Abstract
What is already known about this topic?
Little is known about the epidemiology, natural history, and transmission patterns of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Delta variant. Monitoring the evolution of viral fitness of SARS-CoV-2 in the host population is key for preparedness and response planning.
What is added by this report?
We analyzed a successfully contained local outbreak of Delta that took place in Hunan, China, and provided estimates of time-to-key event periods, infectiousness over time, and risk factors for SARS-CoV-2 infection and transmission for a still poorly understood variant.
What are the implications for public health practice?
Our findings simultaneously shed light on both the characteristics of the Delta variant, by identifying key age groups, risk factors, and transmission pathways, and planning a future response effort against SARS-CoV-2.
Keywords: Delta variant, Transmission dynamics, epidemiological characteristics
Monitoring changes in the epidemiologic features between different severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants is key to understanding the evolution of viral fitness in the host population. Here, we analyzed a successfully contained local outbreak of the Delta variant that took place in Hunan, China, in July–August, 2021. Detailed data on SARS-CoV-2 infections and their contacts were collected during the outbreak. By leveraging these data, we estimated key epidemiological parameters, including the incubation period, serial interval, and generation time. We constructed a generalized linear mixed-effects model (GLMM) to quantify risk factors for Delta infection and transmission. Between July 28 and August 15, 2021, a total of 129 infections and their 2,118 close contacts were identified during the outbreak in Hunan Province. The mean incubation period, serial interval, and generation time were estimated to be 5.3 days [interquartile range (IQR): 3.0–6.5], 4.3 days (IQR: 1.7–6.8), and 4.4 days (IQR: 2.4–5.8), respectively. Infectiousness peaked 1.6 days before symptom onset, with 63.0% of the transmission events occurring during the presymptomatic phase. Household contacts had the highest risk of infection [odds ratio (OR)=6.79, 95% confidence interval (CI): 3.20–14.44]. The susceptibility to Delta infection was higher in children aged 0–9 years than in adults aged 18–64 years (OR=2.44, 95% CI: 1.03–5.80). The effectiveness of the inactivated vaccine against any confirmed infection was 54% (95% CI: 7%–77%). By providing quantitative evidence about key epidemiological features as well as infection and transmission risk factors for Delta, our findings improved our relatively scarce understanding of this variant of concern and, more broadly, the evolutionary trajectory of SARS-CoV-2.
During the 2021 Delta outbreak in Hunan, field epidemiological investigations allowed the prompt identification of the population at risk, including contacts of positive individuals. Here we provided descriptive statistics of positive cases’ characteristics and close contacts. We estimated the incubation period (i.e., the time delay from infection to illness onset), serial interval (i.e., the time interval between the onset of symptoms in a primary infector and their onset in secondary infections), generation time (i.e., the time interval between infection of the primary infector and their secondary infections), and infectiousness profile (i.e., the temporal distribution of the probability of transmission), following the method in reference (1). To evaluate the impact of nonpharmaceutical interventions (NPIs) on the containment of the outbreak, we also compared the distribution of the time delay from symptom onset to hospitalization, to isolation, to the collection of the first positive specimen, and laboratory confirmation before and after the implementation of NPIs using the Kolmogorov-Smirnov test (K-S test).
A GLMM was used to quantify the effects of potential drivers of susceptibility to Delta variant infection and infectivity of infected individuals. To quantify the vaccination effectiveness against the Delta variant infection and transmission, we incorporated the vaccination status of infectors and contacts into the model. Other factors, including age, sex, and the contact setting, could be potential drivers of the susceptibility and infectivity of SARS-CoV-2 ancestral strains in a previous research (1) and thus have also been considered in our models. (Supplementary Material, available in https://weekly.chinacdc.cn/). All vaccinated individuals included in this study received inactivated vaccines. Our primary analysis was based on 2,004 close contacts, and a supplementary analysis was conducted incorporating another 4,131 general contacts aged under 65 years. Statistical analyses were performed in R, version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
The first local infection of Delta in Hunan Province, China, was identified on July 28, 2021: an individual who shared the same boat with domestically infected tourists (2). Between July 28 and August 15, 2021, 129 SARS-CoV-2 Delta infections were identified in Hunan Province, China. According to genomic data, all identified infections in this outbreak were infected by the Delta variant.
The outbreak quickly spread from the original touristic location through other cities within Hunan Province (Supplementary Figure S1, available in https://weekly.chinacdc.cn/). Through epidemiological investigations and active contact tracing, 10,971 individuals categorized as the at-risk population were initially identified, of which 2,118 were further classified as close contacts of infected individuals. Another 4,513 were identified as general contacts (Supplementary Figure S2, available in https://weekly.chinacdc.cn/), and the reconstructed transmission chain was reported in Supplementary Figure S3 (available in https://weekly.chinacdc.cn/). Among all infections, 19 were asymptomatic, and 110 were symptomatic, including 48 mild cases (43.6%), 61 moderate cases (55.5%), and 1 severe case (0.9%), with no critical cases or deaths reported [Table 1, see Supplementary Table S1 (available in https://weekly.chinacdc.cn/) for an analysis of the completeness of the variables].
Table 1. Characteristics of SARS-CoV-2 Delta infected individuals and their close contacts in Hunan Province, China, 2021.
