Abstract
While progress has been made in fighting diseases disproportionally affecting underserved populations, unmet medical needs persist for many neglected tropical diseases. The World Health Organization has encouraged strong public-private partnerships to address this issue and several public and private organizations have set an example in the past showing a strong commitment to combat these diseases. Pharmaceutical companies are contributing in different ways to address the imbalance in research efforts. With this review, we exemplify the role of a public-private partnership in research and development by the journey of our dengue antiviral molecule that is now in early clinical development. We detail the different steps of drug development and outline the contribution of each partner to this process. Years of intensive collaboration resulted in the identification of two antiviral compounds, JNJ-A07 and JNJ-1802, the latter of which has advanced to clinical development.
Keywords: Public-private partnership, Dengue antiviral research and development
Abbreviations: ACTIV, an accelerating COVID-19 therapeutic interventions and vaccines; ADME, absorption distribution metabolism and excretion; BARDA, Biomedical Advanced Research and Development Authority; CARE, Corona Accelerated R&D in Europe; CD3, Center for Drug Design and Discovery; COVID-19, coronavirus disease 2019; DENV, dengue virus; DVI, dengue vaccine initiative; EIF, European Investment Fund; FDA, Food and Drug Administration; GLP, good laboratory practice; GPH, Global Public Health; IMI, Innovative Medicines Initiative; MMV, Medicines for Malaria Venture; MPD, Mectizan® Donation Program; NHP, non-human primates; NME, new molecular entity; NS, non-structural protein; NTD, neglected tropical disease; PPP, public-private partnership; SAR, structure-activity-relationship; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; TB, tuberculosis; UNITE4TB, academia and industry united innovation and treatment for tuberculosis; VLAIO, Vlaams Agentschap voor Innoveren & Ondernemen; WHO, World Health Organization
1. Introduction
The United States (US) Food and Drug Administration (FDA), defines an unmet medical need as a condition which is serious, for which treatment is not addressed adequately by available therapies, and which implies an immediate need for a defined population or a longer-term need for society (US Department of Health and Human ServicesF.a.D.A., 2014). Unmet medical needs exist across a wide range of disease categories including several rare diseases or orphan indications (e.g., cystic fibrosis, amyotrophic lateral sclerosis, and Tourette's syndrome), neglected tropical diseases (NTDs, e.g., Chagas disease, dengue, and leprosy) as well as non-orphan and non-neglected disease categories (e.g., Alzheimer's disease, Parkinson's diseases and major depressive disorder) (Kusynová et al., 2022; Putzeist et al., 2013; Scavone et al., 2019; Schmid and Smith, 2007; Weng et al., 2018; World Health Organization, 2022c). Reasons for unmet needs vary, but commonly unmet needs arise from inadequate investment due to an anticipated lack of financial return (Schmid and Smith, 2007; Weng et al., 2018) or from a scientific bottleneck (Kusynová et al., 2022).
With the recent pandemic caused by the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) entering the spotlight, an unprecedented global effort was undertaken to fight this disease. Collaborative research on the development of new treatments for coronavirus disease 2019 (COVID-19) is unlike anything previously seen (International Federation of Pharmaceutical Manufacturers and Associations, 2020). For instance, the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) partnership between the National Institutes of Health (NIH) in the US, and a number of pharmaceutical companies was built to facilitate the prioritization of therapeutic and vaccine candidates, irrespective of who had developed them. The commitment entailed that industry partners deployed their drug development competence regardless of the agent being studied, and the public partners, including the NIH mobilizing their clinical trial networks, as well as expertise, in a dedicated and prioritized work to resolve research and regulatory issues with unparalleled speed (Buchman et al., 2021; Collins and Stoffels, 2020).
Unfortunately, diseases occurring predominantly in low-income countries have historically experienced a scarcity of resources dedicated to research and development accentuated during the COVID-19 pandemic, especially in resource-limited settings (Akinokun et al., 2021). For instance, the COVID-19 pandemic has set back the progress in preventing tuberculosis (TB)-associated death by years. The year 2020 was registered as the first year-on-year increase (5.6%) in the number of people dying from TB since 2005 (World Health Organization, 2021).
While NTDs cause significant burden of disease (Herricks et al., 2017; Lin et al., 2022), research and development for drugs targeting these diseases receives less attention. Soh et al. (2021) estimated a dengue disease burden of 7645–21,262 disability-adjusted life years from 2010 to 2020 for Singapore using empirically derived disability weights and an economic impact ranging from $1.014 to $2.265 billion. To resolve this problem, several incentives were created to support drug development. For instance, the orphan drug act was enacted by the US government in 1983 to facilitate the development of drugs for rare diseases (Department of Health and Human Services, 2001) and in 2007, the FDA's priority review voucher program was created to encourage the development of new treatments for underfunded diseases (e.g., neglected diseases, rare pediatric diseases, or medical countermeasures) (US Government Accountability Office, 2020). While these initiatives spur research and development activities, they do not provide sufficient incentives for companies to engage in costly, risky and lengthy research and development of new compounds for these diseases (Aerts et al., 2022; Rutschman, 2017).
