Abstract

Supriya Mallick
Introduction Malignant gliomas are the most common primary malignant brain tumors and are typically treated with maximal safe surgical resection followed by chemoradiation. One of the unintended effects of radiation is depletion of circulating lymphocyte pool, which has been correlated with inferior overall survival outcomes.
Methods A comprehensive and systematic searches of the PubMed, Cochrane Central, and Embase databases were done to assess the studies that have reported radiation-related lymphopenia in high-grade gliomas. Hazard ratios (HRs), odds ratios (OR), and mean differences were represented with Forest plots comparing patients with severe lymphopenia and no severe lymphopenia. Review Manager Version 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark) was used for the analysis.
Results Nineteen studies were included in the final systematic review and 12 studies were included in the meta-analysis. The odds of developing severe lymphopenia were 0.39 (95% CI:0.19, 0.81, I 2 = 94%, p = 0.01). Patients with severe lymphopenia were at increased risk of death with a pooled HR = 2.19 (95% CI: 1.70, 2.83, I 2 = 0%, p <0.00001) compared to patients with no severe lymphopenia. The mean difference in survival between patients with severe lymphopenia and no severe lymphopenia was −6.72 months (95% CI: −8.95, −4.49, I 2 = 99%, p <0.00001), with a better mean survival in the no severe lymphopenia group.
Conclusion Radiation-induced severe lymphopenia was associated with poor overall survival and increased risk of death. Photon therapy, larger planning target volume, higher brain dose, higher hypothalamus dose, and female gender were associated with increased risk of severe lymphopenia.
Keywords: radiation, lymphopenia, high-grade gliomas, systematic review
Introduction
Malignant gliomas are the most common primary malignant brain tumors, which account for more than 80% of central nervous system (CNS) tumors. 1 2 Among these, high-grade gliomas (HGG) are the most resistant and challenging solid tumors with dismal long-term outcomes. Despite standard of care multimodality treatment consisting of maximal safe surgical resection and adjuvant concurrent chemoradiation, the median survival is 12 to 24 months. 3 4 5 Adjuvant radiation and chemotherapy are both associated with treatment-induced lymphopenia. Lymphocytes play a crucial role in regulating immune response against cancer cells. 6 7 8 It is estimated that in patients with high-grade glioma, after a single fraction, 0.5 Gy is delivered to 5% of the circulating blood cell pool, and after 30 fractions of conventional treatment 99% of blood cells have received ≥0.5 Gy. 9 Lymphocytes are a highly radiosensitive with a D 10 of approximately 3 Gy and D 50 of approximately 2 Gy. 10 The radiosensitivity among lymphocytes is heterogenous, with B lymphocytes slightly more radiosensitive than T lymphocytes. Irradiation of the circulating pool of blood cells results in lymphopenia and is associated with poor outcomes, including worse survival, in high-grade gliomas and other solid malignancies. Various studies have been attempted and the results have shown a relationship between radiation-induced lymphopenia and poor survival outcomes in high-grade glioma. Here we performed a systematic review and meta-analysis of radiation-induced lymphopenia in high-grade gliomas assessing the impact of lymphopenia on tumor control and survival outcomes.
Methods
Data Search
The PubMed (National Institutes of Health), Cochrane Central (Cochrane collaboration), and Embase (Elsevier) databases were queried with the following search terms – radiation; Gliomas; lymphopenia, and survival. The search strategy with keywords is provided in Supplementary Material S1 (available online only). The duration of the search was from the inception of each database up to September 6, 2020. The search was performed on September 6, 2020.
Non-Database Search Methods
Conference proceedings from the American Society of Radiation Oncology, European Society of Therapeutic Radiation Oncology, European Society of Medical Oncology, and American Society of Clinical Oncology conferences for the timeframe 2000 to 2020 was reviewed to identify additional articles. The search did not have a language filter. B.P.V. and R.U. did the search independently and any disagreements were resolved by mutual discussion.
Eligibility Criteria for Articles
Inclusion criteria were: (1) any prospective clinical trial, retrospective study, cohort study of gliomas in humans, (2) radiation should have been part of treatment and intent had to be in the neoadjuvant, definitive, or adjuvant settings and (3) the study should have data on cancer-specific outcomes and treatment-related lymphopenia.
Exclusion criteria: (1) preclinical models; (2) studies on lymphopenia in patients undergoing immunotherapy or chemotherapy or surgery alone; (3) Any radioactive nucleotides; (4) studies reporting outcomes in HIV positive patients or immunodeficiency states.
