Abstract

Negine Paul
Introduction Traditionally, the concept of complete omentectomy during gastric resection for cancer was based on lymphatic drainage and the occurrence of occult omental metastasis (OM). However, recent emerging evidence has challenged this concept of complete omentectomy. We, therefore, aim to find the incidence and risk factors of occult OM and also evaluate the outcome of patients with and without such metastasis.
Methods This is a single institutional, retrospective study of patients with gastric cancer who underwent curative radical gastrectomy for a period of 3 years (April 1, 2016, to March 31, 2019). A complete omentectomy was performed in all patients and the omentum and nodal stations were dissected in the resected specimen and sent for pathological analysis. Clinical and epidemiological data were collected from the hospital patient database and analysis was done.
Results A total of 185 patients have been included in the study, with a mean age of 53.84 years. Twenty of the 185 patients had OM (10.8%). Age, sex, location of the tumor, and neoadjuvant chemotherapy were not statistically significant in predicting OM. However, tumor size and tumor depth were found to have a significant association with OM. The occurrence of OM was more likely to be associated with disease recurrence, especially in the peritoneum. The mean overall survival was 38.15 months (±3.33 SD), whereas patients with OM had lower survival, 23.31 months (±7.79 SD), with a p -value of 0.012.
Conclusion OM was not encountered in T1 and T2 gastric cancers and the incidence of OM in T3 and T4 tumors was approximately 12.7%. Therefore, complete omentectomy may be omitted in early T1/T2 tumors. OM was associated with poor prognosis, increased peritoneal recurrence, and decreased overall survival, in spite of a complete omentectomy, and may serve as a prognostic indicator for disease recurrence and overall survival.
Keywords: omentectomy, omental metastasis, gastric cancer, gastrectomy, omental nodes
Introduction
Gastric cancer (GC) is the fifth most common cancer and the fourth most common cause of cancer-related mortality in 2020 (GLOBOCON 2020). 1 The omentum is a frequent site for GC metastasis. Omentobursectomy as part of radical gastrectomy was first reported by Groves et al in 1910. 2 Bursectomy has long been controversial, with questions being raised about its role in reducing disease recurrence. Most centers in the world omit the bursectomy part of the gastric resectional surgery, as recent studies have been clear in the fact that bursectomy does not have any survival benefit when done in gastrectomy. 3 4 5 6 The attention has now been turned towards the role of omentectomy in GC. The omentum as such is not an indispensable organ with claims of regenerative properties and its role in reducing postoperative complications. 7 8 Also, in minimally invasive gastrectomy, a complete omentectomy is technically difficult and time-consuming. 9 Hence, there is a trend toward omentum-preserving gastrectomy, especially in early GCs. Recent Japanese guidelines recommend leaving behind the omentum in T1 and T2 tumors, whereas the European Society for Medical Oncology (ESMO) guidelines do not comment on omentectomy at all. 10 11 However, there is still a legitimate concern about oncological safety while leaving part of the omentum behind due to the possibility of occult tumor deposits in the greater omentum. In this context of debate concerning the role of complete omentectomy for GC, it is worthwhile to identify clinicopathological predictors of occult omental metastasis (OM), which may help to tailor the extent of omentectomy during radical gastrectomy. This study aimed to evaluate the incidence of and predictors for OM in patients with GC receiving curative gastrectomy.
Patients and Methods
This is a single institutional, retrospective study of patients with GC who underwent radical gastrectomy in the upper gastrointestinal (GI) surgical unit for a period of 3 years from April 2016 to March 2019. The diagnosis of GC was confirmed using gastroscopy and biopsy. After adequate preoperative staging work-up, patients were discussed in the upper GI multidisciplinary tumor board meeting. All patients underwent a staging laparoscopy as part of their staging work-up and had either perioperative chemotherapy or adjuvant chemotherapy with surgical resection. Patients who had a visible gross peritoneal or omental disease at staging laparoscopy were staged as metastatic and were therefore not included in the study. Also excluded were patients who were found inoperable during the planned gastrectomy and those receiving palliative resection and surgery for stump cancers.
During the study period, our protocol was to dissect the resected specimen and send the omentum, nodal stations, and stomach for pathological analysis separately. The omentum was cut just distal to the gastroepiploic arcade ( Fig. 1 ). Data were collected from the patient database after approval from institutional review board (IRB Min no. 12999) and the need for informed consent was waived, considering the retrospective nature of the study. Follow-up data were collected from the outpatient charts and also from telephonic interviews. Our primary objective was to find out the incidence of OM (number of patients who had tumor deposits in the omentum or metastatic omental nodes). Our secondary objective was to evaluate factors that can predict metastasis to the omentum. The preoperative T stage was calculated based on contrast-enhanced computed tomography (CECT) images and also staging laparoscopy. In case of a discrepancy between the two, the higher T stage was taken. Overall survival (OS) was defined as the time interval between treatment initiation (surgery or neoadjuvant chemotherapy [NACT]) and death and censored at the last day of follow-up.
Fig. 1.
