Abstract
Neuropsychiatric manifestations of coronavirus disease 2019 (COVID-19) have been increasingly recognized. However, the pathophysiology of COVID-19 in the central nervous system remains unclear. Brain organoid models derived from human pluripotent stem cells are potentially useful for the study of complex physiological and pathological processes associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as they recapitulate cellular heterogeneity and function of individual tissues. We identified brain organoid studies that provided insight into the neurotropic properties of SARS-CoV-2. While SARS-CoV-2 was able to infect neurons, the extent of neurotropism was relatively limited. Conversely, choroidal epithelial cells consistently showed a high susceptibility to SARS-CoV-2 infection. Brain organoid studies also elucidated potential mechanism for cellular entry, demonstrated viral replication, and highlighted downstream cellular effects of SARS-CoV-2 infection. Collectively, they suggest that the neuropsychiatric manifestations of COVID-19 may be contributed by both direct neuronal invasion and indirect consequences of neuroinflammation. The use of high throughput evaluation, patient-derived organoids, and advent of “assembloids” will provide a better understanding and functional characterization of the neuropsychiatric symptoms seen in post-acute COVID-19 syndrome. With advancement of organoid technology, brain organoids offer a promising tool for unravelling pathophysiologic clues and potential therapeutic options for neuropsychiatric complications of COVID-19.
Keywords: brain organoids, pluripotent stem cells, COVID-19, SARS-CoV-2, neurotropism, neuropsychiatric
Introduction
The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has many systemic complications (Zheng 2020). Besides prominent respiratory symptoms, COVID-19 may also cause neurological manifestations (weakness, dizziness, headache, and hyposomnia) as well as neuropsychiatric symptoms (confusion, cognitive decline, anxiety, insomnia, and depression) that can be both short and long term (Ellul and others 2020; Fotuhi and others 2020; Taquet and others 2020; Wan and others 2020). A significant proportion of hospitalized COVID-19 patients were found to have acute altered mental status (Varatharaj and others 2020). The risk of getting a psychiatric disorder was also found to be approximately two times higher after a COVID-19 diagnosis with patients having a significantly higher incidence of mood disorders, anxiety, insomnia, and dementia (Taquet and others 2020).
The mechanism and pathophysiology of COVID-19 CNS (central nervous system) involvement remain to be elucidated. Multiple hypotheses, including direct viral invasion of virus into neurons, para-infectious immune-mediated processes, and systemic disease sequelae, have been proposed (Ellul and others 2020; Iadecola and others 2020). Yet, evidence of direct viral invasion into the CNS, from cerebrospinal fluid (CSF) and postmortem histopathological studies, have not been consistent. Some studies detected SARS-CoV-2 RNA in postmortem brains and CSF of patients with encephalitis (Moriguchi and others 2020; Solomon and others 2020) while others had not been able to replicate the findings (Bernard-Valnet and others 2020; Ye and others 2020).
Functional studies used to decipher the behavior and effects of SARS-CoV-2 at the cellular level have attracted attention. Studies using individual cell lines have shown that the SARS-CoV-2 has a spike protein that is primed by the serine protease-transmembrane serine protease 2 (TMPRSS2; Ou and others 2020). The virus then uses the spike protein to interact with the angiotensin converting enzyme 2 (ACE2) receptor to facilitate entry into the host cell (Hoffmann and others 2020). The expression of ACE2 is, however, variable in human tissues due to the presence of different cell types within the tissue (Hikmet and others 2020). As a result, the susceptibility of human tissue to SARS-CoV-2 is not well addressed by in vitro studies using single type of human cell lines (Jacob and others 2020). Organoid models have increasingly been recognized to be potentially useful as they preserve cellular heterogeneity and function of individual cell types (Jo and others 2016), thus improving the study of complex physiological or pathological processes associated with COVID-19.
Brain Organoids
Brain organoids are derived from human pluripotent stem cells, which can be further differentiated into cerebral organoids or brain region-specific organoids through the use of patterning factors (Kelava and Lancaster 2016). Brain organoids have an organized architecture composed of progenitors as well as neuronal and glial cell types (de Melo and others 2020; Qian and others 2019) and have proven to be an excellent model in the study of Zika virus (ZIKV) infections. For example, studies showed that ZIKV specifically infects forebrain cortical neural progenitor cells and, consequently, causes microcephaly-like phenotypes (Ming and others 2016). Besides structural cranial abnormalities, ZIKV infection has also been associated with psychiatric symptoms such as depression and psychosis (Corrêa-Oliveira and others 2017; Zucker and others 2017). A study using brain organoids found that ZIKV resulted in DNA methylation patterns that were associated with psychiatric disorders (Janssens and others 2018). In addition, researchers have used brain organoids to help identify drugs to reduce ZIKV-infection in cortical neural progenitor cells, thereby enabling the treatment of ZIKV infection (Xu and others 2016). Likewise, other CNS viral infections including HIV (dos Reis and others 2020), herpes simplex virus (HSV; D’Aiuto and others 2019), and cytomegalovirus (CMV) infections (Sun and others 2020) have been studied using brain organoid systems.
