy leaf extract and coptidis rhizome (Synatura®) three times daily (fixed dose of 15mL each time) for 8 weeks. Questionnaire completion and blood sampling and testing for inflammatory markers were conducted at baseline and at eight weeks after use of the study drug. During the study period, any unscheduled visits were recorded, and drug adverse events and adherence were assessed at every visit. Concomitant treatment with anti-fibrotic drug and proton-pump inhibitors was allowed, but additional use or change of antitussive drug and mucolytic drug was prohibited.
2.3. Assessment of cough and life quality
All participants were asked to complete a questionnaire of the Korean version of the LCQ and the SGRQ at baseline and at eight weeks, with assistance by a trained nurse. The LCQ was used to evaluate cough-related quality of life and is a 19-item scale organized in three domains (physical, psychological, and social). Each domain has a score ranging from 1 to 7 and the total score varies from 3 to 21. A higher score indicates a better cough-related quality of life. The LCQ has been validated in IPF patients and is well correlated with cough visual analogue scale and cough frequency in IPF patients.[4,14] The minimal clinically important difference (MCID) on the LCQ was defined as an improvement of 1.3 points in total score, as proposed by Raj et al.[15]
The SGRQ was used to evaluate health-related quality of life. The 50 items of the SGRQ comprise the domains of symptoms, activity, and impact, with a total score ranging from 0 to 100. A higher score indicates worse quality of life. The MCID on the SGRQ was defined as a mean four-point change in total score, as proposed by Jones PW et al.[16] The SGRQ has been validated and commonly used in patients with IPF.[17]
2.4. Statistical analysis
The data are presented as frequency with percentage for categorical variables and median and interquartile range for continuous variables. Differences in participant characteristics were compared across subgroups with Fisher exact test for categorical variables and independent t test or Mann-Whitney U test for continuous variables. Paired t test or Wilcoxon signed rank test was performed for comparison between 2 assessment points. To verify a normal distribution, we used Shapiro–Wilk test. Univariate and multivariate analyses, using logistic regression, were performed to identify prognostic factors independently related to responsiveness of the study drug in LCQ and in SGRQ. Mean ± standard error of the mean plots was presented for data visualization. All statistical analyses were carried out using SPSS 24.0 (IBM Corp, Armonk, NY), and p values < 0.05 were considered statistically significant.
3. Results
3.1. Study population and baseline characteristics
Thirty patients were enrolled in and completed this study. Baseline characteristics and clinical information of enrolled patients are summarized in Table 1. Median age was 73.0 years, and 86.7% of participants were male. Among ever-smokers (86.7%), 3 (10.0%) were current smokers, and the median smoking history was 36.5 pack-years. The median weight and height were 65.6 kg and 163 cm, respectively. Most patients exhibited a moderate restrictive insufficiency in spirometry with reduced diffusing capacity of the lung for carbon monoxide (DLco). About one-third of patients had mixed pattern on pulmonary function test. In the gender-age-pulmonary function (GAP) staging for classifying IPF severity, the median GAP stage was grade I (range: I-II). At the beginning of the study, 96.7% of participants had been receiving anti-fibrotic agent with only pirfenidone due to Korean national health insurance coverage, and the median dose of pirfenidone during study period was 1200 mg per day (range: 600–1800 mg) over a median treatment duration of 452 days.
Table 1.
Baseline clinical characteristics of the patients.
| Characteristics | All patients (n = 30) |
|---|---|
| Age, yr | 73.00 (66.75–76.00) |
| Male | 26 (86.7) |
| Weight | 65.50 (59.75–73.00) |
| Height | 163.00 (156.75–167.00) |
| Body mass index | 24.69 (23.38–27.34) |
| Never smoker | 4 (13.3) |
| Ever-smokers | 26 (86.7) |
| Pack-years | 36.50 (19.63–47.75) |
| GAP stage | 1.00 (1.00–2.00) |
| Use of antifibrotic agent | 29 (96.7) |
| Dose of PFD, mg/day | 1200 (600–1800) |
| Duration of PFD | 452.00 (279.50–561.00) |
| Dyspnea, mMRC | 1.00 (1.00–2.00) |
| Pulmonary function test | |
| FVC, % predicted | 77.50 (67.75–92.50) |
| FEV1, % predicted | 85.00 (70.75–97.50) |
| DLco, % predicted | 62.00 (50.00–71.00) |
| Six-minute walk test | |
| Distance (m) | 454.50 (354.75–508.50) |
| Initial SpO2 (%) | 96.00 (95.00–97.75) |
| Nadir SpO2 (%) | 92.50 (89.00–94.00) |
| Arterial oxygen pressure | 87.86 (69.70–100.38) |
| PaCO2 (mm Hg) | 37.90 (35.15–8.17) |
| WBC | 7.40 (6.26–8.17) |
| IL-6 | 5.60 (4.20–9.50) |
| CRP | 0.24 (0.14–0.53) |
Data are presented as median (interquartile range) or number (%), unless otherwise indicated.