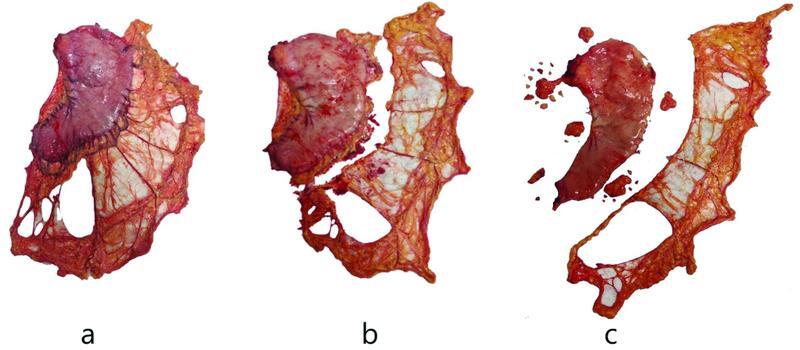
Showing dissection of the omentum from resected stomach. ( a ) Resected stomach with the omentum. ( b ) The omentum cut just distal to the gastroepiploic arcade. ( c ) Dissected stomach, omentum with perigastric lymph nodal stations.
Statistical Analysis
Descriptive statistics such as mean and standard deviation were used for continuous variables. Frequency and percentage were used for categorical variables. To find the association between categorical variables, a chi-square test/Fisher's exact test was used. The cumulative probability of survival was estimated using the Kaplan–Meier method for OS, and to compare two or more survival curves, a log-rank test was used. The study variables that were significant at <0.05 levels in univariate analysis were included in a multivariate Cox proportional hazards model. The model assumption was verified using log–log S ( t ) plots and a global test. p -Value < 0.05 was considered statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS), version 21.0 (IBM Corp., Armonk, NY, USA)/STATA 16.
Results
Of the total 185 patients in the study, 127 were male and 58 were female patients. The mean age of the patient group was 53.84 ± 11.82 years. OM was found in 20 patients (10.8%), of whom 10 had positive omental nodes and 10 had tumor deposits in the omentum. The primary tumors were frequently located in the distal stomach (65.9%), were predominantly cT3/4 (87.3%) or pN + ve (70.3%), and were largely poorly differentiated type (60.0%). Seventy-one (38.4%) patients received NACT. All operations were performed through an open approach. The baseline demographic characteristics of the study cohort are summarized in Table 1 .
Table 1. Baseline clinicodemographic, operative, and pathological characteristics of the study cohort.
| Variables | N = 185 |
|---|---|
| Age (y) | 53.84 ± 11.82 |
| Sex (male:female) | 127:58 |
| Clinical “T” stage | |
| cT1 + cT2 | 27 (14.7%) |
| cT3 | 79 (43.2%) |
| cT4 | 77 (42.1%) |
| Pathological “T” stage | |
| pT0 + pT1 + pT2 | 42 (22.7%) |
| pT3 | 58 (31.35%) |
| pT4 | 85 (45.95%) |
| Pathological “N” stage | |
| pN0 | 55 (29.7%) |
| pN+ | 130 (70.3%) |
| Perioperative chemotherapy | |
| Yes | 71 (38.4%) |
| No | 114 (61.6%) |
| Greater curvature tumors | |
| Yes | 105 (56.8%) |
| No | 80 (43.2%) |
| Subtotal vs total gastrectomy | |
| Subtotal | 122 (65.9%) |
| Total | 63 (34.1%) |
| Completeness of the resection | |
| R0 | 159 (86%) |
| R1 + R2 | 26 (14%) |
| Tumor size (cm) | 4.47 ± 2.63 |
| Tumor depth (cm) | 1.18 ± 0.69 |
| Lymphovascular invasion | |
| Yes | 54 (29.4%) |
| No | 131 (70.6%) |
| Perineural invasion | |
| Yes | 110 (59.5%) |
| No | 75 (40.5%) |
| Perinodal extension | |
| Yes | 22 (11.9%) |
| No | 163 (88.1%) |
| Omental metastasis | |
| Yes | 20 (10.8%) |
| No | 165 (89.2%) |
| Signet ring cell tumor | |
| Yes | 14 (7.6%) |
| No | 171 (92.4%) |
| Histological grade of the tumor | |
| Well/moderately differentiated | 74 (40.0%) |
| Poorly differentiated | 111 (60.0%) |
| Recurrence | |
| Yes | 56 (30.3%) |
| No | 129 (69.7%) |
| Peritoneal recurrence | |
| Yes | 33 (17.8%) |
| No | 152 (82.2%) |
Note: Values expressed as n (%) or mean ± SD.
Age, sex, and location of tumor were not statistically significant in predicting OM. None of the patients with clinical T1 and T2 stage GC had occult omental deposits or nodes. Ninety percent of OM were found in clinical T4 disease and the remaining 10% in T3 disease. Similarly, 85% of pathological T4 stage and 15% of T3 stage showed OM. In patients with cT4 or pT4 disease, the incidence of OM was 23.4 and 20%, respectively. All patients with omental disease had pathological node-positive disease ( Table 2 ).