In an attempt to further address this issue, the World Health Organization (WHO) has encouraged a strong public-private partnership (PPP) engagement since the early 2000s (World Health Organization, 2000, 2022a). An important milestone was the London Declaration on Neglected Tropical Diseases in 2012, the largest coordinated effort to date to eradicate, eliminate, or control 10 NTDs by 2020 (Uniting to Combat NTDs, 2022b). The commitment was renewed in the Kigali Declaration on NTDs to eradicate, eliminate, or control NTDs by 2030 (Uniting to Combat NTDs, 2022a). Thanks to these initiatives, access to and research of medicines for treating NTDs has improved in the past decade (Bradley et al., 2021; Engels and Zhou, 2020; Weng et al., 2018). In this context, the pharmaceutical sector has executed their largest drug donations in history, improving their delivery logistics and engaging in research and development of efficient medications (Bradley et al., 2021; Engels and Zhou, 2020; World Health Organization, 2017). An example is Merck's Mectizan® Donation Program (MDP) to help control river blindness and, as later also included in the program, lymphatic filariasis (i.e., elephantiasis) (Mectizan® Donation Program, 2022). It is critical to further build on this success and strengthen current and future PPPs, as they form a crucial foundation for the research and development of efficacious drugs that target diseases disproportionally affecting underserved populations.
In this review, we will focus on the role of PPPs to accelerate research and development of innovative drugs to treat NTDs. The role of a PPP in research and development will be exemplified by the journey of our dengue antiviral molecule that is now in early clinical development. We particularly aim at describing the importance of the collaboration with different partners to support drug discovery and development.
2. Public-private partnership in R&D for neglected tropical diseases
Research and development underfunding is a major bottleneck to improve advancement of medications for NTDs. A shift from market-driven to needs-driven research and development programs is required to alleviate the disregard of these diseases (Trouiller et al., 2002). Recently, notable progress for NTDs has been made in the context of PPPs (Engels and Zhou, 2020). In this partnership, “private” refers to pharmaceutical companies (for profit) or charities, foundations, and philanthropic institutions (for non-profit). The “public” sector includes international organizations, development and aid agencies, governments, and academia (Trouiller et al., 2002).
The opportunities but also the challenges of a PPP lie in the goal of the partners involved. On the one hand, leveraging ideas, resources and expertise of the different partners may propel the innovation in the drug development process; on the other hand, timelines, resourcing, common objectives, responsibilities, and ethics need to be defined upfront to guarantee a successful and productive partnership.
The Medicines for Malaria Venture (MMV) (Medicines for Malaria Venture, 2022; Van Voorhis et al., 2016) and the dengue vaccine initiative (DVI) (International Vaccine Institute, 2022) are examples of fruitful partnerships that have advanced the field. If private partners and public institutions collaborate, parties will bring complementary resources and expertise to a shared mission accelerating research and development processes, which will translate into novel therapeutics. Following the example of private organizations (i.e., Bill & Melinda Gates Foundation (Bill, 2022)) and the World Bank (The World Bank, 2022), pharmaceutical companies are contributing to address the inequity in access to care, as exemplified by the Global Public Health (GPH) initiative from Johnson & Johnson (Johnson & Johnson, 2022a). The Johnson & Johnson's GPH initiative was launched in late 2014 to discover, develop, and deliver transformational medicines to address the world's greatest unmet public health needs to make a significant impact on the lives of people around the world. Its key focus areas include but are not limited to tuberculosis, leprosy, coronavirus, dengue and other flaviviruses. For this global strategy to thrive, GPH has opted for a multidisciplinary approach comprising world-class research and development, external affairs capabilities, local implementation and impact teams to generate healthier futures for the most vulnerable populations (Johnson & Johnson, 2022a). To ensure that the required critical solutions are within reach of those who need them, GPH relies on global and local partnerships with governments, donors, non-profits, and multilateral institutions. Within GPH different PPPs have been established to contribute to support research and development processes (Table 1). Another PPP critically involved in advancing the research and development of NTDs is the Novartis Institute for Tropical Diseases (NITD). The research facility was founded in 2003 as a Singapore-based PPP between Novartis and the Singapore Economic Development Board (EDB) (Normile, 2003), which moved in 2017 next to the Novartis' infectious diseases research headquarters in Emeryville, California (The Straits Times, 2016). Among the many accomplishments during the roughly 20 years of NITD's existence are the discovery of two antimalaria drug candidates now in clinical development (Novartis, 2022) and commitments entail to advance research and development for Chagas disease, leishmaniasis, dengue, and cryptosporidiosis. Several PPPs have been established in the past to tackle NTDs, e.g. dengue, Chagas disease, Chikungunya and others as summarized in Aerts et al. (2017).
Table 1.