CRP = C-reactive protein, DLco = diffusing capacity of the lungs for carbon monoxide, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, GAP = gender-age-pulmonary function, IL-6 = interleukin-6, mMRC = modified medical research council, PaCO2 = partial pressure of carbon dioxide, PFD = pirfenidone, SpO2 = saturation of percutaneous oxygen, WBC = white blood cell.
3.2. Primary outcome
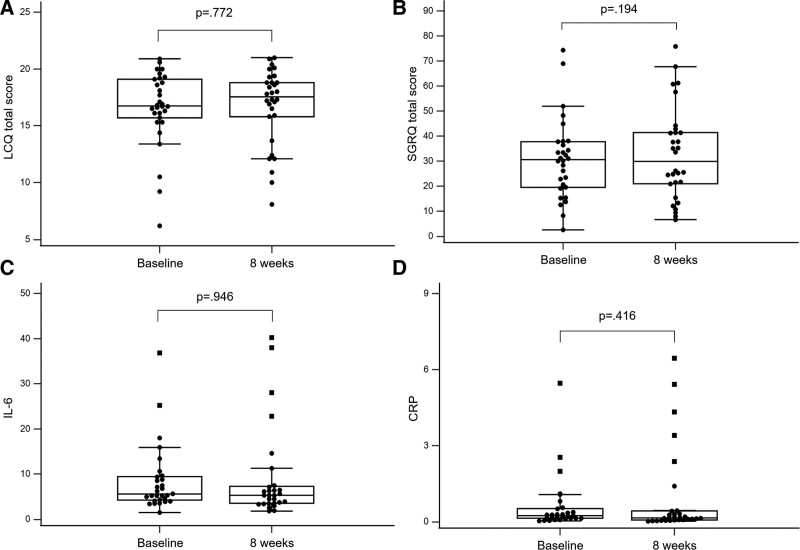
The primary outcome of this study was clinical improvement of cough-related quality of life after use of the study drug. In the assessment of cough-related quality of life, the median total baseline score of LCQ was 16.77. In comparison between baseline and eight weeks, there was no statistically significant change in total LCQ (median difference 0.12, range: -0.73 to 0.97, P = .772) (Fig. 1) or any physical, psychological, or social domain (Table 2). Defining clinically relevant improvement as MCID ≥ 1.3 points, one-third (33.3%) of patients achieved MCID after eight weeks of treatment, and the median difference of total LCQ score was 2.38 (range: 1.79–3.32).
Figure 1.
Comparison of questionnaire scores and inflammatory markers at baseline and after 8 weeks. (A) Total LCQ score at baseline and after 8 weeks. (B) Total SGRQ score at baseline and after 8 weeks. (C) IL-6 at baseline and after 8 weeks. (D) CRP at baseline and after 8 weeks. CRP = C-reactive protein, IL-6 = interleukin-6, LCQ = Leicester cough questionnaire, SGRQ = St. George’s respiratory questionnaire.
Table 2.
Comparison data of questionnaire and inflammatory marker at baseline and after 8 weeks.
| Characteristics | Baseline | After 8 weeks | Difference (95% CI) | P value |
|---|---|---|---|---|
| LCQ | ||||
| Physical | 5.44 (4.88–6.00) | 5.50 (4.78–5.91) | 0.10 (−0.20, 0.41)a | .492* |
| Psychological | 5.57 (4.96–6.61) | 5.79 (4.93–6.43) | 0.01 (−0.35, 0.37)a | .957* |
| Social | 6.00 (5.50–6.75) | 6.00 (5.31–7.00) | 0.00 (0.00, 0.25)b | .720** |
| Total | 16.77 (15.63–19.13) | 17.55 (15.24–18.94) | 0.12 (−0.73, 0.97)a | .772* |
| SGRQ | ||||
| Symptoms | 46.44 (35.08–55.96) | 46.76 (28.70–57.14) | −1.78 (−8.62, 5.06)a | .599* |
| Activity | 45.10 (28.52–61.34) | 50.62 (28.68–63.19) | −0.86 (−5.25, 5.72)b | .876** |
| Impact | 13.55 (6.08–23.78) | 15.95 (6.05–33.08) | 1.81 (−2.26, 8.33)b | .058** |
| Total | 30.59 (19.41–37.82) | 29.88 (19.57–41.78) | 1.90 (−1.02, 4.83)a | .194* |
| WBC | 7.40 (6.26–8.17) | 7.08 (5.93–8.96) | 0.29 (−0.41, 0.99)b | .364** |
| IL-6 | 5.60 (4.20–9.50) | 5.30 (3.53–7.40) | −0.35 (−1.50, 1.30)b | .946** |
| CRP | 0.24 (0.14–0.53) | 0.15 (0.07–0.43) | −0.02 (−0.18, 0.02)b | .416** |
Data are presented as median (interquartile range); Shapiro–Wilk’s test was employed for test of normality assumption.
