Abstract
Background and Objectives
The thick perirenal fat pad can induce high intracapsular pressure and cause compression of the renal vasculature and resultant congestive nephropathy. The current study investigated the association of perirenal fat thickness with kidney dysfunction in patients with acute decompensated heart failure (ADHF).
Methods
Data from 266 patients hospitalized with ADHF were analyzed. Patients were divided into two groups according to the glomerular filtration rate (GFR) at admission (preserved kidney function [GFR ≥60 mL/min/1.73 m2] and reduced kidney function [GFR <60 mL/min/1.73 m2] groups). Right and left posterior perirenal fat thicknesses were measured using computed tomography, and their average values were calculated. Associated factors with reduced kidney function was assessed by logistic regression model, presenting with odds ratio (OR) and confidence interval (CI).
Results
Increasing age (OR, 1.08; 95% CI, 1.04–1.12; p<0.001), diabetes mellitus (OR, 2.46; 95% CI, 1.18–5.12; p<0.017), increased log N-terminal pro-B-type natriuretic peptide (NT-proBNP) (OR, 1.82; 95% CI, 1.32–2.52; p<0.001), and increased average perirenal fat thickness (OR, 1.11; 95% CI, 1.06–1.16; p<0.001) were independently associated with reduced kidney function. In the subgroup analyses, patients over 70 years old, the ratio of mitral-to-mitral annular velocity >15, elevated log NT-proBNP had a significantly higher association with increased perirenal fat thickness with reduced kidney function.
Conclusions
Thick perirenal fat pads were independently associated with kidney function deterioration in patients hospitalized with ADHF.
Keywords: Heart failure, Kidney, Adipose tissue
INTRODUCTION
The term cardiorenal syndrome describes a spectrum of disorders involving the heart and kidneys, in which acute or chronic dysfunction in the heart or kidneys may induce dysfunction in other organs.1,2) Five phenotypes have been classified, and type 1 cardiorenal syndrome was defined as acute worsening of cardiac function leading to kidney dysfunction. In clinical practice, acute kidney injury frequently occurs in patients with acute decompensated heart failure (ADHF).3) Kidney dysfunction in heart failure (HF) has traditionally been believed to result from decreased kidney perfusion and is related to hormonal and neural changes, and persistent renal venous congestion has been regarded as a more important hemodynamic contributor to the development of kidney dysfunction.4,5,6)
Perirenal fat is a fat pad surrounding the kidneys in the retroperitoneal space and is located between the renal fibrous membrane and the renal fascia.7) Recent epidemiological studies revealed that perirenal fat is a risk predictor of cardiovascular disease (CVD), independent of common clinical variables.8) The thickness of perirenal fat surrounding the kidney and accumulated fat volume in the renal sinus have been associated with various chronic diseases, including chronic kidney disease (CKD), arteriosclerosis, hypertension, and the onset of diabetes mellitus.8,9) It has recently suggested that perirenal fat tissue plays a role in acute kidney function deterioration in patients with ADHF by compressing the renal vasculature, leading to pathologic activation of the renin-angiotensin-aldosterone system and reduced renal perfusion,7) even though its role in patients with ADHF needs further investigation.
It can be speculated that a thick perirenal fat pad might induce high intracapsular pressure more easily and cause compression of the renal vasculature and resultant congestive nephropathy. We therefore hypothesized that ADHF patients with a thick perirenal fat pad might be more vulnerable to kidney function deterioration than those with a thin pad. As perirenal fat thickness can be easily measured using computed tomographic images, the current study aimed to investigate the association between perirenal fat thickness assessed by computed tomography (CT) and kidney dysfunction in patients hospitalized with ADHF. In addition, we assessed the characteristics correlated with perirenal fat thickness in the population.
METHODS
Study population
We analyzed the data of patients hospitalized with ADHF between February 2019 and April 2022 at a single center for CVD in Korea. The exclusion criteria were as follows: acute myocardial infarction, aborted sudden cardiac death, hypertrophic cardiomyopathy, peripartum cardiomyopathy, active infective endocarditis, significant organic valvular heart disease, prosthetic heart valves, isolated right ventricular failure, single kidney, history of kidney transplantation, and known CKD. Among the 365 patients with ADHF without known CKD, 99 who lacked CT data covering fields at the kidney level within 6 months of index admission were also excluded. CKD was defined as glomerular filtration rate (GFR) <60 mL/min/1.73 m2 for 3 months or more prior to the index admission. Ultimately, 266 patients hospitalized with ADHF were included in the final analysis. Patients were divided into 2 groups according to the GFR at admission (preserved kidney function [GFR ≥60 mL/min/1.73 m2] and reduced kidney function [GFR <60 mL/min/1.73 m2] groups).
This study was approved by the ethics committee of our institution. The need for informed consent was waived because of the retrospective nature of the study.