Examples of public-private partnerships within Johnson & Johnson's Global Public Health.
| PPP with Johnson & Johnson's GPH involvement | Indication | |
|---|---|---|
| EU PPPs |
Innovative Medicines Initiative (IMI) Corona Accelerated R&D in Europe (CARE) consortium (https://www.imi-care.eu/) | COVID-19 antiviral small molecules and antibodies |
| IMI2 academia and industry united innovation and treatment for tuberculosis (UNITE4TB) (https://www.imi.europa.eu/projects-results/project-factsheets/unite4tb) | Tuberculosis antimicrobials | |
| Vlaams Agentschap voor Innoveren & Ondernemen (VLAIO) | Dengue antiviral and broad-spectrum flavivirus antiviral |
|
| Wellcome Trust | ||
| The University Leuven (KU Leuven) | ||
| Heidelberg University (HU) | ||
| Asian PPPs |
National Medical Research Council (NMRC) in Singapore | Dengue antiviral |
| National University of Singapore in conjunction with Duke University (UK) (Duke-NUS) | ||
| Johnson & Johnson Satellite Center for Global Health Discovery at Singapore's Duke-NUS Medical School, jointly established by Duke University and the National University of Singapore (Duke-NUS) | Broad-spectrum flavivirus antivirals | |
| USA PPPs | Biomedical Advanced Research and Development Authority (BARDA) (https://aspr.hhs.gov/AboutASPR/ProgramOffices/BARDA/Pages/default.aspx) | COVID-19 antiviral and vaccine |
| Medicines for Malaria Venture (MMV) (https://www.mmv.org/) | Long-acting anti-malarial drug | |
COVID-19, coronavirus disease 2019; EU, European Union; GPH, Global Public Health; PPP, public-private partnership; R&D, research and development; USA, United States of America.
Moving on to our class of dengue virus (DENV) inhibitors, a research collaboration between Janssen Pharmaceutical Inc, Wellcome Trust and the University of Leuven (KU Leuven) was initiated in September 2013 (Janssen, 2013). This collaboration aimed at developing a first-in-class antiviral drug for the prevention and/or treatment of dengue, both for vulnerable populations living in and for people traveling to dengue-endemic regions. KU Leuven initiated a drug discovery project against DENV in 2009 by combining the virology expertise of the Rega Institute with the drug discovery capabilities of its Centre for Drug Design and Discovery (CD3 – www.CD3.be) – the latter being a translational drug discovery unit established by KU Leuven and the European Investment Fund (EIF). The Wellcome Trust decided to support this endeavor at KU Leuven against DENV through its Seeding Drug Discovery Award scheme.
DENV is recognized as a global public health threat (World Health Organization, 2022d). Despite the growing awareness and investments, the health concern persists as dengue epidemics are increasing and the disease is expanding to new geographical regions (Messina et al., 2019; World Health Organization, 2022b). To prevent and cure dengue, an integrated approach is needed, focusing on vaccines, small molecule antivirals, vector control, diagnostics, and education/communication. The GPH dengue project contributes to this integrated approach with the development of a first-in-class small molecule dengue antiviral as detailed below. In the context of this development program, Johnson & Johnson has launched a Johnson & Johnson Satellite Center for Global Health Discovery at Singapore's Duke-NUS Medical School, jointly established by Duke University and the National University of Singapore (Duke-NUS) (Johnson & Johnson, 2022b) (Table 1). The center is focusing on developing broad-spectrum flavivirus antivirals (including dengue, Zika, West Nile, yellow fever and Japanese encephalitis). Singapore is located in a dengue-hyperendemic region (Lee et al., 2012) and is considered a leading innovation and research hub. Leveraging regional networks will empower people locally to drive research and development and efficiently provide help to those who are in need. Uniting the local skills and knowledge with the scientific expertise gained at Janssen through its global network, this interdisciplinary approach will help building a thriving, high quality research and development center and will reinforce mutual learning processes.
Janssen recognizes the value of the knowledge built up in epidemic areas that is not only critical for diseases such as dengue but also for other flavivirus infections. By leveraging and fostering the discovery expertise and knowhow in the Asia-Pacific region, the industry partner is establishing a rapid response framework to tackle future viral disease outbreaks. A key element of this approach is to work closely with various Singaporean agencies – especially with the Health Sciences Authority to ensure the development of a ‘science-based regulatory framework’. The framework may serve as a prime example to be deployed for other unmet medical needs. An effective and accessible oral, antiviral small molecule for the treatment and/or prevention of dengue and related flaviviruses may contribute to reduce the economic and societal burden for the entire Singaporean population and for preventing these infections in travelers to the Asia-Pacific region.
