Abstract
Gadd45a, Gadd45b, and Gadd45g have been implicated in cell cycle arrest, DNA repair, apoptosis, innate immunity, genomic stability, and more recently in senescence. Evidence has accumulated that Gadd45a deficiency results in escape of mouse embryo fibroblasts from senescence, whereas Gadd45b deficiency promotes premature senescence and skin aging. Moreover, recently Gadd45b deficiency was found to promote senescence and attenuate liver fibrosis, whereas Gadd45a was observed to exert a protective effect against hepatic fibrosis. These findings indicate that the Gadd45 stress response proteins play important roles in modulating cellular responses to senescence. Thus, exploring how Gadd45 proteins modulate cellular senescence has the potential to provide new and innovative tools to treat cancer as well as liver disease.
Keywords: Gadd45, Gadd45a, Gadd45b, Gadd45g, Senescence, Liver, Fibrosis, Hepatic fibrosis, carbon tetrachloride, CCl4, Collagen
A. Gadd45 family of stress response genes
Gadd45a, Gadd45b, and Gadd45g constitute a family of genes that encode small (18 kDa) evolutionarily conserved proteins, which are highly homologous to each other (Liebermann DA Hoffman B, 2013). Despite marked similarities, these genes are regulated in a differential manner and exhibit functional diversity. They play a pivotal role in regulating diverse cellular functions such as cell cycle control, survival, and apoptosis and are regulated by the nature of the encountered stress stimulus, its magnitude, and the cell type. Gadd45a, Gadd45b, and Gadd45g have been implicated in cell cycle arrest, DNA repair, apoptosis, innate immunity, genomic stability, and more recently in senescence (Liebermann DA Hoffman B, 2013).
B. Cellular Senescence
Senescence is a cellular state of irreversible growth arrest in response to various types of stresses. Senescence can be triggered within several days in somatic cells that are undergoing stress stimuli (He S, Sharpless NE, 2017). It was shown that in normal primary cells, overexpression of an oncogene, such as activated H-Ras, can trigger growth arrest, with features of cellular senescence, as well as apoptosis in fibroblasts and epithelial cells (Bringold, F., and M. Serrano. 2000. Bulavin, DV et al 2002. Ferbeyre, GE. et al 2002. Lin, AW. Lowe SW. 2001. Serrano, MA et al 1997). These responses have been implicated as protective by removing cycling cells when an oncogene has become active. The mechanism for senescence involves multiple pathways, including those involving p53 and RB. To a large extent, Ras-induced cell cycle arrest is dependent on p53 signaling (Serrano, MA et al 1997). In mouse cells, growth arrest after oncogenic stimulation is dependent on the p19/ARF pathway-mediated stabilization of p53 (Ferbeyre, GE. et al 2002), which is not the case for some human cells in which p14/ARF is not induced (Wei, WR 2001). Although p53 accumulation is an important feature, full activation of p53 involves other events including posttranslational modifications, which involve a variety of regulatory kinases, such as the mitogen-activated protein kinases (MAPK) (Appella, E. Anderson CW. 2001). In addition to activation of the ERK1/2 pathway by oncogenic Ras, which regulates p16/Ink4a levels (Lin, AW 1998. Zhu, JD. 1998), increasing evidence indicate that two other major MAPK pathways, p38 MAPK (p38) and c-Jun N-terminal kinase (JNK), have important roles in the cellular response to oncogenic stress (Pruitt, KWM. 2002). In the case of H-Ras activation, all three (ERK, p38, and JNK) major branches of MAPK signaling are activated. Sequential activation of the ERK pathway and then the p38 pathway has been reported to contribute to the induction of senescence by H-RasG12V (Wang, WJ 2002).
C. Gadd45 Proteins in Cellular Senescence
C.1. Gadd45a deficiency results in escape of mouse embryonic fibroblasts from senescence whereas Gadd45b deficiency promotes premature senescence and skin aging.
