Abstract
Heme-oxygenase 1 (HO-1) is an enzyme with well-known anti-inflammatory and antioxidant properties, whose levels have been previously associated with disease severity in the context of sterile and infectious diseases. Moreover, the heme/HO-1 pathway has been associated with prothrombotic changes in other diseases. Accordingly, the potential of modulating HO-1 levels for the treatment of COVID-19 was extensively speculated during the COVID-19 pandemic, but very few actual data were generated. The aim of our study was to explore the association of HO-1, heme, and hemopexin (HPX) levels with COVID-19 severity and with markers of inflammation and coagulation activation. The study was conducted in 30 consecutive patients with COVID-19 admitted due to hypoxemia, and 30 healthy volunteers matched by sex, age, and geographic region. HO-1 and HPX levels were measured by enzyme immunoassay (ELISA) and heme levels were measured by a colorimetric method. A comprehensive panel of coagulation and fibrinolysis activation was also used. Patients with COVID-19 presented increased levels of HO-1 when compared to controls (5741 ± 2696 vs 1953 ± 612 pg/mL, respectively, P < 0.0001), as well as a trend toward increased levels of HPX (3.724 ± 0.880 vs 3.254 ± 1.022 mg/mL, respectively; P = 0.06). In addition, HO-1 and HPX levels reduced from admission to day + 4. HO-1 levels were associated with duration of intensive care unit stay and with several markers of coagulation activation. In conclusion, modulation of HO-1 could be associated with the prothrombotic state observed in COVID-19, and HO-1 could also represent a relevant biomarker for COVID-19. New independent studies are warranted to explore and expand these findings.
Keywords: Heme-oxygenase 1, hemopexin, heme, COVID-19, inflammation, coagulation
Impact statement
Several authors have speculated about the potential of heme-oxygenase 1 (HO-1) modulation in COVID-19, based on the anti-inflammatory effects of this enzyme in other infectious and inflammatory diseases. However, very little actual data have been produced to support these speculations. Here, we provide data about the behavior of important mediators involved in HO-1 pathway, namely, total heme levels and hemopexin, in the course of COVID-19. Using a well-characterized population of patients from a clinical trial, we demonstrate that HO-1 levels are increased in COVID-19, and consistently associated with coagulation activation. The importance of these results lies on the fact that they represent a demonstration that circulating levels of HO-1 are increased in COVID-19, and that prothrombotic markers are associated with HO-1, paving the way for future studies exploring the role of HO-1 as a biomarker for or mediator of COVID-19.
Introduction
COVID-19 is a disease that primarily affects the respiratory tract caused by the SARS-CoV-2 virus which in severe cases can evolve to an acute respiratory distress syndrome associated with a systemic inflammatory response. This inflammatory response involves several host defense systems such as hemostasis, angiogenesis, and complement.1,2 Comorbidities such as diabetes, hypertension, and obesity, as well as advanced age are associated with higher mortality. 3
Heme-oxygenase 1 (HO-1) is an enzyme with well-known cytoprotective properties, mainly expressed in the liver, whose main role is to metabolize heme, catabolizing it into biliverdin, carbon monoxide, and iron. The circulating protein hemopexin (HPX) is responsible to bind free extracellular heme and to deliver it to cells in which HO-1 is expressed. HO-1 induction has been associated with anti-inflammatory and anti-oxidative effects4,5 in the context of both sterile 6 and infectious diseases,7–10 based on different lines of evidence that include clinical studies showing the association of HO-1 levels with disease severity, 11 genetic association studies 12 and by interventional experiments in animal models.13,14 These protective effects attracted the attention of the scientific community during the COVID-19 pandemic, and several reviews and opinion papers1,15–17 have been written about the potential benefits of modulating this pathway in COVID-19. However, very few studies have actually addressed this pathway in patients and other models.18,19 In a small study with eight COVID-19 patients, lower oxygen saturation was associated with higher HO-1 levels, 19 leading authors to hypothesize whether HO-1 induction could be associated with protective effects in this context.
Coagulation activation is a hallmark of both COVID-19 20 and of conditions associated with high levels of extracellular heme, HPX consumption, and HO-1 induction.21–23 Whether the heme/HPX/HO-1 pathway is associated with coagulation activation in COVID-19 has not been explored.
Using samples from a consecutive cohort of COVID-19 patients enrolled in a clinical trial, we explored the association of HO-1, heme, and HPX levels with COVID-19 severity and with markers of inflammation and coagulation activation.
