Abstract
Background
Pembrolizumab has been shown to be effective and safe in improving the survival of patients with advanced non-small-cell lung cancer (NSCLC). However, the effectiveness and safty of pembrolizumab in the induction treatment of patients with potential resectable clinical stage III NSCLC remains undetermined.
Methods
A total of 25 patients who received neoadjuvant pembrolizumab plus chemotherapy for preoperative stage III NSCLC between August 2020 and November 2021 in Zhongshan Hospital were retrospectively evaluated, and 21 of them were followed by pulmonary resection. The neoadjuvant treatment was as follows: intravenous pembrolizumab (200 mg) on day 1, carboplatin [target area under the curve (AUC) 5 mg/mL] or cisplatin (75 mg/m2) on day 1, and pemetrexed (500 mg/m2 for adenocarcinoma) or nab-paclitaxel (260 mg/m2 for other subtypes) on day 1 of every 21-day cycle up to two or three cycles.
Results
The mean age of all 25 patients was 65 years, of whom 22 were men and 3 were women. Seventeen were diagnosed before treatment as clinical stage IIIA, seven as IIIB, and one as IIB. All received neoadjuvant immunotherapy plus chemotherapy. Following induction therapy, 21 patients with stable disease or partial response (PR) according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) underwent surgical resection without delay. Among the patients who underwent operation, major pathological response (MPR) was achieved in 13 patients, including 6 (28.6%) patients achieved a complete pathological response (CPR). Two patients with partial radiologic remission refused operative treatment, one had progressive disease (PD), and another developed a grade immune pneumonia and could not tolerate surgery. However, none of the adverse events caused surgery delays or deaths.
Conclusions
Neoadjuvant pembrolizumab plus chemotherapy could be considered reliable for clinical stage III NSCLC, but needs to be validated with more robust clinical trials.
Keywords: Non-small-cell lung cancer (NSCLC), neoadjuvant therapy, major pathological response (MPR), complete pathological response (CPR), treatment-related adverse event (trAE)
Highlight box.
Key findings
• Neoadjuvant pembrolizumab combined with chemotherapy is safe and effective for c-stage III NSCLC.
What is known and what is new?
• Neoadjuvant immunotherapy has shown good results in the treatment of NSCLC patients.
• There is a lack of clinical study results related to the exercise of pembrolizumab for the neoadjuvant treatment of NSCLC. We retrospectively analyzed the good efficacy and safety of pembrolizumab in combination with chemotherapy for stage III NSCLC.
What is the implication, and what should change now?
• Neoadjuvant pembrolizumab combined with chemotherapy can be considered safe and effective, with encouraging pathological responses in locally advanced NSCLC. Adequately powered trials to clinically verify the meaningful benefits are awaited.
Introduction
Lung cancer is the principal causation for death globally, and nearly 85% of lung cancers are non-small-cell lung cancer (NSCLC) (1). A significant proportion of patients with NSCLC have locally advanced clinical stage III disease (c-III) at the time of diagnosis (2). Outcomes for this subset of patients remained generally poor over the past few decades (3). Neoadjuvant chemotherapy followed by surgical resection with or without adjuvant chemotherapy and thoracic radiotherapy in selected cases has become the standard treatment for patients resectable with this stage of NSCLC. Chemotherapy or chemoradiotherapy remains the conventional option for preoperative induction therapy. However, only a modest (approximately 5%) increase in 5-year overall survival (OS) has been achieved after perioperative (neoadjuvant or adjuvant) chemotherapy. Moreover, the combination of radiotherapy did not improve the prognosis, but increased the chance of complications (4,5).