Study Review
The search process was done in a systematic manner consistent with the PRISMA flow diagram as shown in Fig. 1 . The articles were reviewed by B.P.V. and R.U. The duplicates were excluded, and the titles of articles were evaluated. The articles found to be relevant to the topic of interest were shortlisted and the full-length paper was assessed for the eligibility criteria. The included study references were cross searched for additional studies.
Fig. 1.
PRISMA flow diagram depicting the search strategy in the systematic review literature search.
Statistical Analysis
Categorical variables were presented as percentages and continuous variables were presented as medians with interquartile range. Hazard ratios (HR), odds ratios (OR), and mean differences were represented with corresponding Forest plots comparing patients with severe lymphopenia and no severe lymphopenia which were reported with 95% CI and p -value <0.05 was considered statistically significant. The Forest plot for HR and mean difference was plotted by Generic inverse variance method, OR by Mantel-Haenszel method and p -value from the Z -test to examine whether the pooled estimate of effect is statistically significant. The random-effects model described by Der Simonian and Laird was used for analysis. Study heterogeneity was assessed using the inconsistency index ( I 2 -statistic) with values of 0 to 30%, 31 to 60%, 61 to 75%, and 76 to 100% indicating low, moderate, substantial, and considerable heterogeneity, respectively. Review Manager Version 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark) was used for the analysis.
Search Results
The systematic search of literature resulted in 1,240 articles of which 101 articles underwent complete review. Nineteen studies were included in the final systematic review and 12 studies were included in the meta-analysis. Table 1 summarizes the details of studies reporting on radiation-related lymphopenia outcomes in HGG.
Table 1. Summarizes the details of studies reporting on radiation related lymphopenia outcomes in high-grade glioma.
| First author, Year |
Study design | No. of patients | Definition of lymphopenia | Dosimetric correlation | Results |
|---|---|---|---|---|---|
| Ishikawa et al 2010 Japan |
Retrospective | 28 | CTC version 3.0 | Pre RT/CT lymphocyte count <1,200/mm 3 predictive of severe lymphopenia during RT/CT | |
| Grossman et al 2011 United States |
Prospective- | 96 | CD4 count <200 cells/m 3 | CD4 count at 2 mo< 200 cells/mm 3 had shorter median survival (13.1 m vs. 19.7) ( p = 0.002) | |
| Huang et al 2015 United States |
Retrospective | 183 | TLC of <500/mL | Higher Brain V25Gy> 56% increased risk of severe lymphopenia |
Worse overall survival in severe lymphopenia (median: 12.5 vs. 20.2 mo,
p
< 0.001).
Female sex, older age, lower baseline TLC significant predictors of ASL |
|
Mendez
45
2017 United States |
Retrospective | 72 | CTCAE V 4.03 | TLC <500 cells/mm 3 at 2 mo had a shorter survival | |
|
Campian et al
46
2017 United States |
Prospective | 20 | CTCAE, CD4< 200 | ||
| Rudra et al 2018 United States |
Retrospective | 217 | <500 cells/µL | Brain V25Gy was an independent predictor of ASL | |
| Byun et al 2019 Korea |
Retrospective | 336 | TLC of <500/μL | IMRT and smaller PTV are associated with decreased severe lymphopenia | |
| Hui et al 2019 United States |
Retrospective | 319 | TLC of <500/μL | High dose dexamethasone (>2 mg/d) was associated with higher rates of ASL and worse OS | |
| Ye et al 2019 China |
Retrospective | 148 | PCL < 500/μL |
WBD
max
≥34 Gy
WBD min ≥2 Gy Hypothalamus D max ≥56 Gy associated with increased risk of severe lymphopenia |
Older age, high-grade glioma, HT D max , WBD min , WBD max associated inferior survival |
|
Lee et al
47
2020 Korea |
Retrospective | 186 | TLC< 1,000/μL | Female sex, dexamethasone > 2 mg/d is associated with acute lymphopenia | |
| Mohan et al 2021 United States |
Prospective | 84 | ALC< 500/μL | Higher WBV20 strongest predictor of G3 lymphopenia | Female, photon therapy, baseline ALC associated with severe lymphopenia |
Abbreviations: ALC, absolute lymphocyte count; PCL, plasma cell leukemia; PTV, planning target volume; TLC, thin-layer chromatography; WBD, whole brain dose.