Materials and methods
Study population
The population of our study consisted of 30 patients with COVID-19 confirmed by reverse transcription polymerase chain reaction (RT-PCR) and who required hospital admission due to Severe Acute Respiratory Syndrome (SARS). Laboratory analysis was performed in samples obtained from patients enrolled in a clinical trial conducted at the University of Campinas University Hospital (Brazilian Clinical Trials Registry: https://ensaiosclinicos.gov.br/rg/RBR-5s2mqg). 24 Baseline samples were obtained within 24 h from the confirmation of COVID-19 diagnosis, before any clinical trial intervention. Thirty healthy individuals matched for age, sex, and geographic region were recruited at the same period and used as a control group. Of note, this population has been described in a previous publication from our group. 25
Sample collection and processing
Samples were collected in 3.2% citrated or ethylenediaminetetraacetic acid (EDTA) K2 tubes and processed within 2 h of collection. Plasma for coagulation assays was obtained from citrated tubes double centrifuged at 1800g at 22°C, while plasma from EDTA tubes was obtained after a single cycle of centrifugation. Plasma samples were immediately stored at 80°C.
Clinical and laboratory data
Clinical and laboratory data were obtained from the medical electronic health records. The extent of lung disease was estimated by computerized tomography at admission using a modified score. 24 The WHO-CPS score (World Health Organization’s Clinical Progression Scale) was calculated as previously reported. 26
Laboratory evaluation of hemostasis markers
Coagulation screening assays (PT, aPTT), coagulation factor (F) activities (fibrinogen and Factor VIII activity), vWF antigen, vWF activity, and antithrombin levels were measured in an automated coagulometer (ACL TOP 550 CTS, Instrumentation Laboratory, USA) using commercially available assays from the same manufacturer (HemosIL reagents). u-PAR (urokinase-type plasminogen activator receptor) levels were measured using a customized Luminex immunoassay (Procarta Plex multiplex panel, Thermo-Fischer Scientific) in a Bioplex 200 instrument (Bio-Rad). All assays were performed in citrate-anticoagulated plasma, except for the Luminex immunoassay, which was performed in EDTA-anticoagulated plasma.
Measurement of HO-1, HPX, and heme levels
The measurement of HO-1 and HPX levels was performed by immunoenzymatic method (ELISA) using commercial kits (Abcam), and total heme levels were measured using a commercial colorimetric kit (QuantiChrom™), in EDTA plasma.
Statistical analysis
Data are presented as mean ± standard deviation (SD) or medians and interquartile ranges (IQRs). Differences in continuous variables were analyzed using Mann–Whitney or Student’s t-test according to data distribution, assessed by the D’Agostino and Pearson normality test. To compare two independent variables, the Mann–Whitney test (for samples with non-normal distribution) or the unpaired t-test (for samples with normal distribution) was used. To compare two dependent variables, the Wilcoxon test (for samples with non-normal distribution) or paired t-test (for samples with normal distribution) was used. Correlation was calculated using Spearman’s correlation coefficient. P value ⩽ 0.05 was considered significant. All statistical analyses were performed using SPSS version 25 (IBM) or GraphPad Prism 8.0 Software (GraphPad Inc).
Results
The laboratory and clinical characteristics of the study population are shown in Table 1.
Table 1.
Laboratory and clinical characteristics of study participants.
| Patients (n = 30) | Healthy individuals (n = 30) | P | |
|---|---|---|---|
| Laboratory data (admission) | |||
| Age* | 52.7 ± 12.3 | 50.3 ± 9.2 | 0.40 |
| Sex, male:female | 16:14 | 16:14 | 1.00 |
| Body mass index* | 30.6 ± 6.6 | 25.9 ± 4.2 | 0.006 |
| Hemoglobin, g/dL* | 13.96 ± 1.91 | 14.30 ± 1.11 | 0.42 |
| Leukocytes, ×109/L* | 8.04 ± 3.91 | 5.58 ± 1.58 | 0.004 |
| Neutrophils, ×109/L* | 6.38 ± 3.77 | 3.09 ± 0.93 | <0.001 |
| Lymphocytes, ×109/L* | 1.20 ± 0.55 | 1.79 ± 0.28 | <0.001 |
| Platelets, ×109/L* | 216.33 ± 93.02 | 245.59 ± 40.34 | 0.12 |
| NLR* | 6.19 ± 4.26 | 1.72 ± 0.61 | <0.001 |
| D-dimer, ng/mL* | 3.609 ± 14.440 | 324 ± 242 | <0.001 |
| C-reactive protein, mg/L* | 115.18 ± 75.74 | 4.15 ± 8.37 | <0.001 |
| Troponin, ng/mL* | 10.96 ± 11.98 | 4.33 ± 2.75 | <0.001 |
| CT score* | 17.8 ± 7.3 | NA | – |
| WHO-CPS score* (median and IQR) | 4919 (4103–6830) | NA | – |
| Outcome data | |||
| Time from symptom onset, days* | 8.1 ± 2.3 | NA | – |
| Length of hospital stay, days* | 12.9 ± 9.8 | NA | – |
| Need for intensive care (%)* | 12/30 (40%) | NA | – |
| Length of intensive care stay, days* | 6.1 ± 9.7 | NA | – |
NLR: neutrophil-to-lymphocyte ratio; NA: not applicable; WHO-CPS: World Health Organization’s Clinical Progression Scale; IQR: interquartile range.
