Abstract
Background
Camrelizumab plus chemotherapy have been approved as standards for the treatment of advanced non-small cell lung cancer (NSCLC) patients based on two phase III trials. However, clinical trial results may not be representative of the general population, as clinical trials often have specific inclusion and exclusion criteria. Our research aims to investigate the real-world effectiveness and safety of camrelizumab in inoperable or advanced NSCLC patients.
Methods
This multicenter retrospective observational study included inoperable or advanced pathologically confirmed NSCLC patients who received at least one dose of camrelizumab at 22 hospitals. Clinical and follow-up data of camrelizumab were collected retrospectively from the medical records. The primary outcome was the objective response rate (ORR) and secondary outcomes were disease control rate (DCR), 6-month progression-free survival (PFS), overall survival (OS), and treatment-related adverse events (TRAEs). Multivariate logistic and Cox regression analyses were applied to identify potential predictive factors of ORR and PFS, respectively.
Results
Between July 2019 and March 2021, 336 patients were included. Adenocarcinoma was seen in 58.4% and stage IV disease in 69.3%. Twenty-nine (8.6%) had liver metastasis at baseline. Most patients received camrelizumab in the first-line setting (74.1%) and in combination with chemotherapy (60.7%). The ORR was 40.2% [95% confidence interval (CI): 34.9–45.6%] and DCR was 85.1% (95% CI: 81.3–88.9%), while the 6-month PFS and OS rates were 73.0% (95% CI: 67.1–78.0%) and 93.1% (95% CI: 89.8–95.4%), respectively. In multivariate analyses, liver metastasis [odds ratio (OR), 0.324; 95% CI: 0.115–0.915; P=0.033] and increasing lines of camrelizumab treatment (vs. first line, second line: OR, 0.347; 95% CI: 0.162–0.741; P=0.006; ≥ third line: OR, 0.126; 95% CI: 0.043–0.367; P<0.001) were negatively associated, while a longer duration of camrelizumab treatment was positively associated with ORR and PFS. TRAEs were recorded in 164 (48.8%) patients, without new safety signal.
Conclusions
We conducted a comprehensive overview of the effectiveness and safety profile of camrelizumab in a broader NSCLC population in real world NSCLC patients, and subgroup analysis indicated the presence of liver metastasis was associated with worse outcomes.
Keywords: Non-small cell lung cancer (NSCLC), samrelizumab, observational study, real-world
Highlight box.
Key findings
• Effectiveness and safety of camrelizumab in real world NSCLC patients.
What is known and what is new?
• Camrelizumab was indicated as the first-line treatment for metastatic lung adenocarcinoma patients combined with chemotherapy in China. However, the effectiveness and safety profiles of camrelizumab need to be evaluated in the real world in a wide population.
• Our paper demonstrated that the presence of liver metastasis and increasing lines of camrelizumab treatment were negatively associated, while a longer duration of camrelizumab treatment was positively associated, with ORR and PFS. The safety profile was consistent with those reported in the pivotal trials. Our study also demonstrated atients with liver metastasis had significantly inferior ORR and 6-month PFS rate when compared with those without.
What is the implication, and what should change now?
• Our study showed the effectiveness and safety of camrelizumab in real world NSCLC patients, and subgroup analysis indicated the presence of liver metastasis was associated with worse outcomes.
Introduction
Lung cancer is the leading cause of cancer-related death worldwide, with 2.2 million new cases and 1.8 million deaths estimated in 2020 (1). Non-small cell lung cancer (NSCLC) is a common subtype (85%), and surgery has been well established as cornerstone for the treatment of early-stage disease. However, most patients are diagnosed at an advanced stage and 5-year survival is scarce (6–32%) (2,3). Immunotherapy has revolutionized the landscape of clinical cancer treatment. Immune checkpoint inhibitors (ICIs) targeting the programmed death 1 (PD-1) axis have shown better patient survival in advanced NSCLC patients treated alone or in combination with chemotherapy in first or second-line settings, when compared with chemotherapy alone (4).
Camrelizumab, also known as SHR-1210, is a humanized monoclonal antibody against PD-1. It was first approved by the National Medical Products Administration (NMPA) for the treatment of patients with relapsed or refractory classical Hodgkin lymphoma in May 2019 in China (5), and has since been extensively investigated in various cancers (6). Camrelizumab plus carboplatin and pemetrexed and plus carboplatin and paclitaxel have been successively approved as new standards for the treatment of advanced non-squamous NSCLC and squamous NSCLC in China, based on the encouraging efficacy and safety data from two pivotal phase III trials (CameL and CameL-sq) (7,8). Additionally, a phase Ib/II trial showed promising efficacy and acceptable toxicity of camrelizumab in combination with apatinib, a highly selective tyrosine kinase inhibitor targeting vascular endothelial growth factor receptor-2, in patients with advanced non-squamous NSCLC previously treated with chemotherapy (9).
However, despite the promising results observed, patients enrolled in clinical trials are generally strictly defined to generate robust evidence and translating this evidence into clinical practice can be challenging, especially given a diverse population we deal with in daily life. Clinical trial results may not be representative of the general population, as clinical trials often have specific inclusion and exclusion criteria. This means that the results may not be generalizable to people who do not meet these criteria. Clinical trials are often conducted over a relatively short period of time, which may not be long enough to fully assess the long-term safety and efficacy of a treatment. Clinical trial results may not fully reflect the real-world context in which a treatment is used, as the controlled environment of a clinical trial may not be representative of everyday practice. To better understand the effectiveness and safety profiles of camrelizumab in real-life NSCLC patients, we conducted this retrospective observational study using data collected from 22 tertiary hospitals in China. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-852/rc) (10).