SARS-CoV-2 Neurotropism
With the increasing evidence of neurological symptoms associated with COVID-19, brain organoids have been utilized to study the potential neurotropic effects of SARS-CoV-2. In this review, we identified studies by searches on PubMed/MEDLINE using the search terms “SARS-CoV-2,” “COVID-19” in combination with the terms “organoid,” “brain,” “3D,” “iPSC.” Only articles published in English and articles up till March 31, 2021, were included.
We found a total of nine studies that used brain organoids (neutrospheres were not included) to study SARS-CoV-2 neurotropism. Both general and region-specific brain organoids were used to study the effects of SARS-CoV-2. Song and others (2020), Pellegrini and others (2020), Tiwari and others (2021), and Wang and others (2021) cultured organoids without the addition of any signaling molecules, allowing the spontaneous development of different brain regions within the same organoid. It has been shown that cerebral organoids are able to form complex brain architectures spontaneously using intrinsic signals (Benito-Kwiecinski and Lancaster 2020). The remaining five studies employed a variety of patterning factors and media that promoted regional specificity of brain organoids like the forebrain, midbrain, hippocampus and hypothalamus organoids (Gleeson and others 2021; Jacob and others 2020; Ramani and others 2020; Yi and others 2020; Zhang and others 2020). Particularly, the generation of choroid plexus containing organoids was interesting given that there were two recent studies that highlighted significant susceptibility of the choroid plexus organoid (CPO) to SARS-CoV-2 (Jacob and others 2020; Pellegrini and others 2020). Notably, the use of patterning factors not only allowed the modelling of targeted brain regions but also seemed to increase consistency and reproducibility of the organoids (Qian and others 2019). However, one potential limitation of using this approach was that external patterning factors may alter the natural and intrinsic development of the organoids, resulting in a loss of important brain features and characteristics (Benito-Kwiecinski and Lancaster 2020). We summarized the characteristics of the brain organoids used in COVID-19 studies in Table 1.
Table 1.
Summary of Brain Organoid Characteristics in COVID-19 Studies.
| S. No. | Reference | Brain Organoid Characteristics | |||||
|---|---|---|---|---|---|---|---|
| Brain Region | Stem Cells | Protocol | Patterning Factors | Organoid Age | Agitation | ||
| 1 | Zhang and others (2020) | Cortex | iPSC | iPSC → EB → Organoid | SB-431542 (TGFβ, Activin and NODAL inhibitor), Dorsomorphin (BMP inhibitor), GDNF, BDNF | 35 days | Yes |
| 2 | Song and others (2020) | Whole brain | iPSC | iPSC → EBs → Matrigel embedding → Agitation → Organoid | Intrinsic | 9 weeks | Yes |
| 3 | Yi and others (2020) | Cortex | hESC | hESC → EBs → Organoid | SB-431542 and IWR1 (WNT inhibitor) | 6 months | Yes |
| 4 | Jacob and others (2020) | Cortex | iPSC | iPSC → EBs → Matrigel embedding → Agitation → Organoid | Dorsomorphin, A83 (BMP and TGFβ inhibitor), SB-431542, CHIR99021 (GSK3β inhibitor) | Not stated | Yes |
| Midbrain | Forebrain factors + LDN-193189 (BMP and SMAD inhibitor), SB-431542, Purmorphamine (Smoothened agonist), SHH (Sonic Hedgehog), FGF8, CHIR99021, BDNF and GDNF | ||||||
| Hippocampus | Forebrain factors + Recombinant human BMP-7 | ||||||
| Hypothalamus | Forebrain factors + SHH, Purmorphamine, IWR-1 and SAG (Smoothened Agonist) | ||||||
| Choroid plexus | LDN-193189 (BMP and SMAD inhibitor), IWP-2 (WNT inhibitor), CHIR-99021, and BMP-7, BDNF, GDNF | ||||||
| 5 | Pellegrini and others (2020) | Whole brain | hESC | hESC → EBs → Matrigel embedding → Agitation → Organoid | Intrinsic | >55 days | Yes |
| Choroid plexus | CHIR99021 + BMP4 | ||||||
| 6 | Ramani and others (2020) | Cortex | iPSC | iPSC → EBs → Matrigel embedding → Agitation → Organoid | Dosmorphin, SB431542, BDNF, GDNF | 15/60 days | Yes |
| 7 | Tiwari and others (2021) | Whole brain | iPSC | iPSC → EBs → Matrigel embedding → Agitation → Organoid | Intrinsic | 80 days | Yes |
| 8 | Gleeson and others (2021) | Cortex | iPSC | iPSC → EB → Organoid | SB-431542, IWR-1, GDNF, BDNF | 60 days | — |
| 9 | Wang and others (2021) | Whole brain | iPSC | iPSC → EBs → Matrigel embedding → Agitation → Organoid | Intrinsic | 60 days | Yes |
Neurotropic Effects of SARS-CoV-2
Among the nine studies, spiked-pseudotyped SARS-CoV-2 virus was used in three studies while live virus was used in eight studies (two studies used both spiked-pseudotyped and live virus). The organoids were analyzed at various time points ranging between 24 and 96 hours post-infection (hpi). We summarized the infection protocols and main findings of these studies in Table 2.
Table 2.
