Abstract
Objective
The novel coronavirus (severe acute respiratory syndrome coronavirus 2) has been spreading worldwide since December 2019, posing a serious danger to human health and socioeconomic development. A large number of clinical trials have revealed that coronavirus disease 2019 (COVID-19) results in multi-organ damage including the urogenital system. This study aimed to explore the potential mechanisms of genitourinary damage associated with COVID-19 infection through bioinformatics and molecular simulation analysis.
Methods
We used multiple publicly available databases to explore the expression patterns of angiotensin-converting enzyme 2 (ACE2), transmembrane serine protease 2 (TMPRSS2), and CD147 in major organs in the healthy and disease-specific populations, particularly the genitourinary organs. Single-cell RNA sequencing was used to analyze the cell-specific expression patterns of ACE2, TMPRSS2, CD147, cytokine receptors, and cytokine interacting proteins in genitourinary organs, such as the bladder, kidney, prostate, and testis. Additionally, gene set enrichment analysis was used to investigate the relationship between testosterone levels and COVID-19 vulnerability in patients with prostate cancer.
Results
The results revealed that ACE2, TMPRSS2, and CD147 were highly expressed in normal urogenital organs. Then, they were also highly expressed in multiple tumors and chronic kidney diseases. Additionally, ACE2, TMPRSS2, and CD147 were significantly expressed in a range of cells in urogenital organs according to single-cell RNA sequencing. Cytokine receptors and cytokine interacting proteins, especially CCL2, JUN, and TIMP1, were commonly highly expressed in urogenital organs. Finally, gene set enrichment analysis results showed that high testosterone levels in prostate cancer patients were significantly related to the JAK-STAT signaling pathway and the Toll-like receptor signaling pathway which were associated with COVID-19.
Conclusion
Our study provides new insights into the potential mechanisms of severe acute respiratory syndrome coronavirus 2 damage to urogenital organs from multiple perspectives, which may draw the attention of urologists to COVID-19 and contribute to the development of targeted drugs.
Keywords: Coronavirus disease 2019, Severe acute respiratory syndrome coronavirus 2, Angiotensin-converting enzyme 2, Transmembrane serine protease 2, CD147, Genitourinary organ, Testosterone
1. Introduction
Since December 2019, several cases of pneumonia with unknown etiology were reported in some hospitals around the world which garnered tremendous attention both domestically and abroad [1]. On January 7, 2020, the Chinese Center for Disease Control and Prevention discovered the pathogen from throat swab samples, naming it severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) subsequently [2]. The World Health Organization named the disease coronavirus disease 2019 (COVID-19) and declared COVID-19 outbreak a pandemic on 11 March 2020, and COVID-19 has since become one of the largest threats to the economy and public health in the twenty-first century [3]. A rising number of clinical trials showed that COVID-19 caused multi-organ damage, and genitourinary organs were no exception, despite the fact that SARS-CoV-2 is known to cause severe lung illness, including pneumonia and acute respiratory distress syndrome [4,5].
The specific pathogenesis of genitourinary damage caused by SARS-CoV-2 is still not clear, and drug treatment targets and specific effective drugs are still under investigation. Related studies have suggested that the critical molecules for SARS-CoV-2 infection of the organism include transmembrane serine protease 2 (TMPRSS2) and angiotensin-converting enzyme 2 (ACE2) [6]. Our previous research showed that ACE2 and TMPRSS2 were highly expressed in the genitourinary organs (especially kidneys and testis), indicating that kidney and testis were potential target organs for SARS-CoV-2 [7]. Additionally, research pointed to CD147 as a potential additional route by which SARS-CoV-2 might infect host cells [8,9]. Su et al. [10] found that COVID-19 patients showed increased CD147 expression in the kidney compared to normal subjects, and that the CD147-CypA axis might play a role in the occurrence of kidney damage in COVID-19 patients. Wang et al. [11] reported a direct interaction between CD147 and the SARS-CoV-2 spike protein, which facilitated viral infection of host cells, revealing that CD147 could be a novel receptor for SARS-CoV-2 to infect organism. Besides, a growing amount of clinical evidence indicated that cytokine storm was linked to the severity of COVID-19 and was the main factor leading to the death of COVID-19 [12,13]. These inflammatory factors hold promise as targets for specific drugs. Testosterone has been shown to influence the expression of ACE2 and TMPRSS2, while TMPRSS2 is highly expressed in androgen-dependent prostate cancer (PCa) cells; therefore, PCa and SARS-CoV-2 may intersect via TMPRSS2 pathway [[14], [15], [16]]. The effectiveness of ADT in patients with PCa combined with SARS-CoV-2 infection is currently controversial and requires further study [17].
In this work, we shed light on the potential mechanisms of urogenital damage induced by SARS-CoV-2. We performed a series of bioinformatic analyses at the protein, mRNA, and single cell levels based on multiple databases. Our findings revealed the potential mechanisms of urogenital damage caused by SARS-CoV-2 from multiple perspectives, simultaneously proposing potentially effective targets for drug therapy.
2. Materials and methods
2.1. Data sources
The mRNA and protein expression values of ACE2, TMPRSS2, and CD147 in normal human tissue were obtained from multiple databases, including Human Protein Atlas (HPA), the Genotype-Tissue Expression, and the Functional Annotation of the Mammalian Genome [[18], [19], [20]]. The mRNA expression data of ACE2, TMPRSS2, and CD147 covering 33 types of tumors and normal controls in The Cancer Genome Atlas were accessed from the UCSC Xena project (https://xenabrowser.net/datapages/). To investigate the expression differences of ACE2, TMPRSS2, and CD147 in kidney tissue of patients with chronic kidney disease (CKD) and the healthy, we selected sample counts which were larger than 20 and downloaded the GSE66494 (kidney tissue; control vs. CKD) dataset from the Gene Expression Omnibus database [21]. The GSE72920 dataset used for Gene Set Enrichment Analysis (GSEA) was also obtained from Gene Expression Omnibus database [22].
2.2. Single-cell RNA sequencing analysis
We predicted COVID-19 associated inflammatory factor receptors and interacting proteins through the STRING database (version 11.5, Department of Molecular Life Sciences and Swiss Institute of Bioinformatics, University of Zurich, Zurich, Switzerland) and screened molecules for evidence from experiments or databases [23]. The expression patterns for ACE2, TMPRSS2, CD147, multiple cytokine receptors, and interacting proteins (including CCL2, CCL4, CXCL8, IFNGR1, IFNGR2, IL10RB, IL1B, IL6ST, JAK1, JUN, MAPK1, STAT3, TIMP1, and TNFRSF1A) in different cell types of bladder, kidney, prostate, and testis were obtained from Human Cell Landscape website (http://bis.zju.edu.cn/HCL/index.html) [24].
