Abstract
“It has been commented by someone that ‘polyoma’ is an adjective composed of a prefix and suffix, with no root between—a meatless linguistic sandwich” (C. J. Dawe). The very name “polyomavirus” is a vague mantel: a name given before our understanding of these viral agents was clear but implying a clear tumor life-style, as noted by the late C. J. Dawe. However, polyomavirus are not by nature tumor-inducing agents. Since it is the purpose of this review to consider the natural function of middle T antigen (MT), encoded by one of the seemingly crucial transforming genes of polyomavirus, we will reconsider and redefine the virus and its MT gene in the context of its natural biology and function. This review was motivated by our recent in vivo analysis of MT function. Using intranasal inoculation of adult SCID mice, we have shown that polyomavirus can replicate with an MT lacking all functions associated with transformation to similar levels to wild-type virus. These observations, along with an almost indistinguishable replication of all MT mutants with respect to wild-type viruses in adult competent mice, illustrate that MT can have a play subtle role in acute replication and persistence. The most notable effect of MT mutants was in infections of newborns, indicating that polyomavirus may be highly adapted to replication in newborn lungs. It is from this context that our current understanding of this well-studied virus and gene is presented.
INTRODUCTION TO THE NATURAL LIFE CYCLE OF VERTEBRATE POLYOMAVIRUSES
Members of the subfamily Polyomavirinae infect an array of vertebrate species. Although the ability of mouse polyomavirus (Py) to induce tumors in inbred mice and hamsters is very well studied and provides a productive transformation model, it nevertheless surprises most readers to learn that none of the Polyomavirinae family members are known to cause tumors in natural settings or natural hosts. In fact, many a review or textbook on polyomavirus tumor biology will introduce the premise that these viruses have two life cycles, a transforming cycle and a lytic cycle, and that transformation requires a nonproductive or abortive infection, i.e., that the viral early transforming genes must hijack host DNA synthesis and induce otherwise quiescent host cells to enter S phase in preparation for the lytic replication of virus. Although seemingly logical, this concept has fatal flaws; most obviously, it completely ignores all our biological observations of naturally infected host populations which fail to support it and can contradict these views. If anything, biological studies support the converse view, i.e., that cells must already be cycling or differentiating in order to support the lytic replication of polyomaviruses. We thus attempt to deduce the natural setting in which Py middle T antigen (MT) must function and hence its role in the virus life cycle. This may shed light on how MT, in some instances, perturbs cellular regulation to lead to transformation when this process is essentially nonexistent in natural settings.
The members of the Polyomavirinae are small, nonenveloped, double-stranded DNA viruses with icosahedral capsids. Following its discovery in 1953 by Gross, the ecology of Py in the wild was first explored by Rowe in the early 1960s. From 1959 to 1960, Rowe and colleagues captured mice throughout New York City and in rural farms in Maryland. Collections from both locations were considered to be wild mice, as opposed to inbred laboratory strains (288, 290). They found that not only was Py infection clinically inapparent and focal, but also it remained persistent in the population at roughly the same frequency. Although virus was found in various organs of the mice and in the excreta and environment (floor sweepings), urine appeared to be the source of persistence and spread of the virus in a population (upwards of 103 50% infective doses /0.2 ml excreted in the urine for months from infected newborns) (289). Additional studies in farms and grain and feed mills of rural Maryland revealed that only areas with sustained breeding conditions had significant Py-positive mice, consistent with infection of newborns (154). Thus, the life cycle of mouse and other mammalian polyomaviruses involves lifelong persistent infections. All mice in these experiments were Mus musculus, but this nomenclature would correspond to Mus musculus domesticus or the more current name of Mus domesticus. Natural Py infection was not found in other rodents when the hemagglutination inhibition assay was used to detect Py in previously collected serum, including a limited survey of wild mice (Mus [rural, not associated with human dwellings], Peromyscus, Microtus, Perognathus, and Reithrodontomus) and rats (Rattus, Neotoma, and Dipodomys), although the level of Py infection in the local mice was unknown at the time (275). However, the more recent discovery of avian polyomaviruses displayed a different life cycle, which appeared to be limited to acute (not persistent) infection (355).
The studies by Rowe also suggested that the virus is most likely to be passed from mother to offspring at birth, which is consistent with subsequent laboratory observations establishing that newborns were much more permissive for Py infection. No tumors were observed in such Py-infected mice. One of the conclusions was that under natural conditions, Py does not induce tumors. Rowe suggested four main reasons to explain this conclusion: the protection from maternally transmitted antibodies, the low probability of infection during the small window of neonatal susceptibility, the low dosage of virus likely to be acquired by inhalation (or ingestion), and the effectiveness of the cell-mediated immunity in adult mice. These findings were also consistent with evaluations of tumor incidence rates in large breeding colonies containing Py-infected and uninfected mice which showed no increase in tumor rates in Py-infected colonies (288, 290). Later studies by Gardner also supported this view (124). He surveyed wild-mouse colonies in the Los Angeles area and found only one colony to be highly infected with Py (numerous separate collections were made). His original theory was that Rowe had not waited long enough in his studies for tumors to develop and that mice may show the higher incidence of Py-induced tumors if kept alive for over 2 years. He observed that Py infection did not increase the number of tumors in old age by comparing a heavily Py-infected colony (40%) with a lightly Py-infected colony (0.6%) for an extended period. In addition, even in immunosuppressed mice, tumors were not observed. His conclusion in the end was the same as Rowe's, namely, that Py does not cause cancer in the natural host under natural conditions.
Similar conclusions would apply to the other members of Polyomavirinae, such as the primate simian virus 40 (SV40), and the human BKV and JCV. Although highly prevalent, persistent, and even productive in their natural host and containing a large T antigen (LT) that binds p53 and Rb, these viruses are not associated with tumors in their natural hosts, although there have been reports of a possible connection between the presence of primate SV40 sequences and human malignant mesotheliomas (221). Considering how many people worldwide (including many with immunosuppressive disease) are infected with these agents and able to produce virus and express viral proteins, the lack of observed transformation is compelling and may suggest that our animal tumor studies may not be evaluating a biologically likely phenomena. In other words, not all mechanisms of transformation may be equally valid or biologically relevant. The Py tumor studies may therefore not identify mechanisms that are prevalent causes of human cancer. Therefore the linguistically “meatless” name “polyomavirus” appears not to describe the life-style of any of these agents but reflects phenomena (tumors) that can be induced in specific laboratory settings, such as the inoculation of specific inbred mouse strains within 24 h of birth (76).
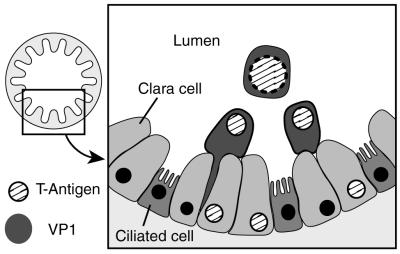
The natural life cycles of the mammalian polyomaviruses, however, do seem similar in many respects. The best studied of these viruses (SV40, BKV, JCV, and Py) all appear to establish primary (and generally inapparent) infections via the respiratory tract or associated organs in the newborn host and then establish persistent inapparent infections in the kidneys, with periodic or episodic shedding of infectious virus from adults into the urine. Only in Py have the specific cell types that support primary and secondary infection been identified. Here, primary infection is almost completely restricted to a very specific cell type, the nonciliated epithelial cells (Clara cells) of the bronchi and bronchioles (131). Newborn immature Clara or Clara-like cells are especially permissive for Py infection, and essentially every Clara cell in the newborn lung will support Py replication. However, 4 days after birth, these cells become considerably less permissive and this reduced permissiveness is maintained in the adult lung. As this period of permissiveness corresponds to active Clara cell division and differentiation (the transition to mature Clara cells and ciliated epithelial cells), Py would appear to replicate best in differentiating cells.
The close connection between Py replication and differentiation was first proposed by us to explain why mice have certain tissues (lung and kidney tissues) which will support efficient Py replication only in newborns, not adults, whereas other tissues which continue to differentiate (bone, skin, and mammary gland) can efficiently replicate Py in adults (381). Subsequent in vivo experiments by us, in which adult kidneys were damaged and induced to differentiate, showing that these differentiating tubular epithelial cells better support Py replication, were consistent with this view (13). In addition, we showed that mice with a genetic form of polycystic kidney disease in which kidney epithelia proliferate but do not differentiate into tubular epithelia were not permissive for Py replication, suggesting that terminal differentiation, not proliferation, is needed in permissive cells (14). The general conclusion from all these studies is that Py resembles the papillomaviruses in its requirement for host cell terminal differentiation for productive infection. If this is so, the prevalent view that a role of the products of the viral early genes is to induce quiescent cells into S phase and the full cell cycle in preparation for viral DNA synthesis must necessarily be wrong in natural settings. It seems more likely that the natural role of these proteins would instead be to alter the programming that normally occurs during terminal differentiation of host cells, in order to maintain in these infected cells the capacity for virus DNA synthesis and replication, which would otherwise normally be suppressed. Given this renewed view of Py biology, we can now consider the specific characteristics of MT in relationship to host cell differentiation.
Origins of Middle T Antigen, an Evolutionary Conundrum
Polyomavirus evolution.
Although Polyomavirinae family members establish prevalent persistent infections in their best-studied host (mouse, human, or primate), it is curious that related virus types (small icosahedral, nonenveloped double-stranded DNA viruses) are not found in nonvertebrate species, given the relative simplicity of this virus family and its parasitic dependence on host replication proteins. In addition, mammalian and avian species of polyomavirus seem to differ significantly in their biology, as noted above, in that the avian versions can infect multiple species and do not appear to establish persistent infections (262, 286, 355). Furthermore, the mammalian but not the avian polyomaviruses appear to be phylogenetically congruent with the evolution of their host, which suggests that polyomaviruses have been infecting their current mammalian host since their divergence from common ancestors (1, 139, 310, 323). Of particular interest to this review, however, is that it appears that only the rodent polyomaviruses code for the production of a MT. All the other polyomaviruses seem able to code for LT and small T antigen (ST) (although the ST for bovine polyomavirus is still questionable) but not MT. Why might this viral gene have evolved in the rodent lineage?
Is middle T antigen needed to offset a lost p53–large T-antigen interaction?
One correlate that may suggest a distinct strategic difference between mouse (rodent) and other mammalian polyomaviruses is that, unlike nonrodent polyomaviruses, mouse LT lacks a p53 binding site, although Rb binding activity is conserved. This could suggest that MT may have evolved after the divergence of common ancestors and that lost p53 binding function was offset by the creation of MT. The bovine LT appears similar (and may encode a protein that appears to be ST) to the primate version. As bovines diverged very early from other mammals (including rodents), it seems likely that the early version of Py had an LT that bound p53 and pRb and had a functional ST, but no MT, and that MT was established only relatively late in the rodent lineage. Although it is apparent that the polyomaviruses of primates and rodents evolved from the same ancestor about 80 million years ago, it seems almost impossible, conceptually, that a mere LT reading frame shift could have fortuitously resulted in the complex, multitask MT protein we know today (316). Since there are no known host analogues of MT, we cannot identify a possible source of MT sequences and currently have no explanation for this conundrum.
Within the rodent MT sequences, some differences are known which might help understand the function and evolution of this gene. The hamster polyomavirus (HaPV) was isolated in 1967 from hair follicle tumors arising spontaneously in young inbred Syrian hamsters (134). The life strategy of the virus appears similar to that of Py in that a majority of the virus was found only in the upper keratinized cell layer, reminiscent of the differentiation strategy used by Py in mammary glands, bone surfaces, and skin (356). However, unlike Py, the tumors that arise do not have virus (HaPV) present, at least not in the lymphomas or leukemias found in the hamsters (133). This difference in tumor profiles between Py and HaPV appears to be associated with the different strategies used by their respective MTs (for a review, see reference 97). One key difference is the lack of a Shc binding site in hamster MT, a site that appears to be quite important for the MT-activated downstream signal transduction pathways (as explained below) (39, 64). This gives us a clue that MT may have evolved along with the rodent lineage, acquiring along the way the complex array of phosphorylation sites that bind and activate signal transduction proteins.
MT is probably a recently evolved protein among polyomaviruses in that comparisons of the genomes among HaPV, SV40, BKV, murine Py, and the monkey lymphotrophic polyomavirus LPV have demonstrated that the highest stretch of conservation is in the late region and portions of LT (264, 356). The 12 types of polyomaviruses identified so far are two human (JCV and BKV), two monkey (SV40 in rhesus monkeys and LPV in African green monkeys), Chacma baboon (simian agent 12 [SA12]), rabbit (rabbit kidney vacuolating virus), two mouse (Py and K virus), rat, hamster (HaPV), bovine, and avian (budgerigar fledgling disease virus) viruses (for a review, see reference 264). Recent papers have shown that the avian polyomaviruses may all belong to one group, but none seem to be very species specific (163, 189, 262). Only the hamster and one of the mouse types, Py, are known to encode an MT (rabbit and rat polyomaviruses remain unsequenced). It is predicated that the rat version should have an MT, since hamsters diverged prior to rats and mice and their polyomavirus (HaPV) encodes an MT. A more thorough examination of polyomavirus throughout the rodent lineage may at least give a time frame for the evolution of MT, since it remains curious that the primate versions and other nonrodent forms of polyomavirus do not even have remnants of coding capabilities for the central portion of the MT sequence.
The nine sequenced versions of polyomavirus fall into categories that closely resemble evolutionary lineages. Based on LT analysis, JCV, BKV, SA12, and SV40 are 75% identical, while HaPV, K virus, LPV, and Py are around 50% identical to each other and to other members, and the most remote evolutionarily, the avian and bovine lineages, also have polyomavirus versions which are less than 15% identical to other members (264). Certain functional domains of LT (ATPase binding domain, DNA binding domains, zinc finger, nucleotide binding fold, nuclear localization signal, and CR1- and CR2-like sequences) have been well conserved except among the avian polyomaviruses, suggesting an absolute requirement for viral function. Other domains (carboxy terminus associated with host range function, general size of LT, and ability to bind p53) have diverged. The members of primate lineage (SV40, BKV, JCV, and SA12) all have a carboxyl-terminal domain in LT not found in the other lineages, but the other lineages without this domain all have an insertion in the amino-terminal half of the early region. The bovine polyomavirus, probably the most ancient among the mammalian polyomaviruses, does not appear to code for an ST and has a smaller coding sequence for LT. The important PP2A binding domain (as mentioned below) of ST is made only upon splicing out the second intron (and not the first from an unusual LT with two introns), which appears to show how evolution could have produced ST (although bovines do not appear to normally produce this protein). However, sequences coding for MT are still a mystery since they do not appear until late in the rodent lineage (303). Interestingly, the polyomaviruses that have LT proteins without the carboxy-terminal domain are those that encode an MT. The p53 binding domain overlaps the ATPase domain and blurs the distinction, leaving us unable to determine the conservation of the region for p53 alone. The inability of Py to bind p53 comes back again as the primary difference in primate versus rodent polyomaviruses and as a potential reason for (or consequence of) the evolution of MT. This would indicate that certain circuits must be reprogrammed by the virus and that the lineages of polyomaviruses simply contain divergent proteins which converge on the same ultimate effects on cell regulation.
Relevance of the p53–Large T-Antigen Interaction and Host Cell Growth Control
Implications of p53 within polyomavirus infections.
The different interactions between mouse and primate LT and p53 might account for the need for mouse MT and the elimination of the p53 binding ability of Py LT. The mammalian polyomaviruses are highly species specific, and it was originally established that the species-specific DNA polymerase α-primase complex was the only factor that determined the tropism of SV40 or Py (240, 241). However, later evidence showed that the murine p53 blocked the ability of SV40 to grow in primate-specific extracts while Py, lacking a p53 binding site, was able to replicate in vitro within mouse-specific extracts in the presence of murine p53 (360). It was concluded that lack of p53 binding of Py LT may be due to an evolutionary need to avoid this repression (360). One example of this need that may have influenced Py evolution is the ability of p53 to repress or transactivate the proliferating-cell nuclear antigen (PCNA) (a requirement for Py DNA replication) promoter depending on the species and the cell type (78, 161, 227, 299, 313, 385). Further experiments demonstrated that human p53 did not prevent SV40 replication in Cos cells (SV40-transformed fibroblast-like cells from CV-1 cells derived from adult African green monkey kidneys) whereas murine p53 did prevent this replication (37). Specifically, murine p53 interferes with the presynthesis stages, namely, the helicase activity of SV40 LT, but not the ATPase activity (360). Furthermore, SV40 LT cannot bind DNA polymerase α when associated with murine p53 (121, 315). The mouse p53 displaces the polymerase α from SV40 T antigen, preventing replication from occurring, which may suggest a role of p53 in initiating or maintaining replicative DNA synthesis (360). This clearly indicates a difference between the mouse and human/simian forms of p53 and perhaps even a role that was acquired specifically in the primate (and bovine, possibly others) lineage but is not found in the mouse lineage. Although the human and mouse p53 proteins are only 78% homologous (with ranges from 37 to 92% among the codons), there are few if any indications that the mouse p53 and its related cell cycle, apoptosis, and other functions are effectively different from the human p53 (31, 249, 318). Thus, it is not obvious why the LT would differ in its ability to bind p53.
Middle T antigen and p53.
Most DNA tumor viruses, including nonrodent polyomaviruses, papillomavirus, and adenovirus, have a mechanism for inactivating p53 (for a review, see reference 338). However, since Py LT does not directly interact with p53, the most obvious possibility, which has frequently been noted in the literature, is that MT function evolved to offset the lost interaction of mouse LT with p53. This would suggest that MT must act on the host p53 regulatory pathway someplace downstream of LT-p53 binding, such as p53 transactivation during cell cycle arrest and/or p53-induced apoptosis. However, there is no clear evidence that this is the case for Py, although there is recent support for the notion that the downstream functions of src may be to counter the negative effect of p53 on growth regulation (41, 89, 107, 232). The expression of MT (or LT) does not coincide with the activity of p53 or the levels of its expression during Py-induced tumorigenesis (within the limited number of tumors analyzed) (266). No conclusion was reached on whether anti-apoptotic functions of p53 were being manipulated by downstream signal transduction targets of MT, but it was clear that p53 transactivation abilities were not changed by the levels of MT (83, 266). Some of the tumors analyzed (sarcomas) did have mutated forms of p53 with affected transactivation abilities, but the number was similar to that of non-virus-associated tumors and could not be accounted for by Py-selected pressure. Furthermore, Py infection of wild-type or p53 knockout (−/−) mice (of those mice susceptible to Py-induced tumors) resulted only in a change in the time of tumorigenesis rather in the type or frequency of tumor found (83). A similar study done with cell cultures showed that the activities of p53 were not affected in the Py transformation of established rat embryo fibroblasts (REF52). Nevertheless, MT alone, as opposed to the entire early region required to transform these cells, could transform the cells in the absence of p53 (dominant negative p53 transfected into the REF52 cells) (232). Although these studies conclude that MT does not affect p53 directly, they do not rule out the possibility that MT is interfering with downstream targets of p53.
Cell culture studies using temperature-sensitive p53 in mouse embryo fibroblasts derived from a p53-null animal demonstrated that MT could not overcome p53-mediated growth arrest (70% of cells remained in G1G0) (88). In addition, these studies showed that MT did not induce apoptosis in these growth-arrested fibroblasts. Later studies with the same system demonstrated that MT associated with pp60c-src, became tyrosine phosphorylated, and was associated with phosphatidylinositol (PI) 3-kinase and Shc, without affecting the p53-mediated growth arrest (89). Furthermore, it was shown that MT did increase the levels of transcriptionally active AP-1 (PEA1) and induced the expression of c-myc in wild-type p53-mediated growth-arrested cells. These studies concluded that p53 does not interfere with MT-induced signal transduction and vice versa but may block the mitogenic signals induced by MT (similar to those of serum). The overall conclusion here seems to be that LT is needed to inactivate the tumor suppressor protein pRb while MT is required to induce the mitogenic signals, but no conclusion on how MT is still able to overcome potential p53 arrest in its ability to induce tumors was drawn. Unfortunately, these studies mainly discuss MT in terms of its function as a tumor-inducing protein only and do not address the natural function of this protein as it relates to acute or persistent infection or its replication in differentiating mouse tissues.
BRIEF HISTORY OF THE STUDY OF POLYOMAVIRUS TRANSFORMATION AND THE ROLE OF MIDDLE T ANTIGEN
“… since its initial discovery the role of middle T-antigen in transformation has been studied almost to the exclusion of any function it might play in the life cycle of the virus” (87)
The relationship between the natural and transforming activities of MT are not at all well known. The early view that MT is important to stimulate quiescent cells into S phase seemed to also explain how these genes would be able to transform cells. However, with our better understanding of the Py life cycle, in which quiescent-cell stimulation is not observed, the relationship to transformation has become less clear. A more recent view consistent with the link between Py replication and host cell differentiation would be that the early genes are needed during terminal differentiation to alter cell programming and allow terminal cells to synthesize viral DNA and make virus. The question then becomes how such an activity would relate to transformation. Although we cannot now answer this question, we can consider established transforming activities of MT from this context.
Requirements of Middle T Antigen for Transformation
The cellular transformation function of Py MT is well established and has been reviewed (159, 280, 342, 343). MT is sufficient to transform established cell lines but requires portions of LT or even ST as well to induce transformation in primary cultures of fibroblasts (72, 191, 280). In addition, increasing the expression of MT over levels normally associated with virus production is the only way to ultimately achieve the full characteristics associated with the transformed state (273, 275). There is a higher stringency in establishing tumorigenesis in vivo, with the large array of tumors associated with Py developing only with the expression of MT in combination with the N-terminal region of LT and the coding region for ST (11). In particular, the MT-associated tyrosine kinase activity (src) has been linked to the ability of Py to transform cells in culture or form tumors in animals (35, 277). The portions of the early region of Py required and the array of tumors that develop also depend on the type of rodent used and the strain of virus, factors that are missed in vitro (12, 77, 94, 109).
Tumor Biology
Tumor biology has been the main focus of Py literature throughout its more than 40-year history of study. This has led to a number of important discoveries, including the understanding of the roles of pRb, MT-associated signal transduction pathways involving PI 3-kinase, Shc, and phospholipase C-γ1 (PLC-γ1), and a number of other proto-oncogene products and tumor suppressor proteins associated with Py tumorigenesis (and non-Py human malignancies). Even more important is that Py allowed researchers to study a mouse model of tumorigenesis induced by known proteins in a controlled, repeatable manner.
Inbred strains, however, have been frequently selected for tumor-producing phenotypes. The 30-year debate over what allows Py to establish tumors in mice is rooted in the very fact that inbred mice have different degrees of tumor induction susceptibility. The earliest experiments maintained that it was the immune response itself that was responsible for the differing effects of Py in different mouse strains (7). Some of these experiments involved removal of the thymus, irradiation of the mice, or the use of knockout mice (double CD4/CD8) and showed that an unsusceptible mouse strain could now be made susceptible to Py-induced tumors (8, 29, 193–195). One of these susceptibility factors relied on the H-2 locus of the mouse. Some mice process portions of Py that are more immunogenic than others, with H-2k (found in AKR and C3H strains of mice) being highly prone to Py-induced tumors (110, 205). Benjamin's group has demonstrated that H-2k mice have a superantigen (sag) derived from Mtv-7 mouse mammary tumor provirus, which eliminates Vβ6-expressing thymocytes, particularly those that recognize amino acids (aa) 389 to 397 of MT (206, 207). Most adult inbred mice can develop Py-induced tumors following radiation, indicating that the block was due to immunological protection against the virus, namely, the particular H-2 locus (49, 110, 192). Some, however, are radiation resistant and appear to block dissemination of the virus throughout the host and thus prevent the establishment of tumors even in newborn inoculated mice (49). This form of resistance may be due to an ability of the mouse to thwart the antiapoptotic mechanisms of MT (49, 74, 225). Others demonstrated that the virus strain itself was largely responsible for the tumor profile and that large-plaque variants, often derived from tumors, were high-tumor strains due to differences in both coding (early or late proteins) and noncoding (enhancer and LT binding sites) sequences of the virus (112). It was later determined that the noncoding sequences are most responsible for the tumor profile, although changes in VP-1, the major capsid protein, significantly after the tumor profile (94, 108, 109, 111). The specific interaction between VP-1 and the sialic acid residues associated with Py cellular tropism has a profound effect on dissemination of the virus and the Py induction of tumors in mice (19, 20, 94, 108, 111). However, the very fact that most, if not all, experiments in which Py was used to study tumorigenesis were done with inbred strains of mice creates and perpetuates the misconceptions about the natural life cycle of Py that we believe should be separated from the tumor biology. As indicated below, immunosuppressed mice are not necessarily prone to Py-induced tumors. It instead seems more likely that other characteristics of inbred mice, such as genomic instability, along with failed immune clearance, are the main determinants of Py tumorigenicity.
IN VIVO VIRUS STUDIES OF MIDDLE T ANTIGEN
Middle T-Antigen Viral Mutants in Newborn and Adult Immunocompetent Mice
Intranasal inoculation of wild-type Py into newborn mice (BALB/c) results in acute replication of the virus in the nonciliated epithelial cells of the bronchioles, with maximum replication during 3 to 6 days postinoculation (p.i.) (131). Interestingly, there is a transition from proliferation to a combination of proliferation and differentiation (based on the decline of peak bromodeoxyuridine (BrdU) incorporation and PCNA expression) in the newborn bronchiolar epithelium at around 12 to 24 h prior to the peak of virus production (5 to 6 days p.i.) (9, 69, 339). Under the same conditions, MT mutants of Py (PTA-1387T, PTA-1178, PTA-250YS, and A3-MOP1033) replicate little or not at all in the bronchiolar epithelium compared to wild-type virus of the same background strain (K. A. Gottlieb and L. P. Villarreal, unpublished data). These mutants either eliminate the key tyrosine phosphorylation sites (PTA-1178 eliminates tyrosine 315, and PTA-250YS eliminates tyrosine 250 [the importance of these is described below]) or truncate MT (PTA-1387T and A3-MOP1033). Although A3-MOP1033 is of a different strain (A3 being a small-plaque variant of Py, while PTA is a large-plaque variant), it is comparable to PTA-1387T in being a truncation mutant (opal termination codon at nucleotides 1033 to 1035 in MT and a consequential proline-to-leucine change in LT) of MT, eliminating the ability of the protein to bind the plasma membrane (335). These results indicate that MT is particularly adapted to function in the nonciliated epithelium of the newborn mouse lung since no other tissue in the mouse (including adult mouse lung) displayed this sensitivity to MT mutants (see below). In addition, since the replication levels of the mutant viruses (measured by in situ hybridization) correlated well with levels of T-antigen expression (as measured by immunofluorescence using an antibody to the shared domain of all three T antigens), this supported the idea that MT plays an autocatalytic role in Py replication in that MT function was associated with levels of early gene expression. MT function is also probably required in maintaining persistence, as indicated by the finding that the MT mutant (PTA-1387T) is unable to persist in the kidneys of mice (113). However, in contrast to their behavior in newborn lungs, the point mutants (PTA-1178 and PTA-250YS) associated with elimination of key phosphorylation sites have minor effects on virus replication in lungs of adult mice, producing subtle, often indistinguishable phenotypes from those produced by wild-type virus. The main difference in the adult is that the overall numbers of cells replicating Py are reduced, but those that are infected show a similar quantity of virus to wild-type infected cells. The lungs of adult mice given an intranasal inoculation of the truncation mutants (PTA-1387T and A3-MOP1033), on the other hand, gave similar phenotypes to the newborn lungs, with only a very few cells showing any infection at all. Since the signal transduction pathways described in the next section are an amalgamation of Py MT effects on many cell types in vitro, some of which are not even part of the life cycle of the virus, it is not clear how these identified interactions might relate to Py replication in vivo or the differences we see in newborn and adult lung infection. Our results indicate that depending on the specific cell type, whether nonciliated epithelium of the newborn or adult lung or tubular epithelium of the kidney, a different but at times redundant subset of signal transduction is required for efficient replication of the virus. On the other hand, the subtle differences in replication of the Py with MT point mutants used in our experiments could indicate either that the associated pathways (Shc/PI 3-kinase) are not required in the bronchiolar epithelium or that one pathway can substitute for the other if necessary.
