Abstract
Systemic lupus erythematosus (SLE) is a chronic systemic autoimmune disease associated with impaired organ functions that can seriously affect the daily life of patients. Recent SLE therapies frequently elicit adverse reactions and side effects in patients, and clinical heterogeneity is considerable. Mesenchymal stromal cells (MSCs) have anti-inflammatory, tissue repair, and immunomodulatory properties. Their ability to treat autoimmune diseases largely depends on secreted extracellular vesicles, especially exosomes. The effects of exosomes and microRNAs (miRNAs) on SLE have recently attracted interest. This review summarizes the applications of MSCs derived from bone marrow, adipocyte tissue, umbilical cord, synovial membrane, and gingival tissue, as well as exosomes to treating SLE and the key roles of miRNAs. The efficacy of MSCs infusion in SLE patients with impaired autologous MSCs are reviewed, and the potential of exosomes and their contents as drug delivery vectors for treating SLE and other autoimmune diseases in the future are briefly described.
Keywords: exosome, mesenchymal stromal cell, microRNA, lupus
Introduction
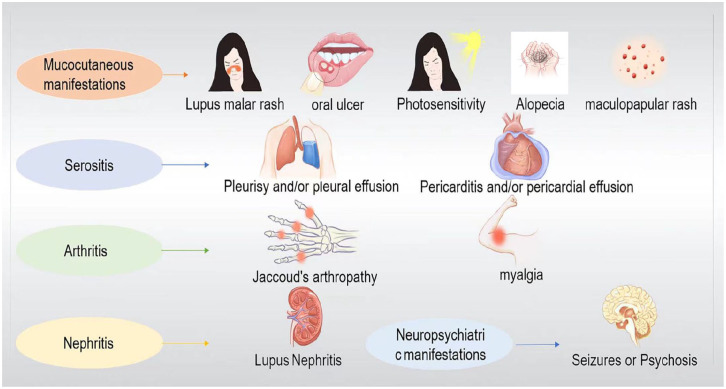
Systemic lupus erythematosus (SLE) is a chronic, systemic inflammatory disease that affects the urinary, motor, circulatory, respiratory, digestive, and nervous systems (Fig. 1). It develops in various populations, especially in women of childbearing age and is particularly prevalent among Africans1. It is generally characterized by alternating periods of remission and relapse, with little progressive deterioration2. The cellular and molecular mechanisms involved in the pathogenesis of SLE are highly complex and are probably affected by intricate interactions among genetic, environmental, and hormonal factors3.
Figure 1.
Main clinical manifestations of systemic lupus erythematosus. Systemic lupus erythematosus is a chronic, systemic inflammatory disease that affects the urinary system, motor system, circulatory system, respiratory system, digestive system, and nervous system. Different patients are often affected by different systems and, so the clinical manifestations are also different. Clinically, skin and mucosal injuries (e.g., lupus malar rash, oral ulcer, photosensitivity, alopecia, maculopapular rash, etc.) and lupus nephritis are most common, but arthritis and serositis may also occur, and the condition is critical when neuropsychiatric manifestation is present. The clinical heterogeneity of the disease highlights the important influence of genetic factors, autoimmunity, and external environment on the disease development model, and brings challenges to the diagnosis and treatment.
A complete cure and universally effective treatment for SLE are not available. Most therapeutic schedules are designed to control and reduce disease activity. Therapeutic pharmaceuticals used to relieve lupus symptoms include non-steroidal anti-inflammatory, antimalarial, immunosuppressant drugs, and glucocorticoids4. However, these drugs carry a high risk of serious adverse reactions. In particular, long-term medication with glucocorticoids is often associated with serious adverse events involving the endocrine, musculoskeletal, hematopoietic, and cardiovascular systems. Osteonecrosis of the femoral head is a common and serious complication of glucocorticoids5.
Considering the severity of the disease, other feasible treatments for SLE have been investigated. Extracellular vesicles (EVs) derived from mesenchymal stromal cells (MSCs)6, exosomes and their micro (mi) RNAs might serve as cell-free approaches to treating SLE. Here, we review the application of potential of exosomes derived from MSCs and miRNAs to treating SLE.
Mesenchymal Stromal Cells
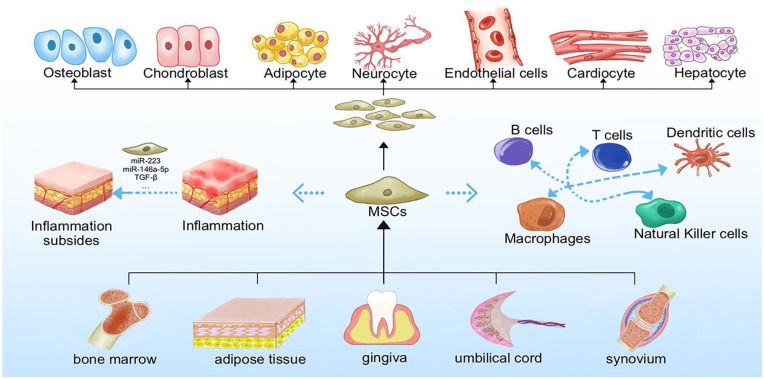
Mesenchymal stromal cells are a subset of non-hematopoietic adult stromal cells7 with remarkable proliferative ability and potential for multiple differentiation, as well as repair, anti-inflammatory, and immunomodulatory properties (Fig. 2). Techniques for isolating and culturing MSCs from tissues, including bone marrow, adipose tissue, synovium, umbilical cord (UC), and gingiva, are gradually being refined.
Figure 2.
General characteristics of mesenchymal stromal cells. Mesenchymal stromal cells can be isolated from a variety of organs and tissues such as bone marrow, adipose tissue, umbilical cord, synovial membrane and gingiva. In addition to their good proliferative capacity and typical tri-lineage differentiation (osteogenic, chondrogenic, and lipogenic differentiation) potential, the potential for differentiation to other cells such as neurocyte, endothelial, cardiocyte, and hepatocyte, which are essential for their tissue repair function, is gradually being explored. In addition, MSCs have received extensive attention for their anti-inflammatory and immunomodulatory roles (both acquired and innate immunity) through secretion of cytokines and extracellular vesicles.
Anti-Inflammatory and Restorative Functions of MSCs
Mesenchymal stromal cells exert anti-inflammatory effects by alleviating inflammation and reducing cytokine production8. Exosomes from bone marrow–derived MSCs (BMMSCs) express miR-146a-5p, which inhibits the expression of interleukin-1 receptor-associated kinase 1 (IRAK1)9, regulates the Th17/Treg cell imbalance, and reduces pro-inflammatory cytokine production. Furthermore, MSCs eliminate the inflammatory effects of neutrophils, inhibit the formation of neutrophil extracellular traps (NETs), and reduce thrombosis, inflammation, and fibrosis induced by NETs10. Mesenchymal stromal cells can migrate and aggregate to sites of inflammation and promote injury repair. For example, allogeneic MSCs can migrate to an injured kidney after infusion. In addition to their immunomodulatory role and suppressing the autoimmune response, MSCs can be differentiated into mesangial cells to treat lupus nephritis (LN)11.
Immunoregulatory Properties of MSCs
Mesenchymal stromal cells exert unique immunosuppressive effects in innate and adaptive immunity by inhibiting the proliferation and activities of immune cells such as macrophages, dendritic cells (DCs), natural killer (NK) cells, B lymphocytes, and T lymphocytes11.
