Abstract
The heart failure (HF) guideline’s purpose is to assist medical professionals while treating patients with HF in accordance with the best current research. Many cases of HF are both, avoidable and treatable thanks to scientific trials. Management is, therefore, based on lifestyle changes, also called non-pharmacological treatment. These, based on lifestyle changes, should be recommended in every patient at risk for HF or with diagnosed of HF, but evidence in itself is scarce. DASH Diet could be clearly beneficial while Mediterranean diet doesn't have enough evidence at the present moment. Smoking should be stopped, and excessive amounts of alcohol drinking avoided, but there is no clinical trial nor registry performed on these aspects. A moderate salt restriction is better than a strict reduction. Exercise and cardiac rehabilitation are beneficial but there are no clear recommendations about type, duration, etc. Most of the evidence that we have in HF patients with obesity is contradictory. Finally, due to the high number of aged frail patients in HF lifestyle changes should be individualized, but again available data is scant. Therefore, due to the lack of current evidence, these gaps need to be considered and need new efforts on investigation in the next future.
Keywords: Sodium, Diet therapy, Heart failure, Exercise
INTRODUCTION
Heart failure (HF) is a complex syndrome with an increasing global incidence and prevalence and remains a leading cause of morbidity and mortality worldwide. However, HF guidelines recommend randomized clinical trials (RCTs) based global management of HF which involves pharmacological and non-pharmacological treatments.1,2,3) hat has shown a significant reduction in HF related mortality and morbidity.
The HF Guidelines’ purpose is to assist medical professionals while treating patients with HF in accordance with the best current research. Nowadays, many cases of HF are both, avoidable and treatable, thanks to the multitude of scientific trials that have helped us choose the best management to improve HF outcomes.1,2,3,4) HF management is, therefore, based on lifestyle changes, also called non-pharmacological treatment, and pharmacological treatment. Pharmacological treatment has proven to be effective in reducing mortality and hospitalizations due to HF. But, when it comes to non-pharmacological treatment, guidelines are only making concise and brief recommendations as for example: Avoid large volumes of fluid intake, avoid excessive salt intake, and maintain a healthy body weight.2,3) On the other hand, exercise is also recommended to improve exercise capacity, functional status, quality of life (QoL) and reduce HF hospitalization.2,3) In Table 1 we have summarized specific recommendations of different HF guidelines in lifestyle modifications in the treatment of HF.
Table 1. Summary of the specific recommendations of different HF guidelines in lifestyle modifications in the treatment of HF1,2,3,4) .
| Recommendations | KSHF | ESC | AHA/ACC |
|---|---|---|---|
| Diet | |||
| Specific diet | None | None | Mediterranean diet DASH Diet |
| Salt restriction | Less than 7–8 g of salt per day. | Avoid >5 g of salt per day | In patients with stage C HF, avoid excessive sodium intake to reduce congestive symptoms |
| Fluid restriction | Education at discharge | Fluid restriction from 1.5 to 2 L in severe HF with Hyponatremia | Benefit of fluid restriction to reduce congestion in advanced HF with hyponatremia is uncertain |
| Weight loss | None | Weight loss is recommended to achieve a BMI <35 kg/m2 pre-HF transplant | None |
| Exercise | Recommended to improve functional status | To undertake regular exercise and be physically active | Cardiac rehabilitation improves functional capacity, exercise tolerance, and health-related QoL. |
| Exercise training is recommended | |||
| Substance abuse | Support patient in stopping smoking, and avoid excessive alcohol drinking | Smoking cessation | None |
| Avoid alcohol if alcohol induced HF |
HF = heart failure; KSHF = The Korean Society of Heart Failure; ESC = The European Society of Cardiology; AHA = American Heart Association; ACC = American College of Cardiology; BMI = body mass index; QoL = quality of life.
One of the main reasons for the different extent of the recommendations, much larger for pharmacological treatment, could be that these have much more weight of evidence as lifestyle changes. On the other hand, besides the lack of evidence, the available is far from offering clear recommendations, being sometimes contradictory, or based on studies with a very small number of participants, or flawed methodology. Therefore, some gaps of evidence of non-pharmacological treatment of HF, following guidelines (1–4) that should be considered in future investigations as indicated in Table 2.
Table 2. Gaps of evidence of non-pharmacological treatment of HF, following guidelines (1–4) that should be considered in future investigations.
| Gaps of evidence of non-pharmacological treatment of HF |
|---|
| 1. Efficacy and safety of specific dietary interventions to prevent and treat HF. |
| 2. Efficacy and safety of nutrition evaluation and intervention, to prevent and treat HF. |
| 3. Efficacy and safety of sodium restriction to prevent and treat HF. |
| 4. Efficacy and safety of fluid restriction to prevent and treat HF. |
| 5. Efficacy and safety of cardiac rehabilitation in patients with HFpEF and HFmrEF. |
HF = heart failure; HFpEF = HF with preserved ejection fraction; HFmrEF = HF with midrange ejection fraction.
