Abstract
Background The clinical results of conservative treatment options for ulnar compression at the elbow have not been clearly determined. The aim of this review was to evaluate available conservative treatment options and their effectiveness for ulnar nerve compression at the elbow.
Methods In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations, a systematic review and meta-analysis of studies was performed. Literature search was performed using Ovid MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL).
Results Of the 1,079 retrieved studies, 20 were eligible for analysis and included 687 cases of ulnar neuropathy at the elbow. Improvement of symptoms was reported in 54% of the cases receiving a steroid/lidocaine injection (95% confidence interval [CI], 41–67) and in 89% of the cases using a splint device (95% CI, 69–99).
Conclusions Conservative management seems to be effective. Both lidocaine/steroid injections and splint devices gave a statistically significant improvement of symptoms and are suitable options for patients who refuse an operative procedure or need a bridge to their surgery. Splinting is preferred over injections, as it shows a higher rate of improvement.
Keywords: ulnar nerve compression syndromes, nerve compression syndromes, conservative treatment
Introduction
Ulnar nerve compression at the elbow is the second most prevalent entrapment neuropathy of the upper limb. The ulnar nerve travels down the medial side of the elbow, through the cubital tunnel, which is the most common location for entrapment of the ulnar nerve. 1 Repeated flexion of the elbow, muscle malformation, or direct compression can be the source of ulnar nerve compression at the elbow. 2 If remained untreated, the ulnar nerve compression at the elbow can lead to chronic loss of sensibility and muscle weakness. 3
Most patients with ulnar nerve compression at the elbow undergo an operative procedure. However, conservative treatments, including splint devices, corticosteroid injections, physical therapy, and nerve gliding movements, have been described. 1 In cases where the risk of operation is high due to patient comorbidities or when patients have to wait a long time before undergoing a procedure, conservative treatment may be a good treatment option or bridge to surgery. The purpose of this article is to evaluate available conservative treatment options for ulnar nerve compression at the elbow and to review their outcomes.
Methods
This review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations. 4
Search Methods for the Identification of Studies
The search strategy was conducted in collaboration with an independent librarian in the databases MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL). The final search was performed in May 2020. In Table 1 , the detailed search methods are displayed. The columns visualize databases that have been used (MEDLINE, Embase, CENTRAL) and the rows are searches with number of hits, stated as results, and the combination of searches. There was no restriction in publication years. Two authors reviewed titles and abstracts of the identified studies, and after selection of relevant studies, the full-text articles were analyzed. Disagreements were resolved by a third reviewer. Cross-referencing took place to identify any additional studies missed in the search.
Table 1. Detailed search methods.
| Ovid MEDLINE In-Process and other nonindexed citations, Ovid MEDLINE Daily, Ovid MEDLINE, and Ovid OLDMEDLINE 1946 to present | Embase 1974 to present | CENTRAL | ||||
|---|---|---|---|---|---|---|
| Search # | Search | Results | Search | Results | Search | Results |
| 1 | Ulnar Nerve.ti,ab,kf. | 6,584 | Ulnar Nerve.ti,ab,kw. | 7,974 | Ulnar nerve | 1,209 |
| 2 | Exp Ulnar Nerve Compression Syndromes/ | 1,053 | Cubital tunnel syndrome/ | 2,583 | ||
| 3 | Exp Nerve Compression Syndromes/ | 21,972 | Exp Nerve Compression Syndromes/ | 13,300 | ||
| 4 | Cubital Tunnel Syndrome*.ti,ab,kf. | 735 | Cubital Tunnel Syndrome*.ti,ab,kw. | 881 | (cubital tunnel syndrome*) | 73 |
| 5 | (Ulnar ADJ5 nerve ADJ5 compress*).ti,ab,kf. | 617 | (Ulnar ADJ5 nerve ADJ5 compress*).ti,ab,kw. | 691 | Ulnar near/5 nerve near/5 compress* | 22 |