Mesenchymal stromal cells derived from human gingiva (GMSCs) reduce the infiltration of CD8+ T, Th1, Th17, and other pro-inflammatory cells, and increase the proportion of immunosuppressive cells such as regulatory T (Treg) cells12. Mesenchymal stromal cells interfere with B cell proliferation and migration, as well as antibody and cytokine production1. They can also indirectly inhibit B cell function by inhibiting T cells13. Macrophages can take on two main phenotypes: inflammatory M1 and anti-inflammatory M2 macrophages. Most MSCs injected intravenously into mouse models of asthma travel through the bloodstream and eventually reach the lungs, where they die and are phagocytosed by macrophages. Such macrophages can differentiate into the immunosuppressive M2 phenotype and decrease the M1/M2 ratio in vivo, which is one mechanism through which MSCs play a regulatory role14.
Extracellular Vesicles Released From MSCs
In addition to classical soluble factors such as cytokines that play a role in paracrine signaling, the role of EVs released from MSCs (MSC-EVs) in intercellular communication should not be ignored15. Extracellular vesicles are a class of cell-derived heterogeneous membrane structures that can be apoptotic bodies, microvesicles, microparticles, or exosomes16. The immunosuppressive effects of MSCs are mainly manifested through their EVs17. Exosomes are rich in proteins, transcription factors, lipids, DNA, miRNAs, mRNAs, and cytokines. They act on target cells by binding to receptors in target tissues or by fusion with plasma membranes18.
Diagnostic Value of Exosomes and Their miRNAs
Exosomes are involved in cell migration, the immune response, cell differentiation, antigen presentation, and tumor invasion, among which miRNAs play important roles19,20. Non-coding miRNAs can regulate pathways associated with gene expression by interacting with specific targets after their extracellular transport by EVs21. Micro RNA entry into exosomes is not random and is mediated by unique miRNA sorting mechanisms22, such as the Endosomal Sorting Complex Required for Transport (ESCRT)23. Exosomes and their miRNAs have potential as diagnostic and therapeutic agents in autoimmune diseases24. For example, primary fibrosis in LN is closely associated with exosomal miR-129 isolated from urine, suggesting a predictive role of miR-129 in disease progression24. The expression of miR-21 and let-7A in urinary exosomes is obviously downregulated in patients with active LN compared with those who have inactive LN, but this rebounds during remission, suggesting that they could serve as biomarkers of active LN activity25. Using MSC-EVs to deliver miRNAs to specific cells is a potential mechanism of alleviating tissue damage26. Elevating the expression of programmed cell death ligand 1 via gene transfection in EVs of MSCs enhances their immunosuppressive ability27. This indicated that enhancing EV function has potential clinical applications.
Advances in exosomes and their miRNAs as biomarkers have suggested their potential therapeutic value. Exosome-based therapies avoid the need to administer live cells6. Therefore, exosome-based therapies might be suitable alternatives to conventional cell therapy for SLE. Exosomes derived from antigen-presenting cells such as DCs and MSCs are currently regarded as potential cell-free approaches to treating autoimmune diseases28.
MSCs in SLE Treatment
Allogenic MSC Transplantation in SLE
Bone marrow, adipose tissues, gingival tissues, UCs, and the synovium are sources of allogenic MSCs that can be used to treat SLE.
Bone marrow–derived MSCs and exosomes
For 40 years since their initial description, BMMSCs have become the most widely studied MSC population and are considered the gold standard for clinical MSC application29. The production of autoantibodies in patients with SLE depends on the activation of T-cell-assisted B cells. Therefore, regulating the activity of upstream T cells might restrict excessive B-cell activity in SLE13. Clinically, the immunological regulation of MSCs is applicable not only as therapy for SLE but also for connective tissue diseases (CTDs), graft-versus-host disease (GVHD), and other immune disorders. For example, a clinical trial found that BMMSCs relieved symptoms in 25 patients with GVHD without significant adverse events30.
Not only do BMMSCs inhibit the differentiation of T cells into T follicular helper (Tfh) cells, they also inhibit differentiated Tfh cell proliferation and interleukin (IL)-21 production, thus alleviating LN in mice31. Human BMMSCs inhibit T-cell-mediated B-cell proliferation, plasma cell differentiation, and antibody production in vitro13. In addition, MSCs secrete exosomes containing miRNAs that inhibit cytokine secretion in macrophages by regulating Toll-like receptor (TLR)-related signaling pathways32. Transfer RNA–derived small RNA (tsRNA)-21109 in BMMSC exosomes inhibits macrophage polarization toward the M1 phenotype in vitro, thus contributing to the development of a new specific therapeutic target for SLE33. Allogeneic BMMSC transplantation also enhances the function of autologous BMMSCs and improves osteopenia in a mouse model of lupus34.
However, bone marrow might not be the optimal source of MSCs. In addition to an invasive and painful isolation procedure, MSCs are scant in bone marrow aspirates, and their qualitative and quantitative properties are affected by donor age35.
Adipose tissue–derived MSCs and exosomes
Differentiation potential, proteomic characteristics, gene expression, and immunological characteristics differ between adipose tissue–derived MSCs (ADSCs) and BMMSCs. Adipose tissue–derived MSCs are easier to isolate and clinically safer to collect than BMMSCs36. Furthermore, ADSCs more effectively inhibit peripheral blood T cells, are more regenerative, and have more adaptable electrokinetic properties than BMMSCs37. Combining ADSCs with exosomes is more effective than injecting them alone and could be considered as a future stromal cell treatment option38,39. The results of treating a patient with SLE in Canada using autologous ADSCs were significant and stable40. A clinical trial in Iran found that ADSC transplantation is effective against LN, whereas a single dose might be insufficient to maintain remission41.
Adipose tissue–derived MSCs inhibit the initial differentiation of T cells into Th17 cells and reduce IL-17 secretion by regulating the protein kinase B/mammalian target of rapamycin complex 1/Ribosomal protein S6 kinase beta-1/Hypoxia-inducible factor 1-alpha/Th17 (AKT/mTORC1/p70S6K/HIF-1A)/Th17 signaling pathway in MLR/lpr mice42. Adipose tissue–derived MSCs also induce the differentiation of macrophages into the M2 phenotype via the exosomal-mediated transfection of active signal transducer and activator of transcription 3 (STAT3)43. Controlled clinical trials of autologous ADSCs for osteoarthritis found that intra-articular injections of ADSCs improved joint function and pain relief in patients44. Exosomes of ADSCs in patients with osteoarthritis are rich in miR-145 and miR-221, which can downregulate pro-inflammatory factor expression in periosteum cells in vitro45.
The properties of ADSCs can be enhanced in several ways. For example, incubating them with glial cell-derived neurotrophic factor promotes their migration and differentiation46. Conditioning ADSCs with interferon-gamma (IFN-γ) improves their immunosuppressive potential in conditioned media and EVs47.
Umbilical cord–derived MSCs and exosomes
Umbilical cords might be an ideal substitute for ADSCs and BMMSCs, as they are discarded after birth and thus can be easily collected. The amplification rate is higher in vitro for umbilical cord–derived mesenchymal stromal cells (UC-MSCs) than BMMSCs48. More importantly, UC-MSCs have similar or superior immunomodulatory characteristics to MSCs derived from other sources48,49.
Clinical trials have revealed that UC-MSCs are significantly effective for treating SLE (Table 1). In fact, MSC therapy can reduce abnormal apoptotic cell accumulation in SLE through phagocytosis, resulting in the promotion of prostaglandin E2 (PGE2)-mediated immunosuppression50. Moreover, MSCs promote CD1c+ DC upregulation in peripheral blood and FLT3L in serum, inhibiting the inflammatory response to lupus51. Furthermore, UC-MSCs can prevent SLE by synthesizing transforming growth factor (TGF)-β1 that promotes the production of IL-10+ B regulatory cells (Bregs) and correct the Treg/Th17/Th1 imbalance in mice52.
Table 1.