| Characteristics |
Infections
(n=129) |
Close contacts
(n=2,118) |
Secondary infection attack rate (%, 95% CI)* |
| Note: “−” means data not available.The last column provided the secondary infection attack rates across different groups of age, sex, and vaccination history. Abbreviation: IQR=interquartile range; CI=confidence interval; SARS-CoV-2=severe acute respiratory syndrome coronavirus 2; COVID-19=coronavirus disease 2019. * The secondary infection attack rate was calculated by dividing the number of infections by the total number of close contacts. † Vaccination history: 1) unvaccinated (i.e., individuals who did not receive any COVID-19 vaccines or received 1 dose of COVID-19 vaccine less than 14 days before the date of the last known contact); 2) partially vaccinated (i.e., individuals who had received either 1 dose of a COVID-19 vaccine or received 2 doses of vaccines with the date of the second dose less than 14 days before the date of the last known contact) and 3) fully vaccinated (i.e., individuals who completed the full 2-dose course vaccination more than 14 days before the date of the last known contact). § For the asymptomatic subjects and the cases diagnosed by imaging characteristics, we used the date of the first sample collection with a positive test instead of the date of symptom onset. | |||
| Age, years | |||
| Median (IQR) | 34 (15, 48) | 34 (20, 46) | |
| Age group, years | |||
| 0–9 | 19 (14.7%) | 140 (6.6%) | 10.7 (6.1, 17.1) |
| 10–17 | 19 (14.7%) | 293 (13.8%) | 4.8 (2.6, 7.9) |
| 18–64 | 87 (67.4%) | 1,595 (75.3%) | 3.2 (2.4, 4.2) |
| >65 | 4 (3.1%) | 90 (4.2%) | 4.4 (1.2, 11) |
| Sex | |||
| Male | 58 (45.0%) | 920 (43.4%) | 4.6 (3.3, 6.1) |
| Female | 71 (55.0%) | 1,198 (56.6%) | 3.5 (2.5, 4.7) |
| Clinical outcome | |||
| Not infected | − | 2,034 (96.0%) | |
| Confirmed asymptomatic infection | 19 (14.7%) | 9 (0.4%) | |
| Confirmed symptomatic infection | 110 (85.3%) | 75 (3.5%) | |
| Mild | 48 (43.6%) | 39 (52.0%) | |
| Moderate | 61 (55.5%) | 35 (46.7%) | |
| Severe | 1 (0.9%) | 1 (1.3%) | |
| Vaccination history† | |||
| Unvaccinated | 66 (51.2%) | 993 (46.9%) | 5.2 (3.9, 6.8) |
| Partially vaccinated | 27 (20.9%) | 290 (13.7%) | 4.8 (2.7, 8.0) |
| Time interval between last vaccination and symptoms onset or last exposure, days§ | |||
| Median (IQR) | 26 (21, 35) | 25 (21, 28) | |
| Fully vaccinated | 36 (27.9%) | 835 (39.4%) | 2.2 (1.3, 3.4) |
| Time interval between last vaccination and symptoms onset or last exposure, days§ | |||
| Median (IQR) | 49 (40, 54) | 48 (41, 56) | |
| Mode of detection | |||
| Passive surveillance | 14 (10.9%) | − | |
| Contact tracing | 80 (62.0%) | − | |
| Community screening | 35 (27.1%) | − | |
We analyzed 71 locally confirmed cases with clear exposure dates or exposure windows to estimate the incubation period. From the best-fitting lognormal distribution, we obtained a mean estimate of the incubation period of 5.3 days (median: 4.4, IQR: 3.0–6.5). Consistent results were obtained when fitting alternative distributions (Supplementary Table S2, available in https://weekly.chinacdc.cn/). The symptom onset date was available for 54 transmission pairs; the resulting serial interval had an estimated mean of 4.3 days (median: 4.2, IQR: 1.7–6.8), based on fitting a Weibull distribution, and consistent results were found by fitting alternative distributions (Supplementary Table S2). Infectiousness was estimated to peak 1.6 days before symptom onset, and the proportion of pre-symptomatic transmission was 63.0%, with 95% of transmission events occurring between −5.6 and 5.8 days after the date of symptom onset. The mean generation time was estimated to be 4.4 days (median: 3.9, IQR: 2.4–5.8) (Figure 1A–B). A marked decreasing trend was observed in all time intervals from symptom onset to hospitalization, to isolation, to the collection of the first positive specimen, and the laboratory diagnosis of symptomatic individuals after the initiation of NPIs. Among them, the time interval from symptom onset to isolation showed the most significant decrease, with a reduction from a median of 4.0 days (IQR: 2.5–7.0) to 0 days (IQR: −1.0–1.0) (Figure 1C), which may have potentially played a crucial role in the successful containment of the outbreak.
Figure 1.
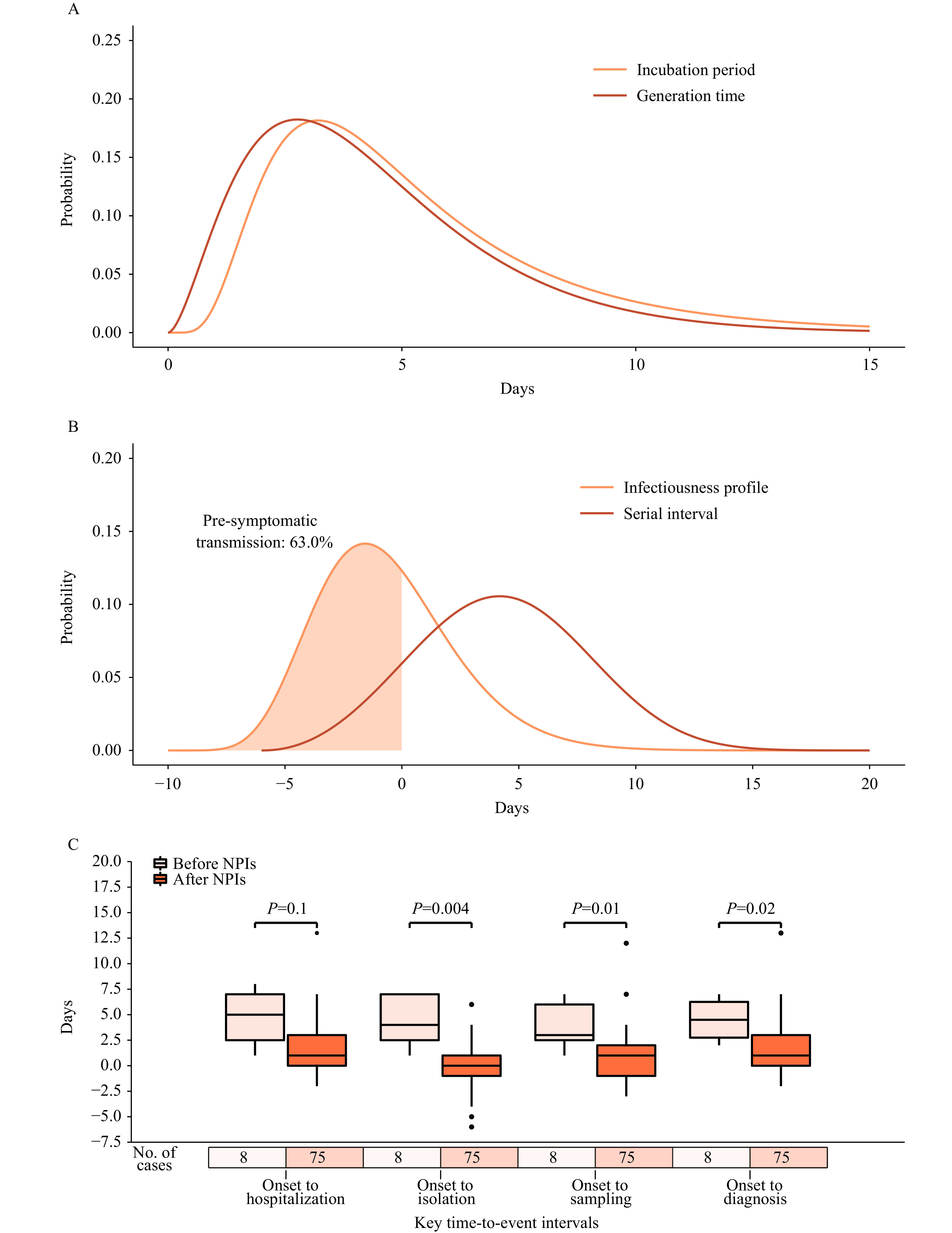
Key time-to-event intervals of SARS-CoV-2 Delta infections in Hunan, China. (A) Estimated incubation period distribution by log-normal distributions and generation time by gamma distributions based on 71 confirmed cases. (B) Estimated distribution of the serial interval by Weibull distributions and of the infectiousness profile by gamma distributions based on 54 transmission pairs. (C) Time intervals from symptom onset to hospitalization, isolation, first time of sampling, and diagnosis of symptomatic cases before and after the implementation of NPIs.
Note: The infectiousness profile describes the infectiousness of an individual over time since the onset of symptoms.
Abbreviation: NPIs=nonpharmaceutical interventions; SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
