Abstract
Background and purpose
Among the most common post-COVID symptoms, many patients experienced subjective cognitive deficit, commonly named “brain fog,” that might be present also in those individuals without severe acute COVID-19 respiratory involvement. Some studies have investigated some of the mechanisms that might be associated with the brain fog with objective techniques including transcranial magnetic stimulation and neuroimaging.
Methods
The aim of this study was to investigate the presence of electroencephalographic (EEG) alterations in people with post-COVID self-reported cognitive deficit.
Results
Out of the 90 patients attending the post-COVID neurology ambulatory service, twenty patients presenting brain fog at least 4 weeks after acute non-severe COVID-19 infection, and without previous history of epilepsy, were investigated with 19-channel EEG, Montreal Cognitive Assessment (MoCA), and magnetic resonance imaging (MRI). EEG was found altered in 65% of the sample, among which 69% presented a slowing activity and 31% were characterized by epileptic discharges principally in the frontal areas. None of the patients showed DWI MRI lesions.
Conclusions
These findings highlight the usefulness of EEG analysis to objectively describe possible neurophysiological abnormalities in post-COVID patients presenting subjective cognitive deficit.
Keywords: COVID-19, Post-COVID-19, EEG, Cognitive impairment
Introduction
Novel coronavirus disease (COVID-19) represents a worldwide burden not only due to the severe respiratory symptoms during the acute phase of the infection, but also for the plethora of complications which may result in the different stages of the disease. During the acute stage, neurological manifestations have been consistently described in a large proportion of COVID-19 patients, ranging from ageusia, anosmia, and headache to more severe conditions such as stroke, seizures, encephalopathy, impaired consciousness, and peripheral nervous system involvement [1–3].
However, neurological involvement seems to persist also after recovery from the acute phase of SARS-CoV-2 infection, which may manifest as cognitive impairment, sleep disturbances, increased fatigue, and autonomic disorders [4–6]. This post-acute phase when different clinical manifestations are still present (or appear ex novo) has been defined “post-acute COVID-19” and defined as a syndrome characterized by persistent symptoms and/or delayed or long-term complications beyond 4 weeks from the onset of COVID-19 [7], although some other authors used the name “long-COVID” with different time-course definitions [8]. These neurological manifestations have been suggested to not depend on the severity of the infection and therefore may manifest also in paucisymptomatic COVID-19 patients [7].
Cognition, in particular, may be impaired after the acute phase of the disease, as follow-up investigations 2 to 4 months after COVID-19 have reported a prevalence of cognitive deficits in 36% of the patients [9, 10]. Despite the burden of these symptoms in the post-acute COVID-19 period, which severely impact individuals quality of life, sometimes these patients are stigmatized as “functional” and not properly assessed [4]. Electroencephalography (EEG) represents an objective non-invasive tool to determine neuronal activity and a prime candidate functional marker of synapse dysfunction and loss in cognitive impairment [11]. During acute COVID-19, EEG has been commonly indicated in those patients with altered mental status and seizure-like events [12, 13], showing frequent abnormal background activity and generalized slowing, and epileptic discharge in up to 20% of the investigated individuals [14]. Therefore, EEG in severe COVID-19 patients has been recommended to monitor brain activity, as commonly performed also in severe intensive care unit patients [15, 16] and acute brain ischemia [17, 18].
The aim of the present short clinical report was to investigate the EEG patterns of the paucisymptomatic COVID-19 patients, who reported a subjective cognitive impairment in the post-acute period.