Clinical Trial and Application of MSCs in Systemic Lupus Erythematosus.
| MSCs | Disease | Patients | Administration | Outcome | Adverse events | Year |
|---|---|---|---|---|---|---|
| BMMSCs (Autologous) | SLE53 | 2 | 1 × 106/kg; intravenous infusion |
CD4+CD25+FoxP3+ cells increased but the disease did not exhibit remission. | Not found. | 2010 |
| ADMSCs (Autologous) | SLE40 | 1 | Intranasal injection (1 × 108), lymph node injection (1 × 108), intravenous transfusions (379 × 106, 234 × 106). | The result was good, and the subsequent physical condition remained stable. | There were only mild adverse reactions and spontaneous recovery. | 2021 |
| UC-MSCs | SLE51 | 21 | 1 × 107/kg; intravenous infusion |
CD1c+ dendritic cells and the cytokine FMS-related Tyrosine kinase 3-ligand increased. Most patients experience varying degrees of remission. | Not found. | 2019 |
| UC-MSCs | Lupus nephritis54 | 12 | 2 × 108; intravenous infusion |
The trial was stopped when it did not show the expected efficacy. | Subcutaneous abscess, leucopenia, and pneumonia. | 2017 |
| BMMSCs | SLE-IV active proliferative nephritis55 | 3 | 9 × 108; intravenous infusion |
Proteinuria levels improved. SLE disease activity index was improved. Symptoms are relieved to varying degrees. | Not found. | 2018 |
| UC-MSCs BMMSCs |
Persistently active SLE56 | 87 | 1 × 106/kg; intravenous infusion |
Disease activity declined. Symptoms were relieved and organ dysfunction improved. | Not found. | 2013 |
| ADMSCs | Refractory lupus nephritis41 | 9 | 2 × 106/kg; intravenous infusion |
Urinary protein levels and disease activity decreased. A single dose may not provide long-term relief. | Not found. | 2021 |
| UC-MSCs | Lupus nephritis57 | 20 | 3 × 107/kg intravenous infusion |
The disease improved significantly and the recurrence rate was low. | Not found. | 2014 |
ADSCs: adipose tissue–derived stem cells; BMMSCs: bone marrow–derived mesenchymal stromal cells; IL: interleukin; MSC: mesenchymal stromal cell; SLE: systemic lupus erythematosus; UC-MSC: umbilical cord–derived mesenchymal stromal cell.
Overactivated complement C5a and C5B-9 might be involved in the progression of LN, and UC-MSCs can improve LN in mice by secreting factor H to inhibit C5 activation58. Current investigations into human UC-MSCs (hUC-MSCs) mainly focus on the application of exosomes. For example, hUC-MSCs induce T-cell senescence and ameliorate lupus by regulating sirtuin 1 (SIRT1)/tumor protein P53 (p53) signaling in CD4+ T cells via miR-199a-5p transfer59. Human UC-MSCs can also release exosomes to induce the anti-inflammatory polarization of macrophages and improve SLE-associated diffuse alveolar hemorrhage in mice60. Exosomes released by UC-MSCs significantly inhibit the production of pro-inflammatory cytokines such as IFN-γ, IL-2, and TNF-α and increase the production of anti-inflammatory cytokines such as IL-1061.
Several clinical trials have confirmed the safety and effectiveness of UC-MSCs in patients with SLE. However, repeated injections of MSCs are necessary to avoid disease recurrence62.
Gingival MSCs and exosomes
A population of human gingival stromal cells (GMSCs) has self-renewal and multipotent differentiation capabilities63. Furthermore, GMSCs have better stem cell properties and immune characteristics than BMMSCs64, are not dependent on growth factor and serum supplements, are non-tumorigenic, and have phenotypic stability and high telomerase activity in long-term culture in vitro65. The phenotype and immunoregulatory functions of GMSCs isolated from patients with active SLE and healthy controls might be similar65. However, clinical trials of GMSCs for treating SLE have not substantially progressed.
Gingival MSCs exert preventive and therapeutic effects on lupus, and their exosomes promote M2 polarization of macrophages66,67. Gingival MSCs convert ADP or ATP to adenosine via elevated CD39 and CD7368 expression. They also promote transformation from an ATP-driven pro-inflammatory to an adenosine-driven anti-inflammatory environment, thus regulating naive CD4+ T-cell differentiation68. Furthermore, GMSCs limit the development of proteinuria as well as autoantibodies, and decrease the frequency of plasma cells and severity of LN by directly inhibiting B-cell activation, proliferation, and differentiation69. Abnormal activation of the mTOR signaling pathway plays a central role in cell aging and GMSC-EVs can significantly inhibit mTOR/PS6 signaling; thus, GMSC-EVs might help to alleviate abnormal cell senescence in lupus tissues70.
Synovium-derived MSCs (SMSCs) and exosomes
Arthritis is a generally non-invasive, common clinical manifestation of SLE. Advances in imaging technology have clarified that chronic synovitis is more prevalent than was previously estimated71. Immune cells, especially T cells, play a critical role in the pathogenesis of lupus arthritis72. Synovium-derived MSCs have powerful immunomodulatory activities73, such as the inhibition of T-cell proliferation. Human synovium–derived mesenchymal stromal cell (hSMSCs) co-cultured with T cells inhibit their proliferation while having high proliferative capacity and limited senescence74. Intra-articular hSMSC injections restore the Th17/Treg and Th1/Th2 cell balance in animal models of rheumatoid arthritis75. Therefore, SMSCs might have similar therapeutic potential against SLE arthritis. However, the effects of SMSCs on NK, antigen-presenting, and B cells await exploration.
Exosomes of hSMSCs have abundant miR-129-5p, which significantly reduces cartilage cell inflammation and apoptosis. Human SMSCs injected into patients with osteoarthritis results in miR-129-5p in exosomes specifically targeting high mobility group box 1 (HMGB1) and attenuating the HMGB1-mediated inflammatory response76.
The pathogenesis of lupus also involves upregulated HMGB177; therefore, HMGB1 might be a potential target for treating SLE. Lupus is often treated using glucocorticoids, but prolonged exposure can lead to necrosis of the femoral head. Infused SMSCs prevent glucocorticoid-induced femoral head necrosis in rats by promoting bone tissue maintenance and regeneration via exosome secretion78. In addition, the extracellular matrix associated with SMSCs enhances anti-inflammatory properties and the proliferative potential of articular cartilage via the SIRT1 pathway79. Therefore, SMSCs are more suitable for treating joint lesions than other sources of MSCs.
Currently, MSCs derived from various sources have shown immunomodulatory effects in animal models, and allogeneic MSCs have exerted substantial clinical effects in SLE treatment. However, the mechanism through which MSCs alleviate SLE remains unclear. Mesenchymal stem cells have immune-regulatory, anti-inflammatory, and tissue repair functions. Specific MSCs might exert superior effects on individual systemic symptoms of SLE, like those of SMSCs in arthritis. Therefore, the feasibility and safety of sampling MSCs must be determined to obtain ethical approval before their clinical application can be promoted. Moreover, the effects and potential side effects of MSCs obtained from various sources should be compared. Like MSCs, exosomes derived from them might be a potential therapeutic approach.
Autologous MSC Transplantation in SLE
Application of autologous MSCs
Therapy is more suitable with autologous cells to prevent immune reactions to allogeneic cells in humans80. Although autologous MSCs to treat SLE does not elicit side effects and increased CD4+CD25+Foxp3+ Treg cells, disease activity is not affected, and later stages of the disease were not prevented53. Lupus MSCs are ineffective against SLE mice, and autologous MSCs are ineffective in treating human SLE80. Thus, autologous MSCs might not produce desirable effects in patients with SLA80.
MSC dysfunction in patients with SLE
Mesenchymal stem cells from patients with SLE have abnormal proliferation, immune regulation, and phenotypes81, as well as an altered intracellular constitution, especially pertaining to miRNA expression.