2.3. GSEA
To explore the association of different testosterone levels in PCa patients with signaling pathways related to COVID-19, Kyoto Encyclopedia of Genes and Genomes term enrichment analysis was carried out using GSEA software (version 4.2.3, Broad Institute, Cambridge, MA, USA) [25,26]. The annotated gene sets for use with GSEA software was c2. cp.kegg.v7.5.1 (https://www.gsea-msigdb.org/gsea/downloads.jsp). Based on the preoperative serum testosterone levels of PCa patients in the GSE72920 dataset, the samples were divided into two groups: the high testosterone level group and the low testosterone level group. Enrichment score (ES) reflects the degree to which a gene set is overrepresented at the extremes (top or bottom) of the entire ranked list. Nominal p-value was used to estimate the statistical significance of the ES. GSEA changed the estimated significance threshold to take multiple hypothesis testing into account when evaluating a database of gene sets as a whole. To considering the size of the set, GSEA initially normalized the ES for each gene set, resulting in a normalized ES (NES), then determined the false discovery rate (FDR) associated with each NES in order to control the percentage of false positives. The GSEA enrichment results were filtered by the following criteria: absolute value of NES >1, nominal p-value <0.05, and FDR <0.25.
3. Results
3.1. ACE2, TMPRSS2, and CD147 expression in human normal tissue from various public databases
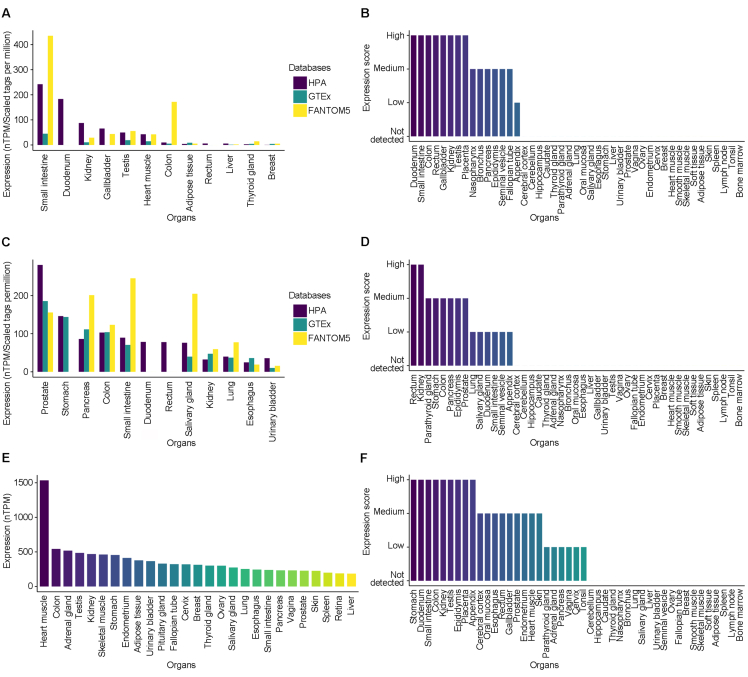
To comprehensively study the mRNA and protein expression of ACE2, TMPRSS2, and CD147 in normal human tissue, we comprehensively analyzed transcriptomic and protein assay datasets from multiple public databases. According to the results, the small intestine, duodenum, kidney, gallbladder, testis, heart muscle, and colon had significant levels of ACE2 mRNA expression, and ACE2 protein was detected at high levels in the tissue of the duodenum, small intestine, colon, rectum, gallbladder, kidney, testis, and placenta (Fig. 1A and B). TMPRSS2 mRNA was highly expressed in the prostate, stomach, pancreas, colon, small intestine, duodenum, rectum, salivary gland, kidney, lung, esophagus, and bladder, and its protein was detected at high levels in the rectum and kidney, detected at medium levels in parathyroid gland, stomach, colon, pancreas, epididymis, and prostate (Fig. 1C and D). CD147 mRNA was highly expressed in heart muscle, colon, adrenal gland, testis, kidney, skeletal muscle, stomach, endometrium, adipose tissue, and urinary bladder, and its protein was detected at high levels in stomach, duodenum, small intestine, colon, kidney, testis, epididymis, placenta, and appendix (Fig. 1E and F).
Figure 1.
The expression of ACE2, TMPRSS2, and CD147 in normal human tissue from multiple public databases. (A) The mRNA expression pattern of ACE2 in HPA, GTEx, and FANTOM5; (B) The protein expression pattern of ACE2 in HPA; (C) The mRNA expression pattern of TMPRSS2 in HPA, GTEx, and FANTOM5; (D) The protein expression pattern of TMPRSS2 in HPA; (E) The mRNA expression pattern of CD147 in GTEx; (F) The protein expression pattern of CD147 in HPA. ACE2, angiotensin-converting enzyme 2; TMPRSS2, transmembrane serine protease 2; HPA, Human Protein Atlas; GTEx, the genotype-tissue Expression; FANTOM5, the Functional Annotation Of The Mammalian Genome.
Combining the above databases, it can be found that ACE2, TMPRSS2, and CD147 are highly expressed in urogenital organs like kidney, testis, prostate, and bladder, indicating that they are likely to be target organs of SARS-CoV-2 other than lung and have the potential to be invaded and infected by SARS-CoV-2.