3. Overview of the development of a small molecule antiviral against dengue
As mentioned above, researchers at the Rega Institute and CD3, funded by Wellcome Trust, discovered an interesting novel compound class blocking DENV replication, which they developed to a lead stage with proof-of-concept in a DENV animal model. In 2013, they entered into a collaboration with Janssen Pharmaceuticals Inc. (Janssen) to further develop candidate antiviral drugs for the prevention and treatment of dengue infection (Janssen, 2013). This research collaboration benefited from the dengue discovery expertise at Janssen on the one hand, as Janssen has brought forward multiple chemical series at lead stage against different dengue viral proteins (e.g., NS2B-NS3 protease, and NS5 polymerase) since 2007and at the Rega Institute (KU Leuven) on the other hand, as also this team had been setting up several dengue assays since 2007. The research collaboration was built on a four-year drug discovery program at KU Leuven in which also a good knowledge was established about the structure-activity-relationship and early absorption, distribution, metabolism, and excretion (ADME)/pharmacokinetic (PK) properties of the compound class thanks to the medicinal chemistry team at CD3. Referring to this research collaboration, Professor Johan Neyts of the Department of Microbiology and Immunology at the Rega Institute of KU Leuven stated: ”We had hoped for a long time to develop efficacious drugs for the treatment and prophylaxis of dengue. Substantial funding from the Wellcome Trust allowed my team and CD3 to develop at KU Leuven, a class of potent dengue inhibitors for which we also demonstrated proof-of-concept in mouse dengue infection models. Once Johnson & Johnson GPH had in-licensed this class of inhibitors, my laboratory together with CD3 in Leuven engaged together with the company in the mission to develop a clinical candidate from this class.”
Starting from thousands of molecules in a compound library of CD3 and a hit compound having been optimized to a lead stage, candidate drugs were identified following further optimization in an iterative process whereby in total ∼2000 analogues of the initial hit were synthesized and tested against the four serotypes of the DENV, and a structure-activity relationship was derived. Years of intensive collaboration resulted in the identification of two compounds, JNJ-A07 and JNJ-1802. These compounds have a novel mechanism of action targeting viral replication, have potent antiviral activity against all four DENV serotypes including clinical isolates, have a high barrier to resistance, and have generated proof-of-concept data in animal models (Goethals et al., 2021; Kaptein et al., 2021). We recently described our findings about compound JNJ-A07, which targets the DENV non-structural protein (NS)4B and thereby prevents the interaction of NS4B with NS3 (Kaptein et al., 2021). JNJ-1802 was selected over JNJ-A07 based on its improved preclinical safety profile and has advanced to clinical development. This compound is being developed for two indications: prophylaxis and treatment of dengue infections. Based on preclinical data obtained in both in vitro models and in vivo mice and non-human primate (NHP) models, JNJ-1802 showed overall potent antiviral activity against all four DENV serotypes and a high specificity toward DENV (Goethals et al., 2021).
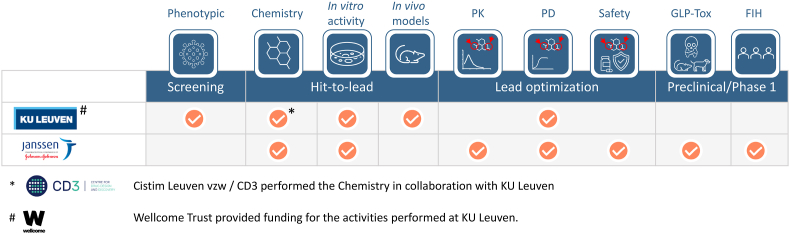
During the discovery phase (2013–2016), the effort contribution to the research work was equally divided between Janssen (50%) and KU Leuven and CD3 (via its partner CISTIM) (50%); CISTIM benefitted from KU Leuven, CD3 and substantial funding from Wellcome Trust, which also provided strategic oversight of the project (Fig. 1). The Rega Institute tested new compounds for antiviral activity against the different DENV serotypes, evaluated the compounds using in vitro resistance selection experiments, explored their activity in dengue mouse infection models and studied their molecular mechanism of action. Both CD3 and Janssen provided medicinal chemistry and early in vitro ADME support. In addition, Janssen and KU Leuven performed the high throughput in vitro profiling of the compounds in cells to achieve the structure-activity-relationship (SAR). In vivo PK profiling and advanced in vitro absorption, distribution, metabolism, and excretion (ADME), good laboratory practice (GLP) and non-GLP toxicity studies were performed by Janssen, and its expertise was applied to achieve new molecular entity (NME) approval for the JNJ-1802. The overall joint effort also included contributions from the Walter Reed Army Institute of Research (WRAIR, US; DENV-1 NHP model), the Biomedical Primate Research Center (BPRC, The Netherlands; DENV-2 NHP model), Heidelberg University (HU, Germany; mechanism of action studies), University of Texas Medical Branch (UTMB, US; DENV-1, 3 and 4 murine models) and Aix-Marseille University (AMU, France; in vitro antiviral activity studies). Johnson & Johnson has exclusive license for the compound and is the main funder of its clinical development. The phase 1a, first-in-human clinical trial showed that JNJ-1802 is generally safe and well tolerated in healthy participants (Ackaert et al., 2021). The clinical development of the compound is currently progressing to phase 2.
Fig. 1.
Overview of the contribution of each partner to the partnership.