A role implicating Gadd45a in senescence was first documented in 2003 (Bulavin DV 2003), which showed that in Gadd45a−/− mouse embryonic fibroblasts (MEF), overexpression of H-Ras activates extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) but not p38 kinase, and this correlates with the loss of H-Ras-induced senescence. Inhibition of p38 mitogen-activated protein kinase (MAPK) activation correlated with the deregulation of p53 activation, and both a p38 MAPK chemical inhibitor and the expression of a dominant-negative p38α inhibited p53 activation in the presence of H-Ras in wild-type MEF. p38, but not ERK or JNK, was found in a complex with Gadd45 proteins. The region of interaction was mapped to amino acids 71 to 96, and the central portion (amino acids 71 to 124) of Gadd45a was required for p38 MAPK activation in the presence of H-Ras. An increase in Gadd45a expression has been correlated to H2O2 stress-induced senescence as well (Furukawa-Hibi Y 2002). The first evidence to suggest a role of Gadd45b in senescence was obtained in the senescence-accelerated mouse (SAMP1) model, revealing that Gadd45b exhibits a higher expression in the aging articular cartilage of SAMP1 mice as compared to the control mice (Shimada H 2011).
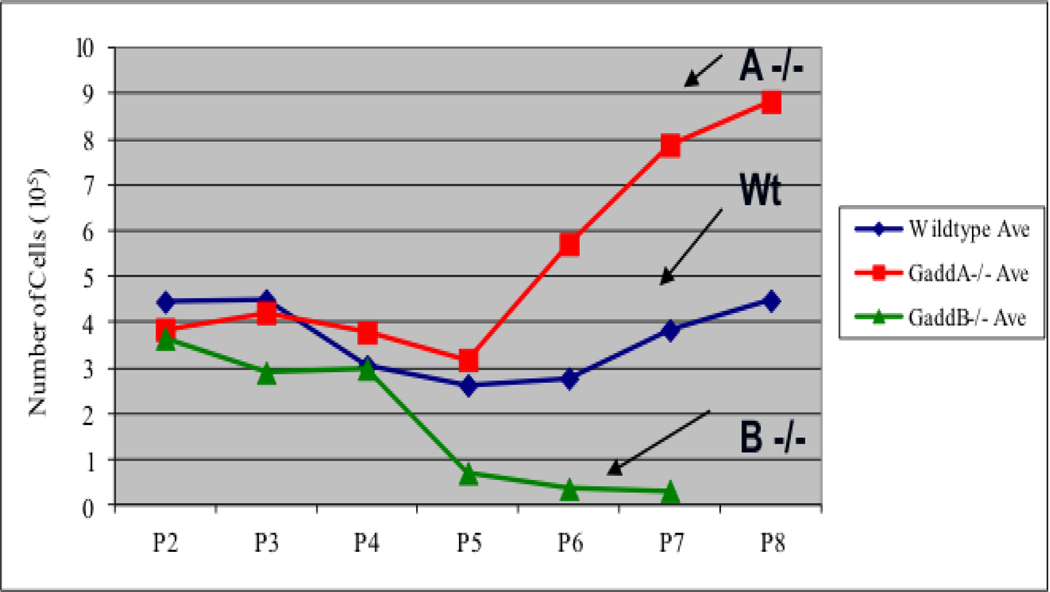
Research conducted in our laboratory has shown that Gadd45a-null MEFs escape senescence, whereas Gadd45b-null MEFs stop proliferating and undergo premature senescence (Fig.1 and Magimaidas A 2016). The impaired proliferation and increased senescence in Gadd45b-null MEFs is partially reversed by culturing at physiological oxygen levels, indicating that Gadd45b deficiency leads to decreased ability to cope with oxidative stress. Interestingly, Gadd45b-null MEFs arrest at the G2/M phase of cell cycle, in contrast to other senescent MEFs, which arrest at G1. FACS analysis of phospho-histone H3 staining showed that Gadd45b-null MEFs are arrested in G2 phase rather than M phase. H2O2 and UV irradiation, known to increase oxidative stress, also triggered increased senescence in Gadd45b-null MEFs as compared to wild-type MEFs. In vivo evidence for increased senescence in Gadd45b-null mice includes the observation that embryos from Gadd45b-null mice exhibit increased senescence staining compared to wild-type embryos (Fig. 2). Furthermore, it has been shown that Gadd45b deficiency promotes senescence and aging phenotypes in mouse skin (Magimaidas A 2016). Together, these results highlight a novel role for Gadd45b in stress-induced senescence and in tissue aging (Fig. 3 and Magimaidas, A 2016).Together, these results highlight a novel role for Gadd45b in stress-induced senescence and in tissue aging.