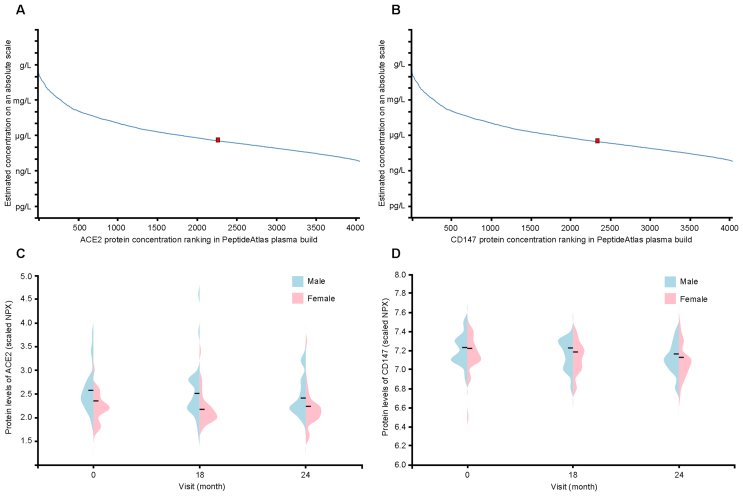
3.2. ACE2 and CD147 protein expressions in human plasma
To explore ACE2 and CD147 protein expressions in plasma, we obtained the abundance and distribution of ACE2 and CD147 proteins from PeptideAtlas based on mass spectrometry proteomics and HPA blood atlas based on proximity extension assays. The concentration of ACE2 protein in plasma was estimated to be 400 ng/L based on the spectrum counts of mass spectrometry-based proteomics in the PeptideAtlas, whereas the concentration of CD147 protein in plasma was around 340 ng/L (Fig. 2A and B). The average concentration of ACE2 and CD147 proteins in plasma was somewhat greater in men than in women during long-term health research involving 76 participants and three visits over 2 years, according to the protein profiling statistics based upon proximity extension assays (Olink, Uppsala, Sweden) (Fig. 2C and D). It can be speculated that men may be more vulnerable to SARS-CoV-2 than women due to the differential expression levels of ACE2 and CD147 proteins in plasma.
Figure 2.
The ACE2 and CD147 protein concentration and distribution between male and female in plasma. (A and B) The concentration of ACE2 and CD147 in human plasma is quantified by mass spectrometry-based plasma proteomics and estimated from spectral counts in a publicly available data set obtained from the PeptideAtlas (ACE2: 400 ng/L, CD147: 340 ng/L); (C and D) Violin plot showing the distribution of ACE2 and CD147 between male and female in plasma, based on proximity extension assays (Olink) for a longitudinal wellness study covering 76 individuals with three visits during 2 years; and protein expression levels are reported as NPX. ACE2, angiotensin-converting enzyme 2; NPX, normalized protein expression.
3.3. ACE2, TMPRSS2, and CD147 expression patterns in specific patient populations
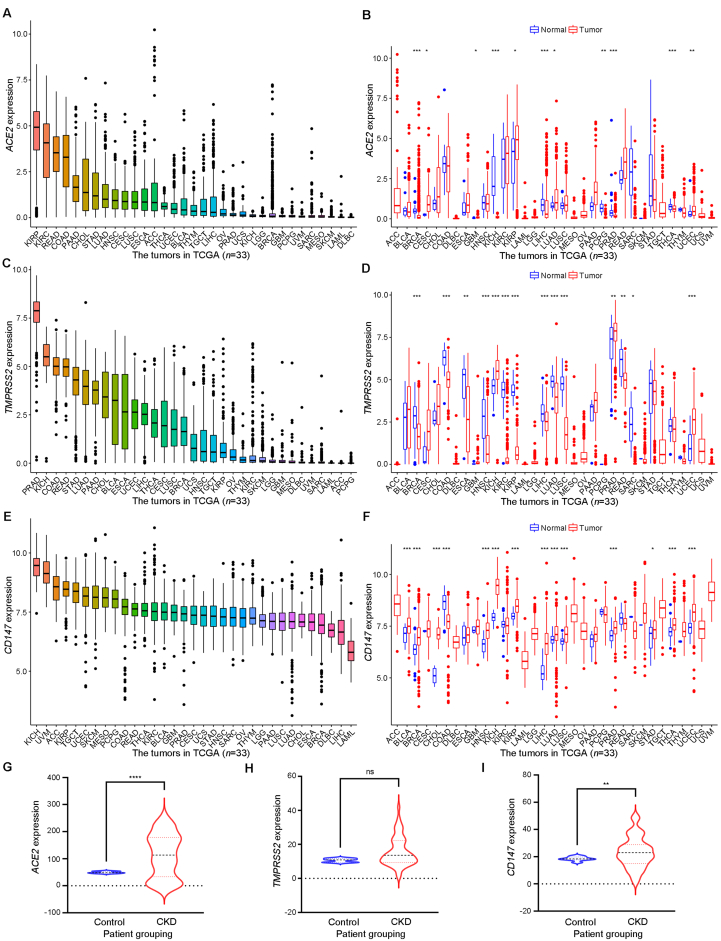
By analyzing the mRNA expression differences of ACE2, TMPRSS2, and CD147 in 33 tumor tissue and their corresponding normal tissue, it was found that ACE2, TMPRSS2, and CD147 were expressed in almost all tumor types. Among them, ACE2 was highly expressed in kidney renal papillary cell carcinoma, kidney renal clear cell carcinoma, rectum adenocarcinoma, and colon adenocarcinoma; TMPRSS2 was highly expressed in prostate adenocarcinoma, kidney chromophobe, colon adenocarcinoma, rectum adenocarcinoma, stomach adenocarcinoma, and lung adenocarcinoma, while CD147 was highly expressed in almost all kinds of tumors (Fig. 3A–F). Besides, compared with the control group, the mRNA expression levels of ACE2 and CD147 were higher in the kidney tissues of patients with CKD, whereas the expression of TMPRSS2 mRNA did not differ in the two groups (Fig. 3G–I).
Figure 3.
Expression patterns of ACE2, TMPRSS2, and CD147 mRNA in specific patient populations. (A) The mRNA expression patterns of ACE2 in 33 kinds of tumors in TCGA; (B) Differential mRNA expression of ACE2 in 33 kinds of tumor tissue and normal tissue from TCGA; (C) The mRNA expression patterns of TMPRSS2 in 33 kinds of tumors in TCGA; (D) Differential mRNA expression of TMPRSS2 in 33 kinds of tumor tissue and normal tissue from TCGA; (E) The mRNA expression patterns of CD147 in 33 kinds of tumors in TCGA; (F) Differential mRNA expression of CD147 in 33 kinds of tumor tissue and normal tissue from TCGA; (G) The mRNA expression patterns of ACE2 in GSE66494; (H) The mRNA expression patterns of TMPRSS2 in GSE66494; (I) The mRNA expression patterns of CD147 in GSE66494. ACE2, angiotensin-converting enzyme 2; TMPRSS2, transmembrane serine protease 2; CKD, chronic kidney disease; ns, no significant. ∗p<0.05, ∗∗p<0.01, ∗∗∗p<0.001, ∗∗∗∗p<0.0001.