Middle T-Antigen Mutants in SCID Mice
A few studies have examined Py infection in SCID or SCID-beige mice, but our study was the first to study Py mutants (MT and LT) in this context (28, 326, 327). The initial studies of others used intraperitoneal inoculation of the virus into adult SCID mice, with or without NK cells, and evaluated the associated myeloproliferative disease that resulted. Mice succumbed to the virus within 16 days after infection after a period of inactivity and severe weight loss. Similar studies by Dalianis and coworkers, also using intraperitoneal inoculation, established the kinetics of viral replication and dissemination in the organs of the mice over an 8-week period. Unlike the previous study, mice did not succumb to a virus-associated disease until after the 8-week period (28). In neither experiment were tumors observed, unlike reports of Py inoculation into adult athymic nude mice (mostly mammary adenocarcinomas in females and osteosarcomas in males) or susceptible strains of newborn mice associated with the depletion of specific thymocytes that recognize MT (94, 381). Although the replication of Py was restricted to a subset of organs in nude mice, namely, skin, bone, and mammary and salivary glands, the tumors were rapid and led to death before other, slow-growing tumors could appear (326, 381). The difference in SCID and nude mouse susceptibility is curious and could possibly be associated with the strain differences, although this remains to be determined.
The myeloproliferative disease observed in histopathological studies of the bone marrow and spleen was characterized as left-shift maturation of myeloid precursors resulting in myeloid hyperplasia and megakaryocyte and erythroid degeneration. Similar to a study in which adult mice were inoculated with a recombinant Moloney leukemia virus expressing MT, the early target for T-antigen expression in SCID mice was found to be megakaryocytes (120). In the SCID mouse study, though, the megakaryocytes were depleted at later time points, perhaps as a result of lytic infection of these cells.
Our studies used intranasally inoculated SCID-bg mice over 6 weeks old with wild-type virus, strain A3. These mice began to show limited replication of the virus in the lungs by 3 weeks after infection, ranging from 5 to 15% of the epithelial cells in any given bronchiole (Gottlieb and Villarreal, unpublished). This replication increased substantially over the next few weeks, eventually encompassing a majority (over 70%) of the epithelium of the bronchioles. The notable delay in Py lung replication relative to that seen in newborns is probably due to the much lower rate of cellular differentiation occurring in the adult lung and suggests that virus replication must await the accumulation of cellular differentiation to generate permissive cells. The mice eventually succumbed to an acute myeloproliferative disease induced by Py about 35 days after infection (reviewed in reference 326). Contrary to the expectations of those who study Py, the MT mutant A3-MOP1033 (MOP), which should lack all MT-associated functions, replicated well in SCID-bg mice. Although the overall numbers of cells infected were decreased, the replication levels on a per-cell basis appeared identical to those in wild-type infection. Surprisingly, dissemination of MOP throughout the mice, including establishing infection and persistence in the kidneys, was indistinguishable from that of wild-type virus, although the numbers of kidney cells replicating Py were also lower. Although MT may still have some immune-related functions which would not be observed in SCID mice, MT function in Py replication in permissive adult tissue is subtle at best. These subtle differences include a slight decrease in virus-infected bronchioles throughout the lungs and, of particular interest, an alteration in the transition time between T-antigen expression and viral DNA replication compared to wild-type virus. The MOP-infected lungs showed a higher percentage of cells only expressing early proteins among those expressing viral proteins (early, late, or both) than did wild-type-infected lungs, indicating a delay in the onset of Py DNA replication. This latter observation indicates that MT acts as a booster to establish a cell capable of virus replication but is not an absolute requirement. However, we have not yet eliminated the possibility that the expression of the truncated MT may retain some functional capabilities that have yet to be described.
The finding of others that a similar MT mutant (PTA-1387T) eventually loses its kidney persistence also suggests a “booster” role for MT (113). Given that MT mutants which do not replicate efficiently also do not express T antigen efficiently, a likely scenario is that an array of cellular transcription factors are induced by MT to establish an environment suitable for both Py early-gene transcription and Py replication but that this permissive state is also dependent on the natural differentiation of infected cells. Over time, permissive adult SCID lung cells accumulate as they enter this window of differentiation, which allows MT to affect Py early expression and replication. Such a delayed and cumulative effect on virus replication would not be observed in a mouse with a functional and normally responsive immune system. Alternatively, since identical levels of MOP replication (with respect to wild type) in the lungs are found only in a subset of cells, MT and its associated signaling may be required to maintain virus replication as the progenitor cells proliferate and differentiate to replace cells lost due to terminal differentiation and virus lytic infection and exfoliation (132). Although we cannot eliminate the possibility that even a truncated MT has remaining activity, these results clearly illustrate that all of the signal transduction pathways associated with transformation (as described below) play a relatively minor role in Py replication in quiescent permissive adult tissue in vivo.
MIDDLE T-ANTIGEN-ACTIVATED SIGNAL TRANSDUCTION PATHWAYS
Contextual Considerations
Although the role of MT in the natural infection is seldom examined, various and numerous cell culture and/or transformation studies have identified specific domains of Py MT which affect cellular signal transduction and may be important for its function in the virus life cycle. However, it is important to remember that these domain studies were often done using high expression levels of only MT (or other viral proteins) instead of a normal virus infection. Caution is warranted when using such approaches since this situation could easily cloud the understanding of the actions of viral proteins. The altered kinetics and high-level expression can result in altered thermodynamic relationships, distorting dissociation and association constants among viral and cellular proteins that in some cases are not biologically relevant. It must not be forgotten that MT evolution is fundamentally linked to that of the other viral proteins, namely, LT and ST, so that MT function apart from these proteins may be aberrant. As will be expanded upon below, transgenic-animal studies readily show that MT acts quite differently when expressed on its own, away from the other early proteins or even from the Py enhancer. Furthermore, viral genes function and evolve in very specific cellular environments, with the biological goal being to establish a persistent, pathologically and immunologically benign infection, not transformation. These specific cellular environments are not assessed in the study of MT signal transduction. The cell types used for these studies are typically established cell lines, often transformed and rarely from the natural host (mouse) of Py. Therefore, the details of MT-activated signal transduction pathways that have been reported and the functions of this viral modulation of cellular proteins may well be misleading. We must await a better understanding in both stoichiometric and thermodynamic terms of the relevance of these highly intricate circuits before we can understand the role of MT in the virus life cycle. This would include the time allotted for early-protein expression, the distinct cell types associated with in vivo acute and persistent replication, and the precise time during which MT is needed to reprogram or regulate cellular protein functions at a particular stage of host cellular differentiation. Finally, it is also important to remember that the plethora of reports focusing on Py-induced transformation and tumorigenesis not only has completely skewed our conception of the true biological character of Py but also has propagated the misconception that the sole purpose of MT is tumorigenic and, in extreme terms, has defined its role as simply a biological anomaly. We therefore outline the detailed highlights of MT and its important regulatory domains and attempt, although often unsuccessfully, to discuss the natural relevance of these findings and their association with the viral infection.
General Structure
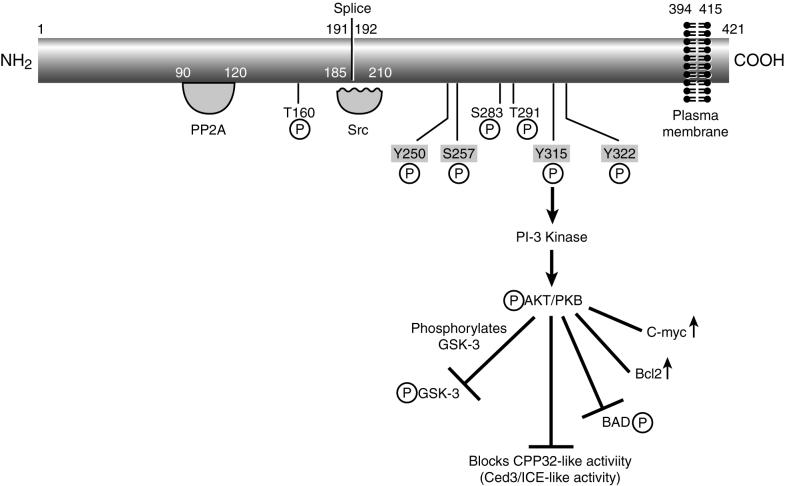
MT is 421 aa long (nucleotides 175 to 748 [aa 1 to 191] spliced to 811 to 1499 [aa 192 to 421]) and is encoded by a splice variant of the early region of Py, which also encodes LT and ST antigens (a general diagram of MT is shown in Fig. 1). MT has a common amino-terminal region (nucleotides 175 to 411) with LT and ST and a further common region with ST (nucleotides 412 to 748). The second exon of MT (nucleotides 811 to 1499, aa 192 to 421) is shared with the LT nucleotide sequence but has a frameshift in the amino acid sequence, astonishingly creating the tyrosines which are phosphorylated through the MT-pp60c-src and result in activation of multiple signal transduction pathways. It has a molecular mass of 55 kDa and contains a tail of 22 hydrophobic amino acids (aa 394 to 415) at its carboxy terminus, bound on either side by clusters rich in basic residues (47). There are multiple phosphorylation sites throughout MT, including the well-studied tyrosines at aa 250, 315, and 322, serines at aa 257 and 283, and threonines at aa 160 and 291 (155, 259). In addition, MT has a protein phosphatase 2A (PP2A) binding domain (aa 90 to 120) and a region associated with pp60c-src (aa 185 to 210). While pp60c-src (and other members of the src family, as mentioned below) serve as the tyrosine kinases, protein kinase C and another, unnamed kinase provide the serine phosphorylation (223). However, in Py-infected cells, only a small portion of MT is phosphorylated, and among this fraction most of the phosphorylation is of serine while a significantly lesser portion is tyrosine or threonine (304).
FIG. 1.
Schematic of MT. MT is 421 aa long and is encoded by nucleotides 175 to 748 (aa 1 to 191) spliced to 811 to 1499 (aa 192 to 421) based upon the sequence numbering of the A2 wild-type strain. The membrane-spanning domain consists of hydrophobic aa 394 to 415. On interaction with the plasma membrane, MT associates with (although does not directly bind to) src, a tyrosine kinase, resulting in the phosphorylation of tyrosines at aa 250, 315, and 322 and in the binding of Shc, PI 3-kinase, and PLCγ-1. In addition, MT contains serine (aa 257 and 283) and threonine (aa 160 and 291) phosphorylation sites. The phosphorylation of serine 257 results in the interaction with the 14-3-3 proteins. Finally, MT associates with both the catalytic subunit C and the regulatory domain A of PP2A at aa 90 to 120.
Cellular Localization
MT was first found associated with the plasma membrane by using antibodies against tumor antigens from Py-induced tumors (158). The insertion of MT into the plasma membrane and the sequences N terminal (RHLRRLGR) to the membrane binding sequence are functionally important for the MT association with pp60c-src (src) and the association with the cytoskeleton needed for the transformation morphology (47, 75, 213, 337). Only a fraction of MT is associated with the plasma membrane; the majority is found in perinuclear compartments that do not colocalize with marker proteins for either the endoplasmic reticulum or other subcellular compartments of the secretory pathway (87, 158, 297, 390). Nocodazole, a drug affecting cytoskeletal structures and vesicular transport, prevents perinuclear localization of MT (228). The localization of MT at these perinuclear locations has been associated with the rearrangement of the actin cytoskeleton seen in transformed cells and the dismantling of tubular endosomes (390). Subfractionation studies show that MT in the perinuclear compartments are complexed with src and Shc and that much of the src from the plasma membrane has been relocalized by MT to these compartments (390). One theory about the discrepancies in localization of MT postulates that MT associates with the membrane skeleton and that this accounts for both the plasma membrane and perinuclear MT fractions (10). There has yet to be a study to determine whether MT complexes not associated with the plasma membrane are functional in the lytic life cycle or in transformation (87). Therefore, since such a small portion of MT is associated with the plasma membrane, it is logical to assume that functions of perinuclear MT currently unclear or speculative must be important for the virus life cycle, but these may be more closely associated with persistence than acute replication.
Activation of the src Family
The importance of various domains within MT was largely determined by the ability of MT mutants to alter or abolish MT-induced transformation (46, 47, 93, 200, 214; for a review, see reference 215). The information below will show, however, that the ability to transform a specific cell line requires not only a certain threshold level of src activity but also an often undetermined subset of MT-activated signal transduction (214). Analysis of a number of MT mutants indicated that the complex with src was necessary but insufficient for cellular transformation (36, 57, 63, 137, 213). src (and to a lesser extent other tyrosine kinases, pp62c-yes [yes] and p59fyn [fyn]) coimmunoprecipitates with MT (56, 58, 67, 68, 138, 152, 184, 188), is associated with and activated by the amino-terminal end (aa 185 to 210) of MT, and phosphorylates three tyrosines in MT, at aa 250, 315, and 322 (143, 155, 296). However, src does not directly bind MT (38), nor does it increase the overall levels of tyrosine phosphorylation in Py-infected cells (97). Although the src family of protein tyrosine kinases includes src, yes, fyn, fgr, lck, hck, lyn, blk, and tkl, as well as other alternatively spliced forms, MT has evolved to only interact with src, fyn, and yes (59, 100, 174, 219, 243, 268, 306, 324, 329, 357, 386, 391). The selection of only this subset may indicate a specialized function of these kinases or a cell type specificity in the evolution of MT. src consists of various domains, including an N-terminal membrane localization SH4 region, a stretch of amino acids unique to each src, an SH3 domain, an SH2 domain, a kinase domain, and a conserved C-terminal tail (42). The exact function (targets) of the tyrosine kinase activity of src is unknown, although src, fyn, and yes have been found in all cell types at approximately the same levels with terminally differentiated cells apparently having higher levels (63, 341). One of the virus associated functions of src activation may be to phosphorylate the threonines found on VP1, one of the capsid proteins of Py, an apparent requirement for efficient viral encapsidation in vivo, although separable from the portions of MT required for transformation (122, 199). In addition, besides using src to phosphorylate important signal transduction activating tyrosines on MT, Py may use the cell cycle progression and/or chemotaxis functions of src, although this relationship has yet to be determined. Unfortunately, as with most of these MT domain studies, the focus of these studies has been on only the aberrant and altered regulation of src by MT in transformation.
As mentioned above, the complex between MT and pp60c-src increases the tyrosine kinase activity of src, but complexes with fyn do not elevate kinase activity, which may be due to the low abundance of MT-fyn complexes formed (56, 188). MT complexes with a population of src that is not phosphorylated at tyrosine 527 (but is phosphorylated at tyrosine 416) (50) and locks it into a configuration in which it cannot be inactivated by the phosphorylation of tyrosine 527 (63). However, already inactivated forms of src (those with intramolecular binding of the SH2 domain to phosphorylated tyrosine 527) cannot be readily bound by MT (98). Specifically, the region bordered by aa 518 and 525 of src are essential for the MT complex and therefore are likely to mask tyrosine 527 (58). This masking of tyrosine 527 removes the normally cell cycle-specific regulation of src (active during mitosis, repressed during interphase), thereby keeping high tyrosine kinase activity throughout the cell cycle (166). Although src contains both a myristylation signal (needed to associate with membranes) and a membrane localization signal in its first 14 aa, its association with MT seems to disable these functions since truncated forms of MT which localize to the cytoplasm instead of the plasma membrane still colocalize with src (66, 383). At any one time, though, only 10 to 15% of MT is associated with the tyrosine kinases of proto-oncogenes, src, yes, or fyn (34, 55), since MT needs the less common open/activated conformation of src to bind successfully (98). Py infections in mice with knockout of any one of the above-mentioned tyrosine kinases are still able to induce tumors, although src is better able to induce the Py-associated tumor profile than is fyn (142, 340). Similar experiments determined that even when one or two of these tyrosine kinases are knocked out, the remaining ones do not compensate for the loss and increase their association with MT or elevate their kinase activity (177). This indicates that the specific stoichiometry of the individual MT and src associations may be important for different cell types, i.e., Clara cells of the lung bronchioles during acute replication, tubular epithelial cells of the kidney during persistence, or other unidentified cells that transport or harbor the virus. Although it seems clear that the MT-src complex is important for the virus life cycle, its in vivo relevance needs to be elucidated further. The shift from the predominant MT-fyn complex in hamsters to the MT-src complex in mice would indicate that pertinent changes were made during evolution, but their nature cannot be fully understood until the evolutionary tree of the MT of polyomaviruses in other species along the rodent lineage is evaluated. However, as mentioned for MT localization, the bulk of MT is not associated with the plasma membrane or with src, which means that although the MT-src complex may be important for transformation or even some facet of the normal virus life cycle, the total activities of MT cannot be simply explained by this minor complex found in both lytically infected and transformed cells (85, 297, 304).
Middle T-antigen tyrosine phosphorylation.
Early experiments established that the phosphorylation of tyrosine 315, long considered the predominant tyrosine-phosphorylated site of MT, is essential for transformation, since without it there is a drastic reduction (20% of wild type) in transforming ability in rat fibroblasts (F-111 cells) (46). However, others demonstrated that tyrosine 315 was not essential for transformation of another rat cell line, Rat 1 (248). Although the conditions were somewhat different, this discrepancy indicated that studying Py in culture can be misleading. However, these experiments produced the first important mutant (Py-1178-T), which replaced tyrosine 315 with a phenylalanine to preserve the character of the wild-type MT (A→T at nucleotide 1178). The residual transforming ability of this mutant was theorized to be in the phosphorylation of the tyrosine at position 322 or other phosphorylation sites but was later shown to remain even after mutation of tyrosine 322 (298) and tyrosine 297 (214). Subsequently, it was shown that the phosphorylation of tyrosine 250 was needed for transformation and that its mutation not only reduced the level of phosphorylation on MT but also weakened the interaction of MT with src (214). These results were supported by the complete lack of transformation by an MT mutant (Py-1387-T, C→T at position 1387) which lacks the ability to bind the plasma membrane by eliminating the last 37 aa of the carboxy terminus and therefore is not phosphorylated by the associated src kinase activity. However, 1387T is able to replicate to almost wild-type levels in culture, although this has been attributed to the ability of the truncated MT to substitute for the ST function (122, 376).
There is some evidence that the N-terminal portion of MT, which has the same sequence as ST (191 of the 195 aa), may act by performing the same Py replication-enhancing function as has been demonstrated for ST during lytic function (336). In contrast, a Py virus that does encode any portion of MT (808A) is both transformation and replication deficient, which definitely raises the possibility that MT has functions not associated with its binding of the plasma membrane (122, 200). Another mutant, NG59, in which the aspartic acid residue at position 179 is replaced by isoleucine-asparagine, associates with the membrane but lacks transforming ability and PI 3-kinase activity, a result that has yet to be fully explained (24, 376). One plausible explanation for NG59 and 808A is their inability to phosphorylate the threonines found on VP1 (aa 63 and 156), which may affect not only packaging but also cellular receptor recognition (19, 20, 94, 199). Again, 1387T did not have this difficulty, thereby supporting strongly the conclusion that MT has functions not associated with its binding of the plasma membrane.
Phosphatidylinositol 3′-Kinase
The phosphorylated tyrosine 315 was then found to bind and result in the phosphorylation and activation of PI 3-kinase (65, 376). MT-src and MT-fyn complexes have an indistinguishable ability to phosphorylate tyrosine 315 and bind PI 3-kinase (55). PI 3-kinase is made of two subunits, the 85-kDa protein, which binds to the phosphorylated tyrosine 315 of MT, and the 110-kDa protein, with both PI 3-kinase and protein serine/threonine kinase activities, which binds the 85-kDa protein and phosphorylates the inositol ring at the D-3 position (84, 169, 250, 375, 376). Specifically, MT associates with one of two SH2 (src homology 2) domains of the p85 subunit of PI 3-kinase (317). The activation of PI 3-kinase has been associated with prevention of apoptosis, promotion of cell division, production of novel lipids which regulate Akt and atypical forms of protein kinase C (PKC), induction of protein synthesis, actin rearrangement, regulation of Ras-dependent signal transduction pathways, vesicle trafficking, and many others (for reviews, see references 16, 196, and 270). Since all of these functions must be well regulated, the cell maintains less than 0.25% of its inositol-containing lipids phosphorylated at the 3 position (270). The association and activation of PI 3-kinase by MT is necessary but not sufficient for transformation (169, 170, 376), another contradiction from the previously reported result that the Py-1178-T was not transformation deficient (215). Py-1178-T is unable to form mammary tumors except in older female mice that had experienced pregnancies, which touches on a potential in vivo role for the MT–PI 3-kinase association (332). The association of PI 3-kinase with the MT-pp60c-src complex results in the phosphorylation of PI at the D-3 position of the inositol ring to produce PI-3-phosphate [PI(3)P], a pathway independent of inositol-1,4,5-triphosphate production (308). Additionally, PI 3-kinase phosphorylates PI(4)P and PI(4,5)P2 to produce PI(3,4)P2, and PIP3, respectively, both novel polyphosphoinositides which are not hydrolyzed by PLC (170, 204, 308, 309). There is also recent evidence that a further phosphatidylinositol, PI(3,5)P2, is formed by the phosphorylation of PI(3)P by PI 5-kinase (Fab1p) and may play roles in vesicle trafficking and stress regulation due to osmotic pressure (61, 125, 151). The production of these phosphatidylinositides has been directly linked to MT-induced transformation (65, 130, 170, 347). PI(3,4)P2 and PIP3 are specifically associated with cell growth since they are found only in subconfluent cultures or MT-transformed cells, not cells at high density or those transfected with a transformation-defective MT (308). Besides its association with the MT-src complexes (src, fyn, and yes), the production of PI(3)P and related phosphatidylinositides is also linked to other protein-tyrosine kinase-activated systems, including platelet-derived growth factor (PDGF) β receptor, epidermal growth factor (EGF) receptor (ErbB2R), colony-stimulating factor 1 receptor, and insulin receptors, and to the oncogenic forms of pp68gag-ros (ros), pp130gag-fps (fps), and pp160gag-abl (abl) (15, 32, 117, 118, 169, 208, 212, 263, 291, 351, 352). Although most of these tyrosine kinase systems are associated with cellular growth, they can also induce the production of PI(3,4)P2 and PI(3,4,5)P3 while stimulating cells to differentiate or even stimulating already terminally differentiated cells, a cellular activation not associated with growth (reviewed in reference 317).
Phosphoinositides.
The plethora of potential functions, including regulation of cell proliferation and survival, glucose metabolism, cytoskeletal reorganization, and vesicle trafficking, of the phosphoinositides PI(3)P, PI(3,4)P2, and PI(3,4,5)P3 has been reviewed extensively, but there is no consensus about how specificity of the signals is established (48, 196, 270, 317). PI(3)P has recently been shown to interact with the FYVE-containing domains, cysteine-rich zinc finger-like motifs named for the proteins Fab1p, YOTB, Vac1p, and EEA1, on numerous proteins which appear to be important for vesicle trafficking including targeting and secretion (reviewed in reference 16). There are few, if any, reports discussing the importance of vesicle trafficking in Py-infected systems, but our own observations in lung tissue show an exfoliation of virus-infected cells from the bronchiolar epithelium without apoptosis or necrosis, indicating that these mechanisms may be utilized. Furthermore, vesicle trafficking may be important during transport of the virus or movement from cell to cell without interference from the immune response. PI(3,4)P2 and to a lesser extent PIP3 bind and activate Akt, a serine/threonine protein kinase, which may account for the ability of the MT–PI 3-kinase complex to inhibit apoptosis, regulate glycolysis, induce protein synthesis through activation of p70S6-K, and numerous other functions outlined below (105, 106).
PIP3 has been associated with vesicle budding, cell migration, and induction of cellular proliferation (reviewed in references 196 and 270). PIP3 alters the organization of the actin cytoskeleton by activating numerous guanine nucleotide exchange factors, including Rac, Rho, and Cdc42 (145). The induction of Rac, as outlined below, by both the MT–PI 3-kinase and MT-Shc complexes was found to be important for MT-induced cellular transformation, which may indicate a possible role for this protein in the virus life cycle (60, 349). As discussed below, hemangiomas form mostly by cellular recruitment in MT transgenic-mouse systems, an indication that the membrane ruffling (actin reorganization) and resulting cell migration and chemotaxis may be associated with this observation (187, 374). Much of the protein interactions of these phosphoinositides is done through binding of pleckstrin homology domains, which are common among the guanine nucleotide exchange factors for small G proteins. The evidence that PIP3 is involved in cellular proliferation comes indirectly in the finding that PTEN (phosphatase and tensin homolog deleted on chromosome 10), a tumor suppressor protein that dephosphorylates PIP3, is often found mutated or deleted in numerous human cancers (209). The vesicular trafficking association is based on the recruitment of Grp1 (general receptor for phosphoinositides) to the membrane by PIP3, which results in the activation of Arf1 (ADP-ribosylation factor 1), a GTPase important for regulating coating and budding from intracellular vesicles (179). All of these responses are aspects that the normal virus life cycle may utilize to some degree and should not be thought of as simply cellular transformation pathways. The context of these actions, though, must also be related to the cross talk among the other MT-induced signal transduction pathways.
Phosphatidylinositol 3-kinase and Akt.
The discovery of the activation of Akt (PKB or RAC-PK) through its phosphorylation by the 110-kDa subunit of PI 3-kinase has resolved some of the apoptotic features associated with MT, as illustrated in Fig. 2 (225). The normal functions of Akt are still unclear, although there are connections with various metabolic events including lipogenesis, glycogen synthesis, and stimulation of glucose transporter GLUT4 translocation, induction of protein synthesis through S6 kinase activation, protection from apoptosis through bcl2 induction and bad phosphorylation, and, finally, cell cycle progression through c-myc induction (for reviews, see references 168 and 325). Early evidence that PI 3-kinase was responsible for the antiapoptotic signal came from an experiment which showed that rat fibroblasts transformed by Py underwent apoptosis when PI 3-kinase was inactivated by wortmannin or 1d-3-deoxy-3-fluoro-myo-inositol, blockers of both the lipid kinase and protein kinase activity of the 110-kDa subunit of PI 3-kinase (74). In addition, mutant 315YF was unable to protect the rat fibroblasts from undergoing apoptosis (74). Akt, a downstream component of the PI 3-kinase pathway, prevents apoptosis by inhibiting Ced3/ICE-like activity which is directly downstream of both the p53 and fas pathways of apoptosis (175). Currently, this is one of the few connections between MT signaling and p53 activities. Other substrates for Akt phosphorylation include bad, which loses its ability to promote apoptosis; forkhead transcription factor, a transcription factor which on phosphorylation is sequestered in the cytoplasm and is unable to activate proapoptotic proteins; glycogen synthase kinase 3, which on phosphorylation results in the maintenance of a dephosphorylated and activated glycogen synthase and cyclin D1; heart 6-phosphofructo-2-kinase, which upon phosphorylation is activated and stimulates glycolysis; endothelial NO synthase, which upon phosphorylation results in activation and production of a high level of nitric oxide; and other proteins mainly associated with the regulation of glucose metabolism, protein synthesis, or apoptosis (for a review, see reference 168). Akt activation in MT-induced transformation was found to be dependent on the MT–PI 3-kinase complex, although it is still unclear whether PI 3-kinase is sufficient to stimulate Akt since there have been reports of PI 3-kinase-independent activation of Akt kinase activity (104, 182, 225, 294, 325). The products of PI 3-kinase, particularly PIP3 and PI(3,4)P2, bind to the pleckstrin homology domain of Akt, resulting in a conformation change and localization to the plasma membrane (23, 105, 162, 180, 321). PDK1, a 3-PI-dependent kinase activated by PI 3-kinase, then phosphorylates threonine 308 of Akt, while a second, less well-defined kinase, tentatively called PDK2, phosphorylates at serine 473; phosphorylation of both threonine 308 and serine 473 results in the full activation of Akt (5, 6, 320, 321). PDK1, though, has the ability to not only protect against apoptosis via its activation of Akt but also induce apoptosis via its dephosphorylation by the MT-PP2A complex and the subsequent activation of the intermediary c-Jun N-terminal kinase (JNK), although JNK can mediate both pro- and anti-apoptotic signals (90, 157, 237, 287, 350). The specific signal seems to depend on the four different phosphorylation sites on PDK1. PDK1 is responsible for the phosphorylation and activation of Akt, pp70S6K (see below), and atypical isoforms of PKC, including PKCλ and PKCζ (4, 5, 265, 320).