A multivariate regression analysis was performed based on close contact data collected. The results showed that the susceptibility to infection of the adult population was lower for fully vaccinated individuals than non-vaccinated individuals (OR=0.46, 95% CI: 0.23–0.93), indicating a vaccine effectiveness of 54% (95% CI: 7%–77%) against any confirmed Delta infection. Among unvaccinated close contacts, susceptibility to Delta infection was higher in children than in adults (OR=2.44, 95% CI: 1.03–5.80). In addition, we found a higher infection risk in the household setting (OR=6.79, 95% CI: 3.20–14.44), while other potential risk factors, such as the sex of the infectors and the contacts, were not statistically significant (Table 2). In Supplementary Table S3 (available in https://weekly.chinacdc.cn/), we present the results of a sensitivity analysis where, when the source of exposure was not resolved, the potential infector was selected at random. The consistency between these results and those of the main analysis supports the robustness of our main conclusions. A supplementary analysis was performed on 6,135 contacts, which included both close contacts and general contacts, and these results are also consistent with those of the main analysis (Supplementary Table S4, available in https://weekly.chinacdc.cn/).
Table 2. Estimating the association of potential risk factors with the risk of acquiring and transmitting the SARS-CoV-2 Delta variant.
| Characteristic | Univariate analysis | Multivariate analysis | |||
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Note: “−” in the column of “OR (95% CI)” indicated that the corresponding variable was not incorporated into the analysis. All children and adolescents (under 18 years) were unvaccinated, as they were not covered by the COVID-19 vaccination program in the Chinese mainland during the Delta outbreak. Vaccinated adults denote fully vaccinated adults, while partially vaccinated adults were deemed unvaccinated adults in the analysis. Abbreviation: CI=confidence interval; OR=odds ratio; COVID-19=coronavirus disease 2019; SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. * P<0.05, ** P<0.01. *** P<0.001. † Contacts who were aged at or over 65 years or whose infectors were aged at or over 65 years were excluded from this analysis due to the small sample size. § For a contact who had interacted with more than one SARS-CoV-2 infection, we chose the first infected individual they had been exposed to as the potential infector. If they had exposed to multiple infectors at the same time, we randomly chose one from among these infectors as the potential infector. | |||||
| Age (years) and vaccination status of contacts† | |||||
| Unvaccinated children (0–9 years) | 3.42 (1.49, 7.89) | 0.004** | 2.44 (1.03, 5.80) | 0.044* | |
| Unvaccinated adolescents (10–17 years) | 1.56 (0.66, 3.69) | 0.310 | 1.20 (0.46, 3.10) | 0.707 | |
| Unvaccinated adults (18–64 years) | Reference | Reference | |||
| Vaccinated adults (18–64 years) | 0.49 (0.25, 0.95) | 0.035* | 0.46 (0.23, 0.93) | 0.030* | |
| Age (years) and vaccination status of infectors§ | |||||
| Unvaccinated children (0–9 years) | 1.79 (0.45, 7.19) | 0.410 | 0.82 (0.19, 3.46) | 0.784 | |
| Unvaccinated adolescents (10–17 years) | 0.32 (0.07, 1.51) | 0.149 | 0.63 (0.16, 2.52) | 0.513 | |
| Unvaccinated adults (18–64 years) | Reference | Reference | |||
| Vaccinated adults (18–64 years) | 0.53 (0.17, 1.61) | 0.260 | 0.51 (0.19, 1.40) | 0.192 | |
| Type of contact | |||||
| Household contact | 7.54 (3.67, 15.48) | <0.001*** | 6.79 (3.20, 14.44) | <0.001*** | |
| Health care | 0.44 (0.10, 1.93) | 0.274 | 0.22 (0.04, 1.11) | 0.067 | |
| Social contact | Reference | Reference | |||
| Workplace contact | 0.64 (0.15, 2.75) | 0.550 | 0.73 (0.17, 3.20) | 0.678 | |
| Other | 0.07 (0.02, 0.20) | <0.001*** | 0.05 (0.02, 0.15) | <0.001*** | |
| Sex of contacts | |||||
| Female | Reference | Reference | |||
| Male | 1.03 (0.62, 1.73) | 0.906 | 0.72 (0.40, 1.28) | 0.261 | |
| Sex of infectors | |||||
| Female | Reference | Reference | |||
| Male | 1.04 (0.39, 2.79) | 0.942 | 0.67 (0.27, 1.67) | 0.391 | |
| Clinical severity of primary cases | |||||
| Asymptomatic infection | Reference | − | − | ||
| Symptomatic infection | 1.05 (0.23, 4.75) | 0.951 | − | − | |
DISCUSSION
Based on case surveillance and contact tracing data, we described the epidemiological characteristics of the SARS-CoV-2 Delta variant outbreak in Hunan Province, China, in July–August 2021. To date, only a few studies have provided estimates of the incubation period, serial interval, and generation time of Delta variant infection. These studies suggest that the mean (or median) incubation period of Delta ranges from 3.0 to 6.0 days (3-4), which contains the mean estimate obtained in our study (5.3 days); also, our estimate of the serial interval (4.3 days) falls inside the range reported in previous studies (range 2.6–5.4 days) (5-6). Regarding the generation time, we found only two studies providing estimates for the Delta variant; the first one was conducted in the UK and found a mean value of 4.7 days (7), while the second one was conducted in Italy and found a mean value of 6.6 days (6). By comparison, we estimated a mean generation time of 4.4 days. However, it is important to stress that here we provide estimates of the “realized” (i.e., observed) generation time. In contrast the two European studies estimate the intrinsic generation time (i.e., what would be observed in an infinitely large and fully susceptible population). Contact tracing could speed up the detection and isolation of infectors, and case isolation and contact quarantine could prevent potential infectors from contacting susceptible individuals, which limited our estimates to the specific conditions of the analyzed outbreak. A strength of our study is that our estimates of the time-to-event distributions are based on transmission pairs of the entire outbreak and are thus insensitive to right-censoring bias mediated by epidemic growth rates (8).
