Abstract
Worldwide, the incidence rate of breast cancer is the highest in women. Approximately 2.3 million people were newly diagnosed and 0.685 million were dead of breast cancer in 2020, which continues to grow. Triple-negative breast cancer (TNBC) is the most aggressive breast cancer subtype with a higher risk of recurrence and metastasis, but disappointly, there are no effective and specific therapies clinically, especially for patients presenting with metastatic diseases. Therefore, it is urgent to develop a new type of cancer therapy for survival improvisation and adverse effects alleviation of breast cancers. Near-infrared photoimmunotherapy (NIR-PIT) is a newly developed, photochemistry-based cancer therapy. It was drive by an antibody–photoabsorber conjugate (APC) which is triggered by near-infrared light. The key part of APC is a cancer-targeting monoclonal antibody (mAb) that can bind to receptors or antigens on the surface of tumor cells. Because of this targeted conjugate accumulation, subsequent deployment of focal NIR-light results in functional damage on the targeted cell membranes without harming the immediately adjacent receptor-negative cells and evokes a kind of photochemical, speedy, and highly specific immunogenic cell death (ICD) of cancer cells with corresponding antigens. Subsequently, immature dendritic cells adjacent to dying cancer cells will become mature, further inducing a host-oriented anti-cancer immune response, complicatedly and comprehensively. Currently, NIR-PIT has progressed into phase 3 clinical trial for recurrent head and neck cancer. And preclinical studies have illustrated strong therapeutic efficacy of NIR-PIT targeting various molecular receptors overexpressed in breast cancer cells, including EGFR, HER2, CD44c, CD206, ICAM-1 and FAP-α. Thereby, NIR-PIT is in early trials, but appears to be a promising breast cancer therapy and moving into the future. Here, we present the specific advantages and discuss the most recent preclinical studies against several transmembrane proteins of NIR-PIT in breast cancers.
Keywords: breast cancer, near-infrared photoimmunotherapy (NIR-PIT), antibody–drug conjugate, mAb-IR700, cancer therapy
Introduction
Breast cancer is the most common malignant cancer among women in China, occupying approximately 15% of new diagnosed cancers.1,2 Compared to other subtypes, triple-negative breast cancer (ER−/PR−/HER2−, TNBC) shows unfriendly characters of poorer prognosis, poor survival rates and higher metastasis rates because of its limited molecular targets and primary or secondary drug chemotherapy resistance.3–6 For surgeons, performing entire tumor resections often present a major burden, particularly when the tumors infiltrate vital organs and vasculature.7 Radiotherapy and chemotherapy also have significant limitations that inevitably cause toxic effects to normal cells including immune cells, which go against patients’ physical recovery and ultimately lead to overall debilitation.8 Current cancer immunotherapies offer hope of overcoming challenges, which destruct cancer cells by activating cytotoxic immune cells to stimulate systemic immune responses and resist tumor metastasis and recurrence, rather than killing cancer cells straight. A therapy that utilizes the host immune system is theoretically possible. But excessive tumor burden may overwhelm the anti-cancer ability of the host immune system. Moreover, non-specific activation of the immune system can cause autoimmune-like damage to normal tissues.9 Therefore, it is urgent for a novel and synergistic cancer treatment to breakthrough these therapeutic bottlenecks.
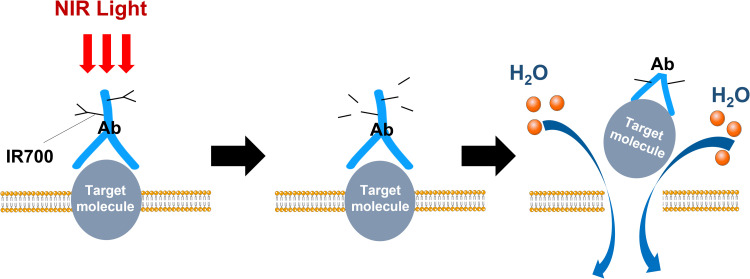
Photoimmunotherapy (PIT), which is a synergistic strategy associated phototherapy with immunotherapy against cancers, demonstrates favorable properties. Near-infrared photoimmunotherapy (NIR-PIT) is dependent on near-infrared (NIR) light, ensuring selectively kill cancer cells while causing no harm to normal tissue.10 This molecularly targeted phototherapy is based on systemically injecting a conjugate of a near-infrared, water-soluble, silicon–phthalocyanine derivative, IRdye700DX (IR700), and a monoclonal antibody (mAb). Because monoclonal antibodies can selectively bind to antigens on cancer cell surfaces, then irradiating NIR light can drives photochemical transformations of the antibody–photoabsorber conjugate (APC) and eventually destruct the cell membrane and cause cell rupture (Figure 1).11–14 Moreover, NIR-PIT can clearly differ from conventional photodynamic therapy (PDT) or photothermal therapy (PTT), which rely on cytotoxic singlet oxygen and hyperthermia, respectively. It mainly activates immunity response through the induction of immunogenic cell death (ICD) of cancer cells to play an anti-cancer role.14,15 Results of the Phase 1/2 clinical trials of NIR-PIT targeting epidermal growth factor receptor (EGFR) against inoperable head and neck squamous cell cancer presents great potential. A global phase 3 clinical trial of that is ongoing. 16 Thus, NIR-PIT appears to be a novel cancer therapy with great potential and remains to be deeply explored. Here we present current evidence on NIR-PIT mechanisms of action and discuss the most recent preclinical studies of NIR-PIT against various effective molecular targets in breast cancers.