Mean ± SD, WHO-CPS.
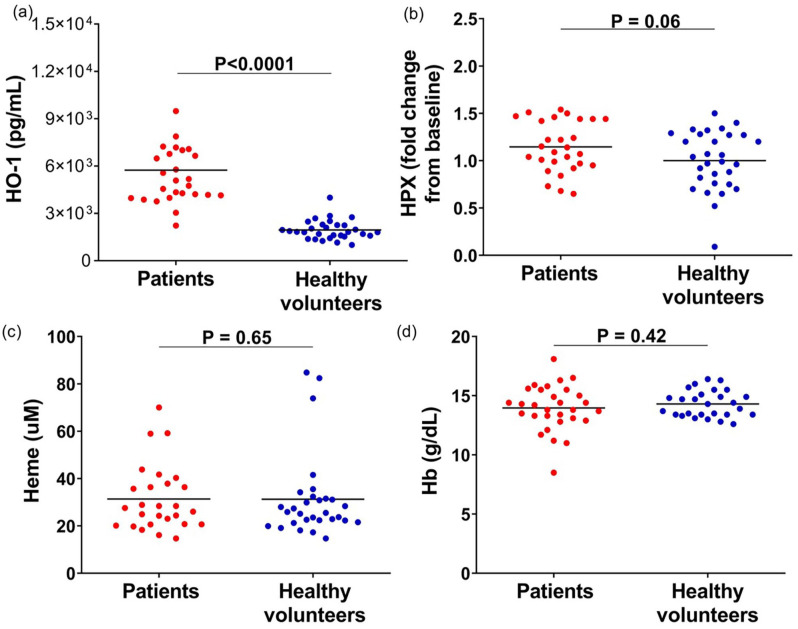
Patients with COVID-19 had increased levels of HO-1 when compared with healthy individuals (P > 0.0001), as well as a trend toward increased HPX (P = 0.06). No differences were observed in total heme levels and hemoglobin between patients and healthy volunteers (Figure 1).
Figure 1.
Plasma levels of (a) HO-1, (b) HPX, (c) heme, and (d) hemoglobin in patients with COVID-19 and healthy volunteers. Results shown as mean and P values are from Mann–Whitney test or unpaired t-test according to data distribution (n = 28–30 per group).
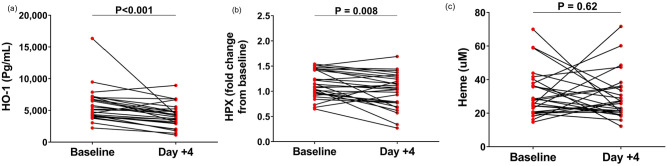
We next evaluated the time-course of HO-1, HPX, and heme levels from admission to day + 4 after admission. Of note, no differences were observed in any of these three markers when patients were compared based on clinical trial arm intervention at baseline, at day + 4, or when the ratio between day 4/baseline was compared (data not shown). Significant decrease of HO-1 and HPX levels could be observed, while total heme levels remained stable (Figure 2).
Figure 2.
Time-course of (a) HO-1, (b) hemopexin, and (c) heme levels on the day of admission and on the fourth day of hospitalization of patients with COVID-19. Results shown as mean and P values are from Wilcoxon test or paired t-test according to data distribution (n = 28–30 per group).