Therefore, in this narrative review we aim to summarize the available evidence of the effect of lifestyle changes on HF related morbidity and mortality as also on QoL, exercise capacity and functional status, and what should be the goal in future studies.
DIET AND HF MANAGEMENT
HF nutritional counselling is, usually, based on sodium and fluid intake restriction. But HF patients, especially those with advanced HF, have, besides congestion, a higher risk of developing cachexia or malnutrition. Therefore, a more wider diet recommendation is needed. On the other hand, available data, show that patients with HF have lower caloric intake and higher micronutrient deficiencies compared with age- and sex-matched healthy older adults.5) and therefore a specific dietetic and nutritional recommendation is also needed. Another crucial question is management of obesity in HF. Although, obesity is related to the development of HF, and to its risk factors, it is not clear if there is a reverse epidemiology with worse outcome in lean HF patients. Besides that, there is a dearth of information on weight loss in obese people with HF.6) As for weight loss, a limited trial has explored the differences in clinical outcomes or adverse events linked weight loss. But, the implementation of lifestyle adjustment was well accepted, although it did not lead to appreciable weight loss.7)
While, neither Korean nor the European Society of Cardiology (ESC) guidelines, do not recommend a specific diet, the AHA/ACC HF recommendations advise The Mediterranean, whole-grain, plant-based diet, and the DASH (Dietary Approaches to Stop Hypertension) diet, that are inversely related with incident HF and may provide some protection against HF development. The DASH diet, on the other hand, is high in antioxidants and potassium, and may be linked to a decrease in HF hospitalizations.3)
And, as usually, HF patients are old and frail, and therefore depend on their caregiver’s diet, another main question is the diet quality of patients with HF and their family caregivers, as they usually consume similar diets. One specific study was aimed to compare diet quality of 40 patients with HF with that of their 40 family caregivers, via a Food Frequency Questionnaire. They found that both, patients, and caregivers, consume poor-quality diets, suggesting that nutrition interventions need to be targeted at the family as a unit.8)
As for Mediterranean diet (MedDiet) some studies have tried to investigate the Ability of MedDiet to prevent HF. But only one prospective study, that included 991 consecutive patients with acute heart failure (AHF) in Spanish emergency department, has evaluated clinical outcomes according to their adherence to the Mediterranean diet (MedDiet) and found that it did not influence long-term mortality after an episode of AHF, but it was associated with decreased rates of rehospitalization during the next year.9)
Other approach could be the Dietary Approaches to Stop Hypertension (DASH) diet interventions. In a recent study, it was attempted to synthesize the most recent research on HF dietary guidelines, including the nutritional treatments of the DASH diet, and to make preliminary suggestions for the use of the DASH diet in outpatient HF therapy. There are just a few studies that involved a small number of patients. For instance, HF with preserved ejection fraction (HFpEF) patients who had DASH sodium-restricted meals for three weeks saw substantial drops in blood pressure and arterial stiffness as well as improvements in ventricular diastolic function and oxidative stress. The GOURMET-HF trial revealed that recently hospitalized HF patients exhibited improvements in symptoms, physical limitations, and hospitalizations when the DASH diet was offered directly to release patients via home-delivered meals. But DASH nutritional interventions in HF have a main limitation for their use and it is the small sample size and non-randomization of interventions, leading to less reliable evidence. To provide conclusive data about the application of the DASH diet in the therapy of HF, randomized controlled trials are required.10)
Weight loss
Guidelines recommendation regarding this aspect recommend weight loss to prevent HF, but they recognise that weight loss has an unknown efficacy for treatment in established HF.1,2,3) Besides that, although obesity increases the risk for development of HF, it appears to exert a protective effect in patients in whom HF has already been diagnosed, as a lower bomy mass index (BMI) is associated with a higher risk of mortality, that situation has been called “obesity paradox.” And it’s observed in HF regardless of comorbidities and HF phenotype.11,12)
The evidence is even scarcer. A small study aimed at investigating whether individuals with HfpEF and obesity would have meaningful weight reduction and an improvement in functional status because of an intensive lifestyle modification program, was conducted in 41 HfpEF patients, with a mean BMI 40.8 kg/m2. The mean 6-minute walk distance rose to 281 m (p=0.001) at 15 weeks before falling to 267 m at 26 weeks. The Minnesota Living With Heart Failure score decreased from 59.9 to 37.3 at 15 weeks (p=0.001) and to 37.06 at 26 weeks. Weight fluctuations related to variations in the Minnesota Living with Heart Failure score and the 6-minute walk distance.13)
In a randomized, attention-controlled, 2×2 factorial trial conducted in an urban academic medical center, 100 older obese HfpEF patients were enrolled. Exercise and diet combined have improved the QoL. Peak VO2 increased also, significantly, by both interventions.14).