Results
Pooled Analysis of Outcomes of Radiation-Related Lymphopenia
Twelve studies reported the rates of severe lymphopenia. The odds of developing severe lymphopenia are 0.39 (95% CI: 0.19, 0.81, I 2 = 94%, p = 0.01). Four studies reported on the survival outcomes in patients with severe lymphopenia. The patients with severe lymphopenia were at increased risk of death with a pooled HR = 2.19 (95% CI: 1.70, 2.83, I 2 = 0%, p <0.00001) compared to patients with no severe lymphopenia. The mean difference in survival between patients with severe lymphopenia and no severe lymphopenia is −6.72 months (95% CI: −8.95, −4.49, I 2 = 99%, p <0.00001), with a 6.72 month better mean survival in the no severe lymphopenia group. The Forest plots of the above reported outcomes are shown in Fig. 2A Fig. 2B Fig. 2C with corresponding funnel plots shown in Supplementary Material S1 (available online only).
Fig. 2.
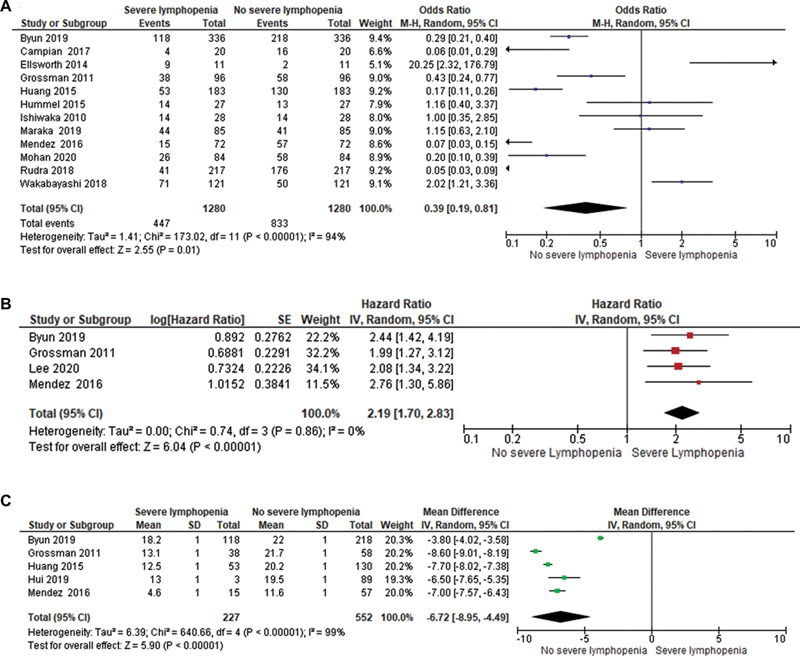
( A ) Forest plot of odds ratio of developing severe lymphopenia. ( B ) Forest plot of pooled hazard ratios of overall survival between patients with severe lymphopenia and no severe lymphopenia. ( C ) Forest plot of mean difference between patients with severe lymphopenia and no severe lymphopenia. The Forest plot for HR and mean difference was plotted by generic inverse variance method; OR by Mantel-Haenszel method and p -value from the Z -test to examine whether the pooled estimate of effect is statistically significant. CI, confidence interval.
Impact of Baseline ALC Value on the Incidence of Lymphopenia
Mohan et al 11 found that baseline ALC was the strongest predictor of G3 + lymphopenia (OR 0.18, 95% CI 0.05‒0.51, p = 0.003). The baseline ALC values in the G3 + L arm was 1.3 ± 0.6 × 103/μL and 1.7 ± 0.5 × 103/μL in the no lymphopenia arm ( p <0.001). Huang et al 12 also revealed lower baseline thin-layer chromatography (TLC) is a predictor of acute severe lymphopenia (ASL) (OR: 0.92; 95% CI: 0.87–0.98, p = 0.009), however, the optimal threshold was not established. Ishikawa et al 13 showed similar results with a lower peripheral lymphocyte count before radiotherapy and temozolomide of <1,200/μL was associated with severe lymphopenia ( p < 0.032).
Impact of Steroid on the Incidence of Lymphopenia
The commonly used steroid during the treatment course of brain tumors is dexamethasone. Dexamethasone has an innate property to reduce lymphocyte count. Hui et al 14 performed retrospective study on 319 patients to analyze the impact of dexamethasone on lymphopenia. It was found that high dose dexamethasone (>2 mg/d) was independently associated with significantly higher ASL. Similar results were seen in the study by Lee et al, which also showed dexamethasone >2 mg/d during chemoradiation and till 4 weeks after completion was associated with lymphopenia (HR: 2.85, p = 0.032). On the contrary, the study by Mohan et al, Huang et al, and Rudra et al 15 did not find any association between lymphopenia and steroid use.