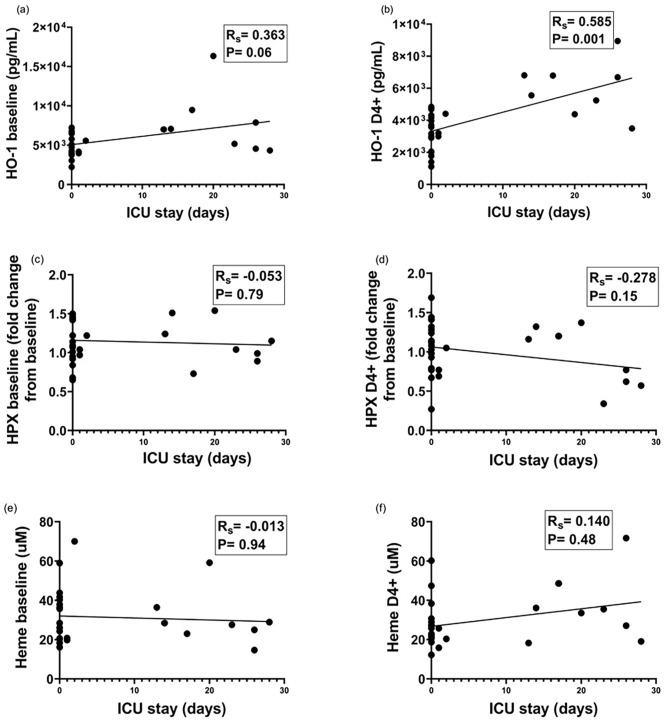
We then explored the association of HO-1 and HPX levels with clinical and laboratory markers of disease severity. As shown in Figure 3, higher HO-1 levels were associated with longer intensive care unit (ICU) stay, yielding a positive correlation (Rs = 0.572; P = 0.001) between the variation of HO-1 levels (day + 4/admission) and ICU length of stay, illustrating that higher increases in HO-1 levels were associated with longer ICU stay. In addition, HO-1 levels of patients with a WHO-CPS score ⩾ 7 (median = 7006, IQR = 4551–7880, ng/mL) were significantly higher than patients with WHO-CPS score below 7 (median = 4278, IQR = 3971–6486, ng/mL). HPX or heme levels at admission or day + 4 did not correlate with length of ICU stay (Figure 3). No significant correlation was observed between HO-1, HPX, or heme levels with other clinical parameters of disease severity such as the magnitude of lung disease (measured by a standardized CT score), overall hospital stay, and oxygen saturation at admission.
Figure 3.
Association between HO-1, HPX, and heme levels with length of ICU stay. (a, b) Association of HO-1 levels at admission and on day 4 + with days of ICU stay. (c, d) Association of hemopexin levels at admission and on day 4 + with days of ICU stay. (e, f) Association of heme levels at admission and on day 4 + with days of ICU stay (Spearman correlation coefficient).
When laboratory parameters were analyzed, a consistent association of HO-1 levels with markers of coagulation activation was observed, illustrated by moderate to strong correlations with several markers. No significant correlation could be observed between HPX or total heme levels with laboratory markers of coagulation activation (Table 2).
Table 2.
Correlation between admission HO-1, HPX, and heme levels and markers of hemostatic activation in COVID-19.
| Parameter | HO-1 | HPX | Heme | |||
|---|---|---|---|---|---|---|
| Rs * | P | Rs * | P | Rs * | P | |
| Platelet count | −0.437 | 0.001 | −0.001 | 0.996 | −0.170 | 0.218 |
| Prothrombin time | 0. 345 | 0.010 | 0.050 | 0.716 | −0.211 | 0.123 |
| aPTT | 0. 372 | 0.005 | 0.101 | 0.458 | −0.233 | 0.086 |
| Fibrinogen | 0. 717 | <0.001 | 0.283 | 0.034 | −0.023 | 0.916 |
| Factor VIII activity | 0. 526 | <0.001 | 0.172 | 0.204 | 0.220 | 0.106 |
| Von Willebrand factor, antigen | 0. 765 | <0.001 | 0.166 | 0.214 | 0.081 | 0.550 |
| Von Willebrand factor, activity | 0. 762 | <0.001 | 0.229 | 0.089 | 0.171 | 0.212 |
| uPAR (urokinase receptor) | 0. 606 | <0.001 | 0.033 | 0.806 | −0.107 | 0.429 |
| D-dimer | 0.263 | 0.184 | −0.246 | 0.206 | 0.024 | 0.906 |
| C-reactive protein | 0.291 | 0.148 | −0.143 | 0.474 | 0.133 | 0.516 |
HO: Heme-oxygenase; HPX: hemopexin; aPTT: activated partial thromboplastin time.
Significant correlations are highlighted in bold.
Rs: Spearman correlation coefficient.
Discussion
The identification of inflammatory pathways associated with disease severity in COVID-19 is a relevant scientific question that can support the development of biomarkers and targeted therapies for this and other related conditions. In this context, the main contribution of our study was the demonstration that HO-1 levels are increased and associated with disease severity and coagulation activation in COVID-19, and that the modulation of HO-1 is not necessarily associated with evidences of increased heme catabolism in this condition.
