Keywords: aperiodic spectral slope, attention deficit hyperactivity disorder, cognition, EEG, event-related potentials
Abstract
Aperiodic spectral slope is a measure of spontaneous neural oscillatory activity that is believed to support regulation of brain responses to environmental stimuli. Compared to typically developing (TD) control participants, children with attention deficit hyperactivity disorder (ADHD) have been shown to have flatter aperiodic spectral slope at rest as well as attenuated event-related potential (ERP) amplitudes in response to environmental stimuli. A small body of research suggests that aperiodic slope may also explain differences in behavioral responses. In this study, we examine associations between prestimulus aperiodic slope, stimulus characteristics, environmental demands, and neural as well as behavioral responses to these stimuli. Furthermore, we evaluate whether ADHD diagnostic status moderates these associations. Seventy-nine children with ADHD and 27 TD school-age children completed two visual ERP experiments with predictable alternating presentations of task-relevant and task-irrelevant stimuli. Aperiodic slope was extracted from prestimulus time windows. Prestimulus aperiodic slope was steeper for the TD relative to ADHD group, driven by task-relevant rather than task-irrelevant stimuli. For both groups, the aperiodic slope was steeper during a task with lower cognitive demand and before trials in which they responded correctly. Aperiodic slope did not mediate the association between ADHD diagnosis and attenuated P300 amplitude. The aperiodic spectral slope is dynamic and changes in anticipation of varying stimulus categories to support performance. The aperiodic slope and P300 amplitude reflect distinct cognitive processes. Background neural oscillations, captured via aperiodic slope, support cognitive behavioral control and should be included in etiological models of ADHD.
NEW & NOTEWORTHY This study constitutes the first investigation of associations between aperiodic spectral slope and three aspects of neurocognition: event-related potential (ERP) amplitudes, cognitive load, and task performance. We find that background oscillatory activity is dynamic, shifting in anticipation of varying levels of task relevance and in response to increasing cognitive load. Moreover, we report that aperiodic activity and ERPs constitute distinct neurophysiological processes. Children with attention deficit hyperactivity disorder (ADHD) show reduced aperiodic dynamics in addition to attenuated ERP amplitudes.
INTRODUCTION
Spontaneous neural oscillatory activity is a critical component of healthy brain functioning (1). This self-organized activity regulates not only the brain’s intrinsic functioning but also its ability to respond efficiently to extrinsic environmental stimuli (2). The frequency and amplitude of the brain’s intrinsic oscillations, in combination with the characteristics of environmental conditions and stimuli, affect the degree to which incoming information is integrated versus segregated, or attended versus ignored, in the brain (3). The distribution of aperiodic neural oscillatory power across a range of frequencies can be measured with electroencephalography (EEG) and quantified as the aperiodic slope (also known as the 1/fβ distribution). Flatter slopes (i.e., smaller β exponents, leading to a less negative slope when the power spectrum is plotted in log-log space) are believed to reflect greater neural expenditure. For example, flatter slopes have been documented during wakefulness compared to sleep states (4), as well as during active compared to resting tasks (5). Developmentally, the aperiodic slope flattens over the course of the human life span (6–8), likely reflecting increased cortical specialization and development of localized neural networks in early childhood (6, 9), followed by age-related cognitive decline in later adulthood (8). In the present study, we investigate the role of aperiodic slope in processing of time-locked visual stimuli.
The association between aperiodic EEG activity and time-locked evoked activity remains a topic of debate among neuroscientists (10). We are not aware of any studies that have directly examined associations between prestimulus aperiodic spectral slope and corresponding stimulus-locked event-related potentials (ERPs), particularly in pediatric and/or clinical samples. However, several studies have investigated associations between aperiodic slope and cognition. Flatter aperiodic slope in adults has been associated with older age as well as poorer performance on tasks of working memory and processing speed, suggesting reduced cognitive efficiency (8, 11, 12). Greater cognitive load likewise appears to elicit a flatter aperiodic slope. For example, flatter slope was found among children during inhibitory trials of a Go-No-Go task (13) and among adults in the context of high attentional demand (14, 15). Thus, there appear to be competing influences on the aperiodic slope wherein a steeper slope may be interpreted as reflecting more efficient neural processing, but a flatter slope is expected in the context of increased cognitive expenditure.
Attention deficit hyperactivity disorder (ADHD) is a common neurodevelopmental disorder associated with difficulties with attention regulation, activity level, and inhibitory control (16). In a previous study, we demonstrated that among typically developing (TD) children the aperiodic slope was steeper in low-contrast visual conditions (i.e., darkness) than in high-contrast visual conditions (i.e., with complex visual input) (17). In contrast, children with ADHD did not demonstrate this dynamic shift in slope across conditions, suggesting that aberrant neural self-tuning represents one possible correlate of behavioral dysregulation and cognitive deficits often associated with ADHD. Another study reported that during a Go-No-Go task children with ADHD had flatter poststimulus aperiodic slope than TD children only during no-go trials, where inhibitory control was required (13). Although this study did not directly examine the association between aperiodic spectral slope and corresponding ERP amplitudes, several studies have reported attenuated no-go ERP amplitudes associated with ADHD (e.g., Ref. 18). Thus, it is plausible that flatter aperiodic slope and reduced ERP amplitudes both reflect reduced efficiency in neural processes supporting attentional and inhibitory control. ADHD also appears to be associated with developmental atypicalities in the resting aperiodic slope (19–21). Infants who go on to develop ADHD have abnormally steep aperiodic slope (20, 21), whereas children and adolescents with ADHD show the opposite pattern (19). These findings suggest that ADHD may relate to atypical maturation of neural circuitries that contribute to the aperiodic slope.