| 6 | (Ulnar ADJ3 neuropat*).ti,ab,kf. OR (ulnar ADJ3 nerve ADJ3 entrap*).ti,ab,kf | 1,322 | (Ulnar ADJ3 neuropat*.ti,ab,kw. OR ulnar ADJ3 nerve ADJ3 entrap*).ti,ab,kw. | 1,714 | Ulnar near/3 neuropat* OR ulnar near/3 nerve near/3 entrap* | 45 |
| 7 | Exp Ulnar Neuropathies/ | 1,681 | ||||
| 8 | Exp Compression neuropathy/ | 8,555 | Exp compression/OR neuropathy/ | 83,947 | ||
| 9 | Exp Elbow/OR elbow.ti,ab,kf. | 33,242 | Elbow/OR elbow.ti,ab,kw. | 43,519 | Elbow | 4,174 |
| 10 | 9 AND (1 OR 2 OR 3 OR 5 OR 6 OR 7 OR 8) | 2,731 | 9 AND (1 OR 3 OR 5 OR 6 OR 8) | 3,653 | 9 AND (1 OR 5 OR 6) | 139 |
| 11 | 4 OR 10 | 3,135 | 2 OR 4 OR 10 | 5,290 | 4 OR 10 | 184 |
| 12 | Exp Conservative Treatment/ | 2,826 | Conservative treatment/ | 79,796 | Conservative next treatment | 4,883 |
| 13 | Exp Splints/ | 8,696 | Exp arm splint/ | 67 | ||
| 14 | Splint*.ti,ab,kf. | 14,464 | Splint*.ti,ab,kw. | 15,653 | Splint* | 2,313 |
| 15 | Surgical casts/ | 8,688 | ||||
| 16 | Cast*.ti,ab,kf. | 102,756 | Cast*.ti,ab,kw. | 120,699 | Cast* | 15,992 |
| 17 | Nonoperative.ti,ab,kf. OR non-operative.ti,ab,kf. | 15,164 | (Nonoperative OR non-operative).ti,ab,kw. | 18,895 | Nonoperative OR non-operative | 1692 |
| 18 | Nonsurgical.ti,ab,kf. OR non-surgical.ti,ab,kf. | 26,613 | (Nonsurgical OR non-surgical).ti,ab,kw. | 34,956 | Non-surgical OR nonsurgical | 3,561 |
| 19 | Brace*.ti,ab,kf. | 7,284 | Brace*.ti,ab,kw. | 9,295 | Brace* | 1,829 |
| 20 | Avoiding pressure.ti,ab,kf. OR activity modification.ti,ab,kf. | 634 | (Avoiding pressure OR activity modification).ti,ab,kw. | 762 | Avoiding next pressure OR activity next modulation | 124 |
| 21 | Immobilization.ti,ab,kf. OR immobilisation.ti,ab,kf. | 51,032 | (Immobilization OR immobilization).ti,ab,kw. | 57,722 | Immobilization OR immobilisation | 2891 |
| 22 | Orthoses.ti,ab,kf OR Orthotic Device*.ti,ab,kf. | 3,551 | (Orthoses OR Orthotic Device*).ti,ab,kw. | 4,737 | Orthoses OR Orthotic next Device* | 1,276 |
| 23 | Nerve tap*.ti,ab,kf. | 2 | Nerve tap*.ti,ab,kw. | 2 | Nerve tap* | 694 |
| 24 | Nerve gliding*.ti,ab,kf. | 60 | Nerve gliding*.ti,ab,kw. | 68 | Nerve gliding* | 63 |
| 25 | Segmental joint manipulation*.ti,ab,kf. | 1 | Segmental joint manipulation*.ti,ab,kw. | 1 | Segmental joint manipulation* | 57 |
| 26 | Exercise*.ti,ab,kf. | 292,975 | Exercise*.ti,ab,kw. | 393,355 | Exercise* | 96,994 |
| 27 | Sliding technique*.ti,ab,kf. | 69 | Sliding technique*.ti,ab,kw. | 107 | Sliding next technique | 19 |
| 28 | Neurodynamic mobilization* OR neurodynamic mobilization*.ti,ab,kf. | 25 | (Neurodynamic mobilization* OR neurodynamic mobilization*).ti,ab,kw. | 29 | Neurodynamic next mobilization OR neurodynamic mobilisation | 85 |
| 29 | Corticosteroid*.ti,ab,kf. | 102,425 | Corticosteroid*.ti,ab,kw. | 153,079 | Corticosteroid* | 20,704 |
| 30 | 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 | 510,884 | Exp external splint/ | 377 | 12 OR 14 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 | 126,293 |
| 31 | Exp arm brace/ | 132 | ||||
| 32 | 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 | 708,965 | ||||
| 33 | 11 AND 29 | 279 | 11 AND 29 | 616 | 11 AND 29 | 184 |
Selection Criteria
All randomized controlled trials, prospective or retrospective cohort study, case–control studies, or case series were eligible for inclusion. Studies were selected if they matched the following inclusion criteria: study groups consisted of a minimum of 5 patients, with a minimal age of 18 years, and patients had received a conservative (nonsurgical) treatment for symptoms of ulnar nerve compression at the elbow. All types of conservative treatment were included. Only studies with clearly described outcomes were selected, with at least a distinction between improvement and no improvement.
Exclusion criteria were studies performing animal experiments, cadaver studies, single case reports, or reviews. In Fig. 1 , the selection process is shown. Table 2 provides a summary of the characteristics of the included studies.
Fig. 1.
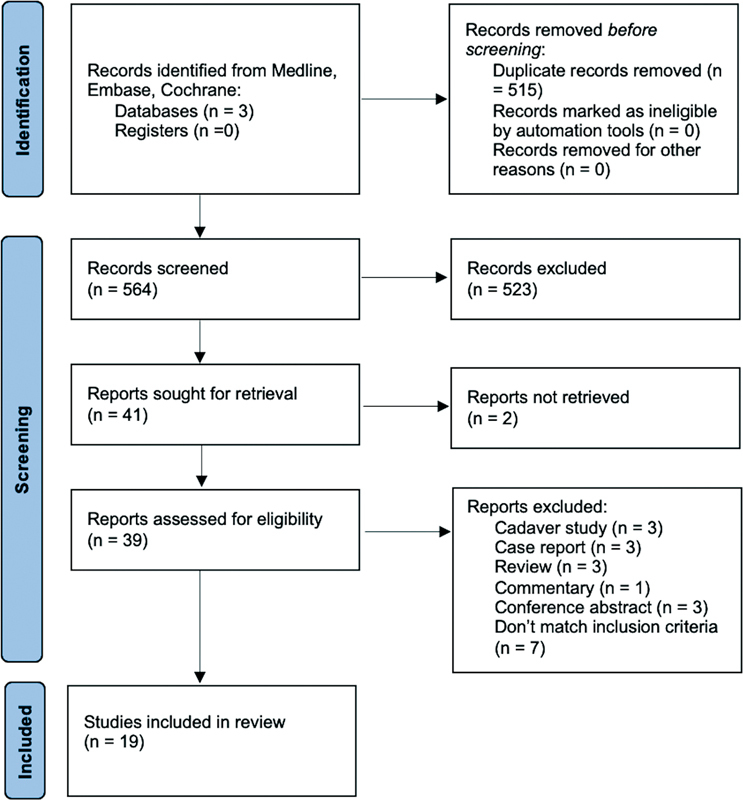
The selection process following the PRISMA 2020 recommendations.
