Abstract
The impact of exercise training and physiotherapy on heart function and pulmonary circulation parameters in heart failure with preserved ejection fraction (HFpEF) patients is uncertain. Hence, we performed a systematic review of published trials studying physical training in HFpEF population, with a focus on exercise and physiotherapy effect on left ventricular (LV), right ventricular (RV) morphological, functional, and pulmonary circulation parameters. We searched Cochrane Library and MEDLINE/PubMed for trials that evaluated the effect of exercise training and/or physiotherapy in adult HFpEF patients (defined as LVEF ≥ 45%), including publications until March 2021. Our systematic review identified eighteen articles (n = 418 trained subjects, 4 to 52 weeks of training) and covered heterogeneous trials with various populations, designs, methodologies, and interventions. Five of twelve trials revealed a significant reduction of mitral E/e’ ratio after the training (− 1.2 to − 4.9). Seven studies examined left atrial volume index; three of them showed its decrease (− 3.7 to − 8 ml/m2). Findings were inconsistent regarding improvement of cardiac output, E/A ratio, and E wave DecT and uncertain for RV function and pulmonary hypertension parameters. For now, no reliable evidence about rehabilitation effect on HFpEF cardiac mechanisms is available. There are some hypotheses generating findings on potential positive effects to parameters of LV filling pressure (E/e’), left atrium size, cardiac output, and RV function. This encourages a broader and more complex assessment of parameters reflecting cardiac function in future HFpEF exercise training studies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10741-022-10259-1.
Keywords: Exercise training, Physiotherapy, Heart failure with preserved ejection fraction, Cardiac imaging, Diastolic function
Introduction
Heart failure with preserved ejection fraction (HFpEF) accounts for approximately half of all heart failure (HF) patients and its burden is increasing [1]. A definite diagnosis of HFpEF can be made by right heart catheterization with pulmonary arterial wedge pressure (PAWP) ≥ 15 mmHg or left ventricular end diastolic pressure (LVEDP) ≥ 16 mmHg at rest in presence of preserved left ventricular systolic function. The hallmark of HFpEF is an elevation in left-sided filling pressures. In some patients, this leads to secondary pulmonary hypertension (PH). Pulmonary arterial pressure (PAP) is a marker of the severity and chronicity of pulmonary venous congestion/hypertension in HFpEF, and, if present, PH is associated with more pronounced symptoms and a poorer outcome [2, 3]. Consistent scientific data show that properly designed exercise interventions alone or as a component of comprehensive cardiac rehabilitation program for HF improved patients’ exercise capacity, symptoms, and health-related quality of life (QOL) and reduced the risk of all-cause and HF hospitalizations [4–7]. Of note, patients with HF with reduced ejection fraction (HFrEF) were predominant in most of the studies. European Society of Cardiology (ESC) guidelines for the diagnosis and treatment of acute and chronic HF recommend exercise rehabilitation not only to improve exercise capacity and QOL, but also to reduce HF hospitalizations, regardless of LV systolic function (Class I recommendation) [4].
The positive impact of exercise training on the functional capacity of HF patients is complex and can be determined by different pulmonary, cardiovascular, skeletal muscle, and metabolic adaptations that increase oxygen delivery and energy production [8]. As shown in several meta-analyses of randomized controlled trials (RCT), aerobic exercise training, especially long-term duration (≥ 6 months), reverses left ventricular (LV) remodeling in clinically stable patients with HFrEF [9–11]. Exercise training was associated with a significant improvement in LV ejection fraction (LV EF), when data from all RCT were pooled in the first meta-analysis (articles were published in 1966–2006; 14 trials; 812 patients) [9]. Later, this finding was confirmed in two updated meta-analyses (including articles published in 2006–2011: 16 trials with 813 patients, and 2007–2017: 18 trials with 1077 patients) [10, 11]. Moreover, in two meta-analyses, aerobic training led to significant improvements in LV end diastolic volume (EDV) (5 trials with 371 patients and 12 trials with 573 patients) and LV end systolic volume (ESV) (5 trials with 371 patients and 11 trials with 548 patients) [10, 11]. There are limited data on the effects of HFrEF patients exercising on more precise structural and functional echocardiographic parameters, such as myocardial velocities, strain and strain rate, stroke volume, right ventricular 3D ejection fraction, estimated systolic pulmonary arterial pressure, and size and collapsibility of inferior vena cava. This knowledge gap is even wider in HFpEF patients.
Earlier HFpEF rehabilitation systematic reviews and meta-analyses demonstrated positive impact of exercise training on functional capacity change, by improving peak oxygen uptake [12–17] and six-minute walk test distance [14, 15]. Moreover, HFpEF training seemed to be safe [12–16, 18, 19] and beneficial for the QOL of patients [12–17]. Five previous meta-analyses assessed the influence of exercising on only several echocardiographic parameters, mostly the mitral E/A ratios, E/e′ ratios, and E wave deceleration time (DecT), and their results were inconsistent [12–16]. Neither of prior systematic reviews evaluated the changes of right ventricular (RV) and pulmonary circulation parameters after the training. The summary of their findings is presented in Table 1.
Table 1.
Results from previous HFpEF exercise training meta-analyses: the changes of left ventricular function and morphology, comparing exercise training vs. control groups
| Meta-analysis | Designs of included studies, n | Participants (n) (training/control) | Results of specific echocardiographic parameter meta-analysis with exercise versus control |
|---|---|---|---|
| Taylor et al. [12] |
1 –observational 1 – non-RCT 1 – RCT |
102/38 | E/e′: − 0.9, 95% CI: − 3.8 to 2.0, P = 0.53*; random effect |
|
1 –observational 1 – non-RCT 1 – RCT |
84/45 | E/A: − 0.02, 95% CI: − 0.11 to 0.06, P = 0.56*; fixed effect | |
|
1 –observational 1 – RCT |
52/24 | LV EDV (ml): 4.5, 95% CI: − 1.8 to 10.9, P = 0.16*; fixed effect | |
|
1 –observational 2 – RCT |
96/44 | LV EF (%): 0.02, 95% CI; − 1.6 to 1.7, P = 0.98*; fixed effect | |
| Pandey et al. [13] | 4 – RCT | 82/81 | E/A: 0.08, 95% CI: − 0.01 to 0.16, P = 0.08#; fixed effect |
| 3 – RCT | 62/70 | DecT (ms): 2.92, 95% CI: − 18.56 to 24.41, P = 0.79#; fixed effect | |
| 5 – RCT | 126/111 | LV EF (%): 1.26, 95% CI: − 0.13 to 2.66%, P = 0.08#; fixed effect | |
| Dieberg et al. [14] | 4 – RCT | 85/60 | E/e′: − 2.3, 95% CI: − 3.44 to − 1.19, P < 0.0001*; fixed effect |
| 3 – RCT | 56/52 | E/A: 0.07, 95% CI: 0.02 to 0.12, P = 0.005*; fixed effect | |
| 3 – RCT | 56/52 | DecT (ms): − 13.2, 95% CI: − 19.8 to − 6.5, P = 0.0001*; fixed effect | |
| Chan et al. [15] | 5 – RCT | 115/89 | E/e′: − 2.38, 95% CI: − 3.47 to − 1.28, P < 0.0001*, fixed effect |
| 4 – RCT | 86/81 | E/A: + 0.07, 95% CI: 0.02 to 0.12, P = 0.006*, fixed effect | |
| 3 – RCT | 56/52 | DecT (ms): − 13.2, 95% CI: − 19.8 to − 6.5, P = 0.0001*, fixed effect | |
| Fukuta et al. [16] | 4 – RCT | 132/109 | E/e′: − 1.20, 95% CI: − 4.07 to 1.66, P = 0.41#, random effect |
| 5 – RCT | 128/124 | E/A: 0.03, 95% CI: − 0.02 to 0.08, P = 0.27#; random effect | |
| 3 – RCT | 102/79 | e′ (cm/s): 0.49, 95% CI: − 1.28 to 2.25, P = 0.59#; random effect | |
| 3 – RCT | 62/69 | DecT (ms): − 2.04, 95% CI: − 26.53 to 22.45, P = 0.87#; random effect | |
| 4 – RCT | 140/120 | LV EDV: − 0.03, 95% CI: − 0.28 to 0.21, P = 0.78#; fixed effect | |
| 3 – RCT | 116/90 | LV mass: 0.07, 95% CI: − 0.21 to 0.35, P = 0.61#; fixed effect | |
| 7 – RCT | 202/174 | LV EF: 0.85, 95% CI: − 0.128 to 1.83, P = 0.09#; fixed effect |
*Mean difference; #weighted mean difference
A single center exercise invasive hemodynamic study revealed that patients with HFpEF, complicated with PH and pulmonary vascular disease, demonstrate unique hemodynamic limitations during exercise that constrain aerobic capacity, including impaired recruitment of LV preload due to excessive right heart congestion (due to afterload) and blunted RV systolic reserve [20]. These conditions are leading to RV and pulmonary artery (PA) uncoupling with further limitation of exercise capacity and poor outcome [21].