With respect to evoked neural activity, amplitude of the P300 ERP component is consistently attenuated in ADHD samples (22). The P300 component reflects stimulus evaluation, categorization, and response preparation; thus, this finding speaks to a neural deficit at the executive stage of information processing (23, 24). At the behavioral level, research has consistently shown that individuals with ADHD have a wide range of neurocognitive challenges, although considerable heterogeneity in performance on cognitive tasks exists (25). Specifically, weaknesses in measures of executive functions (e.g., inhibitory control, processing speed, working memory) have been documented in ADHD groups relative to TD children (26). Critically, there appears to be an association between neural correlates, such as the P300 amplitude, and behavioral measures of executive and cognitive functioning (27, 28). A notable gap in this line of work is whether and to what degree aperiodic EEG activity, as a measure of the brain’s intrinsic background cognitive activity during task preparation, may contribute to ERP and cognitive-behavioral differences in ADHD.
In this study, we examine associations between prestimulus aperiodic slope and expectations of environmental salience (i.e., stimulus task relevance), environmental demands (i.e., task difficulty), time-locked neural responses (ERP amplitude), and behavioral responses (performance accuracy) in a sample of school-age children with ADHD and TD children. Our goal is to examine the role of aperiodic spectral slope in attention and cognitive processes and to evaluate the extent to which aperiodic slope explains individual differences in behavioral and neural indexes of cognition.
We test a series of hypotheses regarding the role of aperiodic slope neurocognition in the present study:
First, we hypothesize that aperiodic exponent will vary depending on the cognitive-behavioral and environmental contexts. Specifically, we hypothesize that 1) for task-relevant trials, prestimulus aperiodic slope will be steeper before accurate compared to inaccurate responses, indicating that effective neural tuning supports efficient behavioral performance. Additionally, we expect that 2) the aperiodic slope will be flatter during a task with greater cognitive and memory load, reflecting increased recruitment of localized networks to support increased cognitive demand. Given the competing influences of neural efficiency and cognitive load, we do not have a specific hypothesis regarding differences in aperiodic slope when the participant is expecting task-relevant versus task-irrelevant stimuli. Finally, we hypothesize that the effects of varying trial types will be weaker among children with ADHD, indicating reduced dynamic aperiodic neural activity in this clinical group.
Second, we hypothesize that prestimulus aperiodic slope facilitates subsequent evoked neural responses, which would be evidenced by a positive association between the prestimulus aperiodic slope exponent and stimulus-locked P300 amplitude. Given the known reduction in P300 amplitude in ADHD, we expect that reduced aperiodic slope in the ADHD group will explain reduced P300 amplitude in this clinical sample.
MATERIALS AND METHODS
Participants
All study procedures were approved by the governing institutional review board (IRB) at the University of Washington. Children ages 7–11 yr were recruited via outreach to pediatric clinics, research registries, and postings on social media and community buildings. Exclusion criteria were a history of seizures, intellectual disability, a diagnosis of autism spectrum disorder, a known genetic syndrome, perinatal complications including premature birth, and prenatal exposure to substances. One hundred seven children with ADHD and 34 TD children (i.e., children without ADHD or an immediate family history of ADHD) were enrolled. ADHD diagnoses were confirmed by a licensed clinical psychologist through standardized clinical interview, review of DSM-5 ADHD rating scales, and/or observation during neuropsychological testing. Twenty-three children were excluded after participation because of IQ scores < 80 (n = 2), suspected autism spectrum disorder (n = 4), unconfirmed ADHD diagnosis or TD status (n = 13), identification of seizures during the EEG (n = 1), failure to abstain from medication before the in-person visit (n = 2), or poor EEG net fit (n = 1). Three additional participants were excluded because they achieved < 60% accuracy on both ERP tasks. Additionally, 2 participants did not achieve 60% accuracy on the easy task only, and 15 did not achieve 60% accuracy on the hard task only; these data points were removed from the respective analyses. Participants whose data were excluded from the hard ERP task did not significantly differ from those whose data were included on full-scale IQ (t[16] = −1.81, P = 0.089), proportion of females (χ2[1] = 0.37, P = 0.544), or proportion of individuals identifying as White (χ2[1] < 0.0001, P = 1.00).The final sample included 82 children with ADHD and 28 TD children. Thirty percent of the sample were assigned female sex at birth, consistent with epidemiological rates in ADHD, and the mean age was 9.58 yr. The TD group had higher IQ than the ADHD group and fewer coexisting psychiatric symptoms but did not differ on age or sex (see Table 1).
Table 1.
Sample demographics
| ADHD | Typically Developing | Difference | |
|---|---|---|---|
| N | 82 | 28 | |
| % Female | 29% | 32% | ns |
| Age, mo (SD) | 116 (17) | 112 (16) | ns |
| Identify as White | 80% | 71% | ns |
| Full-scale IQ (range) | 108 (85–143) | 117 (91–135) | P = 0.001 |
| Easy task accuracy | 0.87 (0.10) | 0.91 (0.10) | ns |
| Hard task accuracy | 0.74 (0.14) | 0.81 (0.12) | P = 0.018 |
| Easy task trials | 135 (7) | 133 (12) | ns |
| Hard task trials | 133 (11) | 137 (8) | ns |
ADHD, attention deficit hyperactivity disorder; female, female sex at birth by parent report; ns, not significant at P = 0.05.
Procedures
Participating children visited a university laboratory for a single 3-h visit, in which they completed EEG recording and comprehensive neuropsychological testing. Written informed consent was obtained from caregivers, and assent was obtained from participating children at the start of the visit, consistent with university institutional review board guidelines. When applicable, participants abstained from taking psychostimulant medications for at least 48 h and nonstimulant or other psychotropic medications for a duration determined by and with the agreement of the child’s prescribing clinician. Caregivers completed behavioral rating scales and a brief medical history on the participating child either remotely online or in person during the visit. The EEG included 5 min of baseline resting (not analyzed) before the two ERP experiments relevant to the present study.