HO-1 is a well-known anti-inflammatory and anti-oxidant enzyme27,28 with tissue-protective effects best illustrated by the dramatic clinical presentation of HO-1 deficiency, which is associated with widespread inflammation, endothelial, and coagulation activation, and early lethality.29,30 It has been previously shown that the expression of HMOX, the gene coding HO-1 can be modulated up to 100 times in the presence of infections or acute lung injury,5,31 with the antiviral properties of HO-1 or of its regulatory gene NRF2 having been demonstrated in models of Influenza, 32 Dengue, 33 and Ebola. 9 Besides, it has also been demonstrated that HO-1 expression is associated with decrease in tissue damage in several inflammatory conditions such as atherosclerosis, 34 sickle cell nephropathy, 35 and neurodegenerative diseases. 36
The modulation of HO-1 can be driven by inflammation and tissue damage, but can also occur in the context of increased heme catabolism, such as observed in hemolytic conditions. In these conditions, the release of extracellular heme leads to the increased delivery of heme-HPX complexes to HO-1 expressing cells in the liver, causing an increase in HO-1 expression and activity. 14 Although the hypothesis that the pathogenesis of COVID-19 could be associated with lower oxygen delivery to tissues caused by derangements of heme function gained massive public attention in the early days of the pandemic, this possibility was ruled out by experimental data showing that COVID-19 is not associated with changes in oxygen dissociation. 37 Besides, anemia and hemolysis are not relevant manifestations of COVID-19. 38 Therefore, the putative association of HO-1 with COVID-19 has been mostly discussed in the context of the anti-inflammatory and anti-oxidant effects of this enzyme. It should be noted, however, that among the 14 peer-reviewed publications addressing this issue, the vast majority were reviews or opinion papers discussing this possibility with only two studies actually addressing HO-1 levels in COVID-19 patients. A recent study demonstrated increased HMOX mRNA expression in circulating leukocytes in COVID-19 patients. 39 Other study which measured circulating HO-1 levels in blood from these patients also demonstrated upregulation of HO-1 levels in a population of eight patients. 19 More recently, a larger study encompassing 64 patients demonstrated that HO-1 is consistently associated with clinical and laboratory markers of disease severity, and that its measurement could refine prediction models for ICU admission. 40
Our demonstration that HO-1 levels are increased in COVID-19 patients compared to healthy individuals and that these results are not related to circulating heme levels is in accordance with the hypothesis that HO-1 modulation in COVID-19 is part of the host inflammatory response. Moreover, the association of higher HO-1 levels with both disease severity (ICU length of stay) and coagulation activation further corroborates the concept that HO-1 increase is an additional element of the host response to COVID-19. Of note, the fact that total heme levels were not different between patients and healthy volunteers, and the mild modulation of HPX levels – a well-known acute phase protein 41 – which were not associated with clinical or laboratory markers of disease severity, reinforces the concept that the pathogenesis of COVID-19 does not involve systemic changes of heme metabolism or function, as previously shown. 37
An original finding of our study was the association of HO-1 levels with several markers of hemostatic activation. Concomitant activation of innate immunity and hemostasis, termed immunothrombosis, is a hallmark of COVID-19. 3 In our patients, HO-1 correlated more strongly with Von Willebrand Factor levels, which is a marker of both hemostasis and endothelial activation, and with fibrinogen and soluble urokinase plasminogen receptor (u-PAR), which are markers of coagulation and fibrinolysis activation. Heme release, which upregulates HO-1 expression, can lead to coagulation activation as shown in both animal models 42 and humans.22,43 While our data rule out the possibility that systemic heme release could be driving HO-1 upregulation in our patients, it has been previously shown that local hemoglobin breakdown products are present in the bronchoalveolar fluid of patients with acute lung injury due to infections. 44 So, one possible explanation for the upregulation of HO-1 is that it occurs due to the inflammatory milieu in the alveolar space of COVID-19 patients, which could include the presence of hemoglobin or heme. In fact, the anti-thrombotic effects of HO-1 activation have been shown in both cell and animal models. In a study using human endothelial cells, carbon monoxide, a byproduct of heme catabolism by HO-1, downregulated tissue factor and plasminogen activator 1 (PAI-1) expression. 45 And in an animal model of venous thrombosis, HO-1 deficiency was associated with larger thrombi. 46 Additional studies are warranted to explore this hypothesis.