3.4. Cell-specific expression of ACE2, TMPRSS2 and CD147 in bladder, kidney, prostate, and testis
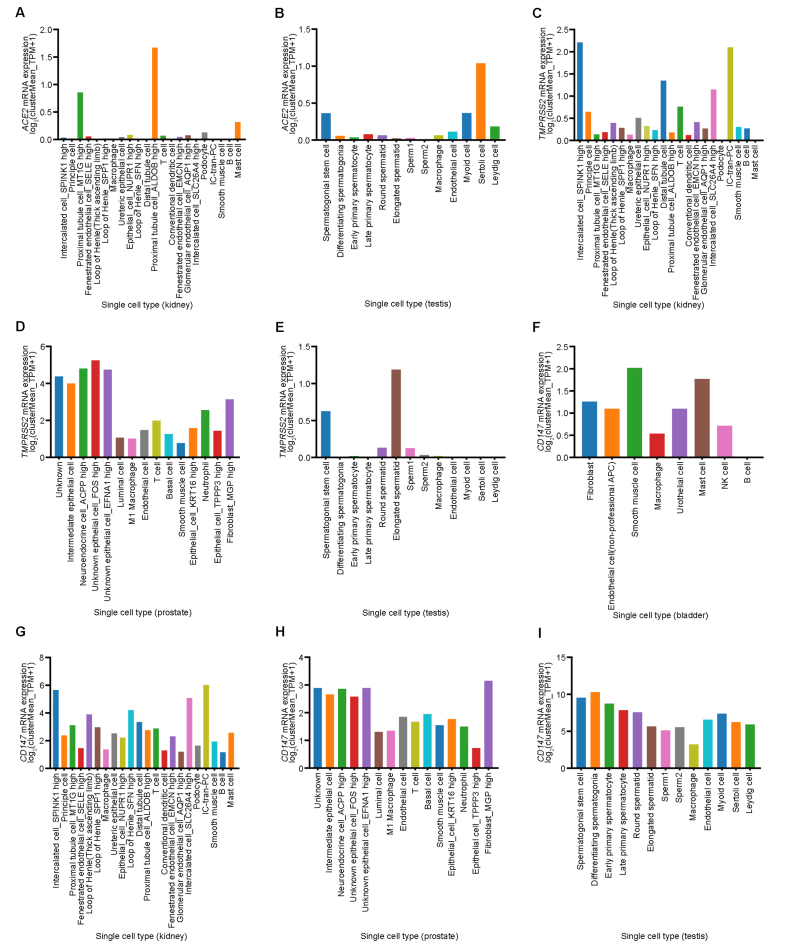
The expression patterns of ACE2, TMPRSS2, and CD147 in single cells of the bladder, kidney, prostate, and testis were visualized by Human Cell Landscape website. In the kidney, ACE2 mRNA was highly expressed in proximal tubule cells and was low or not expressed in other kinds of cells (Fig. 4A). In testis, ACE2 mRNA was highly expressed in Sertoli cells, myoid cells, spermatogonial stem cells, and Leydig cells, while it was low expressed in other types of cells (Fig. 4B). In the kidney, TMPRSS2 mRNA was highly expressed in intercalated cells, IC-tran-PC, distal tubule cells, and T cells, and low expressed in some proximal tubule cells, macrophage, and conventional dendritic cells, but not expressed in podocyte or mast cells (Fig. 4C). In the prostate, TMPRSS2 mRNA was highly expressed in all cell types, especially in epithelial cells, neuroendocrine cells, and fibroblasts (Fig. 4D). In the testis, TMPRSS2 mRNA was expressed at high levels in elongated spermatid and spermatogonial stem cells, and expressed at low levels in early primary spermatocyte, round spermatid, sperm1, sperm2, and macrophages, while not expressed in differentiating spermatogonia, late primary spermatocyte, endothelial cells, myoid cells, Sertoli cells, or Leydig cells (Fig. 4E). In the bladder, CD147 mRNA was highly expressed in all types of cells except B cells, especially in smooth muscle cells and mast cells (Fig. 4F). In contrast, CD147 mRNA was highly expressed in almost all types of cells in the kidney, prostate, and testis (Fig. 4G–I).
Figure 4.
Cell-specific mRNA expressions of ACE2, TMPRSS2, and CD147 in bladder, kidney, prostate, and testis. (A) Cell-specific expression of ACE2 from Human Cell Landscape Kidney2 (http://bis.zju.edu.cn/HCL/search.html); (B) Cell-specific expression of ACE2 from Human Cell Landscape Testis_Guo (http://bis.zju.edu.cn/HCL/search.html); (C) Cell-specific expression of TMPRSS2 from Human Cell Landscape Kidney2 (http://bis.zju.edu.cn/HCL/search.html); (D) Cell-specific expression of TMPRSS2 from Human Cell Landscape Prostate1 (http://bis.zju.edu.cn/HCL/search.html); (E) Cell-specific expression of TMPRSS2 from Human Cell Landscape Testis_Guo (http://bis.zju.edu.cn/HCL/search.html); (F) Cell-specific expression of CD147 from Human Cell Landscape Bladder1 (http://bis.zju.edu.cn/HCL/search.html); (G) Cell-specific expression of CD147 from Human Cell Landscape Kidney2 (http://bis.zju.edu.cn/HCL/search.html); (H) Cell-specific expression of CD147 from Human Cell Landscape Prostate1 (http://bis.zju.edu.cn/HCL/search.html); (I) Cell-specific expression of CD147 from Human Cell Landscape Testis_Guo (http://bis.zju.edu.cn/HCL/search.html). ACE2, angiotensin-converting enzyme 2; TMPRSS2, transmembrane serine protease 2; HPA, Human Protein Atlas; GTEx, the Genotype-Tissue Expression; FANTOM5, the Functional Annotation of The Mammalian Genome.