Table 2. Univariate analysis for factors predicting presence or absence of omental metastasis.
| Variables | Omental metastasis negative ( n = 165) | Omental metastasis positive ( n = 20) | p -Value |
|---|---|---|---|
| Age (y) | 54.15 ± 11.82 | 51.3 ± 11.77 | 0.311 |
| Sex | |||
| Male | 116 (70.3%) | 11 (55.0%) | 0.164 |
| Female | 49 (29.7%) | 9 (45.0%) | |
| Clinical “T” stage | |||
| cT1 + cT2 | 27 (16.6%) | 0 | <0.001 |
| cT3 | 77 (47.2%) | 2 (10%) | |
| cT4 | 59 (36.2%) | 18 (90%) | |
| Pathological “T” stage | |||
| pT0 + pT1 + pT2 | 42 (25.5%) | 0 | 0.001 |
| pT3 | 55 (33.3%) | 3 (15%) | |
| pT4 | 68 (41.2%) | 17 (85%) | |
| Pathological “N” stage | |||
| pN0 | 55 (33.3%) | 0 | 0.002 |
| pN+ | 110 (66.7%) | 20 (100%) | |
| Perioperative chemotherapy | |||
| Yes | 65 (92.5%) | 6 (8.5%) | 0.415 |
| No | 100 (87.7%) | 14 (12.3%) | |
| Subtotal vs total gastrectomy | |||
| Subtotal | 110 (90%) | 12 (10%) | 0.662 |
| Total | 55 (87.3%) | 8 (12.7%) | |
| Greater curvature tumors | |||
| Yes | 91 (86.7%) | 14 (13.3%) | 0.206 |
| No | 74 (92.5%) | 6 (7.5%) | |
| Completeness of the resection | |||
| R0 | 144 (90.6%) | 15 (9.4%) | 0.136 |
| R1 + R2 | 21 (80.8%) | 5 (19.2%) | |
| Tumor size (cm) | 4.21 ± 2.48 | 6.6 ± 2.93 | 0.0001 |
| Tumor depth (cm) | 1.14 ± 0.69 | 1.49 ± 0.68 | 0.038 |
| Lymphovascular invasion | 47 (87%) | 7 (13%) | 0.557 |
| Perineural invasion | 93 (84.5%) | 17 (15.5%) | 0.014 |
| Perinodal extension | 15 (68.2%) | 7 (31.8%) | 0.001 |
| Signet ring cell tumor | 13 (92.9%) | 1 (7.1%) | 0.646 |
| Histological grade of the tumor | |||
| Well/moderately differentiated | 70 (94.6%) | 4 (5.4%) | 0.053 |
| Poorly differentiated | 95 (85.6%) | 16 (14.4%) | |
| Recurrence | 44 (26.67%) | 12 (60%) | 0.002 |
| Peritoneal recurrence | 25 (15.15%) | 8 (40%) | 0.006 |
Note: Values expressed as n (%) or mean ± SD.
As the size and depth of the tumor increase, so does the incidence of OM. Perineural invasion and extranodal extension were associated with an increased risk of OM, while lymphovascular invasion was not. There was no statistical increase in OM with signet ring cells or poorly differentiated tumors. The site of the tumor had no statistical bearing on OM, although the incidence of OM was slightly more in the total gastrectomy group compared with the subtotal gastrectomy group (12.7 vs 10%) and in greater curvature tumors compared with tumors involving other sites (13.3 vs 7.5%). NACT had no statistically significant bearing on the occurrence of OM. The incidence of omental disease was 8.5% in patients who received NACT compared with 12.5% who did not. All patients who had OM in the NACT group had a TRG grade of 2 or higher. Also, the cytology done by peritoneal wash during staging laparoscopy before initiation of NACT was negative for malignant cells in all these patients. Multivariate analysis was not performed owing to the low proportion of patients with OM in each subgroup.
OM patients were more likely to have disease recurrence (60 vs 27%), particularly peritoneal recurrence (40 vs 15%), compared with OM-negative patients. The mean OS of the entire cohort was 38.15 months (±3.33 SD). The 1-year survival rate was 88% and the 2-year survival rate was 69.8%. Patients with OM had a lower mean OS, 23.31 months (±7.79 SD), compared with that of patients without OM, 39.35 months (±3.72 SD) ( Fig. 2a ).
Fig. 2.
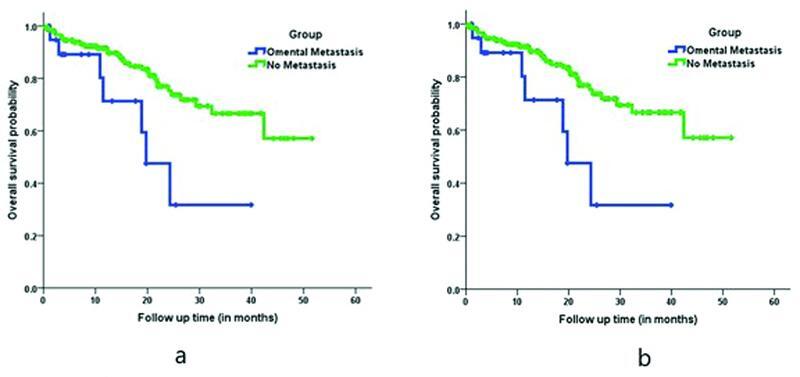
Kaplan–Meier curve. ( a ) Comparison of overall survival in omental metastasis positive versus negative patients. ( b ) Comparison of overall survival in omental metastasis positive versus negative patients in upfront surgery subgroup.