FIG. 2.
MT and apoptosis. A schematic diagram of the potential antiapoptotic effects of the PI 3-kinase activation of Akt/PKB is shown. See the text for details. Akt can prevent apoptosis by inhibiting Ced3/ICE-like activity and phosphorylating and deactivating bad and glycogen synthase kinase-3 (GSK-3). In addition, Akt induces the expression of bcl2 and c-myc, which also helps protect against apoptosis. This pathway resembles those induced by many cellular growth factors, including EGF and PDGF.
Since Py is unable to prevent apoptosis through a direct interaction with p53, a property of other DNA tumor viruses, the MT–PI 3-kinase–Akt pathway would seem to establish the mouse-specific Py evolved method for delaying host cell death (17, 338, 389). The biological relevance of Akt is further discussed below (see “Middle T antigen and apoptosis”). However, many of the nonapoptotic functions of Akt, including upregulation of glycolysis, glucose uptake, and glycogen synthesis, promotion of protein synthesis, and potential induction of the cell cycle by elevating levels of cyclin D1, would appear to be beneficial for virus replication. Unfortunately, the true usefulness of such actions is only conjecture and needs to be supported by assays which can delineate the importance of these functions for Py replication and persistence. It is important to remember again that only a fraction of MT is phosphorylated and found at the plasma membrane even during a lytic infection. Therefore, these multiple functions are probably rather subtle effects during a precise period of virus replication or even throughout its entire life cycle, but they should not be thought of as simply a mechanism for inducing transformation.
40S ribosomal protein S6 kinase.
The ribosomal protein S6 kinase is highly phosphorylated in growing cells and dephosphorylated in growth-arrested cells in culture. Presumably, the 40S ribosomal protein S6 (S6) is also dephosphorylated in terminally differentiated cells in vivo, although this is not well studied. S6 phosphorylation is controlled by pp70S6K (S6K). Genetic studies have shown that S6K controls cell size, growth, and proliferation without affecting any particular phase of the cell cycle (95, 230). S6 is induced by a number of mitogens and oncogene products and results in the translation of a family of mRNA transcripts containing a polypyrimidine tract at the 5′ end (5′TOP family) (73). The 5′TOP family is composed of 100 to 200 genes that encode proteins essential for translation. MT elevates the levels of S6 phosphorylation through two pathways, one of which is activated by src and S6 kinase while the other requires neither kinase (73). The src-dependent activation of S6 kinase requires the phosphorylation of tyrosine 315 (331), while the other pathway may use the binding and inactivation of PP2A by MT to stabilize the phosphates on S6. Activated PI 3-kinase results in the activation of both the cytoplasmic (70-kDa) and nuclear (85-kDa) forms of S6 kinase through the intermediary kinase PDK1 and direct phosphorylation by PI 3-kinase (73). PKCλ and PKCζ, atypical isoforms of PKC induced by the PI 3-kinase/PDK1 pathway, may be responsible for the phosphorylation of S6K in its carboxy terminus necessary for its activation, while mTOR (mammalian Target of Rapamycin), a kinase activated through the PI 3-kinase/Akt pathway, may regulate phosphatase activity (similar to the MT-PP2A complex), act as a kinase upon S6K, or act as a sensor for amino acid availability (3, 95, 305). The transcription and replication of Py would appear to benefit from the activation of S6K by the MT–PI 3-kinase complex, which may result in the upregulation of protein synthesis. However, it remains clear that neither cellular proliferation nor transformation is the ultimate goal but, rather, that the purpose of the S6 activation is only part of the viral program to establish a cell designed specifically to transcribe viral proteins and replicate the viral genome under conditions in which cellular protein synthesis has either been severely curtailed or reprogrammed to express only necessary factors.
Shc/Ras
A mutation (proline 248 to leucine) which was transformation defective but still had the ability to associate with pp60c-src and PI 3-kinase led to the discovery of the association of the tyrosine 250 phosphorylation site of MT with the Shc protein (44, 86). A motif in MT, Asn-Pro-Thr-Tyr (NPTY), was found to be important for the ability of Py to transform cells. Shc (src homology 2 domain-containing protein) is made of three overlapping polypeptides of approximately 46, 52, and 66 kDa. The 52-kDa form contains a phosphotyrosine binding domain (PTB), a central collagen homology domain (CH1), and a carboxyl-terminal SH2 domain (33). Shc has three tyrosine phosphorylation sites, Tyr239, Tyr240, and Tyr317 (considered the principal site) (257). On phosphorylation by the MT-pp60c-src complex, the PTB domain of Shc (specifically, Tyr239/240 is important) associates with the SH2 domain of Grb2 (growth factor receptor-bound protein 2), which leads to the Shc-Grb2-Sos complex, which activates Ras (33, 44, 86). Sos (son of sevenless) is a GDP-GTP exchange factor for Ras. Less than 1% of the 46- and 52-kDa forms are associated with MT, while there is no evidence of association with the least abundant 66-kDa form of Shc (44). Growth factors (EGF, PDGF, etc.) can stimulate the Ras pathway through Shc or directly through Grb2, but it has been shown that the association of MT with Shc and the resulting phosphorylation are needed for full activation of Grb2 and the Ras pathway, although a mimicked binding site for Grb2, similar to that found on Shc, placed into MT can replace the need for Shc. Further discoveries connected the binding of MT to Shc with the transcriptional activation of c-jun (including AP-1 jun-jun homodimers and jun-fos heterodimers) by stimulating the phosphorylation of sites in the transactivation domain of c-jun (serines 63 and 73) through Ras/Raf-1 activation (319). On activation of Ras, a cascade effect results in activation of Raf and MEK which results in nuclear translocation of members of the mitogen-activated protein (MAP) kinase family (ERK1/ERK2/JNK) and subsequently in transcriptional activation of fos, jun, or myc (319, 348). In addition, the activation of PKC by MT-expressing cells may lead to dephosphorylation of sites in the DNA binding domain of c-jun which then leads to an increase in the transcriptional activation of c-jun (319). Activated extracellular signal-regulated kinases (ERKs) also lead to the activation and phosphorylation of transcription factor Elk-1 (ternary complex factor), a member of the Ets family of transcription factors, which then forms a complex with a homodimer of the serum response factor (SRF), activates the transcription of c-fos through its serum response element (SRE), and ultimately leads to increased levels of AP-1 (126). Shc and PI 3-kinase combined Ras- and Raf-dependent signaling results in the binding of Elk-1 to the SRE of the c-fos promoter, while a Raf-independent signal through PI 3-kinase alone results in transduction via Rac1 of a signal to the SRF to bind the SRE of the c-fos promoter (349). c-fos was found to be necessary for MT-induced transformation of rat F111 fibroblasts and can be inhibited by retinoic acid (333). The induction of the Ras signaling pathway and the resulting activation of transcription factors has been associated with cell growth and differentiation, but most, if not all, of the above studies have focused on only the transformation of cells or the tumor profile in mice. Clearly, though, these pathways can result in the regulated expression of AP-1 and members of the Ets-domain (E twenty six, based on homology to v-ets of E26 avian erythroblastosis virus) family of transcription factors. Both of these transcription factors are required for activation of the Py enhancer, as discussed below.
There is some evidence that these interactions may address the LT-p53 interaction. Recent evidence shows a direct connection between Raf activation and cell cycle regulation and mitogenic control, another way in which MT may circumvent the inability of LT to bind p53. Raf1 binds to and regulates the function of cdc25A, a dual-specificity phosphatase involved in cell cycle regulation, and can physically interact with and inhibit the antiapoptotic functions of bcl-2 (176). Furthermore, on activation, Raf1 binds to the functional (hypophosphorylated) form of Rb and, through its intrinsic kinase activity or the action of cdc25A inducing the expression of cyclin D1, cdk4, and cdk6, may lead to the phosphorylation and inhibition of Rb, resulting in activation of E2F complexes and cell proliferation (362). These findings were all established through cell culture, but they illustrate one of the key steps in understanding the complex interaction between the early proteins of Py and host cell machinery. It remains to be determined if such interactions are applicable in vivo.
Cross Talk among Phosphatidylinositol 3-Kinase and Shc Pathways
AP-1 expression.
In some instances, PI 3-kinase and Shc have appeared to be on the same MT molecule, but it has been difficult to tell whether this multiassociation is important for the full activity of MT (44). Studies that used single mutants MT-Y250F or Y315F to measure the mitogenesis (DNA synthesis) effects by determining BrdU incorporation into fibroblasts have demonstrated that neither mutant alone or cotransfected forms of each mutant could restore wild-type levels of BrdU incorporation, providing evidence that Shc and PI 3-kinase may need to be on the same molecule of MT (237). However, it has been shown that the interactions of MT with both PI 3-kinase and Shc, although not necessarily on the same molecule, are needed for the nuclear translocation of MAP kinases (348). While neither Py-1178-T nor a mutant with a mutation in the Shc binding domain (Y250F) resulted in full nuclear translocation of ERK1 or ERK2, the combination of the two in the same infection did (348). The dependence on both MT-Shc and MT–PI 3-kinase complexes for full MAP kinase activity is consistent with other findings indicating a requirement of both the activation of Ras and PI 3-kinase pathways to achieve full MAP kinase activation and thus full AP-1 activity (153, 216, 283). Some studies have shown that MT mutants with mutations in the Shc binding domain result in high AP-1 expression, indicating that only the MT–PI 3-kinase complex was required. These observations may be a result of the cell type used or alternative pathways for activating JNK. The AP-1 expression induced by the combined activation of PI 3-kinase and Shc in 3T3 cells infected with a recombinant retrovirus containing a cDNA encoding MT (or mutant forms of MT) was made up of c-jun and junB, not c-fos or fosB, although the presence of other fos (Fra1, Fra2) and jun (junD) proteins has not been determined (246). This AP-1 composition, which contrasts with earlier findings indicating that MT-induced transformation was dependent on activation of the c-fos promoter, could be a result of either the cell type used (BALB-3T3) or the separation of MT from other components of Py, such as ST, which is important for the activation of the fos promoter (237, 246). The connection between the tyrosine 315 phosphate site and AP-1 has been postulated to be due to the activation of Akt by the PI 3-kinase pathway, which leads to phosphorylation and resultant inactivation of glycogen synthase kinase-3β (GSK-3) (246, 348). GSK-3 is a negative regulator of jun family members and phosphorylates residues in their DNA binding domains (80, 242). The dephosphorylation of the DNA binding domain by the inactivation of GSK-3 and the phosphorylation of the transactivation domain results in c-jun activation in MT-expressing NIH 3T3 cells (319).
Phosphoinositide maintenance.
Further interactions between (and the need for both) Shc and PI 3-kinase activation have been found. Although PI 3-kinase activation results in elevated levels of PI(3,4)P2 and PIP3, MT mutants with mutations in the Shc binding domain do not maintain adequate levels of these modified PIs to allow for transformation (202). The explanation for this connection may involve a specific (PI 3-kinase product) degradative protein blocked by the activation of the Shc pathway. Alternatively, the Shc pathway may be important for specific subcellular localization of the lipid substrates. These complex interactions can be further supported by the finding that mutants with mutations in either the PI 3-kinase binding site (315YF) or Shc binding site (250YS) result in an impaired but not totally deficient ability to induce tumors following intraperitoneal inoculation of newborn mice (C3H/BiDA strain) (40). The 250YS mutant and the 315YF/250YS double mutant are unable to transform in culture but can still develop tumors in vivo. 315YF/250YS, though, is reduced to a minor subset (no epithelial tumors) of wild-type-induced tumors and an increased time until tumors arise (40).
Rho family of GTPases.
The small GTPases of the Rho family, including Rac1, Rho, and Cdc42, have been implicated in Py-induced cellular transformation through their activation by the MT-induced Shc-Ras-Raf and PI 3-kinase pathways (60, 165, 349). The normal biological functions of these proteins include roles in actin cytoskeletal organization (membrane ruffling), formation of stress fibers, regulation of fos and jun expression, cell growth, downstream hormone signaling, and cell cycle progression (62, 149, 150, 229, 247, 282). Both Rac1 and Cdc42 are required for MT-induced cell transformation, although either one alone can activate the c-fos promoter (349). Differentiating the induction of Rac1 by a particular pathway, though, can be difficult, since although PI 3-kinase has been shown to activate Rac1, Ras can also activate PI 3-kinase (reviewed in reference 45). The activities of the MT-Shc complex and the intricate interplay among Rac1 and the ERK cascade leading to high-level production of fos and jun are discussed in more detail above. The problem here is that the association of the Rho family with transformation is even more poorly understood and the known or expected activities of the family members are often in contrast with the observed findings depending on the cell type being studied (reviewed in reference 312). For example, as already mentioned, Akt has a potential antiapoptotic role while Akt and Rac, both activated by PI 3-kinase, result in the activation of NF-κB, an apparent requirement for cellular transformation that can send conflicting signals which can induce and/or inhibit apoptosis depending on the cell type (224, 251, 284). The functions of these proteins are intriguing and may play a role in the natural Py infection, particularly the induced expression of transcription factors and cytoskeletal rearrangement. Unfortunately, the activation of these small GTPases has not yet been linked to either lytic infection or replication in vivo.
Protein kinase C/Ras.
While exploring the roles of PI(3)P in transformation, it was discovered that MT-src also stimulates the activity of membrane-associated PKC and that PKC may be involved in the direct stimulation of Ras and/or a feedback mechanism which enhances transformation through activation of PI 3-kinase (212, 272). It was found that tetradecanoyl phorbol acetate, an inducer of PKC activity, could stimulate the phosphorylation of MT in MT-src complexes and enhance transformation levels in vitro (223, 274, 278). Since diacylglycerols can also stimulate PKC activity, the MT-induced activity of PLCγ-1 may be needed to stimulate PI 3-kinase activity to higher levels by phosphorylation and thus illustrates another cross-feedback mechanism of MT signal transduction (212, 272). The activation of PKC by MT is very sensitive to even low levels of MT expression, while PI 3-kinase is not, indicating a key level of control of the signal transduction pathways induced by MT, at least in the rat F111 cell line (212). As mentioned above, PKC also increases the activity of Ras, but Ras can also feed back to increase the activity of membrane-associated PKC, which promotes the transforming ability of the MT-src complexes (91, 217). Ras activation was shown to be responsible for the accelerated proliferation, focus formation on confluent monolayers of normal cells, and formation of colonies in soft agar (all components of the transformed phenotype of Py) (276). Two theories about the need for Ras in transformation have been put forth: (i) Ras is involved in cell cycle transit in the later stages of G1 and possibly G2 (99), and (ii) Ras interferes with the autocrine factors secreted by Py-transformed cells which allow cells to grow on suspended soft agar (271).
Phospholipase C-γ1
The third important phosphorylated tyrosine of MT, 322, was found to coimmunoprecipitate with PLC-γ1, another SH2 domain-containing signal molecule (322). PLC-γ1 catalyzes a reaction which produces two secondary messengers, IP3 and diacylglycerol. Diacylglycerol results in activation of PKC and therefore is the likely source of high levels of PKC activation seen in MT-expressing cells. Mutants with mutations in residue 322 (322YF) are fully transforming and therefore are probably not important for the full functions of MT-induced signal transduction, but this may again be cell type specific and needs to be further explored in vivo (353). As mentioned above, PKC may be needed to maximize the phosphorylation and resulting activation of PI-3 kinase, but this need may be blurred by cell culture effects, e.g., serum (272, 274, 278).
Protein Phosphatase 2A
Following the observation that a mutant which abolished the ability of MT to associate with PP2A, pp60c-src, and PI 3-kinase (mutation of cysteine 120 to tryptophan) resulted in a transformation-deficient Py (128), the association of MT with PP2A was discovered. The amino terminus of MT prior to the splice junction (aa 191 and 192) contains the site (aa 90 to 120) which coimmunoprecipitates with the A and C components of PP2A (137, 138, 181, 254, 346). PP2A comprises the majority of total phosphatase activity in many tissues and has a broad specificity without the need for divalent cations. It consists of a 36- to 38-kDa catalytic subunit (C) and two additional regulatory subunits of 60 kDa (A) and 55 kDa (and lesser 54-, 72-, and 74-kDa subunits) (B). A nearly 100% conservation of this enzyme is seen across species. MT and ST are associated with the catalytic subunit C and the A subunit of PP2A (254, 359). MT brings PP2A to the plasma membrane and, in some cell types, activates JNK, which results in either a pro- or antiapoptotic signal, although it is not known which (237). MT shares only one sequence motif (sequence DKGG) with the 55-kDa B subunit of PP2A and has no sequence homology to the 74-kDa version of the B subunit (255). However, this motif was found to be dispensable for the complex formation between MT and PP2A and for induction of transformation (129). A cluster of cysteine residues found in MT (aa 120 to 153) and ST of Py and ST of SV40 and BK are important for their interaction with PP2A (129). Along with the cysteine cluster, the first 25 aa and aa 105 to 111 (CRMPLTC motif) are important for the association of MT with PP2A. Interestingly, all mutants which fail to bind PP2A also fail to bind pp60c-src, which may mean that these proteins form a single complex with MT or that structural conformation for binding either PP2A or pp60c-src is similar (remember that MT does not directly bind pp60c-src) (43, 129). The function of the complex between MT and PP2A is still unclear, although it has been suggested that MT reduces PP2A activity and thereby allows for hyperphosphorylation of the Rb protein, which in turn stimulates the cell cycle (52). The most obvious function, though, is to perturb the function of PP2A and thereby prevent dephosphorylation of VP1, which promotes increased encapsidation efficiency of the virus (228). Interestingly, this latter function has been associated with truncated forms of MT, which are made as regular components of the viral life cycle and have the ability to passively diffuse into the nucleus like ST or are actively taken in by nuclear localization signals of the regulatory and catalytic subunits of PP2A (228, 344, 380). These conclusions have been recently countered based on the small amount of MT and ST that binds PP2A, which could never titrate the abundant levels of PP2A (346). It appears that MT-PP2A complexes may be responsible for dephosphorylating critical target proteins rather than inhibiting the function of PP2A (237). Therefore, it is unlikely that MT inhibits the activity of PP2A as a means of promoting the increased threonine phosphorylation of VP1 and increasing encapsidation (199).
14-3-3 Proteins
MT also uses serine phosphorylation at residue 257 to bind proteins of the 14-3-3 family (70, 253). The 14-3-3 family of proteins have been associated with cellular growth regulation and signal transduction, including Raf, PI 3-kinase, cdc25 phosphatase, and PKC and aspects of ADP ribosylation, cell cycle, exocytosis, catecholamine biosynthesis, and numerous other activities (281). These functions could presumably enhance Py replication. The two 14-3-3 proteins that bind MT are 27-kDa (mixture of several 14-3-3 subspecies) and 29-kDa (epsilon subspecies) proteins (253). The kinase responsible for the serine phosphorylation at residue 257 is unknown, although it appears to be stoichiometric (70). Furthermore, the binding of the 14-3-3 proteins at serine 257 does not prevent the binding of Shc at tyrosine 250. Interestingly, 257 mutants were not defective in transformation in vitro, and therefore the 14-3-3 proteins have been proposed to be part of a structural multimerization of MT rather than being involved in functional activity (70). This was further supported by the observation that dl8 mutants of Py, which lack the sequences in MT responsible for binding the 14-3-3 proteins, were not transformation deficient but were actually more efficient at transformation than was wild-type virus (136). The multimerization of MT, at most a dimer based on in vitro data, may be more important in vivo since serine 257 mutants are unusually low in induction of salivary gland and subcutaneous tissue tumors (70, 307). However, the dl8 mutants were more oncogenic in animals and tumors appeared earlier than for wild-type virus (307). The discrepancy is difficult to explain, although these two mutants could be slightly different in their resulting MT conformations. Therefore, the dimerization of MT in conjunction with the 14-3-3 proteins may act to attenuate MT-mediated signaling and thereby allow efficient spread of the virus throughout the host population prior to the complications of tumor formation, although this would support the misconception that tumorigenesis is part of the natural life cycle of polyomavirus (307). Since 14-3-3 proteins have a plethora of potential functions, it may be difficult to ascertain the exact role of the MT–14-3-3 complex without studying Py replication in vivo or at least in the natural target cells for primary and persistent replication. Since both the tumor profile and the activity of MT on certain promoters are altered by the elimination of the 14-3-3 binding site, the MT–14-3-3 complex would appear to be important for virus replication in particular cell types (70). This specificity is a recurring theme since each of the signal transduction pathways illustrated in this section probably acts alone or with cross talk not only in particular cell type but also during a precise time such as in the initiation of terminal differentiation.
Transcription Factor Production
Unfortunately, all of the above information was based on the ability of MT to transform cells in culture or induce tumors in mice and may be completely artificial, although it may indicate that the combined efforts of all of the MT-induced signal transduction pathways is to produce a high level of transcription factors. These transcription factors, then, are important for establishing the cellular environment for which viral replication can take place. The presence of AP-1, whose composition may be different in various cell types or conditions, and other transcription factors at a particular time during cellular differentiation could allow the virus to maintain the expression of the necessary DNA synthesis machinery by competing against nucleosomes or other cellular factors which turn off genes not required in the terminally differentiated cell. In addition, the accumulation of pertinent transcription factors could be involved in not only the viral replication but also the autoregulation of early transcription by downstream effects of MT-activated signal transduction pathways, which in turn would also directly affect viral replication.
As is expanded upon below (see “DNA synthesis”), the Py enhancer contains sites for both AP-1 and the Ets family of transcription factors. In particular, the Ras-responsive element of the Py enhancer has adjacent binding sites for Ets family members and AP-1, although the composition of fos and jun elements required for this site is still unclear. The complexity of these responses is compounded due to the regulation of MT-induced transcription factor expression by other transcription factors regulated by downstream portions of MT-activated signals (potential cross talk), for example the numerous genes, including fos and jun family members, that contain SRE, which are essentially complex Ras-responsive elements. In addition, although Ets family members are widely distributed throughout tissues, recent findings have shown that subsets are found in particular tissues, especially during development (reviewed in reference 363). Since Py probably evolved to contend with a newborn environment, this developmental expression of Ets could be important for efficient acute replication, dissemination, and establishment of persistence. Ets family members and to an extent AP-1 rely not only on differential expression to control their specificity but also on their target nucleotide sequence specificity, interaction with other differential or ubiquitously expressed proteins, and, as detailed above, phosphorylation-controlled activation (reviewed in references 363 and 382). The interaction of DNA and these transcription factors therefore results in a versatile, temporal, and cell-type-specific signal that regulates gene expression (reviewed in references 311 and 364). Therefore, these multilayered and rather elegant responses allow MT to establish a time-specific response in a differentiating cell that establishes a cellular environment needed to elevate viral replication to a level required to establish and maintain persistence. As described above, a Py mutant which contains an MT protein without a membrane binding domain (MOP), thus lacking all the above-described signal transduction, does replicate in a similar pattern to wild-type virus in intranasally inoculated adult SCID-beige mice. Therefore, clearly either these time-specific signals for expression of transcription factors can be mimicked in certain situations by the cellular environment or Py replication, to some degree, does not require them. This illustrates, perhaps, that MT is only an embellisher of replication, not an absolute requirement. This latter conclusion would lead to the possibility that the role of MT may be more important in maintaining persistence than acute replication.
Genes regulated by middle T antigen.
Only a few genes have been shown to be regulated by MT activation of various signal transduction pathways. The shortlist includes the already mentioned genes for fos, jun, myc, and Ets family members, as well as the urokinase-type plasminogen activator (uPA) gene (30, 319, 348). Mice harboring an MT transgene show high levels of uPA in hemangiomas (tumors of the blood vessels), which suggested a relationship between the two (30, 231). uPA is a secreted serine protease that converts zymogen plasminogen to plasmin and plays an important role in tissue remodeling during embryogenesis, cell migration during wound healing, angiogenesis, and tumor metastasis (30). Interestingly, uPA is currently the only activated gene shown to be regulated by MT both in vitro (3T3 cells) and in vivo (MT-transgenic mice). The cis elements involved in uPA gene activation by MT include two composite AP1/Ets sites, which are either activated through the MT-Shc-Ras pathway or independently, perhaps through PI 3-kinase (30).
Unfortunately, AP-1 and Ets binding sites are so ubiquitous that it is difficult to determine which proteins are actually affected by MT. This mind-boggling number is hinted at by the recent finding that many of the roughly 300 genes induced in quiescent fibroblasts after the addition of serum have an AP-1 site in their promoter (160).
Nonphosphorylation Domains
Recently a proline-rich domain in MT has been identified as important for transformation and tumor-inducing ability without disturbing the association with PI 3-kinase or Shc, altering viral replication or immortalization in vitro, or affecting the ability of the virus to grow well in kidneys of infected mice (387). The deletion mutant eliminated amino acids 336 to 338 in MT, a domain which appears as a possible SH3 binding domain. Wild-type virus, in this case the PTA strain of Py, induced a high frequency of salivary gland, mammary gland, thymic, kidney, and hair follicle tumors when given intraperitoneally to newborn C3H/BiDa mice whereas the mutant virus did not induce salivary, kidney or thymic tumors and induced a reduced frequency of mammary and hair follicle tumors than did an equal titer of wild-type virus (387).
Threonines 160 and 291 and Serine 283
Although not well studied, the phosphorylated threonines at 160 and 291 and the serine at 283 of MT have been examined by mutational analysis. A mutation made in the threonine 160 (Thr→Ala) resulted in a transformation-defective mutant that still bound pp60c-src, PI 3-kinase, and PP2A (259). There is some evidence that threonines 160 and 291 and serine 283 are phosphorylated during mitosis by a cdc2-like kinase, possibly by the cell cycle-regulated serine/threonine kinase p34cdc2 (259). Unfortunately, the high expression of MT required for mapping studies occurs only during the lytic viral infection and therefore impedes the ability to synchronize cells and further study this unique phosphorylation. However, no definitive conclusion could be made about the threonine 160 mutant, since phosphorylation at this site does not completely explain the mobility shift of MT during cell cycle progression (259). One theory is that the Thr-to-Ala mutation at threonine 160 results in an MT conformation change that causes the transformation deficiency (260). Interestingly, the threonine 160 mutant is compensated by mutations in the serine 283 site, which may provide evidence for a complex interaction of MT with a cascade of kinases (260). Mutants with mutations at threonine 291 (Thr→Ala) or serine 283 (Ser→Ala) were indistinguishable from wild-type MT (259, 260). However, there is evidence that serine 283 is phosphorylated and accounts for the mobility shift of MT during mitosis (223, 274, 296). During these studies it was discovered that it is not possible to label MT in vivo with a high enough specific activity of 32PO4 for detailing the phosphorylation sites by protease mapping (260). This may impede any detailed study of the importance of these phosphorylations. These studies do present a clear indication that phosphorylation is a key to negatively or positively regulating the signal output of MT and that the cell cycle and probably the cell type may alter the kinases present that control this phosphorylation, resulting in either viral replication or cell transformation (260).