Different from the ancestral strain, we found that children were more likely to be infected with the Delta variant, and similar observations have been found in other settings (9). This finding emphasizes the relevance of young individuals in SARS-CoV-2 outbreaks. In agreement with prior studies (10), the effectiveness of 2 doses of inactivated vaccines against Delta infection was estimated at 54% (OR=0.46, 95% CI: 0.23–0.93), while their effectiveness in preventing forward transmission was not statistically significant, possibly due to the small sample size. Our analysis did not explicitly consider the effect of the waning of vaccine protection as most [86% (31/36)] reported infections among fully vaccinated individuals were infected within two months after receiving their most recent vaccine dose. Nonetheless, our study further supports the key role of vaccination in mitigating coronavirus disease 2019 (COVID-19) burden.
Our study has several limitations. First, the sample size of our study is relatively limited (129 confirmed infections, 2,118 contacts, and 4,513 general contacts). It thus does not allow the analysis for further stratifications, such as the estimation of the key time-to-event intervals by vaccination status. Moreover, it is possible that our sample size is insufficient to provide statistically significant results for reducing forward transmission in vaccinated individuals. Second, due to the small number of asymptomatic infections in our sample, we did not distinguish between asymptomatic and symptomatic infections in our analysis, which may mask the heterogeneity of the two modes of transmission. The low proportion of asymptomatic infections in our sample (14.7%) as compared to more recent studies on outbreaks of the Omicron variants (11) may be due to several factors, including the reliance on repeated city-wide screenings of the population to curb the spread of highly transmissible Omicron variants (12), the progressive increase of population immunity protecting against symptomatic disease, and a possible reduced intrinsic severity of the Omicron variants (13).
Given the rapid adaptive evolution of SARS-CoV-2, having a cohesive picture of the differences and similarities between different variants is key for preparedness planning. As such, our findings not only shed light on the characteristics of the analyzed outbreak but can also be instrumental for planning future response efforts against SARS-CoV-2.
Supplementary Material
The GLMM Model
We performed a generalized linear mixed-effects model (GLMM) to quantify the effects of potential drivers of susceptibility and infectivity of the Delta variant. The GLMM was conducted based on the contact data, where each contact was epidemiologically linked to at least one potential infector. For a contact who had interacted with more than one SARS-CoV-2 infected person, we chose the first infected individuals they had been exposed to as the potential infector in our main analysis. If they had been exposed to multiple individuals at the same time, we randomly chose one from among these infectors as the potential infector. The specifications of the GLMM models were defined as follows:
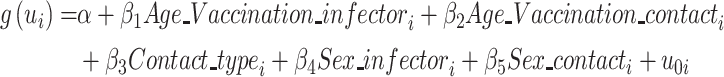 |
where:
· g is a logit link function;
· 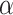 is the intercept;
is the intercept;
· 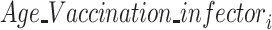 is the fixed-effect of the age group and vaccination status of the infector in the successful (1) or unsuccessful (0) transmission event
is the fixed-effect of the age group and vaccination status of the infector in the successful (1) or unsuccessful (0) transmission event 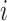 ;
;
· 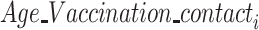 is the fixed-effect of the age group and vaccination status of the contact (potential infectee) in the successful/unsuccessful transmission event
is the fixed-effect of the age group and vaccination status of the contact (potential infectee) in the successful/unsuccessful transmission event 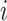 ;
;
· 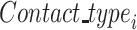 is the type of contact that occurred in the successful/unsuccessful transmission event
is the type of contact that occurred in the successful/unsuccessful transmission event 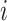 ;
;
· 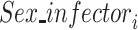 is the sex of the infector in the successful/unsuccessful transmission event
is the sex of the infector in the successful/unsuccessful transmission event 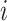 ;
;
· 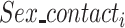 is the sex of the contact in the successful/unsuccessful transmission event
is the sex of the contact in the successful/unsuccessful transmission event 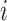 ;
;
·  is the random effect.
is the random effect.
To evaluate the robustness of the regression estimates against the uncertainties in the source of the exposures, we repeated the regression analysis 1,000 times, where the potential infectors were randomly chosen from multiple infectors of contacts in each stochastic realization, regardless of the time order of exposure. We reported the mean and the 2.5–97.5th percentiles of the point estimates of the variables of interest, i.e., the age and vaccination status of both the infectors and contacts, based on all regression results from 1,000 stochastic realizations. The results are reported in Supplementary Table S4.