In healthy subjects, intensive exercise has already shown to cause potentially deleterious remodeling of the RV [22, 23]. As pointed out by Arena et al. there may thus be an exercise training volume/intensity which may be detrimental to the RV in patients with HF and concomitant PH [24].
It is not clear whether changes of heart function and pulmonary circulation parallel improvement in cardiorespiratory fitness, or maybe exercise training may lead to harmful effects or worsening of the disease. We aimed to systematically review existing data on the impact of exercise and physiotherapy in HFpEF trials on LV, RV morphological, functional, and pulmonary circulation parameters.
Methods
We prepared this article by following the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines [25].
We conducted a Cochrane Library and MEDLINE/PubMed search for all types of trials that evaluated the effects of various types of exercise training and/or physiotherapy in adult (> 18 years) HFpEF patients (defined as LVEF ≥ 45%), including all papers published from December 1991 to March 2021. Studies that merely enrolled patients with other cardiac or respiratory diseases were excluded. HFpEF data was extracted from studies if various parameters were included and reported separately. The main outcomes of interest were any reported echocardiographic, MRI, and invasive hemodynamic parameters.
For each database search, we used two groups of keywords and their synonyms for participants and intervention. The search strategy for MEDLINE/PubMed can be found in the Supplementary Material (Table S1). We modified search strategies according to each database to achieve the broadest research.
The search was limited to human studies only, adults (> 18 years), and the results were filtered by “Clinical Trial,” “Meta-Analysis,” and “Systematic Review.” Additionally, for any potential eligible trials, we manually searched in clinicaltrials.gov, Google Scholar, and the references of the identified studies.
Each title and abstract were independently evaluated by 2 reviewers. If at least one of the reviewers considered the trial to be eligible, it was obtained for primary analysis. After initial review, the full texts of selected studies were assessed to verify eligibility criteria.
Two reviewers assessed methodological quality of studies using modified Downs and Black Quality checklist, which is meant to assess the quality for both randomized and non-randomized trials [26]. The sub-domain, estimating the power, was modified (if the study conducted a power analysis to determine the sample size needed to detect a significant difference in effect size, 1 point was added, if not – 0 point). The maximum score in this checklist was 28. The studies were rated as excellent, good, moderate, and poor, based on the percentage of the total score achieved: > 95% (≥ 25), 75–95% (21–24), 55–74% (16–20), and < 55% (≤ 15).
The same reviewers extracted data from the relevant articles, using pre-defined extraction forms, including the aspects of study population, such as mean age and sex, study design, intervention characteristics, follow-up period, and main outcomes. Any disagreements in data extraction were discussed until consensus was reached.
Results
Study identification and selection
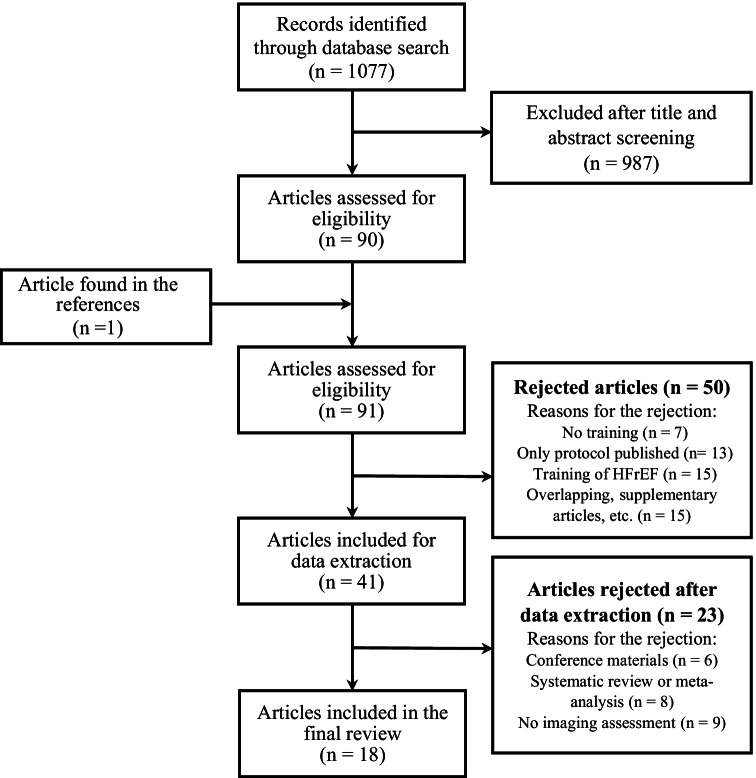
A flow diagram showing the selection of eligible studies is presented in Fig. 1. Initially, our searches in databases identified 1077 relevant publications; after primary review of titles and abstracts, 90 articles were eligible, and one extra paper was later found in the references. After full-text reading, 50 articles were rejected, thus the data were extracted from the rest 41 publications. The reasons for the rejection are described in Fig. 1.
Fig. 1.
Study flow diagram. This flow diagram shows the selection process of eligible studies
Among these 41 papers, 6 were conference materials and 8 were meta-analyses and (or) systematic reviews—they were removed from the final analyses—leaving 27 articles. Nine from 27 articles did not analyze cardiac imaging parameters at all resulting in a final data set of 18 articles for our systematic review (Fig. 1, Table 2). We contacted the corresponding authors of selected trials asking about any additional findings of echocardiographic, MRI, or invasive measurement parameters; nobody could provide any unpublished data.
Table 2.
