Abstract
Background
Particulate matter (PM) is detrimental to the respiratory and circulatory systems. However, no study has evaluated the lag effects of weekly exposure to fine PM during the period from preconception to delivery on the risk of hypertensive disorders of pregnancy (HDPs).
Objective
We set out to investigate the lag effect windows of PM on the risk of HDPs on a weekly scale.
Methods
Data from women with de novo HDPs and normotensive pregnant women who were part of the Peking University Retrospective Birth Cohort, based on the hospital information system of Tongzhou district, were obtained for this study. Meteorological data and data on exposure to fine PM were predicted by satellite remote sensing data based on maternal residential address. The de novo HDP group consisted of pregnant women who were diagnosed with gestational hypertension or preeclampsia. Fine PM was defined as PM2.5 and PM1. The gestational stage of participants was from preconception (starting 12 weeks before gestation) to delivery (before the 42nd gestational week). A distributed-lag nonlinear model (DLNM) was nested in a Cox regression model to evaluate the lag effects of weekly PM exposure on de novo HDP hazard by controlling the nonlinear relationship of exposure–reaction. Stratified analyses by employment status (employed or unemployed), education level (higher or lower), and parity (primiparity or multiparity) were performed.
Results
A total of 22,570 pregnant women (mean age 29.1 years) for whom data were available between 2013 and 2017 were included in this study. The prevalence of de novo HDPs was 6.7% (1520/22,570). Our findings showed that PM1 and PM2.5 were significantly associated with an elevated hazard of HDPs. Exposure to PM1 during the 5th week before gestation to the 6th gestational week increased the hazard of HDPs. A significant lag effect of PM2.5 was observed from the 1st week before gestation to the 6th gestational week. The strongest lag effects of PM1 and PM2.5 on de novo HDPs were observed at week 2 and week 6 (hazard ratio [HR] 1.024, 95% CI 1.007-1.042; HR 1.007, 95% CI 1.000-1.015, respectively, per 10 μg/m3 increase). The stratified analyses indicated that pregnant women who were employed, had low education, and were primiparous were more vulnerable to PM exposure for de novo HDPs.
Conclusions
Exposure to PM1 and PM2.5 was associated with the risk of de novo HDPs. There were significant lag windows between the preconception period and the first trimester. Women who were employed, had low education, and were primiparous were more vulnerable to the effects of PM exposure; more attention should be paid to these groups for early prevention of de novo HDPs.
Keywords: air pollution, PM2.5, PM1, hypertensive disorders of pregnancy, preconceptional period, lag effect, pregnancy, hypertension, hypertensive disorders, risk, pollutants, exposure, maternal health, perinatal health, pollution
Introduction
A risk of multiple adverse health outcomes is attributable to increased exposure to the ambient particulate matter (PM). Fine PM is defined as ambient particles with an aerodynamic diameter ≤2.5 μm or ≤1 μm, that is, PM2.5 and PM1, respectively. PM is derived from a wide range of sources, such as forest fires, coal combustion, industrial processes, and traffic emissions [1]. It is composed of various toxic pollutants, including organic carbon, alkanes, metals, sulfates, and nitrates [2,3]. When people inhale the air, fine PM can easily translocate the abovementioned toxic pollutants across biological membranes from the pulmonary alveoli to the blood circulation, further resulting in systemic and tissue injury due to its physical characteristics of tiny size and large surface area [4]. According to the World Health Organization (WHO) air quality guidelines, about 92% of people in the world are exposed to excessive fine PM. Not long ago, the WHO updated the limit on exposure to fine PM, decreasing it from 10 μg/m3 to 5 μg/m3, based on research findings from the past 15 years. PM exposure is regarded as a risk factor for cardiovascular diseases and adverse perinatal outcomes [5,6]. An accumulating number of studies have used in vitro and in vivo experiments to show that fine PM can induce cardiovascular toxicity [7]. A recent global disease-burden study also indicated that fine PM was associated with risks of low birth weight, preterm birth, and neonatal and infant mortality, particularly in low- and middle-income countries [8].
Emerging evidence indicates a link between PM exposure and hypertensive disorders of pregnancy (HDPs), which are among the leading risk factors for maternal and fetal morbidity and mortality. Gestational hypertension (GH) and preeclampsia (PE) belong to the de novo type of HDP, which accounts for over 85% of HDP diagnoses [9]. HDPs can not only become complicated with proteinuria, edema, seizure, and liver and kidney injury, but also result in a series of adverse pregnancy outcomes, such as intrauterine growth restriction, preterm birth, stillbirth placental abruption, and postpartum hemorrhage [10,11]. In addition, HDPs have grown to become the second leading cause of maternal death in China. Therefore, HDPs are a significant public health problem in China in terms of maternal health [12]. While the etiology of HDPs is still elusive, in recent years an increasing number of researchers have focused on the effects of the environment on cardiovascular health. They have hypothesized that fine PM could result in HDPs via vascular constriction, inflammation, and oxidative stress. However, findings for an association between fine PM and HDPs are inconsistent. A recent Chinese study showed that fine PM exposure during the first trimester was associated with the risk of HDPs [13]. On the other hand, an American study indicated that fine PM exposure increased the development of HDPs during the second trimester rather than the first trimester [14]. This inconsistency may originate from differing study populations and exposure concentrations. Moreover, it has become imperative to draw attention to fine PM exposure before pregnancy. Based on the Developmental Origins of Health and Disease paradigm, the window of sensitivity to fine PM exposure could begin as far back as the preconception period [15]. There is an increasing consensus that preconception exposure to environmental contaminants can affect well-being in pregnancy. Several studies have indicated that air pollution exposure during the preconception period is associated with a risk of termination of pregnancy [16] and gestational diabetes mellitus [17]. The majority of previous studies only focused on the association between air pollution and maternal health in either specific trimesters or the total pregnancy [18,19]. The dynamic association between fine PM exposure and HDPs at different gestational weeks remains to be investigated in Chinese populations. To our knowledge, no study has assessed the association between weekly fine PM exposure, starting during the preconception period, and HDPs, especially de novo HDPs. If an association is shown, preconception precautions for fine PM exposure will become essential practice for lowering the risk of HDPs.
