Abstract
microRNA-146a (miR-146a) plays an essential role in immune anomalies and organ injury of systemic lupus erythematosus (SLE) by regulating the disease’s inflammation and complications. Here, we analyzed the expression of miR-146a in SLE and a panel of pro-inflammatory cytokines (IL-1, IL-6, IL-8, IL-17, and TNF-α). Association between all measured parameters and the disease’s clinical manifestation and response to treatment was monitored. Our study populations were 113 SLE patients and 104 healthy volunteers. miR-146a expression in peripheral blood mononuclear cells (PBMCs) was measured by quantitative real-time PCR (RT-qPCR). The content of the plasma cytokines (IL-1β, IL-6, IL-8, IL-17, and TNF-α) was detected by enzyme-linked immunosorbent assay (ELISA). Compared with healthy controls, miR-146a expression was significantly increased (p < 0.05) in lupus patients. The analysis of the receiver operator characteristic curve (ROC) of miR-146a showed 91% sensitivity and 70% specificity. IL-1β, IL-6, and IL-17 cytokines were significantly increased (p < 0.001), while IL-8 and TNF-α were significantly decreased (p < 0.001) in SLE patients against controls. The expression of miR-146a and TNF-α was upregulated considerably in SLE patients with severe disease activity. miR-146a expression was positively correlated with IL-6. Our results pointed to the elevation of miR-146a as a trade marker of SLE patients. Reduction of IL-8 and TNF-α in combination with an elevation of IL-1β, IL-6, and IL-17 might refer to miR-146a’s dual effect in controlling inflammation in lupus. Although we shed some light on the role of miR-146a in SLE, further study is recommended to improve our results.
Keywords: miR-146a, pro-inflammatory cytokines, systemic lupus erythematosus
Introduction
MicroRNA-146a (miR-146a) is an important member of the micro-ribonucleic acids (miRs) family, with 22–25 nucleotides in length. They are a kind of non-coding single-stranded RNA molecule that participates in gene regulation at the transcriptional level by degrading or blocking translated messenger RNA (mRNA).1,2 Each miR can regulate multiple target genes, while different miRs can also regulate the same target gene. They play essential roles in immune cell differentiation as well as immune-inflammatory response.3,4
miR-146a is located on the long arm of chromosome 5.5,6 The immunological- and hematopoiesis-related miRs participate in hematopoietic cell proliferation and differentiation, immune response, and release of inflammatory mediators.6 It modulates innate immunity by regulating Toll-like receptor (TLR) signaling and cytokine response. Several autoimmune disorders, including psoriasis,7 rheumatoid arthritis (RA),8 osteoarthritis,9 and systemic lupus erythematosus (SLE), have dysregulated miR-146a expression (SLE).10,11
SLE is a complex heterogeneous autoimmune disorder with many clinical and laboratory manifestations. It is triggered by environmental and genetic factors that result in a loss of tolerance toward self-antigens.12 Therefore, auto-reactive antibodies are produced by auto-reactive B cells from the immune complexes causing tissue inflammation and damage in different tissues (like the kidney, skin, joint, vascular, and central nervous system). Consequently, auto-reactive T cells secrete a wide range of pro-inflammatory cytokines with both autocrine and paracrine effects.13,14
Several studies have documented the role of inflammatory cytokines such as IL-1β, IL-6, IL-17, IL-8, and TNF-α in SLE progression by amplifying the inflammatory response.15–22 These pro-inflammatory cytokines can be produced by activating the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) signaling pathway. Interleukin 1 Receptor Associated Kinase-1 (IRAK1) and TNF Receptor Associated Factor-6 (TRAF6) are two key molecules in the NF-kB pathway. Both are targeted to negative feedback regulation by miR-146a.21,23 Therefore, elevation in miR-146a expression can reduce NF-kB activity by inhibiting IRAK-1 and TRAF-6 expressions.6,19,21
Hence, in this study, we measured the expression of miR-146a and its related pro-inflammatory cytokines (IL-1β, IL-6, IL-17, IL-8, and TNF-α) in SLE patients. The implication of these mediators in SLE pathogenicity, disease activity, prognosis, and judging treatment was evaluated. Although several earlier studies have highlighted the significance of miR-146a and pro-inflammatory cytokines in SLE,17–19,24,25 none of them measured the whole panel with a close insight into their correlation with several diseases’ clinical manifestations and therapeutic modalities.
Subjects and methods
Patients and controls
One hundred four healthy volunteers and 113 SLE patients were enrolled in the present cross-sectional case-control study from the Rheumatology and Rehabilitation Department, El-Kasr El-Ainy Hospital, Cairo University, Egypt (from 2017 to 2019). SLE patients were diagnosed in line with the 2012 Systemic Lupus International Collaborating Clinics (SLICC).26 The local ethical committee (Rheumatology and Rehabilitation Department, Faculty of Medicine, Cairo University) approved our current study on lupus patients (following the Declaration of Helsinki). Informed consent was taken from the whole group of participants. Both groups are matched in age and gender.
Complete personal and medical history taking, in addition to clinical examination, were collected, including erythrocyte sedimentation rate (ESR), platelets count, liver and kidney functions, complement component 3 and 4 (C3 and C4) levels, and autoimmune profile tests, including Antinuclear Antibody (ANA) and anti–ds DNA antibodies. The SLE Disease Activity Index (SLEDAI) was used to evaluate the disease activity in lupus patients.27 According to Mosca and Bombardieri,28 SLEDAI scores were classified into six stages (no activity: 0, mild activity: 1–5, moderate activity: 6–10, high activity: 11–19, and severe activity: ≥20). The inclusion criteria involved diagnosis based on SLICC and age of disease onset is more than 18 years. The exclusion criteria were pregnancy, concomitant other autoimmune diseases, oncologic diseases, or chronic infectious diseases.
