Abstract
Background
Significant progress has been made in the investigation of neoadjuvant immune-chemoradiotherapy (NICRT) and neoadjuvant immune-chemotherapy (NICT) on the outcomes of esophageal cancer patients. To summarize the current developments, a systematic review and meta-analysis were conducted to evaluate the efficacy and safety of neoadjuvant immunotherapy combined with chemoradiotherapy or chemotherapy.
Methods
A search strategy of prospective studies on esophageal cancer receiving neoadjuvant immunotherapy was predefined to scan PubMed, Embase, Cochrane, and additional major conferences for prospective studies. Efficacy was assessed by pathological complete response (pCR), major pathological response (MPR), and R0 resection rates. Safety was evaluated based on the incidence of grade ≥ 3 treatment-related adverse events (TRAEs), neoadjuvant therapy completion rate, surgical resection rate, and surgical delay rate. Differences between the NICRT and NICT groups were also analyzed.
Results
A total of 38 studies qualified for the analysis. The pooled pCR, MPR, and R0 resection rates were 30, 58, and 99%, respectively. The pCR and MPR in the NICRT vs. NICT group were 38% vs. 28% (p=0.078) and 67% vs. 57% (p=0.181), respectively. The pooled incidence of grade ≥ 3 TRAEs was 24% (NICRT,58%, I2 = 61% vs. NICT,18%, I2 = 79%; p<0.001). In addition, the pooled neoadjuvant therapy completion and surgical resection rates were 92% and 85%, respectively; the difference was not statistically significant between the NICRT and NICT groups.
Conclusions
Neoadjuvant immunotherapy combined with chemoradiotherapy or chemotherapy is effective and safe in the short term for locally advanced esophageal cancer. However, further randomized trials are needed to confirm which combined model is more favorable.
Systematic review registration
https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021284266, identifier CRD42021284266.
Keywords: efficacy, safety, neoadjuvant, immunotherapy, chemotherapy, chemoradiotherapy, esophageal cancer, meta-analysis
1. Introduction
Esophageal cancer is a serious public health threat, ranking seventh in term of incidence and sixth in term of mortality among all cancers (1). Moreover, the overall 5-year survival rate is about 20% as one of the four cancer types with lowest survival (2).
Neoadjuvant chemoradiotherapy following surgery is considered as one of the standard treatments of choice for local advanced resectable esophageal cancer, with a median overall survival (OS) of 49.4 months, and a pathological complete response (pCR) rate of 29%, as reported in the CROSS trial (3). NEOCRTEC 5010, a phase III randomized controlled trial (RCT), compared neoadjuvant chemoradiation in esophageal squamous cell carcinoma (ESCC) and reported more appealing results with a median OS of 100.1 months and a pCR of 43.2% (4). However, even after neoadjuvant chemoradiotherapy combined with surgery, the 5-year overall survival and progression-free survival (PFS) rates were approximately 47% and 44%, respectively, indicating that a considerable proportion of patients still experience progression of the disease and eventually death (5). In addition, the safety of chemoradiation is a noteworthy problem because some patients cannot tolerate neoadjuvant chemoradiotherapy (4).
Neoadjuvant chemotherapy is an alternative first-line treatment for esophageal cancer, especially in East Asia. A randomized trial, JCOG9907, showed a 5-year OS of 55% in patients with resectable ESCC receiving prior two courses of cisplatin and 5-fluorouracil (6). Perioperative chemotherapy is usually recommended for resectable esophageal adenocarcinoma (EAC). The randomized phase II/III FLOT-4 trial reported a median OS of 50 months for patients receiving the perioperative FLOT regimen, with a pCR of 15% (7). A meta-analysis including three RCTs showed a pCR of < 10% in patients having received neoadjuvant chemotherapy, which was significantly lower than in those who had received neoadjuvant chemoradiotherapy, although survival for neoadjuvant chemotherapy may not be inferior probably due to less toxicity and salvage treatment (8). Briefly, neoadjuvant chemotherapy may achieve acceptable survival rates, but its short-term efficacy is unfavorable for esophageal cancer.
Immune checkpoint inhibitors (ICIs) immunotherapy has been demonstrated to be effective in improving survival and safety in patients with stage IV esophageal cancer (9–11). Neoadjuvant chemoradiation or chemotherapy combined with ICIs for esophageal cancer have been studied in a series of small clinical trials for resectable esophageal cancer (12, 13), showing promising results. Majority of the trials focused on ESCC. A systematic search of the literature and a meta-analysis of multiple clinical trials will help summarize the evidence and provide comprehensive information for clinicians. This meta-analysis aimed to investigate the efficacy and safety of neoadjuvant immunotherapy, and to compare neoadjuvant immunotherapy combined with chemoradiotherapy or chemotherapy for resectable esophageal cancer, therefore providing evidence of neoadjuvant immunotherapy mainly for ESCC. Our main purpose for EAC was to provide a rough reference due to limited available data.
2. Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to conduct systematic reviews and meta-analyses (14).
2.1. Search strategy
A systematic search was conducted in English on PubMed, Embase, and the Cochrane Library to identify articles on neoadjuvant immunotherapy for esophageal cancer reported before July 14, 2022. Abstracts of several important international conferences, such as ASCO, ESMO, and AACR occurring up to July 14, 2022, were also inspected. Keywords included “esophageal cancer”, “neoadjuvant” and “immunotherapy”. The complete search strategy is available in the Supplementary Material. The protocol is registered in the PROSPERO database (CRD42021284266).
2.2. Study selection
The eligibility criteria were as follows: 1) patients with resectable esophageal cancer who received neoadjuvant immunotherapy combined with chemoradiotherapy or chemotherapy, 2) prospective studies with data available on pCR or major pathological response (MPR) rates, and 3) ICIs are currently used in clinical practice or in registered trials.
The exclusion criteria were as follows: 1) the number of patients available for analysis was less than 10, 2) repeated publications, 3) case reports, reviews, and experimental reports and 4) lack of valid data.
2.3. Data extraction
The following information was extracted: first author, publication year, clinical trial registry number, intervention model, study phase, article type, neoadjuvant therapy mode, main inclusion criteria, ICI drug and dose, sample size, sex, median/mean age, pathological complete response (pCR), major pathological response (MPR), R0 resection rate, incidence of ≥ grade 3 treatment-related adverse events (TRAEs), neoadjuvant therapy completion rate (NTCR), surgical resection rate, and surgical delay rate. The rates of pCR, MPR, and R0 resection were calculated based on patients who had undergone surgery. pCR was defined as the absence of residual tumor in all resected specimens (i.e., ypT0N0). MPR referred to 10% or less of residual viable cancer cells after neoadjuvant therapy (15, 16). R0 corresponded to microscopic margin-negative resection. The incidence of ≥grade 3 TRAEs, NTCR and surgical resection rate were calculated based on the intention-to-treat population. TRAEs≥grade 3 were counted based on the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE). NTCR was defined as the ratio of patients who successfully completed the entire course of neoadjuvant therapy within the total number of enrolled patients. The surgical resection rate refered to the ratio of patients receiving surgery to those whose surgery was anticipated. Two authors (LYS and BYX) independently reviewed articles and extracted the data from the included trials. Disagreements were resolved through discussion between the two reviewers until a consensus was reached.
2.4. Statistical analysis
The random effects model using DerSimonian-Laird method was applied because homogeneity might not be validated under the current scenario. The random effects model considers the case in which the effect size is more variable when compared with a homogeneous population. The pooled results were presented as incidence rates with 95% confidence interval (CI). The pooled proportion was calculated with the formula: logit(P)=ln(P/(1-P)). Between-study heterogeneity was assessed using tau2 and I2 statistics. A p-value>0.1 and an I2<50% indicated that the heterogeneity was acceptable. A comparative analysis was carried out between the neoadjuvant immune-chemoradiotherapy (NICRT) group and the neoadjuvant immune-chemotherapy (NICT) group to explore the respective efficacy and safety, as well as acquire a preliminary comparison. The difference between subgroups was tested using the chi-square test, and statistical significance was set at p < 0.05. Notably, some of the studies were still ongoing; we did not include those without all enrolled patients reaching the planned surgery time when pooling the neoadjuvant therapy completion rate, incidence of ≥grade 3 TRAEs, surgical resection rate, and surgical delay rate because of the incomplete incidence of events.
Exploratory subgroup analysis was performed to evaluate the source of heterogeneity and the association between clinical factors and outcomes. Sensitivity analysis was conducted using leave-one-out method by successively omitting each included study, to examine whether the pooled results were affected by a single study. Egger’s and Begg’s tests were performed to evaluate the publication bias of outcomes with no fewer than 10 available studies. Statistical analyses were performed using the R 4.1.0 program.
2.5. Risk of bias and certainty assessment
Since the studies were non-randomized, the risk of bias was assessed following the methodological index for non-randomized studies (MINORS) index (17) which considers the following aspects: a clearly stated aim, inclusion of consecutive patients, prospective collection of data, endpoints appropriate to the aim of the study, unbiased assessment of the study endpoint, follow-up period appropriate to the aim of the study, loss to follow-up of less than 5%, prospective calculation of the study size, adequate control group, contemporary groups, baseline equivalence of groups, and adequate statistical analyses. Certainty of evidence was evaluated using GRADE approach (18), which defines the certainty of evidence into 4 categories: high certainty, moderate certainty, low certainty and very low certainty. The assessments were conducted independently by two researchers, and in case of disagreement, a decision was made through discussion until a consensus was reached.