ROLE OF MIDDLE T ANTIGEN IN VIRAL REPLICATION AND TRANSCRIPTION
DNA Synthesis
“Py-induced cellular DNA synthesis is neither preceded nor accompanied by an overall stimulation of cellular RNA synthesis nor is it followed by mitosis” (261)
Py infection of highly confluent cells in culture has been frequently reported to stimulate an unscheduled round of cellular DNA synthesis (96, 372, 373). This synthesis, however, may occur out of normal cell cycle control, and it does not involve a full round of the cell cycle since mitosis does not follow the S phase (197, 198). However, in vivo studies in our laboratory have provided no evidence for DNA synthesis following Py infection in vivo (233). In addition to cellular DNA synthesis, Py early genes specifically affect viral DNA replication. Reports that MT mutants directly affect the ability of the virus to replicate and maintain persistence are associated with a direct implication that MT is involved in some aspect of the regulation and efficiency of Py replication (54, 114). Before considering the direct effects of MT on Py replication, it should be remembered that the overlapping nature of the Py enhancer makes separation of transcription and replication difficult. Furthermore, MT may actually be acting indirectly on viral replication by inducing the expression of the early proteins, an aspect that can be easily overlooked when considering the effects of MT mutants on viral replication. The details of some of the features of the complex interaction between the control of both replication and transcription by the Py enhancer and the enhancement of transcription factor activation and/or expression through the stimulation of signal transduction pathways by the MT-pp60c-src complex are given below.
Middle T-antigen activation of the polyomavirus enhancer.
MT is needed for high levels of viral replication (113, 343). This requirement is probably associated with an indirect control over the performance of the Py enhancer through induction of transcription factors (enhancer binding proteins). The in vivo results which described a requirement of MT for Py replication in mice (113) have not been directly linked to an in vitro mechanism by which MT may affect Py DNA replication (18, 54). MT-induced signaling was shown to induce transcription from reporter genes which contain PEA1 [polyomavirus enhancer A (α) binding protein 1, the mouse homolog of AP-1 and homologous to the AP-1 recognition sequence of SV40] and PEA3 (mouse homolog of c-ets and homologous to a sequence in the Ad5 EA1 gene promoter) sites. These transcription factors are both components of the α domain of the Py viral enhancer, the portion associated with Py replication activation (220, 366–368, 384). This dependence appears to rely solely on MT (and possibly ST) since Py LT has not been associated with activation or induced expression of transcription factors found on the Py promoter (368).
The replication origin of Py is composed of two functional components, the core component (origin) and the auxiliary component (portions of the viral enhancer named alpha [α] and beta [β]) (238, 239, 345). The origin core, which includes LT binding sites, cannot function on its own even in permissive mouse cells expressing LT (173, 239, 345, 354). The specificity of Py (as opposed to SV40) for mouse cells lies in the binding of a specific primase (DNA polymerase α-primase) within the origin core during the initiation of the replication complex (240, 315). In opposition to this, the auxiliary sequences are interchangeable and can activate either Py or SV40 DNA replication in mouse or monkey cells (25). Both regions are required for replication, although the auxiliary component at first appears redundant, since a functional Py origin requires only the origin core and either α or β, although the redundancy may allow the virus to replicate effectively in multiple cell types with different abundances of binding factors present (147, 238). In contrast, both α and β are required elements of the transcriptional enhancer (236, 238). Binding sites in the α core portion of the enhancer (24 bp) include those for PEA1, PEA2, and PEA3 (236). In addition, a binding site(s) for NF-D (nuclear factor D)/YY1 (yin and yang 1) is located outside the core of the α enhancer (51, 218, 314). At least two of PEA1, PEA3, and PEA2 are required for Py DNA replication, although the specifics, as outlined below, are still unclear. That the majority of the same sequences that drive early transcription also drive DNA replication and that any mutations in these sequences affect both processes may illustrate how MT can be autoregulatory, as illustrated in Fig. 3 (82, 115, 116, 172, 235, 236, 239, 334, 354).
FIG. 3.
Proposed role of MT in polyomavirus replication. Following infection and establishment of persistence, polyomavirus is reactivated following a signal (external) to differentiate in the infected cell (I). This signal triggers the expression of transcription factors (II), which induce early-gene expression, and the production of LT, MT, and ST (III). In an autocatalytic (autoregulatory) loop, MT induces the expression of transcription factors (IV) through its activation of various signal transduction pathways and further induces the expression of the early proteins (V). During this time, the early proteins (LT, MT, and ST) reprogram the infected cell to allow viral DNA replication (IV). There is then a switch from early gene-expression to viral DNA replication (V) and late-region expression (V) at some time during the differentiation of the infected cell. MT and the other early proteins are proposed to establish all the factors required for efficient virus production in a cell during its final stages of differentiation (VI).
Transcription factors, viral replication, and the serum quagmire.
The dependence of Py replication on MT appears to rely on the ability of MT to increase the intracellular concentrations of both c-jun and c-fos family members (components of PEA1) and the c-ets proteins (p54c-ets-1, p68c-ets-1, and/or p58-64c-ets-2) (component of PEA3) by posttranslational modification and transcriptional activation (71, 148, 171, 301, 319, 366–368). Interestingly, as more evidence that Py early proteins evolved to work together, PEA1 has been demonstrated to synergize with LT to activate viral DNA replication (140). Induction of PEA1 would be an expected necessity in vitro, but serum components, common to most Py cell culture experiments described, have been found to elevate PEA1 (PEBP1) and PEA3 (PEBP5) levels in fibroblast cell lines but not F9 EC cells (190, 293, 368). The α domain of the Py viral enhancer is 3.5-fold less efficient in EC cells than in fibroblasts (147, 203). This high level of PEA1 components (c-fos and c-jun) and PEA3 (c-ets) in serum clearly explains the ability of MT mutants to grow well in 3T6 cells under normal cell culture conditions (244). Further evidence is provided by the ability of a Py mutant lacking both transforming and immortalizing functions to grow well in 3T6 cells in serum but not in serum-deprived 3T3 cells (201). That 3T6 cells are also a differentiating cell line only further shows why these cells are so permissive for Py replication even without a functional MT or the ability of LT to bind pRb (135, 334). Interestingly, as mentioned above, the c-ets promoter contains binding sites for PEA1 and c-ets itself, indicating that serum stimulation would result in autoregulation of PEA3 (211). The findings that the Py enhancer is more active due to an increase in c-jun concentration during differentiation and that Py mutants that duplicate PEA1 and PEA3 sites can grow in EC cells further support these conclusions (186, 244). The regulation of the Py enhancer by c-jun, however, is cell type specific, with stimulation occurring in F9 EC cells and repression, due to an unknown mechanism, in LMTK fibroblasts, a potential explanation for the cell type specificity of Py replication (300). Other transforming proteins, v-src, c-HA-ras, v-mos, and v-raf, also induce the expression of PEA1 and PEA3 (367–369). Many of these are downstream portions of MT signal transduction and therefore reiterate the MT induction of PEA1 and PEA3 expression and illustrate how MT signaling can mimic many viral oncogenes in transformation. Interestingly, c-myc, another transcription factor induced by MT signaling, was unable to induce PEA1 levels, but this may be associated with the negative regulation of the c-myc promoter by c-fos and c-jun (146, 330, 368).
The final component of the core of the α domain of the Py enhancer, PEA2, homologous to a sequence found in the fos enhancer, is bound by polyoma enhancer binding protein 2 (PEBP2), or core binding factor, a protein homologous to the Drosophila segmentation gene runt and the human gene AML1, considered a repressor not inducible by oncogene expression, serum components, or TPA (220, 245, 292, 365). Nevertheless, another binding site which binds nuclear factor PEBP4 overlaps the PEA2 site and was determined to be responsible for the inhibition of Py specifically in F9 cells (119). However, mutation of the PEBP4 site and not the overlapping PEBP2 (PEA2) site results in reduced replication of Py DNA in c-jun-overexpressing WOP cells, indicating a direct connection between c-jun expression and a factor which binds the PEA2 site and suppresses Py DNA replication (292). There has been some disagreement about the role of PEBP2, with some results indicating a derepressor of the Py enhancer on differentiation and others, as mentioned above, indicating an inhibitory aspect on the enhancer that is elevated by PEA1 overexpression and binding (119, 365). The discovery that PEBP2 becomes the replication enhancer PEBP3 in transformed cells or after treatment of PEBP2 with acid phosphatase may explain some of the contrasting observations and indicate a regulated control of this change during differentiation (167). PEBP3 consists of various isomers made of α and β subunits, with the exact combination and amount being important in exerting positive or negative effects (328). These findings establish a possible scenario for the replication of Py in vivo. MT appears to open up a longer window of opportunity and/or increase the levels of replication through production of PEA1 and PEA3, but it is not sufficient to do this on its own and needs the cellular changes involved in differentiation to create a proper balance of PEABP proteins, namely the repressor PEABP4 and repressor/enhancer PEABP2/PEABP3, needed for high levels of viral replication (and transcription).
The recent finding that the PEA1 and PEA3 sites exert their effects most strongly during early rounds of replication in vitro is supported by our findings in vivo that although MT mutants do not show high levels of replication immediately, they can eventually replicate and persist once enough cells go through differentiation cycles (as mentioned above for the SCID experiments) (54). This recent study discussed many of the aspects important to our understanding of the ability of MT mutants to grow well in cell culture conditions, namely, high levels of serum, unusual serum conditions, cells recently released from G0, and use of high multiplicities of infection (54). This paper concluded that the regulation of Py DNA replication may be closely linked to the activation of DNA replication proteins by the factors induced by MT. This reiterated earlier observations in which MT (and ST) may control viral replication. In other words, the stimulation of host cell transcription by MT (and ST) results in an increased transcription of viral mRNAs and thus in an increase in viral DNA replication through the efficient activation of the Py enhancer (222, 295, 343). It can easily be seen how this mechanism would allow the autoregulation of early transcription by MT. An example of how the viral early proteins may alter host cell transcription is found in the high expression of PCNA in Py-infected cells of the bronchiolar epithelium (Gottlieb and Villarreal, unpublished). This protein would probably be turned off by p53 when apoptosis is triggered by deregulation of the cell cycle due to the binding of pRb by LT. However, the mouse PCNA promoter includes putative binding sites for the transcription factors PEA3, ATF, Sp1, and E2F (164, 234, 388). Although the presence of p53 may repress the mouse PCNA promoter, the combined regulation of cellular programs by MT and LT (E2F activation) would maintain its expression (385).
c-myc.
There is some evidence that counters the above concepts by demonstrating that MT transformation induces c-myc but not c-fos or c-jun levels (269). This observation, though, was made in experiments with serum-containing culture in which neither c-fos nor c-jun components were likely to be needed (as mentioned above). Others have seen increased levels of c-myc in Py-induced tumors (144). However, this may only be a consequence of transformation and may not be directly related to the replication of the virus in a normal state. One interesting experiment demonstrated that Py integrates into the genome in or around the c-myc gene and by doing so induces oncogenesis, at least in the mammary adenocarcinomas (26). This, however, indicates that tumorigenesis is a consequence of overexpression of a cellular gene by integration of the Py genome and not as a result of the downstream effects of MT signal transduction. This is counterintuitive to the fact that Py not only is selecting for tumor induction but also appears to be selecting for increased viral replication.
Regulation of Transcription
In culture, the induction of c-fos, c-myc, and JE, all early response genes, is MT independent, relying instead on a combination of LT and ST (127, 392). A biphasic response occurs in the infection of quiescent cells. VP1 binding to its receptor on the surface of cells can transiently activate c-fos, c-myc, and c-jun, similar pathways to those for MT, although this activation may not be a requirement for lytic infection (at least in vitro) (54, 123, 392). During an in vitro infection, VP1, the major capsid protein, induces the first peak (between 0 and 1 h after adsorption of the virus) due to an interaction with the as yet unknown receptor and/or binding to the nuclear matrix, while the expression of the early region results in the second peak (12 to 33 h after infection) (392). This latter observation was supported by the finding that Py infections in the presence of DNA synthesis inhibitors still showed the same second peak of early-response genes. Further evidence supported these findings and added c-jun to the list of early-response genes induced by Py infection in a biphasic manner (127). These later experiments also showed that LT is virtually dispensable for the second peak and that ST seems solely responsible. However, neither set of experiments could eliminate the possibility that LT and MT transactivate the early-response gene promoters through signal transduction. Portions of VP1, though, appear to be required for efficient Py transcription and replication since they associate with the nuclear matrix-associated transcription factor YY1 and, along with other as yet identified proteins, may be important for bringing the initial transcription factors required for early transcription to the Py enhancer (252). There are a number of studies indicating a role for the late region, both the sequences and the proteins expressed, in Py transcription and replication, although the connection to MT and the other early proteins is still be elucidated (123, 156, 199, 226).
Although serum induction of early-response genes results in a subsequent repression of transcription, Py infection continues to induce the transcription of the early-response genes for an extended time. Serum induces the early-response genes to a 10- to 20-fold level higher than does Py infection, which may explain why MT mutants (or most early region mutants) of any kind grow equally well in replicating cell (established) cultures in the presence of serum (127). In addition, cellular replication itself induces the expression of the early-response genes at low levels and could be a key to the tropism of Py and the high level of virus production in tumor tissue. All of the above experiments, though, were performed in culture and illustrate again the discrepancies that arise when trying to understand the true functions of the early proteins, an aspect best left to in vivo experiments.
Finally, although MT regulates the levels of PEA1 and PEA3 and these are required transcription factors for Py early transcription, replication, and late transcription, this does not explain how the shift from early to late transcription occurs. It has been postulated that the functions of LT are responsible for this shift, and our recent results (mentioned above in the descriptions of SCID experiments) support this; therefore they will not be included in this review of MT (for review of potential mechanism, see reference 388).
MIDDLE T ANTIGEN AND APOPTOSIS
Because mammalian polyomaviruses persistently infect their host, it is believed that they have devised ways to evade intracellular host defenses. In addition, viral deregulation of normal cell cycle progression is expected to induce apoptosis, suggesting that the virus must subsequently inhibit apoptosis to allow completion of viral replication (for reviews, see references 338 and 389). However, this widely held view is supported by few, if any, observations in vivo. Only a few reports have analyzed apoptosis in conjunction with productive Py infection, and of these, none have shown any in vivo observations of apoptosis in nontumorigenic systems. Our own efforts were unable to observe apoptotic cells using a number of in situ apoptosis detection systems (TUNEL, immunohistochemistry for caspase-3, etc.) on Py-infected lungs from newborn or adult intranasally inoculated mice (unpublished results). Thus, Py-induced apoptosis in vivo remains to be established.
The high level of apoptosis in the mammary epithelial hyperplasias from transgenic mice carrying a mutated MT (Y315/322F) under the control of the mouse mammary tumor virus long terminal repeat provides the only in vivo claim that PI 3-kinase and its downstream activated pathways are responsible for the MT cell survival function (370). In the same study, a dominant negative inhibitor of PI 3-kinase in mammary tumor cells expressing wild-type MT also induced apoptosis. These results, along with a number of in vitro studies, point to the activation of Akt, a protein associated with a number of antiapoptotic mechanisms, by this MT-PI 3-kinase complex as the strategy of preventing apoptosis (175, 225, 267, 325). More recent in vitro studies expressed either MT or ST or a combination of the two in a mouse T-cell lymphoma carrying a temperature-sensitive p53. It was concluded that MT and ST cooperated to protect against p53-induced apoptosis (267). However, the high-level expression well over natural virus infection suggests that this observation may be particular and aberrant to this system. Furthermore, the actions of Py early proteins in a T-cell lymphoma with p53 may be biologically irrelevant, since p53 expression can be quite variable and nonpredictive in Py infections, as noted by the authors of this study (266). However, wortmannin, a specific blocker of PI 3-kinase, prevented the ability of MT to inhibit the p53-induced apoptosis, further supporting the antiapoptotic abilities of MT. This ability of MT to prevent apoptosis might explain the absence of apoptotic cells in wild-type infections. Nevertheless, we were unable to observe apoptotic cells in the lungs of adult or newborn mice intranasally inoculated with Py expressing either truncation or point mutants of MT (Gottlieb and Villarreal, unpublished). Therefore, the biological relevance of MT mediated apoptosis remains to be established.
Virus-infected cells can also die via necrosis. A recent report studied whether an apoptotic or necrotic mechanism was evoked on viral progeny release from Py-infected 3T6 cells, the cell type most commonly used to grow and plaque Py. The study observed that a majority (40%) of the cell lysis was done through necrosis while a minor fraction (10%) was done through apoptosis (detection included electron microscopy, flow cytometry for annexin V and propidium iodide, DNA fragmentation, and caspase-3 activity). However, necrosis has not been documented for Py-infected cells in vivo. Since the capsid proteins were found to be unable to induce cell death on their own, it was proposed that the early proteins must be responsible for these actions. This result may be the first authentic indication that Py induces apoptosis. However, since most cells died of necrosis, necrosis might be important for viral dissemination.
These experiments suggest the following scenario: the binding of Rb by LT leads to the induction of apoptosis, and then because Py LT does not bind p53, the actions induced by MT inhibit apoptosis until the virus life cycle is complete, at which time the cell succumbs to necrosis, allowing the release of viral progeny. Unfortunately, this scenario is likely to be too simplistic, and the actions of MT, as discussed below, may also be responsible for the cascade of signals which ultimately leads to apoptosis. Furthermore, ST, an early protein that is as evolutionarily well conserved as LT, appears to be partly responsible for the alterations in the cell regulatory mechanisms which allow the DNA synthesis machinery to be maintained during terminal differentiation of Py-infected cells (237, 302). In addition, recent evidence demonstrated that LT has the ability to protect against growth-inhibitory functions of beta and gamma interferon, which may help curtail the effects of the cytokine response evoked by Py infection in vivo (92, 371). Thus, the combined actions of all of the early proteins must be considered when determining the mechanisms by which Py alters and/or inhibits apoptosis.
A number of DNA viruses are known to protect against tumor necrosis factor alpha (TNF-α)-induced apoptosis, a cytokine secreted primarily by activated macrophages. TNF-α functions include induction of the inflammatory response, growth inhibition, apoptosis, and other activities (17). The TNF-α studies are based on the ability of the virus to protect the infected host cell from external stimuli. Unfortunately, the only experiments demonstrating the anti-TNF-α functions of Py early proteins were plagued by the same validity questions as those in the above signal transduction section, namely, the overexpression of the individual viral proteins and the use of cycling, established cell lines. Furthermore, the inability of TNF-α to induce apoptosis in culture on its own and the rather blurred understanding of whether the TNF-α-induced signal transduction pathways leads to growth stimulation or apoptosis adds more murkiness to the biological relevance of these experiments, but the results are nevertheless intriguing. Cell lines (C127 and L929, both mammary gland derived) were transfected with the DNA of a replication-deficient Py expressing either an individual early protein or a combination of these (27). Expression of MT on its own was found to increase the susceptibility of the cells to TNF-α-induced apoptosis (all treatments also included actinomycin D). The expression of both MT and ST, though, could protect against the hypersensitivity to TNF-α that was created by MT alone, although ST brought the levels of sensitivity back only to parental (control) levels. The cooperation of early proteins was again shown, since only coordinated expression of MT and ST from the same genome could protect against TNF-α hypersensitivity. The expression of all three early proteins or the expression of LT alone displayed a phenotype similar to the combination of ST and MT, in other words, the same as parental cells. The addition of TNF-α to MT-expressing cells resulted in the release of arachidonic acid, a mediator of the cell inflammatory response and a correlate to TNF-α hypersensitivity. The MT-induced hypersensitivity was explained by the proposal that the mechanism that drives quiescent cells into S phase is the same as or similar to that which increases TNF-α susceptibility, although these experiments showed that LT, which binds Rb and would appear to be the major driving force for S-phase induction, did not increase hypersensitivity. A few mechanisms for the MT-increased TNF-α susceptibility were suggested and include deregulation of c-myc by activation of pp60c-src and the activation of stress genes through intracellular signal amplification by the Ras and/or c-jun pathways mediated by the MT-Shc complex or through association with PP2A (27). The possible mechanisms by which ST returns hypersensitivity to control levels are even less well understood (see references 237 and 302 for a perspective). Signals from TNF-α can result in differentiation, proliferation, or apoptosis, which means that MT-induced TNF-α susceptibility may only be an indication that the pathways used by MT to reprogram the differentiation signaling process are tapping into those induced by TNF-α, but the addition of actinomycin D blurs this distinction. Since actinomycin D inhibits RNA synthesis in these assays, this would indicate that MT-induced signal transduction not only alters the phosphorylation state of numerous proteins but also requires the induction of protein expression, including that of the transcription factors. In other words, downstream functions of the MT complexes with Shc, PI 3-kinase, PLC-γ1, and PP2A have evolved to send a very specific, balanced, and timed signal that probably requires cross talk to properly control its intensity and ultimate function, but the conditions of these in vitro studies are destroying the subtleties of this highly evolved equilibrium.
It is difficult to come to a conclusion about the association of MT with apoptosis, but it remains clear that the MT-associated signal transduction pathways can be demonstrated to be either anti- or proapoptotic by analysis of the wealth of functions connected to these circuits (as summarized in Fig. 2). The main problem with a majority of these studies is that until apoptosis can be shown in a normal or MT mutant Py infection in vivo, there is no reason to assume a priori that the manipulation of circuits which may lead to inhibition of apoptosis evolved for that purpose. Most of these studies have focused on lytic infection or high, unregulated expression of early proteins, but the necessity for control of apoptosis by Py may be more associated with persistence, an aspect rarely studied and poorly funded or understood.
MIDDLE T-ANTIGEN TRANSGENIC MICE AND TUMOR DEVELOPMENT
Hemangiomas
There have been a number of recombinant MT or transgenic (mostly mouse) studies which were originally designed to better understand the association between MT and transformation (for a review, see reference 258). For the most part, the tumor profile of these studies displayed a single type of tumor, hemangiomas, thereby further dismantling the concept that MT on its own is responsible for the broad tumor variety associated with the very name of polyomavirus. The first MT recombinant was made in chickens by using sequences encoding MT under the control of the Rous sarcoma virus long terminal repeat (183). The resulting birds established tumors with a very short latency (1.5 weeks compared to the 2 months or more normally seen in susceptible mice). The predominant tumors were hemangiomas, tumors of vascular endothelium, and hemangiosarcomas in multiple locations including the skin and subcutis of the legs and wings; muscles of the neck, breast, and legs; the intestine; and the kidneys. Since the kidneys are the persistent sites of Py replication (in the mouse), they were closely examined in these transgenic chickens and found to contain multifocal adenocarcinomas. Virus (Rous sarcoma virus expressing MT) from the kidney tumors could be retrieved and used to transform fibroblasts in vitro slightly more efficiently than the virus used to infect the chickens. An interesting outcome of this study was one of the first in vivo measurements of tyrosine phosphorylation in complexes containing pp60c-src. The investigators found an increase in phosphorylation that seemed to match the levels of MT expression in quantified tissues. MT, therefore, was shown to have a clear effect on only a particular cell type, not the expected widespread deregulation of cellular protein expression expected for a transgenic based on the number of circuits that are activated by the MT-pp60c-src complex.
Although the chicken system was important for its initiation of MT transgenics, the mouse system is more relevant to the natural biology of the virus and therefore has been studied in greater detail (2, 21, 258, 378). An initial study used transgenic mice (C57BL/6J × DBA/2J hybrids) with a cDNA of MT under the control of the replication-defective Py early-region regulatory sequences. Similar to the chickens, these mice also developed hemangiomas consisting of blood-filled cysts ranging in size from barely visible to as large as 1.5 cm3 within an average period of 3 months (22). The most common sites for the hemangiomas were the liver, lungs, and subcutaneous tissue. The transgenic MT protein was expressed only in these tumors of the animals, not ubiquitously as would be expected (21). Similarly, although hemangiomas of any type are a rarity and make up only a minor fraction of the large variety of tumors associated with Py tumorigenesis in mice, hemangiomas in similar locations to these transgenics were found when hamsters were inoculated with Py (79, 101, 102). These studies established the difference between the tumor profile of MT and the intact virus in mice. An interesting aside to this mouse transgenic study was the observation that the same construct with cDNA for LT instead of MT resulted in no tumors over an observation period of 15 months. This suggests that the tumor profile caused by high titers of wild-type virus given to susceptible strains of newborn mice, which develop tumors of the parotid and salivary glands, thymus, mammary glands, and bone and, to a lesser extent, hemangiomas, must include cooperation among the early proteins and perhaps a dependence on viral replication. That two different promoters (Rous sarcoma virus and Py regulatory region) in two different strains of animals resulted in hemangiomas raises the issue of the nature of the complementation of endothelial cells for the expression and tumorigenesis of MT.
Another study using a recombinant retrovirus expressing MT under the control of the thymidine kinase promoter was injected into newborn mice and also resulted in the formation of cavernous hemangiomas and subsequent death of the animals within 2 to 4 weeks (178). Other studies using rodents besides mice observed hemangiomas, including Fischer rats (F344), which received fetal brain tissue transplants containing MT; as observed in mouse experiments, MT was found only in the tumorous endothelial cells (2, 377). A contradiction to these results was the finding that transgenic mice carrying the entire early region of Py did not develop hemangiomas on a regular basis but instead developed lymphangiomas, fibrosarcomas, and bone tumors (361). Similar to the chicken study, MT, when expressed, had detectably high levels of kinase activity, and monoclonal antibody tests demonstrated that pp60c-src was present in these complexes (22). These studies also established the testes as another location for MT expression but without the resultant endothelial proliferation or tumor formation. Both LT and ST under control of the virus early regulatory region and the entire Py early region in transgenic mice also displayed testicular expression (22, 361). The expression of LT was later shown to be expressed in Sertoli and germ cells of the testes and eventually caused Sertoli cell tumors in old male mice (256). Another observation was that a cDNA of LT under the control of the same early-region sequences caused uncharacterized pituitary tumors in some mice (22). These latter observations could be related to a potential hormone regulation of the Py enhancer.
The tumor formation in the MT transgenic studies was both focal and of high multiplicity, often in a single organ (21, 183). This stochastic pattern raises the question why only some individual endothelial cells overproliferate and express MT while others do not. One theory is that a second event must occur for the tumor to form, even with transgenic MT expression. This may explain the latency period in some cells or tissues, although the hemangiomas appear too rapidly to allow for a second event. A second theory is that the MT gene is transcriptionally inactive in the endothelial cells but in rare cases MT is expressed and leads to tumorigenesis.
A number of similar studies were performed using an MT-carrying retrovirus to infect embryonic stem cells for use in creating chimeric mice, a necessity due to the inability to yield transgenic mice from embryonic injections (178, 358, 378). These experiments resulted in similar outcomes to the transgenic-mouse experiments above, with mice dying at midgestation from multiple hemangiomas that disrupted blood vessel formation from the yolk sac. Taking these endothelioma (End) cell lines from the chimeric embryos and injecting them into mice, rats, chicks, and quails produced hemangiomas within 10 to 18 h after injection, with 95% of the newly formed tumors originating from host cells (2, 358, 379). This study discovered the connection between MT and the induction of uPA and to a lesser extent the reduction of plasminogen activator inhibitors, namely PAI-1 (358). It was suggested that MT in endothelial cells acted as a single-step oncogene based on the rapidity of tumor formation, practically at the same time as the first appearance of endothelial cells during gestation. However, this ability seems to be masked and in contention with the latency period, typically 2 months in most reported studies, of tumor formation due to inoculation of wild-type virus into newborn mice. This dilemma could be resolved by the demonstration that the hemangiomas caused by injection of MT-transformed endothelial cells were the result of host cell recruitment and not, as originally expected, of the induction of hyperproliferation among the injected endothelial cells or the secretion of a growth-stimulating agent (379).