Nowadays, immune checkpoint inhibitors (ICIs), such as programmed cell death protein 1 (PD-1) and programmed cell death-ligand 1 (ligand PD-L1) inhibitors, have been approved as first-line treatment for metastatic NSCLC patients, either as monotherapy or combined with chemotherapy. Recently, immunotherapy has been assessed for the early-stage curative treatment of NSCLC. As a neoadjuvant treatment, ICIs could stimulate the priming and expansion of neoantigen-specific T cells in the tumor before surgical resection, bringing benefits in long-term tumor control (6,7). Forde and colleagues showed that two cycles of neoadjuvant nivolumab were well tolerated and resulted in a major pathological response (MPR) rate of 45.0% and a complete pathological response (CPR) rate of 10.0% (8). Furthermore, the use of ICIs combined with chemotherapy followed by surgery appear to achieve higher MPR rates in patients with stage IB–IIIA NSCLC (9), probably because of the synergistically boosted effects of chemotherapy in response to ICIs. Pembrolizumab, a humanized IgG4 antibody targeting PD-1, either used alone or in combination with chemotherapy has displayed flexible reliability and anti-tumor action in patients with advanced NSCLC (10). However, pembrolizumab's capacity as a neoadjuvant drug in NSCLC has not been well analyzed. The objective of the study was to evaluate the safety and feasibility of neoadjuvant pembrolizumab plus chemotherapy in patients with potentially operable c-stage-III NSCLC, examining the pathological response and tolerability of this treatment. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-871/rc).
Methods
We retrospectively collected the data of locally advanced NSCLC patients who received neoadjuvant pembrolizumab and chemotherapy at Zhongshan Hospital between August 2020 and November 2021. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The present research was authorized by the Research Ethics Committee of Zhongshan Hospital (No. B2021-128), and written informed consent was acquired from every patients. All eligible patients had tumor staging (TNM 8th edition) at baseline, including diagnostic biopsy and pathological evaluation [endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA)] of mediastinal lymph nodes. In addition, all patients underwent positron emission tomography-computed tomography (PET-CT) and chest enhancement CT or head magnetic resonance imaging (MRI). The tumor response was assessed after two or three cycles of therapy pre-operation. Change in tumor dimension was assessed based on the Response Evaluation Criteria in Solid Tumors (RECIST). The pathological result was assessed by two pathologists and they estimated the residual viability of the primary tumor from each patient. Treatment-related adverse events (trAEs) were classified based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Patients were treated with intravenous pembrolizumab (200 mg) on day 1, carboplatin (target area under the curve 5 mg/mL) or cisplatin (75 mg/m2) on day 1, and pemetrexed (500 mg/m2 for adenocarcinoma) or nab-paclitaxel (260 mg/m2 for other subtypes) on day 1 of every 21-day cycle up to two or three cycles. Patients without disease progression underwent surgery (response of primary tumor and mediastinal lymph node) 2–5 weeks after the first day of the last cycle of therapy. Video-assisted thoracoscopic surgery (VATS) or thoracotomy was chosen according to the preference of surgeon.
The efficacy of neoadjuvant treatment was assessed as follows: (I) MPR was defined as the presence of 10% or fewer viable tumor cells in the primary tumor; (II) incomplete pathological response (IPR) was defined as more than 10% viable tumor cells residual in the primary tumor; (III) CPR was defined as no any viable tumor cells in the resected tumor and removed lymph nodes (11).
The primary endpoint of this retrospective research was the response of neoadjuvant pembrolizumab and chemotherapy in c-III NSCLC. The secondary endpoint was to assess the safety and trAEs of this treatment regimen.
Statistical analysis
We continuously monitor patients for adverse effects, radiological and pathological outcomes. Continuous variables are described by mean ± standard deviation (SD) or median as appropriate, while categorical variables are described by counts and percentages.
Results
Twenty-five eligible patients were enrolled in this study. The baseline characteristics of the patients are listed in Table 1. All patients (22 men and 3 women) had no comorbidities. Among them, 17 (68.0%) were diagnosed as clinical stage IIIA, seven (28.0%) as IIIB, and one (4.0%) as IIB. Five (20.0%) patients had adenocarcinoma, 16 (64.0%) had squamous-cell carcinoma, two (8.0%) had adenosquamous carcinoma, one (4.0%) had large-cell neuroendocrine carcinoma, and one (4.0%) had sarcomatoid carcinoma.