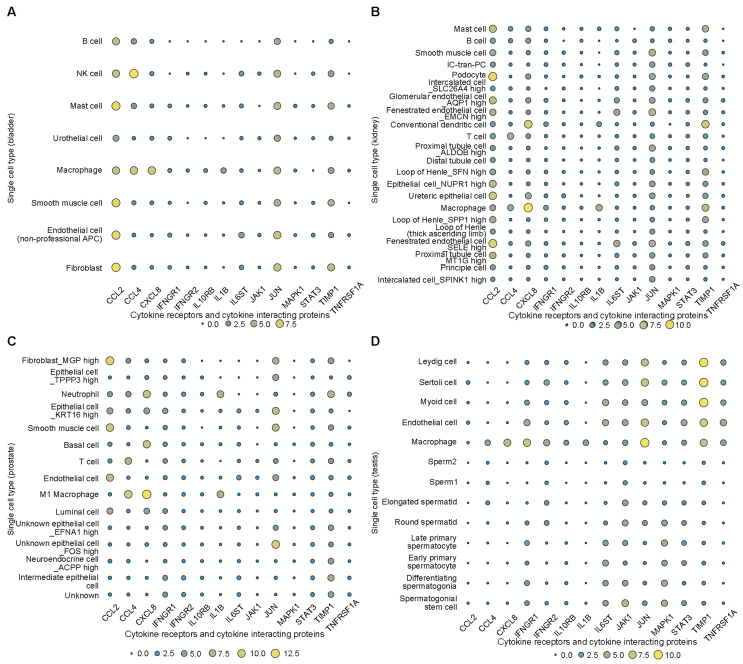
3.5. Cell-specific expression of cytokine receptors and cytokine interacting proteins in bladder, kidney, prostate, and testis
Most cytokine receptors and cytokine interacting proteins were observed to be expressed in one or more urogenital organs (including bladder, kidney, prostate, and testis) according to HPA (Table S1). Taking into account the crucial role of cytokines in the progression of COVID-19, we modeled a signature of cytokine receptors and their interacting proteins including CCL2, CCL4, CXCL8, IFNGR1, IFNGR2, IL10RB, IL1B, IL6ST, JAK1, JUN, MAPK1, STAT3, TIMP1, and TNFRSF1A, and then explored their expression patterns in single cells from urogenital organs (including bladder, kidney, prostate, and testis) by Human Cell Landscape. Visualization showed that CCL2, CCL4, CXCL8, JUN, and TIMP1 were highly expressed in almost all types of bladder cells, and multiple cytokine receptors and cytokine interacting proteins (CCL2, CCL4, CXCL8, IL1B, JUN, and TIMP1) were highly expressed in macrophages (Fig. 5A). CCL2, CCL4, CXCL8, IL6ST, JUN, and TIMP1 were highly expressed in almost all types of renal cells (Fig. 5B). CCL2, CCL4, CXCL8, IFNGR1, JUN, and TIMP1 were highly expressed in almost all types of prostate cells (Fig. 5C). IFNGR1, IFNGR2, IL6ST, JAK1, JUN, MAPK1, STAT3, and TIMP1 were highly expressed in almost all types of testicular cells, meanwhile more than half of the cytokine receptors and cytokine interacting proteins (IFNGR1, IFNGR2, IL10RB, IL6ST, JAK1, JUN, MAPK1, STAT3, TIMP1, and TNFRSF1A) were highly expressed in endothelial cells, myoid cells, Sertoli cells, and Leydig cells. What's more, almost all the cytokine receptors and cytokine interacting proteins were highly expressed in macrophages (Fig. 5D).
Figure 5.
Cell-specific expression of cytokine receptors and cytokine interacting proteins in bladder, kidney, prostate, and testis. (A) Cell-specific expression of cytokine receptors and cytokine interacting proteins from Human Cell Landscape Bladder1 (http://bis.zju.edu.cn/HCL/search.html); (B) Cell-specific expression of cytokine receptors and cytokine interacting proteins from Human Cell Landscape Kidney2 (http://bis.zju.edu.cn/HCL/search.html); (C) Cell-specific expression of cytokine receptors and cytokine interacting proteins from Human Cell Landscape Prostate1 (http://bis.zju.edu.cn/HCL/search.html); (D) Cell-specific expression of cytokine receptors and cytokine interacting proteins from Human Cell Landscape Testis_Guo (http://bis.zju.edu.cn/HCL/search.html).
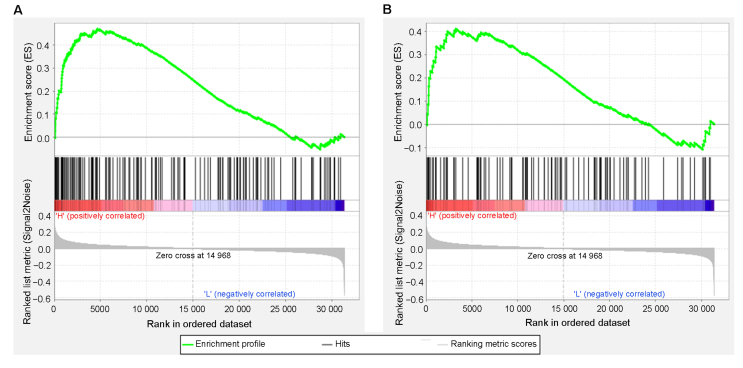
3.6. GSEA enrichment plots of PCa patients with high testosterone level
GSEA was performed on GSE72920 dataset, which contains preoperative serum testosterone levels of PCa patients. The enrichment results were filtered according to the following criteria: absolute value of NES >1, nominal p-value <0.05, and FDR <0.25. Forty-seven signal pathways with significant correlation were screened in the high testosterone level group, while one signal pathway with significant correlation was screened in the low testosterone level group. The enrichment results of GSEA showed that high levels of testosterone in PCa patients were positively correlated with the JAK-STAT signaling pathway and Toll-like receptor signaling pathway (Fig. 6A and B). These results suggested that high level of testosterone in PCa patients may contribute to the activation of the JAK-STAT signaling pathway and Toll-like receptor signaling pathway, which were also reported to be activated in COVID-19 patients [[27], [28], [29], [30]].
Figure 6.
GSEA enrichment plots of GSE72920 dataset. (A) GSEA enrichment plot: JAK-STAT signaling pathway; (B) GSEA enrichment plot: Toll-like receptor signaling pathway. GSEA, Gene Set Enrichment Analysis; JAK-STAT, Janus kinase-signal transducers and activators of transcription.
4. Discussion
With the global epidemic of COVID-19, more and more patients with COVID-19 have urogenital problems, which have prompted people to worry about the potential long-term damage to urogenital organs after SARS-CoV-2 infection. Therefore, our research focused on whether SARS-CoV-2 may directly damage the genitourinary organs, mainly the bladder, kidney, prostate, and testis, as well as its possible pathogenesis. In addition, we also studied the susceptibility difference of SARS-CoV-2 in different populations and the potential relationship between SARS-CoV-2 and PCa.