COVID-19 Infection Protocol and Findings.
| S. No. | Reference | Infection Protocol | SARS-CoV-2 Infection | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Virus Type | MOI | Analysis HPI (hours) | Viral Replication | Neural Stem Cell/Progenitor | Intermediate Neural Progenitor Cells | Immature Neurons | Early Maturation Neurons | Late Maturation Neurons | Other Cells | Apoptosis | ||
| 1 | Zhang and others (2020) | Live | Not stated | 72 | Yes | + (NESTIN) | + (TUJ1) | |||||
| 2 | Song and others (2020) | Live | 1 | 24, 96 | Yes | + (SOX2) | + (TBR1) | + (MAP2/CTIP2) | + (TUNEL) | |||
| 3 | Yi and others (2020) | Pseudo-typed virus | 20 | 48 | + (TUJ1) | |||||||
| 4 | Jacob and others (2020) | Live | 0.001–0.1 | 24, 72 | Yes | + (DCX) | + (GFAP+ astrocyte) + (TTR+ choroid epithelial cell) |
+ (TUNEL) | ||||
| 5 | Pellegrini and others (2020) | Pseudo-typed virus/Live | 0.5 | 24, 48, 96 | Yes | − (SOX2) | − (HuC/D) | − (SMI312+) | + (TTR+ choroid epithelial cell) | |||
| 6 | Ramani and others (2020) | Live | 1.8 × 10−4 8.8 × 10−5 | 48, 96 | No | + (TUJ1) | + (MAP2/Tau) | + (TUNEL) | ||||
| 7 | Tiwari and others (2021) | Pseudo-typed virus/Live | 2 | 24, 48 | Yes | + (SOX2) | + (TUJ1) | |||||
| 8 | Gleeson and others (2021) | Live | 0.5 | 48 | Yes | + (GFP+ Pericyte-like cells) + (GFAP+ astrocyte) |
+ (Cleaved Caspase-3) | |||||
| 9 | Wang and others (2021) | Live | 0.1, 1 | 24, 72 | + (TUJ1) | + (SOX9+ astrocyte) | ||||||
Zhang and others (2020) were the first to show evidence of SARS-CoV-2 neurotropism in a brain organoid model. Using immunofluorescence staining, they demonstrated the presence of SARS-CoV-2 nucleoprotein in TUJ1-positive(+) neurons as well as NESTIN+ neural progenitor cells (NPCs) within the cortical brain organoid, suggesting direct infectivity of SARS-CoV-2 in both cortical neurons and NPCs (Zhang and others 2020). These findings have been replicated by subsequent studies (Song and others 2020). The percentage of invaded neurons seen in the organoids varied across the studies—10% at 48 hpi (Yi and others 2020) and 5% at 48 hpi (Ramani and others 2020). The studies used different neuronal lineage markers for immunostaining of the organoids. Collectively, this allowed for comparison and analysis of the preferential infectivity of SARS-CoV-2 in neuronal cells at different stages of neurogenesis and differentiation.
In contrast with ZIKV that directly targeted NPCs, SARS-CoV-2 has been shown to infect both NPCs and mature neurons (Zhang and others 2020; Song and others 2020; Tiwari and others 2021). Ramani and others (2020) showed, in 15-day-old brain organoids, that SARS-CoV-2 targeted early neurons in the cortical plate rather than the NPCs at the ventricular zones. When comparing the 15- and 60-day-old organoids, the 60-day-old organoid had a significantly greater number of SARS-CoV-2+ cells to the 15-day-old one (Ramani and others 2020). Similarly, Song and others (2020) and Tiwari and others (2021) observed that the majority of SARS-CoV-2 infected cells were the mature neurons rather than the neural progenitor cells (Song and others 2020; Tiwari and others 2021). Collectively, the studies demonstrated that SARS-CoV-2 had a predilection for mature neurons over NPCs.
Using brain organoids with choroid plexus (ChP) and cortical tissue, Pellegrini and others (2020) found that that 13% of ChP epithelial cells were infected and neuronal regions of the organoids were not (Pellegrini and others 2020). The limited infection in neuronal cells was only observed after the viral MOI was increased by 10 times (Pellegrini and others 2020). Jacob and others (2020) used region-specific brain organoids (cortical, hippocampal, hypothalamic, and midbrain) and found only minimal infection of neurons (0.6% at 24 hpi and 1.2% at 72 hpi), while a greater density of infected cells in the choroid plexus regions was observed (Jacob and others 2020). When exposing choroid plexus organoids directly to SARS-CoV-2, 12.6% of transthyretin positive (TTR+) choroidal epithelial cells were infected at 24 hpi and 18.6% of TTR+ choroidal epithelial cells were infected at 72 hpi (Jacob and others 2020). The findings of Pellegrini and others (2020) and Jacob and others (2020) showed that while neuronal invasion by SARS-CoV-2 was possible, the extent of neuron infection was relatively limited as compared to choroid epithelial cells infection.
Cellular Receptors
As ACE2 was known to be an important receptor in SARS-CoV-2 infection, some of the authors further examined the expression of ACE2 in brain organoids to evaluate the reasons behind the differing neurotropic findings. Immunofluorescence staining of ACE2 in brain organoids showed significant expression of ACE2 protein in both mature neurons and cells in neural tube-like structures of the organoids (Song and others 2020). Song and others (2020) further demonstrated the role of ACE2 in SARS-CoV-2 infection. After incubating the organoids with an anti-ACE2 blocking monoclonal antibody, significant inhibition of SARS-CoV-2 infection was noted compared with the isotype control, indicating the important role of ACE2 for infection of brain organoids.