Table 1. Baseline characteristics.
| Characteristic | Patients (n=25) |
|---|---|
| Age (y) | 65 [42–74] |
| Sex, n (%) | |
| Male | 22 (88.0) |
| Female | 3 (12.0) |
| Smoking status, n (%) | |
| Yes | 9 (36.0) |
| No | 16 (64.0) |
| Histology, n (%) | |
| Adenocarcinoma | 5 (20.0) |
| Squamous cell cancer | 16 (64.0) |
| Large cell neuroendocrine carcinoma | 1 (4.0) |
| Adenosquamous carcinoma | 2 (8.0) |
| Sarcomatoid carcinoma | 1 (4.0) |
| Stage at diagnosis, n (%) | |
| IIB | 1 (4.0) |
| IIIA | 17 (68.0) |
| IIIB | 7 (28.0) |
| Tumor lesion size (mm) | 47 [21–80] |
| Clinical nodal status, n (%) | |
| N0 | 3 (12.0) |
| N1 | 1 (4.0) |
| N2 | 21 (84.0) |
| Treatment cycles, n (%) | |
| 2 cycles | 15 (60.0) |
| 3 cycles | 9 (36.0) |
| Unfinished# | 1 (4.0) |
Data are n (%) or median [IQR]. #, failure to complete the neoadjuvant therapy cycle due to severe treatment-related complications; IQR, interquartile range.
Of the 25 patients assessed radiographically according to the RECIST criteria, 20 (80.0%) achieved a partial response (PR) (Figure 1), three (12.0%) had stable disease, one (4.0%) had progressive disease (PD) during neoadjuvant treatment, and one (4.0%) could not be assessed because of interstitial pneumonia. Twenty-one (84.0%) patients voluntarily agreed to surgery. Of the remaining patients, one had PD, and two had a PR but declined surgery, preferring to continue immunotherapy as a maintenance. Another patient could not complete neoadjuvant therapy because of trAEs (interstitial pneumonia) but remained stable after drug withdrawal. There were no treatment-related deaths.
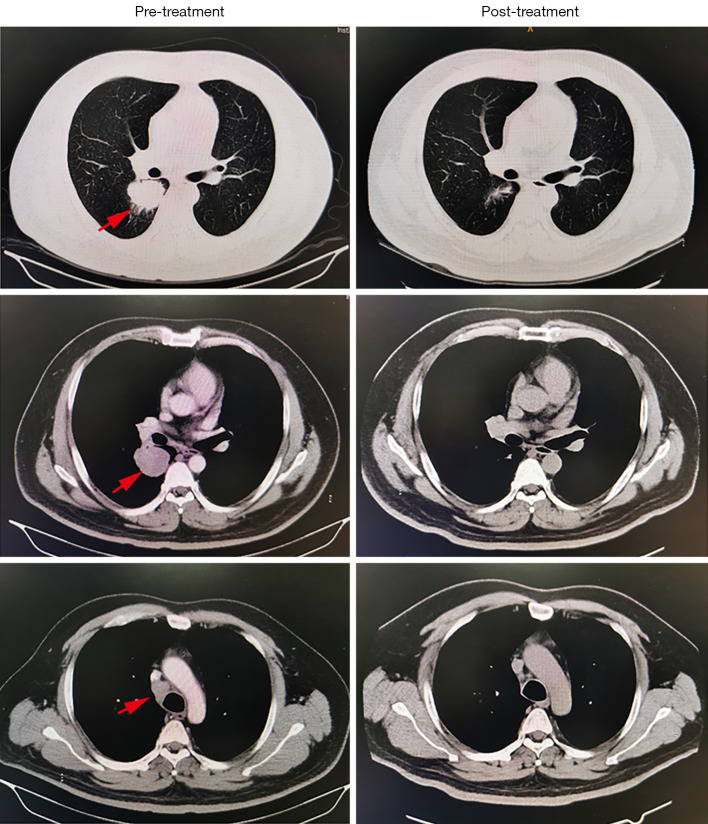
Figure 1.
Disease evolution under neoadjuvant pembrolizumab and chemotherapy. The CT scan shows significant regression of the primary tumor and metastatic mediastinal lymph nodes after neoadjuvant immunochemotherapy. The red arrows indicate tumor and mediastinal lymph node.
Of the 21 surgical patients, no surgical-related delays were identified. The median interval between the last dose of immunochemotherapy and surgical resection was 27 days (range, 16–32 days) (Table 2). Lobectomy was performed for 19 (90.5%) patients, bilobectomy was performed in one (4.0%) patient, and pneumonectomy was performed for another (4.0%) patient. R0 resection was achieved for all patients who had surgery. We observed no in-hospital mortality. As of June 2022, all patients who underwent surgery remained alive and free of disease.
