PURPOSE
Pembrolizumab and pembrolizumab-chemotherapy demonstrated efficacy in recurrent/metastatic head and neck squamous cell carcinoma in KEYNOTE-048. Post hoc analysis of long-term efficacy and progression-free survival on next-line therapy (PFS2) is presented.
METHODS
Patients were randomly assigned (1:1:1) to pembrolizumab, pembrolizumab-chemotherapy, or cetuximab-chemotherapy. Efficacy was evaluated in programmed death ligand 1 (PD-L1) combined positive score (CPS) ≥ 20, CPS ≥ 1, and total populations, with no multiplicity or alpha adjustment.
RESULTS
The median study follow-up was 45.0 months (interquartile range, 41.0-49.2; n = 882). At data cutoff (February 18, 2020), overall survival improved with pembrolizumab in the PD-L1 CPS ≥ 20 (hazard ratio [HR], 0.61; 95% CI, 0.46 to 0.81) and CPS ≥ 1 populations (HR, 0.74; 95% CI, 0.61 to 0.89) and was noninferior in the total population (HR, 0.81; 95% CI, 0.68 to 0.97). Overall survival improved with pembrolizumab-chemotherapy in the PD-L1 CPS ≥ 20 (HR, 0.62; 95% CI, 0.46 to 0.84), CPS ≥ 1 (HR, 0.64; 95% CI, 0.53 to 0.78), and total (HR, 0.71; 95% CI, 0.59 to 0.85) populations. The objective response rate on second-course pembrolizumab was 27.3% (3 of 11). PFS2 improved with pembrolizumab in the PD-L1 CPS ≥ 20 (HR, 0.64; 95% CI, 0.48 to 0.84) and CPS ≥ 1 (HR, 0.79; 95% CI, 0.66 to 0.95) populations and with pembrolizumab-chemotherapy in the PD-L1 CPS ≥ 20 (HR, 0.64; 95% CI, 0.48 to 0.86), CPS ≥ 1 (HR, 0.66; 95% CI, 0.55 to 0.81), and total (HR, 0.73; 95% CI, 0.61 to 0.88) populations. PFS2 was similar after pembrolizumab and longer after pembrolizumab-chemotherapy on next-line taxanes and shorter after pembrolizumab and similar after pembrolizumab-chemotherapy on next-line nontaxanes.
CONCLUSION
With a 4-year follow-up, first-line pembrolizumab and pembrolizumab-chemotherapy continued to demonstrate survival benefit versus cetuximab-chemotherapy in recurrent/metastatic head and neck squamous cell carcinoma. Patients responded well to subsequent treatment after pembrolizumab-based therapy.
INTRODUCTION
Head and neck squamous cell carcinomas (HNSCCs) encompass a heterogenous group of tumors arising from mucosal epithelia of the oral cavity, pharynx, and larynx.1 Most patients present with locally advanced disease, and risk of recurrence and distant metastasis is high.2 Before immunotherapies, the standard of care for recurrent or metastatic (R/M) HNSCC not amenable to surgery was platinum-based chemotherapy with the epidermal growth factor receptor (EGFR) inhibitor cetuximab.2 However, recent success with programmed death 1 inhibitors has led to a paradigm shift in the treatment of HNSCC.3-5 The programmed death 1 inhibitor pembrolizumab is now recommended as first-line treatment for R/M HNSCC as monotherapy in programmed death ligand 1 (PD-L1)–positive disease or with platinum plus fluorouracil independent of PD-L1 status in the United States.6,7 Pembrolizumab and nivolumab are also recommended for second-line treatment of R/M HNSCC after progression on or after platinum-containing therapy.6,7
CONTEXT
Key Objective
On the basis of the results of the phase III KEYNOTE-048 study, pembrolizumab is now the standard of care for the first-line treatment of advanced head and neck squamous cell carcinoma (HNSCC). We present results from long-term follow-up of KEYNOTE-048, including analysis of progression-free survival on next-line therapy.
Knowledge Generated
After a 4-year follow-up, an enduring survival benefit and substantially longer duration of response were observed with pembrolizumab alone and pembrolizumab-chemotherapy compared with cetuximab-chemotherapy in patients with previously untreated recurrent or metastatic HNSCC. Retreatment with pembrolizumab provided benefit in some patients, and patients who received first-line pembrolizumab or pembrolizumab-chemotherapy responded well to subsequent therapy.
Relevance
The results of this analysis support the earlier findings of KEYNOTE-048 and confirm that pembrolizumab and pembrolizumab-chemotherapy are effective first-line treatment options for patients with recurrent or metastatic HNSCC. These results may also help clinical decision making regarding the choice of subsequent therapy.
The inclusion of first-line pembrolizumab in the treatment paradigm is based on results of the phase III KEYNOTE-048 study of pembrolizumab alone and with chemotherapy versus cetuximab with chemotherapy.3 Pembrolizumab alone significantly prolonged overall survival (OS) compared with cetuximab-chemotherapy in patients with PD-L1 combined positive score (CPS) ≥ 20 (hazard ratio [HR], 0.61; 95% CI, 0.45 to 0.83) and CPS ≥ 1 (HR, 0.78; 95% CI, 0.64 to 0.96) and resulted in noninferior OS in the total population (HR, 0.85; 95% CI, 0.71 to 1.03). Pembrolizumab-chemotherapy significantly prolonged OS compared with cetuximab-chemotherapy in all cohorts (PD-L1 CPS ≥ 20, HR, 0.60, 95% CI, 0.45 to 0.82; CPS ≥ 1, HR, 0.65, 95% CI, 0.53 to 0.80; total, HR, 0.77, 95% CI, 0.63 to 0.93). Pembrolizumab alone and pembrolizumab-chemotherapy also demonstrated substantially longer duration of response (DOR) in all populations.3 The safety profile of pembrolizumab alone was favorable versus cetuximab-chemotherapy and was similar for pembrolizumab-chemotherapy and cetuximab-chemotherapy. However, with a median follow-up of approximately 1 year at final analysis, the long-term impact of pembrolizumab-based therapy remained unknown. Here, we present post hoc analysis of KEYNOTE-048 after an approximately 4-year follow-up, including efficacy and progression-free survival on next-line therapy (PFS2).
METHODS
Study Design and Participants
Detailed methods and the protocol for the open-label phase III KEYNOTE-048 study have been published previously.3 Eligible patients were age ≥ 18 years with previously untreated R/M squamous cell carcinoma of the oropharynx (known p16 expression), oral cavity, hypopharynx, or larynx, which was incurable by local therapy (Data Supplement, online only).
The Protocol (online only) and amendments were approved by appropriate institutional review boards or independent ethics committees at each center. The study was conducted in accordance with the protocol and Good Clinical Practice guidelines. All patients provided written informed consent.
Random Assignment and Masking
Patients were randomly allocated 1:1:1 to pembrolizumab alone, pembrolizumab plus platinum and 5-fluorouracil (pembrolizumab-chemotherapy), or cetuximab plus platinum and fluorouracil (cetuximab-chemotherapy). Random assignment was stratified by PD-L1 expression (tumor proportion score ≥ 50% v < 50%), p16 expression for oropharyngeal cancer (positive v negative), and Eastern Cooperative Oncology Group performance status (0 v 1).
Procedures
Patients were randomly allocated to intravenous pembrolizumab (200 mg once every 3 weeks; pembrolizumab alone), pembrolizumab (200 mg once every 3 weeks) plus six cycles of cisplatin (100 mg/m2 once every 3 weeks) or carboplatin (area under the curve 5 once every 3 weeks) and fluorouracil (1,000 mg/m2 per day, 4-day infusion once every 3 weeks; pembrolizumab-chemotherapy), or cetuximab (400-mg/m2 loading dose and then 250 mg/m2 per week) plus six cycles of cisplatin (100 mg/m2 once every 3 weeks) or carboplatin (area under the curve 5 once every 3 weeks) and fluorouracil (1,000 mg/m2 per day, 4-day infusion once every 3 weeks; cetuximab-chemotherapy). After six cycles of cetuximab-chemotherapy, patients could continue cetuximab monotherapy until progression, unacceptable toxicity, or withdrawal. In the pembrolizumab-alone and pembrolizumab-chemotherapy arms, pembrolizumab was administered for ≤ 35 cycles or until disease progression, unacceptable toxicity, or withdrawal. Patients with confirmed complete response (CR) could discontinue pembrolizumab, provided that they had received ≥ 24 weeks of treatment and ≥ 2 doses of pembrolizumab beyond initial CR. Patients in the pembrolizumab-alone or pembrolizumab-chemotherapy arms who stopped pembrolizumab with stable disease (SD) or better were eligible for ≤ 1 year of additional pembrolizumab monotherapy if their disease progressed after stopping treatment (Data Supplement).