Figure S1.
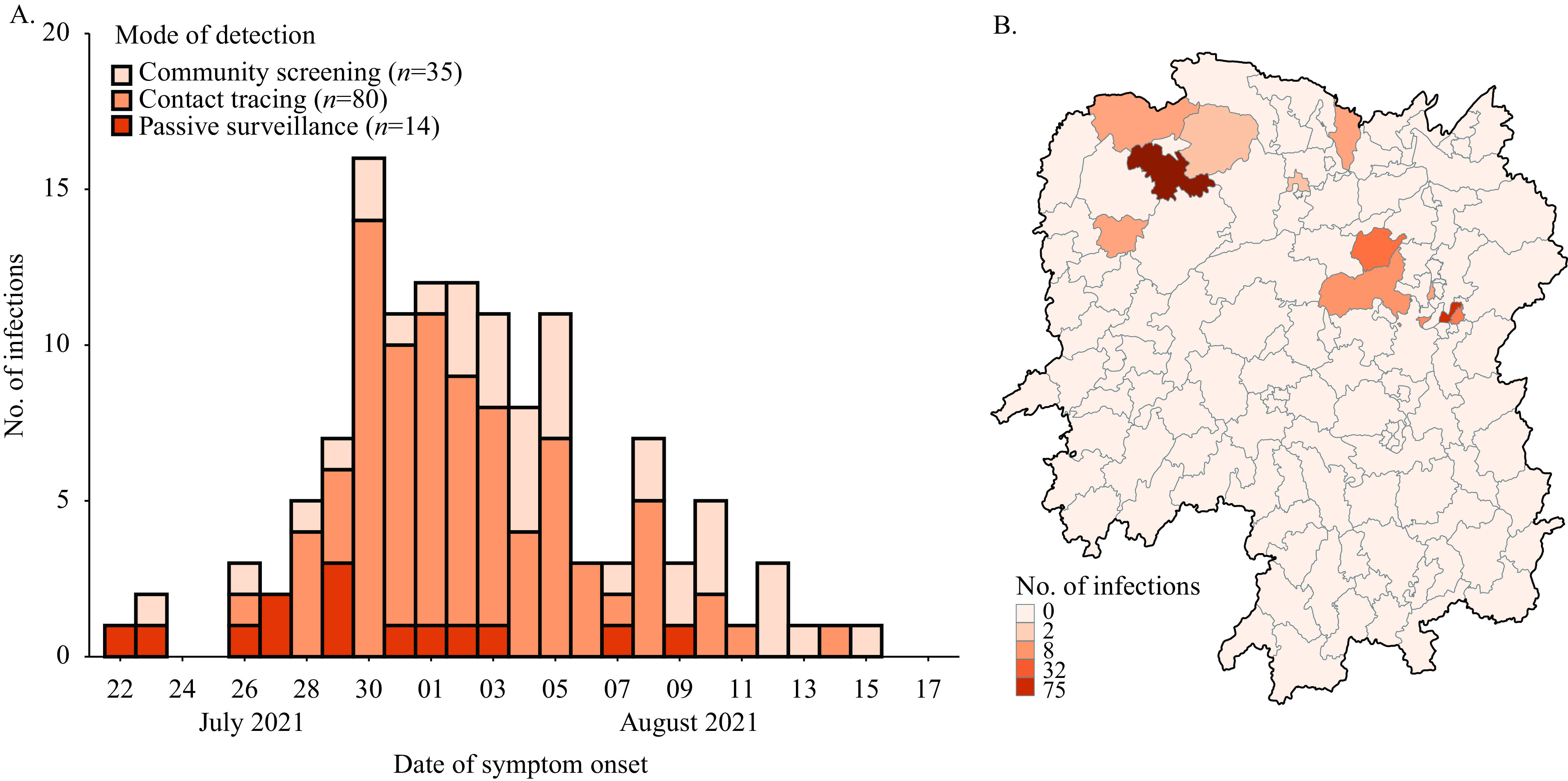
Spatiotemporal distributions of SARS-CoV-2 Delta infections in Hunan Province, China, 2021. (A). Daily number of new infections by the date of symptom onset and the mode of detection. (B). Spatial distribution of infected individuals in each city of Hunan Province.
Note: For the asymptomatic infections and confirmed cases diagnosed by imaging characteristics, we substituted the date of the first positive specimen taken for the date of symptom onset.
Figure S2.