Our study found that ACE2, TMPRSS2, and CD147 mRNAs are highly expressed in urogenital organs (including kidney, testis, bladder, and prostate), which is based on RNA sequence data of human normal tissue in several publicly available databases (HPA, the Genotype-Tissue Expression, and the Functional Annotation of the Mammalian Genome), suggesting that they may be potential target organs for SARS-CoV-2. Acute kidney injury incidence in COVID-19 patients ranged from 1% to 46% according to a retrospective analysis [31]. A prospective cohort study of 701 COVID-19 patients found that, in addition to changes in renal function tests like elevated blood urea nitrogen, reduced glomerular filtration rate, and elevated serum creatinine, which indicated severe damage to renal tissue, 26.7% of the patients had hematuria and 43.9% had proteinuria [32]. By using a real-time reverse transcription-polymerase chain reaction, Wang et al. [33] identified positive SARS-CoV-2 mRNA in the urine sediment of certain patients. These findings suggested that SARS-CoV-2 could directly infect the kidney via ACE2, TMPRSS2, and CD147 molecules, contributing to the development of acute kidney injury. Studies have indicated that 10%–22% of men in the acute infection phase of COVID-19 could develop orchitis or epididymitis, and another research discovered SARS-CoV-2 in testicular tissue specimens of dead patients with COVID-19 through transmission electron microscopy, suggesting that testicular inflammation caused by COVID-19 may be attributable to the direct invasion of SARS-CoV-2 [[34], [35], [36]]. Another study claimed that SARS-CoV-2 was not found in the semen of individuals recovering from COVID-19, but it could not completely exclude the possibility that SARS-CoV-2 was present in semen fluid during acute infection accompanied severe manifestations of COVID-19 [37]. Although ACE2, TMPRSS2, and CD147 mRNAs were expressed in the prostate, especially TMPRSS2 mRNA, SARS-CoV-2 mRNA was not found in the prostate secretions of COVID-19 individuals, which may be attributed to low ACE2 expression [[38], [39], [40]]. Besides, one study focused on the incidence of urinary frequency in individuals with COVID-19 and discovered that seven out of 57 COVID-19 patients receiving treatment had urinary frequency, which the researchers hypothesized might be related to viral cystitis [41]. Another study excluded bacterial urinary tract infections in patients without viral testing also reported urinary symptoms related to COVID-19, including urinary frequency and nocturia, suggesting that the urinary symptoms may be caused by SARS-CoV-2 [42].
Single-cell RNA transcriptomic data revealed that ACE2 mRNA was highly expressed in proximal tubule cells in the kidney. Diffuse proximal tubule damage with loss of brush boundary, non-isometric vacuolar degeneration, and even significant necrosis were found in postmortem renal pathology in COVID-19 individuals [43,44]. In addition, CD147 molecule was shown to participate in kidney injury related to COVID-19. Based on immunohistochemical results, in COVID-19 patients, the distribution of CD147 expanded from the basal to the circumferential pattern, including the interface and the tip, which may facilitate the invasion of SARS-CoV-2 from this luminal surface into the cytoplasm of tubular epithelial cells [10]. In the testis, ACE2 was highly expressed in Sertoli cells, myoid cells, and spermatogonial stem cells. The adhesion of SARS-CoV-2 to ACE2 receptor may hinder the normal function of Sertoli and Leydig cells, which may increase the expression of ACE2 and cause an inflammatory response, affecting male spermatogenesis [45]. Additionally, fever may also be the cause of the poor sperm quality seen in patients with the acute stage of COVID-19 [46]. SARS-CoV-2 may damage the uroepithelial cells of COVID-19 patients since viral mRNAs were found in the urine of the patients, meanwhile ACE2 and CD147 mRNAs were detected in the tissue of bladder. The location of expression, whether the basal or tubular expression is still unclear; therefore, the infection may occur through capillary infection or urinary infection [41].
An increasing mass of clinical evidence indicates that cytokine storm is linked to COVID-19 severity and is a major factor in COVID-19 fatality [13]. In comparison to patients with mild and moderate symptoms, individuals with severe symptoms had greater levels of inflammatory factors, such as IL-2, IL-6, IL-7, IL-10, IP-10, MCP-1, TNF-α, MIP1A, and G-CSF [47]. In light of the crucial role that cytokines play in the pathogenic process of COVID-19, we established the signatures of cytokine receptors and cytokine interacting proteins, including CCL2, CCL4, CXCL8, IFNGR1, IFNGR2, IL10RB, IL1B, IL6ST, JAK1, JUN, MAPK1, STAT3, TIMP1, and TNFRSF1A. It is worth noting that CCL2, JUN, and TIMP1 are observed to be highly expressed in urogenital organs and they may play a greater role in cytokine storm-induced systemic inflammatory responses. In addition, our results found that multiple cytokine receptors and cytokine interacting proteins were expressed at high levels in macrophages that might damage macrophages by interfering with their normal functioning, particularly in the high cytokine microenvironment of critically ill patients. Patients with severe COVID-19 may benefit from medications that prevent and reduce cytokine storms, including corticosteroids, hydroxychloroquine, chloroquine, tocilizumab, mesenchymal stem cells, and others that are currently being tested in clinical trials. Our study suggests that CCL2, JUN, and TIMP1 are promising new targets for drug development to inhibit cytokine storm related to COVID-19. We believe that identifying and treating cytokine storms is a key part of saving patients with severe COVID-19.
Male, cancer, and CKD patients are more vulnerable to experiencing symptoms of COVID-19, according to a number of systematic reviews and meta-analysis studies [[48], [49], [50], [51]]. We found that the average plasma levels of ACE2 and CD147 proteins in males were higher than those in females, which may be one of the factors that lead to men being more susceptible to SARS-CoV-2 and more prone to experience severe symptoms of COVID-19. However, it has been reported that soluble plasma ACE2 levels could not represent the probability of COVID-19 infection, because that SARS-CoV-2 attaches to membrane ACE2, and the expression of membrane ACE2 may provide more pertinent information about this problem [52]. In addition, our findings revealed that ACE2, TMPRSS2, and CD147 mRNAs were detected to be highly expressed in a variety of tumor tissue and patients with CKD, supporting the results of the meta-analysis and systematic reviews that patients suffering malignancy and CKD were more probable to experience severe symptoms of COVID-19. Therefore, the symptoms particular to these populations should receive special clinical attention.