Figure 1.
A scheme for demonstrating cellular cytotoxicity induced by NIR-PIT.
Insights into NIR-PIT Mechanism(s) of Action
Highly Specific Cell Killing
A superiority of NIR-PIT compared with other conventional cancer therapies is specificity and selectivity for killing cancer cells resulting from the nature of mAbs. Since the mAbs tends to bind cancer cells that overexpress the targeted cancer-associated antigens, near-infrared light activation at 690 nm choose to kill those cells rather than adjacent normal cells.10 In addition, as a non-ionizing radiation, NIR light does not cause damage to DNA, and non-target cells suffer no toxic effects because its dosage does not obviously alter tissue temperature. Furthermore, by itself, IR700 is a water-soluble photoreactive dye and is not inherently phototoxic; therefore, unconjugated IR700 dissociated from APC is easily excreted in urine without residue.10,17,18 This highly targeted cancer works unless combining the target-specific APC with the tumor-targeted exposure of NIR light. Actually, many patients have showed significant shrinks in tumor size in the clinical trials while experiencing few side effects.
Immunogenic Cell Death (ICD)
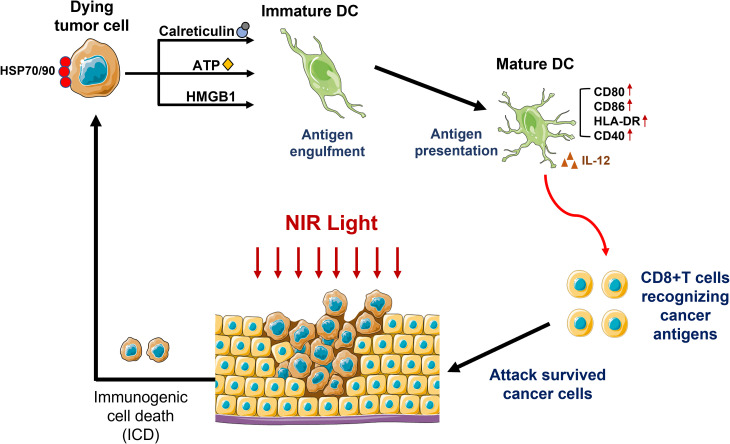
Highly selectively cancer cell death induced by NIR-PIT can evokes multiple tumor-specific immune response. Subsequent deployment of focal NIR-light is invitation to structural changes in APC and some danger signals of membrane destruction. Subsequently, local dendritic cells are initiated, and cancer-specific naïve T cells are stimulated to become mature and proliferate resulting in wider immune response. This process is known as ICD.15,19–21 Moreover, NIR-PIT also rapidly releases various neoantigens which can be recognized and responded by newly primed CD8+ T cells and enhances activation of the adaptive systemic immune response. In addition, the effect is not limited in the NIR-PIT treated tumors, a similar type of tumors located elsewhere in the body also stop growing, which means distant effects.
To further enhance the cancer-targeted NIR-PIT immune response, it is possible to combine conventional systemic immunotherapy. In animal models, the group that received a combination of NIR-PIT and an immune checkpoint inhibitor showed immediate tumor shrinkage and better curation within a few days. Moreover, it was not only presented in situ effects, distant responses such as metastasis also disappeared within days. And the failure in animals that experienced a complete response to re-vaccinate the tumor also suggested that these mice treated with NIR-PIT had acquired immunity against the initial tumor (Figure 2).12,20 Simultaneously, NIR-PIT can take an effective control over immunosuppressive cells in the tumor microenvironment by targeting immunoregulatory cells including Treg and myeloid-derived suppressor cells (MDSCs), contributing to restore normal immune system function and overcome bottlenecks of conventional immunologic drugs including some immune checkpoint inhibitors. Therefore, the combination of NIR-PIT and several adjuvant immunotherapies may be a promising new form of NIR-PIT.
Figure 2.