The first study to explore the age-specific expression of MT in transgenic mice (C57BL/6 × DBA2 hybrids) replaced the Py enhancer with the heavy-chain immunoglobulin E enhancer, which is known to be active only in adult tissues (279). This was done to bypass the premature death of mice due to the MT-induced hemangiomas, which may have squelched the possibility that MT induced other tumors at a later date. MT expression was found to induce tumors in other cell types besides vascular endothelia (liver hemangiomas in this case); they included tumors of the salivary, thyroid, and mammary glands and adenocarcinomas. This tumor profile was still a fraction of those found when susceptible newborn mice are inoculated with wild-type virus. Again, the cell-to-cell replication, including levels of permissivity and cytotoxicity, of the wild-type virus and the cooperation of the three early genes are likely explanations of this difference in tumor development (279). An interesting note in this study was that MT expression did not correspond to previously seen genes controlled by the immunoglobulin E enhancer but, rather, MT again appeared mainly in tumor tissue, as was seen when Py regulatory sequences were used.
Mechanistic Considerations
Although there is no definitive understanding of the reasons for the formation or rapidity of progression of hemangiomas in MT transgenic mice, a number of molecular mechanisms have been proposed. These are based on a number of histological and biochemical observations of MT-transformed endothelial cells and the resulting endotheliomas, including the requirement of a MT-src (src, fyn, or yes) complex, the upregulation of uPA and other alterations of the plasminogen activator system, the release of an endothelial cell derived motility factor, the expression of a number of inflammatory and chemotactic cytokines, and the upregulation of nitric oxide through induction of both endothelial cell NO synthase and inducible NOS (for a review, see reference 258). The best evidence that the signal transduction pathways induced by MT are involved in the formation of hemangiomas comes not from MT studies but from the recent finding that the chicken retrovirus ASV16 carrying an oncogenic form of the catalytic subunit (110-kDa subunit) of PI 3-kinase, v-p3k, induces hemangiosarcomas in chickens (53). Furthermore, the induction of the hemangiosarcomas in chickens was associated with an increase in the activity of Akt and the production of both PI(3,4)P2 and PIP3. Further evidence that either the Shc or PI 3-kinase pathways are involved in the formation of hemangiomas comes from studies identifying the association of MT with induction of uPA. The alterations to the plasminogen activator system were discovered through the use of End cells (MT-transformed endothelial cells) grown in a three-dimensional fibrin gel (231). The changes associated with MT expression included the formation of large cyst-like structures instead of capillary-like tubules and the production of large amounts of uPA and no detectable PAI. As mentioned above, higher expression levels of uPA are probably a result of the increased level of transcription factors, namely, fos, jun, and the ets family, by MT induced signal transduction, in particular the MT-Shc and MT–PI 3-kinase complexes. Since uPA is a serine protease, serine protease inhibitors could reverse the effect of MT and cause the End cells to revert to the formation of branching, capillary-like tubular structures (231). Therefore, MT deregulates the proteolytic balance of endothelial cells and favors increased proteolysis through the use of uPA, which probably degrades the fibrin matrix. This is just one example of how MT can induce hemangiomas and illustrates the complexity of determining the mechanism of action.
Mammary Tumors
The common occurrence of mammary adenocarcinomas due to Py infection in newborn mice led to studies of transgenic mice carrying MT under the control of the murine mammary tumor virus (MMTV) long terminal repeat (141). Tumors in these mice arose in the mammary gland, salivary gland, ovaries, epididymis, and, with highest frequency, the lungs (pulmonary metastases). The unusual aspect of these transgenic mice was the short latency period (less than 30 days), which indicated that MT induces mammary tumors without the need for a secondary event, a now prevalent feature of the hemangiomas of MT transgenic studies. This study also crossed the MMTV/MT transgenic mouse with a disrupted src gene mouse and found that rapid tumor progression no longer occurred, although after a very long latency period some abnormal hyperplasia of the mammary glands did occur (142). However, this hyperplasia was found to be due to the alternative use of MT-associated yes kinase. This finding was supported by another group, which showed similar levels of tyrosine phosphorylation of MT in mice disrupted in the src gene (340). Mice with a disrupted yes gene undergo similar mammary tumorigenesis to MMTV/MT mice, indicating that src is the more commonly used tyrosine kinase in vivo (142). Differing results for the other tumors were found, indicating that MT may utilize specific tyrosine kinases depending on the tissue type. These experiments indicate that there was some evolutionary discretion used in specifically selecting the src interaction, especially in the aspects of cell specificity that was established for acute and persistent replication.
The most extensive study of mammary tumorigenesis used transgenic FVB/N mice expressing various mutants of MT. Using either MT-Y250F or MT-Y315/322F, it was demonstrated that mutants retained the ability to cause mammary gland tumors with secondary tumors in the male reproductive tissues and salivary glands but displayed distinct histology. MT-Y315/322F resulted in high levels of apoptosis in mammary tissue, an important observation since this is the only in vivo proof of the antiapoptotic effects of MT. The most interesting observation, though, was the extensive mammary epithelial hyperplasia, even among the highly apoptotic tissue. In addition, the delayed onset of the mammary tumors illustrated that MT was essential but not sufficient to drive tumorigenesis. The Shc binding site was also established as being important from the observation that over one-third of the metastatic lesions had reversions of the mutant MT-Y250F to wild-type binding capabilities (370). Although this study was one of the first to indicate that downstream portions of both the Shc and PI 3-kinase pathways are important for Py-induced tumorigenesis, it still did not address whether these stimulated pathways, cell survival and proliferation, are important for virus replication or persistence.
A recent study with transgenic mice expressing MT used a truncated MT (only the first 304 of 421 aa) and the full-length ST (103). Instead of using the natural early promoter for Py, an enhancer from a mutant version of Py with a wide host cell range (NB11/1) was substituted (81, 185, 210). The transgene expression was widespread, but a wide variety of tumors or even the hemangiomas normally associated with MT-transgenic mice were not present; instead, the rare medullary thyroid carcinomas developed. This MT lacked the tyrosine phosphorylation sites for both the PI 3-kinase and PLC-γ1 as well as the membrane binding domain, yet it retained the ability to induce tumors, albeit a specific type of tumor rarely associated with the Py tumor profile. This type of experiment further illustrates how muddled and distant the current Py studies are from its natural biology.
CONCLUSIONS
“It is likely that neoplastic transformation of the host is an unintended consequence of the presence of middle T” (285)
The study of Py as a DNA tumor virus and the consistent focus on MT as its instigator of transformation has perpetuated the concept that this virus and its early proteins are designed specifically for tumorigenesis and that this is a natural (or nearly natural) part of the Py life cycle. However, this concept is misleading, as shown by numerous in vivo studies by others and by us. These tumors require a high titer of virus and unnatural sites of inoculations with tumor-derived Py strains into specific strains of tumor-prone inbred newborn mice born of immunologically naive mothers. None of these conditions occur naturally. These points have been nearly lost from the large segment of literature that seeks to narrowly understand the mechanism by which MT induces tumors. Also lost to this literature is an acknowledgment of the complete lack of polyomavirus-associated tumors of any mammalian (or avian) species in the wild. However, the biological reality of the Py life cycle suggests a much more intimate linkage of MT function to the specific circumstances present in newborn mouse lung, which are not likely to be understood in unrelated cell culture or in vitro models.
The initial conclusions made from our own in vivo results is that MT is an accessory factor required for Py to attain high-level virus production in differentiating newborn mouse lung and to subsequently establish persistent infections not only in individual mice but also in mouse populations (Fig. 3). The phased Py replication in the differentiating newborn mouse lung requires a fully functional MT. No other tissue in the mouse has displayed anywhere near this level of phased virus replication or sensitivity to MT function, which includes the adult mouse lung. Although such high-titer phased replication is not likely to occur in nature, it does identify a specific site and temporal window of high cellular susceptibility to the actions of MT. Through the complex and often redundant activation of signal transduction pathways, MT establishes a highly specific cellular environment that can rapidly and effectively replicate Py. It seems clear this is first required during the initial rounds of acute replication for the virus to set up a foothold in the mouse, and then exponential-phase replication is required for rapid (preimmune) dissemination in quantities large enough to “seed” the kidneys for persistence. Finally, the survival of the viral persistence as it passes from phases of latency to lytic replication while consistently maintaining the presence of the virus despite the immune response also requires MT. The activation of the MT-induced signal transduction pathways is likely to be important for the production of transcription factors needed in the unique epithelium of the differentiating newborn bronchioles, but it remains clear that these intricate circuits are not part of a virus life cycle that includes transformation. Figure 4 gives a summary of our in vivo studies showing the relationship between Py infection and the cell types in the mouse bronchioles. Our studies illustrate that only by studying this more natural setting will we understand the functions of MT and the other early proteins as well as the viral strategy of Py.
FIG. 4.
In vivo life cycle of polyomavirus in the mouse lung. A schematic of polyomavirus acute replication in the epithelial cells of the mouse bronchiole is shown. Ciliated cells and Clara cells (nonciliated epithelial cells) which make up a majority of the epithelium in mouse bronchioles, are indicated. In the more basal cells, T antigens (early-gene proteins) are expressed, often at very low levels. The cells then transition from a basal location to more apical position near the bronchiolar lumen. During this transition, capsid proteins (late-gene proteins) are expressed and the nuclei of the cells are found apically as well. The cytoplasm of the cells is squeezed from the basement membrane and then dislodged as the cells exfoliate into the bronchiolar lumen (unattached cell). The cells expressing viral proteins (early or late) do not express markers for either ciliated cells or Clara cells, indicating a new differential pathway.
The study of MT and of polyomavirus in general has been fundamentally important for the understanding of basic cellular processes and has identified many interactions between viral and cellular regulatory processes. These studies, however, now require a recognition of the natural biology of MT function if they are to accurately describe how MT affects cellular regulation.
ACKNOWLEDGMENTS
We thank Edward K. Wagner, Department of Molecular Biology and Biochemistry, and David A. Fruman, Department of Molecular Biology and Biochemistry, for their editorial assistance. We also thank Karin Christensen for rendering the figures.
This work was supported by the Irvine Center for Virus Research.
REFERENCES
- 1.Agostini H T, Yanagihara R, Davis V, Ryschkewitsch C F, Stoner G L. Asian genotypes of JC virus in Native Americans and in a Pacific Island population: markers of viral evolution and human migration. Proc Natl Acad Sci USA. 1997;94:14542–14546. doi: 10.1073/pnas.94.26.14542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguzzi A, Kleihues P, Heckl K, Wiestler O D. Cell type-specific tumor induction in neural transplants by retrovirus-mediated oncogene transfer. Oncogene. 1991;6:113–118. [PubMed] [Google Scholar]
- 3.Akimoto K, Nakaya M, Yamanaka T, Tanaka J, Matsuda S, Weng Q P, Avruch J, Ohno S. Atypical protein kinase Clambda binds and regulates p70 S6 kinase. Biochem J. 1998;335:417–424. doi: 10.1042/bj3350417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akimoto K, Takahashi R, Moriya S, Nishioka N, Takayanagi J, Kimura K, Fukui Y, Osada Si, Mizuno K, Hirai Si, Kazlauskas A, Ohno S. EGF or PDGF receptors activate atypical PKClambda through phosphatidylinositol 3-kinase. EMBO J. 1996;15:788–798. [PMC free article] [PubMed] [Google Scholar]
- 5.Alessi D R, Deak M, Casamayor A, Caudwell F B, Morrice N, Norman D G, Gaffney P, Reese C B, MacDougall C N, Harbison D, Ashworth A, Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- 6.Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 7.Allison A C. Tumour development following immunosuppression. Proc R Soc Med. 1970;63:1077–1080. doi: 10.1177/003591577006301055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allison A C, Monga J N, Hammond V. Increased susceptibility to virus oncogenesis of congenitally thymus-deprived nude mice. Nature. 1974;252:746–747. doi: 10.1038/252746a0. [DOI] [PubMed] [Google Scholar]
- 9.Amy R W, Bowes D, Burri P H, Haines J, Thurlbeck W M. Postnatal growth of the mouse lung. J Anat. 1977;124:131–151. [PMC free article] [PubMed] [Google Scholar]
- 10.Andrews D W, Gupta J, Abisdris G. Evidence that the middle T antigen of polyomavirus interacts with the membrane skeleton. Mol Cell Biol. 1993;13:4703–4713. doi: 10.1128/mcb.13.8.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asselin C, Gelinas C, Bastin M. Role of the three polyoma virus early proteins in tumorigenesis. Mol Cell Biol. 1983;3:1451–1459. doi: 10.1128/mcb.3.8.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asselin C, Gelinas C, Branton P E, Bastin M. Polyoma middle T antigen requires cooperation from another gene to express the malignant phenotype in vivo. Mol Cell Biol. 1984;4:755–760. doi: 10.1128/mcb.4.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atencio I A, Shadan F F, Zhou X J, Vaziri N D, Villarreal L P. Adult mouse kidneys become permissive to acute polyomavirus infection and reactivate persistent infections in response to cellular damage and regeneration. J Virol. 1993;67:1424–1432. doi: 10.1128/jvi.67.3.1424-1432.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atencio I A, Villarreal L P. Polyomavirus replicates in differentiating but not in proliferating tubules of adult mouse polycystic kidneys. Virology. 1994;201:26–35. doi: 10.1006/viro.1994.1262. [DOI] [PubMed] [Google Scholar]
- 15.Auger K R, Serunian L A, Soltoff S P, Libby P, Cantley L C. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989;57:167–175. doi: 10.1016/0092-8674(89)90182-7. [DOI] [PubMed] [Google Scholar]
- 16.Backer J M. Phosphoinositide 3-kinases and the regulation of vesicular trafficking. Mol Cell Biol Res Commun. 2000;3:193–204. doi: 10.1006/mcbr.2000.0202. [DOI] [PubMed] [Google Scholar]
- 17.Barry M, McFadden G. Apoptosis regulators from DNA viruses. Curr Opin Immunol. 1998;10:422–430. doi: 10.1016/s0952-7915(98)80116-7. [DOI] [PubMed] [Google Scholar]
- 18.Baru M, Manor H. Induction of polyomavirus DNA replication by cyclic AMP and a tumor promoter. Intervirology. 1988;29:328–333. doi: 10.1159/000150063. [DOI] [PubMed] [Google Scholar]
- 19.Bauer P H, Bronson R T, Fung S C, Freund R, Stehle T, Harrison S C, Benjamin T L. Genetic and structural analysis of a virulence determinant in polyomavirus VP1. J Virol. 1995;69:7925–7931. doi: 10.1128/jvi.69.12.7925-7931.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer P H, Cui C, Stehle T, Harrison S C, DeCaprio J A, Benjamin T L. Discrimination between sialic acid-containing receptors and pseudoreceptors regulates polyomavirus spread in the mouse. J Virol. 1999;73:5826–5832. doi: 10.1128/jvi.73.7.5826-5832.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bautch V L, Toda S, Hassell J A, Hanahan D. Endothelial cell tumors develop in transgenic mice carrying polyoma virus middle T oncogene. Cell. 1987;51:529–537. doi: 10.1016/0092-8674(87)90122-x. [DOI] [PubMed] [Google Scholar]
- 22.Bautch V L, Toda S, Hassell J A, Hanahan D. Tissue specificity of oncogene action: endothelial cell tumours in polyoma middle T transgenic mice. IARC Sci Publ. 1989;1989:255–266. [PubMed] [Google Scholar]
- 23.Bellacosa A, Chan T O, Ahmed N N, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17:313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 24.Benjamin T. Host range mutants of polyoma virus. Proc Natl Acad Sci USA. 1970;67:394–399. doi: 10.1073/pnas.67.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett E R, Naujokas M, Hassell J A. Requirements for species-specific papovavirus DNA replication. J Virol. 1989;63:5371–5385. doi: 10.1128/jvi.63.12.5371-5385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berebbi M, Cajean-Feroldi C, Apiou F, Couturier J, Garcette M, Emanoil-Ravier R, Cabannes J, Perricaudet M, Blangy D. Integration of viral sequences into the c-myc gene in two mammary adenocarcinomas induced by polyomavirus in athymic nude mice. J Virol. 1995;69:5935–5945. doi: 10.1128/jvi.69.10.5935-5945.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergqvist A, Soderbarg K, Magnusson G. Altered susceptibility to tumor necrosis factor alpha-induced apoptosis of mouse cells expressing polyomavirus middle and small T antigens. J Virol. 1997;71:276–283. doi: 10.1128/jvi.71.1.276-283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berke Z, Dalianis T, Feinstein R, Sandstedt K, Evengard B. Persistence of polyomavirus in adult SCID C.B-17 mice. In Vivo. 1994;8:339–342. [PubMed] [Google Scholar]
- 29.Berke Z, Wen T, Klein G, Dalianis T. Polyoma tumor development in neonatally polyoma-virus-infected CD4−/− and CD8−/− single knockout and CD4−/−8−/− double knockout mice. Int J Cancer. 1996;67:405–408. doi: 10.1002/(SICI)1097-0215(19960729)67:3<405::AID-IJC15>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 30.Besser D, Urich M, Sakaue M, Messerschmitt A, Balimer-Hofer K, Nagamine Y. Urokinase-type plasminogen activator gene regulation by polyomavirus middle-T antigen. Oncogene. 1995;11:2383–2391. [PubMed] [Google Scholar]
- 31.Bienz-Tadmor B, Zakut-Houri R, Libresco S, Givol D, Oren M. The 5′ region of the p53 gene: evolutionary conservation and evidence for a negative regulatory element. EMBO J. 1985;4:3209–3213. doi: 10.1002/j.1460-2075.1985.tb04067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjorge J D, Chan T O, Antczak M, Kung H J, Fujita D J. Activated type 1 phosphatidylinositol kinase is associated with the epidermal growth factor (EGF) receptor following EGF stimulation. Proc Natl Acad Sci USA. 1990;87:3816–3820. doi: 10.1073/pnas.87.10.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blaikie P A, Fournier E, Dilworth S M, Birnbaum D, Borg J P, Margolis B. The role of the Shc phosphotyrosine interaction/phosphotyrosine binding domain and tyrosine phosphorylation sites in polyoma middle T antigen-mediated cell transformation. J Biol Chem. 1997;272:20671–20677. doi: 10.1074/jbc.272.33.20671. [DOI] [PubMed] [Google Scholar]
- 34.Bolen J B, DeSeau V, OShaughnessy J, Amini S. Analysis of middle tumor antigen and pp60c-src interactions in polyomavirus-transformed rat cells. J Virol. 1987;61:3299–3305. doi: 10.1128/jvi.61.10.3299-3305.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolen J B, Israel M A. In vitro association and phosphorylation of polyoma virus middle T antigen by cellular tyrosyl kinase activity. J Biol Chem. 1984;259:11686–11694. [PubMed] [Google Scholar]
- 36.Bolen J B, Thiele C J, Israel M A, Yonemoto W, Lipsich L A, Brugge J S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984;38:767–777. doi: 10.1016/0092-8674(84)90272-1. [DOI] [PubMed] [Google Scholar]
- 37.Braithwaite A W, Sturzbecher H W, Addison C, Palmer C, Rudge K, Jenkins J R. Mouse p53 inhibits SV40 origin-dependent DNA replication. Nature. 1987;329:458–460. doi: 10.1038/329458a0. [DOI] [PubMed] [Google Scholar]
- 38.Brewster C E, Glover H R, Dilworth S M. pp60c-src binding to polyomavirus middle T-antigen (MT) requires residues 185 to 210 of the MT sequence. J Virol. 1997;71:5512–5520. doi: 10.1128/jvi.71.7.5512-5520.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brizuela L, Ulug E T, Jones M A, Courtneidge S A. Induction of interleukin-2 transcription by the hamster polyomavirus middle T antigen: a role for Fyn in T cell signal transduction. Eur J Immunol. 1995;25:385–393. doi: 10.1002/eji.1830250212. [DOI] [PubMed] [Google Scholar]
- 40.Bronson R, Dawe C, Carroll J, Benjamin T. Tumor induction by a transformation-defective polyoma virus mutant blocked in signaling through Shc. Proc Natl Acad Sci USA. 1997;94:7954–7958. doi: 10.1073/pnas.94.15.7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broome M A, Courtneidge S A. No requirement for src family kinases for PDGF signaling in fibroblasts expressing SV40 large T antigen. Oncogene. 2000;19:2867–2869. doi: 10.1038/sj.onc.1203608. [DOI] [PubMed] [Google Scholar]
- 42.Brown M T, Cooper J A. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–149. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 43.Campbell K S, Auger K R, Hemmings B A, Roberts T M, Pallas D C. Identification of regions in polyomavirus middle T and small t antigens important for association with protein phosphatase 2A. J Virol. 1995;69:3721–3728. doi: 10.1128/jvi.69.6.3721-3728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell K S, Ogris E, Burke B, Su W, Auger K R, Druker B J, Schaffhausen B S, Roberts T M, Pallas D C. Polyoma middle tumor antigen interacts with SHC protein via the NPTY (Asn-Pro-Thr-Tyr) motif in middle tumor antigen. Proc Natl Acad Sci USA. 1994;91:6344–6348. doi: 10.1073/pnas.91.14.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell S L, Khosravi-Far R, Rossman K L, Clark G J, Der C J. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- 46.Carmichael G, Schaffhausen B S, Mandel G, Liang T J, Benjamin T L. Transformation by polyoma virus is drastically reduced by substitution of phenylalanine for tyrosine at residue 315 of middle-sized tumor antigen. Proc Natl Acad Sci USA. 1984;81:679–683. doi: 10.1073/pnas.81.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carmichael G G, Schaffhausen B S, Dorsky D I, Oliver D B, Benjamin T L. Carboxy terminus of polyoma middle-sized tumor antigen is required for attachment to membranes, associated protein kinase activities, and cell transformation. Proc Natl Acad Sci USA. 1982;79:3579–3583. doi: 10.1073/pnas.79.11.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carpenter C L, Cantley L C. Phosphoinositide 3-kinase and the regulation of cell growth. Biochim Biophys Acta. 1996;1288:M11–M16. doi: 10.1016/0304-419x(96)00018-2. [DOI] [PubMed] [Google Scholar]
- 49.Carroll J P, Fung J S, Bronson R T, Razvi E, Benjamin T L. Radiation-resistant and radiation-sensitive forms of host resistance to polyomavirus. J Virol. 1999;73:1213–1218. doi: 10.1128/jvi.73.2.1213-1218.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cartwright C A, Kaplan P L, Cooper J A, Hunter T, Eckhart W. Altered sites of tyrosine phosphorylation in pp60c-src associated with polyomavirus middle tumor antigen. Mol Cell Biol. 1986;6:1562–1570. doi: 10.1128/mcb.6.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caruso M, Iacobini C, Passananti C, Felsani A, Amati P. Protein recognition sites in polyomavirus enhancer: formation of a novel site for NF-1 factor in an enhancer mutant and characterization of a site in the enhancer D domain. EMBO J. 1990;9:947–955. doi: 10.1002/j.1460-2075.1990.tb08193.x. . (Erratum, 9:3806.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cayla X, Ballmer-Hofer K, Merlevede W, Goris J. Phosphatase 2A associated with polyomavirus small-T or middle-T antigen is an okadaic acid-sensitive tyrosyl phosphatase. Eur J Biochem. 1993;214:281–286. doi: 10.1111/j.1432-1033.1993.tb17922.x. [DOI] [PubMed] [Google Scholar]
- 53.Chang H W, Aoki M, Fruman D, Auger K R, Bellacosa A, Tsichlis P N, Cantley L C, Roberts T M, Vogt P K. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 54.Chen M C, Redenius D, Osati-Ashtiani F, Fluck M M. Enhancer-mediated role for polyomavirus middle T/small T in DNA replication. J Virol. 1995;69:326–333. doi: 10.1128/jvi.69.1.326-333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng S H, Espino P C, Marshall J, Harvey R, Smith A E. Stoichiometry of cellular and viral components in the polyomavirus middle-T antigen-tyrosine kinase complex. Mol Cell Biol. 1990;10:5569–5574. doi: 10.1128/mcb.10.10.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng S H, Harvey R, Espino P C, Semba K, Yamamoto T, Toyoshima K, Smith A E. Peptide antibodies to the human c-fyn gene product demonstrate pp59c-fyn is capable of complex formation with the middle-T antigen of polyomavirus. EMBO J. 1988;7:3845–3855. doi: 10.1002/j.1460-2075.1988.tb03270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng S H, Markland W, Markham A F, Smith A E. Mutations around the NG59 lesion indicate an active association of polyoma virus middle-T antigen with pp60c-src is required for cell transformation. EMBO J. 1986;5:325–334. doi: 10.1002/j.1460-2075.1986.tb04216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng S H, Piwnica Worms H, Harvey R W, Roberts T M, Smith A E. The carboxy terminus of pp60c-src is a regulatory domain and is involved in complex formation with the middle-T antigen of polyomavirus. Mol Cell Biol. 1988;8:1736–1747. doi: 10.1128/mcb.8.4.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chow L M, Ratcliffe M J, Veillette A. tkl is the avian homolog of the mammalian lck tyrosine protein kinase gene. Mol Cell Biol. 1992;12:1226–1233. doi: 10.1128/mcb.12.3.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Connolly J O, Soga N, Guo X L, Alvarez U, Hruska K A. Rac is essential in the transformation of endothelial cells by polyoma middle T. Cell Adhes Commun. 2000;7:409–422. doi: 10.3109/15419060009109022. [DOI] [PubMed] [Google Scholar]