CI = confidence interval, CRP = C-reactive protein, IL-6 = interleukin-6, LCQ = Leicester cough questionnaire;, SGRQ = St. George respiratory questionnaire, WBC = white blood cell.
Differences with 95% confidence interval were derived from paired t test.
Differences with 95% confidence interval were derived by Wilcoxon’s signed rank test.
P values were derived from paired t test.
P values were derived by Wilcoxon’s signed rank test.
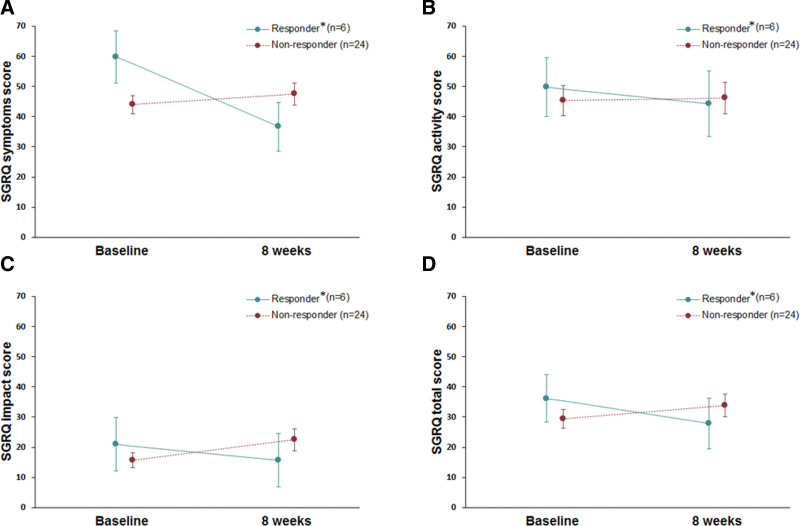
In the assessment of health-related quality of life, the median total baseline score of SGRQ was 30.59 (range: 19.41–37.82). After eight weeks, there was no statistically significant change (median difference 1.90, range: -1.02 to 4.83, P = .194). In the analysis of MCID (4.0) for SGRQ scores, 20% patients met the criteria for such a difference in SGRQ score, with a median difference of -6.92 (range: -12.42 to -6.39). (Fig. 2)
Figure 2.
Median changes of the St. George respiratory questionnaire before and after 8 weeks between responder and non-responders. (A) Symptoms domain (B) Activity domain (C) Impact domain (D) Total domain. *Responders – patients meet criteria of minimal clinically important differences in the LCQ. LCQ = Leicester cough questionnaire.
3.3. Secondary outcome
Baseline serum level of the inflammatory markers white blood cells, interleukin-6, and C-reactive protein were 7.49 (103/mL), 6.00 pg/mL, and 0.20 mg/mL, respectively. In comparison of inflammatory marker levels between baseline and eight weeks of treatment, there was no statistically significant difference.
3.4. Subgroup analysis of achieving MCID in LCQ and SGRQ
Among the 10 patients who achieved MCID in the LCQ, the baseline total score was significantly lower than that of those who did not meet the criteria of MCID (median total LCQ, 16.13 vs 17.38, P = .035), suggesting more severe clinical symptoms and lower cough-specific quality of life in patients with such a change (Table 3). Also, patients achieving MCID showed greater height and more severe smoking history (pack-years) than those who did not show MCID. In multivariable analysis, greater height (odds ratio 1.158, 95% confidence interval: 1.006–1.332, P = .01) was the only independent predictive factor for MCID in the LCQ (Table 4).
Table 3.