Salt restriction
Dietary salt restriction is recommended by many guidelines for patients with HF. Korean HF guidelines recommend that patients with moderate-to-severe HF consume less than 2 g of sodium per day (7–8 g of salt).1) ESGC HF guidelines recommend avoiding excessive salt intake (>5 g/day)2) AHA/ACC Guidelines recommend in patients with stage C HF, avoiding excessive sodium intake to reduce congestive symptoms.3)
There is disagreement about whether salt restriction is appropriate in cases of HF. Due to the stimulation of the neurohormonal system and malnutrition, restricted dietary sodium may have poorer results in people with HF. Contrarily, water retention and, eventually, volume overload in acute HF are primarily induced by positive sodium balance. Recent research has shown links between lower salt intake and greater readmission rates as well as higher mortality. Salt restriction’s role in the treatment of HF is still up for dispute. It seems however, that an individualized nutritional assistance is more beneficial than a standard advice, especially in patients who are at high nutritional risk.15)
A recent review, that included 10 trials (1,011 participants with HF) with 7 days to 83 months of follow-up, and a dietary sodium restriction (from 800 mg/day to 3,000 mg/day) showed no improvement in HF patients’ QoL. A long-term strict dietary sodium restriction did not decrease readmission rates or mortality of patients with HF.16)
Another review including 9 studies involving 479 HF patients concluded that none of the studies provided sufficient data on cardiovascular mortality. Regarding the secondary outcomes of interest, 2 outpatient-based studies found that restricting salt consumption did not improve NYHA functional class, whereas 2 other studies found that it did so significantly. This review concludes that the evidence available is limited, and inconclusive for inpatients.17)
A total of 15 studies, related to salt or fluid restriction, were included in another review. The outcomes of these studies are based on small population groups, using different methodologies between them, and with different restriction targets, but they do demonstrate some benefits in symptoms and QoL as well as a degree of decrease in new hospitalizations. They discovered a lack of convincing data supporting the advantages of sodium/fluid limitation in chronic HF. The data is restricted to a few research that produced contradictory findings. Clinical studies that use randomization are required to close this knowledge gap.18) Finally another review included 13 studies and found that restricting sodium consumption was linked to less adverse clinical outcomes in those who had severe symptoms.19)
Recently, the Study of Dietary Intervention under 100 mmol in Heart Failure (SODIUM-HF Trial) has been published. It was designed to determine if a restriction on dietary salt below 1,500 mg/day, affects clinical outcomes in HF. It was an international, open label, randomized, controlled trial with chronic HF patients getting best tolerated medical therapy in accordance with clinical practice guidelines. 806 participants were randomized to receive standard therapy in accordance with regional recommendations or a low-sodium diet. 15% of those in the low sodium diet group and 17% of those in the usual care group experienced the primary outcome, which is comprised of cardiovascular-related hospital admission, cardiovascular-related emergency department visit, or all-cause death within 12 months (hazard ratio, 0.89; 95% confidence interval [CI], 0.63–1.26; p=0.53). In neither group were any safety incidents associated with the study therapy noted. The authors concluded that dietary salt restriction (to a target of 1,500 mg/day in patients with HF) did not reduce the clinical composite outcome of all-cause mortality, cardiovascular-related hospitalization, or cardiovascular-related emergency department visits compared with usual care over a 12-month period. There was an improvement in the New York Heart Association functional class and patient-reported outcome of QoL, but there was no significant between-group difference in the 6-minute walk distance. Clinicians and patients should think about a dietary intervention comparable to other medical therapies and weigh the possible advantages on an individual basis because the level of dietary salt reduction that would result in a decrease in clinical events has not yet been identified.20)
Fluid restriction
Although usually recommended, there is no clear recommendation of fluid restriction in clinical guidelines. The Korean Society of Heart Failure (KSHF) guidelines recommend to train patient for fluid restriction during education at discharge from a HF hospitalization.1) ESC guidelines recommend considering a fluid restriction of 1.5 to 2 L in patients with severe HF/hyponatremia to relieve symptoms and congestion and increase intake during periods of high heat/humidity and/or nausea/vomiting.2) AHA/ACC guidelines makes a 2b recommendation for it stating that for patients with advanced HF and hyponatremia, the benefit of fluid restriction to reduce congestive symptoms is uncertain.3)
The evidence in which these recommendations are based is very scarce. So, Travers et al.21) carried out small single-blind randomized controlled research to investigate the clinical impact of fluid restriction in class IV HF patients hospitalized. When comparing fluid restricted patients (n=34) with free fluid patients (n=33), this study showed no significant difference in time to clinical stability, time to discontinuation of intravenous diuretic therapy, changes from baseline to achievement of clinical stability in serum urea, serum creatinine, natriuretic peptides, or sodium.