With regard to OS, on univariate analysis, patients with OM were three times more likely to die compared with patients without OM (hazard ratio, 2.89 [1.26–6.65]; p = 0.012). Similarly, pathological T stage, pathological N stage, and grade of tumor adversely affected the OS and were statistically significant. Patients who received chemotherapy were 55% less likely to die compared with the upfront surgery group (hazard ratio, 0.45 [0.22–0.92]; p = 0.030) ( Fig. 3 ). However, on multivariable analysis other than NACT, no other factor was found to affect the OS in a statistically significant way ( Table 3 ).
Fig. 3.
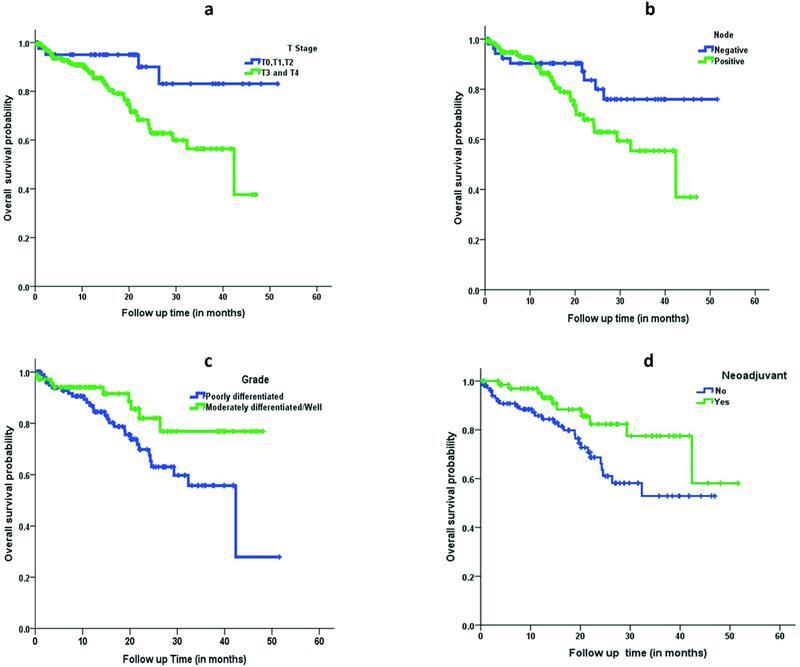
Kaplan–Meier curve. Comparison of overall survival based on ( a ) pathological T stage, ( b ) pathological N stage, ( c ) grade of tumor, and ( d ) neoadjuvant chemotherapy.
Table 3. Univariate and multivariate analysis for factors associated with overall survival.
| Variable | N = 185 | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | p -Value | HR (95% CI) | p -Value | ||
| Age (y) | |||||
| <50 | 65 (35.1%) | 1.00 | 0.124 | – | – |
| ≥50 | 120 (64.9%) | 1.85 (0.85–4.03) | – | ||
| pT stage | |||||
| pT0 + pT1 + pT2 | 42 (22.7%) | 1.00 | 0.018 | 1.00 | 0.152 |
| pT3 + pT4 | 143 (77.3%) | 3.53 (1.24–9.99) | 2.30 (0.74–7.18) | ||
| pN stage | |||||
| pN0 | 55 (29.7%) | 1.00 | 0.056 | 1.00 | 0.773 |
| pN1 + pN2 + pN3 | 130 (70.3%) | 2.08 (0.98–4.42) | 1.13 (0.49–2.62) | ||
| Grade of the tumor | |||||
| Poorly differentiated | 111 (60.0%) | 2.17 (1.02–4.60) | 0.044 | 1.90 (0.86–4.18) | 0.111 |
| Well/moderately differentiated | 74 (40.0%) | 1.00 | 1.00 | ||
| Omental metastasis | |||||
| Yes | 20 (10.8%) | 2.89 (1.26–6.65) | 0.012 | 1.90 (0.80–4.43) | 0.146 |
| No | 165 (89.2%) | 1.00 | 1.00 | ||
| Perioperative chemotherapy | |||||
| Yes | 71 (38.4%) | 0.45 (0.22–0.92) | 0.030 | 0.47 (0.22–0.98) | 0.046 |
| No | 114 (61.6%) | 1.00 | 1.00 | ||
Note: Values expressed as n (%).
After exclusion of the patients who underwent NACT, subgroup analysis was performed on 114 patients who underwent upfront gastrectomy and showed a near-similar trend to that of the entire cohort ( Table 4 ). Clinical T stage, pathological T stage, and pathological nodal stage had a significant predictive value for OM. Also, patients with OM had a high risk of developing recurrence ( p = 0.002) and the OS was less (17.9 months; 95% confidence interval [CI], 13–22.9) compared with patients without OM (34.5 months; 95% CI, 30.2–38.7) ( Fig. 2b ).
