Abstract
FOXC2, a member of the forkhead box family of transcription factors, is an emerging oncogene that has been linked to several hallmarks of cancer progression. Among its many oncogenic functions is the promotion of drug resistance, with evidence supporting roles for FOXC2 in escape from broad classes of chemotherapeutics across an array of cancer types. In this Mini-Review, we highlight the current understanding of the mechanisms by which FOXC2 drives cancer chemoresistance, including its roles in the promotion of epithelial-mesenchymal transition, induction of multidrug transporters, activation of the oxidative stress response, and deregulation of cell survival signaling pathways. We discuss the clinical implications of these findings, including strategies for modulating FOXC2-associated chemoresistance in cancer. Particular attention is given to ways in which FOXC2 and its downstream gene products and pathways can be targeted to restore chemosensitivity in cancer cells. In addition, the utility of FOXC2 expression as a predictor of patient response to chemotherapy is also highlighted, with emphasis on the value of FOXC2 as a novel biomarker that can be used to guide therapeutic choice towards regimens most likely to achieve clinical benefit during frontline therapy.
Keywords: cancer, biomarker, FOXC2, chemotherapy, drug resistance
Introduction
The forkhead box transcription factor FOXC2 is a well-documented oncogene that is capable of driving several hallmarks of cancer progression.1 A critical regulator of diverse physiologic processes in both embryonic and adult tissues, FOXC2 often becomes dysregulated in cancer cells, where its elevated expression and abnormal subcellular localization are poor prognostic factors for many cancer types.2–4 First recognized as a putative oncogene after it was found to play a central role in the epithelial-mesenchymal transition (EMT) program that precedes cancer cell invasion and metastasis,5 FOXC2 has since been linked to a variety of other oncogenic activities, including tumor cell proliferation, deregulated tumor metabolism, angiogenesis, and drug resistance.1 In this mini-review, we bring special attention to FOXC2's emerging role in cancer chemoresistance, highlighting specific mechanisms by which this transcription factor confers resistance to a range of chemotherapeutic agents. We also discuss strategies to improve the chemosensitivity of cancer cells by interfering with or bypassing FOXC2-associated mechanisms of drug resistance.
FOXC2-Associated Mechanisms of Resistance to Chemotherapy
Dysregulated FOXC2 gene expression in tumor tissue is a poor predictor of patient response to diverse families of chemotherapeutic agents, including various antimetabolites and platinum-based as well as non-platinum-based alkylating agents. Indeed, our recent analysis of RNA-sequencing data from The Cancer Genome Atlas revealed elevated FOXC2 gene expression as a poor prognostic indicator of therapeutic responsiveness for several epithelial- and non-epithelial-derived cancers.4 Additionally, though most tissue microarray studies to date have reported only on the link between FOXC2 protein expression/subcellular localization and standard clinicopathological features,1 one group has reported a correlation between elevated FOXC2 protein expression in non-small-cell lung cancer (NSCLC) tissue and resistance to cisplatin.6 In keeping with these clinical observations, several experimental studies have documented a direct role for FOXC2 in tumor cell resistance to a number of widely used chemotherapy drugs (Table 1). With similar data emerging across an array of cancer types, the link between FOXC2 and chemoresistance has propelled research efforts to better understand the mechanisms of FOXC2-associated drug resistance. Collectively, these studies have revealed diverse processes by which FOXC2 and many of its target gene products act to confer broad protection from a range of chemotherapeutic agents (Figure 1).
Table 1.
Chemotherapeutic Agents for Which FOXC2-driven Resistance is Experimentally Validated.