Detection of miR-146a and cytokines
A tube with ethylene-diamine-tetra-acetic acid (EDTA) was used to collect venous blood from all subjects by vein puncture. The separation of human peripheral blood mononuclear cells (PBMCs) from blood was done using Ficoll-Hypaque separation media according to AboEl-Attaet al.29 The concentration of extracted RNA was estimated using nanodropTM2000/2000c (Thermo Fisher Scientific, Waltham, MA, USA). The integrity of RNA was verified by 1% agarose gel electrophoresis. Hs_miR-146a expression was measured using a quantitative real-time polymerase chain reaction.29 The expression of the U6B small nuclear RNA (RNU6B) was used as an endogenous control for data normalization. The cycle threshold (Ct) values variations between the tested miRNA and reference gene were computed to define the relative expression levels and calculated using the 2−ΔΔCt method.29
Measurement of cytokine levels in plasma
According to the guidelines of the DuoSet ELISA development kits (R&D Systems, Inc., Minneapolis, MN, USA), with a few minor modifications, the levels of IL-1β, IL-6, IL-17, IL-8, and TNF-α were measured. The digital data of raw absorbance readings were converted into a standard curve using the ELISA reader-controlling software (Softmax; Molecular Devices, Sunnyvale, CA, USA), from which the cytokine concentrations were calculated. Results were expressed as a picogram of cytokine per milliliter plasma (pg/ml).18
Statistical analysis
SPSS (statistical package version 11) (SPSS, IBM Corporation, USA) was used to perform all statistical calculations. Categorical variables were expressed using counts and percentages. Continuous data were presented as mean with standard deviation (±SE). A two-sided t-test was used to compare continuous data of patients and controls—one-way analysis of variance (ANOVA), followed by Tukey’s Test for Post-Hoc test for multiple comparisons. The correlation between variables was determined using Pearson’s correlation test. For those variables significantly influencing cytokine levels (p < 0.05), a standard linear multiple regression analysis with each cytokine level as the dependent variable was performed. For the regression model, adjusted β and p-values were recorded. The Receiver Operating Characteristic (ROC) curve was used to predict cutoff values of miR-146a as a potential diagnostic marker for SLE. Evaluation of the miR-146a was done by calculating sensitivity and specificity. We computed the adequate sample size and statistical power (https://www.calculator.net/sample-size-calculator.html). The prevalence rate of SLE in Egypt is 6.1 per 100,000,30 and we enrolled 113 SLE patients in our study. Under the assumptions of 6.1/100,000 disease prevalence, 5% margin of error, and 95% confidence level, the smallest sample size to achieve 95% power is 88 cases. Thus, 88 or more lupus patients are needed to have a confidence level of 95%. Using Bonferroni correction, the p-value was corrected for the number of variables (P corrected, Pc).
Results
Characteristics of controls and SLE patients
One hundred four healthy volunteers and 113 SLE patients participated in this study. The mean age of SLE patients and controls were 32.60 ± 9.50 and 27.31 ± 9.70 years, respectively. The mean disease duration was 7.21 ± 0.53 years (M±SE). The mean SLEDAI score of SLE patients was 9.28 ± 0.73 (range from 0 to 39). Supplementary Table 1 shows controls and SLE subjects’ clinical and laboratory data.
Expression of miR-146a in SLE patients
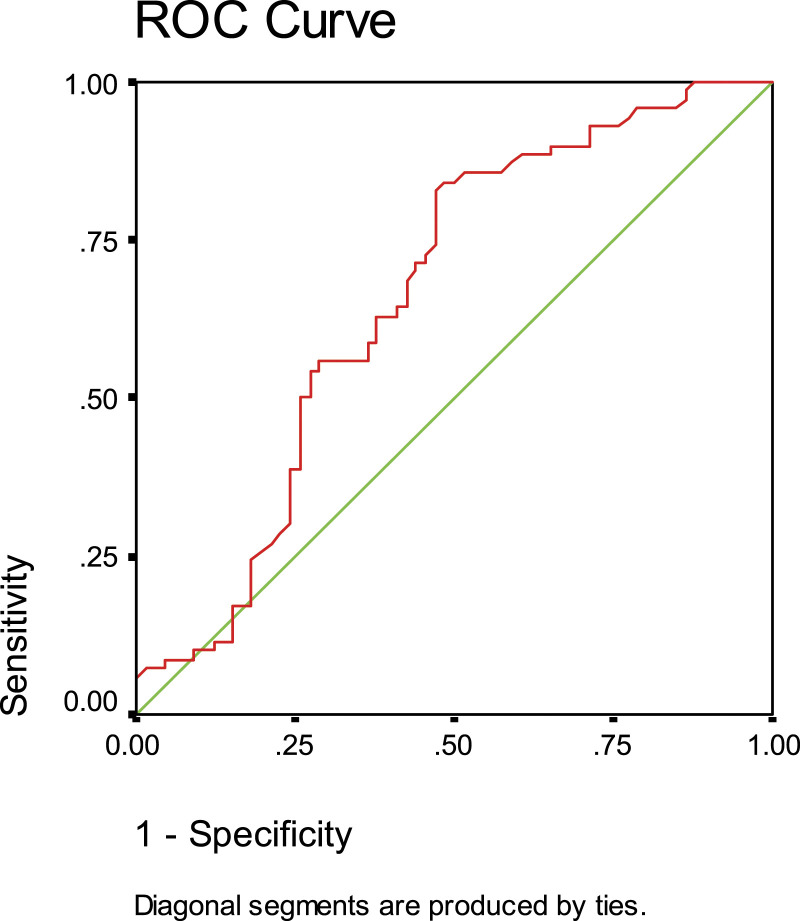
In SLE patients, the expression of miR-146a was significantly upregulated(M ± SE) (1.93 ± 0.17) compared to the controls (1.37 ± 0.17) (p < 0.05). The ROC curve was constructed to evaluate its efficiency as a biomarker for lupus patients. At a cutoff value of 0.66, miR-146a has a sensitivity of 91% and a specificity of 70%. The area under the curve (AUC) value was 0.51 with 95% CI: 0.57–0.75 (p < 0.001) versus the control (Figure 1).
Figure 1.
Receiver operating characteristic (ROC) curve analysis for identification of sensitivity and specificity of the miR-146a in plasma of (SLE) systemic lupus erythematosus patients.
miR-146a was significantly decreased in patients with alopecia (p < 0.01). On the other hand, its expression was significantly increased in SLE patients with several clinical manifestations (p < 0.01), including photosensitivity, vasculitis, Raynaud’s phenomena, neuropsychiatric disorders, pancytopenia, thrombocytopenia, hemolytic anemia, leucopenia, neutropenia, and lymphopenia (Table 1). Significant increase in miR-146a (p < 0.05) with increasing disease activity score (activity score >20) was observed (Table 2). However, miR-146a was significantly reduced with cyclophosphamide treatment (p < 0.01) (Table 3).
Table 1.