Methods
Study design and patients
CTONG2004 is a multicenter retrospective observational study of camrelizumab in real-life inoperable or advanced pathologically confirmed NSCLC patients. Adult (age ≥18 years) NSCLC patients who had received at least one dose of camrelizumab between July 2019 and March 2021 at 22 tertiary hospitals including Cancer Hospital of the University of Chinese Academy of Sciences, The First Affiliated Hospital of Guangzhou Medical University, The Second Affiliated Hospital, Zhejiang University School of Medicine, The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, The First Affiliated Hospital of Nanchang University, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Chongqing University Cancer Hospital, Xuzhou Central Hospital, The First Affiliated Hospital of Chongqing Medical University, The First Affiliated Hospital, Sun Yat-sen University, The First Affiliated Hospital, Zhejiang University School of Medicine, Hunan Cancer Hospital, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Guangdong Provincial People’s Hospital & Guangdong Academy of Medical Sciences, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, The First Affiliated Hospital of Jinan University, Harbin Medical University Cancer Hospital, Zhujiang Hospital of Southern Medical University, The Affiliated Hospital of Xuzhou Medical University, The Second Hospital of Dalian Medical University, First Affiliated Hospital of Gannan Medical University, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, and The Third People’s Hospital of Chengdu were screened. To be eligible, patients must had at least one post-baseline response assessment documented within 6 months of the initiation of camrelizumab. Patients with incomplete data on major variables, such as those for camrelizumab use and clinical outcomes, were excluded. Other exclusion criteria included: (I) patients participating in any ongoing clinical studies; (II) patients receiving other immunotherapies during camrelizumab treatment; or (III) patients who had other malignant tumors. All consecutive patients meeting the eligibility criteria were included.
This study mainly focused on patients with inoperable or advanced NSCLC who had received camrelizumab, and the results of its use in a neoadjuvant setting will be reported elsewhere.
The study protocol was approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital (No. GDREC2020189H(R2)) and the other 21 hospitals were informed and agreed with the study. In accordance with the recommendation from the Institutional Ethics Review Board of each center, written informed consent was obtained at the Cancer Hospital of the University of Chinese Academy of Sciences, The First Affiliated Hospital of Guangzhou Medical University, The Second Affiliated Hospital, Zhejiang University School of Medicine, The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, The First Affiliated Hospital of Nanchang University, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Chongqing University Cancer Hospital, Xuzhou Central Hospital, The First Affiliated Hospital of Chongqing Medical University, The First Affiliated Hospital, Sun Yat-sen University, The First Affiliated Hospital, Zhejiang University School of Medicine, Hunan Cancer Hospital, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Guangdong Provincial People’s Hospital & Guangdong Academy of Medical Sciences, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, The First Affiliated Hospital of Jinan University, Harbin Medical University Cancer Hospital, Zhujiang Hospital of Southern Medical University, The Affiliated Hospital of Xuzhou Medical University, and The Second Hospital of Dalian Medical University, and waived at First Affiliated Hospital of Gannan Medical University, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, and The Third People’s Hospital of Chengdu. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Data collection and management
All patients were managed according to the local institutional clinical practice of each participating center. Data on patient demographic and clinical characteristics, patterns of camrelizumab use, and clinical outcomes were retrospectively collected using an electronic case report form. The baseline demographic and clinical characteristics of patients included age, sex, smoking status, Eastern Cooperative Oncology Group performance status (ECOG PS), comorbidities, concomitant medication, histologic subtype, tumor stage and metastasis, as well as programmed death ligand-1 (PD-L1) expression and epidermal growth factor receptor (EGFR) mutation status. The clinical outcomes analyzed were objective response rate (ORR), disease control rate (DCR), 6-month progression-free survival (PFS) and overall survival (OS), and safety outcome.
All data were extracted from the structured and unstructured electronic medical records of each patient by investigators and/or trained staff. Baseline data were collected within 28 days prior to camrelizumab initiation, and key clinical data were cross-validated to minimize the potential risk of misclassification. All participants were followed up by the investigators according to the criteria of the local hospital and data were extracted from the medical records. Quality control was remotely performed to ensure the reliability of data abstraction.
Clinical outcomes and assessment
The primary outcome was ORR, and secondary outcomes were DCR, 6-month PFS and OS, and treatment-related adverse events (TRAEs). Clinical outcomes were assessed at the discretion of the physician in charge during routine clinical visits. Tumor response was based on the imaging findings and clinical symptoms with reference to the Response Evaluation Criteria in the Solid Tumors (RECIST) criterion (version 1.1). Best overall response was classified as complete response (CR), partial response (PR), stable disease (SD), and progression disease (PD), based on the best response documented in medical records. ORR and DCR were then calculated as the proportions of patients experiencing CR or PR and CR or PR or SD, respectively. PFS was defined as the time interval from camrelizumab initiation to the date of disease progression or death. Patients still alive without disease progression were censored at the date of the last radiological assessment. OS was defined as the time interval from camrelizumab initiation to the date of death, and patients who were alive were censored at the date of last confirmed survival. Safety outcomes included TRAEs and serious adverse events (TRSAEs) and were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Statistical analysis
Descriptive analysis was the main analysis for our study. Continuous variables were expressed by Median (IQR) and categorical variables were presented with frequency (%). For binary endpoints, 95% confidence intervals (CIs) were calculated by Clopper and Pearson method. Since PFS and OS were not yet mature, only 6-month PFS rate and 6-month OS rate were estimated by using Kaplan-Meier method along with 95% CIs by log-log method. Furthermore, univariate logistic regression model and Cox regression model were applied to select the potential prognostic factors of ORR and PFS, respectively. For the factors with missing/unknown rate <30% and P<0.05 identified in the univariate models were included in the multivariate regression models and stepwise selection method were used to select the significant prognostic factors in addition to line of therapy. Moreover, ORR, DCR, 6-months PFS rate and 6-months OS rate in subgroups were explored and forest plot was displayed. Statistical analyses were performed by SAS software, version 9.4 (SAS Institute). Forest plots were presented by R software, version 4.1.0. A two-sided P value <0.05 was considered as nominally significant.