However, another small pilot study, testing the efficacy of an educational and behavioral intervention on compliance with recommended fluid restriction and outcome measures of fluid congestion, symptom distress, and health related QoL revealed that patients receiving the intervention drank marginally less fluid, reported fewer typical HF symptoms, more intense thirst, and maintained HRQOL.22)
Therefore, new randomized control studies are needed to test the efficacy and safety of fluid restriction. The Fluid REStriction in Heart failure vs liberal fluid UPtake (FRESH-UP) study, is aimed to Aims: It is common practice for clinicians to advise fluid restriction in patients with HF, but data from clinical trials are lacking. Furthermore, fluid restriction is connected with thirst discomfort and may have a negative influence on QoL. To address this gap in evidence, the Fluid REStriction in Heart failure vs liberal fluid UPtake (FRESH-UP) study was initiated. Methods: The FRESH-UP study is a randomized, controlled, open-label, multicenter clinical trial that will compare the effects of fluid restriction (1,500 mL/day) against liberal fluid consumption for three months on the QoL of outpatients with chronic HF (New York Heart Association Classes II–III). The main goal is to evaluate the impact on QoL using the Kansas City Cardiomyopathy Questionnaire’s Overall Summary Score after three months (KCCQ). Secondary outcomes include patient-reported fluid intake, safety (death, HF hospitalizations), the KCCQ Clinical Summary Score, each of the KCCQ categories, and clinically relevant improvements in these scores. Thirst distress is measured using the Thirst Distress Scale for patients with HF.23)
EXERCISE
Guidelines recommend exercise as a part of the non-pharmacological treatment of HF but in a rather vague way. The KSHF guidelines recommend the promotion of exercise related activities and the presence of an exercise prescriber in the multidisciplinary team that handles with HF patients.1) In order to increase exercise capacity, QoL, and lower HF hospitalization, ESC recommendations advocate exercise for all patients who are capable of doing so. Moreover supervised, an exercise-based, cardiac rehabilitation program should be considered in patients with more severe disease, frailty, or with comorbidities.2) The 2022 AHA/ACC HF guidelines also recommend in patients with HF who are able to participate, exercise training (ET) (or regular physical activity) to improve functional status, exercise performance, and QoL.3)
Cardiac rehabilitation
Is exercise-based cardiac rehabilitation (ExCR) useful as a form of exercise to improve outcomes in HF? Although current guidelines strongly recommend applying a CR program to all eligible patients with HF, real world data show low participation rates that have been attributed to several barriers, including patient factors, professional factors, and service factors.24)
Two different metanalyses have go through that.
A first analysis was performed on a single dataset, which was obtained from 13 trials with 3,990 patients, (97% with HF with reduced ejection fraction [HFrEF]) where ExCR at least for 3 weeks, was compared with a no exercise control group. It was analyzed to assess the impact of ExCR on health related QoL and exercise capacity in patients with HF, and also differential effects of ExCR across HF patient subgroups. There was a statistically significant difference in favor of ExCR for health related QoL and exercise capacity. At 12-month follow-up, improvements were seen in 6-minute walk test and Minnesota Living with HF score. No consistent evidence was found of differential intervention effects across patient subgroups. The authors conclude that this supports that ExCR should be offered to all HF patients.25)
A second meta-analysis was carried out on 44 trials (January 2013 to January 2018), which included 5,783 patients and a median follow-up of 6 months. Patients reported improved Minnesota Living with Heart Failure questionnaire overall scores (mean difference, 7.1; 95% CI, 10.5–3.7), though ExCR did not lower the risk of all-cause mortality. However, it did lower the risk of all-cause hospitalization (relative risk [RR], 0.70; 95% CI, 0.60–0.83) and HF-specific hospitalization (RR, 0.59; 95% CI, 0.42–0.84).26)
ET
No study has examined the efficacy of ET in preserved (HFpEF) and reduced (HFrEF) ejection fraction HF phenotypes across the same clinically important parameters. An outcome analysis was conducted using a pooled sample size of 11,081 participants, coming from Ninety-three studies (11 HFpEF and 82 HFrEF). ET significantly improved exercise capacity and QoL in both HFpEF and HFrEF patients. HFpEF analysis demonstrated significant improvements in peak VO2 (weighted mean difference: 2.333 mL·min-1·kg-1, p fixed <0.001), 6m walk distance (6MWD) and E/e′ (mean difference: 1.709 m), But such benefits did not translate into significantly reduced hospitalization or mortality after short-term follow-up.27)
As the impact of ET and physiotherapy on heart function and pulmonary circulation parameters in HF with preserved ejection fraction (HFpEF) patients is uncertain, a metanalysis was performed identifying eighteen articles (n=418 trained subjects, 4 to 52 weeks of training). After training, the mitral E/e' ratio significantly decreased in five trials. Findings were inconsistent regarding improvement of cardiac output, E/A ratio, and E wave DecT. Therefore, a broader and more complex assessment of the effect of ET on parameters reflecting cardiac function in future pEF studies is needed.28)
Another meta-analysis was conducted to examine the effects of ET on LV diastolic function, exercise capacity, and QoL in HFpEF patients. This meta-analysis included 8 RCTs with 436 participants (ET 12 to 24 weeks). The findings demonstrated that ET increases exercise capacity and QoL in HFpEF patients without significantly altering LV systolic or diastolic function.29)
A secondary analysis of the intervention arm of the EJECTION-HF trial investigated the association between ET and improvement in 6-min walk distance (6MWD). Being physically active and frequent program attendance were associated with significant improvements in 6MWD.30) Another secondary analysis of the EJECTION-HF trial, analyzed the effects on ET on frail subjects, using a frailty index (FI) that was constructed using cumulative deficits index at randomization and 6-month follow-up. Participants who were frailer had significantly greater improvements in FI and improvement in physical activity, compared with not-frail participants. Neither base-line frailty nor intervention was significantly associated with 12-month death or readmission.31)