Early in the pandemic, the researchers studied the relationship between androgen and susceptibility of SARS-CoV-2, because clinical research results showed that men were relatively likely to have severe symptoms of COVID-19. The risk of infection was considerably lower in patients with PCa receiving ADT than in those who did not receive ADT in a sizable population-based study covering 4532 men with confirmed SARS-CoV-2 infection. The authors came to the conclusion that PCa patients treated with ADT emerged to be partly shielded from SARS-CoV-2 infection [53]. According to our enrichment results of GSEA, high levels of testosterone in PCa patients may contribute to activating signaling pathways related to SARS-CoV-2 infection, including the JAK-STAT signaling pathway and the Toll-like receptor signaling pathway. We hypothesize that ADT for patients with PCa may have a protective effect against SARS-CoV-2. Given that studies have demonstrated that testosterone controls the expression of ACE2 and TMPRSS2, ADT may reduce testosterone levels and TMPRSS2 expression [14,22]. In addition, testosterone may be involved in COVID-19 susceptibility difference between males and females, owing to the hormone differences between them. Children often have fewer COVID-19 symptoms than adults, which may be explained by the correlation between testosterone and COVID-19 symptoms [54].
In summary, our study discloses the possible mechanism of urogenital damage caused by SARS-CoV-2, and provides new targets for the development of targeted drugs. These findings imply that the potential injury caused by SARS-CoV-2 to urogenital organs should be addressed carefully. Furthermore, for male patients recovering from COVID-19, especially those with reproductive problems originally, the function of the urogenital system should be carefully evaluated. However, our research has some limitations: firstly, the data were gathered from several publicly available databases and more experimental and clinical data are required to confirm the bioinformatics analysis findings; secondly, the sample size of the datasets used for GSEA is small, and relevant findings still need to be confirmed in a wider population.
5. Conclusion
In this study, we analyzed the potential targets of SARS-CoV-2 infection from mRNA, protein, and single cells levels, and discussed its possible pathogenic mechanisms. At the same time, we also explored the susceptibility differences of SARS-CoV-2 in different populations, and analyzed the possible reasons for the differences. In conclusion, our findings reveal the possible mechanisms of SARS-CoV-2 damage to the genitourinary organs and provide novel targets for the development of targeted drugs.
Author contributions
Study concept and design: Kai Zhao, Dong Zhang, Xinchi Xu.
Data acquisition: Kai Zhao, Dong Zhang, Xinchi Xu, Shangqian Wang, Zhanpeng Liu, Xiaohan Ren, Xu Zhang, Zhongwen Lu, Shancheng Ren, Chao Qin.
Data analysis: Kai Zhao, Dong Zhang, Xinchi Xu.
Drafting of manuscript: Kai Zhao, Dong Zhang, Xinchi Xu.
Critical revision of the manuscript: Chao Qin, Shancheng Ren, Shangqian Wang.
Conflicts of interest
The relevant data used in our research were obtained from publicly available databases. Ethical approval has been obtained for the patient samples involved in the public database. Users can download relevant data for free for research and publish relevant articles. Our study is based on open source data, so there are no ethical issues and other conflicts of interest.
Footnotes
Peer review under responsibility of Tongji University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajur.2022.12.004.
Contributor Information
Shancheng Ren, Email: renshancheng@gmail.com.
Chao Qin, Email: nmuqinchao@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lu H., Stratton C.W., Tang Y. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P., Yang X., Wang X., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A., et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 5.Nie X., Qian L., Sun R., Huang B., Dong X., Xiao Q., et al. Multi-organ proteomic landscape of COVID-19 autopsies. Cell. 2021;184:775–791.e14. doi: 10.1016/j.cell.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren X., Wang S., Chen X., Wei X., Li G., Ren S., et al. Multiple expression assessments of ACE2 and TMPRSS2 SARS-CoV-2 entry molecules in the urinary tract and their associations with clinical manifestations of COVID-19. Infect Drug Resist. 2020;13:3977–3990. doi: 10.2147/IDR.S270543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenizia C., Galbiati S., Vanetti C., Vago R., Clerici M., Tacchetti C., et al. SARS-CoV-2 entry: at the crossroads of CD147 and ACE2. Cells. 2021;10:1434. doi: 10.3390/cells10061434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radzikowska U., Ding M., Tan G., Zhakparov D., Peng Y., Wawrzyniak P., et al. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy. 2020;75:2829–2845. doi: 10.1111/all.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su H., Wan C., Wang Z., Gao Y., Li Y., Tang F., et al. Expression of CD147 and cyclophilin A in kidneys of patients with COVID-19. Clin J Am Soc Nephrol. 2021;16:618–619. doi: 10.2215/CJN.09440620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang K., Chen W., Zhang Z., Deng Y., Lian J., Du P., et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Targeted Ther. 2020;5:283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93:250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuel R.M., Majd H., Richter M.N., Ghazizadeh Z., Zekavat S.M., Navickas A., et al. Androgen signaling regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men. Cell Stem Cell. 2020;27:876–889.e12. doi: 10.1016/j.stem.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin B., Ferguson C., White J.T., Wang S., Vessella R., True L.D., et al. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res. 1999;59:4180–4184. [PubMed] [Google Scholar]
- 16.Porcacchia A.S., Andersen M.L., Tufik S. Prostate cancer and SARS-CoV-2: possible intersections through the TMPRSS2 pathway. Eur J Cancer Prev. 2021;30:481–483. doi: 10.1097/CEJ.0000000000000696. [DOI] [PubMed] [Google Scholar]
- 17.Ebner B., Volz Y., Mumm J.-N., Stief C.G., Magistro G. The COVID-19 pandemic—what have urologists learned? Nat Rev Urol. 2022;19:344–356. doi: 10.1038/s41585-022-00586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uhlén M., Fagerberg L., Hallström B.M., Lindskog C., Oksvold P., Mardinoglu A., et al. Tissue-based map of the human proteome. Science. 2015;347 doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 19.Kawaji H., Kasukawa T., Forrest A., Carninci P., Hayashizaki Y. The FANTOM5 collection, a data series underpinning mammalian transcriptome atlases in diverse cell types. Sci Data. 2017;4 doi: 10.1038/sdata.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carithers L.J., Moore H.M. The genotype-tissue expression (GTEx) project. Biopreserv Biobanking. 2015;13:307–308. doi: 10.1089/bio.2015.29031.hmm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakagawa S., Nishihara K., Miyata H., Shinke H., Tomita E., Kajiwara M., et al. Molecular markers of tubulointerstitial fibrosis and tubular cell damage in patients with chronic kidney disease. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw G.L., Whitaker H., Corcoran M., Dunning M.J., Luxton H., Kay J., et al. The early effects of rapid androgen deprivation on human prostate cancer. Eur Urol. 2016;70:214–218. doi: 10.1016/j.eururo.2015.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han X., Zhou Z., Fei L., Sun H., Wang R., Chen Y., et al. Construction of a human cell landscape at single-cell level. Nature. 2020;581:303–309. doi: 10.1038/s41586-020-2157-4. [DOI] [PubMed] [Google Scholar]