Summary of articles selected and included in the review
| Study (author, year) | Duration of the training (weeks) | Intervention | Trained patients [N; mean age, years; male gender, N (%)] | Assessed echocardiographic parameters | Significant changes of specific echocardiographic parameters | ||
|---|---|---|---|---|---|---|---|
| Randomized controlled trials (N = 9) | |||||||
| Kitzman et al. [68] | 16 | Outpatient, supervised, endurance training; 60 min 3 times a week |
N = 26; age = 70 ± 6; male 6 (23.1%) 24 patients in the final analysis |
LV EF, E, A, E/A, DecT, IVRT, LVM, LVV, SV | No significant changes of measured echocardiographic parameters | ||
| Edelmann et al. [30] | 12 | Outpatient, supervised, endurance training (supplemented with resistance training from the 5th week); 40 min 3 times a week | N = 44; age = 64 ± 8; male 20 (45.5%) | LV EF, e' (septal), E/e' (septal), S/D ratio, LAVI, LVMI, LVVI |
↓E/e’ from 12.8 ± 3.2 to 10.5 ± 2.5, change − 2.3 (− 3.0 to − 1.6), p < 0.001; between groups − 3.2 (− 4.3 to − 2.1) p < 0.001 ↑e’ from 5.4 ± 1.2 to 6.3 ± 1.3, change 0.9 (0.6 to 1.1), p < 0.001; between groups 1.2 (0.8 to 1.7) p < 0.001 ↓LAVI from 27.9 ± 7.6 to 24.3 ± 6.5, change − 3.7 (− 4.9 to − 2.4), p < 0.001; between groups − 4.0 (− 5.9 to − 2.2) p < 0.001 |
||
| Alves et al. [31] | 26 | Outpatient, supervised, endurance training; 35 min 3 times a week |
N = 20; age = 62.9 ± 10.2; male 22 (71%) mean age and gender distribution of all of the sample size (trained + control) |
LV EF, E/A, DecT, LV dimensions (EDD, ESD) |
↑EF from 56.4 to 57.7, change 1.3% p = 0.01 ↑E/A from 0.93 to 1.05, p < 0.001 ↓DecT from 236.7 to 222.7, p < 0.001 |
||
| Smart et al. [27] | 16 | Outpatient, supervised, endurance training; 30 min 3 times a week | N = 12; age = 67 ± 5.8; male 7 (58.3%) | LV EF, E, A, E/A, E/e' (undetermined), DecT, s’, e’ (undetermined), SV, CO, LV-GLS, LV-GSR | ↑CO from 5.7 ± 2.7 to 7.1 ± 3.1, change 1.4, p = 0.04; | ||
| Haykowsky et al. [28] | 17 | Outpatient, supervised, endurance training; 60 min 3 times a week | N = 22; age = 70 ± 6; male 4 (18.2%) | LVV, SV, CO | No significant changes of measured echocardiographic parameters | ||
| Karavidas et al. [69] | 6 | Outpatient physiotherapy (functional electrical stimulation of the lower limb muscles); 30 min 5 times a week | N = 15; age = 69.4 ± 8.6; male 6 (40.0%) | E, A, E/A, E/e' (undetermined), DecT, LAV, a pulmonary − a mitral duration | No significant changes of measured echocardiographic parameters | ||
| Kitzman et al. [70] | 16 | Outpatient, supervised, endurance training; 60 min 3 times a week |
N = 32; age = 70 ± 7; male 9 (28.1%) 24 patients in the final analysis |
LV EF, E, A, E/A, DecT, IVRT, LVV, SV | No significant changes of measured echocardiographic parameters | ||
| Palau et al. [71] | 12 | At home, unattended, inspiratory muscle training; 20 min 2 times a day | N = 14; age = 68 [60–76]; male 7 (50.0%) | LV EF, e' (septal), E/e' (septal), LAVI, LVMI | No significant changes of measured echocardiographic parameters | ||
| Palau et al. [32] | 12 |
Home, unattended, inspiratory muscle training; 20 min 2 times a day Outpatient, physiotherapy (functional electrical stimulation of the lower limb muscles); 45 min 2 times a week |
IMT (baseline): N = 15; age = 75 ± 10; male 7 (46.7%) FES (baseline): N = 15; age = 72 ± 9; male 6 (40.0%) IMT + FES (baseline): N = 16; age = 73 ± 10; male 8 (50%) 13 patients in each group in the final analysis |
E/e' (undetermined), LAVI |
IMT: After 12 weeks: ↓E/e’ from 18.4 [14.4–28.0] to 17.2 [12.4–23.1], p = 0.015 After 24 weeks3: ↓LAVI from 39 ± 11 to 31 ± 11, p = 0.008 |
FES: After 12 weeks: ↓E/e’ from 20.5 [12–26.4] to 15.7 [11.8–21.8], p = 0.001 After 24 weeks3: No significant changes of measured echocardiographic parameters |
IMT + FES: After 12 weeks: No significant changes of measured echocardiographic parameters After 24 weeks3: No significant changes of measured echocardiographic parameters |
| Randomized parallel group trials (N = 5) | |||||||
| Yeh et al. [72] | 12 | Outpatient and home, endurance training compared with Taichi training; supervised 60 min 2 times a week + exercising at home 35 min 3 times a week |
AT: N = 8; age = 63 ± 11; male 4 (50.0%) Tai Chi: N = 8; age = 68 ± 11; male 4 (50.0%) |
LV EF, E/A, E/e' (undetermined), LA dimension, LAV |
Tai Chi#: ↓LA dimensions decreased more in the Tai Chi (from 3.8 ± 0.4 to 3.7 ± 0.3) comparing with AT (from 3.7 ± 0.3 to 3.8 ± 0.3), p = 0.04 |
AT#: ↓E⁄e’ improved more in the AT (from 17 ± 4 to 14 ± 4), comparing with the Tai Chi (from 14 ± 4 to 13 ± 5), p = 0.01 |
|
| Angadi et al. [33] | 4 | Outpatient, supervised, endurance training; 45 min 3 times a week |
HIT: N = 9; age = 69.0 ± 6.1; male 8 (88.9%) MI-ACT: N = 6; age = 71.5 ± 11.7; male 4 (66.7%) |
LV EF, E, A, e' (septal), E/A, E/e' (septal), DecT, IVRT, diastolic dysfunction grade1, diastolic dysfunction grade distribution2, LAVI |
HIIT: ↓Diastolic dysfunction grade* from 2.1 ± 0.3 to 1.3 ± 0.7, p < 0.01 ↓E from 0.9 ± 0.3 to 0.8 ± 0.3, p = 0.02 ↑DecT from 194 ± 55 to 225 ± 40, p = 0.02 |
MI-ACT: No significant changes of measured echocardiographic parameters |
|
| Angadi et al. [29]** | 4 | Outpatient, supervised, endurance training; 45 min 3 times a week |
HIIT: N = 9; age = 69 ± 6.1; male 8 (88.9%) MI-ACT: N = 6; age = 71.5 ± 11.7; male 4 (66.7%) |
LV EF, LVM, LVMI, SV, SVI, RV-GLS, RV-GSR, LV-GLS, LV-GSR |
HIIT: ↑RV-GLS from − 18.4 ± 3.2 to − 21.4 ± 1.7, p = 0.02 |
MI-ACT: No significant changes of measured echocardiographic parameters |
|
| Silveira et al. [36] | 12 | Outpatient, supervised, endurance training; 38 min (HIIT), 47 min (MCT) 3 times a week |
HIIT: N = 10; age = 60 ± 10; male 3 (30.0%) MI-ACT: N = 9; age = 60 ± 9; male 4 (44.4%) |
LV EF, E, A, e' (average), E/A, E/e' (average), DecT, LV dimensions (EDD, ESD), LAVI, LVM, LVVI, LA diameter, SVI |
HIIT: ↓E/e’ from 13.3 ± 3 to 11.1 ± 2, p < 0.001 |
MI-ACT: ↓E/e’ from 14.2 ± 4 to 11.6 ± 3, p < 0.001 |
|
| Mueller et al. [73] | 52 | Outpatient, supervised (3 months), then continued at home, unattended (for the next 9 months), endurance training; 38 min (HIIT), 47 min (MCT) 3 times a week |
HIIT: N = 58; age = 70 ± 7; male 17 (29.3%) MI-ACT: N = 58; age = 70 ± 8; male 23 (39.7%) 47 patients in the HIIT final and 52 in the MI-ACT final analysis |
E/e' (septal), e' (septal), LAVI | No significant change on measured echocardiographic parameters between HIIT, MI-ACT, and control group# | ||
| Observational trials (N = 4) | |||||||
| Smart et al. [74] | 16 | Outpatient, supervised, endurance training (supplemented with resistance training from the 8th week); 60 min 3 times a week | N = 18; age = 65 ± 5; male 9 (50.0%) | LV EF, E, A, s’, e’ (average), E/A, E/e' (average), DecT, LVV, LV-GLS, LV-GSR, SV | No significant changes of measured echocardiographic parameters | ||
| Fujimoto et al. [37] | 52 | Outpatient, supervised, endurance training; 40 min 3 times a week | N = 7; age = 74.9 ± 6; male 3 (42.9%) | LV EF, E, A, e' (average), a’, E/A, IVRT, LVV, LVVI | ↑E/A from 0.75 ± 0.11 to 0.89 ± 0.14, p = 0.03 | ||
| Nolte et al. [34]* | 24 | Outpatient, supervised, endurance training (supplemented with resistance training from the 5th week); 30–35 min; 3 times a week | N = 24; age = 62 ± 7; male 15 (62.5%) | LV EF, e' (septal), E/e' (septal), S/D ratio, LAVI, LVMI, LVVI |