Clinical variables
Data regarding the presence of hypertension, diabetes mellitus, coronary artery disease, and atrial fibrillation were retrieved from medical records. Medication history before index admission was obtained regarding angiotensin converting enzyme inhibitor (ACEI), angiotensin receptor antagonist (ARB), angiotensin receptor neprilysin inhibitor (ARNI), and diuretics. Height, weight, and blood pressure (BP) were measured during admission, and the first value after admission was used in the analysis. Body mass index (BMI) was calculated using height and weight. HF was categorized according to left ventricular (LV) ejection fraction (EF) as follows: HF with preserved EF (HFpEF), LVEF ≥50%; HF with reduced EF (HFrEF), LVEF ≤40%; and HF with mildly reduced EF (HFmrEF), LVEF 41–49%.10)
Laboratory tests, including hemoglobin, creatinine, aspartate transaminase, alanine transaminase, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels, were obtained at admission. Kidney function was defined by the estimated GFR, calculated using the formula developed and validated in the Modification of Diet in Renal Disease study as follows11): GFR (mL/min/1.73 m2)=186.3×(Serum Creatinine, mg/dL)−1.154×Age−0.203×(0.742, if female). NT-proBNP levels were logarithmically transformed to achieve a normal distribution.
Imaging modalities
Data from the first transthoracic echocardiography after the index admission were used in the analysis. The mean time interval between admission and echocardiography performance was 1.2±1.5 days. Two-dimensional and Doppler echocardiography were performed to assess cardiac structure and function according to the guidelines of the American Society of Echocardiography.12) LV end-diastolic dimension (EDD) and LV end-systolic dimension (ESD) were measured from 2-dimensional images. LVEF was calculated using the LV end-diastolic and end-systolic volumes. LV mass was calculated using a formula proposed by the American Society of Echocardiography guidelines.12) The LV mass index was defined as the LV mass indexed to body surface area. Left atrial volume was calculated using the biplane area–length method. According to the guidelines, 2-dimensional volumetric measurements were based on left atrial area measurements using tracings of the blood-tissue interface and left atrial lengths on apical 4- and 2-chamber views.12) Left atrial volume index (LAVI) was defined as the left atrial volume indexed to body surface area.
The mitral inflow velocities were obtained using pulse-wave Doppler in the apical four-chamber view. Mitral early diastolic (E) velocity and peak early diastolic mitral annular velocity (e′) were measured. We calculated the E/e′ ratio, which is a measure of LV filling pressure, by dividing E velocity by e′ velocity. The calculated systolic pulmonary artery pressure (SPAP) was defined as: 4×(maximum velocity of the tricuspid regurgitation [TR] jet)2+right atrial pressure. Right atrial pressure was estimated by measuring the inferior vena cava diameter and he associated respiratory changes.13)
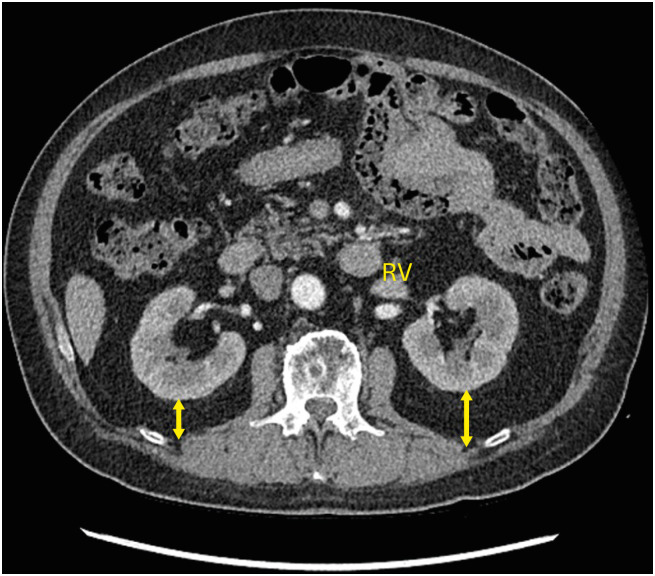
The mean time interval between admission and CT performance was 0.9±0.1 months (median, 0 months; interquartile range, 0–6 months). CT images were used for perirenal fat thickness analysis. The mean time interval between admission and CT acquisition was 0.7±2.8 months. Perirenal fat thickness was defined as the direct distance from the posterior capsule to the posterior abdominal wall at the level of the renal vein (Figure 1).14) The average perirenal fat was calculated using the right and left posterior perirenal fat thicknesses.
Figure 1. Method of measuring posterior perirenal fat thickness. Yellow double-headed arrows indicate the posterior perirenal fat thickness.
RV = renal vein.
Statistics
Demographic characteristics were reported as percentages or means±standard deviations. The patient groups were compared using χ2 statistics for categorical variables and Student’s t-test for continuous variables. To determine potential independent associations between the variables and reduced kidney function, binary logistic regression was applied. Variables with p values <0.1 in the univariable analysis were entered into the multivariable binary logistic regression model, and the odds ratios (ORs) and 95% confidence intervals (CIs) were reported. The interaction between the average perirenal fat thickness and the adjusting factors was evaluated. To determine potential independent correlation between the variables and perirenal fat thickness, linear logistic regression was applied. Variables displaying a p value <0.2 in univariable analysis were entered into the multivariable linear logistic regression model. We fitted a cubic spline model for assessment of correlation between glomerular filtration rate and the mean renal fat thickness. To evaluate intra- and interobserver reproducibility for renal fat thickness measurement, the intraclass correlation coefficient (ICC) was calculated. Good correlation was defined as an ICC >0.8. Statistical significance was set at p<0.05.