In this retrospective birth cohort study, we collected ambient data on PM exposure and applied a distributed-lag nonlinear model (DLNM) to examine the association between weekly PM exposure during the period from preconception to delivery and de novo HDPs. Additionally, we evaluated sensitivity lag windows for fine PM for HDPs across different sociodemographic strata.
Methods
Study Population
The study participants were pregnant women who were part of the Peking University Retrospective Birth Cohort for whom data were available in the hospital information system of Tongzhou for the period between January 2013 and December 2017. All the participants received antenatal examinations and gave birth at the Tongzhou Maternal and Child Health Care Hospital, which is the maternal health center for Tongzhou district, Beijing. The sociodemographic and health details of the participants were recorded at the antenatal examinations, including maternal age (<35 years vs ≥35 years), employment status (employed vs unemployed), parity (nulliparous vs multiparous), prepregnancy weight, height, maternal education level (high school or below, junior college, university or above), maternal ethnicity (Han vs non-Han), first day of last menstrual period, and delivery date. Prepregnancy BMI was calculated by dividing weight (in kilograms) by height (in meters squared) and categorized according to the WHO standards for Asian women: underweight (BMI <18.5 kg/m2), normal weight (BMI 18.5-23 kg/m2), overweight (BMI 23-27.5 kg/m2), or obese (BMI >27.5 kg/m2) [20]. The study data were extracted from the electronic medical records of Tongzhou Maternal and Child Health Care Hospital and were routinely reviewed by in-house professional data engineers every week.
The inclusion criteria of this study were (1) a single gestation, (2) an available residential address, (3) no history of previous HDPs, and (4) a delivery date before the 42nd gestational week. A total of 43,894 pregnant women met the inclusion criteria. We excluded 21,324 pregnant women who met the following exclusion criteria: (1) preexisting conditions, including metabolic syndrome; hemolysis, elevated liver enzymes, slow platelets; or hypertension before the HDP diagnosis; (2) missing data for delivery date; (3) an age at delivery less than 18 years; and (4) missing values for maternal age, ethnicity, education level, employment status, or prepregnancy BMI. Finally, 22,570 women were included in this study. The flowchart in Multimedia Appendix 1 shows the details of participant selection.
Outcome Measurements
The de novo HDP group was defined as patients who experienced GH or PE during the current pregnancy. The normotensive group was defined as those free of any diagnosis of GH or PE and without a history of hypertensive disorders. Information on the history of hypertensive disorders was obtained from initial antenatal examination records. GH or PE diagnoses were made by obstetricians in accord with the Chinese Clinical Practice Guidelines, which are consistent with the guidelines of developed countries [21]. International Classification of Diseases–10 (ICD-10) codes were used to define GH and PE (the ICD codes for GH and PE are described in our previous study [22]). Medical records containing information related to GH and PE were extracted for double confirmation of the diagnosis and to obtain the week of disease diagnosis.
Assessment of Ambient PM Exposure, Temperature, and Relative Humidity
The pregnant women’s residential addresses were collected at the initial antenatal examination. Each residential address was transformed into longitude and latitude values to determine the concentration of PM2.5 or PM1 in the center of the nearest 1 km × 1 km area on a grid. Daily PM concentrations were calculated at a spatial resolution of 1 km × 1 km in Beijing during the study period using satellite remote sensing, meteorology, and land-use information. Details of the calculations and validation of PM daily concentration measurements have been reported previously [23]. Data for daily temperature and relative humidity during the study period were obtained from the National Oceanic and Atmospheric Administration [24]. Average daily values for PM, temperature, and relative humidity from preconception (12 before gestation) to delivery (42 gestational weeks) were used to assess atmospheric exposure in our analysis.
Ethical Considerations
The study was approved by the Institutional Review Board of Peking University Health Science Center (IRB00001052-21023). All the study participants provided written consent. The study data were anonymized to protect the privacy of the study participants.
Statistical Analyses
The Student t test (2-tailed) and chi-square test were applied to assess differences in the distribution of characteristics between the subjects with HDPs and those who were normotensive. The relationships among PM2.5, PM1, temperature and relative humidity were assessed with the Pearson correlation test.
We followed the weekly exposure to PM1 and PM2.5 from the 12th week before gestation to the 42nd gestational week [25]. Cox proportional hazard models with a DLNM were used to estimate the association between the weekly PM2.5 and PM1 exposures and the hazard of de novo HDPs. The basis of the Cox proportional hazard model is the proportional hazards assumption, which means that all the study participants had the same hazard function. Our study complied with this assumption, which means that the ratio of the hazards for any 2 individuals was constant over time [26]. A DLNM was adopted to assess the effects of weekly PM exposures on hazards for HDPs by the “cross basic” function (cb) by constructing a 2D cross matrix that included exposure doses and time to control for the lag effect of PM exposure and the nonlinear relationship of exposure–reaction. A natural spline with 3 degrees of freedom was used to further accommodate the nonlinear effects of PM exposure, temperature, and relative humidity. The maximum number of lag weeks was set at 54 (from the 12th week before gestation to the 42nd gestational week). The lag effect of PM exposure was evaluated by the model as follows: ln(h(t,X)/h0(t)) = β1*Zt + β2*ns(temperature,3) + β3*ns(relative humidity,3) + β4*covariables. “ln(h(t,X)/h0(t))” indicates the hazard of de novo HDPs at the specific lag week (t), and the filed event is de novo HDP, noted as X. Zt represented the cross matrix of PM at specific lag weeks. β2 and β3 are the regression coefficients for temperature and relative humidity with 3 degrees of freedom in the natural spline function. β4 is the coefficient for the covariables, including maternal age, employment status, prepregnancy BMI, maternal education level, parity, maternal ethnicity, and conceptional year.