Material and methods
Participants who referred to the post-COVID neurology ambulatory service of the University Hospital of Trieste from 01.01.2021 to 31.03.2021 were screened for the presence of self-reported cognitive impairment in the post-acute COVID-19 period (diagnosis confirmed by SARS-CoV-2 nasopharyngeal swab). The cognitive impairment had to be present (persistent or ex-novo) at least after 4 weeks from acute COVID-19 symptoms manifestation. Exclusion criteria were: age < 18 y or > 65 year, previous neurological or psychiatric diseases, major vascular alterations to the neuroimaging study, previous history of cognitive deficits, and consumption of agents affecting the nervous system (e.g., antipsychotic, antidepressant or antiepileptic drugs). In addition, for this study, we excluded those patients who suffered from moderate/severe COVID-19 disease, defined as a positive patient to SARS-COV2 with clinical and radiographic evidence of lower respiratory tract disease. All the individuals who presented to the post-COVID neurology ambulatory service reported at least one neurological clinical manifestation, and those reporting a subjective cognitive impairment after the initial clinical evaluation were prospectively assessed with the Montreal Cognitive Assessment (MoCA) screening test and EEG recording. Participants were further investigated with neuroimaging (computed tomography, CT, or magnetic resonance imaging, MRI) and routine blood biochemical analysis. Both EEG and MRI were performed within 20 days from the neurological evaluation. All MRI performed included T1, T2, FLAIR, and DWI sequences.
The MoCA was administered after the first visit by a trained neurologist using the validated Italian version [19] and was further described by domain scores [20] based on single item scores (orientation: spatial and temporal orientation; attention: digit span, letter A tapping, subtraction; executive: trail making, abstraction, word fluency; visuoconstructive: cube copying, clock drawing; language: naming, sentence repetition; memory: delayed word recall). The global MoCA test score was corrected for years of education (YoE; + 1 point if ≤ 12 YoE). Domain scores were not adjusted for YoE. A cut-off of 26 was used to define mild cognitive impairment (MCI) [20]. The fatigue severity scale (FSS), consisting of 9 sentences related to the interference of fatigue with daily activities and subjectively rating its severity on a 7-point scale (1 = “strongly disagree”; 7 = “strongly agree”), was administered during the visit [21].
Nineteen-channel (10–20 system) 20-min standard clinical surface EEG was acquired by using the Be Plus PRO amplifier (EB NEURO, Florence, Italy) and Ag/AgCl electrodes. All patients underwent photic stimulation and hyperventilation during the recording. All electrode impedances were kept below 5 k Ω, and the sampling rate was set to 128 Hz. EEG signals were filtered by second-order band-pass Butterworth filter with 0.1–30 Hz cut-off frequencies, and the epochs containing artifacts were discarded. Brain oscillatory activities were assessed by standard clinical qualitative visual inspection of EEG tracings by two experienced neurologists (G.F. and P.M.), in order to identify clinically significant epileptiform patterns and EEG rhythm alterations. In addition, we also performed the quantitative EEG analysis. The offline analysis was performed by scripts developed in MATLAB (MathWorks Inc., Natick, MA). Power spectral density (PSD) for each channel was estimated on 60 s artifact-free EEG epochs by using Welch’s periodogram. The relative power for each of the spectral bands (delta: 1–4 Hz; theta: 4–8 Hz; alpha: 8–13 Hz; beta: 13–30 Hz) was calculated for each channel. The relative powers were obtained by normalizing with a total power across the 1–30 Hz range. Relative power for each spectral band was averaged over all nineteen channels.
Statistical analysis
Single data were reported for each patient included in the present case series. Descriptive analysis, including medians (25–75th percentile) for continuous variables and proportions (%) for categorical variables, has been used to summarize the results. To explore a possible correlation between equivalent scores on MoCA test with Aiello correction and the presence or absence of EEG abnormalities, a Fisher test was performed as appropriate.
Data availability
The authors confirm that the presented data of this study are saved at the Clinical Unit of Neurology, Trieste University Hospital ASUGI, Italy. They are available upon reasonable request and according to the local institutional and ethics regulation.
Results
From January 2021 to April 2021, 114 individuals with one or more neurological symptoms in the post-acute COVID-19 period attended the post-COVID neurology ambulatory services. Twenty-two reported a self-reported cognitive deficit, and two of them were excluded due to severe COVID-19 symptoms and pre-existing epilepsy. Twenty were then included and performed the cognitive and EEG evaluation (49 ± 11 year, 14 females). Demographic and clinical characteristics are presented in Table 1. All participants underwent magnetic resonance imaging, and none of them presented lesions in DWI. Other comorbidities included migraine (20%), cardiomyopathy (5%), hypertension (10%), asthma (10%), dyslipidemia/diabetes (35%), history of neoplasia (10%), hypothyroidism (10%), and obesity (10%). During the acute phase of SARS-CoV-2 infection, main symptoms were upper respiratory airways involvement (70%), dyspnea (60%), fever (80%), headache (80%), myalgia/arthralgia (65%), hyposmia (45%), tachycardia (15%), and diarrhea/gastrointestinal distress (30%). Only 10% were hospitalized and underwent oxygen antibiotics, and 15% received prophylactic low molecular weight heparin.