EEG Acquisition
Children were seated in a comfortable chair 70 cm from a computer monitor on which the ERP experiments were presented with E-Prime 2.0. Continuous EEG was recorded with a 128-channel Magstim-EGI HydroCel geodesic sensor net and Net Station Acquisition software version 4.5.6, with a 400 series high-impedance amplifier (Magstim-EGI, Plymouth, MN). EEG signals were referenced to the vertex electrode, analog filtered (0.1 Hz high pass, 100 Hz elliptical low pass), amplified, and digitized with a sampling rate of 1,000 Hz. Impedances were reduced to <50 kΩ at the start of the EEG session, and electrodes were rewet throughout the session to maximize the signal-to-noise ratio. Timing of the presentation of visual stimuli was recorded with a Cedrus StimTracker. Responses were recorded with an SRBox.
ERP Experiments
Participants completed two dual-task visual ERP experiments (“easy”/“hard”), adapted from Jonkman and colleagues (29), each lasting ∼8 min. In both experiments, task-relevant “target” stimuli were presented alternately with task-irrelevant, “oddball” stimuli (see Ref. 30). This consistent pattern was explained to the participant and demonstrated during practice trials; thus, participants were able to anticipate the type of stimulus that would be presented next. Target stimuli were solid rectangles presented in one of four colors (blue, green, red, or orange) against a black background, with a visual angle of 49° horizontal and 7.7° vertical arc and a luminosity range of 2.4–10.3%). The oddball stimuli consisted of white brackets (“[”; standard stimuli; 60%) with the same height and width as the target stimuli; identical white brackets oriented in the opposite direction (“]”; deviant; 20%); and nonrepeated white line drawings of vehicles and animals (novel; 20%) presented against a black background. The bracket orientation for standard versus deviant stimuli were randomized. These visual stimuli were identical for both tasks, but the instructions varied such that in the first, “easy” task participants were told to respond to one set of target stimuli with a right button push and to the other set with a left button push. In the second, “hard” experiment, participants were given instructions for a standard 1-back working memory task, such that they were told to push the right button for two consecutive matching target stimuli and the left button for consecutive nonmatching target stimuli. In both experiments, the probability of a left- versus right-button response was equal, and a response was requested for every stimulus. The visual oddball stimuli were passive, in that participants were instructed to “ignore” these images and not push any buttons. Participants completed practice sets of 10 trials each until they appeared to have understood the task, followed by 140 test trials (each trial included a target and oddball stimulus), presented over three blocks, with brief breaks in between. All stimuli were presented against a black background with a duration of 300 ms and an interstimulus interval that varied randomly from 0.8 to 1.4 s.
EEG Processing
EEG data were processed in MATLAB R2019b with additional extensions and functions from the EEGLAB 15 and ERPLab v8.0 packages. To aid with artifact detection and correction, initial processing of EEG data included 5 min of baseline resting EEG data in addition to the 20 min of ERP data. Data were downsampled to 250 Hz and band-pass filtered at 0.3–80 Hz. Electrical line noise from 55 to 65 Hz was corrected with the CleanLine plugin for EEGLAB. Bad channels were automatically detected and subsequently interpolated back into the data set before average referencing, following methods outlined in Ref. 31. Extended independent component (IC) analysis (ICA) (32) with primary component analysis dimension reduction was used to identify and remove artifactual components (i.e., deriving from eyeblinks, line noise, or cardiac signal). ADHD and TD groups marginally differed on the number of retained ICs (TD: mean = 20.93, SD = 12.26; ADHD: mean = 15.98, SD = 9.45; P = 0.054). The number of ICs retained was similar to prior research on neurodevelopmental samples (31, 33) and consistent with what would be expected given the level of motor movement involved in the task.
Measures
Aperiodic slope.
Continuous EEG data were extracted from 500-ms windows occurring immediately before stimulus onset, and data were averaged across trials within each of the stimulus types (standard, deviant, and novel oddball; correct target and error target). A fast Fourier transformation (FFT) was computed with a 0.5-s Hamming window for each of the conditions. The Fitting Oscillations and One-Over-f (FOOOF) MATLAB toolbox (34, 35) was used with a frequency range of 1–50 Hz at each electrode to derive the aperiodic exponent, which was used in subsequent analyses to quantify the aperiodic slope. Based on visual inspection, we did not specify a “knee” in the power spectral density distribution. The remaining FOOOF parameters were specified a priori, consistent with prior publications (17, 30, 35). Peak identification parameters included width limits of 2–12 Hz, minimum peak height of 0.5 µV over and above the aperiodic exponent, and a relative threshold of two standard deviations above the aperiodic exponent. A maximum of eight peaks was set to be identified. Average aperiodic exponents were calculated within each stimulus type for anterior frontal (Afz, Af3, Af4), frontal (Fz, F3, F4), central (Cz, C3, C4), parietal (Pz, P3, P4), and occipital (Oz, O3, O4) electrode clusters.
ERP components.
Based on visual inspection of ERP waveform topography (Fig. 1), the mean amplitude of the P300 component was extracted at a parietal electrode cluster (Pz, P3, P4) at 300–600 ms. P300 amplitudes were computed within each experiment separately for standard, deviant, novel oddball stimuli and for target stimuli for which the participant provided a correct response (correct target) versus an incorrect response (error target). Participants with accuracy < 60% on response trials were excluded from analysis for that particular experiment (easy n = 2; hard n = 14).
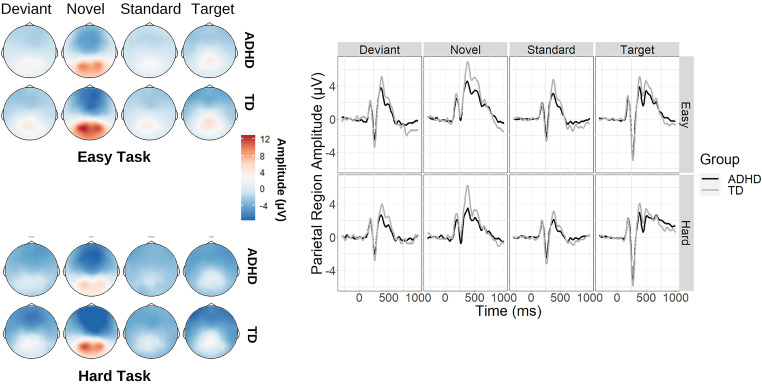
Figure 1.