RESULTS
Patient characteristics
Among the 266 patients hospitalized with ADHF, 198 (87.6%) were initially diagnosed with HF and 28 (12.4%) with acute decompensation of previously diagnosed HF. The mean patient age was 77±12 years, and 135 patients (59.2%) were women. There were 120 patients with preserved kidney function and 106 with reduced kidney function. Mean GFR was 82.1±14.5 mL/min/1.73 m2 and 41.6±10.7 mL/min/1.73 m2 in the preserved and reduced kidney function groups, respectively.
Table 1 summarizes the baseline characteristics of the study population. There were no significant differences in the prevalence of sex, hypertension, diabetes mellitus, atrial fibrillation, BMI, systolic and diastolic BP, medications at admission, etiology of HF, and type of HF between the preserved and reduced kidney function groups (all p>0.05). Patients with reduced kidney function were older than those with preserved kidney function (81±8 vs. 73±14 years, p<0.001). Echocardiographic variables, including LVEDD, LVESD, LVEF, LV mass index, LAVI, E/e′, peak TR velocity, and SPAP, were comparable between the groups. Log NT-proBNP (8.9±1.4 pg/mL vs. 8.4 pg/mL, p<0.001) and average perirenal fat thickness (13.4 mm vs. 8.7 mm, p<0.001) were all higher and hemoglobin was lower (11.5±2.0 g/dL vs. 12.5±2.1 g/dL, p<0.001) in patients with reduced kidney function compared to those with preserved kidney function.
Table 1. Baseline characteristics.
| Variables | Overall | Kidney function at admission | ||||
|---|---|---|---|---|---|---|
| Preserved (GFR ≥60 mL/min/1.73 m2) | Reduced (GFR <60 mL/min/1.73 m2) | p value | ||||
| No. of patients | 226 | 120 | 106 | |||
| Demographics | ||||||
| Age (years) | 77±12 | 73±14 | 81±8 | <0.001* | ||
| Female sex | 135 (59.2) | 68 (56.7) | 67 (63.2) | 0.317 | ||
| Hypertension | 88 (38.9) | 46 (38.3) | 42 (39.6) | 0.843 | ||
| Diabetes mellitus | 59 (26.1) | 25 (20.8) | 34 (32.1) | 0.055 | ||
| Atrial fibrillation | 96 (42.5) | 51 (42.5) | 45 (42.5) | 0.994 | ||
| BMI (kg/m2) | 23.6±5.4 | 23.5±6.2 | 23.6±4.5 | 0.997 | ||
| Systolic BP (mm Hg) | 131.8±21.2 | 130.6±21.5 | 133.2±20.7 | 0.350 | ||
| Diastolic BP (mm Hg) | 76.1±14.4 | 77.7±15.5 | 74.3±12.9 | 0.072 | ||
| HF category | 0.097 | |||||
| HFrEF | 119 (52.7) | 67 (55.8) | 52 (49.1) | |||
| HFmrEF | 37 (16.4) | 23 (19.2) | 14 (13.2) | |||
| HFpEF | 70 (31.0) | 30 (25.0) | 40 (37.7) | |||
| Etiology | 0.876 | |||||
| Ischemic | 54 (23.9) | 28 (23.3) | 26 (24.5) | |||
| Non-ischemic | 172 (76.1) | 92 (76.7) | 80 (75.5) | |||
| NYHA functional class | 0.117 | |||||
| III | 37 (16.4) | 24 (20.0) | 13 (12.3) | |||
| IV | 189 (83.6) | 96 (80.0) | 93 (87.7) | |||
| Medication | ||||||
| ACEI | 5 (2.2) | 3 (2.5) | 2 (1.9) | 0.753 | ||
| ARB | 68 (30.1) | 30 (25.0) | 38 (55.9) | 0.076 | ||
| ARNI | 2 (0.1) | 2 (1.7) | 0 (0) | 0.110 | ||
| Beta blocker | 59 (26.1) | 29 (24.2) | 30 (28.2) | 0.480 | ||
| Diuretics | 53 (3.5) | 22 (18.3) | 31 (29.2) | 0.053 | ||
| Echocardiography | ||||||
| LVEDD (mm) | 54.3±8.5 | 54.9±8.6 | 53.6±8.4 | 0.255 | ||
| LVESD (mm) | 42.2±11.8 | 43.4±11.7 | 40.8±11.7 | 0.095 | ||
| LVEF (%) | 39.6±17.4 | 37.6±16.9 | 41.9±17.9 | 0.068 | ||
| LV mass index (g/m2) | 129.7±40.5 | 127.6±37.3 | 132.0±43.9 | 0.416 | ||
| LAVI (mL/m2) | 58.3±20.5 | 58.5±22.2 | 58.2±18.4 | 0.919 | ||
| E/e′ | 20.7±9.2 | 20.3±9.1 | 21.2±9.3 | 0.464 | ||
| Peak TR velocity (m/s) | 2.7±0.5 | 2.7±0.5 | 2.8±0.5 | 0.226 | ||
| SPAP (mm Hg) | 38.0±13.3 | 37.0±13.6 | 39.2±13.0 | 0.224 | ||
| Laboratory findings | ||||||
| GFR (mL/min/1.73 m2) | 63.1±24.0 | 82.1±14.5 | 41.6±10.7 | <0.001* | ||
| Log NT-proBNP (pg/mL) | 8.6±1.2 | 8.4±1.1 | 8.9±1.4 | <0.001* | ||
| Hemoglobin (g/dL) | 12.0±2.2 | 12.5±2.1 | 11.5±2.0 | <0.001* | ||
| Aspartate transaminase (IU/L) | 49.0±62.4 | 43.6±33.5 | 55.1±83.7 | 0.168 | ||
| Alanine transaminase (IU/L) | 36.2±69.6 | 30.9±35.4 | 42.3±94.2 | 0.219 | ||
| CT | ||||||
| Right perirenal fat thickness (mm) | 10.2±7.9 | 8.1±6.6 | 12.5±8.7 | <0.001* | ||
| Left perirenal fat thickness (mm) | 11.7±8.7 | 9.3±7.5 | 14.4±9.2 | <0.001* | ||
| Average perirenal fat thickness (mm) | 10.9±8.1 | 8.7±6.9 | 13.4±8.7 | <0.001* | ||
Values are presented as number with percentages or means±standard deviations.