Comparison of characteristics between responders and non-responder in the LCQ.
| Variable | Responder† (n = 10) | Non-responder (n = 20) | P value |
|---|---|---|---|
| LCQ total | |||
| Baseline | 16.13 (13.39 to 16.86) | 17.38 (16.41 to 19.41) | .035** |
| 8 wk | 18.39 (17.07 to 19.45) | 17.26 (14.71 to 18.68) | .235** |
| Difference | 2.38 (1.79 to 3.32) | −0.51 (−1.71 to 0.13) | <.001** |
| Age, yr | 74.50 (68.00 to 77.00) | 72.50 (66.50 to 76.00) | .348*** |
| Male | 9 (90.0) | 17 (85.0) | 1.000*** |
| Weight | 69.00 (62.00 to 77.00) | 64.50 (58.50 to 70.50) | .058* |
| Height | 165.0 (162.00 to 171.00) | 160.00 (155.50 to 166.00) | .026* |
| Body mass index | 24.65 (23.84 to 28.28) | 24.75 (22.76 to 27.08) | .522* |
| Never smoker | 0 (0.0) | 4 (20.0) | .353*** |
| Ever-smokers | 10 (100.0) | 16 (80.0) | . |
| Pack-years | 40.00 (38.00 to 50.00) | 27.50 (0.00 to 42.50) | .027* |
| GAP stage | 1.00 (1.00 to 2.00) | 1.00 (1.00 to 1.50) | .100** |
| Antifibrotic agent | 10 (100.0) | 19 (95.0) | 1.000*** |
| Duration of PFD | 463.50 (281.00 to 554.00) | 439.00 (274.00 to 568.00) | .557* |
| Dyspnea, mMRC | 1.50 (1.00 to 2.00) | 1.00 (1.00 to 2.00) | .287** |
| Pulmonary function test | . | ||
| FVC, % predicted | 73.00 (67.00 to 82.00) | 84.50 (68.00 to 93.00) | .569* |
| FEV1, % predicted | 84.00 (71.00 to 95.00) | 85.00 (70.00 to 103.50) | .725** |
| DLco, % predicted | 57.50 (46.0 to 66.00) | 63.00 (53.00 to 72.00) | .318* |
| Six-minute walk test | |||
| Distance (m) | 408.00 (357.00 to 483.00) | 469.50 (354.00 to 528.00) | .435* |
| Initial SpO2 (%) | 96.00 (96.00 to 98.00) | 96.00 (94.00 to 97.00) | .234* |
| Nadir SpO2 (%) | 92.50 (91.00 to 94.00) | 92.50 (85.00 to 94.00) | .754** |
| Arterial oxygen pressure | 85.60 (66.50 to 102.10) | 87.75 (80.40 to 97.00) | .903** |
| PaCO2 (mm Hg) | 37.15 (33.25 to 41.00) | 38.05 (35.65 to 41.55) | .795* |
| WBC | 7.49 (5.45 to 7.79) | 7.35 (6.44 to 8.22) | .468** |
| IL-6 | 6.00 (3.90 to 8.80) | 5.60 (4.60 to 13.40) | .359** |
| CRP | 0.20 (0.13 to 0.29) | 0.27 (0.14 to 1.07) | .271** |
Data are presented as median (interquartile range) or number (%), unless otherwise indicated; Shapiro–Wilk’s test was employed for test of normality assumption.
CI = confidence interval, CRP = C-reactive protein, DLco = diffusing capacity of the lungs for carbon monoxide, FEV1 = forced expiratory volume in one second, FVC = forced vital capacity, GAP = gender-age-pulmonary function, IL-6 = interleukin-6, LCQ = Leicester cough questionnaire, mMRC = modified medical research council, PaCO2 = partial pressure of carbon dioxide, PFD = Pirfenidone, SpO2 = saturation of percutaneous oxygen, WBC = white blood cell.
P values were derived from independent t-test.
P values were derived from Mann-Whitney’s U test.
P values were derived from Fisher’s exact test.
Responders – patients meet criteria of minimal clinically important differences in the LCQ.
Table 4.
Predictive factors for archiving MCID in the LCQ assessed using the Logistic Regression Model.