Left: scalp topography averaged over the P300 window (300–600 ms) following target and oddball stimuli during the easy task (top) and the hard task (bottom). Right: grand average event-related potential (ERP) waveforms extracted from the parietal cluster for each stimulus condition and task, depicted for attention deficit hyperactivity disorder (ADHD) (black) and typically developing (TD) (gray) participants.
Analytic Plan
All analyses were completed in R Studio 2022.07.01 with the R basic, psych, lme4, lmerTest, and lavaan packages. Aperiodic exponents and P300 amplitude demonstrated normal distributions, with skew and kurtosis values < |1|. To test our first hypothesis, that expectation of stimulus task relevance, response accuracy, and cognitive load would moderate the prestimulus aperiodic slope, we ran repeated-measures analysis of covariance (ANCOVA) with aperiodic slope as the dependent variable. Independent categorical variables were stimulus type (correct target, error target, or oddball), experiment (easy or hard), region (frontal, central, parietal, or occipital), and the interaction between stimulus type and experiment. Age (measured in months) was included as a covariate; full-scale IQ was initially included in the model as a covariate but was dropped because it was not a statistically significant predictor and to maximize statistical power to detect effects. Power was estimated for zero between- and three within-person independent variables at an α level of 5% for a medium effect size of 0.5 standard deviation and the ANOVA model. Given a total sample size of n = 110, results indicated that power for the main effects was equal to 99.9%. Similarly, power for the interaction between within-person factors was 99.4%. Power was estimated with PASS22. Power levels were similar after correction of the F tests with the Geisser–Greenhouse correction.
Next, to evaluate whether the effects of stimulus type and cognitive load were weaker in children with ADHD, we tested a second model in which group (ADHD or control), the interaction between group and stimulus type, and the interaction between group and experiment were included as predictors. Power was estimated for one between (ADHD vs. TD)- and two within (stimulus type with 3 levels and experiment with 2 levels)-person independent variables at an α level of 5% for a medium effect size of 0.5 standard deviation and the F test. Given specific group sizes of n = 82 and n = 28, results indicated that power for the main effect was equal to 99.9%. Similarly, power for the interaction between group and the within-person factors ranged between 99.9% and 99.4%. Power levels were similar after correction of the F tests with the Geisser–Greenhouse correction. Power was estimated with PASS22. To test our second hypothesis, that prestimulus aperiodic slope would explain attenuated P300 amplitude in children with ADHD, we tested a mediation model with diagnostic group as the predictor, aperiodic slope as the mediator, and parietal P300 amplitude as the dependent variable. Aperiodic slope was extracted from the parietal electrode cluster to be consistent with the scalp location of the P300 component. First, we used multilevel linear modeling to test for significant a, b, and c′ paths, to justify running the mediation model. In these initial models, experiment, stimulus type, and age were included as covariates. Next, we estimated a multilevel mediation model using the lavaan package in R. Stimulus, exponent, and experiment were modeled on level 1, and the mediation paths were modeled on level 2, with clustering on individual and age as a predictor of both P300 amplitude and aperiodic exponent. Again, full-scale IQ was not a statistically significant predictor and thus not included in subsequent mediation analyses.
RESULTS
Hypothesis 1: Effects of Stimulus Type and Task Difficulty on Prestimulus Aperiodic Slope
Results of the repeated-measures ANCOVAs are reported in Table 2 and depicted in Fig. 2. There were main effects of experiment and stimulus type on aperiodic slope. Additionally, older age was correlated with flatter aperiodic slope (Pearson’s r = −0.18, P < 0.001). Post hoc pairwise comparisons with estimated marginal means indicated that prestimulus aperiodic slope (i.e., aperiodic slope during the 500 ms when participants were predicting and preparing for the next stimulus) differed across oddball, correct target, and incorrect target stimulus trials. As predicted, the aperiodic slope was steeper for the easy compared to the hard task (difference = 0.04, SE = 0.02, P = 0.008). Also consistent with our hypothesis, prestimulus aperiodic slope was steepest before correct target trials. With regard to task relevance of the expected stimuli, the results indicated steeper aperiodic slope before correct target compared to oddball stimulus trials, and aperiodic slope was steeper preceding both of these trial types compared to target trials on which the participant made an error (correct target > oddball difference estimate = 0.06, SE = 0.02, P = 0.004; correct target > error target estimate = 0.16, SE = 0.02, P < 0.001; oddball > error target estimate = 0.10, SE = 0.02, P < 0.001).
Table 2.
Effects of stimulus type and experiment difficulty on aperiodic slope
| df | F | P | |
|---|---|---|---|
| Model 1: Full sample | |||
| Region | 3 | 1.12 | 0.340 |
| Age | 1 | 141.71 | <0.00001 |
| Experiment | 1 | 11.28 | <0.001 |
| Stimulus type | 2 | 27.25 | <0.00001 |
| Experiment × stimulus type | 2 | 0.22 | 0.801 |
| Residuals | 4,030 | ||
| Model 2: Group interaction | |||
| Region | 3 | 1.13 | 0.34 |
| Age | 1 | 142.45 | <0.00001 |
| Experiment | 1 | 11.34 | 0.0008 |
| Stimulus type | 2 | 27.39 | <0.00001 |
| Group | 1 | 18.22 | <0.00001 |
| Experiment × stimulus type | 2 | 0.22 | 0.801 |
| Experiment × group | 1 | 3.031 | 0.082 |
| Stimulus type × group | 2 | 2.68 | 0.069 |
| Experiment × stimulus type × group | 2 | 0.15 | 0.860 |
| Residuals | 4,024 | ||
Group, diagnostic group. Bold values indicate statistically significant effects with P < 0.001.