MicroRNA signaling in BMMSCs from patients with SLE is unique; abnormal miR-663 elevation might be associated with disease activity. Specifically, miR-663 targets TGF-β1 and inhibits BMMSC proliferation and migration, thus inhibiting any decrease in the proportion of Tfh cells or increase in the proportion of Treg cells normally mediated by BMMSCs82. Intracellular miR-153-3p overexpression reduces UC-MSC proliferation and migration, inhibits the UC-MSC-mediated decrease in the population of Tfh cells, and increases that of Tregs by inhibiting PELI183. The onset of lupus is associated with a triggered Treg/Th17 imbalance in BMMSCs due to decreased Let-7f expression84. Furthermore, MSCs in patients with SLE have abnormal differentiation and senescence phenotypes, a senescent morphology, and increased proportions (%) of apoptotic cells. The proliferation potential of MSCs is limited in vitro, and gene expression associated with the senescence secretion phenotype is significantly increased85.
Gingival MSCs isolated from patients with active SLE have phenotypes and persistent immunomodulatory functions like those of healthy controls65. The application of autologous MSCs to SLE requires further investigation.
Conditioned MSCs
Because autologous cell therapy can prevent allogeneic reactions in humans80, effort has been directed to repair autologous MSCs using manipulations in vivo and ex vivo (Table 2).
Table 2.
Effects of Pre-Treated MSCs Combined With Other Cell Types and Drugs.
| Pre-treatment | Type | Response | Specific effect | Year |
|---|---|---|---|---|
| Rapamycin86 | BMMSCs | Improved | Reverse aging phenotype of Lupus BMMSCs Improved immune regulation and increased the proportion of Treg/Th17 cells |
2016 |
| Ethyl pyruvate87 | BMMSCs | Improved | Reverse aging phenotype of BMMSCs Improved immune regulation of Treg |
2019 |
| Acetylsalicylic acid88 | Inflamed GMSCs | Improved | Restore the immunomodulatory properties of impaired inflamed GMSCs. Improve T cell apoptosis mediated by IGMSCs | 2019 |
| HCQ89 | hUC-MSCs | Improved | Enhance MSCs to improve renal morphology and function | 2018 |
| GDNF46 | ADSCs | Improved | Protective effect of GDNF modified ADSC on renal interstitial fibrosis was enhanced. | 2021 |
| Phorbol ester90 | BMMSCs | Improved | Activate mesenchymal stem cells to regulate B cells and improve lupus symptoms | 2020 |
| Metformin91 | ADSCs | Improved | Metformin enhanced STAT1 expression of ADSCs and enhanced its immunomodulatory effect | 2020 |
| Combination therapy | Type | Response | Specific effect | Year |
| Hematopoietic stem cells92 | Amnion-derived MSCs | Improved | Transfusion-related graft-versus-host reaction is rare. Improve kidney function |
2021 |
| CsA93 | ADSCs | Improved | Reduce immunosuppressant dosage while maintaining or improving therapeutic effect | 2017 |
| Rapamycin93 | ADSCs | Improved | Promotes the function of anti-inflammatory Treg lymphocytes. | 2017 |
| MMF93 | ADSCs | Improved | Synergistic effect | 2017 |
| Glucocorticoids93 | ADSCs | Improved | Induced upregulation of Th17-related responses | 2017 |
| HCQ89 | hUC-MSCs | Ineffective | Less effective than HCQ and MSCs alone in mice | 2018 |
| IL-3794 | BMMSCs | Improved | To systemic lupus erythematosus additive therapeutic effect | 2020 |
ADSCs: adipose tissue–derived stem cells; BMMSCs: bone marrow–derived mesenchymal stromal cells; GDNF: glial cell line–derived neurotrophic factor; GMSCs: gingiva-derived mesenchymal stromal cells; HCQ: hydroxychloroquine; HSC: haematopoietic stem cell; MMF: mycophenolate mofetil; IGMSCs: inflammatory gingiva-derived mesenchymal stromal cells; MSC: mesenchymal stromal cell; hUC-MSC: human umbilical cord–derived mesenchymal stromal cell.
Altering aging-related genes or signaling pathways can partially or completely reverse the aging phenotype and the defective immunoregulatory features of MSCs in patients with SLE95. Therefore, reversing MSC senescence might improve its therapeutic effects on SLE81.
The activated mTOR pathway is involved in MSC senescence in patients with SLE. Treating autologous MSCs derived from patients with SLE with the mTOR signaling inhibitor rapamycin (RAPA) ex vivo then infusing them might improve the immune-regulation ability of BMMSCs. Notably, RAPA increases the proportion of Treg/Th17 cells and regulates the secretion of related cytokines86. In addition, treating BMMSCs with ethyl pyruvate reverses BMMSC aging by blocking the HMGB1/TLR4/NF-κB signaling pathway, thus improving Treg-cell-related immune regulation87. Let-7f-5p can somewhat reduce the inflammatory response of SLE-BMMSCs by targeting NOD-like receptor protein 3 (NLRP3)96. Other MSC pre-conditioning methods might be applied to restore the functions of autoimmune MSCs. Such modulators might increase the possibility of applying autoimmune MSCs to autoimmune diseases. For example, incubating inflammatory GMSCs (IGMSCs) that have impaired immune-regulation ability with acetylsalicylic acid upregulates Fas ligand expression, thus enhancing the apoptosis of T cells mediated by IGMSC88.
Development of Combination Therapy
Among the drugs that are currently prescribed to treat SLE, immunosuppressants are often limited due to the risk of side effects. A combination of MSCs and immunosuppressants has shown promise for patients with refractory SLE. Combination therapies decrease the amount of immunosuppressants required to reduce immune activity in SLE93.
Mesenchymal stromal cells exert synergistic effects on immunosuppressants such as the calcineurin inhibitor CsA, the mTOR inhibitor RAPA, mycophenolate mofetil (MMF), dexamethasone (Dex), and prednisone. The combination of MSCs and immunosuppressants significantly alters the activation and balance of T-lymphocyte subsets and reverses the adverse effects of immunosuppressants such as the prednisone-, Dex-, or MMF-induced increase in T-cell differentiation into Th17 cells. However, MSC activity and function have not been significantly inhibited93. The results of combinations of immunosuppressants on MSCs differ based on the included drugs. For example, a combination of tacrolimus and RAPA antagonizes, whereas Dex does not alter the immunosuppressive effects of transplanted MSCs93.
In conclusion, although combining drugs with MSC transplantation is a promising new therapeutic strategy, further studies are warranted to ensure their effectiveness and safety (Table 2).
Prospects and Challenges of Cell-Free Therapy
Although live MSCs have gradually been applied in clinical practice as therapy, dangers include degrees of immune rejection, low cell survival rates, senescence, and carcinogenic potential97. Adequate funding and technology are also required to prepare and preserve live cells, which limits the widespread application of MSCs as therapy in hospital environments. However, the therapeutic effects of MSC-derived EVs, especially exosomes, are similar to those of MSCs. Being cell-free, exosomes have more advantages than MSCs, are easier to preserve and manage, have no immunogenicity, and can easily cross the blood–brain barrier98, which is considered a promising new treatment option. However, many aspects still require optimization before large quantities of such exosomes can serve as human therapies17.