GFR = glomerular filtration rate; BMI =body mass index; BP = blood pressure; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; HFmrEF = heart failure with mildly reduced ejection fraction; HFpEF = heart failure with preserved ejection fraction; NYHA = New York Heart Association; ACEI = Angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; ARNI = angiotensin receptor neprilysin inhibitor; LVEDD = left ventricular end-diastolic dimension; LVESD = left ventricular end-systolic dimension; LVEF = left ventricular ejection fraction; LV = left ventricular; LAVI = left atrial volume index; E/e′ = the ratio of early diastolic mitral inflow to mitral annular velocity; TR = tricuspid regurgitation; SPAP = systolic pulmonary artery pressure; NT-proBNP = N-terminal pro-B-type natriuretic peptide; CT = computed tomography.
*p value <0.05.
Factors associated with reduced kidney function
Table 2 shows the results of the univariable and multivariable logistic regression analyses for factors associated with reduced kidney function in patients hospitalized with ADHF. In multivariate analysis, increasing age (OR, 1.08; 95% CI, 1.04–1.12; p<0.001), diabetes mellitus (OR, 2.46; 95% CI, 1.18–5.12; p<0.017), increased log NT-proBNP (OR, 1.82; 95% CI, 1.32–2.52; p<0.001), and increased average perirenal fat thickness (OR, 1.11; 95% CI, 1.06–1.16; p<0.001) were associated with reduced kidney function, even after adjusting for diastolic BP, HF category, LVESD, and LVEF.
Table 2. Factors associated with reduced kidney function in patients with acute decompensated HF.
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age, per year | 1.07 | 1.04–1.10 | <0.001* | 1.08 | 1.04–1.12 | <0.001* |
| Female sex | 0.76 | 0.45–1.30 | 0.317 | - | - | - |
| Hypertension | 1.06 | 0.62–1.80 | 0.843 | - | - | - |
| Diabetes mellitus | 1.79 | 0.98–3.27 | 0.056 | 2.46 | 1.18–5.12 | 0.017* |
| Atrial fibrillation | 1.00 | 0.95–1.05 | 0.977 | - | - | - |
| BMI | 1.00 | 0.95–1.05 | 0.997 | - | - | - |
| Systolic BP | 1.01 | 0.99–1.02 | 0.349 | - | - | - |
| Diastolic BP | 0.98 | 0.97–1.00 | 0.075 | 0.99 | 0.96–1.01 | 0.987 |
| HFrEF (vs. HFpEF) | 1.72 | 0.95–3.12 | 0.075 | 1.61 | 0.29–8.95 | 0.586 |
| Ischemic etiology | 1.07 | 0.58–1.97 | 0.834 | - | - | - |
| NYHA functional class IV | 1.79 | 0.86–3.72 | 0.120 | - | - | - |
| LVEDD, per 1 mm increase | 0.98 | 0.95–1.01 | 0.982 | - | - | - |
| LVESD, per 1 mm increase | 0.98 | 0.96–1.00 | 0.097 | 1.06 | 1.00–1.12 | 0.051 |
| LVEF, per 1% increase | 1.01 | 1.00–1.03 | 0.069 | 1.05 | 0.99–1.11 | 0.142 |
| LV mass index, per 1 g/m2 increase | 1.00 | 1.00–1.01 | 0.341 | - | - | - |
| LAVI, 1 mL/m2 increase | 1.00 | 0.99–1.01 | 0.919 | - | - | - |
| E/e′, per 1 unit increase | 1.00 | 0.98–1.04 | 0.463 | - | - | - |
| Hemoglobin, per 1 g/dL increase | 0.90 | 0.79–1.01 | 0.182 | - | - | - |
| SPAP, per 1 mmHg increase | 1.01 | 0.99–1.03 | 0.223 | - | - | - |
| Log NT-proBNP, per 1 pg/mL increase | 1.44 | 1.14–1.81 | 0.003* | 1.82 | 1.32–2.52 | <0.001* |
| Average perirenal fat thickness, per 1 mm increase | 1.02 | 1.04–1.13 | <0.001* | 1.11 | 1.06–1.16 | <0.001* |
HF = heart failure; OR = odds ratio; CI = confidence interval; BMI = body mass index; BP = blood pressure; HFrEF = heart failure with reduced ejection fraction; HFpEF = heart failure with preserved ejection fraction; NYHA = New York Heart Association; LVEDD = left ventricular end-diastolic dimension; LVESD = left ventricular end-systolic dimension; LVEF = left ventricular ejection fraction; LV = left ventricular; LAVI = left atrial volume index; E/e′ = the ratio of early diastolic mitral inflow to mitral annular velocity; TR = tricuspid regurgitation; SPAP = systolic pulmonary artery pressure; NT-proBNP = N-terminal pro-B-type natriuretic peptide.