Imaging was performed at baseline, week 9, then every 6 weeks until year 1, and every 9 weeks thereafter. Response was assessed per RECIST, version 1.1, by blinded independent central review (BICR); second-course response was assessed by investigator review. Survival was assessed every 12 weeks after confirmed disease progression or start of new anticancer therapy. Patients were monitored for adverse events (AEs) throughout treatment and for 30 days after stopping treatment (90 days for serious AEs). AEs were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).
Outcomes
Primary end points were progression-free survival (PFS) and OS. Secondary end points included objective response rate (ORR) and safety. DOR was exploratory. Data regarding subsequent treatment were collected. Post hoc analysis of PFS2 (time from random assignment to objective tumor progression on next-line therapy or death from any cause) was not protocol-specified. Per protocol, efficacy was evaluated in PD-L1 CPS ≥ 20, CPS ≥ 1, and total populations. The current analyses were not controlled for multiplicity; no alpha adjustment was applied.
Statistical Analysis
We present post hoc exploratory analyses of the efficacy of pembrolizumab alone versus cetuximab-chemotherapy and pembrolizumab-chemotherapy versus cetuximab-chemotherapy with an approximately 4-year follow-up, PFS2, and efficacy of second-course pembrolizumab. OS, PFS, and ORR were assessed in all patients allocated to treatment (intention-to-treat [ITT] population; Data Supplement). PFS2 was also assessed in the ITT population, as is common in oncology.8 DOR was assessed in all patients with confirmed CR or partial response (PR). Safety was assessed in all patients who received ≥ 1 dose of study treatment.
OS, PFS, PFS2, and DOR were estimated using the Kaplan-Meier method. PFS2 was assessed in treatment groups and by type of subsequent therapy. No formal hypothesis testing was conducted. Nominal one-sided P values were calculated using a stratified log-rank test to assess between-group differences in OS, PFS, and PFS2. HRs and 95% CIs were estimated using a stratified Cox regression model with the Efron method of handling ties with treatment as a covariate. Stratification factors were Eastern Cooperative Oncology Group performance status (0 v 1), p16 expression for oropharyngeal cancer (positive v negative), and PD-L1 tumor expression (tumor proportion score ≥ 50% v < 50%).3 Statistical analyses were performed using SAS version 9.4.
RESULTS
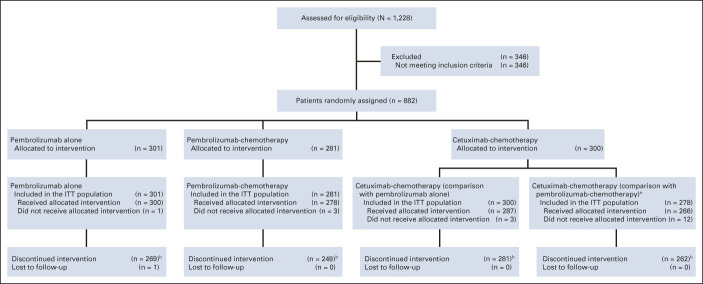
Overall, 882 patients were randomly allocated to treatment (pembrolizumab alone, n = 301; pembrolizumab-chemotherapy, n = 281; and cetuximab-chemotherapy, n = 300). Efficacy populations included all patients allocated to pembrolizumab alone (n = 301) and cetuximab-chemotherapy (n = 300) and all patients allocated to pembrolizumab-chemotherapy (n = 281) and cetuximab-chemotherapy (n = 278) while enrollment for pembrolizumab-chemotherapy was open (Data Supplement). Patient disposition at data cutoff (February 18, 2020) is presented in Figure 1 and the Data Supplement (CONSORT diagrams for PD-L1 CPS ≥ 20, CPS ≥ 1, and total populations are published previously3). Baseline characteristics were similar between treatment groups and across PD-L1 populations.3 The median time from random assignment to data cutoff was 45.0 months (interquartile range, 41.0-49.2; Data Supplement). Median chemotherapy cycles received were 6 (range, 1-11) for pembrolizumab-chemotherapy and 6 (range, 1-9) for cetuximab-chemotherapy.
FIG 1.
Trial profile for the total KEYNOTE-048 population.3,c aEnrollment in the pembrolizumab-chemotherapy arm was temporarily paused after three deaths occurred in the first 14 patients enrolled in the pembrolizumab-chemotherapy arm. Enrollment was later resumed on the advice of the safety monitoring committee. Patients allocated to cetuximab-chemotherapy during this time were excluded from the efficacy analysis population for pembrolizumab-chemotherapy versus cetuximab-chemotherapy analyses. bReasons for discontinuation are provided in the Data Supplement. cReprinted from the study by Burtness et al.3 ITT, intention-to-treat.
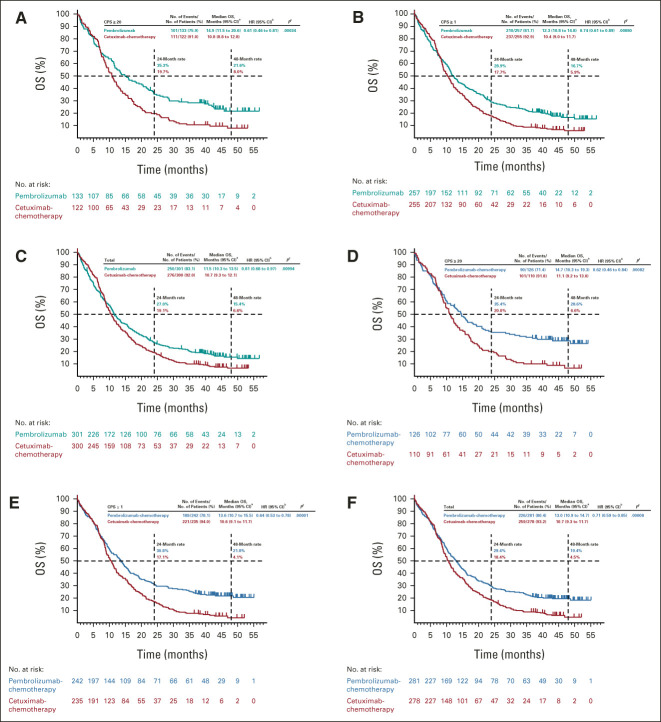
Pembrolizumab alone prolonged OS versus cetuximab-chemotherapy in the PD-L1 CPS ≥ 20 and CPS ≥ 1 populations and was noninferior in the total population (Figs 2A-2C). The median OS was 14.9 months (95% CI, 11.5 to 20.6) for pembrolizumab alone versus 10.8 months (95% CI, 8.8 to 12.8) for cetuximab-chemotherapy in the PD-L1 CPS ≥ 20 population (HR, 0.61; 95% CI, 0.46 to 0.81; nominal one-sided P = .00034), 12.3 months (95% CI, 10.8 to 14.8) versus 10.4 months (95% CI, 9.0 to 11.7) in the CPS ≥ 1 population (HR, 0.74; 95% CI, 0.61 to 0.89; nominal one-sided P = .00080), and 11.5 months (95% CI, 10.3 to 13.5) versus 10.7 months (95% CI, 9.3 to 12.1) in the total population (HR, 0.81; 95% CI, 0.68 to 0.97; nominal one-sided P = .00994; Figs 2A-2C). In subgroup analyses, HRs generally favored pembrolizumab alone except for recurrent-only disease (locally recurrent disease and disease that spread to cervical lymph nodes; Fig 3A and Data Supplement).
FIG 2.