3. Results
3.1. Identification of studies
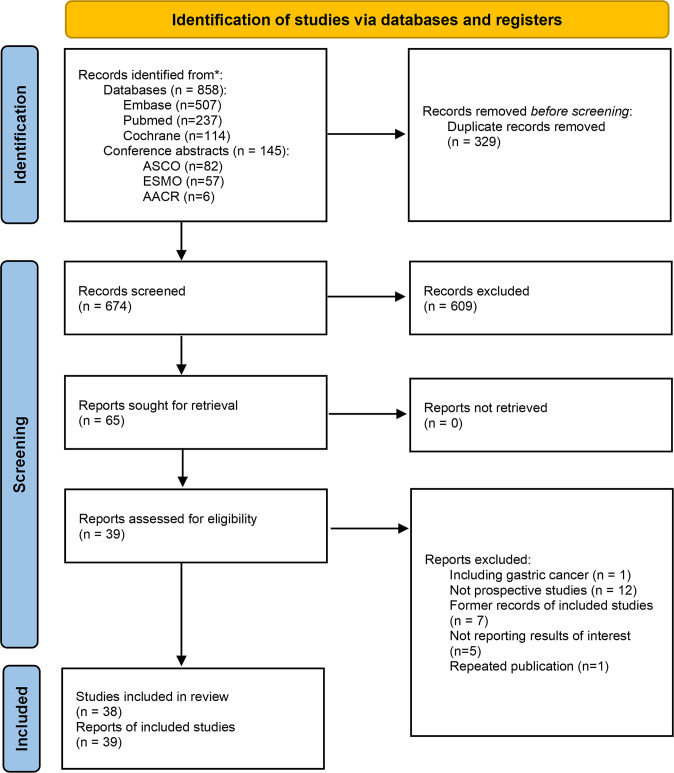
The search identified 1003 records based on the discussed search strategy. First, 329 duplicates were eliminated, and 609 were removed after reviewing the titles and abstracts. 39 records, referring to 38 studies (two records reported different arms of the same trial), were finally selected after full-text review (12, 13, 19–56). The details of the selection process are shown in Figure 1 . The characteristics of the included studies are summarized in Table 1 . The main results of each study are presented in Table 2 . A total of 1252 patients were included in these 38 prospective studies, with 32 single-arm, 3 dual-arm, 1 multi-arm trial and 2 observational cohort studies. 967 patients completed surgery, and reasons for not receiving surgery mainly include not reaching surgery time in ongoing trials, disease progression, toxicity, and patients’ choice. The included trials were similar in terms of the inclusion criteria, age, and sex composition. The median/mean age ranged from 58.3 to 68 years. The proportion of male ranged from 73% to 96% (median 87%). 32 studies enrolled only ESCC (916 patients), 3 studies enrolled only EAC (116 patients) and 3 studies didn’t restrain participants’ histological types (220 patients).
Figure 1.
Flow chart of the selection process. *The literature search were performed up to July 14, 2022.
Table 1.
Characteristics of included studies.
| Authors& Publiation year |
Registration number | Intervention model | Study phase | Type of publication | Group | Modality of neoadjuvant therapy | Chemotherapy regimen | Radiotherapy regimen | Main inclusion criteria | ICI | Dose of ICI | Cycles of ICI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cower, D., et al. (19), 2022 | NCT02962063 | single-arm | II | abstract | NICRT | induced chemotherapy plus concurrent NICRT | induced mFOLFOX; concurrent 5-FU/CAP+OX or paclitaxel/carboplatin | 50.4Gy | resectable cTxN+ or T3-4Nx, M0 esophageal/GEJ adenocarcinoma | durvalumab | 1500mg iv Q4w | 2 |
| Jiang, N., et al. (23), 2022 | ChiCTR2100045104 | single-arm | II | abstract | NICRT | concurrent NICRT | paclitaxel, carboplatin | 30Gy/12f | resectable cT3-4aN0 or cT1-4aN+, M0 ESCC | toripalimab | 240mg iv Q3w | 2 |
| Kelly, R. J., et al. (32), 2022 | NCT03044613 | dual-arm | II | abstract | NICRT | cohort A & original cohort B: induced immunotherapy plus concurrent NICRT; amended cohort B: induced immunotherapy plus concurrent CRT | paclitaxel, carboplatin | 41.1Gy/23f | stage II-III esophageal/GEJ cancer | cohort A: nivolumab; cohort B: nivolumab+relatlimab |
cohort A: 240mg Q2w; cohort B: 240mg Q2w(nivolumab)+80mg Q2w(relatlimab) | cohort A & original cohort B:5; amended cohort B: 2 |
| Lee, S., et al. (33), 2019 | NCT02844075 | single-arm | II | abstract | NICRT | concurrent NICRT | paclitaxel, carboplatin | 44.1Gy/21f | stage IB-III ESCC | pembrolizumab | 200 mg iv Q3w | 2 |
| Li, C., et al. (12), 2021 | NCT03792347 | single-arm | IB | article | NICRT | concurrent NICRT | paclitaxel, carboplatin | 41.4Gy/23f | resectable cT2-T4aNxM0 ESCC | pembrolizumab | 2 mg/kg iv Q3w | 2 |
| Manish A. Shah, et al. (39), 2021 | NCT02998268 | dual-arm | II | abstract | NICRT | cohort A: induced chemotherapy plus concurrent NICRT; cohort B: induced immuno-chemotherapy plus concurrent NICRT | paclitaxel, carboplatin | 41.4Gy/23f | resectable cT3-4Nx or T2N1, M0 esophageal/GEJ adenocarcinoma | pembrolizumab | 200 mg iv Q3w | NA |
| Uboha, NV., et al. (25), 2022 | NCT03490292 | Single-arm | I/II | abstract | NICRT | concurrent NICRT | paclitaxel, carboplatin | 41.4Gy/23f | potentially curable cT1N1 or cT2-3N0-2, M0 esophageal/GEJ SCC/AC | avelumab | 10mg/kg iv Q2w | 3 |
| Qi, W.X., et al. (41), 2020 | NA | single-arm | IB | abstract | NICRT | concurrent NICRT | paclitaxel, carboplatin | 41.4Gy/23f | local advanced resectable ESCC | pembrolizumab | 2 mg/kg iv Q3w | 2 |
| van Den Ende, T., et al. (13), 2021 | NCT03087864 | single-arm | II | article | NICRT | concurrent NICRT | paclitaxel, carboplatin | 41.4Gy/23f | resectable <T4b, NxM0 esophageal/GEJ adenocarcinoma | atezolizumab | 1200 mg iv Q3w | 2 |
| Xu, X., et al. (28), 2022 | NCT04437212 | single-arm | II | abstract | NICRT | concurrent NICRT | paclitaxel, cisplatin | 41.4Gy/23f | resectable cT1-4aN1-2 or T3-4aN0,MO ESCC | toripalimab | 240mg iv Q3w | 2 |
| Duan, H., et al. (29), 2021 | NA | single-arm | NA | article | NICT | concurrent NICT | docetaxel, paclitaxel nedaplatin | resectable cT2-xNxM0 ESCC | sintilimab | 200 mg iv Q3w | 3 | |
| Gao, L., et al. (20), 2022 | ChiCTR2100052784 | single-arm | II | article | NICT | concurrent NICT | docetaxel, cisplatin | resectable cT3-4Nx or cTxN+, M0 ESCC | toripalimab | 240 mg iv Q3w | 2 | |
| Gu, Y., et al. (30), 2020 | NCT03946969 | single-arm | IB/II | abstract | NICT | concurrent NICT | paclitaxel, carboplatin, S1 | resectable cT1b-T3NxM0 ESCC | sintilimab | 200 mg iv Q3w | 2 | |
| Guo, J., et al. (21), 2022 | ChiCTR2000040345 | single-arm | II | abstract | NICT | concurrent NICT | paclitaxel, nedaplatin | resectable cT2-4N1-3M0 ESCC | sintilimab | 200mg iv Q3w | 2 | |
| He, W., et al. (31), 2022 | NCT04177797 | single-arm | II | article | NICT | concurrent NICT | paclitaxel, carboplatin | resectable cT3N1-3M0 ESCC | toripalimab | 240 mg iv Q3w | 2 | |
| Jiang, B., et al. (22), 2022 | NA | single-arm | II | abstract | NICT | concurrent NICT | paclitaxel, cisplatin | resectable stage III ESCC | camrelizumab/sintilimab/tislelizumab | 200mg iv Q3w | 2 | |
| Li, K., et al. (34), 2020 | ChiCTR2000035237 | single-arm | pilot study | abstract | NICT | concurrent NICT | paclitaxel, carboplatin | resectable cT2-T4N0-N2M0 ESCC | toripalimab | 240 mg iv Q3w | 2~3 | |
| Li, Z., et al. (24), 2022 | NA | single-arm | II | abstract | NICT | concurrent NICT | paclitaxel, cisplatin | locally advanced ESCC | sintilimab | 200mg iv Q3w | 2~4 | |