Yi and others (2020) and Tiwari and others (2021) found that ACE2 was predominantly expressed in differentiated neurons rather than undifferentiated neural stem cells which corresponded to the findings before that SARS-CoV-2 preferentially infected mature neurons (Yi and others 2020; Tiwari and others 2021).
ACE2 expression in neurons was, however, relatively low when compared to other cell types. In comparison to human respiratory epithelial cells, ACE-2 expression was 12.5 and 50 times lower in brain organoids and neurons, respectively (Ramani and others 2020). This corresponded with Tiwari and others’ (2021) finding that SARS-CoV-2 infection was six times higher in a lung organoid as compared to a cortical brain organoid. Jacob and others (2020) found expression of ACE2 and TMPRSS2 to be at a much higher level in CPOs than in hippocampal organoids. Pellegrini and others (2020) demonstrated that infection matched ACE2 expression in CPOs, with the infected ChP epithelial cells being the ones that co-stained for ACE2.
Virus Replication
SARS-CoV-2 viral replication was also studied using the brain organoids. Zhang and others (2020) analyzed supernatant samples from infected brain organoids to evaluate SARS-CoV-2 viral particle release. They found that SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) gene copies in the supernatant increased over time, suggesting that the infected cells could release viral particles (Zhang and others 2020). Similarly, Song and others (2020), Tiwari and others (2021), and Gleeson and others (2021) showed evidence of SARS-CoV-2 replication in a brain organoid. Furthermore, viral particles and regions of budding from endoplasmic reticulum-like structures were observed in the organoids using electron microscopy, suggesting that SARS-CoV-2 could even replicate within neurons (Song and others 2020). In contrast, Ramani and others (2020) did not observe an increase in SARS-CoV-2+ cells in the brain organoids nor viral RNA levels in the organoid supernatants from 48 hpi to 96 hpi.
Jacob and others (2020) and Pellegrini and others (2020) showed viral replication in the choroid plexus organoids (CPOs). Both studies demonstrated increase in viral titers in the CPO supernatant and lysates over time (Jacob and others 2020; Pellegrini and others 2020).
Host-Virus Interaction
Neuronal cell death has been consistently observed after SARS-CoV-2 infection. By staining the brain organoids with terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL), a marker of DNA fragmentation and cell death, Ramani and others (2020) detected the presence of cell death in SARS-CoV-2-exposed organoids at 48 hpi. Majority of the SARS-CoV-2+ cells were found to be TUNEL+ as well. Interestingly, while Song and others (2020) also noted extensive neuronal cell death in regions of SARS-CoV-2 infection, the majority of dead cells were not infected with SARS-CoV-2 and only approximately 15% of the cells infected with SARS-CoV-2 were TUNEL+. Song and others (2020) hypothesized that the infected cells may have promoted cell death of adjacent noninfected cells.
Abnormal Tau phosphorylation was noted in some of the SARS-CoV-2+ cells that displayed Caspase-3, an important protease for apoptosis (Ramani and others 2020). The downstream implications are not clear and it remains to be seen if the Tau phosphorylation is directly caused by the virus or an effect of neuronal stress. The possibility for neurodegeneration after SARS-CoV-2 infection was further demonstrated in vitro by Wang and others (2021), who showed a reduction in neurite length and increased synaptic loss in SARS-CoV-2 infected neurons.
Song and others (2020) also performed single-cell RNA sequencing to investigate the transcriptional changes that occurred after SARS-CoV-2 infection of the cells within the organoid. Infected brain organoid cells displayed abnormal expression of genes related to cellular reproduction and upregulated metabolism while noninfected cells showed pathways related to hypoxia-induced cellular response. Song and others (2020) thus hypothesized that the hypermetabolic state in SARS-CoV-2 infected cells may cause a hypoxic microenvironment for surrounding cells and consequently inflict damage on them. Single-cell RNA sequencing also showed extensive transcriptional dysregulation within choroid plexus organoids after SARS-CoV-2 infection (Jacob and others 2020). The upregulated genes were found to be related to inflammation and cytokine release, while the downregulated genes were important for allowing the choroid plexus to function and secrete CSF. Jacob and others (2020) hypothesized that the downregulation of genes important for maintaining cell junctions in the choroid plexus suggested a remodeling or breakdown of normal CSF-blood barrier function (Jacob and others 2020). Indeed, Pellegrini and others (2020) demonstrated a decrease in fluid volume within the CPO and a concomitant dilution of media surrounding the organoid after infection, suggesting a leak of CSF-like fluid from the CPO after infection (Pellegrini and others 2020). This observation may offer a possible mechanism for how SARS-CoV-2 may contact brain parenchyma as the disruption of the choroid epithelial cell lining allows viral particles in the choroid capillaries to filter across the epithelial lining to enter CSF adjacent to the brain parenchyma (Bodnar and others 2021).
Discussion
At present, studies have highlighted some differing neurotropic properties of SARS-CoV-2, possibly contributed by the intrinsic heterogeneity of PSC-derived brain organoids (Shou and others 2020) and the use of different organoids with varying degrees of maturity. The different infection protocols used likely also played a role in the differing results. Viral MOI used, for example, was inconsistent among the studies and ranged widely from 8.8 × 10−5 to 20. The duration that the organoid was exposed to the virus was also not clearly stated in many studies.