Kaplan-Meier estimates of OS. Pembrolizumab alone versus cetuximab with chemotherapy in the (A) PD-L1 CPS ≥ 20, (B) PD-L1 CPS ≥ 1, and (C) total populations at long-term follow-up and pembrolizumab with chemotherapy versus cetuximab with chemotherapy in the (D) PD-L1 CPS ≥ 20, (E) PD-L1 CPS ≥ 1, and (F) total populations. aFrom the product-limit (Kaplan-Meier) method for censored data. bOn the basis of a Cox regression model with the Efron method of handling ties with treatment as a covariate stratified by ECOG PS, HPV status, and PD-L1 status. In case the event count in any stratum was < 5, stratification factors were eliminated in the order of ECOG PS > HPV status > PD-L1 status until the event count in every stratum was ≥ 5. cNominal one-sided P values were calculated using a log-rank test stratified by ECOG PS, HPV status, and PD-L1 status. In case the event count in any stratum was < 5, stratification factors were eliminated in the order of ECOG PS > HPV status > PD-L1 status until the event count in every stratum was ≥ 5. CPS, combined positive score; ECOG PS, Eastern Cooperative Oncology Group performance status; HPV, human papillomavirus; OS, overall survival; PD-L1, programmed death ligand 1.
FIG 3.
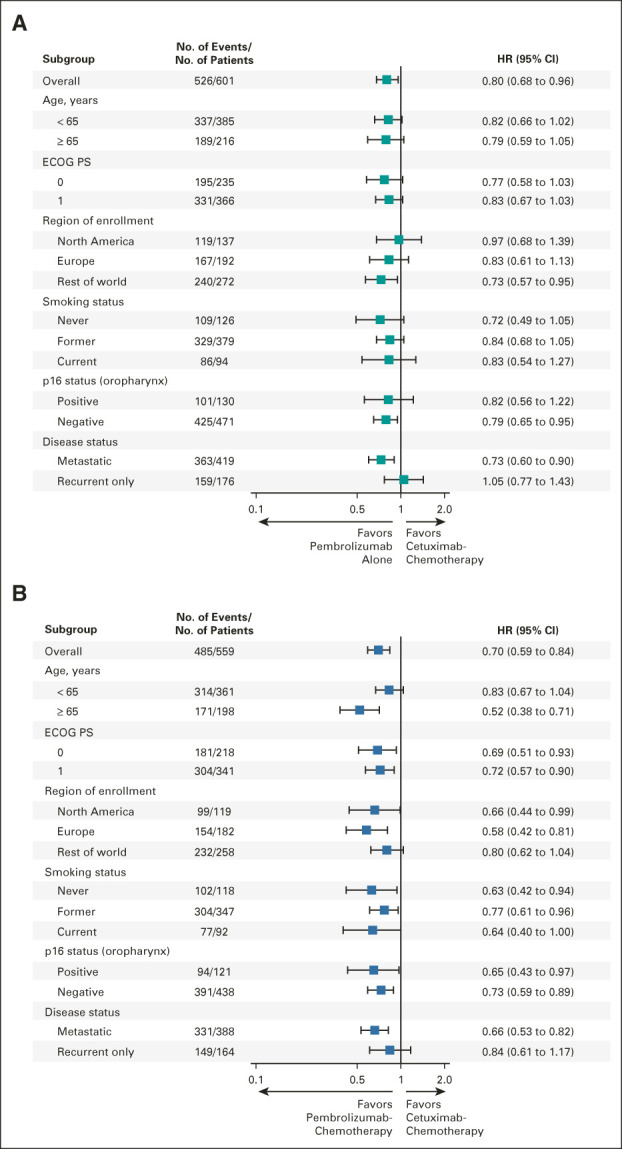
Subgroup analysis of OS. (A) Pembrolizumab alone versus cetuximab with chemotherapy in the total population and (B) pembrolizumab with chemotherapy versus cetuximab with chemotherapy in the total population at long-term follow-up. ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio.
Pembrolizumab-chemotherapy prolonged OS compared with cetuximab-chemotherapy in the PD-L1 CPS ≥ 20, CPS ≥ 1, and total populations (Figs 2D-2F). The median OS was 14.7 months (95% CI, 10.3 to 19.3) for pembrolizumab-chemotherapy versus 11.1 months (95% CI, 9.2 to 13.0) for cetuximab-chemotherapy in the PD-L1 CPS ≥ 20 population (HR, 0.62; 95% CI, 0.46 to 0.84; nominal one-sided P = .00082), 13.6 months (95% CI, 10.7 to 15.5) versus 10.6 months (95% CI, 9.1 to 11.7) in the CPS ≥ 1 population (HR, 0.64; 95% CI, 0.53 to 0.78; nominal one-sided P = .00001), and 13.0 months (95% CI, 10.9 to 14.7) versus 10.7 months (95% CI, 9.3 to 11.7) in the total population (HR, 0.71; 95% CI, 0.59 to 0.85; nominal one-sided P = .00008; Figs 2D-2F). In subgroup analyses, HRs generally favored pembrolizumab-chemotherapy (Fig 3B and Data Supplement).
PFS was similar for pembrolizumab and pembrolizumab-chemotherapy versus cetuximab-chemotherapy (Data Supplement). PFS rates at 24 and 48 months were numerically higher for pembrolizumab alone and pembrolizumab-chemotherapy versus cetuximab-chemotherapy in all populations.
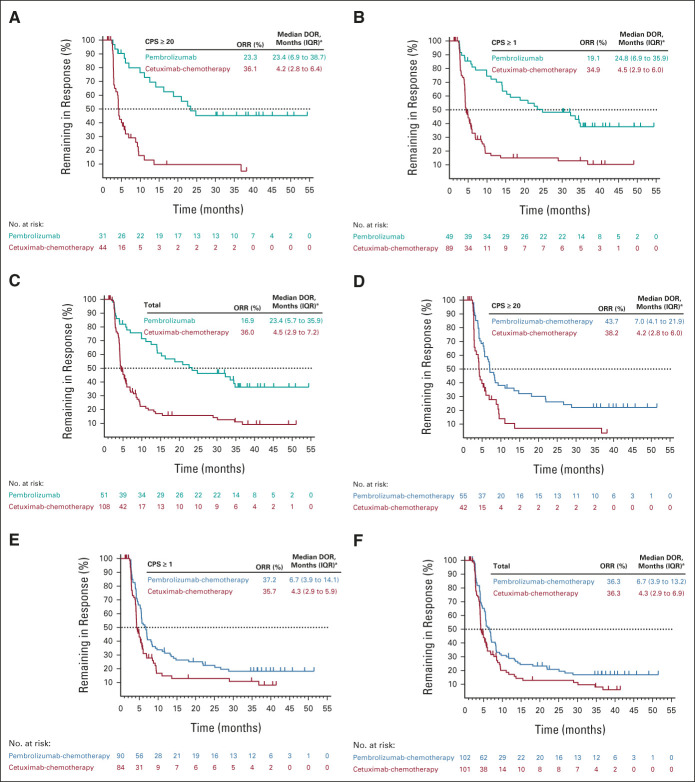
Pembrolizumab alone did not improve ORR compared with cetuximab-chemotherapy in the PD-L1 CPS ≥ 20, CPS ≥ 1, or total populations (Data Supplement). The ORR was 23.3% (11 CR/20 PR) for pembrolizumab alone versus 36.1% (4 CR/40 PR) for cetuximab-chemotherapy in the PD-L1 CPS ≥ 20 population, 19.1% (15 CR/34 PR) versus 34.9% (7 CR/82 PR) in the CPS ≥ 1 population, and 16.9% (15 CR/36 PR) versus 36.0% (8 CR/100 PR) in the total population (Figs 4A-4C and Data Supplement). Median DOR was substantially longer with pembrolizumab alone in all populations (Figs 4A-4C).
FIG 4.