Table 4. Univariate analysis for factors predicting omental metastasis in the upfront surgery group.
| Variables | N = 114 | Omental metastasis negative ( n = 100) | Omental metastasis positive ( n = 14) | p -Value |
|---|---|---|---|---|
| Age (y) | 55.75 ± 12.38 | 55.99 ± 12.40 | 54.0 ± 12.55 | 0.576 |
| Sex | ||||
| Male | 79 (69.3%) | 71 (71.0%) | 8 (57.1%) | 0.356 |
| Female | 35 (30.7%) | 29 (29.0%) | 6 (42.9%) | |
| Clinical “T” stage | ||||
| cT1 + cT2 | 20 (17.5%) | 20 (20.0%) | 0 (0.0) | 0.001 |
| cT3 | 31 (27.2%) | 31 (31.0%) | 0 (0.0) | |
| cT4 | 63 (55.3%) | 49 (49.0%) | 14 (100%) | |
| Pathological “T” stage | ||||
| pT0 + pT1 + pT2 | 20 (17.5%) | 20 (20.0%) | 0 (0.0) | 0.001 |
| pT3 | 31 (27.2%) | 31 (31.0%) | 0 (0.0) | |
| pT4 | 63 (55.3%) | 49 (49.0%) | 14 (100.0%) | |
| Pathological “N” stage | ||||
| pN0 | 27 (23.7%) | 27 (27.0%) | 0 (0.0) | 0.038 |
| pN+ | 87 (76.3%) | 73 (73.0%) | 14 (100%) | |
| Subtotal vs total gastrectomy | ||||
| Subtotal | 85 (74.6%) | 75 (75.0%) | 10 (71.4%) | 0.751 |
| Total | 29 (25.4%) | 25 (25.0%) | 4 (28.6%) | |
| Greater curvature tumors | ||||
| No | 42 (36.8%) | 38 (38.0%) | 4 (28.6%) | 0.493 |
| Yes | 72 (63.2%) | 62 (62.0%) | 10 (71.4%) | |
| Tumor size (cm) | 4 (3–6) | 3.95 (3–5.6) | 6 (4.9–8) | 0.002 |
| Tumor depth (cm) | 1.22 ± 0.60 | 1.17 ± 0.60 | 1.57 ± 0.69 | 0.019 |
| Lymphovascular invasion | ||||
| No | 79 (69.3%) | 70 (70.0%) | 9 (64.3%) | 0.759 |
| Yes | 35 (30.7%) | 30 (30.0%) | 5 (35.7%) | |
| Perineural invasion | ||||
| No | 96 (84.2%) | 88 (88.0%) | 8 (57.1%) | 0.009 |
| Yes | 18 (15.8%) | 12 (12.0%) | 6 (42.9%) | |
| Perinodal extension | ||||
| No | 40 (35.1%) | 38 (38.0%) | 2 (14.3%) | 0.133 |
| Yes | 74 (64.9%) | 62 (62.0%) | 12 (85.7%) | |
| Signet ring cell tumor | ||||
| Yes | 5 (4.4%) | 4 (4.0%) | 1 (7.1%) | 0.487 |
| No | 109 (95.6%) | 96 (6.0%) | 13 (92.9%) | |
| Histological grade of the tumor | ||||
| Well to moderately differentiated | 47 (41.2%) | 59 (59.0%) | 10 (71.4%) | 0.537 |
| Poorly differentiated | 67 (58.8%) | 41 (41.0%) | 4 (28.6%) | |
| Recurrence | ||||
| No | 76 (66.7%) | 72 (72.0%) | 4 (28.6%) | 0.002 |
| Yes | 38 (33.3%) | 28 (28.0%) | 10 (71.4%) | |
Note: Values expressed as n (%) or mean ± SD or median (range).
Discussion
The omentum has long been considered as an organ with regenerative properties and its role in containing infections such as in acute appendicitis is well known. 8 12 The greater omentum develops in the eighth week of gestation from the dorsal mesogastrium. 13 It is composed of two mesothelial sheets, which enclose adipocytes and mononuclear phagocytic cells. 14
The greater omentum has various functions. The macrophages in the omentum act as scavengers helping in the rapid absorption and clearance of bacteria and foreign material from the peritoneal cavity. As a site of B-lymphocyte development, they supply leukocytes to the peritoneal cavity. 15 16 17 Also, the omentum can rapidly produce a layer of fibrin by which it adheres and attempts to seal off areas of contamination. 13 17 The omentum has a high content of progenitor cells as well as growth and lipid angiogenic factors; thus, it helps to increase collateral blood flow by inducing neovascularization. This property has made it an ideal tissue to be used in areas that have a compromised vascular supply. 8 18 Complete omentectomy results in the removal of all these peritoneal defense mechanisms. 15 There are recent studies that show increased postoperative complications in patients undergoing complete omentectomy. 7 19
Laparoscopic gastrectomy is being increasingly performed now as its advantages over open gastrectomy have been shown in many randomized clinical trials. 19 20 21 Complete omentectomy during minimally invasive gastrectomy leads to longer operative time and increased risk of adjacent organ injury. 19 22 23 24 25 26
There is reasonable recent evidence showing that routine complete omentectomy in gastrectomy does not improve recurrence or disease-free survival. 21 26 27 28 29 A retrospective analysis of 3,050 patients by Seo et al showed partial omentectomy patients having superior surgical outcomes and 5-year OS compared with the complete omentectomy group. 30 Similarly, a multicentric retrospective analysis by Ri et al involving 1,758 patients also showed comparable outcomes in locally advanced GC patients. 25 A recent meta-analysis by Ishizuka et al, which included eight retrospective studies, showed no improvement in 5-year OS in patients who underwent complete omentectomy for locally advanced GC. 31 The results of the ongoing large randomized controlled trial JCOG 1711 are much awaited. 32
On the other hand, the reason for concern in preserving the omentum is that of oncological safety. The omentum appears to be capable of supporting not only malignant cells in the milky spots but also free intraperitoneal cells. 6 22 33 Omental milky spots can provide a microenvironment for tumor cells to survive, thereby becoming a cancer stem cell niche. 14 33 34 35 The omentum has been observed to be a frequent site of metastatic disease for many malignancies. 14 35
One more issue to be addressed is the possibility of omental necrosis after partial omentectomy as the entire gastroepiploic arcade is cut off especially in total gastrectomy. However, this is not the usual case as evidenced by studies. In the reported series, the incidence of omental infarction is low after partial omentectomy, and even in patients with omental infarction, the symptoms are often mild. 36 37 There is also the suggestion of omental blood supply by collaterals from the superior mesenteric artery through the middle colic artery. 37 This aspect needs to be looked upon by further studies.