| Chemotherapeutic | Cell Type | Experimental Findings | References |
|---|---|---|---|
| Doxorubicin | Immortalized HMLE cells | FOXC2 overexpression promoted in vitro resistance | Saxena et al.7 |
| Gastric cancer cell lines (SGC7901 variants) | FOXC2 knockdown enhanced in vitro sensitivity; also improved in vivo responsiveness of patient-derived xenografts | Liu et al.8 | |
| Osteosarcoma cell lines (MG-63, KH-OS) | FOXC2 knockdown enhanced in vitro sensitivity; also improved in vivo responsiveness of patient-derived xenografts | Zhang et al.9 | |
| Epirubicin | Basal-like breast cancer cell line (MDA-MB-231) | FOXC2 knockdown enhanced in vitro sensitivity | Cai et al.10 |
| 5-fluoruracil | Gastric cancer cell lines (SGC7901 variants) | FOXC2 knockdown in gastric cancer cell lines enhanced in vitro sensitivity | Liu et al.8 |
| Basal-like breast cancer cell line (MDA-MB-231) | FOXC2 knockdown enhanced in vitro sensitivity | Cai et al.10 | |
| Colon carcinoma cell line (HCT116) | FOXC2 knockdown enhanced in vitro sensitivity | Yang et al.11 | |
| Cisplatin | Basal-like breast cancer cell line (MDA-MB-231) | FOXC2 knockdown enhanced in vitro sensitivity | Cai et al.10 |
| Ovarian cancer cell line (SKOV3) | FOXC2 overexpression enhanced resistance of cell line in vitro and patient-derived xenograft in vivo; FOXC2 knockdown improved drug sensitivity in both settings | Li et al.12 | |
| NSCLC cell lines (A549, H460, H1299) | FOXC2 knockdown enhanced in vitro sensitivity, whereas FOXC2 overexpression promoted in vitro resistance; FOXC2 knockdown also improved in vivo responsiveness of patient-derived xenografts | He et al.6 | |
| Oxaliplatin | Colon carcinoma cell line (HCT116) | FOXC2 overexpression enhanced in vitro resistance; FOXC2 knockdown improved drug sensitivity in vitro and in patient-derived xenografts in vivo | Chen et al.13 |
| Paclitaxel | Basal-like breast cancer cell line (MDA-MB-231) | FOXC2 knockdown enhanced in vitro sensitivity | Cai et al.10 |
| Nasopharyngeal carcinoma cell lines (CNE2) | FOXC2 knockdown enhanced in vitro sensitivity, whereas FOXC2 overexpression promoted in vitro resistance; FOXC2 knockdown also improved in vivo responsiveness of patient-derived xenografts | Zhou et al.14 | |
| RAS-transformed HMLE cells; HMLE cells induced to undergo EMT | FOXC2 knockdown enhanced in vitro sensitivity; FOXC2 overexpression promoted in vitro resistance | Hollier et al.15 | |
| Docetaxel | Prostate cancer cell lines (LNCaP, DU145) | FOXC2 overexpression promoted in vitro resistance; FOXC2 knockdown enhanced in vitro sensitivity | Paranjape et al.16 |
Abbreviations: NSCLC, non-small-cell lung cancer; EMT, epithelial-mesenchymal transition.
Figure 1.
FOXC2-mediated chemoresistance in cancer. Dysregulation of FOXC2 in cancer cells activates a tumor-promoting transcriptional program that drives several hallmarks of cancer progression. Among the protumor functions of this transcription factor, resistance to chemotherapy is achieved through FOXC2-regulated gene products and pathways that activate, EMT, the ATP-binding cassette (ABC) multidrug transport system, the oxidative stress response, and prosurvival signaling.
Promotion of EMT and Cancer Cell Stemness
Phenotypic plasticity is an emerging hallmark of cancer that is regulated by a number of tumor-intrinsic pathways and surrounding microenvironmental factors. Accumulating evidence supports the significant role for nonmutational epigenetic reprograming as a driver of this plasticity, which is critical to various aspects of tumor progression, including acquisition of drug resistance.17 Of note, the EMT program that drives invasion/metastasis and cellular dedifferentiation toward a cancer stem cell (CSC)-like state is a particularly well-studied driver of phenotypic plasticity and drug resistance in cancer.18 Importantly, as one of several EMT transcription factors, FOXC2 supports this program by regulating the expression and activity of several key EMT genes and gene products. First, FOXC2 is a key mediator of the E-cadherin/N-cadherin switch that is characteristic of this program. In addition to functioning as a direct inducer of the CDH2 gene encoding N-cadherin,19 FOXC2 also acts as an indirect negative regulator of E-cadherin by suppressing expression of p120-ctn, a gene whose protein product otherwise stabilizes membrane expression of E-cadherin on epithelial cells.20,21 The significance of these findings to FOXC2-associated drug resistance is underscored by other studies that have specifically documented increased cisplatin resistance in ovarian cancer cells exhibiting a FOXC2-dependent EMT-like phenotype.12,22 Importantly, one of these studies found that the FOXC2-mediated EMT program in drug-resistant cancer cells was driven by activation of the ERK and AKT signaling pathways. It has also been shown that FOXC2 drives stemness in cells undergoing EMT, and Hollier et al15 reported a link between stem-like properties, paclitaxel resistance, and FOXC2-mediated induction of platelet-derived growth factor receptor β (PDGFRβ) signaling in stem cell-enriched immortalized mammary epithelial cells and CSC-like, claudin-low breast cancer cells. Together, these studies suggest that there may be potential for selectively targeting hyperactive signaling pathways in CSC and cells undergoing EMT as a means of reversing FOXC2-mediated drug resistance (described in more detail below).