Table 2. Summary of characteristics of studies included.
| # | Authors and year | Title | Journal | Country | Type of study | Number of patients receiving conservative treatment | Level of evidence | Funding or conflict of interest | Methodological quality assessment |
| Injections: | |||||||||
| 1 | Alblas et al, 2012 8 | Injection with corticosteroids (ultrasound guided) in patients with an ulnar neuropathy at the elbow, feasibility study | European Journal of Neurology | The Netherlands | Case series, prospective | 8 | IV | NM | High |
| 2 | Chen et al, 2020 20 | Perineural dextrose and corticosteroid injections for ulnar neuropathy at the elbow: a randomized double-blind trial | Archives of Physical Medicine & Rehabilitation | China | Case–control, prospective | 33 | III | No | High |
| 3 | Choi et al, 2015 9 | Clinical implications of real-time visualized ultrasound-guided injection for the treatment of ulnar neuropathy at the elbow: a pilot study | Annals of Rehabilitation Medicine | Korea | Caser series, prospective | 10 | IV | No | High |
| 4 | Gronbeck et al, 2021 10 | Ultrasound-guided cubital tunnel injection: a review and exploration of utility as a diagnostic aid in mild or nonclassic cubital tunnel patients | Techniques in Orthopaedics | United States | Case series, retrospective | 63 | IV | NM | High |
| 5 | Pechan and Kredba, 1980 11 | Treatment of cubital tunnel syndrome by means of local administration of cortisonoids. II. Long-term follow-up | Acta Universitatis Carolinae, Medical | Czech Republic | Case series, prospective | 14 | IV | NM | Moderate |
| 6 | Rampen et al, 2011 12 | Ultrasound-guided steroid injection to treat mild ulnar neuropathy at the elbow | Muscle & Nerve | The Netherlands | Case series, prospective | 7 | IV | NM | High |
| 7 | vanVeen et al, 2015 21 | Corticosteroid injection in patients with ulnar neuropathy at the elbow: a randomized, double-blind, placebo-controlled trial | Muscle & Nerve | The Netherlands | Case–control, prospective | 30 | III | NM | High |
| Physical therapy: | |||||||||
| 8 | Oskay et al, 2010 13 | Neurodynamic mobilization in the conservative treatment of cubital tunnel syndrome: long-term follow-up of 7 cases | Journal of Manipulative & Physiological Therapeutics | Turkey | Case series, prospective | 7 | IV | No | Moderate |
| 9 | Ozkan et al, 2015 22 | New treatment alternatives in the ulnar neuropathy at the elbow: ultrasound and low-level laser therapy | Acta Neurologica Belgica | Turkey | Case–control, prospective | 32 | III | No | High |
| Splint devices: | |||||||||
| 10 | Dellon et al, 1993 14 | Nonoperative management of cubital tunnel syndrome: an 8-year prospective study | Neurology | United States | Case series, prospective | 121 | IV | NM | High |
| 11 | Hong et al, 1996 23 | Splinting and local steroid injection for the treatment of ulnar neuropathy at the elbow: clinical and electrophysiological evaluation | Archives of Physical Medicine & Rehabilitation | United States | Case–control, prospective | 10 | III | No | High |
| 12 | Michell and Sesath, 2020 15 | Feasibility trial of treatment of ulnar neuropathy at the elbow using a specifically designed splint | JCR: Journal of Clinical Rheumatology | United Kingdom | Case series, prospective | 15 | IV | NM | Moderate |
| 13 | Seror, 1993 16 | Treatment of ulnar nerve palsy at the elbow with a night splint | Journal of Bone & Joint Surgery | France | Case series, prospective | 22 | IV | NM | High |
| 14 | Shah et al, 2013 17 | Outcomes of rigid night splinting and activity modification in the treatment of cubital tunnel syndrome | Journal of Hand Surgery | United States | Case series, prospective | 19 | IV | NM | High |
| 15 | Svernlöv et al, 2009 24 | Conservative treatment of the cubital tunnel syndrome | Journal of Hand Surgery | Sweden | Case–control, prospective | 51 | III | NM | High |
| Other: | |||||||||
| 16 | Beekman et al, 2004 25 | Ulnar neuropathy at the elbow: follow-up and prognostic factors determining outcome | Neurology | Netherlands | Case– control, prospective | NA | III | NM | High |
| 17 | Nakamichi et al, 2009 18 | Patient education for the treatment of ulnar neuropathy at the elbow | Archives of Physical Medicine & Rehabilitation | Japan | Case series, prospective | 77 | IV | No | High |
| 18 | Omejec and Podnar, 2018 26 | Long-term outcomes in patients with ulnar neuropathy at the elbow treated according to the presumed etiology | Clinical Neurophysiology | Slovenia | Case– control, prospective | 67 | III | Yes | High |
| 19 | Padua et al, 2002 19 | Natural history of ulnar entrapment at elbow | Clinical Neurophysiology | Italy | Case series, retrospective | 30 | IV | NM | High |
Abbreviations: NA, no information available; NM, not mentioned.