Schematic presentation of immune response after NIR-PIT.#
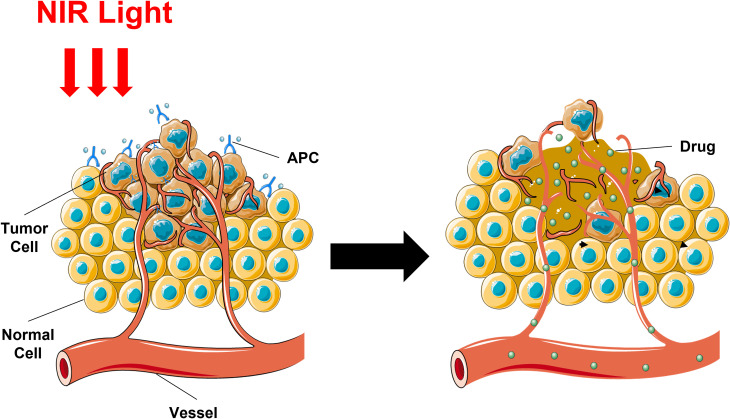
Super-Enhanced Permeability and Retention (SUPR) Effects
The instant effect on tumor vascularity is one of the unique advantages of NIR-PIT. Indeed, a majority of tumors exist vascular leakiness contributing to enhanced permeability and retention (EPR), but NIR-PIT result in a more significant enhancement in vascular permeability because of urgent and enormous cancer cell death adjacent to tumor vessels, a phenomenon named as the super-enhanced permeability and retention (SUPR).22–25 SUFR after NIR-PIT lead to further enhancement of the delivery volume of multiple nano-sized or macromolecular drugs. The core cause is the space enlargement between the blood vessels and the remaining tumor tissues, where the perivascular cancer cells are triggered immediate necrosis and subsequently blood volume and velocity increases and decreases, respectively (Figure 3).
Figure 3.
Schematic demonstration for mechanism of SUPR effects induced by NIR-PIT.#
Therefore, a nano-sized anti-cancer agent combined with NIR-PIT could be more effective than either single therapy.24,26 Other than special drugs, SUPR effects also permit enhanced delivery for other antibodies and APCs, whose intertumoral distribution is improved by enlarged leakage into the tumor bed after NIR-PIT.27–29 A study utilizing FDA approved daunorubicin (DaunoXome) encapsulated with liposome and nanoparticle paclitaxel (nab-paclitaxel; Abraxane) bound with albumin-in mouse xenograft models of cancer, demonstrated that NIR-PIT cooperated with either drug showed greatest advantage.30,31 Furthermore, uncaging reactions induced by NIR light could transmit bioactive compounds encapsuled in particles to any favorable site of the body, illustrating a remarkable chemical progress and prospect of ongoing study.32,33 A research showed that at the absence of NIR, utilizing panitumumab-IR700 (pan-IR700) or CyEt-Panitumumab-Duocarmycin (CyEt-Pan-Duo), a kind of NIR-releasing compound, showed a higher efficiency and more homogeneous distribution in delivered dose because of the SUPR effect, compared with either NIR-PIT or NIR-release therapy alone, which potentially represents a novel combination to achieve stronger antitumor effects.34
The Preclinical Studies of NIR-PIT in Breast Cancers
Recently, several preclinical results have showed latent breakthroughs of NIR-PIT for the treatment of breast cancers, especially TNBC, one of the most malignant tumors in females, whose frequent occurrence of pleural metastasis further worsens patient prognosis.17 The most representative studies are illustrated based on the involved body system, as described in Table 1.
Table 1.
The Main Preclinical Studies Investigating the Potential Breakthroughs of NIR-PIT for Breast Cancers.
| Antigen targeted | Conjugated | Cell lines used in in vivo experiments | Year |
|---|---|---|---|
| EGFR17 | Anti-EGFR (Cetuximab) moAb | MDAMB231, MDAMB468 | 2015 |
| EGFR34 | Anti-EGFR (Panitumumab) moAb | MDA-MB468 | 2018 |
| EGFR35 | Anti-EGFR (Cetuximab) moAb | BT-20, MDA-MB453 | 2021 |
| CD4436 | Anti-CD44 moAb | MDA-MB-231, BT-474 | 2016 |
| HER237 | Anti-HER2 (Trastuzumab) moAb | SKBR3, MDA-MB-231, and MCF7 | 2014 |
| HER238 | Anti-HER2 (Trastuzumab) moAb | BALB/3T3 cells, HCC-1419 cells | 2017 |
| HER239 | HER2 Affibody | SK-BR3, BT474, MDA-MB361 | 2019 |
| HER240 | Anti-HER2 (Trastuzumab) moAb and HER2 Affibody | SK-BR3, MDA-MB361, JIMT1 | 2021 |
| CD20641 | Anti-CD206 moAb | 4T1 | 2016 |
| ICAM-142 | Anti- ICAM-1 moAb | MDAMB468-luc, MDAMB231 cells | 2022 |
| FAP-α43 | Anti-CD44 moAb (AF3715) | MDA-MB-231 | 2022 |
EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor-2; ICAM-1, intercellular adhesion molecule-1; FAP-α, fibroblast activation protein alpha.