Figure 1.
Gadd45a-null MEFs escape senescence, whereas Gadd45b-null MEFs stop proliferating prematurely and undergo premature senescence.
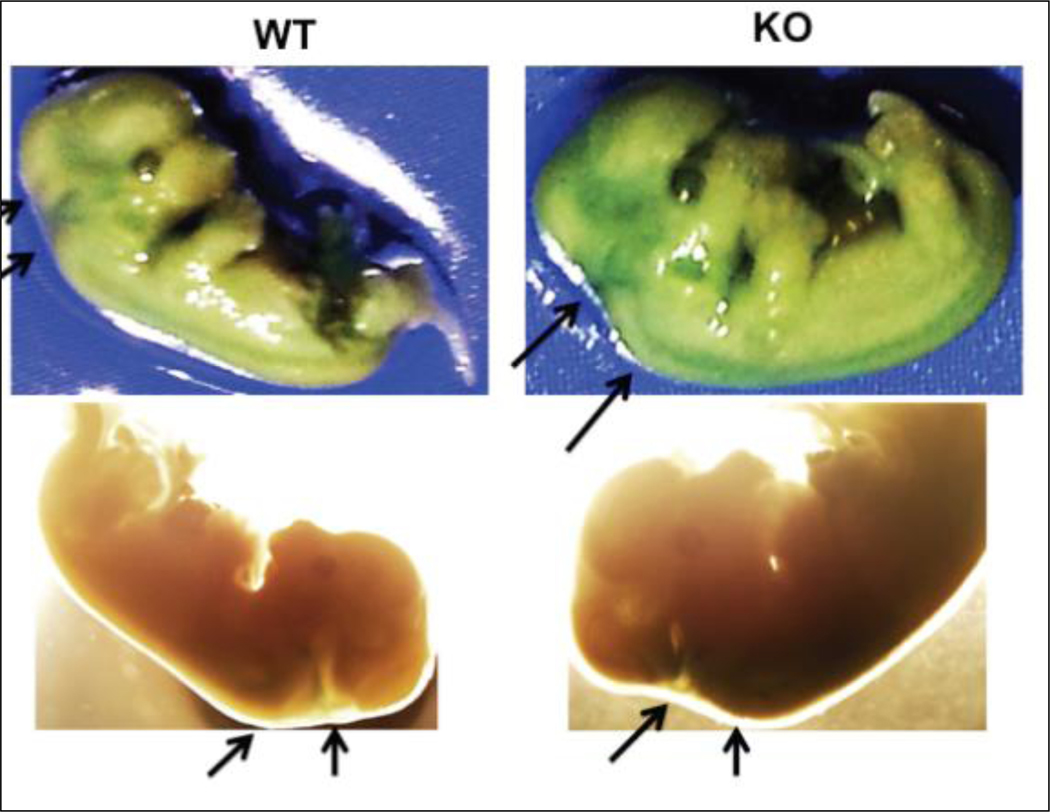
Figure 2.
Photographs of SA-β-gal staining of Gadd45b+/+ and Gadd45b−/− E14 embryos. Arrows indicate embryonic regions with strong senescence staining. Strong staining was found in the head and neck regions of all mutant embryos tested (n=6).
