Abstract
This review describes the various viruses identified in the semen and reproductive tracts of mammals (including humans), their distribution in tissues and fluids, their possible cell targets, and the functional consequences of their infectivity on the reproductive and endocrine systems. The consequences of these viral infections on the reproductive tract and semen can be extremely serious in terms of organ integrity, development of pathological and cancerous processes, and transmission of diseases. Furthermore, of essential importance is the fact that viral infection of the testicular cells may result not only in changes in testicular function, a serious risk for the fertility and general health of the individual (such as a fall in testosteronemia leading to cachexia), but also in the possible transmission of virus-induced mutations to subsequent generations. In addition to providing an exhaustive account of the data available in these domains, this review focuses attention on the fact that the interface between endocrinology and virology has so far been poorly explored, particularly when major health, social and economical problems are posed. Our conclusions highlight the research strategies that need to be developed. Progress in all these domains is essential for the development of new treatment strategies to eradicate viruses and to correct the virus-induced dysfunction of the endocrine system.
Concern about the sexual transmission of viruses in humans and its health consequences has peaked with the appearance and development of the AIDS pandemic. There is also much concern about the possibility of sexual transmission of viruses in animals, particularly in economically important farm animals. Such sexually transmissible diseases (STDs) may cause epidemics, which may be spread further by the worldwide export of the gametes and embryos of these animals. In light of these major health and economic issues concerning sexually transmitted viral diseases (Table 1), it is paradoxical that, to our knowledge, no general review has been published concerning the presence of viruses in human and animal reproductive tracts and semen and the possible effects of these viruses within infected organs and on reproductive endocrinology. This review aims to address this subject by describing the various viruses identified in the semen and reproductive tracts of mammals, their distribution in tissues and fluids, their possible targets, and the functional consequences of their infectivity on the reproductive and endocrine systems.
TABLE 1.
Matters of concern relating to viral infection of the reproductive tract or semen
| Spreading of diseases |
| Infertility/sterility resulting from: |
| Changes in one or more testicular compartments (e.g., germ cells, Sertoli cells, Leydig cells) |
| Infiltration into the reproductive tract or semen of leukocytes causing a T-cell-mediated response to spermatozoa |
| Cachexia induced by a drop in testosterone production |
| Incorporation of the viral genome into the germ cell genome (risk of transmission to subsequent generations) |
| Infection of ova and embryo, miscarriage, and embryonic and fetal abnormalities |
VIRUSES AND SEMEN
Human Semen
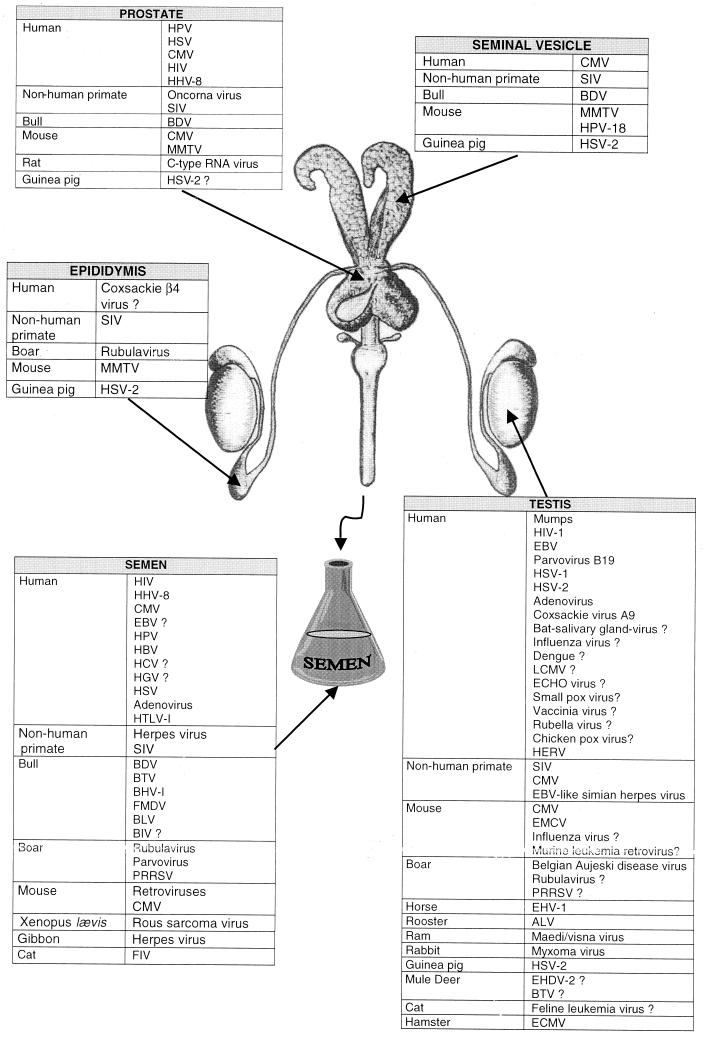
The viruses present in human semen and their consequences are listed in Table 2 and Fig. 1.
TABLE 2.
Presence of viruses in human semen and its consequences
| Virus-infected cells and semen fractions (reference) | |
|---|---|
| Monocytes/macrophages and lymphocytes | |
| HIV (9, 142, 356) | |
| CMV (265) | |
| HBV (84, 135) | |
| HTLV-1 ? (318) | |
| Spermatotozoa | |
| HIV ? (22, 26, 104, 203) | |
| HBV (84) | |
| HSV (172) | |
| Cellular fraction (no specific cell type identified) | |
| Papillomavirus (181, 183, 184) | |
| Adenovirus (80) | |
| Abnormalities detected in the presence of virus | |
|---|---|
| Infertility | |
| HSV (109, 172) | |
| Adenovirus (80) | |
| Azoospermia, oligospermia | |
| HIV (205) | |
| HSV (172) | |
| Morphologically abnormal spermatozoa | |
| HIV (AIDS) (175) | |
| Hematospermia | |
| CMV (169) | |
| Pyospermia | |
| HIV (AIDS) (175) | |
| Decrease in the number of CD4+ cells | |
| CMV (190) | |
| Asthenospermia | |
| Papillomavirus (183) |
FIG. 1.
Summary of the viruses found in the genital tract and semen of mammals.
Human immunodeficiency virus type 1.
Human immunodeficiency virus (HIV) is now the most extensively studied sexually transmitted virus. Its presence in the semen was rapidly established, but the nature of the cells infected remains unclear. One key question is whether the virus strains present in semen arise from the same compartment as those detected in blood cells, since new multidrug therapies are still unable to eradicate the virus completely (71).
The virus was initially isolated from the mononuclear cell fraction of the semen of two men in the process of developing the disease and from one HIV-1-seropositive man (142, 355, 356). It has been shown to be transmitted via the semen of asymptomatic carriers (14, 19, 311). HIV-1 has also been detected in the cell-free seminal fluid of both an AIDS patient and an asymptomatic HIV-positive individual (47). Detection of the virus in this location led to the suggestion that the epididymal epithelial cells may become infected and release HIV into the epididymal fluid. The prostate and seminal vesicles may also act as a virus reservoir and release HIV into the semen. This contamination would occur in addition to that of macrophages and lymphocytes, which are often present in the semen and are natural targets for HIV (9). The possible presence of HIV-1 in the spermatozoa themselves is now a matter of debate and has recently been reviewed (8), raising questions about the vertical transmission of HIV. This problem is very acute because spermatozoa from HIV-positive men, cleared of seminal plasma and infected mononuclear cells, are used for medically assisted reproduction in serodiscordant couples (293–295). Thus, although semen washing on a density gradient before artificial insemination may reduce the risk of HIV transmission from the infected man to an uninfected woman (166, 188), the virus may still be detected in the fraction of motile spermatozoa used for insemination (70, 203, 316). Insemination in such cases is, of course, not performed. These positive results may be false positives due to the use of a single set of primers in the PCR detection of HIV (203) or due to the presence of contaminating cells of nonseminal origin (the mean proportion of such cells is 1/1,000) (316). However, electron microscopy and immunocytochemistry studies have provided evidence that HIV-1 can attach to the surface of spermatozoa and enter these cells through the intact plasma membrane (24–26). An in vitro study has also demonstrated that spermatozoa from healthy donors may carry HIV-1 on their surface and subsequently transmit it to lymphocytes in culture (104). Although still controversial (258), several lines of evidence suggest that HIV is able to bind to spermatozoa. However, it is unclear whether the virus penetrates and replicates in these cells or simply integrates into them. In another study (23), in situ PCR was used to determine HIV-1 provirus levels in seminal cells from 94 HIV-1-infected men at various stages of the clinical disease. Both seminal mononuclear cells and spermatozoa (35 and 33% of samples studied, respectively) were found to harbor HIV-1 proviral sequences. HIV DNA was also detected by PCR in the motile sperm fraction of all three samples tested (288). However, these results are not consistent with those of three other studies using PCR to detect HIV DNA (22, 213, 259). Experimental HIV penetration into the spermatozoa of healthy donors has been reported by Baccetti et al. (22), who used immunocytochemistry and in situ hybridization with electron microscopy to detect antigens and viral RNA. HIV RNA was detected within spermatozoa, but no viral DNA was detected in this study, indicating that if HIV did penetrate spermatozoa, it did not integrate and replicate within these cells. Consistent with this, it has been found that spermatozoa do not represent a significant source of HIV in semen (353). Indeed, a study has shown that vasectomy has little effect on the infectivity of semen, leading to the conclusion that most cell-free HIV in seminal plasma arises distal to the vas deferens (176). Recently, semen samples from 52 men, 21 of whom were receiving antiviral therapy, were tested for HIV and the amount of virus present was quantified (316). HIV RNA was detected in 86% of semen plasma samples and in 14% of spermatozoon fractions (as stated above, the authors suggested possible contamination by nonspermatozoon cells at a mean frequency of 1/1,000), whereas HIV DNA was present in only 57% of nonspermatozoon cell fractions.
It is unknown what prevents HIV replication in spermatozoa and which receptors are used by the virus to enter these cells. The CD4 receptor is present on semen lymphocytes and monocytes (125, 126), but it is unclear whether it is present on spermatozoa. Ashida and Scofield (21) were the first to describe a sperm ligand that reacts with CD4 antibodies and interactions between sperm and HLA-DR-positive cells, providing strong evidence that CD4 is expressed on spermatozoa. CD4 molecules were detected on mouse sperm heads by both immunofluorescence and western Blotting (189). Their presence was also suggested, indirectly, in a study using a semen protein (gp17) that binds to CD4+ T cells and soluble recombinant CD4, as well as spermatozoa (38). However, the CD4 antigen has never been detected on the surface of human spermatozoa or on CD45− ejaculate cells (epithelial and germinal cells) (15, 110, 125, 166, 240, 352). Neither of the two main HIV coreceptors (CXCR4 and CCR5) were detected on the surface of spermatozoa in flow cytometry experiments (166), although the possibility that they are expressed at a very low level cannot be ruled out. It has been suggested that other receptors are responsible for the entry of HIV-1 into spermatozoa. Thus, several studies have described a glycolipid that may function as an HIV receptor on the surface of spermatozoa and that may be involved in transmission of the virus (21, 22, 52, 53, 289). Sperm proteins that bind HLA-DR and are therefore likely to interact with somatic cells have also been described (276, 289). HIV may also infect germ cells early in spermatogenesis, resuting in the clonal transmission of the virus into spermatozoa; this is discussed below (see “Viruses and the testis,” below).
The effect of HIV infection on semen characteristics has been investigated. HIV was isolated from 15 (30%) of 50 specimens from asymptomatic individuals and from 1 of 3 specimens from patients with AIDS (175). The men with AIDS all had pyospermia and grossly abnormal spermatozoa. In contrast, the semen specimens from other seropositive men did not differ significantly from those of healthy seronegative donors. No abnormality in sperm count, morphology, number or type of leukocytes in semen, or any other semen characteristic was associated with HIV shedding into semen. In another study, a significant positive correlation was found between blood CD4+ cell number and sperm motility in seropositive men, and a significant inverse correlation was found between CD4+ cell number and sperm abnormalities. The authors suggested that this may be due to a decrease in testosteronemia, resulting in defective epididymal sperm maturation (102).
Several studies have investigated whether the level of HIV-1 in semen varies with the stage of infection. It has been reported that the isolation of HIV from semen does not correlate with CD4+ or CD8+ T-lymphocyte counts and that seropositive men may shed HIV in semen early in the course of infection (175). Other studies have also reported that the presence of HIV DNA in semen is not related to the CD4+ cell count or disease status (198, 213). Indeed, although HIV-1 is more common in the semen of men with advanced HIV-1 infection and seminal leukocytosis, it can also be isolated from the semen of men with neither of these conditions (16, 342). Furthermore, men with HIV-1 infection are already potentially infectious through sexual relations during the first few weeks after infection (329). It has now been established that HIV-1 may be present in semen in both cell-free and cell-associated forms (and that it may be isolated from both asymptomatic individuals and AIDS patients) and that both forms are transmissible (360). Surprisingly, HIV seems to be shed intermittently into semen (50, 174). Concomitant STDs such as cytomegalovirus (CMV) (174), chancroid, syphilis, gonorrhea, and Chlamydia infections (112) may affect the level of HIV shedding (206, 312). Herpes simplex virus (HSV) increases plasma HIV levels severalfold (221), and this increase may be reflected in seminal fluid. Also, the membrane proteins of CMV and human T-lymphotropic virus type I (HTLV-I) have large regions of similarity to CD4 (276), suggesting that cells infected by these viruses may be more susceptible to HIV infection.
Cohen et al. (72) compared two groups of HIV+ patients, consulting for dermatological problems or genital infections (urethritis). The median HIV RNA level in the semen of the group with dermatological infections was one-eighth that in the group with genital infections. In the group with genital infections, the subgroup of patients with gonorrhea had the highest seminal viral load. Antibiotic treatment of urethritis reduced the viral load in the semen but did not affect the plasma viral load. This study clearly establishes that local infections of the male reproductive tract are important cofactors of HIV load in the semen.
The question of viral compartmentalization was raised in a longitudinal analysis of eight subjects who went on to develop AIDS. The seminal viral load increased in most cases, but the viral load was consistently higher in blood plasma than in semen (132). An absence of correlation between plasma and semen loads was also reported in another study and suggests that the semen and blood are separate viral compartments (198). A comparison of HIV-1 gp120 sequences from five recent seroconverters with those from their corresponding sexual partners (transmitters) revealed that in each couple studied, the variant transmitted corresponded to a minor population in the semen of the transmitter, providing evidence that HIV-1 selection occurs during sexual transmission (360). Protease gene sequences also differ in semen and blood (55, 165), as do viral phenotypes (342) and the ratio of infected to uninfected leukocytes (165). Further evidence of viral compartmentalization is provided by the following observations: the lack of association between the culturability of the virus in semen and viral RNA levels in blood, the discordant distributions of viral phenotypes, the discordant viral RNA levels, the absence of correlation between viral RNA levels in semen and CD4+ cell counts in blood, differences in the biological variability of viral RNA levels, and differences in viral load following antiretroviral treatment (75). HIV-1 comprises two main phenotypic strains: NSI (non-syncitium inducing) strains, which infect macrophages, use CCR5 as a coreceptor for cell entry, and are preferentially transmitted; and SI (syncitium-inducing) strains, which use the CXCR4 coreceptor, appear later in the disease, and are poorly transmitted. It has therefore been suggested that there is selection between NSI and SI strains in the genital tract. However, restriction of SI variants in the male genital tract, such as would account for the observed NSI transmission bias, remains to be established, because SI and NSI strains do not seem to be compartmentalized in semen (90). The precise identification of the viral reservoir in the body is now of the utmost importance for antiretroviral treatment. Thus, although the development of potent treatments raises the hope that HIV-1 eradication might be possible, we do not yet know whether all the compartments in which the virus replicates are accessible to antiretroviral compounds. Several studies have investigated whether drug-resistant strains develop in seminal plasma and whether patients undergoing treatment carry infectious variants in their semen. A study by van't Wout et al. (340) analyzed the relationship between HIV-1 quasispecies extracted from the blood at various times and from various organs at autopsy. The brain quasispecies were very homogeneous and differed from the peripheral blood mononuclear cell variants, suggesting compartmentalization and the early spread of HIV-1 to the brain. Tissue-specific quasispecies were also observed in the testis, but in this case they were suggestive of a later invasion, possibly secondary to lymphocyte infiltration due to the disease (340). An evaluation of blood and genital secretions from HIV-infected men under treatment showed no genotypic changes consistent with protease inhibitor resistance in semen, despite the presence of these agents in blood plasma. This therefore suggests that protease inhibition may have limited penetration into the male genital tract (111). Replication-competent virus was detected in the semen of HIV-infected men receiving antiretroviral treatment, although there was no detectable virus in the peripheral plasma (358). In light of these studies, it appears that treatment strategies for the complete elimination of HIV-1 from the genital tract should now be a public health priority. It is therefore urgent to study the penetration of antiretroviral compounds into the male genital tract and to establish a correlation with the presence of virus and virus subtypes.
Human T-lymphotropic virus type I.
HTLV-I, like HIV, is a retrovirus that infects T cells. It is epidemiologically linked to adult T-cell leukemia-lymphoma (230). HTLV-I is sexually transmitted by semen (231, 317, 318), most probably via contaminated lymphocytes in the semen.
Human herpesvirus 8.
Kaposi's sarcoma (KS) is frequently associated with AIDS and occurs mainly in homosexual men. The epidemiology of KS in HIV-infected patients suggests that it may be caused by a sexually transmitted infectious agent (37, 144). This agent has recently been identified as a new human herpesvirus called human herpesvirus 8 (HHV-8) or KS-associated herpesvirus. According to many studies using extremely sensitive serologic techniques (76, 97, 133, 145, 223, 321, 322), the prevalence of HHV-8 in the general population is low. However, it is generally accepted that the virus can be detected in the semen of KS-positive patients. A recent study detected HHV-8 DNA in 12% of semen samples from KS patients, with a mean copy number per microgram of positive target DNA of 300, versus 9,000 in blood cells (182). Significant amounts of HHV-8 DNA were also detected in semen samples from 28 HIV-1-infected individuals and were shown to infect the mononuclear cell fraction (45). A recent multicenter comparison study concluded that HHV-8 DNA is present in semen but at concentrations that are probably too low to facilitate its consistent detection (251). Thus, the semen viral load should be measured to determine whether it is high enough for sexual transmission to occur. In addition, the prevalence of HHV-8 in the semen of healthy men has not yet been accurately established. It should, however, been borne in mind that the incidence of KS in HIV-negative individuals is much higher in Italy than, for example, in the United Kingdom (30 times higher) or the United States (20 times higher). In Denmark, a recent study performed on 100 healthy donors did not detect any seminal HHV-8 DNA (161). Therefore, the prevalence of HHV-8 in semen may be higher in some geographical areas than in others. The nature of the infected cells in semen and the origin of the virus also remain to be determined.
Cytomegalovirus.
CMV also belongs to the Herpesviridae family. It is very common, with 50% of the otherwise healthy population being infected (349). Infection is spread by intimate contact with infected body fluids including semen (185), and 40% of the semen from healthy donors is infected. This virus was previously erroneously reported to be cold labile, whereas it in fact survives in frozen and thawed semen (137). CMV is thought to be a possible causative agent of hematospermia (169). The virus generally remains in a latent form and causes a lifelong infection, but it may be activated either by a primary infection, for example after organ transplantation, or by the impairment of cellular immunity. Prospective studies in the United States have demonstrated that CMV is responsible for more prenatal and perinatal virus infections than is any other transmissible agent identified to date. The impact of these infections on fetal and neonatal health is unclear. However, preliminary data suggest that CMV is probably the most important agent responsible for congenital infection and damage (185). The French government decided some time ago to reject CMV-seropositive donors for artificial insemination. It then pulled back from this decision, deciding instead to reject only donors with recent seroconversion.
Two independent laboratories tested for CMV in 178 cryopreserved sperm samples from 97 healthy donors, 34% of whom were CMV seronegative, 52.6% of whom were seropositive with no recent contamination (absence of immunoglobulin M), and 13.4% of whom had unknown serological status. They detected CMV in 2.8% of the samples after culture and in 5.6% by PCR, thereby demonstrating that CMV can be detected in the semen in the absence of recent contamination (200).
CMV is also one of the most common opportunistic infections in AIDS patients, and it has been suggested that persistent CMV infection of the semen increases the risk of AIDS, possibly by activating CD4+ cells such that HIV-1 is produced (93). CMV has been isolated from the semen of homosexual men (202, 301), into which it was excreted intermittently (73), and CMV levels are associated with HIV seropositivity (186, 270). However, Rinaldo et al. found no association between CMV shedding and a higher risk of developing AIDS (269). The shedding of HIV was more closely associated with the concomitant shedding of CMV than with the CD4+ cell count (174). In a study investigating the relationship between CMV infection and the progression of HIV-1 disease, a group of 234 asymptomatic HIV-1 antibody-positive homosexual men were tested for CMV. CMV was isolated from the semen of 45% of the men. CD4+ cell levels were significantly lower in those in whom CMV had been isolated from semen. Similarly, an inverse relationship was observed between the concentration of CMV in semen and the CD4+ cell levels (190). Leach et al. (191) later concluded from junctional hybridization experiments that the presence of multiple CMV strains in HIV-1-positive homosexual men was associated with the progression to AIDS, possibly via activation of HIV-1-infected CD4+ cells. Hematopoietic cells are the only cells in the semen to have been identified as being infected (265).
Epstein-Barr virus.
Epidemiological studies have shown that Epstein-Barr virus (EBV) infection is most common in the age group at which sexual activity begins, strongly indicating that it is sexually transmitted (334). The presence of EBV in semen has not yet been investigated, but several studies have reported that human seminal plasma activates replication of this virus. Thus, EBV early-antigen expression is stronger in infected cells cultured in the presence of semen (149, 151, 330, 357). In vitro inhibition by seminal plasma of both the T- and B-lymphocyte responses to infection has also been described (193, 334). These results suggest that seminal plasma may facilitate EBV replication in the cervix of the uterus and may therefore have some relevance to the etiology of cervical cancer.
Papillomavirus.
Papillomaviruses, like EBV, cause cancer. There has been extensive testing for these viruses in the male genital tract, especially in the prostate, since they are thought to be a possible cause of prostate cancer; this is discussed below (see “Viruses and the prostate and other accessory glands”). Human papillomavirus (HPV) DNA was detected in the semen of three patients, and the data obtained were consistent with the contention that HPV can be transmitted sexually via semen, as suggested by epidemiological data on the sexual transmission of HPV (247). Since that first report, papillomaviruses have been detected in semen in several studies (130, 131, 150, 181, 184). These results conflict with early work by Nieminen, which claimed that papillomavirus DNA was not transmitted by semen since the semen of the sexual partners of 17 women positive for HPV DNA was uninfected (238). However, these results actually show only that transmission from the woman to the man is rather inefficient. Indeed, other studies have indicated that HPV type 16 and 18 DNA is present in sperm cells and may be transmitted to the partner (64, 181, 183, 184). Thus, HPV type 16 DNA and RNA were detected in the semen of 25 and 8% of 24 randomly selected patients, respectively, whereas the prevalence of detection for HPV type 18 DNA and RNA was higher (46 and 21%, respectively). The incidence of asthenozoospermia is significantly higher in patients with HPV in their semen (183).