Table 1.
Demographics and clinical characteristics of the included post-acute COVID-19 neurological patients
| Subject | Sex | Age | Years of study | Time from onset of COVID-19 (days) | Post-acute COVID-19 neurological symptoms |
|---|---|---|---|---|---|
| 1 | F | 39 | 8 | 80 | |
| 2 | F | 48 | 16 | 88 | |
| 3 | F | 43 | 13 | 33 | Hyposmia |
| 4 | F | 48 | 12 | 79 | Headache, hyposmia, dizziness, paresthesia, tinnitus |
| 5 | M | 58 | 15 | 59 | |
| 6 | M | 21 | 13 | 121 | Tinnitus |
| 7 | F | 51 | 12 | 97 | |
| 8 | M | 47 | 16 | 113 | Hyposmia |
| 9 | F | 48 | 16 | 66 | |
| 10 | F | 52 | 13 | 42 | Headache, dizziness |
| 11 | M | 60 | 10 | 76 | |
| 12 | F | 58 | 10 | 360 | |
| 13 | M | 57 | 8 | 160 | |
| 14 | F | 60 | 13 | 155 | Hyposmia |
| 15 | F | 55 | 12 | 149 | Myalgia |
| 16 | F | 52 | 17 | 159 | Headache, dizziness |
| 17 | F | 34 | 13 | 394 | |
| 18 | F | 64 | 17 | 126 | |
| 19 | F | 38 | 13 | 128 | |
| 20 | M | 40 | 16 | 76 | Hyposmia, myalgia |
Subjective cognitive impairment was reported to manifest from 2 weeks to 2 months after SARS-CoV-2 infection. At the time of the evaluation, MoCA score was found indicative of mild cognitive impairment in 14 subjects, with a median score of 24.3 (22.0–26.9). Fatigue severity scale ranges from 2.90 to 6.80. Abnormal EEG was found in 65% of the sample, among which 69% presented a slowing activity and 31% were characterized by epileptic discharges. Among the abnormal EEG, 46% were characterized by an asymmetric pattern, and 62% presented a prevalent frontal involvement (Table 2). After EEG evaluation, 15% of the sample presented one episode of focal seizure and started antiepileptic therapy. The median (IQR) values of delta, theta, alpha, and beta relative powers in our sample were δ = 0.28 (0.16–0.38), θ = 0.15 (0.13–0.18), α = 0.32 (0.19–0.46), and β = 0.16 (0.14–0.26), respectively.
Table 2.