Induction of Multidrug Transport Pumps
ATP-binding cassette (ABC) transport pumps comprise a large family of membrane proteins that play significant roles in xeniobiotic detoxification and efflux.23 Often upregulated in multidrug-resistant cancer cells, ABC transporters themselves are particularly challenging to target due to the toxicity associated with inhibitors that also disrupt their normal metabolic and transport functions in healthy cells. As a result, efforts have been made to identify factors that induce ABC transporter expression in cancer cells so that alternative targets that can more specifically limit ABC activity in the context of cancer might be found. In this regard, bioinformatic analyses have shown that promoter sequences for several ABC transporter genes harbor consensus sequences for forkhead-binding domains (Table 2). Consistent with this finding, retroviral overexpression of FOXC2 in both the MCF7 breast cancer cell line and in immortalized human mammary epithelial cells induced expression of many of these genes (including ABCC1, ABCC3, and ABCC5) and drove resistance to the anthracycline drug doxorubicin.7 Another study found that FOXC2 knockdown in human osteosarcoma cells results in loss of ABCG2 expression.24 Our group has also reported reduced Abcb4 gene expression in a gene-edited, FOXC2-deficient murine melanoma model.25 Others have reported anecdotal observations that likewise implicate FOXC2 in the regulation of various ABC transporters. For instance, FOXC2 upregulation in oral cancer cell lines that acquire stem-like properties following exposure to areca nut extract coincides with increased ABCG2 gene expression.26 Similarly, in vivo studies with gastric cancer patient-derived xenografts have shown that treatment with various chemotherapeutics triggers expression of the lncRNA FENDRR, a known positive regulator of FOXC2 expression, and this induction correlated with enhanced expression of ABCB1 in tumor tissue. Though the specific role of the ABCB1 transporter in chemoresistance was not directly evaluated in this study, it is worth noting that FOXC2 expression did drive multidrug resistance against doxorubicin and 5-fluorouracil in both in vitro and in vivo settings.8 In related work, FOXC2 stabilization by another lncRNA, FOXC2-AS1, also led to increased ABCB1 expression and supported doxorubicin resistance in osteosarcoma cells.9,27 Together, these findings highlight the FOXC2-ABC transporter axis as a broad mechanism by which cancer cells develop multidrug chemoresistance during tumor progression.
Table 2.
ATP-Binding Cassette (ABC) Transporter Genes With Promoter Consensus Sequences for Forkhead Binding Domains.
| ABC Transporter gene | # of Forkhead Consensus Sequences in Promotera | Regulation by FOXC2 Validated | Method of Validation | Reference |
|---|---|---|---|---|
| ABCA2 | 2 | ✓ | Induced by overexpression of FOXC2 in MCF7 cells | Saxena et al.7 |
| ABCA3 | 6 | |||
| ABCB1 | 22 | ✓ | Suppressed by FOXC2 knockdown in MG63/DXR and KH-OS/DXR cells | Zhang et al.9 |
| ABCB4 | 7 | ✓ | Diminished expression in FOXC2-deficient, gene-edited B16-F1 cells | Hargadon et al.25 |
| ABCB5 | 13 | |||
| ABCB11 | 12 | |||
| ABCC1 | 12 | ✓ | Induced by overexpression of FOXC2 in MCF7 and HMLE cells | Saxena et al.7 |
| ABCC2 | 13 | |||
| ABCC3 | 2 | ✓ | Induced by overexpression of FOXC2 in MCF7 and HMLE cells | Saxena et al.7 |
| ABCC4 | 3 | ✓ | Induced by overexpression of FOXC2 in MCF7 and HMLE cells | Saxena et al.7 |
| ABCC5 | 9 | ✓ | Induced by overexpression of FOXC2 in MCF7 cells | Saxena et al.7 |
| ABCC6 | 11 | |||
| ABCC10 | 14 | |||
| ABCC11 | 23 | |||
| ABCC12 | 9 | |||
| ABCG2 | 12 | ✓ | Suppressed by FOXC2 knockdown in U2OS cells | Gozo et al.24 |
Data on forkhead binding domain consensus sequences from Saxena et al.7
Regulation of the Oxidative Stress Response
Another means by which FOXC2 facilitates xenobiotic metabolism is through activation of the oxidative stress response. As many chemotherapeutics rely at least in part on mechanisms of action that involve the induction of oxidative stress, FOXC2-mediated detoxification of reactive oxygen species (ROS) is particularly relevant to cancer chemoresistance. Interestingly, ROS accumulation itself enhances the DNA-binding potential and transcriptional activity of FOXC2 by activating SENP3, a redox-sensitive protease that de-SUMOylates the transcription factor at 2 critical lysine residues in the protein's central regulatory domain.19 FOXC2 in turn drives the expression of several genes known to contribute to the oxidative stress response. Indeed, FOXC2 directly activates the expression of the gene encoding HIF-1α,28 a critical regulator of chemoresistance that has specifically been shown to protect cells from oxidative stress-induced apoptosis.29,30 Other oxidative stress response genes that are regulated, either directly or indirectly, by FOXC2 in cancer cells include the oxidoreductase gene NQO1 and genes encoding glutathione S-transferases (Gstk1, Gstm4, Gstm5, Gstt1), glutathione reductase (Gsr), peroxiredoxin 2 (Prdx2), and paraoxonase 3 (Pon3).25,31
Work in non-tumor models also supports important roles for FOXC2 in the oxidative stress response. In macrophages cultured in the presence of oxidized low-density lipoprotein, FOXC2 overexpression was found to enhance glutathione peroxidase activity, reduce ROS accumulation, and protect cells from oxidative stress-induced cell death.32 FOXC2 also alleviated myocardial injury in a rat model of myocardial ischemia-reperfusion by activating the superoxide dismutase genes SOD1 and SOD2, both of which have well-characterized antioxidant functions. This same study also found that oxidative stress-related cellular injury to H9c2 cardiomyocytes following hypoxia/reoxygenation could be alleviated by FOXC2 through its activation of the NRF2/heme oxygenase-1 signaling pathway.33 Though future studies are necessary to determine whether these specific mechanisms also confer resistance to chemotherapy-induced oxidative stress in cancer cells, it is clear that FOXC2 is capable of activating a variety of detoxification mechanisms that have the potential to limit the efficacy of ROS-inducing chemotherapeutics.