EGFR ubiquitously regulates epithelial tissue development and homeostasis with a high overexpression in 70% of TNBCs, so anti-EGFR is an ideal target for NIR-PIT. In this aspect, a remarkable tumor suppression and a prolonged survival rate was presented in athymic nude mice suffering from EGFR-positive human TNBCs with a treatment of cetuximab-IR700 or panitumumab-IR700 and in combination with NIR-light.17,34,35 CyEt-Panitumumab-Duocarmycin degree of labeling 4, CyEt-Pan-Duo, bonds tightly in EGFR receptor expressing in specific cancer cells and subsequently releases duocarmycin after NIR light inducement. Previous researches about NIR-PIT demonstrated enhanced micro-distribution of CyEt-Pan-Duo and therapeutic responses on MDAMB468 tumors with EGFR-expression, illustrating the greatest therapeutic effect of the combination of NIR-PIT and NIR-release therapy.34 Moreover, the comprehensive application of nanotherapy and NIR-PIT is appealing to researchers. A novel type of cetuximab-targeted nanoparticle, named as CTX-AuNR, on the condition of NIR irradiation, could fully exert the abilities of ROS generation and confrontation with hypoxia microenvironment in TAM-embedded BT-20 spheroids, and regulation of reprograming TAM to the anti-tumor M1 phenotype in TME, which brought an inspiration for strengthening therapeutic effects and overcoming conventional drug resistance to patients with EGFR-overexpressing and TAM infiltrated TNBC.35 In addition, a study performed bioluminescence imaging depended on ATP in MDA-MB-468luc cell line with the expression of luciferase and EGFR, to assess the therapeutic effectiveness of different light power and dose of PIT components. Results showed that increasing the dose of Pan-IR700 could offset lowering effects caused by the lessen light power to achieve the equal level of efficacy, which provide a method to reduce cytotoxicity as possible.14
Similarly, CD44, a famous cancer stem cells (CSCs) marker, is a non-kinase transmembrane glycoprotein to mediate uncontrollable cell growth and viciously cell migration, which has been generally acknowledged as a meaningful diagnostic and prognostic biomarker.44,45 And a study demonstrated that either moAbs or small protein mimetic affibodies conjugated to the phthalocyanine dye IRDye700DX could result in the selective injury of breast cancer cells and dramatic reduction of tumor growth when exposed under NIR-light at 690 nm.36
For patients suffering with breast cancers, the incidence of HER2 positivity have been reported ranging from 15% to 20%.45 Nowadays, HER2 is an accepted therapeutic target of breast cancers and trastuzumab has been authorized for treating HER2-positive breast cancer by the FDA.36 However, trastuzumab-dependent therapy has limitations in the clinical application. Firstly, HER2-expressing tumors only accounted for approximately one fourth of primary breast cancers, resulting in a small range application. Moreover, many patients with therapeutic response to trastuzumab at first inevitably develop drug resistance to cause disease progression. Therefore, a research used a adenoviral vector with deficiency in replication, bore a gene that encoded the HER2 extracellular domain (Ad-HER2-ECD). When synergized with trastuzumab-IR700, the vector could transduce HER2-ECD into HER2-negative human cancer cells and selectively killed HER2-negative cells in vitro to further broad the range of clinical application. With the help of viral transduction technology, the indication of antibody-oriented therapy will expand even to the target-negative patients, which represents a potential method to break the limitations of, and overcome resistance to, cancer therapy.37 Moreover, the extent of damage to cell viability depends on NIR-light-intensity and HER2 affibody–IR700 conjugate concentration. The application of affibody molecules may provide unique opportunities in the clinical translation of NIR-PIT because of their rapid tumor clearance and excellent tissue penetration.39 Currently, the combination of NIR-PIT using both HER2 Affibody–IR700Dye conjugate and trastuzumab–IR700Dye conjugate maximized the proportion of necrotic cell death in HER2-positive breast cancer cells and rarely killed HER2-negative breast cancer cells. In addition, it was also exciting that this combination of NIR-PIT exerted significantly therapeutic effects against cancer cells with low expression of HER2, trastuzumab-resistant cells (JIMT1), and brain metastatic cells of breast cancer (MDA-MB361).40 These findings demonstrate that affibody-based PIT is a favorable alternative to moAb-based options, especially for patients with acquired resistance to other conventional anti-HER2 therapies. Due to limits in depth penetration of NIR light, trastuzumab emtansine (T-DM1), an antibody–drug conjugate composed with the monoclonal antibody trastuzumab bonded to the cytotoxic agent maytansinoid DM1, and IR700 plus NIR light treatment, was employed to trigger greater cytotoxic effects for HER2-expressing cells in vitro than T–IR700 plus NIR light treatment, which represents a novel NIR-PIT agent for treating large tumors that always cause failure in sufficient NIR light irradiation to activate the photoabsorber IR700.38
