Abstract
Hepatocellular carcinoma is the most prevalent form of primary liver cancer with a multifactorial aetiology comprising genetic, environmental, and behavioural factors. Evading cell death is a defining hallmark of hepatocellular carcinoma, underpinning tumour growth, progression, and therapy resistance. Ferroptosis is a form of nonapoptotic cell death driven by an array of cellular events, including intracellular iron overload, free radical production, lipid peroxidation and activation of various cell death effectors, ultimately leading to rupture of the plasma membrane. Although induction of ferroptosis is an emerging strategy to suppress hepatocellular carcinoma, malignant cells manage to develop adaptive mechanisms, conferring resistance to ferroptosis and ferroptosis-inducing drugs. Herein, we aim at elucidating molecular mechanisms and signalling pathways involved in ferroptosis and offer our opinions on druggable targets and new therapeutic strategy in an attempt to restrain the growth and progression of hepatocellular carcinoma through induction of ferroptotic cell death.
Subject terms: Hepatocellular carcinoma, Targeted therapies
Introduction
Hepatocellular carcinoma (HCC) is the primary form of liver malignancy with the highest prevalence in patients with chronic liver disease [1]. Liver cancer ranks the 3rd among all cancer mortalities globally, next to lung and colorectal cancers, and HCC constitutes 75% of liver cancers [2]. The 5-year survival rate for localised HCC is 32.6%, compared with 10.8% and 2.4% for regional and metastatic HCC, respectively, underscoring the necessity of novel HCC treatment options [3]. Chronic hepatitis “(see the Glossary)” resulting from persistent infections with hepatotropic viruses often ending in HCC [4]. Moreover, the incidence of HCC caused by metabolic stress is believed to rise dramatically in comparison with virus-induced HCC owing to worldwide pandemics of obesity, diabetes mellitus, and metabolic diseases [5, 6]. Indeed, non-alcoholic steatohepatitis (NASH) is now considered the main risk factor of HCC in developed countries [7]. In the United States, there are 34,800 HCC cases associated with NASH each year, and this number is projected to reach a toll of 44,800 annual cases by 2025 [3]. Considering the high prevalence of HCC worldwide, it is pertinent to elucidate HCC pathogenesis in sufficient detail to eventually identify useful therapeutic approaches [8]. The main goal of targeted therapy is to kill cancer cells without harming otherwise healthy cells. Selective induction of distinct cell death is a possible strategy to eliminate malignant hepatocytes [9, 10]. Cell death can be categorised as either accidental or regulated [11]. Accidental cell death displays no defined molecular mechanisms, regulators, or signals, whereas regulated cell death (RCD) is characterised by tightly regulated molecular and signalling machineries [12]. Reactive oxygen species (ROS) act as the prime drivers of RCD under impairmed cellular antioxidant systems [13]. Broadly speaking, ferroptosis refers to a form of ROS-mediated RCD, which is executed by dynamic signalling cascades (Figs. 1–3) [14]. Ferroptosis is instigated by intracellular iron accumulation and subsequent ROS generation, resulting in lipid peroxidation and membrane disruption, which ignites an array of downstream cell signal pathways and activates suicidal effector proteins (Figs. 1–3) [15]. A plethora of studies have demonstrated that ferroptosis abrogates the growth and proliferation of HCC cells in in vitro and in vivo xenograft models [16, 17]. However, HCC cells may develop adaptive/protective machineries to counteract ferroptosis, thus maintaining tumour growth. Specific molecules that determine the susceptibility to ferroptosis have been identified. Here, we aim at deciphering possible mechanisms underpinning ferroptotic cell death or ferroptosis resistance in HCC cells, as well as at highlighting targetable components and potential therapeutics in the clinical management of HCC.
Fig. 1. Iron metabolism, overload and ferroptosis.
The antiporter SLC7A11 governs GSH production, which is utilised by GPX4 to neutralise lipid peroxidation. Extracellular Fe3+ binds to transferrin, which in turn, binds to TFR1 for endocytosis. In endosomes, Fe3+ is converted into Fe2+ prior to release into the cytosol via SLC11A2, leading to Fe2+ overload, ROS production, lipid peroxidation, ultimately, ferroptosis. Lipid peroxidation also enjoys participation of lipid metabolism enzymes such as PLA2G2A, ALOX15, LPCAT3, and ACSL4. Intracellular Fe2+ is also exported through SLC40A1 or stored as Fe3+ within ferritin. Mitochondrial Fe2+ overload, a direct result of cytosolic Fe2+ overload, triggers ROS generation, thus, promoting lipid peroxidation and ferroptosis. Moreover, ferritinophagy is another process leading to Fe2+ overload. The transcriptional factor NRF2 upregulates many genes encoding proteins with a role in ROS and lipid peroxidation suppression. Under ER stress, activation of the ATF4 transcriptional factor upregulates HSPA5 chaperone, which suppresses lipid peroxidation, likely through promoting GSH folding in the ER.
Fig. 3. Signalling pathways and regulation of ferroptosis.
Cell signalling pathways play a role in the regulation of ferroptosis by modulating the activity and transcription of SLC7A11, labile iron pool, and lipid peroxidation. ER stress and activation of PERK signalling also affect GPX4 and, therefore, lipid peroxidation.
Iron metabolism and its linkage with molecular mechanisms of ferroptosis
Iron is a vital trace element for all living organisms and exists in two major forms in the human body, namely, ferrous iron (Fe2+, absorbable) and ferric iron (Fe3+, less soluble) [18, 19]. Iron metabolism is a complex process involving four main phases: absorption, distribution, storage, and excretion (Fig. 1) [20]. Both endogenous and exogenous (nutritional) sources supply the iron needed for maintaining homoeostasis [21]. Duodenal mucosal cells absorb nutritional iron to either release it into circulation or store as ferritin (Fig. 1) [20]. In conditions of iron deficiency, uptake of exogenous iron and utilisation of endogenous iron rises, while ferritin storage plummets. On the other hand, in conditions of iron sufficiency, intestinal iron absorption declines, while ferritin storage increases, in an effort to avoid excessive iron accumulation [22]. Transferrin is a plasma glycoprotein that binds and transports circulatory Fe3+ to various organs [23]. Serum transferrin-bound Fe3+ is discerned by TFR1 receptors (encoded by TFRC) on cell membranes, mediating endosomal delivery of Fe3+ into cytosols (Figs. 1 and 2) [24]. Subsequently, Fe3+ is reduced to Fe2+ by endosomal STEAP3 enzyme, prior to cytoplasmic release via SLC11A2 transporters (Fig. 1) [25]. Cytosolic Fe2+ supplies multiple biological functions, including DNA biosynthesis, oxygen transport, and regulation of metabolic pathways. Ferritin, a cytosolic protein that consists of two subunits (FTL and FTH1), is the primary protein for iron storage (in the form of Fe3+) [26]. FTH1 and FTL share high sequence homology, albeit they differ in iron utilisation and storage [26]. Importantly, mammalian cells employ a unique cellular mechanisms termed ferritinophagy, a selective form of autophagy that facilitates the degradation of ferritin, to liberate Fe2+ from its carrier (Figs. 1, 3 and 4) [27]. Excessive intracellular Fe2+ can be extruded by the carrier SLC40A1 towards extracellular space, where it is converted back into Fe3+, to balance redox state and iron homoeostasis (Figs. 1 and 2) [28, 29]. In theory, each step of this dynamic process might be therapeutically targeted for combating iron-related diseases, as well as excessive or deficient ferroptosis. Despite the availability of transferrin-bound iron, the majority of mammalian cells possess a feature of uptaking non-transferrin-bound iron (NTBI) [30]. Under conditions of iron overload (e.g., hemochromatosis) iron load may exceed the transferrin carrying capacity, thereby, floating in circulation as NTBI [31]. It is thought that cell surface ferrireductases such as CYBRD1 and cell-secreted reductants such as ascorbate mediate the uptake of NTBI [31]. Mechanistically, ferrireductases and ascorbate convert NTBI into Fe2+, which is subsequently imported into the cytosol via transporters including SLC39A14, SLC39A8 and SLC11A2 (Fig. 1) [32]. Ensuing erastin challenge (a ferroptosis inducer), expression of SLC11A2 and TFRC was upregulated in HT1080 cells [33], indicating contribution of NTBI and transferrin-bound iron in ferroptosis induction. Similarly, liver-specific SLC39A14 knockout inhibited ferroptosis in mouse livers [34], suggesting a role for NTBI in ferroptosis induction. Thus, inhibition of its transport may reverse ferroptosis. Despite obscurity in iron metabolic anomalies in the induction of ferroptosis, ample evidence has depicted compelling evidence of iron metabolic involvement in ferroptosis [35]. For instance, (i) inhibition and prevention of ferroptotic cell death with iron chelators both in vivo and in vitro [35], (ii) ferroptosis induction is accompanied by elevated intracellular labile iron [27] and (iii) iron supplementation enhanced ferroptosis induction upon treatment with erastin [36]. Hence, it is sensible to assume that regulators of iron storage, influx, efflux and utilisation might be involved in the regulation of ferroptosis. The scenario behind iron metabolic anomaly and linkage to ferroptosis begins with excessive extracellular or intracellular iron prompting iron overload, resulting in constitutive oxidative stress and organ damage [37, 38]. Iron overload triggers ferroptosis through two mechanisms, namely (i) excessive ROS generation culminating in oxidative damage of lipids and DNA, and (ii) activation of lipoxygenases and other enzymes containing non-haem iron, resulting in lipid peroxidation (Figs. 1–3) [39–42]. Two remarkable ROS species causing lipid peroxidation are the hydroperoxyl (OOH•) and hydroxyl (OH•) radicals, which are generated by the Fenton reaction resulting from the interaction between Fe2+ and H2O2 (Figs. 1 and 2) [43]. In this regard, impairment of iron efflux (e.g., upon SLC40A1 knockdown) and storage (e.g., upon induction of ferritinophagy or knockdown of either of the two ferritin subunits FTL or FTH1), as well as elevated iron intake (e.g., due to TFRC overexpression) would impose cells at a high risk of ferroptosis [29, 39–41]. Moreover, iron transportation across lysosomes, mitochondria, and cytosol, as well as activation of IREB2 (an RNA-binding protein regulating iron homoeostasis) may influence the organismal or cellular susceptibility to ferroptosis [44, 45]. In particular, iron overload triggers ferroptosis through excess ROS generation, lipid peroxidation, and many yet undefined signalling molecules and effectors.
Fig. 2. Ferroptosis mechanisms and regulations.
The anti-transporter systems of SLC7A11 and SLC3A2 are primarily responsible for cystine uptake in oxidative extracellular environment. With the reductive extracellular environment, SLC7A10 mediates direct uptake of cysteine into the cytosol, leading to activation of GPX4. Moreover, intracellular cysteine levels can be replenished by conversion of methionine amino acid into cysteine through transulfurylation mechanism. The FAT/FATP system mediates uptake of arachidonic acid and adrenoyltic acid, which are key substrates for the initiation of lipid peroxidation. Finally, various compounds can target GPX4, Fe2+ overload and SLC7A11-SLC3A2 system thereby, manipulating ferroptotic fate of the cell.
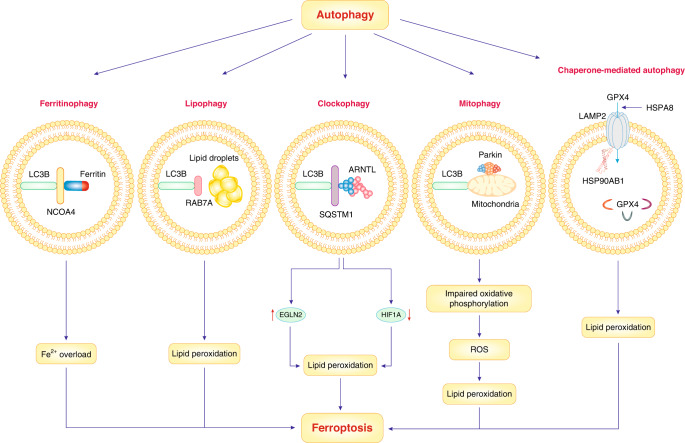
Fig. 4. Autophagy and ferroptosis.
