PURPOSE
Providing a geriatric assessment (GA) summary with management recommendations to oncologists reduces clinician-rated toxicity in older patients with advanced cancer receiving treatment. This secondary analysis of a national cluster randomized clinical trial (ClinicalTrials.gov identifier: NCT02054741) aims to assess the effects of a GA intervention on symptomatic toxicity measured by Patient-Reported Outcomes Common Terminology Criteria for Adverse Events (PRO-CTCAE).
METHODS
From 2014 to 2019, the study enrolled patients age ≥ 70 years, with advanced solid tumors or lymphoma and ≥ 1 GA domain impairment, who were initiating a regimen with high prevalence of toxicity. Patients completed PRO-CTCAEs, including the severity of 24 symptoms (11 classified as core symptoms) at enrollment, 4-6 weeks, 3 months, and 6 months. Symptoms were scored as grade ≥ 2 (at least moderate) and grade ≥ 3 (severe/very severe). Symptomatic toxicity was determined by an increase in severity during treatment. A generalized estimating equation model was used to assess the effects of the GA intervention on symptomatic toxicity.
RESULTS
Mean age was 77 years (range, 70-96 years), 43% were female, and 88% were White, 59% had GI or lung cancers, and 27% received prior chemotherapy. In 706 patients who provided PRO-CTCAEs at baseline, 86.1% reported at least one moderate symptom and 49.7% reported severe/very severe symptoms at regimen initiation. In 623 patients with follow-up PRO-CTCAE data, compared with usual care, fewer patients in the GA intervention arm reported grade ≥ 2 symptomatic toxicity (overall: 88.9% v 94.8%, P = .035; core symptoms: 83.4% v 91.7%, P = .001). The results for grade ≥ 3 toxicity were comparable but not significant (P > .05).
CONCLUSION
In the presence of a high baseline symptom burden, a GA intervention for older patients with advanced cancer reduces patient-reported symptomatic toxicity.
INTRODUCTION
More than 25% of all new cancer cases are diagnosed in patients age 75+ years.1,2 Older patients remain under-represented in cancer clinical trials, limiting knowledge of the safety and efficacy of treatments.3 Older patients with aging-related conditions (eg, disability and comorbidity) and advanced cancer experience a high prevalence of treatment-related symptomatic toxicities.4-7
CONTEXT
Key Objective
Can geriatric assessment (GA)–based recommendations provided to oncologists improve patient-reported symptomatic toxicities in older adults initiating a new systemic treatment regimen?
Knowledge Generated
In a clinical trial of older adults with advanced cancer initiating a new treatment regimen with a high prevalence of toxicity, 86% of patients reported at least one symptom of moderate or greater severity before initiating treatment. Patients who received GA recommendations had decreased symptomatic toxicities compared with usual care. However, the majority of patients in both treatment arms reported new or worsening symptomatic toxicities over 6 months following treatment initiation. The results support the use of GA recommendations in older adults with advanced cancer and reinforce the feasibility of collecting patient-reported toxicity data longitudinally from this population.
Relevance (S.B. Wheeler)
-
GAs can inform treatment selection in older adults with advanced cancer, and high symptom burden in this population underscores the need to monitor patient-reported outcomes on an ongoing basis.*
*Relevance section written by JCO Associate Editor Stephanie B. Wheeler, PhD, MPH.
Increasingly, the importance of partnering with patients to better understand symptomatic toxicity has been recognized.4,8 The US Food and Drug Administration has identified symptom burden and symptomatic toxicities as core concepts of interest.9-11 The National Cancer Institute (NCI) developed the patient-reported outcome version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) as a complement to clinician-rated CTCAE.12-14 The content validity,12 feasibility,15,16 reliability,17 and construct validity17,18 of PRO-CTCAE items have been established. The NCI PRO-CTCAE Library19 includes 78 symptom items that evaluate symptom presence/absence, frequency, severity, and interference with daily activities. Although feasibility data of PRO-CTCAE in clinical trials are growing,15,20 the optimal interpretation of patient-reported symptomatic toxicities is less established.14
Aging-related conditions influence the prevalence and reporting of symptomatic toxicities and their impact on treatment tolerability.4,21-23 Patients' ratings often differ from clinicians' ratings, and provide complementary but unique information on tolerance.24-26 In older adults with advanced cancer, it is particularly important to include the patient-reported perspective because goals of treatment often prioritize palliation of symptoms.27 Moreover, even mild or moderate levels of toxicities may have a negative effect on function.28,29 PRO-CTCAE research has included limited numbers of adults age 70+ years.17,18,30,31 No prior studies have incorporated PRO-CTCAEs in a randomized trial of older adults with advanced cancer and aging-related conditions.4
Geriatric assessment (GA) uses patient-reported and objective measures to evaluate aging-related domains.32 The Geriatric Assessment for Patients 70 Years and Older (GAP70+; ClinicalTrials.gov identifier: NCT02054741) cluster randomized trial demonstrated that providing a GA summary with GA-guided management recommendations to community oncologists significantly reduces serious treatment toxicity (as measured by clinician-rated CTCAE) in older patients with advanced cancer and aging-related conditions.7 The aims of this secondary analysis of the GAP70+ trial are (1) to evaluate patient-reported symptoms at the initiation of a new regimen and (2) to assess the effect of the GA intervention on patient-reported symptomatic toxicities as measured by PRO-CTCAE. The analysis was guided by the NCI Cancer Moonshot Cancer Treatment Tolerability consortium, which provided the opportunity to share methods and resources across investigative groups.4,33
METHODS
Study Design and Participants
This secondary analysis uses data from the GAP70+ trial, a nationwide study conducted in the University of Rochester NCI Community Oncology Research Program (UR NCORP). Community oncology practices were randomized to GA intervention or usual care. Eligible patients were age ≥ 70 years, had incurable solid tumors or lymphoma, at least one GA domain impairment other than polypharmacy, and were initiating a new systemic treatment regimen with a > 50% prevalence of grade 3-5 toxicity. Because of the high prevalence of polypharmacy in older adults34 and unclear association of polypharmacy with toxicity, an additional GA domain impairment was required. Patients in both arms underwent a GA32 before the initiation of the planned treatment regimen. In the intervention arm, oncologists were provided with GA summary and management recommendations for each enrolled patient. In the usual care arm, oncologists did not receive recommendations, but were alerted to positive screens for depression and cognitive impairment. The study was approved by the institutional review boards at all participating sites, and all patients provided informed consent. The study enrolled patients from 2014 to 2019; details on study design, participant demographic, disease, and treatment characteristics, and the effect of intervention on primary and secondary outcomes have been published previously.7 Briefly, along with reduced CTCAE toxicity, a greater proportion of patients in the GA intervention arm, compared with usual care, initiated treatment with a dose-reduced regimen. However, more patients in the usual care arm experienced dose reductions during treatment .7
Measures
Demographics, including age, sex, ethnicity, and race, were collected as self-report measures. Cancer type and stage, history of prior chemotherapy, planned treatment, and physician-reported Karnofsky performance score35 were extracted from the medical record. Before the initiation of the treatment, GA domain impairments for function, objective physical performance, comorbidity, polypharmacy, cognition, social support, and psychologic status were assessed by a series of validated instruments. The GA measures are aligned with the ASCO geriatric oncology guidelines32 and were published previously.7
Symptoms were evaluated with PRO-CTCAE to assess symptomatic toxicity and provide complementary data to CTCAE. During the design of the GAP 70+ study in 2013, the investigators selected 27 PRO-CTCAE symptoms on the basis of relevance to older adults and prevalence of treatment toxicities. The items were reviewed by the NCI, geriatric oncology experts in the Cancer and Aging Research Group,36 patient advocates,37 and clinicians in the UR NCORP network. This analysis concentrates on the attribute of severity. Severity was selected since responses range from none, to mild, moderate, severe, or very severe; these responses correspond to scoring for many CTCAE items.38 PRO-CTCAE items with a severity attribute (24/27) are included in this analysis. Responses were scored as grade ≥ 2 (moderate or higher) or grade ≥ 3 (severe or very severe).