Kaplan-Meier estimates of DOR in patients with a best objective response of CR or PR. Pembrolizumab alone versus cetuximab with chemotherapy in the (A) PD-L1 CPS ≥ 20, (B) PD-L1 CPS ≥ 1, and (C) total populations at long-term follow-up and pembrolizumab with chemotherapy versus cetuximab with chemotherapy in the (D) PD-L1 CPS ≥ 20, (E) PD-L1 CPS ≥ 1, and (F) total populations. aFrom the product-limit (Kaplan-Meier) method for censored data. CPS, combined positive score; CR, complete response; DOR, duration of response; IQR, interquartile range; ORR, objective response rate; PD-L1, programmed death ligand 1; PR, partial response.
Pembrolizumab-chemotherapy resulted in a numerically higher ORR compared with cetuximab-chemotherapy in the PD-L1 CPS ≥ 20 population and similar ORRs in the CPS ≥ 1 and total populations (Data Supplement). The ORR was 43.7% (13 CR/42 PR) for pembrolizumab-chemotherapy versus 38.2% (4 CR/38 PR) for cetuximab-chemotherapy in the PD-L1 CPS ≥ 20 population, 37.2% (17 CR/73 PR) versus 35.7% (7 CR/77 PR) in the CPS ≥ 1 population, and 36.3% (18 CR/84 PR) versus 36.3% (8 CR/93 PR) in the total population (Figs 4D-4F and Data Supplement). Median DOR was numerically longer with pembrolizumab-chemotherapy in all populations (Figs 4D-4F).
Any-grade treatment-related AEs occurred in 58.3% (n = 175) of patients in the pembrolizumab-alone group, 95.7% (n = 264) in the pembrolizumab-chemotherapy group, and 96.9% (n = 278) in the cetuximab-chemotherapy group (Data Supplement). Grade ≥ 3 treatment-related AEs were reported in 17.0% (n = 51), 71.7% (n = 198), and 69.3% (n = 199) of patients, respectively (Data Supplement).
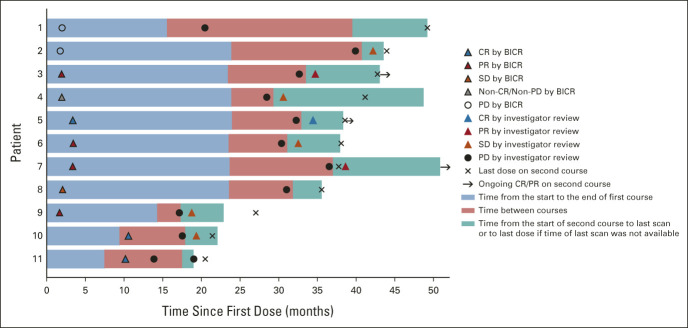
Eleven patients received second-course pembrolizumab; six received first-course pembrolizumab, and five received pembrolizumab-chemotherapy. Of these, three had CR, four had PR, one had SD, one had non-CR/nonprogressive disease (PD), and two had PD as the first objective response per RECIST v1.1 by BICR. Three patients maintained their first-course objective response during or after second-course pembrolizumab; one had CR and two had PR by investigator review (ORR, 27.3%; 95% CI, 6.0 to 61.0; Fig 5). Five patients had SD per investigator review during second-course pembrolizumab; one had CR, two had PR, one had non-CR/non-PD, and one had PD by BICR with first-course pembrolizumab-based therapy (Fig 5).
FIG 5.
Pembrolizumab second-course response characteristics.a-c Each bar represents one patient who received a second course of pembrolizumab. Shown here are first-course first objective response per RECIST v1.1 by BICR, progressive disease after stopping first course per RECIST v1.1 by investigator review, and second-course objective response per RECIST v1.1 by investigator review. Eligibility for second course and response during second course were assessed by the investigator (not by BICR). aAt data cutoff, patients 1 and 8 did not have available last scan on second-course pembrolizumab. bPatients 2, 4, 5, 6, 7, and 8 received first-course treatment of pembrolizumab alone. Patients 1, 3, 9, 10, and 11 received first-course pembrolizumab-chemotherapy. cPatient 1 discontinued first-course pembrolizumab-chemotherapy with CR before PD occurred. At the time of PD, the patient's lesions were smaller than 1 cm and the patient did not have any symptoms of progression. Therefore, the investigator's plan, as agreed on by the study sponsor, was to repeat scans per the protocol schedule and start second-course pembrolizumab once lesions were larger than 1 cm. BICR, blinded independent central review; CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
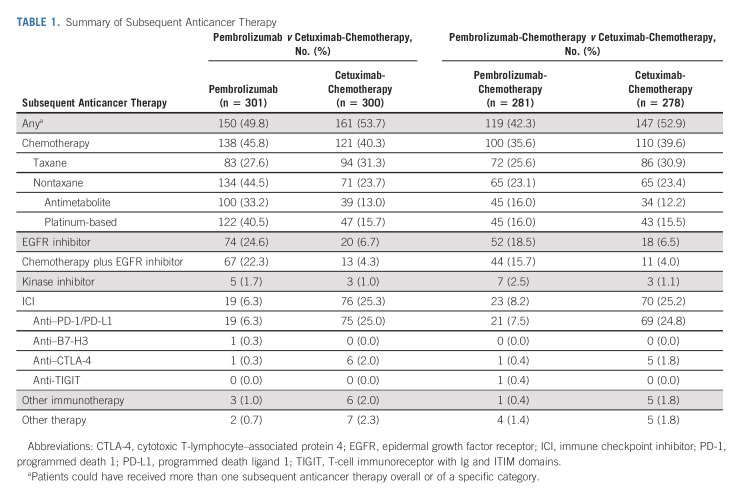
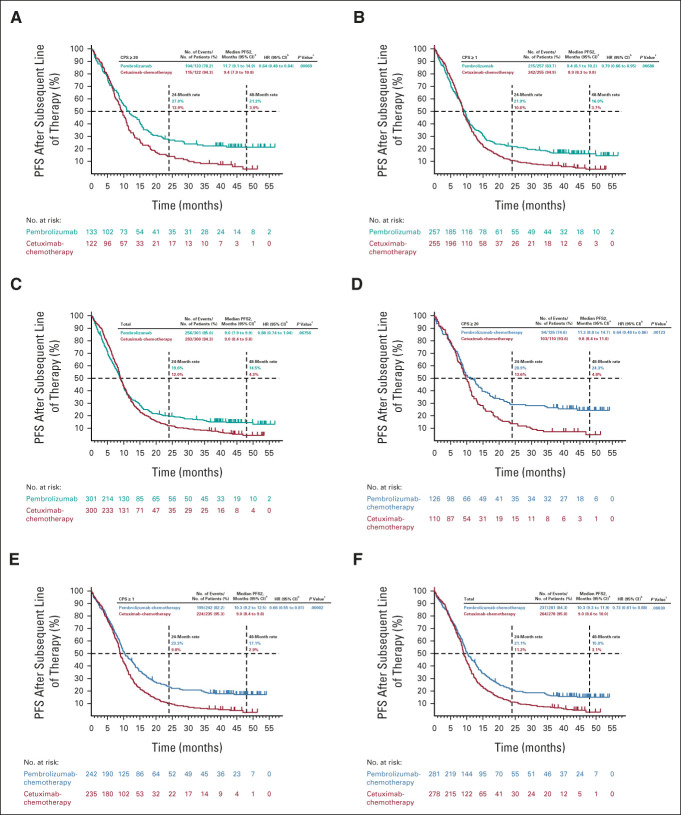
After study drug discontinuation, 150 (49.8%) patients in the pembrolizumab-alone group and 161 (53.7%) in the cetuximab-chemotherapy group received ≥ 1 subsequent therapy (Table 1). PFS2, which was assessed in all patients in the ITT population regardless of receipt of subsequent therapy, was longer for pembrolizumab alone versus cetuximab-chemotherapy in the PD-L1 CPS ≥ 20 and CPS ≥ 1 populations and was similar between treatment groups in the total population (Figs 6A-6C). Subgroup analyses indicated that PFS2 on taxane-containing second-line therapy was similar for pembrolizumab alone versus cetuximab-chemotherapy (HR, 0.96; 95% CI, 0.70 to 1.32; nominal one-sided P = .40360), whereas PFS2 on non–taxane-containing second-line therapy was numerically shorter for pembrolizumab alone versus cetuximab-chemotherapy (HR, 1.38; 95% CI, 1.02 to 1.87; nominal one-sided P = .98221; Data Supplement).