Our study has several limitations that need to be considered. First, the relatively low number of patients, which require independent confirmation. However, we believe that the fact that patients were recruited as part of a clinical study is a strength of our study since it resulted in prospectively and systematically collected data and blood samples. Second, patients had higher body mass index compared to healthy subjects. However, HO-1 was not correlated with body mass index (BMI) in patients or in healthy individuals. Third, we were not able to explore heme or free hemoglobin levels in other tissues or fluids, which were beyond the scope of our original project.
Conclusions
In conclusion, HO-1 is upregulated in COVID-19 and is associated with markers of endothelial and hemostatic activation. Further studies are warranted to explore the pathways involved in this upregulation, as well as the role of HO-1 as a marker of disease severity and as a therapeutic target in COVID-19.
Footnotes
Authors’ Contributions: FL obtained and processed samples, performed coagulation factor assays and ELISA, contributed to data analysis, and drafted the manuscript; CRPM obtained and processes samples; MSB contributed in coagulation factor assays; BB and ACP recruited and managed patients and obtained patient data; MLM, EM, and LAV designed and conducted the clinical trial from which patients were recruited; SSJD analyzed and scored lung tomography images; FAO contributed to study design and data analysis; JMA provided laboratory support and infrastructure for classical coagulation assays; EVDP designed the study, obtained, and processed samples, oversaw and provided resources and infrastructure for coagulation and ELISA analysis, contributed to data analysis, and drafted the manuscript. All collaborators revised and approved the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Sao Paulo Research Foundation (FAPESP), grants 2016/14172-6 and 2020/05985-9, FAEPEX-Unicamp grant 2404/2020; Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior – Brasil (CAPES).
ORCID iDs: Franciele de Lima  https://orcid.org/0000-0002-8698-2388
https://orcid.org/0000-0002-8698-2388
Maria Luiza Moretti  https://orcid.org/0000-0002-2280-5649
https://orcid.org/0000-0002-2280-5649
Eli Mansour  https://orcid.org/0000-0001-6450-6930
https://orcid.org/0000-0001-6450-6930
Erich Vinicius De Paula  https://orcid.org/0000-0003-1539-7912
https://orcid.org/0000-0003-1539-7912
References
- 1.Maiti BK.Heme/heme oxygenase-1 system is a potential therapeutic intervention for COVID-19 patients with severe complications. ACS Pharmacol Transl Sci 2020;3:1032–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hooper PL.COVID-19 and heme oxygenase: novel insight into the disease and potential therapies. Cell Stress Chaperones 2020;25:707–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, Dentali F, Montecucco F, Massberg S, Levi M, Abbate A.Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol 2021;21(5):319–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn LL, Midwinter RG, Ni J, Hamid HA, Parish CR, Stocker R.New insights into intracellular locations and functions of heme oxygenase-1. Antioxidants Redox Signal 2014;20:1723–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi AM, Alam J.Heme oxygenase-1: function, regulation and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol 1996;15(1):9–19 [DOI] [PubMed] [Google Scholar]
- 6.Fredenburgh LE, Merz AA, Cheng S.Haeme oxygenase signalling pathway: Implications for cardiovascular disease. Eur Heart J 2015;36: 1512–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinoza JA, González PA, Kalergis AM.Modulation of antiviral immunity by heme oxygenase-1. Am J Pathol 2017;187(3):487–93 [DOI] [PubMed] [Google Scholar]
- 8.Ma LL, Wang HQ, Wu P, Hu J, Yin JQ, Wu S, Ge M, Sun WF, Zhao JY, Aisa HA, Li YH, Jiang JD.Rupestonic acid derivative YZH-106 suppresses influenza virus replication by activation of heme oxygenase-1-mediated interferon response. Free Radic Biol Med 2016;96:347–61 [DOI] [PubMed] [Google Scholar]