Core symptoms commonly occur across diseases and treatments.39,40 The NCI Symptom Management and Health-Related Quality of Life Steering Committee identified a set of 12 core symptoms that includes fatigue, insomnia, pain, anorexia (appetite loss), dyspnea, cognitive problems, anxiety, nausea, depression, sensory neuropathy, constipation, and diarrhea.40 Among PRO-CTCAE items in GAP70+, 10 core symptoms were included. PRO-CTCAE items of anxiety and depression were not included (because they were captured by GA measures). Cognitive problems were represented by two items (memory and concentration problems), so that 11 core items were collected in total. Patients completed PRO-CTCAE questionnaires on paper at baseline, 4-6 weeks, 3 months, and 6 months.
Statistical Analysis
All patients who provided PRO-CTCAE data at baseline were included in the analysis of symptom burden at the initiation of the regimen, and those who also provided data for at least one postbaseline assessment were included in the analysis of symptomatic toxicity during treatment (Fig 1). For both analyses, two summary approaches were planned: (1) on the basis of all 24 severity items and (2) restricted to 11 core items.
FIG 1.
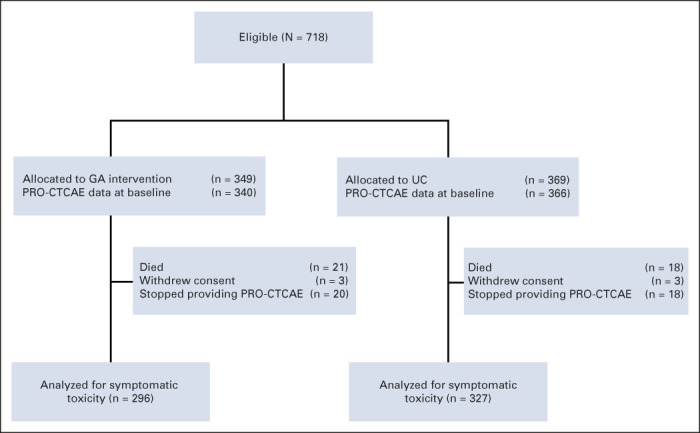
CONSORT flow diagram for symptomatic toxicity as measured by PRO-CTCAE. GA, geriatric assessment; PRO-CTCAE, Patient-Reported Outcome Version of the Common Terminology Criteria for Adverse Events; UC, usual care.
Descriptive statistics, means, standard deviation, range for continuous measures, and n (%) for categorical measures were used to characterize the sample. To appraise baseline severity, the distribution of each symptom was visualized according to study arm. The balance in distribution of symptom severity across the two study arms was assessed using the Wilcoxon rank test. To characterize symptom burden at baseline, the overall proportion of patients who reported any symptom with moderate or higher severity were evaluated as well as proportions of patients who reported severe/very severe symptoms. The same proportions were evaluated for cores symptoms. Additionally, differences between patients who provided only baseline PRO-CTCAE data and those who provided at least one additional time point were compared by chi-square test.
To evaluate the effects of the GA intervention on the outcomes of patient-reported symptomatic toxicity, a two-step process was followed. First, following the baseline-adjusted method developed by Basch et al41 for each PRO-CTCAE item, the maximum severity grade reported after baseline was compared with baseline severity. For each PRO-CTCAE item, a patient was classified as having a grade ≥ 2 symptomatic toxicity event if the maximum grade reported after baseline was both grade ≥ 2 and greater than the baseline grade. Events across symptoms were combined; the binary outcome of grade ≥ 2 symptomatic toxicity was calculated as the patient experiencing a grade ≥ 2 symptomatic toxicity event for any of the 24 symptoms. The grade ≥ 2 core symptomatic toxicity was defined as patient experiencing grade ≥ 2 symptomatic toxicity event for any of the 11 core items. The definition of grade ≥ 3 symptomatic toxicity followed an analogous process. Both grade ≥ 2 and grade ≥ 3 symptomatic toxicity outcomes were prespecified before the analysis.
Between-arm differences in symptomatic toxicities were compared using the chi-square test. To account for correlations between patients from the same practice cluster, we further assessed the effect of the intervention on symptomatic toxicity using a generalized estimating equation42 regression model with the binary outcome and log link. The adjusted risk ratio (ARR) from the generalized estimating equation model together with 95% robust CI is reported. To better understand the contribution of individual PRO-CTCAE items, the between-arm differences for each symptom were assessed using the same analytical approach. Sensitivity analyses assessing the effect of the intervention on symptomatic toxicity in prespecified subgroups by cancer type, treatment type, and history were also performed. Statistical significance was set at a two-sided alpha = .05 level. The findings reported in this manuscript should be considered as hypothesis-generating. Data were analyzed using SAS version 9.4 (SAS Inc, Cory, NC).