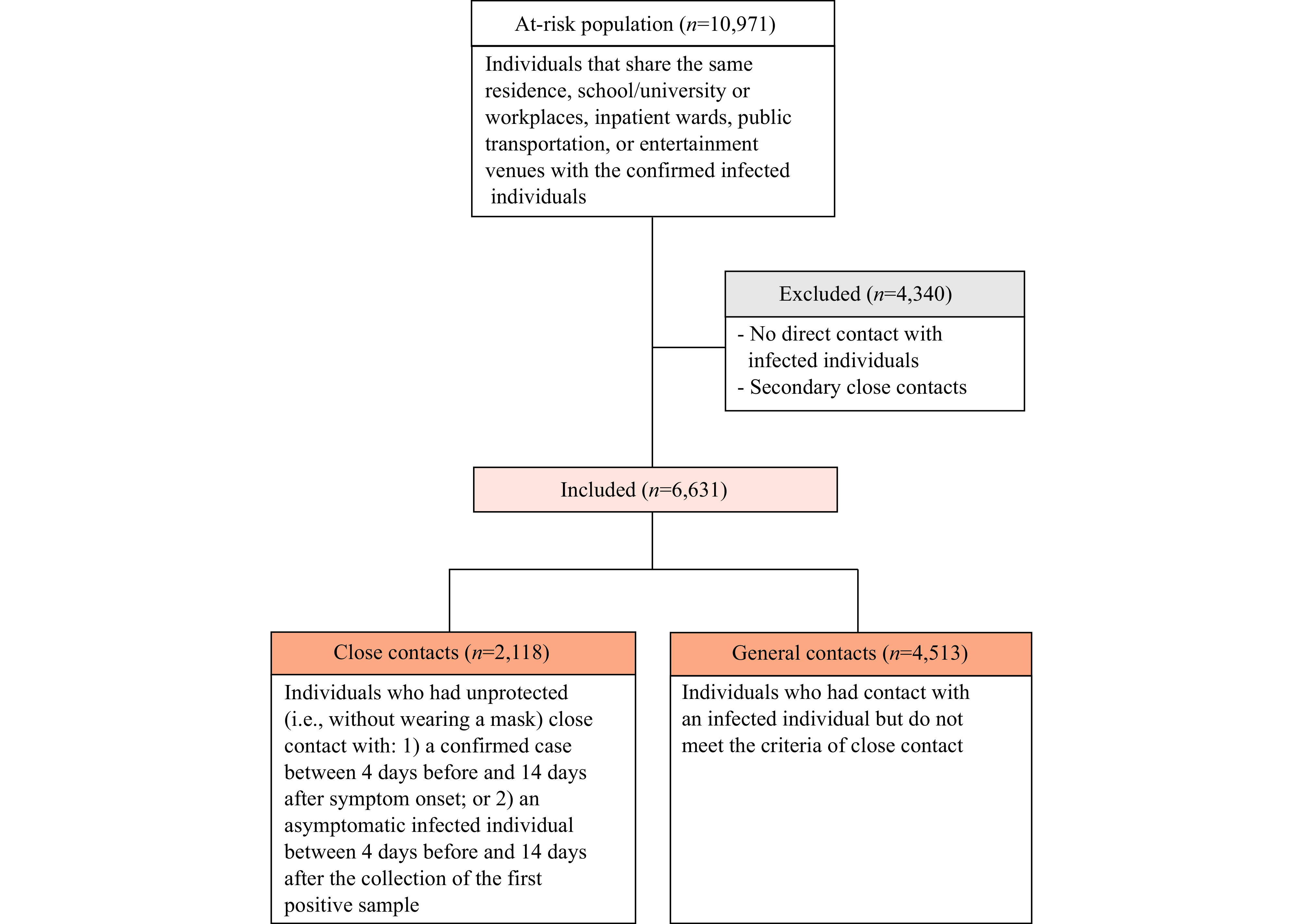
The flowchart of the selection of study participants.
Figure S3.
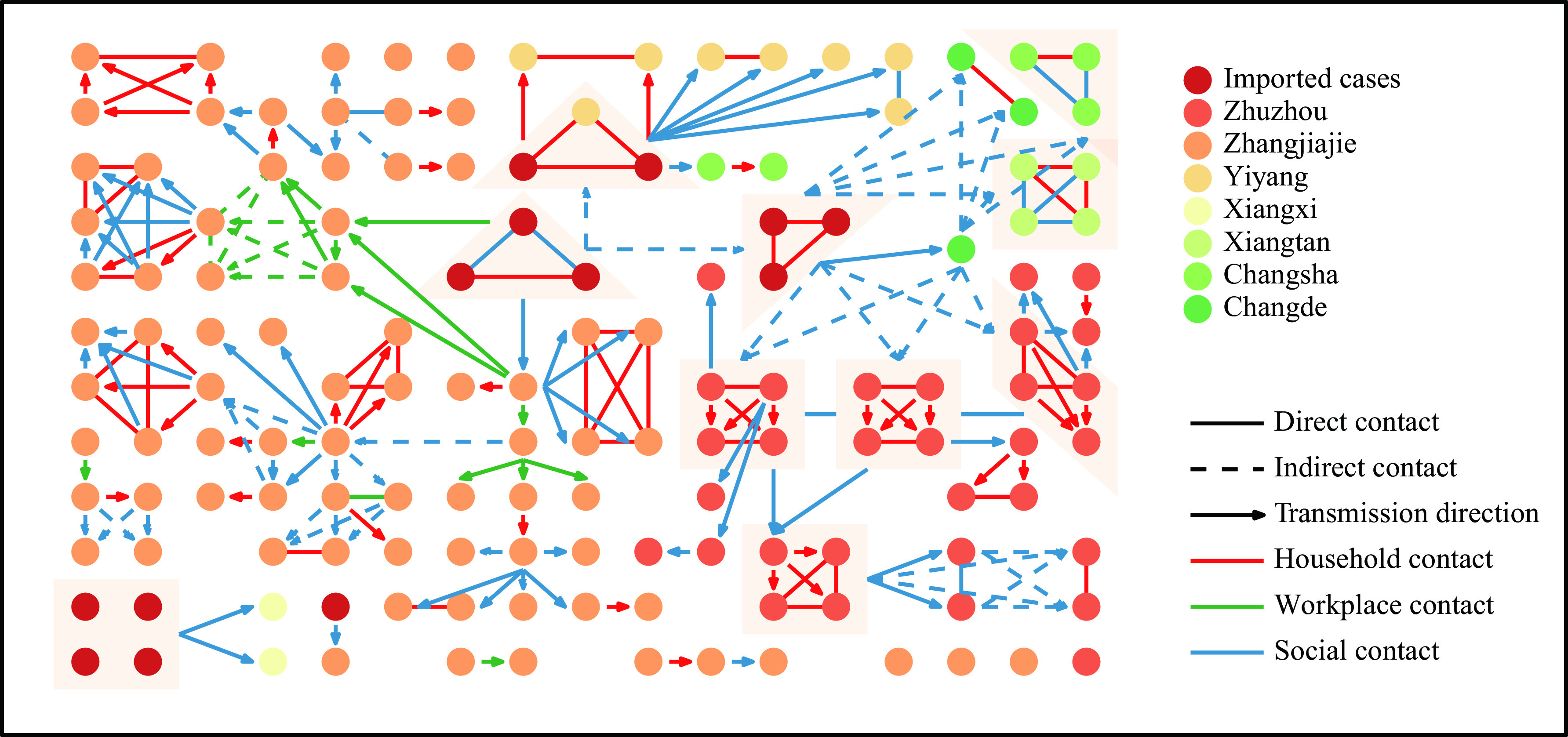
Epidemiological transmission network of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Delta transmission in Hunan Province, China.