Impact of Sex on Incidence of Lymphopenia
In a study by Huang et al, female sex was a significant predictor of ASL, with a higher risk of G3 + L. The ASL at 3 months, for females with brain V25 ≥56Gy, is 56 versus 25% for males and for females with V25 <56% was 39 versus 10% for males, respectively. Similar results were seen in the study by Mohan et al where women had higher rates of G3 + L, 51 versus 15% ( p < 0.001), and multivariate analysis revealed female gender as a significant predictor of G3 + L. Lee et al also found female sex was independently associated with lymphopenia ( p = 0.003). Rudra et al showed women to be an independent predictor of ASL. Byun et al 16 also had similar results with female sex being an independent factor associated with increased ASL.
Impact of Dosimetric Parameters and Incidence of Lymphopenia
Impact of Target Volumes on Lymphopenia
Byun et al showed a direct correlation with planning target volume (PTV) size and ASL. Larger PTV volume was independently associated with increased risk of ASL ( p = 0.042) and use of IMRT was independently associated with decreased risk of ASL ( p = 0.015). The rate of ASL with PTV of <200 and > 600 mL was 14 and 33% in IMRT as compared to 25% arm 53% in the 3DCRT arm, respectively. After propensity score matching the ASL in IMRT was 20.4 and 37.2% in the 3DCRT arm ( p = 0.005).
Impact of Brain Dose on Incidence of Lymphopenia
In a phase II randomized study by Mohan et al with 84 patients, the Pearson correlation matrix showed whole brain mean dose (WBMD) and brain volumes V5-V30 were associated with risk of ASL. Multivariate logistic regression analysis showed brain V20 as a significant predictor for ASL ( p = 0.002). The investigators developed a model for plan optimization which showed the risk of G3+ L could be restricted to 20% by limiting V20 to 32% for women and 58% for men. Rudra et al also showed a similar association, with higher brain V25 having a higher risk of ASL. Rate of ASL with V25< 40% was 12.6% compared with 35.7% for V25 >40% ( p = 0.01). The study by Ye et al, 17 showed a positive correlation with high WBMD mean ( p = 0.006), high WBMD mean >34 Gy ( p = 0.022), and high hypothalamus maximum point dose ≥56 Gy ( p = 0.022) with significantly increased risk of ASL. Retrospective analysis of 183 patients by Huang et al also showed higher brain V25 as the most significant predictor for ASL. Multivariable logistic regression analysis showed brainV25 <56% to be associated with lesser ASL ( p = 0.03). V25 <56% has an ASL rates of 20 versus 38% if above 56% ( p = 0.006).
Impact of Proton Therapy on the Incidence of Lymphopenia
Proton beam therapy has the unique nature to minimize the low dose bath to non-target tissue and thus reduces the integral dose. This may help to spare the circulating peripheral blood pool containing lymphocytes from radiation dose. In a study by Mohan et al, it was shown that G3+ lymphopenia was strongly associated with WBMD and brain V5-40. The proton therapy was able to reduce the WBMD ( p <0.001) and brain V5-V30 significantly as compared to the photon group and was associated with a lower G3+ lymphopenia. The G3+ lymphopenia was lower in proton therapy (14%) as compared to photon (39%) ( p = 0.024). The decline in mean ALC from baseline over time was greater for photons, but recovery after treatment was at the same rate for both proton and photon. Table 2 provides a summary of the prognostic factors associated with increased risk of lymphopenia in Brain tumors.
Table 2. Prognostic factors associated with increased risk of severe lymphopenia.
| Photon (39%) treatment as compared to proton (14%) therapy |
| Larger PTV |
| 3 DCRT (37%) as compared to IMRT (20%) |
| Higher brain V 25 >40 Gy |
| High whole brain mean dose (WBMD) >34 Gy |
| Higher whole brain minimum dose |
| Higher hypothalamus D max ≥56 Gy |
| Female gender |
| Lower baseline TLC |
Abbreviations: PTV, planning target volume; TLC, thin-layer chromatography.