There have been recent theories suggesting the omentum to be a part of peritoneum and hence OM to be similar to peritoneal metastasis, thereby making it a metastatic disease. 33 38 The recent Union for International Cancer Control (UICC) TNM classification categorizes noncontiguous omental disease as metastatic disease. 39
The incidence of occult omental disease in gastrectomy patients in our study population (10.8%) was significantly more than those of the Omega study (5%) and Barchi et al (1.8%). 40 41 However, Haverkamp et al reported a similar incidence as ours (10%) in the European population, whereas Metwally et al reported a higher incidence in the Middle Eastern population (31.3%). 24 42 This variability is probably due to differences in patient cohort and inclusion criteria. For example, the Omega study had a lower proportion of T3 and T4 disease compared with our patient cohort, whereas the study by Metwally et al included patients with diffuse carcinomatosis. 40 42 All these studies had one fact in common—none of the patients with T1 or T2 disease had OM. 24 40 41 42
When it comes to OS in patients with OM, Haverkamp et al reported a significant difference in OS, whereas Metwally et al reported no survival difference. 24 42 Especially, none of the patients with OM in the study by Metwally et al developed peritoneal recurrence. 42 Both these studies had a low sample size, which could explain the totally different conclusions.
The primary objective of our study was to look at the incidence of OM in patients undergoing curative surgery for GC. Our hypothesis was that if this number was insignificant, then a complete omentectomy may be unnecessary. Alternatively, if there was a significant number with OM, then there is a compelling case to do complete omentectomy in all patients. The secondary objective was to evaluate the factors predicting OM, thereby helping to preoperatively predict the patients who are likely to have OM, warranting a complete omentectomy.
This study has evidence to suggest that:
Early T-stage tumors, i.e., T1 and T2, are not associated with occult OM.
As the depth of the tumor increases, so does the incidence of OM (T3 vs T4 = 5 vs 20%).
The anatomical location of the tumor in the stomach had no influence on the rate of OM, reiterating the fact that this was a metastatic spread and not a contiguous spread.
The incidence of OM was almost similar regardless of whether the patient underwent NACT or upfront surgery.
In the subgroup of patients who underwent NACT, all patients who developed OM had a poor response (in spite of the NACT) (TRG 2 or 3).
OM was associated with poor prognosis, increased peritoneal recurrence, and decreased OS.
There were a few limitations to our study.
The retrospective nature of the study and the small sample size are some of the limitations of the study. Due to the low proportion of patients with OM in subgroups, a multivariate analysis could not be performed. Also, the proportion of patients with T1 and T2 disease was significantly less compared with T3 and T4 disease in our patient cohort.
A larger randomized controlled trial comparing partial versus complete omentectomy may help substantiate the results of this study.
Conclusion
In this study, occult OM was not seen in T1 and T2 GCs. However, the incidence of OM in T3 and T4 tumors was approximately 12.7%. Therefore, a complete omentectomy may be omitted in early T stage (T1/T2) tumors but may be necessary for T3 and T4 disease. OM was associated with a poor prognosis, with increased potential of peritoneal recurrence and decreased OS. This trend was seen in spite of a complete omentectomy being performed and hence the presence of OM may be used as a prognostic indicator in predicting disease recurrence and OS after resectional surgery for GC.
Acknowledgments
None.
Funding Statement
Funding There was no funding source for this work.
Conflict of Interest None declared.
Ethics Committee Approval
Obtained (IRB Min. No. 12999 dated June 24, 2020).
Consent to Participate
Waiver obtained from the Ethics Committee, considering the retrospective nature of the study.
Authors' Contribution
Conception and design: N. P., I. S.
Acquisition of data: N. P.
Data analysis and interpretation: N. P., S. S., M. T., I. S.
Drafting of the manuscript: N. P., I. S.
Critical appraisal and revision: S. S., M. Y., S. C., I. S.
Overall guarantor of the work: I. S.
Final approval of the version to be published: All authors.