The potential of jointly achieving a shared goal lies in the general notion that together a multidisciplinary team with various backgrounds is more than the sum of its parts. More specifically, in our PPP neither partner alone would have had the abilities and capacities or resources to successfully accelerate the program to the same extent as via a collaborative approach. Timothy Jinks, Head of Interventions for the Infectious Disease team at Wellcome Trust, stated that with this partnership, we were able to bring together groups with complementary skills and capabilities which allowed the project to pursue a robust drug discovery and development campaign. We benefited from leveraging scientific expertise and approaches, which resulted in the development of a highly promising clinical candidate. KU Leuven's long-lasting expertise in the different virology models together with CD3's knowledge about the medicinal chemistry aspects of this class of molecules laid the foundation of the development program and was indispensable for the success of this collaborative effort. Janssen's profound expertise in drug development allowed the program to move forward rapidly. However, the inter-institutional collaboration also presents several challenges. Creating an appropriate framework of this partnership prior to the start of the collaboration was difficult. Yet, as all partners shared a common vision about the purpose and the goal of the project, the required dedication and persistence was ensured to establish a practical framework aligned with the principles agreed on by all stakeholders. As with every research and development program, scientific issues that arose along the way added to the complexity of the project. However, given the interdisciplinary nature of this strong collaboration, the team succeeded in working out a solution and overcoming multiple hurdles. When setting up a partnership, it is critical to agree upfront on the key principles. Those core principles should then be used to guide discussions and negotiations to ultimately enable legal agreements covering the partnership. Throughout the duration of this partnership, we were reminded of the value of investing in people and creating a productive work environment by encouraging seamless and open communication as well as fostering creativity and empowerment. Thus, combining the unique expertise, competencies, and resources was pivotal to advance JNJ-1802 to clinical development.
PPPs are key drivers for global health and this partnership is a prime example on how to identify innovative solutions to deliver transformational medicines. Our goal is to address the world's greatest unmet public health needs through a sustainable platform that will make a significant impact on the lives of people around the world.
Funding
This work was supported by a Seeding Drug Discovery Award from the Wellcome Trust (grant 089328/Z/09 and grant 106327/Z/14) and received funding from the Flanders Agency Innovation & Entrepreneurship (VLAIO O&O grants IWT 150863 and HBC.2019.2906). Part of this research work was performed using the ‘Caps-It’ research infrastructure (project ZW13-02) that was financially supported by the Hercules Foundation (FWO) and Rega Foundation, KU Leuven. Part of this work was funded from CD3. Studies conducted at the University of Texas Medical Branch were undertaken with U.S. Federal funds from the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN272201700040I.
Declaration of competing interest
O. G., N. V., B. K., T. D. M., A. K., M.V.L. are all full-time employees of Janssen and potential stockholder of Johnson and Johnson. Some of the authors are named inventors in patent applications relating to the dengue virus replication inhibitors described in the article. The other authors declare no financial or competing interests.
Acknowledgments
The authors thank Anne-Theres Henze (Modis, on behalf of Janssen Pharmaceutica NV) who provided writing support and coordinated the manuscript development.
Data availability
The data described in this article is published in different manuscripts
References
- Ackaert O., Vanhoutte F., Verpoorten N., Buelens A., Lachau-Durand S., Lammens L., Van Loock M., Herrera-Taracena G. The 70th American Society of Tropical Medicine & Hygiene (ASTMH) Annual Meeting, November 17-21, 2021, Virtual Meeting. 2021. Safety, tolerability and pharmacokinetics of a novel pan-serotyp dengue antiviral small molecule in a phase 1, double-blind, randomized, dose-escalation study.https://www.astmh.org/getmedia/59a95de8-1a06-49ca-9fd0-286454cc241a/ASTMH-2021-Annual-Meeting-Abstract-Book.pdf Presented at: Abstract 0582. [Google Scholar]