ICAM-1 is a kind of transmembrane proteins as well as a molecule of the Ig superfamily with a function of cell adhesion. TNBC possess higher ICAM-1 expression compared with other subtypes of breast cancers.42,46–48 The application of ICAM-1-targeted NIR-PIT killed TNBC cells in a dose-dependent way in vitro. Furthermore, under the treatment of ICAM-1-targeted NIR-PIT, targeted cells showed early histological signs of cell injury, including widespread cytoplasmic degeneration. The inhabitation of subsequent tumor growth and improvement of survival rate also revealed the destruction of cancer cells caused by NIR-PIT.42
In the process of cancer development, cancer cells are infiltrated by myeloid cells, including tumor-associated macrophages (TAMs), which are recruited from the peripheral blood to the microenvironment of tumor in the shape of monocyte precursor form.49 CD206, named as macrophage mannose receptor, is specifically expressed on the surface of non-inflammatory M2 macrophages on the condition of alternative activation.50–52 A research has reported that a CD206-oriented PIT probe conjugating a monoclonal anti-CD206 antibody with IRDye700, could not only significantly suppress the growth of tumor subcutaneously injected with 4T1 cancer cells resistant to sorafenib, but prevent the metastasis to lungs under NIR exposure, suggesting the potential role of TAM-targeted PIT in the treatment of tumors with congenital or acquaint resistance to conventional drugs.41
Fibroblast activation protein alpha (FAP-α) is a crucial target mainly expressed by cancer-associated fibroblasts (CAFs), exerting the protumorigenic and immune suppressive functions. Compared with adjacent normal tissue, invasive breast cancer tissue had the higher proportion of FAP-α + CAFs. And when co-cultured with cancer cells, FAP-α expression in fibroblasts was further improved. Therefore, a research tried to conjugate AF3715, a kind of antibody with high binding affinity to FAP-α, and the phthalocyanine dye IR700, to achieve a complex, FAP-α-IR700. Subsequently, its effectiveness in specifically reducing FAP-α expressing cell populations with PIT was estimated, with results showing that FAP-α-IR700-PIT effectively inhibited tumor growth, which provide a new promising strategy to eliminate FAP-α + CAFs.43
Limits and Future Perspectives for NIR-PIT
NIR-PIT shows great superiority over conventional cancer treatments. However, some limitations of NIR-PIT remain to be settled. Firstly, NIR-light penetration may restrict the application of NIR-PIT, especially in large tumor sizes, which retard the intraoperative clearance of minimal residual focus or lymph node metastases. Therefore, NIR light exposure through intestines into depth may be an effective method, which also requires the helps of fibreoptical diffusers to realize.53,54 The combinational employment of external and interstitial exposure of NIR light can be readily accessible to clinical practice and realize a more stable and homogeneous light distribution. Secondly, initiatively occurring cancers are not always expressing a single tumor-specific antigen, suggesting the efficiency of simultaneously targeting various antigens. In the current studies, a cocktail of APCs against different tumor antigens could greatly solve this dilemma.55 Thirdly, most studies in vivo have been performed in immunodeficient mice, most commonly athymic nude mice, which still exist a certain gap between clinical researches and animal xenografts. Therefore, what require substantial further exploration are that how host immune cells and systemic antitumor immunity activated by NIR-PIT exactly contribute to the clearance of distant metastases and the prevention of tumor relapse in details. Lastly, despite of the ambiguity of the most suitable treatment domains of NIR-PIT for different types of tumors, it is crucial factors that the expressional level of targeted molecule on the tumor cell surface or distribution of immune cells with anti-tumorigenic or pro-tumorigenic effects in TME, in order to select ideal patients and combinations of NIR-PIT. However, further investigation remains to be explored to identify the best possible combination strategy with NIR-PIT in different tumor types.
Conclusion
Breast cancer is the most common malignant cancer among women both in China and worldwide. For nonmetastatic breast cancer, the main goals of therapy are to clear tumor from the breast and regional lymph nodes as thoroughly as possible, and prevent metastatic recurrence. However, for patients suffering from metastatic breast cancers, therapeutic goals are prolonging life and symptom palliation.4 NIR-PIT is a currently novel type of molecular antigen-oriented and photochemistry-based cancer treatment with the ability of selectively killing tumor cells and highly enhancing the host immune response, including promoting maturation of DCs and initiating cytotoxic T cells to interplay with cancer-related antigens released from destroyed cancer cells. In addition, the systemic antitumor immunity initiated by NIR-PIT contributes to the elimination of distant metastases and the prevention of tumor recurrences. When employed in combination with immunostimulatory therapies, NIR-PIT enable exert more extensive anti-cancer effects and offer a chance to estimate the potential applicability of follow-on or concomitant treatments by both fluorescent imaging and particularly immediate quantitative assessment on antigen release and immune cell activation induced by NIR-PIT. Thus, NIR-PIT is a potential therapeutic approach to breast cancers, indicating a bright development prospect of NIR-PIT.