We also performed several sensitivity analyses to test the consistency of the main results. First, we further used natural splines with 4 and 5 degrees of freedom in the cross matrix to assess the hazards of de novo HDPs. Second, considering the clinical differences between GH and PE, we repeated our analyses excluding GH patients to explore the independent association between PM and PE. Meanwhile, stratified analyses were conducted across strata for differences in employment status, parity, and maternal education level.
All statistical analyses were performed with R (version 4.0.0; R Foundation for Statistical Computing). A 2-sided P value <.05 was considered a significant difference.
Results
Characteristics of the Participants
There were 1520 participants with de novo HDPs and 21,050 normotensive participants in this study. Table 1 shows the characteristics of the participants. The mean maternal age of the pregnancies was 29.1 (SD 4.1) years. There were 3802/22,570 (16.8%) unemployed women, and 21,222/22,570 (94%) women of Chinese Han ethnicity. A higher percentage of de novo HDPs was observed in the follow sociodemographic strata: low education level, primiparity, and prepregnancy overweight or obesity.
Table 1.
Characteristics of mothers in the birth cohorts.
| Characteristics | Total (n=22,570) | Normotensive (n=21,050) | Hypertensive disorders of pregnancy (n=1520) | P value | |||||
| Maternal age (years), mean (SD) | 29.1 (4.1) | 29.1 (4.1) | 29.4 (4.4) | .13 | |||||
| Maternal age groups (years), n (%) | <.001 | ||||||||
|
|
Younger than 35 | 20,126 (89.2) | 18,812 (89.4) | 1314 (86.4) |
|
||||
|
|
35 or older | 2444 (10.8) | 2238 (10.6) | 206 (13.6) |
|
||||
| Maternal ethnicity, n (%) | .80 | ||||||||
|
|
Han | 21,222 (94) | 19,790 (94) | 1432 (94.2) |
|
||||
|
|
Non-Han | 1348 (6) | 1260 (6) | 88 (5.8) |
|
||||
| Parity, n (%) | <.001 | ||||||||
|
|
Primiparous | 15,320 (67.9) | 14,222 (67.6) | 1098 (72.2) |
|
||||
|
|
Multiparous | 7250 (32.1) | 6828 (32.4) | 422 (27.8) |
|
||||
| Maternal education level, n (%) | <.001 | ||||||||
|
|
Low | 6890 (30.5) | 6357 (30.2) | 533 (35.1) |
|
||||
|
|
High | 15,680 (69.5) | 14,693 (68.8) | 987 (64.9) |
|
||||
| Employment status, n (%) | .80 | ||||||||
|
|
Employed | 18,768 (83.2) | 17,501 (83.1) | 1267 (83.4) |
|
||||
|
|
Unemployed | 3802 (16.8) | 3549 (16.9) | 253 (16.6) |
|
||||
| Prepregnancy BMI, n (%) | <.001 | ||||||||
|
|
Underweight | 2372 (10.5) | 2293 (10.9) | 79 (5.2) |
|
||||
|
|
Normal weight | 12,032 (53.3) | 11,455 (54.4) | 577 (38) |
|
||||
|
|
Overweight | 6490 (28.8) | 5901 (28) | 589 (38.8) |
|
||||
|
|
Obesity | 1676 (7.4) | 1401 (6.7) | 275 (18.1) |
|
||||
Characteristics of PM Exposure
The distribution of PM2.5, PM1, temperature, and relative humidity is summarized in Multimedia Appendix 2. The average concentration of PM2.5 was 74.2 (SD 53.2) μg/m3, ranging from 8.5 to 398 μg/m3. The concentration of PM1 ranged from 16.0 to 75.1 μg/m3. Mean temperature and relative humidity were 13.5 °C (SD 11.0 °C) and 54.7% (SD 18.2%). Figure 1 presents the daily fluctuation in PM2.5 and PM1 concentration from January 2013 to December 2017, revealing seasonal fluctuations in PM2.5 and PM1 exposure. Meanwhile, PM2.5 and PM1 were strongly correlated (r=0.80). The Pearson correlations among PM2.5, PM1, temperature, and relative humidity are depicted in Multimedia Appendix 3.
Figure 1.
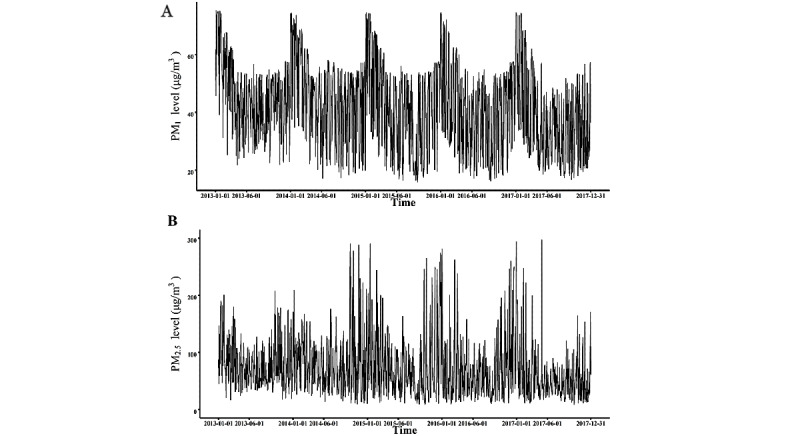
The level of particulate concentration by week. A and B show the daily level of PM1 and PM2.5, respectively, from 2013 to 2017. PM: particulate matter.
Association of Weekly Exposure to PM With HDPs
Figure 2 shows that a 10 μg/m3 increase in PM1 and PM2.5 was associated with de novo HDPs in 2D and 3D plots. The hazard of de novo HDPs was associated with PM1 exposure from the 5th week before gestation to the 6th gestational week, and the strongest effect of PM1 exposure was observed in the 2nd gestational week (HR 1.024, 95% CI 1.007-1.042; Figure 2A). Figure 2B depicts the effects of PM1 exposure on de novo HDPs with different exposure levels and different lag weeks, with a significant single peak by lag week from preconception to the first trimester (from lag 36 weeks to lag 47 weeks). For PM2.5, the significant lag window was from the 1st week before gestation to the 6th gestational week and the maximum lag effect of PM2.5 was found in the 6th gestational week (HR 1.007, 95% CI 1.000-1.015, Figure 2C). Similarly, we also observed a significant single peak from lag 36 weeks to lag 43 weeks when we assessed the association between PM2.5 exposure and de novo HDPs for different exposure doses and lag weeks (Figure 2D).