Cognitive evaluation (MoCA score), fatigue (FSS), and qualitative EEG in the post-acute COVID-19 neurological patients
| Subject | MoCA | FSS | EEG | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C. score | U. score | Visuocons | Executive | Attention | Language | Memory | Orientation | |||
| 1 | 26.98 | 26 | 4 | 3 | 6 | 4 | 3 | 6 | 4.10 | Normal |
| 2 | 24.15 | 27 | 4 | 4 | 6 | 5 | 3 | 6 | 6.20 | Bilateral frontal slow wave |
| 3 | 24.15 | 25 | 4 | 4 | 6 | 6 | 0 | 6 | 6.78 | Normal |
| 4 | 23.15 | 24 | 2 | 4 | 3 | 5 | 4 | 6 | 6.20 | Bilateral frontotemporal slow wave |
| 5 | 21.52 | 24 | 4 | 3 | 6 | 4 | 3 | 4 | 6.80 | Normal |
| 6 | 21.59 | 23 | 2 | 4 | 4 | 5 | 4 | 5 | 4.50 | Left hemisphere slow wave |
| 7 | 24.52 | 25 | 4 | 4 | 4 | 4 | 2 | 6 | 2.90 | Right frontal epileptiform discharges |
| 8 | 22.15 | 25 | 4 | 4 | 5 | 6 | 2 | 6 | 2.90 | Normal |
| 9 | 30 | 30 | 4 | 4 | 6 | 6 | 5 | 6 | 4.30 | Bilateral epileptiform discharges |
| 10 | 24.52 | 25 | 4 | 4 | 3 | 4 | 4 | 6 | 6.80 | Bilateral slow waves |
| 11 | 19.98 | 20 | 2 | 3 | 4 | 4 | 1 | 6 | 6.50 | Bilateral slow waves |
| 12 | 27.52 | 28 | 4 | 3 | 5 | 5 | 5 | 6 | 6.20 | Normal |
| 13 | 27.65 | 26 | 4 | 4 | 6 | 6 | 4 | 6 | 4.50 | Bilateral frontotemporal slow waves |
| 14 | 26.52 | 27 | 4 | 4 | 4 | 6 | 4 | 6 | 6.40 | Left frontal epileptic discharges |
| 15 | 28.52 | 29 | 4 | 3 | 6 | 6 | 5 | 6 | 4.50 | Normal |
| 16 | 25.52 | 28 | 4 | 4 | 5 | 6 | 4 | 6 | 5.00 | Right frontotemporal slow waves |
| 17 | 21.98 | 21 | 3 | 2 | 3 | 5 | 2 | 6 | 6.00 | Normal |
| 18 | 21.98 | 24 | 3 | 2 | 4 | 5 | 5 | 6 | 5.00 | Bilateral frontotemporal slow waves |
| 19 | 23.15 | 26 | 4 | 3 | 6 | 5 | 2 | 6 | 4.70 | Left temporal slow waves |
| 20 | 25.15 | 29 | 4 | 3 | 6 | 6 | 5 | 6 | 6.0 | Left frontal epileptic discharges |
After adjusting MoCA results with Aiello correction for Northern Italian population [22], among the 13 patients with EEG abnormalities, 5 had an equivalent score (ES) = 4, 4 patients had an ES = 3, 2 patients had an ES = 2, and 2 scored 1. In the normal EEG group (7 patients), 3 had an ES of 4, none had an ES = 3, 3 patients had an ES = 2, and the remaining one scored 1 (Table 3). After statistical analysis (Fisher test), no correlation was found between equivalent scores on MoCA test with Aiello correction and the presence or absence of EEG abnormalities (p = 0.40) (Table 3). The mean FSS for patients with EEG abnormalities was 5.31 ± 1.14 (2.90–6.80), while mean FSS for patients with normal EEG was 5.33 ± 1.50 (2.90–6.80). No significant difference in FSS values among these cohorts was found after statistical analysis (p = 0.98). In addition, no significant correlation was observed between uncorrected/corrected MoCA and FSS (r = − 0.17, p = 0.484, and r = − 0.19, p = 0.418, respectively).
Table 3.
Number (%) of patients with/without EEG abnormalities divided for equivalent score (ES) after Aiello’s MoCA correction
| Abnormal EEG group | Normal EEG group | |
|---|---|---|
| ES = 4 | 5 (25%) | 3 (15%) |
| ES = 3 | 4 (20%) | 0 (0%) |
| ES = 2 | 2 (10%) | 3 (15%) |
| ES = 1 | 2 (10%) | 1 (5%) |
Discussion
As the COVID-19 pandemic progresses, a growing number of reports describe the frequent neurological involvement during the acute and post-acute phase of the infection [7, 23]. Most of these reports evaluate these neurological manifestations through clinical observations and subjective reporting.