Type of exercise
Another relevant question is the type of exercise that is more beneficial for HF patients, that means interval training (IT) or continuous training (CT). A recent metanalysis compared the effects of IT vs. CT on cardiorespiratory fitness and exercise tolerance of patients with HF. A total of seventeen randomized controlled trials with 617 patients were included. The meta-analysis showed that IT can improve a patient’s peak oxygen uptake, left ventricular ejection fraction (LVEF), and 6MWD as compared with CT. However, there were no statistical significance on respiratory exchange ratio, CO2 ventilation equivalent slope and resting heart rate. This data demonstrates that IT is superior to CT for enhancing patients with HF’s cardiorespiratory fitness and exercise tolerance. However, additional well-planned investigations are required.32)
Another research compared the effects of high-intensity interval training (HIIT) and moderate-intensity continuous training on exercise capacity and a number of prognostic indicators in people with HF and coronary artery disease (CAD). A total of 15 studies were included comprising 664 patients, HIIT significantly improved prognostic markers, as LVEF in patients with HF.33)
Another meta-analysis that included 28 publications and 2,563 participants compared the impact of aerobic, resistance, or concurrent exercise on natriuretic peptide, to a control group. According to the findings, ET, particularly aerobic exercise, can increase natriuretic peptide levels, which may indicate that the body has adapted positively to aerobic exercise.34)
A further metanalysis was performed to evaluate the effects of aerobic exercise on peak oxygen uptake (peak VO2), minute ventilation/carbon dioxide production (VE/VCO2 slope), and health related QoL among patients with HF and HfpEF. Ten intervention studies were included providing a total of 399 patients. Compared with control, aerobic exercise moderately improves peak VO2 and health related QoL in HfpEF.35)
Alternative exercise
Two studies were performed to identify and summarize the existing evidence and to systematically determine the clinical effectiveness of Tai Chi in the management of HF. However, the results showed that further rigorous and comprehensive RCTs are required to provide robust evidence for definitive conclusions.36,37) A another research examined how traditional Chinese exercise affected HF. The findings suggest that it could be a successful adjuvant treatment for HF patients. However, more rigorous investigations are urgently required to corroborate these findings due to the inclusion of the low-quality elucidations.38) A further study analyzed the effect of Buddhist Walking Meditation (BWM) versus aerobic exercise. A six-week BWM program did not improve the functional capacity, QoL, or hemodynamic characteristics of the HF patients, compared with the values of the patients in the aerobic exercise program.39)
SUBSTANCE ABUSE
Substance abuse is widespread among people with HF, and it’s linked to worse clinical results. The usage of drugs such alcohol, cigarettes, cannabis, and cocaine can hasten the onset and progression of HF. While moderate alcohol use may reduce the incidence of HF, heavy alcohol consumption might cause dilated cardiomyopathy. Substance abuse disorders are substantial causes of morbidity that are independently related HF worsening and hospitalizations, even though they are less frequent than typical medical HF comorbidities. Therefore, HF patients may benefit from better detection and treatment of drug misuse.40)
How much alcohol is required to induce disease is unclear, and the epidemiological mechanisms that connect alcohol use to cardiomyopathy and HF are also poorly understood,41) however patients should receive advice on how to stay away from heavy drinking and binge drinking. Patients who now consume 1–2 drinks per week may be allowed to continue. It’s not a good idea to start treating folks who don’t drink. An important focus of treatment for people with alcoholic cardiomyopathy is to get them to drink less, ideally never at all. Through CAD and processes unrelated to CAD, tobacco raises the risk of HF. Smoking impairs HF patient outcomes, and quitting is linked to a lower incidence of serious adverse cardiac events. Strong focus should be put on quitting smoking among HF patients who are currently smokers. Even a slight reduction in cigarette smoking may provide some therapeutic advantages for those with HF. Future research is required to better understand how these drugs affect HF development as well as how they affect those who already have the disease.42)
MANAGEMENT OF SLEEP APNEA
In patients with sleep apnea, weight loss and HF treatment may help to reduce it. Nevertheless, data are far from being clear.43) In a RCT 1,325 patients with HFrEF and sleep apnoea were randomized to receive guideline-based medical treatment with adaptive servo-ventilation or guideline-based medical treatment alone. In the adaptive servo-ventilation group, all-cause mortality and cardiovascular mortality were considerably greater than in the control group.44)
Small randomized clinical investigations have shown some beneficial effects of Continuous Positive Airway Pressure (CPAP) in HFrEF, namely in Apnoea Hypopnoea Index, number of arousals per night, and daytime systolic BP and heart rate in conjunction with a decrease in LV end-systolic diameter and increase in LVEF. As for mortality and morbidity I n HFrEF related to CPAP treatment in HFrEF, small studies showed tendency toward decreased mortality rate in treated vs untreated In HFpEF CPAP therapy has shown improvement in LV diastolic function in patients with SA but without HFpEF.45)
LIFESTYLE MODIFICATION IN ELDERLY FRAIL PATIENTS
Lifestyle modification in also needed in elderly frail patients as they are more and more common.31) As for physical activity a small post-hoc analysis aimed to analyse the effects of ET on frail and non-frail individuals showed that frailer participant exhibited lower 6WMDs at enrolment but similar 6-month improvements.31) There are no data on fluid nor salt restriction, but Frail patients could not benefit of a severe restriction, due to adverse events as worsening renal function, hyponatremia etc.