Acknowledgements
The author would like to thank everyone who helped me during the writing of this paper.
Abbreviations:
- APC
antibody–photoabsorber conjugate
- CSCs
cancer stem cells
- DCs
dendritic cells
- EGFR
epidermal growth factor receptor
- EPR
enhanced permeability and retention
- FAP-α
fibroblast activation protein alpha
- HER2
EGFR type
- ICAM-1
intercellular adhesion molecule-1
- ICD
immunogenic cell death
- IR700
IRDye700DX
- mAb
monoclonal antibodies
- MDSCs
myeloid-derived suppressor cells
- NIR
near-infrared
- PIT
photoimmunotherapy
- PTT
photothermal therapy
- PDT
photodynamic therapy
- SUPR
super-enhanced permeability and retention
- TNBC
triple-negative breast cancer
Footnotes
Contributors: YC and YX wrote the manuscript. YS and YL prepared paper materials. JH designed and produced the figures. XL and JJ outlined and supervised and edited the draft and approved of the final version to be submitted. All authors approved the final version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Statements: This review did not include animal or human studies. Ethics statements are not applicable in this review.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Yingshu Cui https://orcid.org/0000-0001-7978-727X
Xiaosong Li https://orcid.org/0000-0002-5731-8488
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115-132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 2.Liu Q, Song X, Liu Z, Yu Z. Investigation of candidate genes and pathways in basal/TNBC patients by integrated analysis. Technol Cancer Res Treat. 2021;20:15330338211019506. doi: 10.1177/15330338211019506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desmedt C, Ruíz-García E, André F. Gene expression predictors in breast cancer: current status, limitations and perspectives. Eur J Cancer. 2008;44(18):2714-2720. doi: 10.1016/j.ejca.2008.09.011 [DOI] [PubMed] [Google Scholar]
- 4.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22(8):1736-1747. doi: 10.1093/annonc/mdr304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429-4434. doi: 10.1158/1078-0432.CCR-06-3045 [DOI] [PubMed] [Google Scholar]
- 6.Borri F, Granaglia A. Pathology of triple negative breast cancer. Semin Cancer Biol. 2021;72:136-145. doi: 10.1016/j.semcancer.2020.06.005 [DOI] [PubMed] [Google Scholar]
- 7.Paraboschi I, Turnock S, Kramer-Marek G, et al. Near-InfraRed PhotoImmunoTherapy (NIR-PIT) for the local control of solid cancers: challenges and potentials for human applications. Crit Rev Oncol Hematol. 2021;161:103325. doi: 10.1016/j.critrevonc.2021.103325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qu J, Mei Q, Liu L, et al. The progress and challenge of anti-PD-1/PD-L1 immunotherapy in treating non-small cell lung cancer. Ther Adv Med Oncol. 2021;13:1758835921992968. doi: 10.1177/1758835921992968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375-2391. doi: 10.1093/annonc/mdv383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011;17(12):1685-1691. doi: 10.1038/nm.2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato K, Ando K, Okuyama S, et al. Photoinduced ligand release from a silicon phthalocyanine dye conjugated with monoclonal antibodies: a mechanism of cancer cell cytotoxicity after near-infrared photoimmunotherapy. ACS Cent Sci. 2018;4(11):1559-1569. doi: 10.1021/acscentsci.8b00565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi H, Choyke PL. Near-infrared photoimmunotherapy of cancer. Acc Chem Res. 2019;52(8):2332-2339. doi: 10.1021/acs.accounts.9b00273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitsunaga M, Nakajima T, Sano K, Choyke PL, Kobayashi H. Near-infrared theranostic photoimmunotherapy (PIT): repeated exposure of light enhances the effect of immunoconjugate. Bioconjug Chem. 2012;23(3):604-609. doi: 10.1021/bc200648m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakajima T, Sato K, Hanaoka H, et al. The effects of conjugate and light dose on photo-immunotherapy induced cytotoxicity. BMC Cancer. 2014;14:389. doi: 10.1186/1471-2407-14-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura Y, Nagaya T, Sato K, et al. Alterations of filopodia by near infrared photoimmunotherapy: evaluation with 3D low-coherent quantitative phase microscopy. Biomed Opt Express. 2016;7(7):2738-2748. doi: 10.1364/BOE.7.002738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cognetti DM, Johnson JM, Curry JM, et al. Phase 1/2a, open-label, multicenter study of RM-1929 photoimmunotherapy in patients with locoregional, recurrent head and neck squamous cell carcinoma. Head Neck. 2021;43(12):3875-3887. doi: 10.1002/hed.26885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagaya T, Sato K, Harada T, Nakamura Y, Choyke PL, Kobayashi H. Near infrared photoimmunotherapy targeting EGFR positive triple negative breast cancer: optimizing the conjugate-light regimen. PLoS One. 2015;10(8):e0136829. doi: 10.1371/journal.pone.0136829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okuyama S, Nagaya T, Ogata F, et al. Avoiding thermal injury during near-infrared photoimmunotherapy (NIR-PIT): the importance of NIR light power density. Oncotarget. 