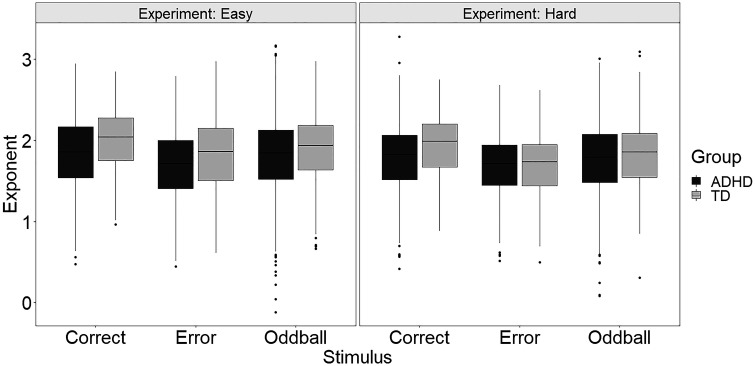
Figure 2.
There were stronger effects of experiment (cognitive load) and stimulus type on prestimulus aperiodic slope exponent for the typically developing (TD) group compared with the attention deficit hyperactivity disorder (ADHD) group.
Next, we added main and interaction effects of group to the model. As expected, ADHD participants had flatter aperiodic slopes than TD participants (difference = 0.08, SE = 0.02, P < 0.001). The group × experiment and group × stimulus type interactions approached significance; because these interactions informed our a priori hypotheses and knowing that the pairwise tests likely have more power than the omnibus test, we subsequently examined pairwise comparisons. Results showed that for the TD group there was an effect of experiment wherein aperiodic slope was steeper during the easy task (difference = 0.09, SE = 0.03, P = 0.021), whereas the ADHD group aperiodic slope did not differ across experiments (difference = 0.03, SE = 0.02, P = 0.428). We found similar results with respect to stimulus type. Within the TD group, aperiodic slope differed significantly across all three stimulus types, consistent with the overall findings. In contrast, the ADHD group did not show a difference in aperiodic slope between correct target and oddball stimuli (difference = 0.03, SE = 0.02, P = 0.638). Flatter aperiodic slope in the ADHD compared with TD group was statistically significant for the easy experiment (difference = −0.11, SE = 0.02, P = 0.001) and correct target trials (difference = ÷0.14, SE = 0.04, P = 0.001) but not the hard experiment (difference = −0.05, SE = 0.03, P = 0.232) or error target (difference = −0.05, SE = 0.04, P = 0.753) or oddball (difference = −0.05, SE = 0.02, P = 0.162) stimuli.
Hypothesis 2: Parietal Prestimulus Aperiodic Slope Explains Group Differences in P300 Amplitude
To test the hypothesis that aperiodic slope would mediate the association between reduced P300 amplitude and ADHD diagnosis, we first confirmed that the associations among P300 amplitude, aperiodic slope, and the binary ADHD diagnostic variable were independently significant. The a path (aperiodic exponent regressed on diagnostic group) approached statistical significance (unstandardized B = 0.10, SE = 0.05, P = 0.060) when controlling for experiment, stimulus type, and age. The b path showed a strong association between aperiodic exponent and P300 amplitude (unstandardized B = 1.00, SE = 0.17, P < 0.0001) after controlling for covariates. Finally, the association between P300 amplitude and group (c path) was statistically significant (unstandardized B = 0.93, SE = 0.35, P = 0.010) after controlling for covariates.
Finally, we tested our hypothesis that aperiodic slope would mediate the association between ADHD status and reduced P300 amplitude. Contrary to our hypothesis, the indirect effect was not statistically significant (B = 0.21, SE = 0.17, P = 0.21) and accounted for only 23% of the total effect (B = 0.93, SE = 0.35, P = 0.007). The direct effect (i.e., c′) remained statistically significant (B = 0.70, SE = 0.32, P = 0.030), further indicating that aperiodic slope did not explain the association between ADHD diagnosis and P300 amplitude; see Fig. 3.
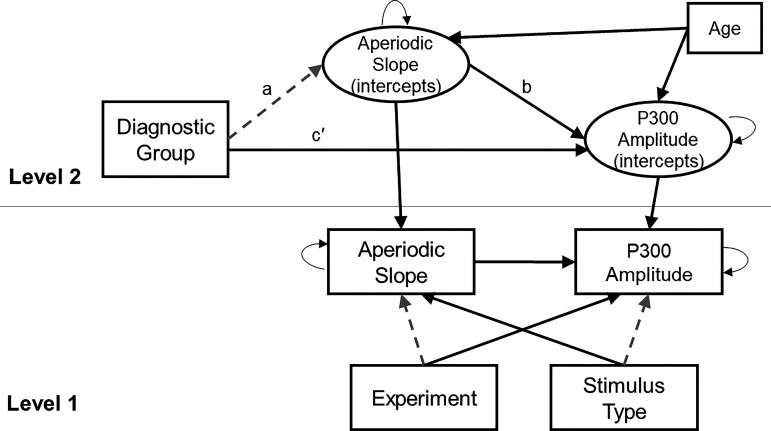
Figure 3.
Visual depiction of the multilevel mediation model. Solid black lines indicate statistically significant path coefficients at P < 0.05. Gray dashed lines indicate nonsignificant path coefficients. Within-person variables experiment (easy vs. hard) and stimulus type (correct target versus oddball) were modeled at level 1. Between-person mediation with age as a covariate was modeled at level 2. The indirect effect (paths a and b) was not statistically significant, whereas the direct effect (path c′) remained statistically significant.
DISCUSSION
In the present study, we investigated the role of dynamic shifts in neural oscillatory activity preceding sensory and higher-order cognitive processes among children with ADHD and TD control children. Consistent with our hypotheses, the prestimulus aperiodic slope varied depending on ongoing cognitive load, as well as the participant’s expectation of stimulus task relevance and performance accuracy. We expanded on our previous research, in which we found reduced resting-state aperiodic dynamics among children with ADHD (17), by demonstrating that the aperiodic slope in children with ADHD is likewise not as dynamic as that of control participants during cognitive tasks.