Quality Assessment
The quality of the included case–control studies was assessed using the “JBI Critical Appraisal Checklist for case-control studies” and the “JBI Critical Appraisal checklist for case series.” 5 These checklists pay attention to selection of the study groups, evaluation of the exposure, and statistical analysis. In the checklist for case–control studies, the comparability of the groups and confounding factors are evaluated. Quality assessment is performed using a score ranging from 0 to 10 points. Studies with a score of 7 to 10 points were considered as high quality, 4 to 6 points as moderate quality, and 0 to 3 as low quality. Two reviewers conducted the quality appraisal. Any disagreements during the process were discussed and resolved by adjudication by a third reviewer.
Data Extraction
Data were independently extracted by two reviewers. The following data were extracted from the studies: total number of patients, gender, affected arm (dominant/nondominant), duration of symptoms until the start of treatment, type of conservative treatment, total duration of treatment, subjective and objective outcome measurements for pain, sensory or motor function improvement after the conservative treatment, advantages and disadvantages described by the authors, complications, and other features. In cases of different interpretations, the results were discussed again by the two reviewers and resolved by involvement of a third reviewer.
Statistical Analysis
The I 2 statistic was determined to measure study heterogeneity. The cutoff value for low, moderate, and high heterogeneity is set at 25, 50, and 75%, respectively. 6 When possible and appropriate, a random-effects model was used to pool proportions of individual studies in the subgroups. This was done for the subgroup injections and splint devices, with the exception of studies reporting no individual response rates. Because I 2 was moderate to high in both subgroups, random-effects models were used for further analyses. Results are presented as mean values or 95% confidence intervals. A p -value of ≤0.05 was considered statistically significant. Forest and funnel plots for both subgroup analyses were created for optimal visualization of the results. No additional analyses were done. Statistical analyses were performed using MedCalc for Windows, version 19.3.1 (MedCalc Software, Ostend, Belgium). 7
Results
Initially, 1,079 studies were identified. A total of 515 duplicates were removed, and the remaining 564 titles and abstracts were screened for suitability. Forty-one studies were selected and the full texts were read. Nineteen papers were included in the final analysis. Screening the reference lists did not provide inclusion of additional studies. The selection process flow diagram with reasons for exclusion is shown in Fig. 1 . Of the included studies, 12 were level IV evidence, 8 9 10 11 12 13 14 15 16 17 18 19 whereas 7 were level III 20 21 22 23 24 25 26 ( Table 2 ). Methodological quality varied among the studies: 16 studies were considered as high quality and 3 studies as moderate quality. Also, 63% ( n = 12) of the studies did not mention if they were funded, while 32% ( n = 6) of the studies explicitly stated no funding. One study reported funding, but declared this had no role in collection, analysis, and interpretation of data and in writing of the manuscript. 26 A total of 682 patients, including 684 arms, were followed-up after receiving a treatment for ulnar nerve compression at the elbow. One study included a patient group receiving surgical management, without stating the exact number of patients involved. 25 In studies describing the following parameters, patients had a mean age of 48.7 years, the dominant side was involved in 64% of patients (198 of 313), and the minimal follow-up period was an average of 6.2 months (range: 1–124 months). Six studies included patients with mild-to-moderate symptoms, 10 12 15 17 20 24 while 13 studies included patients with any severity of symptoms. 8 9 11 13 14 16 18 19 21 22 23 25 26 The most common interventions, from most to least common, included education and activity modification, steroid/lidocaine injection, splinting, physical therapy, pulsed ultrasound (US), or laser therapy. The most commonly reported outcomes included subjective clinical and patient-reported outcomes, such as patient-reported VAS scores, symptoms, questionnaires, and clinical signs, followed by nerve conduction studies and US examination. Two studies only reported subjective outcomes, 10 15 while 17 studies reported on a combination of subjective and objective outcome measurements. 8 9 11 12 13 14 16 17 18 19 20 21 22 23 24 25 26 Subgroup meta-analyses were performed on the injection and the splint devices studies. Oskay et al reported that 100% of patients ( n = 7) had improvement of symptoms after physical therapy with an average follow-up period of 12 months, specifically after neurodynamic mobilization therapy in combination with US therapy. 13 Ozkan et al stated that 69% of the patients ( n = 32) had improvement of symptoms 3 months after starting US or low-level laser therapy (LLLT). 22 The duration of symptoms was between 5 weeks and 6 months at the start of these physical therapies. Nakamichi et al stated that 59% of arms ( n = 80) had improvement of symptoms 3 months after education about the pathophysiology and activity modification. 18 Beekman et al and Omejec et al reported that, respectively, 35 and 82% of the arms ( n = 46 and 67) had improvement of symptoms after an average period of 22,8 months after starting to avoid risky positioning of the affected limb, and Padua et al described that 40% of the arms ( n = 30) had improvement of their symptoms after 6 to 19 months of only giving information about what ulnar nerve entrapment at the elbow is and how to avoid risky positioning. 19 25 26 Beekman et al reported an average duration of symptoms of 3.5 months before the start of activity modification. 25 Omejec et al and Padua et al did not mention the duration of symptoms. 19 26 Study data are presented in Table 3 .