Hepatitis viruses.
Hepatitis viruses may cause acute diseases (fulminant hepatitis) or chronic diseases such as liver cancer and cirrhosis. The ability of human semen to transmit hepatitis B virus (HBV) was first demonstrated by the inoculation of gibbons (291). HBV antigens were subsequently detected in human semen (155), and it is now well established that this biological fluid is a vector for the spread of hepatitis B (84, 107, 115, 153, 158). However, few studies have tried to identify the contaminated cells within semen. Hadchouel et al. (135) showed that HBV DNA was integrated into the DNA of spermatozoa in two of three patients with acute hepatitis, suggesting that there may be true transmission of HBV via the germ line. Another study of chronic HBV antigen carriers showed that HBV DNA was present in all of the semen samples tested with the infected cells being both spermatozoa and mononuclear cells (85). Persistent free HBV DNA has also been detected in the semen of patients with no markers of viral replication in serum, indicating that the genital tract may act as a reservoir and that these patients may transmit the virus sexually (85).
The transmission of hepatitis C virus (HCV) is a major health concern since the disease is asymptomatic in three-quarters of cases. Half of the infected patients become chronic carriers, and 10% develop liver cancer or cirrhosis. Although HCV transmission via parenteral exposure is well documented, the sexual transmission of this virus is more contentious (for reviews, see references 275 and 347).
To determine whether HCV could be sexually transmitted, the frequency of HCV infection was studied in heterosexuals with multiple partners. The frequency of HCV infection in these individuals was found to be much higher than that in healthy women with a stable partner (359). In Egypt, where the rate of seropositivity for HCV is particularly high (13 to 22% versus 0.04 to 1.2% in the United States and Europe), a study suggested that HCV is transmitted within couples (180). Thus, viral nucleotide sequence analysis for 33 husband-and-wife pairs revealed significantly high levels of similarity (97 to 100%) in 32 of the 33 pairs studied. Although the role of sexual contact in such transmission is unclear, these and other studies (5, 11–13, 114, 157, 196, 326) indicate that the sexual transmission of HCV cannot be ruled out.
The search for HCV RNA in semen has generated conflicting results, with about half the studies demonstrating the presence of HCV in seminal fluid (173, 195, 197, 209, 319) and the other half demonstrating its absence (56, 121, 147, 296, 324) or finding its prevalence to be low (117). Thus, in the group of studies supporting the hypothesis of a sexual transmission, a study by Liou et al. showed by nested PCR that among 34 patients with chronic liver disease positive for anti-HCV antibodies and with HCV RNA in serum, the prevalence of HCV RNA in body fluids was 100% (7 of 7) in ascites, 48% (15 of 31) in saliva, 7% (2 of 29) in urine, and 24% (4 of 17) in semen (195). HCV-specific antigens have also been detected in semen (173), and HCV RNA was detected in the supernatant of spermatozoa and spermatids from five patients with chronic hepatitis C (197). In contrast, several studies argue against a sexual transmission of the virus, since they have detected no HCV RNA in the semen of chronically infected patients (56, 121, 147, 296, 324) or have found its prevalence to be low (117). The risk of hepatitis C transmission during artificial insemination has been investigated, and although HCV RNA was detected in the semen of some donors, no viral RNA remained following purification (209). In conclusion, although HCV may be present in semen, the viral load is probably extremely low, and therefore the virus represents a minor risk of sexual transmission.
One group recently presented preliminary evidence that hepatitis G virus (HGV) is present in semen (296), whereas another detected no HGV RNA in semen samples obtained from a cohort of 54 HIV-1-infected homosexual men (143). The pathological significance of HGV is unknown.
Herpesvirus and adenovirus.
HSV is a sexually transmitted agent that may be associated with cervical cancer and may cause high morbidity and mortality in perinatal infection (7, 233). Centifanto et al. (60) were the first to suggest that it was present in the male genital tract: inclusion bodies that resembled those of herpesvirus were found in tissue cultures of semen from two subjects, but no infectious virus could be cultured directly from the samples (94). Moore et al. (226) described an individual in a therapeutic donor insemination program who asymptomatically acquired a primary HSV-2 infection and transmitted it to one of two HSV-seronegative partners, in whom a primary HSV-2 infection developed, providing evidence for the sexual transmission of HSV-2. HSV DNA was recently detected in the semen of men with genital HSV-2 infection, mainly during recurrences of herpes (345). Concerning the type of cell infected within semen, an early electron microscopy study found no ultrastructural relationship between the virion and the spermatozoon (302). However, HSV DNA has recently been detected in human spermatozoa by in situ hybridization (172). The association of HSV DNA with spermatozoa has been linked to fertility problems, because HSV infection was found to be almost three times as common in the semen of patients with a low sperm count attending an infertility clinic as in individuals with a normal sperm count (172). A significant association was also found between infertility and positive tests for HSV in another study performed on 153 men (109). A possible relationship between infertility and the presence of the adenovirus in semen has also been a matter of concern. Csata and Kulcsar (80) detected HSV or adenovirus in the semen of 40% of infertile patients tested and found that these viruses were present in a latent form in 60% of their spermatozoa.
Further studies are required to confirm these data and to eludidate the link between the presence of these viruses in semen and fertility problems.
Animal Semen
Several viruses have been detected in the semen of a number of animal species (Table 3). DNA from Rous sarcoma virus binds to Xenopus laevis spermatozoa and is transferred to ova during fertilization, inducing developmental malformations in 25 to 30% of embryos (134). In gibbons, sperm abnormalities are frequently encountered and have often been shown to be related to the presence of herpesvirus (36, 327).
TABLE 3.
Presence of viruses in animal semen and its consequences
| Virus and species infected (reference) | Cells infected | Effects |
|---|---|---|
| BTV (bull) (51, 146, 250, 252) | Spermatozoa (118) | Semen abnormalities (118) early embryonic death, abortion, malformed fetal calves (245); transient infertility (245) |
| BHV-1 (bull) (106) | Seminal plasma rather than cells (337) | Decrease in semen quality (106); fertility disturbances (337–339) |
| Foot-and-mouth disease virus (bull) (78, 292, 307) | NAa | Transmitted by artificial insemination (35, 58, 86, 285) |
| BLV (bull) (271) | NA | Does not seem to be transmitted by semen; presence of a factor inhibiting virus replication? (35, 225, 271, 313, 332) |
| BIV (bull) (234) | Leukocyte fraction | NA |
| BDV (bull) (28) | NA | Sexual transmission (214, 215, 285); no apparent sperm defect (170) |
| SIV (monkey) (218) | Rarely recovered from mononuclear cells (217) | Sexual transmission |
| PRRSV (pig) (341) | Macrophages (69) | Sexual transmission (6, 237) |
| Porcine parvovirus (pig) (128) | DNA binding to spermatozoa (128) | NA |
| Porcine rubulavirus (pig) (263) | NA | Reduction in spermatozoon mobility and concentration (263) |
| CMV (mouse) (31) | Spermatozoa (31) | Sexual transmission (235) |
| Retroviruses (mouse) (162, 164, 254) | Macrophages (164, 194) | NA |
| Spermatozoa (164, 194) | ||
| FIV (cat) (154) | NA | NA (sexual transmission) |
| Herpesvirus (gibbon) (36, 327) | NA | Sperm abnormalities |
| Rous sarcoma virus (Xenopus laevis) (134) | Spermatozoa | Transfer to ova, developmental malformations (134) |
NA, not available.
Feline immunodeficiency virus is shed into the semen of both experimentally and naturally infected cats (154). Simian immunodeficiency virus (SIV) is also present in the semen of monkeys and is transmitted by this vector (217).
In mice, high concentrations of retroviral particles have been detected in the epididymal fluid (162–164). These viruses are mostly endogenous retroviruses, but some exogenous infectious retroviruses (mouse ecotropic virus and Friend ecotropic virus) have also been described (254), and the association of retroviral particles with spermatozoa may lead to congenital infection. Murine leukemia virus has been found free in the seminal fluid, fixed to spermatozoa, and associated with macrophages (164, 194). The main site of virus synthesis within the male genital tract is the epithelial cells lining the epididymis duct. Murine CMV DNA has also been detected in spermatozoa, and the affected mice remained fertile (31).
The porcine reproductive respiratory syndrome (PRRS) was first recognized in the United States in 1987 and in Europe in 1990 and has now spread worldwide. The PRRS virus (PRRSV) has been detected in semen (341) and is transmitted sexually (6, 237). Attempts have been made to determine the origin of PRRSV in semen by inoculating vasectomized and nonvasectomized boars intranasally with the virus. PRRSV RNA was detected in semen from all the boars and was most consistently found within macrophages. Therefore, macrophages in semen are probably infected via the infection of local tissue macrophages or via infected circulating monocytes or macrophages (69). In contrast, porcine parvovirus DNA, detected in the epididymal semen of oronasally inoculated boars, was found to bind to spermatozoa (128) but seemed to have no negative effect. Lastly, porcine rubulavirus has been reported to reduce sperm quality in sexually mature boars by decreasing spermatozoon motility and concentration (263).
Since viral contamination of semen is common in bulls and since frozen semen is widely distributed and is of major economic importance, national and international organizations have laid down guidelines aimed at establishing disease-free bull studs producing semen free from potential pathogens. Many different viruses have been detected in bull semen. In particular, bovine diarrhea virus has been detected at high titers in semen (28) and is transmitted by semen (214, 215, 285). This virus does not seem to cause sperm defects (170). Bluetongue virus (BTV) has also been isolated from bull semen (51, 146, 250, 252), and a positive relationship has been found between the infectivity of semen samples from bulls infected with latent BTV and semen abnormalities (118), with virus-like particles occasionally observed in the heads of affected spermatozoa (118). BTV is associated with reproductive disorders including early embryo death, abortion, malformation of fetal calves and lambs, and transient infertility in bulls and rams coinciding with the shedding of virus into semen (245). Bulls were experimentally inoculated with BTV to investigate the frequency, duration, and pathogenesis of virus shedding into the semen. The shedding of BTV into semen in infected bulls closely followed peak virus titers in blood, and in no case was the virus isolated from semen without also being isolated from blood. Three of nine heifers inseminated with semen from a bull shedding BTV became viremic, seroconverted, and became pregnant. However, there was no evidence of fetal infection (48, 49). Bovine herpesvirus 1 (BHV-1), one of the most common viral pathogens in bovine semen, may also be associated with a decrease in semen quality, and bull semen is therefore systematically tested for this virus before being used for insemination (106). BHV-1 is present in the seminal plasma rather than the cell fraction (337). The risk of transmitting BHV-1 to cows by insemination has been investigated, and such transmission has been shown to lead to fertility disturbances (337–339). Foot-and-mouth disease virus has also been detected in bull semen and is transmitted by artificial insemination (35, 58, 78, 86, 285, 292, 300, 307). In contrast, bovine leukemia virus is present in semen but does not seem to be transmitted by it (35, 225, 271, 313, 332), possibly due to the presence of an inhibitory factor in fresh semen that prevents bovine leukemia virus infection from becoming established (271). Finally, bovine immunodeficiency virus, a lentivirus, is associated with a lymphoproliferative disease and is prevalent in dairy and beef cattle in the southeastern United States. Its mode of transmission is unknown, but semen is suspected to be a potential vector of infection. Indeed bovine immunodeficiency virus DNA has been detected in the leukocyte fraction of cryopreserved semen specimens (234).
VIRUSES AND THE TESTIS
Semen is known to be an important vector in the dissemination of viral diseases, and several viruses are present in cell-free semen and in seminal cells including spermatozoa, macrophages and lymphocytes (see “Viruses and semen” above). Paradoxically, testing for viruses in the testis has not been extensive despite the possibility that they are involved in some of the disorders of this organ, such as orchitis, decrease in semen quality, azoospermia, and testicular carcinoma. The blood-testis barrier makes it likely that the testis acts as a reservoir for viruses (which may be protected against antiviral treatments), and this is another crucial reason for considering the testis to be an organ of special interest in the context of viral infection and STDs. Viruses present in the testis are listed in Fig. 1.
The mammalian testis has two main compartments: (i) the interstitium, which contains the Leydig cells responsible for testosterone production, macrophages, fibroblasts, blood, and lymphatic vessels; and (ii) the seminiferous tubules, which are bordered by the peritubular myoid cells and are composed of the various generations of germ cells (from spermatogonia [the stem germ cells] to the meiotic spermatocytes and the haploid spermatids that differentiate into spermatozoa) that are associated with the somatic Sertoli cells (152). Viruses have been found in both these compartments in humans and other mammals.
The Human Testis
The best-known viruses causing testicular disorders in humans are mumps virus and HIV. A few studies have considered the role of oncogenic viruses, such as EBV or papillomavirus, in the etiology of testicular carcinoma even though testicular germ cell tumors are the most common malignant tumors in young adults and the incidence of testicular cancer has been steadily increasing in all countries for which epidemiological data are available (2, 39, 331). The viruses found in the human testis are listed in Table 4; see also Table 6.
TABLE 4.
Viruses found in the human testis and their consequences
| Virus (reference) | Cells infected | Effects |
|---|---|---|
| Mumps virus | Leydig cells (4), germ cells ? | Orchitis (29, 34, 120), testicular atrophy (29, 34), sterility (3, 34, 159), decrease in androgen secretion (4), testicular cancer ? (34, 243) |
| HIV | Lymphocytes and macrophages (257), germ cells (228, 240) | Orchitis, interstitial fibrosis, lymphocyte infiltration, change in Leydig cell number, decrease in germ cell number (63, 220, 236, 266, 348), change in spermatogenesis (63, 82, 83, 92, 257, 273, 354) |
| EBV (67) | NAa | Orchitis (268), testicular cancer ? (10, 262, 305) |
| Parvovirus B19 (129) | NA | Testicular cancer ? (129) |
| HSV-2 (95) | NA | Viral reservoir ? (95) |
| HSV-1 (80) | NA | Infertility ? (80) |
| Adenovirus (80) | NA | Infertility ? (80) |
| Coxsackie virus ? | NA | Orchitis (216) |
| Influenza virus ? | NA | Orchitis (216) |
| Endogenous retroviruses | Germ cells (103, 187) | Testicular cancer ? (320) |
NA, not available.
TABLE 6.
Effects of viruses on the reproductive endocrine system in men and animals
| Virus (species affected) | Effect (reference) |
|---|---|
| Mumps (human) | Decrease in androgen production (4) |
| HIV (human) | Increase in testosterone level at the early stage of infection (68, 212); decrease in testosterone level and increase in LH and FSH production (79) in men with AIDS |
| FeLV (cat) | Decrease in testosterone level (346); decrease in LH, FSH, and LHRH levels (346) |
| EHDV-2/BTV ? (Mule deer) | Decrease in testosterone production (328); increase in LH and FSH levels (328) |
| Myxoma virus (rabbit) | Impaired steroidogenesis (119) |
| EMCV (hamster) | Apparent increase in Leydig cell number (141) |
| CMV (mouse) | Leydig cell infection (30) |
| Murine leukemia virus (mouse) | Leydig cell infection (249) |
| Aujesky's virus (boar) | Interstitial cell infection (219) |
| ALV (rooster) | Interstitial cell infection (99) |
| Retrotransposons (human) | Activation in testosterone-producing Leydig cells (284) |
Mumps virus.
Mumps is caused by an RNA virus of the paramyxovirus group. In prepubertal boys, the symptoms of mumps are usually limited to infectious parotitis, but, in men, orchitis is the most common complication (29, 34). Orchitis develops in 5 to 37% of all adult patients infected with mumps (120). Within the first few days of infection, the virus directly attacks the testes, destroying the testicular parenchyma (44, 242) and decreasing androgen production (4). This accounts for the testicular atrophy observed in 40 to 70% of patients with orchitis (29, 34). Unilateral involvement is the most common, while bilateral involvement occurs in 15 to 30% of the patients with orchitis (201). Bilateral orchitis leads to hypofertility with oligospermia and testicular atrophy in 13% of those patients (59). Morphological studies of mumps-associated orchitis were carried out in the 1940s by Gall (122) and Charny and Meranze (65). They showed the focused nature of the inflammation and described the sequential stages of the disease. During the initial stage, an interstitial edema was commonly detected. Blood vessels were congested and surrounded by lymphocytes. Increased permeability of the blood vessels was found to lead to local interstitial hemorrhage and to exudation of leukocytes and fibrin. The seminiferous epithelium degenerated, but the Sertoli cells seemed little affected. Repair of the damage caused by infection involved the deposition of collagen within the interstitium, followed by tubular atrophy and peritubular concentric fibrosis, the usual residue of mumps orchitis. Sterility may be transient or definitive and is due to the loss of the germinal epithelium (290). There are several possible explanations for the mumps-induced degeneration of germ cells. Since mumps virus does not seem to induce germ cell transformation or proliferation in vitro, it may have an indirect effect (243): (i) high fever associated with the disease leads to a change in testicular temperature, contributing to germ cell degeneration (the most common hypothesis); (ii) germ cell degeneration may be caused by seminiferous tubule congestion following the interstitial edema (201); or (iii) a modification of testosterone production by Leydig cells may have a deleterious effect on seminiferous tubule function. Data concerning the consequences of orchitis on testicular endocrine function are rare. Adamopoulos et al. (3) described a severe alteration of Leydig cell function during the acute phase of the disease. These authors observed a drop in the testosterone level together with an increase in luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels in 27 patients suffering from mumps orchitis, suggesting a testicular failure. In another study (4) examining three patients, testicular atrophy, libido decrease, impotence, and gynecomasty were associated with the reduction of testosterone and the increase in LH and FSH levels. Thus, in some cases, the Leydig cell function seems to be damaged by the mumps infection. Whether this alteration is due to a direct or indirect effect of the virus on the cells is unknown. Such a dysfunction would also account for the higher incidence of testicular cancer in men who have had mumps than in control men (34). Two publications suggested that mumps virus replicates within the testis: Bigazzi et al. (42, 43) showed viral replication in the interstitial tissue in organotypic culture of monkey testis following virus inoculation through the testicular vein, and Bjorvatn et al. (44) rescued mumps virus by needle aspiration of testis biopsy specimens from mumps-infected patients. Two studies (113, 177) showed that systemic treatment with interferon (IFN) prevented the testicular atrophy caused by mumps orchitis in all treated patients (4 of 4 and 13 of 13, respectively). In the second study, where patients were randomly assigned into two groups, atrophy of the testes was observed in three of eight men in the control group. However, asthenospermia was still detected in four patients 7 years after interferon treatment (177).
Human immunodeficiency virus type 1.
Several endocrine and testicular dysfunctions have been reported in men infected with HIV-1, depending, in part, on the stage of the disease.
High levels of testosterone have been found in men during the early stages of HIV infection (68, 212), whereas low testosterone levels have been found in men with AIDS (100, 261, 287, 343). Hypogonadism is common in HIV disease, and the decrease in testosterone levels certainly contributes to the weight loss observed in AIDS patients. Indeed, in men with low testosterone levels, testosterone replacement via a transdermal system is followed by a gain in lean body mass (41). The testosteronemia probably results from lymphocyte infiltration and fibrosis of the interstitial tissue and from a decrease in Leydig cell number (82, 83, 257, 273).
AIDS patients often suffer from orchitis, hypogonadism, oligospermia, or azoospermia (20, 100, 253, 257, 325) and, in some cases, from testicular germ cell tumor or lymphoma (54, 116, 244, 351). Among 3,015 HIV-positive men, the incidence of testis tumors has been reported to be 0.2%, which is 57 times that of US. average of 3.5 cases/100,000 men (351). Several autopsy reports for deceased AIDS patients have shown a high rate of testicular abnormalities (63, 220, 236, 266, 348). Several groups have studied testis histology in men with AIDS and spermatogenesis dysfunction is invariably reported. This was first described by Chabon et al. (63), who showed major changes in the testes of 20 patients, resulting in a “Sertoli cell only” pattern, and interstitial inflammation in 53% of the patients. Rogers and Klatt (273) also described germ cell depletion, a decrease in tubule diameter, and an increase in the thickness of the tubule boundary wall. Yoshikawa et al. (354) set up a classification system for the disorders observed in the testes of 31 patients who had died from AIDS. Five categories were established: Sertoli cell only (42%), germ cell degeneration (27%), peritubular fibrosis associated with tubular hyalinization (15%), maturation arrest (12%), and normal appearance (3%). These observations were confirmed in 57 autopsy cases in another study (92). Since then, other authors have described the arrest of spermatogenesis at various points, numerous foci of degenerating germ cells, and epididymis block. Interestingly, a study of 140 testicular autopsy specimens from AIDS patients with and without antiviral treatment showed that treatment and prolongation of survival in AIDS patients is associated with a shift in the histologic findings for testes toward a more pronounced loss of germ cells (304).
These morphological observations have raised questions about the mode of action of HIV on the testis. The virus was first thought to have an indirect effect due to the chronic debilitating illness and cachexia of the patients. It has also been suggested that opportunistic infections are involved. However, another study indicated that only 32% of patients with opportunistic infections had CMV, Mycobacterium avium-intracellulare, or Toxoplasma gondii in the testes. This suggested that these infections were probably not the key factors causing the observed hypogonadism (91). HIV may also act indirectly via changes in the hypothalamic-pituitary axis. Men with AIDS often have low testosteronemia (see above), and a dysfunction in hypothalamic gonadotropin releasing hormone secretion may account for this phenomenon (100). However, other studies found no significant abnormality in the hypothalamic-pituitary axis (253, 286). The low serum testosterone levels in men with AIDS are in some cases associated with high serum LH and FSH levels, implying that there is primary testicular failure (79). A possible reason for the testosterone secretory defect in these men is suggested by reports that cytokines released by the activated phagocytic cells of the immune system inhibit the steroidogenic response to human chorionic gonadotropin in vitro and presumably also the response to LH in vivo (57). Recent studies have shown that physiologic testosterone replacement in HIV-infected men with weight loss who have low testosterone levels can increase muscle mass and effort-dependent strength (for a review, see reference 40). Testosterone therapy also helps to alleviate the symptoms of hypogonadism (260). However, further studies are needed to determine whether androgen therapy can significantly and durably improve physical function and health-related outcomes in HIV-infected men.
