Abstract
Objective
To investigate the influence of different finishing/polishing techniques and in situ aging on the flexural strength (σ), surface roughness, and Candida albicans adherence of 5 mol% yttria-stabilized zirconia (ultratranslucent zirconia).
Materials and methods
A total of 120 zirconia bars (Prettau Anterior, Zirkonzahn) with dimensions of 8 × 2 × 0.5 mm were divided into 8 groups (n = 15) according to two factors: “in situ aging” (non-aged and aged (A)) and “finishing/polishing” (control (C), diamond rubber polishing (R), coarse grit diamond bur abrasion (B), and coarse grit diamond bur abrasion + diamond rubber polishing (BR)). Half of the samples from each group were subjected to a 60-day in situ aging by fixing the bars into cavities prepared in the posterior region of the base of complete or partial dentures of 15 patients. The samples were then subjected to the mini flexural (σ) test (1 mm/min). A total of 40 zirconia blocks (5 × 5 × 2 mm) were prepared and subjected to roughness (Ra) analyses and fungal adherence and complementary analyses (X-ray diffraction (XRD) and scanning electron microscopy (SEM)). The data of mean σ (MPa) and roughness Ra (μm) were statistically analyzed by two-way and one-way ANOVA, respectively, and Tukey’s test. The Weibull analysis was performed for σ data. The fungal adhesion (Log CFU/mL) data were analyzed by Kruskal–Wallis tests.
Results
For flexural resistance, the “finishing/polishing” factor was statistically significant (P = 0.0001); however, the “in situ aging” factor (P = 0.4458) was not significant. The non-aged (507.3 ± 115.7 MPa) and aged (487.6 ± 118.4 MPa) rubber polishing groups exhibited higher mean σ than the other techniques. The non-aged (260.2 ± 43.3 MPa) and aged (270.1 ± 48.8 MPa) bur abrasion groups presented lower σ. The coarse-grit diamond bur abrasion group (1.82 ± 0.61 μm) presented the highest roughness value (P = 0.001). Cell adhesion was not different among groups (P = 0.053). Group B presented the most irregular surface and the highest roughness Ra of 0.61 m.
Conclusions
The finishing of ultratranslucent zirconia might be preferably done with a diamond rubber polisher. Moreover, the protocols used did not interfere with Candida albicans adhesion.
Clinical relevance
Coarse-grit diamond burs might be avoided for finishing ultratranslucent monolithic zirconia, which might be preferably performed with a diamond rubber polisher.
Keywords: Dental ceramics, Zirconia, Biofilm, Aging, Flexural strength, Polishing
Introduction
Zirconia is one of the materials of choice for monolithic restorations due to its excellent mechanical properties [1], biocompatibility, resistance to corrosion [2], and aesthetics [3–7]. The optical properties of zirconia were improved by changes in the microstructure and composition of conventional first-generation zirconia [8], which is opaque and indicated for the fabrication of the coping of indirect restorations [2]. The reduced concentration of the aluminum oxide (A12O3) sintering aid and the lower porosity obtained by high-temperature sintering [8, 9] increased in translucency of second-generation zirconia [10]. The third-generation ultratranslucent (UT) zirconia (5 mol% yttria-stabilized zirconia) is characterized by a greater amount of yttrium oxide stabilizer and optically isotropic cubic phase, with reduced light scattering and greater translucency [8].
Although UT zirconia offers more aesthetic results [5, 6, 11], its mechanical properties are reduced [5, 6, 12] compared to earlier generations of zirconia. The cubic phase is not able to promote the tetragonal to monoclinic phase (T → M) transformation, an important transformation toughening mechanism in zirconia that contributes to the high fracture strength of the first- and second-generation zirconia [2]. As UT zirconia has different microstructural characteristics from previous generations of zirconia, several studies have been conducted to investigate its mechanical performance [3–6, 10, 12–15].
The finishing and polishing technique can influence the roughness [13, 16] and mechanical properties [4, 13, 16] of UT zirconia. In addition, the smoothness of the restoration surface directly interferes with bacterial adhesion [17–19] and wear of the opposite dentition [19]. Concerning cell adhesion, Candida albicans is the most prevalent type of fungus in the oral cavity, besides being identified as an opportunistic species in periodontal and peri-implant injuries [19]. Few studies have evaluated the effect of finishing/polishing on the mechanical properties of UT zirconia [4, 13, 16]. Polishing with rubber polishers seems to improve mechanical properties of the material [4] and generate a more uniform surface [16], while finishing with a diamond bur followed by glazing compromises the strength of UT zirconia [13, 16].
The mechanical properties of UT zirconia subjected to aging are also investigated [10, 12, 20]. The greater quantity of the yttrium oxide stabilizer and increased cubic phase give stability to UT zirconia and increase its resistance to hydrothermal degradation, so that the T → M transformations are drastically reduced [20] and the mechanical strength is not significantly affected by hydrothermal aging [10, 12, 20]. However, no study has evaluated the effect of in situ aging of UT zirconia.
Thus, the objective of the present study was to evaluate the influence of different finishing and polishing techniques with and without in situ aging on surface roughness, flexural strength, and cell adhesion of UT zirconia bars. The hypotheses tested were (1) the finishing and polishing protocols affect the flexural strength of UT zirconia; (2) in situ aging does not influence the resistance to flexural fracture; and (3) finishing and polishing protocols affect the roughness and fungal adhesion to UT zirconia.
Materials and methods
The information about the materials used in the present study is given in Table 1.
Table 1.
Materials used in the study
| Material | Company | Manufacturer | Composition | Batch No |
|---|---|---|---|---|
| Polycrystalline tetragonal zirconia partially stabilized by yttria (ultratranslucent) | Prettau Anterior | Zirkonzahn, Gais, Italy | ZrO2 > 85 wt% Y2 O3 < 12 wt% Al2 O3 < 1 wt% SiO2 max. 0.02 wt% Fe2O3 max. 0.02 wt% |
ZB6229E ZB4235E ZB5261D ZB5121B |
| Cylindrical diamond bur # 4135 | KG Sorensen | KG Sorensen, Cotia, Brazil | Diamond particles | 039,910 |
| Diamond rubber polishers for zirconia finishing and polishing | Kit Premium Compact | Dhpro, Paraná, Brazil | Polyurethane rubber impregnated with high hardness diamond | OP166 |
| Applic | Maquira | Maquira, Paraná, Brazil | Fluorine, resinous matrix of dimethacrylates | P098/19 |
| Adhesive | Âmbar-FGM | FGM, Joinvile, SC, Brazil | MDP | 010,518 |
Fabrication of ceramic bars
One hundred and twenty bars were cut from presintered UT zirconia milling discs (ø: 95 mm; thickness: 18 mm) (Prettau Anterior, Zirkonzahn, Gais Italy), using double-sided diamond discs (22 mm × 0.15 mm, Dhpro, Paraná, Brazil) mounted on a micromotor straight handpiece. This assembly was mounted on a device to fix the position of the handpiece and to aid the cut of the zirconia. A pilot study was conducted to detect the shrinkage from sintering, reduction in the thickness of the bars from finishing/polishing protocols, the duration of the cutting capacity of the burs, and the superficial aspect after rubber polishing according to the manufacturer’s recommendations (N=4 0, n = 5). The control group bars had 10 × 2.5 × 0.7 mm so that the samples presented a thickness of 0.5 mm after the approximate 20% shrinkage from sintering. The bars of the experimental groups had 10 × 2.5 × 0.9–1.0 mm and after the contraction from sintering of approximately 20%, they presented thickness of 0.7–0.8 mm. The bars were polished with 800, 1000, and 1200 grit SiC abrasive papers, cleaned in an ultrasonic bath with distilled water for 5 min, and air dried.