↓E/e’ from 12.2 ± 3.5 to 10.1 ± 3.0, change − 2.1 (− 3.3 to − 0.9), p = 0.002 ↑e’ from 5.9 ± 1.3 to 6.8 ± 1.4, change 0.9 (0.4 to 1.4), p = 0.001 ↓LAVI from 30.0 ± 7.9 to 25.1 ± 8.7, change − 4.9 (− 6.7 to − 3.2), p < 0.001 |
||
| Fu et al. [35] | 12 | Outpatient, supervised, endurance training; 30 min 3 times a week | N = 30; age = 60.5 ± 2.7; male 20 (66.7%) | LV EF, E/A, E/e’(septal), LV dimensions (EDD, ESD) | ↓E/e ‘ (septal) from 21.0 ± 2.2 to 16.1 ± 1.8, p < 0.05 | ||
Data are expressed by number, mean ± standard deviation, median (interquartile range)
A (m/s) late mitral inflow velocity, a’ (m/s) tissue Doppler mitral annular late diastolic velocity, AT aerobic training, CO (l/min.) cardiac output, DecT (ms) mitral flow E wave deceleration time, E (m/s) early mitral inflow velocity, e’ (m/s) tissue Doppler mitral annular early diastolic velocity, E/A E and A ratio, EDD (mm) end diastolic diameter, E/e’ E and e’ ratio, FES functional electrical stimulation, EF (%) ejection fraction, ESD (mm) end systolic diameter, HIIT high-intensity interval training, IMT inspiratory muscle training, IVRT (ms) isovolumetric relaxation time, LA left atrium, LA dimensions (cm) the measurement was not clearly defined, LAV (ml) left atrium volume, LAVI (ml/m2) left atrium volume index, LV left ventricle, LV-GLS (%) left ventricle global longitudinal strain, LV-GSR (s−1) left ventricle global longitudinal strain rate, LVM (g) left ventricle mass, LVMI (g/m2) left ventricle mass index, LVV (ml) left ventricle volume, LVVI (ml/m2) left ventricle volume index, MI-ACT moderate-intensity aerobic continuous training, RV-GLS (%) right ventricle global longitudinal strain, RV-GSR (s−1) right ventricle global longitudinal strain rate, s’(m/s) tissue Doppler mitral annular systolic velocity, S/D pulmonary vein peak systolic velocity and peak diastolic velocity ratio, SV (ml) stroke volume, SVI (ml/m2) stroke volume index
#p values comparing the changes between the groups (changes before and after training in separate groups were not published)
*In this trial, the same patients that participated in the study of Edelmann et al. [30] were enrolled. Authors presented the same data of trained group changes after 12 weeks of training in both articles, but this article was supplemented by additional data after longer exercise training period (24 weeks); **In this study, the same patients that participated in the study Angadi et al. [33] were assessed, but different echocardiographic parameters were measured—secondary analyses to explore the effects of HIIT on biventricular strain characteristics was carried out
aFour grades of diastolic dysfunction were used (0—no diastolic dysfunction, 1—abnormal relaxation pattern, 2—pseudonormal pattern, 3—restrictive filling pattern)
bNumber of patients in each of the four diastolic dysfunction grades
cFollow-up was extended to 24 weeks with the aim of exploring the sustainability of the 12-week training results
Characteristics of included studies and patients
We extracted the data from nine randomized controlled trials, five randomized parallel group trials (no control, all patients trained, but different training protocols were used), and four observational studies (one group, no control).
All studies included stable patients, diagnosed with HFpEF. The trials were performed in different years (1994–2018) and the definition of HFpEF used in each study varied, but in all trials, LV EF of the participants was ≥ 45%. Echocardiography was applied to measure LV EF in all studies: 7 trials used Simpson biplane, 1—Teichholz method (by M-mode echocardiography), and the remaining 10 studies did not specify the methodology. Detailed information about HFpEF definition used in each study is provided in Supplementary Material (Table S2). All incorporated studies measured at least one cardiac imaging parameter, reflecting LV diastolic function, RV function, or pulmonary hemodynamics.
Our study covered heterogeneous trials with various designs, populations, methodologies, and interventions. The majority of studies were small in sample size—more than 25 patients were trained in only four of the eighteen trials. All training programs were held out-patient, and in sixteen of them, intervention was supervised by healthcare professionals. Eleven programs consisted of endurance training alone, and four, endurance and resistance workouts in combination. Two studies applied resistance training, and in one of them, functional electric stimulation (FES) was added. Only FES was used in a single trial. Various research appraised multifarious echocardiographic parameters and none of them provided random variability in the data for these outcomes. In the selected trials, overall 418 patients (mean age 60.0 to 75.0, 57% female, training duration 4 to 52 weeks) were trained. The components of exercise training and (or) physiotherapy, together with other characteristics and results of these studies, are summarized in Table 2.
The methodological quality of trials, assessed by modified Downs and Black Quality checklist, varied between excellent (n = 3), moderate (n = 5), good (n = 9), and poor (n = 1), as summarized in Table 3.
Table 3.
The quality assessment of included studies by modified Downs and Black Quality checklist
| Study type | Reporting (11) | External validity (3) | Internal validity | Power (1) | Total (28) | Quality* | ||
|---|---|---|---|---|---|---|---|---|
| Bias (7) | Confounding (6) | |||||||
| Kitzman et al. [70] | RCT | 10 | 3 | 6 | 5 | 1 | 25 | Excellent |
| Donelli da Silveira et al. [36] | Randomized parallel group | 10 | 3 | 6 | 5 | 1 | 25 | Excellent |
| Mueller et al. [73] | Randomized parallel group | 10 | 3 | 6 | 5 | 1 | 25 | Excellent |
| Kitzman et al. [68] | RCT | 10 | 2 | 6 | 4 | 1 | 23 | Good |
| Edelmann et al. [30] | RCT | 10 | 1 | 6 | 5 | 1 | 23 | Good |
| Alves et al. [31] | RCT | 10 | 1 | 6 | 5 | 1 | 23 | Good |
| Smart et al. [27] | RCT | 9 | 3 | 5 | 5 | 1 | 23 | Good |
| Palau et al. [71] | RCT | 9 | 2 | 6 | 5 | 1 | 23 | Good |
| Karavidas et al. [69] | RCT | 8 | 3 | 5 | 5 | 1 | 22 | Good |
| Palau et al. [32] | RCT | 9 | 1 | 5 | 5 | 1 | 21 | Good |
| Yeh et al. [72] | Randomized parallel group | 9 | 1 | 6 | 5 | 0 | 21 | Good |
| Angadi et al. [29] | Randomized parallel group | 9 | 1 | 5 | 5 | 1 | 21 | Good |
| Haykowsky et al. [28] | RCT | 10 | 1 | 5 | 4 | 0 | 20 | Moderate |
| Angadi et al. [33] | Randomized parallel group | 8 | 1 | 4 | 5 | 1 | 19 | Moderate |
| Smart et al. [74] | Observational | 10 | 0 | 5 | 1 | 0 | 16 | Moderate |
| Nolte et al. [34] | Observational | 9 | 1 | 5 | 4 | 0 | 19 | Moderate |
| Fu et al. [35] | Observational | 9 | 1 | 6 | 3 | 1 | 20 | Moderate |
| Fujimoto et al. [37] | Observational | 9 | 1 | 3 | 2 | 0 | 15 | Poor |
RCT randomized controlled trial
*Evaluated by the total score number: ≥ 25 – excellent, 21–24 – good, 16–20 – moderate, ≤ 15 – poor. Studies with no significant changes of assessed echocardiographic parameters after the intervention are marked in gray
Echocardiographic assessment
All included studies analyzed the changes of echocardiography as secondary endpoints. Various studies appraised multifarious echocardiographic parameters (Table 2). The quantity of studies that assessed specific parameters along with the number of trained patients is shown in Table 4.
Table 4.