Discussion
For high-grade glioma, surgical resection and adjuvant chemoradiation followed by chemotherapy for 6 to 12 cycles remains the standard of care. Despite recent advances in treatment modality, there was no significant improvement in outcomes for high-grade glioma. Recent studies have shown that radiation-induced lymphopenia is correlated with poor prognosis and inferior survival outcomes in patients with HGG. Similar results have been seen in other solid tumors such as cancers of the esophagus, 18 lung, 19 breast, 20 pancreas, 21 22 head and neck 23 and pelvis. 24 25 Multiple rationales have been proposed for radiation-induced lymphopenia or depletion of T cells. Some of these are irradiation of bone marrow, thymic irradiation, and irradiation of a circulating pool of lymphocytes. For brain tumors with limited radiation fields, the bone marrow and thymus are not in the irradiated area, and thus both these reasons do not hold responsible for any lymphopenia seen in high-grade gliomas. Given the fact that in gliomas we have historically moved away from whole brain or hemi brain irradiation to small margins as well as evolution of IMRT for radiation fields, the degree of radiation dose spill off to the skull bone marrow may be minimal to cause significant lymphocyte depletion. However, irradiation of circulating pool of lymphocytes can be proposed for severe lymphopenia, 26 thereby influencing the tumor infiltrating lymphocytes (TILs).
Tumor cells can be attached by the host immune system in a process called immunological surveillance. The brain was considered immune privileged because of blood–brain barrier, 27 and tumor cells can proliferate without any resistance from the immune system. With newer studies the brain is no longer considered as immune-privileged. It is considered that immune cells can traverse through epithelial layer of choroid plexus, through endothelial fenestration in meninges or directly through the meningeal blood vessels. The glioma tumor microenvironment consists of tumor cells, immune cells/lymphocytes, endothelial cells, and cytokines. The TIL is mainly composed of CD8+ cytotoxic T lymphocytes (CTLs), CD4 + T helper cells, regulatory T cells (Tregs), and other immune cells including glioma infiltrating microglia and macrophage (GAMs), natural killer) cells, myeloid-derived suppressor cells (MDSCs), and dendritic cells. 6 28 In the initial phase of tumor development immune system is capable of identifying and eliminating the cancer cells. But in later phase by immunoediting and release of immunosuppressive agents by tumor cells the cancer cells escape the immune surveillance mechanism.
In high-grade glioma an immuno-suppressive tumor microenvironment is created by high levels of Treg cells, 29 30 inhibitory cytokines, 31 checkpoint molecules, 32 and lack of T-cell activation. GAMs and MDSCs are reported in higher density in HGGs and have the ability to attract Treg lymphocytes and correlate inversely with survival. CD4+ cells are associated with tumor grade with 39% in WHO grade II tumors to 73% in grade III and 98% in grade IV tumors. 33 In glioblastoma tumor infiltration with T cells (CD3 + , CD8 + ) is associated with increased survival ( p = 0.027) and is independent of age, postoperative status, and MGMT promoter methylation status. 34 On the other hand, Tregs are immunosuppressive cells which have an important role in tumor recurrence and poor survival. 29 35 Treg is not seen in normal brain but seen in increased number in the tumor microenvironment of gliomas. 36 Tregs act through various direct and indirect mechanisms in the downregulation of T lymphocytes (CD4 + , CD8 + ). There is higher expression of Tregs in GBM with a mean of 24.7% Treg among the glioma-infiltrating lymphocytes and these were absent in control brain specimen ( p < 0.01). 37 The fraction of Tregs in peripheral blood was 2.63 times higher than control group ( p = 0.004) and patients with elevated Treg fraction had significant CD4 + T cell proliferative dysfunction ( p < 0.0001). 29 The immune checkpoint programmed cell death ligand 1 (PD-L1) causes direct inhibition of TIL and thereby increased expression of PD-L1 correlates with poor outcome. 38 39 In glioblastoma PD-L1 expression is seen in 2.8 to 32.2% of tumors. 40 41 Studies are ongoing with nivolumab and pembrolizumab (checkpoint inhibitors) in primary and recurrent GBM. Similarly, dendritic cell vaccines have been tried in GBM treatment, which showed an increase in survival from 15.7 to 35.9 months. Recent analysis showed a positive objective response of 15.6% with DC vaccine in patients with high-grade glioma. 42
Systemic T-cell number and function is depleted in GBM. 24.7% of treatment naïve GBM patients had lymphopenia compared to the control group (lymphocyte count< 1,000 cells/µL). There was a significant reduction approximately 15% in both CD4+ and CD8+ T cell counts (CD4 <200 cells/µL) in peripheral blood pool. 43 In study by Grossman et al, 44 about 40% of patients had CD4+ count less than 200 cells/mm 3 and patients with CD4+ count <200 cells/mm 3 at 2 months after chemoradiation had shorter survival ( p = 0.002) and early death from tumor progression. The adjusted HR for death was 1.66 for the patient with ASL.