- 9.Hill-Batorski L, Halfmann P, Neumann G, Kawaoka Y.The cytoprotective enzyme heme oxygenase-1 suppresses ebola virus replication. J Virol 2013;87(24):13795–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olagnier D, Peri S, Steel C, van Montfoort N, Chiang C, Beljanski V, Slifker M, He Z, Nichols CN, Lin R, Balachandran S, Hiscott J.Cellular oxidative stress response controls the antiviral and apoptotic programs in dengue virus-infected dendritic cells. Plos Pathog 2014; 10(12):e1004566–100418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan T, Qi J, You T, Han S, Yang L, Miao W, Wu D, Ruan C, Zhu L, Han Y.Circulating heme oxygenase-1 and complement activation in transplant-associated thrombotic microangiopathy. Biol Blood Marrow Transplant 2019;25(8):1486–91 [DOI] [PubMed] [Google Scholar]
- 12.Hsieh YH, Chen CW, Schmitz SF, King CC, Chen WJ, Wu YC, Ho MS.Candidate genes associated with susceptibility for SARS-coronavirus. Bull Math Biol 2010;72(1):122–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, Zhao D, Zaky J, Melinek J, Lassman CR, Kolls JK, Alam J, Ritter T, Volk HD, Farmer DG, Ghobrial RM, Busuttil RW, Kupiec-Weglinski JW.Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest 1999;104(11):1631–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belcher JD, Chen C, Nguyen J, Abdulla F, Zhang P, Nguyen H, Nguyen P, Killeen T, Miescher SM, Brinkman N, Nath KA, Steer CJ, Vercellotti GM.Haptoglobin and hemopexin inhibit vaso-occlusion and inflammation in murine sickle cell disease: role of heme oxygenase-1 induction. PLoS ONE 2018;13(4):e0196455–1019620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh D, Wasan H, Reeta KH.Heme oxygenase-1 modulation: a potential therapeutic target for COVID-19 and associated complications. Free Radic Biol Med 2020;161:263–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi M, Piagnerelli M, Van Meerhaeghe A, Zouaoui Boudjeltia K.Heme oxygenase-1 (HO-1) cytoprotective pathway: a potential treatment strategy against coronavirus disease 2019 (COVID-19)-induced cytokine storm syndrome. Med Hypotheses 2020;144:110242–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rapozzi V, Juarranz A, Habib A, Ihan A, Strgar R.Is haem the real target of COVID-19? Photodiagnosis Photodyn Ther 2021;35:102381–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maestro S, Córdoba KM, Olague C, Argemi J, Ávila MA, González-Aseguinolaza G, Smerdou C, Fontanellas A.Heme oxygenase-1 inducer hemin does not inhibit SARS-CoV-2 virus infection. Biomed Pharmacother 2021;137:111384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su WL, Lin CP, Hang HC, Wu PS, Cheng CF, Chao YC.Desaturation and heme elevation during COVID-19 infection: a potential prognostic factor of heme oxygenase-1. J Microbiol Immunol Infect 2021;54(1):113–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackman N, Antoniak S, Wolberg AS, Kasthuri R, Key NS.Coagulation abnormalities and thrombosis in patients infected with SARS-CoV-2 and other pandemic viruses. Arterioscler Thromb Vasc Biol 2020; 40(9):2033–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conran N, de Paula EV.Thromboinflammatory mechanisms in sickle cell disease – challenging the hemostatic balance. Haematologica 2020;105:2380–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Souza GR, Hounkpe BW, Fiusa MML, Colella MP, Annichino-Bizzacchi JM, Traina F, Costa FF, De Paula EV.Tissue factor-dependent coagulation activation by heme: a thromboelastometry study. Plos One 2017;12(4):e0176505–1017610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardoso EC, Silva-Neto PV, Hounkpe BW, Chenou F, Albuquerque CCMX, Garcia NP, Silva-Junior AL, Malheiro A, Cesar P, de Lima F, De Paula Fraiji EVNA. Changes in heme levels during acute vaso-occlusive crisis in sickle cell anemia. Hematol Oncol Stem Cell Ther. Epub ahead of print 21 August 2021. DOI: 10.1016/j.hemonc.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Mansour E, Palma AC, Ulaf RG, Ribeiro LC, Bernardes AF, Nunes TA, Agrela MV, Bombassaro B, Monfort-pires M, Camargo RL, Araujo EP, Brunetti NS, Farias AS, Falc E, Santos TM, Trabasso P, Dertkigil RP, Dertkigil SS, Moretti ML, Velloso LA.Safety and outcomes associated with the pharmacological inhibition of the kinin – kallikrein system in severe COVID-19. Viruses 2021;13:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henderson MW, Lima F, Moraes CRP, Ilich A, Huber SC, Barbosa MS, Santos I, Palma AC, Nunes TA, Ulaf RG, Ribeiro LC, Bernardes AF, Bombassaro B, Dertkigil SSJ, Moretti ML, Strickland S, Annichino-Bizzacchi JM, Orsi FA, Mansour E, Velloso LA, Key NS, De Paula EV.Contact and intrinsic coagulation pathways are activated and associated with adverse clinical outcomes in COVID-19. Blood Adv 2022;6:3367–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020;20(8):e192–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapturczak MH, Wasserfall C, Brusko T, Campbell-Thompson M, Ellis TM, Atkinson MA, Agarwal A.Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol 2004;165(3):1045–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell NK, Fitzgerald HK, Dunne A.Regulation of inflammation by the antioxidant haem oxygenase 1. Nat Rev Immunol 2021;21:411–25 [DOI] [PubMed] [Google Scholar]