*p value <0.05.
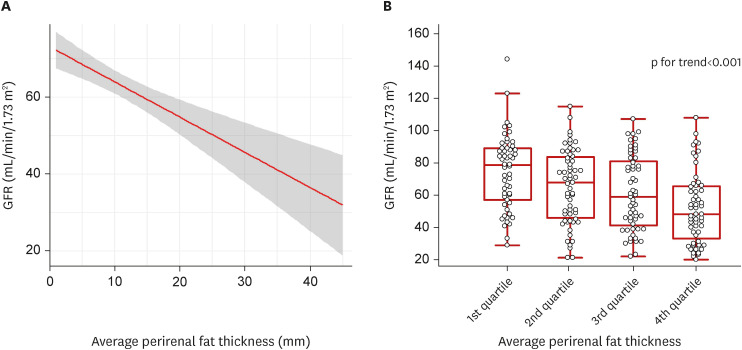
Correlation between GFR and the mean perirenal fat thickness are shown in Figure 2. Cubic spline analysis showed a negative linear correlation between the mean perirenal fat thickness and the GFR. The GFR demonstrated statistically significant association according to the quartiles of the mean perirenal fat thickness (p for trend<0.001).
Figure 2. Correlation between glomerular filtration rate and average perirenal fat thickness.
(A) Cubic spline regression analysis. (B) Changes in glomerular filtration rate according to quartile of average perirenal fat thickness. Shadow area is the 95% confidence interval of the predicted GFR (solid red line). Vertical bars represent 5th to 95th percentiles, boxes represent interquartile range, and horizontal lines represent the median.
GFR = glomerular filtration rate.
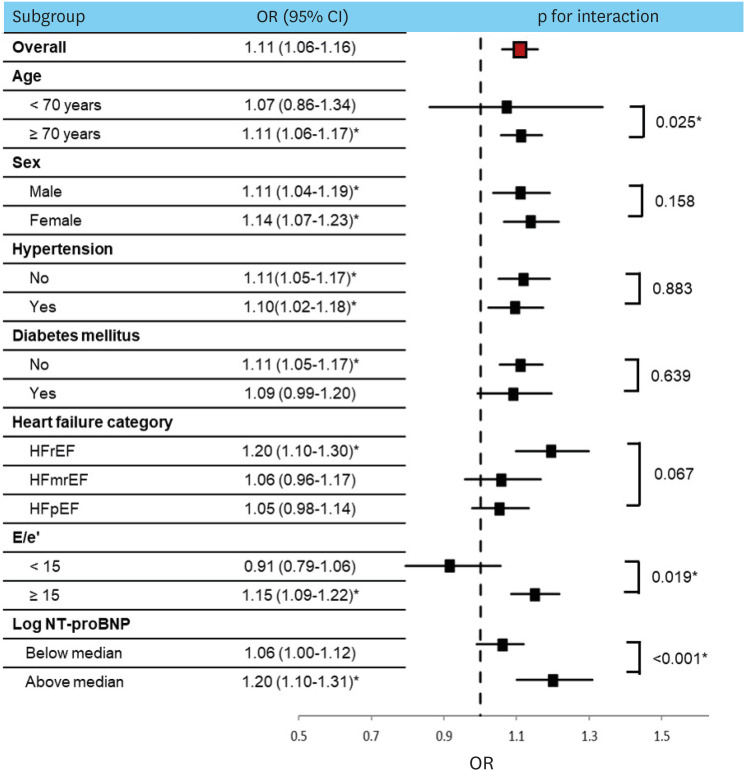
Figure 3 illustrates the subgroup analysis of the association between perirenal fat and reduced kidney function. There were no statistically significant differences when the groups were stratified according to sex, hypertension, diabetes mellitus, or HF categories (all p for interaction>0.05). In the subgroup analyses based on age, patients over 70 years old (OR, 1.11; 95% CI, 1.06–1.17; p<0.001) had a significantly higher association with increased perirenal fat thickness with reduced kidney function compared to younger patients (OR, 1.07; 95% CI, 0.86–1.34; p=0.526) (p for interaction=0.025). Patients with E/e′ >15 (OR, 1.15; 95% CI, 1.09–1.22; p<0.001) demonstrated a significantly higher association with increased perirenal fat thickness and reduced kidney function compared to those with lower E/e′ (OR, 0.91; 95% CI, 0.79–1.06; p=0.347) (p for interaction=0.019). In the subgroup analyses based on log NT-proBNP (median value, 8.6 pg/mL), patients with log NT-proBNP above median (OR, 1.20; 95% CI, 1.10–1.31; p<0.001) demonstrated a significantly higher association with increased perirenal fat thickness and reduced kidney function compared to those with log NT-proBNP below median (p for interaction<0.001).