Pooled analysis of the impact of severe lymphopenia on overall survival in our study showed that patients with severe lymphopenia were at increased risk of death with HR of 2.19 when compared to patients with no severe lymphopenia. The mean survival was increased in no severe lymphopenia group by 6.72 months. By identifying the factors influencing severe lymphopenia and modifying them to reduce the incidence of ASL can result in improved survival outcomes. We found various clinical and treatment-related factors influencing ASL. Understanding dosimetric parameters that can predict the incidence of severe lymphopenia can assist during plan optimization to reduce the impact on lymphopenia and thereby the outcomes.
A larger PTV and photon beam therapy was significantly associated with higher rates of severe lymphopenia. IMRT showed significantly lesser lymphopenia compared to 3DCRT. Higher brain V25Gy dose and higher WBMD were correlated with ASL. Low dose brain volumes V5-V30 were associated with severe lymphopenia. Apart from the whole brain dose, a higher hypothalamus dose was significantly associated with lymphopenia and had an impact on inferior overall survival in a single study. Use of high dose steroids also correlated with significantly higher severe lymphopenia, but studies were conflicting. IMRT has the ability to sculpt the radiation to the given target of interest and hence may reduce the high dose regions but the low dose spill off may be more compared to 3DCRT. Given the fact that higher dose regions V25Gy and WBMD are the factors predictive of lymphopenia, IMRT can lessen the propensity for lymphocyte depletion.
This study is not without limitations. The analysis included retrospective studies, which can bring bias and heterogeneity among the studies present. There was no clear data on the impact of chemotherapy on lymphopenia which might be a potential confounding variable as well as there was not enough data on the impact of hypofractionated radiotherapy on lymphopenia outcomes. The impact of chemotherapy such as TMZ on lymphopenia and survival outcomes would not be assessed from the data available. The studies also have significant heterogeneity which is again a limitation of the study. But this present study forms the first meta-analysis conducted on the effect of lymphopenia in high-grade gliomas. Future randomized prospective studies can be conducted, taking into consideration various predictive factors mentioned in the study.
Conclusion
Radiation-induced severe lymphopenia was associated with poor overall survival and increased risk of death in patients with HGGs. Photon therapy, IMRT compared to proton therapy, larger PTV, higher brain dose, higher hypothalamus dose, and female gender were associated with increased risk of severe lymphopenia. Severe lymphopenia and reduced immune cells (CD4 + , CD8 + ) following chemoradiation correlated with worse outcomes. These findings, if validated, may offer an avenue to explore to improve outcomes in this patient population.
Acknowledgment
None.
Funding Statement
Funding None.
Footnotes
Conflict of Interest None declared. A.A.S. served on advisory boards for Novocure in 2019.
Supplementary Material
References
- 1.Dolecek T A, Propp J M, Stroup N E, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro-oncol. 2012;14 05:v1–v49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Omuro A, DeAngelis L M. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 3.European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups ; National Cancer Institute of Canada Clinical Trials Group . Stupp R, Mason W P, van den Bent M J. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups ; National Cancer Institute of Canada Clinical Trials Group . Stupp R, Hegi M E, Mason W P. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(05):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 5.Dong X, Noorbakhsh A, Hirshman B R. Survival trends of grade I, II, and III astrocytoma patients and associated clinical practice patterns between 1999 and 2010: a SEER-based analysis. Neurooncol Pract. 2016;3(01):29–38. doi: 10.1093/nop/npv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohr J, Ratliff T, Huppertz A. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clin Cancer Res. 2011;17(13):4296–4308. doi: 10.1158/1078-0432.CCR-10-2557. [DOI] [PubMed] [Google Scholar]