Selective types of autophagy, including lipophagy, ferritinophagy, mitophagy, chaperone-mediated autophagy and clockophagy, can promote ferroptosis via increased lipid peroxidation, ROS generation and Fe2+ overload.
Upon lipid peroxidation, membrane polyunsaturated fatty acids (PUFAs) are oxidised, thus, dysfunction of cell antioxidant systems may accelerate lipid peroxidation. Therefore, the application of synthetic antioxidants including liproxstatin-1 and ferrostatin-1 has been shown to hinder lipid peroxidation [46, 47]. Lipid peroxidation of phosphatidyl PUFAs (PUFAs-PL) engages specific enzymes, such as ACSL4 and ALOXs (Figs. 1 and 2) [45, 48, 49]. ACSL4 catalysers the synthesis and enrichment of PUFAs, particularly, arachidonic acid in cell membranes, thereby, acting as a pro-ferroptotic factor [50]. With regards to the ACSL family, upregulation of ACSL4 is considered a vital biomarker and driver for ferroptotic death in cancer cells [50]. Moreover, upregulation of LPCAT3, an essential ACSL4 downstream signal molecule, may foster ferroptosis [51]. ACSL4 and LPCAT3 participate in incorporation of arachidonic acid into cell membranes, thereby, paving the way for ferroptosis induction [52, 53]. For oxidation of PUFAs, several models have been proposed. In the first model, membrane-bound NOXs enzymes catalyse PUFAs oxidation to produce superoxides, thereby, contributing to the build-up of lipid peroxides and ferroptosis induction [54]. In the second model, ALOXs enzymes directly catalyse PUFAs oxygenation to drive ferroptosis [55, 56]. Also, cytochrome P450 oxidoreductase plays a cardinal role in ferroptosis of cancer cells by providing ROS for phospholipid peroxidation [57]. Moreover, electron leakage from the mitochondrial electron chain can lead to ROS generation and lipid peroxidation [58]. As described above, ACSL4 participates in PUFAs-PL production, while ALOXs mediate PUFAs-PL oxidisation into hydroperoxy products. The ALOX family includes ALOX15B, ALOX15, ALOX12B, ALOX12, ALOX5 and ALOXE3, all of which participating in ferroptosis in a cell type-or tissue-specific manner [59, 60]. Alternatively, cytochrome P450 oxidoreductase may mediate lipid peroxidation in ferroptosis in an ALOX-independent manner [61], whereas lipophagy-mediated degradation of lipid droplets provides fatty acids for subsequent lipid peroxidation in HCC cells during ferroptosis [61]. In response to pre-ferroptotic stress, mammalian cells have adapted antioxidant systems, such as the antiporter system xc−, which exports glutamate to import cysteine into the cell, thus facilitating the synthesis of glutathione (GSH), a major scavenger of intracellular free radicals (Figs. 1 and 2) [62]. Mechanistically, specific ferroptosis inducers, such as sulfasalazine and erastin ignite ferroptosis via blockade of system xc− [36, 63]. Of note, sorafenib, an approved drug for HCC treatment, has been repeatedly evinced to ignite ferroptosis through the blockade of system xc− [36, 63]. However, controversy exists as sorafenib was reported to be incapable of igniting ferroptosis like erastin and sulfasalazine in a number of tumour cell lines, indicating that sorafenib may not be a bona fide inducer of ferroptosis [64].
System xc− is a transmembrane protein complex containing light chain SLC7A11 and heavy chain SLC3A2 (Fig. 1). SLC7A11 (a.k.a., xCT) is a transmembrane protein with a twelve-pass that is attached to the regulatory single-pass SLC3A2 protein (a.k.a., CD98hc and 4F2hc) via a disulfide bond [65]. It has been evinced that upregulation of SLC7A11-mediated cystine influx can replace the glutathione peroxidase 4 (GPX4)-GSH antioxidant system (described later in the text) in certain malignant cells both in vivo and in vitro [66, 67]. Besides, upregulation of TP53 has been shown to nullify SLC7A11 expression, thereby, predisposing to ferroptosis [68]. System xc− mediates cystine influx into the cytosol, where it is chemically reduced to cysteine for GSH synthesis (a low-molecular-weight antioxidant). GPX4 contains selenocysteine and employs GSH as a cofactor, thereby, mediating reduction of lipid peroxides into non-toxic alcohols. In other words, GPX4 reduces lipid-OOH (hydroperoxide) into lipid–OH (alcohol) to retard the production of lipid-O• (alkoxy radicals) from lipid-OOH [69]. Importantly, cells exposed to lipid-generated ROS are under the danger of destruction, as suppressed activity or genetic ablation of GPX4 was reported to induce massive accumulation of lipid-generated ROS and cell demise in in vitro and mouse embryo, respectively [70, 71]. RSL3 is a GPX4 inhibitor, and an activator of ferroptosis. Hence, GPX4 overexpression reversed RSL3-mediated ferroptotic cell death, while GPX4 knockdown using short hairpin RNA triggered ferroptotic cell death in malignant HRAS cells [71]. Also, iron chelators and ferrostatin-1 (a lipophilic antioxidant) prevented ferroptotic cell death induced by GPX4 deletion in murine cells [70, 72]. In addition, both GPX4 and GPX7 were remarkably expressed in HCC tissues and this overexpression was more accentuated in grade III HCC, suggesting that GPX4 and GPX7 overexpression play a key role in ferroptosis resistance in advanced stages of HCC [73]. Collectively, these data indicate that GPX4 is cardinal for prevention of ferroptosis. In other words, GPX4 is an important antioxidant component acting downstream of system xc− to remove toxic phospholipid hydroperoxide from membranes [71]. Regarding the precise role of lipid peroxidation in ferroptosis, a decline in the GPX4-GSH antioxidant system provokes ferroptosis [69]. Hence, inhibition of GPX4 by small-molecule compounds such as RSL3, FIN56 or ML210, and pharmacological inhibition of system xc− using erastin stimulates ferroptosis (Figs. 1 and 2) [71]. In addition, AIFM2, GCH1 and DHODH-ignited intracellular antioxidant pathways play an alternative or synergistic role in combating ferroptotic injury [74]. AIFM2 (a.k.a., FSP1) is an endogenous suppressor of ferroptosis that functions independent of GPX4-GSH. Mechanistically, AIFM2 myristoylation fosters its recruitment to the plasma membrane. Subsequently, AIFM2, as an oxidoreductase, reduces coenzyme Q10 into ubiquinol functioning as an antioxidant to trap lipid radicals and avert dispersion of lipid peroxides [75]. Alternatively, our group revealed that AIFM2 mediated plasma membrane recruitment of ESCRT III complex conferring resistance against ferroptosis through regulation of membrane fission and budding and ultimate repairment of the damaged membrane independent of ubiquinol (Fig. 1) [76]. Collectively, these findings indicate that several membrane-repairing systems and antioxidants co-exist to restrain ferroptosis.
Downstream effectors of ferroptosis in HCC: molecular mechanisms, signalling pathways and therapeutic targets
NRF2
NRF2, a transcription factor encoded by the NFE2L2 gene, is tightly regulated by E3-ubiquitin ligase systems including Keap1-CUL3-RBX1, bTrCP and synoviolin [77–79]. As a result of endogenous/exogenous stress or interaction with binding proteins, NRF2 translocates to the nucleus, where it transactivates target genes containing antioxidant response element (ARE) sites [80]. Intriguingly, most proteins involved in the detoxification of lipid peroxidation properties are encoded by NRF2 target genes (Figs. 1, 3 and 5) [81]. NRF2-transactivated genes are also heavily involved in the regulation of iron/haem metabolism [82]. For example, FTL and FTH1, as well as SLC40A1, are upregulated by NRF2 (Figs. 1, 3 and 5) [83, 84]. Moreover, HMOX1, FECH, ABCB6 (critical players in haem biosynthesis), and SLC48A1 (a transporter of haem) are all transactivated by NRF2 (Fig. 5) [85–87]. Therefore, NRF2 exerts vital roles in iron/haem homoeostasis, and in the alleviation of lipid peroxidation and ferroptosis. Our group found that NRF2 confers protection against ferroptosis in HCC cells [88]. In particular, exposure of HCC cells to ferroptosis inducers (e.g., buthionine sulfoximine, sorafenib, and erastin) upregulated p62, which inactivated Keap1, and averted NRF2 degradation, leading to its nuclear translocation (Fig. 3) [88]. Subsequently, nuclear NRF2 interacted with small MAF transcriptional coactivator proteins (such as MafG), resulting in the upregulation of NQO1, HMOX1, FTH1 or MT1G and suppressed ferroptosis [88, 89]. Thus, knockdown of SQSTM1 (encoding p62), NQO1, HMOX1, FTH1 or MT1G sensitised HCC cells to ferroptosis upon exposure to sorafenib and erastin [88, 89]. Moreover, knockdown of NFE2L2 or pharmacological inhibition of NRF2 intensified the anticancer activity of sorafenib and erastin in cell cultures and murine tumour xenograft models [88]. Thus HCC cells hijack the p62-Keap1-NRF2 signalling to deter ferroptosis through upregulation of antioxidant or detoxification genes. Therefore, inhibitory targeting of the p62-Keap1-NRF2 axis by genetic or pharmacological inhibition along with sorafenib or erastin could be a potential therapeutic strategy to trigger the ferroptotic elimination of HCC cells. It is believed that certain HCC cell lines exhibit resistance to sorafenib-induced ferroptosis [90]. GSTZ1, an enzyme involved in phenylalanine/tyrosine catabolism, is dysregulated in various malignancies [91]. Examination of a possible role for GSTZ1 in sorafenib-evoked ferroptosis revealed that sorafenib-resistant HCC cells substantially downregulate the GSTZ1 gene, leading to upregulation of NFE2L2 and GPX4, thereby preventing ferroptosis [92]. Furthermore, inhibition of GPX4 by RSL3 fostered sorafenib-induced ferroptosis in Gstz1−/− mice, hinting to the feasibility of combination therapy with sorafenib and RSL3 against sorafenib-resistant cells [92]. Moreover, forced upregulation of GSTZ1 gene provokes ferroptosis by disrupting NRF2-GPX4 signalling. Hence, genetic modulation or pharmacological manoeuvres affecting the GSTZ1-NRF2-GPX4 axis could be a potential therapeutic avenue in HCC therapy. In addition, ABCC5, a member of ATP-binding cassette (ABC) proteins, belongs to the family of ATP-dependent transporters [93]. The main biological function of ABCC5 is to extrude xenobiotics and certain endogenous metabolites such as folic acid and cyclic GMP/AMP from cells [94]. Of note, ABCC5 is responsible for sorafenib resistance and the prevention of ferroptosis in HCC. In line with this notion, Huang et al. revealed markedly elevated ABCC5 levels in HCC cells exhibiting sorafenib resistance [95]. Activation of the PI3K-AKT1-NRF2 signalling cascade was suggested to facilitate ABCC5 upregulation [95]. Moreover, ABCC5 upregulation promoted SLC7A11 expression, downgraded lipid peroxidation and boosted intracellular GSH levels, resulting in suppressed ferroptosis [95]. Of note, inhibition or downregulation of ABCC5 promoted ferroptosis in vitro and in vivo [95]. These findings denote an essential role for the PI3K-AKT1-NRF2-ABCC5-SLC7A11 signalling cascade in the ferroptosis resistance of HCC. Therefore, pharmacological or genetic manipulation of NRF2, ABCC5 or SLC7A11 could be attempted to kill HCC cells by ferroptosis. Furthermore, QSOX1 perturbs redox homoeostasis through suppression of NRF2/NFE2L2 as this has been noted in human HCC [96]. QSOX1 is an enzyme catalysing thiol oxidisation during protein folding, thus reducing oxygen to hydrogen peroxide [97]. From a mechanistic point of view, QSOX1 mediates ubiquitin-dependent degradation of EGFR and enhances its endosomal trafficking in the cytosol, resulting in the suppression of NFE2L2 [96]. Due to NRF2 inhibition, QSOX1 expression facilitates sorafenib-triggered ferroptosis both in vitro and in vivo [96]. Hence, activation/upregulation of QSOX1 (genetically or pharmacologically) along with sorafenib administration may induce ferroptosis in HCC and other EGFR-dependent tumours. Besides, radiation therapy is the gold standard treatment in patients with unresectable HCC [98]. Nonetheless, this remedy might be accompanied by radiation resistance in certain individuals. In an attempt to reverse radiation resistance, Yuan and colleagues explored the potential of collectrin (CLTRN) to promote the radiosensitivity of HCC [99]. Based on the key role of CLTRN in amino acid transportation through the regulation of membrane distribution and solute carrier (SLC) transporters [100], these authors postulated a relationship between CLTRN and glutathione metabolism and ferroptosis [99]. Indeed, NRF2-RAN-DLD signalling upregulated CLTRN mRNA and modulated glutathione metabolism in a ferroptosis-dependent manner, thereby, enhancing the radiosensitivity of HCC cells both in vitro and in vivo [100]. Thus, NRF2-mediated upregulation of CLTRN may predispose to ferroptosis, a notion that collides with the notion for an anti-ferroptotic role of NRF2. Nevertheless, targeting CLTRN/collectrin along with radiotherapy may be an effective combination therapy in combat with HCC. Overall, these findings support a central role for NRF2 in the transcriptional regulation of anti-ferroptosis genes in HCC cells. However, the exact molecular mechanisms through which the NRF2 target genes regulate ferroptotic and non-ferroptotic cell death domains remain unclear.