RESULTS
Patient Characteristics
Of 718 patients enrolled onto GAP70+, 706 (98.3%) provided PRO-CTCAEs at baseline. Of 706 patients, 83 (11.8%) patients provided PRO-CTCAE data only at baseline and could not be included in the analyses of symptomatic toxicity. Of these 83 patients, 39 (47.0%) died within 4-6 weeks, six (7.2%) withdrew from the study, and 38 (45.8%) declined or were not further able to complete PRO measures (Fig 1). The death of nine patients was classified as toxicity-related (five in usual care and four in GA intervention arm). Proportions of patients reporting PRO-CTCAE data at each time point by arm were similar (Appendix Table A1, online only). The average age of the 706 patients was 77 years (standard deviation = 5.4, range, 70-96 years), 305 (43.2%) were female, and 182 (26.5%) had prior chemotherapy (Table 1). Patients with GI cancers (P = .043), lower performance status (Karnofsky performance score ≤ 80, P = .002), and those who had GA domain impairments in nutrition (P = .002), psychologic status (P = .002), and cognitive status (P = .048) were more likely to not provide PRO-CTCAE data after baseline (Table 1).
TABLE 1.
Patients Characteristics at Initiation of Current Regimen
Baseline Symptom Burden as Measured by PRO-CTCAE
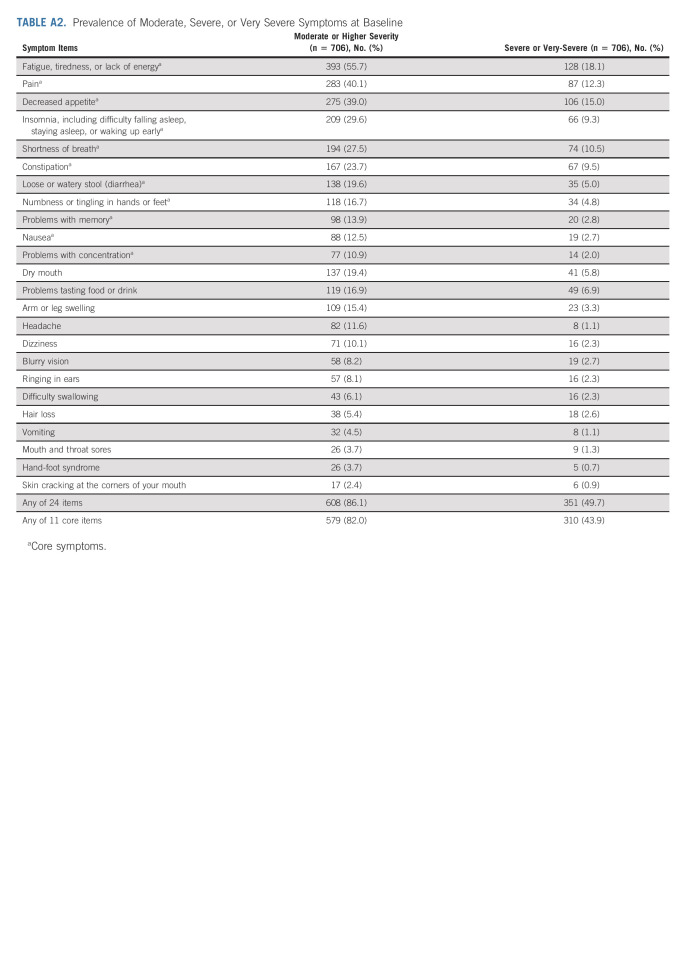
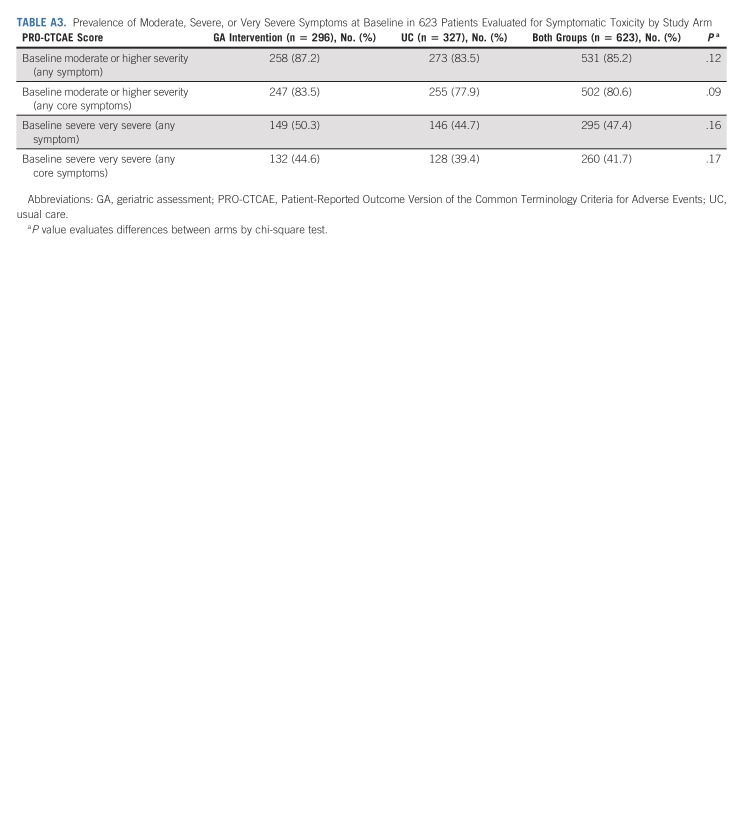
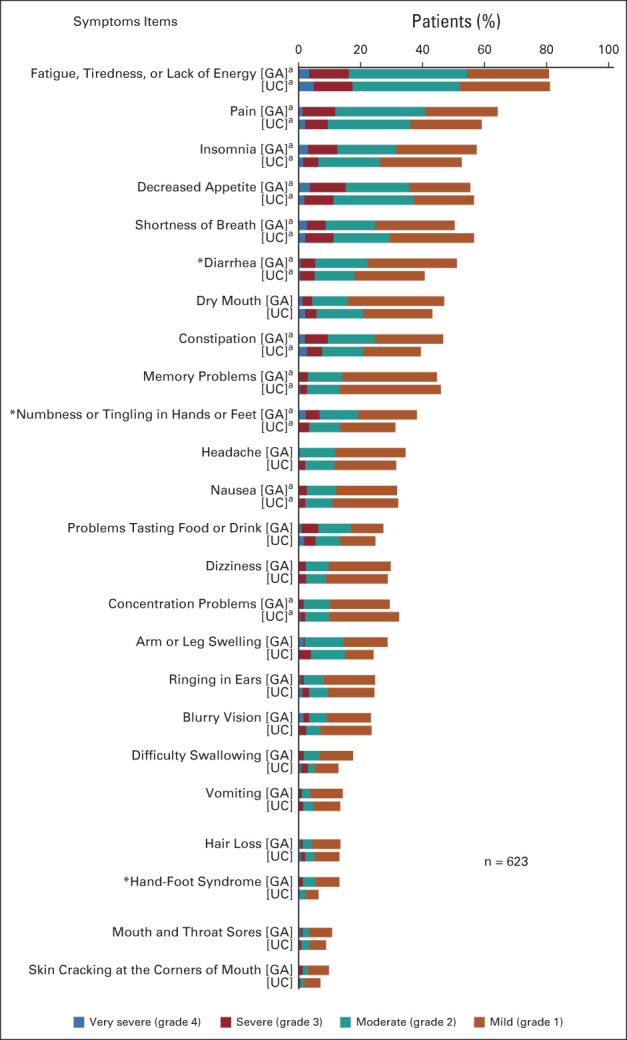
Figure 2 presents the distribution of severity of symptoms by study arm before treatment initiation. The baseline distribution was well balanced between the arms, with a statistically significant difference detected only for numbness/tingling (P = .027) and hand-foot syndrome (P = .002). The most prevalent