Despite the differences, study findings suggest that while SARS-CoV-2 may have the ability to infect neurons, replicate within them and cause cell death, the susceptibility of neurons to SARS-CoV-2 infection was not high. Comparing between neural progenitor cells and mature neurons, NPCs demonstrated an even lower susceptibility to SARS-CoV-2 invasion which was consistent with findings of lower ACE2 receptor expression in the progenitor cells. In contrast with neurons, the choroidal epithelial cells have consistently shown high susceptibility to SARS-CoV-2 infection, which likewise was consistent with the greater expression of ACE2 receptors in choroidal epithelial cells.
Neuronal invasion has been demonstrated in vitro using brain organoids and downstream effects of hypermetabolism and cell death were observed. This corroborates postmortem findings that SARS-CoV-2 proteins were identified in the brain cells of some patients with COVID-19 (Matschke and others 2020). However, in view of the relatively low susceptibility of neurons to SARS-CoV-2 infection, it is likely that other mechanisms for neuroinflammation are present as well. The same autopsy study noted that neuropathological findings of microglia and T-cell infiltration were present regardless of whether SARS-CoV-2 was detected in the CNS, suggesting that the neuropathological changes and neuronal damage may be immune mediated rather than as a result of direct viral invasion. The use of CPO organoids has drawn attention to the possible role of the choroid plexus in contributing to the pathophysiology of neuropsychiatric complications. The breakdown of the blood-CNS barrier with infection of the choroid plexus epithelial cells could facilitate and introduce SARS-CoV-2 particles into the CNS (Fig. 1). Besides the direct introduction of viral particles, a breakdown in blood-CNS barrier may possibly also introduce systemic immune cells and cytokines into the CNS (Pellegrini and others 2020). Many studies have shown increased levels of pro-inflammatory factors and cytokines in the CSF of patients with chronic fatigue syndrome (Yang and others 2019), a condition with symptoms similar to “post-acute COVID-19 syndrome” (Nalbandian and others 2021). While current brain organoid studies have not fully uncovered the neuroinflammatory processes caused by SARS-CoV-2, the possibility of CNS cytokine storm causing neuropsychiatric symptoms of “post-acute COVID-19 syndrome” is intriguing (Fotuhi and others 2020) as Jacob and others (2020) demonstrated that choroid plexus organoids expressed higher levels of cytokines and pro-inflammatory factors after SARS-CoV-2 infection. Collectively, the brain organoid studies demonstrated the possibility that neuropsychiatric manifestations of COVID-19 may be contributed by both direct neuronal invasion and indirect consequences of neuroinflammation facilitated by the breakdown of support cells.
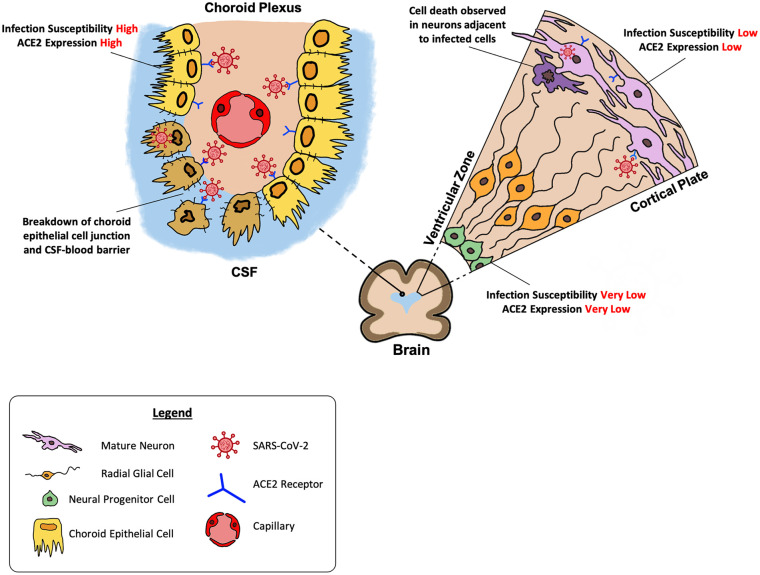
Figure 1.
Brain organoid findings of SARS-CoV-2 effect on human brain. Neurons demonstrated a relatively low susceptibility to SARS-CoV-2 as compared to choroid epithelial cells. SARS-CoV-2 showed a predilection for mature neurons at the cortical plate over the NPCs at the ventricular zone (Right). This corresponded with greater expression of ACE2 receptors in mature neurons as compared to NPCs. Neuronal cell death was observed in neurons adjacent to the infected cells. Break down of choroidal epithelial cell junction and CSF-blood barrier was observed in the choroid plexus following SARS-CoV-2 infection (Left).
