Abstract
Objective
To perform a meta-analysis of controlled studies assessing the relationship between left atrial appendage(LAA) electrical isolation(EI) and recurrent atrial fibrillation(AF).
Background
LAA triggers may play an important role in AF and can be treated with complete EI of the LAA via surgical or percutaneous approaches.
Methods
We conducted a meta-analysis of all controlled studies published as of November 21, 2016, assessing the relationship between LAAEI and recurrent AF. The primary endpoint was atrial tachycardia(AT) or AF recurrence after the post-procedure blanking period. The association between LAAEI and AT/AF was estimated using random-effects modeling. Odds ratios with 95% confidence intervals were calculated using the DerSimonian and Laird method.
Results
We identified 7 studies including 1,037 patients; LAAEI was performed in 566(55%) patients. LAAEI was associated with a significantly lower rate of AT/AF recurrence in the primary analysis (OR 0.38, CI 0.16-0.90, p=0.02). The association between LAAEI and recurrent AT/AF was strongest in a sensitivity analysis restricted to studies of percutaneous LAAEI (OR 0.22, CI 0.11-0.46, p<0.001; 5 studies, n=623). LAAEI was not associated with thromboembolism (OR 0.50, CI 0.18-1.39, p=0.18; n=5 studies, n=767) although these studies either incorporated LAA occlusion (3 studies, n=552) or follow-up echocardiography to assess LAA function (2 studies, n=215) to inform antithrombotic strategies. There was no association between LAAEI and mortality (4 studies, n=725).
Conclusions
LAAEI is associated with a significant reduction in recurrent AT/AF. Randomized trials are required to confirm the efficacy and long-term safety of LAAEI and to determine the optimal concomitant antithrombotic strategy.
Keywords: Left atrial appendage, atrial fibrillation, ablation
Condensed Abstract
We performed a meta-analysis of 7 studies (n=1,037 patients) to assess the association between left atrial appendage electrical isolation (LAAEI) and recurrent atrial tachycardia (AT) or atrial fibrillation (AF) after AF ablation. LAAEI was associated with a ~60% reduction in recurrent AT/AF (OR 0.38, CI 0.16-0.90, p=0.02) and the association was strongest in studies of percutaneous LAAEI (OR 0.22, CI 0.11-0.46, p<0.001). There was no association between LAAEI and thromboembolism or all-cause mortality. Randomized trials are required to confirm the efficacy of LAAEI and to determine the best concomitant antithrombotic strategy.
Introduction
Atrial fibrillation(AF) is the most common sustained cardiac arrhythmia and is associated with substantial symptoms, morbidity (heart failure, myocardial infarction, and stroke), and mortality.(1) The substantial public health impact of AF has made the development of successful treatment strategies a priority. Early work(2) demonstrated that AF is frequently triggered by ectopic activity originating from the pulmonary veins(PVs); this observation led to the development of the modern AF ablation, which involves the electrical isolation of the PVs from the left atrial chamber. Although PV isolation can achieve long term maintenance of sinus rhythm(SR), patients with non-paroxysmal AF and structural heart disease experience frequent recurrences of AF and only approximately 50% are AF free after 1 year.(3) These recurrences are likely due to a combination of PV reconnection and non-PV triggers. Many studies(3-7) have assessed adjunctive ablation of fractionated electrograms, rotors, and empiric linear lesions, but to date, the most rigorous studies have not clearly identified the best approach to AF ablation.
Recently, the left atrial appendage(LAA) has received increasing attention as a potentially important source of AF triggers, particularly among patients with structural heart disease, non-paroxysmal AF, and AF recurrence after AF ablation.(8,9) LAA electrical isolation(LAAEI) has been proposed as a viable adjunctive strategy to PV isolation, although data are limited. LAAEI can be performed with catheter ablation, surgical excision, or via LARIAT (SentreHEART, Inc., Redwood City, CA) exclusion(which infarcts the LAA). Concerns regarding limited evidence on safety(e.g. thromboembolism) and efficacy have limited adoption of routine LAAEI at most centers. Herein, we report the results of a meta-analysis assessing LAAEI vs. no LAAEI as an adjunctive strategy to PV isolation for maintenance of SR.
Methods
This meta-analysis was conducted according to the PRISMA guidelines.
Literature Search
An electronic PubMed literature search was performed by three investigators (DJF, EWB-M, ASB) on November 21, 2016, using the broad search terms of "atrial fibrillation", "left atrial appendage", and “catheter ablation.” No filters were applied. All resulting abstracts were manually screened with subsequent full review of candidate study manuscripts. Clinicaltrials.gov was searched to identify relevant ongoing or unpublished clinical trials. We additionally reviewed the bibliography from each included study to identify relevant studies not identified by other strategies.
Study Selection
We included all randomized and non-randomized studies that (1)compared outcomes among patients with and without LAAEI and (2)reported freedom from AT/AF after a 2-3 month blanking period. We included studies where LAAEI occurred via catheter ablation, LARIAT exclusion, or surgical amputation(Figure 1). We excluded case reports, case series, single armed studies of LAAEI without a comparison group, and studies that included patients with WATCHMAN (Boston Scientific, Natick, MA) LAA occlusion only as there is no evidence that WATCHMAN occlusion leads to electrical isolation. We additionally excluded surgical studies where LAA occlusion was performed via clipping without amputation or heterogeneous or vague methods, as the extent of LAAEI could not be clearly determined in these situations. All studies were reviewed individually by three investigators (DJF, EWB-M, ASB) and selected based on consensus with additional consultation, as needed, by a fourth investigator (JPP).