The echocardiographic parameters, assessed in selected studies
| Echocardiographic parameter | Number of the studies with assessment of parameter | Number of trained patients with assessment of parameter |
|---|---|---|
| LV EF | 14 | 292 |
| E/e’ | 12 | 330 |
| E/e’ (septal) | 6 | 211 |
| E/e’ (average) | 2 | 37 |
| E/e’ (undetermined) | 4 | 82 |
| e’ | 9 | 261 |
| e’ (septal) | 5 | 205 |
| e’ (average) | 3 | 44 |
| e’(undetermined) | 1 | 12 |
| LAVI | 7 | 250 |
| E/A | 11 | 210 |
| E | 8 | 144 |
| A | 8 | 144 |
| E wave DecT | 8 | 144 |
| LVMI | 4 | 92 |
| SV | 6 | 113 |
| LVVI | 4 | 87 |
| LV IVRT | 4 | 80 |
| SVI | 3 | 46 |
| LV GLS | 3 | 45 |
| LV GRS | 3 | 45 |
| CO | 2 | 34 |
| LV tissue S vel | 2 | 30 |
| RV GLS | 1 | 15 |
| RV GSR | 1 | 15 |
Following parameters were NOT assessed in the included studies: RV diameter, SPAP, TV laterals’, TAPSE, RV FAC, RA area, RA pressure, and IVC diameters
A (m/s) late mitral inflow velocity, a’ (m/s), tissue Doppler mitral annular late diastolic velocity, CO (l/min.) cardiac output, DecT (ms) mitral flow E wave deceleration time, E (m/s) early mitral inflow velocity, e’ (m/s) tissue doppler mitral annular early diastolic velocity, E/A E and A ratio, E/e’ E and e’ ratio, EF (%) ejection fraction, IVRT (ms) isovolumetric relaxation time, LAVI (ml/m2) left atrium volume index, LV left ventricle, LV-GLS (%) left ventricle global longitudinal strain, LV-GSR (s-1) left ventricle global longitudinal strain rate, LVMI (g/m2) left ventricle mass index, LVVI (ml/m2) left ventricle volume index, RA right atrium, RV-GLS (%) right ventricle global longitudinal strain, RV-GSR (s-1) right ventricle global longitudinal strain rate, s’(m/s) tissue doppler mitral annular systolic velocity, SPAP (mmHg) systolic pulmonary artery pressure, SV (ml) stroke volume, SVI (ml/m2) stroke volume index, TAPSE (mm) tricuspid annular plane systolic excursion
As it is shown in Table 2, different studies demonstrated controversial results of the training impact on echocardiographic change. Variations of echocardiographic measurements of trained patients before and after intervention were published in sixteen articles (two trials declared only the changes comparing different training modalities). LV EF and E/e’ were parameters most frequently analyzed—in 14 and 12 studies, respectively.
Five of nine RCTs, four of five randomized parallel group trials, and three of four observational trials reported significant changes of different echocardiographic parameters by training, while the other studies detected no changes in the assessed parameters (Table 2). Significant reduction of mitral E/e’ ratio after the training was reported in 5 of 12 studies, ranging from − 1.2 to − 4.9; significant decrease of LAVI was observed in 3 of 7 trials, ranging from − 3.7 to − 8 ml/m2. All but one study showed no significant change of LV EF after the intervention.
Inconsecutive findings were also reported for the change of E/A ratio (9/11 studies showed no change, two statistically significant increase) and E wave DecT (8 studies, one significant increase, one significant decrease).
Furthermore, the impact of exercise training on cardiac output (CO) was reported with inconsistent results, including improvement of CO by 24.5% in one small (n = 12), good quality study, after 16 weeks of endurance exercise training, organized 30 min 3 times a week [27]. Another moderate quality trial with older patients (n = 22) demonstrated no significant changes of CO after similar duration endurance exercise training, 60 min 3 times a week [28].
The effect of exercising on RV function was assessed in one study [29]. RV global longitudinal strain and RV global longitudinal strain rate were measured before and after 4 weeks of high intensity interval training (HIIT) (n = 9) and moderate intensity aerobic continuous training (MI-ACT) (n = 6). HIIT group patients demonstrated the increase of RV global longitudinal strain by 3% (from − 18.4 ± 3.2 to − 21.4 ± 1.7), p = 0.02. The changes between MI-ACT group patients were insignificant.
As it is shown in Table 4, any other right heart and pulmonary hypertension parameters were not evaluated in the included trials.
Not all studies were well-balanced by the gender of trained participants (Table 4) and neither of them compared echocardiographic changes after the intervention according to sex. However, the majority of trials that revealed significant changes of specific echocardiographic parameters (E/e’, LAVI, DecT, CO, EF, RV-GLS) included predominantly males, or males amounted at least 45% (Table 4) [27, 29–35]. The study of Silveira et al. was the only one with female predominance (63.2%) and significant decrease of E/e’; in this study, a multivariate model was created to adjust E/e’ differences for age, BMI, and sex; the effect of training on E/e’ remained statistically significant after the adjustment [36].
Invasive hemodynamic assessment
Invasive hemodynamic measurements were performed in only one very small (n = 7) poor quality study [37]. Right heart catheterization was performed at baseline and after a year of endurance exercise training. The results revealed that pulmonary artery wedge pressure (PAWP) was unaffected by exercise training in HFpEF patients (16.1 ± 5.6 vs. 15.2 ± 3.6 mmHg, p = 0.65). A year of training had no effect on Starling curves (stroke volume index/PAWP) or stroke work-LVEDV relations (as a parameter of LV contractile function), suggesting no change in LV filling and contractile function.
Cardiac magnetic resonance imaging
None of the eighteen eligible studies assessed the impact of exercise rehabilitation to cardiac magnetic resonance imaging parameters.
Discussion
To the best of our knowledge, this is the first systematic literature review, which investigated the exercise training and physiotherapy impact not only on LV, but also RV morphology, function, and pulmonary circulation parameters in HFpEF patients. The results of our extended literature review were inconsistent. Eleven of 18 (61.1%) of the considered studies reported a positive impact of exercise training on at least one left and/or right heart function echocardiographic parameter. In seven of all eligible studies (38.9%, five RCTs), no significant changes were observed. Neither of the trials revealed negative effects of exercise training or physiotherapy on heart function (the outcome was positive or indifferent). These findings are in line with previous meta-analyses reporting inconsistent effects of exercise training in HFpEF [12–16].
LV diastolic dysfunction in HFpEF
Twelve studies assessed mitral E/e′ ratio, and significant decrease of it was observed in five of them [30, 32, 34–36]. Mitral E/e’ ratio is the measure widely accepted as an index of LV filling pressure, but it also has limitations that are relevant in clinical practice, and it is not recommended to use as a single follow-up echocardiographic parameter in HFpEF [38–41].
LAVI is a further echocardiographic parameter, reflecting LV filling pressure, which is crucially important to measure, when assessing LV diastolic function [41]. We found seven articles with published LAVI assessments; three of these studies revealed statistically significant decrease of LAVI after the intervention [30, 32, 34]. Two out of three studies observed significant decrease of LAVI together with substantial decrease of mitral E/e’ ratio, strengthening the tendency of positive training impact to LV diastolic function. When these two parameters were evaluated together, no significant differences of mitral E/e’ ratio or LAVI changes were identified, when comparing the usual care (control) group with any of active treatment groups (IMT; FES; IMT + FES). However, when the analysis of each interventional group was performed separately, significant changes were observed [32]. After 12 weeks of IMT, statistically significant decrease of median mitral E/e’ ratio was observed, while the change of median LAVI was insignificant. After 24 weeks follow-up period, statistically significant decrease of median LAVI was noticed, but the decrease of median mitral E/e’ ratio became insignificant, comparing with the baseline data [32]. These findings could either be explained by the necessity of longer time for the remodeling of LA, as LAVI reflects the cumulative effects of increased LV filling pressures over time [41], or it could be an accidental finding, due to small sample size.
Improvements in mitral E/e’ ratio were more often detected in the trials with larger sample size, which may be a hint that the other studies were underpowered for the effect size of E/e’ changes. Significant decrease in LAVI was more common in studies with a longer follow-up period, which supports the theory of a longer period required to induce reverse atrial remodeling.