- 61.Cooke F T, Dove S K, McEwen R K, Painter G, Holmes A B, Hall M N, Michell R H, Parker P J. The stress-activated phosphatidylinositol 3-phosphate 5-kinase Fab1p is essential for vacuole function in S. cerevisiae. Curr Biol. 1998;8:1219–1222. doi: 10.1016/s0960-9822(07)00513-1. [DOI] [PubMed] [Google Scholar]
- 62.Coso O A, Chiariello M, Yu J C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 63.Courtneidge S A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985;4:1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Courtneidge S A, Goutebroze L, Cartwright A, Heber A, Scherneck S, Feunteun J. Identification and characterization of the hamster polyomavirus middle T antigen. J Virol. 1991;65:3301–3308. doi: 10.1128/jvi.65.6.3301-3308.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Courtneidge S A, Heber A. An 81 kd protein complexed with middle T antigen and pp60c-src: a possible phosphatidylinositol kinase. Cell. 1987;50:1031–1037. doi: 10.1016/0092-8674(87)90169-3. [DOI] [PubMed] [Google Scholar]
- 66.Courtneidge S A, Read M, Wilson J B, Fried M. Cytoplasmic interaction between pp60c-src and a truncated polyoma virus middle T antigen. Oncogene Res. 1989;4:75–80. [PubMed] [Google Scholar]
- 67.Courtneidge S A, Smith A E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983;303:435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- 68.Courtneidge S A, Smith A E. The complex of polyoma virus middle-T antigen and pp60c-src. EMBO J. 1984;3:585–591. doi: 10.1002/j.1460-2075.1984.tb01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crocker T T, Teeter A, Nielsen B. Postnatal cellular proliferation in mouse and hamster lung. Cancer Res. 1970;30:357–361. [PubMed] [Google Scholar]
- 70.Cullere X, Rose P, Thathamangalam U, Chatterjee A, Mullane K P, Pallas D C, Benjamin T L, Roberts T M, Schaffhausen B S. Serine 257 phosphorylation regulates association of polyomavirus middle T antigen with 14-3-3 proteins. J Virol. 1998;72:558–563. doi: 10.1128/jvi.72.1.558-563.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Curran T, Franza B R J. Fos and Jun: the AP-1 connection. Cell. 1988;55:395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- 72.Cuzin F. The polyoma virus oncogenes. Coordinated functions of three distinct proteins in the transformation of rodent cells in culture. Biochim Biophys Acta. 1984;781:193–204. doi: 10.1016/0167-4781(84)90084-8. [DOI] [PubMed] [Google Scholar]
- 73.Dahl J, Freund R, Blenis J, Benjamin T L. Studies of partially transforming polyomavirus mutants establish a role for phosphatidylinositol 3-kinase in activation of pp70 S6 kinase. Mol Cell Biol. 1996;16:2728–2735. doi: 10.1128/mcb.16.6.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dahl J, Jurczak A, Cheng L A, Baker D C, Benjamin T L. Evidence of a role for phosphatidylinositol 3-kinase activation in the blocking of apoptosis by polyomavirus middle T antigen. J Virol. 1998;72:3221–3226. doi: 10.1128/jvi.72.4.3221-3226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dahl J, Thathamangalam U, Freund R, Benjamin T L. Functional asymmetry of the regions juxtaposed to the membrane-binding sequence of polyomavirus middle T antigen. Mol Cell Biol. 1992;12:5050–5058. doi: 10.1128/mcb.12.11.5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dawe C J, Freund R, Barncastle J P, Dubensky T W, Mandel G, Benjamin T L. Necrotizing arterial lesions in mice-bearing tumors induced by polyoma virus. J Exp Pathol. 1987;3:177–201. [PubMed] [Google Scholar]
- 77.Dawe C J, Freund R, Mandel G, Ballmer Hofer K, Talmage D A, Benjamin T L. Variations in polyoma virus genotype in relation to tumor induction in mice. Characterization of wild type strains with widely differing tumor profiles. Am J Pathol. 1987;127:243–261. [PMC free article] [PubMed] [Google Scholar]
- 78.Deb S, Jackson C T, Subler M A, Martin D W. Modulation of cellular and viral promoters by mutant human p53 proteins found in tumor cells. J Virol. 1992;66:6164–6170. doi: 10.1128/jvi.66.10.6164-6170.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Defendi V, Lehman J M. The nature of the hemorrhagic lesions induced by polyoma virus in hamsters. Cancer Res. 1964;24:329–334. [PubMed] [Google Scholar]
- 80.de Groot R P, Auwerx J, Bourouis M, Sassone-Corsi P. Negative regulation of Jun/AP-1: conserved function of glycogen synthase kinase 3 and the Drosophila kinase shaggy. Oncogene. 1993;8:841–847. [PubMed] [Google Scholar]
- 81.De Simone V, Amati P. Replicative cis-advantage of polyomavirus regulatory region mutants in different murine cell lines. J Virol. 1987;61:1615–1620. doi: 10.1128/jvi.61.5.1615-1620.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Villiers J, Schaffner W, Tyndall C, Lupton S, Kamen R. Polyoma virus DNA replication requires an enhancer. Nature. 1984;312:242–246. doi: 10.1038/312242a0. [DOI] [PubMed] [Google Scholar]
- 83.Dey D C, Bronson R P, Dahl J, Carroll J P, Benjamin T L. Accelerated development of polyoma tumors and embryonic lethality: different effects of p53 loss on related mouse backgrounds. Cell Growth Differ. 2000;11:231–237. [PubMed] [Google Scholar]
- 84.Dhand R, Hiles I, Panayotou G, Roche S, Fry M J, Gout I, Totty N F, Truong O, Vicendo P, Yonezawa K. PI 3-kinase is a dual specificity enzyme: autoregulation by an intrinsic protein-serine kinase activity. EMBO J. 1994;13:522–533. doi: 10.1002/j.1460-2075.1994.tb06290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dilworth S M. Protein kinase activities associated with distinct antigenic forms of polyoma virus middle T-antigen. EMBO J. 1982;1:1319–1328. doi: 10.1002/j.1460-2075.1982.tb01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dilworth S M, Brewster C E, Jones M D, Lanfrancone L, Pelicci G, Pelicci P G. Transformation by polyoma virus middle T-antigen involves the binding and tyrosine phosphorylation of Shc. Nature. 1994;367:87–90. doi: 10.1038/367087a0. [DOI] [PubMed] [Google Scholar]
- 87.Dilworth S M, Hansson H A, Darnfors C, Bjursell G, Streuli C H, Griffin B E. Subcellular localisation of the middle and large T-antigens of polyoma virus. EMBO J. 1986;5:491–499. doi: 10.1002/j.1460-2075.1986.tb04238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Doherty J, Freund R. Polyomavirus large T antigen overcomes p53 dependent growth arrest. Oncogene. 1997;14:1923–1931. doi: 10.1038/sj.onc.1201025. [DOI] [PubMed] [Google Scholar]
- 89.Doherty J, Freund R. Middle T antigen activation of signal transduction pathways does not overcome p53-mediated growth arrest. J Virol. 1999;73:7882–7885. doi: 10.1128/jvi.73.9.7882-7885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dong L Q, Zhang R B, Langlais P, He H, Clark M, Zhu L, Liu F. Primary structure, tissue distribution, and expression of mouse phosphoinositide-dependent protein kinase-1, a protein kinase that phosphorylates and activates protein kinase Czeta. J Biol Chem. 1999;274:8117–8122. doi: 10.1074/jbc.274.12.8117. [DOI] [PubMed] [Google Scholar]
- 91.Dotto G P, Parada L F, Weinberg R A. Specific growth response of ras-transformed embryo fibroblasts to tumour promoters. Nature. 1985;318:472–475. doi: 10.1038/318472a0. [DOI] [PubMed] [Google Scholar]
- 92.Drake D R, Knoepp L, Lukacher A E, Actor J K. Patterns of expression of viral and cytokine gene transcripts during mouse polyoma virus infection. Comb Chem High Throughput Screen. 2000;3:329–341. doi: 10.2174/1386207003331562. [DOI] [PubMed] [Google Scholar]
- 93.Druker B J, Ling L E, Cohen B, Roberts T M, Schaffhausen B S. A completely transformation-defective point mutant of polyomavirus middle T antigen which retains full associated phosphatidylinositol kinase activity. J Virol. 1990;64:4454–4461. doi: 10.1128/jvi.64.9.4454-4461.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dubensky T W, Freund R, Dawe C J, Benjamin T L. Polyomavirus replication in mice: influences of VP1 type and route of inoculation. J Virol. 1991;65:342–349. doi: 10.1128/jvi.65.1.342-349.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dufner A, Thomas G. Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res. 1999;253:100–109. doi: 10.1006/excr.1999.4683. [DOI] [PubMed] [Google Scholar]
- 96.Dulbecco R, Hartwell L H, Vogt M. Induction of cellular DNA synthesis by polyoma virus. Proc Natl Acad Sci USA. 1965;53:403–410. doi: 10.1073/pnas.53.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dunant N, Ballmer-Hofer K. Signalling by Src family kinases: lessons learnt from DNA tumour viruses. Cell Signal. 1997;9:385–393. doi: 10.1016/s0898-6568(97)00040-5. [DOI] [PubMed] [Google Scholar]
- 98.Dunant N M, Senften M, Ballmer-Hofer K. Polyomavirus middle-T antigen associates with the kinase domain of Src-related tyrosine kinases. J Virol. 1996;70:1323–1330. doi: 10.1128/jvi.70.3.1323-1330.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Durkin J P, Whitfield J F. Characterization of the mitogenic signal from an oncogene ras protein. Anticancer Res. 1989;9:1313–1323. [PubMed] [Google Scholar]
- 100.Dymecki S M, Niederhuber J E, Desiderio S V. Specific expression of a tyrosine kinase gene, blk, in B lymphoid cells. Science. 1990;247:332–336. doi: 10.1126/science.2404338. [DOI] [PubMed] [Google Scholar]
- 101.Eddy B E. Viral infectivity or oncogenicity due to viruses in hamsters. Prog Exp Tumor Res. 1972;16:454–496. doi: 10.1159/000393385. [DOI] [PubMed] [Google Scholar]
- 102.Eddy B E. Polyomavirus. In: Foster H L, Small D J, Fox J G, editors. The mouse in biomedical research. New York, N.Y: Academic Press, Inc.; 1982. pp. 293–311. [Google Scholar]
- 103.Felici A, Giorgio M, Krauzewicz N, Della Rocca C, Santoro M, Rovere P, Manni I, Amati P, Pozzi L. Medullary thyroid carcinomas in transgenic mice expressing a polyoma carboxyl-terminal truncated middle-T and wild type small-T antigens. Oncogene. 1999;18:2387–2395. doi: 10.1038/sj.onc.1202578. [DOI] [PubMed] [Google Scholar]
- 104.Filippa N, Sable C L, Filloux C, Hemmings B, Van Obberghen E. Mechanism of protein kinase B activation by cyclic AMP-dependent protein kinase. Mol Cell Biol. 1999;19:4989–5000. doi: 10.1128/mcb.19.7.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Franke T F, Kaplan D R, Cantley L C, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 106.Frech M, Andjelkovic M, Ingley E, Reddy K K, Falck J R, Hemmings B A. High affinity binding of inositol phosphates and phosphoinositides to the pleckstrin homology domain of RAC/protein kinase B and their influence on kinase activity. J Biol Chem. 1997;272:8474–8481. doi: 10.1074/jbc.272.13.8474. [DOI] [PubMed] [Google Scholar]
- 107.Freund R, Bauer P H, Crissman H A, Bradbury E M, Benjamin T L. Host range and cell cycle activation properties of polyomavirus large T-antigen mutants defective in pRB binding. J Virol. 1994;68:7227–7234. doi: 10.1128/jvi.68.11.7227-7234.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Freund R, Calderone A, Dawe C J, Benjamin T L. Polyomavirus tumor induction in mice: effects of polymorphisms of VP1 and large T antigen. J Virol. 1991;65:335–341. doi: 10.1128/jvi.65.1.335-341.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Freund R, Dawe C J, Benjamin T L. The middle T proteins of high and low tumor strains of polyomavirus function equivalently in tumor induction. Virology. 1988;167:657–659. [PubMed] [Google Scholar]
- 110.Freund R, Dubensky T, Bronson R, Sotnikov A, Carroll J, Benjamin T. Polyoma tumorigenesis in mice: evidence for dominant resistance and dominant susceptibility genes of the host. Virology. 1992;191:724–731. doi: 10.1016/0042-6822(92)90248-n. [DOI] [PubMed] [Google Scholar]
- 111.Freund R, Garcea R L, Sahli R, Benjamin T L. A single-amino-acid substitution in polyomavirus VP1 correlates with plaque size and hemagglutination behavior. J Virol. 1991;65:350–355. doi: 10.1128/jvi.65.1.350-355.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Freund R, Mandel G, Carmichael G G, Barncastle J P, Dawe C J, Benjamin T L. Polyomavirus tumor induction in mice: influences of viral coding and noncoding sequences on tumor profiles. J Virol. 1987;61:2232–2239. doi: 10.1128/jvi.61.7.2232-2239.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Freund R, Sotnikov A, Bronson R T, Benjamin T L. Polyoma virus middle T is essential for virus replication and persistence as well as for tumor induction in mice. Virology. 1992;191:716–723. doi: 10.1016/0042-6822(92)90247-m. [DOI] [PubMed] [Google Scholar]
- 114.Freund R, Sotnikov A, Bronson R T, Benjamin T L. Polyoma virus middle T is essential for virus replication and persistence as well as for tumor induction in mice. Virology. 1992;191:716–723. doi: 10.1016/0042-6822(92)90247-m. [DOI] [PubMed] [Google Scholar]
- 115.Fujimura F, Deininger P, Friedmann T, Linney E. Mutation near the polyoma DNA replication origin permits productive infection of F9 embryonal carcinoma cells. Cell. 1981;23:809–814. doi: 10.1016/0092-8674(81)90445-1. [DOI] [PubMed] [Google Scholar]
- 116.Fujimura F K, Linney E. Polyoma mutants that productively infect F9 embryonal carcinoma cells do not rescue wild-type polyoma in F9 cells. Proc Natl Acad Sci USA. 1982;79:1479–1483. doi: 10.1073/pnas.79.5.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fukui Y, Hanafusa H. Phosphatidylinositol kinase activity associates with viral p60src protein. Mol Cell Biol. 1989;9:1651–1658. doi: 10.1128/mcb.9.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fukui Y, Kornbluth S, Jong S M, Wang L H, Hanafusa H. Phosphatidylinositol kinase type I activity associates with various oncogene products. Oncogene Res. 1989;4:283–292. [PubMed] [Google Scholar]
- 119.Furukawa K, Yamaguchi Y, Ogawa E, Shigesada K, Satake M, Ito Y. A ubiquitous repressor interacting with an F9 cell-specific silencer and its functional suppression by differentiated cell-specific positive factors. Cell Growth Differ. 1990;1:135–147. [PubMed] [Google Scholar]
- 120.Fusco A, Portella G, Grieco M, Tajana G, Di Minno G, Polli N, Pinto A. A retrovirus carrying the polyomavirus middle T gene induces acute thrombocythemic myeloproliferative disease in mice. J Virol. 1988;62:361–365. doi: 10.1128/jvi.62.1.361-365.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gannon J V, Lane D P. p53 and DNA polymerase alpha compete for binding to SV40 T antigen. Nature. 1987;329:456–458. doi: 10.1038/329456a0. [DOI] [PubMed] [Google Scholar]
- 122.Garcea R L, Talmage D A, Harmatz A, Freund R, Benjamin T L. Separation of host range from transformation functions of the hr-t gene of polyomavirus. Virology. 1989;168:312–319. doi: 10.1016/0042-6822(89)90271-7. [DOI] [PubMed] [Google Scholar]
- 123.Garcia M I, Perez M, Caruso M, Sthandier O, Ferreira R, Cermola M, Macchia C, Amati P. A mutation in the DE loop of the VP1 protein that prevents polyomavirus transcription and replication. Virology. 2000;272:293–301. doi: 10.1006/viro.2000.0351. [DOI] [PubMed] [Google Scholar]
- 124.Gardner M B, Henderson B E, Menck H, Parker J, Estes J D, Huebner R. Spontaneous tumors and C-type virus in polyoma-infected aging wild house mice. J Natl Cancer Inst. 1974;52:979–981. doi: 10.1093/jnci/52.3.979. [DOI] [PubMed] [Google Scholar]
- 125.Gary J D, Wurmser A E, Bonangelino C J, Weisman L S, Emr S D. Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J Cell Biol. 1998;143:65–79. doi: 10.1083/jcb.143.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gille H, Kortenjann M, Thomae O, Moomaw C, Slaughter C, Cobb M H, Shaw P E. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 1995;14:951–962. doi: 10.1002/j.1460-2075.1995.tb07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Glenn G M, Eckhart W. Transcriptional regulation of early-response genes during polyomavirus infection. J Virol. 1990;64:2193–2201. doi: 10.1128/jvi.64.5.2193-2201.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Glenn G M, Eckhart W. Mutation of a cysteine residue in polyomavirus middle T antigen abolishes interactions with protein phosphatase 2A, pp60c-src, and phosphatidylinositol-3 kinase, activation of c-fos expression, and cellular transformation. J Virol. 1993;67:1945–1952. doi: 10.1128/jvi.67.4.1945-1952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Glenn G M, Eckhart W. Amino-terminal regions of polyomavirus middle T antigen are required for interactions with protein phosphatase 2A. J Virol. 1995;69:3729–3736. doi: 10.1128/jvi.69.6.3729-3736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gorga F R, Riney C E, Benjamin T L. Inositol trisphosphate levels in cells expressing wild-type and mutant polyomavirus middle T antigens: evidence for activation of phospholipase C via activation of pp60c-src. J Virol. 1990;64:105–112. doi: 10.1128/jvi.64.1.105-112.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gottlieb K, Villarreal L P. The distribution and kinetics of polyomavirus in lungs of intranasally infected newborn mice. Virology. 2000;266:52–65. doi: 10.1006/viro.1999.0030. [DOI] [PubMed] [Google Scholar]
- 132.Gottlieb K, Villarreal L P. The differentiating epithelial cells of the bronchiolar epithelium eliminate virally infected cells from the mouse lung by hyperplasia and sloughing. J Clin Virol. 2000;18:28. [Google Scholar]
- 133.Graffi A, Bender E, Schramm T, Graffi I, Bierwolf D. Studies on the hamster papilloma and the hamster virus lymphoma. Bibl Haematol. 1970;1970:293–303. doi: 10.1159/000391720. [DOI] [PubMed] [Google Scholar]
- 134.Graffi A, Schramm T, Bender E, Bierwolf D, Graffi I. Uber einen neuen virushaltigen Hauttumor beim Goldhamster. Arch Geschwulstforsch. 1967;30:277–283. [PubMed] [Google Scholar]
- 135.Green H, Goldberg B, Todaro G J. Differentiated cell types and the regulation of collagen synthesis. Nature. 1966;212:631–633. doi: 10.1038/212631b0. [DOI] [PubMed] [Google Scholar]
- 136.Griffin B E, Ito Y, Novak U, Spurr N, Dilworth S, Smolar N, Pollack R, Smith K, Rifkin D B. Early mutants of polyoma virus (dl8 and dl23) with altered transformation properties: is polyoma virus middle T antigen a transforming gene product? Cold Spring Harbor Symp Quant Biol. 1980;44:271–283. doi: 10.1101/sqb.1980.044.01.031. [DOI] [PubMed] [Google Scholar]
- 137.Grussenmeyer T, Carbone Wiley A, Scheidtmann K H, Walter G. Interactions between polyomavirus medium T antigen and three cellular proteins of 88, 61, and 37 kilodaltons. J Virol. 1987;61:3902–3909. doi: 10.1128/jvi.61.12.3902-3909.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Grussenmeyer T, Scheidtmann K H, Hutchinson M A, Eckhart W, Walter G. Complexes of polyoma virus medium T antigen and cellular proteins. Proc Natl Acad Sci USA. 1985;82:7952–7954. doi: 10.1073/pnas.82.23.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guo J, Sugimoto C, Kitamura T, Ebihara H, Kato A, Guo Z, Liu J, Zheng S P, Wang Y L, Na Y Q, Suzuki M, Taguchi F, Yogo Y. Four geographically distinct genotypes of JC virus are prevalent in China and Mongolia: implications for the racial composition of modern China. J Gen Virol. 1998;79:2499–2505. doi: 10.1099/0022-1317-79-10-2499. [DOI] [PubMed] [Google Scholar]
- 140.Guo W, Tang W J, Bu X, Bermudez V, Martin M, Folk W R. AP1 enhances polyomavirus DNA replication by promoting T-antigen-mediated unwinding of DNA. J Virol. 1996;70:4914–4918. doi: 10.1128/jvi.70.8.4914-4918.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guy C T, Cardiff R D, Muller W J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guy C T, Muthuswamy S K, Cardiff R D, Soriano P, Muller W J. Activation of the c-Src tyrosine kinase is required for the induction of mammary tumors in transgenic mice. Genes Dev. 1994;8:23–32. doi: 10.1101/gad.8.1.23. [DOI] [PubMed] [Google Scholar]
- 143.Harvey R, Oostra B A, Belsham G J, Gillett P, Smith A E. An antibody to a synthetic peptide recognizes polyomavirus middle-T antigen and reveals multiple in vitro tyrosine phosphorylation sites. Mol Cell Biol. 1984;4:1334–1342. doi: 10.1128/mcb.4.7.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Haslam S Z, Wirth J J, Counterman L J, Fluck M M. Characterization of the mammary hyperplasia, dysplasia and neoplasia induced in athymic female adult mice by polyomavirus. Oncogene. 1992;7:1295–1303. [PubMed] [Google Scholar]
- 145.Hawkins P T, Eguinoa A, Qiu R G, Stokoe D, Cooke F T, Walters R, Wennstrom S, Claesson-Welsh L, Evans T, Symons M. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- 146.Hay N, Takimoto M, Bishop J M. A FOS protein is present in a complex that binds a negative regulator of MYC. Genes Dev. 1989;3:293–303. doi: 10.1101/gad.3.3.293. [DOI] [PubMed] [Google Scholar]
- 147.Herbomel P, Bourachot B, Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984;39:653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- 148.Herrlich P, Ponta H. ‘Nuclear’ oncogenes convert extracellular stimuli into changes in the genetic program. Trends Genet. 1989;5:112–115. doi: 10.1016/0168-9525(89)90041-3. [DOI] [PubMed] [Google Scholar]
- 149.Hill C S, Treisman R. Differential activation of c-fos promoter elements by serum, lysophosphatidic acid, G proteins and polypeptide growth factors. EMBO J. 1995;14:5037–5047. doi: 10.1002/j.1460-2075.1995.tb00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hill C S, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 151.Hinchliffe K, Irvine R. Inositol lipid pathways turn turtle. Nature. 1997;390:123–124. doi: 10.1038/36458. [DOI] [PubMed] [Google Scholar]
- 152.Horak I D, Kawakami T, Gregory F, Robbins K C, Bolen J B. Association of p60fyn with middle tumor antigen in murine polyomavirus-transformed rat cells. J Virol. 1989;63:2343–2347. doi: 10.1128/jvi.63.5.2343-2347.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hu Q, Klippel A, Muslin A J, Fantl W J, Williams L T. Ras-dependent induction of cellular responses by constitutively active phosphatidylinositol-3 kinase. Science. 1995;268:100–102. doi: 10.1126/science.7701328. [DOI] [PubMed] [Google Scholar]
- 154.Huebner R J, Rowe W P, Hartley J W, Lane W T. Mouse polyoma virus in a rural ecology. Ciba Found Symp. 1962;11:314–328. [Google Scholar]
- 155.Hunter T, Hutchinson M A, Eckhart W. Polyoma middle-sized T antigen can be phosphorylated on tyrosine at multiple sites in vitro. EMBO J. 1984;3:73–79. doi: 10.1002/j.1460-2075.1984.tb01763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Iacoangeli A, Melucci-Vigo G, Risuleo G, Santi E. Role of mouse polyomavirus late region in the control of viral DNA replication: a review. Biochimie. 1995;77:780–786. doi: 10.1016/0300-9084(96)88196-x. [DOI] [PubMed] [Google Scholar]
- 157.Ip Y T, Davis R J. Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 158.Ito Y, Brocklehurst J R, Dulbecco R. Virus-specific proteins in the plasma membrane of cells lytically infected or transformed by pol-oma virus. Proc Natl Acad Sci USA. 1977;74:4666–4670. doi: 10.1073/pnas.74.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ito Y, Spurr N, Griffin B E. Middle T antigen as primary inducer of full expression of the phenotype of transformation by polyoma virus. J Virol. 1980;35:219–232. doi: 10.1128/jvi.35.1.219-232.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Iyer V R, Eisen M B, Ross D T, Schuler G, Moore T, Lee J C F, Trent J M, Staudt L M, Hudson J J, Boguski M S, Lashkari D, Shalon D, Botstein D, Brown P O. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- 161.Jackson P, Ridgway P, Rayner J, Noble J, Braithwaite A. Transcriptional regulation of the PCNA promoter by p53. Biochem Biophys Res Commun. 1994;203:133–140. doi: 10.1006/bbrc.1994.2159. [DOI] [PubMed] [Google Scholar]
- 162.James S R, Downes C P, Gigg R, Grove S J, Holmes A B, Alessi D R. Specific binding of the Akt-1 protein kinase to phosphatidylinositol 3,4,5-trisphosphate without subsequent activation. Biochem J. 1996;315:709–713. doi: 10.1042/bj3150709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Johne R, Muller H. Avian polymavirus in wild birds: genome analysis of isolates from Falconiformes and Psittaciformes. Arch Virol. 1998;143:1501–1512. doi: 10.1007/s007050050393. [DOI] [PubMed] [Google Scholar]
- 164.Jones N C, Rigby P W, Ziff E B. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988;2:267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- 165.Joneson T, White M A, Wigler M H, Bar-Sagi D. Stimulation of membrane ruffling and MAP kinase activation by distinct effectors of RAS. Science. 1996;271:810–812. doi: 10.1126/science.271.5250.810. [DOI] [PubMed] [Google Scholar]
- 166.Kaech S, Covic L, Wyss A, Ballmer-Hofer K. Association of p60c-src with polyoma virus middle-T antigen abrogating mitosis-specific activation. Nature. 1991;350:431–433. doi: 10.1038/350431a0. [DOI] [PubMed] [Google Scholar]
- 167.Kamachi Y, Ogawa E, Asano M, Ishida S, Murakami Y, Satake M, Ito Y, Shigesada K. Purification of a mouse nuclear factor that binds to both the A and B cores of the polyomavirus enhancer. J Virol. 1990;64:4808–4819. doi: 10.1128/jvi.64.10.4808-4819.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kandel E S, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res. 1999;253:210–229. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- 169.Kaplan D R, Whitman M, Schaffhausen B, Pallas D C, White M, Cantley L, Roberts T M. Common elements in growth factor stimulation and oncogenic transformation: 85 kd phosphoprotein and phosphatidylinositol kinase activity. Cell. 1987;50:1021–1029. doi: 10.1016/0092-8674(87)90168-1. [DOI] [PubMed] [Google Scholar]