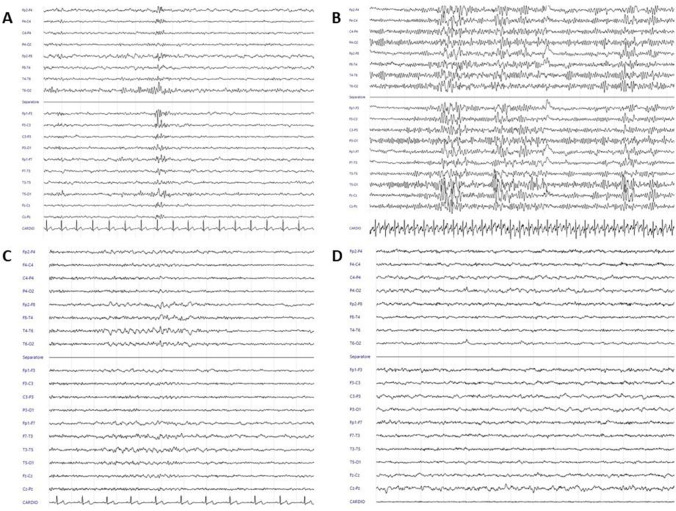
This study proposes EEG analysis as an objective tool to identify abnormal neurophysiological function in people with self-reported cognitive impairment in the post-COVID period. In particular, of 20 individuals who participated in the data collection, EEG was found indicative of epileptic discharges in 4 subjects, while 9 individuals presented a delta-slowing pattern (example in Fig. 1). As such, about two-thirds of the overall sample were characterized by unexpected abnormal EEG patterns.
Fig. 1.
A Male, 40 years, Montreal Cognitive Assessment 25.15, left frontal epileptiform discharge with contiguous and contralateral channels diffusion. B Female, 51 years, Montreal Cognitive Assessment 24.52, bilateral frontotemporal epileptiform discharges. C Female, 52 years, Montreal Cognitive Assessment 25.52, bilateral frontotemporal theta slowing with slight right prevalence. D Male, 60 years, Montreal Cognitive Assessment 19.98, diffuse theta-delta slowing
To the best of the authors’ knowledge, this is the first report presenting EEG pattern abnormalities in people with self-reported post-COVID cognitive impairment. Indeed, “brain fog” is among the most commonly reported post-COVID neurological manifestations [23], and among the patients who referred to the post-COVID neurology ambulatory service, 24% complained about a cognitive deficit, which was confirmed by a MoCA score indicative of MCI in 14 individuals (around 2/3 of the sample). Previous reports suggest a prevalence of post-COVID cognitive impairment in 7.5–31% of the post-COVID individuals [24], but it could be one of the more frequent complaints in those presenting post-COVID symptoms [23, 25]. A high proportion of our sample was characterized by elevated scores of the FSS, confirming fatigue as one of the most common symptoms during the post-COVID period [26, 27]. A previous neurophysiological investigation using transcranial magnetic stimulation (TMS) in post-COVID individuals with fatigue and dysexecutive function showed a markedly reduced GABAergic inhibition in the primary motor cortex (M1) and a slightly reduced short-latency afferent inhibition (SAI) that represents central cholinergic transmission [5, 28]. Taken together, these findings suggest a possible effect of COVID-19 syndrome on cortical transmission which might be objectively measured by neurophysiological techniques. Indeed, the association between cortical excitability measured by TMS and brain oscillatory activity measured by EEG has been reported in physiological brain aging and in different neurological diseases, including epilepsy and MCI [29–31].
SARS-CoV-2 have been rarely detected in cerebrospinal fluid and brain tissue; thus, so far many authors suggest widespread glial cell activation, possibly related metabolic dysfunction, and further exacerbated inflammatory response and blood–brain barrier dysfunction, as key mechanisms to central nervous system involvement. Other factors have been suggested to potentially result in post-COVID cognitive impairment, as microvascular infarcts and hemorrhages, or the effects of respiratory distress, hypoxia, prolonged mechanical ventilation, and sedation [7, 32]; however, in our sample, it is reasonable to exclude such mechanisms based on neuroimaging findings (none of the participant presented vascular lesions on MRI) and the exclusion of severely affected acute COVID-19 patients.