| Variable | Univariate analysis | Multivariate analysis* | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age, yr | 1.081 (0.922–1.269) | .337 | ||
| Male | 1.588 (0.144–17.561) | .706 | ||
| Weight | 1.096 (0.991–1.212) | .073 | ||
| Height | 1.158 (1.006–1.332) | .041 | 1.158 (1.006–1.332) | .041 |
| Body mass index | 1.106 (0.820–1.493) | .508 | ||
| Never smoker | Ref. | . | ||
| Ever-smokers | N/E | N/E | ||
| Pack-yr | 1.037 (0.993–1.083) | .098 | ||
| GAP point | 2.313 (0.838–6.385) | .105 | ||
| Antifibrotic agent | N/E | .N/E | ||
| Duration of PFD | 1.001 (0.997–1.005) | .543 | ||
| Dyspnea, mMRC | 1.717 (0.656–4.491) | .271 | ||
| Pulmonary function test | ||||
| FVC, % predicted | 0.987 (0.945–1.031) | .555 | ||
| FEV1, % predicted | 0.990 (0.946–1.035) | .648 | ||
| DLco, % predicted | 0.966 (0.904–1.032) | .308 | ||
| Six-minute walk test | ||||
| Distance (m) | 0.998 (0.992–1.004) | .421 | ||
| Initial SpO2 (%) | 1.303 (0.843–2.015) | .233 | ||
| Nadir SpO2 (%) | 1.034 (0.912–1.174) | .601 | ||
| Arterial oxygen pressure | 0.997 (0.968–1.026) | .821 | ||
| PaCO2 (mm Hg) | 0.973 (0.800–1.183) | .784 | ||
| WBC | 0.774 (0.495–1.212) | .263 | ||
| IL-6 | 0.888 (0.731–1.080) | .235 | ||
| CRP | 0.203 (0.014–2.961) | .244 | ||
CI = confidence interval, CRP = C-reactive protein, DLco = diffusing capacity of the lungs for carbon monoxide, FEV1 = forced expiratory volume in one second, FVC = forced vital capacity, GAP = gender-age-pulmonary function, IL-6 = interleukin-6, LCQ = Leicester cough questionnaire, MCID = minimal clinically important differences, mMRC = modified medical research council, N/E = not estimable since none was observed in a certain subgroup, OR = odds ratio, PaCO2 = partial pressure of carbon dioxide, PFD = Pirfenidone, Ref = reference group, SpO2 = saturation of percutaneous oxygen, WBC = white blood cell.
In a multivariate logistic regression, the statistically significant variables were selected in a backward elimination method with 0.05 alpha level.
Six patients who met the criteria of MCID in SGRQ showed more severe history of smoking compared to those not achieving MCID (Table 5). However, there was no significant predictive factor in the multivariable analysis.
Table 5.
Comparison of characteristics between responders and non-responders in the SGRQ.
| Variable | Responder† (n = 6) | Non-responder (n = 24) | P value |
|---|---|---|---|
| SGRQ total | |||
| Baseline | 31.81 (26.19 to 33.38) | 30.04 (17.21 to 37.87) | .568** |
| 8 wk | 23.24 (20.96 to 25.19) | 35.18 (18.42 to 42.15) | .300** |
| Difference | −6.92 (−12.42 to −6.39) | 4.37 (−0.31 to 7.39) | <.001* |
| Age, years | 73.00 (66.00 to 75.00) | 73.00 (67.50 to 76.50) | .879* |
| Male | 6 (100.0) | 20 (83.3) | .557*** |
| Weight | 61.50 (59.00 to 77.00) | 66.50 (61.00 to 72.50) | .767* |
| Height | 162.50 (157.00 to 165.00) | 163.00 (156.50 to 167.00) | .970* |
| Body mass index | 24.29 (23.14 to 26.33) | 24.83 (23.57 to 27.40) | .685* |
| Never smoker | 0 (0.0) | 4 (16.7) | .111*** |
| Ever-smokers | 4 (66.7) | 19 (79.2) | |
| Current smokers | 2 (33.3) | 1 (4.2) | |
| Pack-yr | 40.00 (40.00 to 50.00) | 30.00 (9.25 to 46.00) | .047* |
| GAP point | 3.50 (3.00 to 4.00) | 3.00 (3.00 to 4.00) | .655** |
| Antifibrotic agent | 6 (100.0) | 23 (95.8) | 1.000*** |
| Duration of PFD | 414.50 (281.00 to 493.00) | 452.00 (274.00 to 582.00) | .610* |
| Dyspnea, mMRC | 1.00 (1.00 to 1.00) | 1.00 (1.00 to 2.00) | .124** |
| Pulmonary function test | |||
| FVC, % predicted | 76.00 (69.00 to 94.00) | 77.50 (67.50 to 91.50) | .938* |
| FEV1, % predicted | 79.50 (72.00 to 94.00) | 87.00 (69.00 to 102.50) | .636* |
| DLco, % predicted | 70.00 (62.00 to 72.00) | 57.00 (46.00 to 70.00) | .074* |
| Six-minute walk test | |||
| Distance (m) | 481.50 (396.00 to 492.00) | 441.00 (354.00 to 528.00) | .779** |
| Initial SpO2 (%) | 96.00 (95.00 to 97.00) | 96.00 (95.00 to 98.00) | .596* |
| Nadir SpO2 (%) | 93.50 (92.00 to 94.00) | 92.00 (85.00 to 94.00) | .272** |
| Arterial oxygen pressure | 117.00 (77.40 to 161.10) | 87.75 (66.50 to 97.00) | .394** |
| PaCO2 (mm Hg) | 34.35 (30.60 to 40.60) | 37.95 (35.65 to 41.45) | .375* |
| WBC | 6.54 (5.24 to 8.59) | 7.40 (6.51 to 8.09) | .534** |
| IL-6 | 4.65 (4.00 to 8.80) | 6.20 (4.59 to 9.60) | .590** |
| CRP | 0.26 (0.21 to 0.81) | 0.18 (0.10 to 0.51) | .269** |
Data are presented as median (interquartile range) or number (%), unless otherwise indicated; Shapiro–Wilk’s test was employed for test of normality assumption.