symptoms were fatigue (82.0%), pain (63.3%), and decreased appetite (58.2%). The six most prevalent symptoms were core symptoms (Fig 2, Appendix Fig A1, online only). Of 706 patients, 608 (86.1%) reported at least one symptom with moderate or higher severity, and 579 (82.0%) reported at least one core symptom with moderate or higher severity. Almost half (351; 49.7%) reported at least one severe or very severe symptom, and 310 (43.9%) reported at least one core symptom as severe/very severe (Appendix Table A2, online only). The proportions did not significantly differ by study arm. Patients who provided only baseline PRO-CTCAE (n = 83) were more likely to report a core symptom with moderate or higher severity (92.8% v 80.6%, P = .007) and severe or very severe core symptom (67.5% v 47.4%, P = .001). Specifically, they reported a higher prevalence of moderate or greater severity for fatigue, pain, and decreased appetite (Figs 3A and 3B), and of severe/very severe constipation (all P < .05; Fig 3B). The proportions reporting moderate or higher severity at baseline did not differ significantly by arm in 706 patients who had baseline PRO-CTCAE data or in 623 patients who provided data analyzed for symptomatic toxicity (Appendix Table A3, online only).
FIG 2.
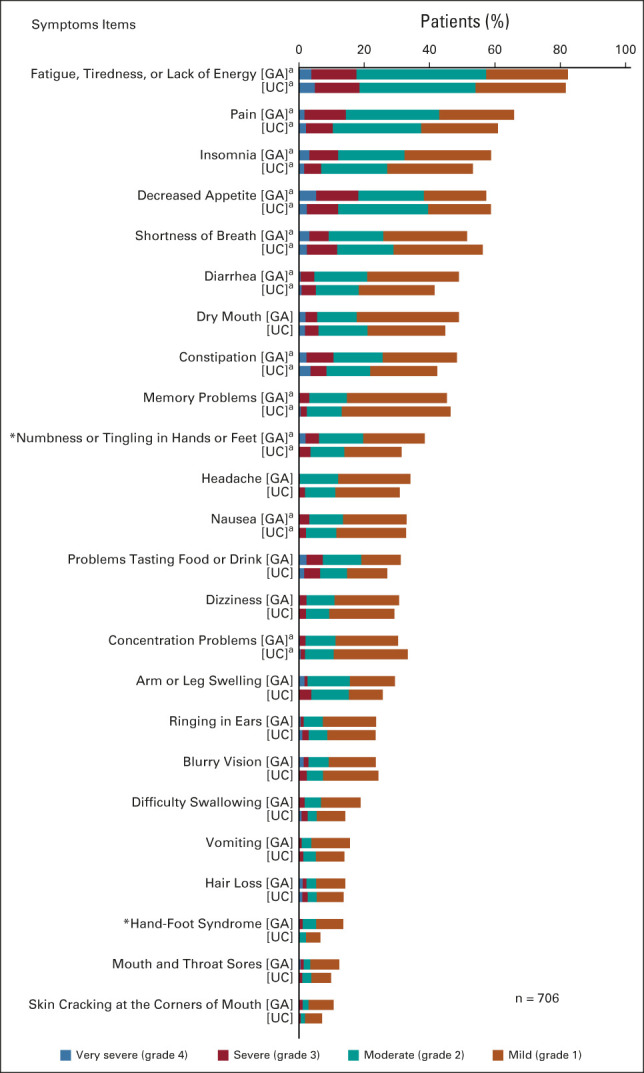
Baseline symptom severity by arm. aCore symptom. *P < .05. GA, geriatric assessment intervention; UC, usual care.
FIG 3.
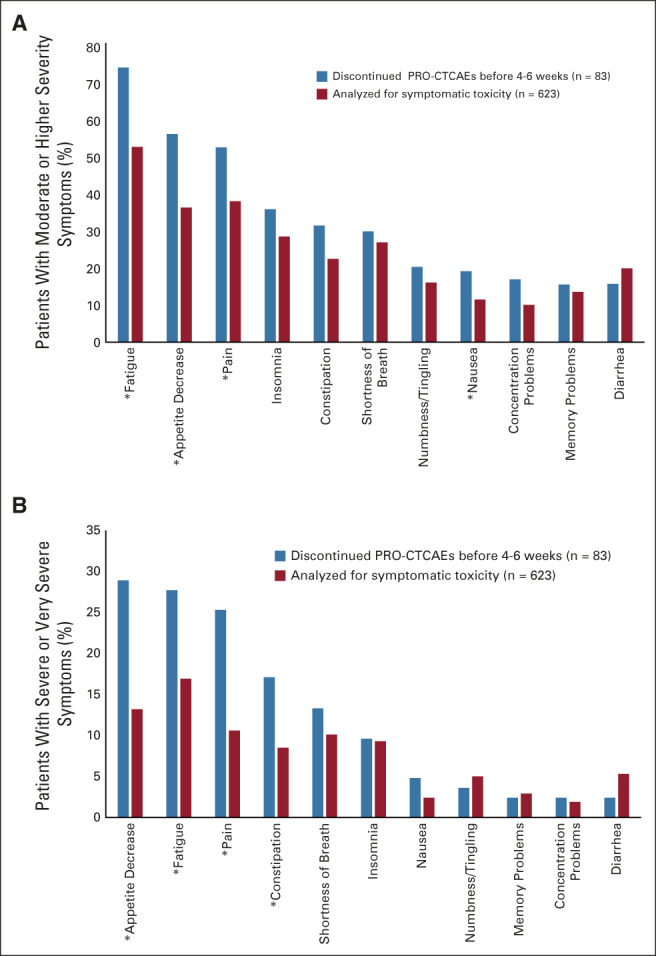
Patients reporting of symptom severity at baseline: (A) moderate or higher severity and (B) severe and very severe. *P < .05. PRO-CTCAE, Patient-Reported Outcome Version of the Common Terminology Criteria for Adverse Events.