- Aerts C., Barrenho E., Miraldo M., Sicuri E. The impact of the priority review voucher on research and development for tropical diseases. Pharmaceut. Med. 2022;36:189–197. doi: 10.1007/s40290-022-00427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aerts C., Sunyoto T., Tediosi F., Sicuri E. Are public-private partnerships the solution to tackle neglected tropical diseases? A systematic review of the literature. Health Pol. 2017;121:745–754. doi: 10.1016/j.healthpol.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Akinokun R.T., Ilesanmi E.B., Adebisi Y.A., Akingbade O. The status of neglected tropical diseases amidst covid-19 in africa: current evidence and recommendations. Health Promot. Perspect. 2021;11:430–433. doi: 10.34172/hpp.2021.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bill, Melinda Gates Foundation . 2022. Neglected Tropical Diseases.https://www.gatesfoundation.org/our-work/programs/global-health/neglected-tropical-diseases [Google Scholar]
- Bradley M., Taylor R., Jacobson J., Guex M., Hopkins A., Jensen J., Leonard L., Waltz J., Kuykens L., Sow P.S., Madeja U.-D., Hida T., Ole-Moiyoi K., King J., Argaw D., Mohamed J., Polo M.R., Yajima A., Ottesen E. Medicine donation programmes supporting the global drive to end the burden of neglected tropical diseases. Trans. R. Soc. Trop. Med. Hyg. 2021;115:136–144. doi: 10.1093/trstmh/traa167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman T.G., Draghia-Akli R., Adam S.J., Aggarwal N.R., Fessel J.P., Higgs E.S., Menetski J.P., Read S.W., Hughes E.A. Accelerating coronavirus disease 2019 therapeutic interventions and vaccines-selecting compounds for clinical evaluation in coronavirus disease 2019 clinical trials. Crit. Care Med. 2021;49:1963–1973. doi: 10.1097/CCM.0000000000005295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F.S., Stoffels P. Accelerating covid-19 therapeutic interventions and vaccines (activ): an unprecedented partnership for unprecedented times. JAMA. 2020;323:2455–2457. doi: 10.1001/jama.2020.8920. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services . 2001. The Orphan Drug Act. Implementation and Impact.https://oig.hhs.gov/oei/reports/oei-09-00-00380.pdf accessed. [Google Scholar]
- Engels D., Zhou X.-N. Neglected tropical diseases: an effective global response to local poverty-related disease priorities. Infectious Diseases of Poverty. 2020;9:10. doi: 10.1186/s40249-020-0630-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goethals O., Kaptein S.J., Kesteleyn B., Bonfanti J.F., Jonckers T.H.M., Marchand A., Verschoor E., Verstrepen B., Van Wesenbeeck L., Ackaert O., Straetemans R., Lachau-Durand S., Geluykens P., Crabbe M., Thys K., Bardiot D., Stoops B., Dallmeier K., Putnak R.J., McCracken M.K., Gromowski G.D., Rutvisuttinunt W., Jarman R.G., Karasavvas N., Touret F., Querat G., de Lamballerie X., Kiemel D., Chatel-Chaix L., Münster M., Milligan G., Beasley D., Bourne N., Barrett A.D.T., Raboisson P., Simmen K., Chaltin P., Bartenschlager R., Bogers W., Neyts J., Van Loock M. 2021. Unprecedented Preclinical Efficacy of Jnj-1802, a Novel Pan-Serotype Dengue Antiviral Small Molecule, against Dengue Virus in Non-human Primates and Murine Models Presented at: the 70th American Society of Tropical Medicine & Hygiene (ASTMH) Annual Meeting, November 17-21, 2021, Virtual Meeting.http://mesamalaria.org/sites/default/files/2021-11/ASTMH-2021-Annual-Meeting-Abstract-Book.pdf Abstract 1291. [Google Scholar]
- Herricks J.R., Hotez P.J., Wanga V., Coffeng L.E., Haagsma J.A., Basáñez M.G., Buckle G., Budke C.M., Carabin H., Fèvre E.M., Fürst T., Halasa Y.A., King C.H., Murdoch M.E., Ramaiah K.D., Shepard D.S., Stolk W.A., Undurraga E.A., Stanaway J.D., Naghavi M., Murray C.J.L. The global burden of disease study 2013: what does it mean for the ntds? PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Federation of Pharmaceutical Manufacturers & Associations . 2020. Global Pharma Update on Unprecedented Efforts to Collaborate in Speeding up the Search for Safe and Effective Covid-19 Therapies.https://www.ifpma.org/resource-centre/working-together-to-beat-covid-19-pharma-industrys-unprecedented-efforts-to-collaborate-in-speeding-up-the-search-for-covid-19-therapies/ accessed. [Google Scholar]
- International Vaccine Institute . 2022. Dengue and Aedes-Transmitted Diseases.https://www.ivi.int/what-we-do/disease-areas/dengue/ accessed. [Google Scholar]
- Janssen . 2013. Report to the Community. Collaboration in the Fight against Dengue Fever.http://www.janssen-verslag-samenleving-2013.be/en/economic-sustainability/collaboration-in-the-fight-against-dengue-fever.htm accessed. [Google Scholar]
- Johnson & Johnson . 2022. Global Public Health.https://www.jnj.com/global-public-health accessed. [Google Scholar]
- Johnson & Johnson 2022. https://www.jnj.com/johnson-johnson-opens-first-satellite-center-for-global-health-discovery-in-asia-pacific-at-duke-nus-to-advance-dengue-research Johnson & johnson opens first satellite center for global health discovery in asia pacific at duke-nus to advance dengue research. accessed.