Table 3. Summary of data in studies included in the systematic review.
| # | Authors and year | No. of patients in conservative group/no. of cases | Males (no. [%])/females (no. [%]) | Mean age in years (range) | Severity of the included cases a | Mean FU in years (range) | No. of cases improved (%)/no. of patients not improved (%) |
|---|---|---|---|---|---|---|---|
| Injections | |||||||
| 1 | Alblas et al, 2012 8 | 8/9 | 4 (50)/4 (50) | 53 (43–67) | U | 0.25 (NA) | 5 (56)/4 (44) |
| 2 | Chen et al, 2020 20 | 33/33 | 11 (33)/22 (67) | 56 (32–77) | Mild-to-moderate | 0.5 (NA) | 17 (52)/16 (48) |
| 3 | Choi et al, 2015 9 | 10/10 | 7 (70)/3 (30) | 63 (57–58) | U | 0.1 (NA) | NA (significant drop in VAS) |
| 4 | Gronbeck et al, 2021 10 | NA/56 | NA | 47 (NA) | Mild | NA (0.1–0.25) | 38 (68)/18 (32) |
| 5 | Pechan and Kredba, 1980 11 | 14/22 | 6 (43)/8 (57) | 41 (25–65) | Mild | 1.2 (0.5–NA) | 14 (64)/8 (36) |
| 6 | Rampen et al, 2011 12 | 7/7 | 6 (86)/1 (14) | 43 (32–54) | Mild | 0.13 (NA) | 4 (57)/3 (43) |
| 7 | vanVeen et al, 2015 21 | 30/30 | 18 (60)/12 (40) | 56 (29–91) | U | 0.25 (NA) | 9 (30)/21 (70) |
| Physical therapy: | |||||||
| 8 | Oskay et al, 2010 13 | 7/7 | NA | NA (35–70) | U | 1.0 (NA) | 7 (100)/0 (0) |
| 9 | Ozkan et al, 2015 22 | 32/32 | 16 (50)/16 (50) | 44 (NA) | U | 0.25 (NA) | 22 (69)/10 (31) |
| Splint devices: | |||||||
| 10 | Dellon et al, 1993 14 | 121/121 | 23 (19)/98 (81) | 44 (15–72) | U | 4.9 (1.0–10.3) | 85 (70)/36 (30) |
| 11 | Hong et al, 1996 23 | 10/12 | 10 (100)/0 (0) | 59 (37–70) | U | 0.5 (NA) | NA (significant improvement in symptoms) |
| 12 | Michell and Sesath, 2020 15 | 15/15 | 4 (27)/11 (73) | 41 (21–84) | Mild-to-moderate | 0.15 (0.13–0.4) | 11 (73)/4 (27) |
| 13 | Seror, 1993 16 | 22/22 | 12 (55)/10 (45) | 52 (39–81) | U | 0.9 (0.3–2.5) | 22 (100)/0 (0) |
| 14 | Shah et al, 2013 17 | 19/24 | 8 (42)/11 (58) | 43 (21–72) | Mild-to-moderate | 2.0 (1.3–2.7) | 21 (88)/3 (12) |
| 15 | Svernlöv et al, 2009 24 | 51/51 | 24 (47)/27 (53) | 43 (17–72) | Mild-to-moderate | 0.5 (NA) | 51 (100)/0 (0) |
| Other: | |||||||
| 16 | Beekman et al, 2004 25 | NA/46 | NA | 51 (39–60) | U | 1.2 (6–NA) | 16 (35)/30 (65) |
| 17 | Nakamichi et al, 2009 18 | 77/80 | 56 (73)/21 (27) | 52 (19–77) | U | NA (3–NA) | 59 (74)/21 (26) |
| 18 | Omejec and Podnar, 2018 26 | 67/67 | 33 (49)/34 (51) | 47 (19–75) | U | 2.4 (2.2–3.4) | 55 (82)/12 (18) |
| 19 | Padua et al, 2002 19 | 27/30 | 11 (41)/16 (59) | 57 (32–76) | U | NA (0.5–1.6) | 12 (40)/18 (60) |
Abbreviations: FU, follow-up; NA, no information available; U, unspecified; VAS, visual analog scale.
The severity of the included cases was defined by the authors of the included studies.
Physical Therapy, Ultrasound, and Laser Therapy
Neurodynamic mobilization in combination with US therapy was reported to be a beneficial therapy for all patients. Oskay et al stated that these therapies are viable options for the treatment of ulnar neuropathy at the elbow. 13 Ozkan et al saw significant improvement in patients after treating them with either US or LLLT. 22 More severely affected patients were pooled in the US group, so they reason that this therapy might be superior to LLLT.