TABLE 1.
Summary of Subsequent Anticancer Therapy
FIG 6.
Kaplan-Meier estimates of progression-free survival on the next line of therapy. Pembrolizumab alone versus cetuximab with chemotherapy in the (A) PD-L1 CPS ≥ 20, (B) PD-L1 CPS ≥ 1, and (C) total populations at long-term follow-up and pembrolizumab with chemotherapy versus cetuximab with chemotherapy in the (D) PD-L1 CPS ≥ 20, (E) PD-L1 CPS ≥ 1, and (F) total populations. aFrom the product-limit (Kaplan-Meier) method for censored data. bOn the basis of a Cox regression model with the Efron method of handling ties with treatment as a covariate stratified by ECOG PS, HPV status, and PD-L1 status. In case the event count in any stratum was < 5, stratification factors were eliminated in the order of ECOG PS > HPV status > PD-L1 status until the event count in every stratum was ≥ 5. cNominal one-sided P values were calculated using a log-rank test stratified by ECOG PS, HPV status, and PD-L1 status. In case the event count in any stratum was < 5, stratification factors were eliminated in the order of ECOG PS > HPV status > PD-L1 status until the event count in every stratum was ≥ 5. CPS, combined positive score; ECOG PS, Eastern Cooperative Oncology Group performance status; HPV, human papillomavirus; PD-L1, programmed death ligand 1; PFS2, progression-free survival on the next line of therapy.
After study drug discontinuation, ≥ 1 subsequent therapy was received by 119 (42.3%) patients in the pembrolizumab-chemotherapy group and 147 (52.9%) patients in the cetuximab-chemotherapy group (Table 1). PFS2 was longer for pembrolizumab-chemotherapy versus cetuximab-chemotherapy in the PD-L1 CPS ≥ 20, CPS ≥ 1, and total populations (Figs 6D-6F). Subgroup analyses indicated that PFS2 on taxane-containing therapy was longer for pembrolizumab-chemotherapy versus cetuximab-chemotherapy (HR, 0.67; 95% CI, 0.48 to 0.93; nominal one-sided P = .00788), whereas PFS2 on non–taxane-containing therapy was similar for pembrolizumab-chemotherapy and cetuximab-chemotherapy (HR, 0.87; 95% CI, 0.61 to 1.24; nominal one-sided P = .22177; Data Supplement).
DISCUSSION
With long-term follow-up of KEYNOTE-048, first-line pembrolizumab alone and pembrolizumab-chemotherapy showed enduring survival benefits compared with cetuximab-chemotherapy in R/M HNSCC. Consistent with earlier analysis, pembrolizumab alone continued to prolong OS compared with cetuximab-chemotherapy in the PD-L1 CPS ≥ 20 and CPS ≥ 1 populations and pembrolizumab-chemotherapy continued to prolong OS compared with cetuximab-chemotherapy in the PD-L1 CPS ≥ 20, CPS ≥ 1, and total populations.3 With an almost 4-year follow-up, 48-month OS rates were higher for pembrolizumab and pembrolizumab-chemotherapy in all populations. A survival plateau at approximately 20% became apparent around the 4-year landmark for all patients receiving pembrolizumab alone. For patients receiving pembrolizumab-chemotherapy, a survival plateau at approximately 30% was observed for the PD-L1 CPS ≥ 20 population and at approximately 20% for the CPS ≥ 1 and total populations. These results highlight that some patients have long-term response. This was also reflected in DOR, which was longer for pembrolizumab and pembrolizumab-chemotherapy versus cetuximab-chemotherapy in all populations. In subgroup analysis of OS, HRs generally favored pembrolizumab and pembrolizumab-chemotherapy over cetuximab-chemotherapy, except for patients with recurrent-only disease. Overall, these results represent unprecedentedly favorable outcomes for patients with R/M HNSCC and demonstrate the broad benefit of pembrolizumab-based therapy, including in patients with poor prognostic markers such as smoking history and HPV-negative oropharyngeal cancer.9,10
As previously presented, neither pembrolizumab nor pembrolizumab-chemotherapy improved PFS over cetuximab-chemotherapy in any population.3 However, although 6-month PFS rates were lower for pembrolizumab and similar for pembrolizumab-chemotherapy versus cetuximab-chemotherapy in earlier analysis,3 PFS rates at later time points were consistently higher for pembrolizumab and pembrolizumab-chemotherapy in all populations.
ORRs were generally similar to those reported previously.3 There was no meaningful change in ORRs with pembrolizumab or pembrolizumab-chemotherapy because few additional responses occurred with long-term follow-up. Compared with the final analysis, one patient with PD-L1 CPS ≥ 20 receiving pembrolizumab improved from PR to CR, one with CPS ≥ 20 receiving pembrolizumab-chemotherapy improved from SD to CR, and one with CPS ≥ 1 receiving pembrolizumab-chemotherapy improved from SD to PR.3
In the current analysis, three (27.3%) patients who received second-course pembrolizumab achieved PR or CR, suggesting that retreatment may be effective in some patients. This is consistent with the CheckMate 141 report of an ORR of 16% among patients with HNSCC who received nivolumab beyond first progression.11
In the current study, PFS2 was longer for patients initially treated with pembrolizumab compared with cetuximab-chemotherapy in the PD-L1 CPS ≥ 20 and CPS ≥ 1 populations and was similar between treatment groups in the total population. PFS2 was longer for those initially treated with pembrolizumab-chemotherapy compared with cetuximab-chemotherapy in all populations. The analysis of PFS2 was conducted in all patients who were allocated to treatment (ITT population), regardless of receipt of subsequent therapy. Although this means that patients who did not receive subsequent therapy are included in the analysis, this statistical approach allows random assignment to be preserved, enabling controlled comparison between treatment arms. Subgroup analysis by type of next-line therapy suggested treatment benefit with subsequent taxanes in patients who received first-line pembrolizumab-based therapy; however, the choice to use subsequent taxanes or nontaxanes might have been influenced by the non–taxane-based chemotherapy (platinum plus fluorouracil) used in KEYNOTE-048. This is illustrated by a higher proportion of patients receiving subsequent platinum-based chemotherapy in the pembrolizumab-alone group compared with the pembrolizumab-chemotherapy and cetuximab-chemotherapy groups. Although data are limited regarding response to subsequent therapies for HNSCC, there is some indication that immune checkpoint inhibitors (ICIs) may increase tumor sensitivity to subsequent treatment. In KEYNOTE-040, which investigated pembrolizumab versus standard-of-care therapy in R/M HNSCC that had progressed on platinum-based therapy, PFS2 was longer for those previously treated with pembrolizumab versus standard of care (6.6 v 5.4 months; HR, 0.75; 95% CI, 0.62 to 0.91; P = .002).12 In a retrospective study of patients with R/M HNSCC who progressed on ICIs and subsequently received salvage chemotherapy, the ORR was 30% (n = 25) in the overall population and 40% (n = 8) among patients who received first-line ICIs, which are higher than rates reported in historic trials investigating second-line chemotherapy.13 The median PFS among patients who received salvage chemotherapy after first-line ICIs was 5.2 months. A retrospective study of outcomes among patients with R/M HNSCC who received cytotoxic or biologic therapy after ICIs showed similar results, with an ORR of 27% (n = 14) and a median PFS of 3.3 months.14 A high response rate was reported for subsequent fluorouracil-containing (63%), platinum-containing (50%), or taxane-containing regimens (36%). The results of these and two additional retrospective studies also indicate that cetuximab-based regimens may be effective after immunotherapy in R/M HNSCC, with ORRs of 32%-53% reported.13-16 The PFS2 results from the current analysis are consistent with the notion that first-line ICIs may potentiate response to subsequent treatment.
The safety profiles of pembrolizumab alone, pembrolizumab-chemotherapy, and cetuximab-chemotherapy were similar to those reported previously, as few patients were continuing to receive treatment at final data cutoff.3 No new safety signals were observed.