- 7.Swann J B, Smyth M J. Immune surveillance of tumors. J Clin Invest. 2007;117(05):1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellman I, Coukos G, Dranoff G.Cancer immunotherapy comes of age Nature 2011480(7378):480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yovino S, Kleinberg L, Grossman S A, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31(02):140–144. doi: 10.3109/07357907.2012.762780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trowell O A. The sensitivity of lymphocytes to ionising radiation. J Pathol Bacteriol. 1952;64(04):687–704. doi: 10.1002/path.1700640403. [DOI] [PubMed] [Google Scholar]
- 11.Mohan R, Liu A Y, Brown P D. Proton therapy reduces the likelihood of high-grade radiation-induced lymphopenia in glioblastoma patients: phase II randomized study of protons vs photons. Neuro-oncol. 2021;23(02):284–294. doi: 10.1093/neuonc/noaa182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang J, DeWees T A, Badiyan S N. Clinical and dosimetric predictors of acute severe lymphopenia during radiation therapy and concurrent temozolomide for high-grade glioma. Int J Radiat Oncol Biol Phys. 2015;92(05):1000–1007. doi: 10.1016/j.ijrobp.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa E, Yamamoto T, Sakamoto N. Low peripheral lymphocyte count before focal radiotherapy plus concomitant temozolomide predicts severe lymphopenia during malignant glioma treatment. Neurol Med Chir (Tokyo) 2010;50(08):638–644. doi: 10.2176/nmc.50.638. [DOI] [PubMed] [Google Scholar]
- 14.Hui C Y, Rudra S, Ma S, Campian J L, Huang J. Impact of overall corticosteroid exposure during chemoradiotherapy on lymphopenia and survival of glioblastoma patients. J Neurooncol. 2019;143(01):129–136. doi: 10.1007/s11060-019-03146-7. [DOI] [PubMed] [Google Scholar]
- 15.Rudra S, Hui C, Rao Y J. Effect of radiation treatment volume reduction on lymphopenia in patients receiving chemoradiotherapy for glioblastoma. Int J Radiat Oncol Biol Phys. 2018;101(01):217–225. doi: 10.1016/j.ijrobp.2018.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byun H K, Kim N, Yoon H I. Clinical predictors of radiation-induced lymphopenia in patients receiving chemoradiation for glioblastoma: clinical usefulness of intensity-modulated radiotherapy in the immuno-oncology era. Radiat Oncol. 2019;14(01):51. doi: 10.1186/s13014-019-1256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye L L, Fan X W, Hu C S. Dosimetry of the brain and hypothalamus predicting acute lymphopenia and the survival of glioma patients with postoperative radiotherapy. Cancer Med. 2019;8(06):2759–2768. doi: 10.1002/cam4.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davuluri R, Jiang W, Fang P. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2017;99(01):128–135. doi: 10.1016/j.ijrobp.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 19.Campian J L, Ye X, Brock M, Grossman S A. Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer. Cancer Invest. 2013;31(03):183–188. doi: 10.3109/07357907.2013.767342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okita R, Saeki T, Takashima S, Yamaguchi Y, Toge T. CD4+CD25+ regulatory T cells in the peripheral blood of patients with breast cancer and non-small cell lung cancer. Oncol Rep. 2005;14(05):1269–1273. [PubMed] [Google Scholar]
- 21.Wild A T, Ye X, Ellsworth S G. The association between chemoradiation related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma. Am J Clin Oncol. 2015;38(03):259–265. doi: 10.1097/COC.0b013e3182940ff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balmanoukian A, Ye X, Herman J, Laheru D, Grossman S A. The association between treatment-related lymphopenia and survival in newly diagnosed patients with resected adenocarcinoma of the pancreas. Cancer Invest. 2012;30(08):571–576. doi: 10.3109/07357907.2012.700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campian J L, Sarai G, Ye X, Marur S, Grossman S A. Association between severe treatment-related lymphopenia and progression-free survival in patients with newly diagnosed squamous cell head and neck cancer. Head Neck. 2014;36(12):1747–1753. doi: 10.1002/hed.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho O, Chun M, Chang S J, Oh Y T, Noh O K. Prognostic value of severe lymphopenia during pelvic concurrent chemoradiotherapy in cervical cancer. Anticancer Res. 2016;36(07):3541–3547. [PubMed] [Google Scholar]
- 25.Wu E S, Oduyebo T, Cobb L P. Lymphopenia and its association with survival in patients with locally advanced cervical cancer. Gynecol Oncol. 2016;140(01):76–82. doi: 10.1016/j.ygyno.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harisiadis L, Kopelson G, Chang C H. Lymphopenia caused by cranial irradiation in children receiving craniospinal radiotherapy. Cancer. 1977;40(03):1102–1108. doi: 10.1002/1097-0142(197709)40:3<1102::aid-cncr2820400319>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 27.Medawar P B. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29(01):58–69. [PMC free article] [PubMed] [Google Scholar]