CONCLUSION
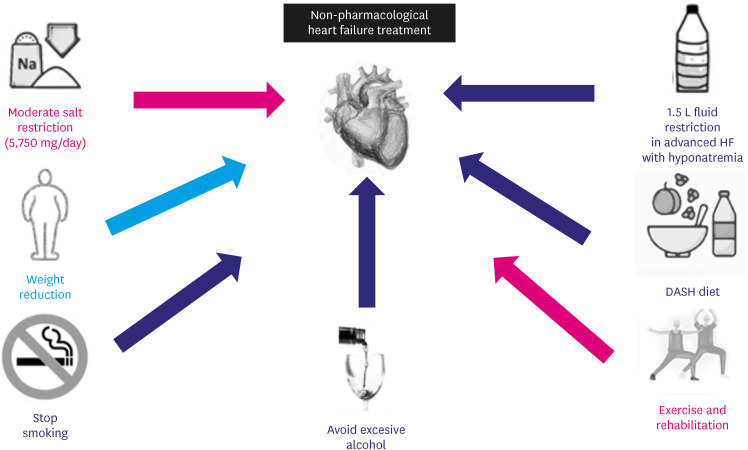
Although current evidence on lifestyle changes in HF is scant, data available show some aspect that could be introduced on daily practice in HF management, as summarized in Figure 1. As for diet, DASH Diet could be clearly beneficial while Mediterranean diet doesn’t have enough evidence now.
Figure 1. Lifestyle Modifications recommended in heart failure and their respective level of evidence. In blue those recommendations that are based on randomized clinical trials. In purple those recommendations with some data, but that need new studies. In red those recommendations that have shown no clear benefit.
As for salt restriction a moderate restriction (below 5,750 mg of salt per day) is better than a strict reduction (below 3,750 mg/day).
Exercise and cardiac rehabilitation are beneficial for HF. Nevertheless, there are no clear recommendations about type of exercise, duration, etc. Although fluid restriction has been a part of the recommendations for a long time, a certain number of small clinical trials has failed to show improvement in congestion by fluid restriction. Smoking should be stopped, and excessive amounts of alcohol drinking avoided, but there is a clear need for clinical trial or registry on these subjects.
Evidence in HF and obesity is contradictory, however, a nutritional assessment approach could help.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Camafort-Babkowski M, Park SM, Kang SM.
- Data curation: Camafort-Babkowski M.
- Investigation: Camafort-Babkowski M.
- Methodology: Camafort-Babkowski M.
- Supervision: Camafort-Babkowski M, Park SM, Kang SM.
- Validation: Camafort-Babkowski M, Park SM.
- Visualization: Camafort-Babkowski M.
- Writing - original draft: Camafort-Babkowski M.
- Writing - review & editing: Park SM, Kang SM.