Figure 1. Procedures leading to electrical isolation of the LAA.
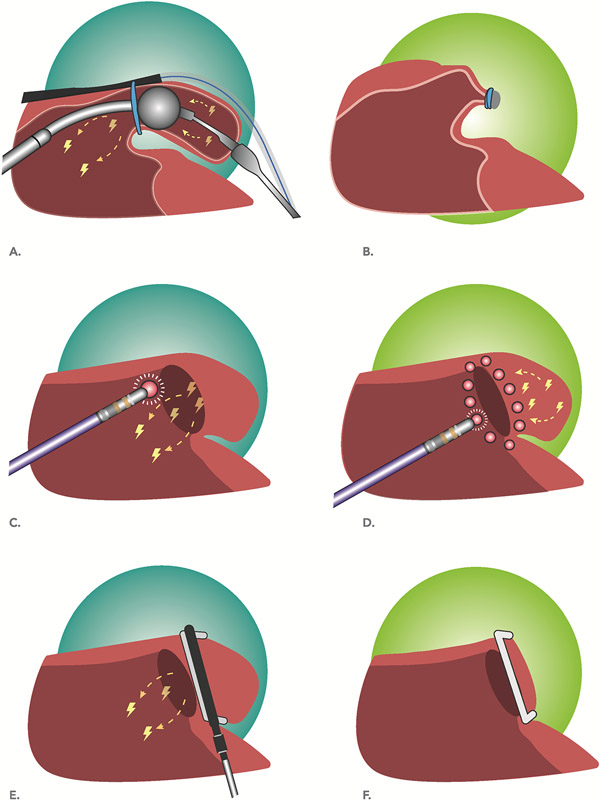
(a)LAA occlusion using the LARIAT is achieved via securing a suture at the neck of the LAA resulting in acute occlusion and infarction of the LAA. (b)Suture induced infarction of the LAA typically results in eventual regression of the LAA, leaving a small residual LAA stump and sometimes a diminutive LAA remnant. Circumferential radiofrequency ablation around the LAA ostium(c) can lead to conduction block with preservation of the distal LAA tissue(d), analogous to when circumferential ablation is performed around the ostia of the pulmonary veins. Successful ablation based LAAEI is associated with impaired LAA mechanical function because the atrial depolarization wavefront can no longer reach the LAA. Surgical LAA occlusion(e) of the LAA with the Atriclip(Atricure, West Chester, Ohio) leads to acute closure and infarction of the LAA with a small residual stump(f). Surgical LAA occlusion can cause electrical isolation of the LAA with other approaches, including oversewing and amputation(not shown).
Data Extraction
All included studies were reviewed by two investigators (DJF and EWB-M or ASB) using a standardized data acquisition form generated specifically for this study by two investigators (DJF, JPP). Disagreements were resolved through discussion or consultation with a third investigator (JPP). The primary outcome for this study was freedom from AT/AF after a blanking period. Secondary outcomes included thromboembolism (stroke or transient ischemic attack) and all-cause mortality. When available, periprocedural outcomes were additionally examined. Details regarding post LAAEI ambulatory monitoring protocols were documented and reported in Table 1.
Table 1.
Controlled Studies Comparing LAAEI versus no LAAEI for the Maintenance of SR
| 1st author | Year | LAAEI (n) | Control (n) | Study Design |
Study Description |
Arrhythmia follow-up Protocol |
Arrhythmia Endpoint |
Mean Follow-up (mos) |
|---|---|---|---|---|---|---|---|---|
| Di Biase(8)* | 2010 | 167 | 43 | Retrospective observational |
Patients with LAA triggers during re-do AF ablation managed with no ablation, LAAEI, or focal ablation | Holter (48hr or 7d) was obtained at 3, 6, 9, 13, 15 months after ablation. Pts had event recorder for 5 mos and had periodic asymptomatic transmissions per protocol | AT/AF > 32s after 2 month blanking period | 12 |
| Lee(15) | 2014 | 119 | 119 | Retrospective observational study with propensity matching | Mitral valve surgery with cryomaze +/− LAA excision at the surgeon’s discretion. | ECG at 1, 3, 6, 12, 24, 36 months. Holter employed for asymptomatic patients but use was not standardized. | AF/AT after 3 month blanking period† | 62 |
| Lakkireddy(14) | 2015 | 69 | 69 | Prospective observational with matched controls | Prospective arm underwent LARIAT LAA occlusion followed by AF ablation during a separate procedure. | 2 month event monitor beginning 2 weeks post procedure, 7 day monitor at 6 and 12 month visits, or additionally as clinically indicated. Device interrogation (when applicable) at 2, 6, and 12 month clinic appointments. | AT/AF >30s after 2 month blanking | 12 |
| DiBiase(13) | 2016 | 85 | 88 | Randomized trial | LAAEI vs. no LAAEI at the time of AF ablation | Holter (48hr or 7d) at 3, 6, 9, 12, 15 mos post ablation with event recorder for symptomatic evens and periodic asymptomatic transmissions | AT/AF >30s after 12 week blanking period | 24 |
| Panikker(12) | 2016 | 20 | 40 | Prospective observational with matched controls | LAAEI with WATCHMAN occlusion at the time of AF ablation compared to AF ablation alone | 7 day continuous ECG monitoring at 3, 6, 9, 12 months | AT/AF >30s after 3 month blanking period | 12 |
| Park(10)‡ | 2016 | 18 | 24 | Retrospective observational | Stepwise approach to AF ablation with reporting of those with and without LAAEI at the conclusion of the procedure | Holter monitoring (48hr) at 3, 6, 9, 12 mos after ablation | >30s AT/AF after 3 month blanking period | 21 |
| Romanov(11) | 2016 | 88 | 88 | Randomized trial | Surgical AF ablation (PV isolation, box lesion, ganglionated plexi ablation) +/− LAA excision. | Holter at 3, 6, 9, 12, 18 mos with implantable monitors in ~80% | >30s recurrent atrial arrhythmia after 3 month blanking period | 18 |
Study included a 3rd arm with focal ablation of LAA triggers (i.e. without complete isolation) that was not included in the current study
Minimum arrhythmia duration was not specified
Study included a 3rd arm with delayed (but ongoing) LAA conduction (i.e. without complete isolation) that was not included in the current study
AF = atrial fibrillation; AT = atrial tachycardia; LAA = left atrial appendage; LAAEI = left atrial appendage electrical isolation; PV = pulmonary vein
Outcome Definitions
A recurrence of AT/AF was defined as AT or AF documented on either standardized or clinically driven ambulatory monitoring after a 2-3 month post-procedure blanking period. A minimum AT/AF duration of 30(10-14) or 32(8) seconds was specified in all but one study.(15) A thromboembolic event was classified clinically by the treating physician and the definition was not standardized.