- Kaptein S.J.F., Goethals O., Kiemel D., Marchand A., Kesteleyn B., Bonfanti J.F., Bardiot D., Stoops B., Jonckers T.H.M., Dallmeier K., Geluykens P., Thys K., Crabbe M., Chatel-Chaix L., Munster M., Querat G., Touret F., de Lamballerie X., Raboisson P., Simmen K., Chaltin P., Bartenschlager R., Van Loock M., Neyts J. A pan-serotype dengue virus inhibitor targeting the ns3-ns4b interaction. Nature. 2021;598:504–509. doi: 10.1038/s41586-021-03990-6. [DOI] [PubMed] [Google Scholar]
- Kusynová Z., Pauletti G.M., van den Ham H.A., Leufkens H.G.M., Mantel-Teeuwisse A.K. Unmet medical need as a driver for pharmaceutical sciences – a survey among scientists. J. Pharmacol. Sci. 2022;111:1318–1324. doi: 10.1016/j.xphs.2021.10.002. [DOI] [PubMed] [Google Scholar]
- Lee K.S., Lo S., Tan S.S., Chua R., Tan L.K., Xu H., Ng L.C. Dengue virus surveillance in Singapore reveals high viral diversity through multiple introductions and in situ evolution. Infect. Genet. Evol. 2012;12:77–85. doi: 10.1016/j.meegid.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Lin Y., Fang K., Zheng Y., Wang H.L., Wu J. Global burden and trends of neglected tropical diseases from 1990 to 2019. J. Trav. Med. 2022;29 doi: 10.1093/jtm/taac031. [DOI] [PubMed] [Google Scholar]
- Mectizan® Donation Program . 2022. History of the Program.https://mectizan.org/history/ [Google Scholar]
- Medicines for Malaria Venture . 2022. Developing Antimalarials to Save Lives.https://www.mmv.org/ [Google Scholar]
- Messina J.P., Brady O.J., Golding N., Kraemer M.U.G., Wint G.R.W., Ray S.E., Pigott D.M., Shearer F.M., Johnson K., Earl L., Marczak L.B., Shirude S., Davis Weaver N., Gilbert M., Velayudhan R., Jones P., Jaenisch T., Scott T.W., Reiner R.C., Jr., Hay S.I. The current and future global distribution and population at risk of dengue. Nat Microbiol. 2019;4:1508–1515. doi: 10.1038/s41564-019-0476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normile D. Novartis kicks off institute for neglected diseases. Science. 2003;299 doi: 10.1126/science.299.5608.811. 811-811. [DOI] [PubMed] [Google Scholar]
- Novartis Malaria. 2022. https://www.novartis.com/diseases/malaria
- Putzeist M., Mantel-Teeuwisse A.K., Llinares J., Gispen-De Wied C.C., Hoes A.W., Leufkens H.G.M. Eu marketing authorization review of orphan and non-orphan drugs does not differ. Drug Discov. Today. 2013;18:1001–1006. doi: 10.1016/j.drudis.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Rutschman A. vol. 125. All Faculty Scholarship; 2017. (The Priority Review Voucher Program at the Fda: from Neglected Tropical Diseases to the 21st Century Cures Act). [Google Scholar]
- Scavone C., di Mauro G., Mascolo A., Berrino L., Rossi F., Capuano A. The new paradigms in clinical research: from early access programs to the novel therapeutic approaches for unmet medical needs. Front. Pharmacol. 2019;10:111. doi: 10.3389/fphar.2019.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid E.F., Smith D.A. Pharmaceutical r&d in the spotlight: why is there still unmet medical need? Drug Discov. Today. 2007;12:998–1006. doi: 10.1016/j.drudis.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Soh S., Ho S.H., Seah A., Ong J., Dickens B.S., Tan K.W., Koo J.R., Cook A.R., Tan K.B., Sim S., Ng L.C., Lim J.T. Economic impact of dengue in Singapore from 2010 to 2020 and the cost-effectiveness of wolbachia interventions. PLOS Global Public Health. 2021;1 doi: 10.1371/journal.pgph.0000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Straits Times . 2016. Novartis Moving Research Facility Out of singapore.https://www.straitstimes.com/business/economy/novartis-moving-research-facility-out-of-singapore [Google Scholar]
- The World Bank . 2022. History.https://www.worldbank.org/en/about/history [Google Scholar]
- Trouiller P., Olliaro P., Torreele E., Orbinski J., Laing R., Ford N. Drug development for neglected diseases: a deficient market and a public-health policy failure. Lancet. 2002;359:2188–2194. doi: 10.1016/S0140-6736(02)09096-7. [DOI] [PubMed] [Google Scholar]
- Uniting to Combat NTDs . 2022. The Kigali Declaration.https://unitingtocombatntds.org/kigali-declaration/ [Google Scholar]
- Uniting to Combat NTDs . 2022. London Declaration on Neglected Tropical Diseases.https://unitingtocombatntds.org/resource-hub/who-resources/london-declaration-neglected-tropical-diseases/ accessed. [Google Scholar]