References
- 1.Kim MS, Lee JH, Kim EJ, et al. Korean guidelines for diagnosis and management of chronic heart failure. Korean Circ J. 2017;47:555–643. doi: 10.4070/kcj.2017.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 4.Kim KJ, Cho HJ, Kim MS, et al. Focused update of 2016 Korean Society of Heart Failure guidelines for the management of chronic heart failure. Int J Heart Fail. 2019;1:4–24. doi: 10.36628/ijhf.2019.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang J, Moser DK, Biddle MJ, Oh G, Lennie TA. Age- and sex-matched comparison of diet quality in patients with heart failure to similarly aged healthy older adults. J Nutr Sci. 2021;10:e65. doi: 10.1017/jns.2021.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vest AR, Chan M, Deswal A, et al. Nutrition, obesity, and cachexia in patients with heart failure: a consensus statement from the Heart Failure Society of America Scientific Statements Committee. J Card Fail. 2019;25:380–400. doi: 10.1016/j.cardfail.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Pritchett AM, Deswal A, Aguilar D. Lifestyle modification with diet and exercise in obese patients with heart failure - a pilot study. J Obes Weight Loss Ther. 2012;2:1–8. doi: 10.4172/2165-7904.1000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung ML, Lee SJ, Moser DK, Kang J, Lennie TA. A comparison of diet quality of patients with heart failure and their family caregivers. J Cardiovasc Nurs. 2020;35:101–106. doi: 10.1097/JCN.0000000000000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miró Ò, Estruch R, Martín-Sánchez FJ, et al. Adherence to mediterranean diet and all-cause mortality after an episode of acute heart failure: results of the MEDIT-AHF study. JACC Heart Fail. 2018;6:52–62. doi: 10.1016/j.jchf.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Wickman BE, Enkhmaa B, Ridberg R, et al. Dietary management of heart failure: dash diet and precision nutrition perspectives. Nutrients. 2021;13:4424. doi: 10.3390/nu13124424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carbone S, Lavie CJ, Arena R. Obesity and heart failure: focus on the obesity paradox. Mayo Clin Proc. 2017;92:266–279. doi: 10.1016/j.mayocp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Hwang IC, Choi HM, Yoon YE, et al. Body mass index, muscle mass, and all-cause mortality in patients with acute heart failure: the obesity paradox revisited. Int J Heart Fail. 2022;4:95–109. doi: 10.36628/ijhf.2022.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Hajj EC, El Hajj MC, Sykes B, et al. Pragmatic weight management program for patients with obesity and heart failure with preserved ejection fraction. J Am Heart Assoc. 2021;10:e022930. doi: 10.1161/JAHA.121.022930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2016;315:36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chrysohoou C, Mantzouranis E, Dimitroglou Y, Mavroudis A, Tsioufis K. Fluid and salt balance and the role of nutrition in heart failure. Nutrients. 2022;14:1386. doi: 10.3390/nu14071386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu C, Cheng M, Su Y, Ma T, Lei X, Hou Y. Effect of dietary sodium restriction on the quality of life of patients with heart failure. J Cardiovasc Nurs. 2022;37:570–580. doi: 10.1097/JCN.0000000000000880. [DOI] [PubMed] [Google Scholar]
- 17.Mahtani KR, Heneghan C, Onakpoya I, et al. Reduced salt intake for heart failure: a systematic review. JAMA Intern Med. 2018;178:1693–1700. doi: 10.1001/jamainternmed.2018.4673. [DOI] [PubMed] [Google Scholar]
- 18.García-García A, Alvarez-Sala-Walther LA, Lee HY, Sierra C, Pascual-Figal D, Camafort M. Is there sufficient evidence to justify changes in dietary habits in heart failure patients? A systematic review. Korean J Intern Med. 2022;37:37–47. doi: 10.3904/kjim.2020.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sousa MM, Gouveia BL, Almeida TD, Freire ME, Melo FA, Oliveira SH. Evidence related to sodium restriction in patients with heart failure. Rev Bras Enferm. 2020;73:e20180874. doi: 10.1590/0034-7167-2018-0874. [DOI] [PubMed] [Google Scholar]
- 20.Ezekowitz JA, Colin-Ramirez E, Ross H, et al. Reduction of dietary sodium to less than 100 mmol in heart failure (SODIUM-HF): an international, open-label, randomised, controlled trial. Lancet. 2022;399:1391–1400. doi: 10.1016/S0140-6736(22)00369-5. [DOI] [PubMed] [Google Scholar]
- 21.Travers B, O’Loughlin C, Murphy NF, et al. Fluid restriction in the management of decompensated heart failure: no impact on time to clinical stability. J Card Fail. 2007;13:128–132. doi: 10.1016/j.cardfail.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Reilly CM, Higgins M, Smith A, Culler SD, Dunbar SB. Isolating the benefits of fluid restriction in patients with heart failure: a pilot study. Eur J Cardiovasc Nurs. 2015;14:495–505. doi: 10.1177/1474515114541729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrmann JJ, Beckers-Wesche F, Baltussen LE, et al. Fluid restriction in heart failure vs liberal fluid uptake: rationale and design of the randomized FRESH-UP study. J Card Fail. 2022;28:1522–1530. doi: 10.1016/j.cardfail.2022.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Chun KH, Kang SM. Cardiac rehabilitation in heart failure. Int J Heart Fail. 2020;3:1–14. doi: 10.36628/ijhf.2020.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor RS, Walker S, Smart NA, et al. Impact of exercise rehabilitation on exercise capacity and quality-of-life in heart failure: individual participant meta-analysis. J Am Coll Cardiol. 