2017;8(68):113194-113201. doi: 10.18632/oncotarget.20179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato K, Sato N, Xu B, et al. Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy. Sci Transl Med. 2016;8(352):352ra110. doi: 10.1126/scitranslmed.aaf6843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagaya T, Friedman J, Maruoka Y, et al. Host immunity following near-infrared photoimmunotherapy is enhanced with PD-1 checkpoint blockade to eradicate established antigenic tumors. Cancer Immunol Res. 2019;7(3):401-413. doi: 10.1158/2326-6066.CIR-18-0546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogawa M, Tomita Y, Nakamura Y, et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget. 2017;8(6):10425-10436. doi: 10.18632/oncotarget.14425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi H, Choyke PL. Super enhanced permeability and retention (SUPR) effects in tumors following near infrared photoimmunotherapy. Nanoscale. 2016;8(25):12504-12509. doi: 10.1039/c5nr05552k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi H, Watanabe R, Choyke PL. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics. 2013;4(1):81-89. doi: 10.7150/thno.7193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alsaab HO, Alghamdi MS, Alotaibi AS, et al. Progress in clinical trials of photodynamic therapy for solid tumors and the role of nanomedicine. Cancers (Basel). 2020;12(10):2793. doi: 10.3390/cancers12102793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sano K, Nakajima T, Choyke PL, Kobayashi H. The effect of photoimmunotherapy followed by liposomal daunorubicin in a mixed tumor model: a demonstration of the super-enhanced permeability and retention effect after photoimmunotherapy. Mol Cancer Ther. 2014;13(2):426-432. doi: 10.1158/1535-7163.MCT-13-0633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanaoka H, Nagaya T, Sato K, et al. Glypican-3 targeted human heavy chain antibody as a drug carrier for hepatocellular carcinoma therapy. Mol Pharm. 2015;12(6):2151-2157. doi: 10.1021/acs.molpharmaceut.5b00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang CP, Nakajima T, Watanabe R, et al. Real-time monitoring of hemodynamic changes in tumor vessels during photoimmunotherapy using optical coherence tomography. J Biomed Opt. 2014;19(9):98004. doi: 10.1117/1.JBO.19.9.098004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang Q, Nagaya T, Liu Y, et al. 3D mesoscopic fluorescence tomography for imaging micro-distribution of antibody-photon absorber conjugates during near infrared photoimmunotherapy in vivo. J Control Release. 2018;279:171-180. doi: 10.1016/j.jconrel.2018.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Q, Nagaya T, Liu Y, et al. Real-time monitoring of microdistribution of antibody-photon absorber conjugates during photoimmunotherapy in vivo. J Control Release. 2017;260:154-163. doi: 10.1016/j.jconrel.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sano K, Nakajima T, Choyke PL, Kobayashi H. Markedly enhanced permeability and retention effects induced by photo-immunotherapy of tumors. ACS Nano. 2013;7(1):717-724. doi: 10.1021/nn305011p [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanaoka H, Nakajima T, Sato K, et al. Photoimmunotherapy of hepatocellular carcinoma-targeting glypican-3 combined with nanosized albumin-bound paclitaxel. Nanomedicine (Lond). 2015;10(7):1139-1147. doi: 10.2217/nnm.14.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorka AP, Schnermann MJ. Harnessing cyanine photooxidation: from slowing photobleaching to near-IR uncaging. Curr Opin Chem Biol. 2016;33:117-125. doi: 10.1016/j.cbpa.2016.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Šolomek T, Wirz J, Klán P. Searching for improved photoreleasing abilities of organic molecules. Acc Chem Res. 2015;48(12):3064-3072. doi: 10.1021/acs.accounts.5b00400 [DOI] [PubMed] [Google Scholar]
- 34.Nagaya T, Gorka AP, Nani RR, et al. Molecularly targeted cancer combination therapy with near-infrared photoimmunotherapy and near-infrared photorelease with duocarmycin–antibody conjugate. Mol Cancer Ther. 2018;17(3):661-670. doi: 10.1158/1535-7163.MCT-17-0851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emami F, Pathak S, Nguyen TT, et al. Photoimmunotherapy with cetuximab-conjugated gold nanorods reduces drug resistance in triple negative breast cancer spheroids with enhanced infiltration of tumor-associated macrophages. J Control Release. 2021;329:645-664. doi: 10.1016/j.jconrel.2020.10.001 [DOI] [PubMed] [Google Scholar]