Activation of Pro-Survival Cell Signaling Pathways
In addition to its direct influence over drug metabolism and efflux, FOXC2 also interferes with the antitumor activities of various chemotherapies by supporting the activation of pro-survival signaling pathways. Li et al12 found that FOXC2-mediated activation of both the PI3K-AKT and MAPK pathways drives ovarian cancer resistance to cisplatin. FOXC2 expression levels did not influence the overall expression of AKT or ERK but did increase the extent of their phosphorylation, which was accompanied by elevated expression of the anti-apoptotic factor BCL-2 and downregulation of the pro-apoptotic factors BAX and cleaved caspase 3. This FOXC2-associated shift in apoptosis-regulating factors in cisplatin-resistant cells was dependent on the activation of the PI3K-AKT and MAPK pathways, as these effects were completely reversed when cells were treated with AKT and MAPK/ERK inhibitors. Similar results have also been reported in models of NSCLC and colorectal cancer, where AKT- and/or MAPK-dependent resistance to cisplatin, oxaliplatin, and 5-fluorouracil has been found to be mediated by FOXC2.6,11,13 Though the mechanism(s) by which FOXC2 promotes MAPK signaling has yet to be elucidated, insight into FOXC2-dependent activation of PI3K-AKT signaling was recently provided by Lin et al,34 who found that FOXC2 binds directly to the promoter of the STC1 gene encoding stanniocalcin 1, a glycoprotein that interacts with the integrin ITGB6 to stimulate PI3K signaling. Of note, silencing of either STC1 or ITGB6 expression in ovarian cancer cell lines increased their sensitivity to cisplatin, highlighting stanniocalcin 1 and ITGB6 as important intermediaries in a FOXC2-STC1-ITGB6-PI3K signaling axis that promotes cancer chemoresistance.
Strategies to Combat FOXC2-driven Chemoresistance in Cancer
Insight into the prognostic value of FOXC2 expression in cancer in recent years has highlighted the pervasive significance of this transcription factor as a correlate of chemoresistance and cancer progression. These findings have important implications for cancer therapy going forward, offering a guide to new strategies that aim either to overcome or to bypass FOXC2-mediated drug resistance in cancer cells (Figure 2).
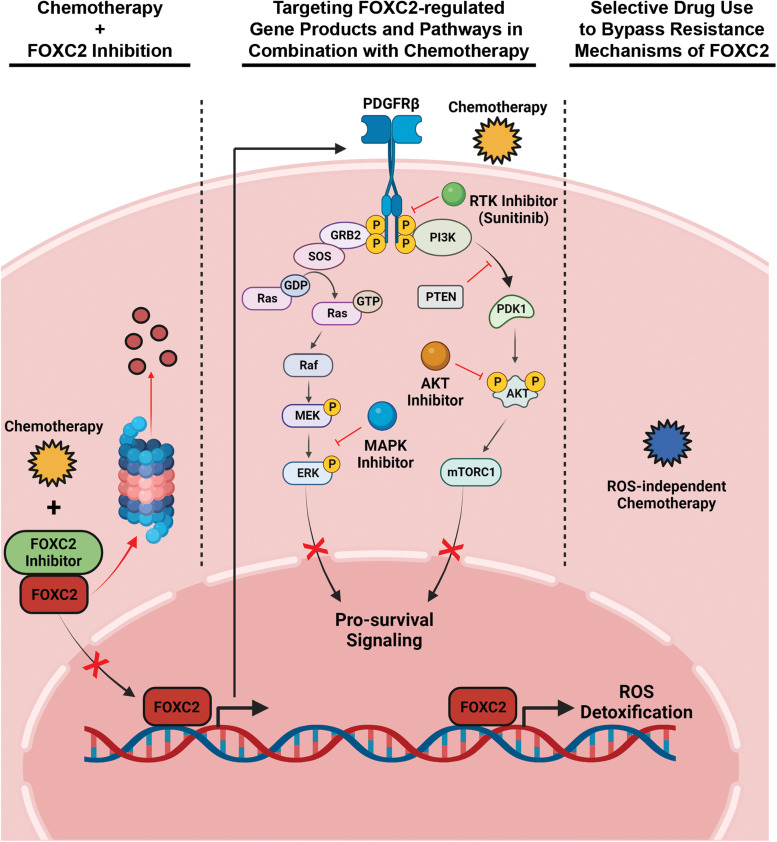
Figure 2.