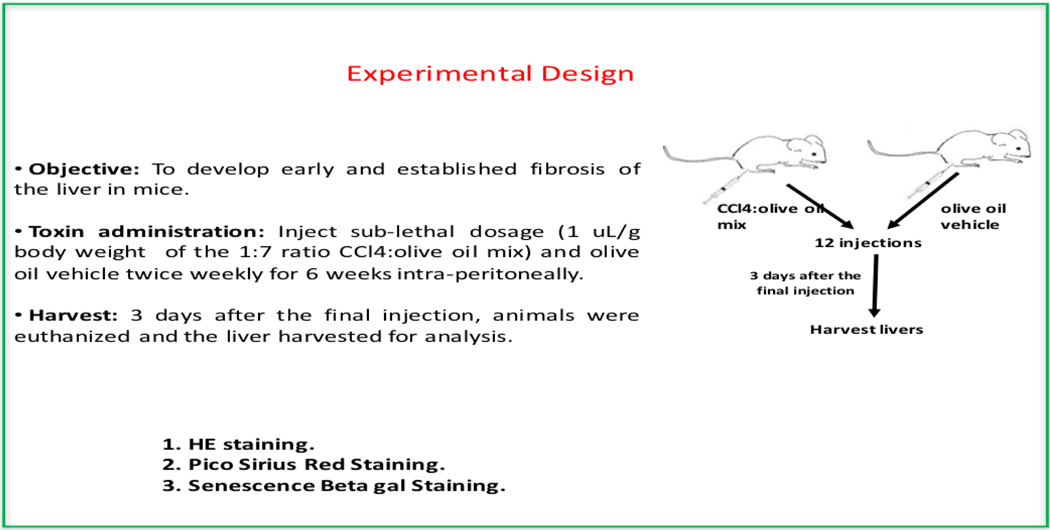
To investigate the role of Gadd45b in liver fibrosis, Gadd45b+/+ and Gadd45b−/− mice were injected intraperitoneally twice weekly for 6 weeks with sub-lethal doses of CCl4 in olive oil or an equal volume of olive oil alone as vehicle control. 3 days after the final injection, livers were harvested and processed for sectioning and histopathological evaluation.
Figure 3.
Proposed role of Gadd45b in premature senescence.
C.2. Gadd45b deficiency promotes senescence and attenuates hepatic fibrosis whereas Gadd45a exerts a protective effect against it.
Fibrosis is a wound healing process characterized by deposition of extracellular matrix components including collagens, leading to encapsulation of the injury site. Liver fibrosis, a pathological feature that is a precursor of cirrhosis, is characterized by the accumulation of fibrotic tissue and the concomitant loss of liver function. It can be triggered by chronic liver damage associated with hepatitis virus infection, alcohol abuse or liver steatosis (fatty liver disease; Friedman SL. 2003. Bataller R Brenner DA. 2005). It has been shown that during chronic damage, hepatic stellate cells (HSCs) become activated and abnormally proliferate as myofibroblasts (damage-activated fibroblasts) (Gäbele E 2003. Moreira RK 2007). Recent studies have shown that myofibroblasts become senescent and produce a stable fibrotic scar with abundant collagen and other extracellular matrix components (Kornelia Kis et al 2011). In human patients, SA β-gal-positive cells accumulate in the periphery of the fibrotic scar. In rodents, chronic treatment with carbon tetrachloride (CCl4, a liver-damaging agent) produces liver fibrosis, which is characterized by positive SA β-gal-positive cells that are derived from activated HSCs and show increased p53, p21 and p16 and other senescence markers (Wiemann SU 2002). Mice deficient in these genes have demonstrated the beneficial role of senescence in restricting liver fibrosis. In response to liver damage, mice lacking Trp53 and/or Cdkn2a present senescence negative fibrotic areas that are larger than those in senescence-competent mice. Similarly, the extracellular matrix protein CCN1 produced by damaged hepatocytes has been shown to be a key mediator of senescence induction in HSCs. Accordingly, mice with Ccn1 deficient hepatocytes do not execute HSC senescence leading to an exacerbated fibrotic response (Kim KH 2013). Taken together, these observations suggest that the Gadd45a-mediated promotion of HSC senescence plays a role in protecting liver cells from fibrosis and could be a potential therapeutic strategy to limit liver fibrosis.