Association of SLE clinical manifestations with miR-146a and pro-inflammatory cytokines in SLE patients.
| Measured parameter | miR-146a (M±SE) | IL-1β (M±SE) | IL-6 (M±SE) | IL-17 (M±SE) | IL-8 (M±SE) | TNF-α (M±SE) |
|---|---|---|---|---|---|---|
| Mucocutaneous manifestation | ||||||
| Yes [93 (83%)] | 1.92 ± 0.20 | 19.83 ± 1.86**/** | 14.12 ± 1.14 | 982.44 ± 213.46 | 76.76 ± 12.51 | 20.68 ± 2.63 |
| No [20 (17%)] | 1.91 ± 0.37 | 29.41 ± 3.24 | 17.73 ± 2.88 | 1494.28 ± 710.99 | 82.44 ± 38.72 | 29.66 ± 5.31 |
| Malar rash | ||||||
| Yes [82 (73%)] | 1.99 ± 0.23 | 19.78 ± 2.10 | 14.51 ± 1.26 | 1104.48 ± 251.44 | 92.55 ± 16.08 | 18.37 ± 2.32*/* |
| No [31 (27%)] | 1.73 ± 0.21 | 24.89 ± 2.69 | 15.36 ± 2.07 | 966.26 ± 394.49 | 41.03 ± 12.02 | 31.47 ± 5.57 |
| Photosensitivity | ||||||
| Yes [60 (54%)] | 2.61 ± 0.21*/* | 19.18 ± 2.11 | 14.56 ± 1.55 | 980.57 ± 248.84 | 91.49 ± 19.01 | 16.91 ± 1.89*/* |
| No [53 (46%)] | 1.81 ± 0.25 | 23.45 ± 2.57 | 14.97 ± 1.49 | 1169.09 ± 393.45 | 62.78 ± 14.62 | 28.11 ± 4.43 |
| Oral ulcers | ||||||
| Yes [57 (51%)] | 1.91 ± 0.25 | 21.97 ± 2.12 | 14.46 ± 1.27 | 1077.36 ± 232.09 | 29.38 ± 3.60 | 21.60 ± 3.21 |
| No [56 (49%)] | 1.98 ± 0.25 | 27.48 ± 3.33 | 15.61 ± 2.03 | 1074.31 ± 437.60 | 28.21 ± 1.86 | 23.33 ± 3.43 |
| Alopecia | ||||||
| Yes [34 (30%)] | 1.73 ± 0.20*/NS | 20.51 ± 1.96 | 13.44 ± 1.85 | 585.21 ± 195.09 | 36.47 ± 6.65*/* | 24.11 ± 3.52 |
| No [79 (70%)] | 2.06 ± 0.25 | 25.87 ± 2.53 | 15.44 ± 1.32 | 1370.91 ± 312.96 | 25.42 ± 1.18 | 21.39 ± 3.09 |
| Serositis | ||||||
| Yes [45 (40%)] | 1.95 ± 0.28 | 20.41 ± 2.11 | 16.64 ± 1.90 | 560.98 ± 249.92 | 77.66 ± 18.52 | 21.39 ± 2.80 |
| No [68 (60%)] | 1.90 ± 0.23 | 22.01 ± 2.41 | 13.47 ± 1.24 | 1378.97 ± 294.61 | 77.70 ± 16.04 | 22.72 ± 3.49 |
| Vasculitis | ||||||
| Yes [30 (26%)] | 2.50 ± 0.22**/* | 27.20 ± 3.95*/* | 15.90 ± 1.97 | 2066.03 ± 516.50 | 85.75 ± 27.58 | 26.58 ± 5.33 |
| No [83 (74%)] | 1.70 ± 0.17 | 18.97 ± 1.66 | 14.32 ± 1.28 | 965.61 ± 214.93 | 74.72 ± 13.96 | 20.48 ± 2.57 |
| Raynaud’s phenomena | ||||||
| Yes [20 (17%)] | 2.71 ± 0.12**/* | 28.07 ± 5.32*/NS | 16.40 ± 2.30 | 633.89 ± 502.11 | 89.02 ± 31.73 | 24.11 ± 5.48 |
| No [93 (83%)] | 1.90 ± 0.16 | 19.69 ± 1.58 | 14.38 ± 1.21 | 1154.61 ± 231.89 | 75.15 ± 13.13 | 21.74 ± 2.65 |
| Neuropsychiatric disorders | ||||||
| Yes [14 (12%)] | 2.65 ± 0.11**/* | 22.83 ± 3.56 | 25.51 ± 3.24***/** | 765.27 ± 549.81 | 47.22 ± 19.79 | 28.75 ± 5.02 |
| No [99 (88%)] | 1.80 ± 0.13 | 21.12 ± 1.88 | 13.12 ± 1.02 | 1119.13 ± 229.15 | 83.22 ± 13.84 | 21.17 ± 2.63 |
| Pancytopenia | ||||||
| Yes [26 (23%)] | 2.21 ± 0.12*/NS | 24.87 ± 3.88 | 15.47 ± 2.51 | 1512.25 ± 539.10 | 75.20 ± 22.11 | 21.90 ± 3.80 |
| No [87 (77%)] | 1.82± 0.19 | 20.26 ± 1.83 | 14.60 ± 2.51 | 917.07 ± 216.37 | 78.47 ± 14.44 | 22.26 ± 2.79 |
| Thrombocytopenia | ||||||
| Yes [27 (24%)] | 2.56 ± 0.10*/NS | 22.33 ± 3.16 | 14.01 ± 1.74 | 1263.14 ± 374.87 | 34.13 ± 6.57 | 19.87 ± 2.64 |
| No [86 (76%)] | 1.80 ± 0.22 | 25.30 ± 2.27 | 15.07 ± 1.34 | 937.62 ± 250.76 | 26.35 ± 1.12 | 23.21 ± 3.22 |
| Hemolytic anemia | ||||||
| Yes [17 (15%)] | 2.80 ± 0.20*/* | 25.54 ± 3.93 | 17.14 ± 2.61 | 1658.46 ± 681.29 | 33.19 ± 3.80 | 31.39 ± 8.86 |
| No [96 (85%)] | 1.86 ± 0.18 | 24.03 ± 2.09 | 14.32 ± 1.17 | 920.93 ± 202.99 | 27.93 ± 2.64 | 20.48 ± 2.27 |
| Leucopenia | ||||||
| Yes [34 (30%)] | 2.46 ± 0.13*/* | 26.77 ± 3.01 | 15.99 ± 1.78 | 1501.54 ± 416.28 | 36.07 ± 6.17*/NS | 25.85 ± 4.16 |
| No [79 (70%)] | 1.74 ± 0.21 | 22.93 ± 2.03 | 14.70 ± 1.14 | 1137.69 ± 233.41 | 26.42 ± 1.26 | 21.94 ± 2.64 |
| Neutropenia | ||||||