- 25.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mootha V.K., Lindgren C.M., Eriksson K.F., Subramanian A., Sihag S., Lehar J., et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 27.Yang L., Xie X., Tu Z., Fu J., Xu D., Zhou Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct Targeted Ther. 2021;6:255. doi: 10.1038/s41392-021-00679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khanmohammadi S., Rezaei N. Role of Toll-like receptors in the pathogenesis of COVID-19. J Med Virol. 2021;93:2735–2739. doi: 10.1002/jmv.26826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aboudounya M.M., Heads R.J. COVID-19 and toll-like receptor 4 (TLR4): SARS-CoV-2 may bind and activate TLR4 to increase ACE2 expression, facilitating entry and causing hyperinflammation. Mediat Inflamm. 2021;2021 doi: 10.1155/2021/8874339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catanzaro M., Fagiani F., Racchi M., Corsini E., Govoni S., Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct Targeted Ther. 2020;5:84. doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farouk S.S., Fiaccadori E., Cravedi P., Campbell K.N. COVID-19 and the kidney: what we think we know so far and what we don't. J Nephrol. 2020;33:1213–1218. doi: 10.1007/s40620-020-00789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L., Li X., Chen H., Yan S., Li D., Li Y., et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020;51:343–348. doi: 10.1159/000507471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L., Huang X., Yi Z., Deng Q., Jiang N., Feng C., et al. Ultrasound imaging findings of acute testicular infection in patients with coronavirus disease 2019: a single-center-based study in wuhan, China. J Ultrasound Med. 2021;40:1787–1794. doi: 10.1002/jum.15558. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y., Song L., Zheng N., Shi J., Wu H., Yang X., et al. A urinary proteomic landscape of COVID-19 progression identifies signaling pathways and therapeutic options. Sci China Life Sci. 2022;65:1866–1880. doi: 10.1007/s11427-021-2070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez D.C., Khodamoradi K., Pai R., Guarch K., Connelly Z.M., Ibrahim E., et al. A systematic review on the investigation of SARS-CoV-2 in semen. Res Rep Urol. 2020;12:615–621. doi: 10.2147/RRU.S277679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan F., Xiao X., Guo J., Song Y., Li H., Patel D.P., et al. No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020;113:1135–1139. doi: 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang S., Wang X., Zhang H., Xu A., Fei G., Jiang X., et al. The absence of coronavirus in expressed prostatic secretion in COVID-19 patients in Wuhan city. Reprod Toxicol. 2020;96:90–94. doi: 10.1016/j.reprotox.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruan Y., Hu B., Liu Z., Liu K., Jiang H., Li H., et al. No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: a perspective and urogenital evaluation. Andrology. 2021;9:99–106. doi: 10.1111/andr.12939. [DOI] [PubMed] [Google Scholar]
- 40.Nassau D.E., Best J.C., Kresch E., Gonzalez D.C., Khodamoradi K., Ramasamy R. Impact of the SARS-CoV-2 virus on male reproductive health. BJU Int. 2022;129:143–150. doi: 10.1111/bju.15573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mumm J.N., Osterman A., Ruzicka M., Stihl C., Vilsmaier T., Munker D., et al. Urinary frequency as a possibly overlooked symptom in COVID-19 patients: does SARS-CoV-2 cause viral cystitis? Eur Urol. 2020;78:624–628. doi: 10.1016/j.eururo.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhar N., Dhar S., Timar R., Lucas S., Lamb L.E., Chancellor M.B. De novo urinary symptoms associated with COVID-19: COVID-19-associated cystitis. J Clin Med Res. 2020;12:681–682. doi: 10.14740/jocmr4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su H., Yang M., Wan C., Yi L., Tang F., Zhu H., et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santoriello D., Khairallah P., Bomback A.S., Xu K., Kudose S., Batal I., et al. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol. 2020;31:2158–2167. doi: 10.1681/ASN.2020050744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Illiano E., Trama F., Costantini E. Could COVID-19 have an impact on male fertility? Andrologia. 2020;52 doi: 10.1111/and.13654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carlsen E., Andersson A.M., Petersen J.H., Skakkebaek N.E. History of febrile illness and variation in semen quality. Hum Reprod. 2003;18:2089–2092. doi: 10.1093/humrep/deg412. [DOI] [PubMed] [Google Scholar]
- 47.Catanzaro M., Fagiani F., Racchi M., Corsini E., Govoni S., Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct Targeted Ther. 2020;5:84. doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ambrosino I., Barbagelata E., Ortona E., Ruggieri A., Massiah G., Giannico O.V., et al. Gender differences in patients with COVID-19: a narrative review. Monaldi Arch Chest Dis. 2020:90. doi: 10.4081/monaldi.2020.1389. [DOI] [PubMed] [Google Scholar]
- 49.Mukherjee S., Pahan K. Is COVID-19 gender-sensitive? J Neuroimmune Pharmacol. 2021;16:38–47. doi: 10.1007/s11481-020-09974-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh A.K., Gillies C.L., Singh R., Singh A., Chudasama Y., Coles B., et al. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: a systematic review and meta-analysis. Diabetes Obes Metabol. 2020;22:1915–1924. doi: 10.1111/dom.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X., Zhong X., Wang Y., Zeng X., Luo T., Liu Q. Clinical determinants of the severity of COVID-19: a systematic review and meta-analysis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0250602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leow M.K.S. Clarifying the controversial risk-benefit profile of soluble ACE2 in COVID-19. Crit Care. 2020;24:396. doi: 10.1186/s13054-020-03097-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montopoli M., Zumerle S., Vettor R., Rugge M., Zorzi M., Catapano C.V., et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (n=4532) Ann Oncol. 2020;31:1040–1045. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mjaess G., Karam A., Aoun F., Albisinni S., Roumeguère T. COVID-19 and the male susceptibility: the role of ACE2, TMPRSS2 and the androgen receptor. Prog Urol. 2020;30:484–487. doi: 10.1016/j.purol.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.