References
- 1.Sung H, Ferlay J, Siegel R L. Global Cancer Statistics 2020 GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(03):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Zhang C D, Yamashita H, Seto Y. Gastric cancer surgery: historical background and perspective in Western countries versus Japan. Ann Transl Med. 2019;7(18):493. doi: 10.21037/atm.2019.08.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurokawa Y, Doki Y, Mizusawa J. Bursectomy versus omentectomy alone for resectable gastric cancer (JCOG1001): a phase 3, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2018;3(07):460–468. doi: 10.1016/S2468-1253(18)30090-6. [DOI] [PubMed] [Google Scholar]
- 4.Osaka University Clinical Research Group for Gastroenterological Study . Hirao M, Kurokawa Y, Fujita J. Long-term outcomes after prophylactic bursectomy in patients with resectable gastric cancer: final analysis of a multicenter randomized controlled trial. Surgery. 2015;157(06):1099–1105. doi: 10.1016/j.surg.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Kochi M, Fujii M, Kanamori N. D2 gastrectomy with versus without bursectomy for gastric cancer. Am J Clin Oncol. 2014;37(03):222–226. doi: 10.1097/COC.0b013e31825eb734. [DOI] [PubMed] [Google Scholar]
- 6.Yamamura Y, Ito S, Mochizuki Y, Nakanishi H, Tatematsu M, Kodera Y. Distribution of free cancer cells in the abdominal cavity suggests limitations of bursectomy as an essential component of radical surgery for gastric carcinoma. Gastric Cancer. 2007;10(01):24–28. doi: 10.1007/s10120-006-0404-5. [DOI] [PubMed] [Google Scholar]
- 7.Ha T K, An J Y, Youn H G, Noh J H, Sohn T S, Kim S. Omentum-preserving gastrectomy for early gastric cancer. World J Surg. 2008;32(08):1703–1708. doi: 10.1007/s00268-008-9598-5. [DOI] [PubMed] [Google Scholar]
- 8.Shah S, Lowery E, Braun R K. Cellular basis of tissue regeneration by omentum. PLoS One. 2012;7(06):e38368. doi: 10.1371/journal.pone.0038368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinoshita T, Kaito A. Current status and future perspectives of laparoscopic radical surgery for advanced gastric cancer. Transl Gastroenterol Hepatol. 2017;2:43. doi: 10.21037/tgh.2017.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ESMO Guidelines Committee . Smyth E C, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27 05:v38–v49. doi: 10.1093/annonc/mdw350. [DOI] [PubMed] [Google Scholar]
- 11.Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2018 (5th edition) Gastric Cancer. 2021;24(01):1–21. doi: 10.1007/s10120-020-01042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Gomez I, Pancholi N, Patel J. Activated omentum slows progression of CKD. J Am Soc Nephrol. 2014;25(06):1270–1281. doi: 10.1681/ASN.2013040387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Platell C, Cooper D, Papadimitriou J M, Hall J C. The omentum. World J Gastroenterol. 2000;6(02):169–176. doi: 10.3748/wjg.v6.i2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koppe M J, Nagtegaal I D, de Wilt J HW, Ceelen W P. Recent insights into the pathophysiology of omental metastases. J Surg Oncol. 2014;110(06):670–675. doi: 10.1002/jso.23681. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Hu J N, Luo N. The essential involvement of the omentum in the peritoneal defensive mechanisms during intra-abdominal sepsis. Front Immunol. 2021;12:631609. doi: 10.3389/fimmu.2021.631609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimotsuma M, Simpson-Morgan M W, Takahashi T, Hagiwara A. Activation of omental milky spots and milky spot macrophages by intraperitoneal administration of a streptococcal preparation, OK-432. Cancer Res. 1992;52(19):5400–5402. [PubMed] [Google Scholar]
- 17.Liebermann-Meffert D.The greater omentum. Anatomy, embryology, and surgical applications Surg Clin North Am 20008001275–293., xii [DOI] [PubMed] [Google Scholar]
- 18.Bhat M A, Dar M A, Lone G N, Dar A M. Use of pedicled omentum in esophagogastric anastomosis for prevention of anastomotic leak. Ann Thorac Surg. 2006;82(05):1857–1862. doi: 10.1016/j.athoracsur.2006.05.101. [DOI] [PubMed] [Google Scholar]
- 19.Kim D J, Lee J H, Kim W. A comparison of total versus partial omentectomy for advanced gastric cancer in laparoscopic gastrectomy. World J Surg Oncol. 2014;12:64. doi: 10.1186/1477-7819-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamabe A, Omori T, Tanaka K, Nishida T. Comparison of long-term results between laparoscopy-assisted gastrectomy and open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surg Endosc. 2012;26(06):1702–1709. doi: 10.1007/s00464-011-2096-0. [DOI] [PubMed] [Google Scholar]
- 21.Olmi S, Uccelli M, Oldani A. Laparoscopic surgery of gastric cancer with D2 lymphadenectomy and omentum preservation: our 10 years experience. J Laparoendosc Adv Surg Tech A. 2020;30(07):749–758. doi: 10.1089/lap.2019.0781. [DOI] [PubMed] [Google Scholar]
- 22.Lawrance R J, Loizidou M, Cooper A J, Alexander P, Taylor I. Importance of the omentum in the development of intra-abdominal metastases. Br J Surg. 1991;78(01):117–119. doi: 10.1002/bjs.1800780135. [DOI] [PubMed] [Google Scholar]