Among TD children, the aperiodic slope was flatter in the context of greater working memory load during the hard task compared with the easy task, indicating increased recruitment of localized neural networks to support simultaneous visual memory maintenance and task execution. In contrast, aperiodic slope was steepest before correct responses to target stimuli, suggesting that increased engagement of global neural networks may facilitate attention to salient visual stimuli as well as better task performance. Together, these two results provide insight into the delicate balance of internal and external influences on aperiodic slope: whereas flatter aperiodic slope is associated with increased cognitive load (36, 37), steeper aperiodic slope is associated with greater cognitive efficiency (11).
Our results suggest not only that the brain adjusts its oscillatory activity in the context of ongoing cognitive demands but also that it modulates the aperiodic slope in anticipation of varying levels of stimulus salience. Among TD children, aperiodic slope was steeper during task-relevant trials (with a correct response) than on task-irrelevant trials. Interestingly, we found that across the whole sample, aperiodic slope before an incorrect response was flatter than when it preceded task-irrelevant oddball stimuli. In other words, error trials occurred subsequent to times when the aperiodic slope reflected an emphasis on local, fast oscillatory activity and reduced slow, global activity (38). This finding suggests that a relatively flatter aperiodic slope may reflect an inefficient neural response to cognitive-behavioral demands and underscores the importance of neural self-tuning for cognitive-behavioral control.
A primary goal of this study was to determine whether differences in aperiodic spectral slope helped to explain diagnostic and neurophysiological differences among children with and without ADHD. As expected, the ADHD group had flatter aperiodic slope overall, suggesting that affected children have less efficient neural processing than control participants during cognitive tasks. This hypothesis is consistent with the small body of existing literature on aperiodic slope in school-age children with ADHD, which has demonstrated that school-age children with ADHD have flatter slopes than TD children at rest and during cognitive tasks (13, 17, 19). Interestingly, pairwise comparisons showed that this group effect was driven by aperiodic slope measurements that preceded correct target trials. Thus, for children with ADHD, execution of a correct response appears to require greater engagement of neural resources, similar to the effects of increased task demand or aging on TD populations. Moreover, in addition to showing flatter aperiodic slope overall, the moderating effects of experiment difficulty and stimulus salience on aperiodic slope were weaker in the ADHD group. This, in combination with our prior work (17), supports our theory that aperiodic oscillatory dynamics, and not simply overall aperiodic slope, contribute to the cognitive behavioral impairment seen in children with ADHD. However, given that these pairwise comparisons were investigated after nonsignificant omnibus tests (P = 0.069 and P = 0.082), these findings will need to be replicated to substantiate our preliminary conclusions.
A substantial body of research has suggested that the ADHD is due at least in part to delayed brain maturation (39). However, more recent prospective evidence indicates that up to 90% of children with ADHD show some symptom persistence into adulthood (40), challenging the notion that neurocognitive differences represent a temporary delay as opposed to a true deviation. Possibly, early childhood differences in neural maturation associated with ADHD lead to a cascade of neurocognitive sequelae, including flatter aperiodic slope.
An additional aim of the present study was to investigate associations between prestimulus aperiodic slope and poststimulus evoked potentials. As expected, steeper aperiodic slope was correlated with greater P300 amplitude. However, contrary to our prediction, aperiodic slope did not explain reduced P300 amplitude in children with ADHD. The P300 component, and particularly the P3a subcomponent, has previously been described as a neural index of novelty processing (24), with greater amplitudes observed in response to infrequent or incongruous stimuli (41). Likewise, P300 differences in ADHD have been attributed to reduced allocation of neural resources during attention orienting and stimulus categorization phases (42). In contrast, the aperiodic slopes of ADHD and control participants differed most notably for correct target rather than oddball stimuli. Thus, our findings may be consistent with the results of Virtue-Griffiths et al. (37), who reported that aperiodic and evoked neural activity had distinct associations with cognition and behavior. Moreover, in a prior study involving a subset of the present sample, we demonstrated that opposing profiles of P300 amplitude and resting aperiodic slope predicted retrospective report of methylphenidate response (30). Individual differences in P300 amplitude and aperiodic slope could help explain heterogeneity in the neurobiological and phenotypic profiles of children with ADHD.
It is worth noting that EEG data in the ADHD group were based on fewer retained ICs than in the TD group. Although this difference was only marginal, exclusion of ICs that may have contained brain data in addition to artifact could potentially explain reduced P3 amplitude in this group. However, this is unlikely given that prior literature has consistently found that individuals with ADHD have attenuated P3 amplitude, including studies that did not use ICA for artifact detection (42). Additional limitations of the study include the narrow age band, which limits the generalizability of our results to school-age children. Prior studies of resting-state aperiodic slope in children with ADHD suggest a quadratic developmental effect wherein infants and young children at risk for ADHD have steeper aperiodic slope than typically developing peers, whereas older children show the opposite effect (19–21). Our findings align with those prior reports. Future research should investigate whether the effects of stimulus type, response accuracy, and cognitive load on aperiodic spectral slope likewise vary across the life span.
Conclusions
Our findings indicate that the aperiodic exponent shifts with changing cognitive demands and in anticipation of task-relevant environmental characteristics and supports behavioral performance. On average, children with ADHD have flatter aperiodic slopes as well as reduced aperiodic dynamics, and this neurobiological difference appears to be distinct from one of the most consistent EEG findings in prior ADHD research: reduction in P300 amplitude. The aperiodic exponent therefore represents a promising biomarker to be included in future etiological models of ADHD.
GRANTS
This research was funded by grants to A.B.A. from the National Institute of Mental Health (K99MH116064-01A1 and R00MH116064-01A1).