| Liu, D., et al. (35), 2021 | ChiCTR1900025318 | single-arm | II | abstract | NICT | concurrent NICT | paclitaxel, cisplatin | resectable cT1-cT2N+ or cT3-cT4aNx ESCC | toripalimab | 240 mg iv Q3w | 2 | |
| Liu, J.; Li, J., et al. (36), 2022 | NCT04225364 | single-arm | II | article | NICT | concurrent NICT | paclitaxel, cisplatin | resectable stage II-IVA ESCC | camrelizumab | 200 mg iv Q3w | 2 | |
| Liu, J.; Yang, Y., et al. (37), 2022 | ChiCTR1900026240 | single-arm | II | article | NICT | concurrent NICT | paclitaxel, carboplatin | resectable ESCC, staged as T1b-4a, N2-3 (≥ 3 stations), M0 or M1 lymph node metastasis (confined to the supraclavicular lymph nodes) | camrelizumab | 200 mg iv Q3w | 2 | |
| Ma, J., et al. (38), 2021 | ChiCTR2000033761 | single-arm | II | abstract | NICT | concurrent NICT | paclitaxel, nedaplatin | resectable IIA-IIIB ESCC | camrelizumab | 200 mg iv Q3w | 2~4 | |
| Shang, X., et al. (42), 2021 | NCT 04389177 | single-arm | II | abstract | NICT | concurrent NICT | paclitaxel, cisplatin | potentially resectable cT3N1 or cT1-3N2, M0 ESCC | pembrolizumab | 200 mg iv Q3w | 3 | |
| Shen, D., et al. (43), 2021 | NA | single-arm | pilot study | article | NICT | concurrent NICT | paclitaxel, carboplatin | potentially curable cT1N1-3 or cT2-4aNx, M0 ESCC | nivolumab/pembrolizumab/camrelizumab | nivolumab 3mg/kg iv Q3w, pembrolizumab 2mg/kg iv Q3w, camrelizumab 200mg iv Q3w | 2 | |
| Wang, F., et al. (55), 2021 | NCT03917966 | single-arm | II | abstract | NICT | induced immunotherapy plus concurrent NICT | docetaxel, nedaplatin | stage II-IVA ESCC | camrelizumab | 200 mg iv Q3w | 1+2 | |
| Wang, W., et al. (26), 2022 | NA | single-arm | II | abstract | NICT | concurrent NICT | docetaxel, cisplatin/carboplatin | resectable stage IIB-IVA ESCC | pembrolizumab | 200mg iv Q3w | 4 | |
| Wang, Z., et al. (44), 2021 | ChiCTR1900023880 | single-arm | IB | abstract | NICT | concurrent NICT | paclitaxel, nedaplatin, apatinib | resectable cT1N2 or T2-3N0-2 ESCC | camrelizumab | 200 mg iv Q2w | 2~4 | |
| Xing, W., et al. (45), 2021 | NCT03985670 | dual-arm | II | article | NICT | concurrent NICT | paclitaxel, cisplatin | resectable stage II-IVA ESCC | toripalimab | 240 mg iv Q3w | 2 | |
| Xu, W., et al. (56), 2022 | NCT04506138 | single-arm | II | abstract | NICT | concurrent NICT | paclitaxel, carboplatin | resectable cT2-4aNx or cT1-3N+, M0 ESCC | camrelizumab | 200 mg iv Q3w | 2 | |
| Yamamoto, S.; Matsuda, S., et al. (40, 46), 2021&2022 | NCT03914443 | multi-arm | I | abstract | NICT | cohort A&C: concurrent NICT; cohort B&D: induced immunotherapy plus concurrent NICT | cohort A&B: cisplatin, 5-FU; cohort C&D: cisplatin, 5-FU, docetaxel | resectable cT1N1-3 or cT2-3Nx, M0 ESCC | nivolumab | cohort A&C: 360mg iv Q3w; cohort B&D: 240 mg lead-in followed by 360 mg iv Q3w | cohort A: 2; cohrot B&C: 3; cohort D: 4 | |
| Yan, X., et al. (47), 2021 | ChiCTR2000037488 | single-arm | II | abstract | NICT | concurrent NICT | paclitaxel, carboplatin | resectable stage II-IVA ESCC | tislelizumab | 200 mg iv Q3w | 3 | |
| Yang, P., et al. (48), 2021 | ChiCTR2100051903 | single-arm | pilot study | article | NICT | concurrent NICT | paclitaxel, carboplatin | potentially curable cT1N1-3 or cT2-4aNx, M0 ESCC | camrelizumab | 200 mg iv Q3w | 2 | |
| Yang, W., et al. (49), 2021 | ChiCTR2000028900 | single-arm | pilot study | article | NICT | concurrent NICT | paclitaxel, carboplatin | resectable stage II-III ESCC | camrelizumab | 200 mg iv Q3w | 2 | |
| Zhang, G., et al. (50), 2020 | ChiCTR1900027160 | NA | observational study | abstract | NICT | concurrent NICT | paclitaxel, S1 | resectable stage I-III ESCC | toripalimab | NA | 2~4 | |
| Zhang, X (51)., 2021 | ChiCTR2000029807 | single-arm | II | abstract | NICT | concurrent NICT | paclitaxel, S1 | resectable stage II-III thoracic ESCC | camrelizumab | 200mg iv Q3w | 3 | |
| Zhang, Y., et al. (52), 2022 | ChiCTR2000039170 | NA | Observational study | abstract | NICT | NA | primarily paclitaxel and nedaplatin | resectable EC | camrelizumab | NA | NA | |
| Zhang, Z.; Hong, Z., et al. (53), 2021 | ChiCTR2100045659 | single-arm | II | article | NICT | concurrent NICT | paclitaxel, cisplatin | cT3-4aN0-3 or cT1-2N1-3, M0 ESCC | sintilimab | 200 mg iv Q3w | 2 | |
| Zhang, Z.; Ye, J., et al. (54), 2021 | ChiCTR1900026593 | single-arm | II | abstract | NICT | concurrent NICT | paclitaxel, carboplatin | resectable stage II-IVA ESCC | sintilimab | 200 mg iv Q3w | 2 |
ICI, immune checkpoint inhibitors; NICRT, neoadjuvant immune-chemoradiotherapy; NICT, neoadjuvant immune-chemotherapy; GEJ, gastroesophageal junction; EC, esophageal cancer; ESCC, esophageal squamous cell carcinoma; NA, not available; CAP, capecitabine; OX, oxaliplatin.
Table 2.
Results of included studies.
| Authors | Sample size | Male | Median/Mean age | pCR | MPR | R0 resection rate | Incidence of ≥ grade 3 TRAEs | Neoadjuvant therapy completion rate | Surgical resection rate | Surgical delay rate | Whether all patients reached surgery time |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cower, D., et al. (19) | 36 | NA | NA | 8/33 | 22/33 | NA | NA | NA | 33/36 | NA | yes |
| Jiang, N., et al. (23) | 23 | NA | NA | 11/20 | 16/20 | NA | 16/23 | 21/23 | 20/23 | NA | yes |
| Kelly, R. J., et al. (32) | 32 | 81% | 65 | 9/31 | 16/31 | NA | NA | NA | 31/32 | NA | not mentioned |
| Lee, S., et al. (33) | 28 | 89% | 60 | 6/26 | NA | 25/26 | NA | NA | 26/28 | NA | yes |
| Li, C., et al. (12) | 20 | 95% | 62 | 10/18 | 16/18 | 17/18 | 13/20 | 19/20 | 18/20 | 0 | yes |
| Manish A. Shah, et al. (39) | 40 | 80% | 68 | NA | 15/31 | NA | NA | NA | NA | NA | no |
| Uboha, NV., et al. (25) | 22 | 91% | 64 | 5/19 | NA | 15/19 | NA | NA | 19/22 | NA | yes |
| Qi, W.X., et al. (41) | 20 | 95% | 61.2 | 9/14 | NA | NA | 3/20 | NA | 14/20 | NA | no |
| van Den Ende, T., et al. (13) | 40 | 88% | 63 | 10/33 | NA | 33/33 | 17/40 | 34/40 | 33/40 | 0 | yes |
| Xu, X., et al. (28) | 20 | NA | NA | 7/13 | 10/13 | NA | 7/13 | NA | 13/10 | NA | no |
| Duan, H., et al. (29) | 23 | 91% | 63.5 | 6/17 | 9/17 | 16/17 | 7/23 | 21/23 | 17/23 | 0 | yes |