Symptomatic Toxicity
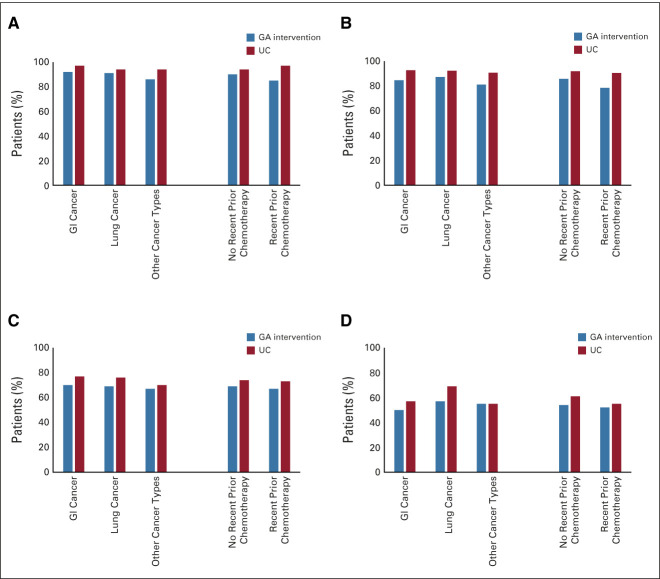
After baseline, compared with usual care (n = 327), a lower proportion of patients who received the GA intervention (n = 296) reported grade ≥ 2 symptomatic toxicity (88.9% v 94.8%; ARR = 0.937, 95% CI, 0.882 to 0.996; P = .035). The proportion of grade ≥ 2 core symptomatic toxicity was also lower in the GA intervention arm compared with usual care (83.4% v 91.7%; ARR = 0.907, 95% CI, 0.860 to 0.962; P = .001; Fig 4). A similar pattern was observed for grade ≥ 3 toxicities; however, the results did not reach statistical significance (Fig 4). In stratified analysis by cancer type and prior chemotherapy, the overall pattern of higher severity of symptomatic toxicity in the usual care arm persisted (Appendix Fig A2, online only).
FIG 4.
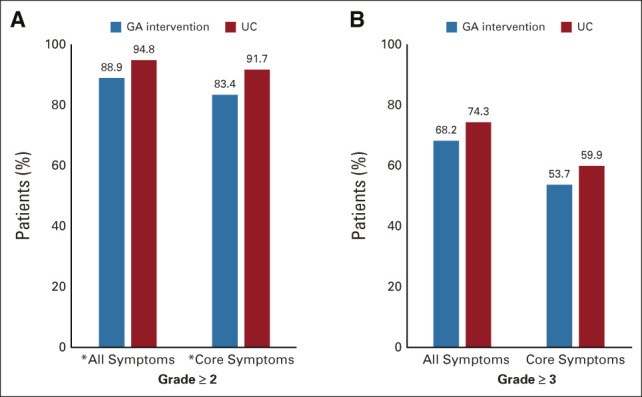
Effect of the GA intervention on patient-reported symptomatic toxicity reported over 6 months: (A) grade ≥ 2 and (B) grade ≥ 3. *P < .05. GA, geriatric assessment; UC, usual care.
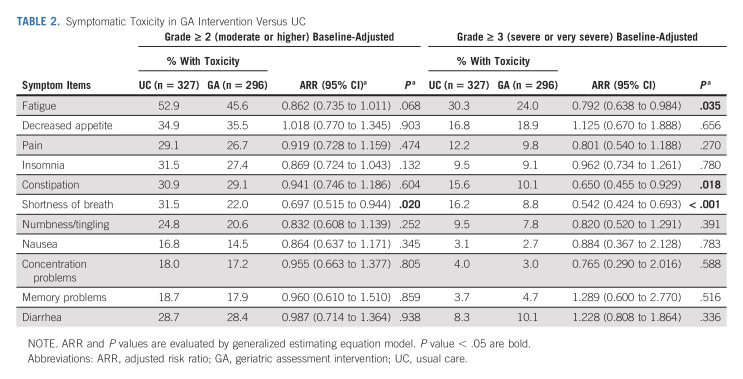
When evaluating individual toxicities, a lower proportion of patients who received the GA intervention reported grade ≥ 2 symptomatic toxicity on all core symptoms except for decreased appetite (Table 2).
TABLE 2.
Symptomatic Toxicity in GA Intervention Versus UC
DISCUSSION
These analyses address important gaps in understanding the symptom experience of older adults with advanced cancer receiving systemic therapy. First, Gap70+ is the first nationwide cluster-randomized trial to demonstrate that a GA intervention can decrease patient-reported symptomatic toxicities. Second, the findings establish high baseline symptom burden and a high prevalence of developing new or worsening symptomatic toxicities over 6 months. Third, to our knowledge, it is the first study to systematically describe baseline symptom burden as measured by PRO-CTCAE in older adults with advanced cancer. Fourth, this study is one of a very few to report patient-reported symptomatic toxicities as an outcome in a randomized controlled trial. Previously, PRO-CTCAEs were evaluated in trials examining the efficacy of therapeutic agents in advanced prostate cancer and non–small-cell lung cancer.26,43 Last, the results reinforce the feasibility of collecting PRO-CTCAE longitudinally from older adults with advanced cancer and aging-related conditions.