The first issue is the high cost and low efficiency of current methods of extracting exosomes from cells, and related processes have not been standardized99. Differential ultracentrifugation can generate the purest exosomes, but it is time-consuming100. Artificial exosomes might guarantee production and facilitate widespread commercialization. Attempts are ongoing to increase EV production by modifying culture conditions and conducting specific interventions for MSCs101. Another issue is the efficiency of exosome homing to specific lesion sites. Extracellular vesicles such as exosomes can be homed to a portion of injured tissue dependent on the expression of CD44, CD29, or CD73102. However, most intravenously injected exosomes accumulate at sites where the mononuclear phagocyte system (MPS) is active103. Artificial modification of exosome membranes to increase the proportions of them that reach target cells is essential to improve bioavailability17. Because the pathogenesis of lupus is different caused by different factors, conventional drugs are only effective for a certain type of patients. Most current studies of potential therapeutic mechanisms of miRNAs in MSC exosomes target a specific miRNA, which provides great support for the development of targeted therapy. However, whether transfecting several miRNAs together into exosomes or inhibiting them will increase their effectiveness or cause side effects is unknown. A single miRNA can target multiple mRNAs, so exosomes carrying specific miRNAs delivered intravenously might elicit side effects104. Further studies are needed to decode the complexity of exosome components to better understand the safety of their combined effects on target tissues. Bioengineering efforts are also needed to achieve the amplification and safe production of compositionally homogeneous exosomes containing specific miRNAs or inhibitors105 of miRNAs106. The optimal dose and frequency of exosome administration should also be determined to maximize efficiency107.
Conclusions
The anti-inflammatory repair potential, immunomodulatory, and specific immunological properties of MSCs hold promise for using allogeneic MSCs to treat SLE with abnormal autologous MSC function. Investigations into the effects of chemical and other manipulations to improve MSCs before delivery and the application of exosomes as drug delivery vectors in treating SLE are currently underway. Roles for exosomes and their miRNAs in the diagnosis and treatment of SLE have been demonstrated. Tissue engineering, biomaterials, and stem cell biology offer a brighter future for patients with SLE through the large-scale preparation of specific exosomes/miRNAs.
Acknowledgments
We are grateful to the Second Affiliated Hospital of Fujian Medical University for providing infrastructure facilities.
Footnotes
Author Contributions: YJL, ZC, and SL: concept and design of manuscript. YJL drafted and finalized the manuscript. ZC and SL drafted the manuscript. ZC, HBM, and SL revised the manuscript and provided critical advice about the content. All authors contributed to the article and approved the submitted version.
Availability of Data and Materials: Not applicable
Ethical Approval: Not applicable.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Science and Technology Bureau of Quanzhou (grant 2020CT003), the Natural Science Foundation of Fujian Province (grant 2020J01219), and the Science and Technology Bureau of Quanzhou (grant 2017Z009).
ORCID iD: Zhen Chen  https://orcid.org/0000-0002-9655-1944
https://orcid.org/0000-0002-9655-1944
References
- 1. Tang WY, Liu JH, Peng CJ, Liao Y, Luo JS, Sun X, Tang Y-L, Luo X-Q. Functional characteristics and application of mesenchymal stem cells in systemic lupus erythematosus. Arch Immunol Ther Exp (Warsz). 2021;69(1):7. [DOI] [PubMed] [Google Scholar]
- 2. Khan F, Granville N, Malkani R, Chathampally Y. Health-related quality of life improvements in systemic lupus erythematosus derived from a digital therapeutic plus tele-health coaching intervention: randomized controlled pilot trial. J Med Internet Res. 2020;22(10):e23868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ukadike KC, Mustelin T. Implications of endogenous retroelements in the etiopathogenesis of systemic lupus erythematosus. J Clin Med. 2021;10(4):856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kariburyo F, Xie L, Sah J, Li N, Lofland JH. Real-world medication use and economic outcomes in incident systemic lupus erythematosus patients in the United States. J Med Econ. 2020;23(1):1–9. [DOI] [PubMed] [Google Scholar]
- 5. Caplan A, Fett N, Rosenbach M, Werth VP, Micheletti RG. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017; 76(1):1–9. [DOI] [PubMed] [Google Scholar]
- 6. Eleuteri S, Fierabracci A. Insights into the secretome of mesenchymal stem cells and its potential applications. Int J Mol Sci. 2019;20(18):4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Radmanesh F, Mahmoudi M, Yazdanpanah E, Keyvani V, Kia N, Nikpoor AR, Zafari P, Esmaeili SA. The immunomodulatory effects of mesenchymal stromal cell-based therapy in human and animal models of systemic lupus erythematosus. IUBMB Life. 2020;72(11):2366–81. [DOI] [PubMed] [Google Scholar]
- 8. Liu B, Ding F, Hu D, Zhou Y, Long C, Shen L, Zhang Y, Zhang D, Wei G. Human umbilical cord mesenchymal stem cell conditioned medium attenuates renal fibrosis by reducing inflammation and epithelial-to-mesenchymal transition via the TLR4/NF-kappaB signaling pathway in vivo and in vitro. Stem Cell Res Ther. 2018;9(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He Y, Ji D, Lu W, Li F, Huang X, Huang R, Chen G. Bone marrow mesenchymal stem cell-derived exosomes induce the Th17/Treg imbalance in immune thrombocytopenia through miR-146a-5p/IRAK1 axis. Hum Cell. 2021;34(5):1360–74. [DOI] [PubMed] [Google Scholar]
- 10. Magana-Guerrero FS, Dominguez-Lopez A, Martinez-Aboytes P, Buentello-Volante B, Garfias Y. Human amniotic membrane mesenchymal stem cells inhibit neutrophil extracellular traps through TSG-6. Sci Rep. 2017;7(1):12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li W, Chen W, Sun L. An update for mesenchymal stem cell therapy in lupus nephritis. Kidney Dis (Basel). 2021;7(2):79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao J, Chen J, Huang F, Wang J, Su W, Zhou J, Qi Q, Cao F, Sun B, Liu Z, Bellanti JA, et al. Human gingiva tissue-derived MSC ameliorates immune-mediated bone marrow failure of aplastic anemia via suppression of Th1 and Th17 cells and enhancement of CD4+Foxp3+ regulatory T cells differentiation. Am J Transl Res. 2019;11(12):7627–43. [PMC free article] [PubMed] [Google Scholar]
- 13. Rosado MM, Bernardo ME, Scarsella M, Conforti A, Giorda E, Biagini S, Cascioli S, Rossi F, Guzzo I, Vivarelli M, Strologo LD, et al. Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev. 2015;24(1):93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Braza F, Dirou S, Forest V, Sauzeau V, Hassoun D, Chesné J, Cheminant-Muller MA, Sagan C, Magnan A, Lemarchand P. Mesenchymal stem cells induce suppressive macrophages through phagocytosis in a mouse model of asthma. Stem Cells. 2016;34(7):1836–45. [DOI] [PubMed] [Google Scholar]