Challenges and Future Directions
Current published studies have several limitations. First, the direct exposure of the SARS-CoV-2 virus to the brain organoid does not represent physiological conditions, especially when the viral mode of entry to the brain remains elusive, with numerous pathways including hematogenous, transneuronal, and meningeal-lymphatic routes (Bostancıklıoğlu 2020; Kumar and others 2020). Second, there is no established optimal MOI or duration of viral exposure on neuronal cells. Third, brain organoids currently lack physiological properties such as blood-brain barrier and innate immune cells that are most critical to model process of viral infection. Last, due to limited culture time in vitro, brain organoids largely resemble fetal brain tissue rather than adult brains (Sun and others 2018). These limitations should be addressed with modifications and improvements of organoid protocols in the near future.
COVID-19 patients can continue to complain of persistent symptoms after the acute-phase of the illness, leading to “post-acute COVID-19 syndrome” (Baig 2020, Nalbandian and others 2021). The potential impact of this syndrome is attracting considerable attention from the medical community and will only be clarified in the coming years. A wide range of neurological related symptoms such as fatigue, cognitive difficulties, dysautonomia, and “brain fog” have been reported (Nath and Smith 2020). It is not clear if some of these chronic symptoms may be related to early neurodegeneration, even though there have been case reports of Parkinson’s disease following COVID-19 infection (Li and others 2020). The findings of abnormal Tau phosphorylation in some SARS-CoV-2 infected cells (Ramani and others 2020) is an intriguing observation and may potentially provide a link between chronic neurologic symptoms and subsequent neurodegeneration. Brain organoids are useful for further investigation of the pathophysiology behind “post-acute COVID-19 syndrome” and the longer term neuropsychiatric effects of COVID-19. Region-specific brain organoids may be used to study the susceptibility of the basal ganglia and limbic system to the SARS-CoV-2 virus and single-cell RNA may be used to elucidate the downstream effects of SARS-CoV-2 in these brain regions.
Besides shedding light on the pathophysiology of long-term neuropsychiatric effects, brain organoids can also be used as screening and validation tools for potential therapeutic agents, as seen in the case of ZIKV where compounds identified from a high-content chemical screen in hiPS cell-derived hNPCs were tested on brain organoids (Amin and Paşca 2018). Wang and others (2021) found that the antiviral drug remdesivir not only effectively inhibited SARS-CoV-2 infection in hiPSC-derived neurons but also resulted in a significant improvement in neurite lengths of the infected neurons (Wang and others 2021). Such studies may be furthered using the brain organoid platform to evaluate the downstream cellular effects of the medication.
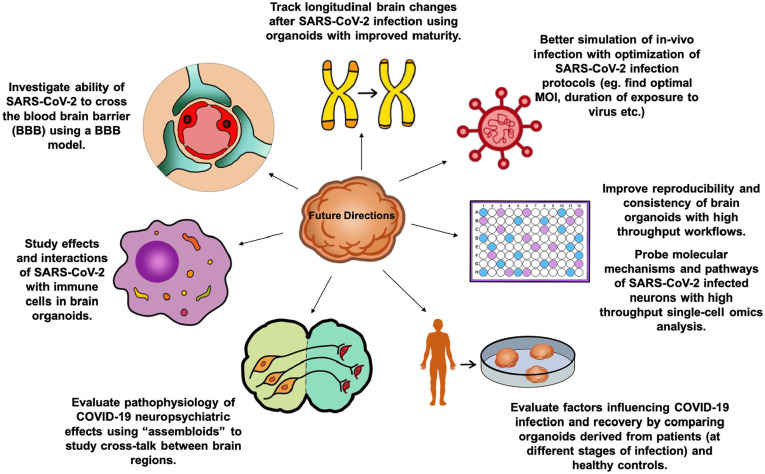
Other future directions to further develop brain organoids as models for neuro-COVID-19 (Fig. 2) include the development of high throughput evaluation to analyze the transcriptomes, epigenetics, and proteomics of infected brain organoids and the use of patient-derived organoids for different stages of COVID-19 infection and recovery to draw correlation between pathophysiology and neuropsychiatric clinical findings. Gleeson and others (2021) integrated perivascular cells into cortical organoids and found that cortical organoids with the pericyte-like cells had more extensive SARS-CoV-2 infection than the traditional cortical organoids. It would be interesting to evaluate SARS-CoV-2 infection in choroid plexus organoids with perivascular cells. The advent of such “assembloids” would facilitate the modelling of more sophisticated brain functions and the “cross-talk” between different brain regions to study neuropsychiatric symptoms seen as a sequelae of COVID-19.
Figure 2.
Future directions for using brain organoids to model neuro-COVID-19.
Conclusions
While the field of organoid technology is still at an infant stage of development, brain organoid models have provided several crucial pieces of evidence on the effects of SARS-CoV-2 on the brain. Current studies have identified neurotropic properties, highlighted potential mechanism for cellular entry, and also demonstrated the ability of the virus in replicating and interacting with neurons, resulting in several downstream cellular effects. However, these studies are still not able to explain specific clinical manifestations of neuro-COVID-19, and further study to investigate selective tropism and molecular pathways is required to identify the pathophysiology underpinning the neuropsychiatric complications of COVID-19. With advancement of organoid technology, the use of brain organoids offers a promising tool for unravelling novel pathophysiologic clues and potential therapeutic options for the neurological and psychiatric complications of COVID-19 and provides a better understanding and functional characterization of chronic COVID-19 syndrome.
Acknowledgments
We would like to thank the National Medical Research Council, Singapore, for their support.