Results
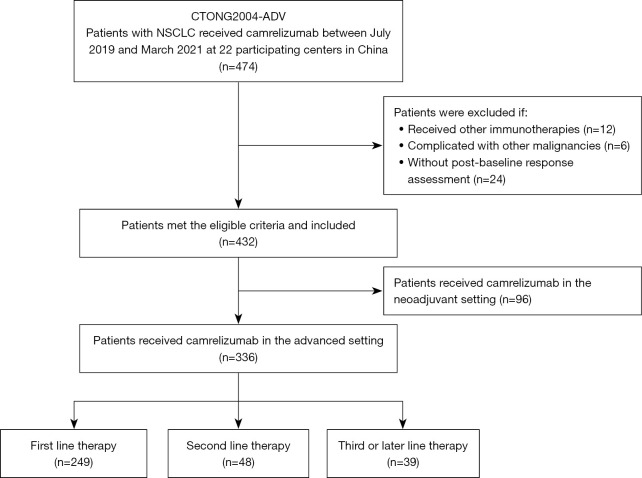
Between July 2019 and March 2021, 474 consecutive patients were identified to have received at least one dose of camrelizumab at the 22 participating centers. Of these, 42 were excluded based on the eligibility criteria and 92 were excluded as they had received camrelizumab in the neoadjuvant setting, resulting in a final sample of 336 patients for analysis (Figure 1).
Figure 1.
Patient flowchart. NSCLC, non-small cell lung cancer.
Patient characteristics and treatment
Among the 336 patients, 249 (74.1%) received camrelizumab in the first-line setting, 48 (14.3%) in the second-line setting, and 39 (11.6%) in third- or later-line settings. The demographic and clinical characteristics of patients and those stratified by line of camrelizumab treatment are summarized in Table 1. The median age of patients was 62 years, ranging from 27 to 87 years, and approximately 17.6% (59/336) were aged 70 years or older. Most patients were male (76.2%), had adenocarcinoma (58.4%) and stage IV disease (69.3%), while 42 (12.5%) patients with early-stage (IB–IIIA) diseases received camrelizumab. Of the 42 patients, five with medically inoperable stage I–II NSCLC received camrelizumab-based therapy, and none received prior or concurrent radiotherapy. ECOG PS was recorded in 166 (49.4%) patients, and most (94.0%) had a PS of 0–1. Eighty-three (24.7%) patients underwent tests for PD-L1 expression, and 64 (77.1%) patients showed positive staining (≥1%). The presence of EGFR mutation was tested in 121 (37.2%) patients, and 19 (5.7%) harbored sensitizing mutations. Brain metastasis was seen in 49 (14.6%) patients and 29 (8.6%) had liver metastasis at baseline. Ninety-nine (29.5%) patients were on baseline steroids and 34 (10.1%) were on baseline antibiotics, primarily for prophylactic purposes. The most common comorbidities recorded were hypertension in 77 (22.9%) patients, diabetes in 34 (10.1%) patients, and HBV infection in 21 (6.3%) patients.
Table 1. Baseline patient characteristics at the time of camrelizumab initiation (n=336).
| Variables | Total (n=336) | First line (n=249) | Second line (n=48) | ≥ Third line (n=39) |
|---|---|---|---|---|
| Age, years | ||||
| Median (IQR) | 62.0 (55.0, 67.0) | 62 (55.0, 67.0) | 60.5 (55.5, 66.5) | 60.0 (52.0, 65.0) |
| ≥70 years, n (%) | 59 (17.6) | 44 (17.7) | 9 (18.8) | 6 (15.4) |
| Sex, n (%) | ||||
| Male | 256 (76.2) | 200 (80.3) | 33 (68.8) | 23 (59.0) |
| Female | 80 (23.8) | 49 (19.68) | 15 (31.2) | 16 (41.0) |
| Smoking status, n (%) | ||||
| Current/former | 168 (50.0) | 137 (55.0) | 18 (37.5) | 13 (33.3) |
| Never | 134 (39.9) | 90 (36.1) | 24 (50.0) | 20 (51.3) |
| Unknown | 34 (10.1) | 22 (8.8) | 6 (12.5) | 6 (15.4) |
| ECOG PS, n (%) | ||||
| 0 | 37 (11.0) | 28 (11.2) | 6 (12.5) | 3 (7.7) |
| 1 | 119 (35.4) | 80 (32.1) | 22 (45.8) | 17 (43.6) |
| ≥2 | 10 (3.0) | 6 (2.4) | 1 (2.1) | 3 (7.7) |
| Unknown | 170 (50.6) | 135 (54.2) | 19 (39.6) | 16 (41.0) |
| Histology, n (%) | ||||
| Adenocarcinoma | 195 (58.0) | 135 (54.2) | 33 (68.7) | 27 (69.2) |
| Squamous cell carcinoma | 131 (39.0) | 105 (42.2) | 14 (29.2) | 12 (30.8) |
| Other/unspecified | 10 (3.0) | 9 (3.6) | 1 (2.1) | 0 (0.0) |
| Tumor stage, n (%) | ||||
| I–II | 8 (2.4) | 8 (3.2) | 0 (0.0) | 0 (0.0) |
| III | 95 (28.3)* | 87 (34.9) | 5 (10.4)* | 3 (7.7) |
| IIIA | 34 (10.1) | 33 (13.2) | 1 (2.1) | 0 (0.0) |
| IIIB–IIIC | 60 (17.9) | 54 (21.7) | 3 (6.2) | 3 (7.7) |
| IV | 233 (69.3) | 154 (61.9) | 43 (89.6) | 36 (92.3) |
| Metastatic sites, n (%) | ||||
| Brain | 49 (14.6) | 30 (12.1) | 9 (18.6) | 10 (25.6) |
| Liver | 29 (8.6) | 15 (6.0) | 9 (18.6) | 5 (12.8) |
| PD-L1 expression, n (%) | ||||
| <1% | 19 (5.7) | 13 (5.2) | 2 (4.2) | 4 (10.3) |
| ≥1% | 64 (19.1) | 50 (20.1) | 12 (25.0) | 2 (5.2) |
| 1–49% | 30 (8.9) | 19 (7.6) | 9 (18.8) | 2 (5.2) |
| ≥50% | 34 (10.1) | 31 (12.5) | 3 (6.3) | 0 (0.0) |
| Unknown | 253 (75.3) | 186 (74.7) | 34 (70.8) | 33 (84.6) |
| Sensitizing EGFR mutation, n (%) | ||||
| Yes | 19 (5.7) | 3 (1.2) | 5 (10.4) | 11 (28.2) |
| No | 102 (30.4) | 78 (31.3) | 15 (31.3) | 9 (23.1) |
| Unknown | 215 (64.0) | 168 (67.5) | 28 (58.3) | 19 (48.7) |
*, substage of one patient treated for stage III disease in the second setting was not specified. ECOG PS, Eastern Cooperative Oncology Group performance status; PD-L1, programmed death ligand-1; EGFR, epidermal growth factor receptor; IQR, interquartile range.