Meta-analysis
Injections
In our meta-analysis of the outcomes of conservative therapy for ulnar nerve compression at the elbow, a statistically significant proportion of patients improved after a steroid/lidocaine injection.
Pooled results of six studies in the injections subgroup showed that 54% of the patients (95% confidence interval [CI], 41–67) improved after an average period of 4.3 months after receiving a steroid/lidocaine injection for ulnar nerve compression at the elbow. The duration of symptoms before injection was 2 to 36 months. The I 2 was 59% (95% CI, 0–83). Forest and funnel plot are shown in Fig. 2 , and detailed calculations are shown in Table 4 .
Fig. 2.
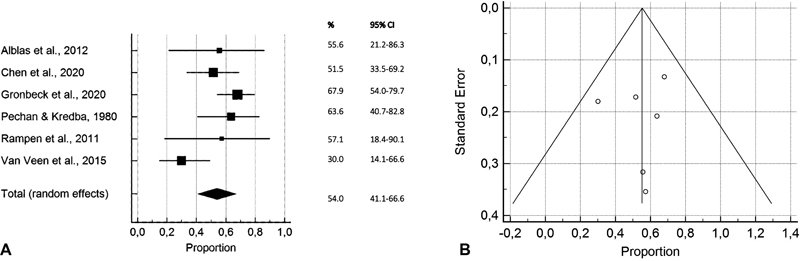
Forest plot ( A ) and funnel plot ( B ) showing pooled results of overall symptomatic improvement in proportions of improved patients in the injections subgroup with 95% CIs per included study.
Table 4. Exact calculations and tests for heterogeneity corresponding with Figs. 2 and 3 .
| Author and year | Sample size (no. of cases) | Proportion (%) | 95% CI | Weight (%) random effects |
|---|---|---|---|---|
| Fig. 2 : pooled results of overall symptomatic improvement in proportions of improved patients in the injections subgroup | ||||
| Alblas et al, 2012 8 | 9 | 55,556 | 21,201–86,300 | 11.02 |
| Chen et al, 2020 20 | 33 | 51,515 | 33,544–69,204 | 19.93 |
| Gronbeck et al, 2021 10 | 56 | 67,857 | 54,036–79,715 | 23.07 |
| Pechan and Kredba, 1980 11 | 22 | 63,636 | 40,658–82,802 | 17.17 |
| Rampen et al, 2011 12 | 7 | 57,143 | 18,405–90,101 | 9.51 |
| vanVeen et al, 2015 21 | 30 | 30,000 | 14,735–49,396 | 19.30 |
| Total (random effects) | 157 | 54,009 | 41,135–66,617 | 100.00 |
| Fig. 3 : pooled results of overall symptomatic improvement in proportions of improved patients in the splint devices subgroup | ||||
| Dellon et al, 1993 14 | 121 | 70,248 | 61,262–78,215 | 21.77 |
| Michell and Sesath, 2020 15 | 15 | 73,333 | 44,900–92,213 | 18.30 |
| Seror, 1993 16 | 22 | 100,000 | 84,563–100,000 | 19.38 |
| Shah et al, 2013 17 | 24 | 87,500 | 67,639–97,344 | 19.59 |
| Svernlöv et al, 2009 24 | 51 | 100,000 | 93,022–100,000 | 20.96 |
| Total (random effects) | 235 | 89,000 | 69,729–99,128 | 100 |
Splinting
Pooled results of five studies in the splint devices subgroup showed that 89% of the patients (95% CI, 69–99) improved using a splint device for ulnar nerve compression at the elbow for an average period of 18.7 months. 14 I 2 was 92% (95% CI, 84–96). Forest and funnel plot are shown in Fig. 3 , and detailed calculations are shown in Table 4 . The duration of symptoms before starting the usage of a splint was 0.5 to 72 months. All studies used an elbow brace that prevented elbow flexion. Dellon et al, Seror, Shah et al, and Svernlöv et al used a nighttime splint, 14 16 17 24 while Hong et al and Michell and Sesath recommended to wear the splints as much as possible. 15 23 The splints consisted of a variety of materials, including neoprene, polyform, and thermoplastic. 14 23 24 Michell and Sesath designed the Cambridge Ulnar Splint, with a plastic exoskeleton, for their study. 15
Fig. 3.
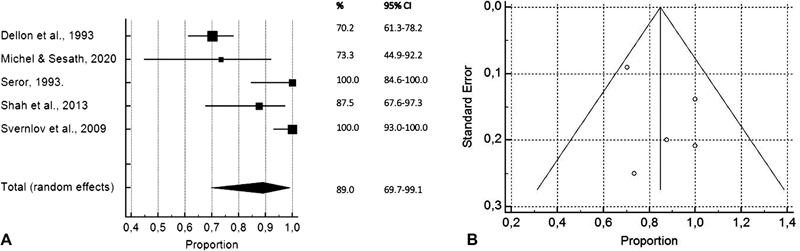
Forest plot ( A ) and funnel plot ( B ) showing pooled results of overall symptomatic improvement in proportions of improved patients in the splint devices subgroup with 95% CIs per included study.
Discussion
In this systematic review, we evaluated available conservative treatment options for ulnar nerve compression at the elbow and reviewed the effectiveness and complications of the options. Of the 1,079 retrieved studies, 19 were eligible for analysis and included a total of 682 patients and 684 cases of ulnar neuropathy at the elbow. Improvement of symptoms was reported in 54% of the cases receiving a steroid/lidocaine injection (95% CI, 41–67). Improvement of symptoms was reported in 89% of the cases using a splint device (95% CI, 69–99).