HIV infection of the testis has now been described in several studies, indicating that direct local action may be responsible for the observed damage within the gonads. The HIV p17 protein was first detected within the testis by immunohistochemistry using monoclonal antibodies (83). Pudney and Anderson (257) detected the CD4 receptor on the cell surface of lymphocytes and macrophages infiltrating the testis, which suggests that these cells have the potential to be infected by HIV. Indeed, in 9 of the 23 cases in which immunocytochemistry was used to test for HIV-1 protein, HIV-infected cells of the lymphocytic/monocytic type were found in the seminiferous tubules and interstitium of the testis. Such cells were also found in the semen (257). Using in situ PCR, several other studies have detected HIV-1 DNA within testicular germ cells. (i) The infection of spermatogonia, spermatocytes, and a small number of spermatids in 11 of 12 men with AIDS was described by Nuovo et al. (240). (ii) Another study with seropositive asymptomatic subjects reported the presence of HIV-1 DNA in the nuclei of spermatogonia and other germ cells at all stages of differentiation, suggesting clonal infection (228). In these subjects, the presence of provirus did not cause cell damage and was associated with normal spermatogenesis, whereas in AIDS patients, spermatogenesis was arrested and there were few infected spermatogonia and spermatocytes. (iii) HIV-1 DNA was recently found in 25 to 33% of the residual germ cells in the testes of AIDS patients, with testicular changes including all variants from decreased spermatogenesis to the Sertoli-cell-only pattern (303). In contrast, HIV-1 DNA was not detected in the testes of any of three preadolescent boys who acquired HIV in utero (303).
The way in which HIV enters germ cells is unknown. The CD4 receptor has still not been unequivocally detected on the germ cell surface, nor have the chemokine coreceptors triggering HIV entry in other cell types (e.g., monocytes/macrophages, lymphocytes and microglial cells). The virus may also enter germ cells via an alternative galactoglycerolipid receptor, as suggested (52). Tissue-specific HIV-1 quasispecies have been identified in the testis, indicating that there may be a tissue tropism for this organ (341). This is a very important issue for HIV treatment because triple therapy seems to be unable to eradicate the virus completely. Thus, the testis could act as a viral reservoir isolated by the blood-testis barrier which cannot be reached by drugs (71) (see the section on HIV-1 in “Human semen” above).
Oncogenic viruses.
Orchitis has been described as a symptom of infectious mononucleosis caused by EBV, indicating that this virus may have a direct effect on the testis (268). EBV DNA was detected in the testes of three individuals with no EBV-related disease, showing that the testis may be a target organ (67). Rajpert De Meyts et al. (262) found that EBV was not directly involved in the testicular germ cell tumors of 20 patients but thought that germ cell proliferation might be stimulated by testicular EBV-transformed lymphocytes. However, more recently, Shimakage et al. (305) detected EBV RNA and EBV-related proteins in all of the 27 seminomas they tested but in none of 25 nonmalignant testes, providing further support for the hypothesis that EBV is associated with testicular tumors. In addition, transgenic mice expressing papillomavirus genes develop germ cell tumors resembling human seminoma (171). Recently, 39 patients with testicular germ cell tumors were screened for EBV, CMV, and parvovirus B19 DNA by PCR. Neither EBV nor CMV DNA was detected in the testis. In contrast, parvovirus B19 DNA sequences were found in the testicular tissues of 85% of patients with testicular cancers but in none of the normal testicular tissue samples (129). However, the cellular targets of parvovirus B19 within normal and cancerous testes are unknown and the possible role (direct or indirect) of this virus in the development of testicular germ cell tumors is unclear.
Other viruses.
HSV-2 was detected in the testes of 4 of 10 corpses at autopsy, suggesting that this organ acts as a reservoir for transmission of the virus (95). Csata and Kulcsar (80) studied the relationship between male infertility and the presence in the semen or testis of HSV-1 or adenovirus. In 40% of patients with infertility either HSV-1 or adenovirus was present within the testis. Testicular cells were also infected in vitro by either HSV-1 or adenovirus, and these two viruses were found to penetrate and replicate (80).
Several viruses are suspected of inducing orchitis, based on epidemiological or clinical association. There is, however, little evidence that any known human viral pathogen, apart from mumps virus, has selective or exclusive organotropism for the testis. The association between viral infection and orchitis was reviewed in 1962 (268) and in 1982 (216). It appears that coxsackievirus, dengue virus, and bat salivary gland virus infections are associated with a significant incidence of clinical orchitis. Other viruses, such as influenza virus, EBV, lymphocytic choriomeningitis virus, phlebotomus fever virus, adenovirus, echovirus, smallpox virus, vaccinia virus, rubella virus, and chickenpox virus, also cause orchitis, albeit more rarely (216, 268). Atrophy of the testis is common in viral orchitis, but since atrophy is unilateral in most cases, sterility is extremely rare. Rubella virus is thought to alter the development of some aspects of the reproductive tract, as shown by a study reporting that 12% of 316 boys with congenital rubella suffered from cryptorchidism (256). The virus may also affect the adult testis: testalgia, an indicator of orchitis, was found in 5 of 68 adults during an epidemic of rubella (255). However, it is unclear whether this clinical symptom does indeed reflect testicular inflammation or is just a nonspecific manifestation of viremia.
Endogenous retroviruses.
Endogenous retroviruses (ERV) are chromosomal elements that have a genomic organization analogous to that of exogenous retroviruses. Their relevance to this review is that they may originate from ancient infections of germ cells by exogenous retroviruses (199), thus demonstrating that viral genomes can be incorporated into the germinal genome. Retrotransposons are closely related to ERV, but differ from them in that they do not have the env gene and, therefore, produce particles that are intracellular and not infectious (178).
The first human ERV (HERV) was cloned in 1981 (204). Since then, more than 20 HERV families have been identified, cloned, and partially characterized (199, 336). No HERV has ever been shown to be infectious, but they may be associated with germ cell tumors (320). HERV genes are often highly expressed in the testis due to the hypomethylation of testicular genes. Hence, HERV-K, the most biologically active member of the HERV family in terms of the coding of viral protein and particles, preferentially expressed in cell lines derived from teratocarcinomas (199, 336), is also closely associated with active seminomas (199, 283). One study also reported the detection of antibodies against HERV-K10 Gag protein in 45% of seminoma patients (283), whereas no positive tests were observed in seminoma patients tested months or years after treatment. The HERV-K Gag protein was detected only in tumor cells, with no reaction being observed in the surrounding testicular tissue (283). However, the authors found no conclusive association between HERV and other testicular neoplasms, including teratomas (283). In contrast, HERV gag and env were detected by in situ hybridization in 100% of a series of germ cell tumor specimens (140). Samples of testicular carcinoma, a precursor of germ cell tumor, also consistently tested positive for HERV-K mRNA in situ (140), whereas the surrounding testicular tissues mostly tested negative. However, HERV-K mRNA has been detected in normal testicular parenchyma in other studies using the more sensitive reverse transcription-PCR technique (272). Herbst et al. (140) observed that a consistent absence of transcripts was observed in a series of immature and mature teratomas. They suggested that this inhibition of expression might result from the higher level of differentiation of the cells, a characteristic that distinguishes teratomas from other germ cell tumors. Indeed, the HERV gag sequence was found to be more highly methylated in primary germ cell testicular tumors (127). It is therefore possible that HERV-K may be involved in seminomas, due to the high level of gene expression resulting from demethylation. Another endogenous retrovirus, ERV-3, also has high levels of gene expression, at least at the mRNA level, in germ cells during the early phases of spermatogenesis but not in the somatic Sertoli cells or Leydig cells (187). However, ERV-3 env gene expression was almost undetectable in the two cases of seminoma studied so far (187).
The transcription of cellular retrotransposons is induced by a variety of physiological stimuli. A study reported that VL30 retrotransposons are expressed in steroidogenic cells within all four endocrine tissues engaged in the synthesis of steroid hormones in response to pituitary-derived trophic hormones (284). In the testis, the transcription of VL30 retrotransposons is activated in the testosterone-producing Leydig cells. Specific activation of retrotransposons has also been observed in spermatogenic stem cells. This may help to increase genomic 2plasticity within the germ line (103). Indeed, the testis is the only organ in which retrotransposon long terminal repeats LTRs are hypomethylated (320).
The Animal Testis
The viruses found in the animal testis and their consequences are listed in Table 5. The effects of viruses on the reproductive endocrine systems in men and animals are listed in Table 6.
TABLE 5.
Viruses found in the animal testis and their consequences
| Virus and species infected | Cells infected | Effects |
|---|---|---|
| ECMV (mouse, hamster) | Sertoli cells (335), germ cells (335) | Orchitis (335), necrosis and degeneration of germ cells including spermatogonia (141), increase in Leydig cell number ? (141) |
| Influenza virus (mouse) | Spermatocytes (299) | Chromosomal abnormalities (299) |
| CMV (mouse) | Germ cells (31, 105), Leydig cells (30) | No effect on fertility (31) |
| PRRSV (pig) | Germ cells (315), macrophages (315) | Germ cell apoptosis (315) |
| Myxoma virus (rabbit) | Interstitial cells (119) | Orchitis (119), impaired steroidogenesis (119), impaired spermatogenesis (119) |
| SIV (monkey) | Lymphocytes and macrophages in interstitium (32) | Degeneration of seminiferous tubules, hypospermatogenesis (32) |
| EHV-1 (pony) | Endothelial cells (323) | Necrotizing vasculitis, thrombosis (323) |
| Porcine rubulavirus (pig) | NAa | Interstitial monuclear cell-infiltration (263), seminiferous tubule degeneration (263), infertility (263) |
| Maedi/visna virus (ram) | NA | Interstitial mononuclear cell infiltration and fibrosis (248), atrophy of seminiferous tubules (248), disturbances in spermatogenesis (248) |
NA, not available.
Viruses and the seminiferous epithelium.
In rhesus monkeys experimentally infected with SIV, many seminiferous tubules were found to be necrotic and infiltrated with neutrophils due to opportunist CMV infection. No inclusion-bearing cells (characteristic of CMV infection) were observed in the interstitial tissue, but considerably more were present in the seminiferous tubules than anywhere else in the body (33). CMV antigens were detected by immunohistochemistry in the testes of SIV-infected monkeys in another recent study (179). Malignant lymphoma associated with SIV-induced immunodeficiency in macaques is a high-grade malignant tumor that most frequently affects the viscera, skin, central nervous system, and testes. It has been associated with an EBV-like simian herpesvirus (116). Testicular atrophy is a relative common finding in monkeys infected by SIV (218).
Several studies have investigated CMV infection in mice because of its similarity to disorders in humans. Dukto and Oldstone (105) detected murine CMV in a latent form in the testes of mice. Acutely infected male adult mice homozygous for the nude gene produced infectious virus in their testes, whereas their heterozygous littermates contained significantly less virus than did nude/nude mice. Viral DNA was restricted to the testicular germ cells and spermatozoa. This demonstrates that murine CMV may be harbored in the testes during both acute and latent infections and may replicate in male germ cells. In another study (235), CMV was recovered from both epididymal sperm and seminal vesicles. CMV DNA was detected by in situ hybridization in the spermatozoa and spermatocytes of infected immunocompetent mice, but their fertility was not impaired (31).
Encephalomyocarditis virus (EMCV) is also known to cause orchitis in mice. An initial study in hamsters showed that intraperitoneal injection of EMCV led to necrosis and degeneration of spermatogonia and other germ cell types. The number of Leydig cells may have increased at the same time (141), but it is not clear whether this increase was real or the result of the shrinkage of the seminiferous tubules, causing an impression of enlargement of the interstitial tissue. No regeneration of the seminiferous epithelium was observed 6 weeks after injection of the virus (141). The relationship between the distribution of viral RNA and histological changes during the early stages of EMCV-induced orchitis was investigated in another study (335). Viral RNA was first detected in a few Sertoli cells in almost intact seminiferous epithelium 2 days after inoculation. Two days later, viral DNA was detected not only in Sertoli cells but also in a small number of germ cells including spermatogonia. Mild to moderate degenerative changes were found to have occurred in the seminiferous epithelium, and virus-like particles were observed by electron microscopy in the degenerated and desquamated germ cells (335).
Influenza virus also has cytogenetic effects on male mouse germ cells, inducing the induction of chromosomal abnormalities in spermatocytes (299). Similarly, PRRSV, which is shed in semen (see “Animal semen” above), was able to replicate in pig testicular germ cells and macrophages, affecting spermatogenesis by inducing germ cell apoptosis (315).
Avian leukosis virus (ALV) is frequently congenitally transmitted by hens to their progeny. Interestingly, although the testes of congenitally infected roosters contain large amounts of the virus, ALV does not seem to be transmitted by semen (277). A more thorough study tried to resolve this apparent contradiction by attempting to identify the infected testicular cells involved and to detect free virus particles in the male genital duct system (99). The authors used virus assays and electron microscopy and showed that the viral buds were seen only in Sertoli cells and never on germ cells. This may account for the lack of male congenital transmission, although the techniques used do not exclude the possibility of latent viral infection of the germ cells. Very few free virus particles were shed into the duct system (99).
Viruses and the interstitial compartment.
The induction of feline acquired immune deficiency syndrome by feline leukemia virus (FeLV) in cats alters hormone production in the hypothalamic-pituitary-gonadal system. Thus, 12 weeks after the infection of male cats with FeLV, much lower levels of testicular testosterone production were observed following the injection of human chorionic gonadotropin in infected cats than in uninfected cats (346). Other glands, such as the hypothalamus and pituitary were also affected, with lower levels of release of LH, FSH, and luteinizing hormone-releasing hormone LHRH (346).
The monitoring of mule deer on a former plutonium production site revealed that 27% of the 116 adult males had abnormally developed testicles associated with low levels of testosterone in serum and compensatory high levels of LH and FSH (328). The severity of the atrophy and the absence of other affected tissues suggested that radiation might not be the cause. Indeed, 90% of 10 affected animals were seropositive for epizootic hemorrhagic disease virus and BTV, versus only 63% of 19 unaffected animals.
One of the most common lesions observed in SIV-infected monkeys is hypospermatogenesis with degeneration of the seminiferous tubules (32). Staining for SIV antigen identified small numbers of positive lymphocytes and macrophages in the interstitium (32). In contrast, a study of macaques inoculated with SIV concluded that spermatogenesis was impaired but that this was due to cachexia rather than to a direct effect of the virus on the testes (232). However, this conclusion may not be fully justified because the study was performed on prepubertal monkeys in which spermatogenesis was not fully established and the authors did not test for SIV in the testes.
Furthermore, CMV is a common opportunistic infection in rhesus monkeys experimentally infected with SIV. CMV causes changes in several organs including the testis (33). In 3 of 11 SIV-infected monkeys with CMV infection, the testicles were found to be severely affected: massive infiltration of the interstitial area was observed with patchy areas in which all elements were necrotic.
Intratesticular or intraperitoneal injection of CMV in mice leads to Leydig cell infection (30), as does injection of the murine leukemia retrovirus (249).
In boars experimentally infected with porcine rubulavirus, interstitial mononuclear cell infiltration was observed in atrophic testes and was identified as the most probable cause of seminiferous tubule degeneration (263). Similar testicular lesions were previously observed in rams infected with maedi/visna virus, in which the atrophy of seminiferous tubules was associated with disturbances in spermatogenesis (248). In contrast, intratesticular inoculation of boars with a high titer of a virulent strain of Belgian Aujesky's disease virus had much less dramatic effects (219). Only a few small foci of viruses were detected by immunofluorescence in the interstitium, and no viral replication, necrosis, or inflammatory lesions were detected in the seminiferous tubules (219).
Rabbits infected with an attenuated strain of myxoma virus develop interstitial orchitis and have impaired steroidogenesis and spermatogenesis, with the virus being restricted to the interstitial cells (119).
Equine herpesvirus 1 was found to replicate in the testes of ponies and to be shed into semen (323). Endothelial cells are the main testicular target cells of this virus, and there is associated necrotizing vasculitis and thrombosis, but no productive viral infection of the seminiferous epithelium (323). Venereal shedding may thus be facilitated by focal tissue ischemia and leakage of free virus of infected cells across the disrupted blood-testis barrier. Finally, ALV has been found to multiply in the connective tissue stroma and interstitial cells of the testis of mature roosters (99).
VIRUSES AND THE PROSTATE AND OTHER ACCESSORY GLANDS
The effects of viruses on the human and animal prostate are listed in Table 7.
TABLE 7.
Viruses and the human and animal prostate
| Viruses found in the human prostate | Putative effect or consequence in humans | Viruses found in the animal prostate |
|---|---|---|
| HPV | Inducer of prostate cancer ? | Oncorna virus (baboons) |
| DNA (18, 81, 101, 148, 210, 211, 282, 333, 350) | Viral particles (156) | |
| HSV | Viral reservoir ? | CMV (mouse) |
| Viral particles (60, 227) | Viral particles (66) | |
| Antigens (27, 62) | ||
| Mammary tumor virus (mouse) | ||
| Viral particles (138, 241) | ||
| CMV | C-type virus (rat) | |
| Antigens (123, 264) | RNA (17) | |
| DNA and RNA (46) | ||
| HIV | ||
| Antigens (83) | ||
| DNA (240) | ||
| KSHV/HHV-8 | ||
| DNA, only in HIV-infected patients (77) |
Human Prostate
In terms of incidence, prostate cancer is one of the most important cancers in men throughout the world. Putative causative agents have therefore been extensively studied. The etiology of prostate cancer is still unknown, but there are two main hypotheses. The first and most popular is the hormonal hypothesis, i.e., that the disease is caused directly or indirectly by endogenous or exogenous androgenic hormonal factors. The other hypothesis is that prostate cancer is caused by an oncogenic virus that may be transmitted venereally. There is indeed some evidence supporting an association between prostate cancer and STD, in particular the link between a high level of sexual activity and prostate cancer reported in some studies. Before reviewing the various viruses detected in the prostate, we should bear in mind that there are two different clinical entities: benign prostate hyperplasia and carcinoma of the prostate. It is unknown whether benign prostate hyperplasia coexists with prostate cancer and has a common etiology or whether it is a totally separate condition, etiologically unrelated to prostate cancer. According to Sokol-Roseman et al. (306), latent carcinoma of the prostate and prostate cancer are etiologically related and the latent carcinoma may correspond to a stage in the natural history of prostate cancer.
Papillomavirus.
A link between HPV, a family of oncogenic viruses, and prostate cancer has been sought. McNicol and Dodd (210) detected type 16 and 18 HPV DNA by Southern blotting in prostate tissue from 4 of 12 Canadian patients with benign prostate hyperplasia and in 3 of 4 patients with prostate carcinoma. The viral DNA was in an episomal or integrated form, and the authors concluded that this may have important implications for the etiology of prostate disease. In the following year, they published their results with a larger series of 88 prostate biopsy specimens and found a much higher prevalence of type 16 HPV than of type 18 (211). However, no significant difference was observed between patients with benign disease and those with evidence of malignancy, with nearly half of the patients testing positive in each case. Furthermore, the prevalence of type 16 HPV DNA in diseased prostates did not differ significantly from that in normal prostates. Several studies on the prevalence of HPV in the prostate were published in 1992 and are reviewed in detail elsewhere (81, 279). When the data were combined, 32% of the cancers tested positive for HPV, versus 49% of the benign lesions and 9% of the normal tissue samples. The highest rates of detection were found in earlier studies (probably due to problems of PCR contamination), whereas more recent results have been predominantly negative (81). In particular, one study of 84 American patients carried out using PCR and in situ hybridization (148) reported a low prevalence of type 16 HPV in both normal and cancerous prostates and, in two other studies, no HPV DNA was detected in 30 benign and malignant prostate tissues samples, even by PCR (108, 297). A secondary adult human prostate epithelial cell culture, on transfection with a plasmid containing the entire type 18 HPV genome, acquired an indefinite, life span in culture but did not undergo malignant conversion (267). Thus, although there are strong arguments in favor of the prostate being an important reservoir for the transmission of HPV (18, 101, 282, 333, 350), the involvement of HPV in the pathogenesis of prostate cancer is still a subject of great controversy. For example, two laboratories with extensive experience in HPV research recently tested specimens from two populations with different risks of prostate carcinoma, using three different PCR assays and two serologic assays for HPV. They concluded that HPV was not associated with prostate carcinoma (239, 314). However, another group found that a subgroup of prostate tumors (10 of 47) had a significantly larger number of copies of type 16 HPV sequence than did control tissue samples (1 of 37) (298). Thus, we cannot rule out the possibility that HPV is involved in the etiology of a minority of prostate cancers, as recently suggested by a serological and epidemiological study (98).
Herpes simplex virus.
The importance of HSV in prostate disease was first suggested when the virus was isolated from 2 men with prostatitis and from none of 10 men with no prostatic symptoms (227). A later study in which HSV was isolated from the prostate tissue of 15% of the men in a randomly selected population of 190 men suggested that the prostate acts as a reservoir of the virus (60). HSV antigens have also been found in prostate cancer cells (62), and antibodies against an HSV antigen were detected in the sera of all eight patients with prostate cancer tested (280). However, a larger serological epidemiological study investigating whether there was an association between antibodies against HSV-2 and cancer of the prostate showed no significant difference in the level of HSV-2 antibodies between patients with cancer of the prostate and those with benign prostate hypertrophy (139). Specific immunofluorescence was used to test for HSV-2 in prostate carcinoma in 305 patients: 5% of the patients had HSV-2 antigen in the prostate, and a higher prevalence was observed in patients with carcinoma of the prostate than in controls with benign prostate hypertrophy (27). This higher prevalence of HSV-2 was not confirmed in another study of 27 prostate carcinoma and 33 benign prostate hyperplasia patients (136). It therefore seems that HSV-2 infection of the prostate is common (25% in the most recent study), but no causal relationship with prostate carcinoma has yet been demonstrated. However, in vitro transformation of hamster embryo cells by tumor-associated HSV-2 from a human prostate carcinoma has been reported (61).
Cytomegalovirus.
CMV was first reported to be present in the prostate in a 3-year-old male donor from whom a cell line was obtained that expressed CMV-specific antigen at the cell surface (264). Geder et al. (124) reported that human prostate cancer cell lines expressed CMV-specific membrane antigen and suggested that CMV might be associated with prostate cancer. Primary cells were then transformed in vitro by a strain of CMV isolated from a normal prostate (123). These authors also showed that prostate cancer cells in culture possessed intracellular antigens specific for CMV but produced no infectious virus particles. However, normal human prostate tissue yielded a CMV isolate that transformed cells, and lymphocytes from prostate cancer patients were found to have specific reactivity against CMV-transformed cells (123). Antibodies against CMV have also been found at higher titers in patients with prostate cancer than in controls with benign prostate hyperplasia (123). Although CMV DNA has been detected in both normal and cancerous prostates, CMV RNA and antigens have only been detected in benign prostate hyperplasia and prostate carcinoma. This suggests that CMV may be associated with prostatic abnormality and that latent CMV may be harbored by normal prostates (46). However, in another study, CMV antigens were not detected in any of the 21 tissue samples from patients with benign prostate hyperplasia (309). Finally, CMV has been detected in the prostate of a patient with prostatitis (208).