Limitations of KEYNOTE-048 are its open-label treatment and that it was not designed to compare pembrolizumab alone with pembrolizumab-chemotherapy. The current analysis is limited by its post hoc nature, the small number of patients who received second-course pembrolizumab, and the small size of some recurrent-only subgroups. Although the current analysis did not show treatment benefit with pembrolizumab-based therapy in recurrent-only disease, previous analysis of KEYNOTE-048 has suggested a benefit with pembrolizumab-based therapy on pooling of all patients with locoregional recurrence, regardless of the presence of metastases.17 An additional limiting factor is that the PFS2 analysis might have been affected by nonstandardized imaging intervals and choice of second-line therapy, which likely differed between centers.
The results of this long-term follow-up of KEYNOTE-048 confirm that pembrolizumab alone improved OS in the PD-L1 CPS ≥ 20 and CPS ≥ 1 populations and pembrolizumab-chemotherapy improved OS in the PD-L1 CPS ≥ 20, CPS ≥ 1, and total populations. The DOR was also substantially longer with pembrolizumab and pembrolizumab-chemotherapy in all populations. Retreatment with pembrolizumab may provide benefit in some patients. Patients who received first-line pembrolizumab alone or pembrolizumab-chemotherapy responded well to subsequent therapy. These results support earlier findings of KEYNOTE-048 and reaffirm that pembrolizumab alone or pembrolizumab-chemotherapy are appropriate first-line therapies for R/M HNSCC. These findings suggest that clinicians have two treatment options: pembrolizumab monotherapy and pembrolizumab combined with chemotherapy. These results may help clinicians select appropriate treatment on the basis of patient disease state and characteristics.
ACKNOWLEDGMENT
We thank the patients and their families and caregivers for participating in this trial, all investigators and site personnel, and the following employees of MSD: Nati Lerman and Adela Maragoto for clinical study support. Medical writing and/or editorial assistance was provided by Jemimah Walker, PhD, and Doyel Mitra, PhD, CMPP, of ApotheCom (Yardley, PA). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ.
Kevin J. Harrington
Honoraria: Arch Oncology (Inst), AstraZeneca (Inst), BMS (Inst), Boehringer Ingelheim (Inst), Merck Serono (Inst), MSD (Inst), Oncolys Biopharma (Inst), Pfizer (Inst), Replimune (Inst), Inzen Therapeutics (Inst), Codiak Biosciences (Inst)
Consulting or Advisory Role: Arch Oncology (Inst), AstraZeneca (Inst), BMS (Inst), Boehringer Ingelheim (Inst), Merck Serono (Inst), MSD (Inst), Oncolys BioPharma (Inst), Replimune (Inst), Inzen Therapeutics (Inst)
Speakers' Bureau: BMS (Inst), Merck Serono (Inst), MSD (Inst)
Research Funding: AstraZeneca (Inst), Merck Sharp & Dohme (Inst), Replimune (Inst), Boehringer Ingelheim (Inst)
Barbara Burtness
Leadership: Merck (Inst), Exelixis (Inst), CUE Biopharma (Inst), Eisai, AstraZeneca, Merck
Richard Greil
Honoraria: Celgene, MSD, Sandoz, AbbVie, Gilead Sciences, Daiichi Sankyo
Consulting or Advisory Role: Celgene, Novartis, Roche, Bristol Myers Squibb, Takeda, AbbVie, AstraZeneca, Janssen, MSD, Merck, Gilead Sciences, Daiichi Sankyo
Research Funding: Celgene (Inst), Merck (Inst), Takeda (Inst), AstraZeneca (Inst), Novartis (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), Sandoz (Inst), Gilead Science (Inst), Roche (Inst), Daiichi Sankyo Europe GmbH (Inst)
Patient, Royalties, Other Intellectual Property: Daiichi Sankyo Europe GmbH (Inst)
Travel, Accommodations, Expenses: Roche, Amgen, Janssen-Cilag, AstraZeneca, Novartis, MSD, Celgene, Gilead Science, Bristol Myers Squibb, AbbVie, Daiichi Sankyo
Denis Soulières
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Merck, Novartis, Pfizer, AstraZeneca, Ipsen, Bristol Myers Squibb, Eisai, Adlai Nortye
Consulting or Advisory Role: Merck, Pfizer, Ipsen, Adlai Nortye, Eisai
Research Funding: Novartis (Inst), Merck (Inst), Pfizer (Inst), Roche/Genentech (Inst), Bristol-Myers Squibb (Inst), Lilly (Inst), Adlai Nortye (Inst)
Makoto Tahara
Honoraria: Merck Serono, Bristol Myers Squibb, Eisai, Ono Pharmaceutical, MSD
Consulting or Advisory Role: Ono Pharmaceutical, MSD, Novartis, Pfizer, Bristol Myers Squibb, AstraZeneca
Speaker's Bureau: Novartis
Research Funding: Pfizer, Bristol Myers Squibb (Inst), Rakuten Medical (Inst), Bayer (Inst), GlaxoSmithKline (Inst), Lilly (Inst)
Gilberto de Castro Jr
Honoraria: AstraZeneca, Pfizer, Merck Sharp & Dohme, Bristol Myers Squibb, Novartis, Roche, Amgen, Janssen, Merck Serono
Consulting or Advisory Role: Boehringer Ingelheim, Pfizer, Bayer, Roche, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Yuhan, Merck Serono, Janssen, Libbs, Sanofi, Novartis
Speakers' Bureau: AstraZeneca, Bayer, Novartis, Roche, Merck Serono, Bristol Myers Squibb, Merck Sharp & Dohme, Boehringer Ingelheim, Pfizer, Janssen, Amgen
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Novartis, Pfizer, Roche, AstraZeneca, Boehringer Ingelheim, Bayer, Bristol Myers Squibb, Merck Serono
Amanda Psyrri
Honoraria: Merck Serono, Roche, BMS, MSD Oncology, Genesis Pharmaceuticals, Bayer, Rakuten Medical, AstraZeneca, Pfizer
Consulting of Advisory Role: AstraZeneca, MSD Oncology, Pfizer, Bristol Myers Squibb, Amgen, Rakuten Medical, eTheRNA immunotherapies
Research Funding: Kura oncology, Bristol Myers Squibb, Roche, Amgen, Boehringer Ingelheim, Pfizer, Demo pharmaceutical, Pharmathen
Travel, Accommodations, Expenses: Roche, MSD Oncology, Ipsen
Uncompensated Relationships: AstraZeneca
Irene Brana
Consulting of Advisory Role: Merck sharp & Dohme, Sanofi, Achilles Therapeutics, eTheRNA Immunotherapies, Cancer Expert Now, Boehringer Ingelheim
Speakers' Bureau: Bristol Myers Squibb, Merck Serono, Roche, MSD
Research Funding: AstraZeneca (Inst), Bristol Myers Squibb (Inst), Celgene (Inst), Gliknik (Inst), GlaxoSmithKline (Inst), Janssen Oncology (Inst), Kura Oncology (Inst), Merck Sharp & Dohme (Inst), Novartis (Inst), Pfizer (Inst), Roche (Inst), Shattuck labs (Inst), Nanobiotix (Inst), Seattle Genetics (Inst), Immutep (Inst), Debiopharm Group (Inst), Regeneron (Inst), Boehringer Ingelheim (Inst), ISA Pharmaceuticals (Inst), Merck Serono (Inst), Seattle Genetics (Inst), Northern Biologics (Inst), VCN Biosciences (Inst)
Travel, Accommodations, Expenses: MSD Oncology
Neus Basté
Consulting or Advisory Role: Merck Serono, Sanofi, Bristol Myers Squibb Foundation, BioNTech, MSD Oncology, Eisai
Travel, Accommodations, Expenses: Merck Serono, Bristol Myers Squibb Foundation
Prakash Neupane
Research Funding: Merck Sharp & Dohme
Åse Bratland
Honoraria: MSD Oncology, Bristol Myers Squibb, Sanofi, Merck Serono
Research Funding: Bristol Myers Squibb (Inst), MSD Oncology (Inst)
Travel, Accommodations, Expenses: MSD Oncology
Thorsten Fuereder
Honoraria: MSD, Bristol Myers Squibb/Celgene, Roche, Merck KGaA, Pfizer, Boehringer Ingelheim, Amgen
Consulting or Advisory Role: Merck KGaA, MSD, Amgen, Boehringer Ingelheim, Janssen, Takeda