| Yes [18 (16%)] | 2.59 ± 0.12*/* | 32.40 ± 4.23 | 16.22 ± 3.43 | 761.59 ± 548.78 | 44.91 ± 14.79**/* | 25.32 ± 4.83 |
| No [95 (84%)] | 1.80 ± 0.20 | 22.93 ± 2.03 | 14.70 ± 1.14 | 1137.69 ± 233.43 | 26.42 ± 1.26 | 21.94 ± 1.64 |
| Lymphopenia | ||||||
| Yes [35 (31%)] | 2.48 ± 0.13*/* | 27.59 ± 3.12 | 15.50 ± 1.91 | 1290.37 ± 370.97 | 36.03 ± 6.19*/* | 26.80 ± 4.24 |
| No [78 (69%)] | 1.75 ± 0.20 | 22.13 ± 2.38 | 14.61 ± 1.35 | 924.41 ± 252.80 | 25.11 ± 1.25 | 20.38 ± 2.99 |
| Arthritis | ||||||
| Yes [63 (56%)] | 1.95 ± 0.27 | 21.89 ± 2.75 | 13.55 ± 1.34 | 972.95 ± 316.69 | 104.89 ± 19.95*/* | 22.70 ± 3.85 |
| No [50 (44%)] | 1.89 ± 0.24 | 21.08 ± 2.21 | 14.96 ± 1.71 | 1204.35 ± 301.79 | 45.49 ± 10.42 | 21.95 ± 3.03 |
| Renal disorders | ||||||
| Yes [53 (47%)] | 1.97 ± 0.22 | 20.19 ± 2.01 | 13.77 ± 1.33 | 1225.16 ± 265.60 | 71.17 ± 15.69 | 21.67 ± 2.81 |
| No [60 (53%)] | 1.80 ± 0.30 | 22.98 ± 2.95 | 16.86 ± 1.82 | 753.92 ± 274.51 | 87.56 ± 16.69 | 23.53 ± 4.51 |
| Anti-dsDNA Ab | ||||||
| Yes [113 (100%)] | 1.93 ± 0.20 | 24.31 ± 2.17 | 13.98 ± 1.24 | 1123.87 ± 254.53 | 59.54 ± 12.86**/** | 26.26 ± 3.10*/NS |
| No [0 (0%)] | 1.76 ± 0.44 | 28.86 ± 5.84 | 16.36 ± 4.28 | 784.97 ± 420.64 | 147.45 ± 32.49 | 13.22 ± 2.50 |
PC: P corrected; *: p < 0.05; **: p < 0.01; ACR: American College of Rheumatology; M: Mean; SE: Standard Error; Anti-dsDNA; Ab; Anti-double-stranded DNA.
Table 2.
Association of disease activity with miR-146a and pro-inflammatory cytokines in SLE patients.
| Disease activity (N) | miR-146a (M±SE) | IL-1β (M±SE) | IL-6 (M±SE) | IL-17 (M±SE) | IL-8 (M±SE) | TNF-α (M±SE) |
|---|---|---|---|---|---|---|
| Inactive17 | 1.90 ± 0.35 | 19.27 ± 2.55 | 11.19 ± 1.10 | 920.30 ± 355.38 | 34.84 ± 10.02 | 12.97 ± 1.56 |
| Mild activity31 | 2.00 ± 0.57 | 17.11 ± 2.98 | 15.17 ± 2.45 | 1873.89 ± 537.23 | 120.62 ± 34.72 | 19.14 ± 6.12 |
| Moderate activity22 | 1.94 ± 0.31 | 25.35 ± 5.57 | 15.66 ± 3.57 | 1139.69 ± 619.61 | 107.59 ± 32.12 | 26.71 ± 9.87 |
| High activity26 | 1.75 ± 0.25 | 25.70 ± 3.75 | 19.23 ± 2.87*/* | 936.29 ± 466.99 | 77.47 ± 27.18 | 29.71 ± 3.82**/** |
| Severe activity17 | 2.50 ± 0.30*/* | 20.46 ± 4.55 | 18.11 ± 3.89 | 171.49 ± 47.3*/Ns | 91.01 ± 54.95 | 37.35 ± 5.87**/** |
PC: P corrected; *: p < 0.05; ***: p < 0.001; Significant value compared with the inactive group; N: Number; M: Mean; SE: Standard Error.
Table 3.
Association of treatment with miR-146a and pro-inflammatory cytokines in SLE patients.
| Treatment | miR-146a (M±SE) | IL-1β (M±SE) | IL-6 (M±SE) | IL-17 (M±SE) | IL-8 (M±SE) | TNF-α (M±SE) |
|---|---|---|---|---|---|---|
| Corticosteroid | ||||||
| Yes [91 (81%)] | 1.90 ± 0.18 | 21.30 ± 1.70 | 14.72 ± 1.08 | 1091.75 ± 217.04 | 75.55 ± 12.08 | 22.64 ± 2.44 |
| No [22 (19%)] | 1.93 ± 0.13 | 44.79 ± 3.03 | 10.10 ± 1.02 | 279.60 ± 85.31 | 29.24 ± 2.54 | 13.93 ± 1.11 |
| Hydroxychloroquine | ||||||
| Yes [95 (84%)] | 1.82 ± 0.19 | 22.07 ± 1.82 | 15.11 ± 1.16 | 1117.08 ± 228.13 | 27.53 ± 1.49** | 22.69 ± 2.63 |
| No [18 (16%)] | 1.96 ± 0.41 | 23.84 ± 6.95 | 9.86 ± 1.16 | 559.47 ± 338.83 | 46.52 ± 20.81 | 23.71 ± 4.21 |
| Cyclophosphamide | ||||||
| Yes [54 (48%)] | 1.74 ± 0.15** | 27.59 ± 3.07* | 12.58 ± 1.30* | 1031.69 ± 244.87 | 56.13 ± 12.56* | 20.96 ± 2.25 |
| No [59 (52%)] | 2.23 ± 0.44 | 20.39 ± 2.76 | 17.32 ± 2.30 | 1181.76 ± 434.83 | 27.30 ± 1.54 | 33.49 ± 7.41 |
| Azathioprine | ||||||
| Yes [71 (63%)] | 1.91 ± 0.19 | 21.04 ± 2.20 | 14.33 ± 1.28 | 1063.64 ± 265.03 | 61.0 ± 13.46 | 23.52 ± 2.66 |
| No [42 (37%)] | 1.87 ± 0.43 | 27.77 ± 3.87 | 15.67 ± 2.63 | 1118.09 ± 327.94 | 35.39 ± 8.82 | 28.74 ± 7.99 |
PC: P corrected; *: p < 0.05; **: p < 0.01; M: Mean; SE: Standard Error.