Some studies of our systematic review appraised intervention impact on LV diastolic function by evaluating the changes of mitral E wave, A wave, E/A ratio. However, the increase of mitral E wave or E/A ratio can be associated both with improvement and deterioration of diastolic function (a shift from impaired relaxation pattern to normal diastolic function, but also with a shift from normal diastolic function or pseudonormalized pattern to the restrictive filling pattern). For the same reasons and bidirectional interpretation, the changes of mitral E wave DecT and IVRT should not be used for pooled data analysis as well. A solution could be the graduation of LV diastolic function, as it was done by Angadi et al. [33], or use of unidirectional LV diastolic function indices such as mitral e’ and E/e’ ratio.
RV dysfunction and pulmonary hypertension in HFpEF
Though LV diastolic dysfunction is considered to be the cornerstone of HFpEF, the pathophysiology of the disease is complex. It consists not only of variable contributions of diastolic dysfunction, but also of impaired atrial function, impaired contractile reserve, ventriculo–arterial uncoupling, RV dysfunction, and pulmonary hypertension (PH) [42–44]. Despite variable reports, methods, and criteria, the best available current data indicate that RV dysfunction is present in up to 30–50% of HFpEF. It appears to be present in 18%, 28%, and 21% of HFpEF patients using RV FAC, TAPSE, and RV S’ measurements, respectively [45, 46]. Increased LV filling pressure (> 12 mmHg) in HFpEF promotes symptoms of dyspnea [47], impairs exercise capacity [48], and leads to pulmonary venous congestion and secondary PH, which are associated with worse symptoms and overall prognosis of HFpEF [2]. PH is common in HFpEF—a population-based study reported echocardiographic signs of PH in 83% of HFpEF patients [2]. In a prospective invasive hemodynamic assessment study, 77% of HFpEF patients were diagnosed with PH, and 12% of them had combined post- and pre-capillary PH (CpcPH) [49]. According the recent recommendations to define PH as mean pulmonary artery pressure > 20 mmHg is considered to be abnormal [50]; these numbers probably are even higher.
As LV diastolic dysfunction parameters are not the only ones that are relevant to the symptoms and prognosis of HFpEF patients [2, 44–47, 51, 52], we believe that evaluating effectiveness of exercise training on cardiac mechanisms should not be limited by the estimation of LV function single parameters. Instead, it should be more inclusive, by additionally assessing structural and functional measurements of both atria (LAVI, RA area, RA pressure), LV (LVMI, EDV, ESV, SV), RV, and pulmonary circulation (RV area, RV FAC, TAPSE, RV S’, estimated PAP) and using more precise methods, including 3D and speckle tracking echocardiography. Even applying extended inclusion criteria for the trials, we found very few studies assessing change of indicated LA and LV echocardiographic parameters after the training; almost no studies analyzed specific right heart and pulmonary circulation parameters. This implies the need to evaluate them in the future HFpEF rehabilitation studies.
Impact of exercise training on HFpEF with PH
There are scientific insights on heterogeneity of HFpEF patients, recommending to look for specific phenotypes [53]. Previously, PH was considered to be limited to the end-stage HFpEF patients, but the study by Borlaug BA et al. revealed abnormalities in PA vasodilation and dynamic RV-PA coupling even in the earliest stages of HFpEF [43]. HFpEF patients with PH (HFpEF-PH) differ in hemodynamics and exercise intolerance, compared with HFpEF patients without PH. Phenotyping HFpEF patients according to the presence of PH in the exercise training studies could be beneficial in gaining a better understanding of the workouts’ role on pulmonary circulation changes and finding the optimal exercise training modality for an individual patient. Significant impact of pulmonary vascular disease on the pathophysiology of exercise intolerance was already proven. During symptom limited peak exercise, CpcPH-HFpEF patients, comparing with non-PH-HFpEF and isolated post capillary PH, demonstrated greater increase in right atrial pressure, enhanced ventricular interdependence, and displayed an inability to enhance cardiac output together with blunted augmentation in RV systolic performance; these changes were coupled with marked limitation in aerobic capacity [20].
Extra-cardiac mechanisms of exercise intolerance in HFpEF and PH
Though major reasons for reduced physical capacity in many patients with HFpEF seem to be cardiac, non-cardiac factors are also very important. Reduced peripheral oxygen extraction during exercise in these patients was observed [54–56] that can be related to adverse changes in leg muscle mass and volume [57]. The role of extra-cardiac mechanisms of exercise intolerance in PH is probably even more prominent; they include respiratory muscle weakness, dynamic hyperinflation and mechanical constraints [58], poor skeletal muscle and cerebral oxygenation, hyperventilation, and enhanced sympathetic drive [59–61]. Skeletal muscles represent the largest pool of proteins in the organism, and its proper function is essential for locomotion and breathing [62]. Loss of skeletal muscle mass, that is characteristic in advanced HFpEF and PH, directly contributes to exercise intolerance. Exercise training provides benefits at the molecular and physiological level preventing muscle wasting and reduction in force generation [62, 63].
Limitations
Our systematic review included trials that were conducted in different years (1994–2018). The definition of HFpEF used in each study was not the same. The lowest limit of LV EF in this review was 45%, and according to the very recent ESC guidelines, one of the definition criteria for HFpEF diagnosis is LV EF ≥ 50%, while LV EF 41–49% is considered to be a diagnostic criteria for HFmrEF [4]. Involving a lot of studies with different designs and various statistics, we did not perform a meta-analysis, but previous systematic reviews and meta-analyses of RCT revealed controversial echocardiographic changes after exercise training [12–16]. Potential reasons for these inconsistent results could be related with pooled evaluation of studies with different populations, methodologies, and protocols, when different exercise training modalities and durations of training period and unequal HFpEF diagnostic criteria were used, as well as small sample sizes of the trials. Moreover, the mechanisms of exercise intolerance in HFpEF are complex and the improvement of cardiorespiratory fitness after exercise training might be mediated by cardiac and extra-cardiac mechanisms, being only partially dependent on LV function [28, 64, 65].
Future research
Our work encourages future HFpEF rehabilitation trials to be supplemented by right heart function and pulmonary circulation evaluation in addition with more precise assessments of LV parameters. Further studies that consider echocardiographic changes after exercising according to sex could be beneficial. Moreover, estimation of specialized rehabilitation influence in HFpEF-PH phenotype would be useful, as until now we have no information about training safety and effectiveness in these patients, while the effectiveness of standardized exercise training in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension was already demonstrated [66, 67].
Conclusions
This systematic literature review that aimed to evaluate and summarize existing data of exercise training and physiotherapy impact on LV, RV morphological, functional, and pulmonary circulation parameters in HFpEF revealed a gap in this area. There are some hypotheses generating findings on potential positive effects on parameters of LV filling pressure (E/e’), left atrial size, cardiac output, and right ventricular function (RV-GLS). However, no reliable evidence about rehabilitation effect to HFpEF cardiac mechanisms is available for now. This encourages a broader and more complex assessment of parameters reflecting cardiac function in the future HFpEF exercise training studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Ethics approval
Due to the design of the trial (literature review), the study was exempted from requiring ethics approval.