Although EEG activity has been scarcely investigated in the post-COVID period, more data are available during the acute phase of COVID-19. A recent meta-analysis of the literature found a prevalence of abnormal background activity in 96.1% of the acute COVID-19 patients, with 20.3% of epileptiform discharges and 92.3% of slowing activity [14]. In those patients with altered mental status and seizure, SARS-CoV-2-related encephalitis might be hypothesized due to a cytokine release syndrome [33] or autoimmune mechanisms [34]. EEG can support this diagnosis, showing improved background activity after intravenous immunoglobulin treatment [13]. Unfortunately, in our sample, EEG was not performed during the acute phase of the infection, and it is not possible to determine how many were characterized by electrocortical abnormalities which were already present during the early stage of the disease.
Some reports suggest a cognitive deficit characterized by a predominant executive dysfunction [23, 28, 35, 36]. A further involvement of the frontal lobe structures has been corroborated by positron emission tomography (PET) findings, showing glucose hypometabolism in the fronto-parietal cortex which slowly improved with time and was correlated with MoCA score [9]. EEG findings from our study showed among the abnormal patterns, 62% presented a prevalent frontal involvement, in line with both the reported neuropsychological assessments and the above-mentioned brain metabolism results. The EEG slowing observed by qualitative EEG analysis is supported by the results of quantitative analysis. Indeed, the relative delta power values in our sample were higher than those reported in literature for the healthy subjects [17, 37]. EEG slowing has been suggested to reflect brain hypoperfused areas in acute ischemic stroke patients and might be considered as an index of neurovascular coupling [17]; nevertheless, there is a lack of data about brain perfusion in post-COVID patients presenting cognitive impairment, and future studies should address the association between brain metabolism, perfusion, and oscillatory activity.
Taken together, these findings confirm previous observations suggesting a high prevalence of cognitive impairment among the most reported post-COVID symptoms, and most of those reporting a subjective cognitive deficit were characterized by MoCA scores suggestive of MCI. This study provides evidence of altered EEG traces in most of these individuals, which might be predominant in the frontal areas. Among the four patients who were found with an epileptiform EEG, three of them presented at least one focal seizure in the following 4 weeks (never presented before COVID-19). As known from the previous literature, the presence of IEDs is related with decreased attention and altered cognition faculties [38]. This finding encourages the use of EEG analysis in the post-COVID period, and in particular in those with subjective cognitive deficit, to detect possible abnormal traces that might predict the occurrence of new seizures. A limitation of this study is represented by the small number of subjects who performed both the neuropsychological evaluation and the EEG recording, as well as the qualitative nature of the EEG analysis. Another limitation was represented by the lack of a control group of cognitively healthy post-COVID patients who underwent EEG. Further studies on larger study samples are mandatory to confirm the results and to investigate the nature of cognitive disorders in post-COVID-19 syndrome. Despite these limitations, this study presents data from an acute respiratory pauci-asymptomatic sample of COVID-19 patients, who developed a wide range of post-COVID symptoms, including “brain fog.”
Conclusions
In conclusion, these findings suggest the use of objective techniques (e.g., EEG) to better understand the neurophysiological alterations underlying post-COVID cognitive deficit. Indeed, in these patients with mild cognitive symptoms, EEG pattern might be characterized by a predominant slowing, and to a lesser extent, epileptiform discharges, in particular in the frontal areas.
Acknowledgements
The authors would like to thank Matteo di Franza for editorial assistance and english proof-reading.
Declarations
Ethical approval
All procedures performed in study were approved by the CEUR FVG ethical committee in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Giovanni Furlanis and Alex Buoite Stella have contributed equally.