The zirconia bars were sintered in a specific oven (Zirkonofen 600, zirkonzahn, Gais, Italy) according to the manufacturer’s instructions: final temperature, 1450 °C; heating rate (from room temperature): 5 °C/min; waiting time, 2 h; and cooling rate: 5 °C/min until room temperature. After sintering, the bars had the final dimensions of 8 × 2 × 0.5 mm for the control groups and 8 × 2 × 0.7–0.8 mm for experimental groups.
Considering that the wear generated by the finishing and polishing protocols reduces the thickness of the bars of the experimental groups (pilot study), they were manufactured with higher thickness (0.7–0.8 mm) than the bars of the control group (0.5 mm), which will not be subjected to the finishing and polishing procedures. After the bars of the test groups were subjected to finishing/polishing techniques, they presented a final thickness of 0.5 mm, equaling the control groups. The measurements were performed using a digital caliper 150/6 in. MM Starrett 799A-6/150 in three different locations of the bar. The samples were then divided into 8 groups (2 control groups and 6 experimental groups) according to the factors: “finishing and polishing” (4 levels) and “in situ aging” (2 levels) for 3-point mini-flexural strength test (Fig. 1).
Fig. 1.
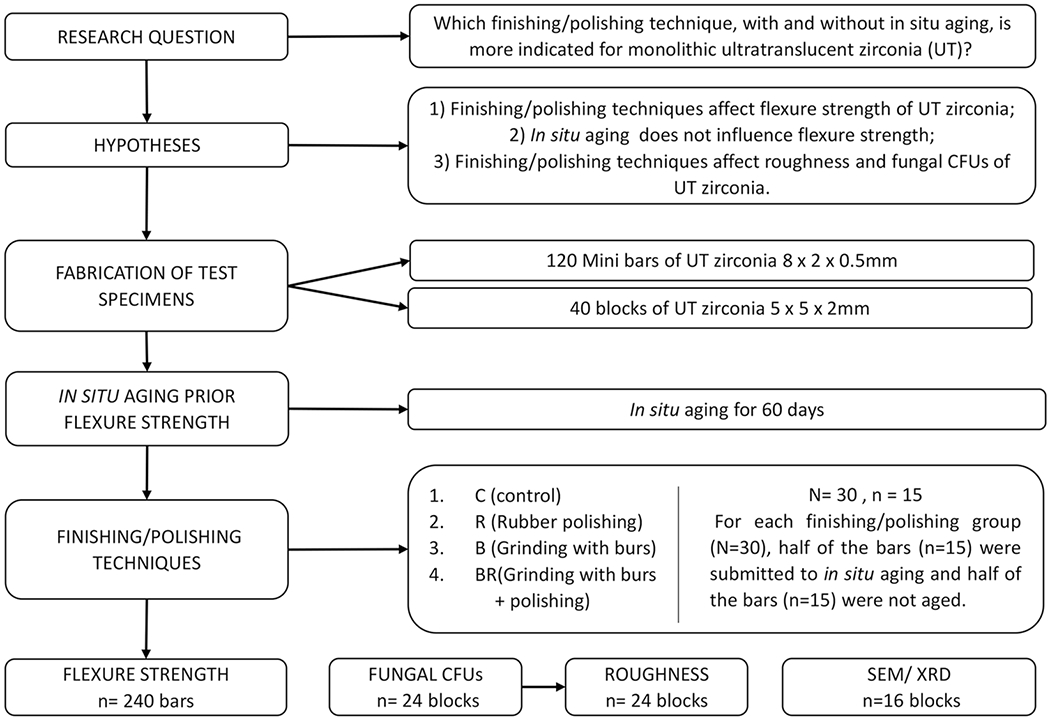
Flowchart of the study design
A total of 40 blocks of 6.5 × 6.5 × 2.5 mm were fabricated for the fungal adherence, roughness (Ra), and complementary analyses, and after contraction from sintering (approximately 20%), they presented 5 × 5 × 2 mm. The cutting and polishing of the blocks were performed using the same protocol for the fabrication of the bars. The blocks for the fungal adherence and roughness (Ra) were divided into four groups (n = 6) according to the factors finishing and polishing (4 levels); the blocks for the complementary analyzes were divided into eight groups (n = 2) according to the factors finishing and polishing (4 levels) and in situ aging (2 levels) (Fig. 1).
Finishing and polishing
The bars were adapted to a silicone index to assist the wear from the finishing/polishing protocols [16]. The protocols were performed by a single operator according to the experimental group:
Control (C): without treatment.
Coarse-grit diamond bur abrasion (B): grinding was performed with regular cylindrical coarse-grit diamond burs (# 4135-FG, 90–120 μm, KG Sorensen, Cotia, Brazil) mounted to a high-speed pen (505C, Kavo, Joinville, SC, Brazil) with abundant water cooling, in one-direction movements, on the whole surface, along the length of the bar. The bur was replaced with a new one every 10 samples. This protocol was adopted considering the study by Vila Nova et al. [16] and based on the findings of the pilot study regarding the surface analysis and cutting capacity of the burs. A reduction of diamonds in the burs was detected after 10 samples. This analysis was performed using a stereomicroscope (10 ×).
Diamond rubber polishing (R): polishing was performed using a kit of abrasive rubbers of polyurethane impregnated with high hardness diamonds (100 Shore A) (Premium Compact kit, Dhpro, Paraná, Brazil) mounted to a handpiece and micromotor (500, Kavo, Joinville, SC, Brazil) and applied according to the manufacturer’s recommendations (HZ1DL—adjustment, HZ2DL—pre-polishing, and HC3DL—high gloss or glaze) at 12,000 rpm (approximately 20 s per each grade of rubber polishing point), in one-direction movements on the whole surface along the length of the bar. This protocol was tested in a previous pilot study in which a visual increase in smoothness was observed after the application of all tips for 20 s each.
Coarse-grit diamond bur abrasion followed by Rubber polishing (BR): a combination of both methods.
The bars of the group B and R presented 0.7 mm of thickness (0.2 mm more than the control) and the bars of the BR presented 0.8 mm of thickness (0.3 mm more than the control). The amount of wear from finishing/polishing protocols was measured in a previous pilot study. For all experimental groups, the finishing and polish protocols were applied until the thickness of the samples was equal to 0.5 mm. The thickness of the bars was frequently measured in three locations of the bars with a digital caliper.
In situ aging
This research was approved by the Research Ethics Committee (No. 3.133.187). A total of 15 patients who were undergoing complete or partial denture replacement were selected. Patients with active caries, periodontal disease, gastroesophageal reflux, or who used regular medication were excluded from the research.