Figure 2.
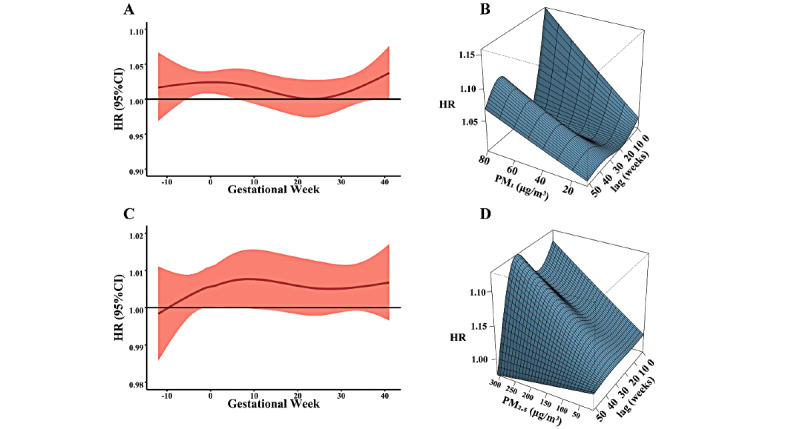
The lag effect of PM on hypertensive disorders of pregnancy in 2D and 3D plots. A and B show the association between PM1 and de novo hypertensive disorders of pregnancy in 2D and 3D plots, respectively. C and D show the association between PM2.5 and de novo hypertensive disorders of pregnancy in 2D and 3D plots, respectively. HR: hazard ratio; PM: particulate matter.
Sensitivity Analyses
We conducted two sensitivity analyses to assess the consistency of the sensitivity windows for PM1 and PM2.5 for de novo HDP hazard. First, using natural splines with 4 and 5 degrees of freedom for the lag constraint, we observed similar results, that is, the significant lag windows for PM1 and PM2.5 spanned the preconception period and the first trimester (Multimedia Appendix 4). Furthermore, we excluded patients with GH to explore the independent association between PM and PE. Multimedia Appendix 5 shows a result consistent with our primary analyses, that is, that the lag windows for PM1 and PM2.5 spanned the preconception period and the first trimester.
Stratified Analyses
In the stratified analyses, we estimated the effects of PM on de novo HDPs across differences in employment status, parity, and maternal education level. In terms of PM1, a significant sensitivity window for de novo HDPs was observed for employed, primiparous, and low educational status women (Figure 3). Similar findings are shown in Figure 4: women who were employed, primiparous, or had a low education level were more sensitive to PM2.5 exposure across the preconception period and the first trimester.
Figure 3.
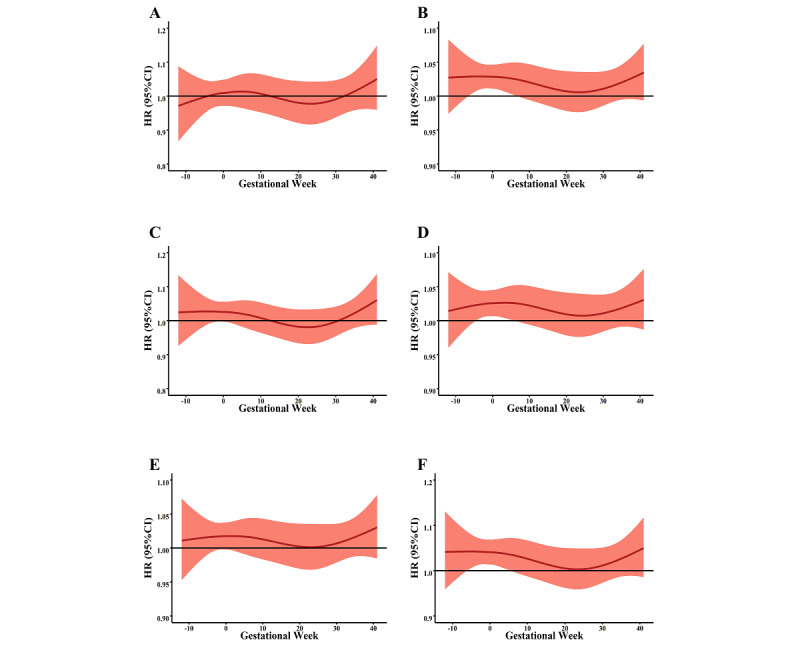
The stratified associations between PM2.5 and de novo hypertensive disorders of pregnancy for different maternal characteristics. A and B show the association in unemployed and employed women, respectively. C and D show the association in multiparous and primiparous women, respectively. E and F show the association in women with higher and lower education status, respectively. HR: hazard ratio; PM: particulate matter.
Figure 4.