- 170.Kaplan D R, Whitman M, Schaffhausen B, Raptis L, Garcea R L, Pallas D, Roberts T M, Cantley L. Phosphatidylinositol metabolism and polyoma-mediated transformation. Proc Natl Acad Sci USA. 1986;83:3624–3628. doi: 10.1073/pnas.83.11.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 172.Katinka M, Vasseur M, Montreau N, Yaniv M, Blangy D. Polyoma DNA sequences involved in control of viral gene expression in murine embryonal carcinoma cells. Nature. 1981;290:720–722. doi: 10.1038/290720a0. [DOI] [PubMed] [Google Scholar]
- 173.Katinka M, Yaniv M. DNA replication origin of polyoma virus: early proximal boundary. J Virol. 1983;47:244–248. doi: 10.1128/jvi.47.1.244-248.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kawakami T, Pennington C Y, Robbins K C. Isolation and oncogenic potential of a novel human src-like gene. Mol Cell Biol. 1986;6:4195–4201. doi: 10.1128/mcb.6.12.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kennedy S G, Wagner A J, Conzen S D, Jordan J, Bellacosa A, Tsichlis P N, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 176.Kerkhoff E, Rapp U R. Cell cycle targets of Ras/Raf signalling. Oncogene. 1998;17:1457–1462. doi: 10.1038/sj.onc.1202185. [DOI] [PubMed] [Google Scholar]
- 177.Kiefer F, Anhauser I, Soriano P, Aguzzi A, Courtneidge S A, Wagner E F. Endothelial cell transformation by polyomavirus middle T antigen in mice lacking Src-related kinases. Curr Biol. 1994;4:100–109. doi: 10.1016/s0960-9822(94)00025-4. [DOI] [PubMed] [Google Scholar]
- 178.Kiefer F, Courtneidge S A, Wagner E F. Oncogenic properties of the middle T antigens of polyomaviruses. Adv Cancer Res. 1994;64:125–157. doi: 10.1016/s0065-230x(08)60837-4. [DOI] [PubMed] [Google Scholar]
- 179.Klarlund J K, Rameh L E, Cantley L C, Buxton J M, Holik J J, Sakelis C, Patki V, Corvera S, Czech M P. Regulation of GRP1-catalyzed ADP ribosylation factor guanine nucleotide exchange by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:1859–1862. doi: 10.1074/jbc.273.4.1859. [DOI] [PubMed] [Google Scholar]
- 180.Klippel A, Kavanaugh W M, Pot D, Williams L T. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol Cell Biol. 1997;17:338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Koch W, Carbone A, Walter G. Purified polyoma virus medium T antigen has tyrosine-specific protein kinase activity but no significant phosphatidylinositol kinase activity. Mol Cell Biol. 1986;6:1866–1874. doi: 10.1128/mcb.6.6.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Konishi H, Matsuzaki H, Tanaka M, Takemura Y, Kuroda S, Ono Y, Kikkawa U. Activation of protein kinase B (Akt/RAC-protein kinase) by cellular stress and its association with heat shock protein Hsp27. FEBS Lett. 1997;410:493–498. doi: 10.1016/s0014-5793(97)00541-3. [DOI] [PubMed] [Google Scholar]
- 183.Kornbluth S, Cross F R, Harbison M, Hanafusa H. Transformation of chicken embryo fibroblasts and tumor induction by the middle T antigen of polyomavirus carried in an avian retroviral vector. Mol Cell Biol. 1986;6:1545–1551. doi: 10.1128/mcb.6.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kornbluth S, Sudol M, Hanafusa H. Association of the polyomavirus middle-T antigen with c- yes protein. Nature. 1987;325:171–173. doi: 10.1038/325171a0. [DOI] [PubMed] [Google Scholar]
- 185.Krippl B, Griep A E, Mahon K A, Bohnlein E, Gruss P, Westphal H. Expression and amplification in transgenic mice of a polyoma virus mutant regulatory region. Nucleic Acids Res. 1988;16:8963–8976. doi: 10.1093/nar/16.18.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kryszke M H, Piette J, Yaniv M. Induction of a factor that binds to the polyoma virus A enhancer on differentiation of embryonal carcinoma cells. Nature. 1987;328:254–256. doi: 10.1038/328254a0. [DOI] [PubMed] [Google Scholar]
- 187.Kundra V, Escobedo J A, Kazlauskas A, Kim H K, Rhee S G, Williams L T, Zetter B R. Regulation of chemotaxis by the platelet-derived growth factor receptor-beta. Nature. 1994;367:474–476. doi: 10.1038/367474a0. [DOI] [PubMed] [Google Scholar]
- 188.Kypta R M, Hemming A, Courtneidge S A. Identification and characterization of p59fyn (a src-like protein tyrosine kinase) in normal and polyoma virus transformed cells. EMBO J. 1988;7:3837–3844. doi: 10.1002/j.1460-2075.1988.tb03269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lafferty S L, Fudge A M, Schmidt R E, Wilson V G, Phalen D N. Avian polyomavirus infection and disease in a green aracaris (Pteroglossus viridis) Avian Dis. 1999;43:577–585. [PubMed] [Google Scholar]
- 190.Lamph W W, Wamsley P, Sassone-Corsi P, Verma I M. Induction of proto-oncogene JUN/AP-1 by serum and TPA. Nature. 1988;334:629–631. doi: 10.1038/334629a0. [DOI] [PubMed] [Google Scholar]
- 191.Land H, Parada L F, Weinberg R A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983;304:596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- 192.Law L L, Dawe C J. Influence of the total body X-irradiation on tumor induction by parotid tumor agent in adult mice. Proc Soc Exp Biol Med. 1960;105:414–419. doi: 10.3181/00379727-105-26127. [DOI] [PubMed] [Google Scholar]
- 193.Law L W. Immunologic responsiveness and the induction of experimental neoplasms. Cancer Res. 1966;26:1121–1134. [PubMed] [Google Scholar]
- 194.Law L W, Ting R C. Immunologic competence and induction of neoplasms by polyoma virus. Proc Soc Exp Biol Med. 1965;119:823–830. doi: 10.3181/00379727-119-30311. [DOI] [PubMed] [Google Scholar]
- 195.Law L W, Ting R C, Leckband E. Prevention of virus-induced neoplasms in mice through passive transfer of immunity by sensitized syngeneic lymphoid cells. Proc Natl Acad Sci USA. 1967;57:1068–1075. doi: 10.1073/pnas.57.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Leevers S J, Vanhaesebroeck B, Waterfield M D. Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr Opin Cell Biol. 1999;11:219–225. doi: 10.1016/s0955-0674(99)80029-5. [DOI] [PubMed] [Google Scholar]
- 197.Lehman J M, Laffin J, Friedrich T D. DNA content distribution of mouse cells following infection with polyoma virus. Cytometry. 1994;16:138–143. doi: 10.1002/cyto.990160207. [DOI] [PubMed] [Google Scholar]
- 198.Lehman J M, Laffin J, Friedrich T D. Simian virus 40 induces multiple S phases with the majority of viral DNA replication in the G2 and second S phase in CV-1 cells. Exp Cell Res. 2000;258:215–222. doi: 10.1006/excr.2000.4927. [DOI] [PubMed] [Google Scholar]
- 199.Li M, Garcea R L. Identification of the threonine phosphorylation sites on the polyomavirus major capsid protein VP1: relationship to the activity of middle T antigen. J Virol. 1994;68:320–327. doi: 10.1128/jvi.68.1.320-327.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liang T J, Carmichael G G, Benjamin T L. A polyoma mutant that encodes small T antigen but not middle T antigen demonstrates uncoupling of cell surface and cytoskeletal changes associated with cell transformation. Mol Cell Biol. 1984;4:2774–2783. doi: 10.1128/mcb.4.12.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Linder S, Nilsson M, Martens I, Magnusson G. A viable mouse polyomavirus mutant without immortalizing or transforming activities. Virology. 1990;179:78–86. doi: 10.1016/0042-6822(90)90276-w. [DOI] [PubMed] [Google Scholar]
- 202.Ling L E, Druker B J, Cantley L C, Roberts T M. Transformation-defective mutants of polyomavirus middle T antigen associate with phosphatidylinositol 3-kinase (PI 3-kinase) but are unable to maintain wild-type levels of PI 3-kinase products in intact cells. J Virol. 1992;66:1702–1708. doi: 10.1128/jvi.66.3.1702-1708.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Linney E, Donerly S. DNA fragments from F9 PyEC mutants increase expression of heterologous genes in transfected F9 cells. Cell. 1983;35:633–699. doi: 10.1016/0092-8674(83)90102-2. [DOI] [PubMed] [Google Scholar]
- 204.Lips D L, Majerus P W, Gorga F R, Young A T, Benjamin T L. Phosphatidylinositol 3-phosphate is present in normal and transformed fibroblasts and is resistant to hydrolysis by bovine brain phospholipase C II. J Biol Chem. 1989;264:8759–8763. [PubMed] [Google Scholar]
- 205.Lukacher A E, Freund R, Carroll J P, Bronson R T, Benjamin T L. Pyvs: a dominantly acting gene in C3H/BiDa mice conferring susceptibility to tumor induction by polyoma virus. Virology. 1993;196:241–248. doi: 10.1006/viro.1993.1472. [DOI] [PubMed] [Google Scholar]
- 206.Lukacher A E, Ma Y, Carroll J P, Abromson-Leeman S R, Laning J C, Dorf M E, Benjamin T L. Susceptibility to tumors induced by polyoma virus is conferred by an endogenous mouse mammary tumor virus superantigen. J Exp Med. 1995;181:1683–1692. doi: 10.1084/jem.181.5.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lukacher A E, Wilson C S. Resistance to polyoma virus-induced tumors correlates with CTL recognition of an immunodominant H-2Dk-restricted epitope in the middle T protein. J Immunol. 1998;160:1724–1734. [PubMed] [Google Scholar]
- 208.Macara I G, Marinetti G V, Balduzzi P C. Transforming protein of avian sarcoma virus UR2 is associated with phosphatidylinositol kinase activity: possible role in tumorigenesis. Proc Natl Acad Sci USA. 1984;81:2728–2732. doi: 10.1073/pnas.81.9.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Maehama T, Dixon J E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 210.Maione R, Passananti C, De Simone V, Delli Bovi P, Augusti Tocco G, Amati P. Selection of mouse neuroblastoma cell-specific polyoma virus mutants with stage differentiative advantages of replication. EMBO J. 1985;4:3215–3221. doi: 10.1002/j.1460-2075.1985.tb04068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Majerus M A, Bibollet-Ruche F, Telliez J B, Wasylyk B, Bailleul B. Serum, AP-1 and Ets-1 stimulate the human ets-1 promoter. Nucleic Acids Res. 1992;20:2699–2703. doi: 10.1093/nar/20.11.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Marcellus R, Whitfield J F, Raptis L. Polyoma virus middle tumor antigen stimulates membrane-associated protein kinase C at lower levels than required for phosphatidylinositol kinase activation and neoplastic transformation. Oncogene. 1991;6:1037–1040. [PubMed] [Google Scholar]
- 213.Markland W, Cheng S H, Oostra B A, Smith A E. In vitro mutagenesis of the putative membrane-binding domain of polyomavirus middle-T antigen. J Virol. 1986;59:82–89. doi: 10.1128/jvi.59.1.82-89.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Markland W, Oostra B A, Harvey R, Markham A F, Colledge W H, Smith A E. Site-directed mutagenesis of polyomavirus middle-T antigen sequences encoding tyrosine 315 and tyrosine 250. J Virol. 1986;59:384–391. doi: 10.1128/jvi.59.2.384-391.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Markland W, Smith A E. Mutants of polyomavirus middle-T antigen. Biochim Biophys Acta. 1987;907:299–321. doi: 10.1016/0304-419x(87)90011-4. [DOI] [PubMed] [Google Scholar]
- 216.Marra F, Pinzani M, DeFranco R, Laffi G, Gentilini P. Involvement of phosphatidylinositol 3-kinase in the activation of extracellular signal-regulated kinase by PDGF in hepatic stellate cells. FEBS Lett. 1995;376:141–145. doi: 10.1016/0014-5793(95)01261-0. [DOI] [PubMed] [Google Scholar]
- 217.Marshall C J, Lloyd A C, Morris J D, Paterson H, Price B, Hall A. Signal transduction by p21ras. Int J Cancer Suppl. 1989;4:29–31. doi: 10.1002/ijc.2910440708. [DOI] [PubMed] [Google Scholar]
- 218.Martelli F, Iacobini C, Caruso M, Felsani A. Characterization of two novel YY1 binding sites in the polyomavirus late promoter. J Virol. 1996;70:1433–1438. doi: 10.1128/jvi.70.3.1433-1438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Marth J D, Peet R, Krebs E G, Perlmutter R M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985;43:393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- 220.Martin M E, Piette J, Yaniv M, Tang W J, Folk W R. Activation of the polyomavirus enhancer by a murine activator protein 1 (AP1) homolog and two contiguous proteins. Proc Natl Acad Sci USA. 1988;85:5839–5843. doi: 10.1073/pnas.85.16.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Matker C M, Rizzo P, Pass H I, Di Resta I, Powers A, Mutti L, Kast W M, Carbone M. The biological activities of simian virus 40 large-T antigen and its possible oncogenic effects in humans. Monaldi Arch Chest Dis. 1998;53:193–197. [PubMed] [Google Scholar]
- 222.Matter J M, Tiercy J M, Weil R. Sequential stimulation of cellular RNA synthesis in polyoma-infected mouse kidney cell cultures. Nucleic Acids Res. 1983;11:6611–6629. doi: 10.1093/nar/11.19.6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Matthews J T, Benjamin T L. 12-O-Tetradecanoylphorbol-13-acetate stimulates phosphorylation of the 58,000-Mr form of polyomavirus middle T antigen in vivo: implications for a possible role of protein kinase C in middle T function. J Virol. 1986;58:239–246. doi: 10.1128/jvi.58.2.239-246.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mayo M W, Wang C Y, Cogswell P C, Rogers-Graham K S, Lowe S W, Der C J, Baldwin A S J. Requirement of NF-kappaB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 225.Meili R, Cron P, Hemmings B A, Ballmer-Hofer K. Protein kinase B/Akt is activated by polyomavirus middle-T antigen via a phosphatidylinositol 3-kinase-dependent mechanism. Oncogene. 1998;16:903–907. doi: 10.1038/sj.onc.1201605. [DOI] [PubMed] [Google Scholar]
- 226.Melucci-Vigo G, Magnusson G, Risuleo G. Mouse polyomavirus late region mutants expressing a defective VP2 capsid protein exhibit an enhanced viral DNA replication. Virus Genes. 1994;8:137–141. doi: 10.1007/BF01703070. [DOI] [PubMed] [Google Scholar]
- 227.Mercer W E, Shields M T, Lin D, Appella E, Ullrich S J. Growth suppression induced by wild-type p53 protein is accompanied by selective down-regulation of proliferating-cell nuclear antigen expression. Proc Natl Acad Sci USA. 1991;88:1958–1962. doi: 10.1073/pnas.88.5.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Messerschmitt A, Disela C, Dilworth S, Marti A G, Ballmer-Hofer K. Polyomavirus middle-T antigen lacking a membrane anchor sequence accumulates in the nucleus. J Gen Virol. 1996;77:17–26. doi: 10.1099/0022-1317-77-1-17. [DOI] [PubMed] [Google Scholar]
- 229.Minden A, Lin A, Claret F X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 230.Montagne J, Stewart M J, Stocker H, Hafen E, Kozma S C, Thomas G. Drosophila S6 kinase: a regulator of cell size. Science. 1999;285:2126–2129. doi: 10.1126/science.285.5436.2126. [DOI] [PubMed] [Google Scholar]
- 231.Montesano R, Pepper M S, Mohle-Steinlein U, Risau W, Wagner E F, Orci L. Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell. 1990;62:435–445. doi: 10.1016/0092-8674(90)90009-4. [DOI] [PubMed] [Google Scholar]
- 232.Mor O, Read M, Fried M. p53 in polyoma virus transformed REF52 cells. Oncogene. 1997;15:3113–3119. doi: 10.1038/sj.onc.1201549. [DOI] [PubMed] [Google Scholar]
- 233.Moreno J P, Villarreal L P. Analysis of cellular DNA synthesis during polyoma virus infection of mice: acute infection fails to induce cellular DNA synthesis. Virology. 1992;186:463–474. doi: 10.1016/0042-6822(92)90011-d. [DOI] [PubMed] [Google Scholar]
- 234.Mudryj M, Hiebert S W, Nevins J R. A role for the adenovirus inducible E2F transcription factor in a proliferation dependent signal transduction pathway. EMBO J. 1990;9:2179–2184. doi: 10.1002/j.1460-2075.1990.tb07387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mueller C R, Mes Masson A M, Bouvier M, Hassell J A. Location of sequences in polyomavirus DNA that are required for early gene expression in vivo and in vitro. Mol Cell Biol. 1984;4:2594–2609. doi: 10.1128/mcb.4.12.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Mueller C R, Muller W J, Hassell J A. The polyomavirus enhancer comprises multiple functional elements. J Virol. 1988;62:1667–1678. doi: 10.1128/jvi.62.5.1667-1678.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Mullane K P, Ratnofsky M, Cullere X, Schaffhausen B. Signaling from polyomavirus middle T and small T defines different roles for protein phosphatase 2A. Mol Cell Biol. 1998;18:7556–7564. doi: 10.1128/mcb.18.12.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Muller W J, Dufort D, Hassell J A. Multiple subelements within the polyomavirus enhancer function synergistically to activate DNA replication. Mol Cell Biol. 1988;8:5000–5015. doi: 10.1128/mcb.8.11.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Muller W J, Mueller C R, Mes-Masson A-M, Hassell J A. Polyomavirus origin for DNA replication comprises multiple genetic elements. J Virol. 1983;47:586–599. doi: 10.1128/jvi.47.3.586-599.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Murakami Y, Eki T, Yamada M, Prives C, Hurwitz J. Species-specific in vitro synthesis of DNA containing the polyoma virus origin of replication. Proc Natl Acad Sci USA. 1986;83:6347–6351. doi: 10.1073/pnas.83.17.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Murakami Y, Wobbe C R, Weissbach L, Dean F B, Hurwitz J. Role of DNA polymerase alpha and DNA primase in simian virus 40 DNA replication in vitro. Proc Natl Acad Sci USA. 1986;83:2869–2873. doi: 10.1073/pnas.83.9.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Nikolakaki E, Coffer P J, Hemelsoet R, Woodgett J R, Defize L H. Glycogen synthase kinase 3 phosphorylates Jun family members in vitro and negatively regulates their transactivating potential in intact cells. Oncogene. 1993;8:833–840. [PubMed] [Google Scholar]
- 243.Nishizawa M, Semba K, Yoshida M C, Yamamoto T, Sasaki M, Toyoshima K. Structure, expression, and chromosomal location of the human c-fgr gene. Mol Cell Biol. 1986;6:511–517. doi: 10.1128/mcb.6.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Nothias J Y, Weinmann R, Blangy D, Melin F. Analysis of transcription factors binding to the duplicated PEA1 and PEA3 sites that are required for polyomavirus mutant expression in PCC4 embryonic carcinoma cells. J Virol. 1993;67:3036–3047. doi: 10.1128/jvi.67.6.3036-3047.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ogawa E, Inuzuka M, Maruyama M, Satake M, Naito-Fujimoto M, Ito Y, Shigesada K. Molecular cloning and characterization of PEBP2 beta, the heterodimeric partner of a novel Drosophila runt-related DNA binding protein PEBP2 alpha. Virology. 1993;194:314–331. doi: 10.1006/viro.1993.1262. [DOI] [PubMed] [Google Scholar]
- 246.Oliveira M L, Roberts T M, Druker B J, Armelin M C. Mapping of polyomavirus middle T domain that is responsible for AP-1 activation. Oncogene. 1998;16:2975–2982. doi: 10.1038/sj.onc.1201841. [DOI] [PubMed] [Google Scholar]
- 247.Olson M F, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 248.Oostra B A, Harvey R, Ely B K, Markham A F, Smith A E. Transforming activity of polyoma virus middle-T antigen probed by site-directed mutagenesis. Nature. 1983;304:456–459. doi: 10.1038/304456a0. [DOI] [PubMed] [Google Scholar]
- 249.Oren M. The p53 cellular tumor antigen: gene structure, expression and protein properties. Biochim Biophys Acta. 1985;823:67–78. doi: 10.1016/0304-419x(85)90015-0. [DOI] [PubMed] [Google Scholar]
- 250.Otsu M, Hiles I, Gout I, Fry M J, Ruiz-Larrea F, Panayotou G, Thompson A, Dhand R, Hsuan J, Totty N. Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell. 1991;65:91–104. doi: 10.1016/0092-8674(91)90411-q. [DOI] [PubMed] [Google Scholar]
- 251.Ozes O N, Mayo L D, Gustin J A, Pfeffer S R, Pfeffer L M, Donner D B. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 252.Palkova Z, Spanielova H, Gottifredi V, Hollanderova D, Forstova J, Amati P. The polyomavirus major capsid protein VP1 interacts with the nuclear matrix regulatory protein YY1. FEBS Lett. 2000;467:359–364. doi: 10.1016/s0014-5793(00)01170-4. [DOI] [PubMed] [Google Scholar]
- 253.Pallas D C, Fu H, Haehnel L C, Weller W, Collier R J, Roberts T M. Association of polyomavirus middle tumor antigen with 14-3-3 proteins. Science. 1994;265:535–537. doi: 10.1126/science.8036498. [DOI] [PubMed] [Google Scholar]
- 254.Pallas D C, Shahrik L K, Martin B L, Jaspers S, Miller T B, Brautigan D L, Roberts T M. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990;60:167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- 255.Pallas D C, Weller W, Jaspers S, Miller T B, Lane W S, Roberts T M. The third subunit of protein phosphatase 2A (PP2A), a 55- kilodalton protein which is apparently substituted for by T antigens in complexes with the 36- and 63-kilodalton PP2A subunits, bears little resemblance to T antigens. J Virol. 1992;66:886–893. doi: 10.1128/jvi.66.2.886-893.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Paquis-Flucklinger V, Michiels J F, Vidal F, Alquier C, Pointis G, Bourdon V, Cuzin F, Rassoulzadegan M. Expression in transgenic mice of the large T antigen of polyomavirus induces Sertoli cell tumours and allows the establishment of differentiated cell lines. Oncogene. 1993;8:2087–2094. [PubMed] [Google Scholar]
- 257.Pelicci G, Lanfrancone L, Grignani F, McGlade J, Cavallo F, Forni G, Nicoletti I, Pawson T, Pelicci P G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992;70:93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- 258.Pepper M S, Tacchini-Cottier F, Sabapathy T K, Montesano R, Wagner E F. Endothelial cells transformed by polyomavirus middle T oncogene: a model for haemangiomas and other vascular tumours. In: Bicknell R J, Lewis C E, Ferrara N, editors. Tumour angiogenesis. Oxford, United Kingdom: Oxford University Press; 1997. pp. 309–331. [Google Scholar]
- 259.Perez L, Paasinen A, Schnierle B, Kach S, Senften M, Ballmer-Hofer K. Mitosis-specific phosphorylation of polyomavirus middle-sized tumor antigen and its role during cell transformation. Proc Natl Acad Sci USA. 1993;90:8113–8117. doi: 10.1073/pnas.90.17.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Perez L, Urich M, Paasinen A, Senften M, Meili R, Ballmer-Hofer K. Domains in middle-T antigen that cooperate in polyomavirus-mediated oncogenic transformation. Virology. 1995;208:26–37. doi: 10.1006/viro.1995.1126. [DOI] [PubMed] [Google Scholar]
- 261.Petursson G, Weil R. A study on the mechanism of polyoma-induced activation of the cellular DNA-synthesizing apparatus. Synchronization by FUdR of virus-induced DNA synthesis. Arch Gesamte Virusforsch. 1968;24:1–29. doi: 10.1007/BF01242898. [DOI] [PubMed] [Google Scholar]
- 262.Phalen D N, Wilson V G, Gaskin J M, Derr J N, Graham D L. Genetic diversity in twenty variants of the avian polyomavirus. Avian Dis. 1999;43:207–218. [PubMed] [Google Scholar]
- 263.Pignataro O P, Ascoli M. Epidermal growth factor increases the labeling of phosphatidylinositol 3,4-bisphosphate in MA-10 Leydig tumor cells. J Biol Chem. 1990;265:1718–1723. [PubMed] [Google Scholar]
- 264.Pipas J M. Common and unique features of T antigens encoded by the polyomavirus group. J Virol. 1992;66:3979–3985. doi: 10.1128/jvi.66.7.3979-3985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Pullen N, Dennis P B, Andjelkovic M, Dufner A, Kozma S C, Hemmings B A, Thomas G. Phosphorylation and activation of p70s6k by PDK1. Science. 1998;279:707–710. doi: 10.1126/science.279.5351.707. [DOI] [PubMed] [Google Scholar]
- 266.Qian W, Kashuba E, Magnusson K P, Pokrovskaja K, Okan I, Klein G, Wiman K G. Role of p53 mutation in polyomavirus-induced tumorigenesis. J Gen Virol. 1997;78:893–903. doi: 10.1099/0022-1317-78-4-893. [DOI] [PubMed] [Google Scholar]
- 267.Qian W, Wiman K G. Polyoma virus middle T and small t antigens cooperate to antagonize p53-induced cell cycle arrest and apoptosis. Cell Growth Differ. 2000;11:31–39. [PubMed] [Google Scholar]
- 268.Quintrell N, Lebo R, Varmus H, Bishop J M, Pettenati M J, Le Beau M M, Diaz M O, Rowley J D. Identification of a human gene (HCK) that encodes a protein-tyrosine kinase and is expressed in hemopoietic cells. Mol Cell Biol. 1987;7:2267–2275. doi: 10.1128/mcb.7.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Rameh L E, Armelin M C. T antigens' role in polyomavirus transformation: c-myc but not c-fos or c-jun expression is a target for middle T. Oncogene. 1991;6:1049–1056. [PubMed] [Google Scholar]
- 270.Rameh L E, Cantley L C. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 271.Raptis L. Polyomavirus middle tumor antigen increases responsiveness to growth factors. J Virol. 1991;65:2691–2694. doi: 10.1128/jvi.65.5.2691-2694.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Raptis L, Bell J, Whitfield J F. Protein kinase C increases the activity of the polyoma virus middle T antigen-associated phosphatidylinositol kinase. Biochem Biophys Res Commun. 1988;154:306–311. doi: 10.1016/0006-291x(88)90685-7. [DOI] [PubMed] [Google Scholar]
- 273.Raptis L, Bolen J B. Polyomavirus transforms rat F111 and mouse NIH 3T3 cells by different mechanisms. J Virol. 1989;63:753–758. doi: 10.1128/jvi.63.2.753-758.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Raptis L, Boynton A L, Whitfield J F. Protein kinase C promotes the phosphorylation of immunoprecipitated middle T antigen from polyoma-transformed cells. Biochem Biophys Res Commun. 1986;136:995–1000. doi: 10.1016/0006-291x(86)90431-6. [DOI] [PubMed] [Google Scholar]
- 275.Raptis L, Lamfrom H, Benjamin T L. Regulation of cellular phenotype and expression of polyomavirus middle T antigen in rat fibroblasts. Mol Cell Biol. 1985;5:2476–2486. doi: 10.1128/mcb.5.9.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Raptis L, Marcellus R, Corbley M J, Krook A, Whitfield J, Anderson S K, Haliotis T. Cellular ras gene activity is required for full neoplastic transformation by polyomavirus. J Virol. 1991;65:5203–5210. doi: 10.1128/jvi.65.10.5203-5210.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Raptis L, Marcellus R C, Whitfield J F. Transforming signals generated by the polyoma virus tumor antigens. Adv Enzyme Regul. 1990;30:133–142. doi: 10.1016/0065-2571(90)90014-s. [DOI] [PubMed] [Google Scholar]
- 278.Raptis L, Whitfield J F. Protein kinase C stimulation increases the transforming ability of the polyoma virus middle T antigen. Biochem Biophys Res Commun. 1986;140:1106–1112. doi: 10.1016/0006-291x(86)90749-7. [DOI] [PubMed] [Google Scholar]
- 279.Rassoulzadegan M, Courtneidge S A, Loubiere R, el Baze P, Cuzin F. A variety of tumours induced by the middle T antigen of polyoma virus in a transgenic mouse family. Oncogene. 1990;5:1507–1510. [PubMed] [Google Scholar]
- 280.Rassoulzadegan M, Cowie A, Carr A, Glaichenhaus N, Kamen R, Cuzin F. The roles of individual polyoma virus early proteins in oncogenic transformation. Nature. 1982;300:713–718. doi: 10.1038/300713a0. [DOI] [PubMed] [Google Scholar]
- 281.Reuther G W, Pendergast A M. The roles of 14-3-3 proteins in signal transduction. Vitam Horm. 1996;52:149–175. doi: 10.1016/s0083-6729(08)60410-0. [DOI] [PubMed] [Google Scholar]