| Gao, L., et al. (20) | 20 | 85% | 58.3 | 2/12 | 5/12 | 12/12 | NA | 20/20 | 12/20 | 0 | yes |
| Gu, Y., et al. (30) | 17 | 76% | 65 | 4/15 | 8/15 | 15/15 | 6/17 | 17/17 | 15/17 | NA | no |
| Guo, J., et al. (21) | 15 | NA | NA | NA | 6/11 | 11/11 | NA | NA | 11/15 | 0 | yes |
| He, W., et al. (31) | 20 | 75% | 61.4 | 3/16 | 7/16 | 14/16 | 4/20 | 18/20 | 16/20 | 0 | yes |
| Jiang, B., et al. (22) | 10 | NA | NA | 4/10 | 6/10 | 10/10 | NA | NA | 10/10 | 0 | yes |
| Li, K., et al. (34) | 17 | 94% | 64 | 2/12 | 7/12 | 12/12 | 2/17 | NA | 12/17 | 0 | yes |
| Li, Z., et al. (24) | 20 | 80% | NA | 3/20 | 7/20 | 20/20 | 2/20 | NA | 20/20 | NA | yes |
| Liu, D., et al. (35) | 23 | NA | NA | 6/18 | NA | 18/18 | 2/23 | NA | 18/23 | NA | yes |
| Liu, J.; Li, J., et al. (36) | 56 | 75% | 61 | 16/51 | 30/51 | 51/51 | 6/56 | 51/56 | 51/56 | NA | yes |
| Liu, J.; Yang, Y., et al. (37) | 60 | 83% | 65 | 20/51 | 35/51 | 50/51 | 34/60 | 55/60 | 51/60 | 8/60 | yes |
| Ma, J., et al. (38) | 42 | NA | 63 | 6/16 | NA | NA | NA | NA | 16/42 | NA | no |
| Shang, X.,et al. (42) | 49 | NA | NA | 12/29 | 21/29 | 29/29 | 0/29 | NA | 29/42 | 0 | no |
| Shen, D., et al. (43) | 28 | 96% | 62.2 | 9/28 | NA | 26/27 | 2/28 | 28/28 | 27/28 | 0 | yes |
| Wang, F., et al. (55) | 26 | 65% | 63 | 3/12 | 5/12 | 12/12 | 1/26 | 17/26 | 12/17 | NA | no |
| Wang, W., et al. (26) | 27 | NA | NA | 4/14 | NA | 14/14 | NA | 24/27 | 14/27 | NA | no |
| Wang, Z., et al. (44) | 30 | 80% | 62 | 7/29 | 15/29 | NA | 11/30 | 29/30 | 29/30 | 5/30 | yes |
| Xing, W., et al. (45) | 30 | 73% | experiment group:63.8; control group:63.13 | 5/24 | NA | 24/24 | NA | NA | 24/30 | NA | yes |
| Xu, W., et al. (56) | 46 | NA | NA | 8/37 | 18/37 | 37/37 | 7/46 | 45/46 | 37/46 | NA | not mentioned |
| Yamamoto, S.; Matsuda, S., et al. (40, 46) | 25 | NA | cohort A&B:62; cohort C&D:60 | 6/25 | NA | 23/25 | NA | 25/25 | 25/25 | 0 | yes |
| Yan, X., et al. (47) | 45 | NA | NA | 18/36 | 26/36 | NA | 15/45 | NA | 36/45 | NA | yes |
| Yang, P., et al. (48) | 16 | 88% | 60.5 | 5/16 | NA | 15/16 | NA | NA | 16/16 | NA | yes |
| Yang, W., et al. (49) | 23 | 96% | 58.6 | 5/20 | 10/20 | 20/20 | NA | 23/23 | 20/23 | 0 | yes |
| Zhang, G., et al. (50) | 24 | NA | NA | 3/18 | 9/18 | NA | NA | NA | 18/24 | NA | no |
| Zhang, X (51). | 25 | NA | NA | 8/25 | 16/25 | NA | 2/25 | NA | 25/25 | 0 | yes |
| Zhang, Y., et al. (52) | 166 | 84% | 62.9 | 15/81 | 51/81 | 9/82 | 13/166 | NA | 82/166 | NA | no |
| Zhang, Z.; Hong, Z., et al. (53) | 30 | 87% | 58.3 | 4/23 | 12/23 | 23/23 | 1/30 | 30/30 | 23/30 | 0 | yes |
| Zhang, Z.; Ye, J., et al. (54) | 40 | NA | NA | 10/40 | 19/40 | 39/40 | NA | NA | NA | 0 | yes |
pCR, pathological complete response; MPR, major pathological response; TRAEs, treatment-related adverse events. NA, not available.
3.2. Quality assessment
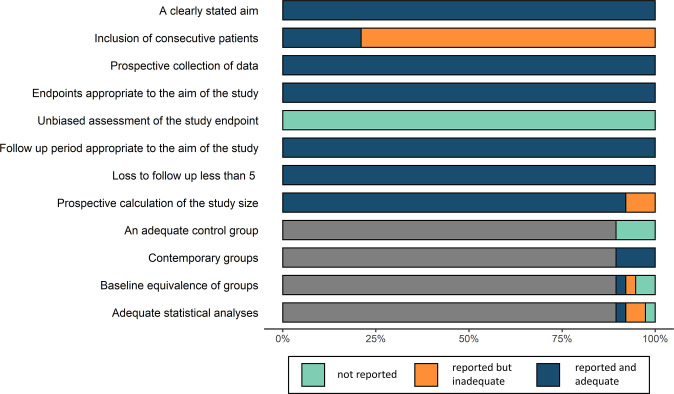
The 38 studies were scored from 13 to 18 using the MINORS index, with a low risk of summary bias, indicating acceptable quality for the present meta-analysis ( Figure 2 ; Supplementary Table 1 ).
Figure 2.
Quality assessment by the methodological index for non-randomized studies (MINORS) index.
3.3. Efficacy
3.3.1. Pathological complete responses
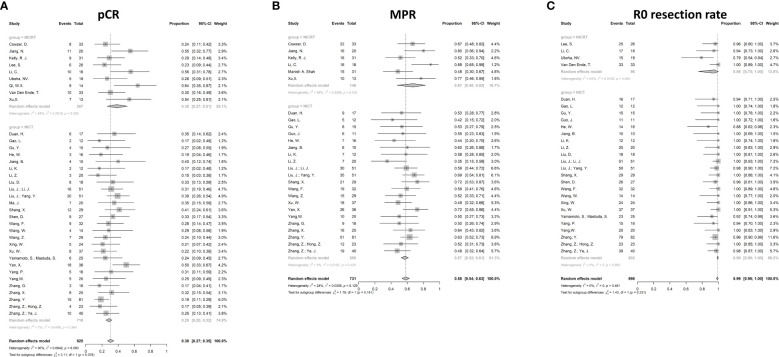
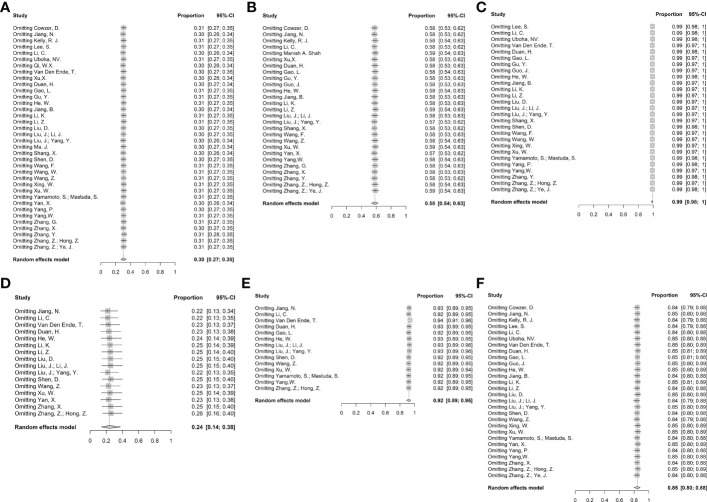
The pooled pCR for 925 patients in 36 studies with available pCR data was 30% (95%CI 27%-35%) with potential heterogeneity (I2 = 30%, p=0.050) ( Figure 3A ). The pCR in the NICRT group and NICT group was 38% (95%CI 27%-51%) and 28% (95%CI 25-32%), respectively. Although numerically higher in the NICRT group, no significant difference was shown between the two groups (p=0.078). In addition, high heterogeneity was observed in the NICRT group (I2 = 60%, p=0.026) however not in the NICT group (I2 = 11%, p=0.364).
Figure 3.
Forest plots for efficacy. (A) Pathological complete response (pCR), (B) Major pathological response (MPR), and (C) R0 resection rate. NICRT, neoadjuvant immune-chemoradiotherapy; NICT, neoadjuvant immune-chemotherapy.
3.3.2. Major pathological responses
The MPR data were available in 27 studies. The pooled MPR was 58% (95%CI 54%-63%, I2 = 24%, p=0.129) ( Figure 3B ). The pooled MPR in the NICRT group was 67% (95%CI 48%-82%, I2 = 59%, p=0.031)., while the pooled MPR in the NICT group was 57% (95%CI 52%-61%, I2 = 3%, p=0.425). There were no statistical differences between the two groups (p=0.181).
3.3.3. R0 resection rate
Pooled analysis showed a high R0 resection rate reaching 99% in 27 studies (95%CI 98%-100%, I2 = 0%, p=0.862) ( Figure 3C ). Both the NICRT and NICT groups achieved a high chance of R0 resection (95% and 99%, respectively).
3.4. Safety
3.4.1. Incidence of ≥grade 3 TRAEs
As the overall incidence of TRAEs has not been widely reported, we analyzed ≥grade 3 TRAEs. The pooled incidence was 24% (95%CI 14%-38%) ( Figure 4A ). 16 studies with 506 patients provided valid data. Significant heterogeneity was observed (I2 = 82%, p<0.001). Most of the reported ≥grade 3 TRAEs were hematologic, including lymphopenia, leukopenia, thrombocytopenia, and anemia. Non-hematological TRAEs, such as anorexia, rash, vomiting, and diarrhea, were less common. Grade 5 TRAEs were rare and were mainly due to hemorrhage. Although significant differences were shown between the NICRT and NICT groups (58%, 95%CI 23%-87% vs. 18%, 95%CI 11%-29%, p<0.001), high heterogeneity was found in both groups (I2 = 61%, p=0.075 vs. I2 = 79%, p<0.001).
Figure 4.
Forest plots for safety. (A) Incidence of ≥grade 3 TRAEs, (B) Neoadjuvant therapy completion rate, (C) Surgical resection rate, and (D) Surgical delay rate. NICRT, neoadjuvant immune-chemoradiotherapy; NICT, neoadjuvant immune-chemotherapy.
3.4.2. Neoadjuvant therapy completion rates
Valid data were reported in 14 studies. The NTCR had a pooled value of 92% (95%CI 89%-95%, I2 = 0%; p=0.605) ( Figure 4B ). The pooled NTCR was similarly high in both the NICRT and NICT groups (88% vs. 94%, p=0.061).