These results provide further support for GA-based models of care.32 A significant decrease in the proportion of patients reporting the development of grade ≥ 2 symptomatic toxicity over 6 months was found for patients receiving the GA intervention. Older individuals may have a decreased ability to tolerate even low-grade symptomatic toxicities because of concurrent functional decline and competing comorbidities.28,29 The overall effect on symptomatic toxicity was smaller than the effect found for CTCAE7 and was significant for grade 2 but not grade 3. This may be because GA intervention was administered at baseline only and did not specifically address symptoms. Furthermore, the sample size may not provide sufficient power to establish statistical significance for symptomatic grade ≥ 3 toxicity. A smaller difference for patient-reported PRO-CTCAE compared with clinician-rated CTCAE is consistent with the complementary purpose of these measurement systems.44 Prior reports indicate that patient-reported symptoms are higher in prevalence and greater in severity than clinician-rated, which may explain the smaller difference found for PRO-CTCAE results.26,45,46
Symptomatic toxicities were highly prevalent in both arms over 6 months. At baseline, patients had a high prevalence (86%) of having at least one moderate to severe symptom, and also the majority (92%) experienced worsening or new symptomatic toxicities. Given that an important treatment goal in the advanced cancer setting is to decrease tumor burden and improve symptom control,47 it is troubling that the symptomatic toxicity was so prevalent. This result reinforces the need to integrate guideline-supported palliative care48 with GA-informed care; the preliminary efficacy of one integrated model was recently reported in a promising pilot trial.49,50 In the GAP70+ trial, although oncologists reduced treatment intensity at cycle 1 and provided management for aging-related conditions, there was no systematic provision of symptom management recommendations.7 Emerging consensus on the efficacy of routine electronic symptom reporting and follow-up interventions could also strengthen symptom reduction in this population.20,51-53 The efficacy of weekly electronic PRO monitoring for adults with metastatic cancer receiving treatment (including symptoms with alerts to clinicians for severe or worsening symptoms) on improving symptoms has recently been reported.54 A similar symptom-specific intervention that integrates GA could be evaluated for older patients with aging-related conditions. Further research is needed to assess the feasibility of digital methods for capture of PRO data from older adults with advanced cancer and aging-related conditions.
The specific symptoms of fatigue, pain, insomnia, decreased appetite, and dyspnea were reported by more than 50% of older adults before treatment initiation. These results align with past research on commonly experienced symptom prevalence in individuals of all ages with advanced cancer.55-57 In GAP70+, older adults with advanced cancer who had the highest baseline symptom burden more often did not complete PRO-CTCAE at follow-up. This result is consistent with other research that has found individuals who are ill are less likely to complete PRO measures.15,16 It is important to offer assistance to older adults who need help with completing PRO measures.21 Other studies have evaluated the benefit of capturing symptom data from older adults using different PRO questionnaires. For example, Battisti et al58 found that the symptoms increased on the European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaires (EORTC-QLQ) C30 in older patients with early breast cancer receiving chemotherapy. Symptom assessment instruments need to be selected for clinical trials on the basis of specific study aims, ease of use in setting, and interpretation. For the GAP 70+ study, PRO-CTCAE was included because it was designed as a complement to clinician-rated CTCAE.
Ongoing efforts are underway to determine best approaches for analyzing and reporting PRO-CTCAE data.59,60 An aggregate measure, summing data for PRO-CTCAE items, was used. This summary measure parallels reporting for CTCAE by clinicians in clinical trials.7 The baseline-adjusted method was used to estimate symptomatic toxicity,41 and although these methods were successfully implemented26,33 for this analysis, methodologic challenges remain. The method does not capture toxicity for patients entering treatment with the highest symptom severity scores. Thus, the method may underestimate symptomatic toxicity for symptoms that are severe and highly prevalent at baseline.26 Also unique in this examination is a focus on core symptoms as recommended by the NCI.40
There were several study limitations. The sample was primarily White, limiting generalizability. The selected time intervals were a limitation since the PRO-CTAE items were not collected weekly, and thus may not have captured the period when symptomatic toxicities were present or most severe.61 The study was not fully powered for the outcome of symptomatic toxicity. Causal attribution of symptoms to treatment alone is particularly challenging in older patients with a high prevalence of aging-related conditions. Even with these limitations, this study adds to growing evidence that PRO-CTCAEs are feasible for patients to report symptoms during treatment. This analysis demonstrates their value for older patients with advanced cancer and aging-related conditions being cared for in community oncology clinics.
This analysis provides evidence that a GA intervention can decrease the prevalence of symptomatic toxicities as measured by patient-reported outcomes. Future trials should examine whether GA-based models of care that integrate symptom monitoring and management can further improve outcomes of older patients with advanced cancer and aging-related conditions.49
ACKNOWLEDGMENT
The authors would like to thank Susan Rosenthal, MD, for editing and Shuhan Yang, MS, for formatting of figures. The authors would like to thank the geriatric oncology research staff and University of Rochester NCI Community Oncology Research Base staff. The authors would also like to thank the patients, community oncologists, and staff who participated in the study.
APPENDIX
FIG A1.
Baseline symptom severity by study arm in patients analyzed for symptomatic toxicity. aCore symptom. *P < .05. GA, geriatric assessment intervention; UC, usual care.
FIG A2.
Patient-reported symptomatic toxicity over 6 months stratified by cancer type and prior treatment history: (A) grade ≥ 2 (all symptoms), (B) grade ≥ 2 (core symptoms), (C) grade ≥ 3 (all symptoms), and (D) grade ≥ 3 (core symptoms). GA, geriatric assessment intervention; UC, usual care.
TABLE A1.
Availability of PRO-CTCAE Data by Study Arm
TABLE A2.
Prevalence of Moderate, Severe, or Very Severe Symptoms at Baseline
TABLE A3.
Prevalence of Moderate, Severe, or Very Severe Symptoms at Baseline in 623 Patients Evaluated for Symptomatic Toxicity by Study Arm
Luke Peppone
Consulting or Advisory Role: Ajna Biosciences
Erika Ramsdale
Honoraria: Flatiron Health
Travel, Accommodations, Expenses: Flatiron Health
Megan Wells
Stock and Other Ownership Interests: Agenus, ADRx, Corbus Pharmaceuticals, X4 Pharma, MindMed
Rachael Tylock
Employment: Ortho Clinical Diagnostics
Kah Poh Loh
Honoraria: Pfizer
Consulting or Advisory Role: Pfizer, Seattle Genetics
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2020 ASCO Quality Care Symposium, virtual, October 9-10, 2020.
SUPPORT
Supported by the National Cancer Institute (R01CA177592, U01CA233167, UG1CA189961, and R00CA237744) and the National Institute on Aging (K24AG056589 and P30-AG024832). The investigators were independent in the conduct of the analysis and presentation of the results.
CLINICAL TRIAL INFORMATION
E.C. and S.G.M. contributed equally to this work as co‐first authors.
DATA SHARING STATEMENT
The study protocol, statistical analysis plan, informed consent form, and clinical study reports are available on the Cancer and Aging Research Group website (https://www.mycarg.org/). These documents will be available beginning 6 months and with no end date following publication of the article. The above data and materials are made available to anyone who wishes to use the data. For any further data or materials, research proposals can be directed to SM (supriya_mohile@urmc.rochester.edu). Opportunities for further analyses will be made available to investigators of the Cancer and Aging Research Group. There is no cost to be a member of the Cancer and Aging Research Group (see https://www.mycarg.org/ for membership information).