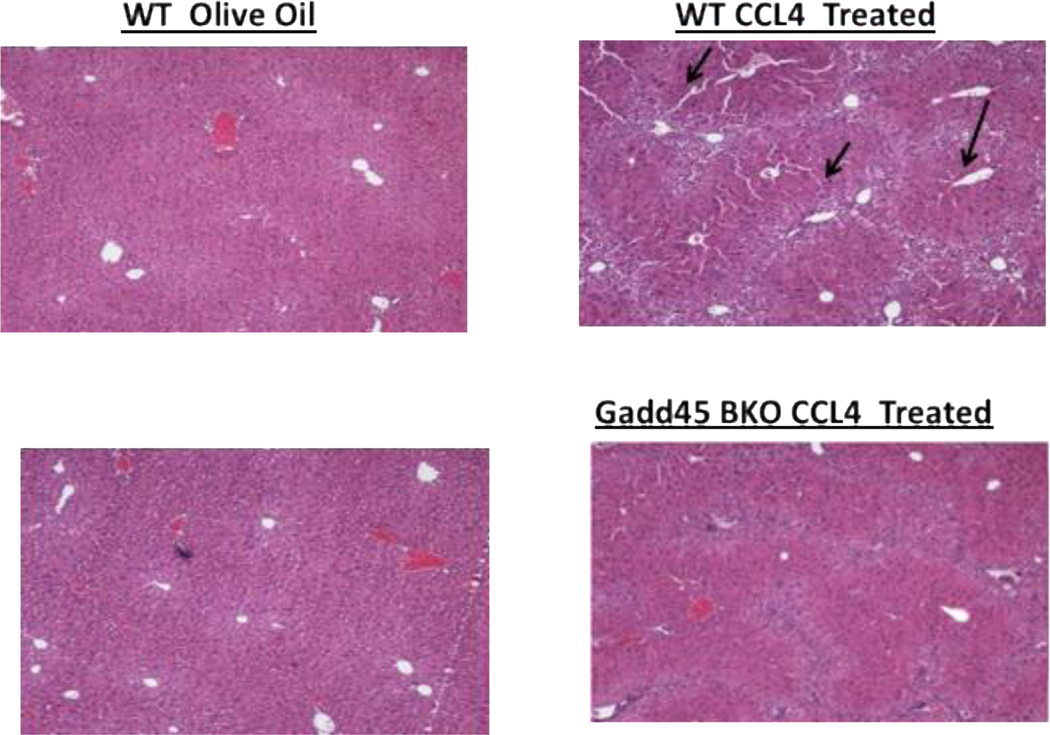
Mouse models of liver fibrosis have proven invaluable to the investigation of liver fibrogenesis and chronic hepatic injury. Thus, to investigate the role of Gadd45b in liver fibrosis, Gadd45b+/+ and Gadd45b−/− mice were injected intra-peritoneally twice weekly for 6 weeks with sub-lethal doses of CCl4 in olive oil or an equal volume of olive oil alone as vehicle control. Three days after the final injection, livers were harvested and processed for sectioning and histopathological evaluation (Fig. 4). Interestingly, Histopathological analysis using H&E staining revealed reduced cyto-architectural damage (fibrotic scars) in Gadd45b−/− mice compared with Gadd45b+/+ mice. In order to further characterize this fibrotic phenotype and analyze the collagen deposition, Sirius red staining was carried out. Gadd45b−/− mice showed reduced hepatic collagen accumulation as compared with Gadd45b+/+ mice after the chronic CCl4 challenge. These data indicate that loss of Gadd45b protected against CCl4-induced chronic hepatic injury (Fig. 5).