CRP = C-reactive protein, DLco = diffusing capacity of the lungs for carbon monoxide, FEV1 = forced expiratory volume in one second, FVC = forced vital capacity, GAP = gender-age-pulmonary function, IL-6 = interleukin-6, mMRC = modified medical research council, PaCO2 = partial pressure of carbon dioxide, PFD = Pirfenidone, SGRQ = St. George’s respiratory questionnaire, SpO2 = saturation of percutaneous oxygen, WBC = white blood cell.
P values were derived from independent t-test.
P values were derived from Mann-Whitney’s U test.
P values were derived from Fisher’s exact test.
Responders—patients meet criteria of minimal clinically important differences in the SGRQ.
4. Discussion
This was the first study to assess the effect of a mixture of ivy leaf extract and coptidis rhizome in IPF patients with chronic bronchitis. For this, 30 patients who met the inclusion criteria completed the eight-week study protocol. In comparison of subjective measures of cough-specific quality of life and levels of systemic inflammatory markers between baseline and eight weeks, there was no statistically significant improvement. However, there was clinically relevant improvement in 1-third and 1-fifth of patients reaching an MCID of the LCQ and SGRQ, respectively. Patients achieving MCID in the LCQ showed a lower baseline of LCQ, a greater height, and a larger number of pack-years of smoking. Greater height was the only independent predictive factor of MCID in the LCQ.
The study drug (Synatura®) is a liquid formula of a mixture of ivy extract and coptidis rhizome. Ivy leaf extract preparations have been used in respiratory medicine as herbal medicinal products and were accepted officially by the European Medicines Agency in 2011.[18,19] Coptidis rhizome has been used in Asian countries and shows therapeutic characteristics of suppression of inflammation by inhibiting liposaccharide-stimulated pro-inflammatory cytokines such as interleukin-6.[20] Recently, Lee et al reported that this mixture improved quality of life and reduced level of fibrinogen in patients with chronic bronchitis-type chronic obstructive pulmonary disease (COPD).[11] Xu et al showed that berberine, a natural product of coptidis rhizome, attenuates cigarette smoke-agent-induced airway inflammation and mucus overproduction by significantly reducing IL-1β, TNF-α, and monocyte chemoattractant protein-1 levels in a mouse model.[21] Therefore, we hypothesized that this study mixture will play a role in clinical improvement in IPF patients with chronic bronchitis and smoking history.
Generally, cough in IPF is nonproductive and dry, with greater frequency in the advanced state of IPF and in never-smokers.[22] This might be related with a possible mechanism of IPF, reflecting increased cough reflex sensitivity. Jone et al suggested that mechanical changes that could influence the sensitivity or quantity by traction forces of the fibrosis might increase cough reflex sensitivity, supporting more frequent cough in advanced IPF.[14] However, cough with sputum or bronchitis symptoms can be observed in patients with IPF due to complications caused by various confounding comorbidities and general health status with smoking history. Recently, Lee et al reported that, in a study of 1536 IPF patients, half presented sputum as an initial symptom, and sputum was more common in IPF with COPD compared to IPF alone (60.6% vs 48.3%, P = .023).[23] The authors suggested that IPF and COPD have common risk factors, such as aging and tobacco use, and that patients with IPF would show a combined pattern of airway obstruction. Among the 30 patients in our study, 11 (36.7%) had decreased lung function consistent with COPD, defined as forced expiratory volume in 1 second/ forced vital capacity ratio < 0.7.