Fig. 5. NRF2-keap1 pathway dictates degradation or nuclear translocation of NRF2.
Under normal conditions, keap1 in association with CUL3 induce proteasomal degradation of NRF2. However, under stress conditions, keap1 cysteine residues are altered, leading to NRF2 dissociation from Keap1. As a result, NRF2 translocates to the nucleus and transactivates genes involved in the suppression of lipid peroxidation and ferroptosis.
YAP1/TAZ and METTL14
Gao et al. evaluated sorafenib resistance in HCC cell lines and xenograft models and found that YAP1 and TAZ transcriptional factors drastically promoted sorafenib resistance via suppression of ferroptosis [101]. Mechanistically, YAP1/TAZ transcriptional factors upregulated SLC7A11 in a manner contingent upon TEAD1 activation [101]. SLC7A11, a crucial amino acid transporter (Fig. 1), favours intracellular glutathione production, and thereby, elicits resistance to sorafenib-triggered ferroptosis [101]. Moreover, YAP1/TAZ transcriptional factors promoted nuclear localisation and activation of ATF4, which in turn, transactivated SLC7A11, conferring resistance to ferroptosis [101]. This finding denotes a role for YAP1/TAZ transcriptional factors in repressed ferroptosis in HCC cells, mainly through activation of the TEAD1-SLC7A11 and the ATF4-SLC7A11 networks. Therefore, the YAP1/TAZ transcriptional factors, as well as the TEAD1-SLC7A11/ATF4-SLC7A11 axes, may serve as targets to reverse sorafenib resistance, thus favoring ferroptotic cell death in HCC cells. To corroborate the role of SLC7A11 in ferroptosis inhibition and resistance, Fan et al. evaluated METTL14 levels in HCC cells under hypoxia, revealing that hypoxia suppressed METTL14 through HIF-1α activation, which then resulted in ferroptosis inhibition through upregulation of SLC7A11 mRNA [102]. These investigators revealed that METTL14 induced N6-methyladenosine (refers to the methylation of adenosine at the N6-position on eukaryotic mRNA) on SLC7A11 mRNA at 5’ UTR (untranslated region), predisposing it to degradation dependent on the YTHDF2 signal in vitro and in vivo [102, 103]. These findings suggest that hypoxia blocks ferroptosis in HCC cells through activation of the HIF-1α-METTL14-YTHDF2-SLC7A11 signalling axis. Therapeutic targeting of this pathway may elicit ferroptotic cell death of HCC cells, contrasting with the finding that HIF-1α plays an active role in promoting ferroptosis in renal cancers [104, 105]. More studies are needed to determine the cell-specific role of hypoxia signalling in shaping ferroptosis sensitivity.
Circular and long non-coding RNAs
Single-stranded RNAs form a covalently bound loop, commonly referred to as circular RNAs (circRNAs) [106]. Such non-coding RNAs participate in tumorigenesis and may also affect ferroptosis [106]. Circular-interleukin-4 receptor (circIL4R) is highly expressed in HCC cells [107]. Xu and colleagues studied the role of circIL4R in HCC progression and ferroptosis and revealed that circIL4R conferred protection against ferroptosis [108]. Knockdown of circIL4R elicited ferroptosis and thereby impeded oncogenesis [108]. Mechanistically, circIL4R directly suppressed miR-541-3p, leading to increased expression of GPX4 mRNA (a direct target of miR-541-3p) and disabled ferroptosis [108]. These data insinuate an oncogenic role for circIL4R that is largely imputed to ferroptosis inhibition through activation of circIL4R-miR-541-3p-GPX4 axis in HCC. Hence, silencing circIL4R or pharmacological blockade of the circIL4R-miR-541-3p-GPX4 axis could facilitate ferroptosis in HCC. In a recent study, Lyu and coworkers reported that circ0097009 was remarkably increased, leading to enhanced invasion and proliferation of HCC cells in murine xenograft models. However, these effects were reversed by circ0097009 knockdown [109]. Further analysis disclosed that SLC7A11 and circ0097009 directly and concurrently bound to microRNA (miR)-1261 [109]. Mechanistically, circ0097009 directly binds and blocks miR-1261, thereby inducing SLC7A11 upregulation and ferroptosis inhibition, leading to the proliferation and invasion of HCC cells [109]. These data suggest that HCC cells activate the circ0097009-miR-1261-SLC7A11 axis to revert ferroptosis, hence revealing yet another potential target for expediting ferroptosis in HCC cells. Moreover, examining causal correlations among circ0013731, miR-877-3p, and SLC7A11 showed that circ0013731 was significantly upregulated in an E2F1-dependent fashion [110]. circ0013731 boosted cell growth due to suppressed ferroptosis in HCC cells (QGY-7703, SMMC-7721) and xenograft models [110]. Mechanistically, circ0013731 upregulates SLC7A11 by direct binding to and sponging miR-877-3p [110]. These findings suggest that HCC cells adapt the E2F1-circ0013731-miR-877-3p-SLC7A11 axis to prevent ferroptosis. Qi et al. reported that erastin induced upregulation of the long non-coding RNA (lncRNA) GABPB1-AS1, which favours ferroptosis induction [111]. Mechanistically, GABPB1-AS1-induced downregulation of GABPB1 resulted in PRDX5 downregulation, thus, reducing the cellular antioxidant potential and inducing ferroptosis in HepG2 cells [111]. Hence, bolstering the GABPB1-AS1-GABPB1-PRDX5 axis by genetic or pharmacological means might be a potential approach to sensitise HCC to ferroptosis. Taken together, current research might inspire RNA-based ferroptosis therapeutics in HCC pending additional mechanistic and translational exploration.
SPARC, ATP-binding cassette and PERK
SPARC participates in interactions and communications between cells and extracellular matrix (ECM), to govern cellular functions, including differentiation, proliferation and adhesion [112]. Nonetheless, its role in HCC progression and sorafenib sensitivity remains largely elusive. Wei et al. reported that SPARC enhanced sorafenib cytotoxicity in HCC cell lines (e.g., HepG2 and Hep3B) [113]. Thus, abrogation of SPARC expression diminished sorafenib-mediated killing of these cell lines [113]. Conversely, SPARC overexpression promoted ferroptosis through excessive oxidative stress, thus, enhancing sorafenib cytotoxicity [113]. These findings indicate that SPARC is a potential target to further sensitise HCC cells to sorafenib-induced ferroptosis. ATP-binding cassette (ABC) transporters belong to a family of membrane proteins, the function of which remains largely elusive in HCC [114]. Bioinformatic analyses by Zhang et al. uncovered that ABCA8 and ABCA9 are markedly downregulated, while ABCA6 is upregulated in HCC cells [114]. Further analysis showed that ABCB6 participates in the regulation of ferroptosis [114]. This finding spurs further research to explore the mechanisms underlying ABCB6-regulated ferroptosis and its role in oncogenesis. In particular, the identification of ABC substrates may provide a new framework for understanding the metabolic basis of ferroptosis. PERK is an endoplasmic reticulum (ER) stress sensor and ER transmembrane protein [115]. Of note, in HCC cells irradiation triggered ER stress with consequent PERK signalling, which in turn, induced expression of ATF4 and DRAM1. Moreover, PERK-induced expression of p53 stimulated apoptosis and ferroptosis via downregulation of SLC7A11, hence sensitising HepG2 cells to irradiation [116]. The combination of carbon ion irradiation with sorafenib triggered a mixed type of apoptotic and ferroptotic cell death [116]. Thus, induction of ER stress, in particular activation of the PERK-p53 pathway, may promote ferroptosis in HCC cells.
S1R, ceruloplasmin and retinoblastoma
The ligand-operated sigma-1 receptor (S1R) is a multi-functional protein residing in the endoplasmic reticulum (ER) membranes [117]. S1R is assumed to regulate oxidative stress in an NRF2-dependent manner [118], and thereby governs the regulation of ferroptosis. Bai and coworkers reported that sorafenib triggered nucleus translocation of S1Rs, while inhibition of ferroptosis with ferrostatin-1 reversed this translocation in HCC cells, suggesting a role for enhanced S1R levels in ferroptosis inhibition [119]. Abrogation of S1R promoted sorafenib-induced ferroptosis due to repression of GPX4, the elevation of lipid peroxidation, and iron metabolism in HCC in vitro and in vivo [119]. Interestingly, NRF2 inhibition promoted S1R expression and conferred protection against ferroptosis [119]. Overall, these findings suggest that in response to sorafenib, HCC cells upregulate S1R to resist ferroptosis and maintain survival. Meanwhile, inhibition of NRF2, which predisposes to ferroptosis, leads to S1R upregulation and subsequent inhibition of ferroptosis, suggesting that HCC cells would employ this “smart system” (NRF2-S1R) to resist ferroptosis under conditions of NRF2 inhibition. A more profound exploration of the role of S1R in ferroptosis is warranted. On the other hand, the glycoprotein ceruloplasmin plays a cardinal role in iron homoeostasis [120]. In HCC cells, ceruloplasmin blocks ferroptosis by modulating iron homoeostasis [121]. Ceruloplasmin depletion accelerates ferroptosis by increasing intracellular accumulation of Fe2+ and lipid-associated ROS in HCC cells under treatment with RSL3 or erastin [121]. Conversely, overexpression of CP prevents ferroptosis [121]. These data indicate that upregulation of CP/ceruloplasmin fends off ferroptosis via reduction of intracellular Fe2+, [121]. Since ceruloplasmin plays an important role in storing and carrying copper, it remains to be seen whether copper affects ferroptosis resistance as well. Besides, retinoblastoma denotes a tumour suppressor protein, which is inactivated in several malignancies [122]. Retinoblastoma also plays a crucial role in liver carcinogenesis, and depletion of retinoblastoma protein promotes sorafenib-induced ferroptosis in human HCC cells both in vitro and in xenograft settings [123]. Hence, as a potential combination therapy, suppression of retinoblastoma along with sorafenib administration might abrogate HCC progression.