Figure 4.
Experimental design for the liver fibrosis analysis in mice.
Figure 5.
Gadd45b deficiency attenuates CCl4-induced collagen accumulation in liver. Histopathological analysis using Pico-Sirius Red staining of liver sections from Gadd45b+/+ and Gadd45b−/− mice treated with sub-lethal doses of CCl4 in olive oil or olive oil control showed a significant decrease in collagen accumulation in CCl4 treated Gadd45b−/− mice as compared with their wild-type counterparts. Arrows indicate liver sections with collagen accumulation.
Given that senescence has been shown to be a protective mechanism against liver fibrosis, and that Gadd45b−/− MEFs show increased senescence as compared to Gadd45b+/+ MEFs, it was of interest therefore to investigate the level of cellular senescence within the liver samples. To identify senescent cells in situ, liver sections from CCl4 and vehicle-treated mice were subjected to senescence associated β-galactosidase staining. As predicted, Gadd45b−/− mice showed increased senescence staining in livers of CCl4-treated mice compared with Gadd45b+/+ mice. These data indicate that loss of Gadd45b leads to increased senescence, which is protective against CCl4-induced liver fibrosis, leading to reduced fibrotic scars and reduced collagen deposition.
In conclusion, the results obtained highlight a novel and significant role for Gadd45b in the senescence response to CCl4-induced liver injury providing the impetus to further investigate the role of Gadd45 proteins in physiological and pathological conditions that trigger senescence in liver.
In contrast, data have been obtained to indicate that Gadd45a exerts a protective effect against hepatic fibrosis induced by CCl4, via the inhibition of canonical transforming growth factor-β/Smad signaling and fibrogenic gene expression, as well as by exerting ROS scavenging effects via upregulation of expression of antioxidant enzymes (Liang H 2016).
Moreover, recent data have shown that Gadd45a-null mice have more severe hepatic inflammation and fibrosis, higher levels of mRNAs encoding pro-inflammatory, pro-fibrotic, and pro-apoptotic proteins, and greater oxidative and endoplasmic reticulum (ER) stress compared with WT mice, where Gadd45a was induced in response to ER stress in primary hepatocytes, indicating that Gadd45a plays protective roles against methionine and choline-deficient diet (MCD)-induced nonalcoholic steatohepatitis (NASH) (Tanaka N 2017).
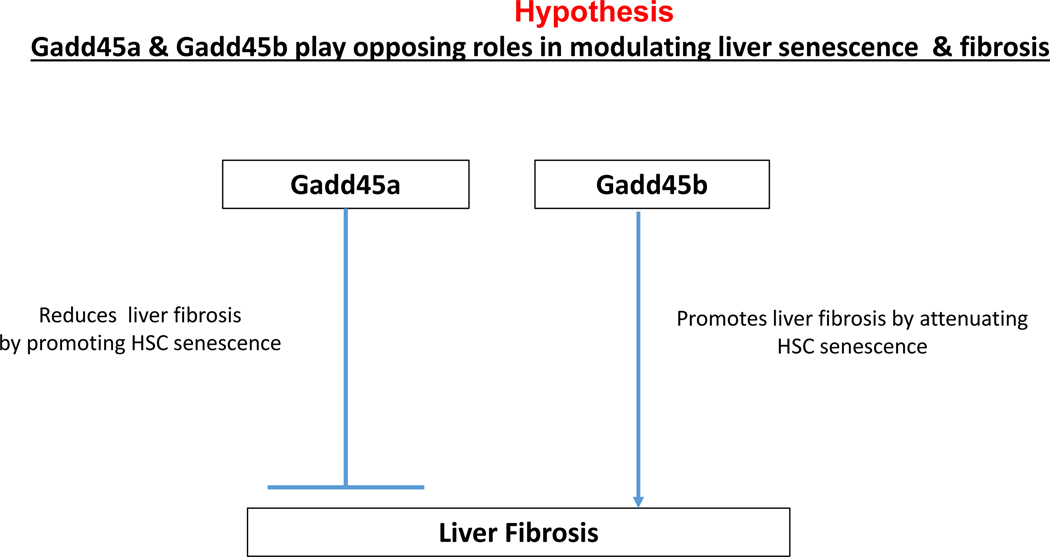
Taken together, these data suggest that Gadd45a and Gadd45b play opposing roles in modulating liver senescence and fibrosis. It is plausible that Gadd45a exerts a protective effect against hepatic fibrosis, whereas Gadd45b plays a role in sensitizing the liver to hepatic injury and fibrosis. It would be important to elucidate how this novel nexus of Gadd45 stress sensors cooperate in modulating liver homoeostasis upon injury and fibrosis (Fig. 6).