DISCLAIMERS
The funders were not involved in the study design, collection, analysis, or interpretation of data; nor were they involved in the writing of the manuscript or the decision to submit the article for publication.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.B.A., V.P., and A.R.L. conceived and designed research; A.B.A. performed experiments; A.B.A. analyzed data; A.B.A., V.P., and A.R.L. interpreted results of experiments; A.B.A. prepared figures; A.B.A. drafted manuscript; A.B.A., V.P., and A.R.L. edited and revised manuscript; A.B.A., V.P., and A.R.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Georgios Sideridis for support with the power analyses.
REFERENCES
- 1. Uddin LQ. Bring the noise: reconceptualizing spontaneous neural activity. Trends Cogn Sci 24: 734–746, 2020. doi: 10.1016/j.tics.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Palva JM, Palva S. Roles of multiscale brain activity fluctuations in shaping the variability and dynamics of psychophysical performance. Prog Brain Res 193: 335–350, 2011. doi: 10.1016/B978-0-444-53839-0.00022-3. [DOI] [PubMed] [Google Scholar]
- 3. Palva S, Palva JM. Roles of brain criticality and multiscale oscillations in temporal predictions for sensorimotor processing. Trends Neurosci 41: 729–743, 2018. doi: 10.1016/j.tins.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 4. Lendner JD, Helfrich RF, Mander BA, Romundstad L, Lin JJ, Walker MP, Larsson PG, Knight RT. An electrophysiological marker of arousal level in humans. eLIfe 9: e55092, 2020. doi: 10.7554/eLife.55092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. He BJ, Zempel JM, Snyder AZ, Raichle ME. The temporal structures and functional significance of scale-free brain activity. Neuron 66: 353–369, 2010. doi: 10.1016/j.neuron.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schaworonkow N, Voytek B. Longitudinal changes in aperiodic and periodic activity in electrophysiological recordings in the first seven months of life. Dev Cogn Neurosci 47: 100895, 2021. doi: 10.1016/j.dcn.2020.100895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cellier D, Riddle J, Petersen I, Hwang K. The development of theta and alpha neural oscillations from ages 3 to 24 years. Dev Cogn Neurosci 50: 100969, 2021. doi: 10.1016/j.dcn.2021.100969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Voytek B, Kramer MA, Case J, Lepage KQ, Tempesta ZR, Knight RT, Gazzaley A. Age-related changes in 1/f neural electrophysiological noise. J Neurosci 35: 13257–13265, 2015. doi: 10.1523/JNEUROSCI.2332-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnson MH. Interactive specialization: a domain-general framework for human functional brain development? Dev Cogn Neurosci 1: 7–21, 2011. doi: 10.1016/j.dcn.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shin J. A unifying theory on the relationship between spike trains, EEG, and ERP based on the noise shaping/predictive neural coding hypothesis. Biosystems 67: 245–257, 2002. doi: 10.1016/s0303-2647(02)00101-6. [DOI] [PubMed] [Google Scholar]
- 11. Ouyang G, Hildebrandt A, Schmitz F, Herrmann CS. Decomposing alpha and 1/f brain activities reveals their differential associations with cognitive processing speed. NeuroImage 205: 116304, 2020. doi: 10.1016/j.neuroimage.2019.116304. [DOI] [PubMed] [Google Scholar]
- 12. Pathania A, Euler MJ, Clark M, Cowan RL, Duff K, Lohse KR. Resting EEG spectral slopes are associated with age-related differences in information processing speed. Biol Psychol 168: 108261, 2022. doi: 10.1016/j.biopsycho.2022.108261. [DOI] [PubMed] [Google Scholar]
- 13. Pertermann M, Bluschke A, Roessner V, Beste C. The modulation of neural noise underlies the effectiveness of methylphenidate treatment in attention-deficit/hyperactivity disorder. Biol Psychiatry Cogn Neurosci Neuroimaging 4: 743–750, 2019. doi: 10.1016/j.bpsc.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 14. Gyurkovics M, Clements GM, Low KA, Fabiani M, Gratton G. Stimulus-induced changes in 1/f-like background activity in EEG. J Neurosci 42: 7144–7151, 2022. doi: 10.1523/JNEUROSCI.0414-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Waschke L, Donoghue T, Fiedler L, Smith S, Garrett DD, Voytek B, Obleser J. Modality-specific tracking of attention and sensory statistics in the human electrophysiological spectral exponent. eLIfe 10: e70068, 2021. doi: 10.7554/eLife.70068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry 57: 1336–1346, 2005. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 17. Arnett AB, Fearey M, Peisch V, Levin AR. Absence of dynamic neural oscillatory response to environmental conditions marks childhood attention deficit hyperactivity disorder. Child Psychol Psychiatry. In press. doi: 10.1111/jcpp.13645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wiersema JR, Roeyers H. ERP correlates of effortful control in children with varying levels of ADHD symptoms. J Abnorm Child Psychol 37: 327–336, 2009. doi: 10.1007/s10802-008-9288-7. [DOI] [PubMed] [Google Scholar]
- 19. Ostlund BD, Alperin BR, Drew T, Karalunas SL. Behavioral and cognitive correlates of the aperiodic (1/f-like) exponent of the EEG power spectrum in adolescents with and without ADHD. Dev Cogn Neurosci 48: 100931, 2021. doi: 10.1016/j.dcn.2021.100931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robertson MM, Furlong S, Voytek B, Donoghue T, Boettiger CA, Sheridan MA. EEG power spectral slope differs by ADHD status and stimulant medication exposure in early childhood. J Neurophysiol 122: 2427–2437, 2019. doi: 10.1152/jn.00388.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karalunas SL, Ostlund BD, Alperin BR, Figuracion M, Gustafsson HC, Deming EM, Foti D, Antovich D, Dude J, Nigg J, Sullivan E. Electroencephalogram aperiodic power spectral slope can be reliably measured and predicts ADHD risk in early development. Dev Psychobiol 64: e22228, 2022. doi: 10.1002/dev.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaiser A, Aggensteiner PM, Baumeister S, Holz NE, Banaschewski T, Brandeis D. Earlier versus later cognitive event-related potentials (ERPs) in attention-deficit/hyperactivity disorder (ADHD): a meta-analysis. Neurosci Biobehav Rev 112: 117–134, 2020. doi: 10.1016/j.neubiorev.2020.01.019. [DOI] [PubMed] [Google Scholar]