- 36.Jin J, Krishnamachary B, Mironchik Y, Kobayashi H, Bhujwalla ZM. Phototheranostics of CD44-positive cell populations in triple negative breast cancer. Sci Rep. 2016;6:27871. doi: 10.1038/srep27871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimoyama K, Kagawa S, Ishida M, et al. Viral transduction of the HER2-extracellular domain expands trastuzumab-based photoimmunotherapy for HER2-negative breast cancer cells. Breast Cancer Res Treat. 2015;149(3):597-605. doi: 10.1007/s10549-015-3265-y [DOI] [PubMed] [Google Scholar]
- 38.Ito K, Mitsunaga M, Nishimura T, et al. Near-infrared photochemoimmunotherapy by photoactivatable bifunctional antibody–drug conjugates targeting human epidermal growth factor receptor 2 positive cancer. Bioconjug Chem. 2017;28(5):1458-1469. doi: 10.1021/acs.bioconjchem.7b00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamaguchi H, Pantarat N, Suzuki T, Evdokiou A. Near-infrared photoimmunotherapy using a small protein mimetic for HER2-overexpressing breast cancer. Int J Mol Sci. 2019;20(23):5835. doi: 10.3390/ijms20235835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamaguchi H, On J, Morita T, et al. Combination of near-infrared photoimmunotherapy using trastuzumab and small protein mimetic for HER2-positive breast cancer. Int J Mol Sci. 2021;22(22):12213. doi: 10.3390/ijms222212213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang C, Gao L, Cai Y, et al. Inhibition of tumor growth and metastasis by photoimmunotherapy targeting tumor-associated macrophage in a sorafenib-resistant tumor model. Biomaterials. 2016;84:1-12. doi: 10.1016/j.biomaterials.2016.01.027 [DOI] [PubMed] [Google Scholar]
- 42.Fukushima H, Kato T, Furusawa A, et al. Intercellular adhesion molecule-1-targeted near-infrared photoimmunotherapy of triple-negative breast cancer. Cancer Sci. 2022. doi: 10.1111/cas.15466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin J, Barnett JD, Krishnamachary B, et al. Evaluating near-infrared photoimmunotherapy for targeting fibroblast activation protein-α expressing cells in vitro and in vivo. Cancer Sci. 2022. doi: 10.1111/cas.1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen C, Zhao S, Karnad A, Freeman JW. The biology and role of CD44 in cancer progression: therapeutic implications. J Hematol Oncol. 2018;11(1):64. doi: 10.1186/s13045-018-0605-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan Y, Zuo X, Wei D. Concise review: emerging role of CD44 in cancer stem cells: a promising biomarker and therapeutic target. Stem Cells Transl Med. 2015;4(9):1033-1043. doi: 10.5966/sctm.2015-0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo P, Huang J, Wang L, et al. ICAM-1 as a molecular target for triple negative breast cancer. Proc Natl Acad Sci USA. 2014;111(41):14710-14715. doi: 10.1073/pnas.1408556111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei H, Wang Z, Kuang Y, et al. Intercellular adhesion molecule-1 as target for CAR-T-cell therapy of triple-negative breast cancer. Front Immunol. 2020;11:573823. doi: 10.3389/fimmu.2020.573823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo P, Yang J, Liu D, et al. Dual complementary liposomes inhibit triple-negative breast tumor progression and metastasis. Sci Adv. 2019;5(3):eaav5010. doi: 10.1126/sciadv.aav5010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Movahedi K, Laoui D, Gysemans C, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70(14):5728-5739. doi: 10.1158/0008-5472.CAN-09-4672 [DOI] [PubMed] [Google Scholar]
- 50.Allavena P, Chieppa M, Bianchi G, et al. Engagement of the mannose receptor by tumoral mucins activates an immune suppressive phenotype in human tumor-associated macrophages. Clin Dev Immunol. 2010;2010:547179. doi: 10.1155/2010/547179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi KM, Kashyap PC, Dutta N, et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138(7):2399-2409.e1. doi: 10.1053/j.gastro.2010.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reeves AR, Spiller KL, Freytes DO, Vunjak-Novakovic G, Kaplan DL. Controlled release of cytokines using silk-biomaterials for macrophage polarization. Biomaterials. 2015;73:272-283. doi: 10.1016/j.biomaterials.2015.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okuyama S, Nagaya T, Sato K, et al. Interstitial near-infrared photoimmunotherapy: effective treatment areas and light doses needed for use with fiber optic diffusers. Oncotarget. 2018;9(13):11159-11169. doi: 10.18632/oncotarget.24329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maruoka Y, Nagaya T, Sato K, et al. Near infrared photoimmunotherapy with combined exposure of external and interstitial light sources. Mol Pharm. 2018;15(9):3634-3641. doi: 10.1021/acs.molpharmaceut.8b00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakajima T, Sano K, Choyke PL, Kobayashi H. Improving the efficacy of photoimmunotherapy (PIT) using a cocktail of antibody conjugates in a multiple antigen tumor model. Theranostics. 2013;3(6):357-365. doi: 10.7150/thno.5908 [DOI] [PMC free article] [PubMed] [Google Scholar]