G6PD, CISD2 and CISD1, and NUPR1
G6PD is an enzyme in the pentose phosphate pathway, a sequence of biochemical reactions that convert glucose into ribose-5-phosphate (Fig. 5) [124]. G6PD is often upregulated in HCC. Cao et al. reported that G6PD buttressed invasion, migration, and proliferation of HCC in vivo by inhibiting ferroptosis through downregulation of POR, a type of cytochrome P450 oxidoreductase [125]. Indeed, G6PD knockdown resulted in the abatement of tumour weight and volume [125]. Hence, the G6PD-POR axis might constitute yet another potential manoeuverable target to ignite ferroptosis in HCC, pending confirmation and further mechanistic exploration of this pathway. In addition, the protein CISD2 is localised at the ER and mitochondrial outer membrane (MOM) [126]. CISD2 serves as a key regulator for cellular processes, including intracellular Ca2+ homoeostasis, mitochondrial function, and ER integrity (Fig. 3) [126]. It has been evinced that CISD2 is associated with HCC progression. Li et al. demonstrated that CISD2 was overly upregulated in HCC cells, contributing to disease progression and sorafenib resistance [127]. Thus, CISD2 knockdown reversed sorafenib resistance, reduced viability, and provoked ferroptosis in HCC cells [127]. Mechanistically, CISD2 knockdown triggered excessive Beclin1-dependent autophagy and ferritinophagy, which spurred ferroptosis in sorafenib-resistant HCC cells [127]. Moreover, genetic inhibition of CISD1 favoured iron-mediated intramitochondrial lipid peroxidation, contributing to erastin-induced ferroptosis in human HCC cells [128]. Hence both CISD1 and CISD2 may affect ferroptosis sensitivity in HCC cells. Besides, NUPR1 is a small chromatin-binding protein that activates and regulates gene expression in response to various types of cellular stress. NUPR1 was originally identified as a ferroptosis suppressor in pancreatic cancer cells, promoting iron export by activating LCN2 expression [129]. Subsequent studies confirmed a role for NUPR1 in suppressing ferroptosis by reducing mitochondrial oxidative damage in HCC cells [130]. Consequently, the NUPR1 inhibitor ZZW-115 can increase the sensitivity of pancreatic cancer cells and HCC cells to ferroptosis [129, 130]. These findings establish a nuclear factor pathway to limit oxidative damage during ferroptosis.
Other effectors
Examination of miRNA-related epigenetic modifications and their clinical relevance revealed that miRNA (miR)-23a-3p is predominantly overexpressed in HCC, in association with sorafenib resistance, HCC relapse, poor survival, as well as suppression of ferroptosis, lipid peroxidation, and iron accumulation [131]. Mechanistically, activation of ETS1 contributed to miR-23a-3p upregulation, which in turn, targeted 3′-UTR of ACSL4 mRNA, resulting in ferroptosis suppression [131]. Notably, CRISPR/Cas9-mediated miR-23a-3p knockout boosted the sorafenib response in HCC cells and orthotopic HCC xenografts [131]. Ferroptosis is linked to selective autophagy categories including chaperone-mediated autophagy, clockophagy, lipophagy, and ferritinophagy (Fig. 6). However, selective autophagy of ER fragments, which is referred to as reticulophagy or ER-phagy [132], is less explored regarding its possible role in ferroptosis. In a recent study, Liu and coworkers described that sorafenib induced FAM134B-dependent reticulophagy, conferring resistance to ferroptosis in HCC in vitro and in vivo [133]. PABPC1 was found to interact with FAM134B mRNA, thereby upregulating its translation [133]. FAM134B knockdown suppressed reticulophagy and overtly promoted ferroptosis sensitivity [133]. These data suggest that activation of PABPC1-FAM134B-reticulophagy in response to sorafenib treatment may serve as an adaptive mechanism of HCC cells to prevent ferroptosis. Therefore, targeting this pathway might reverse sorafenib resistance. Also, HCC radioresistance is coupled to reduced COMMD10 expression post-irradiation, leading to enhanced intracellular copper levels, thus suppressing ferroptosis [134]. Mechanistically, cytosolic copper accumulation rescued HIF-1-α from ubiquitin degradation, favouring its translocation to the nucleus and transactivation of CP (encoding ceruloplasmin) and SLC7A11, resulting in ferroptosis suppression. Conversely, COMMD10 overexpression triggered radiosensitivity via induction of ferroptosis both in vitro and in vivo [134]. Moreover, a recent CRISPR/Cas9 screening uncovered a role for PSTK in reducing the therapeutic sensitivity of HCC cells [135]. PSTK suppressed sorafenib-induced ferroptosis, while its depletion inactivated GPX4 and GSH metabolism as well as the synthesis of cysteine and selenocysteine, hence sensitising cells to sorafenib in vitro and in vivo [135]. Similarly, the PSTK inhibitor punicalin reverted sorafenib resistance of HCC [135], supporting future translational studies of PSTK as a potential target for ferroptosis sensitisation.
Fig. 6. Potential compounds targeting NRF2 at different stages of HCC progression.
Given the role of NRF2 in preventing HCC cells from ferroptotic cell death, potential natural/pharmacological therapeutics inhibiting transcription, translation, nuclear translocation, and DNA binding of NRF2, or promoting its degradation could be promising tools in the neutralisation of NRF2 oncogenic activities.
Potential ferroptosis inducers in HCC therapy
As indicated by the aforementioned studies, ferroptosis induction suppresses the growth and progression of HCC and promotes chemotherapeutic sensitisation. Hence, small molecules that upregulate ferroptosis might be useful for HCC therapy. Here, we provide a short list of natural and synthetic agents that might be considered as lead compounds for the future development of ferroptosis inducers. Artesunate, which is primarily employed for malaria treatment, is clinically well-tolerated. [136]. Li et al. reported that artesunate synergistically boosted the anticancer effects of sorafenib through enhancement of lipid peroxidation and ferroptosis in HCC cells (SNU-182, SNU-449, Huh7) and murine xenograft models (Balb/c nude) [137]. Moreover, the combination treatment of artesunate and sorafenib elevated oxidative stress and ferritinophagy, thereby, upregulating ferroptosis in Huh7 cells [137]. In this system, artesunate induced activation of lysosomal cathepsin B/L, lipid peroxidation and ferritinophagy [137]. Pending additional preclinical confirmation, these findings suggest that artesunate could be utilised in combination with sorafenib. Also, the anticancer agent atractylodin [138] inhibits invasion, migration, and proliferation of HCC cells, including Hccm and Huh7 [139]. Moreover, atractylodin elevated ROS levels, downregulated GPX4 and FTL, and upregulated TFRC and ACSL4, thus contributing to ferroptosis [139]. Formosanin C is a natural saponin used in Chinese herbal medicine to alleviate inflammation [140]. As described earlier, a rise in iron pool in response to ferritinophagy promotes ferroptosis (Figs. 1 and 4) [44]. Su and colleagues found that formosanin C inhibited HCC cell growth (Hep3B, HepG2) and that administration of the ferroptosis inhibitor ferrostatin-1 reversed this effect [141]. Mechanistically, formosanin C induced NCOA4-dependent ferritinophagy, leading to Fe2+ overload and ferroptosis [141]. Moreover, formosanin C triggered ferroptosis and autophagic flux in HepG2 cells while enhancing NCOA4 and reducing FTH1 mRNA levels [142]. Moreover, formosanin C-induced ferritinophagy triggers ferroptosis in HA59T and HA22T cells [142], confirming its potential therapeutic utility. In addition, haloperidol, a drug used for the treatment of schizophrenia [143], may substantially potentiate sorafenib- or erastin-induced ferroptosis in HCC [144]. At a relatively low dose, haloperidol effectively triggered intracellular Fe2+ overload and lipid peroxidation [144]. Therefore, haloperidol is a potential ferroptosis inducer that should be further investigated at the preclinical level. Heteronemin, derived from the marine sponge Hippospongia sp., has anticancer properties against myeloid leukaemia, prostate cancer, and cholangiocarcinoma [145]. Heteronemin also blocks the proliferation of HA59T and HA22T cell lines through initiation of caspase-dependent apoptosis [16]. Moreover, heteronemin induces ROS production and GPX4 downregulation, resulting in ferroptotic cell death [16]. Hence, ferroptosis inhibition attenuated heteronemin-mediated cell death [16]. Table 1 lists newly identified ferroptosis inducers in the HCC therapy.
Table 1.
Recently identified ferroptosis inducers for HCC therapy.
| Compounds | Mechanisms of action | Refs |
|---|---|---|
| DSF (disulfiram) | Remarkably inhibits angiogenesis, invasion, and migration, induces mitochondrial impairment, lipid peroxidation, and intracellular iron overload, resulting in ferroptotic cell death | [146] |
| Heteronemin (natural marine Terpenoid derived from Hippospongia sp) | Inhibits HCC cell lines (HA22T and HA59T) proliferation by inducing apoptosis and ferroptosis via inhibition of GPX4 and ERK expression | [16] |
| Necrostatin-1 (TNF-α inhibitor) | Blocks ferroptosis due to its antioxidant capacity independent of RIPK1 inhibition in HCC cells (SK-HEP-1 and Huh7) | [157] |
| Solasonine (a glycoalkaloid) | Remarkably suppresses proliferation, invasion, and migration in HepRG and HepG2 cells and in mouse xenograft models via ferroptosis and GPX4 suppression | [158] |
| Triapine (lactose-decorated nanomicelles loaded with triapine and photosensitizer Ce6) | Activates fenton reaction, ROS generation, and ferroptosis both in vitro and in HCC orthotopic mice | [159] |
Targeting NRF2 in HCC therapy
Ample evidence has indicated that NRF2 inhibition remarkably boosts anticancer effects of ferroptosis inducers in HCC and other cancers. For instance, trigonelline, a natural alkaloid abundantly found in plants (e.g., oats, hemp seed, garden peas and coffee beans), promotes ferroptosis under sorafenib treatment due to NRF2 inhibition in HCC cells and xenograft models [88]. Although trigonelline cannot be considered as a specific NRF2 inhibitor, targeting NRF2 seems to be promising for ferroptotic elimination of HCC cells, and more specific NRF2 inhibitors should be developed. Disulfiram, which is in clinical use for alcoholism therapy, remarkably inhibited angiogenesis, invasion and migration in HCC cell lines, as it induced mitochondrial impairment, lipid peroxidation, and intracellular iron overload, resulting in ferroptotic cell death [146]. The elevation of NRF2 expression induced resistance to disulfiram, while NRF2 inhibition by RNA interference remarkably accelerated lipid peroxidation and facilitated disulfiram-triggered ferroptosis [146], suggesting the benefit of NRF2 inhibition. The use of high-throughput screening (HTS) in association with cell-based assays has made it possible to discover potential anticancer drugs and to identify new applications for already FDA-approved drugs [147]. Using this technique, AEM1 has been revealed as a small molecule that interferes with transcriptional activity of NRF2, resulting in growth suppression and enhanced chemosensitivity of A549 cancer cells both in vitro and in vivo [148]. Moreover, Singh et al. screened 400,000 small molecules and found that the compound ML385 targets NRF2 with high selectivity and specificity. Indeed, combination of ML385 with carboplatin exhibited remarkable anticancer efficacy in preclinical models of NRF2-addicted non-small cell lung cancer (NSLC) [149]. Recently, Matthews et al. screened several RNA interference and chemical libraries to identify actin disturbing agents, STAT3 inhibitors, and cardiac glycosides as NRF2 inhbitors that and synergised with anticancer drugs in killing A549 NSLC cells [150]. These findings broaden the list of potential compounds with NRF2-inhibitory effects, and pave the way for the characterisation and identification of new NRF2 inhibitors with more specificity and sensitivity. Figure 6 briefly summarises potential natural and synthetic therapeutics targeting NRF2 in HCC. Box 1 also represents the utility of nanotechnology in ferroptosis induction.
Box 1 Nanotechnology for ferroptosis induction in HCC therapy.