The results of the subgroup meta-analyses show the proportions of patients with improvement of symptoms, but not how much they improved. The inability to determine the amount of the improvement is due to the wide variety of outcome measures used in the included studies (e.g., subjective clinical and patient-reported outcomes, nerve conduction studies, and US examination).
All the studies included in this systematic review described improvement in symptoms after education, information about avoiding risky positioning of the elbow, or both. Nakamichi et al described this treatment to be effective, inexpensive, and simple, with no contraindications. It can be started immediately after diagnosis. 18 Since there were no control groups in any of these studies, where patients received no information at all, improvement due to the natural course of ulnar neuropathy at the elbow cannot be ruled out.
In our meta-analysis, a statistically significant proportion of patients using a splint device for ulnar nerve compression at the elbow improved. Michell and Sesath presented it to be a comfortable, effective, and cost-effective treatment option. 15 Seror and Svernlöv et al report that even patients with severe and long-lasting symptoms benefited from wearing a splint. 16 24 Hong et al compared wearing a splint with an additional steroid injection and detected no supplementary effect of the injection. 23 This brings us to a curious point where it is the question if the placebo effect or natural course of ulnar nerve compression at the elbow might not be inadequate.
A major flaw of the study is the lack of preoperative clinical data. The severity of the clinical situation is not exactly known. Six studies only included patients with mild-to-moderate symptoms, while 13 studies included patients with any severity of symptoms. However, it is possible that patients with more severe symptoms were offered or opted for surgery earlier. Different patient populations are compared, and different treatment durations, follow-up periods, compliances, and outcome measures are reported in the included studies. Duration of symptoms in the included studies is not clearly stated, so no conclusion could be drawn on the natural course of ulnar neuropathy at the elbow.
It cannot be denied that bias might be introduced especially due to the lack of a proper control group and small samples. Dropouts in the included studies are likely to be patients who are experiencing no effect from conservative treatment options, so effectiveness of the investigated treatment could be overrated in some of the included studies. This might be overcome by developing a proper randomized clinical trial comparing some kind of conservative treatment with no treatment.
Conservative management for ulnar neuropathy at the elbow seems to improve symptoms in up to 9 out of 10 patients. Both lidocaine/steroid injections and splint devices gave a significant improvement in symptoms and are suitable options for patients who refuse an operative procedure or need a bridge to this treatment. Physical therapy also seems to be a promising option but needs to be investigated further in larger samples to draw any conclusions on the overall effectiveness. Also, the education and activity modification gave a positive effect on the symptoms and form a simple way to start any treatment for ulnar neuropathy at the elbow. In cases where surgical treatment is not applicable to patients due to comorbidities, it is tempting to advise education in combination with activity modification. This might be followed or combined with further splinting. However, the limitations of this study should be taken into consideration.
Acknowledgments
We thank Alice Tillema for her assistance with database searches.
Conflict of Interest None declared.
Note
The authors received no financial support for the research, authorship, and/or publication of this article.
Authors' Contributions
T.N.: conceptualization, data curation, formal analysis, investigation, methodology, writing – original draft, writing – review and editing.
M.S.v.d.W.: Conceptualization, data curation, formal analysis, investigation, methodology, writing – original draft, writing – review and editing.
E.P.H.: Conceptualization, methodology, writing – original draft, writing – review and editing.
N.J.S.: Data curation, formal analysis, investigation, methodology, writing – original draft.
E.T.W.: Conceptualization, project administration, supervision, writing – review and editing.
R.H.M.A.B.: Conceptualization, investigation, methodology, project administration, resources, supervision, writing – review and editing.