Overcoming FOXC2-mediated chemoresistance in cancer. Mechanistic insights into FOXC2's regulation of chemotherapy resistance suggest several strategies for improving drug sensitivity in cancer cells. Chemosensitivity may be increased by directly targeting FOXC2 with molecular inhibitors such as MC-1-F2, which interferes with FOXC2 nuclear localization and promotes proteasomal degradation (left). Alternatively, FOXC2-regulated gene products or pathways can be targeted to support responsiveness to chemotherapy, as has been achieved with RTK, MAPK, and AKT inhibitors (middle). Finally, FOXC2-mediated resistance mechanisms can be bypassed in cancer cells by screening biopsy samples for overexpression of this transcription factor and selectively employing chemotherapeutics whose mode of action is independent of the biological consequences triggered by hyperactive FOXC2 (right).
Targeting the FOXC2 Transcription Factor to Restore Cancer Chemosensitivity
Based on FOXC2's ability to drive chemoresistance by diverse mechanisms, one approach to restore the chemosensitivity of cancer cells expressing this transcription factor is to couple standard chemotherapy regimens with an agent that specifically targets FOXC2 itself. An attractive prospect, therapeutically targeting transcription factors has historically proven difficult for a number of reasons. First, unlike the deep pockets of enzyme active sites that have traditionally been the focal point of targeted therapies, the more flattened and heavily charged surfaces that mediate the critical protein–protein and protein–DNA interactions of transcription factors are challenging topologies to target with small molecule inhibitors. Nevertheless, several strategies for inhibiting transcription factors in the context of cancer have emerged in recent years,35,36 and FOXC2 is now one of an increasing number of transcription factors for which targeted drugs have been developed. MC-1-F2 is a small molecule inhibitor of FOXC2 recently described by Castaneda et al,37 who reported several antitumor functions of the compound in breast cancer cell lines expressing high levels of FOXC2. Specifically, MC-1-F2 treatment impaired nuclear localization of FOXC2 and triggered its proteasomal degradation. In various in vitro assays, these effects led to decreases in cancer cell viability, colony-forming capacity, migration, and invasion. In addition to these functional consequences, exposure to this inhibitor also promoted a phenotypic reversal of properties normally associated with EMT and CSC.
A second challenge associated with targeting transcription factors for therapeutic purposes arises from the fact that these proteins often regulate a vast array of target genes. As such, there is concern that drugs that disrupt such pleiotropic functions may have unintended and deleterious consequences in healthy tissues. In this regard, the fact that MC-1-F2 shows minimal activity in breast cancer cells that express only low levels of FOXC2 suggests that this inhibitor may exhibit little to no toxicity in cells with steady-state levels of FOXC2, making this drug potentially well-suited for selective use against cancers with elevated FOXC2 expression.37 Though the drug has yet to be evaluated in vivo, and though it has not been assessed for its impact on cancer cell chemosensitivity to date, current data suggest that MC-1-F2 may indeed have a tolerable safety profile, and it is reasonable to expect that this inhibitor will interfere at least to some degree with FOXC2-dependent drug resistance mechanisms. Therefore, future studies that assess whether this and related drugs might improve cancer responsiveness to chemotherapy are warranted, and it will be interesting to assess whether such combinatorial approaches might even reduce chemotherapy-associated toxicities by lowering the dosing threshold required for certain chemotherapeutics to achieve their anticancer effects.
Finally, the functional redundancy of many transcription factors has the potential to compromise the efficacy of therapeutic strategies targeting any one specific protein. Functional redundancy has indeed been reported among the 50 members of the FOX transcription factor family in humans. While it is not always the case, this redundancy is most typically seen across members of specific subfamilies, though, and there are only 2 such members (FOXC1 and FOXC2) within the FOXC subfamily.38 Nevertheless, FOXC1 dysregulation in cancer has indeed been associated with many of the same oncogenic properties of FOXC2, including being implicated in the acquisition of drug resistance.39–42 Therefore, comprehensive expression profiling of both FOXC1 and FOXC2 (as well as other FOX family members that have been linked to drug-resistant phenotypes) may be necessary to determine whether targeting FOXC2 specifically is likely to achieve any clinical benefit.