Most patients received camrelizumab in the first-line setting (74.1%) and in combination regimens (91.4%), primarily in combination with chemotherapy (60.7%) (Table 2). The median duration of camrelizumab treatment was 20.6 (range, 0.1–25.9) weeks, and the median number of cycles was six (range, 1–11). At six months after camrelizumab initiation, 120 (35.7%) patients discontinued the treatment, mostly due to disease progression (43.3%). Twenty-four (20.0%) patients were recorded to receive subsequent treatments, primarily chemotherapy (n=7), anti-angiogenesis (n=7), or their combination therapy (n=4), while three patients were re-challenged with immunotherapy (alone or plus anti-angiogenesis).
Table 2. Patterns of camrelizumab use in patients with inoperable or advanced NSCLC (n=336).
| Treatment patterns | Total (n=336) | First line (n=249) | Second line (n=48) | ≥ Third line (n=39) |
|---|---|---|---|---|
| No. of patients still taking camrelizumab treatments, n (%) | 216 (64.3) | 175 (70.3) | 25 (52.1) | 16 (41.0) |
| Duration of camrelizumab treatments | ||||
| Median [range], weeks | 20.6 [0.1–25.9] | 20.9 [0.1–25.9] | 13.7 [0.1–25.9] | 14.0 [0.1–25.4] |
| Cycle of camrelizumab use | ||||
| Median [range] | 6 [1–11] | 6 [1–11] | 4 [1–10] | 5 [1–9] |
| ≥6 cycles, n (%) | 178 (53.0) | 143 (57.4) | 16 (33.3) | 19 (48.7) |
| Patterns of camrelizumab use, n (%) | ||||
| Monotherapy | 29 (8.6) | 13 (5.2) | 9 (18.8) | 7 (17.9) |
| Plus chemotherapy | 204 (60.7) | 169 (67.9) | 25 (52.1) | 10 (25.6) |
| Plus anti-angiogenesis | 30 (8.9) | 16 (6.4) | 4 (8.3) | 10 (25.6) |
| Plus chemotherapy + anti-angiogenesis | 73 (21.7) | 51 (20.5) | 10 (20.8) | 12 (30.8) |
NSCLC, non-small cell lung cancer.
Effectiveness
The best overall response was CR in 1 (0.3%) patient, PR in 134 (39.9%) patients, SD in 151 (44.9%) patients, and PD in 47 (14.0%) patients. The ORR was 40.2% (95% CI: 34.9–45.6%) and DCR was 85.1% (95% CI: 81.3–88.9%), while 81 (24.1%) patients had progressive disease or died within 6 months after camrelizumab initiation. The objective response rate in first-line therapy was 48.6% which was higher than that of second-line or further lines (Figures 2,3). The 6-month PFS and OS rates were 73.0% (95% CI: 67.1–78.0%) and 93.1% (95% CI: 89.8–95.4%), respectively.
Figure 2.
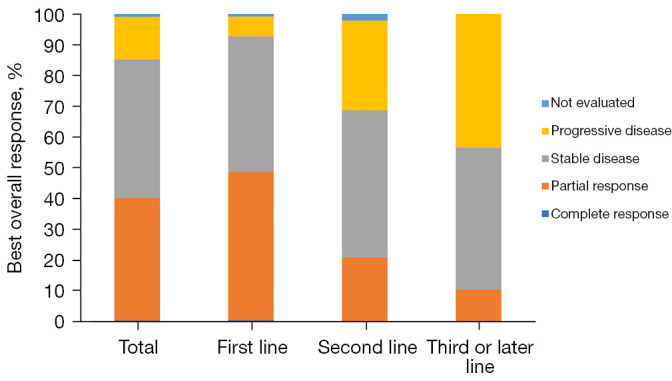
Best overall response to systemic treatments with camrelizumab in overall patients and those stratified by the line of therapy.
Figure 3.
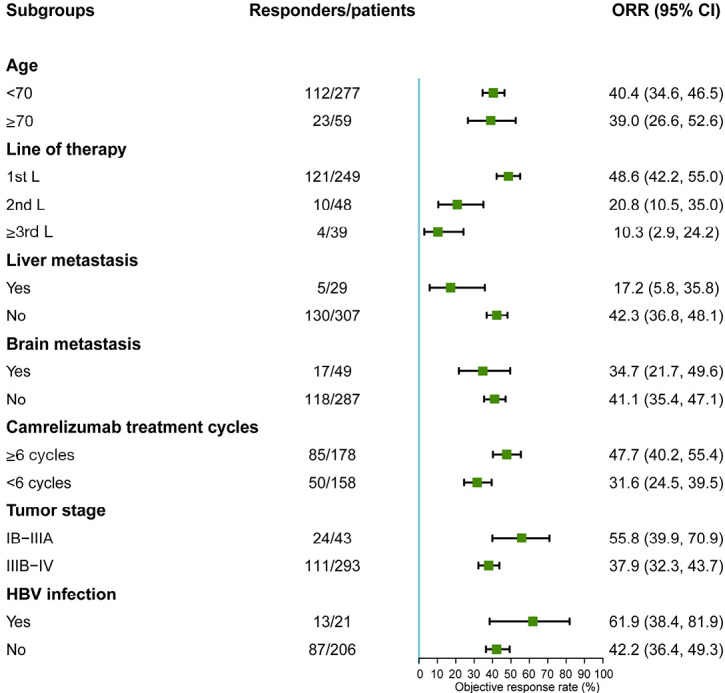
Subgroup analysis of ORR in NSCLC patients treated with camrelizumab. ORR, objective response rate; CI, confidence interval; NSCLC, non-small cell lung cancer; HBV, hepatitis B virus.