- 28.Bowman R L, Klemm F, Akkari L. Macrophage ontogeny underlies differences in tumor-specific education in brain malignancies. Cell Rep. 2016;17(09):2445–2459. doi: 10.1016/j.celrep.2016.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fecci P E, Mitchell D A, Whitesides J F. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(06):3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 30.El Andaloussi A, Lesniak M S. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro-oncol. 2006;8(03):234–243. doi: 10.1215/15228517-2006-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nitta T, Hishii M, Sato K, Okumura K.Selective expression of interleukin-10 gene within glioblastoma multiforme Brain Res 1994649(1-2):122–128. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs J F, Idema A J, Bol K F. Regulatory T cells and the PD-L1/PD-1 pathway mediate immune suppression in malignant human brain tumors. Neuro-oncol. 2009;11(04):394–402. doi: 10.1215/15228517-2008-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heimberger A B, Abou-Ghazal M, Reina-Ortiz C. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14(16):5166–5172. doi: 10.1158/1078-0432.CCR-08-0320. [DOI] [PubMed] [Google Scholar]
- 34.Kmiecik J, Poli A, Brons N HC.Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level J Neuroimmunol 2013264(1-2):71–83. [DOI] [PubMed] [Google Scholar]
- 35.Sayour E J, McLendon P, McLendon R. Increased proportion of FoxP3+ regulatory T cells in tumor infiltrating lymphocytes is associated with tumor recurrence and reduced survival in patients with glioblastoma. Cancer Immunol Immunother. 2015;64(04):419–427. doi: 10.1007/s00262-014-1651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grauer O M, Nierkens S, Bennink E. CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int J Cancer. 2007;121(01):95–105. doi: 10.1002/ijc.22607. [DOI] [PubMed] [Google Scholar]
- 37.Sonabend A M, Rolle C E, Lesniak M S.The role of regulatory T cells in malignant glioma Anticancer Res 200828(2B):1143–1150. [PubMed] [Google Scholar]
- 38.Nduom E K, Wei J, Yaghi N K. PD-L1 expression and prognostic impact in glioblastoma. Neuro-oncol. 2016;18(02):195–205. doi: 10.1093/neuonc/nov172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hao C, Chen G, Zhao H. PD-L1 expression in glioblastoma, the clinical and prognostic significance: a systematic literature review and meta-analysis. Front Oncol. 2020;10:1015. doi: 10.3389/fonc.2020.01015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berghoff A S, Kiesel B, Widhalm G. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro-oncol. 2015;17(08):1064–1075. doi: 10.1093/neuonc/nou307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z, Zhang C, Liu X. Molecular and clinical characterization of PD-L1 expression at transcriptional level via 976 samples of brain glioma. OncoImmunology. 2016;5(11):e1196310. doi: 10.1080/2162402X.2016.1196310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao J-X, Zhang X-Y, Liu J-L. Clinical efficacy of tumor antigen-pulsed DC treatment for high-grade glioma patients: evidence from a meta-analysis. PLoS One. 2014;9(09):e107173. doi: 10.1371/journal.pone.0107173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chongsathidkiet P, Jackson C, Koyama S. S1P1 loss mediates T-cell sequestration in bone marrow amidst glioblastoma. Nat Med. 2018;24(09):1459–1468. doi: 10.1038/s41591-018-0135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.NABTT CNS Consortium . Grossman S A, Ye X, Lesser G. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendez J S, Govindan A, Leong J, Gao F, Huang J, Campian J L.Association between treatment-related lymphopenia and overall survival in elderly patients with newly diagnosed glioblastoma J Neurooncol [Internet] 2016. 2016 Apr [cited 2022 Jul 3]1270232935.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4783226/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campian J L, Ye X, Gladstone D E, Ambady P, Nirschl T R, Borrello I.Pre-radiation lymphocyte harvesting and post-radiation reinfusion in patients with newly diagnosed high grade gliomasJ Neurooncol [Internet] 2015 Sep [cited 2022 Jul 3];124(2):30716. Available from:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4696006/ [DOI] [PMC free article] [PubMed]
- 47.Lee C, Ahn S, Park J S, Song J H, Hong Y K, Jeun S S.Effect of Cumulative Dexamethasone Dose during Concomitant Chemoradiation on Lymphopenia in Patients with Newly Diagnosed Glioblastoma Brain Tumor Res Treat [Internet] 2020. Oct [cited 2021 Mar 20]802716.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7595853/ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