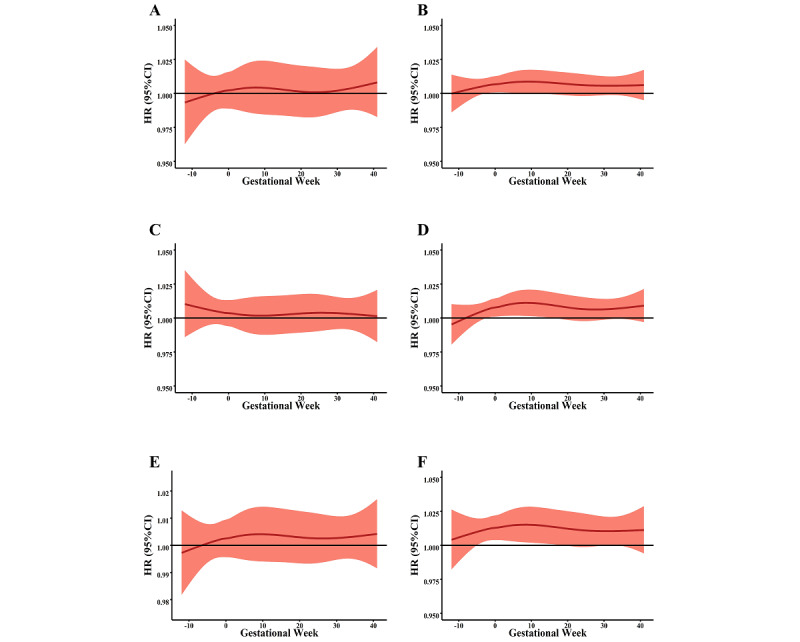
The stratified associations between PM1 exposure and de novo hypertensive disorders of pregnancy for different maternal characteristics. A and B show the association in unemployed and employed women, respectively. C and D show the association in multiparous and primiparous women, respectively. E and F show the association in women with higher and lower education status, respectively. HR: hazard ratio; PM: particulate matter.
Discussion
Principal Results
This study found that the significant lag windows for PM1 and PM2.5 were from the 5th week before gestation to the 6th gestational week and the 1st week before gestation to the 6th gestational week, respectively, in terms of the de novo HDP hazard. The findings remained robust in sensitivity analyses, which reexamined the associations using different degrees of freedom and after excluding patients with GH. Stratified analyses indicated that pregnant women who were employed, primiparous, or had a lower education level were more sensitive to PM1 and PM2.5 exposure in the prepregnancy period and the first trimester.
In recent years, numerous studies have evaluated the association between air pollution and cardiovascular health during pregnancy. However, there has been no study to assess the adverse effect of preconception air pollution on gestational health in terms of de novo HDPs. We adopted a time-serial study design to explore the weekly effects of PM1 and PM2.5 from the preconception period to delivery. To our knowledge, this is the first study to focus on the detrimental association between PM during both the preconception and gestational periods and de novo HDPs. During the study period, the average exposure to PM2.5 was 74.2 μg/m3, which is more than 2-fold higher than the recommended maximum level of 35 μg/m3 set by the National Ambient Air Quality Standards organization [27]. Moreover, exposure to PM showed seasonal fluctuations, peaking in winter and spring. Considering that our participants were mainly northern Chinese living in Beijing, the capital of China, the high concentration of PM can be attributed to central heating and a dense population.
In this study, the prevalence of de novo HDPs was 6.7% (1520/22,570). However, a lower prevalence (5%) of HDPs was reported in a recent study conducted in Shanghai [13]. In comparison with the Shanghai study, the pregnant subjects in our study were exposed to a higher concentration of PM pollution. Since both Beijing and Shanghai have populations of over 24 million and similar demographic compositions, the prevalence of HDPs is unlikely to have been influenced by differences in industrialization or urbanization between these two megacities. The difference in HDP prevalence might be partially explained by the higher PM exposure in the north due to the climate difference and use of central heating.
In our time-serial study, we observed that the women were sensitive to PM1 and PM2.5 during the preconception period and the first trimester. Our PM exposure measurements were at the week scale, which is more precise than previous studies that have used the trimester scale. Previous studies have made inconsistent findings on the association between fine PM and HDPs. Among 4 US studies, one in California [28] and one in Florida [14] observed that PM2.5 exposure was associated with HDPs during pregnancy. However, studies conducted in Rhode Island [29] and New York [30] failed to observe significant associations. There are some potential explanations for these inconsistent results. First, the latitudinal difference may have influenced the temperature and humidity in the different regions. A recent national cohort study indicated that temperature was positively associated with the risk of HDPs during pregnancy [31]. Second, exposure measurement bias may have resulted in a false-negative association. Third, ethnicity differences can affect the association between PM and HDPs. A national cross-sectional study from 2007 to 2018 showed that there was a racial difference in the prevalence of HDPs in the United States [32]. Our study participants mainly lived in Beijing, and 94% were members of the Chinese Han population. Meanwhile, we described our method for high-precision air pollution measurement in a previous study [23]. Our results are consistent with those of the Shanghai study, which had a similar ethnicity and urbanization profile. Thus, climate, measurement, and ethnicity differences were well controlled in our study.
Furthermore, we used a DLNM model, which allowed an examination of the lag effect of PM on HDPs at the week scale, rather than the previously used trimester scale, to improve the precision of the search for the most sensitive gestational week. We also considered potential bias in the model parameters by testing the consistency of our results with different degrees of freedom. Based on the robust results from our sensitivity analyses, we consider that bias from model parameters was not likely. Additionally, our second sensitivity analysis excluding the patients with GE further showed that exposure to fine PM during the preconception period and first trimester was independently associated with the risk of PE. PE is one of the leading causes of adverse pregnancy outcomes, accounting for 10% to 15% of maternal deaths and 15% to 20% of preterm births [33,34]. Early identification of PE risk in clinical practice remains a difficult problem [35]. Our environmental evidence for an association between fine PM exposure and PE might indicate the need for preconception PE prevention to consider environmental hazards.
Considering the strong link with maternal characteristics and gestational blood pressure [36], we further investigated the effects of PM1 and PM2.5 on HDPs across different maternal characteristics, including employment status, parity, and education level. Among employed women, exposure to PM was significantly associated with the risk of HDPs. PM exposure also elevated the risk of HDPs in women who were primiparous and had a low education level, which indicates that women with these maternal characteristics have a higher risk of HDPs with exposure to PM.