Patients with liver metastasis had a significantly inferior ORR [OR: 0.284 (95% CI: 0.105–0.763), P=0.013], and were also those with advanced disease [stage IIIB–IV vs. stage IB–IIIA: OR: 0.483 (95% CI: 0.253–0.922), P=0.027] (Table 3 and Figure 3). However, there was no significant difference in ORR in other specific subgroups, including elderly patients [≥70 vs. <70 years: OR: 0.941 (95% CI: 0.529–1.674), P=0.837], those with brain metastasis [OR: 0.761 (95% CI: 0.404, 1.434), P=0.398], or those taking baseline antibiotics [OR: 1.559 (95% CI: 0.776–3.174), P=0.221] or steroids [OR: 0.749 (95% CI: 0.461–1.218), P=0.244]. HBV infection showed no obvious effect on tumor response, with an ORR of 61.9% (95% CI: 38.4–81.9%) in patients with HBV infection and 42.2% (95% CI: 36.4–49.3%) in those without.
Table 3. Logistic regression analysis of ORR in NSCLC patients treated with camrelizumab.
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Camrelizumab treatment line | |||||
| First line | Ref | ||||
| Second line | 0.278 (0.133–0.583) | 0.001 | 0.347 (0.162–0.741) | 0.006 | |
| ≥ Third line | 0.121 (0.042–0.35) | <0.001 | 0.126 (0.043–0.367) | <0.001 | |
| Age | |||||
| <70 years | Ref | ||||
| ≥70 years | 0.941 (0.529–1.674) | 0.837 | |||
| Sex | |||||
| Male | Ref | ||||
| Female | 0.649 (0.382–1.103) | 0.11 | |||
| Histology | |||||
| Adenocarcinoma | Ref | ||||
| Squamous cell carcinoma | 1.543 (0.982–2.425) | 0.06 | |||
| Other/unspecified | 2.739 (0.747–10.038) | 0.128 | |||
| Smoking status | |||||
| Never | Ref | ||||
| Current/former | 1.154 (0.726–1.834) | 0.544 | |||
| Brain metastasis | |||||
| No | Ref | ||||
| Yes | 0.761 (0.404–1.434) | 0.398 | |||
| Liver metastasis | |||||
| No | Ref | ||||
| Yes | 0.284 (0.105–0.763) | 0.013 | 0.324 (0.115–0.915) | 0.033 | |
| Baseline antibiotics | |||||
| No | Ref | ||||
| Yes | 1.559 (0.766–3.174) | 0.221 | |||
| Baseline steroids | |||||
| No | Ref | ||||
| Yes | 0.749 (0.461–1.218) | 0.244 | |||
| Tumor stage | |||||
| IB–IIIA | Ref | ||||
| IIIB–IV | 0.483 (0.253–0.922) | 0.027 | |||
| Duration of camrelizumab use | |||||
| <6 cycles | Ref | ||||
| ≥6 cycles | 1.974 (1.264–3.084) | 0.003 | 1.843 (1.149–2.956) | 0.011 | |
| Patterns of camrelizumab use | |||||
| Monotherapy | Ref | ||||
| Plus CT | 2.114 (0.895–4.995) | 0.088 | |||
| Plus anti-angiogenesis | 0.955 (0.303–3.009) | 0.937 | |||
| Plus CT + anti-angiogenesis | 1.633 (0.637–4.186) | 0.307 | |||
ORR, objective response rate; NSCLC, non-small cell lung cancer; OR, odds ratio; CI, confidence interval; CT, chemotherapy.
In multivariate analyses, liver metastasis remained significantly associated with inferior ORR (OR, 0.324; 95% CI: 0.115–0.915; P=0.033). In addition, increasing lines of camrelizumab treatment (vs. first line, second line: OR, 0.347; 95% CI: 0.162–0.741; P=0.006; ≥third line: OR, 0.126; 95% CI: 0.043–0.367; P<0.001) were negatively associated, while the longer duration of camrelizumab treatments (≥6 vs. <6 cycles: OR, 1.843; 95% CI: 1.149–2.956; P=0.011) was positively associated with ORR (Table 3).
These results were confirmed by subgroup analyses of the 6-month PFS rate (Figure S1). Multivariate analyses indicated liver metastasis (HR, 2.416; 95% CI: 1.27–4.595; P=0.007) and increasing lines of camrelizumab treatment (vs. first line, second line: HR, 2.177; 95% CI: 1.238, 3.828; P=0.007; ≥ third line: HR, 7.533; 95% CI: 4.308–13.172; P<0.001) were associated with higher risks of PFS, while a longer duration of camrelizumab treatments (≥6 vs. <6 cycles: HR, 0.192; 95% CI: 0.114–0.325; P<0.001) was associated with a lower risk (Table S1).
Safety
TRAEs of any grade were observed in 164 (48.8%) patients, and those of grade 3 or worse were seen in 42 (12.5%). The most common TRAEs were anaemia (17.6%), followed by abnormal hepatic transaminase (13.4%), decreased white blood cell count (12.2%), and decreased neutrophil count (10.1%). Reactive cutaneous capillary endothelial proliferation (RCCEP) was recorded in 23 (6.9%) patients and most (95.7%) were mild (grade 1–2) (Table 4). TRSAEs were noted in seven (2.1%) patients, including immune-mediated pneumonia (n=2), infections (n=2), RCCEP, respiratory failure, arrhythmia, decreased white blood cell count, diarrhea, and autoimmune hepatitis. Eleven patients required dose delay, while 15 discontinued camrelizumab due to TRAEs. There were no treatment-related deaths. The safety profiles of specific subgroups of patients, including those with brain metastasis, liver metastasis, HBV infection, and early-stage disease were generally consistent with the overall population, with no new safety signal.