In our study, patients with clinically relevant improvement in LCQ had a larger number of pack-years of smoking, suggesting a dose-response relationship between smoking intensity and efficacy of the study drug. However, there was no difference in GAP stage, use of pirfenidone, or lung function reflecting severity of IPF. Although further studies are needed, this result suggests that the study drug might have an impact in clinical improvement in IPF patients with a history of smoking. Smoking is the most studied and well-known risk factor of IPF, and the prevalence of smoking in IPF ranges from 41% to 83%.[24–26] Recently, Bellou et al[27] reported in a prospective cohort study of 4,37,453 men and women that smoking has an independent detrimental effect on risk of IPF, with a dose-response relationship observed between smoking intensity expressed in pack-years and risk of IPF (hazard ratio per 1-pack year increase, 1.013; 95% confidence interval, 1.009–1.016). In agreement with our results, previous studies have posited that the study drug was effective for smoking-induced lung inflammation, and smoking is a potential risk factor for development of IPF and its detrimental effect on survival.[21,27,28]
This study has some limitations. First, it was conducted in a single center with a small number of patients. However, the results of this study would be helpful in conducting a full-scale study to evaluate the actual efficacy of the study drug in IPF patients with smoking history. We are preparing an additional trial to address the limitations of this study. Second, this study was conducted in IPF patients with chronic bronchitis, for which the study drug has been approved by the Korean Ministry of Food and Drug Safety. There is no characteristic symptom of chronic bronchitis in IPF patients, and it was difficult to distinguish whether improvement associated with the study drug was due to improvement in IPF or chronic bronchitis. Thus, a study in general IPF patients without chronic bronchitis is needed. Third, there was no control group. Therefore, the actual efficacy of the study drug should be evaluated and validated in a large, randomized, controlled trial.
In conclusion, a mixture of ivy leaf extract and coptidis rhizome did not show a statistically significant improvement of cough-related quality of life or systemic inflammatory markers in IPF patients with chronic bronchitis. To confirm the clinical effect of this mixture, a large-scale, double-blind, randomized, controlled study is needed.
Author contributions
Conceptualization: Jae Ha Lee, Hang-Jea Jang.
Data curation: Ji Hoon Jang, Jin Han Park, So Young Jung, Ji Yeon Kim, Junghae Ko, Hee Eun Choi, Tae-Hoon No.
Formal analysis: Ji Hoon Jang, Jin Han Park, So Young Jung, Sunggun Lee, Seong-Ho Kim, Ji Yeon Kim, Hang-Jea Jang.
Methodology: Jae Ha Lee, Jin Han Park.
Validation: So Young Jung, Sunggun Lee, Seong-Ho Kim, Ji Yeon Kim, Junghae Ko, Hee Eun Choi, Hang-Jea Jang.
Visualization: Sunggun Lee, Seong-Ho Kim, Junghae Ko, Hee Eun Choi, Tae-Hoon No.
Writing – original draft: Jae Ha Lee, Hang-Jea Jang.
Writing – review & editing: Jae Ha Lee, Ji Hoon Jang, Jin Han Park, So Young Jung, Sunggun Lee, Seong-Ho Kim, Ji Yeon Kim, Junghae Ko, Hee Eun Choi, Tae-Hoon No, Hang-Jea Jang.
Abbreviations:
- COPD
- chronic obstructive pulmonary disease
- GAP
- gender-age-pulmonary function
- IPF
- idiopathic pulmonary fibrosis
- LCQ
- Leicester cough questionnaire
- MCID
- minimal clinically important difference
- SGRQ
- St. George respiratory questionnaire
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
The authors have no conflicts of interest to disclose.
This study was supported by the Ahn-Gook Pharmaceuticals Co., Ltd., Republic of Korea. The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
How to cite this article: Lee JH, Jang JH, Park JH, Jung SY, Lee S, Kim S-H, Kim JY, Ko J, Choi HE, No T-H, Jang H-J. The clinical efficacy of a mixture of ivy leaf extract and coptidis rhizome in patients with idiopathic pulmonary fibrosis. Medicine 2023;102:5(e32786).
Contributor Information
Jae Ha Lee, Email: sglee@paik.ac.kr.
Ji Hoon Jang, Email: okabango21@gmail.com.
Jin Han Park, Email: 1982han@hanmail.net.
So Young Jung, Email: docjsy@hanmail.net.
Sunggun Lee, Email: sglee@paik.ac.kr.
Seong-Ho Kim, Email: h00200@paik.ac.kr.
Ji Yeon Kim, Email: h00200@paik.ac.kr.
Junghae Ko, Email: arrioph1@gmail.com.
Hee Eun Choi, Email: solideogloria@paik.ac.kr.
Tae-Hoon No, Email: tae-hoon.no@paik.ac.kr.