Figure 3. Subgroup analysis for association of average perirenal fat thickness with reduced kidney function.
OR = odds ratio; CI = confidence interval; E/e′ = the ratio of early diastolic mitral inflow to mitral annular velocity; NT-proBNP = N-terminal pro-B-type natriuretic peptide.
To reconfirm our data, we performed additional analysis with different cutoff values of GFR of 35 mL/min/1.32 m2 (Supplementary Tables 1 and 2), which showed similar results performed with the cutoff value of GFR 60 mL/min/1.32 m2.
Factors correlated with perirenal fat thickness
The clinical characteristics correlated with the average perirenal fat thickness are presented in Table 3. In the univariate analysis, BMI, systolic BP, and HFpEF were positively correlated with perirenal fat thickness. However, the only factor showing an independent correlation with increasing perirenal fat thickness was BMI, even after adjusting for sex, coronary artery disease, systolic BP, and HF categories in the multivariable analysis (p<0.001).
Table 3. Characteristics correlated with perirenal fat thickness.
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| Beta | t | p value | Beta | t | p value | |
| Age | 0.052 | 0.775 | 0.439 | - | - | - |
| Female sex | −0.092 | −1.380 | 0.169 | −0.093 | −1.456 | 0.147 |
| Hypertension | 0.034 | 0.508 | 0.612 | - | - | - |
| Diabetes mellitus | 0.058 | 0.868 | 0.386 | - | - | - |
| Atrial fibrillation | −0.025 | −0.371 | 0.711 | - | - | - |
| BMI | 0.297 | 4.647 | <0.001* | 0.269 | 3.973 | <0.001* |
| Systolic BP | 0.142 | 2.146 | 0.033* | 0.049 | 0.735 | 0.463 |
| Diastolic BP | 0.045 | 0.680 | 0.497 | - | - | - |
| HFpEF | 0.137 | 2.067 | 0.040* | 0.108 | 1.685 | 0.093 |
| Ischemic etiology | 0.086 | 1.294 | 0.197 | 0.096 | 1.504 | 0.134 |
BMI = body mass index; BP = blood pressure; HFpEF = heart failure with preserved ejection fraction.
*p value <0.05.
Reproducibility of renal fat measurement
The ICCs for intra-observer and interobserver reproducibility of renal fat measurement were 0.998 (95% CI, 0.993–1.000) and 0.994 (95% CI, 0.977–0.999), respectively (all p<0.001).
DISCUSSION
The principal findings of the current study are that 1) a thick posterior perirenal fat pad was independently associated with kidney function deterioration in patients hospitalized with ADHF; 2) the association was more prominent in the subgroups of patients over 70 years and those with evidences of congestion reflected by high E/e′ and NT-proBNP; and 3) the BMI was the only factor correlated with perirenal fat thickness in those populations.
The heart and kidneys are closely related and interdependent; the term cardiorenal syndrome describes this phenomenon.15) Type 1 acute cardiorenal syndrome is characterized by acute worsening of cardiac function, leading to renal dysfunction.1) Traditionally, the pathophysiology of kidney dysfunction in HF is believed to result from decreased renal perfusion and associated hormonal and neural changes.16) However, recently, persistent venous congestion has been identified as a main contributor to cardiorenal syndrome4,5) and animal models support that congestion is a more important determinant of kidney dysfunction than renal perfusion.17) Elevated central venous pressure has been reported to result in renal venous hypertension, increased renal resistance, and ultimately impaired intrarenal blood flow in patients with ADHF.4,18) Boorsma et al.7) recently suggested the renal tamponade hypothesis and renal decapsulation therapy, which have demonstrated benefits in animals,19) making it an interesting, novel treatment for HF, that requires further investigation.
Although congestive nephropathy is a potentially reversible form of renal dysfunction due to effective decongestion, its diagnosis is challenging. There is no gold standard for assessing renal venous congestion.6) The invasive assessment using a wireless implantable hemodynamic monitoring system has been suggested, but limited availability, high cost, and invasive nature of the procedure limit its application.20)
Perirenal fat is a fat pad surrounding the kidneys in the retroperitoneal space, located between the renal fibrous membrane and the renal fascia.7) Perirenal fat shares the same developmental origin as typical visceral fat, but differs regarding histology, physiology, and functions.8) Perirenal fat is separated from the peritoneum by the renal fascia, whereas visceral fat is typically located in the intraperitoneal space.21)
The kidneys are low-resistance circuits that are known to receive a disproportionately large fraction of cardiac output.1) It has been suggested that elevated intra-abdominal pressure in the presence of ADHF may contribute to renal dysfunction by causing renal compression and reduced perfusion22) and the rigidity of the renal capsule plays a central role in congestion-induced kidney damage.7) Our study suggests that a thick perirenal fat pad might load more burden to the kidney in the setting of ADHF by occupying a large intracapsular space. That is, the thick perirenal fat pad can accelerate the increase in renal pressure by prohibiting the expansion of the kidney within the renal capsule and causing pressure to be reflected inward.