Table 4. TRAEs occurred in >2% of patients (n=336).
| AEs | TRAEs n (%) | |||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3/4 | All grade | |
| Anaemia | 24 (7.1) | 20 (6.0) | 15 (4.5) | 59 (17.6) |
| Abnormal hepatic transaminase | 33 (9.8) | 10 (3.0) | 2 (0.6) | 45 (13.4) |
| White blood cell count decreased | 9 (2.7) | 18 (5.4) | 14 (4.2) | 41 (12.2) |
| Neutrophil count decreased | 12 (3.6) | 9 (2.7) | 13 (3.9) | 34 (10.1) |
| Platelet count decreased | 8 (2.4) | 7 (2.1) | 16 (4.8) | 31 (9.2) |
| Lymphocyte count decreased | 6 (1.9) | 8 (2.4) | 12 (3.6) | 26 (7.7) |
| RCCEP | 17 (5.1) | 4 (1.2) | 1 (0.3) | 23 (6.9) |
| Electrolyte imbalance | 5 (1.5) | 4 (1.2) | 5 (1.5) | 14 (4.2) |
| Thyroid dysfunction | 9 (2.7) | 2 (0.6) | 0 (0.0) | 11 (3.3) |
| Infection | 2 (0.6) | 1 (0.3) | 6 (1.8) | 9 (2.7) |
| Blood bilirubin increased | 8 (2.4) | 0 (0.0) | 1 (0.3) | 9 (2.7) |
| Myelosuppression | 0 (0.0) | 5 (1.5) | 3 (0.9) | 8 (2.4) |
| Hypoalbuminemia | 4 (1.2) | 3 (0. 9) | 0 (0.0) | 7 (2.1) |
| Liver injury | 5 (1.5) | 1 (0.3) | 1 (0.3) | 7 (2.1) |
| Elevated blood glucose | 7 (2.1) | 0 (0.0) | 0 (0.0) | 7 (2.1) |
TRAEs, treatment-related adverse events; AEs, adverse events; RCCEP, reactive cutaneous capillary endothelial proliferation.
Discussion
This was a retrospective study (CTONG2004-ADV) investigating the effect of camrelizumab on 336 NSCLC patients. An overall ORR of 40.2%, DCR of 85.1%, and 6-month PFS and OS rates of 73.0% and 93.1%, respectively were observed, while patients with liver metastasis had significantly inferior ORR and PFS. Those of older age, with brain metastasis, or taking baseline antibiotics or steroids showed comparable ORR and 6-month PFS rates. In multivariate analyses, the presence of liver metastasis and increasing lines of camrelizumab treatment were negatively associated, while a longer duration of camrelizumab treatment was positively associated, with ORR and PFS. The safety profile in our real-world population was generally consistent with those reported in the pivotal trials, although the frequency of TRAEs was comparatively lower. To our best knowledge, the CTONG2004-ADV is one of the largest reported clinical evaluations of camrelizumab in real-world NSCLC patients to date, and our findings provide a comprehensive overview of effectiveness and safety profiles of camrelizumab in a broader population from routine clinical practice.
Camrelizumab showed an inferior ORR, while a comparable DCR in real-world settings. The ORR (40.2%) observed in our cohort was lower than observed in first-line trials (CameL: 60.5% for non-squamous NSCLC; CameL-sq: 64.8% for squamous NSCLC) (7,8). However, it was higher than the results reported periodically in the two prospective observational studies (25% and 26%) (11,12). Interestingly, despite the comparatively lower ORR observed in real-world settings, the DCR (85.1%) was generally comparable among the pivotal trials (CameL: 87.8% for non-squamous NSCLC; CameL-sq: 88.1% for squamous NSCLC) (7,8) and observational studies (83.40% and 89.30%) (11,12). These variations may be partially explained by different patient characteristics and treatment variables. As an example, the increasing line of camrelizumab treatment was found to independently associate with a deteriorated tumor response in the current study. Most (74%) of our patients received camrelizumab in the first-line setting, whereas more (70% and 75%) in the two prospective observational studies received it in second or later line settings. Considering the context of cross-study comparison and the difference in patient characteristics and treatments, the ORR observed herein was generally comparable to that seen in the pivotal trials (7,8). Unfortunately, only the 6-month survival outcomes were available in this study. The tumor response to nivolumab was found to associate with prolonged survival in an observational study (13). However, whether the anti-tumor activity observed in the current study will translate into the long-term survival benefits is unknown.
Our study also adds novel insights into the effectiveness of camrelizumab in specific subgroups of patients who were excluded from or underrepresented in the pivotal trials. Patients with liver metastasis were found to have significantly inferior ORR and 6-month PFS rate when compared with those without. Consistent results of comparatively poor response and survival were reported in patients with liver metastasis receiving other ICIs. However, contrary results of comparable efficacy irrespective of liver metastasis status were also noted (14,15). Despite this, a recent study demonstrated a restrained efficacy of immunotherapy in both preclinical models and clinical cancer patients with liver metastasis in eight cohorts, including NSCLC, with the authors suggesting a combination with radiotherapy may improve immunotherapy efficacy in such patients (16). Patients with liver metastasis were also found to benefit from immunotherapy as part of a treatment modality, albeit with a poor response, and those with adenocarcinoma metastasis to the liver seemed to benefit more from immunotherapy than their squamous cell carcinoma counterparts (17). Additionally, the therapeutic effects of ICIs were found to relate to treatment patterns in patients with liver metastasis, with stronger effects derived from the ICI-based combination therapy. The latter was suggested to be able to overcome intrinsic resistance of liver metastasis to ICIs (18). However, our study showed no significant impact of camrelizumab treatment patterns on tumor response both in overall patients and those with liver metastasis [ORR: 20% (1/5) monotherapy vs. 16.7% (4/24) combination therapy]. Liver metastasis was found to independently associate with inferior clinical outcomes after adjusting for other factors, including patterns of camrelizumab use. Future studies of immunotherapy in patients with liver metastasis may focus on the patterns of ICI use (combination therapy e.g., radiotherapy) or identification of specific patients more likely to benefit.