- 15. Dabrowska S, Andrzejewska A, Lukomska B, Janowski M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J Neuroinflammation. 2019;16(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8(7):727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. 2019;8(12):1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seo Y, Kim HS, Hong IS. Stem cell-derived extracellular vesicles as immunomodulatory therapeutics. Stem Cells Int. 2019;2019:5126156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li I, Nabet BY. Exosomes in the tumor microenvironment as mediators of cancer therapy resistance. Mol Cancer. 2019;18(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yan C, Chen J, Wang C, Yuan M, Kang Y, Wu Z, Li W, Zhang G, Machens HG, Rinkevich Y, Chen Z, et al. Milk exosomes-mediated miR-31-5p delivery accelerates diabetic wound healing through promoting angiogenesis. Drug Deliv. 2022;29(1):214–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leavitt RJ, Limoli CL, Baulch JE. miRNA-based therapeutic potential of stem cell-derived extracellular vesicles: a safe cell-free treatment to ameliorate radiation-induced brain injury. Int J Radiat Biol. 2019;95(4):427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garcia-Martin R, Wang G, Brandão BB, Zanotto TM, Shah S, Kumar Patel S, Schilling B, Kahn CR. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature. 2022;601(7893):446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Juan T, Fürthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin Cell Dev Biol. 2018; 74:66–77. [DOI] [PubMed] [Google Scholar]
- 24. Mirzaei R, Zamani F, Hajibaba M, Rasouli-Saravani A, Noroozbeygi M, Gorgani M, Hosseini-Fard SR, Jalalifar S, Ajdarkosh H, Abedi SH, Keyvani H, et al. The pathogenic, therapeutic and diagnostic role of exosomal microRNA in the autoimmune diseases. J Neuroimmunol. 2021;358:577640. [DOI] [PubMed] [Google Scholar]
- 25. Tangtanatakul P, Klinchanhom S, Sodsai P, Sutichet T, Promjeen C, Avihingsanon Y, Hirankarn N. Down-regulation of let-7a and miR-21 in urine exosomes from lupus nephritis patients during disease flare. Asian Pac J Allergy Immunol. 2019;37(4):189–97. [DOI] [PubMed] [Google Scholar]
- 26. Chen L, Lu FB, Chen DZ, Wu JL, Hu ED, Xu LM, Zheng MH, Li H, Huang Y, Jin XY, Gong YW, et al. BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol Immunol. 2018;93: 38–46. [DOI] [PubMed] [Google Scholar]
- 27. Xu F, Fei Z, Dai H, Xu J, Fan Q, Shen S, Zhang Y, Ma Q, Chu J, Peng F, Zhou F, et al. Mesenchymal stem cell-derived extracellular vesicles with high PD-L1 expression for autoimmune diseases treatment. Adv Mater. 2022;34(1):e2106265. [DOI] [PubMed] [Google Scholar]
- 28. Elashiry M, Elsayed R, Cutler CW. Exogenous and endogenous dendritic cell-derived exosomes: lessons learned for immunotherapy and disease pathogenesis. Cells. 2021;11(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Batsali AK, Kastrinaki MC, Papadaki HA, Pontikoglou C. Mesenchymal stem cells derived from Wharton’s Jelly of the umbilical cord: biological properties and emerging clinical applications. Curr Stem Cell Res Ther. 2013;8(2):144–155. [DOI] [PubMed] [Google Scholar]
- 30. Muroi K, Miyamura K, Okada M, Yamashita T, Murata M, Ishikawa T, Uike N, Hidaka M, Kobayashi R, Imamura M, Tanaka J, et al. Bone marrow-derived mesenchymal stem cells (JR-031) for steroid-refractory grade III or IV acute graft-versus-host disease: a phase II/III study. Int J Hematol. 2016;103(2):243–50. [DOI] [PubMed] [Google Scholar]
- 31. Yang X, Yang J, Li X, Ma W, Zou H. Bone marrow-derived mesenchymal stem cells inhibit T follicular helper cell in lupus-prone mice. Lupus. 2018;27(1):49–59. [DOI] [PubMed] [Google Scholar]
- 32. Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, Stolz DB, Watkins SC, Di YP, Leikauf GD, Kolls J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dou R, Zhang X, Xu X, Wang P, Yan B. Mesenchymal stem cell exosomal tsRNA-21109 alleviate systemic lupus erythematosus by inhibiting macrophage M1 polarization. Mol Immunol. 2021;139:106–14. [DOI] [PubMed] [Google Scholar]
- 34. Liu S, Liu D, Chen C, Hamamura K, Moshaverinia A, Yang R, Liu Y, Jin Y, Shi S. MSC transplantation improves osteopenia via epigenetic regulation of notch signaling in lupus. Cell Metab. 2015;22(4):X606–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82(4):583–90. [DOI] [PubMed] [Google Scholar]
- 36. Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21(14):2724–52. [DOI] [PubMed] [Google Scholar]
- 37. El-Badawy A, Amer M, Abdelbaset R, Sherif SN, Abo-Elela M, Ghallab YH, Abdelhamid H, Ismail Y, El-Badri N. Adipose stem cells display higher regenerative capacities and more adaptable electro-kinetic properties compared to bone marrow-derived mesenchymal stromal cells. Sci Rep. 2016;6:37801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen KH, Chen CH, Wallace CG, Yuen CM, Kao GS, Chen YL, Shao P-L, Chen Y-L, Chai H-T, Lin K-C, Liu C-F, et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget. 2016;7(46):74537–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin KC, Yip HK, Shao PL, Wu SC, Chen KH, Chen YT, Yang C-C, Sun C-K, Kao G-S, Chen S-Y, Chai H-T, et al. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int J Cardiol. 2016;216:173–85. [DOI] [PubMed] [Google Scholar]
- 40. Wong SC, Medrano LC, Hoftman AD, Jones OY, McCurdy DK. Uncharted waters: mesenchymal stem cell treatment for pediatric refractory rheumatic diseases; a single center case series. Pediatr Rheumatol Online J. 2021;19(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ranjbar A, Hassanzadeh H, Jahandoust F, Miri R, Bidkhori HR, Monzavi SM, Sanjar-Moussavi N, Matin MM, Shariati-Sarabi Z. Allogeneic adipose-derived mesenchymal stromal cell transplantation for refractory lupus nephritis: results of a phase I clinical trial. Curr Res Transl Med. 2022;70(2):103324. [DOI] [PubMed] [Google Scholar]
- 42. Xu K, Ma D, Zhang G, Gao J, Su Y, Liu S, Liu Y, Han J, Tian M, Wei C, Zhang L. Human umbilical cord mesenchymal stem cell-derived small extracellular vesicles ameliorate collagen-induced arthritis via immunomodulatory T lymphocytes. Mol Immunol. 2021;135:36–44. [DOI] [PubMed] [Google Scholar]
- 43. Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H, Zhang Q, Guo C, Zhang L, Wang Q. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. 2018;67(2):235–47. [DOI] [PubMed] [Google Scholar]
- 44. Lee WS, Kim HJ, Kim KI, Kim GB, Jin W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med. 2019;8(6):504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhao C, Chen JY, Peng WM, Yuan B, Bi Q, Xu YJ. Exosomes from adiposederived stem cells promote chondrogenesis and suppress inflammation by upregulating miR145 and miR221. Mol Med Rep. 2020;21(4):1881–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li S, Wang Y, Wang Z, Chen L, Zuo B, Liu C, Sun D. Enhanced renoprotective effect of GDNF-modified adipose-derived mesenchymal stem cells on renal interstitial fibrosis. Stem Cell Res Ther. 2021;12(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Serejo TRT, Silva-Carvalho AE, Braga L, Neves FAR, Pereira RW, Carvalho JL, Saldanha-Araujo F. Assessment of the immunosuppressive potential of INF-gamma licensed adipose mesenchymal stem cells, their secretome and extracellular vesicles. Cells. 2019;8(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McGuirk JP, Smith JR, Divine CL, Zuniga M, Weiss ML. Wharton’s jelly-derived mesenchymal stromal cells as a promising cellular therapeutic strategy for the management of graft-versus-host disease. Pharmaceuticals (Basel). 2015; 8(2):196–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Najar M, Raicevic G, Boufker HI, Fayyad Kazan H, De Bruyn C, Meuleman N, Bron D, Toungouz M, Lagneaux L. Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: combined comparison of adipose tissue, Wharton’s Jelly and bone marrow sources. Cell Immunol. 2010;264(2):171–79. [DOI] [PubMed] [Google Scholar]