Half of the zirconia bars (n = 60) and blocks (n = 8) were mounted to the buccal surface of dentures. A channel (19 × 4 × 3 mm) was prepared on the left and right sides of the prostheses with a maxicut bur (American Burs, RS, Brazil). Approximately four bars (for 3-point mini flexural strength) and one block (for scanning electron microscopy (SEM) and X-ray diffraction (XRD)) were mounted onto each denture with a transparent light-cured temporary restorative material (Applic, Maquira, Paraná, Brazil), with prior application of an adhesive system (Âmbar-FGM, Joinvile, SC, Brazil), with the polished surface of the samples exposed to the oral cavity (Fig. 2). The participants wore the dentures with the embedded samples for 60 days [21, 22], which was the treatment period for the fabrication of new dentures. The patients were instructed to clean the dentures with a brush and toothpaste and store the dentures in water during the night. The patients wore the denture for approximately 16 h a day and 960 h during the 60-day period. When the patients attended the appointment for treatments and for the new dentures, they were questioned about the denture wearing and if they had any difficulties. All the patients participated in the study regularly wore the denture with zirconia samples.
Fig. 2.
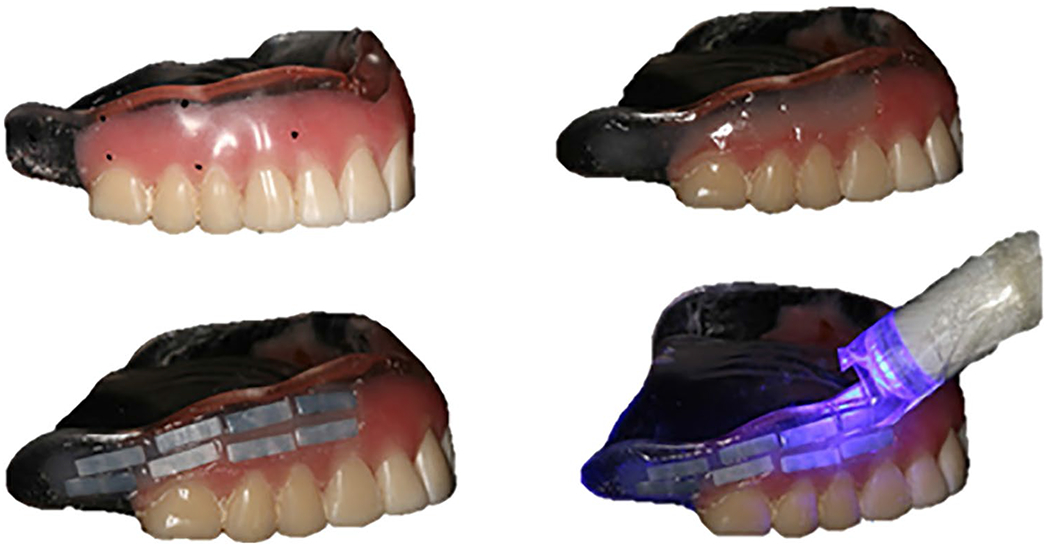
Embedding of samples in dentures
Mini-flexural strength test
The 120 bars were subjected to the three-point mini flexural strength test in a universal testing machine (ODEME-ISO150, Anchieta, Santa Catarina, Brazil). The thickness of the center of the bars was measured with a digital caliper before the test. A metal device [16, 21] adjusted for the dimensions of the bar was used. The bar was supported by two pins 6 mm apart with the treated side facing downwards, and a loading pin was placed at the center of the upper surface with a displacement speed of 1.0 mm/min and a load cell of 100 kgf. Flexural strength (σ) in MPa was recorded based on the critical load at the time of specimen failure, according to the equation below:
where l is the distance (mm) between the lower pins, F is the critical load (N) applied at the time of specimen failure, h is the thickness (mm) of the specimen, and w is the width of the specimen.
Candida albicans adherence
Six blocks of 5 × 5 × 2 mm from each finishing/polishing group (control (C), diamond rubber polishing (R); coarsegrit diamond burs abrasion (B); coarse-grit diamond bur abrasion + diamond rubber polishing (BR)) were made (N = 24, n = 6). The polishing/finishing protocols were applied to the blocks by the same operator and following the same parameters as the protocols for the bars. The samples were fixed to the bottom of a 24-compartment plate with wax, sterilized by ethylene oxide, and kept at room temperature. Microbiological adherence to specimens was assessed using a microbial cell viability assay. A strain of Candida albicans (ATCC 90,028), cultivated in RPMI 1640 medium at 37 °C for 24 h, was used. C. albicans was used in this study as it is one of the microorganisms that colonize biofilm in dentures and glazed or polished zirconia samples [23, 24]. The cell suspension was centrifuged at 1200 rpm for 5 min, and the cell pellet was resuspended in 5 mL of sterile saline (NaCl 0.9%). Then, the cell concentration was determined with a spectrophotometer at 600 nm wavelength. Cell density was established at an absorbance of 0.1, equivalent to a concentration of 1 × 106 cells/mL. To adjust the final solution, RPMI 1640 medium was used [22].
The samples were immersed in artificial saliva (2.5 g/L mucin, 0.25 g/L sodium chloride, 0.2 g/L potassium chloride, 0.2 g/L calcium chloride, 2.0 g/L yeast extract, 5.0 g/L protease peptone) [25] for film formation (2 mL of saliva/ specimen) and incubated at 37 °C for 60 min. The artificial saliva is composed of 2.5 g/L mucin, 0.25 g/L sodium chloride, 0.2 g/L potassium chloride, 0.2 g/L calcium chloride, 2.0 g/L yeast extract, 5.0 g/L peptone protease, and 1.25 m L/L of 40% urea.
Afterwards, the specimens were covered with 500 μL of the cell suspension and the system was incubated at 37 °C for 48 h in aerobic conditions. The specimens (n = 6) were transferred to tubes containing 1.0 mL of sterile saline and vortexed for 60 s each to collect the biofilms. Then, serial dilution of the aliquots was performed to determine the number of viable microorganisms (100 to 104). Aliquots of 10 μL were seeded in triplicate in dextrose agar plates in each serial dilution, and the plates incubated at 37 °C for 24 h. The number of viable cells was counted and multiplied by the serial dilution and converted to a logarithmic scale.
Roughness
Six samples from each group (the same samples used for the Candida albicans adherence test) were used for the roughness analysis. Each sample was fixed using a double-sided tape on a test table, and a digital rugosimeter (Surftest, Model SJ-2010, Mitutoyo, Japan) was used to determine the surface roughness. The arithmetic mean (Ra) between the peaks and valleys obtained by the active tip of the device, which covered a distance of 0.25 mm, was used. Three readings were taken in different areas of the surface. The data were averaged to obtain the value for each treatment of each block.