3.4.3. Surgical resection rate
28 trials provided valid data, leading to a pooled rate of 85% (95%CI 80%-88%, I2 = 38%, p=0.022) ( Figure 4C ). The surgical resection rates in the NICRT vs NICT groups were 88% (95%CI 82%-93%, I2 = 0%, p=0.633) vs 83% (95%CI 77%-88%, I2 = 44%, p=0.018), with no significant differences (p=0.088).
3.4.4. Surgical delay rate
Among the 16 valid studies, only 2 reported a surgical delay rate of 13% and 17%, and the others reported no surgical delay. The pooled surgical delay rate was 1% (95%CI 0%-3%, I2 = 44%, p=0.032) ( Figure 4D ).
3.5. Efficacy and safety in ESCC and EAC
32 (84%) of the included studies enrolled patients only with ESCC. A pooled analysis was performed, specifically for ESCC patients ( Figure 5 ). 5 studies were in the NICRT group and 27 were in the NICT group. The overall pooled pCR was 32% (95%CI 27%-36%, I2 = 30%, p=0.062). The pooled pCR in the NICRT and NICT groups was 49% (95%CI 29%-70%, I2 = 51%, p=0.084) and 30% (95%CI 26%-34%, I2 = 0%, p=0.552), respectively, with significant differences (p=0.011). The pooled MPR was 58% (95%CI 52%-63%, I2 = 28%, p=0.101). The pooled MPR in the NICRT and NICT groups was 82% (95%CI 61%-93%, I2 = 0%, p=0.657) and 56% (95%CI 51%-61%, I2 = 1%, p=0.440), respectively, with significant differences (p<0.001). The R0 resection rate was 96% (95%CI 95%-97%, I2 = 0%, p=0.998), and no significant differences were shown between the NICRT and NICT groups (95% vs. 96%, p=0.429). The pooled incidence of ≥ grade 3 TRAEs was 23% (95%CI 13%-37%, I2 = 83%, p<0.001). Incidence of ≥ grade 3 TRAEs in the NICRT and NICT group was 67% (95%CI 36%-89%, I2 = 0%, p=0.750) and 18% (95%CI 11%-29%, I2 = 79%, p<0.001), respectively, with a significant difference (p<0.001). The pooled NTCR was 94% (95%CI 91%-96%, I2 = 0%, p=0.830), with 93% for the NICRT group and 94% for NICT group. The surgical resection rate was 84% (95%CI 79%-88%, I2 = 40%, p=0.022). The NICRT group had a higher surgical resection rate than did the NICT group (90% vs. 83%, p=0.024). The surgical delay rate was 1% (95%CI 0%-3%, I2 = 45%, p=0.031).
Figure 5.

Forest plots of efficacy and safety in ESCC. (A) Pathological complete response (pCR), (B) Major pathological response (MPR), (C) R0 resection rate, (D) Incidence of ≥grade 3 TRAEs, (E) Neoadjuvant therapy completion rate, (F) surgical resection rate, and (G) Surgical delay rate. NICRT, neoadjuvant immune-chemoradiotherapy; NICT, neoadjuvant immune-chemotherapy.
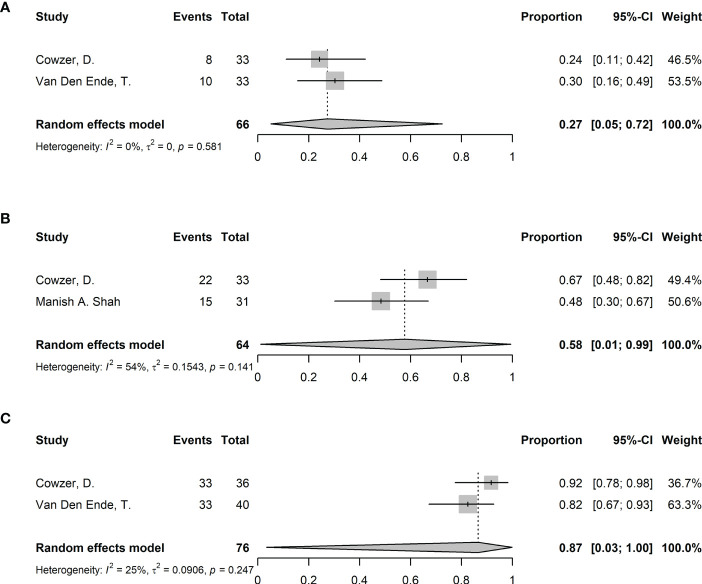
3 (8%) of the included studies enrolled patients only with EAC. All 3 studies adopted NICRT. The pooled analysis was only performed for outcomes with more than 1 available study (i.e., pCR, MPR and surgical resection rate) ( Figure 6 ). The pooled pCR was 27% (95%CI 5%-72%, I2 = 0%, p=0.581). The pooled MPR was 58% (95%CI 1%-99%, I2 = 54%, p=0.141). The pooled surgical resection rate was 87% (95%CI 3%-100%, I2 = 25%, p=0.247). The R0 resection rate, incidence of ≥ grade 3 TRAEs, NTCR and surgical delay rate were reported in single study as 100%, 42.5%, 85%, 0%.
Figure 6.
Forest plots of efficacy and safety in EAC. (A) Pathological complete response (pCR), (B) Major pathological response (MPR), and (C) surgical resection rate.
3.6. Exploratory subgroup analysis
Subgroup analysis was performed based on types of publication (abstract or article), types of ICI and cycles of ICI. The cycles of ICI were categorized into 2 subgroups, i.e., 2 cycles and >2 cycles (including studied adopting 2 or more cycles, such as 2-4 cycles). Studies not reporting relevant factors were excluded. Due to the level of heterogeneity and incidence of outcomes, only pCR, MPR, incidence of ≥ grade 3 TRAEs and surgical resection rate underwent subgroup analysis. Findings indicate the types of publication and the cycles of ICI were not source of heterogeneity ( eFigure 1 and eFigure 2 in Supplement). The types of ICI might affect MPR (p=0.017), with relatively high MPR for tislelizumab (72%, 95%CI 55%-86%) and low MPR for sintilimab (49%, 95%CI 41%-56%) ( eFigure 3B in Supplement). The types of ICI might also contribute to the heterogeneity of incidence of ≥ grade 3 TRAEs (p=0.023), with relatively high incidence for pembrolizumab (65%, 95%CI 41%-85%) and atezolizumab (42%, 95%CI 27-59%) and low incidence for sintilimab (13%, 95%CI 1-77%) ( eFigure 3C in Supplement). Surgical resection rate might also be affected by types of ICI (p<0.001), with relatively high rate for nivolumab (97%, 95%CI 65%-100%) and low rate for toripalimab (76%, 95%CI 65%-84%) ( eFigure 3D in Supplement).
3.7. Survival
The survival data were mostly incomplete or immature. Only a few trials published limited data, which was insufficient for the pooled analysis. According to the reported data ( Table 3 ), one study reported a median OS of 29.7 months (13), and the median DFS ranged from 19.4 to 35.4 months (13, 32). The 1-year OS rate ranged from 77% to 92% (19, 25, 33, 48), and the 2-year OS rate ranged from 73.1% to 85% (19, 33).The 1-year DFS rate ranged from 67% to 83% (19, 25, 48). Onestudy reported a 2-year DFS rate of 71% (19).
Table 3.
Survival data.
| Study | Median follow-up, months | Median OS, months (95%CI) | Median DFS, months (95%CI) |
1y OS (95%CI) |
1y DFS (95%CI) |
2y OS (95%CI) |
2y DFS (95%CI) |
|---|---|---|---|---|---|---|---|
| Cowzer, D., et al. (19) | 30 | NA | NA | 92% (83%-100%) | 81% (69%-95%) | 85% (74%-98%) | 71% (58%-88%) |
| Kelly, R. J., et al (32), | 30 | NA | 35.4 (24.7-NA) |
NA | 79.1% (65.5-95.6%) | NA | NA |
| Lee, S., et al. (33) | NA | NA | NA | 80.80% | NA | 73.10% | NA |
| Uboha, NV., et al. (25) | 9.8 | NA | NA | 77% | 67% | NA | NA |
| van den Ende, T., et al. (13) | 24 | 29.7 | 19.4 | NA | NA | NA | NA |
| Yang, P., et al. (48) | NA | NA | NA | 90.90% | 83% | NA | NA |
OS, overall survival; DFS, disease-free survival; NA, not available.
3.8. Sensitivity analysis, publication bias and certainty assessment
Sensitivity analysis revealed no obvious changes regarding efficacy and safety when excluding any single study ( Figure 7 ). Results of Egger’s and Begg’s tests were shown in Supplementary Table 2 . No publication bias was detected in the pCR or MPR groups. The incidence of ≥3 TRAEs, NTCR, and surgical resection rates exhibited a publication bias in both tests. Furthermore, there was a discrepancy between the Begg’s test and Egger’s test in terms of the surgical delay rate (p=0.005 vs. p=0.093) and R0 resection rate (p=0.030 vs. p=0.372). Considering that Egger’s test is more sensitive (57), potential publication bias might not exist in these outcomes. The details of certainty assessment are shown in eTable 1 in Supplement. Notably, due to no RCTs were included, the certainty was graded from low certainty, and downgraded if certain limitations were met. Overall, the results of pCR, MPR and R0 resection rate had low certainty of evidence, and the results of incidence of ≥ grade 3 TRAEs, NTCR and surgical resection rate had very low certainty of evidence.