Statistical Analyses
We performed random-effects modeling and evaluated the relationship between LAAEI and recurrent AT/AF using the method of DerSimonian and Laird to compute odds ratios (OR) with 95% confidence intervals (CI) based on event counts. Sensitivity analyses were performed by serial exclusion of individual studies with additional analyses based on procedure characteristics, including method of LAAEI and concomitant LAA excision or closure. When no events were observed in both groups, a 1 was added to each group’s count to allow for analysis(16); this approach was not possible for Panniker et al(12) due to differences in the size of the study arms. Heterogeneity between studies was assessed using I2 index(17) and τ2 values. To assess for the possibility of reporting bias, funnel plots were generated by plotting the standard error versus the DerSimonian and Laird log odds ratio for each study. Analyses were conducted using Comprehensive Meta-Analysis Software™(BIOSTAT, Englewood, NJ, USA). All tests were two-tailed and a p-value of <0.05 was considered statistically significant.
Results
Study Summary
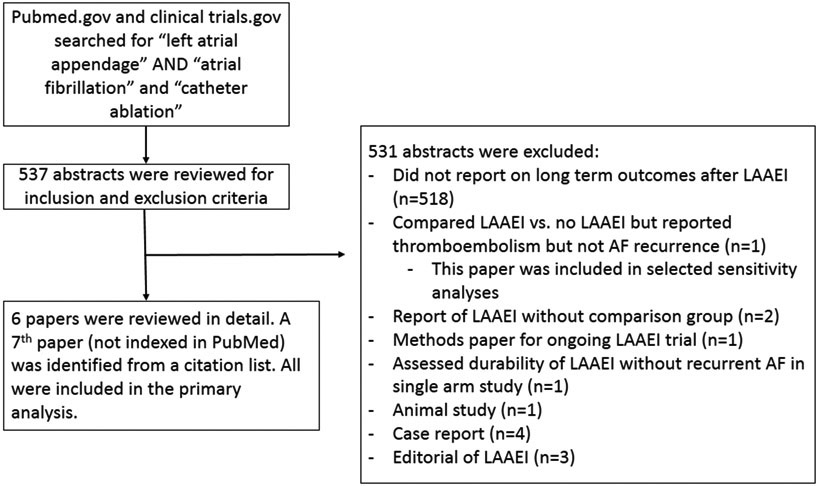
A total of 537 abstracts were identified via PubMed and reviewed. Seven studies were identified that reported on AT/AF recurrence after LAAEI and did not meet any exclusion criteria(Figure 2): six were identified from the PubMed search(8,10-13,15) and one was identified from a bibliography search(14). LAAEI was achieved via catheter ablation in four studies(8,10,12,13), LARIAT exclusion in one study(14), and surgical excision in two studies.(11,15) Two studies were randomized controlled trials(11,13) and the remainder were observational studies with control comparator groups(Table 1).(8,10,12,14,15) The ablation strategy in all studies included PV isolation (or re-isolation) with adjunctive ablation that varied by study. All studies employed arrhythmia monitoring protocols with adjunctive clinically indicated monitoring. Key details regarding each study are presented in Table 1.
Figure 2. PRISMA algorithm.
Patient Characteristics
A total of 1,037 patients were included; LAAEI was performed in 566(55%) patients. The majority(60%, n=623) of patients underwent percutaneous ablation and 349(34%) patients were from a randomized trial. Patients were generally older men and had hypertension, non-paroxysmal AF, a preserved ejection fraction, and an enlarged left atrium. The baseline characteristics of the study patients, when stratified by study and LAAEI versus no LAAEI, are detailed in Table 2.
Table 2.