Owing to the peculiar physicochemical characteristics of nanomaterials, nanotechnology might open the avenue to future antineoplastic approaches. Although research on nanotechnology-based cancer treatment constitutes an expanding area, so far no clinical translation of these approaches are at sight [160]. Given that some characteristics (such as pH, oxygen tension and blood flow) of the tumour microenvironment (TME) are distinct from those of healthy tissues, nanomaterials can be designed to specifically reach tumour cells without affecting normal cells [161–163]. In recent years, nanomedicine has participated to the development of ferroptosis inducers [164, 165]. The construction of nano ferroptosis inducers constitutes conjugation or incorporation of small ferroptosis inducers on or into nanocarriers that are supposed to boost the biocompatibility, solubility, and tumour localisation of the cytotoxic drugs [166]. Alternatively, nanomaterials can be developed with the scope to generate hybrid molecules that combine ferroptosis induction with malignant cell targeting [166, 167]. For the treatment of HCC, Sorafenib is the only FDA-approved chemotherapeutic and ferroptosis inducer. Nonetheless, sorafenib administration is associated with many pitfalls, including toxic side effects, poor bioavailability and water solubility [168]. In a recent study, Yue and colleagues incorporated sorafenib into pH-sensitive HMPB (hollow mesoporous Prussian blue) nanoparticles, thereby generating a sorafenib nanocarrier with facilitated drug release [169]. They also conjugated pH-responsive chitosan to the HMPB surface to boost the biocompatibility of sorafenib [169]. The resulting tripartite HMPB-sorafenib-chitosan nanocomposite exhibited enhanced specificity for HCC cells and less toxicity to normal tissues due to improved permeability and retention in vitro and in vivo [169]. Mechanistically, nanocomposite ignited excessive ROS generation and ferroptosis, resulting in HCC shrinkage in mice [169]. In another study, MMSNs (manganese-silica nanoparticles) induced ferroptosis after uptake of the particles into HCC cells and their degradation, which resulted in rapid scavenging of intracellular GSH [170]. Moreover, loading MMSNs with sorafenib facilitated specific targeting of the HCC TME [170]. Thus, MMSNs can act as a ferroptosis inducer, as well as a sorafenib carrier. Altogether, nanomaterials may be advantageous for the development of novel ferroptotic inducers with favourable galenic properties. Clinical trials are needed to validate their efficacies.
Ferroptosis and the efficacy of HCC immunotherapy
In a clinical study, a risk assessment model was established based on differential expression of ferroptosis-related genes to predict survival and prognosis of immunotherapy efficacy in patients with HCC [151]. Based on sample analyses, patients were categorised into two groups; the low-ferrscore group (harbouring less differentially expressed ferroptosis-related genes) and the high-ferrscore group (harbouring high differentially expressed ferroptosis-related genes). As a result, the high-ferrscore group exhibited a remarkably lower survival rate than the other group. Besides, expression of PD-1 and PD-L1 was markedly greater in the high-ferrscore than the low-ferrscore group. Thus, immunotherapy efficacy was unsatisfactory in the high-ferrscore group following treatment with PD-1 and CTLA4 inhibitors (immune checkpoint inhibitors) [151]. Collectively, these clinical findings indicate that highly differentiated expression of ferroptotic genes in HCC cells renders resistance to immunotherapy. Also, in an independent study, transcriptome and methylome analyses of ferroptosis-related genes were carried out in 374 patients with HCC to determine ferroptosis-associated HCC subtypes [152]. Subsequently, ferroptosis-associated HCC subtypes were explored for their link with tumour immune microenvironment. As a result, two distinct phenotypes including ferroptosis-L and ferroptosis-H were recognised based on methylation and expression of ferroptotic genes in HCC patients. The ferroptosis-H group had a lower survival rate and different microenvironmental immune status [152]. Besides, 15 ferroptosis-associated genes were identified as a prognostic and risk stratification model for accurate decision-making in clinical treatment and immunotherapy of HCC [152]. In another setting, HCC patients were analysed using bioinformatic techniques to reveal a ferroptosis-associated diagnostic/prognostic model useful for prediction of immune activity [153]. Hence, a prognostic model was developed based on SLC1A5 and SLC7A11 mRNAs and 8 ferroptosis-associated long non-coding RNAs (lncRNAs), which were proven to function better than pathological characteristics [153]. Collectively, this finding represented a ferroptosis-associated model based on mRNA and lncRNA molecules for prognosis and prediction of immune activity, thereby, offering a guideline for successful immunotherapies.
Concluding remarks and future perspectives
Within the last 5 years, research on ferroptosis in HCC has advanced considerably, and many small-molecule compounds or drugs were developed to induce ferroptosis to suppress tumour growth. Despite these prominent advances in the field, ferroptosis resistance remains a challenge for translational application. Following treatment with ferroptosis activators, the surviving fraction of HCC cells can undergo a process of “redox reset” with a stronger antioxidant system. Notably, transcriptional factor NRF2 orchestrates the stress-induced activation of numerous cytoprotective genes to suppress ferroptosis. Understanding the regulation of NRF2 activity and its downstream signalling may contribute to the pharmacological subversion of this “redox reset” and hence to the avoidance of ferroptosis resistance. Based on the available literature, we speculate that the ignition of a single pathway of ferroptosis is unlikely to achieve a durable ferroptotic response that affects the vast majority of HCC cells within a tumour. Instead, combination efforts should be sought in which several pathways are concurrently turned on. A better understanding of tumour microenvironment, especially the communication between immune cells and cancer cells, may provide new insights into the immunological signature of ferroptosis, which can aid in the design of new drugs and combination therapies [154]. Indeed, in an ideal scenario, induction of ferroptosis should stimulate or at least be compatible with an anticancer immune response that keeps residual HCC cells. Last but not least, the search for specific, user-friendly biomarkers to predict ferroptosis responses will be important for the design of targeted therapies [155]. Thus, identification of drivers and genetic or metabolic vulnerabilities of ferroptosis that affect malignant but not normal cells, may gear customised interventions in individual patients [156].
Acknowledgements
The authors wish to express our sincere apology to those authors whose important work cannot be discussed and cited due to page limitations. GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR)—Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Fondation pour la Recherche Médicale (FRM); a donation by Elior; Equipex Onco-Pheno-Screen; European Joint Programme on Rare Diseases (EJPRD); Gustave Roussy Odyssea, the European Union Horizon 2020 Projects Oncobiome and Crimson; Fondation Carrefour; Institut National du Cancer (INCa); Institut Universitaire de France; LabEx Immuno-Oncology (ANR-18-IDEX-0001); a Cancer Research ASPIRE Award from the Mark Foundation; the RHU Immunolife; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumour Immune Elimination (SOCRATE); and SIRIC Cancer Research and Personalised Medicine (CARPEM). This study contributes to the IdEx Université de Paris ANR-18-IDEX-0001.
Glossary
- Autophagy
An evolutionarily conserved process in eukaryote cells mediating engulfment of damaged organelles or cellular components within transitory organelles, so-called, autophagosomes, which subsequently fuse with lysosomes for ultimate degradation of the engulfed cargo.
- Cathepsin B/L
Cathepsin B/L are lysosomal cysteine proteases playing a role in cellular functions, including intracellular proteolysis.
- Chronic hepatitis
Refers to liver inflammation and impairment inflicted by hepatitis B and C viruses and drug toxicity.
- Ferrireductases
A group of enzymes that mediate the reduction of Fe3+ to Fe2+.
- Hemochromatosis
A disorder caused by accumulation and build-up of extra iron in the body.
- Methylome
A technique analysing the distribution of 5-methylcytosine in the entire genome.
- Myristoylation
A lipid modification mechanism in which a fatty acid known as myristic acid binds to the N-terminal domain of a protein.
- Non-alcoholic steatohepatitis (NASH)
Refers to liver inflammation and impairment induced by fat accumulation in the liver.
- Saponin
Natural glycosides, which constitute sugars like apiose, arabinose, galactose, and glucose, are found abundantly in plants.
- Transcriptome
Refers to a thorough range/record of expressed mRNAs within an organism.
- Xenobiotics
Refers to chemical compounds that are naturally produced or exists within an organism.
Author contributions
AA has written the initial draft of the manuscript, and DT, GK and JR have contributed to the revising, editing and finalising of the manuscript.
Funding
Not applicable.
Data availability
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
GK has been holding research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Samsara, Sanofi, Sotio, Tollys, Vascage and Vasculox/Tioma. GK has been consulting for Reithera. GK is on the Board of Directors of the Bristol Myers Squibb Foundation France. GK is a scientific co-founder of everImmune, Osasuna Therapeutics, Samsara Therapeutics and Therafast Bio. GK is the inventor of patents covering therapeutic targeting of ageing, cancer, cystic fibrosis and metabolic disorders. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Daolin Tang, Email: daolin.tang@utsouthwestern.edu.
Guido Kroemer, Email: kroemer@orange.fr.
Jun Ren, Email: jren_aldh2@outlook.com.