- 23.Hagiwara A, Sawai K, Sakakura C. Complete omentectomy and extensive lymphadenectomy with gastrectomy improves the survival of gastric cancer patients with metastases in the adjacent peritoneum. Hepatogastroenterology. 1998;45(23):1922–1929. [PubMed] [Google Scholar]
- 24.Haverkamp L, Brenkman H JF, Ruurda J P, Ten Kate F JW, van Hillegersberg R. The oncological value of omentectomy in gastrectomy for cancer. J Gastrointest Surg. 2016;20(05):885–890. doi: 10.1007/s11605-016-3092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ri M, Nunobe S, Honda M. Gastrectomy with or without omentectomy for cT3-4 gastric cancer: a multicentre cohort study. Br J Surg. 2020;107(12):1640–1647. doi: 10.1002/bjs.11702. [DOI] [PubMed] [Google Scholar]
- 26.Kim M C, Kim K H, Jung G J, Rattner D W. Comparative study of complete and partial omentectomy in radical subtotal gastrectomy for early gastric cancer. Yonsei Med J. 2011;52(06):961–966. doi: 10.3349/ymj.2011.52.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young S, DiFronzo L A, Ahuja A. Performing omentectomy during gastrectomy does not improve survival: a multi-center analysis of 471 patients with gastric adenocarcinoma. J Gastrointest Surg. 2020;24(12):2856–2858. doi: 10.1007/s11605-020-04772-7. [DOI] [PubMed] [Google Scholar]
- 28.Sakimura Y, Inaki N, Tsuji T, Kadoya S, Bando H. Long-term outcomes of omentum-preserving versus resecting gastrectomy for locally advanced gastric cancer with propensity score analysis. Sci Rep. 2020;10(01):16305. doi: 10.1038/s41598-020-73367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasegawa S, Kunisaki C, Ono H. Omentum-preserving gastrectomy for advanced gastric cancer: a propensity-matched retrospective cohort study. Gastric Cancer. 2013;16(03):383–388. doi: 10.1007/s10120-012-0198-6. [DOI] [PubMed] [Google Scholar]
- 30.Seo W J, Choi S, Roh C K. Omentum preservation as an oncologically comparable and surgically superior alternative to total omentectomy during radical gastrectomy for T3-T4 gastric cancer. Surgery. 2021;170(02):610–616. doi: 10.1016/j.surg.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Ishizuka M, Shibuya N, Takagi K. Omentectomy does not affect the postoperative outcome of patients with locally advanced gastric cancer: a systematic review and meta-analysis. J Surg Res. 2021;264:287–295. doi: 10.1016/j.jss.2021.02.033. [DOI] [PubMed] [Google Scholar]
- 32.Stomach Cancer Study Group/Japan Clinical Oncology Group . Sato Y, Yamada T, Yoshikawa T. Randomized controlled phase III trial to evaluate omentum preserving gastrectomy for patients with advanced gastric cancer (JCOG1711, ROAD-GC) Jpn J Clin Oncol. 2020;50(11):1321–1324. doi: 10.1093/jjco/hyaa113. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Geng X, Li Y. Milky spots: omental functional units and hotbeds for peritoneal cancer metastasis. Tumour Biol. 2016;37(05):5715–5726. doi: 10.1007/s13277-016-4887-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao L, Hu X, Zhang Y, Sun X T. Omental milky spots in screening gastric cancer stem cells. Neoplasma. 2011;58(01):20–26. doi: 10.4149/neo_2011_01_20. [DOI] [PubMed] [Google Scholar]
- 35.Oosterling S J, van der Bij G J, Bögels M. Insufficient ability of omental milky spots to prevent peritoneal tumor outgrowth supports omentectomy in minimal residual disease. Cancer Immunol Immunother. 2006;55(09):1043–1051. doi: 10.1007/s00262-005-0101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh J Y, Cho J H, Kang M J. Omental infarction caused by laparoscopy-assisted gastrectomy for gastric cancer: CT findings. Clin Radiol. 2011;66(10):966–973. doi: 10.1016/j.crad.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Park K E, Chung D J, Kim W, Hahn S T, Lee J M. Secondary omental infarction related to open and laparoscopic-assisted distal gastrectomy: report of two cases. Korean J Radiol. 2011;12(06):757–760. doi: 10.3348/kjr.2011.12.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J Y, Yuan J P, Geng X F, Qu A P, Li Y. Morphological study and comprehensive cellular constituents of milky spots in the human omentum. Int J Clin Exp Pathol. 2015;8(10):12877–12884. [PMC free article] [PubMed] [Google Scholar]
- 39.TNM Classification of Malignant Tumours 8th edition | UICCAccessed February 2, 2022 at:https://www.uicc.org/resources/tnm-classification-malignant-tumours-8th-edition
- 40.Jongerius E J, Boerma D, Seldenrijk K A. Role of omentectomy as part of radical surgery for gastric cancer. Br J Surg. 2016;103(11):1497–1503. doi: 10.1002/bjs.10149. [DOI] [PubMed] [Google Scholar]
- 41.Barchi L C, Ramos M FKP, Dias A R. Total omentectomy in gastric cancer surgery: is it always necessary? Arq Bras Cir Dig. 2019;32(01):e1425. doi: 10.1590/0102-672020180001e1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Metwally I H, Abdelkhalek M, Shetiwy M. Significance of omental infiltration in gastric cancer patients: a retrospective cohort study. J Gastrointest Cancer. 2020;51(03):861–867. doi: 10.1007/s12029-019-00310-0. [DOI] [PubMed] [Google Scholar]