Strengths and Limitations
There are several strengths of this study. First, to our knowledge, this was the first to adopt the Cox proportion model with a DLNM to estimate the week-scale sensitivity window to PM for HDPs in a Chinese population. HDPs are a major cause of adverse maternal and prenatal outcomes [37]. However, the identification of risk factors for HDPs has been limited due to complex confounders. Previous studies primarily explored the association between PM and HDPs in a specific trimester [38,39]. Our study analyzed the specific gestational weeks and improved the precision of the sensitivity window. Second, this was the first study to cover the effect of air pollution, starting from the preconception period, on de novo HDPs. Our findings reconfirmed the necessity of focusing on air pollution before pregnancy in terms of de novo HDPs. Third, the large sample size of this study made it possible to determine the effects of PM on subtypes of HDP in stratified analyses.
Some limitations must be noted. First, all the participants were residents of Beijing. The lag windows for PM1 and PM2.5 should be tested in different regions to increase generalizability. Second, PM is a mixture of types of air pollution. The effects of specific PM components should be further explored in future studies.
Conclusions
Exposure to PM1 and PM2.5 was associated with the risk of de novo HDPs. The significant lag windows for PM exposure were between the preconception period and the first trimester. Women who were employed, had a lower education level, or were primiparous were more vulnerable to the effects of PM exposure on the risk of HDPs; more attention should be paid to these groups for the early prevention of de novo HDPs.
Acknowledgments
We sincerely thank the research group of the Peking University Retrospective Birth Cohort, in Tongzhou, for their help with the hospital information system. We appreciate the health professionals at Tongzhou Maternal and Child Health Care Hospital of Beijing for the data collection and management. This study was sponsored by the Beijing Natural Science Foundation (7212144) and National Natural Science Foundation of China (92046019 and 82204055).
Abbreviations
- DLNM
distributed lag nonlinear model
- GE
gestational hypertension
- HDP
hypertensive disorder of pregnancy
- HR
hazard ratio
- ICD-10
International Classification of Diseases–10
- PE
preeclampsia
- PM
particulate matter
- WHO
World Health Organization
Study flow chart.
Distribution of PM2.5, PM1, temperature and relative humidity.
The correlation between ambient exposure.
The association between PM and de novo HDP with 4 and 5 degrees of freedom for the lag constraint. HDP: hypertensive disorders of pregnancy; PM: particulate matter.
The association between PM1 and PM2.5, and preeclampsia.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Miller M, Newby David E. Air pollution and cardiovascular disease: car sick. Cardiovasc Res. 2020 Feb 01;116(2):279–294. doi: 10.1093/cvr/cvz228.5579822 [DOI] [PubMed] [Google Scholar]
- 2.Tian Y, Liu X, Huo R, Shi Z, Sun Y, Feng Y, Harrison RM. Organic compound source profiles of PM2.5 from traffic emissions, coal combustion, industrial processes and dust. Chemosphere. 2021 Sep;278:130429. doi: 10.1016/j.chemosphere.2021.130429.S0045-6535(21)00899-7 [DOI] [PubMed] [Google Scholar]
- 3.Curtis L. PM2.5, NO2, wildfires, and other environmental exposures are linked to higher Covid 19 incidence, severity, and death rates. Environ Sci Pollut Res Int. 2021 Oct;28(39):54429–54447. doi: 10.1007/s11356-021-15556-0.10.1007/s11356-021-15556-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang L, Chong WT, Wang X, Pei F, Zhang X, Wang T, Wang C, Pan S. Recent progress in research on PM2.5 in subways. Environ Sci Process Impacts. 2021 May 26;23(5):642–663. doi: 10.1039/d1em00002k. [DOI] [PubMed] [Google Scholar]
- 5.Lin C, Wan C, Liu W, Wang H. PM2.5 induces early epithelial mesenchymal transition in human proximal tubular epithelial cells through activation of IL-6/STAT3 pathway. Int J Mol Sci. 2021 Nov 25;22(23):12734. doi: 10.3390/ijms222312734. https://www.mdpi.com/resolver?pii=ijms222312734 .ijms222312734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michikawa T, Morokuma Seiichi, Takeda Yuki, Yamazaki Shin, Nakahara Kazushige, Takami Akinori, Yoshino Ayako, Sugata Seiji, Saito Shinji, Hoshi Junya, Kato Kiyoko, Nitta Hiroshi, Nishiwaki Yuji. Maternal exposure to fine particulate matter over the first trimester and umbilical cord insertion abnormalities. Int J Epidemiol. 2022 Feb 18;51(1):191–201. doi: 10.1093/ije/dyab192.6370659 [DOI] [PubMed] [Google Scholar]
- 7.Zhao T, Qi W, Yang P, Yang L, Shi Y, Zhou L, Ye L. Mechanisms of cardiovascular toxicity induced by PM2.5: a review. Environ Sci Pollut Res Int. 2021 Dec;28(46):65033–65051. doi: 10.1007/s11356-021-16735-9.10.1007/s11356-021-16735-9 [DOI] [PubMed] [Google Scholar]