- 50. Zhang Z, Huang S, Wu S, Qi J, Li W, Liu S, Cong Y, Chen H, Lu L, Shi S, Wang D, et al. Clearance of apoptotic cells by mesenchymal stem cells contributes to immunosuppression via PGE2. Ebiomedicine. 2019;45:341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yuan X, Qin X, Wang D, Zhang Z, Tang X, Gao X, Chen W, Sun L. Mesenchymal stem cell therapy induces FLT3L and CD1c(+) dendritic cells in systemic lupus erythematosus patients. Nat Commun. 2019;10(1):2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chun W, Tian J, Zhang Y. Transplantation of mesenchymal stem cells ameliorates systemic lupus erythematosus and upregulates B10 cells through TGF-beta1. Stem Cell Res Ther. 2021;12(1):512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Carrion F, Nova E, Ruiz C, Diaz F, Inostroza C, Rojo D, Mönckeberg G, Figueroa FE. Autologous mesenchymal stem cell treatment increased T regulatory cells with no effect on disease activity in two systemic lupus erythematosus patients. Lupus. 2010;19(3):317–22. [DOI] [PubMed] [Google Scholar]
- 54. Deng D, Zhang P, Guo Y, Lim TO. A randomised double-blind, placebo-controlled trial of allogeneic umbilical cord-derived mesenchymal stem cell for lupus nephritis. Ann Rheum Dis. 2017;76(8):1436–39. [DOI] [PubMed] [Google Scholar]
- 55. Barbado J, Tabera S, Sánchez A, García-Sancho J. Therapeutic potential of allogeneic mesenchymal stromal cells transplantation for lupus nephritis. Lupus. 2018;27(13):2161–65. [DOI] [PubMed] [Google Scholar]
- 56. Wang D, Zhang H, Liang J, Li X, Feng X, Wang H, Hua B, Liu B, Lu L, Gilkeson GS, Silver RM, et al. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant. 2013;22(12):2267–77. [DOI] [PubMed] [Google Scholar]
- 57. Yang GX, Pan LP, Zhou QY, Song W, Chen ZQ, Wang CX, Wu YB, Wang X, Chen Q. [Therapeutic effects of umbilical cord mesenchymal stem cells transplantation on systemic lupus erythematosus]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2014;45(2):338–41, 350. [PubMed] [Google Scholar]
- 58. Ma H, Liu C, Shi B, Zhang Z, Feng R, Guo M, Lu L, Shi S, Gao X, Chen W, Sun L. Mesenchymal stem cells control complement C5 activation by factor H in lupus nephritis. Ebiomedicine. 2018;32:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cheng T, Ding S, Liu S, Li Y, Sun L. Human umbilical cord-derived mesenchymal stem cell therapy ameliorates lupus through increasing CD4+ T cell senescence via MiR-199a-5p/Sirt1/p53 axis. Theranostics. 2021;11(2):893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen X, Wei Q, Sun H, Zhang X, Yang C, Tao Y, Nong G. Exosomes derived from human umbilical cord mesenchymal stem cells regulate macrophage polarization to attenuate systemic lupus erythematosus-associated diffuse alveolar hemorrhage in mice. Int J Stem Cells. 2021;14(3):331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang L, Gu Z, Zhao X, Yang N, Wang F, Deng A, Zhao S, Luo L, Wei H, Guan L, Gao Z, et al. Extracellular vesicles released from human umbilical cord-derived mesenchymal stromal cells prevent life-threatening acute graft-versus-host disease in a mouse model of allogeneic hematopoietic stem cell transplantation. Stem Cells Dev. 2016;25(24):1874–83. [DOI] [PubMed] [Google Scholar]
- 62. Wang D, Li J, Zhang Y, Zhang M, Chen J, Li X, Hu X, Jiang S, Shi S, Sun L. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis Res Ther. 2014;16(2):R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, Shi S, Le AD. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183(12):7787–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38(6):758–68. [DOI] [PubMed] [Google Scholar]
- 65. Huang F, Chen M, Chen W, Gu J, Yuan J, Xue Y, Dang J, Su W, Wang J, Zadeh HH, He X, et al. Human gingiva-derived mesenchymal stem cells inhibit xeno-graft-versus-host disease via CD39-CD73-adenosine and IDO signals. Front Immunol. 2017;8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang X, Huang F, Li W, Dang JL, Yuan J, Wang J, Zeng DL, Sun CX, Liu YY, Ao Q, Tan H, et al. Human gingiva-derived mesenchymal stem cells modulate monocytes/macrophages and alleviate atherosclerosis. Front Immunol. 2018;9:878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang R, Ji Q, Meng C, Liu H, Fan C, Lipkind S, Wang Z, Xu Q. Role of gingival mesenchymal stem cell exosomes in macrophage polarization under inflammatory conditions. Int Immunopharmacol. 2020;81:106030. [DOI] [PubMed] [Google Scholar]
- 68. Zhang W, Zhou L, Dang J, Zhang X, Wang J, Chen Y, Liang J, Li D, Ma J, Yuan J, Chen W, et al. Human gingiva-derived mesenchymal stem cells ameliorate streptozoticin-induced T1DM in mice via suppression of T effector cells and up-regulating Treg subsets. Sci Rep. 2017;7(1):15249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dang J, Xu Z, Xu A, Liu Y, Fu Q, Wang J, Huang F, Zheng Y, Qi G, Sun B, Bellanti JA, et al. Human gingiva-derived mesenchymal stem cells are therapeutic in lupus nephritis through targeting of CD39(-)CD73 signaling pathway. J Autoimmun. 2020;113:102491. [DOI] [PubMed] [Google Scholar]
- 70. Shi HZ, Zeng JC, Shi SH, Giannakopoulos H, Zhang QZ, Le AD. Extracellular vesicles of GMSCs alleviate aging-related cell senescence. J Dent Res. 2021;100(3):283–92. [DOI] [PubMed] [Google Scholar]
- 71. Zayat AS, Mahmoud K, Md Yusof MY, Mukherjee S, D’Agostino MA, Hensor EMA, Wakefield RJ, Conaghan PG, Edwards CJ, Emery P, Vital EM. Defining inflammatory musculoskeletal manifestations in systemic lupus erythematosus. Rheumatology (Oxford). 2019;58(2):304–12. [DOI] [PubMed] [Google Scholar]
- 72. Sippl N, Faustini F, Rönnelid J, Turcinov S, Chemin K, Gunnarsson I, Malmström V. Arthritis in systemic lupus erythematosus is characterized by local IL-17A and IL-6 expression in synovial fluid. Clin Exp Immunol. 2021;205(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. De Bari C, Dell’Accio F, Vandenabeele F, Vermeesch JR, Raymackers JM, Luyten FP. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J Cell Biol. 2003;160(6):909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–42. [DOI] [PubMed] [Google Scholar]
- 75. Yan M, Liu X, Dang Q, Huang H, Yang F, Li Y. Intra-articular injection of human synovial membrane-derived mesenchymal stem cells in murine collagen-induced arthritis: assessment of immunomodulatory capacity in vivo. Stem Cells Int. 2017; 2017:9198328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Qiu M, Liu D, Fu Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1beta induced osteoarthritis via targeting HMGB1. Life Sci. 2021;269:118987. [DOI] [PubMed] [Google Scholar]
- 77. Liu T, Son M, Diamond B. HMGB1 in systemic lupus erythematosus. Front Immunol. 2020;11:1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Guo SC, Tao SC, Yin WJ, Qi X, Sheng JG, Zhang CQ. Exosomes from human synovial-derived mesenchymal stem cells prevent glucocorticoid-induced osteonecrosis of the femoral head in the rat. Int J Biol Sci. 2016;12(10):1262–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yan J, Chen X, Pu C, Zhao Y, Liu X, Liu T, Pan G, Lin J, Pei M, Yang H, He F. Synovium stem cell-derived matrix enhances anti-inflammatory properties of rabbit articular chondrocytes via the SIRT1 pathway. Mater Sci Eng C Mater Biol Appl. 2020;106:110286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Collins E, Gu F, Qi M, Molano I, Ruiz P, Sun L, Gilkeson GS. Differential efficacy of human mesenchymal stem cells based on source of origin. J Immunol. 2014;193(9):4381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cheng RJ, Xiong AJ, Li YH, Pan SY, Zhang QP, Zhao Y, Liu Y, Marion TN. Mesenchymal stem cells: allogeneic MSC may be immunosuppressive but autologous MSC are dysfunctional in lupus patients. Front Cell Dev Biol. 2019;7:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Geng L, Tang X, Zhou K, Wang D, Wang S, Yao G, Chen W, Gao X, Chen W, Shi S, Shen N, et al. MicroRNA-663 induces immune dysregulation by inhibiting TGF-beta1 production in bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Cell Mol Immunol. 2019; 16(3):260–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li D, Li X, Duan M, Dou Y, Feng Y, Nan N, Zhang W. MiR-153-3p induces immune dysregulation by inhibiting PELI1 expression in umbilical cord-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Autoimmunity. 2020;53(4):201–209. [DOI] [PubMed] [Google Scholar]