Baseline Characteristics Stratified by Study and Treatment
| Di Biase (8) |
Lee (15) |
Lakkirredy (14) |
Romanov (11) |
Panniker (12) |
Di Biase (13) |
Park (10) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LAAEI | No LAAEI | LAAEI | No LAAEI | LAAEI | No LAAEI | LAAEI | No LAAEI | LAAEI | No LAAEI | LAAEI | No LAAEI | LAAEI | No LAAEI | |
| N | 167 | 43 | 119 | 119 | 69 | 69 | 88 | 88 | 20 | 40 | 85 | 88 | 18 | 24 |
| Age, mean | 64 | 61 | 53 | 54 | 67 | 67 | 57 | 58 | 68 | 67 | 64 | 64 | 57 | 56 |
| Male, % | 73 | 74 | 38 | 41 | 70 | 70 | 81 | 77 | 65 | 65 | 88 | 83 | 83 | 88 |
| HTN, % | 47 | 40 | 19 | 19 | 74 | 78 | 52 | 47 | n/a | n/a | 68 | 68 | 33 | 38 |
| DM, % | 8 | 7 | 8 | 8 | 29 | 26 | 11 | 9 | n/a | n/a | 20 | 21 | 6 | 4 |
| Non-paroxysmal AF, % | 87 | 72 | 91 | 91 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 83 | 88 |
| EF, mean | 59 | 58 | 57 | 57 | 53 | 53 | 58 | 56 | n/a | n/a | 54 | 55 | 51 | 52 |
| LA size in mm, mean | 43 | 41 | 59 | 60 | 50 | 48 | 47 | 47 | 46 | 45 | 48 | 48 | 41 | 44 |
AF = atrial fibrillation, DM = diabetes mellitus, EF = ejection fraction, HTN = hypertension, LA = left atrium, LAAEI = left atrial appendage electrical isolation
Recurrent Atrial Arrhythmias after LAAEI
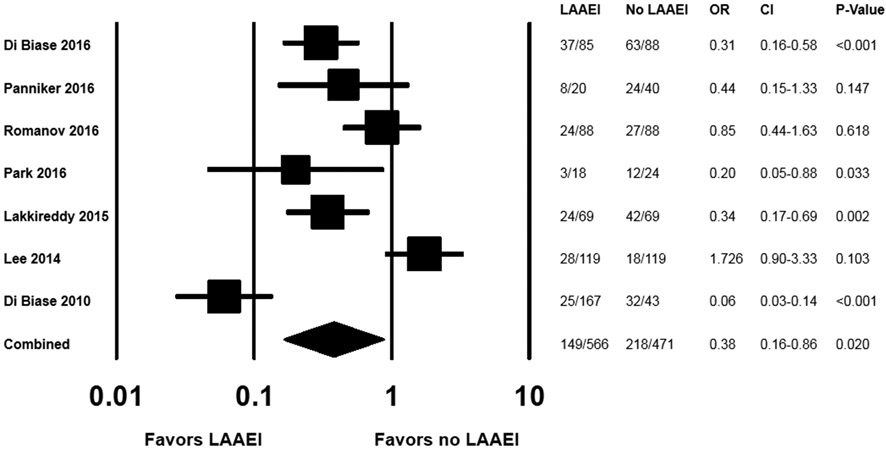
Recurrent AT/AF was significantly less common among patients who received LAAEI compared with those who did not (OR 0.375, CI 0.164-0.895, p=0.02;Figure 3). Heterogeneity was noted for the primary endpoint (I2=87.1; τ2=1.054). A plot of the standard error versus the DerSimonian and Laird log odds ratio for the primary endpoint for each study suggested the possibility of bias(Supplemental Figure 1).
Figure 3. Odds ratio (LAAEI vs. no-LAAEI) for AT/AF recurrence among all studies (n=7).
In a series of sensitivity analyses with sequential removal of individual studies, the results were consistent with the primary analysis(Supplemental Figure 2). In a subgroup analysis of percutaneous LAAEI(8,10,12-14) (5 studies, n=623), LAAEI was associated with a particularly robust reduction in recurrent AT/AF (OR 0.223, CI 0.108-0.463, p<0.001;Supplemental Figure 3). Results were consistent, but not statistically significant, when exclusively considering the two randomized trials(11,13)(OR 0.507, CI 0.187-1.377, p=0.183).
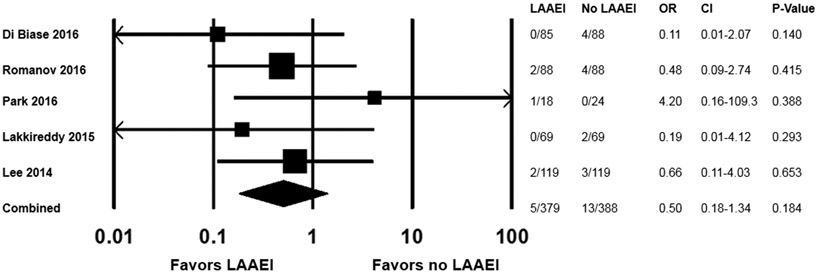
Thromboembolism after LAAEI
Thromboembolism during follow-up was reported in 6 studies and was rare. Overall, there were 18 thromboembolic events among 977 patients (across both arms) from 6 studies.(10-15) There was no association between LAAEI and thromboembolism (OR 0.5, CI 0.18-1.389, p=0.184;Figure 4), and no heterogeneity was noted (I2=0%, τ2=0). Notably, among the 6 studies that reported long-term thromboembolism, the LAA was either occluded or excised in 4 studies,(10-12,14) and follow-up transesophageal echocardiography(TEE) was performed to assess LAA mechanical function in the other 2 studies(10,13) and may have influenced antithrombotic strategies including LAA occlusion. A sensitivity analysis restricted to studies with concomitant LAA occlusion or excision (3 studies, n=552; Supplemental Figure 4) demonstrated consistent results. A study by Rillig et al(18) reported rates of thromboembolism among patients with LAAEI vs. no-LAAEI, but did not report on arrhythmia recurrence and therefore did not meet inclusion criteria for the current study; inclusion of the results from Rillig et al into a sensitivity analysis of LAAEI and thromboembolism did not change the results(Supplemental Figure 5). In an analysis including studies without LAA occlusion or excision(10,13,18)(3 studies, n=315), there was no association with thromboembolism, although the CIs were wide and the upper bound was greater than 21 (OR 1.451, CI 0.10-21.138, p=0.785; Supplemental Figure 6).