References
- 1.Caliandro P, La Torre G, Padua R, Giannini F, Padua L. Treatment for ulnar neuropathy at the elbow. Cochrane Database Syst Rev. 2016;11:CD006839. doi: 10.1002/14651858.CD006839.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson L, Dengler J, Moore A M. Nerve entrapments. Clin Plast Surg. 2020;47(02):267–278. doi: 10.1016/j.cps.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Boone S, Gelberman R H, Calfee R P.The management of cubital tunnel syndrome J Hand Surg Am 201540091897–1904., quiz 1904 [DOI] [PubMed] [Google Scholar]
- 4.Page M J, McKenzie J E, Bossuyt P M. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moola S, Munn Z, Tufanaru C . The Joanna Briggs Institute; 2017. Systematic reviews of etiology and risk. [DOI] [PubMed] [Google Scholar]
- 6.Higgins J PT, Thompson S G, Deeks J J, Altman D G.Measuring inconsistency in meta-analyses BMJ 2003327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostend, Belgium: MedCalc Software Ltd;2020
- 8.Alblas C L, van Kasteel V, Jellema K. Injection with corticosteroids (ultrasound guided) in patients with an ulnar neuropathy at the elbow, feasibility study. Eur J Neurol. 2012;19(12):1582–1584. doi: 10.1111/j.1468-1331.2012.03676.x. [DOI] [PubMed] [Google Scholar]
- 9.Choi C K, Lee H S, Kwon J Y, Lee W J. Clinical implications of real-time visualized ultrasound-guided injection for the treatment of ulnar neuropathy at the elbow: a pilot study. Ann Rehabil Med. 2015;39(02):176–182. doi: 10.5535/arm.2015.39.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gronbeck C, Wolf J, Rodner C M. Ultrasound-guided cubital tunnel injection: a review and exploration of utility as a diagnostic aid in mild or nonclassic cubital tunnel patients. Tech Orthop. 2021;36(03):301–306. [Google Scholar]
- 11.Pechan J, Kredba J.Treatment of cubital tunnel syndrome by means of local administration of cortisonoids. II. Long-term follow-up Acta Univ Carol [Med] (Praha) 198026(3–4):135–140. [PubMed] [Google Scholar]
- 12.Rampen A J, Wirtz P W, Tavy D L. Ultrasound-guided steroid injection to treat mild ulnar neuropathy at the elbow. Muscle Nerve. 2011;44(01):128–130. doi: 10.1002/mus.22091. [DOI] [PubMed] [Google Scholar]
- 13.Oskay D, Meriç A, Kirdi N, Firat T, Ayhan C, Leblebicioğlu G. Neurodynamic mobilization in the conservative treatment of cubital tunnel syndrome: long-term follow-up of 7 cases. J Manipulative Physiol Ther. 2010;33(02):156–163. doi: 10.1016/j.jmpt.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Dellon A L, Hament W, Gittelshon A. Nonoperative management of cubital tunnel syndrome: an 8-year prospective study. Neurology. 1993;43(09):1673–1677. doi: 10.1212/wnl.43.9.1673. [DOI] [PubMed] [Google Scholar]
- 15.Michell A W, Sesath H GR. Feasibility trial of treatment of ulnar neuropathy at the elbow using a specifically designed splint. J Clin Rheumatol. 2020;26(01):37–39. doi: 10.1097/RHU.0000000000000828. [DOI] [PubMed] [Google Scholar]
- 16.Seror P. Treatment of ulnar nerve palsy at the elbow with a night splint. J Bone Joint Surg Br. 1993;75(02):322–327. doi: 10.1302/0301-620X.75B2.8444959. [DOI] [PubMed] [Google Scholar]
- 17.Shah C M, Calfee R P, Gelberman R H, Goldfarb C A. Outcomes of rigid night splinting and activity modification in the treatment of cubital tunnel syndrome. J Hand Surg Am. 2013;38(06):1125–11300. doi: 10.1016/j.jhsa.2013.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamichi K, Tachibana S, Ida M, Yamamoto S. Patient education for the treatment of ulnar neuropathy at the elbow. Arch Phys Med Rehabil. 2009;90(11):1839–1845. doi: 10.1016/j.apmr.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Padua L, Aprile I, Caliandro P, Foschini M, Mazza S, Tonali P. Natural history of ulnar entrapment at elbow. Clin Neurophysiol. 2002;113(12):1980–1984. doi: 10.1016/s1388-2457(02)00295-x. [DOI] [PubMed] [Google Scholar]
- 20.Chen L C, Ho T Y, Shen Y P. Perineural dextrose and corticosteroid injections for ulnar neuropathy at the elbow: a randomized double-blind trial. Arch Phys Med Rehabil. 2020;101(08):1296–1303. doi: 10.1016/j.apmr.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 21.vanVeen K E, Alblas K C, Alons I M. Corticosteroid injection in patients with ulnar neuropathy at the elbow: a randomized, double-blind, placebo-controlled trial. Muscle Nerve. 2015;52(03):380–385. doi: 10.1002/mus.24551. [DOI] [PubMed] [Google Scholar]
- 22.Ozkan F U, Saygı E K, Senol S. New treatment alternatives in the ulnar neuropathy at the elbow: ultrasound and low-level laser therapy. Acta Neurol Belg. 2015;115(03):355–360. doi: 10.1007/s13760-014-0377-9. [DOI] [PubMed] [Google Scholar]
- 23.Hong C Z, Long H A, Kanakamedala R V, Chang Y M, Yates L. Splinting and local steroid injection for the treatment of ulnar neuropathy at the elbow: clinical and electrophysiological evaluation. Arch Phys Med Rehabil. 1996;77(06):573–577. doi: 10.1016/s0003-9993(96)90297-x. [DOI] [PubMed] [Google Scholar]
- 24.Svernlöv B, Larsson M, Rehn K, Adolfsson L. Conservative treatment of the cubital tunnel syndrome. J Hand Surg Eur Vol. 2009;34(02):201–207. doi: 10.1177/1753193408098480. [DOI] [PubMed] [Google Scholar]
- 25.Beekman R, Wokke J H, Schoemaker M C, Lee M L, Visser L H. Ulnar neuropathy at the elbow: follow-up and prognostic factors determining outcome. Neurology. 2004;63(09):1675–1680. doi: 10.1212/01.wnl.0000142535.24626.90. [DOI] [PubMed] [Google Scholar]
- 26.Omejec G, Podnar S. Long-term outcomes in patients with ulnar neuropathy at the elbow treated according to the presumed aetiology. Clin Neurophysiol. 2018;129(08):1763–1769. doi: 10.1016/j.clinph.2018.04.753. [DOI] [PubMed] [Google Scholar]