Figure 7.
Sensitivity analysis. (A) pCR, (B) MPR, (C) R0 resection rate, (D) Incidence of ≥ grade 3 TRAEs, (E) Neoadjuvant therapy completion rate, and (F) Surgical resection rate.
4. Discussion
Overall, these results support the acceptable efficacy and safety of neoadjuvant immunotherapy combined with chemoradiotherapy or chemotherapy for esophageal cancer. To our knowledge, this is the largest meta-analysis of neoadjuvant immunotherapy for esophageal cancer based on prospective studies.
The pooled pCR reached 30%, a value much higher than the pCR of 0%-9% of neoadjuvant chemotherapy mentioned in previous RCTs (58, 59), and even higher than the latest DCF neoadjuvant chemotherapy regimen discussed in JCOG1109, mentioning a pCR of 19.8% (60). Moreover, the pooled pCR was comparable to the 29% in the neoadjuvant chemoradiotherapy group in the CROSS trial (3). A high pooled MPR (58%) also demonstrated good efficacy. The pooled R0 resection rate reached 99%, which is close to the 92%-98.4% for neoadjuvant chemoradiotherapy in the CROSS and NEOCRTEC5010 trials (3, 4), and higher than the reported 74%-89% for neoadjuvant chemotherapy (8), indicating a satisfying guarantee for high-quality surgery. These results showed that the short-term efficacy of neoadjuvant immunotherapy was favorable.
It remains unclear how pCR or MPR affect the survival of patients with esophageal cancer receiving neoadjuvant immunotherapy. Due to insufficient follow-up, survival data were not available for most trials. Based on the limited data, the 1-year and 2-year OS for neoadjuvant immunotherapy were comparable to the 1-year and 2-year OS being 81-90% and 67-75.1% for neoadjuvant chemoradiotherapy in different RCTs (4, 5). DFS rates were similar to those of neoadjuvant chemoradiotherapy. In the PERFECT trial, no significant difference in OS or DFS was observed between the neoadjuvant immunotherapy cohort and the propensity score-matched neoadjuvant chemoradiotherapy cohort (13). Briefly, neoadjuvant immunotherapy may achieve a short-term survival similar to that achieved with neoadjuvant chemoradiotherapy. However, large-scale RCTs with long follow-up periods are necessary to further investigate survival, particularly long-term survival.
The adverse event profiles were acceptable. The pooled overall incidence of ≥grade 3 TRAEs was 24%. In comparison, the incidence of ≥ grade 3 leukopenia in the neoadjuvant chemotherapy group in Intergroup0113 trial was 29% (59), and the incidence of ≥ grade 3 neutropenia related to preoperative chemotherapy was 23.8% in the MAGIC trial (61), indicating that the addition of immunotherapy might not increase the incidence of serious adverse events. Regarding neoadjuvant chemoradiotherapy, the incidence of ≥ grade 3 TRAEs was 17%-27.6% in the FFCD9901 and CROSS trials (3, 62), and 54.3% of patients developed ≥ grade 3 hematologic toxicity in the NEOCRTEC 5010 trial (4), supporting the fact that the incidence of severe adverse events for neoadjuvant immunotherapy may not be significantly higher than that of chemoradiotherapy. Meanwhile, the incidence of ≥ grade 3 immune-related adverse events was as low as 2.5%-17.8% in the included studies, which supported the safety of immunotherapy. In addition, almost all patients (92%) completed the entire course of neoadjuvant immunotherapy, indicating that the addition of ICIs may not affect the overall administration of the full regimen, and adverse events may be clinically rectifiable to continue the treatment course. The pooled surgical resection rate was close to 82%-93% reported in studies on neoadjuvant chemoradiotherapy (3, 4, 62), and surgery delay was rare. Neoadjuvant immunotherapy combined with chemoradiotherapy or chemotherapy is generally safe. Nevertheless, publication bias and heterogeneity made the results unstable, so caution should be exercised when drawing conclusions. Large clinical trials are required to validate its safety.
The pCR and MPR of the NICRT group were both higher than those of the NICT group numerically. Although a significant difference between the two groups was not observed, it might have been underestimated. First, the efficacy of neoadjuvant treatment may be better in ESCC than in EAC (3), and most patients in the NICT group had ESCC, whereas some patients in the NICRT group had EAC. This can also explain why the pCR and MPR of the NICRT group showed apparent heterogeneity among the included studies. Second, the p-value may have been affected by the number of patients in the NICRT group. Notably, the incidence of ≥ grade 3 TRAEs was significantly higher in the NICRT group than in the NICT group, indicating that the intense treatment modality might increase the risk of adverse events. However, high heterogeneity existed, which may have been caused by differences in the treatment regimen and follow-up time.
ESCC had a higher pCR than EAC in the CROSS trial (3). The KEYNOTE-181 and CHECKMATE-577 trials showed that ESCC responded better to immunotherapy than EAC (11, 63). In addition, ESCC is more radiosensitive (64); therefore, it may benefit more from neoadjuvant immunotherapy, especially in the NICRT group. Our specifical subgroup analysis of ESCC showed that neoadjuvant immunotherapy was also effective. In the ESCC subgroup, the pooled pCR and MPR were 49% and 82%, respectively, which were significantly higher than NICT, and close to the pCR of 43.2%-49% for ESCC patients receiving neoadjuvant chemoradiotherapy (3, 4). Notably, except for the pCR of 23% reported by Lee et al. (33), all studies in the NICRT group had a pCR above 54%. Whether NICRT can lead to better short-term efficacy than neoadjuvant chemoradiotherapy requires further investigation in RCTs. Patients with EAC undergoing NICRT had pooled pCR of 27%, comparing to 23% for EAC in the CROSS trial (3), with satisfactory MPR and surgical resection rate. However, the available studies were too few, and more research must be conducted to explore the value of neoadjuvant immunotherapy for EAC.
Exploratory subgroup analysis indicated that the types of ICI might be associated with the efficacy and safety of neoadjuvant immunotherapy for esophageal cancer, which may cause heterogeneity of outcomes. However, due to the limited sample size in each drug group, more relevant clinical trials are necessary to explore the correlation further.
Neoadjuvant immunotherapy combined with radiotherapy is not a common choice for esophageal cancer, as only one trial has published the results in an abstract. Of the 14 patients who underwent surgery, seven (50%) achieved pCR and nine (64.3%) achieved MPR. Only 1 patient developed ≥ grade 3 TRAEs among the 22 patients, although some patients were still waiting for surgery (65). This modality can possibly achieve favorable efficacy with a low incidence of adverse events. Notably, it has been demonstrated that neoadjuvant immunotherapy combined with radiotherapy achieved higher pCR (26.7%) and MPR (53.3%) than immunotherapy alone, with similar toxicity (66). This modality is promising, but the exploration and selection of suitable specific patient groups based on biomarkers (e.g., PD-L1 expression) is required.
This meta-analysis had several limitations. Although the number of trials was adequate, most studies were early phase trials with a relatively small sample size. The risk of selection bias existed due to the selected cohorts adopted in the trials, which constrained reliability. The results of outcomes can only provide low or very low certainty of evidence. Some trials are still ongoing; therefore, the final outcome may not be consistent with the interim readout. The majority of trials were published in abstracts, making it difficult to access complete data. Furthermore, because of the relatively small number of studies and patients in the NICRT group, the statistical differences between NICRT and NICT should be interpreted with caution. Most trials have focused on ESCC; therefore, the interpretation was not reliable with regard to EAC. Although a specifical analysis were performed for EAC, the results were not able to lead to any meaningful deduction due to too few data. In addition, variations exist among different trials in the treatment regimen, study design, and patients’ characteristics. However, most outcomes showed acceptable heterogeneity and produced relatively stable results in sensitivity analysis, supporting the reliability of results. Finally, another limitation is the lack of long-term survival data, which has not yet been reported in most trials. Due to the small sample size and short follow-up time, it is hard to obtain reliable survival results based on current data.
This meta-analysis illustrates that neoadjuvant immunotherapy combined with chemoradiotherapy or chemotherapy can be effective and safe in the short term for locally advanced esophageal cancer, especially for ESCC, and can be used as a reference for future trials. The value of neoadjuvant immunotherapy for EAC needs more research to explore. NICRT may achieve better short-term efficacy with possibly higher risk of adverse events than NICT for ESCC. However, whether NICRT or NICT is more favorable for locally advanced esophageal cancer merits further investigation.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author contributions
ZH and YL conceived the study. YL and YB performed the searches and collected raw data. XY, SS, MY, ZM and YW checked the data and performed statistical analysis. WZ and YZ made substantial contributions to interpretation of data. YL, WZ, YB, XY, SS, MY, ZM and YW drafted the manuscript. YZ, YM, JQ, LX, JW and ZH provided important expertise on design and revised the whole manuscript critically. All authors contributed to the article and approved the submitted version.
Acknowledgments
We deeply thank Prof. Zhongxing Liao, Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA for generously providing us with the expertise and writing assistance.