- 23. Polich J. Neuropsychology of P300. In: The Oxford Handbook of Event-Related Potential Components, edited by Luck SJ, Kappenman ES.. Oxford, UK: Oxford University Press, 2012, p. 159–188. [Google Scholar]
- 24. Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol 118: 2128–2148, 2007. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kofler MJ, Irwin LN, Soto EF, Groves NB, Harmon SL, Sarver DE. Executive functioning heterogeneity in pediatric ADHD. J Abnorm Child Psychol 47: 273–286, 2019. doi: 10.1007/s10802-018-0438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pennington BF. From single to multiple deficit models of developmental disorders. Cognition 101: 385–413, 2006. doi: 10.1016/j.cognition.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 27. Kim MS, Kim JJ, Kwon JS. Frontal P300 decrement and executive dysfunction in adolescents with conduct problems. Child Psychiatry Hum Dev 32: 93–106, 2001. doi: 10.1023/a:1012299822274. [DOI] [PubMed] [Google Scholar]
- 28. Downes M, Bathelt J, De Haan M. Event‐related potential measures of executive functioning from preschool to adolescence. Dev Med Child Neurol 59: 581–590, 2017. doi: 10.1111/dmcn.13395. [DOI] [PubMed] [Google Scholar]
- 29. Jonkman LM, Kemner C, Verbaten MN, Koelega HS, Camfferman G, Vd Gaag RJ, Buitelaar JK, van Engeland H. Event-related potentials and performance of attention-deficit hyperactivity disorder: children and normal controls in auditory and visual selective attention tasks. Biol Psychiatry 41: 595–611, 1997. doi: 10.1016/s0006-3223(96)00073-x. [DOI] [PubMed] [Google Scholar]
- 30. Arnett AB, Rutter TM, Stein MA. Neural markers of methylphenidate response in children with attention deficit hyperactivity disorder. Front Behav Neurosci 16: 887622, 2022. doi: 10.3389/fnbeh.2022.887622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gabard-Durnam LJ, Mendez Leal AS, Wilkinson CL, Levin AR. The Harvard Automated Processing Pipeline for Electroencephalography (HAPPE): standardized processing software for developmental and high-artifact data. Front Neurosci 12: 97, 2018. doi: 10.3389/fnins.2018.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee TW, Lewicki MS, Sejnowski TJ. Unsupervised classification with non-Gaussian mixture models using ICA. In: NIPS'98: Proceedings of the 11th International Conference on Neural Information Processing Systems, edited by Kearns MJ, Solla SA, Cohn DA.. Cambridge, MA: MIT Press, 1999, p. 508–514. [Google Scholar]
- 33. Piazza C, Cantiani C, Akalin-Acar Z, Miyakoshi M, Benasich AA, Reni G, Bianchi AM, Makeig S. ICA-derived cortical responses indexing rapid multi-feature auditory processing in six-month-old infants. Neuroimage 133: 75–87, 2016. doi: 10.1016/j.neuroimage.2016.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Donoghue T, Dominguez J, Voytek B. Electrophysiological frequency band ratio measures conflate periodic and aperiodic neural activity. eNeuro 7: ENEURO.0192-20.2020, 2020. doi: 10.1523/ENEURO.0192-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Donoghue T, Haller M, Peterson EJ, Varma P, Sebastian P, Gao R, Noto T, Lara AH, Wallis JD, Knight RT, Shestyuk A, Voytek B. Parameterizing neural power spectra into periodic and aperiodic components. Nat Neurosci 23: 1655–1665, 2020. doi: 10.1038/s41593-020-00744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Podvalny E, Noy N, Harel M, Bickel S, Chechik G, Schroeder CE, Mehta AD, Tsodyks M, Malach R. A unifying principle underlying the extracellular field potential spectral responses in the human cortex. J Neurophysiol 114: 505–519, 2015. doi: 10.1152/jn.00943.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Virtue-Griffiths SE, Fornito A, Thompson S, Biabani M, Tiego J, Thapa T, Rogasch NC. Task-related changes in aperiodic activity are related to visual working memory capacity independent of event-related potentials and alpha oscillations (Preprint). bioRxiv 2022.01.18.476852, 2022. doi: 10.1101/2022.01.18.476852. [DOI]
- 38. Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci 9: 474–480, 2005. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 39. Rubia K. Neuro-anatomic evidence for the maturational delay hypothesis of ADHD. Proc Natl Acad Sci USA 104: 19663–19664, 2007. doi: 10.1073/pnas.0710329105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sibley MH, Arnold LE, Swanson JM, Hechtman LT, Kennedy TM, Owens E, Molina BS, Jensen PS, Hinshaw SP, Roy A, Chronis-Tuscano A, Newcorn JH, Rohde LA; MTA Cooperative Group. Variable patterns of remission from ADHD in the multimodal treatment study of ADHD. Am J Psychiatry 179: 142–151, 2022. doi: 10.1176/appi.ajp.2021.21010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Dinteren R, Arns M, Jongsma ML, Kessels RP. P300 development across the lifespan: a systematic review and meta-analysis. PLoS One 9: e87347, 2014. doi: 10.1371/journal.pone.0087347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barry RJ, Johnstone SJ, Clarke AR. A review of electrophysiology in attention-deficit/hyperactivity disorder: II. Event-related potentials. Clin Neurophysiol 114: 184–198, 2003. doi: 10.1016/s1388-2457(02)00363-2. [DOI] [PubMed] [Google Scholar]