However, other specific subgroups, such as older age (≥70 years), presence of brain metastasis, and baseline antibiotics or steroids administration were not found to significantly impact the clinical outcomes in this study. While similar results were observed in previous studies of other ICIs (19-21), other studies showed equivocal benefits of ICIs in older patients (22,23), or those taking baseline steroids (24,25) or antibiotics (26-28). A recent study indicated patients with HBV/HCV infection could be treated safely with anti-PD-1 inhibitors (29,30), and consistent with those results, our study showed no significant impact of HBV infection on tumor response and survival outcomes. Regrettably, we failed to include HBV infection in the multivariate analyses, and future studies in specific subgroups of interest are still needed to better define the long-term therapeutic effects of camrelizumab in real-world NSCLC patients.
The most common TRAEs in our study were anaemia, abnormal hepatic transaminase, white blood cell count decrease, and neutrophil count decrease, which are generally consistent with the profiles reported in the pivotal trials. Notably, TRAEs were less commonly reported in the real-world setting, especially mild symptoms (e.g., RCCEP). A similar phenomenon was noted in previous studies of other ICIs (31,32). One reasonable explanation is the less stringent monitoring and reporting of low-grade AEs in the real-world setting, although direct reporting to other agencies such as regional pharmacovigilance centers may also play a role. Nevertheless, no new safety signal was observed in NSCLC patients treated with camrelizumab in routine clinical practice.
Some limitations must be acknowledged when interpreting the results. Primarily, the retrospective nature of the study design lends to potential selection and misclassification bias, although we have tried to include all consecutive patients eligible for inclusion and to conduct data cross-validation. Secondly, some major patient characteristics such as PD-L1 expression and ECOG PS were not routinely assessed/recorded. Thirdly, the small subsets of patients made it difficult to evaluate their clinical significance in the real-world setting, and finally, all patients were enrolled from tertiary hospitals, and only the short-term outcomes were available. Accordingly, a prospective observational study with a larger sample size (~1,000) is underway to further evaluate camrelizumab use in real world NSCLC patients (NCT04793139).
Conclusions
Our study showed the effectiveness and safety of camrelizumab in real world NSCLC patients, and subgroup analysis indicated the presence of liver metastasis was associated with worse outcomes. Future studies are required to provide an in-depth insight into the long-term effects of camrelizumab in real-world NSCLC patients.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported by the Jiangsu Hengrui Pharmaceuticals Co., Ltd.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital [No. GDREC2020189H(R2)] and the other 21 hospitals were informed and agreed with the study. In accordance with the recommendation from the Institutional Ethics Review Board of each center, written informed consent was obtained at the Cancer Hospital of the University of Chinese Academy of Sciences, The First Affiliated Hospital of Guangzhou Medical University, The Second Affiliated Hospital, Zhejiang University School of Medicine, The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, The First Affiliated Hospital of Nanchang University, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Chongqing University Cancer Hospital, Xuzhou Central Hospital, The First Affiliated Hospital of Chongqing Medical University, The First Affiliated Hospital, Sun Yat-sen University, The First Affiliated Hospital, Zhejiang University School of Medicine, Hunan Cancer Hospital, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Guangdong Provincial People’s Hospital & Guangdong Academy of Medical Sciences, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, The First Affiliated Hospital of Jinan University, Harbin Medical University Cancer Hospital, Zhujiang Hospital of Southern Medical University, The Affiliated Hospital of Xuzhou Medical University, and The Second Hospital of Dalian Medical University, and waived at First Affiliated Hospital of Gannan Medical University, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, and The Third People’s Hospital of Chengdu. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-852/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-852/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-852/coif). All authors report this research was supported and funded by Jiangsu Hengrui Pharmaceuticals Co., Ltd. YW reports that he received grants or contracts from Boehringer Ingelheim (Inst), Roche (Inst), Pfizer (Inst), and BMS (Inst), and consulting fees from AstraZeneca, Roche, Boehringer Ingelheim, and Takeda, and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Lilly, Roche, Pfizer, Boehringer Ingelheim, MSD Oncology, Bristol Myers Squibb/China, and Hengrui Pharmaceutical. The authors have no other conflicts of interest to declare.