Targeting FOXC2-regulated Gene Products and Pathways to Restore Cancer Chemosensitivity
As an alternative to directly targeting FOXC2 itself, it is also possible to target many of the gene products and pathways regulated by this transcription factor. Indeed, molecular insights into the basic biology of FOXC2 have revealed a number of potential downstream targets that are already “druggable” with FDA-approved small molecule inhibitors. For instance, sunitinib is an FDA-approved inhibitor of PDGFRβ, a growth factor receptor directly regulated by FOXC2, and this drug reduces both primary tumor outgrowth and metastatic burden in mice challenged with FOXC2-expressing tumor cells.15 Though this study did not evaluate whether sunitinib treatment of FOXC2-expressing tumors might also enhance chemotherapeutic efficacy, other studies have found that targeting FOXC2-activated pathways does enhance cancer chemosensitivity. For instance, targeting the PI3K-AKT pathway with an AKT inhibitor enhances the sensitivity of FOXC2-overexpressing lung adenocarcinoma cells to cisplatin,6 and a MAPK/ERK inhibitor has similarly been shown to enhance the responsiveness of FOXC2-overexpressing colorectal cancer cells to oxaliplatin.13 Collectively, these studies highlight the potential for synergism between chemotherapy and targeted regimens that specifically antagonize FOXC2-activated pathways. As we continue to advance this era of precision oncology, selective use of such combinatorial regimens may prove particularly beneficial against cancers screened for elevated FOXC2 expression.
Using FOXC2 Expression as a Biomarker to Guide Therapeutic Choice
As an alternative to those approaches that aim to overcome FOXC2-mediated drug resistance, insight into FOXC2 expression levels in tumor biopsy specimens may at the very least serve as a useful biomarker to inform therapeutic choice for clinicians. Several studies have documented the prognostic significance of FOXC2 RNA and protein expression levels for patient response to particular chemotherapeutics,1,4 and these data can guide oncologists toward regimens for which FOXC2 is not associated with resistance. For example, because FOXC2 has been shown to confer resistance to several drugs that rely at least in part on the induction of oxidative stress, patients whose tumors express high levels of FOXC2 might respond better to frontline treatment with chemotherapies that achieve anti-cancer activity by ROS-independent mechanisms of action. In this way, rather than overcoming FOXC2-associated mechanisms of drug resistance, such an approach may instead bypass FOXC2-mediated resistance to specific chemotherapies, thus increasing the likelihood of a successful response to frontline treatment.
Conclusions
Since it was first described as an oncogenic transcription factor in 2007,5 FOXC2 has emerged as a critical regulator of several hallmarks of cancer progression.1 In addition to supporting processes that include tumor cell proliferation, metastasis, and metabolic adaptation, FOXC2's ability to drive resistance to broad classes of chemotherapeutics is particularly problematic in the clinical management of cancer. Importantly, work over the last decade has provided useful insight into the genes and pathways regulated by this oncogenic transcription factor, and these studies have revealed diverse mechanisms by which FOXC2 mediates cancer chemoresistance. These research efforts are now paving the way for novel approaches to enhance cancer cell chemosensitivity, either by interfering with or by bypassing FOXC2-associated mechanisms of drug resistance. Going forward, preclinical and clinical studies designed to evaluate the therapeutic strategies described herein will improve our ability to (1) target FOXC2 and its associated pathways as part of combinatorial regimens with chemotherapy and (2) utilize FOXC2 as a predictive biomarker to guide appropriate treatment at the time of diagnosis. Together, advances in these areas of precision medicine are likely to improve clinical outcomes for many cancer patients in the future.
Acknowledgements
BioRender software was used for figure preparation.
Abbreviations
- ABC
ATP-binding cassette
- CSC
cancer stem cell
- EMT
epithelial-mesenchymal transition
- NSCLC
non-small-cell lung cancer
- PDGFRβ
platelet-derived growth factor receptor β
- ROS
reactive oxygen species
Footnotes
Author Contributions: Kristian M. Hargadon handeled the conceptualization, literature review, manuscript organization, and writing. Elijah W. Strong handeled the conceptualization, literature review, and manuscript organization.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a Mary Louise Andrews Award for Cancer Research from the Virginia Academy of Science.