Footnotes
Author Contributions: JHN and EKT contributed to the conception and design of the review; JHN and EKT contributed to the acquisition and analysis of data; JHN, EKT, AS, and SJ contributed to the drafting of the manuscript; EKT supervised the completion of this work.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Medical Research Council (STaR and PD-LCG-002).
ORCID iD: Jing-Han Ng  https://orcid.org/0000-0001-8195-5025
https://orcid.org/0000-0001-8195-5025
References
- Amin ND, Paşca SP. 2018. Building models of brain disorders with three-dimensional organoids. Neuron 100(2):389–405. doi: 10.1016/j.neuron.2018.10.007 [DOI] [PubMed] [Google Scholar]
- Baig AM. 2020. Deleterious outcomes in long-hauler COVID-19: the effects of SARS-CoV-2 on the CNS in chronic COVID syndrome. ACS Chem Neurosci 11(24):4017–20. doi: 10.1021/acschemneuro.0c00725 [DOI] [PubMed] [Google Scholar]
- Benito-Kwiecinski S, Lancaster MA. 2020. Brain organoids: human neurodevelopment in a dish. Cold Spring Harb Perspect Biol 12(8):1–18. doi: 10.1101/cshperspect.a035709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard-Valnet R, Pizzarotti B, Anichini A, Demars Y, Russo E, Schmidhauser M, and others. 2020. Two patients with acute meningoencephalitis concomitant with SARS-CoV-2 infection. Eur J Neurol 27(9):e43–e44. doi: 10.1111/ene.14298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar B, Patel K, Ho W, Luo JJ, Hu W.2021. Cellular mechanisms underlying neurological/neuropsychiatric manifestations of COVID-19. J Med Virol 93(4):1983–98. doi: 10.1002/jmv.26720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostancıklıoğlu M. 2020. SARS-CoV2 entry and spread in the lymphatic drainage system of the brain. Brain Behav Immun 87:122–3. doi: 10.1016/j.bbi.2020.04.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa-Oliveira GE, Do Amaral JL, Da Fonseca BAL, Del-Ben CM. 2017. Zika virus infection followed by a first episode of psychosis: another flavivirus leading to pure psychiatric symptomatology. Rev Bras Psiquiatr 39(4):381–2. doi: 10.1590/1516-4446-2017-2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aiuto L, Bloom DC, Naciri JN, Smith A, Edwards TG, McClain L, and others. 2019. Modeling herpes simplex virus 1 infections in human central nervous system neuronal cells using two- and three-dimensional cultures derived from induced pluripotent stem cells. J Virol 93(9):e00111–19. doi: 10.1128/jvi.00111-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo BAG, Benincasa JC, Cruz EM, Maricato JT, Porcionatto MA. 2020. 3D culture models to study SARS-CoV-2 infectivity and antiviral candidates: from spheroids to bioprinting. Biomed J 44(1):31–42. doi: 10.1016/j.bj.2020.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Reis RS, Sant S, Keeney H, Wagner MCE, Ayyavoo V.2020. Modeling HIV-1 neuropathogenesis using three-dimensional human brain organoids (hBORGs) with HIV-1 infected microglia. Sci Rep 10(1):15209. doi: 10.1038/s41598-020-72214-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, and others. 2020. Neurological associations of COVID-19. Lancet Neurol 19(9):767–83. doi: 10.1016/S1474-4422(20)30221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotuhi M, Mian A, Meysami S, Raji CA. 2020. Neurobiology of COVID-19. J Alzheimers Dis 76(1):3–19. doi: 10.3233/JAD-200581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson J, Wang L, Sievert D, Clark A, Federman H, Gastfriend B, and others. 2021. A human 3D neural assembloid model for SARS-CoV-2 infection. Res Sq. Preprint. doi: 10.21203/rs.3.rs-214352/v1 [DOI] [Google Scholar]
- Hikmet F, Méar L, Edvinsson Å, Micke P, Uhlén M, Lindskog C.2020. The protein expression profile of ACE2 in human tissues. Mol Syst Biol 16(7):e9610. doi: 10.15252/msb.20209610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, and others. 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271–80.e8. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Anrather J, Kamel H.2020. Effects of COVID-19 on the nervous system. Cell. 183(1):16–27.e1. doi: 10.1016/j.cell.2020.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F, Pather SR, Huang WK, Zhang F, Wong SZH, Zhou H, and others. 2020. Human pluripotent stem cell-derived neural cells and brain organoids reveal SARS-CoV-2 neurotropism predominates in choroid plexus epithelium. Cell Stem Cell 27(6):937–950.e9. doi: 10.1016/j.stem.2020.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens S, Schotsaert M, Karnik R, Balasubramaniam V, Dejosez M, Meissner A, and others. 2018. Zika virus alters DNA methylation of neural genes in an organoid model of the developing human brain. mSystems 3(1):e00219–17. doi: 10.1128/msystems.00219-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J, Xiao Y, Sun AX, Cukuroglu E, Tran HD, Göke J, and others. 2016. Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell 19(2):248–57. doi: 10.1016/j.stem.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelava I, Lancaster MA. 2016. Stem cell models of human brain development. Cell Stem Cell 18(6):736–48. doi: 10.1016/j.stem.2016.05.022 [DOI] [PubMed] [Google Scholar]
- Kumar A, Pareek V, Prasoon P, Faiq MA, Kumar P, Kumari C, and others. 2020. Possible routes of SARS-CoV-2 invasion in brain: in context of neurological symptoms in COVID-19 patients. J Neurosci Res 98(12):2376–83. doi: 10.1002/jnr.24717 [DOI] [PubMed] [Google Scholar]