Funding Statement
This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS: 2020-I2M-C&T-B-074) and Beijing Xisike Clinical Oncology Research Foundation (Y-HR2020ZD-0779 and Y-2019AZMS-0426).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1117448/full#supplementary-material
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 3. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med (2012) 366(22):2074–84. doi: 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 4. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): A phase III multicenter, randomized, open-label clinical trial. J Clin Oncol (2018) 36(27):2796–803. doi: 10.1200/JCO.2018.79.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol (2015) 16(9):1090–8. doi: 10.1016/S1470-2045(15)00040-6 [DOI] [PubMed] [Google Scholar]
- 6. Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol (2012) 19(1):68–74. doi: 10.1245/s10434-011-2049-9 [DOI] [PubMed] [Google Scholar]
- 7. Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet (2019) 393(10184):1948–57. doi: 10.1016/S0140-6736(18)32557-1 [DOI] [PubMed] [Google Scholar]
- 8. Jing SW, Qin JJ, Liu Q, Zhai C, Wu YJ, Cheng YJ, et al. Comparison of neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy for esophageal cancer: a meta-analysis. Future Oncol (2019) 15(20):2413–22. doi: 10.2217/fon-2019-0024 [DOI] [PubMed] [Google Scholar]
- 9. Bang YJ, Kang YK, Catenacci DV, Muro K, Fuchs CS, Geva R, et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer (2019) 22(4):828–37. doi: 10.1007/s10120-018-00909-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: The phase 2 KEYNOTE-180 study. JAMA Oncol (2019) 5(4):546–50. doi: 10.1001/jamaoncol.2018.5441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol (2020) 38(35):4138–48. doi: 10.1200/JCO.20.01888 [DOI] [PubMed] [Google Scholar]
- 12. Li C, Zhao S, Zheng Y, Han Y, Chen X, Cheng Z, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer (Oxford England: 1990) (2021) 144:232–41. doi: 10.1016/j.ejca.2020.11.039 [DOI] [PubMed] [Google Scholar]
- 13. van den Ende T, de Clercq NC, van Berge Henegouwen MI, Gisbertz SS, Geijsen ED, Verhoeven RHA, et al. Neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: A single-arm phase II feasibility trial (PERFECT). Clin Cancer Res (2021) 27(12):3351–9. doi: 10.1158/1078-0432.CCR-20-4443 [DOI] [PubMed] [Google Scholar]
- 14. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med (2015) 162(11):777–84. doi: 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 15. Hellmann MD, Chaft JE, William WN, Rusch V, Pisters KMW, Kalhor N, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol (2014) 15(1):e42–50. doi: 10.1016/S1470-2045(13)70334-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pataer A, Kalhor N, Correa AM, Raso MG, Erasmus JJ, Kim ES, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol (2012) 7(5):825–32. doi: 10.1097/JTO.0b013e318247504a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg (2003) 73(9):712–6. doi: 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 18. Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the journal of clinical epidemiology. J Clin Epidemiol (2011) 64(4):380–2. doi: 10.1016/j.jclinepi.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 19. Cowzer D, Wu AJ-C, Sihag S, Walch HS, Park BJ, Jones DR, et al. Durvalumab (D) and PET-directed chemoradiation (CRT) after induction FOLFOX for esophageal adenocarcinoma: Final results. J Clin Oncol (2022) 40(16_suppl):4029. doi: 10.1200/JCO.2022.40.16_suppl.4029 [DOI] [Google Scholar]
- 20. Gao L, Lu J, Zhang P, Hong ZN, Kang M. Toripalimab combined with docetaxel and cisplatin neoadjuvant therapy for locally advanced esophageal squamous cell carcinoma: a single-center, single-arm clinical trial (ESONICT-2). J Gastrointest Oncol (2022) 13(2):478–87. doi: 10.21037/jgo-22-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guo J. ed. Neoadjuvant sintilimab combined with chemotherapy in patients with resectable esophageal squamous cell carcinoma (ESCC): Preliminary results from a phase II study2022, in: ASCO Annual Meeting, JCO: American Society of Clinical Oncology. (2022) 40(16_suppl):e16008. [Google Scholar]
- 22. Jiang B, Yang X, Zhang J, Huang M. Abstract 5230: Neoadjuvant programmed cell death protein 1 inhibitors combined with chemotherapy in resectable esophageal squamous carcinoma: an open-label, single-arm study. Cancer Res (2022) 82(12_Supplement):5230. doi: 10.1158/1538-7445.AM2022-5230 [DOI] [Google Scholar]
- 23. Jiang N. ed. SCALE-1: Safety and efficacy of short course neoadjuvant chemo-radiotherapy plus toripalimab for locally advanced resectable squamous cell carcinoma of esophagus2022, in: ASCO Annual Meeting, JCO: American Society of Clinical Oncology; (2022) 40(16_suppl):4063. [Google Scholar]
- 24. Li Z. ed. A study of neoadjuvant sintilimab combined with chemotherapy TP for locally advanced esophageal squamous cell carcinoma (ESCC)2022, in: ASCO Annual Meeting, JCO: American Society of Clinical Oncology; (2022) 40(16_suppl):e16038. [Google Scholar]
- 25. Uboha NV, Eickhoff JC, Maloney JD, McCarthy D, DeCamp M, Deming DA, et al. Phase I/II trial of perioperative avelumab in combination with chemoradiation (CRT) in the treatment of stage II/III resectable esophageal and gastroesophageal junction (E/GEJ) cancer. J Clin Oncol (2022) 40(16_suppl):4034. doi: 10.1200/JCO.2022.40.16_suppl.4034 [DOI] [Google Scholar]
- 26. Wang W. ed. Neoadjuvant pembrolizumab plus chemotherapy for resectable locally advanced esophageal squamous cell carcinoma (ESCC): Interim results2022, in: ASCO Annual Meeting, JCO: American Society of Clinical Oncology; (2022) 40(16_suppl):e16011. [Google Scholar]
- 27. Xu L, Qi Y, Jiang Y, Ji Y, Zhao Q, Wu J, et al. Crosstalk between the gut microbiome and clinical response in locally advanced thoracic esophageal squamous cell carcinoma during neoadjuvant camrelizumab and chemotherapy. Ann Trans Med (2022) 10(6):325. doi: 10.21037/atm-22-1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu X. ed. Neoadjuvant chemoradiotherapy combined with perioperative toripalimab in locally advanced esophageal cancer2022, in: ASCO Annual Meeting, JCO: American Society of Clinical Oncology; (2022) 40(16_suppl):e16065. [Google Scholar]
- 29. Duan H, Wang T, Luo Z, Wang X, Liu H, Tong L, et al. A multicenter single-arm trial of sintilimab in combination with chemotherapy for neoadjuvant treatment of resectable esophageal cancer (SIN-ICE study). Ann Trans Med (2021) 9(22):1700. doi: 10.21037/atm-21-6102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gu Y, Chen X, Wang D, Ding M, Xue L, Zhen F, et al. A study of neoadjuvant sintilimab combined with triplet chemotherapy of lipo-paclitaxel, cisplatin, and s-1 for resectable esophageal squamous cell carcinoma (ESCC). Ann Oncol (2020) 31:S1307–S8. doi: 10.1016/j.annonc.2020.10.196 [DOI] [Google Scholar]
- 31. He W, Leng X, Mao T, Luo X, Zhou L, Yan J, et al. Toripalimab plus paclitaxel and carboplatin as neoadjuvant therapy in locally advanced resectable esophageal squamous cell carcinoma. oncologist (2022) 27(1):e18–28. doi: 10.1093/oncolo/oyab011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kelly RJ, Zaidi AH, van Liere Canzoniero J, Feliciano JL, Hales RK, Voong KR, et al. Multicenter phase II study of neoadjuvant nivolumab or nivolumab plus relatlimab (antiLAG3 antibody) plus chemoradiotherapy in stage II/III esophageal/gastroesophageal junction (E/GEJ) carcinoma. J Clin Oncol (2022) 40(4 SUPPL):321. doi: 10.1200/JCO.2022.40.4_suppl.321 [DOI] [Google Scholar]
- 33. Lee S, Ahn BC, Park SY, Kim DJ, Lee CG, Cho J, et al. A phase II trial of preoperative chemoradiotherapy and pembrolizumab for locally advanced esophageal squamous cell carcinoma (ESCC). Ann Oncol (2019) 30:v754. doi: 10.1093/annonc/mdz065.004 [DOI] [Google Scholar]
- 34. Li K, Yang X, Luo W, Ma Q, Wang Y, Xiong Y, et al. Toripalimab plus nab-paclitaxel and carboplatin as neoadjuvant therapy for patients with esophageal squamous cell carcinoma at clinical stage t2-t4/n0-n2/m0: A single-arm, single-center clinical study. J ImmunoTher Cancer (2020) 8(SUPPL 3):A253. doi: 10.1136/jitc-2020-SITC2020.0415 [DOI] [Google Scholar]
- 35. Liu D, Zhang Q, Zhu J, Qian T, Yin R, Fan Z, et al. Phase-II study of toripalimab combined with neoadjuvant chemotherapy for the treatment of resectable esophageal squamous cell carcinoma. J Clin Oncol (2021) 39(15 SUPPL):e16029. doi: 10.1200/JCO.2021.39.15_suppl.e16029 [DOI] [Google Scholar]
- 36. Liu J, Li J, Lin W, Shao D, Depypere L, Zhang Z, et al. Neoadjuvant camrelizumab plus chemotherapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): A multicenter, phase 2 study. Int J Cancer (2022) 151(1):128–37. doi: 10.1002/ijc.33976 [DOI] [PubMed] [Google Scholar]