Secretion of pro-inflammatory cytokines and SLE clinical manifestations
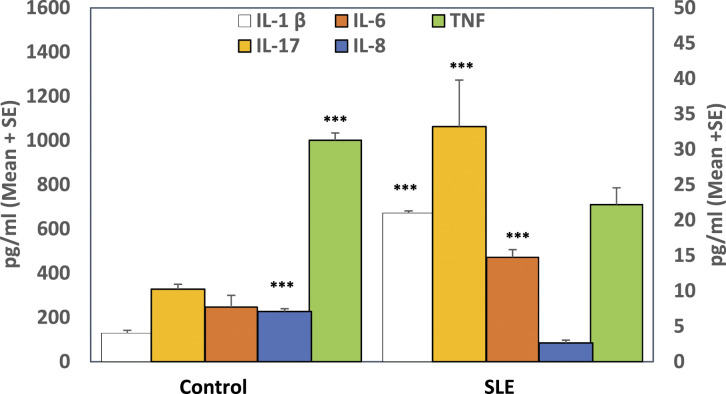
There was a significant elevation in IL-1β, IL-6, and IL-17 levels (p < 0.001) coincided with a significant reduction (p < 0.001) in IL-8 and TNF-α plasma levels in SLE patients in relation to healthy subjects (Figure 2). As shown in Table 1, IL-1β level was significantly decreased in SLE patients with mucocutaneous manifestation (p < 0.01), while it was significantly elevated with vasculitis(p < 0.01) and Raynaud’s phenomena (p < 0.01). Patients with neurological disorder tended to have a high level of IL-6 (p < 0.001). IL-8 was elevated in SLE patients with alopecia, leucopenia, lymphopenia, arthritis (p < 0.05), and neutropenia (p < 0.001), while it was decreased in patients with positive Anti-dsDNA Ab (p < 0.05). Patients with malar rash and photosensitivity manifestations (p < 0.05) had a low level of TNF-α, whereas this cytokine was raised in patients with Anti-dsDNA Ab (p < 0.05). However, our study couldn’t detect any significant correlation between IL-17 cytokine and SLE clinical manifestations; there was a slight elevation in IL-17 levels in a patient with hemolytic anemia and renal disorder.
Figure 2.
Detection of inflammatory cytokines in plasma of SLE patients and controls. Results are expressed as mean ± standard error. IL-17 and IL-8 were presented on the Y1 axis, while IL-1β, IL-6, and TNF-α were presented on the Y2 axis. ***: Refer to significance (p < 0.001).
Correlation analysis between measured parameters and SLE clinical manifestations revealed that IL-8 is positively correlated with alopecia (r = 0.245, p < 0.05), leucopenia (r = 0.246, p < 0.05), and neutropenia (r = 0.295, p < 0.05). IL-6 is negatively correlated with mucocutaneous manifestation (r = −0.288; p < 0.05) while it is positively correlated with a neurological disorder (r = 0.469; p < 0.001). TNF-α is negatively correlated with molar rash (r = −0.305; p < 0.05) and photosensitivity (r = −0.408; p < 0.01). IL-1β was positively correlated with vasculitis (r = 0.269; p < 0.05) and Raynaud’s phenomena (r = 0.249, p < 0.05).
Since patients with SLE usually do not present with only one clinical manifestation, we decided to perform a multivariate analysis. Using multiple linear regression, disease association remained significant (β = 0.238, p < 0.05) between IL-8 level, taken as the dependent variable (R2 = 0.152), for the presence of alopecia. On the other hand, other independent variables (leucopenia and neutropenia) were not significant in the multivariate analysis. We also found disease association remained significant (β = −0.232, p < 0.05 and β = 0.439, p < 0.001) between IL-6 level, taken as the dependent variable (R2 = 0.273), for the presence of mucocutaneous and neuropsychiatric manifestations, respectively. Also, disease association remained significant (β = −0.347, p < 0.05) between TNF-α level, taken as the dependent variable (R2 = 0.174), for the presence of photosensitivity. On the other hand, the malar rash was not significant. Looking at IL-1β, disease association is insignificant between IL-1β level, taken as the dependent variable, for the presence of vasculitis and Raynaud’s phenomena
As shown in Table 2, TNF-α was significantly increased with increasing disease activity score. In contrast, IL-17 was decreased by increasing the disease severity score. On the other hand, patients with no disease activity had reduced IL-6, IL-8, and TNF-α levels. IL-6 (r = 0.333; p < 0.01) and TNF-α (r = 0.269; p < 0.05) were positively correlated with SLEDAI scores.
SLE patients treated with cyclophosphamide exhibited a significant elevation in IL-1β and IL-8 levels (p < 0.05) and a significant reduction in IL-6 (p < 0.05). Hydroxychloroquine treatment reduced IL-8 production levels (p < 0.01) in SLE patients (Table 3).
Correlations between miR-146a and pro-inflammatory cytokines in lupus patients
IL-6 was directly correlated with miR-146a expression (r = 0.25; p < 0.01), IL-17 (r = 0.27; p < 0.01), IL-1β (r = 0.34; p < 0.001) and negatively correlated with IL-8 (r = −0.26; p < 0.001). On the other hand, IL-8 was negatively correlated with IL-1β (r = −0.43; p < 0.001) and IL-17 (r = −0.21; p < 0.05) in SLE patients. In SLE patients with elevated miR-146a expression compared to controls, IL-1, IL-6, and IL-17 levels were increased (20%, 52%, and 31%, respectively), but TNF- and IL-8 levels were decreased (70% and 37%, respectively).
Discussion
SLE patients frequently experience a wide range of heterogeneous phenotypes, from mild manifestations (like skin rashes, inflammatory arthritis, leucopenia/lymphopenia, a non-scarring alopecia, and oral ulcers) to life-threatening major organ involvement (e.g., vasculitis, renal, nervous system). This established heterogeneity in clinical SLE presentation is influenced by several variables, including an individual’s ancestry, environment, sex, age, and genetic background.23,26
One of the attractive features of miR-146a is that it can target multiple molecules and regulate various signaling pathways, collectively regulating the same or related physiological functions.13 IRAK1 and TRAF6 (two of the regulatory targets of miR-146a) are the critical signaling molecules involved in the TLR4 signal transduction pathway.21,23 From previous studies, the aggregation of immune complexes activates TLR leading to stimulation of the IRAK1/TRAF6/IΚKβ/NF-κB pathway.16 Then, the activation of NF-κB promotes the expression of pro-inflammatory cytokines (IL-1β, IL-6, IL-8, IL-17, and TNF-α), which are increased in SLE patients.16–22 Also, the dysregulation in pro-inflammatory cytokines was associated with disease development and progression.16,21,31 As a negative feedback regulation of NF-κB signaling, NF-κB gives the spark for producing miR-146a level, which will reduce pro-inflammatory cytokines by blocking IRAK1/TRAF6 proteins.5,13 Accordingly, our study is designed to measure miR-146a expression level and a panel of pro-inflammatory cytokines (IL-1β, IL-6, IL-8, IL-17, and TNF-α) in SLE patients.