References
- 1.Sharifian-Dorche M, Huot P, Osherov M, et al. Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic. J Neurol Sci. 2020;417:117085. doi: 10.1016/j.jns.2020.117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manganotti P, Pesavento V, Buoite Stella A, et al. Miller Fisher syndrome diagnosis and treatment in a patient with SARS-CoV-2. J Neurovirol. 2020;26(4):605–606. doi: 10.1007/s13365-020-00858-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manganotti P, Bellavita G, D’Acunto L, et al. Clinical neurophysiology and cerebrospinal liquor analysis to detect Guillain-Barré syndrome and polyneuritis cranialis in COVID-19 patients: a case series. J Med Virol. 2021;93(2):766–774. doi: 10.1002/jmv.26289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nath A. Long-Haul COVID. Neurology. 2020;95(13):559–560. doi: 10.1212/WNL.0000000000010640. [DOI] [PubMed] [Google Scholar]
- 5.Ortelli P, Ferrazzoli D, Sebastianelli L, et al. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: Insights into a challenging symptom. J Neurol Sci. 2021;420:117271. doi: 10.1016/j.jns.2020.117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buoite Stella A, Furlanis G, Frezza NA, Valentinotti R, Ajcevic M, Manganotti P (2021) Autonomic dysfunction in post-COVID patients with and witfhout neurological symptoms: a prospective multidomain observational study. J Neurol :1–10. 10.1007/s00415-021-10735-y [DOI] [PMC free article] [PubMed]
- 7.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alwan NA, Johnson L. Defining long COVID: going back to the start. Med (New York, NY) 2021;2(5):501–504. doi: 10.1016/j.medj.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blazhenets G, Schröter N, Bormann T, et al. Slow but evident recovery from neocortical dysfunction and cognitive impairment in a series of chronic COVID-19 patients. J Nucl Med. 2021 doi: 10.2967/jnumed.121.262128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Borst B, Peters JB, Brink M, et al. Comprehensive health assessment three months after recovery from acute COVID-19. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2020 doi: 10.1093/cid/ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koenig T, Smailovic U, Jelic V. Past, present and future EEG in the clinical workup of dementias. Psychiatry Res Neuroimaging. 2020;306:111182. doi: 10.1016/j.pscychresns.2020.111182. [DOI] [PubMed] [Google Scholar]
- 12.Antony AR, Haneef Z. Systematic review of EEG findings in 617 patients diagnosed with COVID-19. Seizure. 2020;83:234–241. doi: 10.1016/j.seizure.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manganotti P, Furlanis G, Ajčević M et al (2021) Intravenous immunoglobulin response in new-onset refractory status epilepticus (NORSE) COVID-19 adult patients. J Neurol :1–5. 10.1007/s00415-021-10468-y [DOI] [PMC free article] [PubMed]
- 14.Kubota T, Gajera PK, Kuroda N. Meta-analysis of EEG findings in patients with COVID-19. Epilepsy Behav. 2021;115:107682. doi: 10.1016/j.yebeh.2020.107682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grippo A, Amantini A. Continuous EEG on the intensive care unit: terminology standardization of spectrogram patterns will improve the clinical utility of quantitative EEG. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2020;131(9):2281–2283. doi: 10.1016/j.clinph.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Grippo A, Assenza G, Scarpino M et al (2020) Electroencephalography during SARS-CoV-2 outbreak: practical recommendations from the task force of the Italian Society of Neurophysiology (SINC), the Italian League Against Epilepsy (LICE), and the Italian Association of Neurophysiology Technologists (A. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol :1–7. 10.1007/s10072-020-04585-1 [DOI] [PMC free article] [PubMed]
- 17.Ajčević M, Furlanis G, Miladinović A, et al. Early EEG alterations correlate with CTP Hypoperfused volumes and neurological deficit: a wireless EEG study in hyper-acute ischemic stroke. Ann Biomed Eng. 2021 doi: 10.1007/s10439-021-02735-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stragapede L, Furlanis G, Ajcevic M et al (2019) Brain oscillatory activity and CT perfusion in hyper-acute ischemic stroke. J Clin Neurosci :pii: S0967–5868(19)30948–8. 10.1016/j.jocn.2019.07.068 [DOI] [PubMed]
- 19.Pirrotta F, Timpano F, Bonanno L, et al. Italian validation of Montreal Cognitive Assessment. Eur J Psychol Assess. 2014;31(2):131–137. doi: 10.1027/1015-5759/a000217. [DOI] [Google Scholar]