Table 2. Surgery and pathological response.
| Patient No. | Histology | c-stage | Preoperative interval (d) | Surgical resection | CPR | MPR | IPR |
|---|---|---|---|---|---|---|---|
| 1 | SCC | IIIB | 28 | RUL | + | ||
| 2 | LCNEC | IIB | 23 | RLL | + | ||
| 3 | SCC | IIIA | 27 | Lower-bilobectomy | + | ||
| 4 | SCC | IIIA | 21 | LUL | + | ||
| 5 | ASCC | IIIA | 22 | L-pneumonectomy | + | ||
| 6 | ADC | IIIA | 21 | RUL | + | ||
| 7 | SCC | IIIA | 30 | RLL | + | ||
| 8 | SCC | IIIA | 29 | RML | + | ||
| 9 | SCC | IIIA | 23 | RUL | + | ||
| 10 | ADC | IIIA | 32 | LUL | + | ||
| 11 | ADC | IIIA | 26 | LUL | + | ||
| 12 | SCC | IIIB | 22 | LUL | + | ||
| 13 | SCC | IIIB | 30 | LUL | + | ||
| 14 | SCC | IIIA | 30 | RUL | + | ||
| 15 | SCC | IIIA | 26 | RLL | + | ||
| 16 | SCC | IIIB | 28 | LLL | + | ||
| 17 | SAC | IIIA | 16 | RUL | + | ||
| 18 | SCC | IIIA | 27 | LLL | + | ||
| 19 | ADC | IIIA | 28 | LUL | + | ||
| 20 | SCC | IIIA | 30 | LLL | + | ||
| 21 | ASCC | IIIA | 31 | RUL | + |
“+” means the column where the patient’s final pathological reactivity is located. CPR, complete pathological response; MPR, major pathological response; IPR, incomplete pathological response; SCC, squamous cell carcinoma; LCNEC, large cell neuroendocrine carcinoma; ADC, adenocarcinoma; SAC, sarcomatoid carcinoma; ASCC, adenosquamous carcinoma; RUL, right upper lobe; RLL, right lower lobe; LUL, left upper lobe; RML, right middle lobe; LLL, left lower lobe.
The pathologic outcomes for the 21 surgical patients are shown in Table 2. Thirteen patients (61.9%) achieved an MPR to neoadjuvant pembrolizumab and chemotherapy, including six patients (28.6%) who achieved CPR. One patient achieved CPR of the primary tumor but had parabronchial lymph node metastasis after treatment, defined as MPR. The pathological findings from patients who responded to treatment usually showed hyperplasia of bronchial and peribronchial fibrous tissue with transparent degeneration, necrosis, a large number of foam cells, lipid crystallization, and multinucleated giant cell reactions in some areas. There was minimal or no residual tumor.
The trAEs induced by neoadjuvant therapy are listed in Table 3, and nausea and neutropenia are the most common trAEs, in 68.0% and 64.0% of the patients, respectively. One patient failed to undergo surgery because of grade 4 pneumonia that developed during treatment. There were no treatment-related deaths.
Table 3. Treatment-related adverse events during neoadjuvant treatment.
| Patients (n=25) | Grades 1–2, n (%) | Grade 3, n (%) | Grade 4, n (%) |
|---|---|---|---|
| Nausea | 17 (68.0) | 0 | 0 |
| Vomiting | 5 (20.0) | 0 | 0 |
| Diarrhea | 5 (20.0) | 1 (4.0) | 0 |
| Anorexia | 8 (32.0) | 0 | 0 |
| Fatigue | 9 (36.0) | 1 (4.0) | 0 |
| Pruritus | 4 (16.0) | 0 | 0 |
| Rash | 2 (8.0) | 0 | 0 |
| Arthralgia or myalgia | 5 (20.0) | 0 | 0 |
| Pneumonia | 1 (4.0) | 0 | 1 (4.0) |
| Peripheral sensory neuropathy | 1 (4.0) | 0 | 0 |
| Alopecia | 11 (44.0) | 0 | 0 |
| Increased aminotransferases | 6 (24.0) | 1 (4.0) | 0 |
| Neutropenia | 16 (64.0) | 0 | 0 |
| Hepatitis | 0 | 1 (4.0) | 0 |
| Increased creatinine | 2 (8.0) | 0 | 0 |
| Thrombocytopenia | 3 (12.0) | 0 | 0 |
| Hypothyroidism | 5 (20.0) | 0 | 0 |
No grade 5 treatment-related adverse events were reported.