We found that the miR-146a level was significantly escalated in SLE patients compared with healthy controls. Hence, it might be used as a diagnostic biomarker in patients with SLE, where these results were confirmed by previous studies.11,32–34 In agreement, Perez-Hernandez et al.35 found that miR-146a expression was the biggest augmented with a 100-fold change in the urine of SLE patients compared to healthy controls. In contrast to our study, previous studies documented that miR-146a was reduced in SLE patients compared to controls.36–39 Moreover, Zhu et al.19 revealed that miR-146a was dropped in lupus nephritis (LN) patients against controls.
miR-146a was positively upregulated with severe disease activity. Although an elevation in miR-146a expression was detected in SLE patients and was associated with several clinical manifestations, miR-146a was decreased with alopecia. In agreement, Perez-Hernandez et al.35 documented that the elevated miR-146a level was associated with disease activity causing disease progression to LN. On the other hand, Wang et al.37 previously reported that miR-146a expression was inversely correlated with disease activity.
Additionally, we found that the reduction in miR-146a expression was associated with the cyclophosphamide drug. Similarly, Shahid et al.40 found a substantial drop in miR-146a expression in the plasma of acute lymphoblastic leukemia (ALL) patients after treatment. On the contrary, Labib et al.11 found that miRNA146a tended to be elevated in SLE patients with LN after treatment. In contrast to our study, Tang et al.32 observed that miR-146a is upregulated in treated lupus against non-treated ones. Unlike us, Wang et al.37 showed that the main increment in miR-146a expression was associated with treatment.
Looking at cytokine production, our study found an elevation in the secretion of IL-1β, IL-6, and IL-17 in SLE patients against controls, in agreement with previously documented data.15,17-19,22 Similarly, Paquissi and Abensur41 demonstrated that elevated IL-1β and IL-6 are the critical cytokines for Th17 cell differentiation, increasing IL-17 production. In line with our study, Rodriguez et al.42 showed that IL-6 was constitutively higher in human umbilical vein endothelial cells (HUVEC) from lupus patients. In disagreement with our results, Mende et al.43 reported that serum IL-1β level was not significantly raised in SLE patients compared to controls.
In contrast with Mende et al.,43 our study found that IL-1β expression was significantly elevated in SLE patients with vasculitis and Raynaud’s phenomena, whereas it was reduced with mucocutaneous manifestation. IL-6 level was increased in SLE patients with neuropsychiatric disorders. These results were like those previously reported by Talaat et al.18 Although we could not detect any significant association between IL-17 expression and different clinical manifestations, IL-17 was slightly elevated in patients with hemolytic anemia and renal disorder. Similarly, Paquissi and Abensur41 found that IL-17 was increased in lupus patients with hemolytic anemia and renal disorder.
Concerning the disease activity, IL-6, IL-1β, and IL-17 were down-regulated in patients with no disease activity, which was previously reported.17,44 We have reported an association between disease activity score and IL-17 production in a former study.18 In disagreement with our study, Mende et al.43 reported that IL-1β wasn’t associated with SLE activity.
Although IL-6 was increased with almost all treatment regimens, it was reduced with cyclophosphamide. On the other hand, IL-1β was elevated with cyclophosphamide, while it was decreased with the other treatments. These results might confirm that cyclophosphamide alone didn’t restore the levels of these cytokines in SLE patients. Our data agreed with other studies recorded that rituximab and cyclophosphamide have been shown to restore the function of inflammatory cytokines in patients with Mycobacterium infection and SLE.45–47
Our results showed that IL-8 and TNF-α were down-regulated in patients with SLE. In agreement with us, Zhu et al.48 showed that LN patients with HLA-DQwl genotype had a low level of TNF-α. In the same line, Rodriguez et al.42 showed that IL-8 was down-regulated upon activating HUVEC (from SLE patients) with TNF-α. Similarly, Bhaumik et al.49 found that a reduction of IL-8 accompanied the decreased level of IRAK1 in HCA2 human fibroblasts. In contrast, other researchers found controversial results.15,19,50,51
Moreover, IL-8 level was elevated in SLE patients with alopecia, leucopenia, neutropenia, lymphopenia, and arthritis. On the other hand, the level of IL-8 was decreased in patients with positive Anti-dsDNA Ab. Mao et al.20 reported that IL-8 escalated with several clinical manifestations of SLE. The reduced level of TNF-α was associated with malar rash and photosensitivity. At the same time, its expression was exacerbated in patients with Anti-dsDNA Ab, and these results were similar to Postal and Appenzeller.52
IL-8 and TNF-α levels were elevated in SLE patients with high disease activity. Similarly, Robinson and Werth50 reported that the disease activity of cutaneous lupus erythematosus (CLE) was significantly associated with elevated IL-8 and TNF-α. Also, the study of Zhu et al.19 indicated that IL-8 and TNF-α were elevated with disease activity, leading to increased disease complications such as LN. Furthermore, we found a reduction in IL-8 in patients treated with hydroxychloroquine, whereas it was elevated with cyclophosphamide treatment. According to Mao et al.,20 elevated IL-8 in neuropsychiatric SLE patients (NPSLE) was reduced after treatment.
Conclusion
To the best of our knowledge, the present study is the first to analyze the miR-146a expression and pro-inflammatory cytokines (IL-1β, IL-6, IL-8, IL-17, and TNF-α) together in the same patients SLE (no previous study on Egyptians). Our data demonstrated a significant increase in miR-146a, which coincides with the elevation of certain pro-inflammatory cytokines (IL-1β, IL-6, and IL-17)and a reduction in IL-8 and TNF-α in SLE patients. This may suggest that miR-146a has dual effects on controlling lupus inflammation. miR-146a has a moderate diagnostic potential and might be used as a diagnostic marker to differentiate between SLE patients. Moreover, miR-146a is associated with the appearance of certain disease manifestations and increased disease activity scores. This supports the role of miR-146a and pro-inflammatory cytokines in the immunopathology of SLE. A better understanding of miRNAs’ physiologic and pathologic roles will aid in the diagnosis and/or prognosis of SLE and the effectiveness of therapeutics to slow disease manifestations. Each identified miRNA in lupus opens the way to finding predictors of disease outcomes and giving hope to millions of sufferers. Furthermore, mimic miRNA/antagomir in disease treatment and control could open a new era of research.