- 29.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S.Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest 1999;103(1):129–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radhakrishnan N, Yadav SP, Sachdeva A, Pruthi PK, Sawhney S, Piplani T, Wada T, Yachie A.Human heme oxygenase-1 deficiency presenting with hemolysis, nephritis, and asplenia. J Pediatr Hematol Oncol 2011;33(1):74–8 [DOI] [PubMed] [Google Scholar]
- 31.Soares MP, Bach FH.Heme oxygenase-1: from biology to therapeutic potential. Trends Mol Med 2009;15(2):50–8 [DOI] [PubMed] [Google Scholar]
- 32.Cummins NW, Weaver EA, May SM, Croatt AJ, Foreman O, Kennedy RB, Poland GA, Barry MA, Nath KA, Badley AD. Heme oxygenase-1 regulates the immune response to influenza virus infection and vaccination in aged mice. FASEB J 2012;26(7):2911–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tseng CK, Lin CK, Wu YH, Chen YH, Chen WC, Young KC, Lee JC.Human heme oxygenase 1 is a potential host cell factor against dengue virus replication. Sci Rep 2016;6:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durante W.Protective role of heme oxygenase-1 against inflammation in atherosclerosis. Front Biosci 2017;16:2372–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nath KA, Grande JP, Haggard JJ, Croatt AJ, Katusic ZS, Solovey A, Hebbel RP.Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am J Pathol 2001;158(3):893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Z, Zhou T, Ziegler AC, Dimitrion P, Zuo L.Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxid Med Cell Longev 2017;2017:2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeMartino AW, Rose JJ, Amdahl MB, Dent MR, Shah FA, Bain W, McVerry BJ, Kitsios GD, Tejero J, Gladwin MT.No evidence of hemoglobin damage by SARS-CoV-2 infection. Haematologica 2020;105: 2769–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B.Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Detsika MG, Nikitopoulou I, Veroutis D, Vassiliou AG, Jahaj E, Tsipilis S, Athanassiou N, Gakiopoulou H, Gorgoulis VG, Dimopoulou I, Orfanos SE, Kotanidou A.Increase of HO-1 expression in critically Ill COVID-19 patients is associated with poor prognosis and outcome. Antioxidants 2022;11:1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hara Y, Tsukiji J, Yabe A, Onishi Y, Hirose H, Yamamoto M, Kudo M, Kaneko T, Ebina T.Heme oxygenase-1 as an important predictor of the severity of COVID-19. PLoS ONE 2022;17(8):e0273500–1027314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tolosano E, Fagoonee S, Morello N, Vinchi F, Fiorito V.Heme scavenging and the other facets of hemopexin. Antioxid Redox Signal 2010;12(2):305–20 [DOI] [PubMed] [Google Scholar]
- 42.Sparkenbaugh EM, Chantrathammachart P, Wang S, Jonas W, Kirchhofer D, Gailani D, Gruber A, Kasthuri R, Key NS, Mackman N, Pawlinski R.Excess of heme induces tissue factor-dependent activation of coagulation in mice. Haematologica 2015;100(3):308–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hounkpe BW, Moraes CRP, Lanaro C, Santos MNN, Costa FF, De Paula EV.Evaluation of the mechanisms of heme-induced tissue factor activation: contribution of innate immune pathways. Exp Biol Med 2022;247:1542–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaver CM, Grove BS, Clune JK, Mackman N, Ware LB, Bastarache JA.Myeloid tissue factor does not modulate lung inflammation or permeability during experimental acute lung injury. Sci Rep 2016;6:22249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maruyama K, Morishita E, Yuno T, Sekiya A, Asakura H, Ohtake S, Yachie A.Carbon monoxide (CO)-releasing molecule-derived CO regulates tissue factor and plasminogen activator inhibitor type 1 in human endothelial cells. Thromb Res 2012;130(3):e188–93 [DOI] [PubMed] [Google Scholar]
- 46.Tracz MJ, Juncos JP, Grande JP, Croatt AJ, Ackerman AW, Katusic ZS, Nath KA.Induction of heme oxygenase-1 is a beneficial response in a murine model of venous thrombosis. Am J Pathol 2008;173(6): 1882–90 [DOI] [PMC free article] [PubMed] [Google Scholar]