ORCID iD: Kristian M. Hargadon https://orcid.org/0000-0002-8668-5228
References
- 1.Hargadon KM, Goodloe TB, Lloyd ND. Oncogenic functions of the FOXC2 transcription factor: a hallmarks of cancer perspective. Cancer Metastasis Rev. 2022;41(4):833-852. doi: 10.1007/S10555-022-10045-3. [DOI] [PubMed] [Google Scholar]
- 2.Kume T, Shackour T. Meta-analysis of the likelihood of FOXC2 expression in early and late-stage tumors. Oncotarget. 2018;9(70):33396-33402. doi: 10.18632/oncotarget.26087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu B, Tian Y, Liu L. Meta-analysis of the prognostic significance of FOXC2 in various tumors. J Int Med Res. 2020;48(3):300060519891648. doi: 10.1177/0300060519891648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hargadon KM, Győrffy B, Strong EW. The prognostic significance of FOXC2 gene expression in cancer: a comprehensive analysis of RNA-seq data from the cancer genome atlas. Cancer Genet. 2021;254–255:58-64. doi: 10.1016/J.CANCERGEN.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Mani SA, Yang J, Brooks M, et al. Mesenchyme forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci USA. 2007;104(24):10069-10074. doi: 10.1073/pnas.0703900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Y, Xie H, Yu P, Jiang S, Wei L. FOXC2 promotes epithelial–mesenchymal transition and cisplatin resistance of non-small cell lung cancer cells. Cancer Chemother Pharmacol. 2018;82(6):1049-1059. doi: 10.1007/s00280-018-3697-2. [DOI] [PubMed] [Google Scholar]
- 7.Saxena M, Stephens MA, Pathak H, Rangarajan A. Transcription factors that mediate epithelial-mesenchymal transition lead to multidrug resistance by upregulating ABC transporters. Cell Death Dis. 2011;2(7):e179. doi: 10.1038/cddis.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H, Zhang Z, Han Y, et al. The FENDRR/FOXC2 axis contributes to multidrug resistance in gastric cancer and correlates with poor prognosis. Front Oncol. 2021;11:634579. doi: 10.3389/FONC.2021.634579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang C-L, Zhu K-P, Ma X-L. Antisense lncRNA FOXC2-AS1 promotes doxorubicin resistance in osteosarcoma by increasing the expression of FOXC2. Cancer Lett. 2017;396:66-75. doi: 10.1016/j.canlet.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Cai J, Tian A-X, Wang Q-S, et al. FOXF2 suppresses the FOXC2-mediated epithelial–mesenchymal transition and multidrug resistance of basal-like breast cancer. Cancer Lett. 2015;367(2):129-137. doi: 10.1016/j.canlet.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Yang C, Cui X, Dai X, Liao W. Downregulation of Foxc2 enhances apoptosis induced by 5-fluorouracil through activation of MAPK and AKT pathways in colorectal cancer. Oncol Lett. 2016;11(2):1549-1554. doi: 10.3892/ol.2016.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C, Ding H, Tian J, et al. Forkhead box protein C2 (FOXC2) promotes the resistance of human ovarian cancer cells to cisplatin in vitro and in vivo. Cell Physiol Biochem. 2016;39(1):242-252. doi: 10.1159/000445620. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Deng G, Fu Y, et al. FOXC2 promotes oxaliplatin resistance by inducing epithelial-mesenchymal transition via MAPK/ERK signaling in colorectal cancer. Onco Targets Ther. 2020;13:1625-1635. doi: 10.2147/OTT.S241367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Z, Zhang L, Xie B, et al. FOXC2 promotes chemoresistance in nasopharyngeal carcinomas via induction of epithelial mesenchymal transition. Cancer Lett. 2015;363(2):137-145. doi: 10.1016/j.canlet.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Hollier BG, Tinnirello AA, Werden SJ, et al. FOXC2 Expression links epithelial-mesenchymal transition and stem cell properties in breast cancer. Cancer Res. 2013;73(6):1981-1992. doi: 10.1158/0008-5472.CAN-12-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paranjape AN, Soundararajan R, Werden SJ, et al. Inhibition of FOXC2 restores epithelial phenotype and drug sensitivity in prostate cancer cells with stem-cell properties. Oncogene. 2016;35(46):5963-5976. doi: 10.1038/onc.2015.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31-46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 18.Shibue T, Weinberg RA. EMT CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14(10):611-629. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren YH, Liu KJ, Wang M, et al. De-SUMOylation of FOXC2 by SENP3 promotes the epithelial-mesenchymal transition in gastric cancer cells. Oncotarget. 2014;5(16):7093-7104. doi: 10.18632/ONCOTARGET.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mortazavi F, An J, Dubinett S, Rettig M. p120-catenin is transcriptionally downregulated by FOXC2 in non-small cell lung cancer cells. Mol Cancer Res. 2010;8(5):762-774. doi: 10.1158/1541-7786.MCR-10-0004. [DOI] [PubMed] [Google Scholar]