Discussion
The role of surgery in treating of c-III NSCLC is controversial, and several studies have shown that surgical treatment alone cannot improve the prognosis of patients (12,13). Currently, neoadjuvant therapy plays an important role in the treatment of patients with c-III NSCLC (14-17). Neoadjuvant therapy not only contributes to the regression of the primary tumor and metastatic lymph nodes, decreases the tumor stage, and ameliorates the surgical R0 resection rate but also removes micrometastases and reduces the risk of postoperative recurrence and metastasis, thus improving the prognosis of patients (18). It is also important to note that preoperative patients are generally in good physical condition, have good compliance with systemic therapy, and are able to complete a full course of treatment. Unfortunately, neoadjuvant or adjuvant chemotherapy gains only 5% improvement in 5-year OS and is highly toxicity (4). In recent years, the exploration of new and optimal neoadjuvant therapy has become the focus of research for c-III NSCLC (19). ICIs have significantly improved clinical prognosis in patients with locally advanced and metastatic NSCLC and have also showed prospective as neoadjuvant therapy for resectable early-stage disease (20). In our study only one patient with stage IIB lung cancer was pathologically diagnosed as large-cell neuroendocrine carcinoma, a cancer type with a high degree of malignancy and high metastasis rate; thus, we started the neoadjuvant therapy with excellent results.
In the case of neoadjuvant therapy, ICIs provide a chance to prime the antitumor immune response and root out micrometastases early when tumor-specific antigens seem to show less heterogeneity and attract more radical antitumor response. Furthermore, neoadjuvant therapy also allows rapid assessment of tumor sensitivity or resistance to therapy, particularly when there are evaluable surrogate endpoints at the time of surgery that can correlate with hard endpoints of clinical efficacy (20).
MPR is proposed as a surrogate endpoint for survival, as it has been associated with improved survival. It could shorten the period needed to evaluate neoadjuvant therapies and provide a faster means of comparing different neoadjuvant treatment regimens (21,22). In previously published trials, MPR rates were reported to be as high as 45% for patients in a monotherapy ICI cohort (8). Moreover, in neoadjuvant ICI and chemotherapy cohorts, two phase 2 trials (NCT02716038 and NADIM) showed higher MPR rates (57–84%) (9,11,23), much higher than the 16% in the chemotherapy alone group (24). To date, no published studies assessed the response to pembrolizumab in combination with chemotherapy for locally advanced NSCLC in a neoadjuvant setting. The MPR rate of our study, the first in literature for the type of treated patients, was 61.9%. Among the MPR group, six patient (28.6%) achieved CPR, comparable to other previous trials ranging between 12% and 63% (8), which is higher than the 10.5% reported after neoadjuvant chemotherapy (25). The mechanism of the high response rate in the ICI plus chemotherapy group remains unknown. Cytotoxic chemotherapy might function as a sensitizer for immune checkpoint blockade, and it deserves further research for clarification.
However, the benefits of ICIs exist only for a small group of patients (6,26), and identifying and developing predictive biomarkers of ICI response is necessary for effective patient selection. The Food and Drug Administration (FDA) has approved a companion diagnostic biomarker test for assessing PD-L1 expression in determining which patients are eligible for pembrolizumab therapy. Currently, the results of clinical studies suggest that PD-L1 expression has considerable variability as a biomarker when assessing the response to ICIs. In fact, patients with high or low expressions of PD-L1 may receive both benefits (20,27). Therefore, PD-L1 expression remains an imprecise marker of response. Consequently, we conducted this study using neoadjuvant pembrolizumab and chemotherapy regardless of PD-L1 expression levels.