X-ray diffraction
To detect the presence of the monoclinic, tetragonal, and cubic phases, as well as to determine the volume percentage of the M phase (VM%), X-ray diffraction analysis was performed. The block (5 × 5 × 2 mm) samples (N = 16; n = 2) were analyzed on the diffractometer (D8 ADVANCE-Bruker) using copper radiation (CuKα, λ = 1.54 Å). The scans were performed at a current of 10 mA and voltage of 30 kV, using a Lynxeye detector, 0.02°/step, and acquisition time of 0.1 s. The graphs were generated using Origin 8 software. The phase percentages were subsequently determined, where (− 111)M, 2θ = 28°; (111)M, 2θ = 31.2°; (101)T, 2θ = 30°, representing the integrated intensity of the diffracted peaks [26–28]. The equations used were
| (1) |
| (2) |
Scanning electron microscopy
The samples used in the XDR analysis (N = 16, n = 2) were gold sputtered (BAL-TEC SCD 005) for 130 s with a current of 15 mA to obtain an 80-Å-thick layer. The surface features were observed at 10,000 × magnification in a SEM (FEG-ZEISS, Jena, Germany) and micrographs were obtained.
Statistical analysis
Statistical assumptions were evaluated before statistical analysis. Two-way analysis of variance (ANOVA) and Tukey’s test (5%) were performed to compare flexural strength (MPa) and one-way ANOVA and Tukey’s test (5%) to compare roughness Ra (μm) among groups. The computer program STATISTIX (Analytical Software Inc., version 8.0, 2003) was used for analyses.
The Kruskal–Wallis test was used to assess C. albicans adherence (Log CFU/mL). The computer program STATISTIX (Analytical Software Inc., version 8.0, 2003) was used.
Weibull modulus (m) and characteristic strength (σ0) were obtained with Weibull analysis, which indicated the microstructural homogeneity of the material considering strength variation. Characteristic strength is the strength at a failure probability of approximately 63.3%. Weibull modulus and characteristic strength with a 95% confidence interval were calculated by the ln{ln [1/(1 − F(σc)]} vs. lnσc diagram (according to ENV 843–5):
Statistical analysis was performed in the Minitab software (version 17, 2013, Minitab, State College, PA). The level of significance was 5%.
Results
Flexural strength
A sample power of 100% was obtained. Two-way ANOVA revealed that only the finishing and polishing factor (P < 0.0001) was significant (Table 2). When the finishing and polishing factor was evaluated alone, all groups differed statistically from each other, with group R (497.4 MPa) having the highest flexure strength followed by groups C (428.3 MPa), BR (348.0 MPa), and B (265.1 MPa). The flexural strength data are summarized in Table 3.
Table 2.
Two-way ANOVA results for flexural resistance of the test groups
| Effect | df | SQ | QM | F | p |
|---|---|---|---|---|---|
| Finishing | 3 | 907,615 | 302,538 | 37.80 | 0.0001* |
| Aging | 1 | 4686 | 4686 | 0.59 | 0.4458 |
| Finishing × aging | 3 | 546 | 182 | 0.02 | 0.9953 |
| Residuals | 112 | 896,508 | 8005 | ||
| Total | 119 | 1,809,355 |
df, degrees of freedom; SQ, sum of squares; MQ, mean squares.
p < 0.05, significant effect
Table 3.
Flexural strength means and standard deviations (MPa) of the experimental groups
| Finishing/polishing | In situ aging |
|
|---|---|---|
| Without aging | With aging | |
| Control (C) | 434.1 ± 74.3 AB | 422.6 ± 129.1 AB |
| Coarse diamond burs (B) | 270.1 ± 48.8 C | 260.2 ± 43.3 C |
| Diamond rubber polishers (R) | 507.3 ± 115.7 A | 487.6 ± 118.4 A |
| Burs + diamond rubber polishers (BR) | 352.4 ± 55.0 BC | 343.6 ± 84.3 BC |
Different uppercase letters (A–C) indicate significant differences among groups for flexure strength
When considering all experimental groups, R (507.3 ± 115.7 MPa) and RA (487.6 ± 118.4 MPa) had higher values compared to the other groups and similar to control groups. Groups B (270.1 ± 48.8 MPa) and BA (260.2 ± 43.3 MPa) had the lowest means of flexural strength and were significantly lower than control groups.
Weibull analysis
The results of the Weibull analysis are shown in Table 4 and Fig. 3. The characteristic strength (σ0) was statistically significant (P < 0.0001). The groups R (555.0 MPa) and RA (534.6 MPa) were significantly higher than all groups, except for the control groups. There was no statistically significant difference in the Weibull modulus (m) among the experimental groups.
Table 4.
Weibull modulus (m), characteristic strength (σ0), and 95% confidence intervals (95% CI) for flexural strength according to groups
| In situ aging | Groups | m | CI | σ 0 | 95% CI |
|---|---|---|---|---|---|
| Non-aged | C | 6.3 AB | 3.7–10.6 | 465.3 a | 427.6–506.4 |
| B | 6.4 AB | 4.4–9.2 | 289.2 d | 266.0–314.4 | |
| R | 5.5 AB | 3.2–9.4 | 555.0 a | 504.2–611.0 | |
| BR | 7.9 AB | 5.9–10.5 | 373.0 c | 348.1–399.8 | |
| Aged | CA | 4.3 B | 3.4–5.5 | 460.1 ab | 405.2–522.4 |
| BA | 7.0 AB | 4.8–10.0 | 277.3 d | 256.8–299.4 | |
| RA | 4.3 AB | 2.6–7.0 | 534.6 a | 472.6–604.7 | |
| BRA | 4.5 AB | 3.0–6.9 | 375.2 bc | 333.6–421.9 |
Different uppercase letters (A and B) indicate statistical differences between groups for Weibull modulus (m).
Different lowercase letters (a–d) indicate statistical differences between groups for characteristic strength (σ0).
C, control; R, diamond rubber polishing; B, coarse diamond burs abrasion; BR, coarse diamond bur abrasion + diamond rubber polishing; A, aged specimens
Fig. 3.
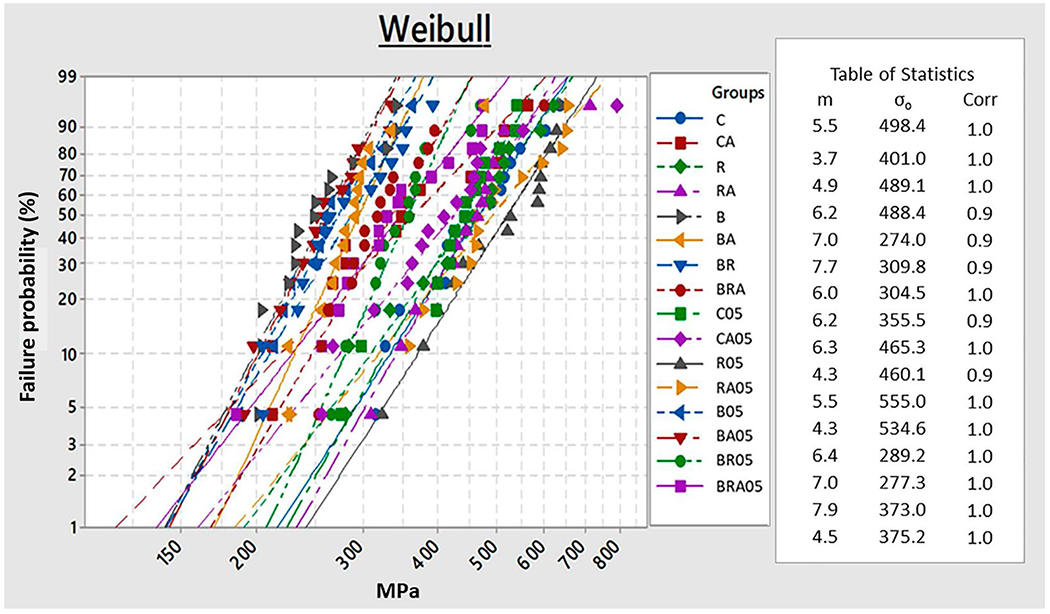
Weibull analysis graph
Candida albicans adherence
C. albicans adhesion was similar for the four groups, ranging from 6.3 to 7.1 Log CFU/mL (P = 0.053) (Fig. 4).
Fig. 4.