References
- 1.Röcken C, Carl-McGrath S. Pathology and pathogenesis of hepatocellular carcinoma. Digestive Dis. 2001;19:269–78. doi: 10.1159/000050693. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed O, Liu L, Gayed A, Baadh A, Patel M, Tasse J, et al. The changing face of hepatocellular carcinoma: forecasting prevalence of nonalcoholic steatohepatitis and hepatitis C cirrhosis. J Clin Exp Hepatol. 2019;9:50–55. doi: 10.1016/j.jceh.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanwal F, Hoang T, Kramer JR, Asch SM, Goetz MB, Zeringue A, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140:1182–8. e1181. doi: 10.1053/j.gastro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer: Interdiscip Int J Am Cancer Soc. 2009;115:5651–61. doi: 10.1002/cncr.24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gan L, Liu Z, Sun C. Obesity linking to hepatocellular carcinoma: a global view. Biochimica et Biophysica Acta (BBA)-Rev Cancer. 2018;1869:97–102. doi: 10.1016/j.bbcan.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Cholankeril G, Patel R, Khurana S, Satapathy SK. Hepatocellular carcinoma in non-alcoholic steatohepatitis: current knowledge and implications for management. World J Hepatol. 2017;9:533. doi: 10.4254/wjh.v9.i11.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaki MYW, Mahdi AK, Patman GL, Whitehead A, Maurício JP, McCain MV, et al. Key features of the environment promoting liver cancer in the absence of cirrhosis. Sci Rep. 2021;11:1–17. doi: 10.1038/s41598-021-96076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y, Peng Z. Programmed cell death and cancer. Postgrad Med J. 2009;85:134–40. doi: 10.1136/pgmj.2008.072629. [DOI] [PubMed] [Google Scholar]
- 10.Aizawa S, Brar G, Tsukamoto H. Cell death and liver disease. Gut liver. 2020;14:20. doi: 10.5009/gnl18486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–64. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dionísio P, Amaral J, Rodrigues C. Oxidative stress and regulated cell death in Parkinson’s disease. Ageing Res Rev. 2021;67:101263. [DOI] [PubMed]
- 14.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–85. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2020; 10.1080/15548627.2020.1810918. [DOI] [PMC free article] [PubMed]
- 16.Chang W-T, Bow Y-D, Fu P-J, Li C-Y, Wu C-Y, Chang Y-H, et al. A marine terpenoid, heteronemin, induces both the apoptosis and ferroptosis of hepatocellular carcinoma cells and involves the ROS and MAPK pathways. Oxid Med Cell Longev. 2017;2021:7689045. doi: 10.1155/2021/7689045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nie J, Lin B, Zhou M, Wu L, Zheng T. Role of ferroptosis in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2018;144:2329–37. doi: 10.1007/s00432-018-2740-3. [DOI] [PubMed] [Google Scholar]
- 18.Lakhal-Littleton S. Mechanisms of cardiac iron homeostasis and their importance to heart function. Free Radic Biol Med. 2019;133:234–7. doi: 10.1016/j.freeradbiomed.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bi Y, Ajoolabady A, Demillard LJ, Yu W, Hilaire ML, Zhang Y, et al. Dysregulation of iron metabolism in cardiovascular diseases: from iron deficiency to iron overload. Biochem Pharmacol. 2021;190:114661. [DOI] [PubMed]
- 20.Aisen P, Wessling-Resnick M, Leibold EA. Iron metabolism. Curr Opin Chem Biol. 1999;3:200–6. doi: 10.1016/S1367-5931(99)80033-7. [DOI] [PubMed] [Google Scholar]
- 21.Wang C-Y, Babitt JL. Liver iron sensing and body iron homeostasis. Blood, J Am Soc Hematol. 2019;133:18–29. doi: 10.1182/blood-2018-06-815894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochemical J. 2011;434:365–81. doi: 10.1042/BJ20101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponka P, Beaumont C, Richardson DR. Function and regulation of transferrin and ferritin. Semin Hematol. 1998;35:35–54. [PubMed]
- 24.Newman R, Schneider C, Sutherland R, Vodinelich L, Greaves M. The transferrin receptor. Trends Biochemical Sci. 1982;7:397–400. [Google Scholar]
- 25.Montalbetti N, Simonin A, Kovacs G, Hediger MA. Mammalian iron transporters: families SLC11 and SLC40. Mol Asp Med. 2013;34:270–87. doi: 10.1016/j.mam.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Munro HN, Linder MC. Ferritin: structure, biosynthesis, and role in iron metabolism. Physiological Rev. 1978;58:317–96. doi: 10.1152/physrev.1978.58.2.317. [DOI] [PubMed] [Google Scholar]
- 27.Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ, 3rd, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–8. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayr R, Griffiths WJ, Hermann M, McFarlane I, Halsall DJ, Finkenstedt A, et al. Identification of mutations in SLC40A1 that affect ferroportin function and phenotype of human ferroportin iron overload. Gastroenterology. 2011;140:2056–63. e2051. doi: 10.1053/j.gastro.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Liu J, Xu Y, Wu R, Chen X, Song X, et al. Tumor heterogeneity in autophagy-dependent ferroptosis. Autophagy. 2021; 10.1080/15548627.2021.1872241. [DOI] [PMC free article] [PubMed]
- 30.Breuer W, Hershko C, Cabantchik Z. The importance of non-transferrin bound iron in disorders of iron metabolism. Transfus Sci. 2000;23:185–92. doi: 10.1016/s0955-3886(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 31.Lane D, Merlot A, Huang M-H, Bae D-H, Jansson P, Sahni S, et al. Cellular iron uptake, trafficking and metabolism: key molecules and mechanisms and their roles in disease. Biochimica et Biophysica Acta (BBA)-Mol Cell Res. 2015;1853:1130–44. doi: 10.1016/j.bbamcr.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Knutson MD. Non-transferrin-bound iron transporters. Free Radic Biol Med. 2019;133:101–11. doi: 10.1016/j.freeradbiomed.2018.10.413. [DOI] [PubMed] [Google Scholar]
- 33.Song X, Zhu S, Chen P, Hou W, Wen Q, Liu J, et al. AMPK-mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system Xc–activity. Curr Biol. 2018;28:2388–99. e2385. doi: 10.1016/j.cub.2018.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu Y, Jiang L, Wang H, Shen Z, Cheng Q, Zhang P, et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood. 2020;136:726–39. doi: 10.1182/blood.2019002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Yu C, Kang R, Tang D. Iron metabolism in ferroptosis. Front Cell Dev Biol. 2020;8:590226. [DOI] [PMC free article] [PubMed]
- 36.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paterek A, Mackiewicz U, Mączewski M. Iron and the heart: a paradigm shift from systemic to cardiomyocyte abnormalities. J Cell Physiol. 2019;234:21613–29. doi: 10.1002/jcp.28820. [DOI] [PubMed] [Google Scholar]
- 38.Vela D. Keeping heart homeostasis in check through the balance of iron metabolism. Acta Physiologica. 2020;228:e13324. doi: 10.1111/apha.13324. [DOI] [PubMed] [Google Scholar]
- 39.Bogdan AR, Miyazawa M, Hashimoto K, Tsuji Y. Regulators of iron homeostasis: new players in metabolism, cell death, and disease. Trends biochemical Sci. 2016;41:274–86. doi: 10.1016/j.tibs.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du J, Wang T, Li Y, Zhou Y, Wang X, Yu X, et al. DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radic Biol Med. 2019;131:356–69. doi: 10.1016/j.freeradbiomed.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26:1021–32. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X, Yu C, Kang R, Tang D. Iron metabolism in ferroptosis. Front Cell Dev Biol. 2020;8:590226. doi: 10.3389/fcell.2020.590226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Higdon A, Diers AR, Oh JY, Landar A, Darley-Usmar VM. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. Biochemical J. 2012;442:453–64. doi: 10.1042/BJ20111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Latunde-Dada GO. Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochimica et Biophysica Acta (BBA)-Gen Subj. 2017;1861:1893–1900. doi: 10.1016/j.bbagen.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 45.Guo J, Xu B, Han Q, Zhou H, Xia Y, Gong C, et al. Ferroptosis: a novel anti-tumor action for cisplatin. Cancer Res Treat. 2018;50:445. doi: 10.4143/crt.2016.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheng X, Shan C, Liu J, Yang J, Sun B, Chen D. Theoretical insights into the mechanism of ferroptosis suppression via inactivation of a lipid peroxide radical by liproxstatin-1. Phys Chem Chem Phys. 2017;19:13153–9. doi: 10.1039/c7cp00804j. [DOI] [PubMed] [Google Scholar]
- 47.Zilka O, Shah R, Li B, Friedmann Angeli JP, Griesser M, Conrad M, et al. On the mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Cent Sci. 2017;3:232–43. doi: 10.1021/acscentsci.7b00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci USA. 2016;113:E4966–75. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochemical biophysical Res Commun. 2016;478:1338–43. doi: 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- 51.Dixon SJ, Winter GE, Musavi LS, Lee ED, Snijder B, Rebsamen M, et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol. 2015;10:1604–9. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soupene E, Kuypers FA. Mammalian long-chain acyl-CoA synthetases. Exp Biol Med. 2008;233:507–21. doi: 10.3181/0710-MR-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shindou H, Shimizu T. Acyl-CoA: lysophospholipid acyltransferases. J Biol Chem. 2009;284:1–5. doi: 10.1074/jbc.R800046200. [DOI] [PubMed] [Google Scholar]
- 54.Yang W-H, Huang Z, Wu J, Ding C-KC, Murphy SK, Chi J-T. A TAZ–ANGPTL4–NOX2 axis regulates ferroptotic cell death and chemoresistance in epithelial ovarian cancerTAZ promotes ferroptosis in OvCa. Mol Cancer Res. 2020;18:79–90. doi: 10.1158/1541-7786.MCR-19-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kagan VE, Mao G, Qu F, Angeli JPF, Doll S, Croix CS, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kreft H, Jetz W. Global patterns and determinants of vascular plant diversity. Proc Natl Acad Sci USA. 2007;104:5925–30. doi: 10.1073/pnas.0608361104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zou Y, Li H, Graham ET, Deik AA, Eaton JK, Wang W, et al. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat Chem Biol. 2020;16:302–9. doi: 10.1038/s41589-020-0472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, et al. Role of mitochondria in ferroptosis. Mol Cell. 2019;73:354–63. e353. doi: 10.1016/j.molcel.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hinman A, Holst CR, Latham JC, Bruegger JJ, Ulas G, McCusker KP, et al. Vitamin E hydroquinone is an endogenous regulator of ferroptosis via redox control of 15-lipoxygenase. PLoS ONE. 2018;13:e0201369. doi: 10.1371/journal.pone.0201369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuang F, Liu J, Tang D, Kang R. Oxidative damage and antioxidant defense in ferroptosis. Front Cell Dev Biol. 2020;8:969. doi: 10.3389/fcell.2020.586578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bai Y, Meng L, Han L, Jia Y, Zhao Y, Gao H, et al. Lipid storage and lipophagy regulates ferroptosis. Biochem Biophys Res Commun. 2019;508:997–1003. doi: 10.1016/j.bbrc.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 62.Xu X, Zhang X, Wei C, Zheng D, Lu X, Yang Y, et al. Targeting SLC7A11 specifically suppresses the progression of colorectal cancer stem cells via inducing ferroptosis. Eur J Pharm Sci. 2020;152:105450. doi: 10.1016/j.ejps.2020.105450. [DOI] [PubMed] [Google Scholar]
- 63.Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18:280–96. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 64.Zheng J, Sato M, Mishima E, Sato H, Proneth B, Conrad M. Sorafenib fails to trigger ferroptosis across a wide range of cancer cell lines. Cell death Dis. 2021;12:1–10. doi: 10.1038/s41419-021-03998-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274:11455–8. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- 66.Harris IS, Treloar AE, Inoue S, Sasaki M, Gorrini C, Lee KC, et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell. 2015;27:211–22. doi: 10.1016/j.ccell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 67.Banjac A, Perisic T, Sato H, Seiler A, Bannai S, Weiss N, et al. The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene. 2008;27:1618–28. doi: 10.1038/sj.onc.1210796. [DOI] [PubMed] [Google Scholar]