AUTHOR CONTRIBUTIONS
Conception and design: Eva Culakova, Supriya G. Mohile, Paul R. Duberstein, Marie Anne Flannery
Financial support: Supriya G. Mohile, William Dale
Administrative support: Supriya G. Mohile, William Dale, Marie Anne Flannery
Provision of study materials or patients: Marie Anne Flannery
Collection and assembly of data: Eva Culakova, Supriya G. Mohile, Mostafa Mohamed, Megan Wells, Rachael Tylock, Leah Jamieson, Victor Vogel, William Dale
Data analysis and interpretation: Eva Culakova, Supriya G. Mohile, Luke Peppone, Erika Ramsdale, Huiwen Xu, Jim Java, Kah Poh Loh, Allison Magnuson, Paul R. Duberstein, Benjamin P. Chapman, William Dale, Marie Anne Flannery
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Effects of a Geriatric Assessment Intervention on Patient-Reported Symptomatic Toxicity in Older Adults with Advanced Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Luke Peppone
Consulting or Advisory Role: Ajna Biosciences
Erika Ramsdale
Honoraria: Flatiron Health
Travel, Accommodations, Expenses: Flatiron Health
Megan Wells
Stock and Other Ownership Interests: Agenus, ADRx, Corbus Pharmaceuticals, X4 Pharma, MindMed
Rachael Tylock
Employment: Ortho Clinical Diagnostics
Kah Poh Loh
Honoraria: Pfizer
Consulting or Advisory Role: Pfizer, Seattle Genetics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2020. CA Cancer J Clin 70:7-30, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Smith BD, Smith GL, Hurria A, et al. : Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol 27:2758-2765, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Sedrak MS, Freedman RA, Cohen HJ, et al. : Older adult participation in cancer clinical trials: A systematic review of barriers and interventions. CA Cancer J Clin 71:78-92, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flannery MA, Culakova E, Canin BE, et al. : Understanding treatment tolerability in older adults with cancer. J Clin Oncol 39:2150-2163, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurria A, Mohile S, Gajra A, et al. : Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol 34:2366-2371, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klepin HD, Sun CL, Smith DD, et al. : Predictors of unplanned hospitalizations among older adults receiving cancer chemotherapy. JCO Oncol Pract 17:e740-e752, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohile SG, Mohamed MR, Xu H, et al. : Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): A cluster-randomised study. Lancet 398:1894-1904, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basch E, Campbell A, Hudgens S: A Friends of Cancer Research White Paper: Broadening the Definition of Tolerability in Cancer Clinical Trials to Better Measure the Patient Experience, 2018. https://www.focr.org/sites/default/files/Comparative%20Tolerability%20Whitepaper_FINAL.pdf [Google Scholar]
- 9.Kim JW, Kim YJ, Lee KW, et al. : The early discontinuation of palliative chemotherapy in older patients with cancer. Support Care Cancer 22:773-781, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Kluetz PG, Papadopoulos EJ, Johnson LL, et al. : Focusing on core patient-reported outcomes in cancer clinical trials-response. Clin Cancer Res 22:5618, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kluetz PG, Slagle A, Papadopoulos EJ, et al. : Focusing on core patient-reported outcomes in cancer clinical trials: Symptomatic adverse events, physical function, and disease-related symptoms. Clin Cancer Res 22:1553-1558, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Basch E, Reeve BB, Mitchell SA, et al. : Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 106, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruner DW, Hanisch LJ, Reeve BB, et al. : Stakeholder perspectives on implementing the national cancer Institute's patient-reported outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Transl Behav Med 1:110-122, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kluetz PG, Chingos DT, Basch EM, et al. : Patient-reported outcomes in cancer clinical trials: Measuring symptomatic adverse events with the National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Am Soc Clin Oncol Ed Book 35:67-73, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Basch E, Dueck AC, Rogak LJ, et al. : Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol 3:1043-1050, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basch E, Dueck AC, Rogak LJ, et al. : Feasibility of implementing the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events in a multicenter trial: NCCTG N1048. J Clin Oncol 36:3120-3125, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dueck AC, Mendoza TR, Mitchell SA, et al. : Validity and reliability of the US National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol 1:1051-1059, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atkinson TM, Hay JL, Dueck AC, et al. : What do "none," "mild," "moderate," "severe," and "very severe" mean to patients with cancer? Content validity of PRO-CTCAE response scales. J Pain Symptom Manage 55:e3-e6, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Cancer Institute : Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE™). https://healthcaredelivery.cancer.gov/pro-ctcae/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basch E, Stover AM, Schrag D, et al. : Clinical utility and user perceptions of a digital system for electronic patient-reported symptom monitoring during routine cancer care: Findings from the PRO-TECT trial. JCO Clin Cancer Inform 4:947-957, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flannery MA, Mohile S, Culakova E, et al. : Completion of patient-reported outcome questionnaires among older adults with advanced cancer. J Pain Symptom Manage 63:301-310, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao JJ, Armstrong K, Bowman MA, et al. : Symptom burden among cancer survivors: Impact of age and comorbidity. J Am Board Fam Med 20:434-443, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Mohile SG, Hurria A, Cohen HJ, et al. : Improving the quality of survivorship for older adults with cancer. Cancer 122:2459-2568, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atkinson TM, Rogak LJ, Heon N, et al. : Exploring differences in adverse symptom event grading thresholds between clinicians and patients in the clinical trial setting. J Cancer Res Clin Oncol 143:735-743, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atkinson TM, Ryan SJ, Bennett AV, et al. : The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): A systematic review. Support Care Cancer 24:3669-3676, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dueck AC, Scher HI, Bennett AV, et al. : Assessment of adverse events from the patient perspective in a phase 3 metastatic castration-resistant prostate cancer clinical trial. JAMA Oncol 6:e193332, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnipper LE, Davidson NE, Wollins DS, et al. : American Society of Clinical Oncology statement: A conceptual framework to assess the value of cancer treatment options. J Clin Oncol 33:2563-2577, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalsi T, Babic-Illman G, Fields P, et al. : The impact of low-grade toxicity in older people with cancer undergoing chemotherapy. Br J Cancer 111:2224-2228, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Q, Lee JW, Wang XS, et al. : Testing symptom severity thresholds and potential alerts for clinical intervention in patients with cancer undergoing chemotherapy. JCO Oncol Pract 16:e893-e901, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett AV, Dueck AC, Mitchell SA, et