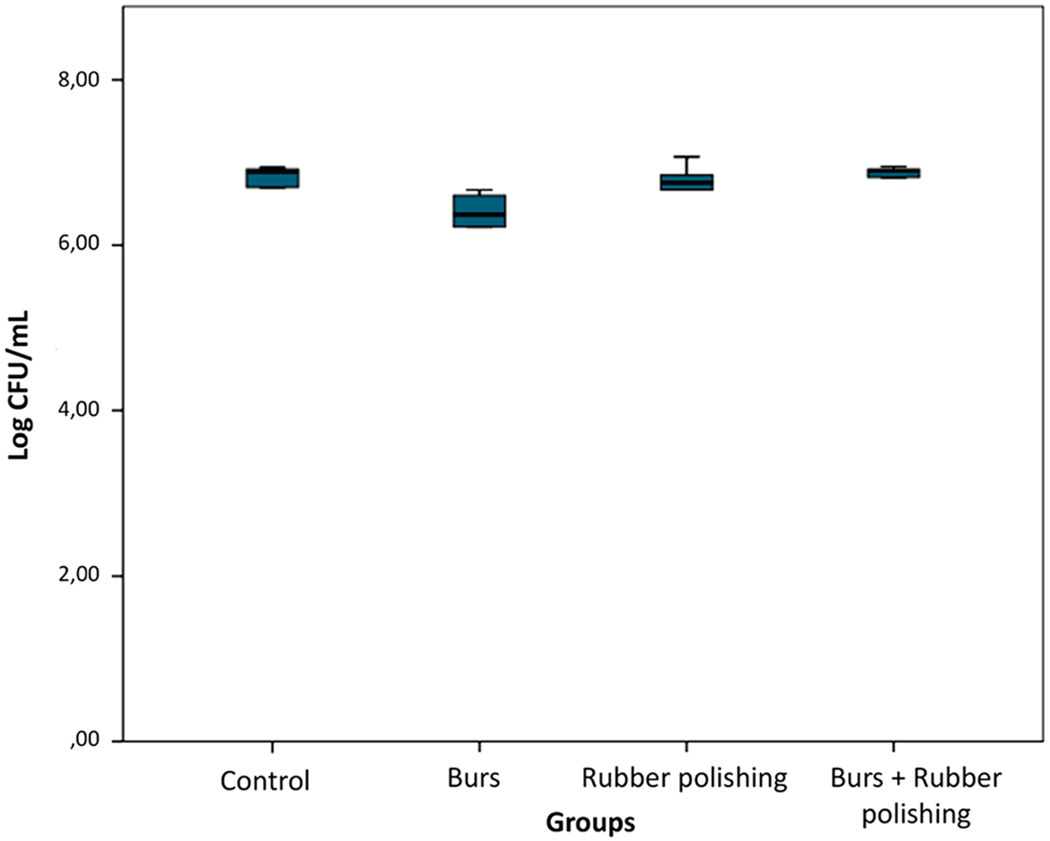
Log CFU/mL count graph
Roughness
The finishing and polishing factor was significant (P = 0.001) for roughness values. The means of groups ranged from 0.34 to 1.82 m Ra, with the coarse-grit diamond bur abrasion group having the highest value (B = 1.82 ± 0.61 m). The mean roughness value for the bur and rubber group (BR = 0.34 ± 0.16 m) and the rubber group (R = 0.36 ± 0.32 m) were significantly lower than the control group (C = 1.01 ± 0.20 m).
X-ray diffraction
Figure 5 shows graphs of the crystalline phase for each group. For all groups, peaks corresponding to the tetragonal and cubic phases were detected at 2¸ angles of ~ 30°, 35°, and 50°. However, no significant monoclinic peaks were detected at 28° and 31°, in all cases.
Fig. 5.
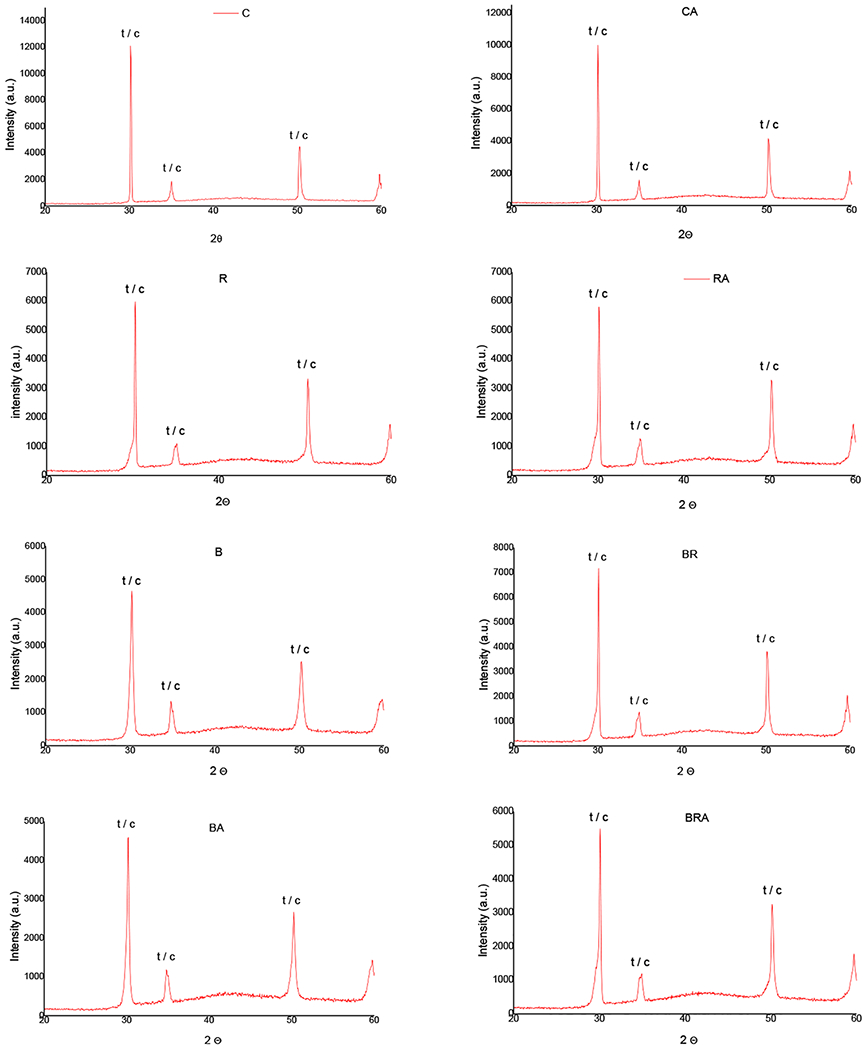
Representative graphics of the XRD spectra: C, control; CA, control with aging; B, coarse diamond bur abrasion; BA, coarse diamond bur abrasion bur abrasion with aging; R, diamond rubber polishing; RA, diamond rubber polishing with aging; BR, bur abrasion + rubber polishing; BRA, bur abrasion + rubber polishing with aging
Scanning electron microscopy
The groups subjected to finishing/polishing showed superficial changes in relation to the control group (Fig. 6). A greater uniformity of the surface was seen in the R groups. Cracks and craters were detected in the B groups. In the BR groups, a smoother surface was observed in comparison to the B groups. The images of the aged and non-aged samples were similar, very different from previously reported aging behaviors of finished/polished 3Y-TZP tetragonal zirconia [29].