Previous studies suggested that a high tumor mutational burden (TMB) plays a major role in tumor response to ICIs (28), though the response to ICIs is yet far more nuanced (26). TMB means the quantity of somatic mutations within the coding region of a tumor, but there is a lack of a standard cutoff for high TMB (20). Due to the small size of the puncture specimen before treatment, we could not perform whole exome sequencing (WES) on the tumor tissues of each patient to evaluate TMB. We believe that further studies are needed to explore the relationship between TMB and the efficacy of immunotherapy.
It is interesting to note that most of the neoadjuvant therapy patients in our study were male patients. According to our database analysis, a considerable number of female patients with advanced-stage NSCLC had positive driver gene mutations and were enrolled in clinical studies of neoadjuvant-targeted therapy. Although preliminary clinical results revealed that blocking PD-1 can prolong survival in epidermal rowth factor receptor (EGFR)-driven lung adenocarcinoma mice by promoting effector T-cell function, patients with EGFR-mutated NSCLC had a poor response to ICIs (29,30). More importantly, the combined use of ICIs and tyrosine kinase inhibitors (TKIs) did not produce synergistic antitumor effects in patients and caused a high incidence of toxicity (30,31). In our study, four patients with lung adenocarcinoma and two with adenosquamous carcinoma were EGFR-negative.
According to a previous report, the rate of grade 3–4 trAEs to ICIs combined with chemotherapy ranged from 0% to 67% (9). In our study, most trAEs were grade1–2, and 24 patients successfully completed the treatment. One patient experienced grade 4 pneumonia after the first treatment cycle and was forced to stop immunotherapy. There were no treatment-related deaths.
The limitations of this study are as follows: First, this was a retrospective research with a small sample size. Second, it did not include WES or single-cell sequencing techniques to explore predictive biomarkers. Third, the follow-up period was short.
Conclusions
Our results suggest that neoadjuvant pembrolizumab and chemotherapy can be considered safe and feasible, with encouraging pathological responses in locally advanced NSCLC. Further research is needed to identify patients who may benefit most from this approach, and adequately powered trials to clinically verify the meaningful benefits are awaited.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: The study was supported by Science and Technology Commission of Shanghai Municipality (No. 22Y31920403).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This report was approved by the Research Ethics Committee of Zhongshan Hospital, Fudan University (No. B2021-128). Written informed consent was obtained from all patients.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-871/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-871/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-871/coif). FG reports that he serves as Advisory Role in Eli Lilly, Roche, Boehringer Ingelheim, AstraZeneca, Pierre Fabre, BMS, MSD, Novartis, Merck, Otsuka, Novartis, Takeda, Bayer; and honoraria from Eli Lilly, Roche, Boehringer Ingelheim, AstraZeneca, Pierre Fabre, AMGEN, Celgene, BMS, MSD. The other authors have no conflicts of interest to declare.
(English Language Editor: D. Fitzgerald)
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:1675-84. [DOI] [PubMed] [Google Scholar]
- 3.Tong BC, Gu L, Wang X, et al. Perioperative outcomes of pulmonary resection after neoadjuvant pembrolizumab in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 2022;163:427-36. 10.1016/j.jtcvs.2021.02.099 [DOI] [PubMed] [Google Scholar]
- 4.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. 10.1200/JCO.2007.13.9030 [DOI] [PubMed] [Google Scholar]
- 5.Arriagada R, Dunant A, Pignon JP, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol 2010;28:35-42. 10.1200/JCO.2009.23.2272 [DOI] [PubMed] [Google Scholar]
- 6.Benitez JC, Remon J, Besse B. Current Panorama and Challenges for Neoadjuvant Cancer Immunotherapy. Clin Cancer Res 2020;26:5068-77. 10.1158/1078-0432.CCR-19-3255 [DOI] [PubMed] [Google Scholar]