2019;73:1430–1443. doi: 10.1016/j.jacc.2018.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor RS, Long L, Mordi IR, et al. Exercise-based rehabilitation for heart failure: cochrane systematic review, meta-analysis, and trial sequential analysis. JACC Heart Fail. 2019;7:691–705. doi: 10.1016/j.jchf.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 27.Edwards JJ, O’Driscoll JM. Exercise training in heart failure with preserved and reduced ejection fraction: a systematic review and meta-analysis. Sports Med Open. 2022;8:76. doi: 10.1186/s40798-022-00464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palevičiūtė E, Šimbelytė T, Eichstaedt CA, et al. The effect of exercise training and physiotherapy on left and right heart function in heart failure with preserved ejection fraction: a systematic literature review. Heart Fail Rev. 2022 doi: 10.1007/s10741-022-10259-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukuta H, Goto T, Wakami K, Kamiya T, Ohte N. Effects of exercise training on cardiac function, exercise capacity, and quality of life in heart failure with preserved ejection fraction: a meta-analysis of randomized controlled trials. Heart Fail Rev. 2019;24:535–547. doi: 10.1007/s10741-019-09774-5. [DOI] [PubMed] [Google Scholar]
- 30.Adsett JA, Morris NR, Mudge AM. Impact of exercise training program attendance and physical activity participation on six minute walk distance in patients with heart failure. Physiother Theory Pract. 2021;37:1051–1059. doi: 10.1080/09593985.2019.1669232. [DOI] [PubMed] [Google Scholar]
- 31.Mudge AM, Pelecanos A, Adsett JA. Frailty implications for exercise participation and outcomes in patients with heart failure. J Am Geriatr Soc. 2021;69:2476–2485. doi: 10.1111/jgs.17145. [DOI] [PubMed] [Google Scholar]
- 32.Li D, Chen P, Zhu J. The effects of interval training and continuous training on cardiopulmonary fitness and exercise tolerance of patients with heart failure—a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18:6761. doi: 10.3390/ijerph18136761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C, Xing J, Zhao B, et al. The effects of high-intensity interval training on exercise capacity and prognosis in heart failure and coronary artery disease: a systematic review and meta-analysis. Cardiovasc Ther. 2022;2022:4273809. doi: 10.1155/2022/4273809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malandish A, Ghadamyari N, Karimi A, Naderi M. The role of exercise training on cardiovascular peptides in patients with heart failure: a systematic review and meta-analysis. Curr Res Physiol. 2022;5:270–286. doi: 10.1016/j.crphys.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomes-Neto M, Durães AR, Conceição LS, et al. Effect of aerobic exercise on peak oxygen consumption, VE/VCO2 slope, and health-related quality of life in patients with heart failure with preserved left ventricular ejection fraction: a systematic review and meta-analysis. Curr Atheroscler Rep. 2019;21:45. doi: 10.1007/s11883-019-0806-6. [DOI] [PubMed] [Google Scholar]
- 36.Huang J, Qin X, Shen M, Xu Y, Huang Y. The effects of tai chi exercise among adults with chronic heart failure: an overview of systematic review and meta-analysis. Front Cardiovasc Med. 2021;8:589267. doi: 10.3389/fcvm.2021.589267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor-Piliae R, Finley BA. Benefits of tai chi exercise among adults with chronic heart failure: a systematic review and meta-analysis. J Cardiovasc Nurs. 2020;35:423–434. doi: 10.1097/JCN.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 38.Yao F, Zhang Y, Kuang X, et al. Effects of cardiac rehabilitation training in patients with heart failure based on traditional Chinese exercise: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2021;2021:1068623. doi: 10.1155/2021/1068623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srisoongnern S, Pajareya K, Sriboon R, Thanakiatpinyo T, Chirakarnjanakorn S, Thirapatarapong W. Effects of Buddhist walking meditation on exercise capacity and quality of life of patients with chronic heart failure: a randomized controlled trial. Heart Lung. 2021;50:363–368. doi: 10.1016/j.hrtlng.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Nishimura M, Bhatia H, Ma J, et al. The impact of substance abuse on heart failure hospitalizations. Am J Med. 2020;133:207–213.e1. doi: 10.1016/j.amjmed.2019.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andersson C, Schou M, Gustafsson F, Torp-Pedersen C. Alcohol intake in patients with cardiomyopathy and heart failure: consensus and controversy. Circ Heart Fail. 2022;15:e009459. doi: 10.1161/CIRCHEARTFAILURE.121.009459. [DOI] [PubMed] [Google Scholar]
- 42.Grubb AF, Greene SJ, Fudim M, Dewald T, Mentz RJ. Drugs of abuse and heart failure. J Card Fail. 2021;27:1260–1275. doi: 10.1016/j.cardfail.2021.05.023. [DOI] [PubMed] [Google Scholar]
- 43.Vazir A, Sundaram V. Management of sleep apnea in heart failure. Heart Fail Clin. 2018;14:635–642. doi: 10.1016/j.hfc.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373:1095–1105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Camafort M, Kario K. Hypertension, heart failure, and frailty in older people: a common but unclear situation. J Clin Hypertens (Greenwich) 2020;22:1763–1768. doi: 10.1111/jch.14004. [DOI] [PMC free article] [PubMed] [Google Scholar]