Human immunodeficiency virus type 1.
HIV-1-related proteins have been found in several adjacent glandular epithelial cells in prostate sections from patients who died from AIDS (82), indicating that this organ may act as a reservoir of the virus (1). However, morphologic analyses of reproductive tissues obtained at autopsy from the bodies of 43 male AIDS patients revealed no major abnormalities of the prostate (257). In another study, viral nucleic acids were not detected by PCR amplification in the epithelium of the prostate but a few macrophages infected with the virus were observed (240).
Human herpesvirus 8.
Initially, two studies from the same group claimed to have detected HHV-8 in normal, hyperplastic, and neoplastic prostate glands from HIV-negative individuals (222, 224), but other studies did not find HHV-8 to be associated with this site in HIV-negative individuals (76, 77, 192, 278, 321). However, HHV-8 was detected by PCR in the prostates of all five HIV-infected KS-positive patients tested (76), whereas most other organs tested negative. This indicates that the prostate gland may be a preferential site of viral latency and persistence. HHV-8 was found not to establish latent infection in prostate cancer cell lines (229), indicating that it is probably not involved in prostate pathogenesis. A study recently identified the location of the infected cells more precisely within the prostate: HHV-8 DNA was detected in the nuclei of more than 90% of glandular epithelial cells, and in situ hybridization showed that the virus was mostly latent, with only 1 to 5% of cells harboring viral transcripts produced by HHV-8 replication (96).
Human Seminal Vesicles and Epididymis
Although malignant lymphoma of the testis involves the adjacent epididymis in approximately 60% of patients, isolated epididymal lymphoma is rare. One case report, however, described bilateral epididymal enlargement in a 34-year-old man, associated with an increase in the level of immunoglobulin M antibodies against coxsackie B4 virus (207). Biopsy revealed follicular lymphoproliferation with minimal involvement of the testes. CMV inclusions were found in the epithelia of the seminal vesicles and ductus deferens of a 32-year-old man with papillary adenocarcinoma of the lung (167), and the epithelial cells were shown to produce viral nucleocapsids resembling those of the Herpesviridae family.
Animal Prostate
Animal studies provide strong evidence that the prostate is a potential reservoir, if not an infection site, for viruses. Oncorna-like virus particles were observed in the prostate tissue of 2 of 11 baboons injected 3 years previously with a chemical carcinogen. Biopsy specimens from the animals showed no evidence of neoplasia, and electron microscopy suggested that this virus should be classed as a B-type oncorna virus (156).
Following the infection of adult mice, CMV was detectable, in an apparently latent form, in explants of prostate glands and in cell lines derived from these explants (66). Prostate tissue samples from a total of 61 normal mice of 10 strains were examined by electron microscopy for the presence of virus particles. Type B virus particles were present in normal prostate tissues of old mice from the mammary cancer-prone strains and these virus particles were morphologically and immunologically similar to the mouse mammary tumor virus (MMTV). Thus, the prostate tissues of mice are a potential source of horizontally transmitted mammary tumor virus, at least in some mammary cancer-prone strains (241). In another study, MMTV expression was also detected in the prostate gland and seminal vesicles of mice containing various endogenous proviruses (138). In vivo expression of a C-type RNA virus has also been detected in rat prostate epithelial cells (17).
Animal Seminal Vesicles and Epididymis
In SIV-infected animals, the most common type of lesion, observed in 18 of 22 monkeys, was focal lymphoid infiltration in the epididymis, prostate, or seminal vesicles (32). Another study found that within the male genital tract of infected monkeys, the SIV-infected T cells and macrophages were present mostly in the epididymis (218). Atrophy of the glandular elements and an interstitial fibrosis of the prostate were also observed (218).
In bulls, bovine diarrhea virus is transmitted by semen (see “Animal semen” above). Virological studies of the reproductive tracts of bulls during acute transient infection suggest that the most productive sites for virus replication are the seminal vesicles and the prostate gland (168).
Swelling of the head of the epididymis was observed in boars inoculated with porcine rubulavirus, which causes “blue eye” disease, resulting in infertility in sows and boars and corneal opacity in pigs of all ages (263). The lesion was associated with mononuclear cell infiltration and interstitial fibroplasia. Porcine rubulavirus antigen was detected by immunofluorescence in the head of the epididymis in pigs killed during acute infection (263). Mice transgenic for the type 18 HPV long control region were found to have enlarged seminal vesicles due to distension by fluid, suggesting that the transcript is specifically produced in the genital tract of mice (74).
The inoculation of guinea pigs with HSV-2 causes vesicular lesions in the genital area. During acute infection, the virus has been detected in the epididymis, seminal vesicles, prostate, and testis, but persistent detection of the virus is rare in these organs (except the prostate) (310).
Endogenous MMTV is present not only in the mammary glands and the prostate but also in the seminal vesicles, the vas deferens, and the epididymis, as shown by electron microscopy (274), radioimmunoassay (246), immunocytochemistry (344), and in situ hybridization (138).
TESTICULAR ANTIVIRAL DEFENSE SYSTEM
Viral infection of the testis, as described above, can have damaging effects on spermatogenesis and may lead to testicular cancers. It may also be sexually transmitted. Therefore, elucidation of the natural mechanisms of defense of the testis against viral attack is of major importance (Fig. 2).
FIG. 2.
Schematic representation of the topography of IFNs and IFN-induced proteins (2′5′AS, PKR, and Mx) expression within the rat testis following Sendai virus stimulation.
IFNs and IFN-induced proteins were the first elements of the testicular defense system to be investigated. IFNs are small proteins well known for their crucial involvement in cellular antiviral defenses. They bind to specific cellular receptors, either on the producing cells themselves or on neighboring cells. They then induce the synthesis of several proteins, three of which, 2′,5′-oligoadenylate synthetase (2′,5′-AS), double-stranded RNA-activated protein kinase (PKR), and Mx protein, have attracted a great deal of attention due to their ability to generate an antiviral state (for reviews, see references 281 and 308).
Attempts were first made to detect these molecules within the rat seminiferous tubules. The protection of the germ cells in these seminiferous tubules is of prime importance because their infection may result in the total destruction of the germ line or the dissemination of the virus or viral DNA to all germ cell classes, including the spermatozoa themselves. Spermatogonia are one of the most important potential targets because, unlike the meiotic and postmeiotic germ cells, they are not isolated from the molecules and agents produced by the interstitial compartment. The basal tubular position of these germ line precursors, a priori, exposes them to the risk of contamination in cases of viral infection. Despite the high potential risk of infection of spermatogonia, these cells do not constitutively express biologically active IFNs, and they respond only weakly to stimulation by Sendai virus in vitro (89). Similarly, early spermatids and pachytene spermatocytes, although expressing low levels of IFNs under basal conditions, respond very weakly if at all to exposure to Sendai virus (88). It was therefore suggested that the germ cell antiviral protection system may involve mainly the somatic peritubular and Sertoli cells within the seminiferous tubules and the somatic Leydig cells and macrophages within the interstitium, all of which have subsequently been shown to express high levels of IFN-α/β in response to viral infection (88). Of all the testicular cell types tested, Leydig cells and Sertoli cells have by far the strongest antiviral potential.
The synthesis of the main antiviral IFN-induced proteins, 2′,5′-AS, PKR, and Mx, was then studied. 2′5′-AS was not detected in meiotic and postmeiotic germ cells, but it was found in Sertoli cells under basal conditions, and its synthesis was increased by IFN and Sendai virus. A pattern similar to that in Sertoli cells was observed in peritubular cells, except that there was no basal expression (87).
No PKR protein or mRNA was detected in pachytene spermatocytes and early spermatids, even after exposure to IFNs or to Sendai virus. In contrast, Sertoli and peritubular cells constitutively expressed the PKR gene, and this expression was stimulated by IFN-α/β and IFN-γ (87).
Sertoli cells constitutively synthesize small amounts of Mx2 and Mx3 but no Mx1 (87). Mx1 was detected only after IFN or Sendai virus stimulation, and the synthesis of Mx2 and Mx3 was increased by these stimuli. This was the first time that Mx proteins were reported to be constitutively synthesized in a particular cell type, and the pattern of synthesis seemed to differ for the three proteins. Moreover, IFN-γ usually has no effect on Mx protein regulation, but it induced Mx1 gene expression and stimulated Mx2 and Mx3 gene expression in Sertoli cells. In contrast to what was observed in Sertoli cells, no Mx protein or mRNA was detected in peritubular cells cultured under basal conditions. However, Mx1, Mx2, and Mx3 were also detected in peritubular cells after IFN or Sendai virus exposure. As with 2′5′-AS and PKR, no Mx mRNA or protein was detected in pachytene spermatocytes and early spermatids (87). Our latest results establish that Leydig cells and testicular macrophages produce high levels of 2′5′-AS, PKR, and Mx while late spermatids and spermatogonia express only Mx (N. Melaine, unpublished data).
Thus, the first line of testicular defense against viruses arriving in the bloodstream is the Leydig cells and testicular macrophages. The second line of defense involves the myoid cells lining the seminiferous tubules and Sertoli cells. These two barriers may be fundamental for the protection of both spermatogenesis and androgen production, which is essential not only for testicular function but also for other crucial functions such as the development and maintenance of the secondary male sex characteristics, control of the musculoskeletal system, and key aspects of the nervous and cardiovascular systems.
CONCLUSION
A number of viruses are able to infect both the reproductive tract tissues and the semen in humans and animals (see the summary in Fig. 1), and the consequences of these viral infections may be extremely serious in terms of organ integrity, the development of diseases, and changes in the reproductive and endocrine systems. The immunosuppressive properties of the seminal plasma (designed to prevent a reaction against sperm antigens in the female reproductive tract) may play a critical role in STDs, not only because semen is a vector of viral propagation but also because the immune response of the female genital tract to infectious agents present in this biological fluid is certainly affected by the high level of immunosuppressive factors present in seminal plasma (160). In all animal species, but particularly in humans, viral infection of the germ cells may result not only in changes in testicular function (a serious risk for the fertility and general health of the individual) but also in the possible transmission of virus-induced mutations to subsequent generations. There are several obvious examples illustrating the lack of attention paid to studies of viral infection of the genital tract in men: (i) although the deleterious effects of mumps virus on fertility have been known for at least five decades, the mechanism by which the seminiferous epithelium is destroyed in testes infected with mumps virus is still unknown, and (ii) similarly, although the presence of HIV within the testis and spermatozoa themselves is still a matter for debate, key questions have been raised about the safety of insemination with spermatozoa from HIV-positive men and the possible existence of a testicular or genital tract reservoir for HIV. Another consequence of the superficial attention paid to viral infection of the testis in all species is the relative paucity of data concerning its effects on the endocrine system.
It is therefore urgent for both health and economic reasons to develop the scientific and medical interface between endocrinology and virology. In our opinion, the key priorities include (i) identification of the routes of entry of viruses into the male genital tract, (ii) identification and characterization of viral receptors in the various cell populations, (iii) studies of the infectivity of the virus in the male genital tract and its precise consequences on the endocrine system, (iv) determination of the mode of replication of the virus in the corresponding tissues, (v) determination of the nature of possible genital reservoirs, (vi) elucidation of the antiviral defense system of the reproductive tract and investigation of why this system is permissive for a number of viruses, and (vii) assessment of the efficacy of antiretroviral therapies in the genital tract and of the possible side effects of these treatments on testicular cells.
All this requires the establishment of close cooperation between clinicians/andrologists, virologists and biologists. Progress in these domains is essential for the development of new treatment strategies to eradicate viruses and to correct the virus-induced dysfunction of the endocrine system.
ACKNOWLEDGMENTS
We thank Louis Bujan and Michel Samson for their most valuable advice during the writing of this review as well as Simon Rainsford for proofreading.
REFERENCES
- 1.Ablin R J. HIV-related protein in the prostate: a possible reservoir of virus. Am J Clin Pathol. 1991;95:759–760. [PubMed] [Google Scholar]
- 2.Adami H O, Bergstrom R, Mohner M, Zatonski W, Storm H, Ekbom A, Tretli S, Teppo L, Ziegler H, Rahu M, et al. Testicular cancer in nine northern European countries. Int J Cancer. 1994;59:33–38. doi: 10.1002/ijc.2910590108. [DOI] [PubMed] [Google Scholar]
- 3.Adamopoulos D A, Lawrence D M, Vassilopoulos P, Contoyiannis P A, Swyer G I. Pituitary-testicular interrelationships in mumps orchitis and other viral infections. Br Med J. 1978;1:1177–1180. doi: 10.1136/bmj.1.6121.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aiman J, Brenner P F, MacDonald P C. Androgen and estrogen production in elderly men with gynecomastia and testicular atrophy after mumps orchitis. J Clin Endocrinol Metab. 1980;50:380–386. doi: 10.1210/jcem-50-2-380. [DOI] [PubMed] [Google Scholar]
- 5.Akahane Y, Kojima M, Sugai Y, Sakamoto M, Miyazaki Y, Tanaka T, Tsuda F, Mishiro S, Okamoto H, Miyakawa Y, et al. Hepatitis C virus infection in spouses of patients with type C chronic liver disease. Ann Intern Med. 1994;120:748–752. doi: 10.7326/0003-4819-120-9-199405010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Albina E. Epidemiology of porcine reproductive and respiratory syndrome (PRRS): an overview. Vet Microbiol. 1997;55:309–316. doi: 10.1016/s0378-1135(96)01322-3. [DOI] [PubMed] [Google Scholar]
- 7.Alexander H. Herpes simplex virus: a cause for concern. Am J Med Technol. 1982;48:241–245. [PubMed] [Google Scholar]
- 8.Alexander N J. HIV and germinal cells: how close an association? J Reprod Immunol. 1998;41:17–26. doi: 10.1016/s0165-0378(98)00046-1. [DOI] [PubMed] [Google Scholar]
- 9.Alexander N J. Sexual transmission of human immunodeficiency virus: virus entry into the male and female genital tract. Fertil Steril. 1990;54:1–18. doi: 10.1016/s0015-0282(16)53628-0. [DOI] [PubMed] [Google Scholar]
- 10.Algood C B, Newell G R, Johnson D E. Viral etiology of testicular tumors. J Urol. 1988;139:308–310. doi: 10.1016/s0022-5347(17)42394-9. [DOI] [PubMed] [Google Scholar]
- 11.Alter M J. The detection; transmission, and outcome of hepatitis C virus infection. Infect Agents Dis. 1993;2:155–166. [PubMed] [Google Scholar]
- 12.Alter M J. Epidemiology of hepatitis C in the West. Semin Liver Dis. 1995;15:5–14. doi: 10.1055/s-2007-1007259. [DOI] [PubMed] [Google Scholar]
- 13.Alter M J, Hadler S C, Judson F N, Mares A, Alexander W J, Hu P Y, Miller J K, Moyer L A, Fields H A, Bradley D W, et al. Risk factors for acute non-A, non-B hepatitis in the United States and association with hepatitis C virus infection. JAMA. 1990;264:2231–2235. [PubMed] [Google Scholar]
- 14.Anderson D. HIV can be transmitted from asymptomatic carriers. Am Fam Physician. 1991;44:936. [PubMed] [Google Scholar]
- 15.Anderson D, Wolff H, Pudney J, Wenhao A, Martinez A, Mayer K. Presence of HIV in semen. In: Alexander N J, Spieler J M, editors. Heterosexual transmission of AIDS. New York, N.Y: Wiley-Liss; 1990. pp. 167–180. [Google Scholar]
- 16.Anderson D J, O'Brien T R, Politch J A, Martinez A, Seage G R, Padian N, Horsburgh C R, Jr, Mayer K H. Effects of disease stage and zidovudine therapy on the detection of human immunodeficiency virus type 1 in semen. JAMA. 1992;267:2769–2774. [PubMed] [Google Scholar]
- 17.Anderson K M, Rubenstein M, Seed T M. In-vivo expression of a C-type RNA virus in rat ventral prostate epithelial cells. Biochem Biophys Res Commun. 1979;86:402–406. doi: 10.1016/0006-291x(79)90879-9. [DOI] [PubMed] [Google Scholar]
- 18.Anderson M, Handley J, Hopwood L, Murant S, Stower M, Maitland N J. Analysis of prostate tissue DNA for the presence of human papillomavirus by polymerase chain reaction, cloning, and automated sequencing. J Med Virol. 1997;52:8–13. doi: 10.1002/(sici)1096-9071(199705)52:1<8::aid-jmv2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Araneta M R, Mascola L, Eller A, O'Neil L, Ginsberg M M, Bursaw M, Marik J, Friedman S, Sims C A, Rekart M L, et al. HIV transmission through donor artificial insemination. JAMA. 1995;273:854–858. [PubMed] [Google Scholar]
- 20.Armenakas N A, Schevchuk M M, Brodherson M, Fracchia J A. AIDS presenting as primary testicular lymphoma. Urology. 1992;40:162–164. doi: 10.1016/0090-4295(92)90519-3. [DOI] [PubMed] [Google Scholar]
- 21.Ashida E R, Scofield V L. Lymphocyte major histocompatibility complex-encoded class II structures may act as sperm receptors. Proc Natl Acad Sci USA. 1987;84:3395–3399. doi: 10.1073/pnas.84.10.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baccetti B, Benedetto A, Burrini A G, Collodel G, Ceccarini E C, Crisa N, Di Caro A, Estenoz M, Garbuglia A R, Massacesi A, et al. HIV-particles in spermatozoa of patients with AIDS and their transfer into the oocyte. J Cell Biol. 1994;127:903–914. doi: 10.1083/jcb.127.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bagasra O, Farzadegan H, Seshamma T, Oakes J W, Saah A, Pomerantz R J. Detection of HIV-1 proviral DNA in sperm from HIV-1-infected men. AIDS. 1994;8:1669–1674. doi: 10.1097/00002030-199412000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Bagasra O, Freund M. In vitro and in vivo studies of HIV-1 and human sperm. In: Alexander N J, Spieler J M, editors. Heterosexual transmission of AIDS. New York, N.Y: Wiley-Liss; 1990. pp. 155–166. [Google Scholar]
- 25.Bagasra O, Freund M, Condoluci D, Heins B, Whittle P, Weidmann J, Comita J. Presence of HIV-1 in sperm of patients with HIV/AIDS. Mol Androl. 1990;2:109–125. [Google Scholar]