Note: A total of 13 infected visitors and 129 local infections are shown in the network, indicated by the dots. The lines and arrows indicate potential transmission routes. Direct contact refers to unprotected close contact with a confirmed SARS-CoV-2 infection. Indirect contact refers to potential contact with a confirmed SARS-CoV-2 infection through sharing the same residential communities, study or workplaces, inpatient wards, public transportation, or entertainment venues.
Table S1. The completeness of the variables for SARS-CoV-2 Delta infections and the at-risk population.
| Variables |
Percentage of infections, %
(n=129) |
Percentage of risk population, %
(n=10,971) |
| Note: “−” means data not available. Abbreviation: SARS-CoV-2=severe acute respiratory syndrome coronavirus 2; COVID-19=coronavirus disease 2019. * The three-dose regimen of a tandem-repeat dimeric RBD protein-based COVID-19 vaccine ZF2001 was selected for use in real-world practice. | ||
| Demographic information | ||
| Age | 100 (129/129) | 96.5 (10,586/10,971) |
| Sex | 100 (129/129) | 97.3 (10672/10,971) |
| Vaccination information | ||
| Vaccine manufacturer (1st dose) | 100 (67/67) | 100 (6,724/6,724) |
| Vaccination date (1st dose) | 100 (67/67) | 100 (6,723/6,724) |
| Vaccine manufacturer (2nd dose) | 100 (44/44) | 100 (5,348/5,348) |
| Vaccination date (2nd dose) | 100 (44/44) | 100 (5,348/5,348) |
| Vaccine manufacturer (3rd dose)* | 100 (1/1) | 100 (604/604) |
| Vaccination date (3rd dose) | 100 (1/1) | 100 (604/604) |
| Exposure information | ||
| Exposure start date | 74.4 (96/129) | 56.1 (6,152/10,971) |
| Exposure end date | 80.6 (104/129) | 88.6 (9,716/10,971) |
| Contact type | 100 (129/129) | 92.1 (10,102/10,971) |
| Clinical information | ||
| Date of symptom onset | 83.6 (92/110) | − |
| Date of the first positive sample collection for PCR testing | 100 (129/129) | − |
| Date of the laboratory confirmation | 100 (129/129) | − |
| Clinical severity | 100 (129/129) | − |
| Type of detection | 100 (129/129) | − |
Table S2. Estimates of the incubation period and serial interval.
| Distribution |
Sample size for
estimation |
Parameters
[mean (SD)] |
Mean
(days) |
Quantiles
(0.025–0.975, days) |
AIC |
| Abbreviation: SD=standard deviation; AIC=Akaike information criterion. | |||||
| Incubation period | |||||
| Gamma | 71 | shape=3.02(0.60), rate=0.57(0.12) | 5.3 | 1.1–12.9 | 217.9 |
| Weibull | 71 | shape=1.69(0.17), scale=5.94(0.49) | 5.3 | 0.6–12.8 | 222.4 |
| Log-normal | 71 | meanlog=1.50(0.08), sdlog=0.58(0.06) | 5.3 | 1.5–13.9 | 216.3 |
| Serial interval | |||||
| Gamma | 54 | shape=6.09(1.17), rate=0.59(0.12), shift=6 | 4.3 | −2.2–14.0 | 274.5 |
| Weibull | 54 | shape=3.12(0.34), scale=11.52(0.53), shift=6 | 4.3 | −2.4–11.5 | 265.7 |
| Log-normal | 54 | meanlog=2.25(0.07), sdlog=0.47(0.05), shift=6 | 4.6 | −2.2–18.0 | 288.0 |
Table S3. A sensitivity analysis of GLMM-logit regression projecting uncertainties of the fixed-effects of age (years) and vaccination status induced by those contacts with multiple infectors.
| Age and vaccination status | Susceptibility odds ratio* | Transmissibility odds ratio* | ||||
| Mean | (Quantiles, 0.025–0.975th) | Mean | (Quantiles, 0.025–0.975th) | |||
| Note: All children and adolescents (under 18 years) were unvaccinated, as they were not covered by the COVID-19 vaccination program in the Chinese mainland during the Delta outbreak. Vaccinated adults denote fully vaccinated adults, while partially vaccinated adults were deemed unvaccinated adults in the analysis. Contacts who were aged at or over 65 years or whose infectors were aged at or over 65 years were excluded from this analysis due to the small sample size. * The GLMM-logit regression was repeated 1,000 times, where one single infector was randomly chosen for those contacts with multiple infectors in each stochastic realization. The mean odds ratio and 2.5–97.5th percentiles were summarized based on all results from 1,000 stochastic realizations. The odds ratios of age and vaccination status were adjusted by contact setting and the sex of the infector and contact. | ||||||
| Unvaccinated children (0–9 years) | 2.13 | (1.79, 2.57) | 1.34 | (0.52, 2.78) | ||
| Unvaccinated adolescents (10–17 years) | 1.29 | (1.04, 1.58) | 0.72 | (0.38, 1.19) | ||
| Unvaccinated adults (18–64 years) | Reference | Reference | ||||
| Vaccinated adults (18–64 years) | 0.47 | (0.41, 0.52) | 0.59 | (0.35, 0.95) | ||
Table S4. Estimating the association of potential risk factors with the risk of acquiring and transmitting the SARS-CoV-2 Delta variant based on 6,135 contacts in Hunan, China.