Research Funding: MSD (Inst), Merck KGaA (Inst), Bristol Myers Squibb/Celgene (Inst), Kura Oncology (Inst)
Travel, Accommodations, Expenses: MSD, Bristol Myers Squibb/Celgene, Merck KGaA
Brett G.M. Hughes
Consulting or Advisory Role: MSD Oncology, Bristol Myers Squibb, Roche, Pfizer, AstraZeneca, Eisai, Takeda, Sanofi/Aventis
Research Funding: Amgen (Inst)
Ricard Mesia
Consulting or Advisory Role: Bristol Myers Squibb, MSD, Merck KGaA, Roche, Amgen, Bayer, Nanobiotix, Seagan, Boehringer Ingelheim, Bristol Myers Squibb
Speakers' Bureau: Merck KGaA, MSD, Boehringer Ingelheim
Travel, Accommodations, Expenses: Bristol Myers Squibb
Nuttapong Ngamphaiboon
Consulting or Advisory Role: MSD, Novartis, Amgen, Eisai, Merck,
Speakers' Bureau: Roche, Eisai, MSD, Novartis
Research Funding: MSD (Inst), Pfizer (Inst), Roche (Inst), Exelixis (Inst), RAPT Therapeutics (Inst), BeiGene (Inst)
Travel, Accommodations, Expenses: Roche
Wan Zamaniah Wan Ishak
Honoraria: Roche/Genentech, Amgen, Eisai, Merck Sharp & Dohme, Pfizer, Merck Serono, Novartis
Consulting or Advisory Role: Eisai, Merck Sharp & Dohme, Novartis
Speakers' Bureau: Merck Sharp & Dohme, Amgen, Eisai
Research Funding: Merck Sharp & Dohme, Roche/Genentech, Novartis
Jianxin Lin
Employment: Merck
Stock and Other Ownership Interests: Merck
Burak Gumuscu
Employment: Merck
Stock and Other Ownership Interests: Merck
Travel, Accommodations, Expenses: Merck
Ramona F. Swaby
Employment: Merck, Prelude Therapeutics, Carisma Therapeutics
Stock and Other Ownership Interests: Merck, Carisma Therapeutics
Travel, Accommodations, Expenses: Merck
Danny Rischin
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Research Funding: Genentech/Roche (Inst), Merck (Inst), Regeneron (Inst), Bristol Myers Squibb (Inst), GlaxoSmithKline (Inst), Sanofi (Inst), Kura Oncology (Inst), Merck KGaA (Inst)
No other potential conflicts of interest were reported.
See accompanying Oncology Grand Rounds on page 736
PRIOR PRESENTATION
Presented in part at the annual meeting of the European Society for Medical Oncology Virtual Congress 2020, September 19‐21, 2020. Results from a previous data cutoff (not reported in the current draft being submitted) were reported at the annual meeting of the American Society of Clinical Oncology Conference 2020, May 29-31, 2020, and at the annual meeting of the Chinese Society of Clinical Oncology 2020, September 19-26, 2020.
SUPPORT
Supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country-specific or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
AUTHOR CONTRIBUTIONS
Conception and design: Kevin J. Harrington, Barbara Burtness, Denis Soulières, Amanda Psyrri, Ricard Mesia
Provision of study materials or patients: Barbara Burtness, Amanda Psyrri, Irene Brana, Gilberto de Castro Jr, Neus Basté, Prakash Neupane, Åse Bratland, Thorsten Fuereder, Brett G.M. Hughes, Ricard Mesia, Wan Zamaniah Wan Ishak, Ramona F. Swaby, Danny Rischin
Collection and assembly of data: Kevin J. Harrington, Richard Greil, Denis Soulières, Gilberto de Castro Jr, Amanda Psyrri, Irene Brana, Neus Basté, Prakash Neupane, Åse Bratland, Thorsten Fuereder, Ricard Mesia, Nuttapong Ngamphaiboon, Tamara Rordorf, Wan Zamaniah Wan Ishak, Burak Gumuscu, Ramona F. Swaby, Danny Rischin
Data analysis and interpretation: Kevin J. Harrington, Barbara Burtness, Makoto Tahara, Gilberto de Castro Jr, Irene Brana, Neus Basté, Prakash Neupane, Åse Bratland, Thorsten Fuereder, Brett G.M. Hughes, Ricard Mesia, Tamara Rordorf, Jianxin Lin, Burak Gumuscu, Ramona F. Swaby, Danny Rischin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pembrolizumab With or Without Chemotherapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Updated Results of the Phase III KEYNOTE-048 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kevin J. Harrington
Honoraria: Arch Oncology (Inst), AstraZeneca (Inst), BMS (Inst), Boehringer Ingelheim (Inst), Merck Serono (Inst), MSD (Inst), Oncolys Biopharma (Inst), Pfizer (Inst), Replimune (Inst), Inzen Therapeutics (Inst), Codiak Biosciences (Inst)
Consulting or Advisory Role: Arch Oncology (Inst), AstraZeneca (Inst), BMS (Inst), Boehringer Ingelheim (Inst), Merck Serono (Inst), MSD (Inst), Oncolys BioPharma (Inst), Replimune (Inst), Inzen Therapeutics (Inst)
Speakers' Bureau: BMS (Inst), Merck Serono (Inst), MSD (Inst)
Research Funding: AstraZeneca (Inst), Merck Sharp & Dohme (Inst), Replimune (Inst), Boehringer Ingelheim (Inst)
Barbara Burtness
Leadership: Merck (Inst), Exelixis (Inst), CUE Biopharma (Inst), Eisai, AstraZeneca, Merck
Richard Greil
Honoraria: Celgene, MSD, Sandoz, AbbVie, Gilead Sciences, Daiichi Sankyo
Consulting or Advisory Role: Celgene, Novartis, Roche, Bristol Myers Squibb, Takeda, AbbVie, AstraZeneca, Janssen, MSD, Merck, Gilead Sciences, Daiichi Sankyo
Research Funding: Celgene (Inst), Merck (Inst), Takeda (Inst), AstraZeneca (Inst), Novartis (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), Sandoz (Inst), Gilead Science (Inst), Roche (Inst), Daiichi Sankyo Europe GmbH (Inst)
Patient, Royalties, Other Intellectual Property: Daiichi Sankyo Europe GmbH (Inst)
Travel, Accommodations, Expenses: Roche, Amgen, Janssen-Cilag, AstraZeneca, Novartis, MSD, Celgene, Gilead Science, Bristol Myers Squibb, AbbVie, Daiichi Sankyo
Denis Soulières
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Merck, Novartis, Pfizer, AstraZeneca, Ipsen, Bristol Myers Squibb, Eisai, Adlai Nortye
Consulting or Advisory Role: Merck, Pfizer, Ipsen, Adlai Nortye, Eisai
Research Funding: Novartis (Inst), Merck (Inst), Pfizer (Inst), Roche/Genentech (Inst), Bristol-Myers Squibb (Inst), Lilly (Inst), Adlai Nortye (Inst)
Makoto Tahara
Honoraria: Merck Serono, Bristol Myers Squibb, Eisai, Ono Pharmaceutical, MSD
Consulting or Advisory Role: Ono Pharmaceutical, MSD, Novartis, Pfizer, Bristol Myers Squibb, AstraZeneca
Speaker's Bureau: Novartis
Research Funding: Pfizer, Bristol Myers Squibb (Inst), Rakuten Medical (Inst), Bayer (Inst), GlaxoSmithKline (Inst), Lilly (Inst)
Gilberto de Castro Jr
Honoraria: AstraZeneca, Pfizer, Merck Sharp & Dohme, Bristol Myers Squibb, Novartis, Roche, Amgen, Janssen, Merck Serono
Consulting or Advisory Role: Boehringer Ingelheim, Pfizer, Bayer, Roche, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Yuhan, Merck Serono, Janssen, Libbs, Sanofi, Novartis
Speakers' Bureau: AstraZeneca, Bayer, Novartis, Roche, Merck Serono, Bristol Myers Squibb, Merck Sharp & Dohme, Boehringer Ingelheim, Pfizer, Janssen, Amgen
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Novartis, Pfizer, Roche, AstraZeneca, Boehringer Ingelheim, Bayer, Bristol Myers Squibb, Merck Serono
Amanda Psyrri
Honoraria: Merck Serono, Roche, BMS, MSD Oncology, Genesis Pharmaceuticals, Bayer, Rakuten Medical, AstraZeneca, Pfizer
Consulting of Advisory Role: AstraZeneca, MSD Oncology, Pfizer, Bristol Myers Squibb, Amgen, Rakuten Medical, eTheRNA immunotherapies
Research Funding: Kura oncology, Bristol Myers Squibb, Roche, Amgen, Boehringer Ingelheim, Pfizer, Demo pharmaceutical, Pharmathen