Figure 4. Odds ratio (LAAEI vs. no-LAAEI) for thromboembolism (n=5 studies).
Thromboembolism was not reported in one study(8) and did not occur in another study with asymmetric arms,(12) which precluded the substitution of a 1 count in each arm.(16)
Peri-procedural Complications
The heterogeneous approaches to LAAEI in this study preclude a comparison of peri-procedural outcomes among all studies. Among the individual surgical studies,(11,15) there was no difference in peri-procedural re-operation for bleeding or peri-procedural mortality among patients with and without LAAEI. Among the studies with LAAEI via radiofrequency ablation,(8,10,12,13) there were no reports of phrenic nerve damage or circumflex artery injury; there were insufficient data by treatment group to determine whether LAAEI was associated with different rates of pericardial effusion and tamponade.
Discussion
This meta-analysis demonstrates a number of important findings. First, LAAEI was associated with a significant reduction in recurrent AF/AF and this association was particularly dramatic among studies where LAAEI was performed percutaneously. Second, although there was no significant association between LAAEI and thromboembolic events, all studies either occluded or excised the LAA or utilized echocardiographic follow-up to determine the intensity of antithrombotic therapy. Finally, peri-procedural complications were not consistently reported across studies, precluding meaningful analysis and conclusions regarding safety.
The LAA has been known to be a site of AT and ectopic activity that could trigger AF although the contribution of the LAA to the development and maintenance of AF has been unclear. Although some studies have demonstrated that LAA triggers are common among patients undergoing AF ablation(8,9,19), others suggest that LAA triggers are rare.(20-22) The results from this meta-analysis support the role of the LAA in recurrent AF, particularly among patients with persistent AF. However, this analysis cannot determine whether the observed association is due to isolation of triggers, elimination of rotors or rotational propagation, debulking of an arrhythmogenic substrate, ganglionic plexus ablation, or a combination thereof. The inferiority of LAA trigger ablation when compared with LAAEI suggests the antiarrhythmic effect of LAAEI may be more complicated than simply abolishing a trigger.(8) It is also important to consider that ablation aimed at isolation of the LAA may lead to more extensive ablation and/or isolation of the adjacent Ligament of Marshall which contains extensive autonomic innervation and is an important source of focal AF in certain patients.(23) The antiarrhythmic effect of LAAEI in the The LAA Isolation in Patients With Longstanding Persistent AF Undergoing Catheter Ablation (BELIEF) trial is unexpected given the high reported rates of LAA reconnection, supporting the hypothesis that catheter ablation around the base of the LAA, but not durable LAAEI per se, may be the minimum requirement for benefit.(13) Finally, the majority of LAA tachycardias appear to originate from the base of the LAA(24); this suggests that adjunctive ablation may be necessary when there is a residual stump after surgical or LARIAT based LAA occlusion (see Figure 1 for an overview of the different approaches to LAAEI).
Several studies have documented the acute impact of LAAEI on the electrical substrate. One study of 68 patients undergoing LARIAT occlusion demonstrated that suture tightening led to an acute reduction in unipolar and bipolar voltages and an inability to capture LAA tissue in the majority of patients.(25) Another study of 15 patients undergoing LARIAT occlusion reported acute significant decreases in P-wave duration, the PQ interval, and P-wave dispersion, which were stable during short term follow-up.(26) In contrast, PV isolation, when performed after LARIAT LAAEI, did not cause an acute change in P-wave duration or P-wave dispersion.(27) The acute antiarrhythmic potential of LAAEI has been emphasized by an report of 162 patients with ≥3 months continuous AF undergoing LARIAT LAAEI. With LAAEI, 13 patients (12 had continuous AF >1 year) converted from AF to SR. Eight of these patients converted to SR during the procedure; 5 converted to SR within 2 days.(28)
Antiarrhythmic benefit of LAAEI is invariably dependent on the extent to which the mechanism of an individual’s AF depends upon triggers and/or substrate within the LAA. Patients with paroxysmal AF, which is most commonly due to PV triggers, are unlikely to benefit from LAAEI. In contrast, patients with longer standing AF and patients with recurrences after AF ablation may be more likely to have LAA-dependent AF. These hypotheses are supported by a study demonstrating that LAAEI was associated with decreased AF burden and that the magnitude of association was strongest among those with non-paroxysmal and those with known LAA triggers.(29) Notably, among the studies in this meta-analysis, those with a higher proportion of patients with longstanding persistent AF were the studies that demonstrated the most favorable association between LAAEI and AF recurrence.