- 26.Bagasra O, Freund M, Weidmann J, Harley G. Interaction of human immunodeficiency virus with human sperm in vitro. J Acquired Immune Defic Syndr. 1988;1:431–435. [PubMed] [Google Scholar]
- 27.Baker L H, Mebust W K, Chin T D, Chapman A L, Hinthorn D, Towle D. The relationship of herpesvirus to carcinoma of the prostate. J Urol. 1981;125:370–374. doi: 10.1016/s0022-5347(17)55039-9. [DOI] [PubMed] [Google Scholar]
- 28.Barlow R M, Nettleton P F, Gardiner A C, Greig A, Campbell J R, Bonn J M. Persistent bovine virus diarrhoea virus infection in a bull. Vet Rec. 1986;118:321–324. doi: 10.1136/vr.118.12.321. [DOI] [PubMed] [Google Scholar]
- 29.Bartak V. Sperm count, morphology and motility after unilateral mumps orchitis. J Reprod Fertil. 1973;32:491–494. doi: 10.1530/jrf.0.0320491. [DOI] [PubMed] [Google Scholar]
- 30.Baskar J, Stanat S, Huang E. Cytomegalovirus infection of murine testicular interstitial Leydig cells. Infect Immun. 1983;40:726–732. doi: 10.1128/iai.40.2.726-732.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baskar J F, Stanat S C, Huang E S. Murine cytomegalovirus infection of mouse testes. J Virol. 1986;57:1149–1154. doi: 10.1128/jvi.57.3.1149-1154.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baskerville A, Cook R W, Dennis M J, Cranage M P, Greenaway P J. Pathological changes in the reproductive tract of male rhesus monkeys associated with age and simian AIDS. J Comp Pathol. 1992;107:49–57. doi: 10.1016/0021-9975(92)90095-c. [DOI] [PubMed] [Google Scholar]
- 33.Baskin G B. Disseminated cytomegalovirus infection in immunodeficient rhesus monkeys. Am J Pathol. 1987;129:345–352. [PMC free article] [PubMed] [Google Scholar]
- 34.Beard C M, Benson R C, Jr, Kelalis P P, Elveback L R, Kurland L T. The incidence and outcome of mumps orchitis in Rochester, Minnesota, 1935 to 1974. Mayo Clin Proc. 1977;52:3–7. [PubMed] [Google Scholar]
- 35.Belev N, Mateva V, Milanov M L, Arnaudov K, Ignatov G. The spread of enzootic bovine leukemia via the seminal fluid in certain breeds of bulls. Vet Med Nauki. 1986;23:3–10. [PubMed] [Google Scholar]
- 36.Benfield D A, Adldinger H K. Latent herpesvirus infection of testes and spinal ganglia of turkeys with semen abnormalities. Arch Virol. 1984;82:195–209. doi: 10.1007/BF01311163. [DOI] [PubMed] [Google Scholar]
- 37.Beral V. Epidemiology of Kaposi's sarcoma. Cancer Surv. 1991;10:5–22. [PubMed] [Google Scholar]
- 38.Bergamo P, Balestrieri M, Cammarota G, Guardiola J, Abrescia P. CD4-mediated anchoring of the seminal antigen gp17 onto the spermatozoon surface. Hum Immunol. 1997;58:30–41. doi: 10.1016/s0198-8859(97)00213-9. [DOI] [PubMed] [Google Scholar]
- 39.Bergstrom R, Adami H O, Mohner M, Zatonski W, Storm H, Ekbom A, Tretli S, Teppo L, Akre O, Hakulinen T. Increase in testicular cancer incidence in six European countries: a birth cohort phenomenon. J Natl Cancer Inst. 1996;88:727–733. doi: 10.1093/jnci/88.11.727. [DOI] [PubMed] [Google Scholar]
- 40.Bhasin S, Javanbakht M. Can androgen therapy replete lean body mass and improve muscle function in wasting associated with human immunodeficiency virus infection? J Parenter Enteral Nutr. 1999;23(6 Suppl.):195–201. doi: 10.1177/014860719902300605. [DOI] [PubMed] [Google Scholar]
- 41.Bhasin S, Storer T W, Asbel-Sethi N, Kilbourne A, Hays R, Sinha-Hikim I, Shen R, Arver S, Beall G. Effects of testosterone replacement with a nongenital, transdermal system, Androderm, in human immunodeficiency virus-infected men with low testosterone levels. J Clin Endocrinol Metab. 1998;83:3155–3162. doi: 10.1210/jcem.83.9.5079. [DOI] [PubMed] [Google Scholar]
- 42.Bigazzi P L, Barron A L, Flanagan T D, Andrada J A, Witebsky E. Growth of mumps virus in organ cultures of rhesus testis. J Infect Dis. 1968;118:411–421. doi: 10.1093/infdis/118.4.411. [DOI] [PubMed] [Google Scholar]
- 43.Bigazzi P L, Barron A L, Flanagan T D, Andrada J A, Witebsky E. Multiplication of mumps virus in organotypical cultures of monkey testes. Prog Immunobiol Stand. 1970;4:650–656. [PubMed] [Google Scholar]
- 44.Bjorvatn B. Mumps virus recovered from testicles by fine-needle aspiration biopsy in cases of mumps orchitis. Scand J Infect Dis. 1973;5:3–5. doi: 10.3109/inf.1973.5.issue-1.02. [DOI] [PubMed] [Google Scholar]
- 45.Bobroski L, Bagasra A U, Patel D, Saikumari P, Memoli M, Abbey M V, Wood C, Sosa C, Bagasra O. Localization of human herpesvirus type 8 (HHV-8) in the Kaposi's sarcoma tissues and the semen specimens of HIV-1 infected and uninfected individuals by utilizing in situ polymerase chain reaction. J Reprod Immunol. 1998;41:149–160. doi: 10.1016/s0165-0378(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 46.Boldogh I, Baskar J F, Mar E C, Huang E S. Human cytomegalovirus and herpes simplex type 2 virus in normal and adenocarcinomatous prostate glands. JNCI. 1983;70:819–826. [PubMed] [Google Scholar]
- 47.Borzy M S, Connell R S, Kiessling A A. Detection of human immunodeficiency virus in cell-free seminal fluid. J Acquired Immune Defic Syndr. 1988;1:419–424. [PubMed] [Google Scholar]
- 48.Bowen R A, Howard T H, Entwistle K W, Pickett B W. Seminal shedding of bluetongue virus in experimentally infected mature bulls. Am J Vet Res. 1983;44:2268–2270. [PubMed] [Google Scholar]
- 49.Bowen R A, Howard T H, Pickett B W. Seminal shedding of bluetongue virus in experimentally infected bulls. Prog Clin Biol Res. 1985;178:91–96. [PubMed] [Google Scholar]
- 50.Brechard N, Galea P, Silvy F, Amram M, Chermann J C. HIV virus detection in ejaculates collected at different times in seropositive patients. Contracept Fertil Sex. 1997;25:725–729. [PubMed] [Google Scholar]
- 51.Breckon R D, Luedke A J, Walton T E. Bluetongue virus in bovine semen: viral isolation. Am J Vet Res. 1980;41:439–442. [PubMed] [Google Scholar]
- 52.Brogi A, Presentini R, Piomboni P, Collodel G, Strazza M, Solazzo D, Costantino Ceccarini E. Human sperm and spermatogonia express a galactoglycerolipid which interacts with gp120. J Submicrosc Cytol Pathol. 1995;27:565–571. [PubMed] [Google Scholar]
- 53.Brogi A, Presentini R, Solazzo D, Piomboni P, Costantino-Ceccarini E. Interaction of human immunodeficiency virus type 1 envelope glycoprotein gp120 with a galactoglycerolipid associated with human sperm. AIDS Res Hum Retroviruses. 1996;12:483–489. doi: 10.1089/aid.1996.12.483. [DOI] [PubMed] [Google Scholar]
- 54.Buzelin F, Karam G, Moreau A, Wetzel O, Gaillard F. Testicular tumor and the acquired immunodeficiency syndrome. Eur Urol. 1994;26:71–76. doi: 10.1159/000475346. [DOI] [PubMed] [Google Scholar]
- 55.Byrn R A, Zhang D, Eyre R, McGowan K, Kiessling A A. HIV-1 in semen: an isolated virus reservoir. Lancet. 1997;350:1141. doi: 10.1016/S0140-6736(97)24042-0. [DOI] [PubMed] [Google Scholar]
- 56.Caldwell S H, Sue M, Bowden J H, Dickson R C, Driscoll C J, Yeaton P, Stevenson W C, Ishitani M B, McCullough C S, Pruett T L, Lovell M A. Hepatitis C virus in body fluids after liver transplantation. Liver Transplant Surg. 1996;2:124–129. doi: 10.1002/lt.500020207. [DOI] [PubMed] [Google Scholar]
- 57.Calkins J H, Sigel M M, Nankin H R, Lin T. Interleukin-1 inhibits Leydig cell steroidogenesis in primary culture. Endocrinology. 1988;123:1605–1610. doi: 10.1210/endo-123-3-1605. [DOI] [PubMed] [Google Scholar]
- 58.Callis J J. Evaluation of the presence and risk of foot and mouth disease virus by commodity in international trade. Rev Sci Technol. 1996;15:1075–1085. doi: 10.20506/rst.15.3.974. [DOI] [PubMed] [Google Scholar]
- 59.Casella R, Leibundgut B, Lehmann K, Gasser T C. Mumps orchitis: report of a mini-epidemic. J Urol. 1997;158:2158–2161. doi: 10.1016/s0022-5347(01)68186-2. [DOI] [PubMed] [Google Scholar]
- 60.Centifanto Y M, Drylie D M, Deardourff S L, Kaufman H E. Herpesvirus type 2 in the male genitourinary tract. Science. 1972;178:318–319. doi: 10.1126/science.178.4058.318. [DOI] [PubMed] [Google Scholar]
- 61.Centifanto Y M, Kaufman H E. In vitro transformation by HSV-2 from a human prostatic carcinoma. IARC Sci Publ. 1975;11:195–197. [PubMed] [Google Scholar]
- 62.Centifanto Y M, Kaufman H E, Zam Z S, Drylie D M, Deardourff S L. Herpesvirus particles in prostatic carcinoma cells. J Virol. 1973;12:1608–1611. doi: 10.1128/jvi.12.6.1608-1611.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chabon A B, Stenger R J, Grabstald H. Histopathology of testis in acquired immune deficiency syndrome. Urology. 1987;29:658–663. doi: 10.1016/0090-4295(87)90118-x. [DOI] [PubMed] [Google Scholar]
- 64.Chan P J, Seraj I M, Kalugdan T H, King A. Evidence for ease of transmission of human papillomavirus DNA from sperm to cells of the uterus and embryo. J Assist Reprod Genet. 1996;13:516–519. doi: 10.1007/BF02066536. [DOI] [PubMed] [Google Scholar]
- 65.Charny C W, Meranze D R. Pathology of mumps orchitis. J Urol. 1948;60:140. doi: 10.1016/S0022-5347(17)69213-9. [DOI] [PubMed] [Google Scholar]
- 66.Cheung K S, Lang D J. Detection of latent cytomegalovirus in murine salivary and prostate explant cultures and cells. Infect Immun. 1977;15:568–574. doi: 10.1128/iai.15.2.568-574.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheung W Y, Chan A C, Loke S L, Srivastava G, Pittaluga S, Lim L Y, Ho F C. Latent sites of Epstein-Barr virus infection. Am J Clin Pathol. 1993;100:502–506. doi: 10.1093/ajcp/100.5.502. [DOI] [PubMed] [Google Scholar]
- 68.Christeff N, Gharakhanian S, Thobie N, Rozenbaum W, Nunez E A. Evidence for changes in adrenal and testicular steroids during HIV infection. J Acquired Immune Defic Syndr. 1992;5:841–846. [PubMed] [Google Scholar]
- 69.Christopher-Hennings J, Nelson E A, Nelson J K, Rossow K D, Shivers J L, Yaeger M J, Chase C C, Garduno R A, Collins J E, Benfield D A. Identification of porcine reproductive and respiratory syndrome virus in semen and tissues from vasectomized and nonvasectomized boars. Vet Pathol. 1998;35:260–267. doi: 10.1177/030098589803500404. [DOI] [PubMed] [Google Scholar]
- 70.Chrystie I L, Mullen J E, Braude P R, Rowell P, Williams E, Elkington N, de Ruiter A, Rice K, Kennedy J. Assisted conception in HIV discordant couples: evaluation of semen processing techniques in reducing HIV viral load. J Reprod Immunol. 1998;41:301–306. doi: 10.1016/s0165-0378(98)00066-7. [DOI] [PubMed] [Google Scholar]
- 71.Cohen J. Exploring how to get at—and eradicate—hidden HIV. Science. 1998;279:1854–1855. doi: 10.1126/science.279.5358.1854. [DOI] [PubMed] [Google Scholar]
- 72.Cohen M S, Hoffman I F, Royce R A, Kazembe P, Dyer J R, Daly C C, Zimba D, Vernazza P L, Maida M, Fiscus S A, Eron J J., Jr Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 73.Collier A C, Meyers J D, Corey L, Murphy V L, Roberts P L, Handsfield H H. Cytomegalovirus infection in homosexual men. Relationship to sexual practices, antibody to human immunodeficiency virus, and cell-mediated immunity. Am J Med. 1987;82(3 Spec. No.):593–601. doi: 10.1016/0002-9343(87)90105-7. [DOI] [PubMed] [Google Scholar]
- 74.Comerford S A, Maika S D, Laimins L A, Messing A, Elsasser H P, Hammer R E. E6 and E7 expression from the HPV 18 LCR: development of genital hyperplasia and neoplasia in transgenic mice. Oncogene. 1995;10:587–597. [PubMed] [Google Scholar]
- 75.Coombs R W, Speck C E, Hughes J P, Lee W, Sampoleo R, Ross S O, Dragavon J, Peterson G, Hooton T M, Collier A C, Corey L, Koutsky L, Krieger J N. Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis. 1998;177:320–330. doi: 10.1086/514213. [DOI] [PubMed] [Google Scholar]
- 76.Corbellino M, Bestetti G, Galli M, Parravicini C. Absence of HHV-8 in prostate and semen. N Engl J Med. 1996;335:1237–1239. doi: 10.1056/NEJM199610173351614. [DOI] [PubMed] [Google Scholar]
- 77.Corbellino M, Poirel L, Bestetti G, Pizzuto M, Aubin J T, Capra M, Bifulco C, Berti E, Agut H, Rizzardini G, Galli M, Parravicini C. Restricted tissue distribution of extralesional Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS patients with Kaposi's sarcoma. AIDS Res Hum Retroviruses. 1996;12:651–657. doi: 10.1089/aid.1996.12.651. [DOI] [PubMed] [Google Scholar]
- 78.Cottral G E, Gailiunas P, Cox B F. Foot-and-mouth disease virus in semen of bulls and its transmission by artificial insemination. Arch Gesamte Virusforsch. 1968;23:362–377. doi: 10.1007/BF01242132. [DOI] [PubMed] [Google Scholar]
- 79.Croxson T S, Chapman W E, Miller L K, Levit C D, Senie R, Zumoff B. Changes in the hypothalamic-pituitary-gonadal axis in human immunodeficiency virus-infected homosexual men. J Clin Endocrinol Metab. 1989;68:317–321. doi: 10.1210/jcem-68-2-317. [DOI] [PubMed] [Google Scholar]
- 80.Csata S, Kulcsar G. Virus-host studies in human seminal and mouse testicular cells. Acta Chir Hung. 1991;32:83–90. [PubMed] [Google Scholar]
- 81.Cuzick J. Human papillomavirus infection of the prostate. Cancer Surv. 1995;23:91–95. [PubMed] [Google Scholar]
- 82.Dalton A D, Harcourt-Webster J N. The histopathology of the testis and epididymis in AIDS—a post-mortem study. J Pathol. 1991;163:47–52. doi: 10.1002/path.1711630109. [DOI] [PubMed] [Google Scholar]
- 83.da Silva M, Shevchuk M M, Cronin W J, Armenakas N A, Tannenbaum M, Fracchia J A, Ioachim H L. Detection of HIV-related protein in testes and prostates of patients with AIDS. Am J Clin Pathol. 1990;93:196–201. doi: 10.1093/ajcp/93.2.196. [DOI] [PubMed] [Google Scholar]
- 84.Davidson A J, Freeman S A, Crosier K E, Wood C R, Crosier P S. Expression of murine interleukin 11 and its receptor alpha-chan in adult and embryonic tissues. Stem Cells. 1997;15:119–124. doi: 10.1002/stem.150119. [DOI] [PubMed] [Google Scholar]
- 85.Davison F, Alexander G J, Trowbridge R, Fagan E A, Williams R. Detection of hepatitis B virus DNA in spermatozoa, urine, saliva and leucocytes, of chronic HBsAg carriers. A lack of relationship with serum markers of replication. J Hepatol. 1987;4:37–44. doi: 10.1016/s0168-8278(87)80007-7. [DOI] [PubMed] [Google Scholar]
- 86.Deas D W, Johnston W S. The isolation and transmission of the virus of infectious bovine rhinotracheitis-infectious pustular vulvo-vaginitis. Vet Rec. 1973;92:636–639. doi: 10.1136/vr.92.24.636. [DOI] [PubMed] [Google Scholar]
- 87.Dejucq N, Chousterman S, Jegou B. The testicular antiviral defense system: localization, expression, and regulation of 2′5′ oligoadenylate synthetase, double-stranded RNA-activated protein kinase, and Mx proteins in the rat seminiferous tubule. J Cell Biol. 1997;139:865–873. doi: 10.1083/jcb.139.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dejucq N, Dugast I, Ruffault A, van der Meide P H, Jegou B. Interferon-alpha and -gamma expression in the rat testis. Endocrinology. 1995;136:4925–4931. doi: 10.1210/endo.136.11.7588226. [DOI] [PubMed] [Google Scholar]
- 89.Dejucq N, Lienard M O, Guillaume E, Dorval I, Jegou B. Expression of interferons-alpha and -gamma in testicular interstitial tissue and spermatogonia of the rat. Endocrinology. 1998;139:3081–3087. doi: 10.1210/endo.139.7.6083. [DOI] [PubMed] [Google Scholar]
- 90.Delwart E L, Mullins J I, Gupta P, Learn G H, Jr, Holodniy M, Katzenstein D, Walker B D, Singh M K. Human immunodeficiency virus type 1 populations in blood and semen. J Virol. 1998;72:617–623. doi: 10.1128/jvi.72.1.617-623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Paepe M E, Guerri C, Waxman M. Opportunistic infections of the testis in the acquired immunodeficiency syndrome. Mt Sinai J Med. 1990;57:25–29. [PubMed] [Google Scholar]
- 92.De Paepe M E, Waxman M. Testicular atrophy in AIDS: a study of 57 autopsy cases. Hum Pathol. 1989;20:210–214. doi: 10.1016/0046-8177(89)90125-1. [DOI] [PubMed] [Google Scholar]
- 93.Deteis R, Leach C T, Hennessey K, Liu Z, Visscher B R, Cherry J D, Giorgi J V. Persistent cytomegalovirus infection of semen increases risk of AIDS. J Infect Dis. 1994;169:766–768. doi: 10.1093/infdis/169.4.766. [DOI] [PubMed] [Google Scholar]
- 94.Deture F A, Drylie D M, Kaufman H E, Centifanto Y M. Herpesvirus type 2: study of semen in male subjects with recurrent infections. J Urol. 1978;120:449–451. doi: 10.1016/s0022-5347(17)57225-0. [DOI] [PubMed] [Google Scholar]
- 95.Deture F A, Drylie D M, Kaufman H E, Centifanto Y N. Herpesvirus type 2: isolation from seminal vesicle and testes. Urology. 1976;7:541–544. doi: 10.1016/0090-4295(76)90204-1. [DOI] [PubMed] [Google Scholar]
- 96.Diamond C, Brodie S J, Krieger J N, Huang M L, Koelle D M, Diem K, Muthui D, Corey L. Human herpesvirus 8 in the prostate glands of men with Kaposi's sarcoma. J Virol. 1998;72:6223–6227. doi: 10.1128/jvi.72.7.6223-6227.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Diamond C, Huang M L, Kedes D H, Speck C, Rankin G W, Jr, Ganem D, Coombs R W, Rose T M, Krieger J N, Corey L. Absence of detectable human herpesvirus 8 in the semen of human immunodeficiency virus-infected men without Kaposi's sarcoma. J Infect Dis. 1997;176:775–777. doi: 10.1086/517299. [DOI] [PubMed] [Google Scholar]
- 98.Dillner J, Knekt P, Boman J, Lehtinen M, Af Geijersstam V, Sapp M, Schiller J, Maatela J, Aromaa A. Sero-epidemiological association between human-papillomavirus infection and risk of prostate cancer. Int J Cancer. 1998;75:564–567. doi: 10.1002/(sici)1097-0215(19980209)75:4<564::aid-ijc12>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 99.Di Stefano H S, Dougherty R M. Multiplication of avian leukosis virus in the reproductive system of the rooster. J Natl Cancer Inst. 1968;41:451–464. [PubMed] [Google Scholar]
- 100.Dobs A S, Dempsey M A, Ladenson P W, Polk B F. Endocrine disorders in men infected with human immunodeficiency virus. Am J Med. 1988;84:611–616. doi: 10.1016/0002-9343(88)90144-1. [DOI] [PubMed] [Google Scholar]
- 101.Dodd J G, Paraskevas M, McNicol P J. Detection of human papillomavirus 16 transcription in human prostate tissue. J Urol. 1993;149:400–402. doi: 10.1016/s0022-5347(17)36103-7. [DOI] [PubMed] [Google Scholar]
- 102.Dondero F, Rossi T, D'Offizi G, Mazzilli F, Rosso R, Sarandrea N, Pinter E, Aiuti F. Semen analysis in HIV seropositive men and in subjects at high risk for HIV infection. Hum Reprod. 1996;11:765–768. doi: 10.1093/oxfordjournals.humrep.a019251. [DOI] [PubMed] [Google Scholar]
- 103.Dupressoir A, Heidmann T. Germ line-specific expression of intracisternal A-particle retrotransposons in transgenic mice. Mol Cell Biol. 1996;16:4495–4503. doi: 10.1128/mcb.16.8.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dussaix E, Guetard D, Dauguet C, D'Almeida M, Auer J, Ellrodt A, Montagnier L, Auroux M. Spermatozoa as potential carriers of HIV. Res Virol. 1993;144:437–445. doi: 10.1016/s0923-2516(06)80064-6. [DOI] [PubMed] [Google Scholar]
- 105.Dutko F J, Oldstone M B. Murine cytomegalovirus infects spermatogenic cells. Proc Natl Acad Sci USA. 1979;76:2988–2991. doi: 10.1073/pnas.76.6.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eaglesome M D, Garcia M M. Disease risks to animal health from artificial insemination with bovine semen. Rev Sci Technol. 1997;16:215–225. doi: 10.20506/rst.16.1.1017. [DOI] [PubMed] [Google Scholar]
- 107.Eastlund T. Infectious disease transmission through cell, tissue, and organ transplantation: reducing the risk through donor selection. Cell Transplant. 1995;4:455–477. doi: 10.1177/096368979500400507. [DOI] [PubMed] [Google Scholar]
- 108.Effert P J, Frye R A, Neubauer A, Liu E T, Walther P J. Human papillomavirus types 16 and 18 are not involved in human prostate carcinogenesis: analysis of archival human prostate cancer specimens by differential polymerase chain reaction. J Urol. 1992;147:192–196. doi: 10.1016/s0022-5347(17)37195-1. [DOI] [PubMed] [Google Scholar]
- 109.el Borai N, Inoue M, Lefevre C, Naumova E N, Sato B, Yamamura M. Detection of herpes simplex DNA in semen and menstrual blood of individuals attending an infertility clinic. J Obstet Gynaecol Res. 1997;23:17–24. doi: 10.1111/j.1447-0756.1997.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 110.El-Dermiry M, Hargreave T, Busuttil A, James K, Ritchie A, Chrisholm G. Lymphocyte subpopulations in the male genital tract. Br J Urol. 1985;57:769. doi: 10.1111/j.1464-410x.1985.tb07051.x. [DOI] [PubMed] [Google Scholar]