Travel, Accommodations, Expenses: Roche, MSD Oncology, Ipsen
Uncompensated Relationships: AstraZeneca
Irene Brana
Consulting of Advisory Role: Merck sharp & Dohme, Sanofi, Achilles Therapeutics, eTheRNA Immunotherapies, Cancer Expert Now, Boehringer Ingelheim
Speakers' Bureau: Bristol Myers Squibb, Merck Serono, Roche, MSD
Research Funding: AstraZeneca (Inst), Bristol Myers Squibb (Inst), Celgene (Inst), Gliknik (Inst), GlaxoSmithKline (Inst), Janssen Oncology (Inst), Kura Oncology (Inst), Merck Sharp & Dohme (Inst), Novartis (Inst), Pfizer (Inst), Roche (Inst), Shattuck labs (Inst), Nanobiotix (Inst), Seattle Genetics (Inst), Immutep (Inst), Debiopharm Group (Inst), Regeneron (Inst), Boehringer Ingelheim (Inst), ISA Pharmaceuticals (Inst), Merck Serono (Inst), Seattle Genetics (Inst), Northern Biologics (Inst), VCN Biosciences (Inst)
Travel, Accommodations, Expenses: MSD Oncology
Neus Basté
Consulting or Advisory Role: Merck Serono, Sanofi, Bristol Myers Squibb Foundation, BioNTech, MSD Oncology, Eisai
Travel, Accommodations, Expenses: Merck Serono, Bristol Myers Squibb Foundation
Prakash Neupane
Research Funding: Merck Sharp & Dohme
Åse Bratland
Honoraria: MSD Oncology, Bristol Myers Squibb, Sanofi, Merck Serono
Research Funding: Bristol Myers Squibb (Inst), MSD Oncology (Inst)
Travel, Accommodations, Expenses: MSD Oncology
Thorsten Fuereder
Honoraria: MSD, Bristol Myers Squibb/Celgene, Roche, Merck KGaA, Pfizer, Boehringer Ingelheim, Amgen
Consulting or Advisory Role: Merck KGaA, MSD, Amgen, Boehringer Ingelheim, Janssen, Takeda
Research Funding: MSD (Inst), Merck KGaA (Inst), Bristol Myers Squibb/Celgene (Inst), Kura Oncology (Inst)
Travel, Accommodations, Expenses: MSD, Bristol Myers Squibb/Celgene, Merck KGaA
Brett G.M. Hughes
Consulting or Advisory Role: MSD Oncology, Bristol Myers Squibb, Roche, Pfizer, AstraZeneca, Eisai, Takeda, Sanofi/Aventis
Research Funding: Amgen (Inst)
Ricard Mesia
Consulting or Advisory Role: Bristol Myers Squibb, MSD, Merck KGaA, Roche, Amgen, Bayer, Nanobiotix, Seagan, Boehringer Ingelheim, Bristol Myers Squibb
Speakers' Bureau: Merck KGaA, MSD, Boehringer Ingelheim
Travel, Accommodations, Expenses: Bristol Myers Squibb
Nuttapong Ngamphaiboon
Consulting or Advisory Role: MSD, Novartis, Amgen, Eisai, Merck,
Speakers' Bureau: Roche, Eisai, MSD, Novartis
Research Funding: MSD (Inst), Pfizer (Inst), Roche (Inst), Exelixis (Inst), RAPT Therapeutics (Inst), BeiGene (Inst)
Travel, Accommodations, Expenses: Roche
Wan Zamaniah Wan Ishak
Honoraria: Roche/Genentech, Amgen, Eisai, Merck Sharp & Dohme, Pfizer, Merck Serono, Novartis
Consulting or Advisory Role: Eisai, Merck Sharp & Dohme, Novartis
Speakers' Bureau: Merck Sharp & Dohme, Amgen, Eisai
Research Funding: Merck Sharp & Dohme, Roche/Genentech, Novartis
Jianxin Lin
Employment: Merck
Stock and Other Ownership Interests: Merck
Burak Gumuscu
Employment: Merck
Stock and Other Ownership Interests: Merck
Travel, Accommodations, Expenses: Merck
Ramona F. Swaby
Employment: Merck, Prelude Therapeutics, Carisma Therapeutics
Stock and Other Ownership Interests: Merck, Carisma Therapeutics
Travel, Accommodations, Expenses: Merck
Danny Rischin
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Research Funding: Genentech/Roche (Inst), Merck (Inst), Regeneron (Inst), Bristol Myers Squibb (Inst), GlaxoSmithKline (Inst), Sanofi (Inst), Kura Oncology (Inst), Merck KGaA (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Johnson DE, Burtness B, Leemans CR, et al. : Head and neck squamous cell carcinoma. Nat Rev Dis Primers 6:92, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow LQM: Head and neck cancer. N Engl J Med 382:60-72, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Burtness B, Harrington KJ, Greil R, et al. : Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 394:1915-1928, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Cohen EEW, Soulieres D, Le Tourneau C, et al. : Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 393:156-167, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Ferris RL, Blumenschein G, Jr, Fayette J, et al. : Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375:1856-1867, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Machiels JP, René Leemans C, Golusinski W, et al. : Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 31:1462-1475, 2020 [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology. Head and Neck Cancers. Version.1 2021. Plymouth Meeting, PA, National Comprehensive Cancer Network, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Woodford RG, Zhou DD, Kok PS, et al. : The validity of progression-free survival 2 as a surrogate trial end point for overall survival. Cancer 128:1449-1457, 2022 [DOI] [PubMed] [Google Scholar]
- 9.Ang KK, Harris J, Wheeler R, et al. : Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24-35, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterson LA, Bellile EL, Wolf GT, et al. : Cigarette use, comorbidities, and prognosis in a prospective head and neck squamous cell carcinoma population. Head Neck 38:1810-1820, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haddad R, Concha-Benavente F, Blumenschein G, Jr, et al. : Nivolumab treatment beyond RECIST-defined progression in recurrent or metastatic squamous cell carcinoma of the head and neck in CheckMate 141: A subgroup analysis of a randomized phase 3 clinical trial. Cancer 125:3208-3218, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Tourneau C, Cohen EEW, Harrington KJ, et al. : Pembrolizumab for recurrent head and neck squamous cell carcinoma (HNSCC): Post hoc analyses of treatment options from the phase 3 KEYNOTE-040 trial. Ann Oncol 29 :viii373-viii374, 2018. (suppl 8) [Google Scholar]
- 13.Saleh K, Daste A, Martin N, et al. : Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Eur J Cancer 121:123-129, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Kacew AJ, Harris EJ, Lorch JH, et al. : Chemotherapy after immune checkpoint blockade in patients with recurrent, metastatic squamous cell carcinoma of the head and neck. Oral Oncol 105:104676, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Kurosaki T, Mitani S, Tanaka K, et al. : Safety and efficacy of cetuximab-containing chemotherapy after immune checkpoint inhibitors for patients with squamous cell carcinoma of the head and neck: A single-center retrospective study. Anticancer Drugs 32:95-101, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pestana RC, Becnel M, Rubin ML, et al. : Response rates and survival to systemic therapy after immune checkpoint inhibitor failure in recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol 101:104523, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Burtness B, Zhang Y, Harrington KJ, et al. : Further clinical interpretation and implications of KEYNOTE-048 findings—Authors' reply. Lancet 396:379-380, 2020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country-specific or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.