| Characteristic | Multivariate analysis | |
| OR (95% CI) | P value | |
| Note: All children and adolescents (under 18 years) were unvaccinated, as they were not covered by the COVID-19 vaccination program in mainland China during the Delta outbreak. Vaccinated adults denote fully vaccinated adults, while partially vaccinated adults were deemed unvaccinated adults in the analysis. Abbreviation: CI=confidence interval; OR=odds ratio. * P<0.05; ** P<0.01; *** P<0.001; † Contacts who were aged at or over 65 years or whose infectors were aged at or over 65 years were excluded from this analysis due to the small sample size. § For a contact who had interacted with more than one SARS-CoV-2 infection, we chose the first infected individuals they had been exposed to as the potential infector. If they had been exposed to multiple infectors at the same time, we randomly chose one from among these infectors as the potential infector. | ||
| Age (years) and vaccination status of contacts† | ||
| Unvaccinated children (0–9 years) | 2.40 (1.12, 5.18) | 0.025** |
| Unvaccinated adolescents (10–17 years) | 1.38 (0.59, 3.25) | 0.458 |
| Unvaccinated adults (18–64 years) | Reference | |
| Vaccinated adults (18–64 years) | 0.54 (0.29, 0.99) | 0.048* |
| Age (years) and vaccination status of infectors§ | ||
| Unvaccinated children (0–9 years) | 2.19 (0.52, 9.23) | 0.285 |
| Unvaccinated adolescents (10–17 years) | 1.29 (0.31, 5.39) | 0.728 |
| Unvaccinated adults (18–64 years) | Reference | |
| Vaccinated adults (18–64 years) | 0.92 (0.32, 2.65) | 0.875 |
| Type of contact | ||
| Household contacts | 6.90 (3.24, 14.68) | <0.001*** |
| Health care | 0.29 (0.06, 1.34) | 0.113 |
| Social contact | Reference | |
| Workplace contact | 0.58 (0.13, 2.50) | 0.461 |
| Other close contact | 0.04 (0.01, 0.14) | <0.001*** |
| General contact | 0.10 (0.05, 0.21) | <0.001*** |
| Sex of contacts | ||
| Female | Reference | |
| Male | 0.68 (0.41, 1.13) | 0.138 |
| Sex of infectors | ||
| Female | Reference | |
| Male | 0.42 (0.16, 1.10) | 0.078 |
Acknowledgments
All the teams of field investigators of the Hunan Provincial Center for Disease Control and Prevention for their contribution.
Funding Statement
Supported by grants from the Key Program of the National Natural Science Foundation of China (82130093), Shanghai Municipal Science and Technology Major Project (ZD2021CY001), Hunan Provincial Innovative Construction Special Fund: Emergency response to COVID-19 outbreak (No. 2020SK3012), and Chinese Academy of Medical Sciences Coronavirus Disease 2019 Science and Technology Research Project in 2020 (No. 2020HY320003)
Contributor Information
Shixiong Hu, Email: 379788967@qq.com.
Hongjie Yu, Email: yhj@fudan.edu.cn.
References
- 1.Hu SX, Wang W, Wang Y, Litvinova M, Luo KW, Ren LS, et al Infectivity, susceptibility, and risk factors associated with SARS-CoV-2 transmission under intensive contact tracing in Hunan, China. Nat Commun. 2021;12(1):1533. doi: 10.1038/s41467-021-21710-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Office of Changde Municipal People’s Government. An asymptomatic case was reported in Changde City, Hunan Province. https://www.changde.gov.cn/cdzx/cdyw/content_850758. [2022-7-26].
- 3.Kang M, Xin HL, Yuan J, Ali ST, Liang ZM, Zhang JY, et al Transmission dynamics and epidemiological characteristics of SARS-CoV-2 Delta variant infections in Guangdong, China, May to June 2021. Euro Surveill. 2022;27(10):2100815. doi: 10.2807/1560-7917.ES.2022.27.10.2100815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogata T, Tanaka H, Irie F, Hirayama A, Takahashi Y Shorter incubation period among unvaccinated delta variant coronavirus disease 2019 patients in Japan. Int J Environ Res Public Health. 2022;19(3):1127. doi: 10.3390/IJERPH19031127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Águila-Mejía J, Wallmann R, Calvo-Montes J, Rodríguez-Lozano J, Valle-Madrazo T, Aginagalde-Llorente A Secondary attack rate, transmission and incubation periods, and serial interval of SARS-CoV-2 omicron variant, spain. Emerg Infect Dis. 2022;28(6):1224–8. doi: 10.3201/eid2806.220158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manica M, Litvinova M, De Bellis A, Guzzetta G, Mancuso P, Vicentini M, et al. Estimation of the incubation period and generation time of SARS-CoV-2 Alpha and Delta variants from contact tracing data. Epidemiol Infect 2022. https://doi.org/10.1017/S0950268822001947.
- 7.Hart WS, Miller E, Andrews NJ, Waight P, Maini PK, Funk S, et al Generation time of the alpha and delta SARS-CoV-2 variants: an epidemiological analysis. Lancet Infect Dis. 2022;22(5):603–10. doi: 10.1016/S1473-3099(22)00001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park SW, Sun KY, Champredon D, Li M, Bolker BM, Earn DJD, et al Forward-looking serial intervals correctly link epidemic growth to reproduction numbers. Proc Natl Acad Sci USA. 2021;118(2):e2011548118. doi: 10.1073/pnas.2011548118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riley S, Wang HW, Eales O, Haw D, Walters CE, Ainslie KEC, et al. REACT-1 round 12 report: resurgence of SARS-CoV-2 infections in England associated with increased frequency of the Delta variant. medRxiv 2021. http://medrxiv.org/content/early/2021/06/21/2021.06.17.21259103.abstract.
- 10.Kang M, Yi Y, Li Y, Sun LM, Deng AP, Hu T, et al Effectiveness of inactivated COVID-19 vaccines against illness caused by the B. 1.617.2 (Delta) variant during an outbreak in Guangdong, China: a cohort study. Ann Intern Med. 2022;175(4):533–40. doi: 10.7326/M21-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen XH, Yan XM, Sun KY, Zheng N, Sun RJ, Zhou JX, et al. Estimation of disease burden and clinical severity of COVID-19 caused by Omicron BA.2 in Shanghai, February-June 2022. Emerg Microbes Infect 2022;11(1):2800-7. http://dx.doi.org/10.1080/22221751.2022.2128435.
- 12.Chen ZY, Deng XW, Fang LQ, Sun KY, Wu YP, Che TL, et al Epidemiological characteristics and transmission dynamics of the outbreak caused by the SARS-CoV-2 Omicron variant in Shanghai, China: a descriptive study. Lancet Reg Health West Pacific. 2022;29:100592. doi: 10.1016/j.lanwpc.2022.100592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bager P, Wohlfahrt J, Bhatt S, Stegger M, Legarth R, Møller CH, et al Risk of hospitalisation associated with infection with SARS-CoV-2 omicron variant versus delta variant in Denmark: an observational cohort study. Lancet Infect Dis. 2022;22(7):967–76. doi: 10.1016/S1473-3099(22)00154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