- 7.Caushi JX, Zhang J, Ji Z, et al. Transcriptional programs of neoantigen-specific TIL in anti-PD-1-treated lung cancers. Nature 2021;596:126-32. 10.1038/s41586-021-03752-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018;378:1976-86. 10.1056/NEJMoa1716078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulas EB, Dickhoff C, Schneiders FL, et al. Neoadjuvant immune checkpoint inhibitors in resectable non-small-cell lung cancer: a systematic review. ESMO Open 2021;6:100244. 10.1016/j.esmoop.2021.100244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 11.Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:1413-22. 10.1016/S1470-2045(20)30453-8 [DOI] [PubMed] [Google Scholar]
- 12.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. 10.1016/S0140-6736(09)60737-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007;99:442-50. 10.1093/jnci/djk093 [DOI] [PubMed] [Google Scholar]
- 14.Martins RG, D'Amico TA, Loo BW, Jr, et al. The management of patients with stage IIIA non-small cell lung cancer with N2 mediastinal node involvement. J Natl Compr Canc Netw 2012;10:599-613. 10.6004/jnccn.2012.0062 [DOI] [PubMed] [Google Scholar]
- 15.Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst 1994;86:673-80. 10.1093/jnci/86.9.673 [DOI] [PubMed] [Google Scholar]
- 16.Pisters KM, Vallières E, Crowley JJ, et al. Surgery with or without preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer: Southwest Oncology Group Trial S9900, an intergroup, randomized, phase III trial. J Clin Oncol 2010;28:1843-9. 10.1200/JCO.2009.26.1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cascone T, Weissferdt A, Godoy MCB, et al. Nodal immune flare mimics nodal disease progression following neoadjuvant immune checkpoint inhibitors in non-small cell lung cancer. Nat Commun 2021;12:5045. 10.1038/s41467-021-25188-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilligan D, Nicolson M, Smith I, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet 2007;369:1929-37. 10.1016/S0140-6736(07)60714-4 [DOI] [PubMed] [Google Scholar]
- 19.Saw SPL, Ong BH, Chua KLM, et al. Revisiting neoadjuvant therapy in non-small-cell lung cancer. Lancet Oncol 2021;22:e501-16. 10.1016/S1470-2045(21)00383-1 [DOI] [PubMed] [Google Scholar]
- 20.Cascone T, Fradette J, Pradhan M, et al. Tumor Immunology and Immunotherapy of Non-Small-Cell Lung Cancer. Cold Spring Harb Perspect Med 2022;12:a037895. 10.1101/cshperspect.a037895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hellmann MD, Chaft JE, William WN, Jr, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42-50. 10.1016/S1470-2045(13)70334-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weissferdt A, Pataer A, Vaporciyan AA, et al. Agreement on Major Pathological Response in NSCLC Patients Receiving Neoadjuvant Chemotherapy. Clin Lung Cancer 2020;21:341-8. 10.1016/j.cllc.2019.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:786-95. 10.1016/S1470-2045(20)30140-6 [DOI] [PubMed] [Google Scholar]
- 24.Qu Y, Emoto K, Eguchi T, et al. Pathologic Assessment After Neoadjuvant Chemotherapy for NSCLC: Importance and Implications of Distinguishing Adenocarcinoma From Squamous Cell Carcinoma. J Thorac Oncol 2019;14:482-93. 10.1016/j.jtho.2018.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol 2010;28:3138-45. 10.1200/JCO.2009.27.6204 [DOI] [PubMed] [Google Scholar]
- 26.Morad G, Helmink BA, Sharma P, et al. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021;184:5309-37. 10.1016/j.cell.2021.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lantuejoul S, Sound-Tsao M, Cooper WA, et al. PD-L1 Testing for Lung Cancer in 2019: Perspective From the IASLC Pathology Committee. J Thorac Oncol 2020;15:499-519. 10.1016/j.jtho.2019.12.107 [DOI] [PubMed] [Google Scholar]
- 28.Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 2019;19:133-50. 10.1038/s41568-019-0116-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiao M, Jiang T, Liu X, et al. Immune Checkpoint Inhibitors in EGFR-Mutated NSCLC: Dusk or Dawn? J Thorac Oncol 2021;16:1267-88. 10.1016/j.jtho.2021.04.003 [DOI] [PubMed] [Google Scholar]
- 30.Lee CK, Man J, Lord S, et al. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol 2017;12:403-7. 10.1016/j.jtho.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 31.Oxnard GR, Yang JC, Yu H, et al. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol 2020;31:507-16. 10.1016/j.annonc.2020.01.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as