- US Department of Health and Human Services, F.a.D.A. 2014. Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER)https://www.fda.gov/files/drugs/published/Expedited-Programs-for-Serious-Conditions-Drugs-and-Biologics.pdf Guidance for industry expedited programs for serious conditions – drugs and biologics. [Google Scholar]
- US Government Accountability Office . 2020. Drug Development: Fda's Priority Review Voucher Programs.https://www.gao.gov/products/gao-20-251 [Google Scholar]
- Van Voorhis W.C., Adams J.H., Adelfio R., Ahyong V., Akabas M.H., Alano P., Alday A., Alemán Resto Y., Alsibaee A., Alzualde A., Andrews K.T., Avery S.V., Avery V.M., Ayong L., Baker M., Baker S., Ben Mamoun C., Bhatia S., Bickle Q., Bounaadja L., Bowling T., Bosch J., Boucher L.E., Boyom F.F., Brea J., Brennan M., Burton A., Caffrey C.R., Camarda G., Carrasquilla M., Carter D., Belen Cassera M., Chih-Chien Cheng K., Chindaudomsate W., Chubb A., Colon B.L., Colón-López D.D., Corbett Y., Crowther G.J., Cowan N., D'Alessandro S., Le Dang N., Delves M., DeRisi J.L., Du A.Y., Duffy S., Abd El-Salam El-Sayed S., Ferdig M.T., Fernández Robledo J.A., Fidock D.A., Florent I., Fokou P.V., Galstian A., Gamo F.J., Gokool S., Gold B., Golub T., Goldgof G.M., Guha R., Guiguemde W.A., Gural N., Guy R.K., Hansen M.A., Hanson K.K., Hemphill A., Hooft van Huijsduijnen R., Horii T., Horrocks P., Hughes T.B., Huston C., Igarashi I., Ingram-Sieber K., Itoe M.A., Jadhav A., Naranuntarat Jensen A., Jensen L.T., Jiang R.H., Kaiser A., Keiser J., Ketas T., Kicka S., Kim S., Kirk K., Kumar V.P., Kyle D.E., Lafuente M.J., Landfear S., Lee N., Lee S., Lehane A.M., Li F., Little D., Liu L., Llinás M., Loza M.I., Lubar A., Lucantoni L., Lucet I., Maes L., Mancama D., Mansour N.R., March S., McGowan S., Medina Vera I., Meister S., Mercer L., Mestres J., Mfopa A.N., Misra R.N., Moon S., Moore J.P., Morais Rodrigues da Costa F., Müller J., Muriana A., Nakazawa Hewitt S., Nare B., Nathan C., Narraidoo N., Nawaratna S., Ojo K.K., Ortiz D., Panic G., Papadatos G., Parapini S., Patra K., Pham N., Prats S., Plouffe D.M., Poulsen S.A., Pradhan A., Quevedo C., Quinn R.J., Rice C.A., Abdo Rizk M., Ruecker A., St Onge R., Salgado Ferreira R., Samra J., Robinett N.G., Schlecht U., Schmitt M., Silva Villela F., Silvestrini F., Sinden R., Smith D.A., Soldati T., Spitzmüller A., Stamm S.M., Sullivan D.J., Sullivan W., Suresh S., Suzuki B.M., Suzuki Y., Swamidass S.J., Taramelli D., Tchokouaha L.R., Theron A., Thomas D., Tonissen K.F., Townson S., Tripathi A.K., Trofimov V., Udenze K.O., Ullah I., Vallieres C., Vigil E., Vinetz J.M., Voong Vinh P., Vu H., Watanabe N.A., Weatherby K., White P.M., Wilks A.F., Winzeler E.A., Wojcik E., Wree M., Wu W., Yokoyama N., Zollo P.H., Abla N., Blasco B., Burrows J., Laleu B., Leroy D., Spangenberg T., Wells T., Willis P.A. Open source drug discovery with the malaria box compound collection for neglected diseases and beyond. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng H.-B., Chen H.-X., Wang M.-W. Innovation in neglected tropical disease drug discovery and development. Infectious Diseases of Poverty. 2018;7:67. doi: 10.1186/s40249-018-0444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Who/htp/edm; Geneva: 2000. Who Medicines Strategy: Framework for Action in Essential Drugs and Medicines Policy 2000–2003.https://apps.who.int/iris/bitstream/handle/10665/66503/WHO_EDM_2000.1.pdf?sequence=1&isAllowed=y [Google Scholar]
- World Health Organization . 2017. Crossing the Billion. Lymphatic Filariasis, Onchocerciasis, Schistosomiasis, Soil-Transmitted Helminthiases and Trachoma: Preventive Chemotherapy for Neglected Tropical Diseases.https://apps.who.int/iris/handle/10665/255498 [Google Scholar]
- World Health Organization . 2021. Global Tuberculosis Report 2021.https://www.who.int/publications/i/item/9789240037021 [Google Scholar]
- World Health Organization . 2022. Control of Neglected Tropical Diseases.https://www.who.int/teams/control-of-neglected-tropical-diseases/collaboration [Google Scholar]
- World Health Organization . 2022. Dengue and Severe Dengue. Fact Sheet.https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue [Google Scholar]
- World Health Organization . 2022. Negleted Tropical Diseases.https://www.who.int/health-topics/neglected-tropical-diseases#tab=tab_1 [Google Scholar]
- World Health Organization . 2022. Ten Threats to Global Health in 2019.https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data described in this article is published in different manuscripts