Fig. 6.
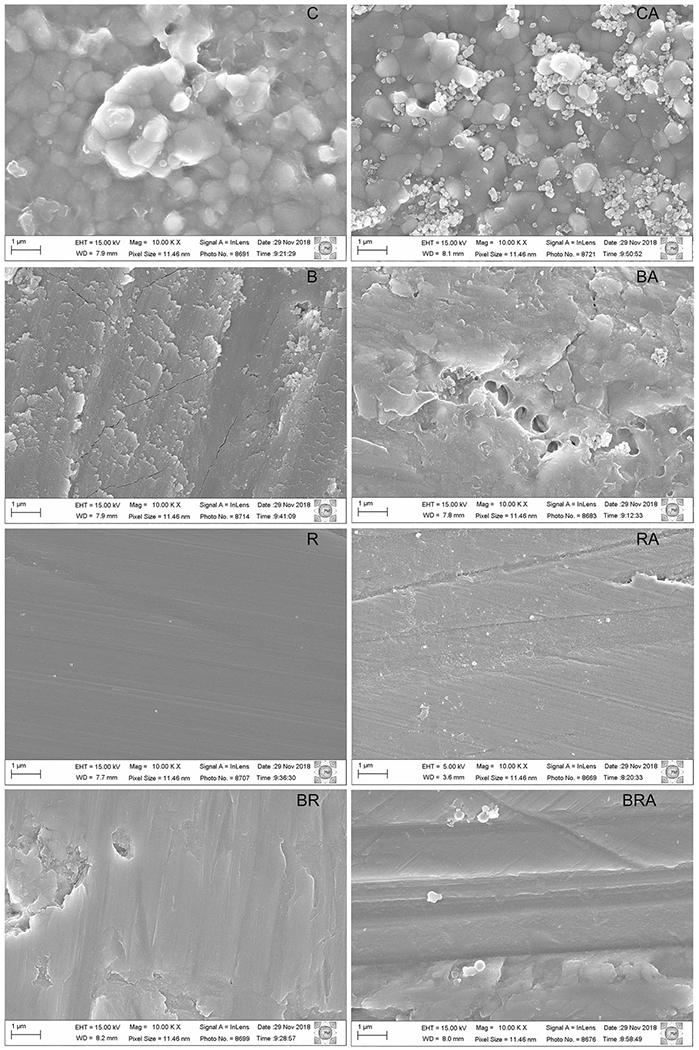
Micrographs (10,000×) of the ultra-translucent zirconia surface with different finishing and polishing protocols. Left, show non-aged groups and right panels show aged groups. C, control; CA, control with aging; B, coarse diamond bur abrasion; BA, coarse diamond bur abrasion with aging; R, diamond rubber polishing; RA, diamond rubber polishing with aging; BR, bur abrasion + rubber polishing; BRA, bur abrasion + rubber polishing with aging
Discussion
In the present study, the specimens were made smaller than those recommended by the ISO (International Organization for Standardization, 6872/2015), and tested using a miniature three-point bending jig, to be able to perform the in situ aging by embedding the zirconia samples in the denture of patients. This methodology was based on the studies by Miragaya et al. [21] and Vila-Nova et al. [16]. In the research developed by Vila-Nova et al. [16], the authors also used the mini flexural test and reported that the control group showed a fracture strength (450.8 ± 79.5 MPa) very similar to that of our control group (C = 434.1 ± 74.3 MPa). According to Vila-Nova et al. [16], as some of the ISO 6872/2015 specifications could not be met in mini flexural test, the present flexural strength data of zirconia with various surface treatments shall not be used for direct comparison with results reported in other studies with different sample specifications.
The hypothesis that the finishing and polishing protocols affect the flexural strength of zirconia was partially rejected, since the rubber polishing group had the highest flexural strength values, being statistically similar to the control group. A similar result was reported by Vila-Nova et al. [16], in which the rubber polishing group (791.8 ± 169.4 MPa) presented a higher strength value and similar to the aged control group (678.7 ± 225.4 MPa). The SEM images indicated that rubber polishing promotes the compression of the zirconia grains on the surface, which, although superficial, was effective in reducing crack propagation, optimizing the material strength. Mao et al. [4] also observed that polishing increased flexural strength.
The groups subjected to coarse-grit diamond bur abrasion showed a lower flexural strength in relation to the other groups. This result corroborates that from the study by Vila-Nova et al. [16], in which the diamond bur finishing group (429.9 ± 182.3 MPa) had less resistance when compared to the control group (678.7 ± 225.4 MPa) or the rubber polishing group (791.8 ± 169.4 MPa). Different results have been reported by Hatanaka et al. [13], in which diamond bur finishing did not significantly reduce the flexural strength of UT zirconia. This may be related to differences in the finishing/polishing technique, such as polishing time, motor rotating speed, presence or absence of cooling, among other factors. In addition, the SEM images of this study showed that in the bur finishing groups there was chipping and dislodgement of the zirconia grains, which may have caused the loss of mechanical properties of UT zirconia. Also, the characteristic strength of the bur groups was the lowest in comparison to the other groups. The greater presence of superficial irregularities detected in this group may have influenced the origin of cracks, decreasing the material’s strength.
Unlike surface damage and reduced mechanical properties, finishing with burs did not induce notable T → M phase transformation, as shown by the XRD spectra. The present finding is in good agreement with previous study on bur finishing of UT 5Y-TZP zirconia [4], but in stark contrast to bur finishing of 3Y-TZP tetragonal zirconia, where significant amounts of T → M phase transformation were reported [29].
No studies evaluating the influence of in situ aging of UT zirconia samples were found. in situ aging aims at assessing the behavior of the material intraorally. Miragaya et al. [21] carried out a 60-day in situ aging of conventional and translucent zirconia samples (Lava Frame, 3 M ESPE and Lava Plus, 3 M ESPE) using an intraoral device. The results indicated an increase in surface roughness and proportion of monoclinic phase. In addition, the three-point flexural strength of both zirconia (conventional and translucent) reduced significantly compared to the control (non-aged). In the present study, in situ aging for 60 days did not cause significant changes in the mechanical properties of UT zirconia, and thus our second study hypothesis was accepted. This result corroborates studies that carried out hydrothermal aging of UT zirconia samples [10, 12, 16, 20]. In the study by Vila-Nova et al. [16], minibar UT zirconia samples subjected to hydrothermal aging (127 °C, 1.7 bar/24 h) were similarly prepared to the present study, which may enable a comparison of the effect of the two types of aging on UT zirconia. As in the present study, hydrothermal aging did not promote a significant difference in the 3-point mini flexural test of UT zirconia. On the other hand, mechanical aging (mechanical cycling) and its association with hydrothermal aging reduced the biaxial flexural strength of UT zirconia [20]. Therefore, in situ aging for 60 days as well as other hydrothermal aging methods may not cause significant changes in UT zirconia due to its high phase stability and thus good resistance to low temperature degradation [20].