- Li WS, Chan LL, Chao YX, Tan EK. 2020. Parkinson’s disease following COVID-19: causal link or chance occurrence? J Transl Med 18(1):493. doi: 10.1186/s12967-020-02670-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, and others. 2020. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol 19(11):919–29. doi: 10.1016/S1474-4422(20)30308-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming G, Tang H, Song H.2016. Advances in Zika virus research: stem cell models, challenges, and opportunities. Cell Stem Cell 19(6):690–702. doi: 10.1016/j.stem.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, and others. 2020. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis 94:55–8. doi: 10.1016/j.ijid.2020.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, and others. 2021. Post-acute COVID-19 syndrome. Nat Med 27(4):601–5. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Smith B.2020. Neurological issues during COVID-19: an overview. Neurosci Lett 742:135533. doi: 10.1016/j.neulet.2020.135533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, and others. 2020. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 11(1):1620. doi: 10.1038/s41467-020-15562-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini L, Albecka A, Mallery DL, Kellner MJ, Paul D, Carter AP, and others. 2020. SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell 27(6):951–61.e5. doi: 10.1016/j.stem.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Song H, Ming GL. 2019. Brain organoids: advances, applications and challenges. Development 146(8):dev166074. doi: 10.1242/dev.166074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani A, Müller L, Ostermann PN, Gabriel E, Abida-Islam P, Müller-Schiffmann A, and others. 2020. SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J 39(20):e106230. doi: 10.15252/embj.2020106230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou Y, Liang F, Xu S, Li X.2020. The application of brain organoids: from neuronal development to neurological diseases. Front Cell Dev Biol 8:579659. doi: 10.3389/fcell.2020.579659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, and others. 2020. Neuropathological features of Covid-19. N Engl J Med 383(10):989–92. doi: 10.1056/nejmc2019373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S, and others. 2020. Neuroinvasion of SARS-CoV-2 in human and mouse brain. bioRxiv Prepr Serv Biol. doi: 10.1101/2020.06.25.169946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun AX, Ng HH, Tan EK. 2018. Translational potential of human brain organoids. Ann Clin Transl Neurol 5(2):226–35. doi: 10.1002/acn3.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Chiuppesi F, Chen X, Wang C, Tian E, Nguyen J, and others. 2020. Modeling human cytomegalovirus-induced microcephaly in human iPSC-derived brain organoids. Cell Rep Med. 1(1):100002. doi: 10.1016/j.xcrm.2020.100002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M, Luciano S, Geddes JR, Harrison PJ. 2020. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 8(2):130–40. doi: 10.1016/S2215-0366(20)30462-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari SK, Wang S, Smith D, Carlin AF, Rana TM. 2021. Revealing tissue-specific SARS-CoV-2 infection and host responses using human stem cell-derived lung and cerebral organoids. Stem Cell Rep 16(3):437–45. doi: 10.1016/j.stemcr.2021.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA, Tenorio EL, and others. 2020. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry 7(10):875–82. doi: 10.1016/S2215-0366(20)30287-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YM, Deng X, Tan EK. 2020. Olfactory dysfunction and COVID-19. Lancet Psychiatry 7(8):663. doi: 10.1016/S2215-0366(20)30253-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhang M, Garcia G, Tian E, Cui Q, Chen X, and others. 2021. ApoE-isoform-dependent SARS-CoV-2 neurotropism and cellular response. Cell Stem Cell 28(2):331–42.e5. doi: 10.1016/j.stem.2020.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Lee EM, Wen Z, Cheng Y, Huang WK, Qian X, and others. 2016. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med 22(10):1101–7. doi: 10.1038/nm.4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Yang Y, Wang D, Li C, Qu Y, Guo J, and others. 2019. The clinical value of cytokines in chronic fatigue syndrome. J Transl Med 17(1):213. doi: 10.1186/s12967-019-1948-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M, Ren Y, Lv T.2020. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun 88:945–6. doi: 10.1016/j.bbi.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SA, Nam KH, Yun J, Gim D, Joe D, Kim YH, and others. 2020. Infection of brain organoids and 2D cortical neurons with SARS-CoV-2 pseudovirus. Viruses. 12(9):1004. doi: 10.3390/v12091004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang BZ, Chu H, Han S, Shuai H, Deng J, Hu Y, and others. 2020. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res 30(10):928–31. doi: 10.1038/s41422-020-0390-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J. 2020. SARS-coV-2: an emerging coronavirus that causes a global threat. Int J Biol Sci 16(10):1678–85. doi: 10.7150/ijbs.45053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker J, Neu N, Chiriboga CA, Hinton VJ, Leonardo M, Sheikh A, and others. 2017. Zika virus–associated cognitive impairment in adolescent, 2016. Emerg Infect Dis 23(6):1047–8. doi: 10.3201/eid2306.162029 [DOI] [PMC free article] [PubMed] [Google Scholar]