- 21.Pham TND, Perez White BE, Zhao H, Mortazavi F, Tonetti DA. Protein kinase C α enhances migration of breast cancer cells through FOXC2-mediated repression of p120-catenin. BMC Cancer. 2017;17(1):832. doi: 10.1186/s12885-017-3827-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, Ding H, Tian J, et al. Forkhead box protein C2 promotes epithelial-mesenchymal transition, migration and invasion in cisplatin-resistant human ovarian cancer cell line (SKOV3/CDDP). Cell Physiol Biochem. 2016;39(3):1098-1110. doi: 10.1159/000447818. [DOI] [PubMed] [Google Scholar]
- 23.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48-58. doi: 10.1038/NRC706. [DOI] [PubMed] [Google Scholar]
- 24.Gozo MC, Jia D, Aspuria P-J, et al. FOXC2 augments tumor propagation and metastasis in osteosarcoma. Oncotarget. 2016;7(42):68792-68802. doi: 10.18632/oncotarget.11990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hargadon KM, Györffy B, Strong EW, et al. The FOXC2 transcription factor promotes melanoma outgrowth and regulates expression of genes associated with drug resistance and interferon responsiveness. Cancer Genomics Proteomics. 2019;16(6):491-503. doi: 10.21873/cgp.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li YC, Chang JT, Chiu C, et al. Areca nut contributes to oral malignancy through facilitating the conversion of cancer stem cells. Mol Carcinog. 2016;55(5):1012-1023. doi: 10.1002/MC.22344. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C-L, Zhu K-P, Shen G-Q, Zhu Z-S. A long non-coding RNA contributes to doxorubicin resistance of osteosarcoma. Tumor Biol. 2016;37(2):2737-2748. doi: 10.1007/S13277-015-4130-7/METRICS. [DOI] [PubMed] [Google Scholar]
- 28.Liu M, Zhong J, Zeng Z, et al. Hypoxia-induced feedback of HIF-1α and lncRNA-CF129 contributes to pancreatic cancer progression through stabilization of p53 protein. Theranostics. 2019;9(16):4795-4810. doi: 10.7150/THNO.30988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samanta D, Gilkesa DM, Chaturvedia P, Xiang L, Semenza GL. Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proc Natl Acad Sci USA. 2014;111(50):E5429-E5438. doi: 10.1073/PNAS.1421438111/SUPPL_FILE/PNAS.201421438SI.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Li HS, Zhou YN, Li L, et al. HIF-1α protects against oxidative stress by directly targeting mitochondria. Redox Biol. 2019;25:101109. doi: 10.1016/J.REDOX.2019.101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hargadon KM, Williams CJ. RNA-seq analysis of wild-type vs. FOXC2–deficient melanoma cells reveals a role for the FOXC2 transcription factor in the regulation of multiple oncogenic pathways. Front Oncol. 2020;10:267. doi: 10.3389/fonc.2020.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L, Li T, Zha L. Foxc2 alleviates ox-LDL-induced lipid accumulation, inflammation, and apoptosis of macrophage via regulating the expression of Angptl2. Inflammation. 2020;43(4):1397-1410. doi: 10.1007/S10753-020-01217-W. [DOI] [PubMed] [Google Scholar]
- 33.Wang R, Wu Y, Jiang S. FOXC2 alleviates myocardial ischemia-reperfusion injury in rats through regulating Nrf2/HO-1 signaling pathway. Dis Markers. 2021;2021:9628521. doi: 10.1155/2021/9628521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin F, Li X, Wang X, Sun H, Wang Z, Wang X. Stanniocalcin 1 promotes metastasis, lipid metabolism and cisplatin chemoresistance via the FOXC2/ITGB6 signaling axis in ovarian cancer. J Exp Clin Cancer Res. 2022;41(1):129. doi: 10.1186/S13046-022-02315-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bushweller JH. Targeting transcription factors in cance – from undruggable to reality. Nat Rev Cancer. 2019;19(11):611-624. doi: 10.1038/s41568-019-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen A, Koehler AN. Transcription factor inhibition: lessons learned and emerging targets. Trends Mol Med. 2020;26(5):508-518. doi: 10.1016/J.MOLMED.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castaneda M, Chen L, Pradhan L, et al. A forkhead box protein C2 inhibitor: targeting epithelial-mesenchymal transition and cancer metastasis. Chembiochem. 2018;19(13):1359-1364. doi: 10.1002/cbic.201800022. [DOI] [PubMed] [Google Scholar]
- 38.Benayoun BA, Caburet S, Veitia RA. Forkhead transcription factors: key players in health and disease. Trends Genet. 2011;27(6):224-232. doi: 10.1016/J.TIG.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Lu YT, Xu T, Iqbal M, et al. FOXC1 binds enhancers and promotes cisplatin resistance in bladder cancer. Cancers (Basel). 2022;14(7):1717. doi: 10.3390/CANCERS14071717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng C, Li P, Yang M, Chen D, Huang Y. FOXC1 Knockdown reverses gefitinib resistance in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2021;24(8):538-547. doi: 10.3779/J.ISSN.1009-3419.2021.103.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar U, Hu Y, Masrour N, et al. MicroRNA-495/TGF-β/FOXC1 axis regulates multidrug resistance in metaplastic breast cancer cells. Biochem Pharmacol. 2021;192:114692. doi: 10.1016/J.BCP.2021.114692. [DOI] [PubMed] [Google Scholar]
- 42.Hsu HH, Kuo WW, Shih HN, et al. FOXC1 regulation of miR-31-5p confers oxaliplatin resistance by targeting LATS2 in colorectal cancer. Cancers (Basel). 2019;11(10):1576. doi: 10.3390/CANCERS11101576. [DOI] [PMC free article] [PubMed] [Google Scholar]