Competing interests
The author E.P. reports speaker honoraria fees from Johnson and Johnson and Medis Pharma outside this work. The authors T.Š., C.A.E, N.B., and B.E. have no competing interests to declare. The author E.G. reports research grants and speaker honoraria/consultancy fees from Actelion, Janssen, Bayer, MSD, Merck, and Ferrer and research grants to the institution from Acceleron, Actelion, Bayer, MSD, Janssen, Liquidia, United Therapeutics, and OMT outside the submitted work. The author J.Č. reports personal fees from AstraZeneca, Boehringer Ingelheim, Pfizer, Bayer, and Novartis outside this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reddy YNV, Borlaug BA. Heart failure with preserved ejection fraction. Curr Probl Cardiol. 2016;41:145–188. doi: 10.1016/j.cpcardiol.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Lam CSP, Roger VL, Rodeheffer RJ, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Opitz CF, Hoeper MM, Gibbs JSR, et al. Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol. 2016;68:368–378. doi: 10.1016/j.jacc.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 4.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 5.Pelliccia A, Sharma S, Gati S, et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42:17–96. doi: 10.1093/eurheartj/ehaa605. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor RS, Long L, Mordi IR, et al. Exercise-based rehabilitation for heart failure: Cochrane systematic review, meta-analysis, and trial sequential analysis. JACC Heart Fail. 2019;7:691–705. doi: 10.1016/j.jchf.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Crimi E, Ignarro LJ, Cacciatore F, Napoli C. Mechanisms by which exercise training benefits patients with heart failure. Nat Rev Cardiol. 2009;6:292–300. doi: 10.1038/nrcardio.2009.8. [DOI] [PubMed] [Google Scholar]
- 9.Haykowsky MJ, Liang Y, Pechter D, et al. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J Am Coll Cardiol. 2007;49:2329–2336. doi: 10.1016/j.jacc.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 10.Chen YM, Li ZB, Zhu M, Cao YM. Effects of exercise training on left ventricular remodelling in heart failure patients: an updated meta-analysis of randomised controlled trials. Int J Clin Pract. 2012;66:782–791. doi: 10.1111/j.1742-1241.2012.02942.x. [DOI] [PubMed] [Google Scholar]
- 11.Tucker WJ, Beaudry RI, Liang Y, et al. Meta-analysis of exercise training on left ventricular ejection fraction in heart failure with reduced ejection fraction: a 10-year update. Prog Cardiovasc Dis. 2019;62:163–171. doi: 10.1016/j.pcad.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor RS, Davies EJ, Dalal HM, et al. Effects of exercise training for heart failure with preserved ejection fraction: a systematic review and meta-analysis of comparative studies. Int J Cardiol. 2012;162:6–13. doi: 10.1016/j.ijcard.2012.05.070. [DOI] [PubMed] [Google Scholar]
- 13.Pandey A, Parashar A, Kumbhani D, et al. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail. 2015;8:33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dieberg G, Ismail H, Giallauria F, Smart NA. Clinical outcomes and cardiovascular responses to exercise training in heart failure patients with preserved ejection fraction: a systematic review and meta-analysis. J Appl Physiol Bethesda Md. 2015;119:726–733. doi: 10.1152/japplphysiol.00904.2014. [DOI] [PubMed] [Google Scholar]
- 15.Chan E, Giallauria F, Vigorito C, Smart NA. Exercise training in heart failure patients with preserved ejection fraction: a systematic review and meta-analysis. Monaldi Arch Chest Dis Arch Monaldi Mal Torace. 2016;86:759. doi: 10.4081/monaldi.2016.759. [DOI] [PubMed] [Google Scholar]
- 16.Fukuta H, Goto T, Wakami K, et al. Effects of exercise training on cardiac function, exercise capacity, and quality of life in heart failure with preserved ejection fraction: a meta-analysis of randomized controlled trials. Heart Fail Rev. 2019;24:535–547. doi: 10.1007/s10741-019-09774-5. [DOI] [PubMed] [Google Scholar]
- 17.Gomes-Neto M, Durães AR, Conceição LSR, et al. Effect of aerobic exercise on peak oxygen consumption, VE/VCO2 slope, and health-related quality of life in patients with heart failure with preserved left ventricular ejection fraction: a systematic review and meta-analysis. Curr Atheroscler Rep. 2019;21:45. doi: 10.1007/s11883-019-0806-6. [DOI] [PubMed] [Google Scholar]
- 18.Leggio M, Fusco A, Loreti C, et al. Effects of exercise training in heart failure with preserved ejection fraction: an updated systematic literature review. Heart Fail Rev. 2020;25:703–711. doi: 10.1007/s10741-019-09841-x. [DOI] [PubMed] [Google Scholar]
- 19.Palau P, Núñez E, Domínguez E, et al. Physical therapy in heart failure with preserved ejection fraction: a systematic review. Eur J Prev Cardiol. 2016;23:4–13. doi: 10.1177/2047487314562740. [DOI] [PubMed] [Google Scholar]
- 20.Gorter TM, Obokata M, Reddy YNV, et al. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J. 2018;39:2825–2835. doi: 10.1093/eurheartj/ehy331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rommel K-P, von Roeder M, Oberueck C, et al. Load-independent systolic and diastolic right ventricular function in heart failure with preserved ejection fraction as assessed by resting and handgrip exercise pressure-volume loops. Circ Heart Fail. 2018;11:e004121. doi: 10.1161/CIRCHEARTFAILURE.117.004121. [DOI] [PubMed] [Google Scholar]
- 22.La Gerche A, Heidbüchel H, Burns AT, et al. Disproportionate exercise load and remodeling of the athlete’s right ventricle. Med Sci Sports Exerc. 2011;43:974–981. doi: 10.1249/MSS.0b013e31820607a3. [DOI] [PubMed] [Google Scholar]
- 23.La Gerche A, Burns AT, Mooney DJ, et al. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J. 2012;33:998–1006. doi: 10.1093/eurheartj/ehr397. [DOI] [PubMed] [Google Scholar]
- 24.Arena R, Lavie CJ, Borghi-Silva A, et al. Exercise training in group 2 pulmonary hypertension: which intensity and what modality. Prog Cardiovasc Dis. 2016;59:87–94. doi: 10.1016/j.pcad.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smart NA, Haluska B, Jeffriess L, Leung D. Exercise training in heart failure with preserved systolic function: a randomized controlled trial of the effects on cardiac function and functional capacity. Congest Heart Fail Greenwich Conn. 2012;18:295–301. doi: 10.1111/j.1751-7133.2012.00295.x. [DOI] [PubMed] [Google Scholar]
- 28.Haykowsky MJ, Brubaker PH, Stewart KP, et al. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angadi SS, Jarrett CL, Sherif M, et al. The effect of exercise training on biventricular myocardial strain in heart failure with preserved ejection fraction. ESC Heart Fail. 2017;4:356–359. doi: 10.1002/ehf2.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edelmann F, Gelbrich G, Düngen H-D, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 31.Alves AJ, Ribeiro F, Goldhammer E, et al. Exercise training improves diastolic function in heart failure patients. Med Sci Sports Exerc. 2012;44:776–785. doi: 10.1249/MSS.0b013e31823cd16a. [DOI] [PubMed] [Google Scholar]
- 32.Palau P, Domínguez E, López L, et al. Inspiratory muscle training and functional electrical stimulation for treatment of heart failure with preserved ejection fraction: the TRAINING-HF trial. Rev Espanola Cardiol Engl Ed. 2019;72:288–297. doi: 10.1016/j.rec.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Angadi SS, Mookadam F, Lee CD et al (2015) High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: a pilot study. J Appl Physiol Bethesda Md 1985 119:753–758. 10.1152/japplphysiol.00518.2014 [DOI] [PubMed]
- 34.Nolte K, Schwarz S, Gelbrich G, et al. Effects of long-term endurance and resistance training on diastolic function, exercise capacity, and quality of life in asymptomatic diastolic dysfunction vs. heart failure with preserved ejection fraction. ESC Heart Fail. 2014;1:59–74. doi: 10.1002/ehf2.12007. [DOI] [PubMed] [Google Scholar]
- 35.Fu T-C, Yang N-I, Wang C-H, et al. Aerobic interval training elicits different hemodynamic adaptations between heart failure patients with preserved and reduced ejection fraction. Am J Phys Med Rehabil. 2016;95:15–27. doi: 10.1097/PHM.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 36.Donelli da Silveira A, Beust de Lima J, da Silva PD, et al. High-intensity interval training is effective and superior to moderate continuous training in patients with heart failure with preserved ejection fraction: a randomized clinical trial. Eur J Prev Cardiol. 2020;27:1733–1743. doi: 10.1177/2047487319901206. [DOI] [PubMed] [Google Scholar]