- 8.Ghosh R, Causey K, Burkart K, Wozniak S, Cohen A, Brauer M. Ambient and household PM2.5 pollution and adverse perinatal outcomes: A meta-regression and analysis of attributable global burden for 204 countries and territories. PLoS Med. 2021 Sep;18(9):e1003718. doi: 10.1371/journal.pmed.1003718. https://dx.plos.org/10.1371/journal.pmed.1003718 .PMEDICINE-D-21-00737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garovic VD, White WM, Vaughan L, Saiki M, Parashuram S, Garcia-Valencia O, Weissgerber TL, Milic N, Weaver A, Mielke MM. Incidence and long-term outcomes of hypertensive disorders of pregnancy. J Am Coll Cardiol. 2020 May 12;75(18):2323–2334. doi: 10.1016/j.jacc.2020.03.028. https://linkinghub.elsevier.com/retrieve/pii/S0735-1097(20)34633-7 .S0735-1097(20)34633-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah NS, Harrington KA, Huang X, Cameron NA, Yee LM, Khan SS. Trends in de novo hypertensive disorders of pregnancy among Asian and Hispanic population subgroups in the United States, 2011 to 2019. JAMA Cardiol. 2022 Jul 01;7(7):742–746. doi: 10.1001/jamacardio.2022.1378.2793313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garovic VD, Dechend R, Easterling T, Karumanchi SA, McMurtry Baird S, Magee LA, Rana S, Vermunt JV, August P, American Heart Association Council on Hypertension; Council on the Kidney in Cardiovascular Disease‚ Kidney in Heart Disease Science Committee; Council on Arteriosclerosis‚ ThrombosisVascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Peripheral Vascular Disease;Stroke Council Hypertension in pregnancy: diagnosis, blood pressure goals, and pharmacotherapy: a scientific statement from the American Heart Association. Hypertension. 2022 Feb;79(2):e21–e41. doi: 10.1161/HYP.0000000000000208. https://www.ahajournals.org/doi/abs/10.1161/HYP.0000000000000208?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng X, Zhu J, Zhang L, Song L, Hipgrave D, Guo S, Ronsmans C, Guo Y, Yang Q. Socio-economic disparities in maternal mortality in China between 1996 and 2006. BJOG. 2010 Nov;117(12):1527–36. doi: 10.1111/j.1471-0528.2010.02707.x. [DOI] [PubMed] [Google Scholar]
- 13.Su X, Zhao Y, Yang Y, Hua J. Correlation between exposure to fine particulate matter and hypertensive disorders of pregnancy in Shanghai, China. Environ Health. 2020 Sep 17;19(1):101. doi: 10.1186/s12940-020-00655-1. https://ehjournal.biomedcentral.com/articles/10.1186/s12940-020-00655-1 .10.1186/s12940-020-00655-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X, Hu H, Ha S, Roth J. Ambient air pollution and hypertensive disorder of pregnancy. J Epidemiol Community Health. 2014 Jan;68(1):13–20. doi: 10.1136/jech-2013-202902. https://europepmc.org/abstract/MED/24022815 .jech-2013-202902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grandjean P, Barouki Robert, Bellinger David C, Casteleyn Ludwine, Chadwick Lisa H, Cordier Sylvaine, Etzel Ruth A, Gray Kimberly A, Ha Eun-Hee, Junien Claudine, Karagas Margaret, Kawamoto Toshihiro, Paige Lawrence B, Perera Frederica P, Prins Gail S, Puga Alvaro, Rosenfeld Cheryl S, Sherr David H, Sly Peter D, Suk William, Sun Qi, Toppari Jorma, van den Hazel Peter, Walker Cheryl L, Heindel Jerrold J. Life-long implications of developmental exposure to environmental stressors: new perspectives. Endocrinology. 2015 Oct;156(10):3408–15. doi: 10.1210/EN.2015-1350. https://europepmc.org/abstract/MED/26241067 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun S, Wang X, Ding L, Zhang Q, Li N, Sui X, Li C, Ju L, Zhao Q, Chen H, Ding R, Cao J. Association between preconceptional air pollution exposure and medical purposes for selective termination of pregnancy. Environ Res. 2021 Nov;202:111743. doi: 10.1016/j.envres.2021.111743.S0013-9351(21)01037-9 [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, Wang X, Yang X, Dong T, Hu W, Guan Q, Tun HM, Chen Y, Chen R, Sun Z, Chen T, Xia Y. Increased risk of gestational diabetes mellitus in women with higher prepregnancy ambient PM2.5 exposure. Sci Total Environ. 2020 Aug 15;730:138982. doi: 10.1016/j.scitotenv.2020.138982.S0048-9697(20)32499-2 [DOI] [PubMed] [Google Scholar]
- 18.Leung M, Weisskopf MG, Laden F, Coull BA, Modest AM, Hacker MR, Wylie BJ, Wei Y, Schwartz J, Papatheodorou S. Exposure to PM2.5 during pregnancy and fetal growth in Eastern Massachusetts, USA. Environ Health Perspect. 2022 Jan;130(1):17004. doi: 10.1289/EHP9824. https://ehp.niehs.nih.gov/doi/10.1289/EHP9824?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang K, Tian Y, Zheng H, Shan S, Zhao X, Liu C. Maternal exposure to ambient fine particulate matter and risk of premature rupture of membranes in Wuhan, Central China: a cohort study. Environ Health. 2019 Nov 14;18(1):96. doi: 10.1186/s12940-019-0534-y. https://ehjournal.biomedcentral.com/articles/10.1186/s12940-019-0534-y .10.1186/s12940-019-0534-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004 Jan 10;363(9403):157–63. doi: 10.1016/S0140-6736(03)15268-3.S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 21.Hypertensive Disorders in Pregnancy Subgroup‚ Chinese Society of Obstetrics and Gynecology‚ Chinese Medical Association [Diagnosis and treatment of hypertension and pre-eclampsia in pregnancy: a clinical practice guideline in China(2020)] Zhonghua Fu Chan Ke Za Zhi. 2020 Apr 25;55(4):227–238. doi: 10.3760/cma.j.cn112141-20200114-00039. [DOI] [PubMed] [Google Scholar]
- 22.Yuan Z, Wang H, Su T, Yang J, Chen J, Peng Y, Zhou S, Bao H, Luo S, Wang H, Liu J, Han N, Ji Y. The first-trimester gestational weight gain associated with de novo hypertensive disorders during pregnancy: mediated by mean arterial pressure. Front Nutr. 2022;9:862323. doi: 10.3389/fnut.2022.862323. https://europepmc.org/abstract/MED/35495902 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou S, Lin L, Bao Z, Meng T, Wang S, Chen G, Li Q, Liu Z, Bao H, Han N, Wang H, Guo Y. The association of prenatal exposure to particulate matter with infant growth: A birth cohort study in Beijing, China. Environ Pollut. 2021 May 15;277:116792. doi: 10.1016/j.envpol.2021.116792.S0269-7491(21)00372-9 [DOI] [PubMed] [Google Scholar]