Off course, manipulation with our data must be performed considering some study limitations such as sequestration of patients to one geographical place, absence of cross-sectional design, lack of serial data, very few number of patients to be confident about clinical correlations with disease manifestations, and lack of untreated newly diagnosed patients and difficulty to follow up our patients for a long time and absence of analysis of the role of glucocorticoids in influencing the levels of proteins measured.
Supplemental Material
Supplemental Material for Crosstalk between miR-146a and pro-inflammatory cytokines in patients with systemic lupus erythematosus by Basima A El-Akhras, Roba M Talaat, Samir A El-Masry, Iman H Bassyouni, Ibrahim H El-Sayed and Yasser BM Ali in International Journal of Immunopathology and Pharmacology
Acknowledgement
This work was performed in Immunology Lab. at the Genetic Engineering and Biotechnology Research Institute (GEBRI), University of Sadat City (USC).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Part of this work was funded by the Science and Technological Development Fund (STDF), Ministry of Scientific Research, Egypt (Grant No: 15,123).
Ethics approval: The local ethical committee (Rheumatology and Rehabilitation Department, Faculty of Medicine, Cairo University) approved our current study.
Informed consent: Written informed consent was obtained from all subjects before the study.
Supplemental Material: Supplementary material for this article is available on the online.
ORCID iD
Roba M Talaat https://orcid.org/0000-0002-1176-2727
References
- 1.Honarpisheh M, Köhler P, von Rauchhaupt E, et al. (2018) The involvement of microRNAs in modulation of innate and adaptive immunity in systemic lupus erythematosus and lupus nephritis. Journal of Immunology Research 2018: 4126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagy D, Shaheen NH, Selim HM, et al. (2020) MicroRNA-126 and 146a as potential biomarkers in systemic lupus erythematosus patients with secondary antiphospholipid syndrome. The Egyptian Rheumatologist 42: 201–206. [Google Scholar]
- 3.Hamam R, Ali AM, Alsaleh KA, et al. (2016) microRNA expression profiling on individual breast cancer patients identifies novel panel of circulating microRNA for early detection. Scientific Reports 6: 25997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Contreras J, Rao DS. (2012) MicroRNAs in inflammation and immune responses. Leukemia 26: 404–413. [DOI] [PubMed] [Google Scholar]
- 5.Taganov KD, Boldin MP, Chang KJ, et al. (2006) NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proceedings of the National Academy of Sciences of the United States of America 103: 12481–12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boldin MP, Taganov KD, Rao DS, et al. (2011) miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. The Journal of Experimental Medicine 208: 1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonkoly E, Wei T, Janson PCJ, et al. (2007) MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PLoS One 2: e610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayeldeen G, Nassar Y, Ahmed H, et al. (2018) Possible use of miRNAs-146a and -499 expression and their polymorphisms as diagnostic markers for rheumatoid arthritis. Molecular and Cellular Biochemistry 449: 145–156. DOI: 10.1007/s11010-018-3351-7 [DOI] [PubMed] [Google Scholar]
- 9.Zakaria SS, Gaballah HH, El Saadany HM. (2016) Micro RNA-146a expression, NF-jB/P65 activity and serum pentosidine levels as potential biomarkers for disease severity in primary knee osteoarthritis patients. The Egyptian Rheumatologist 42: 201–206. [Google Scholar]
- 10.Labib DA, Shaker OG, El Refai RM, et al. (2019) Association between miRNA-146a and polymorphisms of its target gene, IRAK1, regarding susceptibility to and clinical features of systemic lupus erythematous and multiple sclerosis. Laboratory Medicine 50: 34–41. [DOI] [PubMed] [Google Scholar]
- 11.Labib DA, Koptan D, Ghoniem S, et al. (2020) dysregulation of microRNA146a-5p expression in systemic lupus erythematosus females: diagnostic potential and association with ocular manifestations. The Egyptian Rheumatologist 42: 117–121. [Google Scholar]
- 12.Tan G, Baby B, Zhou Y, et al. (2021) Emerging molecular markers towards potential diagnostic panels for lupus. Frontiers in Immunology 12: 808839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li B, Wang X, Choi IY, et al. (2017) miR-146a modulates autoreactive Th17 cell differentiation and regulates organ-specific autoimmunity. The Journal of Clinical Investigation 127: 3702–3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radwan N, Hamza MT, Ghareeb I, et al. (2021) Serum interleukin-17 expression in a group of Egyptian patients with juvenile systemic lupus erythematosus. The Egyptian Journal of Pediatric Allergy and Immunology 19: 97–103. [Google Scholar]
- 15.Tsai CY, Wu TH, Yu CL, et al. (2000) Increased excretions of beta2-microglobulin, IL-6, and IL-8 and decreased excretion of Tamm-Horsfall glycoprotein in urine of patients with active lupus nephritis. Nephron 85: 207–214. [DOI] [PubMed] [Google Scholar]
- 16.Nahid MA, Satoh M, Chan EK. (2011) MicroRNA in TLR signaling and endotoxin tolerance. Cellular & Molecular Immunology 8: 388–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umare V, Pradhan V, Nadkar M, et al. (2014) Effect of proinflammatory cytokines (IL-6, TNF-α, and IL-1β) on clinical manifestations in Indian SLE patients. Mediators of Inflammation 2014: 385297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talaat RM, Mohamed SF, Bassyouni IH, et al. (2015) Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: correlation with disease activity. Cytokine 72: 146–153. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y, Xue Z, Di L. (2017) Regulation of MiR-146a and TRAF6 in the diagnose of lupus nephritis. Medical Science Monitor 23: 2550–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao YM, Zhao CN, Liu LN, et al. (2018) Increased circulating interleukin-8 levels in systemic lupus erythematosus patients: a meta-analysis. Biomarkers in Medicine 12: 1291–1302. [DOI] [PubMed] [Google Scholar]
- 21.Zhou C, Zhao L, Wang K, et al. (2019) MicroRNA-146a inhibits NF-κB activation and pro-inflammatory cytokine production by regulating IRAK1 expression in THP-1 cells. Experimental and Therapeutic Medicine 18: 3078–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen HH, Fan Y, Wang YN, et al. (2020) Elevated circulating interleukin-17 levels in patients with systemic lupus erythematosus: a meta-analysis. Immunological Investigations 49: 662–675. [DOI] [PubMed] [Google Scholar]