- 84. Geng L, Tang X, Wang S, Sun Y, Wang D, Tsao BP, Feng X, Sun L. Reduced Let-7f in bone marrow-derived mesenchymal stem cells triggers Treg/Th17 imbalance in patients with systemic lupus erythematosus. Front Immunol. 2020;11:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gao L, Bird AK, Meednu N, Dauenhauer K, Liesveld J, Anolik J, Looney RJ. Bone marrow-derived mesenchymal stem cells from patients with systemic lupus erythematosus have a senescence-associated secretory phenotype mediated by a mitochondrial antiviral signaling protein-interferon-beta feedback loop. Arthritis Rheumatol. 2017;69(8):1623–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gu Z, Tan W, Ji J, Feng G, Meng Y, Da Z, Guo G, Xia Y, Zhu X, Shi G, Cheng C. Rapamycin reverses the senescent phenotype and improves immunoregulation of mesenchymal stem cells from MRL/lpr mice and systemic lupus erythematosus patients through inhibition of the mTOR signaling pathway. Aging (Albany NY). 2016;8(5):1102–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ji J, Fu T, Dong C, Zhu W, Yang J, Kong X, Zhang Z, Bao Y, Zhao R, Ge X, Sha X, et al. Targeting HMGB1 by ethyl pyruvate ameliorates systemic lupus erythematosus and reverses the senescent phenotype of bone marrow-mesenchymal stem cells. Aging (Albany NY). 2019;11(13):4338–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yu T, Yan B, Li J, Zhang T, Yang R, Wang X, Liu Y, Liu D. Acetylsalicylic acid rescues the immunomodulation of inflamed gingiva-derived mesenchymal stem cells via upregulating FasL in mice. Stem Cell Res Ther. 2019;10(1):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mai S, Zou L, Tian X, Liao X, Luan Y, Han X, Wei Y, Wu Y, Kuang S, Yang Y, Ma J, et al. Double-edged effect of hydroxychloroquine on human umbilical cord-derived mesenchymal stem cells treating lupus nephritis in MRL/lpr mice. Mol Pharm. 2018;15(5):1800–13. [DOI] [PubMed] [Google Scholar]
- 90. Lee HK, Kim HS, Pyo M, Park EJ, Jang S, Jun HW, Lee TY, Kim KS, Bae SC, Kim Y, Hong JT, et al. Phorbol ester activates human mesenchymal stem cells to inhibit B cells and ameliorate lupus symptoms in MRL.Fas(lpr) mice. Theranostics. 2020;10(22):10186–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jang SG, Lee J, Hong SM, Kwok SK, Cho ML, Park SH. Metformin enhances the immunomodulatory potential of adipose-derived mesenchymal stem cells through STAT1 in an animal model of lupus. Rheumatology (Oxford). 2020;59(6): 1426–38. [DOI] [PubMed] [Google Scholar]
- 92. Tago Y, Kobayashi C, Ogura M, Wada J, Yamaguchi S, Yamaguchi T, Hayashi M, Nakaishi T, Kubo H, Ueda Y. Human amnion-derived mesenchymal stem cells attenuate xenogeneic graft-versus-host disease by preventing T cell activation and proliferation. Sci Rep. 2021;11(1):2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hajkova M, Hermankova B, Javorkova E, Bohacova P, Zajicova A, Holan V, Krulova M. Mesenchymal stem cells attenuate the adverse effects of immunosuppressive drugs on distinct T cell subopulations. Stem Cell Rev Rep. 2017;13(1):104–15. [DOI] [PubMed] [Google Scholar]
- 94. Xu J, Chen J, Li W, Lian W, Huang J, Lai B, Li L, Huang Z. Additive therapeutic effects of mesenchymal stem cells and IL-37 for systemic lupus erythematosus. J Am Soc Nephrol. 2020;31(1):54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tan W, Gu Z, Shen B, Jiang J, Meng Y, Da Z, Liu H, Tao T, Cheng C. PTEN/Akt-p27(kip1) signaling promote the BM-MSCs senescence and apoptosis in SLE patients. J Cell Biochem. 2015;116(8):1583–94. [DOI] [PubMed] [Google Scholar]
- 96. Tan W, Gu Z, Leng J, Zou X, Chen H, Min F, Zhou W, Zhang L, Li G. Let-7f-5p ameliorates inflammation by targeting NLRP3 in bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Biomed Pharmacother. 2019;118:109313. [DOI] [PubMed] [Google Scholar]
- 97. Lee OJ, Luk F, Korevaar SS, Koch TG, Baan CC, Merino A, Hoogduijn MJ. The importance of dosing, timing, and (in)activation of adipose tissue-derived mesenchymal stromal cells on their immunomodulatory effects. Stem Cells Dev. 2020;29(1):38–48. [DOI] [PubMed] [Google Scholar]
- 98. Sharma J, Hampton JM, Valiente GR, Wada T, Steigelman H, Young MC, Spurbeck RR, Blazek AD, Bösh S, Jarjour WN, Young NA. Therapeutic development of mesenchymal stem cells or their extracellular vesicles to inhibit autoimmune-mediated inflammatory processes in systemic lupus erythematosus. Front Immunol. 2017;8:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Li X, Corbett AL, Taatizadeh E, Tasnim N, Little JP, Garnis C, Daugaard M, Guns E, Hoorfar M, Li ITS. Challenges and opportunities in exosome research —perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019;3(1):011503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Xu D, Di K, Fan B, Wu J, Gu X, Sun Y, Khan A, Li P, Li Z. MicroRNAs in extracellular vesicles: sorting mechanisms, diagnostic value, isolation, and detection technology. Front Bioeng Biotechnol. 2022;10:948959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gowen A, Shahjin F, Chand S, Odegaard KE, Yelamanchili SV. Mesenchymal stem cell-derived extracellular vesicles: challenges in clinical applications. Front Cell Dev Biol. 2020;8:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Xiang H, Su W, Wu X, Chen W, Cong W, Yang S, Liu C, Qiu C, Yang SY, Wang Y, Zhang G, et al. Exosomes derived from human urine-derived stem cells inhibit intervertebral disc degeneration by ameliorating endoplasmic reticulum stress. Oxid Med Cell Longev. 2020;2020:6697577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wan Z, Zhao L, Lu F, Gao X, Dong Y, Zhao Y, Wei M, Yang G, Xing C, Liu L. Mononuclear phagocyte system blockade improves therapeutic exosome delivery to the myocardium. Theranostics. 2020;10(1):218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liu H, Chen Y, Yin G, Xie Q. Therapeutic prospects of MicroRNAs carried by mesenchymal stem cells-derived extracellular vesicles in autoimmune diseases. Life Sci. 2021; 277:119458. [DOI] [PubMed] [Google Scholar]
- 105. Momen-Heravi F, Bala S, Bukong T, Szabo G. Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomedicine. 2014;10(7):1517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Elshaer SL, Bahram SH, Rajashekar P, Gangaraju R, El-Remessy AB. Modulation of mesenchymal stem cells for enhanced therapeutic utility in ischemic vascular diseases. Int J Mol Sci. 2021;23(1):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Matheakakis A, Batsali A, Papadaki HA, Pontikoglou CG. Therapeutic implications of mesenchymal stromal cells and their extracellular vesicles in autoimmune diseases: from biology to clinical applications. Int J Mol Sci. 2021;22(18):10132. [DOI] [PMC free article] [PubMed] [Google Scholar]