- 282.Ridley A J, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 283.Rodriguez-Viciana P, Warne P H, Dhand R, Vanhaesebroeck B, Gout I, Fry M J, Waterfield M D, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 284.Romashkova J A, Makarov S S. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 285.Rondinelli R H, Haslam S Z, Fluck M M. The role of ovarian hormones, age and mammary gland development in polyomavirus mammary tumorigenesis. Oncogene. 1995;11:1817–1827. [PubMed] [Google Scholar]
- 286.Rott O, Kroger M, Muller H, Hobom G. The genome of budgerigar fledgling disease virus, an avian polyomavirus. Virology. 1988;165:74–86. doi: 10.1016/0042-6822(88)90660-5. [DOI] [PubMed] [Google Scholar]
- 287.Roulston A, Reinhard C, Amiri P, Williams L T. Early activation of c-Jun N-terminal kinase and p38 kinase regulate cell survival in response to tumor necrosis factor alpha. J Biol Chem. 1998;273:10232–10239. doi: 10.1074/jbc.273.17.10232. [DOI] [PubMed] [Google Scholar]
- 288.Rowe W P. The epidemiology of mouse polyoma virus infection. Bacteriol Rev. 1961;25:18–31. doi: 10.1128/br.25.1.18-31.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Rowe W P, Hartley J W, Brodsky I. Observations on the spread of mouse polyoma virus infection. Nature. 1958;182:1617. doi: 10.1038/1821617a0. [DOI] [PubMed] [Google Scholar]
- 290.Rowe W P, Huebner R J, Hartley J W. Ecology of a mouse tumor virus. In: Pollard M, editor. Perspectives in virology II. Minneapolis, Minn: Burgess Publishing Co.; 1961. pp. 177–193. [Google Scholar]
- 291.Ruderman N B, Kapeller R, White M F, Cantley L C. Activation of phosphatidylinositol 3-kinase by insulin. Proc Natl Acad Sci USA. 1990;87:1411–1415. doi: 10.1073/pnas.87.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Rutberg S E, Fuchs S Y, Ronai Z. Ultraviolet irradiation and c-jun over-expression regulates replication of polyoma sequences in WOP cells through a PEBP2 binding site. Biochim Biophys Acta. 1995;1261:90–98. doi: 10.1016/0167-4781(94)00230-z. [DOI] [PubMed] [Google Scholar]
- 293.Ryder K, Nathans D. Induction of protooncogene c-jun by serum growth factors. Proc Natl Acad Sci USA. 1988;85:8464–8467. doi: 10.1073/pnas.85.22.8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sable C L, Filippa N, Hemmings B, Van Obberghen E. cAMP stimulates protein kinase B in a Wortmannin-insensitive manner. FEBS Lett. 1997;409:253–257. doi: 10.1016/s0014-5793(97)00518-8. [DOI] [PubMed] [Google Scholar]
- 295.Salomon C, Turler H, Weil R. Polyoma-induced stimulation of cellular RNA synthesis is paralleled by changed expression of the viral genome. Nucleic Acids Res. 1977;4:1483–1503. doi: 10.1093/nar/4.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Schaffhausen B, Benjamin T L. Comparison of phosphorylation of two polyoma virus middle T antigens in vivo and in vitro. J Virol. 1981;40:184–196. doi: 10.1128/jvi.40.1.184-196.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Schaffhausen B S, Dorai H, Arakere G, Benjamin T L. Polyoma virus middle T antigen: relationship to cell membranes and apparent lack of ATP-binding activity. Mol Cell Biol. 1982;2:1187–1198. doi: 10.1128/mcb.2.10.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Schaffhausen B S, Liang T J, Carmichael G G, Benjamin T L. Residual transforming activity of PY1178T, a mutant lacking the principal in vitro tyrosine phosphorylation site, is not affected by removal of the secondary tyrosine phosphorylation site at residue 322. Virology. 1985;143:671–675. doi: 10.1016/0042-6822(85)90410-6. [DOI] [PubMed] [Google Scholar]
- 299.Scheffner M, Munger K, Byrne J C, Howley P M. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci USA. 1991;88:5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Schneikert J, Imler J L, Wasylyk B. Repression by Jun of the polyoma-virus enhancer overrides activation in a cell specific manner. Nucleic Acids Res. 1991;19:783–787. doi: 10.1093/nar/19.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Schonthal A, Srinivas S, Eckhart W. Induction of c-jun protooncogene expression and transcription factor AP-1 activity by the polyoma virus middle-sized tumor antigen. Proc Natl Acad Sci USA. 1992;89:4972–4976. doi: 10.1073/pnas.89.11.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Schuchner S, Wintersberger E. Binding of polyomavirus small T antigen to protein phosphatase 2A is required for elimination of p27 and support of S-phase induction in concert with large T antigen. J Virol. 1999;73:9266–9273. doi: 10.1128/jvi.73.11.9266-9273.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Schuurman R, Jacobs M, van Strien A, van der Noordaa J, Sol C. Analysis of splice sites in the early region of bovine polyomavirus: evidence for a unique pattern of large T mRNA splicing. J Gen Virol. 1992;73:2879–2886. doi: 10.1099/0022-1317-73-11-2879. [DOI] [PubMed] [Google Scholar]
- 304.Segawa K, Ito Y. Differential subcellular localization of in vivo-phosphorylated and nonphosphorylated middle-sized tumor antigen of polyoma virus and its relationship to middle-sized tumor antigen phosphorylating activity in vitro. Proc Natl Acad Sci USA. 1982;79:6812–6816. doi: 10.1073/pnas.79.22.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Sekulic A, Hudson C C, Homme J L, Yin P, Otterness D M, Karnitz L M, Abraham R T. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–3513. [PubMed] [Google Scholar]
- 306.Semba K, Nishizawa M, Miyajima N, Yoshida M C, Sukegawa J, Yamanashi Y, Sasaki M, Yamamoto T, Toyoshima K. yes-related protooncogene, syn, belongs to the protein-tyrosine kinase family. Proc Natl Acad Sci USA. 1986;83:5459–5463. doi: 10.1073/pnas.83.15.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Senften M, Dilworth S, Ballmer-Hofer K. Multimerization of polyomavirus middle-T antigen. J Virol. 1997;71:6990–6995. doi: 10.1128/jvi.71.9.6990-6995.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Serunian L A, Auger K R, Roberts T M, Cantley L C. Production of novel polyphosphoinositides in vivo is linked to cell transformation by polyomavirus middle T antigen. J Virol. 1990;64:4718–4725. doi: 10.1128/jvi.64.10.4718-4725.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Serunian L A, Haber M T, Fukui T, Kim J W, Rhee S G, Lowenstein J M, Cantley L C. Polyphosphoinositides produced by phosphatidylinositol 3-kinase are poor substrates for phospholipases C from rat liver and bovine brain. J Biol Chem. 1989;264:17809–17815. [PubMed] [Google Scholar]
- 310.Shadan F F, Villarreal L P. The evolution of small DNA viruses of eukaryotes: past and present considerations. Virus Genes. 1995;11:239–257. doi: 10.1007/BF01728663. [DOI] [PubMed] [Google Scholar]
- 311.Sharrocks A D, Brown A L, Ling Y, Yates P R. The ETS-domain transcription factor family. Int J Biochem Cell Biol. 1997;29:1371–1387. doi: 10.1016/s1357-2725(97)00086-1. [DOI] [PubMed] [Google Scholar]
- 312.Shields J M, Pruitt K, McFall A, Shaub A, Der C J. Understanding Ras: ‘it ain’t over 'til it's over'. Trends Cell Biol. 2000;10:147–154. doi: 10.1016/s0962-8924(00)01740-2. [DOI] [PubMed] [Google Scholar]
- 313.Shivakumar C V, Brown D R, Deb S, Deb S P. Wild-type human p53 transactivates the human proliferating cell nuclear antigen promoter. Mol Cell Biol. 1995;15:6785–6793. doi: 10.1128/mcb.15.12.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Shivakumar C V, Das G C. The A enhancer of polyomavirus: protein-protein interactions for the differential early and late promoter function under nonreplicating conditions. Intervirology. 1998;41:103–109. doi: 10.1159/000024921. [DOI] [PubMed] [Google Scholar]
- 315.Smale S T, Tjian R. T-antigen-DNA polymerase alpha complex implicated in simian virus 40 DNA replication. Mol Cell Biol. 1986;6:4077–4087. doi: 10.1128/mcb.6.11.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Soeda E, Maruyama T, Arrand J R, Griffin B E. Host-dependent evolution of three papova viruses. Nature. 1980;285:165–167. doi: 10.1038/285165a0. [DOI] [PubMed] [Google Scholar]
- 317.Soltoff S P, Carpenter C L, Auger K R, Kapeller R, Schaffhausen B, Cantley L C. Phosphatidylinositol-3 kinase and growth regulation. Cold Spring Harbor Symp Quant Biol. 1992;57:75–80. doi: 10.1101/sqb.1992.057.01.010. [DOI] [PubMed] [Google Scholar]
- 318.Soussi T, Caron de Fromentel C, May P. Structural aspects of the p53 protein in relation to gene evolution. Oncogene. 1990;5:945–952. [PubMed] [Google Scholar]
- 319.Srinivas S, Schonthal A, Eckhart W. Polyomavirus middle-sized tumor antigen modulates c-Jun phosphorylation and transcriptional activity. Proc Natl Acad Sci USA. 1994;91:10064–10068. doi: 10.1073/pnas.91.21.10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter G F, Holmes A B, Gaffney P R, Reese C B, McCormick F, Tempst P, Coadwell J, Hawkins P T. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 321.Stokoe D, Stephens L R, Copeland T, Gaffney P R, Reese C B, Painter G F, Holmes A B, McCormick F, Hawkins P T. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 322.Su W, Liu W, Schaffhausen B S, Roberts T M. Association of polyomavirus middle tumor antigen with phospholipase C-gamma 1. J Biol Chem. 1995;270:12331–12334. doi: 10.1074/jbc.270.21.12331. [DOI] [PubMed] [Google Scholar]
- 323.Sugimoto C, Kitamura T, Guo J, Al-Ahdal M N, Shchelkunov S N, Otova B, Ondrejka P, Chollet J Y, El-Safi S, Ettayebi M, Gresenguet G, Kocagoz T, Chaiyarasamee S, Thant K Z, Thein S, Moe K, Kobayashi N, Taguchi F, Yogo Y. Typing of urinary JC virus DNA offers a novel means of tracing human migrations. Proc Natl Acad Sci USA. 1997;94:9191–9196. doi: 10.1073/pnas.94.17.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Sukegawa J, Semba K, Yamanashi Y, Nishizawa M, Miyajima N, Yamamoto T, Toyoshima K. Characterization of cDNA clones for the human c-yes gene. Mol Cell Biol. 1987;7:41–47. doi: 10.1128/mcb.7.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Summers S A, Lipfert L, Birnbaum M J. Polyoma middle T antigen activates the Ser/Thr kinase Akt in a PI3-kinase-dependent manner. Biochem Biophys Res Commun. 1998;246:76–81. doi: 10.1006/bbrc.1998.8575. [DOI] [PubMed] [Google Scholar]
- 326.Szomolanyi-Tsuda E, Dundon P L, Joris I, Shultz L D, Woda B A, Welsh R M. Acute, lethal, natural killer cell-resistant myeloproliferative disease induced by polyomavirus in severe combined immunodeficient mice. Am J Pathol. 1994;144:359–371. [PMC free article] [PubMed] [Google Scholar]
- 327.Szomolanyi-Tsuda E, Welsh R M. T cell-independent antibody-mediated clearance of polyoma virus in T cell-deficient mice. J Exp Med. 1996;183:403–411. doi: 10.1084/jem.183.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Takahashi A, Satake M, Yamaguchi-Iwai Y, Bae S C, Lu J, Maruyama M, Zhang Y W, Oka H, Arai N, Arai K. Positive and negative regulation of granulocyte-macrophage colony-stimulating factor promoter activity by AML1-related transcription factor, PEBP2. Blood. 1995;86:607–616. [PubMed] [Google Scholar]
- 329.Takeya T, Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983;32:881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- 330.Takimoto M, Quinn J P, Farina A R, Staudt L M, Levens D. fos/jun and octamer-binding protein interact with a common site in a negative element of the human c-myc gene. J Biol Chem. 1989;264:8992–8999. [PubMed] [Google Scholar]
- 331.Talmage D A, Blenis J, Benjamin T L. Polyomavirus middle T antigen induces ribosomal protein S6 phosphorylation through pp60c-src-dependent and -independent pathways. Mol Cell Biol. 1988;8:2309–2315. doi: 10.1128/mcb.8.6.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Talmage D A, Freund R, Young A T, Dahl J, Dawe C J, Benjamin T L. Phosphorylation of middle T by pp60c-src: a switch for binding of phosphatidylinositol 3-kinase and optimal tumorigenesis. Cell. 1989;59:55–65. doi: 10.1016/0092-8674(89)90869-6. [DOI] [PubMed] [Google Scholar]
- 333.Talmage D A, Listerud M. Retinoic acid suppresses polyoma virus transformation by inhibiting transcription of the c-fos proto-oncogene. Oncogene. 1994;9:3557–3563. [PubMed] [Google Scholar]
- 334.Tang W J, Berger S L, Triezenberg S J, Folk W R. Nucleotides in the polyomavirus enhancer that control viral transcription and DNA replication. Mol Cell Biol. 1987;7:1681–1690. doi: 10.1128/mcb.7.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Templeton D, Eckhart W. Mutation causing premature termination of the polyoma virus medium T antigen blocks cell transformation. J Virol. 1982;41:1014–1024. doi: 10.1128/jvi.41.3.1014-1024.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Templeton D, Simon S, Eckhart W. Truncated forms of the polyomavirus middle T antigen can substitute for the small T antigen in lytic infection. J Virol. 1986;57:367–370. doi: 10.1128/jvi.57.1.367-370.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Templeton D, Voronova A, Eckhart W. Construction and expression of a recombinant DNA gene encoding a polyomavirus middle-size tumor antigen with the carboxyl terminus of the vesicular stomatitis virus glycoprotein G. Mol Cell Biol. 1984;4:282–289. doi: 10.1128/mcb.4.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Thaete L G, Ahnen D J, Malkinson A M. Proliferating cell nuclear antigen (PCNA/cyclin) immunocytochemistry as a labeling index in mouse lung tissues. Cell Tissue Res. 1989;256:167–173. doi: 10.1007/BF00224731. [DOI] [PubMed] [Google Scholar]
- 340.Thomas J E, Aguzzi A, Soriano P, Wagner E F, Brugge J S. Induction of tumor formation and cell transformation by polyoma middle T antigen in the absence of Src. Oncogene. 1993;8:2521–2529. [PubMed] [Google Scholar]
- 341.Thomas S M, Brugge J S. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 342.Treisman R, Novak U, Favaloro J, Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981;292:595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- 343.Turler H, Salomon C. Small and middle T antigens contribute to lytic and abortive polyomavirus infection. J Virol. 1985;53:579–586. doi: 10.1128/jvi.53.2.579-586.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Turowski P, Fernandez A, Favre B, Lamb N J, Hemmings B A. Differential methylation and altered conformation of cytoplasmic and nuclear forms of protein phosphatase 2A during cell cycle progression. J Cell Biol. 1995;129:397–410. doi: 10.1083/jcb.129.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Tyndall C, La Mantia G, Thacker C M, Favaloro J, Kamen R. A region of the polyoma virus genome between the replication origin and late protein coding sequences is required in cis for both early gene expression and viral DNA replication. Nucleic Acids Res. 1981;9:6231–6250. doi: 10.1093/nar/9.23.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Ulug E T, Cartwright A J, Courtneidge S A. Characterization of the interaction of polyomavirus middle T antigen with type 2A protein phosphatase. J Virol. 1992;66:1458–1467. doi: 10.1128/jvi.66.3.1458-1467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Ulug E T, Hawkins P T, Hanley M R, Courtneidge S A. Phosphatidylinositol metabolism in cells transformed by polyomavirus middle T antigen. J Virol. 1990;64:3895–3904. doi: 10.1128/jvi.64.8.3895-3904.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Urich M, el Shemerly M Y, Besser D, Nagamine Y, Ballmer-Hofer K. Activation and nuclear translocation of mitogen-activated protein kinases by polyomavirus middle-T or serum depend on phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:29286–29292. doi: 10.1074/jbc.270.49.29286. [DOI] [PubMed] [Google Scholar]
- 349.Urich M, Senften M, Shaw P E, Ballmer-Hofer K. A role for the small GTPase Rac in polyomavirus middle-T antigen-mediated activation of the serum response element and in cell transformation. Oncogene. 1997;14:1235–1241. doi: 10.1038/sj.onc.1200982. [DOI] [PubMed] [Google Scholar]
- 350.Vanhaesebroeck B, Alessi D R. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346 Pt 3:561–576. [PMC free article] [PubMed] [Google Scholar]
- 351.Varticovski L, Daley G Q, Jackson P, Baltimore D, Cantley L C. Activation of phosphatidylinositol 3-kinase in cells expressing abl oncogene variants. Mol Cell Biol. 1991;11:1107–1113. doi: 10.1128/mcb.11.2.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Varticovski L, Druker B, Morrison D, Cantley L, Roberts T. The colony stimulating factor-1 receptor associates with and activates phosphatidylinositol-3 kinase. Nature. 1989;342:699–702. doi: 10.1038/342699a0. [DOI] [PubMed] [Google Scholar]
- 353.Vasudevan C, Freund R, Gorga F R. The elevation of cellular phosphatidic acid levels caused by polyomavirus transformation can be disassociated from the activation of phospholipase D. Virology. 1997;233:392–401. doi: 10.1006/viro.1997.8630. [DOI] [PubMed] [Google Scholar]
- 354.Veldman G M, Lupton S, Kamen R. Polyomavirus enhancer contains multiple redundant sequence elements that activate both DNA replication and gene expression. Mol Cell Biol. 1985;5:649–658. doi: 10.1128/mcb.5.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Villarreal L P, DeFilippis V R, Gottlieb K A. Acute and persistent viral life strategies and their relationship to emerging diseases. Virology. 2000;272:1–6. doi: 10.1006/viro.2000.0381. [DOI] [PubMed] [Google Scholar]
- 356.Vogel F, Rhode K, Scherneck S, Bastien C, Delmas V, Feunteun J. The hamster papovavirus: evolutionary relationships with other polyomaviruses. Virology. 1986;154:335–343. doi: 10.1016/0042-6822(86)90459-9. [DOI] [PubMed] [Google Scholar]
- 357.Voronova A F, Sefton B M. Expression of a new tyrosine protein kinase is stimulated by retrovirus promoter insertion. Nature. 1986;319:682–685. doi: 10.1038/319682a0. [DOI] [PubMed] [Google Scholar]
- 358.Wagner E F, Wang Z Q, Grigoriadis A E, Mohle-Steinlein U, Aguzzi A, Risau W. Origins of human cancer: a comprehensive review. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1991. Analysis of oncogene function in embryonal stem cells and chimeric mice; pp. 815–823. [Google Scholar]
- 359.Walter G, Ruediger R, Slaughter C, Mumby M. Association of protein phosphatase 2A with polyoma virus medium tumor antigen. Proc Natl Acad Sci USA. 1990;87:2521–2525. doi: 10.1073/pnas.87.7.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Wang E H, Friedman P N, Prives C. The murine p53 protein blocks replication of SV40 DNA in vitro by inhibiting the initiation functions of SV40 large T antigen. Cell. 1989;57:379–392. doi: 10.1016/0092-8674(89)90913-6. [DOI] [PubMed] [Google Scholar]
- 361.Wang R, Bautch V L. The polyomavirus early region gene in transgenic mice causes vascular and bone tumors. J Virol. 1991;65:5174–5183. doi: 10.1128/jvi.65.10.5174-5183.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Wang S, Ghosh R N, Chellappan S P. Raf-1 physically interacts with Rb and regulates its function: a link between mitogenic signaling and cell cycle regulation. Mol Cell Biol. 1998;18:7487–7498. doi: 10.1128/mcb.18.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Wasylyk B, Hagman J, Gutierrez-Hartmann A. Ets transcription factors: nuclear effectors of the Ras-MAP-kinase signaling pathway. Trends Biochem Sci. 1998;23:213–216. doi: 10.1016/s0968-0004(98)01211-0. [DOI] [PubMed] [Google Scholar]
- 364.Wasylyk B, Hahn S L, Giovane A. The Ets family of transcription factors. Eur J Biochem. 1993;211:7–18. doi: 10.1007/978-3-642-78757-7_2. . (Erratum, 215:907, 1993.) [DOI] [PubMed] [Google Scholar]
- 365.Wasylyk B, Imler J L, Chatton B, Schatz C, Wasylyk C. Negative and positive factors determine the activity of the polyoma virus enhancer alpha domain in undifferentiated and differentiated cell types. Proc Natl Acad Sci USA. 1988;85:7952–7956. doi: 10.1073/pnas.85.21.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Wasylyk B, Wasylyk C, Flores P, Begue A, Leprince D, Stehelin D. The c-ets proto-oncogenes encode transcription factors that cooperate with c-Fos and c-Jun for transcriptional activation. Nature. 1990;346:191–193. doi: 10.1038/346191a0. [DOI] [PubMed] [Google Scholar]
- 367.Wasylyk C, Flores P, Gutman A, Wasylyk B. PEA3 is a nuclear target for transcription activation by non-nuclear oncogenes. EMBO J. 1989;8:3371–3378. doi: 10.1002/j.1460-2075.1989.tb08500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Wasylyk C, Imler J L, Wasylyk B. Transforming but not immortalizing oncogenes activate the transcription factor PEA1. EMBO J. 1988;7:2475–2483. doi: 10.1002/j.1460-2075.1988.tb03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Wasylyk C, Wasylyk B, Heidecker G, Huleihel M, Rapp U R. Expression of raf oncogenes activates the PEA1 transcription factor motif. Mol Cell Biol. 1989;9:2247–2250. doi: 10.1128/mcb.9.5.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Webster M A, Hutchinson J N, Rauh M J, Muthuswamy S K, Anton M, Tortorice C G, Cardiff R D, Graham F L, Hassell J A, Muller W J. Requirement for both Shc and phosphatidylinositol 3′ kinase signaling pathways in polyomavirus middle T-mediated mammary tumorigenesis. Mol Cell Biol. 1998;18:2344–2359. doi: 10.1128/mcb.18.4.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Weihua X, Ramanujam S, Lindner D J, Kudaravalli R D, Freund R, Kalvakolanu D V. The polyoma virus T antigen interferes with interferon-inducible gene expression. Proc Natl Acad Sci USA. 1998;95:1085–1090. doi: 10.1073/pnas.95.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Weil R, Kara J. Polyoma “tumor antigen”: an activator of chromosome replication? Proc Natl Acad Sci USA. 1970;67:1011–1017. doi: 10.1073/pnas.67.2.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Weil R, Michel M R, Ruschmann G K. Induction of cellular DNA synthesis by polyoma virus. Proc Natl Acad Sci USA. 1965;53:1468–1475. doi: 10.1073/pnas.53.6.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Wennstrom S, Siegbahn A, Yokote K, Arvidsson A K, Heldin C H, Mori S, Claesson-Welsh L. Membrane ruffling and chemotaxis transduced by the PDGF beta-receptor require the binding site for phosphatidylinositol 3′ kinase. Oncogene. 1994;9:651–660. [PubMed] [Google Scholar]
- 375.Whitman M, Downes C P, Keeler M, Keller T, Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988;332:644–646. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- 376.Whitman M, Kaplan D R, Schaffhausen B, Cantley L, Roberts T M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985;315:239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- 377.Wiestler O D, Aguzzi A, Williams R L, Wagner E F, Boulter C A, Kleihues P. Tumour induction in fetal brain transplants exposed to the viral oncogenes polyoma middle T and v-src. IARC Sci Publ. 1989;1989:267–274. [PubMed] [Google Scholar]
- 378.Williams R L, Courtneidge S A, Wagner E F. Embryonic lethalities and endothelial tumors in chimeric mice expressing polyoma virus middle T oncogene. Cell. 1988;52:121–131. doi: 10.1016/0092-8674(88)90536-3. [DOI] [PubMed] [Google Scholar]
- 379.Williams R L, Risau W, Zerwes H G, Drexler H, Aguzzi A, Wagner E F. Endothelioma cells expressing the polyoma middle T oncogene induce hemangiomas by host cell recruitment. Cell. 1989;57:1053–1063. doi: 10.1016/0092-8674(89)90343-7. [DOI] [PubMed] [Google Scholar]
- 380.Wilson J B, Hayday A, Courtneidge S, Fried M. A frameshift at a mutational hotspot in the polyoma virus early region generates two new proteins that define T-antigen functional domains. Cell. 1986;44:477–487. doi: 10.1016/0092-8674(86)90469-1. [DOI] [PubMed] [Google Scholar]
- 381.Wirth J J, Amalfitano A, Gross R, Oldstone M B, Fluck M M. Organ- and age-specific replication of polyomavirus in mice. J Virol. 1992;66:3278–3286. doi: 10.1128/jvi.66.6.3278-3286.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Wisdom R. AP-1: one switch for many signals. Exp Cell Res. 1999;253:180–185. doi: 10.1006/excr.1999.4685. [DOI] [PubMed] [Google Scholar]
- 383.Wyss A, Kaech S, Ballmer-Hofer K. Myristylation of pp60c-src is not required for complex formation with polyomavirus middle-T antigen. J Virol. 1990;64:5163–5166. doi: 10.1128/jvi.64.10.5163-5166.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Xin J H, Cowie A, Lachance P, Hassell J A. Molecular cloning and characterization of PEA3, a new member of the Ets oncogene family that is differentially expressed in mouse embryonic cells. Genes Dev. 1992;6:481–496. doi: 10.1101/gad.6.3.481. [DOI] [PubMed] [Google Scholar]
- 385.Yamaguchi M, Hayashi Y, Matsuoka S, Takahashi T, Matsukage A. Differential effect of p53 on the promoters of mouse DNA polymerase beta gene and proliferating-cell-nuclear-antigen gene. Eur J Biochem. 1994;221:227–237. doi: 10.1111/j.1432-1033.1994.tb18733.x. [DOI] [PubMed] [Google Scholar]
- 386.Yamanashi Y, Fukushige S, Semba K, Sukegawa J, Miyajima N, Matsubara K, Yamamoto T, Toyoshima K. The yes-related cellular gene lyn encodes a possible tyrosine kinase similar to p56lck. Mol Cell Biol. 1987;7:237–243. doi: 10.1128/mcb.7.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Yi X, Freund R. Deletion of proline-rich domain in polyomavirus T antigens results in virus partially defective in transformation and tumorigenesis. Virology. 1998;248:420–431. doi: 10.1006/viro.1998.9246. [DOI] [PubMed] [Google Scholar]
- 388.Yoo W, Martin M E, Folk W R. PEA1 and PEA3 enhancer elements are primary components of the polyomavirus late transcription initiator element. J Virol. 1991;65:5391–5400. doi: 10.1128/jvi.65.10.5391-5400.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Young L S, Dawson C W, Eliopoulos A G. Viruses and apoptosis. Br Med Bull. 1997;53:509–521. doi: 10.1093/oxfordjournals.bmb.a011627. [DOI] [PubMed] [Google Scholar]
- 390.Zhu W, Eicher A, Leber B, Andrews D W. At the onset of transformation polyomavirus middle-T recruits shc and src to a perinuclear compartment coincident with condensation of endosomes. Oncogene. 1998;17:565–576. doi: 10.1038/sj.onc.1201979. [DOI] [PubMed] [Google Scholar]
- 391.Ziegler S F, Marth J D, Lewis D B, Perlmutter R M. Novel protein-tyrosine kinase gene (hck) preferentially expressed in cells of hematopoietic origin. Mol Cell Biol. 1987;7:2276–2285. doi: 10.1128/mcb.7.6.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Zullo J, Stiles C D, Garcea R L. Regulation of c-myc and c-fos mRNA levels by polyomavirus: distinct roles for the capsid protein VP1 and the viral early proteins. Proc Natl Acad Sci USA. 1987;84:1210–1214. doi: 10.1073/pnas.84.5.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]