Thromboembolism after LAAEI is a key concern and safety consideration. Rillig and colleagues’ data is concerning as they found that 10 of 50 patients had evidence of LAA thrombus on TEE imaging after LAA isolation despite oral anticoagulation in 9 of the 10.(18) Electrical isolation of the LAA would be expected to lead to decreased contractile function, increased stasis, and increased risk of thrombus formation, at least among patients without concomitant LAA occlusion or excision. In the overall analysis, there was no association between LAAEI and clinical thromboembolism. Although, the subgroup analysis limited to studies without LAA occlusion or excision(10,13,18)(3 studies, n=315), demonstrated no statistically significant association with thromboembolism, the CIs were wide and we were unable to exclude the possibility of a several fold increased risk of thromboembolism (given the upper bound of the confidence interval). It is possible that the frequent occurrence of LAA reconnection(13) led to an underestimate of the potential risk for thromboembolism associated with durable LAAEI.
Follow-up TEE was reported in a number of studies to assess LAA function and asymptomatic thrombus formation after LAAEI.(8,10,13,18) A TEE was performed in 204 patients who were in SR 6 months after 1st time LAAEI (as index or follow-up procedure) in the Di Biase, et al. study(8); although qualitatively poor LAA contractility was noted in 47% of patients, no thrombus was identified. Of note, Di Biase, et al. (2010) did not report rates of thromboembolism, LAA function, or rates of asymptomatic thrombus among those in AF during follow-up. Sixteen (of 18) patients with LAAEI from Park, et al. underwent follow-up TEE 1 month after the index procedure.(10) Although 50% of patients had qualitatively poor LAA contractility and one patient had LAA orifice stenosis, no asymptomatic thrombus or spontaneous echocardiographic contrast was noted. The BELIEF trial investigators performed routine TEE in the 62 patients who underwent LAAEI and were in SR at 6 months; 56.5% of patients had impaired LAA function, 1 patient with a subtherapeutic INR had an LAA thrombus, and one patient had spontaneous LAA contrast despite therapeutic warfarin.(13) In the BELIEF trial, LAA function and the presence of asymptomatic thrombi were not reported in AF patients after catheter ablation. None of the above studies compared LAA function among patients with and without LAAEI. Rillig et al (2016) compared LAA function among patients with and without LAAEI. TEE was performed in 47/50 cases and 50/50 of matched controls.(18) Patients with LAAEI (vs. controls) demonstrated reduced LAA flow velocity after ablation (0.2m/s vs. 0.5m/s. p<0.01) and increased rates of spontaneous echocardiographic contrast in the LAA (41% vs. 18%, p=0.012) and thromboembolism or asymptomatic thrombus (26% vs. 0%, p<0.01; due to 10 asymptomatic thrombi and 3 thromboembolic events in the LAAEI group). Of note, 9 of the 50 patients had evidence of LAA thrombus despite oral anticoagulation. A retrospective analysis from the same group reported 10%(7/71) of patients undergoing LAAEI had an asymptomatic LAA thrombus identified by TEE prior to a subsequent LA procedure; interpretation of these findings is difficult since anticoagulation use was not reported.(30)
The occasional finding of preserved LAA function after isolation is intriguing and suggests durable LAAEI is often not achieved. Among patients in the BELIEF study who underwent LAAEI and required a repeat procedure, LAA reconnection was observed in 37% of patients.(13) A recent study reported a 27% reconnection rate after LAAEI; however, patients in this report underwent LAAEI through a wide area isolation approach, typically using linear and or complex fractionated electrogram ablation to achieve isolation, often after several procedures.(30) The LAA can be activated via both endocardial propagation and through the distal extensions of Bachmann’s bundle(31); incomplete ablation of this epicardial component of the specialized conduction system may be an important barrier to durable LAAEI.
Limitations
This meta-analysis has several limitations. The majority of the studies were observational, raising the possibility that residual confounding influenced the results.(8,10,12,14,15) Morever, the studies included the meta-analysis have different designs thus limiting the specificity of the findings. Accordingly, we conducted a series of sensitivity analyses, which generally support the main analyses. Although the funnel plot suggests the possibility of underreporting, the primary results reassuringly remained robust in a series of sensitivity analyses, including analyses that excluded studies with the most statistically significant association between LAAEI and recurrent AT/AF. We observed heterogeneity among the studies used in the primary analysis, likely due to several factors, including one study(8) that studied only patients with known LAA triggers, resulting in a relatively larger effect size. We included studies with a variety of approaches to AF ablation and LAAEI which improves the overall generalizability of our findings but limits the precision with which we can estimate clinical benefit for a specific type of patient undergoing a specific procedure There is uncertainty regarding the exact ablation approach employed in the intervention arm of certain studies (8,10,12,14,15). Specifically, it is not clear if interventional arm ablation only differed based on performance of LAAEI. Only one study(11) was blinded, raising the possibility of bias. One study included patients with incidentally noted LAAEI.(10) Finally, there are several important differences in the patient populations across the various studies, including differences in AF subtype, AF duration, and left atrial size.
The infrequency of thromboembolism and the relatively short follow-up intervals may have limited the ability to detect differences in these outcomes among patients who did and did not receive LAAEI. Additionally, the wide confidence intervals observed in the analysis assessing the association between LAAEI without occlusion and thromboembolism suggests these analyses were limited by relatively low power to detect a difference. Peri-procedural complications were not uniformly recorded, precluding robust analyses on peri-procedural safety. Finally, the incidence rates for our primary and secondary outcomes were infrequently available and therefore raw event counts were used for all statistical analyses.
Clinical Implications and Future Directions
The current study suggests that the LAA plays an important role in certain types of AF and that LAAEI may reduce AF recurrence. Although LAAEI does not appear to be associated with increased risk of thromboembolism among patients with concomitant LAA occlusion or excision, there are insufficient data to determine whether this strategy is safe among patients managed exclusively with oral anticoagulation.