- 20.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 21.Siciliano M, Chiorri C, De Micco R, et al. Fatigue in Parkinson’s disease: Italian validation of the Parkinson fatigue scale and the fatigue severity scale using a Rasch analysis approach. Park Relat Disord. 2019;65(April):105–110. doi: 10.1016/j.parkreldis.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 22.Aiello EN, Gramegna C, Esposito A, et al. The Montreal Cognitive Assessment (MoCA): updated norms and psychometric insights into adaptive testing from healthy individuals in Northern Italy. Aging Clin Exp Res. 2022;34(2):375–382. doi: 10.1007/s40520-021-01943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham EL, Clark JR, Orban ZS, et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann Clin Transl Neurol. 2021;8(5):1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walitt B, Bartrum E. A clinical primer for the expected and potential post-COVID-19 syndromes. Pain Rep. 2021;6(1):e887. doi: 10.1097/PR9.0000000000000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sudre CH, Murray B, Varsavsky T et al (2020) Attributes and predictors of long-COVID: analysis of COVID cases and their symptoms collected by the COVID symptoms study App. medRxiv :2020.10.19.20214494. 10.1101/2020.10.19.20214494
- 26.Townsend L, Moloney D, Finucane C, et al. Fatigue following COVID-19 infection is not associated with autonomic dysfunction. PLoS One. 2021;16(2):e0247280. doi: 10.1371/journal.pone.0247280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15(11):e0240784. doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Versace V, Sebastianelli L, Ferrazzoli D, et al. Intracortical GABAergic dysfunction in patients with fatigue and dysexecutive syndrome after COVID-19. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2021;132(5):1138–1143. doi: 10.1016/j.clinph.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guerra A, Rocchi L, Grego A, Berardi F, Luisi C, Ferreri F (2021) Contribution of TMS and TMS-EEG to the understanding of mechanisms underlying physiological brain aging. Brain Sci 11(3). 10.3390/brainsci11030405 [DOI] [PMC free article] [PubMed]
- 30.Del Felice A, Fiaschi A, Bongiovanni GL, Savazzi S, Manganotti P. The sleep-deprived brain in normals and patients with juvenile myoclonic epilepsy: a perturbational approach to measuring cortical reactivity. Epilepsy Res. 2011;96(1–2):123–131. doi: 10.1016/j.eplepsyres.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Benussi A, Grassi M, Palluzzi F, et al. Classification accuracy of TMS for the diagnosis of mild cognitive impairment. Brain Stimul. 2021;14(2):241–249. doi: 10.1016/j.brs.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Ali Awan H, Najmuddin Diwan M, Aamir A et al (2021) SARS-CoV-2 and the brain: what do we know about the causality of ’cognitive COVID? J Clin Med 10(15). 10.3390/jcm10153441 [DOI] [PMC free article] [PubMed]
- 33.Pilotto A, Masciocchi S, Volonghi I, et al. SARS-CoV-2 encephalitis is a cytokine release syndrome: evidences from cerebrospinal fluid analyses. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2021 doi: 10.1093/cid/ciaa1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solomon T (2021) Neurological infection with SARS-CoV-2 - the story so far. Nat Rev Neurol: 1–2. 10.1038/s41582-020-00453-w [DOI] [PMC free article] [PubMed]
- 35.Miners S, Kehoe PG, Love S. Cognitive impact of COVID-19: looking beyond the short term. Alzheimers Res Ther. 2020;12(1):170. doi: 10.1186/s13195-020-00744-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helms J, Kremer S, Merdji H, et al. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit Care. 2020;24(1):491. doi: 10.1186/s13054-020-03200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polverino P, Ajčević M, Catalan M, Mazzon G, Bertolotti C, Manganotti P. Brain oscillatory patterns in mild cognitive impairment due to Alzheimer’s and Parkinson’s disease: an exploratory high-density EEG study. Clin Neurophysiol. 2022;138:1–8. doi: 10.1016/j.clinph.2022.01.136. [DOI] [PubMed] [Google Scholar]
- 38.Kasteleijn-Nolst Trenité DG, Vermeiren R. The impact of subclinical epileptiform discharges on complex tasks and cognition: relevance for aircrew and air traffic controllers. Epilepsy Behav. 2005;6(1):31–34. doi: 10.1016/j.yebeh.2004.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the presented data of this study are saved at the Clinical Unit of Neurology, Trieste University Hospital ASUGI, Italy. They are available upon reasonable request and according to the local institutional and ethics regulation.