- 37.Fujimoto N, Prasad A, Hastings JL, et al. Cardiovascular effects of 1 year of progressive endurance exercise training in patients with heart failure with preserved ejection fraction. Am Heart J. 2012;164:869–877. doi: 10.1016/j.ahj.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lancellotti P, Galderisi M, Edvardsen T, et al. Echo-Doppler estimation of left ventricular filling pressure: results of the multicentre EACVI Euro-Filling study. Eur Heart J Cardiovasc Imaging. 2017;18:961–968. doi: 10.1093/ehjci/jex067. [DOI] [PubMed] [Google Scholar]
- 39.Obokata M, Borlaug BA. The strengths and limitations of E/e’ in heart failure with preserved ejection fraction. Eur J Heart Fail. 2018;20:1312–1314. doi: 10.1002/ejhf.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pieske B, Tschöpe C, de Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) Eur Heart J. 2019;40:3297–3317. doi: 10.1093/eurheartj/ehz641. [DOI] [PubMed] [Google Scholar]
- 41.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507–515. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 43.Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37:3293–3302. doi: 10.1093/eurheartj/ehw241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borlaug BA, Olson TP, Lam CSP, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorter TM, van Veldhuisen DJ, Bauersachs J, et al. Right heart dysfunction and failure in heart failure with preserved ejection fraction: mechanisms and management. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:16–37. doi: 10.1002/ejhf.1029. [DOI] [PubMed] [Google Scholar]
- 46.Gorter TM, Hoendermis ES, van Veldhuisen DJ, et al. Right ventricular dysfunction in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Heart Fail. 2016;18:1472–1487. doi: 10.1002/ejhf.630. [DOI] [PubMed] [Google Scholar]
- 47.Obokata M, Olson TP, Reddy YNV, et al. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J. 2018;39:2810–2821. doi: 10.1093/eurheartj/ehy268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reddy YNV, Olson TP, Obokata M, et al. Hemodynamic correlates and diagnostic role of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. JACC Heart Fail. 2018;6:665–675. doi: 10.1016/j.jchf.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerges M, Gerges C, Pistritto A-M, et al. Pulmonary hypertension in heart failure. Epidemiology, Right Ventricular Function, and Survival. Am J Respir Crit Care Med. 2015;192:1234–1246. doi: 10.1164/rccm.201503-0529OC. [DOI] [PubMed] [Google Scholar]
- 50.Simonneau G, Montani D, Celermajer DS et al (2019) Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 53. 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed]
- 51.Melenovsky V, Hwang S-J, Lin G, et al. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–3462. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gorter TM, van Veldhuisen DJ, Voors AA, et al. Right ventricular-vascular coupling in heart failure with preserved ejection fraction and pre- vs. post-capillary pulmonary hypertension. Eur Heart J Cardiovasc Imaging. 2018;19:425–432. doi: 10.1093/ehjci/jex133. [DOI] [PubMed] [Google Scholar]
- 53.Del Buono MG, Iannaccone G, Scacciavillani R, et al. Heart failure with preserved ejection fraction diagnosis and treatment: an updated review of the evidence. Prog Cardiovasc Dis. 2020;63:570–584. doi: 10.1016/j.pcad.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 54.Haykowsky MJ, Brubaker PH, John JM, et al. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dhakal BP, Malhotra R, Murphy RM, et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015;8:286–294. doi: 10.1161/CIRCHEARTFAILURE.114.001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhella PS, Prasad A, Heinicke K, et al. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tucker WJ, Angadi SS, Haykowsky MJ, et al. Pathophysiology of exercise intolerance and its treatment with exercise-based cardiac rehabilitation in heart failure with preserved ejection fraction. J Cardiopulm Rehabil Prev. 2020;40:9–16. doi: 10.1097/HCR.0000000000000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laveneziana P, Garcia G, Joureau B, et al. Dynamic respiratory mechanics and exertional dyspnoea in pulmonary arterial hypertension. Eur Respir J. 2013;41:578–587. doi: 10.1183/09031936.00223611. [DOI] [PubMed] [Google Scholar]
- 59.Malenfant S, Brassard P, Paquette M, et al. Compromised cerebrovascular regulation and cerebral oxygenation in pulmonary arterial hypertension. J Am Heart Assoc. 2017;6:e006126. doi: 10.1161/JAHA.117.006126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malenfant S, Potus F, Mainguy V, et al. Impaired skeletal muscle oxygenation and exercise tolerance in pulmonary hypertension. Med Sci Sports Exerc. 2015;47:2273–2282. doi: 10.1249/MSS.0000000000000696. [DOI] [PubMed] [Google Scholar]
- 61.Müller-Mottet S, Hildenbrand FF, Keusch S, et al. Effects of exercise and vasodilators on cerebral tissue oxygenation in pulmonary hypertension. Lung. 2015;193:113–120. doi: 10.1007/s00408-014-9667-5. [DOI] [PubMed] [Google Scholar]
- 62.Adams V, Reich B, Uhlemann M, Niebauer J. Molecular effects of exercise training in patients with cardiovascular disease: focus on skeletal muscle, endothelium, and myocardium. Am J Physiol Heart Circ Physiol. 2017;313:H72–H88. doi: 10.1152/ajpheart.00470.2016. [DOI] [PubMed] [Google Scholar]
- 63.Marra AM, Arcopinto M, Bossone E, et al. Pulmonary arterial hypertension-related myopathy: an overview of current data and future perspectives. Nutr Metab Cardiovasc Dis NMCD. 2015;25:131–139. doi: 10.1016/j.numecd.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Adamopoulos S, Parissis J, Kroupis C, et al. Physical training reduces peripheral markers of inflammation in patients with chronic heart failure. Eur Heart J. 2001;22:791–797. doi: 10.1053/euhj.2000.2285. [DOI] [PubMed] [Google Scholar]
- 65.Linke A, Schoene N, Gielen S, et al. Endothelial dysfunction in patients with chronic heart failure: systemic effects of lower-limb exercise training. J Am Coll Cardiol. 2001;37:392–397. doi: 10.1016/s0735-1097(00)01108-6. [DOI] [PubMed] [Google Scholar]
- 66.Grünig E, Eichstaedt C, Barberà J-A, et al. ERS statement on exercise training and rehabilitation in patients with severe chronic pulmonary hypertension. Eur Respir J. 2019;53:1800332. doi: 10.1183/13993003.00332-2018. [DOI] [PubMed] [Google Scholar]
- 67.Grünig E, MacKenzie A, Peacock AJ, et al. Standardized exercise training is feasible, safe, and effective in pulmonary arterial and chronic thromboembolic pulmonary hypertension: results from a large European multicentre randomized controlled trial. Eur Heart J. 2021;42:2284–2295. doi: 10.1093/eurheartj/ehaa696. [DOI] [PubMed] [Google Scholar]
- 68.Kitzman DW, Brubaker PH, Morgan TM, et al. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karavidas A, Driva M, Parissis JT, et al. Functional electrical stimulation of peripheral muscles improves endothelial function and clinical and emotional status in heart failure patients with preserved left ventricular ejection fraction. Am Heart J. 2013;166:760–767. doi: 10.1016/j.ahj.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 70.Kitzman DW, Brubaker PH, Herrington DM, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. J Am Coll Cardiol. 2013;62:584–592. doi: 10.1016/j.jacc.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palau P, Domínguez E, Núñez E, et al. Effects of inspiratory muscle training in patients with heart failure with preserved ejection fraction. Eur J Prev Cardiol. 2014;21:1465–1473. doi: 10.1177/2047487313498832. [DOI] [PubMed] [Google Scholar]
- 72.Yeh GY, Wood MJ, Wayne PM, et al. Tai chi in patients with heart failure with preserved ejection fraction. Congest Heart Fail Greenwich Conn. 2013;19:77–84. doi: 10.1111/chf.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mueller S, Winzer EB, Duvinage A, et al. Effect of high-intensity interval training, moderate continuous training, or guideline-based physical activity advice on peak oxygen consumption in patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2021;325:542–551. doi: 10.1001/jama.2020.26812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smart N, Haluska B, Jeffriess L, Marwick TH. Exercise training in systolic and diastolic dysfunction: effects on cardiac function, functional capacity, and quality of life. Am Heart J. 2007;153:530–536. doi: 10.1016/j.ahj.2007.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.