We found that increasing perirenal fat thickness was associated with deterioration of kidney function in hospitalized patients with ADHF, and this relationship was more pronounced in the population with high E/e′ and NT-proBNP. The E/e′ ratio on echocardiography and NT-proBNP can be used to diagnose congestive states in patients with HF.23) E/e′ directly correlates with pulmonary capillary wedge pressure, with an E/e′ >15 correlating with a pulmonary capillary wedge pressure of ≥15 mmHg.24,25) Interestingly, we found that the association between perirenal fat thickness and reduced kidney function was significant in patients with evidence of high E/e′ (>15) and elevated NT-proBNP above median, while this association was blunted in the population with lower E/e′ ratio and NT-proBNP. This fact might further support our hypothesis that a thick perirenal fat pad induces high intracapsular pressure more easily, facilitating compression of the renal vasculature and resultant congestive nephropathy.
CT is a valuable imaging tool for the diagnosis of acutely ill patients who present with symptoms of chest pain or dyspnea, which are common symptoms of ADHF. Therefore, many patients hospitalized with ADHF frequently undergo CT for various purposes. Posterior perirenal fat thickness can be easily measured using previously acquired CT images without an additional reconstruction process. We found that the measurement of perirenal fat thickness could be a novel surrogate marker for the detection of vulnerable patients with ADHF who are prone to kidney function deterioration. Considering the fact that biomarkers for the differential diagnosis of acute kidney injury in ADHF are scarce, the simple measurement of perirenal fat thickness could provide additional understanding regarding diagnosis, treatment strategies, and prognostic value for renal injury in patients with ADHF. Furthermore, perirenal fat thickness can be measured using other modalities, such as ultrasound26) or magnetic resonance imaging14) further studies are required regarding the role of perirenal fat assessed by various imaging modalities in patients with ADHF.
Obesity is a known risk factor for HF and contributes to the onset of cardiorenal syndrome.27) Visceral adiposity is associated with a greater risk of cardiorenal morbidity than subcutaneous adiposity.28) Perirenal fat has been reported as an indirect measurement that correlates with visceral fat29,30) although perirenal fat and visceral fat differ in various aspects.6) Therefore, the differential role of perirenal fat and intra-abdominal fat in renal function deterioration in HF requires further investigation. Increasing BMI was independently associated with increasing perirenal fat thickness in the current study, and it would be worthwhile to investigate whether weight reduction in patients with high perirenal fat thickness could decrease the perirenal fat pad, resulting in renal decapsulation effect, and finally reducing cardiorenal syndrome in patients with various categories of HF.
The main limitation of the current study is that the results were based on retrospective data analysis. However, the patients’ medical records, echocardiography, and CT findings were carefully reviewed to minimize biases. Second, echocardiography was not performed on the same day in all patients. Hemodynamic variables, especially E/e′, might be influenced by HF treatment, although persistent elevation of E/e′ after a prolonged time interval might be evidence of more advanced congestion. Furthermore, the interval between the GFR measurement and echocardiography performance was not prolonged, as reflected by the mean time interval of approximately one day in our study. Third, CT was performed by each clinician’s decision and the reason for CT was not clearly identified in many cases, due to the retrospective nature of this study. Fourth, the perirenal fat thickness was measured at a single plane in the posterior direction, while the perirenal fat was located around the kidney. Studies using multidirectional or volumetric measurements are warranted for more accurate measurement of the perirenal fat pad. However, perirenal fat thickness measured using CT at the level of the renal veins has been reported as a simple and reliable estimate of perirenal fat volume,31) and therefore, our simple measurement method would be clinically useful and easy to apply. Finally, the number of the study population was not enough to calculate the discrimination power. Further studies are warranted with an enough sample size study population in the future.
In conclusion, a thick perirenal fat pad was independently associated with deterioration of kidney function in patients hospitalized with ADHF and the association was more prominent in subgroups of patients over 70 years and those with the evidence of congestion. The measurement of posterior perirenal fat thickness may be a novel surrogate marker for identifying patients with ADHF who are prone to kidney function deterioration.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Cho IJ.
- Data curation: Lee SE.
- Writing - original draft: Cho IJ.
- Writing - review & editing: Wi J, Kim DH, Pyun WB.