- 68.Jiang L, Kon N, Li T, Wang S-J, Su T, Hibshoosh H, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73:2195–209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seiler A, Schneider M, Förster H, Roth S, Wirth EK, Culmsee C, et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent-and AIF-mediated cell death. Cell Metab. 2008;8:237–48. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 71.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–31. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–91. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guerriero E, Capone F, Accardo M, Sorice A, Costantini M, Colonna G, et al. GPX4 and GPX7 over-expression in human hepatocellular carcinoma tissues. Eur J Histochemistry. 2015;59:2540. doi: 10.4081/ejh.2015.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gan B. Mitochondrial regulation of ferroptosis. J Cell Biol. 2021;220:e202105043. doi: 10.1083/jcb.202105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–92. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dai E, Zhang W, Cong D, Kang R, Wang J, Tang D. AIFM2 blocks ferroptosis independent of ubiquinol metabolism. Biochemical Biophysical Res Commun. 2020;523:966–71. doi: 10.1016/j.bbrc.2020.01.066. [DOI] [PubMed] [Google Scholar]
- 77.Martinez VD, Vucic EA, Pikor LA, Thu KL, Hubaux R, Lam WL. Frequent concerted genetic mechanisms disrupt multiple components of the NRF2 inhibitor KEAP1/CUL3/RBX1 E3-ubiquitin ligase complex in thyroid cancer. Mol Cancer. 2013;12:1–6. doi: 10.1186/1476-4598-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rada P, Rojo AI, Evrard-Todeschi N, Innamorato NG, Cotte A, Jaworski T, et al. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/β-TrCP axis. Mol Cell Biol. 2012;32:3486–99. doi: 10.1128/MCB.00180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang J, Zhang J, Ni H, Wang Y, Katwal G, Zhao Y, et al. Downregulation of XBP1 protects kidney against ischemia-reperfusion injury via suppressing HRD1-mediated NRF2 ubiquitylation. Cell Death Discov. 2021;7:1–13. doi: 10.1038/s41420-021-00425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–5. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dodson M, Castro-Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107. doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kerins MJ, Ooi A. The roles of NRF2 in modulating cellular iron homeostasis. Antioxid Redox Signal. 2018;29:1756–73. doi: 10.1089/ars.2017.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Agyeman AS, Chaerkady R, Shaw PG, Davidson NE, Visvanathan K, Pandey A, et al. Transcriptomic and proteomic profiling of KEAP1 disrupted and sulforaphane-treated human breast epithelial cells reveals common expression profiles. Breast cancer Res Treat. 2012;132:175–87. doi: 10.1007/s10549-011-1536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harada N, Kanayama M, Maruyama A, Yoshida A, Tazumi K, Hosoya T, et al. Nrf2 regulates ferroportin 1-mediated iron efflux and counteracts lipopolysaccharide-induced ferroportin 1 mRNA suppression in macrophages. Arch Biochem biophysics. 2011;508:101–9. doi: 10.1016/j.abb.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 85.Chorley BN, Campbell MR, Wang X, Karaca M, Sambandan D, Bangura F, et al. Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic Acids Res. 2012;40:7416–29. doi: 10.1093/nar/gks409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hübner R-H, Schwartz JD, De BP, Ferris B, Omberg L, Mezey JG, et al. Coordinate control of expression of Nrf2-modulated genes in the human small airway epithelium is highly responsive to cigarette smoking. Mol Med. 2009;15:203–19. doi: 10.2119/molmed.2008.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Campbell MR, Karaca M, Adamski KN, Chorley BN, Wang X, Bell DA. Novel hematopoietic target genes in the NRF2-mediated transcriptional pathway. Oxid Med Cell Longev. 2013;2013:120305. doi: 10.1155/2013/120305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, et al. Activation of the p62‐Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–84. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun X, Niu X, Chen R, He W, Chen D, Kang R, et al. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology. 2016;64:488–500. doi: 10.1002/hep.28574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang H, Lin D, Yu Q, Li Z, Lenahan C, Dong Y, et al. A promising future of ferroptosis in tumor therapy. Front Cell Dev Biol. 2021;9:1255. [DOI] [PMC free article] [PubMed]
- 91.Guo W, Zhou BP. Oncometabolite modification of Keap1 links GSTZ1 deficiency with cancer. Genes Dis. 2019;6:333–4. doi: 10.1016/j.gendis.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Q, Bin C, Xue Q, Gao Q, Huang A, Wang K, et al. GSTZ1 sensitizes hepatocellular carcinoma cells to sorafenib-induced ferroptosis via inhibition of NRF2/GPX4 axis. Cell Death Dis. 2021;12:1–16. doi: 10.1038/s41419-021-03718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jedlitschky G, Burchell B, Keppler D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem. 2000;275:30069–74. doi: 10.1074/jbc.M005463200. [DOI] [PubMed] [Google Scholar]
- 94.Weaver DA, Crawford EL, Warner KA, Elkhairi F, Khuder SA, Willey JC. ABCC5, ERCC2, XPA and XRCC1 transcript abundance levels correlate with cisplatin chemoresistance in non-small cell lung cancer cell lines. Mol Cancer. 2005;4:1–8. doi: 10.1186/1476-4598-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang W, Chen K, Lu Y, Zhang D, Cheng Y, Li L, et al. ABCC5 facilitates the acquired resistance of sorafenib through the inhibition of SLC7A11-induced ferroptosis in hepatocellular carcinoma. Neoplasia. 2021;23:1227–39. doi: 10.1016/j.neo.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun J, Zhou C, Zhao Y, Zhang X, Chen W, Zhou Q, et al. Quiescin sulfhydryl oxidase 1 promotes sorafenib-induced ferroptosis in hepatocellular carcinoma by driving EGFR endosomal trafficking and inhibiting NRF2 activation. Redox Biol. 2021;41:101942. doi: 10.1016/j.redox.2021.101942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lake DF, Faigel DO. The emerging role of QSOX1 in cancer. Antioxid Redox Signal. 2014;21:485–96. doi: 10.1089/ars.2013.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu MJ, Feng M. Radiation therapy in HCC: What Data Exist and What Data Do We Need to Incorporate into Guidelines? Semin Liver Dis. 2019;39:43–52. doi: 10.1055/s-0038-1676098. [DOI] [PubMed] [Google Scholar]
- 99.Yuan Y, Cao W, Zhou H, Qian H, Wang H. CLTRN, regulated by NRF1/RAN/DLD protein complex, enhances radiation sensitivity of hepatocellular carcinoma cells through ferroptosis pathway. Int J Radiat Oncol* Biol* Phys. 2021;110:859–71. doi: 10.1016/j.ijrobp.2020.12.062. [DOI] [PubMed] [Google Scholar]
- 100.Fukui K, Yang Q, Cao Y, Takahashi N, Hatakeyama H, Wang H, et al. The HNF-1 target collectrin controls insulin exocytosis by SNARE complex formation. Cell Metab. 2005;2:373–84. doi: 10.1016/j.cmet.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 101.Gao R, Kalathur RK, Coto-Llerena M, Ercan C, Buechel D, Shuang S, et al. YAP/TAZ and ATF4 drive resistance to Sorafenib in hepatocellular carcinoma by preventing ferroptosis. EMBO Mol Med. 2021;13:e14351. [DOI] [PMC free article] [PubMed]
- 102.Fan Z, Yang G, Zhang W, Liu Q, Liu G, Liu P, et al. Hypoxia blocks ferroptosis of hepatocellular carcinoma via suppression of METTL14 triggered YTHDF2‐dependent silencing of SLC7A11. J Cell Mol Med. 2021;25:10197–212. doi: 10.1111/jcmm.16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.He L, Li H, Wu A, Peng Y, Shu G, Yin G. Functions of N6-methyladenosine and its role in cancer. Mol Cancer. 2019;18:1–15. doi: 10.1186/s12943-019-1109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Feng X, Wang S, Sun Z, Dong H, Yu H, Huang M, et al. Ferroptosis enhanced diabetic renal tubular injury via HIF-1α/HO-1 pathway in Db/Db mice. Front Endocrinol. 2021;12:21. doi: 10.3389/fendo.2021.626390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miess H, Dankworth B, Gouw AM, Rosenfeldt M, Schmitz W, Jiang M, et al. The glutathione redox system is essential to prevent ferroptosis caused by impaired lipid metabolism in clear cell renal cell carcinoma. Oncogene. 2018;37:5435–50. doi: 10.1038/s41388-018-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen S, Zhao Y. Circular RNAs: characteristics, function, and role in human cancer. Histol Histopathol. 2018;33:887–93. doi: 10.14670/HH-11-969. [DOI] [PubMed] [Google Scholar]
- 107.Gaffo E, Boldrin E, Dal Molin A, Bresolin S, Bonizzato A, Trentin L, et al. Circular RNA differential expression in blood cell populations and exploration of circRNA deregulation in pediatric acute lymphoblastic leukemia. Sci Rep. 2019;9:1–12. doi: 10.1038/s41598-019-50864-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu Q, Zhou L, Yang G, Meng F, Wan Y, Wang L, et al. CircIL4R facilitates the tumorigenesis and inhibits ferroptosis in hepatocellular carcinoma by regulating the miR‐541‐3p/GPX4 axis. Cell Biol Int. 2020;44:2344–56. doi: 10.1002/cbin.11444. [DOI] [PubMed] [Google Scholar]
- 109.Lyu N, Zeng Y, Kong Y, Chen Q, Deng H, Ou S, et al. Ferroptosis is involved in the progression of hepatocellular carcinoma through the circ0097009/miR-1261/SLC7A11 axis. Ann Transl Med. 2021;9:675. doi: 10.21037/atm-21-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fang S, Chen W, Ding J, Zhang D, Zheng L, Song J, et al. Oxidative medicine and cellular longevity Hsa_circ_0013731 mediated by E2F1 inhibits ferroptosis in hepatocellular carcinoma cells by sponging miR-877-3p and targeting SLC7A11. Researchsquare, 2021.
- 111.Qi W, Li Z, Xia L, Dai J, Zhang Q, Wu C, et al. LncRNA GABPB1-AS1 and GABPB1 regulate oxidative stress during erastin-induced ferroptosis in HepG2 hepatocellular carcinoma cells. Sci Rep. 2019;9:1–12. doi: 10.1038/s41598-019-52837-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wong SL, Sukkar MB. The SPARC protein: an overview of its role in lung cancer and pulmonary fibrosis and its potential role in chronic airways disease. Br J Pharmacol. 2017;174:3–14. doi: 10.1111/bph.13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hua H-W, Jiang H-S, Jia L, Jia Y-P, Yao Y-L, Chen Y-W, et al. SPARC regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma. Cancer Biomark. 2021;32:425–33. [DOI] [PubMed]
- 114.Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J lipid Res. 2001;42:1007–17. [PubMed] [Google Scholar]
- 115.Ren J, Bi Y, Sowers JR, Hetz C, Zhang Y. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat Rev Cardiol. 2021;18:499–521. doi: 10.1038/s41569-021-00511-w. [DOI] [PubMed] [Google Scholar]
- 116.Zheng X, Liu B, Liu X, Li P, Zhang P, Ye F, et al. PERK regulates the sensitivity of hepatocellular carcinoma cells to high-LET carbon ions via either apoptosis or ferroptosis. J Cancer. 2022;13:669. doi: 10.7150/jca.61622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ryskamp DA, Korban S, Zhemkov V, Kraskovskaya N, Bezprozvanny I. Neuronal sigma-1 receptors: signaling functions and protective roles in neurodegenerative diseases. Front Neurosci. 2019;13:862. doi: 10.3389/fnins.2019.00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weng T-Y, Hung DT, Su T-P, Tsai S-YA. Loss of sigma-1 receptor chaperone promotes astrocytosis and enhances the Nrf2 antioxidant defense. Oxid Med Cell Longev. 2017;2017:4582135. doi: 10.1155/2017/4582135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bai T, Lei P, Zhou H, Liang R, Zhu R, Wang W, et al. Sigma-1 receptor protects against ferroptosis in hepatocellular carcinoma cells. J Cell Mol Med. 2019;23:7349–59. doi: 10.1111/jcmm.14594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roeser H, Lee G, Nacht S, Cartwright G. The role of ceruloplasmin in iron metabolism. J Clin Investig. 1970;49:2408–17. doi: 10.1172/JCI106460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shang Y, Luo M, Yao F, Wang S, Yuan Z, Yang Y. Ceruloplasmin suppresses ferroptosis by regulating iron homeostasis in hepatocellular carcinoma cells. Cell Signal. 2020;72:109633. doi: 10.1016/j.cellsig.2020.109633. [DOI] [PubMed] [Google Scholar]
- 122.Choudhury AD, Beltran H. Retinoblastoma loss in cancer: casting a wider net. Clin Cancer Res. 2019;25:4199–201. doi: 10.1158/1078-0432.CCR-19-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Louandre C, Marcq I, Bouhlal H, Lachaier E, Godin C, Saidak Z, et al. The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells. Cancer Lett. 2015;356:971–7. doi: 10.1016/j.canlet.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 124.Kornberg A, Horecker B, Smyrniotis P. [42] Glucose-6-phosphate dehydrogenase 6-phosphogluconic dehydrogenase. Methods in Enzymology Vol. 1, 323–327 (Academic Press, 1955).