al. : Mode equivalence and acceptability of tablet computer-, interactive voice response system-, and paper-based administration of the U.S. National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Health Qual Life Outcomes 14:24, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hay JL, Atkinson TM, Reeve BB, et al. : Cognitive interviewing of the US National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Qual Life Res 23:257-269, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohile SG, Dale W, Somerfield MR, et al. : Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol 36:2326-2347, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langlais B, Mazza GL, Thanarajasingam G, et al. : Evaluating treatment tolerability using the toxicity index with patient-reported outcomes data. J Pain Symptom Manage 63:311-320, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maggiore RJ, Gross CP, Hurria A: Polypharmacy in older adults with cancer. Oncologist 15:507-522, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schag CC, Heinrich RL, Ganz PA: Karnofsky performance status revisited: Reliability, validity, and guidelines. J Clin Oncol 2:187-193, 1984 [DOI] [PubMed] [Google Scholar]
- 36.Anand M, Magnuson A, Patil A, et al. : Developing sustainable national infrastructure supporting high-impact research to improve the care of older adults with cancer: A Delphi investigation of geriatric oncology experts. J Geriatr Oncol 11:338-342, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilmore NJ, Canin B, Whitehead M, et al. : Engaging older patients with cancer and their caregivers as partners in cancer research. Cancer 125:4124-4133, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_8.5x11.pdf
- 39.Cleeland CS, Zhao F, Chang VT, et al. : The symptom burden of cancer: Evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group Symptom Outcomes and Practice Patterns study. Cancer 119:4333-4340, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reeve BB, Mitchell SA, Dueck AC, et al. : Recommended patient-reported core set of symptoms to measure in adult cancer treatment trials. J Natl Cancer Inst 106, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basch E, Rogak LJ, Dueck AC: Methods for implementing and reporting patient-reported outcome (PRO) measures of symptomatic adverse events in cancer clinical trials. Clin Ther 38:821-830, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ziegler A, Gromping U: The generalised estimating equations: A comparison of procedures available in commercial statistical software packages. Biometrical J 40:245-260, 1998 [Google Scholar]
- 43.Sebastian M, Ryden A, Walding A, et al. : Patient-reported symptoms possibly related to treatment with osimertinib or chemotherapy for advanced non-small cell lung cancer. Lung Cancer 122:100-106, 2018 [DOI] [PubMed] [Google Scholar]
- 44.Kim J, Singh H, Ayalew K, et al. : Use of PRO measures to inform tolerability in oncology trials: Implications for clinical review, IND safety reporting, and clinical site inspections. Clin Cancer Res 24:1780-1784, 2018 [DOI] [PubMed] [Google Scholar]
- 45.Kowalchuk RO, Hillman D, Daniels TB, et al. : Assessing concordance between patient-reported and investigator-reported CTCAE after proton beam therapy for prostate cancer. Clin Transl Radiat Oncol 31:34-41, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nyrop KA, Deal AM, Reeder-Hayes KE, et al. : Patient-reported and clinician-reported chemotherapy-induced peripheral neuropathy in patients with early breast cancer: Current clinical practice. Cancer 125:2945-2954, 2019 [DOI] [PubMed] [Google Scholar]
- 47.Roeland EJ, LeBlanc TW: Palliative chemotherapy: Oxymoron or misunderstanding? BMC Palliat Care 15:33, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrell BR, Temel JS, Temin S, et al. : Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 35:96-112, 2017 [DOI] [PubMed] [Google Scholar]
- 49.Nipp RD, Subbiah IM, Loscalzo M: Convergence of geriatrics and palliative care to deliver personalized supportive care for older adults with cancer. J Clin Oncol 39:2185-2194, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nipp RD, Temel B, Fuh CX, et al. : Pilot randomized trial of a transdisciplinary geriatric and palliative care intervention for older adults with cancer. J Natl Compr Canc Netw 18:591-598, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donovan HS, Sereika SM, Wenzel LB, et al. : Effects of the WRITE symptoms interventions on symptoms and quality of life among patients with recurrent ovarian cancers: An NRG Oncology/GOG Study (GOG-0259). J Clin Oncol 40:1464-1473, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daly B, Nicholas K, Flynn J, et al. : Analysis of a remote monitoring Program for symptoms among adults with cancer receiving antineoplastic therapy. JAMA Netw Open 5:e221078, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thong MSY, Chan RJ, van den Hurk C, et al. : Going beyond (electronic) patient-reported outcomes: Harnessing the benefits of smart technology and ecological momentary assessment in cancer survivorship research. Support Care Cancer 29:7-10, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Basch E, Schrag D, Henson S, et al. : Effect of electronic symptom monitoring on patient-reported outcomes among patients with metastatic cancer: A randomized clinical trial. JAMA 327:2413-2422, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henson LA, Maddocks M, Evans C, et al. : Palliative care and the management of common distressing symptoms in advanced cancer: Pain, breathlessness, nausea and vomiting, and fatigue. J Clin Oncol 38:905-914, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Solano JP, Gomes B, Higginson IJ: A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage 31:58-69, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Soones T, Ombres R, Escalante C: An update on cancer-related fatigue in older adults: A narrative review. J Geriatr Oncol 13:125-131, 2022 [DOI] [PubMed] [Google Scholar]
- 58.Battisti NML, Reed MWR, Herbert E, et al. : Bridging the Age Gap in breast cancer: Impact of chemotherapy on quality of life in older women with early breast cancer. Eur J Cancer 144:269-280, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kluetz PG, King-Kallimanis BL, Suzman D, et al. : Advancing assessment, analysis, and reporting of safety and tolerability in cancer trials. J Natl Cancer Inst 113:507-508, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Otto E, Culakova E, Meng S, et al. : Overview of Sankey flow diagrams: Focusing on symptom trajectories in older adults with advanced cancer. J Geriatr Oncol 13:742-746, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mercieca-Bebber R, King MT, Calvert MJ, et al. : The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas 9:353-367, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study protocol, statistical analysis plan, informed consent form, and clinical study reports are available on the Cancer and Aging Research Group website (https://www.mycarg.org/). These documents will be available beginning 6 months and with no end date following publication of the article. The above data and materials are made available to anyone who wishes to use the data. For any further data or materials, research proposals can be directed to SM (supriya_mohile@urmc.rochester.edu). Opportunities for further analyses will be made available to investigators of the Cancer and Aging Research Group. There is no cost to be a member of the Cancer and Aging Research Group (see https://www.mycarg.org/ for membership information).