The ongoing aMAZE trial is rigorously testing the antiarrhythmic effect of LARIAT based LAAEI by randomizing up to 600 patients (2:1) with non-paroxysmal AF to standard AF ablation with LARIAT LAA exclusion versus standard AF ablation alone.(32) Additional randomized trials are necessary to confirm LAAEI efficacy, define procedural safety, and determine the best concomitant antithrombotic strategies and optimal method for LAAEI.
Conclusions
LAAEI is associated with a significant reduction in recurrent AT/AF. However, the data also raise concern for potential increased risk of LAA stasis and long-term risk of thromboembolic events. Randomized trials are required to confirm safety and efficacy and to determine the best treatment regimen to prevent thromboembolic events after LAAEI.
Supplementary Material
PERSPECTIVES.
Clinical Competency 1.
The LAA plays a critical role in atrial fibrillation pathogenesis in certain patients with atrial fibrillation.
Clinical Competency 2.
Electrical isolation of the LAA is associated with a significant reduction in recurrent atrial fibrillation among patients undergoing atrial fibrillation ablation.
Translational Outlook.
Future studies are needed to understand the anatomic and electrophysiological determinants of successful LAA electrical isolation in order to optimize safety and efficacy of this promising albeit controversial adjunctive strategy.
Funding:
Dr. Friedman is funded by the National Institutes of Health T 32 training grant HL069749
Abbreviations List
- AF
atrial fibrillation
- AT
atrial tachycardia
- CI
confidence interval
- LAA
left atrial appendage
- LAAEI
left atrial appendage electrical isolation
- OR
odds ratio
- SR
sinus rhythm
- PV
pulmonary vein
Footnotes
Disclosures: B.D. Atwater: research grants from St Jude Medical and Boston Scientific and serves as a consultant to Biosense Webster, Biotronik, Boston Scientific, St Jude Medical, and Medtronic. T.D. Bahnson: Research Grants: Medtronic; St. Jude Medical; Consultant to Boehringer Ingelheim, ChanRX, Sequel Pharma, and Sanofi-Aventis. C.R. Ellis has research grants from Atricure, Boston Scientific and Medtronic, consultant to Sentre Heart, Atricure and Boston Scientific. D.J. Friedman: education grants from St. Jude Medical and Boston Scientific, research grants from the National Cardiovascular Data Registry. K.P Jackson: serves as consultant to Medronic, St Jude Medical, and Merit Medical. J.P. Piccini: research grants for clinical research from ARCA biopharma, Boston Scientific, Gilead, Janssen Pharmaceuticals, and St Jude Medical and serves as a consultant to Allergan, Amgen, GSK, Johnson & Johnson, Medtronic, and Spectranetics. S. D. Pokorney reports research grants from the Food and Drug Administration, Boston Scientific, Gilead, Bristol-Myers Squibb, and Janssen Pharmaceuticals; advisory board/consulting support from Medtronic, Boston Scientific, and Bristol-Myers Squibb. The remaining authors report no disclosures.
References
- 1.January CT, Wann LS, Alpert JS et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–76. [DOI] [PubMed] [Google Scholar]
- 2.Haissaguerre M, Jais P, Shah DC et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–66. [DOI] [PubMed] [Google Scholar]
- 3.Verma A, Jiang CY, Betts TR et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 4.Dixit S, Marchlinski FE, Lin D et al. Randomized ablation strategies for the treatment of persistent atrial fibrillation: RASTA study. Circ Arrhythm Electrophysiol 2012;5:287–94. [DOI] [PubMed] [Google Scholar]
- 5.Elayi CS, Verma A, Di Biase L et al. Ablation for longstanding permanent atrial fibrillation: results from a randomized study comparing three different strategies. Heart Rhythm 2008;5:1658–64. [DOI] [PubMed] [Google Scholar]
- 6.Mohanty S, Gianni C, Mohanty P et al. Impact of Rotor Ablation in Nonparoxysmal Atrial Fibrillation Patients: Results From the Randomized OASIS Trial. J Am Coll Cardiol 2016;68:274–82. [DOI] [PubMed] [Google Scholar]
- 7.Willems S, Klemm H, Rostock T et al. Substrate modification combined with pulmonary vein isolation improves outcome of catheter ablation in patients with persistent atrial fibrillation: a prospective randomized comparison. Eur Heart J 2006;27:2871–8. [DOI] [PubMed] [Google Scholar]
- 8.Di Biase L, Burkhardt JD, Mohanty P et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation 2010;122:109–18. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, Di Biase L, Trivedi C et al. Importance of non-pulmonary vein triggers ablation to achieve long-term freedom from paroxysmal atrial fibrillation in patients with low ejection fraction. Heart Rhythm 2016;13:141–9. [DOI] [PubMed] [Google Scholar]
- 10.Park HC, Lee D, Shim J, Choi JI, Kim YH. The clinical efficacy of left atrial appendage isolation caused by extensive left atrial anterior wall ablation in patients with atrial fibrillation. J Interv Card Electrophysiol 2016;46:287–97. [DOI] [PubMed] [Google Scholar]
- 11.Romanov A, Pokushalov E, Elesin D et al. Effect of left atrial appendage excision on procedure outcome in patients with persistent atrial fibrillation undergoing surgical ablation. Heart Rhythm 2016;13:1803–9. [DOI] [PubMed] [Google Scholar]