- 111.Eron J, Vernazza P L, Johnston D M, Seillier-Moiseiwitsch F, Alcorn T M, Fiscus S A, Cohen M S. Resistance of HIV-1 to antiretroviral agents in blood and seminal plasma: implications for transmission. AIDS. 1998;12:181–189. doi: 10.1097/00002030-199815000-00003. [DOI] [PubMed] [Google Scholar]
- 112.Eron J J, Gilliam B, Fiscus S, Dyer J, Cohen M. HIV-1 shedding and chlamydial urethritis. JAMA. 1996;275:36. [PubMed] [Google Scholar]
- 113.Erpenbach K H J. Systemic treatment with interferon-alpha 2 B: an effective method to prevent sterility after bilateral mumps orchitis. J Urol. 1991;146:54–56. doi: 10.1016/s0022-5347(17)37713-3. [DOI] [PubMed] [Google Scholar]
- 114.Everhart J E, Di Bisceglie A M, Murray L M, Alter H J, Melpolder J J, Kuo G, Hoofnagle J H. Risk for non-A, non-B (type C) hepatitis through sexual or household contact with chronic carriers. Ann Intern Med. 1990;112:544–545. doi: 10.7326/0003-4819-112-7-544. [DOI] [PubMed] [Google Scholar]
- 115.Fagan E A, Alexander G J, Davison F, Williams R. Persistence of free HBV DNA in body secretions and liver despite loss of serum HBV DNA after interferon-induced seroconversion. J Med Virol. 1986;20:183–188. doi: 10.1002/jmv.1890200210. [DOI] [PubMed] [Google Scholar]
- 116.Foichtinger H, Kaaya E, Putkonen P, Li S L, Ekman M, Gendelman R, Biberfeld G, Biberfeld P. Malignant lymphoma associated with human AIDS and with SIV-induced immunodeficiency in macaques. AIDS Res Hum Retroviruses. 1992;8:339–348. doi: 10.1089/aid.1992.8.339. [DOI] [PubMed] [Google Scholar]
- 117.Fiore R J, Potenza D, Monno L, Appice A, DiStefano M, Giannelli A, LaGrasta L, Romanelli C, DiBari C, Pastore G. Detection of HCV RNA in serum and seminal fluid from HIV-1 co-infected intravenous drug addicts. J Med Virol. 1995;46:364–367. doi: 10.1002/jmv.1890460412. [DOI] [PubMed] [Google Scholar]
- 118.Foster N M, Alders M A, Luedke A J, Walton T E. Abnormalities and virus-like particles in spermatozoa from bulls latently infected with bluetongue virus. Am J Vet Res. 1980;41:1045–1048. [PubMed] [Google Scholar]
- 119.Fountain S, Holland M K, Hinds L A, Janssens P A, Kerr P J. Interstitial orchitis with impaired steroidogenesis and spermatogenesis in the testes of rabbits infected with an attenuated strain of myxoma virus. J Reprod Fertil. 1997;110:161–169. doi: 10.1530/jrf.0.1100161. [DOI] [PubMed] [Google Scholar]
- 120.Freeman R, Hambling M H. Serological studies on 40 cases of mumps virus infection. J Clin Pathol. 1980;33:28–32. doi: 10.1136/jcp.33.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fried M W, Shindo M, Fong T L, Fox P C, Hoofnagle J H, Di Bisceglie A M. Absence of hepatitis C viral RNA from saliva and semen of patients with chronic hepatitis C. Gastroenterology. 1992;102:1306–1308. [PubMed] [Google Scholar]
- 122.Gall A E. The histopathology of acute mumps orchitis. Am J Pathol. 1947;23:637. [PMC free article] [PubMed] [Google Scholar]
- 123.Geder L, Rapp F. Herpesviruses and prostate carcinogenesis. Arch Androl. 1980;4:71–78. doi: 10.3109/01485018008988282. [DOI] [PubMed] [Google Scholar]
- 124.Geder L, Sanford E J, Rohner T J, Rapp F. Cytomegalovirus and cancer of the prostate: in vitro transformation of human cells. Cancer Treat Rep. 1977;61:139–146. [PubMed] [Google Scholar]
- 125.Gil T, Castilla J A, Hortas M L, Molina J, Redondo M, Samaniego F, Garrido F, Vergara F, Herruzo A. CD4+ cells in human ejaculates. Hum Reprod. 1995;10:2923–2927. doi: 10.1093/oxfordjournals.humrep.a135821. [DOI] [PubMed] [Google Scholar]
- 126.Gobert B, Amiel C, Tang J Q, Barbarino P, Bene M C, Faure G. CD4-like molecules in human sperm. FEBS Lett. 1990;261:339–342. doi: 10.1016/0014-5793(90)80586-8. [DOI] [PubMed] [Google Scholar]
- 127.Gotzinger N, Sauter M, Roemer K, Mueller Lantzsch N. Regulation of human endogenous retrovirus-K Gag expression in teratocarcinoma cell lines and human tumours. J Gen Virol. 1996;77:2983–2990. doi: 10.1099/0022-1317-77-12-2983. [DOI] [PubMed] [Google Scholar]
- 128.Gradil C, Molitor T, Harding M, Crabo B. Excretion of porcine parvovirus through the genital tract of boars. Am J Vet Res. 1990;51:359–362. [PubMed] [Google Scholar]
- 129.Gray A, Guillou L, Zufferey J, Rey F, Kurt A M, Jichlinski P, Leisinger H J, Benhattar J. Persistence of parvovirus B19 DNA in testis of patients with testicular germ cell tumours. J Gen Virol. 1998;79:573–579. doi: 10.1099/0022-1317-79-3-573. [DOI] [PubMed] [Google Scholar]
- 130.Green J, Monteiro E, Bolton V N, Sanders P, Gibson P E. Detection of human papillomavirus DNA by PCR in semen from patients with and without penile warts. Genitourin Med. 1991;67:207–210. doi: 10.1136/sti.67.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Green J, Monteiro E, Gibson P. Detection of human papillomavirus DNA in semen from patients with intrameatal penile warts. Genitourin Med. 1989;65:357–360. doi: 10.1136/sti.65.6.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gupta P, Mellors J, Kingsley L, Riddler S, Singh M K, Schreiber S, Cronin M, Rinaldo C R. High viral load in semen of human immunodeficiency virus type 1-infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. J Virol. 1997;71:6271–6275. doi: 10.1128/jvi.71.8.6271-6275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gupta P, Singh M K, Rinaldo C, Ding M, Farzadegan H, Saah A, Hoover D, Moore P, Kingsley L. Detection of Kaposi's sarcoma herpesvirus DNA in semen of homosexual men with Kaposi's sarcoma. AIDS. 1996;10:1596–1598. doi: 10.1097/00002030-199611000-00022. [DOI] [PubMed] [Google Scholar]
- 134.Habrova V, Takac M, Navratil J, Macha J, Ceskova N, Jonak J. Association of Rous sarcoma virus DNA with Xenopus laevis spermatozoa and its transfer to ova through fertilization. Mol Reprod Dev. 1996;44:332–342. doi: 10.1002/(SICI)1098-2795(199607)44:3<332::AID-MRD7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 135.Hadchouel M, Scotto J, Huret J L, Molinie C, Villa E, Degos F, Brechot C. Presence of HBV DNA in spermatozoa: a possible vertical transmission of HBV via the germ line. J Med Virol. 1985;16:61–66. doi: 10.1002/jmv.1890160109. [DOI] [PubMed] [Google Scholar]
- 136.Haid M, Sharon N. Immunofluorescent evidence of prior herpes simplex virus type-2 infection in prostate carcinoma. Urology. 1984;24:623–625. doi: 10.1016/0090-4295(84)90118-3. [DOI] [PubMed] [Google Scholar]
- 137.Hammitt D G, Aschenbrenner D W, Williamson R A. Culture of cytomegalovirus from frozen-thawed semen. Fertil Steril. 1988;49:554–557. doi: 10.1016/s0015-0282(16)59793-3. [DOI] [PubMed] [Google Scholar]
- 138.Henrard D, Ross S R. Endogenous mouse mammary tumor virus is expressed in several organs in addition to the lactating mammary gland. J Virol. 1988;62:3046–3049. doi: 10.1128/jvi.62.8.3046-3049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Herbert J T, Birkhoff J D, Feorino P M, Caldwell G G. Herpes simplex virus type 2 and cancer of the prostate. J Urol. 1976;116:611–612. doi: 10.1016/s0022-5347(17)58931-4. [DOI] [PubMed] [Google Scholar]
- 140.Herbst H, Sauter M, Mueller Lantzsch N. Expression of human endogenous retrovirus K elements in germ cell and trophoblastic tumors. Am J Pathol. 1996;149:1727–1735. [PMC free article] [PubMed] [Google Scholar]
- 141.Hirasawa K, Takeda M, Matsuzaki H, Doi K. Encephalomyocarditis (EMC) virus-induced orchitis in Syrian hamsters. Int J Exp Pathol. 1991;72:617–622. [PMC free article] [PubMed] [Google Scholar]
- 142.Ho D D, Schooley R T, Rota T R, Kaplan J C, Flynn T, Salahuddin S Z, Gonda M A, Hirsch M S. HTLV-III in the semen and blood of a healthy homosexual man. Science. 1984;226:451–453. doi: 10.1126/science.6208608. [DOI] [PubMed] [Google Scholar]
- 143.Hollingsworth R C, Jameson C L, Minton J E, Crowe M, Curran R, Rowe T, Grabowska A M, Pillay D, Irving W L, Ball J K. GBV-C/HGV coinfection in HIV-1-positive men: frequent detection of viral RNA in blood plasma but absence from seminal fluid plasma. J Med Virol. 1998;56:321–326. doi: 10.1002/(sici)1096-9071(199812)56:4<321::aid-jmv6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 144.Holmberg S D. Possible cofactors for the development of AIDS-related neoplasms. Cancer Detect Prev. 1990;14:331–336. [PubMed] [Google Scholar]
- 145.Howard M R, Whitby D, Bahadur G, Suggett F, Boshoff C, Tenant Flowers M, Schulz T F, Kirk S, Matthews S, Weller I V, Tedder R S, Weiss R A. Detection of human herpesvirus 8 DNA in semen from HIV-infected individuals but not healthy semen donors. AIDS. 1997;11:15–19. doi: 10.1097/00002030-199702000-00001. [DOI] [PubMed] [Google Scholar]
- 146.Howard T H, Bowen R A, Pickett B W. Isolation of bluetongue virus from bull semen. Prog Clin Biol Res. 1985;178:127–134. [PubMed] [Google Scholar]
- 147.Hsu H H, Wright T L, Luba D, Martin M, Feinstone S M, Garcia G, Greenberg H B. Failure to detect hepatitis C virus genome in human secretions with the polymerase chain reaction. Hepatology. 1991;14:763–767. doi: 10.1002/hep.1840140504. [DOI] [PubMed] [Google Scholar]
- 148.Ibrahim G K, Gravitt P E, Dittrich K L, Ibrahim S N, Melhus O, Anderson S M, Robertson C N. Detection of human papillomavirus in the prostate by polymerase chain reaction and in situ hybridization. J Urol. 1992;148:1822–1826. doi: 10.1016/s0022-5347(17)37040-4. [DOI] [PubMed] [Google Scholar]
- 149.Ida K, Tokuda H, Kanaoka T, Kanzaki H, Noda Y, Yoshida O, Ito Y, Mori T. Epstein-Barr virus activating principle in husbands' semen of cervical cancer patients. Am J Reprod Immunol. 1991;26:89–92. doi: 10.1111/j.1600-0897.1991.tb00978.x. [DOI] [PubMed] [Google Scholar]
- 150.Inoue M, Nakazawa A, Fujita M, Tanizawa O. Human papillomavirus (HPV) type 16 in semen of partners of women with HPV infection. Lancet. 1992;339:1114–1115. doi: 10.1016/0140-6736(92)90708-b. [DOI] [PubMed] [Google Scholar]
- 151.Ito Y, Tokuda H, Morigaki T, Shimizu K, Kawana T, Sanada S, Yoshida O. Epstein-Barr virus-activating principle in human semen. Cancer Lett. 1984;23:129–134. doi: 10.1016/0304-3835(84)90145-9. [DOI] [PubMed] [Google Scholar]
- 152.Jegou B. The Sertoli-germ cell communication network in mammals. Int Rev Cytol. 1993;147:25–96. [PubMed] [Google Scholar]
- 153.Jenison S A, Lemon S M, Baker L N, Newbold J E. Quantitative analysis of hepatitis B virus DNA in saliva and semen of chronically infected homosexual men. J Infect Dis. 1987;156:299–307. doi: 10.1093/infdis/156.2.299. [DOI] [PubMed] [Google Scholar]
- 154.Jordan H L, Howard J, Barr M C, Kennedy-Stoskopf S, Levy J K, Tompkins W A. Feline immunodeficiency virus is shed in semen from experimentally and naturally infected cats. AIDS Res Hum Retroviruses. 1998;14:1087–1092. doi: 10.1089/aid.1998.14.1087. [DOI] [PubMed] [Google Scholar]
- 155.Judson F N. Epidemiology of sexually transmitted hepatitis B infections in heterosexuals: a review. Sex Transm Dis. 1981;8:336–343. [PubMed] [Google Scholar]
- 156.Kalter S S, Shain S A, Smith G C, McCullough B, Heberling R L, Dalton A J. Oncorna-like viruses in baboon prostate tissue. J Natl Cancer Inst. 1975;55:1237–1241. doi: 10.1093/jnci/55.5.1237. [DOI] [PubMed] [Google Scholar]
- 157.Kao J H, Chen P J, Yang P M, Lai M Y, Sheu J C, Wang T H, Chen D S. Intrafamilial transmission of hepatitis C virus: the important role of infections between spouses. J Infect Dis. 1992;166:900–903. doi: 10.1093/infdis/166.4.900. [DOI] [PubMed] [Google Scholar]
- 158.Karayiannis P, Novick D M, Lok A S, Fowler M J, Monjardino J, Thomas H C. Hepatitis B virus DNA in saliva, urine, and seminal fluid of carriers of hepatitis B e antigen. Br Med J Clin Res Ed. 1985;290:1853–1855. doi: 10.1136/bmj.290.6485.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Katamura E, Arai E, Fukuyama T, Komatsu Y. Five cases of mumps orchitis. Hinyokika Kiyo. 1967;13:35–41. [PubMed] [Google Scholar]
- 160.Kelly R W, Critchley H O. Immunomodulation by human seminal plasma: a benefit for spermatozoon and pathogen? Hum Reprod. 1997;12:2200–2207. doi: 10.1093/oxfordjournals.humrep.a019559. [DOI] [PubMed] [Google Scholar]
- 161.Kelsen J, Tarp B, Obel N. Absence of human herpes virus 8 in semen from healthy Danish donors. Hum Reprod. 1999;14:2274–2276. doi: 10.1093/humrep/14.9.2274. [DOI] [PubMed] [Google Scholar]
- 162.Kiessling A A. Evidence that reverse transcriptase is a component of murine epididymal fluid. Proc Soc Exp Biol Med. 1984;176:175–182. doi: 10.3181/00379727-176-41859. [DOI] [PubMed] [Google Scholar]
- 163.Kiessling A A, Crowell R, Fox C. Epididymis is a principal site of retrovirus expression in the mouse. Proc Natl Acad Sci USA. 1989;86:5109–5113. doi: 10.1073/pnas.86.13.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kiessling A A, Crowell R C, Connell R S. Sperm-associated retroviruses in the mouse epididymis. Proc Natl Acad Sci USA. 1987;84:8667–8671. doi: 10.1073/pnas.84.23.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kiessling A A, Fitzgerald L M, Zhang D, Chhay H, Brettler D, Eyre R C, Steinberg J, McGowan K, Byrn R A. Human immunodeficiency virus in semen arises from a genetically distinct virus reservoir. AIDS Res Hum Retroviruses. 1998;14:33–41. [PubMed] [Google Scholar]
- 166.Kim L, Johnson M, Barton S, Nelson M, Sontag G, Smith J, Gotch F, Gilmour J. Evaluation of sperm washing as a potential method of reducing HIV transmission in HIV-discordant couples wishing to have children. AIDS. 1999;13:645–651. doi: 10.1097/00002030-199904160-00004. [DOI] [PubMed] [Google Scholar]
- 167.Kimura M, Maekura S, Satou T, Hashimoto S. Cytomegalovirus inclusions detected in the seminal vesicle, ductus deferens and lungs in an autopsy case of lung cancer. Rinsho Byori. 1993;41:1059–1062. [PubMed] [Google Scholar]
- 168.Kirkland P D, Richards S G, Rothwell J T, Stanley D F. Replication of bovine viral diarrhoea virus in the bovine reproductive tract and excretion of virus in semen during acute and chronic infections. Vet Rec. 1991;128:587–590. doi: 10.1136/vr.128.25.587. [DOI] [PubMed] [Google Scholar]
- 169.Koment R W, Poor P M. Infection by human cytomegalovirus associated with chronic hematospermia. Urology. 1983;22:617–621. doi: 10.1016/0090-4295(83)90309-6. [DOI] [PubMed] [Google Scholar]
- 170.Kommisrud E, Vatn T, Lang Ree J R, Loken T. Bovine virus diarrhoea virus in semen from acutely infected bulls. Acta Vet Scand. 1996;37:41–47. doi: 10.1186/BF03548118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kondoh G, Murata Y, Aozasa K, Yutsudo M, Hakura A. Very high incidence of germ cell tumorigenesis (seminomagenesis) in human papillomavirus type 16 transgenic mice. J Virol. 1991;65:3335–3339. doi: 10.1128/jvi.65.6.3335-3339.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kotronias D, Kapranos N. Detection of herpes simplex virus DNA in human spermatozoa by in situ hybridization technique. In Vivo. 1998;12:391–394. [PubMed] [Google Scholar]
- 173.Kotwal G J, Rustgi V K, Baroudy B M. Detection of hepatitis C virus-specific antigens in semen from non-A, non-B hepatitis patients. Dig Dis Sci. 1992;37:641–644. doi: 10.1007/BF01296416. [DOI] [PubMed] [Google Scholar]
- 174.Krieger J N, Coombs R W, Collier A C, Ho D D, Ross S O, Zeh J E, Corey L. Intermittent shedding of human immunodeficiency virus in semen: implications for sexual transmission. J Urol. 1995;154:1035–1040. [PubMed] [Google Scholar]
- 175.Krieger J N, Coombs R W, Collier A C, Koehler J K, Ross S O, Chaloupka K, Murphy V L, Corey L. Fertility parameters in men infected with human immunodeficiency virus. J Infect Dis. 1991;164:464–469. doi: 10.1093/infdis/164.3.464. [DOI] [PubMed] [Google Scholar]
- 176.Krieger J N, Nirapathpongporn A, Chaiyaporn M, Peterson G, Nikolaeva I, Akridge R, Ross S O, Coombs R W. Vasectomy and human immunodeficiency virus type 1 in semen. J Urol. 1998;159:820–825. [PubMed] [Google Scholar]
- 177.Ku J H, Kim Y H, Jeon Y S, Lee N K. The preventive effect of systemic treatment with interferon-alpha2B for infertility from mumps orchitis. BJU Int. 1999;84:839–842. doi: 10.1046/j.1464-410x.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 178.Kuff E L, Lueders K K. The intracisternal A-particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- 179.Kuhn E M, Stolte N, Matz-Rensing K, Mach M, Stahl-Henning C, Hunsmann G, Kaup F J. Immunohistochemical studies of productive rhesus cytomegalovirus infection in rhesus monkeys (Macaca mulatta) infected with simian immunodeficiency virus. Vet Pathol. 1999;36:51–56. doi: 10.1354/vp.36-1-51. [DOI] [PubMed] [Google Scholar]
- 180.Kumar R M. Interspousal and intrafamilial transmission of hepatitis C virus: a myth or a concern? Obstet Gynecol. 1998;91:426–431. doi: 10.1016/s0029-7844(97)00684-4. [DOI] [PubMed] [Google Scholar]
- 181.Kyo S, Inoue M, Koyama M, Fujita M, Tanizawa O, Hakura A. Detection of high-risk human papillomavirus in the cervix and semen of sex partners. J Infect Dis. 1994;170:682–685. doi: 10.1093/infdis/170.3.682. [DOI] [PubMed] [Google Scholar]
- 182.LaDuca J R, Love J L, Abbott L Z, Dube S, Freidman-Kien A E, Poiesz B J. Detection of human herpesvirus 8 DNA sequences in tissues and bedily fluids. J Infect Dis. 1998;178:1610–1615. doi: 10.1086/314514. [DOI] [PubMed] [Google Scholar]
- 183.Lai Y M, Lee J F, Huang H Y, Soong Y K, Yang F P, Pao C C. The effect of human papillomavirus infection on sperm cell motility. Fertil Steril. 1997;67:1152–1155. doi: 10.1016/s0015-0282(97)81454-9. [DOI] [PubMed] [Google Scholar]
- 184.Lai Y M, Yang F P, Pao C C. Human papillomavirus deoxyribonucleic acid and ribonucleic acid in seminal plasma and sperm cells. Fertil Steril. 1996;65:1026–1030. [PubMed] [Google Scholar]
- 185.Lang D J. The epidemiology of cytomegalovirus infections: interpretation of recent observations. In: Krugman S G A, editor. Infections of the fetus and the newborn infant. Vol. 3. New York, N.Y: Alan R. Liss, Inc.; 1975. pp. 35–45. [Google Scholar]
- 186.Lange M, Klein E B, Kornfield H, Cooper L Z, Grieco M H. Cytomegalovirus isolation from healthy homosexual men. JAMA. 1984;252:1908–1910. [PubMed] [Google Scholar]
- 187.Larsson E, Andersson A C, Nilsson B O. Expression of an endogenous retrovirus (ERV3 HERV-R) in human reproductive and embryonic tissues—evidence for a function for envelope gene products. Upsala J Med Sci. 1994;99:113–120. doi: 10.3109/03009739409179354. [DOI] [PubMed] [Google Scholar]
- 188.Lasheeb A S, King J, Ball J K, Curran R, Barratt C L, Afnan M, Pillay D. Semen characteristics in HIV-1 positive men and the effect of semen washing. Genitourin Med. 1997;73:303–305. doi: 10.1136/sti.73.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lavitrano M, Maione B, Forte E, Francolini M, Sperandio S, Testi R, Spadafora C. The interaction of sperm cells with exogenous DNA: a role of CD4 and major histocompatibility complex class II molecules. Exp Cell Res. 1997;233:56–62. doi: 10.1006/excr.1997.3534. [DOI] [PubMed] [Google Scholar]
- 190.Leach C T, Cherry J D, English P A, Hennessey K, Giorgi J V, Visscher B R, Dudley J P, Detels R. The relationship between T-cell levels and CMV infection in asymptomatic HIV-1 antibody-positive homosexual men. J Acquired Immune Defic Syndr. 1993;6:407–413. [PubMed] [Google Scholar]
- 191.Leach C T, Detels R, Hennessey K, Liu Z, Visscher B R, Dudley J P, Cherry J D. A longitudinal study of cytomegalovirus infection in human immunodeficiency virus type 1-seropositive homosexual men: molecular epidemiology and association with disease progression. J Infect Dis. 1994;170:293–298. doi: 10.1093/infdis/170.2.293. [DOI] [PubMed] [Google Scholar]
- 192.Lebbe C, Pellet C, Tatoud R, Agbalika F, Dosquet P, Desgrez J, Morel P, Calvo F. Absence of human herpesvirus 8 sequences in prostate specimens. AIDS. 1997;11:270. [PubMed] [Google Scholar]