- 37. Liu J, Yang Y, Liu Z, Fu X, Cai X, Li H, et al. Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J immunother Cancer (2022) 10(3):e004291. doi: 10.1136/jitc-2021-004291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ma J, Zhang J, Yang Y, Zheng D, Wang X, Liang H, et al. 65P camrelizumab combined with paclitaxel and nedaplatin as neoadjuvant therapy for locally advanced esophageal squamous cell carcinoma (ESPRIT): A phase II, single-arm, exploratory research. Ann Oncol (2021) 32:S1400. doi: 10.1016/j.annonc.2021.10.083 [DOI] [Google Scholar]
- 39. Manish A, Shah KA. Multicenter, randomized phase II study of neoadjuvant pembrolizumab plus chemotherapy and chemoradiotherapy in esophageal adenocarcinoma (EAC)2021, in: ASCO Annual Meeting, JCO: American Society of Clinical Oncology; (2021) 39(15_suppl):4005. [Google Scholar]
- 40. Matsuda S, Yamamoto S, Kato K, Daiko H, Kojima T, Hara H, et al. FRONTiER: A feasibility trial of nivolumab with neoadjuvant CF or DCF, FLOT therapy for locally advanced esophageal carcinoma (JCOG1804E)-short-term results for cohorts c and d. J Clin Oncol (2022) 40(4 SUPPL):286. doi: 10.1200/JCO.2022.40.4_suppl.286 [DOI] [Google Scholar]
- 41. Qi WX, Zhao S, Li H, Chen J. Safety and tolerability of neoadjuvant chemoradiotherapy combined with pembrolizumab for local advanced, resectable esophageal cancer: preliminary results of a prospective phase IB trial. Int J Radiat Oncol Biol Phys (IJROBP) (2020) 108(3):e576–e7. doi: 10.1016/j.ijrobp.2020.07.1773 [DOI] [Google Scholar]
- 42. Shang X, Zhang C, Zhao G, Zhang W, Liu L, Duan X, et al. LBA3 safety and efficacy of pembrolizumab combined with paclitaxel and cisplatin as a neoadjuvant treatment for locally advanced resectable (stage III) esophageal squamous cell carcinoma (Keystone-001): Interim analysis of a prospective, single-arm, single-center, phase II trial. Ann Oncol (2021) 32:S1428–S9. doi: 10.1016/j.annonc.2021.10.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shen D, Chen Q, Wu J, Li J, Tao K, Jiang Y. The safety and efficacy of neoadjuvant PD-1 inhibitor with chemotherapy for locally advanced esophageal squamous cell carcinoma. J Gastrointest Oncol (2021) 12(1):1–10. doi: 10.21037/jgo-20-599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang Z. ed. Neoadjuvant camrelizumab combined with chemotherapy and apatinib for locally advanced thoracic esophageal squamous cell carcinoma (ESCC): A single-arm, open-label, phase ib study2021, in: ASCO Annual Meeting, JCO: American Society of Clinical Oncology; (2021) 39(15_suppl):4047. [Google Scholar]
- 45. Xing W, Zhao L, Zheng Y, Liu B, Liu X, Li T, et al. The sequence of chemotherapy and toripalimab might influence the efficacy of neoadjuvant chemoimmunotherapy in locally advanced esophageal squamous cell cancer–a phase II study. . Front Immunol (2021) 12:772450. doi: 10.3389/fimmu.2021.772450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamamoto S, Kato K, Daiko H, Kojima T, Hara H, Abe T, et al. FRONTiER: A feasibility trial of nivolumab with neoadjuvant CF or DCF therapy for locally advanced esophageal carcinoma(JCOG1804E)-the short-termresults of cohort a and b. J Clin Oncol (2021) 39(3 SUPPL):202. doi: 10.1200/JCO.2021.39.3_suppl.202 33332191 [DOI] [Google Scholar]
- 47. Yan X, Zhao J, Lei J, Duan H, Ni Y, Zhou Y, et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer (TD-NICE): A single arm, phase II study. Ann Oncol (2021) 32:S1442. doi: 10.1016/j.annonc.2021.10.163 [DOI] [Google Scholar]
- 48. Yang P, Zhou X, Yang X, Wang Y, Sun T, Feng S, et al. Neoadjuvant camrelizumab plus chemotherapy in treating locally advanced esophageal squamous cell carcinoma patients: a pilot study. World J Surg Oncol (2021) 19(1):333. doi: 10.1186/s12957-021-02446-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang W, Xing X, Yeung SJ, Wang S, Chen W, Bao Y, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J immunother Cancer (2022) 10(1):e003497. doi: 10.1136/jitc-2021-003497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang G, Hu Y, Yang B, Xu Q, Li J, Sun S, et al. A single-centre, prospective, open-label, single-arm trial of toripalimab with nab-paclitaxel and s-1 as a neoadjuvant therapy for esophageal squamous cell carcinoma (ESCC). Ann Oncol (2020) 31:S722. doi: 10.1016/j.annonc.2020.08.1178 [DOI] [Google Scholar]
- 51. Zhang X, Yang G, Su X, Luo G, Cai P, Zheng Y, et al. Neoadjuvant programmed death1 blockade plus chemotherapy in locally advanced esophageal squamous cell carcinoma. J Clin Oncol (2021) 39(15 SUPPL):e16076. doi: 10.1200/JCO.2021.39.15_suppl.e16076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang Y, Shen G, Xu R, Huang G, Yang S, Zheng Q, et al. Real-world effectiveness and safety of camrelizumab-based neoadjuvant therapy in resectable esophageal cancer: Initial results of a prospective multicenter observational study. J Clin Oncol (2022) 40(4 SUPPL):250. doi: 10.1200/JCO.2022.40.4_suppl.250 [DOI] [Google Scholar]
- 53. Zhang Z, Hong Z-N, Xie S, Lin W, Lin Y, Zhu J, et al. Neoadjuvant sintilimab plus chemotherapy for locally advanced esophageal squamous cell carcinoma: A single-arm, single-center, phase 2 trial (ESONICT-1). Ann Trans Med (2021) 9(21):1623. doi: 10.21037/atm-21-5381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang Z, Ye J, Li H, Du M, Gu D, Zhang J, et al. 1378P a single-center, prospective, open-label, single-arm trial of sintilimab with paclitaxel and carboplatin as a neoadjuvant therapy for esophageal squamous carcinoma. Ann Oncol (2021) 32:S1042–S3. doi: 10.1016/j.annonc.2021.08.1487 [DOI] [Google Scholar]
- 55. Wang F. ed. Camrelizumab in combination with preoperative chemotherapy for locally advanced esophageal squamous cell carcinoma: A single-arm, open-label, phase II study2021, in: ASCO Annual Meeting, JCO: American Society of Clinical Oncology; (2021) 39(3_suppl):222. [Google Scholar]
- 56. Xu W, Jiang Y, Wang C, Wu J, Li J, Hu Y, et al. The efficacy and safety of neoadjuvant camrelizumab and chemotherapy for locally advanced thoracic esophageal squamous cell carcinoma. J Clin Oncol (2022) 40(4 SUPPL):278. doi: 10.1200/JCO.2022.40.4_suppl.278 [DOI] [Google Scholar]
- 57. Hayashino Y, Noguchi Y, Fukui T. Systematic evaluation and comparison of statistical tests for publication bias. J Epidemiol (2005) 15(6):235–43. doi: 10.2188/jea.15.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhao X, Ren Y, Hu Y, Cui N, Wang X, Cui Y. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or the gastroesophageal junction: A meta-analysis based on clinical trials. PloS One (2018) 13(8):e0202185. doi: 10.1371/journal.pone.0202185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kelsen DP, Ginsberg R, Pajak TF, Sheahan DG, Gunderson L, Mortimer J, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med (1998) 339(27):1979–84. doi: 10.1056/NEJM199812313392704 [DOI] [PubMed] [Google Scholar]
- 60. Kato K, Ito Y, Daiko H, Ozawa S, Ogata T, Hara H, et al. A randomized controlled phase III trial comparing two chemotherapy regimen and chemoradiotherapy regimen as neoadjuvant treatment for locally advanced esophageal cancer, JCOG1109 NExT study. J Clin Oncol (2022) 40(4_suppl):238. doi: 10.1200/JCO.2022.40.4_suppl.238 [DOI] [Google Scholar]
- 61. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med (2006) 355(1):11–20. doi: 10.1056/NEJMoa055531 [DOI] [PubMed] [Google Scholar]
- 62. Mariette C, Dahan L, Mornex F, Maillard E, Thomas PA, Meunier B, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol (2014) 32(23):2416–22. doi: 10.1200/JCO.2013.53.6532 [DOI] [PubMed] [Google Scholar]
- 63. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med (2021) 384(13):1191–203. doi: 10.1056/NEJMoa2032125 [DOI] [PubMed] [Google Scholar]
- 64. Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet (2017) 390(10110):2383–96. doi: 10.1016/S0140-6736(17)31462-9 [DOI] [PubMed] [Google Scholar]
- 65. Sun H, Yang W, Luo J, Lin H, Zhou T, Gong H, et al. Safety and tolerability of neoadjuvant radiotherapy combined with anti-PD-1 antibody toripalimab for locally advanced, resectable esophageal squamous cell cancer: A prospective phase IB trial. Int J Radiat Oncol Biol Phys (2021) 111(3):S102–S3. doi: 10.1016/j.ijrobp.2021.07.238 [DOI] [Google Scholar]
- 66. Altorki NK, McGraw TE, Borczuk AC, Saxena A, Port JL, Stiles BM, et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. Lancet Oncol (2021) 22(6):824–35. doi: 10.1016/S1470-2045(21)00149-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.