- 12.Panikker S, Jarman JW, Virmani R et al. Left Atrial Appendage Electrical Isolation and Concomitant Device Occlusion to Treat Persistent Atrial Fibrillation: A First-in-Human Safety, Feasibility, and Efficacy Study. Circ Arrhythm Electrophysiol 2016;9. [DOI] [PubMed] [Google Scholar]
- 13.Di Biase L, Burkhardt JD, Mohanty P et al. Left Atrial Appendage Isolation in Patients With Longstanding Persistent AF Undergoing Catheter Ablation: BELIEF Trial. J Am Coll Cardiol 2016;68:1929–1940. [DOI] [PubMed] [Google Scholar]
- 14.Lakkireddy D, Mahankali AS, Kanmanthareddy A et al. Left Atrial Appendage Ligation and Ablation for Persistent Atrial Fibrillation: The LAALA-AF Registry. J Am Coll Cardiol EP 2015;1:153–160. [DOI] [PubMed] [Google Scholar]
- 15.Lee CH, Kim JB, Jung SH, Choo SJ, Chung CH, Lee JW. Left atrial appendage resection versus preservation during the surgical ablation of atrial fibrillation. Ann Thorac Surg 2014;97:124–32. [DOI] [PubMed] [Google Scholar]
- 16.Jones DR, Sutton AJ, Abrams KR, Fenty J, Warren F, Rushton L. Systematic review and meta-analysis of mortality in crop protection product manufacturing workers. Occup Environ Med 2009;66:7–15. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 18.Rillig A, Tilz RR, Lin T et al. Unexpectedly High Incidence of Stroke and Left Atrial Appendage Thrombus Formation After Electrical Isolation of the Left Atrial Appendage for the Treatment of Atrial Tachyarrhythmias. Circ Arrhythm Electrophysiol 2016;9:e003461. [DOI] [PubMed] [Google Scholar]
- 19.Hocini M, Shah AJ, Nault I et al. Localized reentry within the left atrial appendage: arrhythmogenic role in patients undergoing ablation of persistent atrial fibrillation. Heart Rhythm 2011;8:1853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerstenfeld EP, Callans DJ, Dixit S, Zado E, Marchlinski FE. Incidence and location of focal atrial fibrillation triggers in patients undergoing repeat pulmonary vein isolation: implications for ablation strategies. J Cardiovasc Electrophysiol 2003;14:685–90. [DOI] [PubMed] [Google Scholar]
- 21.Valles E, Fan R, Roux JF et al. Localization of atrial fibrillation triggers in patients undergoing pulmonary vein isolation: importance of the carina region. J Am Coll Cardiol 2008;52:1413–20. [DOI] [PubMed] [Google Scholar]
- 22.Lin D, Frankel DS, Zado ES et al. Pulmonary vein antral isolation and nonpulmonary vein trigger ablation without additional substrate modification for treating longstanding persistent atrial fibrillation. J Cardiovasc Electrophysiol 2012;23:806–13. [DOI] [PubMed] [Google Scholar]
- 23.Hwang C, Wu TJ, Doshi RN, Peter CT, Chen PS. Vein of marshall cannulation for the analysis of electrical activity in patients with focal atrial fibrillation. Circulation 2000;101:1503–5. [DOI] [PubMed] [Google Scholar]
- 24.Yamada T, Murakami Y, Yoshida Y et al. Electrophysiologic and electrocardiographic characteristics and radiofrequency catheter ablation of focal atrial tachycardia originating from the left atrial appendage. Heart Rhythm 2007;4:1284–91. [DOI] [PubMed] [Google Scholar]
- 25.Han FT, Bartus K, Lakkireddy D et al. The effects of LAA ligation on LAA electrical activity. Heart Rhythm 2014;11:864–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawamura M, Scheinman MM, Lee RJ, Badhwar N. Left atrial appendage ligation in patients with atrial fibrillation leads to a decrease in atrial dispersion. J Am Heart Assoc 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badhwar N, Lakkireddy D, Kawamura M et al. Sequential Percutaneous LAA Ligation and Pulmonary Vein Isolation in Patients with Persistent AF: Initial Results of a Feasibility Study. J Cardiovasc Electrophysiol 2015;26:608–14. [DOI] [PubMed] [Google Scholar]
- 28.Badhwar N, Mittal S, Rasekh A et al. Conversion of persistent atrial fibrillation to sinus rhythm after LAA ligation with the LARIAT device. Int J Cardiol 2016;225:120–122. [DOI] [PubMed] [Google Scholar]
- 29.Afzal MR, Kanmanthareddy A, Earnest M et al. Impact of left atrial appendage exclusion using an epicardial ligation system (LARIAT) on atrial fibrillation burden in patients with cardiac implantable electronic devices. Heart Rhythm 2015;12:52–9. [DOI] [PubMed] [Google Scholar]
- 30.Reissmann B, Rillig A, Wissner E et al. Durability of wide-area left atrial appendage isolation: Results from extensive catheter ablation for treatment of persistent atrial fibrillation. Heart Rhythm 2017;14:314–319. [DOI] [PubMed] [Google Scholar]
- 31.Chan CP, Wong WS, Pumprueg S et al. Inadvertent electrical isolation of the left atrial appendage during catheter ablation of persistent atrial fibrillation. Heart Rhythm 2010;7:173–80. [DOI] [PubMed] [Google Scholar]
- 32.Lee RJ, Lakkireddy D, Mittal S et al. Percutaneous alternative to the Maze procedure for the treatment of persistent or long-standing persistent atrial fibrillation (aMAZE trial): Rationale and design. Am Heart J 2015; 170:1184–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.