- 193.Lee H K, Lee H H, Park Y M, Lee J H, Ha T Y. Regulation of human B cell proliferation and differentiation by seminal plasma. Clin Exp Immunol. 1991;85:174–179. doi: 10.1111/j.1365-2249.1991.tb05700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Levy J A, Joyner J, Borenfreund E. Mouse sperm can horizontally transmit type C viruses. J Gen Virol. 1980;51:439–443. doi: 10.1099/0022-1317-51-2-439. [DOI] [PubMed] [Google Scholar]
- 195.Liou T C, Chang T T, Young K C, Lin X Z, Lin C Y, Wu H L. Detection of HCV RNA in saliva, urine, seminal fluid, and ascites. J Med Virol. 1992;37:197–202. doi: 10.1002/jmv.1890370309. [DOI] [PubMed] [Google Scholar]
- 196.Lissen E, Alter H J, Abad M A, Torres Y, Perez-Romero M, Leal M, Pineda J A, Torronteras R, Sanchez-Quijano A. Hepatitis C virus infection among sexually promiscuous groups and the heterosexual partners of hepatitis C virus infected index cases. Eur J Clin Microbiol Infect Dis. 1993;12:827–831. doi: 10.1007/BF02000402. [DOI] [PubMed] [Google Scholar]
- 197.Liu F H, Tian G S, Fu X X. Detection of plus and minus strand hepatitis C virus RNA in peripheral blood mononuclear cells and spermatid. Chung-Hua I Hsueh Tsa Chih. 1994;74:284–286. [PubMed] [Google Scholar]
- 198.Liuzzi G, Chirianni A, Clementi M, Bagnarelli P, Valenza A, Cataldo P T, Piazza M. Analysis of HIV-1 load in blood, semen and saliva: evidence for different viral compartments in a cross-sectional and longitudinal study. AIDS. 1996;10:51–56. doi: 10.1097/00002030-199612000-00001. [DOI] [PubMed] [Google Scholar]
- 199.Lower R, Lower J, Kurth R. The viruses in all of us: characteristics and biological significance of human endogenous retrovirus sequences. Proc Natl Acad Sci USA. 1996;93:5177–5184. doi: 10.1073/pnas.93.11.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mansat A, Mengelle C, Chalet M, Boumzebra A, Mieusset R, Puel J, Prouheze C, Segondy M. Cytomegalovirus detection in cryopreserved semen samples collected for therapeutic donor insemination. Hum Reprod. 1997;12:1663–1666. doi: 10.1093/humrep/12.8.1663. [DOI] [PubMed] [Google Scholar]
- 201.Manson A L. Mumps orchitis. Urology. 1990;36:355–358. doi: 10.1016/0090-4295(90)80248-l. [DOI] [PubMed] [Google Scholar]
- 202.Manuel F R, Embil J A. Virusemenia after cytomegalovirus mononucleosis. Can Med Assoc J. 1975;112:604. [PMC free article] [PubMed] [Google Scholar]
- 203.Marina S, Marina F, Alcolea R, Exposito R, Huguet J, Nadal J, Verges A. Human immunodeficiency virus type 1—serodiscordant couples can bear healthy children after undergoing intrauterine insemination. Fertil Steril. 1998;70:35–39. doi: 10.1016/s0015-0282(98)00102-2. [DOI] [PubMed] [Google Scholar]
- 204.Martin M A, Bryan T, Rasheed S, Khan A S. Identification and cloning of endogenous retroviral sequences present in human DNA. Proc Natl Acad Sci USA. 1981;78:4892–4896. doi: 10.1073/pnas.78.8.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Martin P M, Gresenguet G, Herve V M, Renom G, Steenman G, Georges A J. Decreased number of spermatozoa in HIV-1-infected individuals. AIDS. 1992;6:130. doi: 10.1097/00002030-199201000-00021. [DOI] [PubMed] [Google Scholar]
- 206.Mayer K H, Anderson D J. Heterosexual HIV transmission. Infect Agents Dis. 1995;4:273–284. [PubMed] [Google Scholar]
- 207.McDermott M B, O'Briain D S, Shiels O M, Daly P A. Malignant lymphoma of the epididymis. A case report of bilateral involvement by a follicular large cell lymphoma. Cancer. 1995;75:2174–2179. doi: 10.1002/1097-0142(19950415)75:8<2174::aid-cncr2820750823>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 208.McKay T C, Albala D M, Sendelbach K, Gattuso P. Cytomegalovirus prostatitis. Case report and review of the literature. Int Urol Nephrol. 1994;26:535–540. doi: 10.1007/BF02767655. [DOI] [PubMed] [Google Scholar]
- 209.McKee T A, Avery S, Majid A, Brinsden P R. Risks for transmission of hepatitis C virus during artificial insemination. Fertil Steril. 1996;66:161–163. doi: 10.1016/s0015-0282(16)58407-6. [DOI] [PubMed] [Google Scholar]
- 210.McNicol P J, Dodd J G. Detection of papillomavirus DNA in human prostatic tissue by Southern blot analysis. Can J Microbiol. 1990;36:359–362. doi: 10.1139/m90-062. [DOI] [PubMed] [Google Scholar]
- 211.McNicol P J, Dodd J G. High prevalence of human papillomavirus in prostate tissues. J Urol. 1991;145:850–853. doi: 10.1016/s0022-5347(17)38476-8. [DOI] [PubMed] [Google Scholar]
- 212.Merenich J A, McDermott M T, Asp A A, Harrison S M, Kidd G S. Evidence of endocrine involvement early in the course of human immunodeficiency virus infection. J Clin Endocrinol Metab. 1990;70:566–571. doi: 10.1210/jcem-70-3-566. [DOI] [PubMed] [Google Scholar]
- 213.Mermin J H, Holodniy M, Katzenstein D A, Merigan T C. Detection of human immunodeficiency virus DNA and RNA in semen by the polymerase chain reaction. J Infect Dis. 1991;164:769–772. doi: 10.1093/infdis/164.4.769. [DOI] [PubMed] [Google Scholar]
- 214.Meyling A, Houe H, Jensen A M. Epidemiology of bovine virus diarrhoea virus. Rev Sci Technol. 1990;9:75–93. doi: 10.20506/rst.9.1.489. [DOI] [PubMed] [Google Scholar]
- 215.Meyling A, Jensen A M. Transmission of bovine virus diarrhoea virus (BVDV) by artificial insemination (A1) with semen from a persistently-infected bull. Vet Microbiol. 1988;17:97–105. doi: 10.1016/0378-1135(88)90001-6. [DOI] [PubMed] [Google Scholar]
- 216.Mikuz G, Damjanov I. Inflammation of the testis, epididymis, peritesticular membranes, and scrotum. Pathol Annu. 1982;17:101–128. [PubMed] [Google Scholar]
- 217.Miller C J, Alexander N J, Sutjipto S, Lackner A A, Gettie A, Hendrickx A G, Lowenstine L J, Jennings M, Marx P A. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J Virol. 1989;63:4277–4284. doi: 10.1128/jvi.63.10.4277-4284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Miller C J, Vogel P, Alexander N J, Dandekar S, Hendrickx A G, Marx P A. Pathology and localization of simian immunodeficiency virus in the reproductive tract of chronically infected male rhesus macaques. Lab Investig. 1994;70:255–262. [PubMed] [Google Scholar]
- 219.Miry C, Pensaert M B, Bonte P, De Geest J. Effect of intratesticular inoculation with Aujeszky's disease virus on genital organs of boars. Vet Microbiol. 1987;14:355–363. doi: 10.1016/0378-1135(87)90027-7. [DOI] [PubMed] [Google Scholar]
- 220.Mobley K, Rotterdam H Z, Lerner C W, Tapper M L. Autopsy findings in the acquired immune deficiency syndrome. Pathol Annu. 1985;20:45–65. [PubMed] [Google Scholar]
- 221.Mole L, Ripich S, Margolis D, Holodniy M. The impact of active herpes simplex virus infection on human immunodeficiency virus load. J Infect Dis. 1997;176:766–770. doi: 10.1086/517297. [DOI] [PubMed] [Google Scholar]
- 222.Monini P, de Lellis L, Fabris M, Rigolin F, Cassai E. Kaposi's sarcoma-associated herpesvirus DNA sequences in prostate tissue and human semen. N Engl J Med. 1996;334:1168–1172. doi: 10.1056/NEJM199605023341805. [DOI] [PubMed] [Google Scholar]
- 223.Monini P, Howard M R, Rimessi P, de Lellis L, Schulz T F, Cassai E. Human herpesvirus DNA in prostate and semen from HIV-negative individuals in Italy. AIDS. 1997;11:1530–1532. [PubMed] [Google Scholar]
- 224.Monini P, Rotola A, de Lellis L, Corallini A, Secchiero P, Albini A, Benelli R, Parravicini C, Barbanti Brodano G, Cassai E. Latent BK virus infection and Kaposi's sarcoma pathogenesis. Int J Cancer. 1996;66:717–722. doi: 10.1002/(SICI)1097-0215(19960611)66:6<717::AID-IJC1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 225.Monke D R. Noninfectivity of semen from bulls infected with bovine leukosis virus. J Am Vet Med Assoc. 1986;188:823–826. [PubMed] [Google Scholar]
- 226.Moore D E, Ashley R L, Zarutskie P W, Coombs R W, Soules M R, Corey L. Transmission of genital herpes by donor insemination. JAMA. 1989;261:3441–3443. [PubMed] [Google Scholar]
- 227.Morriseau P M, Philipps C A, Leadbetter G. Viral prostatitis. J Urol. 1970;103:767–769. doi: 10.1016/s0022-5347(17)62045-7. [DOI] [PubMed] [Google Scholar]
- 228.Muciaccia B, Uccini S, Filippini A, Ziparo E, Paraire F, Baroni C D, Stefanini M. Presence and cellular distribution of HIV in the testes of seropositive subjects: an evaluation by in situ PCR hybridization. FASEB J. 1998;12:151–163. doi: 10.1096/fasebj.12.2.151. [DOI] [PubMed] [Google Scholar]
- 229.Munker R, Tasaka T, Park D, Miller C W, Koeffler H P. HHV-8 (KSHV) does not establish latency in prostate cancer cell lines. Prostate. 1997;33:286–288. doi: 10.1002/(sici)1097-0045(19971201)33:4<286::aid-pros10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 230.Murphy E L, Blattner W A. HTLV-I-associated leukemia: a model for chronic retroviral diseases. Ann Neurol. 1988;23:174–180. doi: 10.1002/ana.410230739. [DOI] [PubMed] [Google Scholar]
- 231.Murphy E L, Figueroa J P, Gibbs W N, Brathwaite A, Holding Cobham M, Waters D, Cranston B, Hanchard B, Blattner W A. Sexual transmission of human T-lymphotropic virus type 1 (HTLV-1) Ann Intern Med. 1989;111:555–560. doi: 10.7326/0003-4819-111-7-555. [DOI] [PubMed] [Google Scholar]
- 232.Nadler R D, Manocha A D, McClure H M. Spermatogenesis and hormone levels in rhesus macaques inoculated with simian immunodeficiency virus. J Med Primatol. 1993;22:325–329. [PubMed] [Google Scholar]
- 233.Nahmias A J, Shore S L, Kohl S, Starr S E, Ashman R B. Immunology of herpes simplex virus infection: relevance to herpes simplex virus vaccines and cervical cancer. Cancer Res. 1976;36:836–844. [PubMed] [Google Scholar]
- 234.Nash J W, Hanson L A, St. Cyr Coats K. Bovine immunodeficiency virus in stud bull semen. Am J Vet Res. 1995;56:760–763. [PubMed] [Google Scholar]
- 235.Neighbour P A, Fraser L R. Murine cytomegalovirus and fertility: potential sexual transmission and the effect of this virus on fertilization in vitro. Fertil Steril. 1978;30:216–222. doi: 10.1016/s0015-0282(16)43463-1. [DOI] [PubMed] [Google Scholar]
- 236.Niedt G W, Schinella R A. Acquired immunodeficiency syndrome. Clinicopathologic study of 56 autopsies. Arch Pathol Lab Med. 1985;109:727–734. [PubMed] [Google Scholar]
- 237.Nielsen T L, Nielsen J, Have P, Baekbo P, Hoff Jorgensen R, Botner A. Examination of virus shedding in semen from vaccinated and from previously infected boars after experimental challenge with porcine reproductive and respiratory syndrome virus. Vet Microbiol. 1997;54:101–112. doi: 10.1016/s0378-1135(96)01272-2. [DOI] [PubMed] [Google Scholar]
- 238.Nieminen P, Koskimies A I, Paavonen J. Human papillomavirus DNA is not transmitted by semen. Int J Sex Teansm Dis AIDS. 1991;2:207–208. doi: 10.1177/095646249100200313. [DOI] [PubMed] [Google Scholar]
- 239.Noda T, Sasagawa T, Dong Y, Fuse H, Namiki M, Inoue M. Detection of human papillomavirus (HPV) DNA in archival specimens of benign prostatic hyperplasia and prostatic cancer using a highly sensitive nested PCR method. Urol Res. 1998;26:165–169. doi: 10.1007/s002400050041. [DOI] [PubMed] [Google Scholar]
- 240.Nuovo G J, Becker J, Simsir A, Margiotta M, Khalife G, Shevchuk M. HIV-1 nucleic acids localize to the spermatogonia and their progeny. A study by polymerase chain reaction in situ hybridization. Am J Pathol. 1994;144:1142–1148. [PMC free article] [PubMed] [Google Scholar]
- 241.Ohtsuki Y, Dmochowski L. Virus particles in normal prostate tissues of mice from ten strains, with special reference to type-B virus particles. Gann. 1979;70:195–202. [PubMed] [Google Scholar]
- 242.Oldstone M B, Dixon F J. Immune complex disease in chronic viral infections. J Exp Med. 1971;134:32–40. [PMC free article] [PubMed] [Google Scholar]
- 243.Oliver R T. Atrophy, hormones, genes and viruses in aetiology germ cell tumours. Cancer Surv. 1990;9:263–286. [PubMed] [Google Scholar]
- 244.Oliver R T. Testis cancer. Curr Opin Oncol. 1997;9:287–294. doi: 10.1097/00001622-199709030-00012. [DOI] [PubMed] [Google Scholar]
- 245.Osburn B I. The impact of bluetongue virus on reproduction. Comp Immunol Microbiol Infect Dis. 1994;17:189–196. doi: 10.1016/0147-9571(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 246.Osterrieth P M, Kozma S, Hendrick J C, Francois C, Calberg-Bacq C M, Franchimont P, Gosselin L. Detection of virus antigens in Swiss albino mice infected by milk-borne mouse mammary tumour virus: the effect of age, sex and reproductive status. II. Radioimmunoassay of two virus components, gp47 and p28 in serum and organ extracts. J Gen Virol. 1979;45:41–50. doi: 10.1099/0022-1317-45-1-41. [DOI] [PubMed] [Google Scholar]
- 247.Ostrow R S, Zachow K R, Niimura M, Okagaki T, Muller S, Bender M, Faras A J. Detection of papillomavirus DNA in human semen. Science. 1986;231:731–733. doi: 10.1126/science.3003908. [DOI] [PubMed] [Google Scholar]
- 248.Palfi V, Glavits R, Hajtos I. Testicular lesions in rams infected by maedi/visna virus. Acta Vet Hung. 1989;37:97–102. [PubMed] [Google Scholar]
- 249.Panthier J J, Gounon P, Condamine H, Jacob F. Pattern of expression of ecotropic murine leukemia virus in gonads of inoculated SWR/J mice. J Virol. 1989;63:2134–2142. doi: 10.1128/jvi.63.5.2134-2142.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Parsonson I M, Della Porta A J, McPhee D A, Cybinski D H, Squire K R, Standfast H A, Uren M F. Isolation of bluetongue virus serotype 20 from the semen of an experimentally-infected bull. Aust Vet J. 1981;57:252–253. doi: 10.1111/j.1751-0813.1981.tb02681.x. [DOI] [PubMed] [Google Scholar]
- 251.Pellett P E, Spira T J, Bagasra O, Boshoff C, Corey L, de Lellis L, Huang M L, Lin J C, Matthews S, Monini P, Rimessi P, Sosa C, Wood C, Stewart J A. Multicenter comparison of PCR assays for detection of human herpesvirus 8 DNA in semen. J Clin Microbiol. 1999;37:1298–1301. doi: 10.1128/jcm.37.5.1298-1301.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Phillips R M, Carnahan D L, Rademacher D J. Virus isolation from semen of bulls serologically positive for bluetongue virus. Am J Vet Res. 1986;47:84–85. [PubMed] [Google Scholar]
- 253.Poretsky L, Can S, Zumoff B. Testicular dysfunction in human immunodeficiency virus-infected men. Metabolism. 1995;44:946–953. doi: 10.1016/0026-0495(95)90250-3. [DOI] [PubMed] [Google Scholar]
- 254.Portis J L, McAtee F J, Hayes S F. Horizontal transmission of murine retroviruses. J Virol. 1987;61:1037–1044. doi: 10.1128/jvi.61.4.1037-1044.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Preblud S R, Dobbs H I, Sedmak G V, Herrmann K L, Nieburg P I. Testalgia associated with rubella infection. South Med J. 1980;73:594–595. doi: 10.1097/00007611-198005000-00013. [DOI] [PubMed] [Google Scholar]
- 256.Priebe C J, Jr, Holahan J A, Ziring P R. Abnormalities of the vas deferens and epididymis in cryptorchid boys with congenital rubella. J Pediatr Surg. 1979;14:834–838. doi: 10.1016/s0022-3468(79)80276-6. [DOI] [PubMed] [Google Scholar]
- 257.Pudney J, Anderson D. Orchitis and human immunodeficiency virus type 1 infected cells in reproductive tissues from men with the acquired immune deficiency syndrome. Am J Pathol. 1991;139:149–160. [PMC free article] [PubMed] [Google Scholar]
- 258.Pudney J, Nguyen H, Xu C, Anderson D J. Microscopic evidence against HIV-1 infection of germ cells or attachment to sperm. J Reprod Immunol. 1999;44:57–77. doi: 10.1016/s0165-0378(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 259.Quayle A J, Xu C, Mayer K H, Anderson D J. Tlymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. J Infect Dis. 1997;176:960–968. doi: 10.1086/516541. [DOI] [PubMed] [Google Scholar]
- 260.Rabkin J G, Wagner G J, Rabkin R. A double-blind, placebo-controlled trial of testosterone therapy for HIV-positive men with hypogonadal symptoms. Arch Gen Psychiatry. 2000;57:141–147. doi: 10.1001/archpsyc.57.2.141. [DOI] [PubMed] [Google Scholar]
- 261.Raffi F, Brisseau J M, Planchon B, Remi J P, Barrier J H, Grolleau J Y. Endocrine function in 98 HIV-infected patients: a prospective study. AIDS. 1991;5:729–733. doi: 10.1097/00002030-199106000-00013. [DOI] [PubMed] [Google Scholar]
- 262.Rajpert De Meyts E, Hording U, Nielsen H W, Skakkebaek N E. Human papillomavirus and Epstein-Barr virus in the etiology of testicular germ cell tumours. APMIS. 1994;102:38–42. doi: 10.1111/j.1699-0463.1994.tb04842.x. [DOI] [PubMed] [Google Scholar]
- 263.Ramirez Mendoza H, Hernandez Jauregui P, Reyes Leyva J, Zenteno E, Moreno Lopez J, Kennedy S. Lesions in the reproductive tract of boars experimentally infected with porcine rubulavirus. J Comp Pathol. 1997;117:237–252. doi: 10.1016/s0021-9975(97)80018-7. [DOI] [PubMed] [Google Scholar]
- 264.Rapp F, Geder L, Murasko D, Lausch R, Ladda R, Huang E S, Webber M M. Long-term persistence of cytomegalovirus genome in cultured human cells of prostatic origin. J Virol. 1975;16:982–990. doi: 10.1128/jvi.16.4.982-990.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Rasmussen L, Hong C, Zipeto D, Morris S, Sherman D, Chou S, Miner R, Drew W L, Wolitz R, Dowling A, Warford A, Merigan T C. Cytomegalovirus gB genotype distribution differs in human immunodeficiency virus-infected patients and immunocompromised allograft recipients. J Infect Dis. 1997;175:179–184. doi: 10.1093/infdis/175.1.179. [DOI] [PubMed] [Google Scholar]
- 266.Reichert C M, O'Leary T J, Levens D L, Simrell C R, Macher A M. Autopsy pathology in the acquired immune deficiency syndrome. Am J Pathol. 1983;112:357–382. [PMC free article] [PubMed] [Google Scholar]
- 267.Rhim S H, Millar S E, Robey F, Luo A M, Lou Y H, Yule T, Allen P, Dean J, Tung K S. Autoimmune disease of the ovary induced by a ZP3 peptide from the mouse zona pellucida. J Clin Investis. 1992;89:28–35. doi: 10.1172/JCI115572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Riggs S, Sanford J P. Viral orchitis. N Engl J Med. 1962;266:990. doi: 10.1056/NEJM196205102661906. [DOI] [PubMed] [Google Scholar]
- 269.Rinaldo C R, Jr, Kingsley L A, Ho M, Armstrong J A, Zhou S Y. Enhanced shedding of cytomegalovirus in semen of human-immunodeficiency virus-seropositive homosexual men. J Clin Microbiol. 1992;30:1148–1155. doi: 10.1128/jcm.30.5.1148-1155.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Rinaldo C R, Jr, Kingsley L A, Lyter D W, Bodner A J, Weiss S H, Saxinger W C. Excretion of cytomegalovirus in semen associated with HTLV-III seropositivity in asymptomatic homosexual men. J Med Virol. 1986;20:17–22. doi: 10.1002/jmv.1890200104. [DOI] [PubMed] [Google Scholar]
- 271.Roberts D H, Lucas M H, Wibberley G, Chasey D. An investigation into the susceptibility of cattle to bovine leukosis virus following inoculation by various routes. Vet Rec. 1982;110:222–224. doi: 10.1136/vr.110.10.222. [DOI] [PubMed] [Google Scholar]
- 272.Roelofs H, van Gurp R J, Oosterhuis J W, Looijenga L H. Detection of human endogenous retrovirus type K-specific transcripts in testicular parenchyma and testicular germ cell tumors of adolescents and adults: clinical and biological implications. Am J Pathol. 1998;153:1277–1282. doi: 10.1016/S0002-9440(10)65672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Rogers C, Klatt E C. Pathology of the testis in acquired immunodeficiency syndrome. Histopathology. 1988;12:659–665. doi: 10.1111/j.1365-2559.1988.tb01990.x. [DOI] [PubMed] [Google Scholar]
- 274.Rongey R W, Abtin A H, Estes J D, Gardner M B. Mammary tumor virus particles in the submaxillary gland, seminal vesicle, and nonmammary tumors of wild mice. J Natl Cancer Inst. 1975;54:1149–1156. doi: 10.1093/jnci/54.5.1149. [DOI] [PubMed] [Google Scholar]
- 275.Rooney G, Gilson R J. Sexual transmission of hepatitis C virus infection. Sex Transm Infect. 1998;74:399–404. doi: 10.1136/sti.74.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Root Bernstein R S, Hobbs S H. Does HIV “piggyback” on CD4-like surface proteins of sperm, viruses, and bacteria? Implications for co-transmission, cellular tropism and the induction of autoimmunity in AIDS. J Theor Biol. 1993;160:249–264. doi: 10.1006/jtbi.1993.1017. [DOI] [PubMed] [Google Scholar]