- 23.Gao M, Wang X, Zhang X, et al. (2015) Attenuation of cardiac dysfunction in polymicrobial sepsis by microRNA-146a is mediated via targeting of IRAK1 and TRAF6 expression. Journal of Immunology 195: 672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stypińska B, Paradowska-Gorycka A. (2015) Cytokines and MicroRNAs as candidate biomarkers for systemic lupus erythematosus. International Journal of Molecular Sciences 16: 24194–24218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aragón CC, Tafúr RA, Suárez-Avellaneda A, et al. (2020) Urinary biomarkers in lupus nephritis. Journal of Translational Autoimmunity 3: 100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petri M, Orbai AM, Alarcón GS, et al. (2012) Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis and Rheumatism 64: 2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bombardier C, Gladman DD, Urowitz MB, et al. (1992) Derivation of the SLEDAI. a disease activity index for lupus patients. The committee on prognosis studies in SLE. Arthritis and Rheumatism 35: 630–640. [DOI] [PubMed] [Google Scholar]
- 28.Mosca M, Bombardieri S. (2006) Assessing remission in systemic lupus erythematosus. Clinical and Experimental Rheumatology 24: 99–99. [PubMed] [Google Scholar]
- 29.Abo ElAtta AS, Ali YBM, Bassyouni IH, et al. (2019) Upregulation of miR-221/222 expression in rheumatoid arthritis (RA) patients: correlation with disease activity. Clinical and Experimental Medicine 19: 47–53. [DOI] [PubMed] [Google Scholar]
- 30.Gheita TA, Noor RA, Abualfadl E, et al. (2021) Adult systemic lupus erythematosus in Egypt: The nation-wide spectrum of 3661 patients and world-wide standpoint. Lupus 30(9): 1526–1535. DOI: 10.1177/09612033211014253 [DOI] [PubMed] [Google Scholar]
- 31.He X, Zheng Y, Liu S, et al. (2018) MiR-146a protects small intestine against ischemia/reperfusion injury by down-regulating TLR4/TRAF6/NF-κB pathway. Journal of Cellular Physiology 233: 2476–2488. [DOI] [PubMed] [Google Scholar]
- 32.Tang Q, Yang Y, Zhao M, et al. (2015) Mycophenolic acid upregulates miR-142-3P/5P and miR-146a in lupus CD4+T cells. Lupus 24: 935–942. [DOI] [PubMed] [Google Scholar]
- 33.Shumnalieva R, Kachakova D, Shoumnalieva-Ivanova V, et al. (2018) Whole peripheral blood miR-146a and miR-155 expression levels in Systemic lupus erythematosus patients. Acta Reumatologica Portuguesa 43: 217–225. [PubMed] [Google Scholar]
- 34.Zununi Vahed S, Nakhjavani M, Etemadi J, et al. (2018) Altered levels of immune-regulatory microRNAs in plasma samples of patients with lupus nephritis. BioImpacts 8: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez-Hernandez J, Forner MJ, Pinto C, et al. (2015) Increased urinary exosomal microRNAs in patients with systemic lupus erythematosus. PLoS One 10: e0138618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang Y, Luo X, Cui H, et al. (2009) MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis and Rheumatism 60: 1065–1075. [DOI] [PubMed] [Google Scholar]
- 37.Wang G, Tam LS, Li EKM, et al. (2010) Serum and urinary cell-free MiR-146a and MiR-155 in patients with systemic lupus erythematosus. The Journal of Rheumatology 37: 2516–2522. [DOI] [PubMed] [Google Scholar]
- 38.Luo X, Yang W, Ye DQ, et al. (2011) A functional variant in microRNA-146a promoter modulates its expression and confers disease risk for systemic lupus erythematosus. PLoS genetics 7: e1002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hashad DI, Abdelmagid MH, Elsherif SH. (2012) microRNA146a expression in lupus patients with and without renal complications. Journal of Clinical Laboratory Analysis 26: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shahid S, Shahid W, Shaheen J, et al. (2021) Circulating miR-146a expression as a non-invasive predictive biomarker for acute lymphoblastic leukemia. Scientific Reports 11: 22783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paquissi FC, Abensur H. (2021) The Th17/IL-17 axis and kidney diseases, with focus on lupus nephritis. Frontiers in Medicine 8: 654912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez E, Guevara J, PAez A, et al. (2008) The altered expression of inflammation-related molecules and secretion of IL-6 and IL-8 by HUVEC from newborns with maternal inactive systemic lupus erythematosus is modified by estrogens. Lupus 17: 1086–1095. [DOI] [PubMed] [Google Scholar]
- 43.Mende R, Vincent FB, Kandane-Rathnayake R, et al. (2018) Analysis of serum interleukin (IL)-1β and IL-18 in systemic lupus erythematosus. Frontiers in Immunology 9: 1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding J, Su S, You T, et al. (2020) Serum interleukin-6 level is correlated with the disease activity of systemic lupus erythematosus: a meta-analysis. Clinics (Sao Paulo) 75: e1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koizumi Y, Sakagami T, Nishiyama N, et al. (2017) Rituximab restores IFN--STAT1 function and ameliorates disseminated mycobacterium avium infection in a patient with anti-interferon- autoantibody. Journal of Clinical Immunology 37: 644–649. [DOI] [PubMed] [Google Scholar]
- 46.Chetchotisakd P, Anunnatsiri S, Nanagara R, et al. (2018) Intravenous cyclophosphamide therapy for anti-IFN-gamma autoantibody-associated mycobacterium abscessus infection. Journal of Immunology Research 2018: 6473629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Howe HS, Leung BPL. (2019) Anti-cytokine autoantibodies in systemic lupus erythematosus. Cells 9: 72. DOI: 10.3390/cells9010072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu LJ, Yang X, Yu XQ. (2010) Anti-TNF-α therapies in systemic lupus erythematosus. Journal of Biomedicine & Biotechnology 2010: 465898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhaumik D, Scott GK, Schokrpur S, et al. (2009) MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging (Albany N Y) 1: 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson ES, Werth VP. (2015) The role of cytokines in the pathogenesis of cutaneous lupus erythematosus. Cytokine 73: 326–334. [DOI] [PubMed] [Google Scholar]
- 51.Park J, Jang W, Park HS, et al. (2020) Cytokine clusters as potential diagnostic markers of disease activity and renal involvement in systemic lupus erythematosus. The Journal of International Medical Research 48: 300060520926882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Postal M, Appenzeller S. (2011) The role of tumor necrosis factor-alpha (TNF-α) in the pathogenesis of systemic lupus erythematosus. Cytokine 56: 537–543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Crosstalk between miR-146a and pro-inflammatory cytokines in patients with systemic lupus erythematosus by Basima A El-Akhras, Roba M Talaat, Samir A El-Masry, Iman H Bassyouni, Ibrahim H El-Sayed and Yasser BM Ali in International Journal of Immunopathology and Pharmacology