Figure 6.
Hypothetical model for the proposed role of Gadd45a and Gadd45b in liver fibrosis.
Finally, Gadd45g expression was shown to directly induce senescence in HCC Sk-Hep1, SMMC-7721, and Hep3B cells. Notably, knockdown of GADD45G in Sk-Hep1 tumor cells by small interfere RNA (siRNA) attenuated MG132- induced senescence (Guiqin X et al 2015)
D. Summary and Future prospects
The stress response Gadd45 proteins appear to play important roles in modulating cellular responses to senescence. How do they function in this capacity has been largely unexplored. Thus, exploring how Gadd45 proteins modulate cellular senescence is important and novel. Furthermore, it has the potential to provide new innovative tools to treat cancer as well as liver disease.
REFERENCES
- Appella E, and Anderson CW. 2001. Post-translational modifications and activation of p53 by genotoxic stresses. Eur. J. Biochem. 268:2764–2772. [DOI] [PubMed] [Google Scholar]
- Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005; 115: 209–218 [PMID: 15690074 DOI: 10.1172/jci24282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringold F, and Serrano M. 2000. Tumor suppressors and oncogenes in cellular senescence. Exp. Gerontol. 35:317–329. [PubMed] [Google Scholar] [DOI] [PubMed] [Google Scholar]
- Bulavin DV, Kovalsky O, Hollander MC, Fornace AJ Jr. Loss of onco- genic H-ras-induced cell cycle arrest and p38 mitogen-activated protein kinase activation by disruption of Gadd45a. Mol Cell Biol 2003;23:3859–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulavin DV, Demidov ON, Saito S, Kauraniemi P, Phillips C, Amundson SA, Ambrosino C, Sauter G, Nebreda AR, Anderson CW, Kallioniemi A, Fornace AJ Jr., and Appella E. 2002. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat. Genet. 31:210–215. [DOI] [PubMed] [Google Scholar]
- Ferbeyre G, de Stanchina E, Lin AW, Querido E, McCurrach ME, Hannon GJ, and Lowe SW. 2002. Oncogenic ras and p53 cooperate to induce cellular senescence. Mol. Cell. Biol. 22:3497–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol 2003; 38 Suppl 1: S38–S53 [PMID: 12591185 DOI: 10.1016/S0168-8278(02)00429-4] [DOI] [PubMed] [Google Scholar]
- Furukawa-Hibi Y, Yoshida-Araki K, Ohta T, Ikeda K, Motoyama N.FOXO forkhead transcription factors induce G(2)-M checkpoint in response to oxidative stress. J Biol Chem 2002;277:26729–32 [DOI] [PubMed] [Google Scholar]
- Gäbele E, Brenner DA, Rippe RA. Liver fibrosis: signals leading to the amplification of the fibrogenic hepatic stellate cell. Front Biosci 2003; 8: d69–d77 [PMID: 12456323 DOI: 10.2741/887] [DOI] [PubMed] [Google Scholar]
- Xu Guiqin, Zhang Li, Ma Aihui, Qian Yu, Ding Qi, Liu Yun, Wang Boshi,Yang Zhaojuan, and Liu Yongzhong., 2015. SIP1 is a downstream effector of GADD45G in senescence induction and growth inhibition of liver tumor cell. Oncotarget. Oct 20; 6(32): 33636–33647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Sharpless NE. Senescence in Health and Disease.Cell. 2017. Jun 1;169(6):1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Chen CC, Monzon RI, Lau LF. Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts. Mol Cell Biol 2013; 33: 2078–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kis Kornelia, Liu Xiaoqiu, and Hagood James S.. 2011. Myofibroblast Differentiation and Survival in Fibrotic Disease. Expert Rev Mol Med. 2011. Aug 23; 13: e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizhanovsky V1, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW Senescence of activated stellate cells limits liver fibrosis.. Cell. 2008. Aug 22;134(4):657–67. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Liang, Sun Qing-Feng, Xu Ting-Yan, Wu Yang-He, Zhang Hui, Fu Rong-Quan, Cai Fu-Jing, Zhou Qing-Qing, Zhou Ke, Du Qing-Wei, Zhang Dong, Xu Shuang, Ding Ji-Guang. New role and molecular mechanism of Gadd45a in hepatic fibrosis. World J Gastroenterol 2016. March 7; 22(9): 2779–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebermann DA Hoffman B Editors. Gadd45 Stress Senssors. Springer book series, Advances in Experimental Medicine and Biology 793, 2013. [DOI] [PubMed] [Google Scholar]
- Lin AW, and Lowe SW. 2001. Oncogenic ras activates the ARF-p53 pathway to suppress epithelial cell transformation. Proc. Natl. Acad. Sci. USA 98:5025–5030. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AW, Barradas M, Stone JC, van Aelst L, Serrano M, and Lowe SW. 1998. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 12:3008–3019. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magimaidas A, Madireddi P, Maifrede S, Mukherjee K, Hoffman B, Liebermann DA. Gadd45b deficiency promotes premature senescence and skin aging. Oncotarget. 2016. May 10;7(19):26935–48. doi: 10.18632/oncotarget.8854. PMID: 27105496 Free PMC Article [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira RK. Hepatic stellate cells and liver fibrosis. Arch Pathol Lab Med 2007; 131: 1728–1734 [PMID: 17979495] [DOI] [PubMed] [Google Scholar]
- Pruitt K, Pruitt WM, Bilter GK, Westwick JK, and Der CJ. 2002.Raf-independent deregulation of p38 and JNK kinases are critical for Rastransformation. J. Biol. Chem. 277:31808–31817. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, and Lowe SW. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593–602. [DOI] [PubMed] [Google Scholar]
- Shimada H, Sakakima H, Tsuchimochi K, Matsuda F, Komiya S,Goldring MB, Ijiri K. Senescence of chondrocytes in aging articularcartilage: GADD45beta mediates p21 expression in association withC/EBPbeta in senescence-accelerated mice. Pathol Res Pract. 2011;207:225–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Takahashi S, Hu X, Lu Y, Fujimori N, Golla S, Fang ZZ, Aoyama T, Krausz KW, Gonzalez FJ. Growth arrest and DNA damage-inducible 45α protects against nonalcoholic steatohepatitis induced by methionine- and choline-deficient diet.Biochim Biophys Acta. 2017. Dec;1863(12):3170–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Chen JX, Liao R, Deng Q, Zhou JJ, Huang S, and Sun P. 2002. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol. Cell. Biol. 22:3389–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Hemmer RM, and Sedivy JM. 2001. Role of p14ARF in replicativeand induced senescence of human fibroblasts. Mol. Cell. Biol. 21:6748–6757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann SU, Satyanarayana A, Tsahuridu M, Tillmann HL, Zender L, Klempnauer J et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2002; 16: 935–942 [DOI] [PubMed] [Google Scholar]
- Zhu J, Woods D, McMahon M, and Bishop JM. 1998. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 12:2997–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]