References
- [1].Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Horton MR, Santopietro V, Mathew L, et al. Thalidomide for the treatment of cough in idiopathic pulmonary fibrosis: a randomized trial. Ann Intern Med. 2012;157:398–406. [DOI] [PubMed] [Google Scholar]
- [3].Ryerson CJ, Abbritti M, Ley B, et al. Cough predicts prognosis in idiopathic pulmonary fibrosis. Respirology. 2011;16:969–75. [DOI] [PubMed] [Google Scholar]
- [4].Key AL, Holt K, Hamilton A, et al. Objective cough frequency in idiopathic pulmonary fibrosis. Cough. 2010;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Raghu G, Rochwerg B, Zhang Y, et al. An Official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. an update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3–19. [DOI] [PubMed] [Google Scholar]
- [6].Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–82. [DOI] [PubMed] [Google Scholar]
- [7].King TE, Jr., Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–92. [DOI] [PubMed] [Google Scholar]
- [8].van Manen MJG, Birring SS, Vancheri C, et al. Effect of pirfenidone on cough in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2017;50:1701157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Azuma A, Taguchi Y, Ogura T, et al. Exploratory analysis of a phase III trial of pirfenidone identifies a subpopulation of patients with idiopathic pulmonary fibrosis as benefiting from treatment. Respir Res. 2011;12:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hong G, Kim YI, Park SJ, et al. Effects of a mixture of ivy leaf extract and coptidis rhizome on patients with chronic bronchitis and bronchiectasis. Int J Environ Res Public Health. 2021;18:4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee EG, Rhee CK. The clinical efficacy of AG NPP709 (Synatura [R]) in patients with chronic bronchitis type stable chronic obstructive pulmonary disease. J Thorac Dis. 2020;12:2435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e44–68. [DOI] [PubMed] [Google Scholar]
- [13].Mejza F, Gnatiuc L, Buist AS, et al. Prevalence and burden of chronic bronchitis symptoms: results from the BOLD study. Eur Respir J. 2017;50:1700621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jones RM, Hilldrup S, Hope-Gill BD, et al. Mechanical induction of cough in Idiopathic Pulmonary Fibrosis. Cough. 2011;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Raj AA, Pavord DI, Birring SS. Clinical cough IV: what is the minimal important difference for the Leicester Cough Questionnaire? Handb Exp Pharmacol. 2009;187:311–20. [DOI] [PubMed] [Google Scholar]
- [16].Jones PS. George’s respiratory questionnaire: MCID. COPD. 2005;2:75–9. [DOI] [PubMed] [Google Scholar]
- [17].O’Brien EC, Hellkamp AS, Neely ML, et al. Disease severity and quality of life in patients with idiopathic pulmonary fibrosis: a cross-sectional analysis of the IPF-PRO registry. Chest. 2020;157:1188–98. [DOI] [PubMed] [Google Scholar]
- [18].Sierocinski E, Holzinger F, Chenot JF. Ivy leaf (Hedera helix) for acute upper respiratory tract infections: an updated systematic review. Eur J Clin Pharmacol. 2021;77:1113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Holzinger F, Chenot JF. Systematic review of clinical trials assessing the effectiveness of ivy leaf (hedera helix) for acute upper respiratory tract infections. Evid Based Complement Alternat Med. 2011;2011:382789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Choi YY, Kim MH, Cho IH, et al. Inhibitory effect of coptis chinensis on inflammation in LPS-induced endotoxemia. J Ethnopharmacol. 2013;149:506–12. [DOI] [PubMed] [Google Scholar]
- [21].Xu D, Wan C, Wang T, et al. Berberine attenuates cigarette smoke-induced airway inflammation and mucus hypersecretion in mice. Int J Clin Exp Med. 2015;8:8641–7. [PMC free article] [PubMed] [Google Scholar]
- [22].van Manen MJ, Birring SS, Vancheri C, et al. Cough in idiopathic pulmonary fibrosis. Eur Respir Rev. 2016;25:278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee SH, Park JS, Kim SY, et al. Clinical features and prognosis of patients with idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2019;23:678–84. [DOI] [PubMed] [Google Scholar]
- [24].Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet. 2017;389:1941–52. [DOI] [PubMed] [Google Scholar]
- [25].Ekstrom M, Gustafson T, Boman K, et al. Effects of smoking, gender and occupational exposure on the risk of severe pulmonary fibrosis: a population-based case-control study. BMJ Open. 2014;4:e004018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ryu JH, Colby TV, Hartman TE, et al. Smoking-related interstitial lung diseases: a concise review. Eur Respir J. 2001;17:122–32. [DOI] [PubMed] [Google Scholar]
- [27].Bellou V, Belbasis L, Evangelou E. Tobacco smoking and risk for pulmonary fibrosis: a prospective cohort study from the UK Biobank. Chest. 2021;160:983–93. [DOI] [PubMed] [Google Scholar]
- [28].Park Y, Ahn C, Kim TH. Occupational and environmental risk factors of idiopathic pulmonary fibrosis: a systematic review and meta-analyses. Sci Rep. 2021;11:4318. [DOI] [PMC free article] [PubMed] [Google Scholar]