SUPPLEMENTARY MATERIALS
Baseline characteristics
Factors associated with reduced kidney function of GFR <35 mL/min/1.73 m2 in patients with acute decompensated HF
References
- 1.Rangaswami J, Bhalla V, Blair JEA, et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2019;139:e840–e878. doi: 10.1161/CIR.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 2.Jang SY, Yang DH. Prognostic and therapeutic implications of renal insufficiency in heart failure. Int J Heart Fail. 2022;4:75–90. doi: 10.36628/ijhf.2021.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sawamura A, Kajiura H, Sumi T, et al. Clinical impact of worsening renal function in elderly patients with acute decompensated heart failure. Int J Heart Fail. 2021;3:128–137. doi: 10.36628/ijhf.2020.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 6.Husain-Syed F, Gröne HJ, Assmus B, et al. Congestive nephropathy: a neglected entity? Proposal for diagnostic criteria and future perspectives. ESC Heart Fail. 2021;8:183–203. doi: 10.1002/ehf2.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boorsma EM, Ter Maaten JM, Voors AA, van Veldhuisen DJ. Renal compression in heart failure: the renal tamponade hypothesis. JACC Heart Fail. 2022;10:175–183. doi: 10.1016/j.jchf.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Liu BX, Sun W, Kong XQ. Perirenal fat: a unique fat pad and potential target for cardiovascular disease. Angiology. 2019;70:584–593. doi: 10.1177/0003319718799967. [DOI] [PubMed] [Google Scholar]
- 9.Fang Y, Xu Y, Yang Y, Liu C, Zhao D, Ke J. The relationship between perirenal fat thickness and reduced glomerular filtration rate in patients with type 2 diabetes. J Diabetes Res. 2020;2020:6076145. doi: 10.1155/2020/6076145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Yao Y, Gong H, Pang Y, et al. Risk factors influencing the thickness and stranding of perinephric fat of mayo adhesive probability score in minimally invasive nephrectomy. Med Sci Monit. 2019;25:3825–3831. doi: 10.12659/MSM.916359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.House AA. Cardiorenal syndrome: new developments in the understanding and pharmacologic management. Clin J Am Soc Nephrol. 2013;8:1808–1815. doi: 10.2215/CJN.02920313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341:577–585. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Liu M, Bedja D, et al. Acute renal venous obstruction is more detrimental to the kidney than arterial occlusion: implication for murine models of acute kidney injury. Am J Physiol Renal Physiol. 2012;302:F519–F525. doi: 10.1152/ajprenal.00011.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damman K, Navis G, Smilde TD, et al. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail. 2007;9:872–878. doi: 10.1016/j.ejheart.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Cruces P, Lillo P, Salas C, et al. Renal decapsulation prevents intrinsic renal compartment syndrome in ischemia-reperfusion-induced acute kidney injury: a physiologic approach. Crit Care Med. 2018;46:216–222. doi: 10.1097/CCM.0000000000002830. [DOI] [PubMed] [Google Scholar]
- 20.Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 21.Coffin A, Boulay-Coletta I, Sebbag-Sfez D, Zins M. Radioanatomy of the retroperitoneal space. Diagn Interv Imaging. 2015;96:171–186. doi: 10.1016/j.diii.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Mullens W, Abrahams Z, Skouri HN, et al. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol. 2008;51:300–306. doi: 10.1016/j.jacc.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 23.Wang AY, Lam CW, Yu CM, et al. N-terminal pro-brain natriuretic peptide: an independent risk predictor of cardiovascular congestion, mortality, and adverse cardiovascular outcomes in chronic peritoneal dialysis patients. J Am Soc Nephrol. 2007;18:321–330. doi: 10.1681/ASN.2005121299. [DOI] [PubMed] [Google Scholar]
- 24.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Nagueh SF, Bhatt R, Vivo RP, et al. Echocardiographic evaluation of hemodynamics in patients with decompensated systolic heart failure. Circ Cardiovasc Imaging. 2011;4:220–227. doi: 10.1161/CIRCIMAGING.111.963496. [DOI] [PubMed] [Google Scholar]
- 26.Lamacchia O, Nicastro V, Camarchio D, et al. Para- and perirenal fat thickness is an independent predictor of chronic kidney disease, increased renal resistance index and hyperuricaemia in type-2 diabetic patients. Nephrol Dial Transplant. 2011;26:892–898. doi: 10.1093/ndt/gfq522. [DOI] [PubMed] [Google Scholar]
- 27.Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:e984–1010. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buliga-Finis ON, Ouatu A, Badescu MC, et al. Beyond the cardiorenal syndrome: pathophysiological approaches and biomarkers for renal and cardiac crosstalk. Diagnostics (Basel) 2022;12:773. doi: 10.3390/diagnostics12040773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin L, Seton G, Aldred B, et al. When body mass index fails to measure up: perinephric and periumbilical fat as predictors of operative risk. Am J Surg. 2016;212:1039–1046. doi: 10.1016/j.amjsurg.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Geraci G, Zammuto MM, Mattina A, et al. Para-perirenal distribution of body fat is associated with reduced glomerular filtration rate regardless of other indices of adiposity in hypertensive patients. J Clin Hypertens (Greenwich) 2018;20:1438–1446. doi: 10.1111/jch.13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Favre G, Grangeon-Chapon C, Raffaelli C, François-Chalmin F, Iannelli A, Esnault V. Perirenal fat thickness measured with computed tomography is a reliable estimate of perirenal fat mass. PLoS One. 2017;12:e0175561. doi: 10.1371/journal.pone.0175561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics
Factors associated with reduced kidney function of GFR <35 mL/min/1.73 m2 in patients with acute decompensated HF