- 125.Cao F, Luo A, Yang C. G6PD inhibits ferroptosis in hepatocellular carcinoma by targeting cytochrome P450 oxidoreductase. Cell Signal. 2021;87:110098. doi: 10.1016/j.cellsig.2021.110098. [DOI] [PubMed] [Google Scholar]
- 126.Shen Z-Q, Huang Y-L, Teng Y-C, Wang T-W, Kao C-H, Yeh C-H, et al. CISD2 maintains cellular homeostasis. Biochimica et Biophysica Acta (BBA)-Molecular Cell Res. 2021;1868:118954. [DOI] [PubMed]
- 127.Li B, Wei S, Yang L, Peng X, Ma Y, Wu B, et al. CISD2 promotes resistance to sorafenib-induced ferroptosis by regulating autophagy in hepatocellular carcinoma. Front Oncol. 2021;11:657723. doi: 10.3389/fonc.2021.657723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yuan H, Li X, Zhang X, Kang R, Tang D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun. 2016;478:838–44. doi: 10.1016/j.bbrc.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 129.Liu J, Song X, Kuang F, Zhang Q, Xie Y, Kang R, et al. NUPR1 is a critical repressor of ferroptosis. Nat Commun. 2021;12:647. doi: 10.1038/s41467-021-20904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huang C, Santofimia-Castano P, Liu X, Xia Y, Peng L, Gotorbe C, et al. NUPR1 inhibitor ZZW-115 induces ferroptosis in a mitochondria-dependent manner. Cell Death Discov. 2021;7:269. doi: 10.1038/s41420-021-00662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lu Y, Chan Y-T, Tan H-Y, Zhang C, Guo W, Xu Y, et al. Epigenetic regulation of ferroptosis via ETS1/miR-23a-3p/ACSL4 axis mediates sorafenib resistance in human hepatocellular carcinoma. J Exp Clin Cancer Res. 2022;41:1–17. doi: 10.1186/s13046-021-02208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ajoolabady A, Wang S, Kroemer G, Klionsky DJ, Uversky VN, Sowers JR, et al. ER stress in cardiometabolic diseases: from molecular mechanisms to therapeutics. Endocr Rev. 2021;42:839–71. doi: 10.1210/endrev/bnab006. [DOI] [PubMed] [Google Scholar]
- 133.Liu Z, Ma C, Wang Q, Yang H, Lu Z, Bi T, et al. Targeting FAM134B-mediated reticulophagy activates sorafenib-induced ferroptosis in hepatocellular carcinoma. Biochemical biophysical Res Commun. 2022;589:247–53. doi: 10.1016/j.bbrc.2021.12.019. [DOI] [PubMed] [Google Scholar]
- 134.Yang M, Wu X, Hu J, Wang Y, Wang Y, Zhang L, et al. COMMD10 inhibits HIF1α/CP loop to enhance ferroptosis and radiosensitivity by disrupting Cu-Fe balance in hepatocellular carcinoma. J Hepatol. 2022;76:1138–50. doi: 10.1016/j.jhep.2022.01.009. [DOI] [PubMed] [Google Scholar]
- 135.Chen Y, Li L, Lan J, Cui Y, Rao X, Zhao J, et al. CRISPR screens uncover protective effect of PSTK as a regulator of chemotherapy-induced ferroptosis in hepatocellular carcinoma. Mol Cancer. 2022;21:1–17. doi: 10.1186/s12943-021-01466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Eling N, Reuter L, Hazin J, Hamacher-Brady A, Brady NR. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience. 2015;2:517. doi: 10.18632/oncoscience.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li Z-J, Dai H-Q, Huang X-W, Feng J, Deng J-H, Wang Z-X, et al. Artesunate synergizes with sorafenib to induce ferroptosis in hepatocellular carcinoma. Acta Pharmacologica Sin. 2021;42:301–10. doi: 10.1038/s41401-020-0478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kotawong K, Chaijaroenkul W, Muhamad P, Na-Bangchang K. Cytotoxic activities and effects of atractylodin and β-eudesmol on the cell cycle arrest and apoptosis on cholangiocarcinoma cell line. J Pharmacol Sci. 2018;136:51–56. doi: 10.1016/j.jphs.2017.09.033. [DOI] [PubMed] [Google Scholar]
- 139.He Y, Fang D, Liang T, Pang H, Nong Y, Tang L, et al. Atractylodin may induce ferroptosis of human hepatocellular carcinoma cells. Ann Transl Med. 2021;9:1535. doi: 10.21037/atm-21-4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yin L, Shi C, Zhang Z, Wang W, Li M. Formosanin C attenuates lipopolysaccharide-induced inflammation through nuclear factor-κB inhibition in macrophages. Korean J Physiol Pharmacol. 2021;25:395–401. doi: 10.4196/kjpp.2021.25.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Su C-L, Lin P-L. Natural saponin formosanin c‐induced ferroptosis in human hepatocellular carcinoma cells involved ferritinophagy. FASEB J. 2020;34:1–1. doi: 10.3390/antiox9080682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lin P-L, Tang H-H, Wu S-Y, Shaw N-S, Su C-L. Saponin formosanin C-induced ferritinophagy and ferroptosis in human hepatocellular carcinoma cells. Antioxidants. 2020;9:682. doi: 10.3390/antiox9080682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Irving CB, Adams CE, Lawrie S. Haloperidol versus placebo for schizophrenia. The Cochrane database of systematic reviews. 2013;Cd003082. 10.1002/14651858.CD003082.pub3. [DOI] [PubMed]
- 144.Bai T, Wang S, Zhao Y, Zhu R, Wang W, Sun Y. Haloperidol, a sigma receptor 1 antagonist, promotes ferroptosis in hepatocellular carcinoma cells. Biochemical Biophysical Res Commun. 2017;491:919–25. doi: 10.1016/j.bbrc.2017.07.136. [DOI] [PubMed] [Google Scholar]
- 145.Schumacher M, Cerella C, Eifes S, Chateauvieux S, Morceau F, Jaspars M, et al. Heteronemin, a spongean sesterterpene, inhibits TNFα-induced NF-κB activation through proteasome inhibition and induces apoptotic cell death. Biochemical Pharmacol. 2010;79:610–22. doi: 10.1016/j.bcp.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 146.Ren X, Li Y, Zhou Y, Hu W, Yang C, Jing Q, et al. Overcoming the compensatory elevation of NRF2 renders hepatocellular carcinoma cells more vulnerable to disulfiram/copper-induced ferroptosis. Redox Biol. 2021;46:102122. doi: 10.1016/j.redox.2021.102122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Markossian S, Ang KK, Wilson CG, Arkin MR. Small-molecule screening for genetic diseases. Annu Rev Genomics Hum Genet. 2018;19:263–88. doi: 10.1146/annurev-genom-083117-021452. [DOI] [PubMed] [Google Scholar]
- 148.Bollong MJ, Yun H, Sherwood L, Woods AK, Lairson LL, Schultz PG. A small molecule inhibits deregulated NRF2 transcriptional activity in cancer. ACS Chem Biol. 2015;10:2193–8. doi: 10.1021/acschembio.5b00448. [DOI] [PubMed] [Google Scholar]
- 149.Singh A, Venkannagari S, Oh KH, Zhang Y-Q, Rohde JM, Liu L, et al. Small molecule inhibitor of NRF2 selectively intervenes therapeutic resistance in KEAP1-deficient NSCLC tumors. ACS Chem Biol. 2016;11:3214–25. doi: 10.1021/acschembio.6b00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Matthews JH, Liang X, Paul VJ, Luesch H. A complementary chemical and genomic screening approach for druggable targets in the Nrf2 pathway and small molecule inhibitors to overcome cancer cell drug resistance. ACS Chem Biol. 2018;13:1189–99. doi: 10.1021/acschembio.7b01025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gao L, Xue J, Liu X, Cao L, Wang R, Lei L. A scoring model based on ferroptosis genes for prognosis and immunotherapy response prediction and tumor microenvironment evaluation in liver hepatocellular carcinoma. Aging. 2021;13:24866. doi: 10.18632/aging.203721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Deng T, Hu B, Jin C, Tong Y, Zhao J, Shi Z, et al. A novel ferroptosis phenotype-related clinical-molecular prognostic signature for hepatocellular carcinoma. J Cell Mol Med. 2021;25:6618–33. doi: 10.1111/jcmm.16666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen Z-A, Tian H, Yao D-M, Zhang Y, Feng Z-J, Yang C-J. Identification of a ferroptosis-related signature model including mRNAs and lncRNAs for predicting prognosis and immune activity in hepatocellular carcinoma. Front Oncol. 2021;11:738477. doi: 10.3389/fonc.2021.738477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen X, Kang R, Kroemer G, Tang D. Ferroptosis in infection, inflammation, and immunity. J Exp Med. 2021;218:e20210518. [DOI] [PMC free article] [PubMed]
- 155.Chen X, Comish P, Tang D, Kang R. Characteristics and biomarkers of ferroptosis. Front. Cell Dev. Biol. 2021; 10.3389/fcell.2021.637162. [DOI] [PMC free article] [PubMed]
- 156.Rodriguez R, Schreiber SL, Conrad M. Persister cancer cells: iron addiction and vulnerability to ferroptosis. Mol Cell. 2021; 10.1016/j.molcel.2021.12.001. [DOI] [PMC free article] [PubMed]
- 157.Yuk H, Abdullah M, Kim D-H, Lee H, Lee S-J. Necrostatin-1 prevents ferroptosis in a RIPK1-and IDO-independent manner in hepatocellular carcinoma. Antioxidants. 2021;10:1347. doi: 10.3390/antiox10091347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jin M, Shi C, Li T, Wu Y, Hu C, Huang G. Solasonine promotes ferroptosis of hepatoma carcinoma cells via glutathione peroxidase 4-induced destruction of the glutathione redox system. Biomedicine Pharmacother. 2020;129:110282. doi: 10.1016/j.biopha.2020.110282. [DOI] [PubMed] [Google Scholar]
- 159.Zhang P, Liu C, Wu W, Mao Y, Qin Y, Hu J, et al. Triapine/Ce6-loaded and lactose-decorated nanomicelles provide an effective chemo-photodynamic therapy for hepatocellular carcinoma through a reactive oxygen species-boosting and ferroptosis-inducing mechanism. Chem Eng J. 2021;425:131543. [Google Scholar]
- 160.Chen H, Zhang W, Zhu G, Xie J, Chen X. Rethinking cancer nanotheranostics. Nat Rev Mater. 2017;2:1–18. doi: 10.1038/natrevmats.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dai Y, Xu C, Sun X, Chen X. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem Soc Rev. 2017;46:3830–52. doi: 10.1039/c6cs00592f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cai Y, Wei Z, Song C, Tang C, Han W, Dong X. Optical nano-agents in the second near-infrared window for biomedical applications. Chem Soc Rev. 2019;48:22–37. doi: 10.1039/c8cs00494c. [DOI] [PubMed] [Google Scholar]
- 163.Liang P, Huang X, Wang Y, Chen D, Ou C, Zhang Q, et al. Tumor-microenvironment-responsive nanoconjugate for synergistic antivascular activity and phototherapy. ACS Nano. 2018;12:11446–57. doi: 10.1021/acsnano.8b06478. [DOI] [PubMed] [Google Scholar]
- 164.Shen Z, Song J, Yung BC, Zhou Z, Wu A, Chen X. Emerging strategies of cancer therapy based on ferroptosis. Adv Mater. 2018;30:1704007. doi: 10.1002/adma.201704007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu M, Liu B, Liu Q, Du K, Wang Z, He N. Nanomaterial-induced ferroptosis for cancer specific therapy. Coord Chem Rev. 2019;382:160–80. [Google Scholar]
- 166.Zhang Y, Tan H, Daniels JD, Zandkarimi F, Liu H, Brown LM, et al. Imidazole ketone erastin induces ferroptosis and slows tumor growth in a mouse lymphoma model. Cell Chem Biol. 2019;26:623–33. e629. doi: 10.1016/j.chembiol.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liang C, Zhang X, Yang M, Dong X. Recent progress in ferroptosis inducers for cancer therapy. Adv Mater. 2019;31:1904197. doi: 10.1002/adma.201904197. [DOI] [PubMed] [Google Scholar]
- 168.Boland P, Wu J. Systemic therapy for hepatocellular carcinoma: beyond sorafenib. Chin Clin Oncol. 2018;7:50–50. doi: 10.21037/cco.2018.10.10. [DOI] [PubMed] [Google Scholar]
- 169.Yue H, Gou L, Tang Z, Liu Y, Liu S, Tang H. Construction of pH-responsive nanocarriers in combination with ferroptosis and chemotherapy for treatment of hepatocellular carcinoma. Cancer Nanotechnol. 2022;13:1–21. [Google Scholar]
- 170.Tang H, Chen D, Li C, Zheng C, Wu X, Zhang Y, et al. Dual GSH-exhausting sorafenib loaded manganese-silica nanodrugs for inducing the ferroptosis of hepatocellular carcinoma cells. Int J Pharmaceutics. 2019;572:118782. doi: 10.1016/j.ijpharm.2019.118782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.