The stability of UT zirconia is related to a higher concentration of yttrium that causes the presence of the stable cubic phase, which is not susceptible to thermal transformation, making UT zirconia more resistant to hydrothermal degradation than previous generations of zirconia [2]. Autoclave aging also does not promote the T → M transformation [10, 12, 16, 20]. The findings indicate that UT zirconia undergoes minimum phase transformation, mostly maintaining its mechanical properties. However, these results must be analyzed with caution. It is important to considerer that the period of 60 days of in situ aging may not be able to promote significant alterations in the properties of UT zirconia, thus, further studies with longer in situ aging duration are important to investigate the performance of this material.
In the present study, the effect of the finishing and polishing techniques on the fungal adherence to zirconia surface was also investigated. C. albicans is an opportunistic pathogen commonly associated not only with denture-related stomatitis, but also with other oral diseases such as peri-implantitis and periodontitis. Previous studies have reported that the number of C. albicans Log CFU/mL present in subgingival oral biofilm was higher in individuals with peri-implantitis than in healthy individuals [30, 31] and also in individuals with severe chronic periodontitis [32–35]. Thus, considering that monolithic zirconia crown can be supported by teeth and implants and that the crown is commonly in contact with gingival tissues and subgingival oral biofilm in both situations, it is clinically relevant to investigate the fungal adherence in zirconia samples.
The third hypothesis, that the finishing and polishing protocols affect the roughness and C. albicans adhesion in UT zirconia, was partially rejected. The group finished with a bur had higher roughness than the other groups, but there was no significant difference in fungal adherence among the groups. This could be explained by the difficulty of C. albicans to adhere to very smooth surfaces due to its hydrophobicity and to the anti-adhesive properties of zirconia compounds [36]. C. albicans adherence to a zirconia surface was previously described in Cepic et al. [23]. The author reported that although the glazed zirconia showed higher roughness (Ra) and surface free energy (SFE) than polishing zirconia, there was no significant difference in C. albicans CFU/mL between these treatments, as detected in the present study. In addition, other tested finishing/polishing protocols also did not influence the fungal adhesion. On the other hand, Dal Piva et al. [17] investigated the biofilm formation in translucent zirconia and lithium silicate submitted to glaze and polishing, and reported that C. albicans formed higher Log CFU/mL in glazed than in polishing groups. The glazed zirconia group showed higher roughness (Ra) and lower surface free energy than the polished zirconia group. The methodological differences between the studies may influence the different results among the studies. Also, the finishing/polishing techniques investigated in these studies were different from this study. Further studies investigating the zirconia surface properties and microorganism adhesion are necessary to clarify the relation between them, and also its effect on the longevity of zirconia restorations.
Additional studies should be conducted to evaluate the effect of mechanical cycling associated with in situ aging on the mechanical properties of UT zirconia. As a study limitation, the 60 days for in situ aging may not be sufficient to promote significant changes in UT zirconia properties. Finally, studies with a longer in situ aging period and randomized clinical trials are needed to confirm the results found in our study in a clinical setting.
Conclusions
Polishing with diamond-impregnated rubber polishers proved to be the best technique for finishing and polishing UT zirconia. Restoration adjustment with coarse-grid diamond burs might be avoided, but if needed, the restoration might be polished with rubber polishers afterwards to minimize the effects on fracture resistance of zirconia.
The strength of UT zirconia was not affected by in situ aging.
Different methods of finishing and polishing do not interfere with C. albicans adhesion to zirconia; however, they affect roughness; polishing with rubber polishers provides the highest surface smoothness.
Acknowledgements
This study was based on a Master of Science thesis submitted to the Federal University of Rio Grande do Norte (UFRN), Natal, RN, Brazil. The authors thank Vagner (São Paulo, Brazil) and LTN Laboratories (Rio Grande do Norte, Brazil) for providing the ceramic materials used in the study.
Funding
This study was partly funded by the Coordination for the Development of Higher Education Personnel—Brazil (CAPES)—Grant Code 001. YZ would like to thank the United States National Institutes of Health/National Institute of Dental and Craniofacial Research for their support (grants No. R01 DE026772 and R01 DE026279).
Footnotes
Presented partially at the 13th GBRPI—Brazilian Group of Recycling in Prosthesis and Implant, Campos do Jordão, SP, Brazil, April, 2019.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the Research Ethics Committee—HUOL (No. 3.133.187).
Informed consent Informed consent was obtained from all individual participants included in the study.
Conflict of interest The authors declare no conflict of interest.
References
- 1.Denry I, Kelly JR (2008) State of the art of zirconia for dental applications. Dent Mater 24:299–307. 10.1016/j.dental.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Lawn BR (2018) Novel zirconia materials in dentistry. J Dent Res 97:140–147. 10.1177/0022034517737483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang F, Inokoshi M, Batuk M et al. (2016) Strength, toughness and aging stability of highly-translucent Y-TZP ceramics for dental restorations. Dent Mater 32:e327–e337. 10.1016/j.dental.2016.09.025 [DOI] [PubMed] [Google Scholar]