- 277.Rubin H, Cornelius A, Fanshier L. The pattern of congenital transmission of an avian leukosis virus. Proc Natl Acad Sci USA. 1961;47:1058–1069. doi: 10.1073/pnas.47.7.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Rubin M A, Parry J P, Singh B. Kaposi's sarcoma associated herpesvirus deoxyribonucleic acid sequences: lack of detection in prostatic tissue of human immunodeficiency virus-negative immunocompetent adults. J Urol. 1998;159:146–148. doi: 10.1016/s0022-5347(01)64038-2. [DOI] [PubMed] [Google Scholar]
- 279.Ruijter E, van de Kaa C, Miller G, Ruiter D, Debruyne F, Schalken J. Molecular genetics and epidemiology of prostate carcinoma. Endocr Rev. 1999;20:22–45. doi: 10.1210/edrv.20.1.0356. [DOI] [PubMed] [Google Scholar]
- 280.Sabin A B, Tarro G. Herpes simplex and herpes genitalis viruses in etiology of some human cancers. Proc Natl Acad Sci USA. 1973;70:3225–3229. doi: 10.1073/pnas.70.11.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Samuel C E. Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology. 1991;183:1–11. doi: 10.1016/0042-6822(91)90112-o. [DOI] [PubMed] [Google Scholar]
- 282.Sarkar F H, Sakr W A, Li Y W, Sreepathi P, Crissman J D. Detection of human papillomavirus (HPV) DNA in human prostatic tissues by polymerase chain reaction. Prostate. 1993;22:171–180. doi: 10.1002/pros.2990220210. [DOI] [PubMed] [Google Scholar]
- 283.Sauter M, Schommer S, Kremmer E, Remberger K, Dolken G, Lemm I, Buck M, Best B, Neumann Haefelin D, Mueller Lantzsch N. Human endogenous retrovirus K10: expression of Gag protein and detection of antibodies in patients with seminomas. J Virol. 1995;69:414–421. doi: 10.1128/jvi.69.1.414-421.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Schiff R, Itin A, Keshet E. Transcriptional activation of mouse retrotransposons in vivo: specific expression in steroidogenic cells in response to trophic hormones. Genes Dev. 1991;5:521–532. doi: 10.1101/gad.5.4.521. [DOI] [PubMed] [Google Scholar]
- 285.Schlafer D H, Gillespie J H, Foote R H, Quick S, Pennow N N, Dougherty E P, Schiff E I, Allen S E, Powers P A, Hall C E, et al. Experimental transmission of bovine viral diseases by insemination with contaminated semen or during embryo transfer. DTW Dtsch Tieraerztl Wochenschr. 1990;97:68–72. [PubMed] [Google Scholar]
- 286.Schlienger J L, Lang J M. Endocrine consequences of infection by human immunodeficiency virus (HIV) Pathol Biol Paris. 1989;37:921–926. [PubMed] [Google Scholar]
- 287.Schurmeyer T H, Muller V, von zur Muhlen A, Schmidt R E. Endocrine testicular function in HIV-infected outpatients. Eur J Med Res. 1997;2:275–281. [PubMed] [Google Scholar]
- 288.Scofield V, Rao B, Broder S, Kennedy C, Wallace M, Graham B, Poiesz B. HIV interaction with sperm. AIDS. 1994;8:1733–1736. [PubMed] [Google Scholar]
- 289.Scofield V L, Clisham R, Bandyopadhyay L, Gladstone P, Zamboni L, Raghupathy R. Binding of sperm to somatic cells via HLA-DR. Modulation by sulfated carbohydrates. J Immunol. 1992;148:1718–1724. [PubMed] [Google Scholar]
- 290.Scott L S. Mumps and male fertility. Br J Urol. 1960;32:183. doi: 10.1111/j.1464-410x.1960.tb03761.x. [DOI] [PubMed] [Google Scholar]
- 291.Scott R M, Snitbhan R, Bancroft W H, Alter H J, Tingpalapong M. Experimental transmission of hepatitis B virus by semen and saliva. J Infect Dis. 1980;142:67–71. doi: 10.1093/infdis/142.1.67. [DOI] [PubMed] [Google Scholar]
- 292.Sellers R F, Burrows R, Mann J A, Dawe P. Recovery of virus from bulls affected with foot-and-mouth disease. Vet Rec. 1968;83:303. doi: 10.1136/vr.83.12.303. [DOI] [PubMed] [Google Scholar]
- 293.Semprini A E. Insemination of HIV-negative women with processed semen of HIV-positive partners. Lancet. 1993;341:1343–1344. doi: 10.1016/0140-6736(93)90850-g. [DOI] [PubMed] [Google Scholar]
- 294.Semprini A E, Fiore S, Pardi G. Reproductive counselling for HIV-discordant couples. Lancet. 1997;349:1401–1402. doi: 10.1016/S0140-6736(05)63250-3. [DOI] [PubMed] [Google Scholar]
- 295.Semprini A E, Levi Setti P, Bozzo M, Ravizza M, Taglioretti A, Sulpizio P, Albani E, Oneta M, Pardi G. Insemination of HIV-negative women with processed semen of HIV-positive partners. Lancet. 1992;340:1317–1319. doi: 10.1016/0140-6736(92)92495-2. [DOI] [PubMed] [Google Scholar]
- 296.Semprini A E, Persico T, Thiers V, Oneta M, Tuveri R, Serafini P, Boschini A, Giuntelli S, Pardi G, Brechot C. Absence of hepatitis C virus and detection of hepatitis G virus/GB virus C RNA sequences in the semen of infected men. J Infect Dis. 1998;177:848–854. doi: 10.1086/515257. [DOI] [PubMed] [Google Scholar]
- 297.Serfling U, Ciancio G, Zhu W Y, Leonardi C, Penneys N S. Human papillomavirus and herpes virus DNA are not detected in benign and malignant prostatic tissue using the polymerase chain reaction. J Urol. 1992;148:192–194. doi: 10.1016/s0022-5347(17)36551-5. [DOI] [PubMed] [Google Scholar]
- 298.Serth J, Panitz F, Paeslack U, Kuczyk M A, Jonas U. Increased levels of human papillomavirus type 16 DNA in a subset of prostate cancers. Cancer Res. 1999;59:823–825. [PubMed] [Google Scholar]
- 299.Sharma G, Polasa H. Cytogenetic effects of influenza virus infection on male germ cells of mice. Hum Genet. 1978;45:179–187. doi: 10.1007/BF00286960. [DOI] [PubMed] [Google Scholar]
- 300.Sheffy B E, Krinsky M. Infectious bovine rhinotracheitis virus in extended bovine semen 1. Proc Annu Meet US Anim Health Assoc. 1973;77:131–137. [PubMed] [Google Scholar]
- 301.Sherertz R J, Peacock J E, Jr, Sixbey J W, Folds J D, Bowdre J H, Huang E S, Hamilton J D, McDowell D L. Nonurban male homosexuals: epidemiologic, immunologic and virologic characteristics. Am J Med Sci. 1984;288:109–113. doi: 10.1097/00000441-198410000-00003. [DOI] [PubMed] [Google Scholar]
- 302.Sherman J K, Morgan P N. Effect of human semen on herpes-simplex virus-2. Fertil Steril. 1989;51:186–189. doi: 10.1016/s0015-0282(16)60454-5. [DOI] [PubMed] [Google Scholar]
- 303.Shevchuk M M, Nuovo G J, Khalife G. HIV in testis: quantitative histology and HIV localization in germ cells. J Reprod Immunol. 1998;41:69–79. doi: 10.1016/s0165-0378(98)00049-7. [DOI] [PubMed] [Google Scholar]
- 304.Shevchuk M M, Pigato J B, Khalife G, Armenakas N A, Fracchia J A. Changing testicular histology in AIDS: its implication for sexual transmission of HIV. Urology. 1999;53:203–208. doi: 10.1016/s0090-4295(98)00463-4. [DOI] [PubMed] [Google Scholar]
- 305.Shimakage M, Oka T, Shinka T, Kurata A, Sasagawa T, Yutsudo M. Involvement of Epstein-Barr virus expression in testicular tumors. J Urol. 1996;156:253–257. [PubMed] [Google Scholar]
- 306.Sokol Roseman D, Ansell J S, Chapman W H. Sexually transmitted diseases and carcinogenesis. Urol Clin North Am. 1984;11:27–43. [PubMed] [Google Scholar]
- 307.Spradbrow P B. The isolation of infectious bovine rhinotracheitis virus from bovine semen. Aust Vet J. 1968;44:410–412. doi: 10.1111/j.1751-0813.1968.tb09134.x. [DOI] [PubMed] [Google Scholar]
- 308.Staeheli P. Interferon-induced proteins and the antiviral state. Adv Virus Res. 1990;38:147–200. doi: 10.1016/s0065-3527(08)60862-3. [DOI] [PubMed] [Google Scholar]
- 309.Stapleton A M F, Williams R H, Timme T L, Yang G, Truong L D, Thompson T C. Human cytomegalovirus is not implicated in benign prostatic hyperplasia: a study using immunohistochemistry and the polymerase chain reaction. J Urol. 1996;156:542–545. doi: 10.1097/00005392-199608000-00079. [DOI] [PubMed] [Google Scholar]
- 310.Stephanopoulos D E, Myers M G, Bernstein D I. Genital infections due to herpes simplex virus type 2 in male guinea pigs. J Infect Dis. 1989;159:89–95. doi: 10.1093/infdis/159.1.89. [DOI] [PubMed] [Google Scholar]
- 311.Stewart G J, Tyler J P, Cunningham A L, Barr J A, Driscoll G L, Gold J, Lamont B J. Transmission of human T-cell lymphotropic virus type III (HTLV-III) by artificial insemination by donor. Lancet. 1985;ii:581–585. doi: 10.1016/s0140-6736(85)90585-9. [DOI] [PubMed] [Google Scholar]
- 312.Stratton P, Alexander N. Heterosexual spread of HIV infection. Reprod Med Rev. 1994;3:113–136. [Google Scholar]
- 313.Straub O C. Enzootic bovine leukosis—a retrovirus disease. Tieraerztl Prax. 1988;16:353–357. [PubMed] [Google Scholar]
- 314.Strickler H D, Burk R, Shah K, Viscidi R, Jackson A, Pizza G, Bertoni F, Schiller J T, Manns A, Metcalf R, Qu W, Goedert J J. A multifaceted study of human papillomavirus and prostate carcinoma. Cancer. 1998;82:1118–1125. [PubMed] [Google Scholar]
- 315.Sur J H, Doster A R, Christian J S, Galeota J A, Wills R W, Zimmerman J J, Osorio F A. Porcine reproductive and respiratory syndrome virus replicates in testicular germ cells, alters spermatogenesis, and induces germ cell death by apoptosis. J Virol. 1997;71:9170–9179. doi: 10.1128/jvi.71.12.9170-9179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Tachet A, Dulioust E, Salmon D, De Almeida M, Rivalland S, Finkielsztejn L, Heard I, Jouannet P, Sicard D, Rouzioux C. Detection and quantification of HIV-1 in semen: identification of a subpopulation of men at high potential risk of viral sexual transmission. AIDS. 1999;13:823–831. doi: 10.1097/00002030-199905070-00012. [DOI] [PubMed] [Google Scholar]
- 317.Tajima K. Malignant lymphomas in Japan: epidemiological analysis of adult T-cell leukemia/lymphoma. Cancer Metastasis Rev. 1988;7:223–241. doi: 10.1007/BF00047753. [DOI] [PubMed] [Google Scholar]
- 318.Tajima K, Cartier L. Epidemiological features of HTLV-I and adult T cell leukemia. Intervirology. 1995;38:238–246. doi: 10.1159/000150438. [DOI] [PubMed] [Google Scholar]
- 319.Tang Z, Yang D, Hao L, Huang Y, Wang S. Detection and significance of HCV RNA in saliva, seminal fluid and vaginal discharge in patients with hepatitis C. J Tongji Med Univ. 1996;16:11–13. doi: 10.1007/BF02889035. [DOI] [PubMed] [Google Scholar]
- 320.Taruscio D, Mantovani A. Human endogenous retroviral sequences: possible roles in reproductive physiopathology. Biol Reprod. 1998;59:713–724. doi: 10.1095/biolreprod59.4.713. [DOI] [PubMed] [Google Scholar]
- 321.Tasaka T, Said J W, Koeffler H P. Absence of HHV-8 in prostate and semen. N Engl J Med. 1996;335:1237–1239. [PubMed] [Google Scholar]
- 322.Tasaka T, Said J W, Morosetti R, Park D, Verbeek W, Nagai M, Takahara J, Koeffler H P. Is Kaposi's sarcoma-associated herpesvirus ubiquitous in urogenital and prostate tissues? Blood. 1997;89:1686–1689. [PubMed] [Google Scholar]
- 323.Tearle J P, Smith K C, Boyle M S, Binns M M, Livesay G J, Mumford J A. Replication of equid herpesvirus-1 (EHV-1) in the testes and epididymides of ponies and venereal shedding of infectious virus. J Comp Pathol. 1996;115:385–397. doi: 10.1016/s0021-9975(96)80073-9. [DOI] [PubMed] [Google Scholar]
- 324.Terada S, Katayama K, Negayama K, Kawanishi K. Analysis of hepatitis C virus RNA from semen and urine of the patients with chronic type C hepatitis. Kansenshogaku Zasshi. 1992;66:106–107. doi: 10.11150/kansenshogakuzasshi1970.66.106. [DOI] [PubMed] [Google Scholar]
- 325.Tessler A N, Catanese A. AIDS and germ cell tumors of testis. Urology. 1987;30:203–204. doi: 10.1016/0090-4295(87)90233-0. [DOI] [PubMed] [Google Scholar]
- 326.Thomas D L, Zenilman J M, Alter H J, Shih J W, Galai N, Carella A V, Quinn T C. Sexual transmission of hepatitis C virus among patients attending sexually transmitted diseases clinics in Baltimore—an analysis of 309 sex partnerships. J Infect Dis. 1995;171:768–775. doi: 10.1093/infdis/171.4.768. [DOI] [PubMed] [Google Scholar]
- 327.Thurston R J, Hess R A, Biellier H V, Adldinger H K, Solorzano R F. Ultrastructural studies of semen abnormalities and Herpesvirus associated with cultured testicular cells from domestic turkeys. J Reprod Fertil. 1975;45:235–241. doi: 10.1530/jrf.0.0450235. [DOI] [PubMed] [Google Scholar]
- 328.Tiller B L, Dagle G E, Cadwell L L. Testicular atrophy in a mule deer population. J Wildl Dis. 1997;33:420–429. doi: 10.7589/0090-3558-33.3.420. [DOI] [PubMed] [Google Scholar]
- 329.Tindall B, Evans L, Cunningham P, McQueen P, Hurren L, Vasak E, Mooney J, Cooper D A. Identification of HIV-1 in semen following primary HIV-1 infection. AIDS. 1992;6:949–952. doi: 10.1097/00002030-199209000-00006. [DOI] [PubMed] [Google Scholar]
- 330.Tokuda H, Ito Y, Kanaoka T, Yoshida O. Tumor-promoting activity of extracts of human semen in SENCAR mice. Int J Cancer. 1987;40:554–556. doi: 10.1002/ijc.2910400420. [DOI] [PubMed] [Google Scholar]
- 331.Toppari J, Larsen J C, Christiansen P, Giwercman A, Grandjean P, Guillette L J, Jr, Jegou B, Jensen T K, Jouannet P, Keiding N, Leffers H, McLachlan J A, Meyer O, Muller J, Rajpert De Meyts E, Scheike T, Sharpe R, Sumpter J, Skakkebaek N E. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996;4:741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Tsutsumanski V, Genov I. Role of vertical transmission and seminal fluid in the infectious leukemia process. Vet Med Nauki. 1984;21:62–67. [PubMed] [Google Scholar]
- 333.Tu H, Jacobs S C, Mergner W J, Kyprianou N. Rare incidence of human papillomavirus types 16 and 18 in primary and metastatic human prostate cancer. Urology. 1994;44:726–731. doi: 10.1016/s0090-4295(94)80215-7. [DOI] [PubMed] [Google Scholar]
- 334.Turner M J, White J O, Soutter W P. Human seminal plasma inhibits the lymphocyte response to infection with Epstein-Barr virus. Gynecol Oncol. 1990;37:60–65. doi: 10.1016/0090-8258(90)90309-9. [DOI] [PubMed] [Google Scholar]
- 335.Ueno A, Takeda M, Hirasawa K, Itagaki S, Doi K. Relation between distribution of viral RNA and development of histopathological changes in encephalomyocarditis virus-induced orchitis in mice. Int J Exp Pathol. 1996;77:25–30. doi: 10.1046/j.1365-2613.1996.959097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Urnovitz H B, Murphy W H. Human endogenous retroviruses: nature, occurrence, and clinical implications in human disease. Clin Microbiol Rev. 1996;9:72–99. doi: 10.1128/cmr.9.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.van Oirschot J T. Bovine herpesvirus 1 in semen of bulls and the risk of transmission: a brief review. Vet Q. 1995;17:29–33. doi: 10.1080/01652176.1995.9694526. [DOI] [PubMed] [Google Scholar]
- 338.van Oirschot J T, Rijsewijk F A, Straver P J, Ruuis R C, Quak J, Davidse A, Westenbrink F, Gielkens A L, van Dijk J E, Moerman A. Virulence and genotype of a bovine herpesvirus 1 isolate from semen of a subclinically infected bull. Vet Rec. 1995;137:235–239. doi: 10.1136/vr.137.10.235. [DOI] [PubMed] [Google Scholar]
- 339.van Oirschot J T, Straver P J, van Lieshout J A, Quak J, Westenbrink F, van Exsel A C. A subclinical infection of bulls with bovine herpesvirus type 1 at an artificial insemination centre. Vet Rec. 1993;132:32–35. doi: 10.1136/vr.132.2.32. [DOI] [PubMed] [Google Scholar]
- 340.van't Wout A B, Ran L J, Kuiken C L, Kootstra N A, Pals S T, Schuitemaker H. Analysis of the temporal relationship between human immunodeficiency virus type 1 quasispecies in sequential blood samples and various organs obtained at autopsy. J Virol. 1998;72:488–496. doi: 10.1128/jvi.72.1.488-496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Van Woensel P, Van der Wouw J, Visser N. Detection of porcine reproductive respiratory syndrome virus by the polymerase chain reaction. J Virol Methods. 1994;47:273–278. doi: 10.1016/0166-0934(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 342.Vernazza P L, Eron J J, Cohen M S, van der Horst C M, Troiani L, Fiscus S A. Detection and biologic characterization of infectious HIV-i in semen of seropositive men. AIDS. 1994;8:1325–1329. doi: 10.1097/00002030-199409000-00017. [DOI] [PubMed] [Google Scholar]
- 343.Villette J M, Bourin P, Doinel C, Mansour I, Fiet J, Boudou P, Dreux C, Roue R, Debord M, Levi F. Circadian variations in plasma levels of hypophyseal, adrenocortical and testicular hormones in men infected with human immunodeficiency virus. J Clin Endocrinol Metab. 1990;70:572–577. doi: 10.1210/jcem-70-3-572. [DOI] [PubMed] [Google Scholar]
- 344.Wajjwalku W, Takahashi M, Miyaishi O, Lu J, Sakata K, Yokoi T, Saga S, Imai M, Matsuyama M, Hoshino M. Tissue distribution of mouse mammary tumor virus (MMTV) antigens and new endogenous MMTV loci in Japanese laboratory mouse strains. Jpn J Cancer Res. 1991;82:1413–1420. doi: 10.1111/j.1349-7006.1991.tb01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Wald A, Matson P, Ryncarz A, Corey L. Detection of herpes simplex virus DNA in semen of men with genital HSV-2 infection. Sex Transm Dis. 1999;26:1–3. doi: 10.1097/00007435-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 346.Wang S W, Teng C S. Induction of feline acquired immune deficiency syndrome by feline leukemia virus: alteration in response to hormones in the hypothalamic- pituitary-gonadal system. Proc Soc Exp Biol Med. 1995;208:404–412. doi: 10.3181/00379727-208-43869. [DOI] [PubMed] [Google Scholar]
- 347.Wejstal R. Sexual transmission of hepatitis C virus. J Hepatol. 1999;31(Suppl. 1):92–95. doi: 10.1016/s0168-8278(99)80382-1. [DOI] [PubMed] [Google Scholar]
- 348.Welch K, Finkbeiner W, Alpers C E, Blumenfeld W, Davis R L, Smuckler E A, Beckstead J H. Autopsy findings in the acquired immune deficiency syndrome. JAMA. 1984;252:1152–1159. [PubMed] [Google Scholar]
- 349.Weller T H, Hanshaw J B. Virologic and clinical observations on cytomegalic inclusion disease. N Engl J Med. 1962;266:1233–1244. doi: 10.1056/NEJM196206142662401. [DOI] [PubMed] [Google Scholar]
- 350.Wideroff L, Schottenfeld D, Carey T E, Beals T, Fu G, Sakr W, Sarkar F, Schork A, Grossman H B, Shaw M W. Human papillomavirus DNA in malignant and hyperplastic prostate tissue of black and white males. Prostate. 1996;28:117–123. doi: 10.1002/(SICI)1097-0045(199602)28:2<117::AID-PROS7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 351.Wilson W T, Frenkel E, Vuitch F, Sagalowsky A I. Testicular tumors in men with human immunodeficiency virus. J Urol. 1992;147:1038–1040. doi: 10.1016/s0022-5347(17)37458-x. [DOI] [PubMed] [Google Scholar]
- 352.Wolff H, Anderson D J. Potential human immunodeficiency virus-host cells in human semen. AIDS Res Hum Retroviruses. 1988;4:1–2. doi: 10.1089/aid.1988.4.1. [DOI] [PubMed] [Google Scholar]
- 353.Xu C, Politch J A, Tucker L, Mayer K H, Seage G R r, Anderson D J. Factors associated with increased levels of human immunodeficiency virus type 1 DNA in semen. J Infect Dis. 1997;176:941–947. doi: 10.1086/516539. [DOI] [PubMed] [Google Scholar]
- 354.Yoshikawa Y, Truong L D, Fraire A E, Kim H S. The spectrum of histopathology of the testis in acquired immunodeficiency syndrome. Mod Pathol. 1989;2:233–238. [PubMed] [Google Scholar]
- 355.Zagury D, Bernard J, Leibowitch J, Safai B, Groopman J E, Feldman M, Sarngadharan M G, Gallo R C. HTLV-III in cells cultured from semen of two patients with AIDS. Science. 1984;226:449–451. doi: 10.1126/science.6208607. [DOI] [PubMed] [Google Scholar]
- 356.Zagury D, Fouchard M, Cheynier R, Bernard J, Cattan A, Salahuddin S Z, Sarin P S. Evidence for HTLV-III in T-cells from semen of AIDS patients: expression in primary cell culture, long-term mitogen-stimulated cell cultures, and cocultures with a permissive T-cell line. Cancer Res. 1985;45:4595–4597. [PubMed] [Google Scholar]
- 357.Zeng Y, Gi Z W, Ito Y. Epstein-Barr virus activation by human semen principle: synergistic effect of culture fluids of bacteria isolated from patients with carcinoma of uterine cervix. Cancer Lett. 1985;28:311–315. doi: 10.1016/0304-3835(85)90040-0. [DOI] [PubMed] [Google Scholar]
- 358.Zhang H, Dornadula G, Beumont M, Livornese L, Van Uitert B, Henning K, Pomerantz R J. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med. 1998;339:1803–1809. doi: 10.1056/NEJM199812173392502. [DOI] [PubMed] [Google Scholar]
- 359.Zhao X P, Yang D L, Tang Z Y, Huang Y C, Hao L J, Cheng L, Zhou W. Infectivity and risk factors of hepatitis C virus transmission through sexual contact. J Tongji Med Univ. 1995;15:147–150. doi: 10.1007/BF02888223. [DOI] [PubMed] [Google Scholar]
- 360.Zhu T, Wang N, Carr A, Nam D S, Moor Jankowski R, Cooper D A, Ho D D. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J Virol. 1996;70:3098–3107. doi: 10.1128/jvi.70.5.3098-3107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]