- 24.Climate Data Online. National Oceanic and Atmospheric Administration. [2023-01-11]. https://www.ncdc.noaa.gov/cdo-web/
- 25.Lawn JE, Blencowe H, Waiswa P, Amouzou A, Mathers C, Hogan D, Flenady V, Frøen J Frederik, Qureshi ZU, Calderwood C, Shiekh S, Jassir FB, You D, McClure EM, Mathai M, Cousens S, Lancet Ending Preventable Stillbirths Series study group. Lancet Stillbirth Epidemiology investigator group Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. 2016 Feb 06;387(10018):587–603. doi: 10.1016/S0140-6736(15)00837-5.S0140-6736(15)00837-5 [DOI] [PubMed] [Google Scholar]
- 26.Moolgavkar SH, Chang ET, Watson HN, Lau EC. An assessment of the Cox proportional hazards regression model for epidemiologic studies. Risk Anal. 2018 Apr;38(4):777–794. doi: 10.1111/risa.12865. [DOI] [PubMed] [Google Scholar]
- 27.Ling H, Qing L, Jian X, Lishu S, Liang L, Qian W, Yangjun W, Chaojun G, Hong Z, Qiang Y, Sen Z, Guozhu Z, Li L. Strategies towards PM2.5 attainment for non-compliant cities in China: A case study. J Environ Manage. 2021 Nov 15;298:113529. doi: 10.1016/j.jenvman.2021.113529.S0301-4797(21)01591-7 [DOI] [PubMed] [Google Scholar]
- 28.Mobasher Z, Salam MT, Goodwin T, Lurmann F, Ingles SA, Wilson ML. Associations between ambient air pollution and hypertensive disorders of pregnancy. Environ Res. 2013 May;123:9–16. doi: 10.1016/j.envres.2013.01.006. https://europepmc.org/abstract/MED/23522615 .S0013-9351(13)00010-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choe S, Kauderer S, Eliot MN, Glazer KB, Kingsley SL, Carlson L, Awad YA, Schwartz JD, Savitz DA, Wellenius GA. Air pollution, land use, and complications of pregnancy. Sci Total Environ. 2018 Dec 15;645:1057–1064. doi: 10.1016/j.scitotenv.2018.07.237.S0048-9697(18)32734-7 [DOI] [PubMed] [Google Scholar]
- 30.Savitz DA, Elston B, Bobb JF, Clougherty JE, Dominici F, Ito K, Johnson S, McAlexander T, Ross Z, Shmool JLC, Matte TD, Wellenius GA. Ambient fine particulate matter, nitrogen dioxide, and hypertensive disorders of pregnancy in New York City. Epidemiology. 2015 Sep;26(5):748–57. doi: 10.1097/EDE.0000000000000349. https://europepmc.org/abstract/MED/26237745 .00001648-201509000-00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong T, Chen P, Mu Y, Li X, Di B, Li J, Qu Y, Tang J, Liang J, Mu D. Association between ambient temperature and hypertensive disorders in pregnancy in China. Nat Commun. 2020 Jun 10;11(1):2925. doi: 10.1038/s41467-020-16775-8. doi: 10.1038/s41467-020-16775-8.10.1038/s41467-020-16775-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bornstein E, Eliner Y, Chervenak FA, Grünebaum Amos. Racial disparity in pregnancy risks and complications in the US: temporal changes during 2007-2018. J Clin Med. 2020 May 10;9(5):1414. doi: 10.3390/jcm9051414. https://www.mdpi.com/resolver?pii=jcm9051414 .jcm9051414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009 Jun;33(3):130–7. doi: 10.1053/j.semperi.2009.02.010.S0146-0005(09)00021-4 [DOI] [PubMed] [Google Scholar]
- 34.Jeyabalan A. Epidemiology of preeclampsia: impact of obesity. Nutr Rev. 2013 Oct;71 Suppl 1(0 1):S18–25. doi: 10.1111/nure.12055. https://europepmc.org/abstract/MED/24147919 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marić I, Tsur A, Aghaeepour N, Montanari A, Stevenson DK, Shaw GM, Winn VD. Early prediction of preeclampsia via machine learning. Am J Obstet Gynecol MFM. 2020 May;2(2):100100. doi: 10.1016/j.ajogmf.2020.100100.S2589-9333(20)30030-6 [DOI] [PubMed] [Google Scholar]
- 36.Jwa SC, Fujiwara T, Hata A, Arata N, Sago H, Ohya Y. BMI mediates the association between low educational level and higher blood pressure during pregnancy in Japan. BMC Public Health. 2013 Apr 25;13(1):389. doi: 10.1186/1471-2458-13-389. https://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-13-389 .1471-2458-13-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinkey RG, Battarbee AN, Bello NA, Ives CW, Oparil S, Tita ATN. Prevention, diagnosis, and management of hypertensive disorders of pregnancy: a comparison of international guidelines. Curr Hypertens Rep. 2020 Aug 27;22(9):66. doi: 10.1007/s11906-020-01082-w. https://europepmc.org/abstract/MED/32852691 .10.1007/s11906-020-01082-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun M, Yan W, Fang K, Chen D, Liu J, Chen Y, Duan J, Chen R, Sun Z, Wang X, Xia Y. The correlation between PM2.5 exposure and hypertensive disorders in pregnancy: A meta-analysis. Sci Total Environ. 2020 Feb 10;703:134985. doi: 10.1016/j.scitotenv.2019.134985.S0048-9697(19)34977-0 [DOI] [PubMed] [Google Scholar]
- 39.Bai W, Li Y, Niu Y, Ding Y, Yu X, Zhu B, Duan R, Duan H, Kou C, Li Y, Sun Z. Association between ambient air pollution and pregnancy complications: A systematic review and meta-analysis of cohort studies. Environ Res. 2020 Jun;185:109471. doi: 10.1016/j.envres.2020.109471.S0013-9351(20)30364-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study flow chart.
Distribution of PM2.5, PM1, temperature and relative humidity.
The correlation between ambient exposure.
The association between PM and de novo HDP with 4 and 5 degrees of freedom for the lag constraint. HDP: hypertensive disorders of pregnancy; PM: particulate matter.
The association between PM1 and PM2.5, and preeclampsia.