- 4.Mao L, Kaizer MR, Zhao M et al. (2018) Graded ultra-translucent zirconia (5Y-PSZ) for strength and functionalities. J Dent Res 97:1222–1228. 10.1177/0022034518771287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrabba M, Keeling AJ, Aziz A et al. (2017) Translucent zirconia in the ceramic scenario for monolithic restorations: a flexural strength and translucency comparison test. J Dent 60:70–76. 10.1016/j.jdent.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 6.Kwon SJ, Lawson NC, McLaren EE et al. (2018) Comparison of the mechanical properties of translucent zirconia and lithium disilicate. J Prosthet Dent 120:132–137. 10.1016/j.prosdent.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 7.Souza R, Barbosa F, Araújo G et al. (2018) Ultrathin monolithic zirconia veneers: reality or future? Report of a clinical case and one-year follow-up. Oper Dent 43:3–11. 10.2341/16-350-t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stawarczyk B, Keul C, Eichberger M et al. (2017) Three generations of zirconia: from veneered to monolithic. Part I. Quintessence Int 48:369–380. 10.3290/j.qi.a38057 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y (2014) Making yttria-stabilized tetragonal zirconia translucent. Dent Mater 30:1195–1203. 10.1016/j.dental.2014.08.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camposilvan E, Leone R, Gremillard L et al. (2018) Aging resistance, mechanical properties and translucency of different yttria-stabilized zirconia ceramics for monolithic dental crown applications. Dent Mater 34:879–890. 10.1016/j.dental.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 11.Kolakarnprasert N, Kaizer MR, Kim DK et al. (2019) New multilayered zirconias: composition, microstructure and translucency. Dent Mater 35:797–806. 10.1016/j.dental.2019.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira GKR, Guilardi LF, Dapieve KS et al. (2018) Mechanical reliability, fatigue strength and survival analysis of new polycrystalline translucent zirconia ceramics for monolithic restorations. J Mech Behav Biomed Mater 85:57–65. 10.1016/j.jmbbm.2018.05.029 [DOI] [PubMed] [Google Scholar]
- 13.Hatanaka GR, Polli GS, Adabo GL (2020) The mechanical behavior of high-translucent monolithic zirconia after adjustment and finishing procedures and artificial aging. J Prosthet Dent 123:330–337. 10.1016/j.prosdent.2018.12.013 [DOI] [PubMed] [Google Scholar]
- 14.Kaizer MR, Kolakarnprasert N, Rodrigues C et al. (2020) Probing the interfacial strength of novel multi-layer zirconias. Dent Mater 36:60–67. 10.1016/j.dental.2019.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan J, Kaizer MR, Zhang Y (2018) Load-bearing capacity of lithium disilicate and ultra-translucent zirconias. J Mech Behav Biomed Mater 88:170–175. 10.1016/j.jmbbm.2018.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vila-Nova TEL, Carvalho IGH, Moura DMD et al. (2020) Effect of finishing/polishing techniques and low temperature degradation on the surface topography, phase transformation and flexural strength of ultra-translucent ZrO2 ceramic. Dent Mater 36:e126–e139. 10.1016/j.dental.2020.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dal Piva A, Contreras L, Ribeiro FC et al. (2018) Monolithic ceramics: effect of finishing techniques on surface properties, bacterial adhesion and cell viability. Oper Dent 43:315–325. 10.2341/17-011-1 [DOI] [PubMed] [Google Scholar]
- 18.Lee DH, Mai HN, Thant PP et al. (2019) Effects of different surface finishing protocols for zirconia on surface roughness and bacterial biofilm formation. J Adv Prosthodont 11:41–47. 10.4047/jap.2019.11.1.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Go H, Park H, Lee J et al. (2019) Effect of various polishing burs on surface roughness and bacterial adhesion in pediatric zirconia crowns. Dent Mater J 38:311–316. 10.4012/dmj.2018-106 [DOI] [PubMed] [Google Scholar]
- 20.Muñoz EM, Longhini D, Antonio SG et al. (2017) The effects of mechanical and hydrothermal aging on microstructure and biaxial flexural strength of an anterior and a posterior monolithic zirconia. J Dent 63:94–102. 10.1016/j.jdent.2017.05.021 [DOI] [PubMed] [Google Scholar]
- 21.Miragaya LM, Guimarães RB, Souza ROA et al. (2017) Effect of intra-oral aging on T→M phase transformation, microstructure, and mechanical properties of Y-TZP dental ceramics. J Mech Behav Biomed Mater 72:14—21. 10.1016/j.jmbbm.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 22.Veríssimo AH, Moura DMD, Dal Piva AMO, Bottino MA et al. (2020) Effect of different repair methods on the bond strength of resin composite to CAD/CAM materials and microorganisms adhesion: an in situ study. J Dent 93: 103266 In Press. 10.1016/j.jdent.2019.103266 [DOI] [PubMed] [Google Scholar]
- 23.Zupancic Cepic L, Dvorak G, Piehslinger E et al. (2020) In vitro adherence of Candida albicans to zirconia surfaces. Oral Dis 26:1072–1080. 10.1111/odi.13319 [DOI] [PubMed] [Google Scholar]
- 24.Al-Fouzan AF, Al-Mejrad LA, Albarrag AM et al. (2017) Adherence of Candida to complete denture surfaces in vitro: a comparison of conventional and CAD/CAM complete dentures. J Adv Prosthodont 9:402–408. 10.4047/jap.2017.9.5.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavalcanti YW, Wilson M, Lewis M et al. (2016) Modulation of Candida albicans virulence by bacterial biofilms on titanium surfaces. Biofouling 32(2):123–134. 10.1080/08927014.2015.1125472 [DOI] [PubMed] [Google Scholar]
- 26.Garvie RC, Nicholson PS (1972) Phase analysis in zirconia systems. J Am Ceram Soc 55:303–305. 10.1111/j.1151-2916.1972.tb11290.x [DOI] [Google Scholar]
- 27.Toraya H, Yoshimura M, Somiya S et al. (1984) Calibration curve for quantitative analysis of the monoclinic-tetragonal ZrO2 system by X-ray diffraction. J Am Ceram Soc 67:C-119–C-121. 10.1111/j.1151-2916.1984.tb19715.x [DOI] [Google Scholar]
- 28.Souza ROA, Valandro LF, Melo RM et al. (2013) Air-particle abrasion on zirconia ceramic using different protocols: effects on biaxial flexural strength after cyclic loading, phase transformation and surface topography. J Mech Behav Biomed Mater 26(2013):155–163. 10.1016/j.jmbbm.2013.04.018 [DOI] [PubMed] [Google Scholar]
- 29.Kim JW, Covel NS, Guess PC, Rekow ED, Zhang Y (2010) Concerns of hydrothermal degradation in CAD/CAM Zirconia. J Dent Res 89(1):91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alrabiah M, Alshagroud RS, Alsahhaf A et al. (2019) Presence of Candida species in the subgingival oral biofilm of patients with peri-implantitis. Clin Implant Dent Relat Res 21(4):781–785. 10.1111/cid.12760 [DOI] [PubMed] [Google Scholar]
- 31.Alsahhaf A, Al-Aali KA, Alshagroud RS et al. (2019) Comparison of yeast species in the subgingival oral biofilm of individuals with type 2 diabetes and peri-implantitis and individuals with periimplantitis without diabetes. J Periodontol 90(12):1383–1389. 10.1002/JPER.19-0091 [DOI] [PubMed] [Google Scholar]
- 32.Urzúa B, Hermosilla G, Gamonal J et al. (2008) Yeast diversity in the oral microbiota of subjects with periodontitis: Candida albicans and Candida dubliniensis colonize the periodontal pockets. Med Mycol 46(8):783–793. 10.1080/13693780802060899 [DOI] [PubMed] [Google Scholar]
- 33.Canabarro A, Valle C, Farias MR et al. (2013) Association of subgingival colonization of Candida albicans and other yeasts with severity of chronic periodontitis. J Periodontal Res 48(4):428–432. 10.1111/jre.12022 [DOI] [PubMed] [Google Scholar]
- 34.De-La-Torre J, Quindós G, Marcos-Arias C et al. (2018) Oral Candida colonization in patients with chronic periodontitis. Is there any relationship? Rev Iberoam Micol 35(3):134–139. 10.1016/j.riam.2018.03.005 [DOI] [PubMed] [Google Scholar]
- 35.Suresh Unniachan A, KrishnavilasomJayakumari N, Sethuraman S (2020) Association between Candida species and periodontal disease: a systematic review. Curr Med Mycol 6(2):63–68. 10.18502/CMM.6.2.3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karygianni L, Jähnig A, Schienle S et al. (2013) Initial bacterial adhesion on different yttria-stabilized tetragonal zirconia implant surfaces in vitro. Materials (Basel) 6:5659–5674. 10.3390/ma6125659 [DOI] [PMC free article] [PubMed] [Google Scholar]