(English Language Editor: B. Draper)
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 4.Thai AA, Solomon BJ, Sequist LV, et al. Lung cancer. Lancet 2021;398:535-54. 10.1016/S0140-6736(21)00312-3 [DOI] [PubMed] [Google Scholar]
- 5.Markham A, Keam SJ. Camrelizumab: First Global Approval. Drugs 2019;79:1355-61. 10.1007/s40265-019-01167-0 [DOI] [PubMed] [Google Scholar]
- 6.Song H, Liu X, Jiang L, et al. Current Status and Prospects of Camrelizumab, A Humanized Antibody Against Programmed Cell Death Receptor 1. Recent Pat Anticancer Drug Discov 2021;16:312-32. 10.2174/22123970MTE09MDYg0 [DOI] [PubMed] [Google Scholar]
- 7.Zhou C, Chen G, Huang Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med 2021;9:305-14. 10.1016/S2213-2600(20)30365-9 [DOI] [PubMed] [Google Scholar]
- 8.Ren S, Chen J, Xu X, et al. Camrelizumab Plus Carboplatin and Paclitaxel as First-Line Treatment for Advanced Squamous NSCLC (CameL-Sq): A Phase 3 Trial. J Thorac Oncol 2022;17:544-57. 10.1016/j.jtho.2021.11.018 [DOI] [PubMed] [Google Scholar]
- 9.Zhou C, Wang Y, Zhao J, et al. Efficacy and Biomarker Analysis of Camrelizumab in Combination with Apatinib in Patients with Advanced Nonsquamous NSCLC Previously Treated with Chemotherapy. Clin Cancer Res 2021;27:1296-304. 10.1158/1078-0432.CCR-20-3136 [DOI] [PubMed] [Google Scholar]
- 10.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 2007;4:e297. 10.1371/journal.pmed.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu K, Bi M, Zhao D, et al. A real-world study of camrelizumab in the treatment of advanced lung cancer patients. Wolters Kluwer Health, 2021. [Google Scholar]
- 12.Shu Y. P81. 01 Efficacy and Safety of Camrelizumab in Patients with Advanced Lung Cancer: A Multicentre, Prospective, Observational Study. J Thorac Oncol 2021;16:S650. 10.1016/j.jtho.2021.01.1190 [DOI] [Google Scholar]
- 13.Schouten RD, Egberink L, Muller M, et al. Nivolumab in pre-treated advanced non-small cell lung cancer: long term follow up data from the Dutch expanded access program and routine clinical care. Transl Lung Cancer Res 2020;9:1736-48. 10.21037/tlcr-19-698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi MG, Choi CM, Lee DH, et al. Different prognostic implications of hepatic metastasis according to front-line treatment in non-small cell lung cancer: a real-world retrospective study. Transl Lung Cancer Res 2021;10:2551-61. 10.21037/tlcr-21-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin BD, Jiao XD, Liu J, et al. The effect of liver metastasis on efficacy of immunotherapy plus chemotherapy in advanced lung cancer. Crit Rev Oncol Hematol 2020;147:102893. 10.1016/j.critrevonc.2020.102893 [DOI] [PubMed] [Google Scholar]
- 16.Yu J, Green MD, Li S, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med 2021;27:152-64. 10.1038/s41591-020-1131-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badin K, Waseem B, Ullah A, et al. Effect of personalized approach to therapy in non-small cell lung cancer (NSCLC) with liver metastasis with immunotherapy on outcomes. J Clin Oncol 2020;38:abstr e21504.
- 18.Chen XJ, Ren A, Zheng L, et al. Pan-Cancer Analysis Identifies Liver Metastases as Negative Predictive Factor for Immune Checkpoint Inhibitors Treatment Outcome. Front Immunol 2021;12:651086. 10.3389/fimmu.2021.651086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortellini A, Tiseo M, Banna GL, et al. Clinicopathologic correlates of first-line pembrolizumab effectiveness in patients with advanced NSCLC and a PD-L1 expression of ≥ 50. Cancer Immunol Immunother 2020;69:2209-21. 10.1007/s00262-020-02613-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaguchi O, Imai H, Minemura H, et al. Efficacy and safety of immune checkpoint inhibitor monotherapy in pretreated elderly patients with non-small cell lung cancer. Cancer Chemother Pharmacol 2020;85:761-71. 10.1007/s00280-020-04055-7 [DOI] [PubMed] [Google Scholar]
- 21.Grossi F, Genova C, Crinò L, et al. Real-life results from the overall population and key subgroups within the Italian cohort of nivolumab expanded access program in non-squamous non-small cell lung cancer. Eur J Cancer 2019;123:72-80. 10.1016/j.ejca.2019.09.011 [DOI] [PubMed] [Google Scholar]
- 22.Naltet C, Besse B. Immune checkpoint inhibitors in elderly patients treated for a lung cancer: a narrative review. Transl Lung Cancer Res 2021;10:3014-28. 10.21037/tlcr-20-1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song P, Zhang J, Shang C, et al. Real-world evidence and clinical observations of the treatment of advanced non-small cell lung cancer with PD-1/PD-L1 inhibitors. Sci Rep 2019;9:4278. 10.1038/s41598-019-40748-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott SC, Pennell NA. Early Use of Systemic Corticosteroids in Patients with Advanced NSCLC Treated with Nivolumab. J Thorac Oncol 2018;13:1771-5. 10.1016/j.jtho.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 25.Ciccarese C, Iacovelli R, Bria E, et al. The Anticancer Efficacy of Immune Checkpoint Inhibitors According to Patients' Age: A Systematic Review and Meta-Analysis. J Immunother 2020;43:95-103. 10.1097/CJI.0000000000000312 [DOI] [PubMed] [Google Scholar]
- 26.Zhao S, Gao G, Li W, et al. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer 2019;130:10-7. 10.1016/j.lungcan.2019.01.017 [DOI] [PubMed] [Google Scholar]
- 27.Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol 2018;29:1437-44. 10.1093/annonc/mdy103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouaknine Krief J, Helly de Tauriers P, Dumenil C, et al. Role of antibiotic use, plasma citrulline and blood microbiome in advanced non-small cell lung cancer patients treated with nivolumab. J Immunother Cancer 2019;7:176. 10.1186/s40425-019-0658-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu F, Zeng Z, Yan B, et al. Safety and efficacy of anti-PD-1 inhibitors in Chinese patients with advanced lung cancer and hepatitis B virus infection: a retrospective single-center study. Transl Lung Cancer Res 2021;10:1819-28. 10.21037/tlcr-21-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pertejo-Fernandez A, Ricciuti B, Hammond SP, et al. Safety and efficacy of immune checkpoint inhibitors in patients with non-small cell lung cancer and hepatitis B or hepatitis C infection. Lung Cancer 2020;145:181-5. 10.1016/j.lungcan.2020.02.013 [DOI] [PubMed] [Google Scholar]
- 31.Barlesi F, Dixmier A, Debieuvre D, et al. Effectiveness and safety of nivolumab in the treatment of lung cancer patients in France: preliminary results from the real-world EVIDENS study. Oncoimmunology 2020;9:1744898. 10.1080/2162402X.2020.1744898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amrane K, Geier M, Corre R, et al. First-line pembrolizumab for non-small cell lung cancer patients with PD-L1 ≥50% in a multicenter real-life cohort: The PEMBREIZH study. Cancer Med 2020;9:2309-16. 10.1002/cam4.2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as